Electron-Induced Decomposition of 5-Bromo-4-thiouracil and 5-Bromo-4-thio-2′-deoxyuridine: The Effect of the Deoxyribose Moiety on Dissociative Electron Attachment
Abstract
1. Introduction
2. Results and Discussion
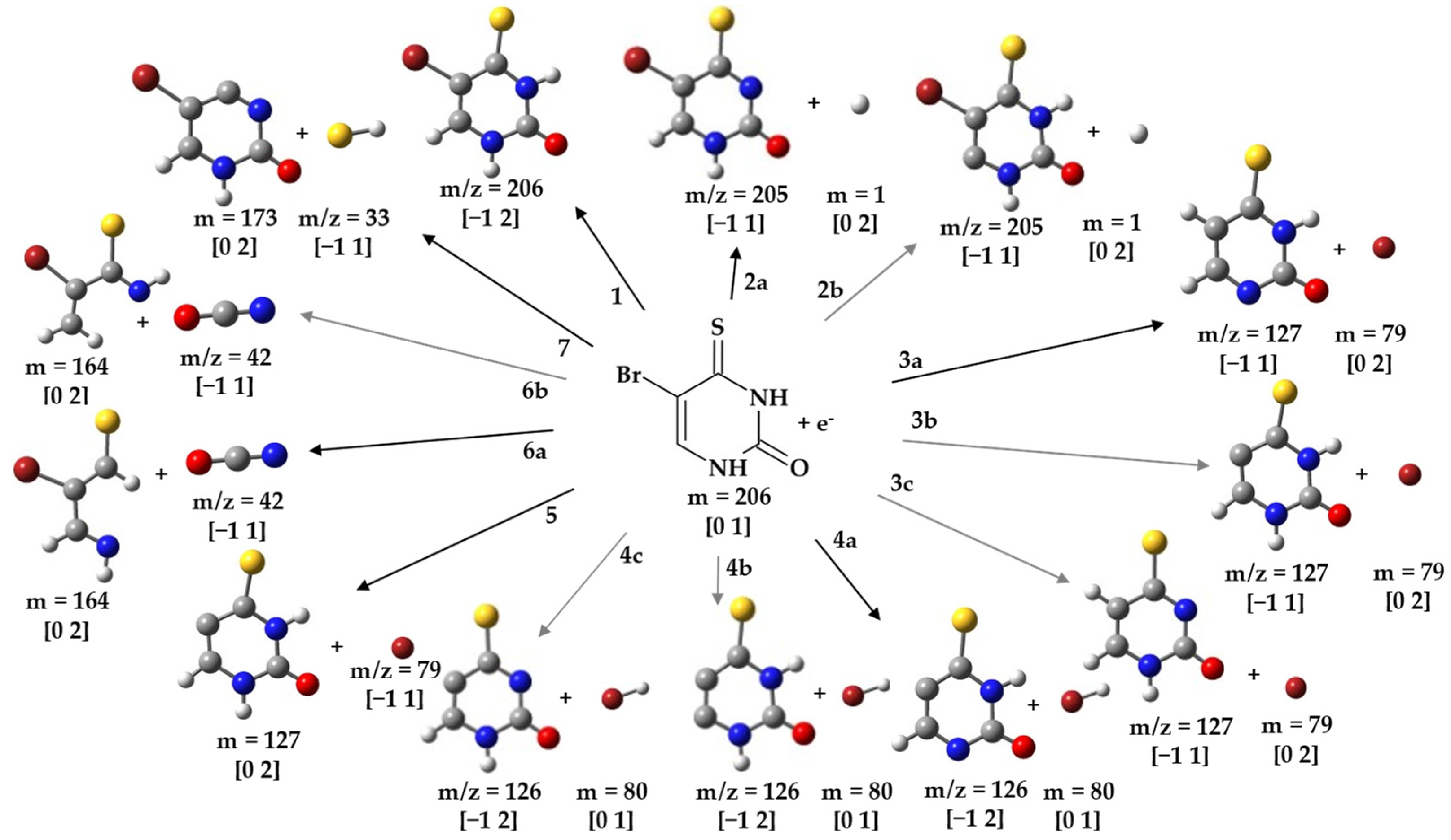
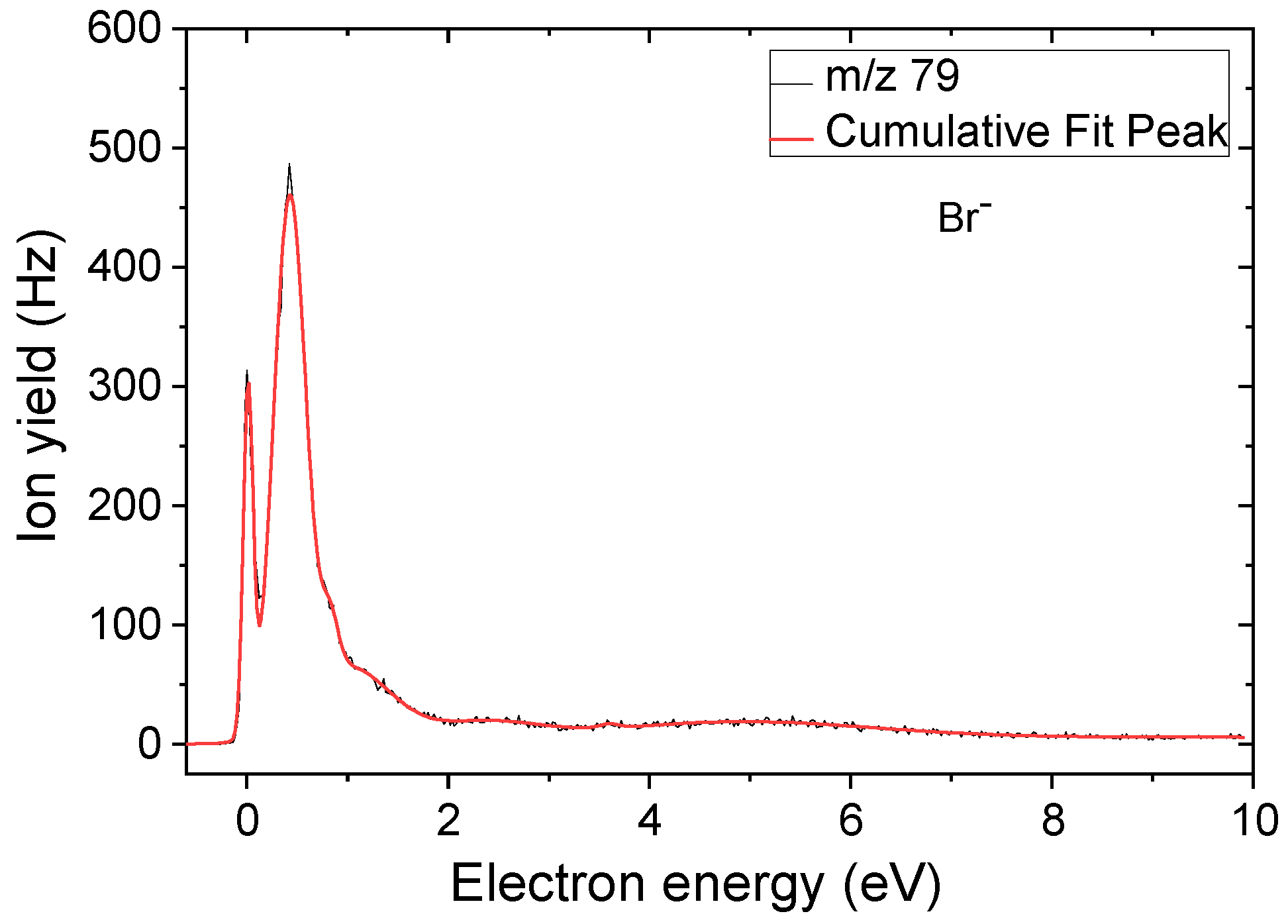
2.1. Formation of BrSU− Anions and the Dehydrogenated (BrSU-H)− Anions—Cleavage of the Br-C5 Bond
2.2. Light Anions Formed upon DEA to BrSU
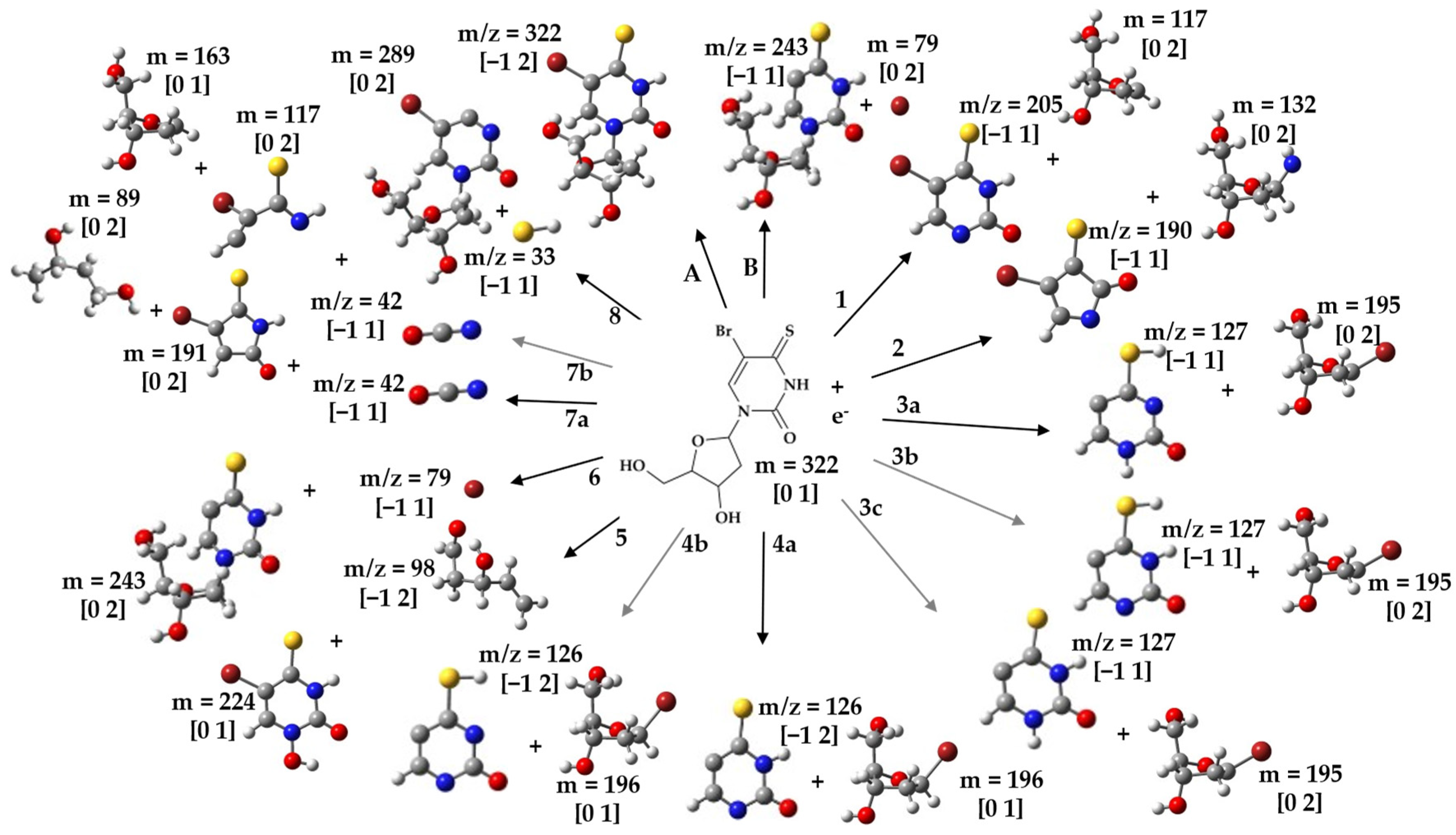
2.3. Possible Dissociation Channels of BrSdU
3. Materials and Methods
3.1. Experiments
3.2. Quantum Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oronsky, B.T.; Knox, S.J.; Scicinski, J. Six Degrees of Separation: The Oxygen Effect in the Development of Radiosensitizers. Transl. Oncol. 2011, 4, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.M.; Wilson, W.R. Exploiting Tumour Hypoxia in Cancer Treatment. Nat. Rev. Cancer 2004, 4, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Mayer, A. Hypoxia in Cancer: Significance and Impact on Clinical Outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Rami, M.; Dubois, L.; Parvathaneni, N.-K.; Alterio, V.; van Kuijk, S.J.A.; Monti, S.M.; Lambin, P.; De Simone, G.; Supuran, C.T.; Winum, J.-Y. Hypoxia-Targeting Carbonic Anhydrase IX Inhibitors by a New Series of Nitroimidazole-Sulfonamides/Sulfamides/Sulfamates. J. Med. Chem. 2013, 56, 8512–8520. [Google Scholar] [CrossRef]
- Pimblott, S.M.; LaVerne, J.A. Production of Low-Energy Electrons by Ionizing Radiation. Radiat. Phys. Chem. 2007, 76, 1244–1247. [Google Scholar] [CrossRef]
- Meißner, R.; Kočišek, J.; Feketeová, L.; Fedor, J.; Fárník, M.; Limão-Vieira, P.; Illenberger, E.; Denifl, S. Low-Energy Electrons Transform the Nimorazole Molecule into a Radiosensitiser. Nat. Commun. 2019, 10, 2388. [Google Scholar] [CrossRef]
- Arthur-Baidoo, E.; Ameixa, J.; Ziegler, P.; Ferreira da Silva, F.; Ončák, M.; Denifl, S. Reactions in Tirapazamine Induced by the Attachment of Low-Energy Electrons: Dissociation Versus Roaming of OH. Angew. Chem.-Int. Ed. 2020, 59, 17177–17181. [Google Scholar] [CrossRef] [PubMed]
- Arthur-Baidoo, E.; Izadi, F.; Guerra, C.; Garcia, G.; Ončák, M.; Denifl, S. Dynamics of Ring-Cleavage Reactions in Temozolomide Induced by Low-Energy Electron Attachment. Front. Phys. 2022, 10, 880689. [Google Scholar] [CrossRef]
