Adsorption Removal of Mo(VI) from an Aqueous Solution by Alumina with the Subsequent Regeneration of the Adsorbent
Abstract
1. Introduction
2. Results and Discussion
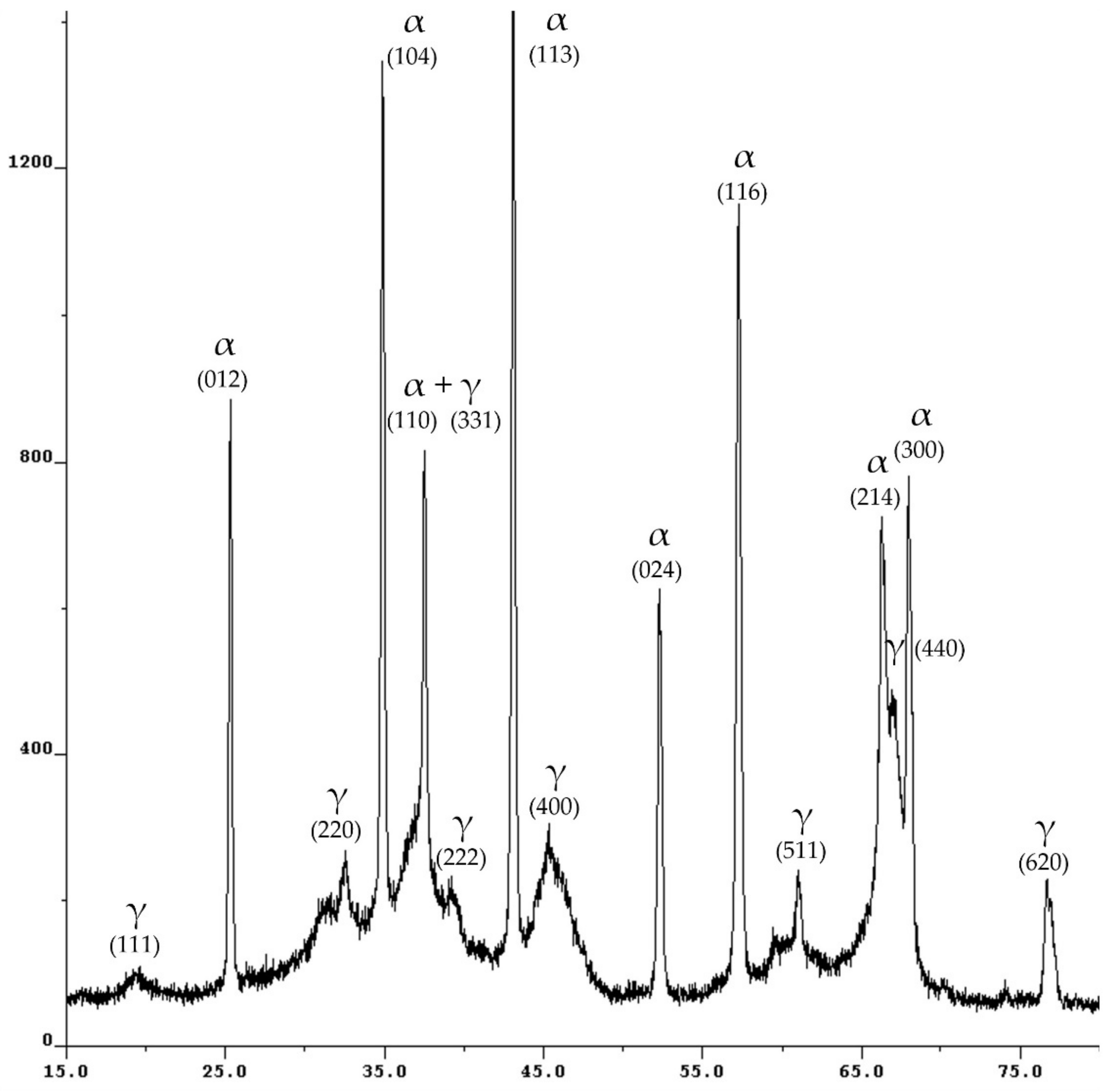
2.1. Characterization of Adsorbent
2.2. Sorption Studies
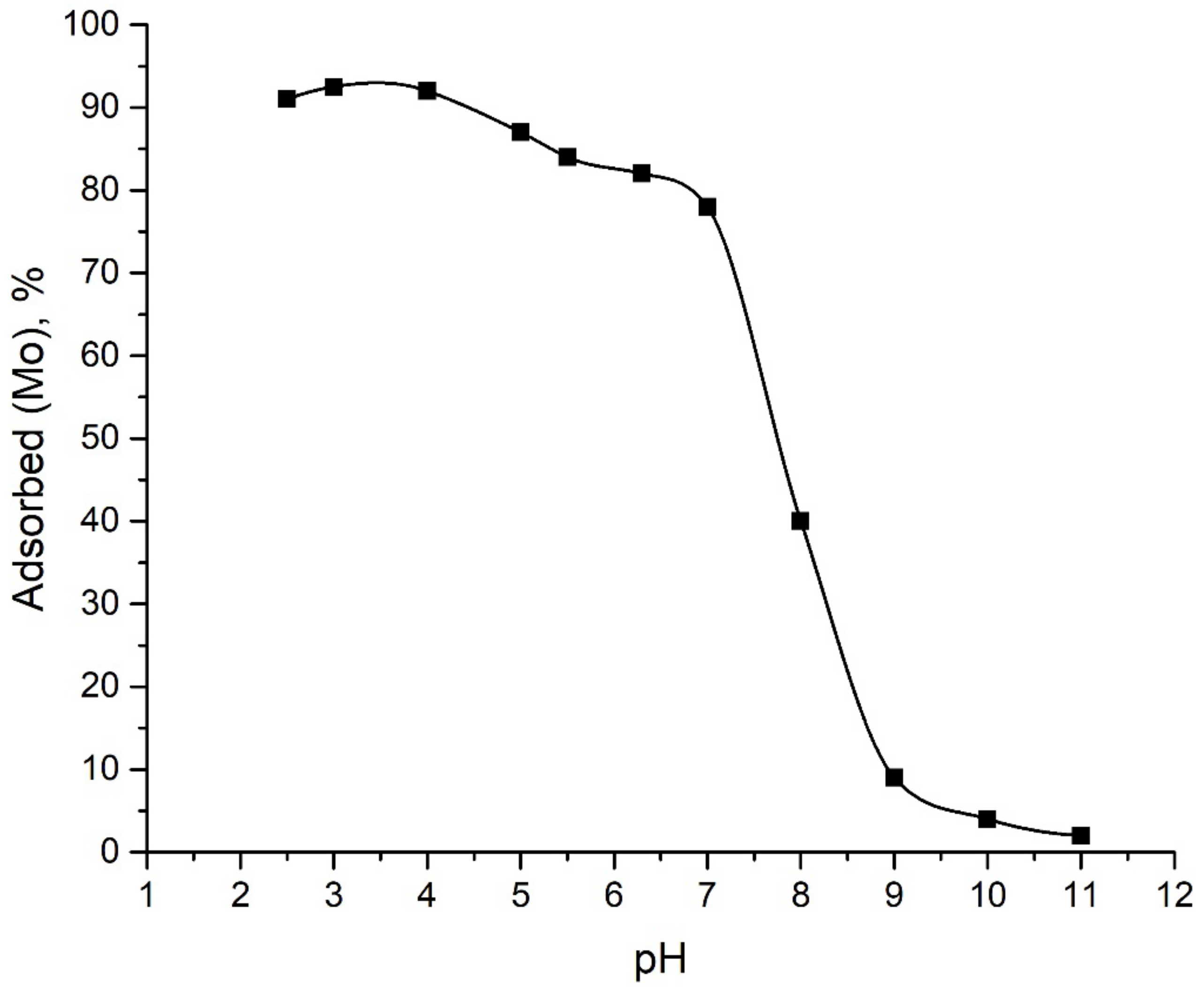
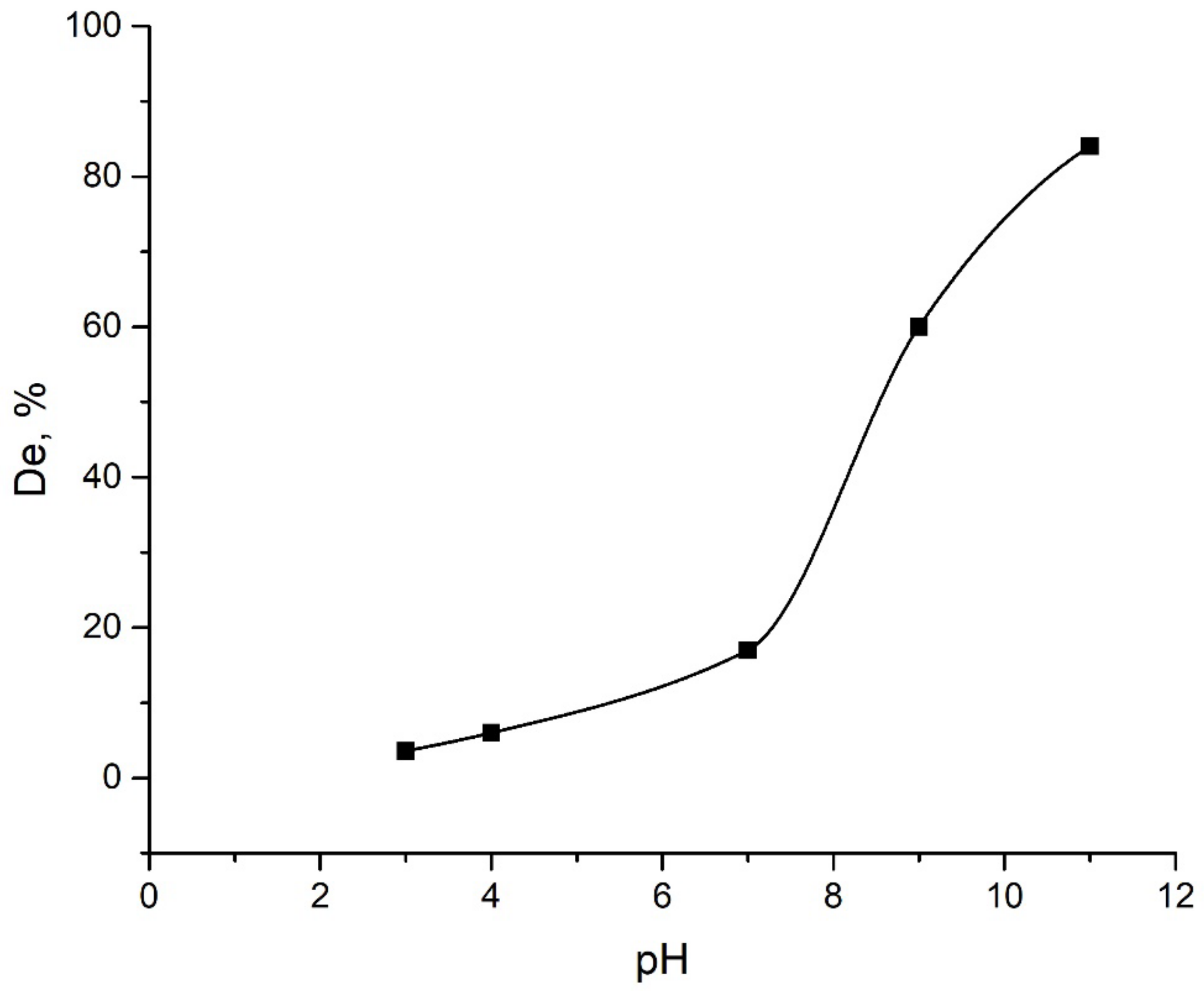
2.2.1. Effect of pH
2.2.2. Adsorption Kinetics
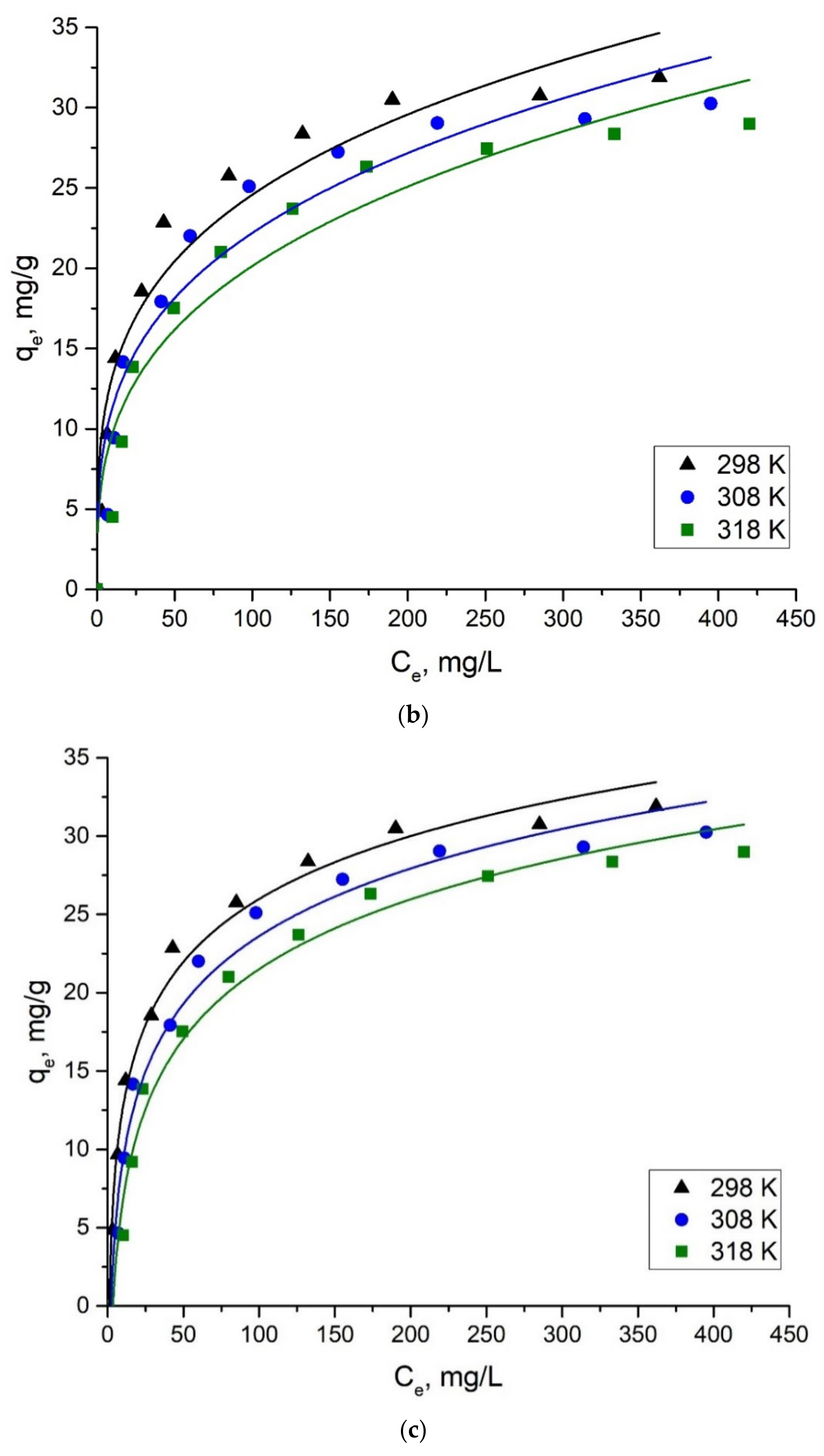
2.2.3. Adsorption Isotherms
2.2.4. Thermodynamics Parameters
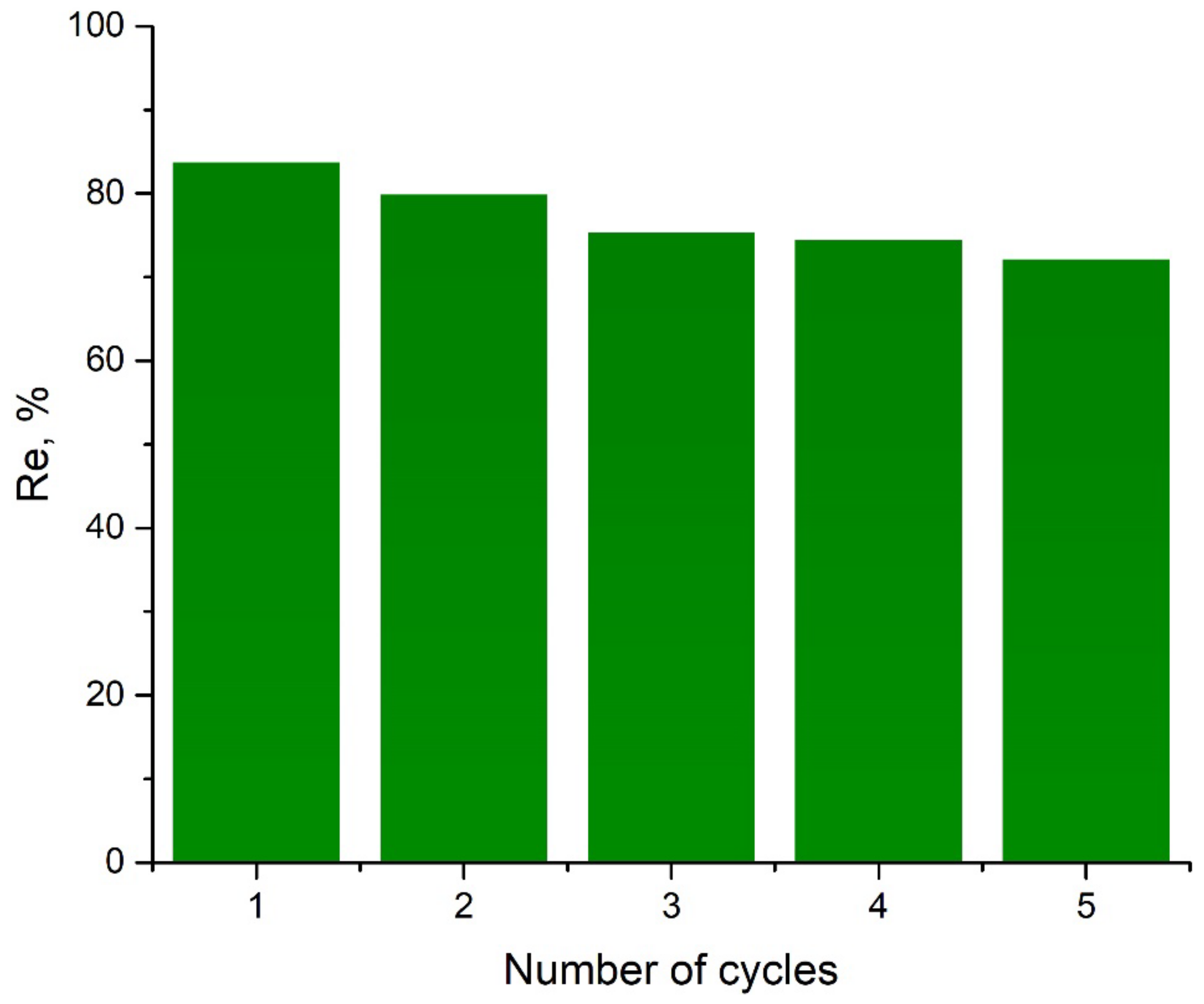
2.3. Desorption Studies
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Adsorbent Characterization
3.3. Adsorption Studies
3.4. Desorption Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abejón, R. An Overview to Technical Solutions for Molybdenum Removal: Perspective from the Analysis of the Scientific Literature on Molybdenum and Drinking Water (1990–2019). Water 2022, 14, 2108. [Google Scholar] [CrossRef]
- Wasekar, N.P.; Verulkar, S.; Vamsi, M.V.N.; Sundararajan, G. Influence of molybdenum on the mechanical properties, elec-trochemical corrosion and wear behavior of electrodeposited Ni-Mo alloy. Surf. Coat. Technol. 2019, 370, 298–310. [Google Scholar] [CrossRef]
- Vereschaka, A.A.; Volosova, M.A.; Grigoriev, S.N.; Vereschaka, A.S. Development of wear-resistant complex for high-speed steel tool when using process of combined cathodic vacuum arc deposition. Procedia CIRP 2013, 9, 8–12. [Google Scholar] [CrossRef]
- Grigoriev, S.N.; Fominski, V.Y.; Gnedovets, A.G.; Romanov, R.I. Experimental and numerical study of the chemical composi-tion of WSex thin films obtained by pulsed laser deposition in vacuum and in a buffer gas atmosphere. Appl. Surf. Sci. 2012, 258, 7000–7007. [Google Scholar] [CrossRef]
