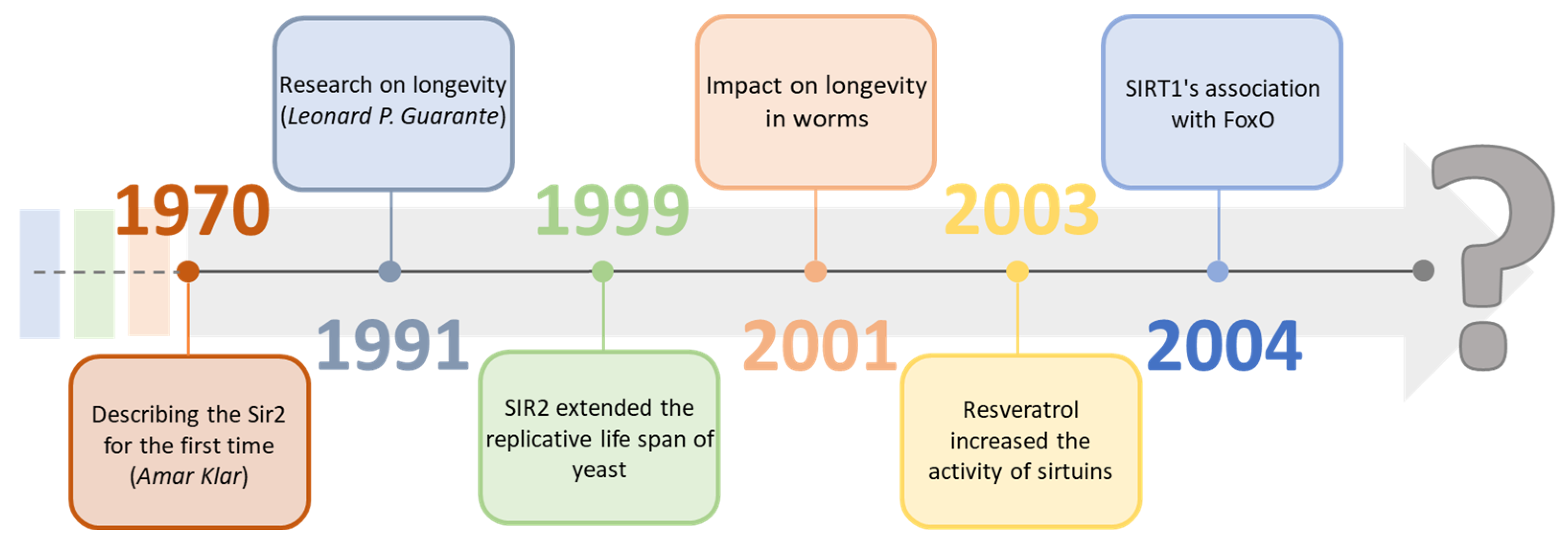
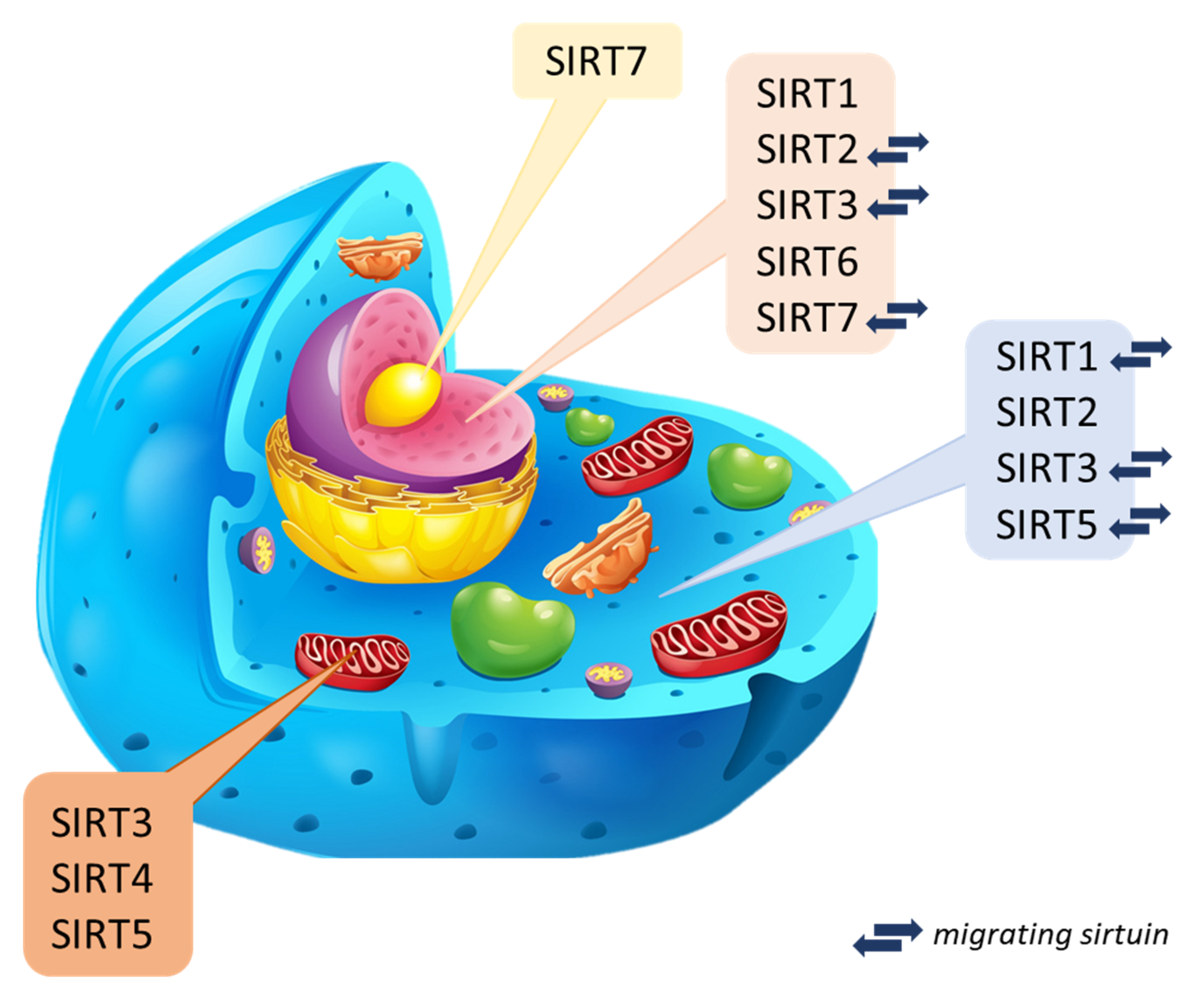
Why Is Longevity Still a Scientific Mystery? Sirtuins—Past, Present and Future
Abstract
1. Introduction
2. General Characteristics of Sirtuins: Classification, Structure, and Localization
2.1. Sirtuin 1 (SIRT1)
2.2. Sirtuin 2 (SIRT2)
2.3. Sirtuin 3 (SIRT3)
2.4. Sirtuin 4 (SIRT4)
2.5. Sirtuin 5 (SIRT5)
2.6. Sirtuin 6 (SIRT6)
2.7. Sirtuin 7 (SIRT7)
2.8. Sirtuin 8—Myth or Fact?
2.9. Sirtuins and Invertebrates
3. Enzymatic Activity
3.1. The Sirtuin Activity in the Light of Exercise Training and Caloric Restriction
3.2. FOXO Proteins and P53 Protein
4. Modulators of Sirtuin Activity
4.1. Resveratrol—An Antidote for Aging?
4.2. Polydatin—An Improved Resveratrol?
4.3. Honokiol—An Effective Antioxidant
4.4. Triclosan-Synthetic Sirtuin Activator
4.5. Cambinol–SIRT 1 and SIRT 2 Inhibitor
4.6. EX-527—A Precursor of Modern Sirtuin Inhibitors
| Modulator | Chemical Formula | Effect | Characteristic | Reference |
|---|---|---|---|---|
| Resveratrol | 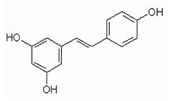 | activator |
| [187,229] |
| Polydatin |  | activator |
| [84,199,204] |
| Honokiol | 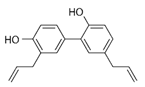 | activator |
| [206,208,213] |
| Triclosan | 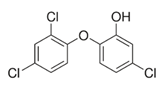 | activator |
| [230,231,232] |
| Cambinol | 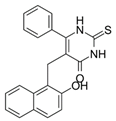 | inhibitor |
| [219,220,221,233] |
| EX-527 |  | inhibitor |
| [155,226,227] |
5. Diseases and Sirtuin-Based Therapies
6. Sirtuins as an Elixir of Youth?
7. The Darkside of Sirtuins
8. Looking into the Future—Are We Ready for Longevity?
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Ahr | aryl hydrocarbon receptor |
| Akt | Type of serine/threonine protein kinase |
| AMPK | AMP-activated protein kinase |
| ATP | Adenosine triphosphate |
| BAX | Bcl-2 Associated X-protein |
| BCAA | branched-chain amino acid |
| Bcl-2 | B-cell lymphoma 2 |
| CAT | Catalase |
| CaMKIIα | Calcium/calmodulin-dependent protein kinase type II subunit alpha |
| CB1 | Cannabinoid type 1 receptor |
| CHD | Coronary heart disease |
| CKD | Chronic kidney disease |
| COX | Cyclooxygenase |
| CRC cell lines | Colorectal cancer cell lines |
| CRP | C Reactive Protein |
| CuE | cucurbitacin E glucoside |
| daf-2 | abnormal dauer formation protein 2 |
| DHP | 1,4-dihydropyridine |
| DIC | Doxorubicin-Induced Cardiotoxicity |
| DNA | deoxyribonucleic acid |
| DOX | doxorubicin |
| dSir2 | Drosophila Sir2 |
| E11/gp38 | transmembrane glycoprotein |
| ERK | Extracellular signal-regulated kinase |
| EX-527 | 6-Chloro-2,3,4,9-tetrahydro-1H-Carbazole-1-carboxamide |
| EZH2 | Enhancer of zeste homolog 2 |
| FATP2 | Fatty acid transport protein 2 |
| FATP5 | Fatty acid transport protein 5 |
| FKHR | Forkhead protein |
| FKHRL1 | Transcription factor |
| FOXC1 | Forkhead Box C1 |
| FOXM1 | Forkhead Box M1 |
| FOXO | Forkhead Box O |
| FoxO | Transcription factor |
| FOXO1 | Forkhead Box protein O1(FKHR) |
| FOXO3A | Forkhead Box protein O3 (FKHRL1) |
| FOXO4 | Forkhead Box Protein O4 (AFX1) |
| FOXO6 | Forkhead Box Protein O6 |
| SOD1-G93A | Transgene |
| GABA | Gamma-aminobutyric acid |
| GADD45 | The Growth Arrest and DNA Damage |
| GLUT2 | Glucose transporter 2 |
| H2O2 | Hydrogen peroxide |
| HDAC | Histone deacetylases |
| Hif-1a | Hypoxia-inducible factor 1 |
| HIV | Human immunodeficiency virus |
| HK2 | Hexokinase 2 |
| HKL | Honokiol |
| HSP | Heat-shock proteins |
| IGF-1 | Insulin-like Growth Factor 1 |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin 6 |
| IRT | Immunoreactive Trypsinogen |
| Keap1 | Kelch-like ECH-associated protein 1 |
| KLF15 | Krüppel-like factor 15 |
| LSK | Lin-Sca1(+) c-kit(+) |
| MAPK | mitogen-activated protein kinase |
| MDL-811 | Chemical drug, SIRT6 activator |
| MDM2 | Mouse double minute 2 homolog |
| miR-323-3p | microRNA-323-3p |
| MnSOD | manganese superoxide dismutase |
| mtSIRT | mitochondrial sirtuins |
| NAD+ | nicotinamide adenine dinucleotide (oxidized form) |
| NADH | nicotinamide adenine dinucleotide (reduced form) |
| NAFLD | Non-alcoholic fatty liver disease |
| NAMPT | nicotinamide phosphoribosyltransferase |
| NBS1 | nibrin |
| NF-κB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NRK1 | Nicotinamide riboside kinase |
| NSC-34 | Neuroblastoma hybrid cell line |
| p21 | cyclin-dependent kinase inhibitor 1 |
| p53 | cellular tumor antigen p53 |
| PCAF | P300/CBP-associated factor |
| PCOS | Polycystic ovary syndrome |
| PD | polydatin (reservatrol-3-β-mono-D-glucoside) |
| PDAC | pancreatic ductal adenocarcinoma |
| PGC-1 α | Peroxisome proliferator–activated receptor gamma coactivator-1 alpha |
| PI3K | Phosphoinositide 3-kinases |
| PKM2 | pyruvate kinase isozymes M1/M2 |
| Pol | DNA polymerase I |
| Prx1 | peroxiredoxin PRX1 |
| PUFA | polyunsaturated fatty acids |
| RSV | resveratrol |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus 2 |
| SIR2 | NAD-dependent histone deacetylase |
| Sir-2 | Yeast Silent Information Regulators II, SIR2 |
| SIRT | sirtuin |
| SOD | superoxide dismutase |
| SOD2 | superoxide dismutase 2 |
| STACs | Sirtuin-activating compounds |
| TBI | Traumatic brain injury |
| TCS | triclosan |
| Teacrine | 1,3,7,9-tetramethyluric acid |
| TERT | telomerase reverse transcriptase |
| Th17 cells | T helper 17 cells |
| TLRS | Toll-like receptors |
| TNF-α | tumor necrosis factor α |
| UCP-1 | Uncoupling protein 1 |
References
- Kaeberlein, M.; McVey, M.; Guarente, L. The SIR2/3/4 Complex and SIR2 Alone Promote Longevity in Saccharomyces Cerevisiae by Two Different Mechanisms. Genes Dev. 1999, 13, 2570–2580. [Google Scholar] [CrossRef]
- Grootaert, M.O.J.; Bennett, M.R. Sirtuins in Atherosclerosis: Guardians of Healthspan and Therapeutic Targets. Nat. Rev. Cardiol. 2022, 19, 668–683. [Google Scholar] [CrossRef]
- Taborsky, B.; Kuijper, B.; Fawcett, T.W.; English, S.; Leimar, O.; McNamara, J.M.; Ruuskanen, S. An Evolutionary Perspective on Stress Responses, Damage and Repair. Horm. Behav. 2022, 142, 105180. [Google Scholar] [CrossRef]
- Naseer, A.; Mir, S.S.; Takacs-Vellai, K.; Nazir, A. Sirtuins and Autophagy in Age-Associated Neurodegenerative Diseases: Lessons from the C. Elegans Model. Int. J. Mol. Sci. 2021, 22, 12263. [Google Scholar] [CrossRef]
- Wang, Y.; He, J.; Liao, M.; Hu, M.; Li, W.; Ouyang, H.; Wang, X.; Ye, T.; Zhang, Y.; Ouyang, L. An Overview of Sirtuins as Potential Therapeutic Target: Structure, Function and Modulators. Eur. J. Med. Chem. 2019, 161, 48–77. [Google Scholar] [CrossRef]
- Haigis, M.C.; Sinclair, D.A. Mammalian Sirtuins: Biological Insights and Disease Relevance. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 253–295. [Google Scholar] [CrossRef]
- Couzin-Frankel, J. Aging Genes: The Sirtuin Story Unravels. Science 2011, 334, 1194–1198. [Google Scholar] [CrossRef]
- Dang, W. The Controversial World of Sirtuins. Drug Discov. Today Technol. 2014, 12, e9–e17. [Google Scholar] [CrossRef]
- Gold, D.A.; Sinclair, D.A. Sirtuin Evolution at the Dawn of Animal Life. Mol. Biol. Evol. 2022, 39, msac192. [Google Scholar] [CrossRef]