- Schürmann, R.; Tsering, T.; Tanzer, K.; Denifl, S.; Kumar, S.V.K.; Bald, I. Resonant Formation of Strand Breaks in Sensitized Oligonucleotides Induced by Low-Energy Electrons (0.5–9 eV). Angew. Chem.-Int. Ed. Engl. 2017, 56, 10952–10955. [Google Scholar] [CrossRef]
- Westphal, K.; Skotnicki, K.; Bobrowski, K.; Rak, J. Radiation Damage to Single Stranded Oligonucleotide Trimers Labelled with 5-Iodopyrimidines. Org. Biomol. Chem. 2016, 14, 9331–9337. [Google Scholar] [CrossRef]
- Westphal, K.; Wiczk, J.; Miloch, J.; Kciuk, G.; Bobrowski, K.; Rak, J. Irreversible Electron Attachment—A Key to DNA Damage by Solvated Electrons in Aqueous Solution. Org. Biomol. Chem. 2015, 13, 10362–10369. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, S.; Dobrucki, I.T.; Kim, E.Y.; Marrison, S.T.; Vu, V.T. Hypoxia and Radiation Therapy: Past History, Ongoing Research, and Future Promise. Curr. Mol. Med. 2009, 9, 442–458. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Mu, X.; He, H.; Zhang, X.-D. Cancer Radiosensitizers. Trends Pharmacol. Sci. 2018, 39, 24–48. [Google Scholar] [CrossRef]
- Am, B.; Lynam-Lennon, N.; O’Neill, H.; O’Sullivan, J. Targeting Hallmarks of Cancer to Enhance Radiosensitivity in Gastrointestinal Cancers. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 298–313. [Google Scholar] [CrossRef]
- Greer, S.; Alvarez, M.; Mas, M.; Wozniak, C.; Arnold, D.; Knapinska, A.; Norris, C.; Burk, R.; Aller, A.; Dauphinée, M. Five-Chlorodeoxycytidine, a Tumor-Selective Enzyme-Driven Radiosensitizer, Effectively Controls Five Advanced Human Tumors in Nude Mice. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 791–806. [Google Scholar] [CrossRef]
- Rak, J.; Chomicz, L.; Wiczk, J.; Westphal, K.; Zdrowowicz, M.; Wityk, P.; Żyndul, M.; Makurat, S.; Golon, Ł. Mechanisms of Damage to DNA Labeled with Electrophilic Nucleobases Induced by Ionizing or UV Radiation. J. Phys. Chem. B 2015, 119, 8227–8238. [Google Scholar] [CrossRef]
- Kopyra, J.; Keller, A.; Bald, I. On the Role of Fluoro-Substituted Nucleosides in DNA Radiosensitization for Tumor Radiation Therapy. RSC Adv. 2014, 4, 6825–6829. [Google Scholar] [CrossRef]