- Glowka, K.; Zubko, M.; Świec, P.; Prusik, K.; Szklarska, M.; Chrobak, D.; Lábár, J.L.; Stróż, D. Influence of Molybdenum on the Microstructure, Mechanical Properties and Corrosion Resistance of Ti20Ta20Nb20(ZrHf)20−xMox (Where: X = 0, 5, 10, 15, 20). High. Entropy Alloys Mater. 2022, 15, 393. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, J.; Lu, X.; Huangfu, X.; Zou, J. High efficient removal of molybdenum from water by Fe2(SO4)3: Effects of pH and affecting factors in the presence of co-existing background constituents. J. Hazard. Mater. 2015, 300, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Weidner, E.; Ciesielczyk, F. Removal of Hazardous Oxyanions from the Environment Using Metal-Oxide-Based Materials. Materials 2019, 12, 927. [Google Scholar] [CrossRef]
- Smedley, P.L.; Kinniburgh, D.G. Molybdenum in natural waters: A review of occurrence, distributions and controls. Appl. Geochem. 2017, 84, 387–432. [Google Scholar] [CrossRef]
- Vistoso, E.; Theng, B.K.G.; Bolan, N.S.; Parfitt, R.L.; Mora, M.L. Competitive sorption of molybdate and phosphate in Andisols. J. Soil Sci. Plant Nutrit. 2012, 12, 59–72. [Google Scholar] [CrossRef]
- Bertine, K.K. The deposition of molybdenum in anoxic waters. Mar. Chem. 1972, 1, 43–53. [Google Scholar] [CrossRef]
- Polowczyk, I.; Cyganowski, P.; Urbano, B.F.; Rivas, B.L.; Bryjak, M.; Kabay, N. Amberlite IRA-400 and IRA-743 chelating resins for the sorption and recovery of molybdenum(VI) and vanadium(V): Equilibrium and kinetic studies. Hydrometallurgy 2017, 169, 496–507. [Google Scholar] [CrossRef]
- Ahmad, A.A.; Al-Ghouti, M.A.; Khraisheh, M.; Zouari, N. Insights into the removal of lithium and molybdenum from groundwater by adsorption onto activated carbon, bentonite, roasted date pits, and modified-roasted date pits. Biores. Technol. Rep. 2022, 18, 101045. [Google Scholar] [CrossRef]
