Evaluation of Astatine-211-Labeled Fibroblast Activation Protein Inhibitor (FAPI): Comparison of Different Linkers with Polyethylene Glycol and Piperazine
Abstract
1. Introduction
2. Results
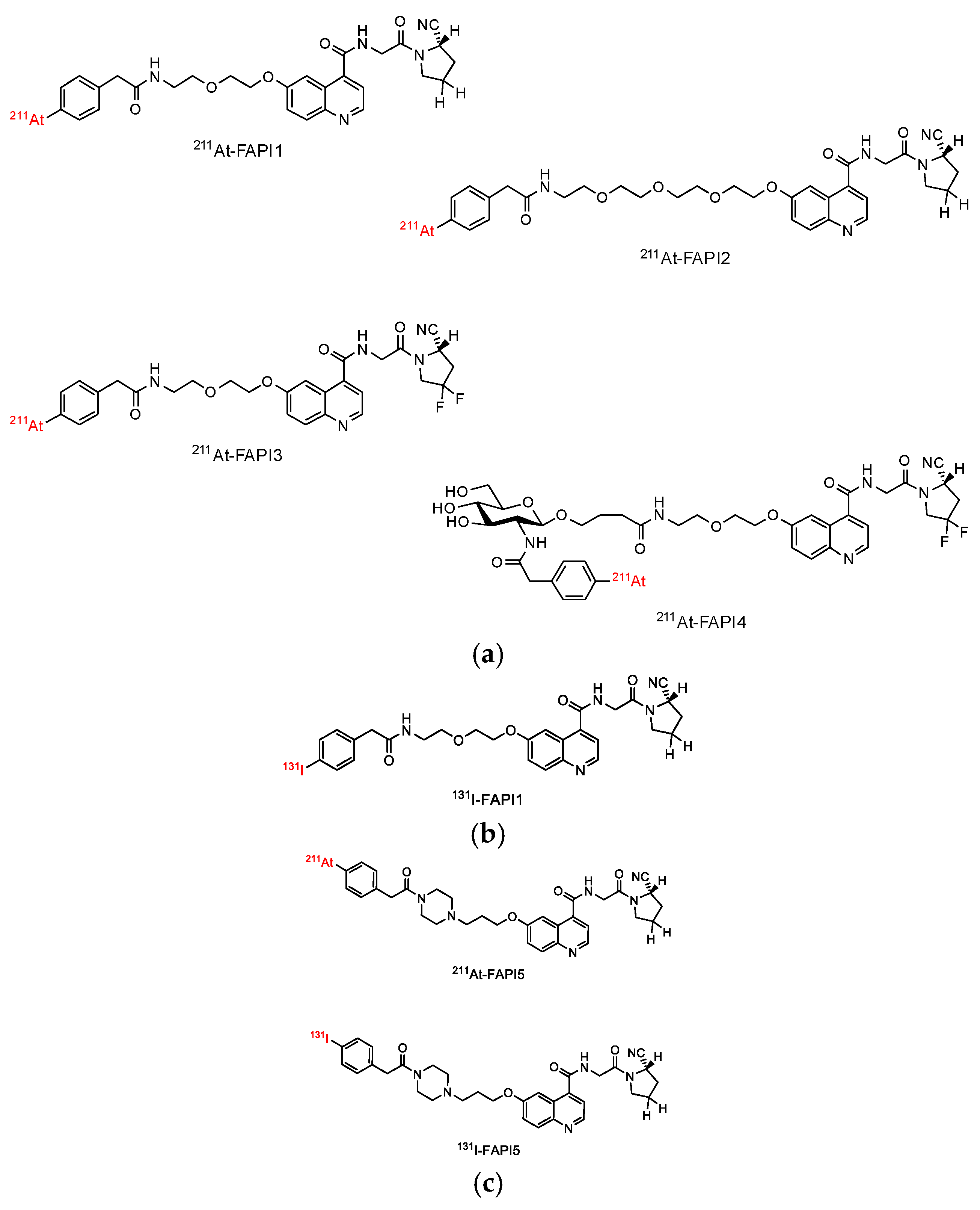
2.1. Establishment of FAPI1 to 5
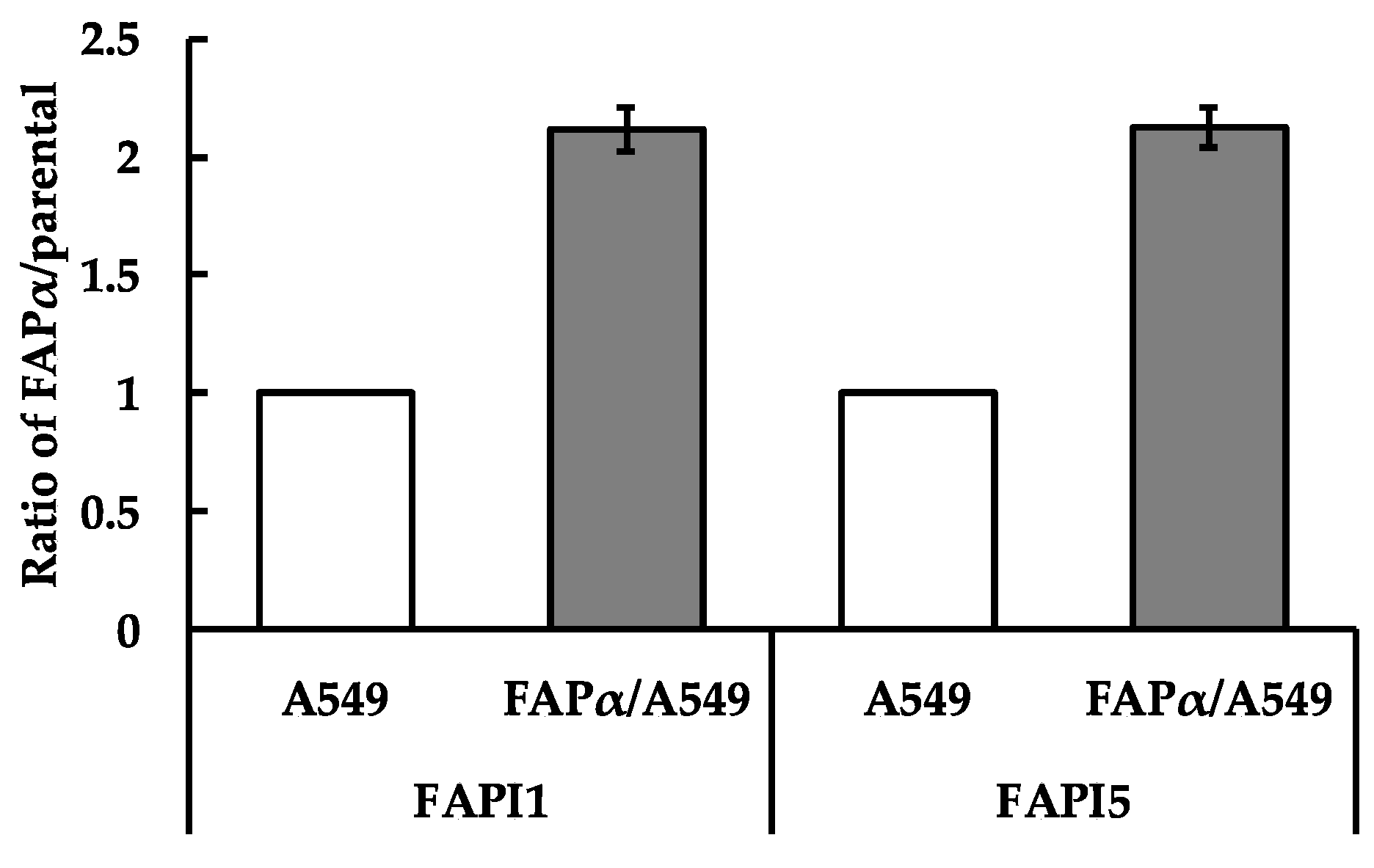
2.2. Comparison of Cellular Uptake between FAPI1 to 5
2.3. Comparison of Body Distributions of FAPI1 or FAPI5 between Two Nuclides
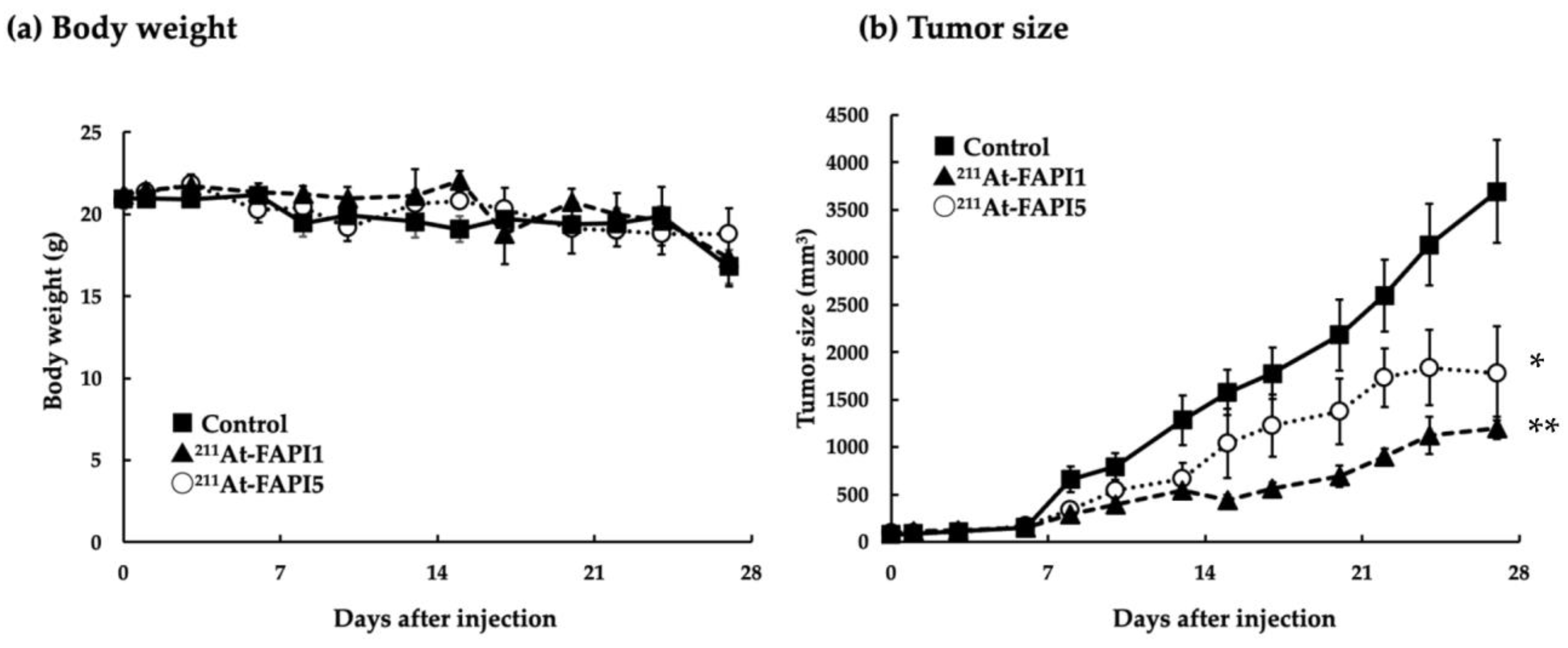
2.4. Comparison of Anti-Tumor Effects due to Differences in Linker
3. Discussion
4. Materials and Methods
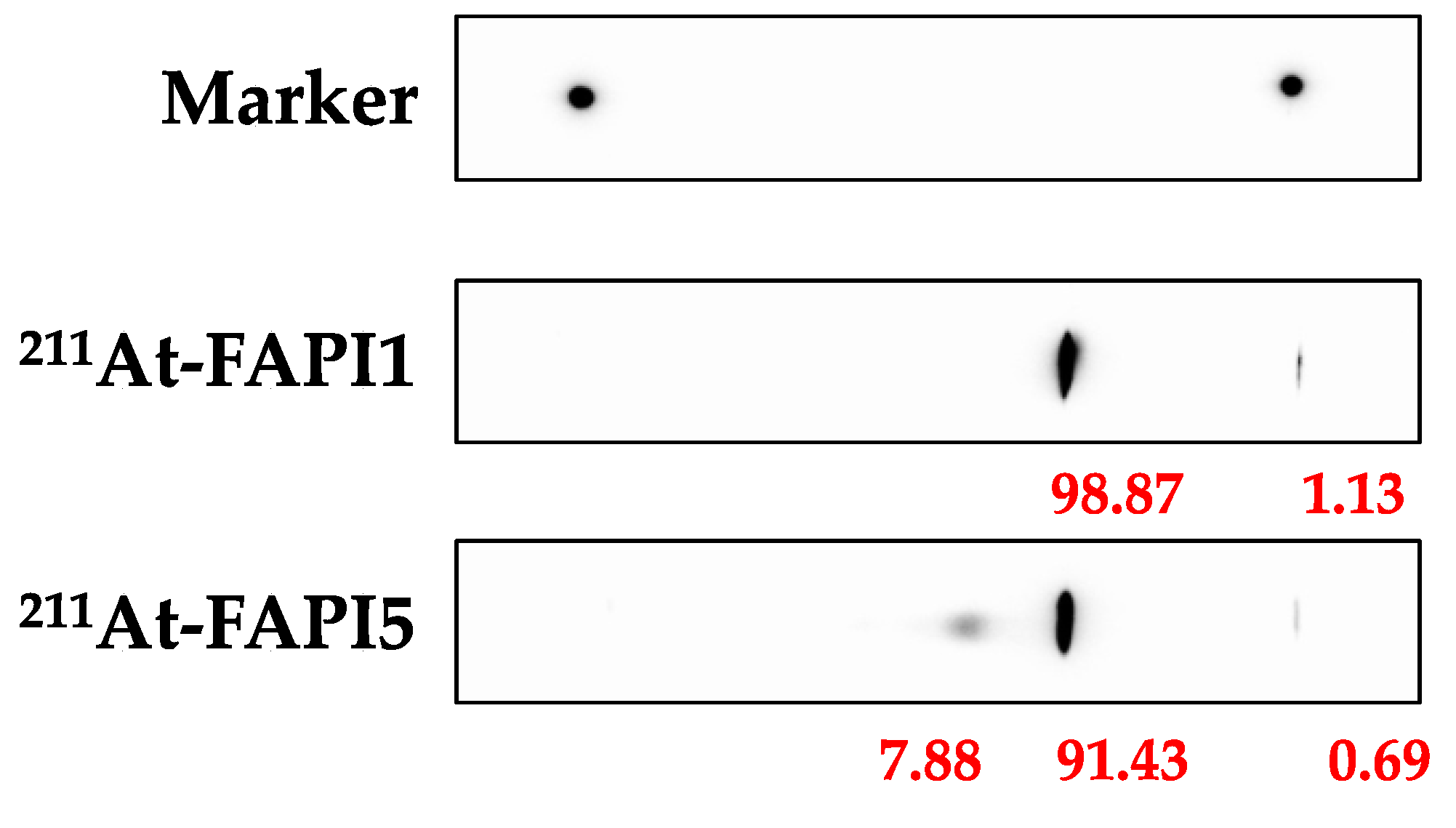
4.1. Synthesis of 211At-FAPI Compounds
4.2. Synthesis of 131I-FAPI Compounds
4.3. In Vitro Cellular Uptake Analysis
4.4. Preparation of Animals
4.5. Measurement of Biodistribution of FAP1 and FAPI5 Labeled with 131I or 211At
4.5.1. Biodistribution of 131I-FAPI1 and 131I-FAPI5
4.5.2. Biodistribution of 211At-FAPI1 and 211At-FAPI5
4.6. Examination of Therapeutic Effect
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ryabtsova, O.; Jansen, K.; Van Goethem, S.; Joossens, J.; Cheng, J.D.; Lambeir, A.M.; De Meester, I.; Augustyns, K.; Van der Veken, P. Acylated Gly-(2-cyano)pyrrolidines as inhibitors of fibroblast activation protein (FAP) and the issue of FAP/prolyl oligopeptidase (PREP)-selectivity. Bioorg. Med. Chem. Lett. 2012, 22, 3412–3417. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.Y.; Yeh, T.K.; Chen, X.; Hsu, T.; Jao, Y.C.; Huang, C.H.; Song, J.S.; Huang, Y.C.; Chien, C.H.; Chiu, Y.H.; et al. Substituted 4- carboxymethylpyroglutamic acid diamides as potent and selective inhibitors of fibroblast activation protein. J. Med. Chem. 2010, 53, 6573–6583. [Google Scholar] [CrossRef]