- Cecchini, S.; Masson, C.; La Madeleine, C.; Huels, M.A.; Sanche, L.; Wagner, J.R.; Hunting, D.J. Interstrand Cross-Link Induction by UV Radiation in Bromodeoxyuridine-Substituted DNA: Dependence on DNA Conformation. Biochemistry 2005, 44, 16957–16966. [Google Scholar] [CrossRef]
- Cooperrider, J.; Chan, H.H.; Gale, J.T.; Park, H.-J.; Baker, K.B.; Machado, A.G. BrdU-Induced Hyperlocomotion in the Stroked Rat. Neurosci. Lett. 2019, 703, 96–98. [Google Scholar] [CrossRef]
- Bald, I.; Langer, J.; Tegeder, P.; Ingólfsson, O. From Isolated Molecules through Clusters and Condensates to the Building Blocks of Life. Int. J. Mass Spectrom. 2008, 277, 4–25. [Google Scholar] [CrossRef]
- Gorfinkiel, J.D.; Ptasinska, S. Electron Scattering from Molecules and Molecular Aggregates of Biological Relevance. J. Phys. B At. Mol. Opt. Phys. 2017, 50, 182001. [Google Scholar] [CrossRef]
- Ameixa, J.; Arthur-Baidoo, E.; Meißner, R.; Makurat, S.; Kozak, W.; Butowska, K.; Ferreira da Silva, F.; Rak, J.; Denifl, S. Low-Energy Electron-Induced Decomposition of 5-Trifluoromethanesulfonyl-Uracil: A Potential Radiosensitizer. J. Chem. Phys. 2018, 149, 164307. [Google Scholar] [CrossRef]
- Spisz, P.; Zdrowowicz, M.; Kozak, W.; Chomicz-Mańka, L.; Falkiewicz, K.; Makurat, S.; Sikorski, A.; Wyrzykowski, D.; Rak, J.; Arthur-Baidoo, E.; et al. Uracil-5-yl O-Sulfamate: An Illusive Radiosensitizer. Pitfalls in Modeling the Radiosensitizing Derivatives of Nucleobases. J. Phys. Chem. B 2020, 124, 5600–5613. [Google Scholar] [CrossRef] [PubMed]
- Meißner, R.; Makurat, S.; Kozak, W.; Limão-Vieira, P.; Rak, J.; Denifl, S. Electron-Induced Dissociation of the Potential Radiosensitizer 5-Selenocyanato-2’-Deoxyuridine. J. Phys. Chem. B 2019, 123, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Denifl, S.; Matejcik, S.; Gstir, B.; Hanel, G.; Probst, M.; Scheier, P.; Märk, T.D. Electron Attachment to 5-Chloro Uracil. J. Chem. Phys. 2003, 118, 4107–4114. [Google Scholar] [CrossRef]
- Denifl, S.; Matejcik, S.; Ptasinska, S.; Gstir, B.; Probst, M.; Scheier, P.; Illenberger, E.; Märk, T.D. Electron Attachment to Chlorouracil: A Comparison between 6-ClU and 5-ClU. J. Chem. Phys. 2004, 120, 704–709. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Huels, M.A.; Illenberger, E.; Sanche, L. Formation of Negative Ions from Gas Phase Halo-Uracils by Low-Energy (0–18 eV) Electron Impact. Int. J. Mass Spectrom. 2003, 228, 703–716. [Google Scholar] [CrossRef]