- Fu, Y.-F.; Xiao, Q.-G.; Gao, Y.-Y.; Ning, P.-G.; Xu, H.-B.; Zhang, Y. Direct extraction of Mo(VI) from acidic leach solution of molybdenite ore by ion exchange resin: Batch and column adsorption studies. Transact. Nonferr. Metals Soc. China 2018, 28, 1660–1669. [Google Scholar] [CrossRef]
- Wu, H.; Kim, S.-Y.; Miwa, M.; Matsuyama, S. Synergistic adsorption behavior of a silica-based adsorbent toward palladium, molybdenum, and zirconium from simulated high-level liquid waste. J. Hazard. Mater. 2021, 411, 125136. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Vasileiadis, A.; Wolterbeek, H.T.; Denkova, A.G.; Crespo, P.S. Adsorption of molybdenum on Zr-based MOFs for po-tential application in the 99Mo/99mTc generator. Appl. Surf. Sci. 2022, 572, 151340. [Google Scholar] [CrossRef]
- Li, G.; Zhang, S.; Mia, X.; Guo, Y.; Guo, Y.; Pak, T. Adsorption of molybdenum(VI) by low-temperature biochar derived from activated sludge and application in reservoir water purification. Desalin. Water Treat. 2021, 226, 292–302. [Google Scholar] [CrossRef]
- Fujita, Y.; Niizeki, T.; Fukumitsu, N.; Ariga, K.; Yamauchi, Y.; Malgras, V.; Kaneti, Y.V.; Liu, C.-H.; Hatano, K.; Suematsu, H.; et al. Mechanisms Responsible for Adsorption of Molybdate ions on Alumina for the Production of Medical. Radioisotopes. Bull. Chem. Soc. Jap. 2022, 95, 129–137. [Google Scholar] [CrossRef]
- Denkova, A.G.; Terpstra, B.E.; Steinbach, O.M.; Dam, J.T.; Wolterbeek, H.T. Adsorption of Molybdenum on Mesoporous Aluminum Oxides for Potential Application in Nuclear Medicine. Separ. Sci. Technol. 2013, 48, 1331–1338. [Google Scholar] [CrossRef]
- Goldberg, S.; Forster, H.S.; Godfrey, C.L. Molybdenum Adsorption on Oxides, Clay Minerals, and Soils. Soil Sci. Soc. Amer. J. 1996, 60, 425–432. [Google Scholar] [CrossRef]
- Yang, P.-T.; Wang, S.-L. Sorption and speciation of molybdate in soils: Implications for molybdenum mobility and availability. J. Hazard. Mater. 2021, 408, 124934. [Google Scholar] [CrossRef]