- Jansen, K.; Heirbaut, L.; Cheng, J.D.; Joossens, J.; Ryabtsova, O.; Cos, P.; Maes, L.; Lambeir, A.M.; De Meester, I.D.; Augustyns, K.; et al. Selective inhibitors of fibroblast activation protein (FAP) with a (4-Quinolinoyl)-glycyl-2-cyanopyrrolidine scaffold. ACS Med. Chem. Lett. 2013, 4, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Lindner, T.; Loktev, A.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Jäger, D.; Mier, W.; Haberkorn, U. Development of quinoline-based theranostic ligands for the targeting of fibroblast activation protein. J. Nucl. Med. 2018, 59, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Pu, Y.; Huang, S.; Yang, C.; Yang, F.; Pu, Y.; Li, J.; Chen, L.; Huang, Y. FAPI-PET/CT in cancer imaging: A potential novel molecule of the century. Front. Oncol. 2022, 12, 854658. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chen, J.; Pang, Y.; Fu, K.; Shang, Q.; Wu, H.; Sun, L.; Lin, Q.; Chen, H. Fibroblast activation protein-based theranostics in cancer research: A state-of-the-art review. Theranostics 2022, 12, 1557–1569. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Mier, W.; Debus, J.; Altmann, A.; Jäger, D.; Giesel, F.; Kratochwil, C.; Barthe, P.; Roumestand, C.; et al. A tumor-imaging method targeting cancer-associated fibroblasts. J. Nucl. Med. 2018, 59, 1423–1429. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef]
- Giesel, F.L.; Adeberg, S.; Syed, M.; Lindner, T.; Jiménez-Franco, L.D.; Mavriopoulou, E.; Staudinger, F.; Tonndorf-Martini, E.; Regnery, S.; Rieken, S.; et al. FAPI-74 PET/CT using either 18F-AlF or cold-kit 68Ga-labeling: Biodistribution, radiation dosimetry and tumor delineation in lung cancer patients. J. Nucl. Med. 2021, 62, 201–207. [Google Scholar] [CrossRef]