- Costantini, S.; Sharma, A.; Raucci, R.; Costantini, M.; Autiero, I.; Colonna, G. Genealogy of an Ancient Protein Family: The Sirtuins, a Family of Disordered Members. BMC Evol. Biol. 2013, 13, 60. [Google Scholar] [CrossRef]
- Curry, A.M.; White, D.S.; Donu, D.; Cen, Y. Human Sirtuin Regulators: The “Success” Stories. Front. Physiol. 2021, 12, 1853. [Google Scholar] [CrossRef]
- He, L.; Wang, J.; Yang, Y.; Li, J.; Tu, H. Mitochondrial Sirtuins in Parkinson’s Disease. Neurochem. Res. 2022, 47, 1491–1502. [Google Scholar] [CrossRef]
- Mei, Z.; Zhang, X.; Yi, J.; Huang, J.; He, J.; Tao, Y. Sirtuins in Metabolism, DNA Repair and Cancer. J. Exp. Clin. Cancer Res. 2016, 35, 182. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, S.; Gan, L.; Vosler, P.S.; Gao, Y.; Zigmond, M.J.; Chen, J. Protective Effects and Mechanisms of Sirtuins in the Nervous System. Prog. Neurobiol. 2011, 95, 373–395. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, H.; Xu, S.; Liu, Z.; Yang, L.; Fang, L.; Huo, Z.; Yan, X. Characterization of the SIRT Genes and Their Distinct Expression Patterns in Manila Clams (Ruditapes Philippinarum) Exposed to Air. Meta Gene 2021, 28, 100892. [Google Scholar] [CrossRef]
- Denu, R.A.; Hematti, P. Sirtuins and Stem Cell Maintenance, Proliferation, and Differentiation. In Sirtuin Biology in Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 175–190. [Google Scholar]
- Liu, T.; Yang, L.; Mao, H.; Ma, F.; Wang, Y.; Li, S.; Li, P.; Zhan, Y. Sirtuins as Novel Pharmacological Targets in Podocyte Injury and Related Glomerular Diseases. Biomed. Pharmacother. 2022, 155, 113620. [Google Scholar] [CrossRef]
- Gámez-García, A.; Vazquez, B.N. Nuclear Sirtuins and the Aging of the Immune System. Genes 2021, 12, 1856. [Google Scholar] [CrossRef]
- Watroba, M.; Szukiewicz, D. Sirtuins at the Service of Healthy Longevity. Front. Physiol. 2021, 12, 724506. [Google Scholar] [CrossRef]
- Chang, H.-C.; Guarente, L. SIRT1 and Other Sirtuins in Metabolism. Trends Endocrinol. Metab. 2014, 25, 138–145. [Google Scholar] [CrossRef]
- Priya, S.H.; Kedari, G.S.R.; Naidu, M.P. Higher Serum Sirtuin 1 Levels and GA Heterozygote of SIRT1 Gene Polymorphism Rs10823108 Serve as Independent Risk Factor for Diabetic Nephropathy in Women. Hum. Gene 2022, 34, 201084. [Google Scholar] [CrossRef]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. DNA Damage, Sirtuins, and Epigenetic Marks. In Epigenetics and DNA Damage; Elsevier: Amsterdam, The Netherlands, 2022; pp. 87–108. [Google Scholar]
- Lagunas-Rangel, F.A. Bioinformatic Analysis of SIRT7 Sequence and Structure. J. Biomol. Struct. Dyn. 2022, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hałasa, M.; Bartuzi, D.; Cieślak, D.; Kaczor, A.A.; Miziak, P.; Stepulak, A.; Matosiuk, D. Role of N-Terminus in Function and Dynamics of Sirtuin 7: An in Silico Study. J. Biomol. Struct. Dyn. 2020, 38, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.; Liu, G.-H.; Qu, J. Mitochondrial Sirtuins, Metabolism, and Aging. J. Genet. Genom. 2022, 49, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; Barman, M.; Bandyopadhyay, U.; Bindu, S. Sirtuins as Endogenous Regulators of Lung Fibrosis: A Current Perspective. Life Sci. 2020, 258, 118201. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.Z.; Haris, M.S.; Khan, M.S.; Mahjabeen, I. Role of Mitochondrial Sirtuins in Rheumatoid Arthritis. Biochem. Biophys. Res. Commun. 2021, 584, 60–65. [Google Scholar] [CrossRef]
- Mahjabeen, I.; Rizwan, M.; Fareen, G.; Waqar Ahmed, M.; Farooq Khan, A.; Akhtar Kayani, M. Mitochondrial Sirtuins Genetic Variations and Gastric Cancer Risk: Evidence from Retrospective Observational Study. Gene 2022, 807, 145951. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Yang, Z.-X.; Ma, P.-F.; Liu, J.-S.; Wang, D.-S.; Yu, H.-C. Overexpression of SLC25A51 Promotes Hepatocellular Carcinoma Progression by Driving Aerobic Glycolysis through Activation of SIRT5. Free Radic. Biol. Med. 2022, 182, 11–22. [Google Scholar] [CrossRef]
- van de Ven, R.A.H.; Santos, D.; Haigis, M.C. Mitochondrial Sirtuins and Molecular Mechanisms of Aging. Trends Mol. Med. 2017, 23, 320–331. [Google Scholar] [CrossRef]
- Tang, X.; Chen, X.-F.; Chen, H.-Z.; Liu, D.-P. Mitochondrial Sirtuins in Cardiometabolic Diseases. Clin. Sci. 2017, 131, 2063–2078. [Google Scholar] [CrossRef]
- Anderson, G.; Maes, M. Sirtuins, Mitochondria, and the Melatonergic Pathway in Alzheimer’s Disease. In Sirtuin Biology in Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 117–135. [Google Scholar]
- Olmos, Y.; Brosens, J.J.; Lam, E.W.-F. Interplay between SIRT Proteins and Tumour Suppressor Transcription Factors in Chemotherapeutic Resistance of Cancer. Drug Resist. Updates 2011, 14, 35–44. [Google Scholar] [CrossRef]
- Bai, W.; Zhang, X. Nucleus or Cytoplasm? The Mysterious Case of SIRT1’s Subcellular Localization. Cell Cycle 2016, 15, 3337–3338. [Google Scholar] [CrossRef] [PubMed]
- Yanagisawa, S.; Baker, J.R.; Vuppusetty, C.; Koga, T.; Colley, T.; Fenwick, P.; Donnelly, L.E.; Barnes, P.J.; Ito, K. The Dynamic Shuttling of SIRT1 between Cytoplasm and Nuclei in Bronchial Epithelial Cells by Single and Repeated Cigarette Smoke Exposure. PLoS ONE 2018, 13, e0193921. [Google Scholar] [CrossRef] [PubMed]
- Kupis, W.; Pałyga, J.; Tomal, E.; Niewiadomska, E. The Role of Sirtuins in Cellular Homeostasis. J. Physiol. Biochem. 2016, 72, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Loharch, S.; Chhabra, S.; Kumar, A.; Swarup, S.; Parkesh, R. Discovery and Characterization of Small Molecule SIRT3-Specific Inhibitors as Revealed by Mass Spectrometry. Bioorg. Chem. 2021, 110, 104768. [Google Scholar] [CrossRef]
- Ramarao, S.; Pothireddy, M.; Venkateshwarlu, R.; Moturu, K.M.V.R.; Siddaiah, V.; Kapavarapu, R.; Dandela, R.; Pal, M. Sonochemical Synthesis and In Silico Evaluation of Imidazo[1,2-a]Pyridine Derivatives as Potential Inhibitors of Sirtuins. Polycycl. Aromat. Compd. 2022, 1–20. [Google Scholar] [CrossRef]
- Smith, J.S. Human Sir2 and the ‘Silencing’ of P53 Activity. Trends Cell Biol. 2002, 12, 404–406. [Google Scholar] [CrossRef]
- Selepe, M.A.; Kunyane, P.; Seboletswe, P.; Nair, S.; Cele, N.; Engelbrecht, M.; Joubert, D.F.; Vandevoorde, C.; Singh, P.; Sonopo, M.S. Synthesis and Evaluation of Benzoylbenzofurans and Isoflavone Derivatives as Sirtuin 1 Inhibitors with Antiproliferative Effects on Cancer Cells. Bioorg. Chem. 2022, 128, 106101. [Google Scholar] [CrossRef]
- Najafi, M.; Nikpayam, O.; Tavakoli-Rouzbehani, O.M.; Papi, S.; Bioky, A.A.; Ahmadiani, E.S.; Sohrab, G. A Comprehensive Insight into the Potential Effects of Resveratrol Supplementation on SIRT-1: A Systematic Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 102224. [Google Scholar] [CrossRef]
- Li, J.; Yang, C.; Ran, J.; Yu, C.; Yin, L.; Li, Z.; Liu, Y. The Age-Dependent Variations for Fatty Acid Composition and Sensory Quality of Chicken Meat and Associations between Gene Expression Patterns and Meat Quality. Livest. Sci. 2021, 254, 104736. [Google Scholar] [CrossRef]