- Mostafa, N.G.; Yunnus, A.F.; Elawwad, A. Adsorption of Pb(II) from Water onto ZnO, TiO2, and Al2O3: Process Study, Ad-sorption Behaviour, and Thermodynamics. Adsorpt. Sci. Technol. 2022, 2022, 7582756. [Google Scholar] [CrossRef]
- Arora, R. Adsorption of Heavy Metals–A Review. Mater. Today Proc. 2019, 18, 4745–4750. [Google Scholar] [CrossRef]
- Lian, J.; Zhou, F.; Chen, B.; Yang, M.; Wang, S.; Liu, Z.; Niu, S. Enhanced adsorption of molybdenum(VI) onto drinking water treatment residues modified by thermal treatment and acid activation. J. Clean. Product. 2020, 244, 118719. [Google Scholar] [CrossRef]
- Tu, Y.-J.; Chan, T.-S.; Tu, H.-W.; Wang, S.-L.; You, C.-F.; Chang, C.-K. Rapid and efficient removal/recovery of molybdenum onto ZnFe2O4 nanoparticles. Chemosphere 2016, 148, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Xu, S.; Yu, C.; Han, C. Removal of Mo(VI) from aqueous solutions using sulfuric acid-modified cinder: Kinetic and thermodynamic studies. Toxic Environ. Chem. 2012, 94, 500–511. [Google Scholar] [CrossRef]
- Xie, R.J.; MacKenzie, A.F. Molybdate sorption-desorption in soils treated with phosphate. Geoderma 1991, 48, 321–333. [Google Scholar] [CrossRef]
- Sun, W.; Selim, H.M. Molybdenum-phosphate retention and transport in soils. Geoderma 2017, 308, 60–68. [Google Scholar] [CrossRef]
- Chauruka, S.R.; Hassanpour, A.; Brydson, R.; Roberts, K.J.; Ghadiri, M.; Stitt, H. Effect of mill type on the size reduction and phase transformation of gamma alumina. Chem. Eng. Sci. 2015, 134, 774–783. [Google Scholar] [CrossRef]
- Mohammed, A.A.; Khodair, Z.T.; Khadom, A.A. Preparation and investigation of the structural properties of α-Al2O3 nanoparticles using the sol-gel method. Chem. Data Coll. 2020, 29, 100531. [Google Scholar] [CrossRef]