- Loktev, A.; Lindner, T.; Burger, E.M.; Altmann, A.; Giesel, F.; Kratochwil, C.; Debus, J.; Marmé, F.; Jäger, D.; Mier, W.; et al. Development of fibroblast activation protein–targeted radiotracers with improved tumor retention. J. Nucl. Med. 2019, 60, 1421–1429. [Google Scholar] [CrossRef]
- Lindner, T.; Altmann, A.; Krämer, S.; Kleist, C.; Loktev, A.; Kratochwil, C.; Giesel, F.; Mier, W.; Marme, F.; Debus, J.; et al. Design and development of 99mTc-labeled FAPI tracers for SPECT imaging and 188Re therapy. J. Nucl. Med. 2020, 61, 1507–1513. [Google Scholar] [CrossRef]
- Glatting, F.M.; Hoppner, J.; Liew, D.P.; van Genabith, A.; Spektor, A.M.; Steinbach, L.; Hubert, A.; Kratochwil, C.; Giesel, F.L.; Dendl, K.; et al. Repetitive early FAPI-PET acquisition comparing FAPI-02, FAPI-46 and FAPI-74: Methodological and diagnostic implications for malignant, inflammatory and degenerative lesions. J. Nucl. Med. 2022, 63, 1844–1851. [Google Scholar] [CrossRef]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of 68Ga-FAPI and 18F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4377–4385. [Google Scholar] [CrossRef]
- Röhrich, M.; Syed, M.; Liew, D.P.; Giesel, F.L.; Liermann, J.; Choyke, P.L.; Wefers, A.K.; Ritz, T.; Szymbara, M.; Schillingsm, L.; et al. 68Ga-FAPI-PET/CT improves diagnostic staging and radiotherapy planning of adenoid cystic carcinomas—Imaging analysis and histological validation. Radiother. Oncol. 2021, 160, 192–201. [Google Scholar] [CrossRef]
- Zhao, L.; Niu, B.; Fang, J.; Pang, Y.; Li, S.; Xie, C.; Sun, L.; Zhang, X.; Guo, Z.; Lin, Q.; et al. Synthesis, preclinical evaluation, and a pilot clinical PET imaging study of 68Ga-labeled FAPI Dimer. J. Nucl. Med. 2022, 63, 862–868. [Google Scholar] [CrossRef]