- Sivakumar, K.K.; Stanley, J.A.; Behlen, J.C.; Wuri, L.; Dutta, S.; Wu, J.; Arosh, J.A.; Banu, S.K. Inhibition of Sirtuin-1 Hyperacetylates P53 and Abrogates Sirtuin-1-P53 Interaction in Cr(VI)-Induced Apoptosis in the Ovary. Reprod. Toxicol. 2022, 109, 121–134. [Google Scholar] [CrossRef]
- Zhao, J.; Tan, Y.; Feng, Z.; Zhou, Y.; Wang, F.; Zhou, G.; Yan, J.; Nie, X. Catalpol Attenuates Polycystic Ovarian Syndrome by Regulating Sirtuin 1 Mediated NF-ΚB Signaling Pathway. Reprod. Biol. 2022, 22, 100671. [Google Scholar] [CrossRef] [PubMed]
- Charążka, B.; Siejka, A. Correlations between Serum Sirtuin Levels and Cardiovascular Risk Factors in Women with Polycystic Ovary Syndrome. Adv. Med. Sci. 2022, 67, 123–128. [Google Scholar] [CrossRef]
- Packer, M. Mutual Antagonism of Hypoxia-Inducible Factor Isoforms in Cardiac, Vascular, and Renal Disorders. JACC Basic Transl. Sci. 2020, 5, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Eldridge, M.J.G.; Pereira, J.M.; Impens, F.; Hamon, M.A. Active Nuclear Import of the Deacetylase Sirtuin-2 Is Controlled by Its C-Terminus and Importins. Sci. Rep. 2020, 10, 2034. [Google Scholar] [CrossRef]
- Hoffmann, G.; Breitenbücher, F.; Schuler, M.; Ehrenhofer-Murray, A.E. A Novel Sirtuin 2 (SIRT2) Inhibitor with P53-Dependent Pro-Apoptotic Activity in Non-Small Cell Lung Cancer. J. Biol. Chem. 2014, 289, 5208–5216. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Hiratsuka, M.; Osaki, M.; Yamada, H.; Kishimoto, I.; Yamaguchi, S.; Nakano, S.; Katoh, M.; Ito, H.; Oshimura, M. SIRT2, a Tubulin Deacetylase, Acts to Block the Entry to Chromosome Condensation in Response to Mitotic Stress. Oncogene 2007, 26, 945–957. [Google Scholar] [CrossRef]
- Wang, H.-L.; Ma, X.; Guan, X.-Y.; Song, C.; Li, G.-B.; Yu, Y.-M.; Yang, L.-L. Potential Synthetic Lethality for Breast Cancer: A Selective Sirtuin 2 Inhibitor Combined with a Multiple Kinase Inhibitor Sorafenib. Pharmacol. Res. 2022, 177, 106050. [Google Scholar] [CrossRef]
- Head, P.E.; Zhang, H.; Bastien, A.J.; Koyen, A.E.; Withers, A.E.; Daddacha, W.B.; Cheng, X.; Yu, D.S. Sirtuin 2 Mutations in Human Cancers Impair Its Function in Genome Maintenance. J. Biol. Chem. 2017, 292, 9919–9931. [Google Scholar] [CrossRef]
- Park, S.; Chung, M.-J.; Son, J.-Y.; Yun, H.H.; Park, J.-M.; Yim, J.-H.; Jung, S.-J.; Lee, S.-H.; Jeong, K.-S. The Role of Sirtuin 2 in Sustaining Functional Integrity of the Liver. Life Sci. 2021, 285, 119997. [Google Scholar] [CrossRef]
- Chen, X.; Lu, W.; Wu, D. Sirtuin 2 (SIRT2): Confusing Roles in the Pathophysiology of Neurological Disorders. Front. Neurosci. 2021, 15, 518. [Google Scholar] [CrossRef]
- Jeong, S.-G.; Cho, G.-W. The Tubulin Deacetylase Sirtuin-2 Regulates Neuronal Differentiation through the ERK/CREB Signaling Pathway. Biochem. Biophys. Res. Commun. 2017, 482, 182–187. [Google Scholar] [CrossRef]
- Chojdak-Łukasiewicz, J.; Bizoń, A.; Waliszewska-Prosół, M.; Piwowar, A.; Budrewicz, S.; Pokryszko-Dragan, A. Role of Sirtuins in Physiology and Diseases of the Central Nervous System. Biomedicines 2022, 10, 2434. [Google Scholar] [CrossRef]
- Boardman, N.T.; Migally, B.; Pileggi, C.; Parmar, G.S.; Xuan, J.Y.; Menzies, K.; Harper, M.-E. Glutaredoxin-2 and Sirtuin-3 Deficiencies Impair Cardiac Mitochondrial Energetics but Their Effects Are Not Additive. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2021, 1867, 165982. [Google Scholar] [CrossRef]
- Jaiswal, A.; Xudong, Z.; Zhenyu, J.; Saretzki, G. Mitochondrial Sirtuins in Stem Cells and Cancer. FEBS J. 2022, 289, 3393–3415. [Google Scholar] [CrossRef]
- Lagunas-Rangel, F.A. Current Role of Mammalian Sirtuins in DNA Repair. DNA Repair 2019, 80, 85–92. [Google Scholar] [CrossRef]
- Shi, T.; Wang, F.; Stieren, E.; Tong, Q. SIRT3, a Mitochondrial Sirtuin Deacetylase, Regulates Mitochondrial Function and Thermogenesis in Brown Adipocytes. J. Biol. Chem. 2005, 280, 13560–13567. [Google Scholar] [CrossRef]
- Bellizzi, D.; Dato, S.; Cavalcante, P.; Covello, G.; di Cianni, F.; Passarino, G.; Rose, G.; de Benedictis, G. Characterization of a Bidirectional Promoter Shared between Two Human Genes Related to Aging: SIRT3 and PSMD13. Genomics 2007, 89, 143–150. [Google Scholar] [CrossRef]
- Li, Q.; Wang, R.; Zhang, Z.; Wang, H.; Lu, X.; Zhang, J.; Kong, A.P.-S.; Tian, X.Y.; Chan, H.-F.; Chung, A.C.-K.; et al. Sirt3 Mediates the Benefits of Exercise on Bone in Aged Mice. Cell Death Differ. 2022. [Google Scholar] [CrossRef]
- Liu, L.; Lu, H.; Loor, J.J.; Aboragah, A.; Du, X.; He, J.; Peng, T.; Su, J.; Wang, Z.; Liu, G.; et al. Sirtuin 3 Inhibits Nuclear Factor-ΚB Signaling Activated by a Fatty Acid Challenge in Bovine Mammary Epithelial Cells. J. Dairy Sci. 2021, 104, 12871–12880. [Google Scholar] [CrossRef]
- Cao, M.; Zhao, Q.; Sun, X.; Qian, H.; Lyu, S.; Chen, R.; Xia, H.; Yuan, W. Sirtuin 3: Emerging Therapeutic Target for Cardiovascular Diseases. Free Radic. Biol. Med. 2022, 180, 63–74. [Google Scholar] [CrossRef]
- Liu, Y.; Shen, X.; Pang, M.; Sun, Z.; Qian, Y.; Xue, W.; Wang, Z.; Li, L. Role of Histone Deacetylase Sirt3 in the Development and Regression of Atherosclerosis. Life Sci. 2021, 272, 119178. [Google Scholar] [CrossRef] [PubMed]
- Min, Z.; Gao, J.; Yu, Y. The Roles of Mitochondrial SIRT4 in Cellular Metabolism. Front. Endocrinol. 2019, 9, 783. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Y.; Wang, F.; Chen, X.; Wang, C.; Wang, J.; Liu, T.; Li, Y.; He, B. SIRT4 Is the Last Puzzle of Mitochondrial Sirtuins. Bioorg. Med. Chem. 2018, 26, 3861–3865. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zheng, W. Mammalian Sirtuins SIRT4 and SIRT7. Prog. Mol. Biol. Transl. Sci. 2018, 154, 147–168. [Google Scholar]
- He, L.; Wang, J.; Yang, Y.; Zou, P.; Xia, Z.; Li, J. SIRT4 Suppresses Doxorubicin-Induced Cardiotoxicity by Regulating the AKT/MTOR/Autophagy Pathway. Toxicology 2022, 469, 153119. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, H.; Luo, G.; Dai, X. SIRT4 Inhibits Cigarette Smoke Extracts-Induced Mononuclear Cell Adhesion to Human Pulmonary Microvascular Endothelial Cells via Regulating NF-ΚB Activity. Toxicol. Lett. 2014, 226, 320–327. [Google Scholar] [CrossRef]
- Molinari, F.; Feraco, A.; Mirabilii, S.; Saladini, S.; Sansone, L.; Vernucci, E.; Tomaselli, G.; Marzolla, V.; Rotili, D.; Russo, M.A.; et al. SIRT5 Inhibition Induces Brown Fat-Like Phenotype in 3T3-L1 Preadipocytes. Cells 2021, 10, 1126. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, H.; He, B.; Du, J.; Lin, H.; Cerione, R.A.; Hao, Q. The Bicyclic Intermediate Structure Provides Insights into the Desuccinylation Mechanism of Human Sirtuin 5 (SIRT5). J. Biol. Chem. 2012, 287, 28307–28314. [Google Scholar] [CrossRef]
- Jung, Y.H.; Chae, C.W.; Chang, H.S.; Choi, G.E.; Lee, H.J.; Han, H.J. Silencing SIRT5 Induces the Senescence of UCB-MSCs Exposed to TNF-α by Reduction of Fatty Acid β-Oxidation and Anti-Oxidation. Free Radic. Biol. Med. 2022, 192, 1–12. [Google Scholar] [CrossRef]
- Korotkov, A.; Seluanov, A.; Gorbunova, V. Sirtuin 6: Linking Longevity with Genome and Epigenome Stability. Trends Cell Biol. 2021, 31, 994–1006. [Google Scholar] [CrossRef]