- Gamal, R.; Rizk, S.E.; El-Hefny, N.E. The adsorptive removal of Mo(VI) from aqueous solution by a synthetic magnetic chromium ferrite nanocomposite using a nonionic surfactant. J. Alloys Comp. 2021, 853, 157039. [Google Scholar] [CrossRef]
- Das, N.K.; Navarathna, C.M.; Alchouron, J.; Arwenyo, B.; Rahman, S.; Hoffman, B.; Lee, K.; Stokes, S.; Anderson, R.; Perez, F.; et al. Efficient aqueous molybdenum removal using commercial Douglas fir biochar and its iron oxide hybrids. J. Hazard. Mater. 2023, 443, 130257. [Google Scholar] [CrossRef]
- Lian, J.J.; Yang, M.; Wang, H.L.; Zhong, Y.; Chen, B.; Huang, W.L.; Peng, P.A. Enhanced molybdenum(VI) removal using sul-fide-modified nanoscale zerovalent iron: Kinetics and influencing factors. Water Sci. Technol. 2021, 83, 297–308. [Google Scholar] [CrossRef]
- Torres, J.; Gonzatto, L.; Peinado, G.; Kremer, C.; Kremer, E. Interaction of molybdenum(VI) oxyanions with +2 metal cations. J. Solut. Chem. 2014, 43, 1687–1700. [Google Scholar] [CrossRef]
- Oyerinde, O.F.; Weeks, C.L.; Anbar, A.D.; Spiro, T.G. Solution structure of molybdic acid from Raman spectroscopy and DFT analysis. Inorg. Chim. Acta 2008, 361, 1000–1007. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetensk. Sakademiens Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Proc. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morriss, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. Am. Soc. Civ. Eng. 1963, 89, 31–60. [Google Scholar] [CrossRef]
- Zeldowitsch, J. The catalytic oxidation of carbon monoxide on manganese dioxide. Acta Physicochim. URSS 1934, 1, 364–449. [Google Scholar]
- Srivastava, V.; Sharma, Y.C.; Sillanpää, M. Application of nano-magnesso ferrite (n-MgFe2O4) for the removal of Co2+ ions from synthetic wastewater: Kinetic, equilibrium and thermodynamic studies. Appl. Surf. Sci. 2015, 338, 42–54. [Google Scholar] [CrossRef]