- Kossoski, F.; Bettega, M.H.F.; Varella, M.T.d.N. Shape Resonance Spectra of Uracil, 5-Fluorouracil, and 5-Chlorouracil. J. Chem. Phys. 2014, 140, 024317. [Google Scholar] [CrossRef]
- Denifl, S.; Candori, P.; Ptasińska, S.; Limão-Vieira, P.; Grill, V.; Märk, T.D.; Scheier, P. Positive and Negative Ion Formation via Slow Electron Collisions with 5-Bromouridine. Eur. Phys. J. D 2005, 35, 391–398. [Google Scholar] [CrossRef]
- Spisz, P.; Zdrowowicz, M.; Makurat, S.; Kozak, W.; Skotnicki, K.; Bobrowski, K.; Rak, J. Why Does the Type of Halogen Atom Matter for the Radiosensitizing Properties of 5-Halogen Substituted 4-Thio-2′-Deoxyuridines? Molecules 2019, 24, 2819. [Google Scholar] [CrossRef]
- Chomicz-Mańka, L.; Czaja, A.; Falkiewicz, K.; Zdrowowicz, M.; Biernacki, K.; Demkowicz, S.; Izadi, F.; Arthur-Baidoo, E.; Denifl, S.; Zhu, Z.; et al. Intramolecular Proton Transfer in the Radical Anion of Cytidine Monophosphate Sheds Light on the Sensitivities of Dry vs Wet DNA to Electron Attachment-Induced Damage. J. Am. Chem. Soc. 2023, 145, 9059–9071. [Google Scholar] [CrossRef] [PubMed]
- Kopyra, J.; Abdoul-Carime, H. Unusual Temperature Dependence of the Dissociative Electron Attachment Cross Section of 2-Thiouracil. J. Chem. Phys. 2016, 144, 034306. [Google Scholar] [CrossRef]
- Rosen, B.M.; Quasdorf, K.W.; Wilson, D.A.; Zhang, N.; Resmerita, A.-M.; Garg, N.K.; Percec, V. Nickel-Catalyzed Cross-Couplings Involving Carbon-Oxygen Bonds. Chem. Rev. 2011, 111, 1346–1416. [Google Scholar] [CrossRef] [PubMed]
- Kopyra, J.; Abdoul-Carime, H.; Kossoski, F.; Varella, M.T.d.N. Electron Driven Reactions in Sulphur Containing Analogues of Uracil: The Case of 2-Thiouracil. Phys. Chem. Chem. Phys. 2014, 16, 25054–25061. [Google Scholar] [CrossRef] [PubMed]
- Desfrançois, C.; Abdoul-Carime, H.; Khelifa, N.; Schermann, J.P. From 1/r to 1/r2 Potentials: Electron Exchange between Rydberg Atoms and Polar Molecules. Phys. Rev. Lett. 1994, 73, 2436–2439. [Google Scholar] [CrossRef] [PubMed]
- Lévy-Leblond, J.-M. Electron Capture by Polar Molecules. Phys. Rev. 1967, 153, 1–4. [Google Scholar] [CrossRef]