- Bian, C.; Zhang, R.; Wang, Y.; Li, J.; Song, Y.; Guo, D.; Gao, J.; Ren, H. Sirtuin 6 Affects Glucose Reabsorption and Gluconeogenesis in Type 1 Diabetes via FoxO1. Mol. Cell. Endocrinol. 2022, 547, 111597. [Google Scholar] [CrossRef] [PubMed]
- Mostoslavsky, R.; Chua, K.F.; Lombard, D.B.; Pang, W.W.; Fischer, M.R.; Gellon, L.; Liu, P.; Mostoslavsky, G.; Franco, S.; Murphy, M.M.; et al. Genomic Instability and Aging-like Phenotype in the Absence of Mammalian SIRT6. Cell 2006, 124, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Carreño, M.; Bresque, M.; Machado, M.R.; Santos, L.; Durán, R.; Vitturi, D.A.; Escande, C.; Denicola, A. Nitro-Fatty Acids as Activators of HSIRT6 Deacetylase Activity. J. Biol. Chem. 2020, 295, 18355–18366. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Z.; Gao, Q. Transfer of MicroRNA-25 by Colorectal Cancer Cell-Derived Extracellular Vesicles Facilitates Colorectal Cancer Development and Metastasis. Mol. Ther. Nucleic Acids 2021, 23, 552–564. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Lu, L.; Huang, T.; Hou, W.; Wang, F.; Yu, L.; Wu, F.; Qi, J.; Chen, X.; et al. Sirt7 Associates with ELK1 to Participate in Hyperglycemia Memory and Diabetic Nephropathy via Modulation of DAPK3 Expression and Endothelial Inflammation. Transl. Res. 2022, 247, 99–116. [Google Scholar] [CrossRef] [PubMed]
- Grummt, I.; Pikaard, C.S. Epigenetic Silencing of RNA Polymerase I Transcription. Nat. Rev. Mol. Cell Biol. 2003, 4, 641–649. [Google Scholar] [CrossRef]
- Li, G.; Tang, X.; Zhang, S.; Deng, Z.; Wang, B.; Shi, W.; Xie, H.; Liu, B.; Li, J. Aging-Conferred SIRT7 Decline Inhibits Rosacea-Like Skin Inflammation by Modulating Toll-Like Receptor 2-NF-ΚB Signaling. J. Investig. Dermatol. 2022, 142, 2580–2590.e6. [Google Scholar] [CrossRef]
- Li, X.-T.; Zhang, Y.-P.; Zhang, M.-W.; Zhang, Z.-Z.; Zhong, J.-C. Sirtuin 7 Serves as a Promising Therapeutic Target for Cardiorenal Diseases. Eur. J. Pharmacol. 2022, 925, 174977. [Google Scholar] [CrossRef]
- Li, X.-T.; Song, J.-W.; Zhang, Z.-Z.; Zhang, M.-W.; Liang, L.-R.; Miao, R.; Liu, Y.; Chen, Y.-H.; Liu, X.-Y.; Zhong, J.-C. Sirtuin 7 Mitigates Renal Ferroptosis, Fibrosis and Injury in Hypertensive Mice by Facilitating the KLF15/Nrf2 Signaling. Free Radic. Biol. Med. 2022, 193, 459–473. [Google Scholar] [CrossRef]
- Hajishengallis, G.; Li, X.; Chavakis, T. Immunometabolic Control of Hematopoiesis. Mol. Aspects Med. 2021, 77, 100923. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Xu, Z. SIRT1 Provides New Pharmacological Targets for Polydatin through Its Role as a Metabolic Sensor. Biomed. Pharmacother. 2021, 139, 111549. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jiang, F.; Yang, P.; Liu, Q.; Wang, X.; Kang, L. Characteristics and Expression Patterns of Histone-Modifying Enzyme Systems in the Migratory Locust. Insect Biochem. Mol. Biol. 2016, 76, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Tissenbaum, H.A.; Guarente, L. Increased Dosage of a Sir-2 Gene Extends Lifespan in Caenorhabditis Elegans. Nature 2001, 410, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Newman, B.L.; Lundblad, J.R.; Chen, Y.; Smolik, S.M. A Drosophila Homologue of Sir2 Modifies Position-Effect Variegation but Does Not Affect Life Span. Genetics 2002, 162, 1675–1685. [Google Scholar] [CrossRef]
- Shukla, N.; Kolthur-Seetharam, U. Drosophila Sirtuin 6 Mediates Developmental Diet-dependent Programming of Adult Physiology and Survival. Aging Cell 2022, 21, e13576. [Google Scholar] [CrossRef]
- Sejour, R.; Sanguino, R.A.; Mikolajczak, M.; Ahmadi, W.; Villa-Cuesta, E. Sirt4 Modulates Oxidative Metabolism and Sensitivity to Rapamycin through Species-Dependent Phenotypes in Drosophila MtDNA Haplotypes. G3 Genes|Genomes|Genetics 2020, 10, 1599–1612. [Google Scholar] [CrossRef]
- Carneiro Dutra, H.L.; Deehan, M.A.; Frydman, H. Wolbachia and Sirtuin-4 Interaction Is Associated with Alterations in Host Glucose Metabolism and Bacterial Titer. PLoS Pathog. 2020, 16, e1008996. [Google Scholar] [CrossRef]
- Wood, J.G.; Schwer, B.; Wickremesinghe, P.C.; Hartnett, D.A.; Burhenn, L.; Garcia, M.; Li, M.; Verdin, E.; Helfand, S.L. Sirt4 Is a Mitochondrial Regulator of Metabolism and Lifespan in Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 2018, 115, 1564–1569. [Google Scholar] [CrossRef]
- Damschroder, D.; Zapata-Pérez, R.; Richardson, K.; Vaz, F.M.; Houtkooper, R.H.; Wessells, R. Stimulating the Sir2–Spargel Axis Rescues Exercise Capacity and Mitochondrial Respiration in a Drosophila Model of Barth Syndrome. Dis. Model Mech. 2022, 15, dmm049279. [Google Scholar] [CrossRef]
- Zhang, M.; Fei, S.; Xia, J.; Wang, Y.; Wu, H.; Li, X.; Guo, Y.; Swevers, L.; Sun, J.; Feng, M. Sirt5 Inhibits BmNPV Replication by Promoting a Relish-Mediated Antiviral Pathway in Bombyx Mori. Front. Immunol. 2022, 13, 2259. [Google Scholar] [CrossRef]
- Hackett, B.A.; Dittmar, M.; Segrist, E.; Pittenger, N.; To, J.; Griesman, T.; Gordesky-Gold, B.; Schultz, D.C.; Cherry, S. Sirtuin Inhibitors Are Broadly Antiviral against Arboviruses. mBio 2019, 10, e01446-19. [Google Scholar] [CrossRef] [PubMed]
- May, M.A.; Feezell, M.K.; Gonzalez, S.J.; Vasquez, M.C.; Todgham, A.E.; Tomanek, L. Sirtuin-Dependent Recovery from Aerial Heat Shock: The Effects of Food Ration, Thermal History, and Sirtuin Inhibition on Clearance Rates and Valve Gape Activity of the California Mussel, Mytilus Californianus (Conrad). J. Exp. Mar. Biol. Ecol. 2021, 536, 151510. [Google Scholar] [CrossRef]
- Shah, S.M.A.; Taju, S.W.; Dlamini, B.B.; Ou, Y.-Y. DeepSIRT: A Deep Neural Network for Identification of Sirtuin Targets and Their Subcellular Localizations. Comput. Biol. Chem. 2021, 93, 107514. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, Y.-M.; Hu, Y.-Y.; Ouyang, L.; Sun, Z.-H.; Yin, X.-F.; Li, N.; He, Q.-Y.; Wang, Y. Inhibition of Nuclear Deacetylase Sirtuin-1 Induces Mitochondrial Acetylation and Calcium Overload Leading to Cell Death. Redox. Biol. 2022, 53, 102334. [Google Scholar] [CrossRef] [PubMed]
- Seifert, T.; Malo, M.; Kokkola, T.; Stéen, E.J.L.; Meinander, K.; Wallén, E.A.A.; Jarho, E.M.; Luthman, K. A Scaffold Replacement Approach towards New Sirtuin 2 Inhibitors. Bioorg. Med. Chem. 2020, 28, 115231. [Google Scholar] [CrossRef]
- Zähringer, S.; Rumpf, T.; Melesina, J.; Lang, A.E.; Aktories, K.; Sippl, W.; Jung, M.; Wagner, G.K. Defined Stereoisomers of 2″-Amino NAD+ and Their Activity against Human Sirtuins and a Bacterial (ADP-Ribosyl) Transferase. Bioorg. Med. Chem. 2022, 68, 116875. [Google Scholar] [CrossRef]
- Frydzińska, Z.; Owczarek, A.; Winiarska, K. Sirtuiny i Ich Rola w Regulacji Metabolizmu. Postepy Biochem. 2019, 65, 31–40. [Google Scholar] [CrossRef]
- Lin, J.-Y.; Kuo, W.-W.; Baskaran, R.; Kuo, C.-H.; Chen, Y.-A.; Chen, W.S.-T.; Ho, T.-J.; Day, C.H.; Mahalakshmi, B.; Huang, C.-Y. Swimming Exercise Stimulates IGF1/ PI3K/Akt and AMPK/SIRT1/PGC1α Survival Signaling to Suppress Apoptosis and Inflammation in Aging Hippocampus. Aging 2020, 12, 6852–6864. [Google Scholar] [CrossRef]