- Wu, C.-H.; Lo, S.-L.; Lin, C.-F. Competitive adsorption of molybdate, chromate, sulfate, selenate, and selenite on γ-Al2O3. Colloids Surf. A Physicochem. Eng. Asp. 2000, 166, 251–259. [Google Scholar] [CrossRef]
- Fallah, N.; Taghizadeh, M.; Hassanpour, S. Selective adsorption of Mo(VI) ions from aqueous solution using a surface-grafted Mo(VI) ion imprinted polymer. Polymer 2018, 144, 80–91. [Google Scholar] [CrossRef]
- Brion-Roby, R.; Gagnon, J.; Nosrati, S.; Deschênes, J.-S.; Chabot, B. Adsorption and desorption of molybdenum(VI) in con-taminated water using a chitosan sorbent. J. Water Proc. Eng. 2018, 23, 13–19. [Google Scholar] [CrossRef]
- Namasivayam, C.; Sangeetha, D. Removal of molybdate from water by adsorption onto ZnCl2 activated coir pith carbon. Biores. Technol. 2006, 97, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-C.; Lu, C. Kinetics, thermodynamics and regeneration of molybdenum adsorption in aqueous solutions with NaOCl-oxidized multiwalled carbon nanotubes. J. Ind. Eng. Chem. 2014, 20, 2521–2527. [Google Scholar] [CrossRef]
- Qian, D.; Su, Y.; Huang, Y.; Chu, H.; Zhou, X.; Zhang, Y. Simultaneous molybdate (Mo(VI)) recovery and hazardous ions im-mobilization via nanoscale zerovalent iron. J. Hazard. Mater. 2018, 344, 698–706. [Google Scholar] [CrossRef]
- Rana, M.S.; Hu, C.X.; Shaaban, M.; Imran, M.; Afzal, J.; Moussa, M.G.; Elyamine, A.M.; Bhantana, P.; Saleem, M.H.; Syaifudin, M.; et al. Soil phosphorus transformation characteristics in response to molybdenum supply in legu-minous. crops. J. Environ. Manag. 2020, 268, 110610. [Google Scholar] [CrossRef]