- Hu, K.; Li, J.; Wang, L.; Huang, Y.; Li, L.; Ye, S.; Han, Y.; Huang, S.; Wu, H.; Su, J.; et al. Preclinical evaluation and pilot clinical study of [18F]AlF-labeled FAPI-tracer for PET imaging of cancer associated fibroblasts. Acta Pharm. Sin. B. 2022, 12, 867–875. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, X.; Xu, X.; Ding, J.; Liu, S.; Hou, X.; Li, N.; Zhu, H.; Yang, Z. Clinical translational evaluation of Al18F-NOTA-FAPI for fibroblast activation protein-targeted tumour imaging. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4259–4271. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Tripathi, M.; ArunRaj, S.T.; Sarswat, S.; Bal, C. Biodistribution, pharmacokinetics, dosimetry of [68Ga]Ga-DOTA.SA.FAPi, and the head-to-head comparison with [18F]F-FDG PET/CT in patients with various cancers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1915–1931. [Google Scholar] [CrossRef]
- Greifenstein, L.; Kramer, C.S.; Moon, E.S.; Rösch, F.; Klega, A.; Landvogt, C.; Müller, C.; Baum, R.P. From Automated Synthesis to In Vivo Application in Multiple Types of Cancer-Clinical Results with [68Ga]Ga-DATA5m.SA.FAPi. Pharmaceuticals 2022, 15, 1000. [Google Scholar] [CrossRef]
- Naka, S.; Watabe, T.; Lindner, T.; Cardinale, J.; Kurimoto, K.; Moore, M.; Tatsumi, M.; Mori, Y.; Shimosegawa, E.; Valla Jr, F.; et al. One-pot and one-step automated radio-synFthesis of [18F]AlF-FAPI-74 using a multi purpose synthesizer: A proof-of-concept experiment. EJNMMI Radiopharm. Chem. 2021, 6, 28. [Google Scholar] [CrossRef]
- Liu, Y.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Naka, S.; Ooe, K.; Toyoshima, A.; Nagata, K.; Haberkorn, U.; Kratochwil, C.; et al. Fibroblast activation protein targeted therapy using [177Lu]FAPI-46 compared with [225Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Shirakami, Y.; Lindner, T.; Ooe, K.; Toyoshima, A.; Nagata, K.; Shimosegawa, E.; Haberkorn, U.; et al. Theranostics targeting fibroblast activation protein