- Sellitto, C.; Corbi, G.; Stefanelli, B.; Manzo, V.; Trucillo, M.; Charlier, B.; Mensitieri, F.; Izzo, V.; Lucariello, A.; Perna, A.; et al. Antioxidant Supplementation Hinders the Role of Exercise Training as a Natural Activator of SIRT1. Nutrients 2022, 14, 2092. [Google Scholar] [CrossRef]
- Vargas-Ortiz, K.; Pérez-Vázquez, V.; Macías-Cervantes, M.H. Exercise and Sirtuins: A Way to Mitochondrial Health in Skeletal Muscle. Int. J. Mol. Sci. 2019, 20, 2717. [Google Scholar] [CrossRef]
- Edgett, B.A.; Hughes, M.C.; Matusiak, J.B.L.; Perry, C.G.R.; Simpson, C.A.; Gurd, B.J. SIRT3 Gene Expression but Not SIRT3 Subcellular Localization Is Altered in Response to Fasting and Exercise in Human Skeletal Muscle. Exp. Physiol. 2016, 101, 1101–1113. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Safdar, A.; Wilkin, G.P.; Tarnopolsky, M.A.; Gibala, M.J. A Practical Model of Low-Volume High-Intensity Interval Training Induces Mitochondrial Biogenesis in Human Skeletal Muscle: Potential Mechanisms. J. Physiol. 2010, 588, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Bori, Z.; Koltai, E.; Fatouros, I.G.; Jamurtas, A.Z.; Douroudos, I.I.; Terzis, G.; Nikolaidis, M.G.; Chatzinikolaou, A.; Sovatzidis, A.; et al. Age-Dependent Changes in 8-Oxoguanine-DNA Glycosylase Activity Are Modulated by Adaptive Responses to Physical Exercise in Human Skeletal Muscle. Free Radic. Biol. Med. 2011, 51, 417–423. [Google Scholar] [CrossRef]
- Wu, Q.; Gao, Z.-J.; Yu, X.; Wang, P. Dietary Regulation in Health and Disease. Signal Transduct. Target Ther. 2022, 7, 252. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, M.; Chen, J.; Duan, H.; Huang, T.; Jiang, G.; Zhong, Y. High Calorie Diet Background Alters the Expression of Sirtuins in the Testes of Mice under Caloric Restriction. Transl. Med. Aging 2021, 5, 10–16. [Google Scholar] [CrossRef]
- Vera, E.; Bernardes de Jesus, B.; Foronda, M.; Flores, J.M.; Blasco, M.A. Telomerase Reverse Transcriptase Synergizes with Calorie Restriction to Increase Health Span and Extend Mouse Longevity. PLoS ONE 2013, 8, e53760. [Google Scholar] [CrossRef]
- Amano, H.; Chaudhury, A.; Rodriguez-Aguayo, C.; Lu, L.; Akhanov, V.; Catic, A.; Popov, Y.V.; Verdin, E.; Johnson, H.; Stossi, F.; et al. Telomere Dysfunction Induces Sirtuin Repression That Drives Telomere-Dependent Disease. Cell Metab. 2019, 29, 1274–1290.e9. [Google Scholar] [CrossRef]
- Yzydorczyk, C.; Li, N.; Rigal, E.; Chehade, H.; Mosig, D.; Armengaud, J.B.; Rolle, T.; Krishnasamy, A.; Orozco, E.; Siddeek, B.; et al. Calorie Restriction in Adulthood Reduces Hepatic Disorders Induced by Transient Postnatal Overfeeding in Mice. Nutrients 2019, 11, 2796. [Google Scholar] [CrossRef]
- Tauriainen, E.; Luostarinen, M.; Martonen, E.; Finckenberg, P.; Kovalainen, M.; Huotari, A.; Herzig, K.-H.; Lecklin, A.; Mervaala, E. Distinct Effects of Calorie Restriction and Resveratrol on Diet-Induced Obesity and Fatty Liver Formation. J. Nutr. Metab. 2011, 2011, 1–10. [Google Scholar] [CrossRef]
- Heindel, J.J.; Howard, S.; Agay-Shay, K.; Arrebola, J.P.; Audouze, K.; Babin, P.J.; Barouki, R.; Bansal, A.; Blanc, E.; Cave, M.C.; et al. Obesity II: Establishing Causal Links between Chemical Exposures and Obesity. Biochem. Pharmacol. 2022, 199, 115015. [Google Scholar] [CrossRef]
- Ahmed, M.A.; O’Callaghan, C.; Chang, E.D.; Jiang, H.; Vassilopoulos, A. Context-Dependent Roles for SIRT2 and SIRT3 in Tumor Development Upon Calorie Restriction or High Fat Diet. Front. Oncol. 2020, 9, 1462. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Xu, D.; Sha, W.; Shen, L.; Lu, G.; Yin, X.; Wang, M. High Glucose Induces Renal Tubular Epithelial Injury via Sirt1/NF-KappaB/MicroR-29/Keap1 Signal Pathway. J. Transl. Med. 2015, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Mastrocola, R.; Nigro, D.; Chiazza, F.; Medana, C.; Dal Bello, F.; Boccuzzi, G.; Collino, M.; Aragno, M. Fructose-Derived Advanced Glycation End-Products Drive Lipogenesis and Skeletal Muscle Reprogramming via SREBP-1c Dysregulation in Mice. Free Radic. Biol. Med. 2016, 91, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Opstad, T.B.; Farup, P.G.; Rootwelt, H.; Aaseth, J.O. Changes in Circulating Sirtuin 1 after Bariatric Surgery. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 2858–2864. [Google Scholar] [CrossRef] [PubMed]
- Savastano, S.; di Somma, C.; Colao, A.; Barrea, L.; Orio, F.; Finelli, C.; Pasanisi, F.; Contaldo, F.; Tarantino, G. Preliminary Data on the Relationship between Circulating Levels of Sirtuin 4, Anthropometric and Metabolic Parameters in Obese Subjects According to Growth Hormone/Insulin-like Growth Factor-1 Status. Growth Horm. IGF Res. 2015, 25, 28–33. [Google Scholar] [CrossRef]
- Dali-Youcef, N.; Lagouge, M.; Froelich, S.; Koehl, C.; Schoonjans, K.; Auwerx, J. Sirtuins: The ‘Magnificent Seven’, Function, Metabolism and Longevity. Ann. Med. 2007, 39, 335–345. [Google Scholar] [CrossRef]
- Peluso, I. Diet and Exercise in Lifestyle Medicine: The Hormetic Effects of Bioactive Compounds on Human Health. Curr. Opin. Toxicol. 2022, 30, 100342. [Google Scholar] [CrossRef]
- Abrescia, P.; Treppiccione, L.; Rossi, M.; Bergamo, P. Modulatory Role of Dietary Polyunsaturated Fatty Acids in Nrf2-Mediated Redox Homeostasis. Prog. Lipid Res. 2020, 80, 101066. [Google Scholar] [CrossRef]
- Tapia, P.C. Sublethal Mitochondrial Stress with an Attendant Stoichiometric Augmentation of Reactive Oxygen Species May Precipitate Many of the Beneficial Alterations in Cellular Physiology Produced by Caloric Restriction, Intermittent Fasting, Exercise and Dietary Phytonutrients: “Mitohormesis” for Health and Vitality. Med. Hypotheses 2006, 66, 832–843. [Google Scholar] [CrossRef]
- Jerome, M.S.; Kuthethur, R.; Kabekkodu, S.P.; Chakrabarty, S. Regulation of Mitochondrial Function by Forkhead Transcription Factors. Biochimie 2022, 198, 96–108. [Google Scholar] [CrossRef]
- Martins, R.; Lithgow, G.J.; Link, W. Long Live FOXO: Unraveling the Role of FOXO Proteins in Aging and Longevity. Aging Cell 2016, 15, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Lam, E.W.-F.; Brosens, J.J.; Gomes, A.R.; Koo, C.-Y. Forkhead Box Proteins: Tuning Forks for Transcriptional Harmony. Nat. Rev. Cancer 2013, 13, 482–495. [Google Scholar] [CrossRef]
- Daitoku, H.; Hatta, M.; Matsuzaki, H.; Aratani, S.; Ohshima, T.; Miyagishi, M.; Nakajima, T.; Fukamizu, A. Silent Information Regulator 2 Potentiates Foxo1-Mediated Transcription through Its Deacetylase Activity. Proc. Natl. Acad. Sci. USA 2004, 101, 10042–10047. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Sun, H.; Lu, J.; Li, X.; Chen, X.; Tao, D.; Huang, W.; Huang, B. Lifespan Extension and Elevated Hsp Gene Expression in Drosophila Caused by Histone Deacetylase Inhibitors. J. Exp. Biol. 2005, 208, 697–705. [Google Scholar] [CrossRef]
- Merksamer, P.I.; Liu, Y.; He, W.; Hirschey, M.D.; Chen, D.; Verdin, E. The Sirtuins, Oxidative Stress and Aging: An Emerging Link. Aging 2013, 5, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Skurk, C.; Maatz, H.; Shiojima, I.; Ivashchenko, Y.; Yoon, S.; Park, Y.; Walsh, K. Akt/FOXO3a Signaling Modulates the Endothelial Stress Response through Regulation of Heat Shock Protein 70 Expression. FASEB J. 2005, 19, 1042–1044. [Google Scholar] [CrossRef]