- Chen, D.; Meng, Z.-W.; Chen, Y.-P. Toxicity assessment of molybdenum slag as a mineral fertilizer: A case study with pakchoi (Brassica chinensis L.). Chemosphere 2019, 217, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Fiol, N.; Villaescusa, I. Determination of sorbent point zero charge: Usefulness in sorption studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Crouthamel, C.E.; Johnson, C.E. Thiocyanate Spectrophotometric Determination of Molybdenum and Tungsten. Anal. Chem. 1954, 26, 1284–1291. [Google Scholar] [CrossRef]
- Larasati, A.; Fowler, G.D.; Graham, N.J.D. Insights into chemical regeneration of activated carbon for water treatment. J. Environ. Chem. Eng. 2021, 9, 105555. [Google Scholar] [CrossRef]

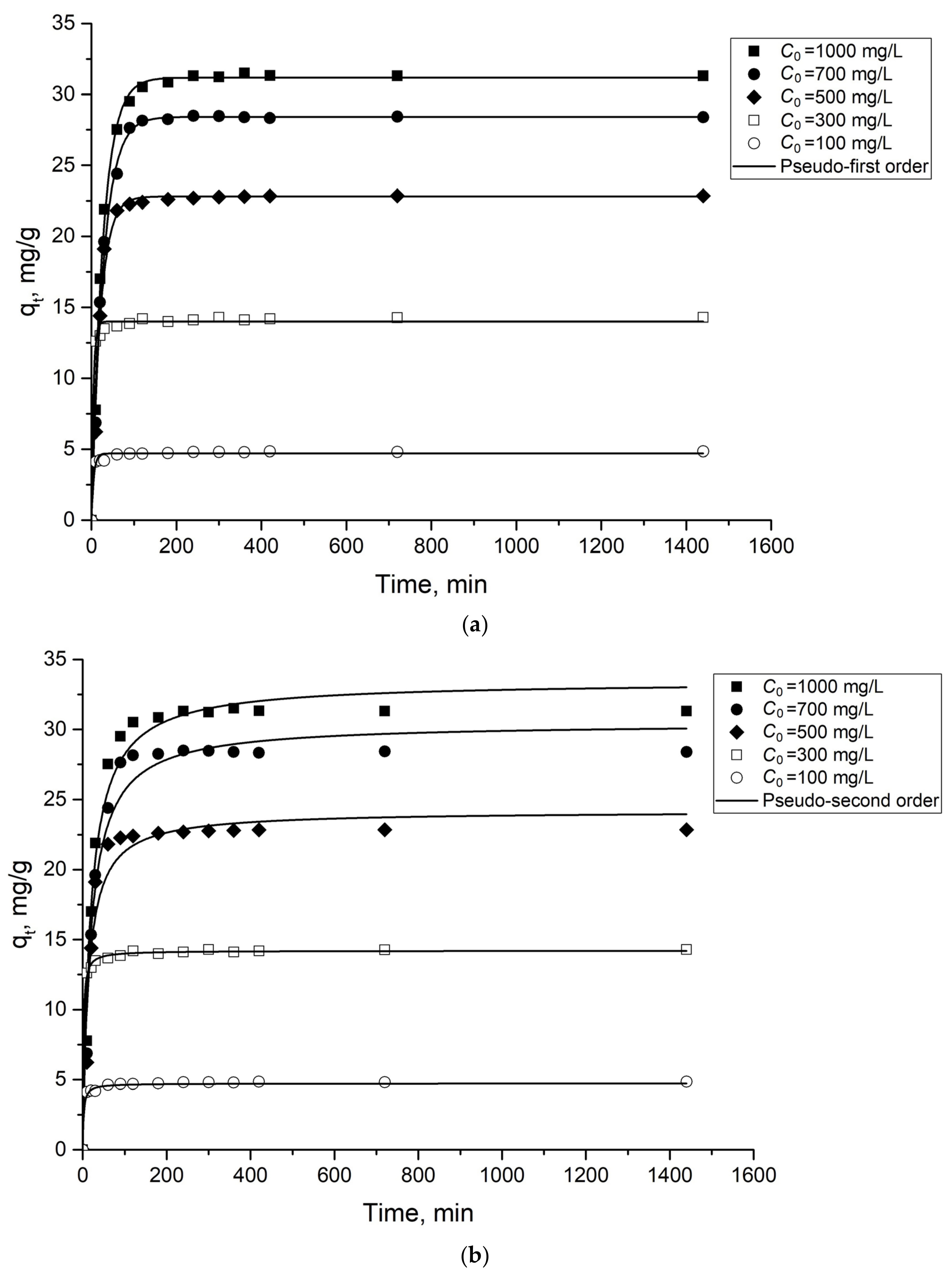



| C0 Mo (VI), mg/L | Pseudo-First Order | Pseudo-Second Order | qe Experimental, mg/g | ||||
|---|---|---|---|---|---|---|---|
| k1 | qe1, mg/g | R2 | k2 | qe2, mg/g | R2 | ||
| 100 | 0.186 | 4.694 | 0.977 | 0.099 | 4.722 | 0.921 | 4.841 |
| 300 | 0.048 | 14.205 | 0.998 | 0.217 | 13.991 | 0.992 | 14.294 |
| 500 | 0.048 | 22.812 | 0.986 | 0.003 | 24.185 | 0.953 | 22.856 |
| 700 | 0.036 | 28.423 | 0.996 | 0.002 | 30.456 | 0.972 | 28.385 |
| 1000 | 0.037 | 31.187 | 0.996 | 0.002 | 33.431 | 0.976 | 31.9 |
| Model Parameters | Temperature, K | |||
|---|---|---|---|---|
| 298 | 308 | 318 | ||
| Langmuir | qm, mg/g | 32.61 | 32.246 | 31.662 |
| bl, L/g | 0.056 | 0.036 | 0.026 | |
| R2 | 0.994 | 0.991 | 0.993 | |
| Freundlich | KF, mg/g | 7.189 | 5.794 | 4.681 |
| 1/n | 0.267 | 0.292 | 0.317 | |
| R2 | 0.923 | 0.902 | 0.912 | |
| Temkin | bT, J/mol | 427.204 | 410.582 | 410.835 |
| A, L/g | 0.883 | 0.441 | 0.283 | |
| R2 | 0.991 | 0.982 | 0.985 | |
| Adsorbent | qe, mg/g | SBET, m2/g | Equilibrium Time, min | Ref. |
|---|---|---|---|---|
| γ-Al2O3 | 31 | 100 | - | [40] |
| Magnetic Cr-ferrite | 26.8 | - | 180 | [30] |
| Mo(VI) ion-imprinted polymer | 126.06 | 307.32 | 10 | [41] |
| chitosan sorbent | 124.34 | - | 15 | [42] |
| Modified drinking water treatment residues | 39.52 | 36.73 | - | [23] |