- Garrett, W.R. Thermally Stable Negative Ions of Polar Molecules. J. Chem. Phys. 1978, 69, 2621–2626. [Google Scholar] [CrossRef]
- Scheer, A.M.; Aflatooni, K.; Gallup, G.A.; Burrow, P.D. Bond Breaking and Temporary Anion States in Uracil and Halouracils: Implications for the DNA Bases. Phys. Rev. Lett. 2004, 92, 068102. [Google Scholar] [CrossRef]
- Sommerfeld, T. Dipole-Bound States as Doorways in (Dissociative) Electron Attachment. J. Phys. Conf. Ser. 2005, 4, 245. [Google Scholar] [CrossRef]
- Sommerfeld, T. Intramolecular Electron Transfer from Dipole-Bound to Valence Orbitals: Uracil and 5-Chlorouracil. J. Phys. Chem. A 2004, 108, 9150–9154. [Google Scholar] [CrossRef]
- Compton, R.N.; Carman, H.S.; Desfrançois, C.; Abdoul-Carime, H.; Schermann, J.P.; Hendricks, J.H.; Lyapustina, S.A.; Bowen, K.H. On the Binding of Electrons to Nitromethane: Dipole and Valence Bound Anions. J. Chem. Phys. 1996, 105, 3472–3478. [Google Scholar] [CrossRef]
- Abdoul-Carime, H.; Limão-Vieira, P.; Gohlke, S.; Petrushko, I.; Mason, N.J.; Illenberger, E. Sensitization of 5-Bromouridine by Slow Electrons. Chem. Phys. Lett. 2004, 393, 442–447. [Google Scholar] [CrossRef]
- Ptasinska, S.; Denifl, S.; Scheier, P.; Illenberger, E.; Märk, T.D. Bond- and Site-Selective Loss of H Atoms from Nucleobases by Very-Low-Energy Electrons (<3 eV). Angew. Chem.-Int. Ed. 2005, 44, 6941. [Google Scholar]
- Abdoul-Carime, H.; Gohlke, S.; Illenberger, E. Site-Specific Dissociation of DNA Bases by Slow Electrons at Early Stages of Irradiation. Phys. Rev. Lett. 2004, 92, 168103. [Google Scholar] [CrossRef] [PubMed]
- Abdoul-Carime, H.; Huels, M.A.; Brüning, F.; Illenberger, E.; Sanche, L. Dissociative Electron Attachment to Gas-Phase 5-Bromouracil. J. Chem. Phys. 2000, 113, 2517–2521. [Google Scholar] [CrossRef]
- Shchukin, P.V.; Muftakhov, M.V.; Khatymov, R.V.; Tuktarov, R.F. Resonant Electron Capture by 5-Br-2′-Deoxyuridine. J. Chem. Phys. 2022, 156, 104304. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.E.; Dutta, A.K.; Izsák, R.; Pantazis, D.A. Systematic High-Accuracy Prediction of Electron Affinities for Biological Quinones. J. Comput. Chem. 2018, 39, 2439–2451. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Account. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Epifanovsky, E.; Kowalski, K.; Fan, P.-D.; Valiev, M.; Matsika, S.; Krylov, A.I. On the Electronically Excited States of Uracil. J. Phys. Chem. A 2008, 112, 9983–9992. [Google Scholar] [CrossRef]