- Greiss, S.; Hall, J.; Ahmed, S.; Gartner, A.C. Elegans SIR-2.1 Translocation Is Linked to a Proapoptotic Pathway Parallel to Cep-1 /P53 during DNA Damage-Induced Apoptosis. Genes Dev. 2008, 22, 2831–2842. [Google Scholar] [CrossRef]
- Giannakou, M.E.; Partridge, L. The Interaction between FOXO and SIRT1: Tipping the Balance towards Survival. Trends Cell Biol. 2004, 14, 408–412. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Furukawa-Hibi, Y.; Chen, C.; Horio, Y.; Isobe, K.; Ikeda, K.; Motoyama, N. SIRT1 Is Critical Regulator of FOXO-Mediated Transcription in Response to Oxidative Stress. Int. J. Mol. Med. 2005, 16, 237–243. [Google Scholar] [CrossRef]
- E Tamura, R.; F de Vasconcellos, J.; Sarkar, D.; A Libermann, T.; B Fisher, P.; F Zerbini, L. GADD45 Proteins: Central Players in Tumorigenesis. Curr. Mol. Med. 2012, 12, 634–651. [Google Scholar] [CrossRef]
- Wang, F.; Nguyen, M.; Qin, F.X.-F.; Tong, Q. SIRT2 Deacetylates FOXO3a in Response to Oxidative Stress and Caloric Restriction. Aging Cell 2007, 6, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Tyner, S.D.; Venkatachalam, S.; Choi, J.; Jones, S.; Ghebranious, N.; Igelmann, H.; Lu, X.; Soron, G.; Cooper, B.; Brayton, C.; et al. P53 Mutant Mice That Display Early Ageing-Associated Phenotypes. Nature 2002, 415, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Xia, N.; Strand, S.; Schlufter, F.; Siuda, D.; Reifenberg, G.; Kleinert, H.; Förstermann, U.; Li, H. Role of SIRT1 and FOXO Factors in ENOS Transcriptional Activation by Resveratrol. Nitric Oxide 2013, 32, 29–35. [Google Scholar] [CrossRef]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-Dependent Regulation of FOXO Transcription Factors by the SIRT1 Deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef]
- Kume, S.; Haneda, M.; Kanasaki, K.; Sugimoto, T.; Araki, S.; Isono, M.; Isshiki, K.; Uzu, T.; Kashiwagi, A.; Koya, D. Silent Information Regulator 2 (SIRT1) Attenuates Oxidative Stress-Induced Mesangial Cell Apoptosis via P53 Deacetylation. Free Radic. Biol. Med. 2006, 40, 2175–2182. [Google Scholar] [CrossRef] [PubMed]
- van der Horst, A.; Tertoolen, L.G.J.; de Vries-Smits, L.M.M.; Frye, R.A.; Medema, R.H.; Burgering, B.M.T. FOXO4 Is Acetylated upon Peroxide Stress and Deacetylated by the Longevity Protein HSir2. J. Biol. Chem. 2004, 279, 28873–28879. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, V.; Vilwanathan, R. Silencing Sirtuin 6 Induces Cell Cycle Arrest and Apoptosis in Non-Small Cell Lung Cancer Cell Lines. Genomics 2020, 112, 3703–3712. [Google Scholar] [CrossRef]
- Kim, U.; Kim, K.S.; Park, J.-K.; Um, H.-D. Hyperacetylation of the C-Terminal Domain of P53 Inhibits the Formation of the P53/P21 Complex. Biochem. Biophys. Res. Commun. 2022, 635, 52–56. [Google Scholar] [CrossRef]
- Chang, H.W.; Lee, M.; Lee, Y.S.; Kim, S.H.; Lee, J.C.; Park, J.J.; Nam, H.Y.; Kim, M.R.; Han, M.W.; Kim, S.W.; et al. P53-Dependent Glutamine Usage Determines Susceptibility to Oxidative Stress in Radioresistant Head and Neck Cancer Cells. Cell Signal 2021, 77, 109820. [Google Scholar] [CrossRef]
- Igase, M.; Fujiki, N.; Shibutani, S.; Sakai, H.; Noguchi, S.; Nemoto, Y.; Mizuno, T. Tenovin-6 Induces the SIRT-Independent Cell Growth Suppression and Blocks Autophagy Flux in Canine Hemangiosarcoma Cell Lines. Exp. Cell Res. 2020, 388, 111810. [Google Scholar] [CrossRef]
- Bordoni, V.; Tartaglia, E.; Sacchi, A.; Fimia, G.M.; Cimini, E.; Casetti, R.; Notari, S.; Grassi, G.; Marchioni, L.; Bibas, M.; et al. The Unbalanced P53/SIRT1 Axis May Impact Lymphocyte Homeostasis in COVID-19 Patients. Int. J. Infect. Dis. 2021, 105, 49–53. [Google Scholar] [CrossRef]
- Wang, P.; Chen, D.; An, J.; Lin, S.; Liu, T.; Li, Y.; Chen, L.; He, B. Development of a Single-Step Fluorogenic Sirtuin Assay and Its Applications for High-Throughput Screening. Org. Biomol. Chem. 2022, 20, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Sanders, B.D.; Jackson, B.; Marmorstein, R. Structural Basis for Sirtuin Function: What We Know and What We Don’t. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2010, 1804, 1604–1616. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Su, H.; Deng, J.; Mou, L.; Wang, H.; Li, R.; Dai, Q.-Q.; Yan, Y.-H.; Qian, S.; Wang, Z.; et al. Discovery of New Human Sirtuin 5 Inhibitors by Mimicking Glutaryl-Lysine Substrates. Eur. J. Med. Chem. 2021, 225, 113803. [Google Scholar] [CrossRef]
- Kumari, S.; Chaurasia, S.N.; Nayak, M.K.; Mallick, R.L.; Dash, D. Sirtuin Inhibition Induces Apoptosis-like Changes in Platelets and Thrombocytopenia. J. Biol. Chem. 2015, 290, 12290–12299. [Google Scholar] [CrossRef]
- Gertz, M.; Fischer, F.; Nguyen, G.T.T.; Lakshminarasimhan, M.; Schutkowski, M.; Weyand, M.; Steegborn, C. Ex-527 Inhibits Sirtuins by Exploiting Their Unique NAD+ -Dependent Deacetylation Mechanism. Proc. Natl. Acad. Sci. USA 2013, 110, E2772–E2781. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, H.; Zhang, X.; Zhang, S.; Zhu, S.; Wang, H. Resveratrol Attenuates Radiation Enteritis through the SIRT1/FOXO3a and PI3K/AKT Signaling Pathways. Biochem. Biophys. Res. Commun. 2021, 554, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Fang, L.; Xie, S.; Hu, Y.; Chen, S.; Amin, N.; Fang, M.; Hu, Z. Resveratrol Alleviates Postpartum Depression-like Behavior by Activating Autophagy via SIRT1 and Inhibiting AKT/MTOR Pathway. Behav. Brain Res. 2022, 438, 114208. [Google Scholar] [CrossRef]
- Yu, D.; Zhao, X.-Y.; Meng, Q.-P.; Teng, D.; Deng, K.; Lin, N. Resveratrol Activates the SIRT1/PGC-1 Pathway in Mice to Improve Synaptic-Related Cognitive Impairment after TBI. Brain Res. 2022, 1796, 148109. [Google Scholar] [CrossRef]
- Alcaín, F.J.; Villalba, J.M. Sirtuin Activators. Expert Opin. Ther. Pat. 2009, 19, 403–414. [Google Scholar] [CrossRef]
- Gozelle, M.; Kaya, S.G.; Aksel, A.B.; Ozkan, E.; Bakar-Ates, F.; Ozkan, Y.; Eren, G. Hit Evaluation Results in 5-Benzyl-1,3,4-Thiadiazole-2-Carboxamide Based SIRT2-Selective Inhibitor with Improved Affinity and Selectivity. Bioorg. Chem. 2022, 123, 105746. [Google Scholar] [CrossRef] [PubMed]
- Laaroussi, H.; Ding, Y.; Teng, Y.; Deschamps, P.; Vidal, M.; Yu, P.; Broussy, S. Synthesis of Indole Inhibitors of Silent Information Regulator 1 (SIRT1), and Their Evaluation as Cytotoxic Agents. Eur. J. Med. Chem. 2020, 202, 112561. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Lou, T.; Lu, J.; Wang, M.; Chen, X.; Xue, L.; Tang, X.; Qi, W.; Zhang, Z.; Su, H.; et al. Major Ginsenosides from Panax Ginseng Promote Aerobic Cellular Respiration and SIRT1-Mediated Mitochondrial Biosynthesis in Cardiomyocytes and Neurons. J. Ginseng Res. 2022, 46, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.E.; Tekiel, V.; Campo, V.A. In Vitro Evaluation of Resveratrol as a Potential Pre-Exposure Prophylactic Drug against Trypanosoma Cruzi Infection. Int. J. Parasitol. Drugs Drug Resist. 2022, 20, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Ibrahim, S.R.M.; El-Agamy, D.S.; Elsaed, W.M.; Sirwi, A.; Asfour, H.Z.; Koshak, A.E.; Elhady, S.S. Cucurbitacin E Glucoside Alleviates Concanavalin A-Induced Hepatitis through Enhancing SIRT1/Nrf2/HO-1 and Inhibiting NF-ĸB/NLRP3 Signaling Pathways. J. Ethnopharmacol. 2022, 292, 115223. [Google Scholar] [CrossRef]