in the tumor stroma: 64Cu- and 225Ac-labeled FAPI-04 in pancreatic cancer xenograft mouse models. J. Nucl. Med. 2020, 61, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Li, F.; Shen, G.; Pan, L.; Liu, W.; Liang, R.; Lan, T.; Yang, Y.; Yang, J.; Liao, J.; et al. In vitro and in vivo evaluation of 211At-labeled fibroblast activation protein inhibitor for glioma treatment. Bioorg. Med. Chem. 2022, 55, 116600. [Google Scholar] [CrossRef] [PubMed]
- Aso, A.; Kaneda-Nakashima, K.; Nabetani, H.; Kadonaga, Y.; Shirakami, Y.; Watabe, T.; Yoshiya, T.; Mochizuki, M.; Koshino, Y.; Ooe, K.; et al. Substrate study for dihydroxyboryl astatine substitution reaction with Fibroblast Activation Protein Inhibitor (FAPI). Chem. Lett. 2022, 51, 1091–1094. [Google Scholar] [CrossRef]
- Shirakami, Y.; Watabe, T.; Obata, H.; Kaneda, K.; Ooe, K.; Liu, Y.; Teramoto, T.; Toyoshima, A.; Shinohara, A.; Shimosegawa, E.; et al. Synthesis of [211At]4-astato-L-phenylalanine by dihydroxyboryl-astatine substitution reaction in aqueous solution. Sci. Rep. 2021, 11, 12982. [Google Scholar] [CrossRef]
- Watabe, T.; Kaneda-Nakashima, K.; Liu, Y.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Shimosegawa, E.; Fukuda, M.; Shinohara, A.; Hatazawa, J. Enhancement of astatine-211 uptake via the sodium iodide symporter by the addition of ascorbic acid in targeted alpha therapy of thyroid cancer. J. Nucl. Med. 2019, 60, 1301–1307. [Google Scholar] [CrossRef]
- King, A.P.; Lin, F.I.; Escorcia, F.E. Why bother with alpha particles? Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 7–17. [Google Scholar] [CrossRef]
- Sathekge, M.; Bruchertseifer, F.; Vorster, M.; Lawal, O.I.; Mokoala, K.; Reed, J.; Maseremule, L.; Ndlovu, H.; Hlongwa, K.; Maes, A.; et al. 225Ac-PSMA-617 radioligand therapy of de novo metastatic hormone-sensitive prostate carcinoma (mHSPC): Preliminary clinical findings. Eur. J. Nucl. Med. Mol. Imaging, 2023; in press. [Google Scholar] [CrossRef]