| Activated carbon | 16.54 | - | 30 | [43] |
| Multiwalled carbon nanotubes | 18.4 | 167 | 360 | [44] |
| Commercial Al2O3 | 31.9 | 65.5 | 100 | This work |
| T, K | ΔG, kJ/mol | ΔH, kJ/mol | ΔS, kJ/mol∙K |
|---|---|---|---|
| 298 | −8.41 | −48.044 | −0.133 |
| 308 | −7.08 | ||
| 318 | −5.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurmysheva, A.Y.; Vedenyapina, M.D.; Kulaishin, S.A.; Podrabinnik, P.; Pinargote, N.W.S.; Smirnov, A.; Metel, A.S.; Bartolomé, J.F.; Grigoriev, S.N. Adsorption Removal of Mo(VI) from an Aqueous Solution by Alumina with the Subsequent Regeneration of the Adsorbent. Int. J. Mol. Sci. 2023, 24, 8700. https://doi.org/10.3390/ijms24108700
Kurmysheva AY, Vedenyapina MD, Kulaishin SA, Podrabinnik P, Pinargote NWS, Smirnov A, Metel AS, Bartolomé JF, Grigoriev SN. Adsorption Removal of Mo(VI) from an Aqueous Solution by Alumina with the Subsequent Regeneration of the Adsorbent. International Journal of Molecular Sciences. 2023; 24(10):8700. https://doi.org/10.3390/ijms24108700
Chicago/Turabian StyleKurmysheva, Alexandra Yu., Marina D. Vedenyapina, Stanislav A. Kulaishin, Pavel Podrabinnik, Nestor Washington Solís Pinargote, Anton Smirnov, Alexander S. Metel, José F. Bartolomé, and Sergey N. Grigoriev. 2023. "Adsorption Removal of Mo(VI) from an Aqueous Solution by Alumina with the Subsequent Regeneration of the Adsorbent" International Journal of Molecular Sciences 24, no. 10: 8700. https://doi.org/10.3390/ijms24108700
APA StyleKurmysheva, A. Y., Vedenyapina, M. D., Kulaishin, S. A., Podrabinnik, P., Pinargote, N. W. S., Smirnov, A., Metel, A. S., Bartolomé, J. F., & Grigoriev, S. N. (2023). Adsorption Removal of Mo(VI) from an Aqueous Solution by Alumina with the Subsequent Regeneration of the Adsorbent. International Journal of Molecular Sciences, 24(10), 8700. https://doi.org/10.3390/ijms24108700