- Chen, L.; Song, C.; Zhang, Y.; Li, Y.; Zhao, Y.; Lin, F.; Han, D.; Dai, M.; Li, W.; Pan, P. Quercetin Protects against LPS-Induced Lung Injury in Mice via SIRT1-Mediated Suppression of PKM2 Nuclear Accumulation. Eur. J. Pharmacol. 2022, 936, 175352. [Google Scholar] [CrossRef]
- Ibrahim, K.A.; Abdelgaid, H.A.; Eleyan, M.; Mohamed, R.A.; Gamil, N.M. Resveratrol Alleviates Cardiac Apoptosis Following Exposure to Fenitrothion by Modulating the Sirtuin1/c-Jun N-Terminal Kinases/P53 Pathway through pro-Oxidant and Inflammatory Response Improvements: In Vivo and in Silico Studies. Life Sci. 2022, 290, 120265. [Google Scholar] [CrossRef]
- Song, Y.; Wang, Z.; Liu, L.; Zhang, S.; Zhang, H.; Qian, Y. 1,4-Dihydropyridine (DHP) Suppresses against Oxidative Stress in Nucleus Pulposus via Activating Sirtuin-1. Biomed. Pharmacother. 2020, 121, 109592. [Google Scholar] [CrossRef]
- Moretti, C.H.; Schiffer, T.A.; Montenegro, M.F.; Larsen, F.J.; Tsarouhas, V.; Carlström, M.; Samakovlis, C.; Weitzberg, E.; Lundberg, J.O. Dietary Nitrite Extends Lifespan and Prevents Age-Related Locomotor Decline in the Fruit Fly. Free Radic. Biol. Med. 2020, 160, 860–870. [Google Scholar] [CrossRef]
- Pan, Y.; Zhang, H.; Zheng, Y.; Zhou, J.; Yuan, J.; Yu, Y.; Wang, J. Resveratrol Exerts Antioxidant Effects by Activating SIRT2 To Deacetylate Prx1. Biochemistry 2017, 56, 6325–6328. [Google Scholar] [CrossRef]
- Lv, X.-T.; Wang, R.-H.; Liu, X.-T.; Ye, Y.-J.; Liu, X.-Y.; Qiao, J.-D.; Wang, G.-E. Theacrine Ameliorates Experimental Liver Fibrosis in Rats by Lowering Cholesterol Storage via Activation of the Sirtuin 3-Farnesoid X Receptor Signaling Pathway. Chem. Biol. Interact. 2022, 364, 110051. [Google Scholar] [CrossRef] [PubMed]
- Si, Y.; Bao, H.; Han, L.; Chen, L.; Zeng, L.; Jing, L.; Xing, Y.; Geng, Y. Dexmedetomidine Attenuation of Renal Ischaemia-Reperfusion Injury Requires Sirtuin 3 Activation. Br. J. Anaesth. 2018, 121, 1260–1271. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, N.T.; de Jesús García Rivas, G.; Montemayor, L.L.E. Oroxylin A Reduces Angiotensin II- Induced Hypertrophy and Mitochondrial Dysfunction by Activating Sirtuin 3 in Cardiac Myocytes. J. Mol. Cell. Cardiol. 2017, 112, 148. [Google Scholar] [CrossRef]
- Zefzoufi, M.; Fdil, R.; Bouamama, H.; Gadhi, C.; Katakura, Y.; Mouzdahir, A.; Sraidi, K. Effect of Extracts and Isolated Compounds Derived from Retama Monosperma (L.) Boiss. on Anti-Aging Gene Expression in Human Keratinocytes and Antioxidant Activity. J. Ethnopharmacol. 2021, 280, 114451. [Google Scholar] [CrossRef]
- Wei, W.; Hu, P.; Qin, M.; Chen, G.; Wang, F.; Yao, S.; Jin, M.; Xie, Z.; Zhang, X. SIRT4 Is Highly Expressed in Retinal Müller Glial Cells. Front. Neurosci. 2022, 16. [Google Scholar] [CrossRef]
- Li, Y.; Gao, M.; Yin, L.-H.; Xu, L.-N.; Qi, Y.; Sun, P.; Peng, J.-Y. Dioscin Ameliorates Methotrexate-Induced Liver and Kidney Damages via Adjusting MiRNA-145-5p-Mediated Oxidative Stress. Free Radic. Biol. Med. 2021, 169, 99–109. [Google Scholar] [CrossRef]
- Xiao, Z.-P.; Lv, T.; Hou, P.-P.; Manaenko, A.; Liu, Y.; Jin, Y.; Gao, L.; Jia, F.; Tian, Y.; Li, P.; et al. Sirtuin 5-Mediated Lysine Desuccinylation Protects Mitochondrial Metabolism Following Subarachnoid Hemorrhage in Mice. Stroke 2021, 52, 4043–4053. [Google Scholar] [CrossRef]
- Yamada, C.; Ho, A.; Akkaoui, J.; Garcia, C.; Duarte, C.; Movila, A. Glycyrrhizin Mitigates Inflammatory Bone Loss and Promotes Expression of Senescence-Protective Sirtuins in an Aging Mouse Model of Periprosthetic Osteolysis. Biomed. Pharmacother. 2021, 138, 111503. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Yang, L.; Liu, Z.-L.; Peng, Y.; Gao, M.; Deng, L.-T.; Liu, X.; Xing, W. Sirtuin 6 Regulates Macrophage Polarization to Alleviate Sepsis-Induced Acute Respiratory Distress Syndrome via Dual Mechanisms Dependent on and Independent of Autophagy. Cytotherapy 2022, 24, 149–160. [Google Scholar] [CrossRef]
- Kang, J.-Y.; Lee, J.-S.; Seol, I.-C.; Kim, Y.-S.; Park, M.S.; Yoo, H.-R. Pharmacological Effects of Gami-Yukmijihwang-Tang on the Lipopolysaccharide-Induced Hippocampus Oxidation and Inflammation via Regulation of Sirt6. Pharmaceuticals 2022, 15, 293. [Google Scholar] [CrossRef]
- Song, J.; Liu, L.; Hao, K.; Mao, S.; Tang, Y.; Tong, X.; Dai, F. Resveratrol Elongates the Lifespan and Improves Antioxidant Activity in the Silkworm Bombyx Mori. J. Pharm. Anal. 2021, 11, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Alshammari, G.M.; Al-Qahtani, W.H.; Alshuniaber, M.A.; Yagoub, A.E.A.; Al-Khalifah, A.S.; Al-Harbi, L.N.; Alhussain, M.H.; AlSedairy, S.A.; Yahya, M.A. Quercetin Improves the Impairment in Memory Function and Attenuates Hippocampal Damage in Cadmium Chloride-Intoxicated Male Rats by Suppressing Acetylcholinesterase and Concomitant Activation of SIRT1 Signaling. J. Funct. Foods 2021, 86, 104675. [Google Scholar] [CrossRef]
- Wei, L.; Liu, B.; Yao, Z.; Yuan, T.; Wang, C.; Zhang, R.; Wang, Q.; Zhao, B. Sirtuin 1 Inhibitor EX527 Suppresses Morphine-Induced Behavioral Sensitization. Neurosci. Lett. 2021, 744, 135599. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.; Lee, Y.; Bae, M.; Park, Y.-K.; Lee, J.-Y. Astaxanthin Inhibits Alcohol-Induced Inflammation and Oxidative Stress in Macrophages in a Sirtuin 1-Dependent Manner. J. Nutr. Biochem. 2020, 85, 108477. [Google Scholar] [CrossRef] [PubMed]
- Wei, R.; Han, C.; He, F.; Xiong, X.; Ye, F.; Liu, H.; Li, L.; Xu, H.; Wei, S.; Zeng, X. Role of Forkhead Box Protein O1 and Insulin on Cell Proliferation Mediated by Sirtuin 1 in Goose Primary Hepatocytes. J. Appl. Poult. Res. 2021, 30, 100144. [Google Scholar] [CrossRef]
- Ceballos, M.P.; Angel, A.; Delprato, C.B.; Livore, V.I.; Ferretti, A.C.; Lucci, A.; Comanzo, C.G.; de Luján Alvarez, M.; Quiroga, A.D.; Mottino, A.D.; et al. Sirtuin 1 and 2 Inhibitors Enhance the Inhibitory Effect of Sorafenib in Hepatocellular Carcinoma Cells. Eur. J. Pharmacol. 2021, 892, 173736. [Google Scholar] [CrossRef]
- Tan, Y.J.; Lee, Y.T.; Mancera, R.L.; Oon, C.E. BZD9L1 Sirtuin Inhibitor: Identification of Key Molecular Targets and Their Biological Functions in HCT 116 Colorectal Cancer Cells. Life Sci. 2021, 284, 119747. [Google Scholar] [CrossRef]
- Hirata, Y.; Nishino, H.; Sasaki, T.; Nagaoka, Y.; Uesato, S.; Taniguchi, M. Sirtuin Inhibition and Neurite Outgrowth Effect as New Biological Activities for Areca Catechu Nut Alkaloids. Phytomedicine Plus 2022, 2, 100294. [Google Scholar] [CrossRef]
- Kato, Y.; Kihara, H.; Fukui, K.; Kojima, M. A Ternary Complex Model of Sirtuin4-NAD+-Glutamate Dehydrogenase. Comput. Biol. Chem. 2018, 74, 94–104. [Google Scholar] [CrossRef]
- Yang, L.-L.; Wang, H.-L.; Yan, Y.-H.; Liu, S.; Yu, Z.-J.; Huang, M.-Y.; Luo, Y.; Zheng, X.; Yu, Y.; Li, G.-B. Sensitive Fluorogenic Substrates for Sirtuin Deacylase Inhibitor Discovery. Eur. J. Med. Chem. 2020, 192, 112201. [Google Scholar] [CrossRef]