- Filippi, L.; Chiaravalloti, A.; Schillaci, O.; Bagni, O. The potential of PSMA-targeted alpha therapy in the management of prostate cancer. Expert Rev. Anticancer Ther. 2020, 20, 823–829. [Google Scholar] [CrossRef]
- Kaneda-Nakashima, K.; Zhang, Z.; Manabe, Y.; Shimoyama, A.; Kabayama, K.; Watabe, T.; Kanai, Y.; Ooe, K.; Toyoshima, A.; Shirakami, Y.; et al. α-Emitting cancer therapy using 211At-AAMT targeting LAT1. Cancer Sci. 2021, 112, 1132–1140. [Google Scholar] [CrossRef]
- Scholz, S.O.; Farney, E.P.; Kim, S.; Bates, D.M.; Yoon, T.P. Spin-Selective Generation of Triplet Nitrenes: Olefin Aziridination through Visible-Light Photosensitization of Azidoformates. Angew. Chem. Int. Ed. 2016, 55, 2239–2242. [Google Scholar] [CrossRef]


| (a) 1 h | (b) 3 h | ||||
|---|---|---|---|---|---|
| 131I-FAPI1 | 131I-FAPI5 | 131I-FAPI1 | 131I-FAPI5 | ||
| Brain | 0.54 ± 0.15 | 0.50 ± 0.10 | Brain | 0.34 ± 0.04 | 0.30 ± 0.02 |
| Thyroid | 7.96 ± 1.99 | 5.66 ± 0.26 | Thyroid | 12.86 ± 0.68 * | 8.40 ± 0.70 |
| Salivary gland | 2.32 ± 0.12 | 2.65 ± 0.36 | Salivary gland | 1.72 ± 0.30 | 1.82 ± 0.11 |
| Heart | 1.33 ± 0.06 | 1.21 ± 0.07 | Heart | 0.91 ± 0.12 | 1.95 ± 0.06 |
| Lung | 3.82 ± 2.11 | 4.43 ± 2.34 | Lung | 2.15 ± 0.47 | 2.33 ± 0.38 |
| Liver | 7.02 ± 0.90 | 7.40 ± 0.71 | Liver | 3.02 ± 0.70 | 2.85 ± 0.29 |
| Stomach | 7.67 ± 3.50 | 7.75 ± 4.99 | Stomach | 2.57 ± 0.66 | 2.10 ± 0.84 |
| Small Intestine | 48.18 ± 23.44 | 38.53 ± 17.18 | Small Intestine | 5.99 ± 1.91 | 5.54 ± 1.90 |
| Colon | 9.63 ± 1.69 | 10.43 ± 1.67 | Colon | 155.39 ± 16.65 | 165.96 ± 15.26 |
| Kidney | 3.68 ± 0.26 | 3.92 ± 0.41 | Kidney | 1.74 ± 0.12 | 1.74 ± 0.08 |