- Illenberger, E.; Momigny, J. Gaseous Molecular Ions: An Introduction to Elementary Processes Induced by Ionization; Topics in physical chemistry; Steinkopff Verlag; Springer: Darmstadt, Germany; New York, NY, USA, 1992; ISBN 978-3-7985-0870-5. [Google Scholar]
- Gallup, G.A.; Fabrikant, I.I. Vibrational Feshbach Resonances in Dissociative Electron Attachment to Uracil. Phys. Rev. A 2011, 83, 012706. [Google Scholar] [CrossRef]
- Izadi, F.; Arthur-Baidoo, E.; Strover, L.T.; Yu, L.-J.; Coote, M.L.; Moad, G.; Denifl, S. Selective Bond Cleavage in RAFT Agents Promoted by Low-Energy Electron Attachment. Angew. Chem.-Int. Ed. 2021, 60, 19128–19132. [Google Scholar] [CrossRef]
- Arthur-Baidoo, E.; Schöpfer, G.; Ončák, M.; Chomicz-Mańka, L.; Rak, J.; Denifl, S. Electron Attachment to 5-Fluorouracil: The Role of Hydrogen Fluoride in Dissociation Chemistry. Int. J. Mol. Sci. 2022, 23, 8325. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Sanche, L.; Sevilla, M.D. Dehalogenation of 5-Halouracils after Low Energy Electron Attachment: A Density Functional Theory Investigation. J. Phys. Chem. A 2002, 106, 11248–11253. [Google Scholar] [CrossRef] [PubMed]
- Woon, D.E.; Dunning, T.H. Gaussian Basis Sets for Use in Correlated Molecular Calculations. III. The Atoms Aluminum through Argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Łapucha, A.R. A Rapid and Efficient Synthesis of Sulfur Analogues of Pyrimidine Bases. Synthesis 1987, 1987, 256–258. [Google Scholar] [CrossRef]
- Gallup, G.A.; Aflatooni, K.; Burrow, P.D. Dissociative Electron Attachment near Threshold, Thermal Attachment Rates, and Vertical Attachment Energies of Chloroalkanes. J. Chem. Phys. 2003, 118, 2562–2574. [Google Scholar] [CrossRef]
- Meißner, R.; Feketeová, L.; Bayer, A.; Postler, J.; Limão-Vieira, P.; Denifl, S. Positive and Negative Ions of the Amino Acid Histidine Formed in Low-Energy Electron Collisions. J. Mass Spectrom. 2019, 54, 802–816. [Google Scholar] [CrossRef]
- Ptasińska, S.; Candori, P.; Denifl, S.; Yoon, S.; Grill, V.; Scheier, P.; Märk, T.D. Dissociative Ionization of the Nucleosides Thymidine and Uridine by Electron Impact. Chem. Phys. Lett. 2005, 409, 270–276. [Google Scholar] [CrossRef]
- Ptasińska, S.; Denifl, S.; Gohlke, S.; Scheier, P.; Illenberger, E.; Märk, T.D. Decomposition of Thymidine by Low-Energy Electrons: Implications for the Molecular Mechanisms of Single-Strand Breaks in DNA. Angew. Chem.-Int. Ed. 2006, 45, 1893–1896. [Google Scholar] [CrossRef]
- Bald, I.; Dąbkowska, I.; Illenberger, E. Probing Biomolecules by Laser-Induced Acoustic Desorption: Electrons at Near Zero Electron Volts Trigger Sugar–Phosphate Cleavage. Angew. Chem.-Int. Ed. 2008, 47, 8518–8520. [Google Scholar] [CrossRef]
- Kendall, R.A.; Dunning, T.H.; Harrison, R.J. Electron Affinities of the First-row Atoms Revisited. Systematic Basis Sets and Wave Functions. J. Chem. Phys. 1992, 96, 6796–6806. [Google Scholar] [CrossRef]
- Hratchian, H.P.; Schlegel, H.B. Theory and Applications of Computational Chemistry: The First 40 Years; Dykstra, C., Frenking, G., Kim, K., Scuseria, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2005; pp. 195–249. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016; p. 29. [Google Scholar]
- Bocková, J.; Rebelo, A.; Ryszka, M.; Pandey, R.; Mészáros, D.; Limão-Vieira, P.; Papp, P.; Mason, N.J.; Townsend, D.; Nixon, K.L.; et al. Thermal Desorption Effects on Fragment Ion Production from Multi-Photon Ionized Uridine and Selected Analogues. RSC Adv. 2021, 11, 20612–20621. [Google Scholar] [CrossRef] [PubMed]
- Kossoski, F.; Varella, M.T.d.N. Negative Ion States of 5-Bromouracil and 5-Iodouracil. Phys. Chem. Chem. Phys. 2015, 17, 17271–17278. [Google Scholar] [CrossRef] [PubMed]
- McAllister, M.; Smyth, M.; Gu, B.; Tribello, G.A.; Kohanoff, J. Understanding the Interaction between Low-Energy Electrons and DNA Nucleotides in Aqueous Solution. J. Phys. Chem. Lett. 2015, 6, 3091–3097. [Google Scholar] [CrossRef] [PubMed]


| Mass (m/z) | Anion | Peak Positions (eV) | Threshold (eV) | |||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Exp. | Theory | ||
| 206 | BrSU− | ≈0 | 0.55 | -- | -- | -- | ≈0 | −1.18 1 |
| 205 | (BrSU-H)− | ≈0 | 0.2 | 0.5 | 0.8 | 1.3 | ≈0 | −0.82 2a 1.54 2b |