| Pancreas | 1.54 ± 0.18 | 1.58 ± 0.30 | Pancreas | 0.63 ± 0.12 | 0.79 ± 0.12 |
| Spleen | 1.42 ± 0.42 | 1.15 ± 0.06 | Spleen | 0.54 ± 0.17 | 0.64 ± 0.05 |
| Testis | 0.56 ± 0.04 | 0.66 ± 0.08 | Testis | 0.76 ± 0.13 | 0.64 ± 0.08 |
| Blood | 1.99 ± 0.20 | 2.36 ± 0.17 | Blood | 2.86 ± 0.46 | 2.32 ± 0.19 |
| Bone | 0.47 ± 0.07 | 0.68 ± 0.09 | Bone | 0.37 ± 0.07 | 0.40 ± 0.01 |
| Tumor | 0.77 ± 0.31 | 1.13 ± 0.10 | Tumor | 0.89 ± 0.44 | 1.13 ± 0.07 |
| (a) 1 h | (b) 3 h | ||||
|---|---|---|---|---|---|
| 211At-FAPI1 | 211At-FAPI5 | 211At-FAPI1 | 211At-FAPI5 | ||
| Brain | 0.38 ± 0.04 | 0.30 ± 0.05 | Brain | 0.20 ± 0.04 | 0.30 ± 0.07 |
| Thyroid | 3.73 ± 0.57 | 3.58 ± 0.71 | Thyroid | 16.12 ± 5.84 | 24.33 ± 6.86 |
| Salivary gland | 4.18 ± 0.72 | 3.27 ± 0.38 | Salivary gland | 8.51 ± 2.25 | 6.43 ± 3.55 |
| Heart | 3.24 ± 0.17 * | 1.51 ± 0.14 | Heart | 2.21 ± 0.13 | 0.90 ± 0.63 |
| Lung | 4.61 ± 0.68 | 2.86 ± 0.24 | Lung | 4.92 ± 1.39 | 3.28 ± 1.95 |
| Liver | 17.06 ± 0.98 * | 6.46 ± 0.62 | Liver | 1.77 ± 0.44 | 0.50 ± 0.61 |
| Stomach | 19.31 ± 1.21 | 14.33 ± 3.00 | Stomach | 9.30 ± 1.27 * | 1.85 ± 1.70 |
| Small Intestine | 33.16 ± 3.61 | 28.20 ± 5.27 | Small Intestine | 4.85 ± 1.28 | 4.89 ± 3.82 |
| Colon | 6.34 ± 0.35 | 4.29 ± 0.44 | Colon | 32.44 ± 14.99 | 58.40 ± 9.91 |
| Kidney | 6.92 ± 1.34 * | 3.07 ± 0.07 | Kidney | 2.22 ± 0.24 | 1.56 ± 0.81 |
| Pancreas | 1.43 ± 0.16 | 1.17 ± 0.12 | Pancreas | 0.63 ± 0.05 | 0.45 ± 0.29 |
| Spleen | 3.93 ± 0.52 | 2.58 ± 0.51 | Spleen | 5.27 ± 1.11 | 4.32 ± 0.21 |
| Testis | 1.19 ± 0.10 | 1.05 ± 0.07 | Testis | 1.51 ± 0.28 | 1.95 ± 0.46 |
| Blood | 3.76 ± 2.69 | 1.32 ± 0.04 | Blood | 1.20 ± 0.11 | 1.33 ± 0.24 |
| Bone | 1.07 ± 0.12 | 0.61 ± 0.13 | Bone | 0.70 ± 0.14 | 1.35 ± 0.51 |