| 127 | (BrSU-Br)− | ≈0 | 0.17 | 0.54 | -- | -- | ≈0 | −1.03 3a 1.17 3b −0.44 3c |
| 126 | (BrSU-HBr)− | ≈0 | 0.25 | 0.7 | -- | -- | ≈0 | 0.21 4a 0.70 4b 1.27 4c |
| 79 | Br− | ≈0 | 0.4 | 1.0 | 2.3 | 4.8 | ≈0 | 0.13 5 |
| 42 | CNO− | ≈0 | 1.0 | 2.5 | 3.4 | 4.6 | ≈0 | 1.13 6a 1.30 6b |
| 33 | SH− | 0.1 | 1.2 | 2.4 | 5.1 | -- | ≈0 | 1.74 7 |
| Mass (m/z) | Anion | T (K) | Threshold (eV) Theory |
|---|---|---|---|
| 205 | (BrSdU-deoxyribose)− | 385.15 | −0.34 1 |
| 190 | (BrSdU-deoxyribose-NH)− | 385.15 | 1.40 2 |
| 127 | (BrSdU-deoxyribose-Br+H)− | 387.15 | 0.45 3a 0.66 3b 1.68 3c |
| 126 | (BrSdU-deoxyribose-Br)− | 387.15 | 0.31 4a 1.23 4b |
| 98 | (C5H6O2)− | 385.15 | 2.71 5 |
| 79 | Br− | 387.15 | 0.14 6 |
| 42 | NCO− | 387.15 | 1.15 7a 2.48 7b |
| 33 | SH− | 387.15 | 1.70 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izadi, F.; Szczyrba, A.; Datta, M.; Ciupak, O.; Demkowicz, S.; Rak, J.; Denifl, S. Electron-Induced Decomposition of 5-Bromo-4-thiouracil and 5-Bromo-4-thio-2′-deoxyuridine: The Effect of the Deoxyribose Moiety on Dissociative Electron Attachment. Int. J. Mol. Sci. 2023, 24, 8706. https://doi.org/10.3390/ijms24108706
Izadi F, Szczyrba A, Datta M, Ciupak O, Demkowicz S, Rak J, Denifl S. Electron-Induced Decomposition of 5-Bromo-4-thiouracil and 5-Bromo-4-thio-2′-deoxyuridine: The Effect of the Deoxyribose Moiety on Dissociative Electron Attachment. International Journal of Molecular Sciences. 2023; 24(10):8706. https://doi.org/10.3390/ijms24108706
Chicago/Turabian StyleIzadi, Farhad, Adrian Szczyrba, Magdalena Datta, Olga Ciupak, Sebastian Demkowicz, Janusz Rak, and Stephan Denifl. 2023. "Electron-Induced Decomposition of 5-Bromo-4-thiouracil and 5-Bromo-4-thio-2′-deoxyuridine: The Effect of the Deoxyribose Moiety on Dissociative Electron Attachment" International Journal of Molecular Sciences 24, no. 10: 8706. https://doi.org/10.3390/ijms24108706
APA StyleIzadi, F., Szczyrba, A., Datta, M., Ciupak, O., Demkowicz, S., Rak, J., & Denifl, S. (2023). Electron-Induced Decomposition of 5-Bromo-4-thiouracil and 5-Bromo-4-thio-2′-deoxyuridine: The Effect of the Deoxyribose Moiety on Dissociative Electron Attachment. International Journal of Molecular Sciences, 24(10), 8706. https://doi.org/10.3390/ijms24108706