| Tumor | 2.15 ± 0.24 * | 1.24 ± 0.14 | Tumor | 3.04 ± 0.69 * | 1.48 ± 0.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aso, A.; Nabetani, H.; Matsuura, Y.; Kadonaga, Y.; Shirakami, Y.; Watabe, T.; Yoshiya, T.; Mochizuki, M.; Ooe, K.; Kawakami, A.; et al. Evaluation of Astatine-211-Labeled Fibroblast Activation Protein Inhibitor (FAPI): Comparison of Different Linkers with Polyethylene Glycol and Piperazine. Int. J. Mol. Sci. 2023, 24, 8701. https://doi.org/10.3390/ijms24108701
Aso A, Nabetani H, Matsuura Y, Kadonaga Y, Shirakami Y, Watabe T, Yoshiya T, Mochizuki M, Ooe K, Kawakami A, et al. Evaluation of Astatine-211-Labeled Fibroblast Activation Protein Inhibitor (FAPI): Comparison of Different Linkers with Polyethylene Glycol and Piperazine. International Journal of Molecular Sciences. 2023; 24(10):8701. https://doi.org/10.3390/ijms24108701
Chicago/Turabian StyleAso, Ayaka, Hinako Nabetani, Yoshifumi Matsuura, Yuichiro Kadonaga, Yoshifumi Shirakami, Tadashi Watabe, Taku Yoshiya, Masayoshi Mochizuki, Kazuhiro Ooe, Atsuko Kawakami, and et al. 2023. "Evaluation of Astatine-211-Labeled Fibroblast Activation Protein Inhibitor (FAPI): Comparison of Different Linkers with Polyethylene Glycol and Piperazine" International Journal of Molecular Sciences 24, no. 10: 8701. https://doi.org/10.3390/ijms24108701
APA StyleAso, A., Nabetani, H., Matsuura, Y., Kadonaga, Y., Shirakami, Y., Watabe, T., Yoshiya, T., Mochizuki, M., Ooe, K., Kawakami, A., Jinno, N., Toyoshima, A., Haba, H., Wang, Y., Cardinale, J., Giesel, F. L., Shimoyama, A., Kaneda-Nakashima, K., & Fukase, K. (2023). Evaluation of Astatine-211-Labeled Fibroblast Activation Protein Inhibitor (FAPI): Comparison of Different Linkers with Polyethylene Glycol and Piperazine. International Journal of Molecular Sciences, 24(10), 8701. https://doi.org/10.3390/ijms24108701