- Liu, Y.; Debnath, B.; Kumar, S.; Lombard, D.B.; Neamati, N. Identification of 2-Hydroxybenzoic Acid Derivatives as Selective SIRT5 Inhibitors. Eur. J. Med. Chem. 2022, 241, 114623. [Google Scholar] [CrossRef] [PubMed]
- Collins, J.A.; Kapustina, M.; Bolduc, J.A.; Pike, J.F.W.; Diekman, B.O.; Mix, K.; Chubinskaya, S.; Eroglu, E.; Michel, T.; Poole, L.B.; et al. Sirtuin 6 (SIRT6) Regulates Redox Homeostasis and Signaling Events in Human Articular Chondrocytes. Free Radic. Biol. Med. 2021, 166, 90–103. [Google Scholar] [CrossRef]
- Chudzińska, M.; Rogowicz, D.; Wołowiec, Ł.; Banach, J.; Sielski, S.; Bujak, R.; Sinkiewicz, A.; Grześk, G. Resveratrol and Cardiovascular System—The Unfulfilled Hopes. Ir. J. Med. Sci. 2021, 190, 981–986. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Santos, B.; Chorilli, M. The Uses of Resveratrol for Neurological Diseases Treatment and Insights for Nanotechnology Based-Drug Delivery Systems. Int. J. Pharm. 2020, 589, 119832. [Google Scholar] [CrossRef]
- Li, K.-X.; Ji, M.-J.; Sun, H.-J. An Updated Pharmacological Insight of Resveratrol in the Treatment of Diabetic Nephropathy. Gene 2021, 780, 145532. [Google Scholar] [CrossRef]
- Okudaira, N.; Ishizaka, Y.; Tamamori-Adachi, M. Resveratrol Blocks Retrotransposition of LINE-1 through PPAR α and Sirtuin-6. Sci. Rep. 2022, 12, 7772. [Google Scholar] [CrossRef]
- Griñán-Ferré, C.; Bellver-Sanchis, A.; Izquierdo, V.; Corpas, R.; Roig-Soriano, J.; Chillón, M.; Andres-Lacueva, C.; Somogyvári, M.; Sőti, C.; Sanfeliu, C.; et al. The Pleiotropic Neuroprotective Effects of Resveratrol in Cognitive Decline and Alzheimer’s Disease Pathology: From Antioxidant to Epigenetic Therapy. Ageing Res. Rev. 2021, 67, 101271. [Google Scholar] [CrossRef]
- Miguel, C.A.; Noya-Riobó, M.V.; Mazzone, G.L.; Villar, M.J.; Coronel, M.F. Antioxidant, Anti-Inflammatory and Neuroprotective Actions of Resveratrol after Experimental Nervous System Insults. Special Focus on the Molecular Mechanisms Involved. Neurochem. Int. 2021, 150, 105188. [Google Scholar] [CrossRef] [PubMed]
- Kinra, M.; Ranadive, N.; Mudgal, J.; Zhang, Y.; Govindula, A.; Anoopkumar-Dukie, S.; Davey, A.K.; Grant, G.D.; Nampoothiri, M.; Arora, D. Putative Involvement of Sirtuin Modulators in LPS-Induced Sickness Behaviour in Mice. Metab. Brain Dis. 2022, 37, 1969–1976. [Google Scholar] [CrossRef]
- Chou, X.; Li, X.; Min, Z.; Ding, F.; Ma, K.; Shen, Y.; Sun, D.; Wu, Q. Sirtuin-1 Attenuates Cadmium-Induced Renal Cell Senescence through P53 Deacetylation. Ecotoxicol. Environ. Saf. 2022, 245, 114098. [Google Scholar] [CrossRef]
- Zhao, X.-Y.; Yu, D.; Shi, X.; Hou, S.; Teng, D. Resveratrol Reduces P38 Mitogen-Activated Protein Kinase Phosphorylation by Activating Sirtuin 1 to Alleviate Cognitive Dysfunction after Traumatic Brain Injury in Mice. Neuroreport 2022, 33, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, C.; Yang, J.; Yuan, F.; Cheng, R.; Chen, R.; Shen, Y.; Huang, L. Impairment of Sirtuin 1-Mediated DNA Repair Is Involved in Bisphenol A-Induced Aggravation of Macrophage Inflammation and Atherosclerosis. Chemosphere 2021, 265, 128997. [Google Scholar] [CrossRef] [PubMed]
- Zada, N.S.; Belduz, A.O.; Güler, H.I.; Sahinkaya, M.; Khan, S.I.; Saba, M.; Bektas, K.I.; Kara, Y.; Kolaylı, S.; Badshah, M.; et al. Cloning, Biochemical Characterization and Molecular Docking of Novel Thermostable β-Glucosidase BglA9 from Anoxybacillus Ayderensis A9 and Its Application in de-Glycosylation of Polydatin. Int. J. Biol. Macromol. 2021, 193, 1898–1909. [Google Scholar] [CrossRef] [PubMed]
- Sunsong, R.; Du, T.; Etim, I.; Zhang, Y.; Liang, D.; Gao, S. Development of a Novel UPLC-MS/MS Method for the Simultaneously Quantification of Polydatin and Resveratrol in Plasma: Application to a Pharmacokinetic Study in Rats. J. Chromatogr. B 2021, 1185, 123000. [Google Scholar] [CrossRef] [PubMed]
- Fakhri, S.; Gravandi, M.M.; Abdian, S.; Akkol, E.K.; Farzaei, M.H.; Sobarzo-Sánchez, E. The Neuroprotective Role of Polydatin: Neuropharmacological Mechanisms, Molecular Targets, Therapeutic Potentials, and Clinical Perspective. Molecules 2021, 26, 5985. [Google Scholar] [CrossRef]
- Tong, Y.; Wang, K.; Sheng, S.; Cui, J. Polydatin Ameliorates Chemotherapy-Induced Cognitive Impairment (Chemobrain) by Inhibiting Oxidative Stress, Inflammatory Response, and Apoptosis in Rats. Biosci. Biotechnol. Biochem. 2020, 84, 1201–1210. [Google Scholar] [CrossRef]
- Bao, H.-L.; Chen, C.-Z.; Ren, C.-Z.; Sun, K.-Y.; Liu, H.; Song, S.-H.; Fu, Z.-R. Polydatin Ameliorates Hepatic Ischemia-Reperfusion Injury by Modulating Macrophage Polarization. Hepatobiliary Pancreat. Dis. Int. 2022. [Google Scholar] [CrossRef]
- Jin, W.; Fan, M.; Zhang, Y.; Zhang, Q.; Jing, C.; Jiang, R.; Piao, C.; Sun, L. Polydatin Prevents Lipotoxicity-Induced Dysfunction in Pancreatic β-Cells by Inhibiting Endoplasmic Reticulum Stress and Excessive Autophagy. Phytomedicine 2022, 106, 154410. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, Y.; Pei, Z.; Gao, H.; Shi, W.; Sun, M.; Xu, Q.; Zhao, J.; Meng, W.; Xiao, K. Protective Effects of Polydatin against Sulfur Mustard-Induced Hepatic Injury. Toxicol. Appl. Pharmacol. 2019, 367, 1–11. [Google Scholar] [CrossRef]
- Cao, K.; Lei, X.; Liu, H.; Zhao, H.; Guo, J.; Chen, Y.; Xu, Y.; Cheng, Y.; Liu, C.; Cui, J.; et al. Polydatin Alleviated Radiation-Induced Lung Injury through Activation of Sirt3 and Inhibition of Epithelial-Mesenchymal Transition. J. Cell. Mol. Med. 2017, 21, 3264–3276. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, S.; Cheng, Z.; Xiong, Z.; Lv, J.; Yang, Z.; Li, T.; Jiang, S.; Gu, J.; Sun, D.; et al. Polydatin Ameliorates Diabetic Cardiomyopathy via Sirt3 Activation. Biochem. Biophys. Res. Commun. 2017, 493, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, P.; Alvi, S.S.; Iqbal, D.; Khan, M.S. Insights into Pharmacological Mechanisms of Polydatin in Targeting Risk Factors-Mediated Atherosclerosis. Life Sci. 2020, 254, 117756. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, H.; Zhao, N.; Du, E.; Jin, F.; Fan, Q.; Guo, W.; Huang, S.; Wei, J. Effects of Magnolol and Honokiol Blend on Performance, Egg Quality, Hepatic Lipid Metabolism, and Intestinal Morphology of Hens at Late Laying Cycle. Animal 2022, 16, 100532. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Hayashi, T.; Suzuki, R.; Kitamura, M.; Inoue, Y. Inhibitory Effect of Honokiol on Furin-like Activity and SARS-CoV-2 Infection. J. Tradit. Complement. Med. 2022, 12, 69–72. [Google Scholar] [CrossRef]
- Zhou, Y.; Tang, J.; Lan, J.; Zhang, Y.; Wang, H.; Chen, Q.; Kang, Y.; Sun, Y.; Feng, X.; Wu, L.; et al. Honokiol Alleviated Neurodegeneration by Reducing Oxidative Stress and Improving Mitochondrial Function in Mutant SOD1 Cellular and Mouse Models of Amyotrophic Lateral Sclerosis. Acta Pharm. Sin. B 2022. [Google Scholar] [CrossRef]
- Borgonetti, V.; Governa, P.; Manetti, F.; Miraldi, E.; Biagi, M.; Galeotti, N. A Honokiol-Enriched Magnolia Officinalis Rehder & E.H. Wilson. Bark Extract Possesses Anxiolytic-like Activity with Neuroprotective Effect through the Modulation of CB1 Receptor. J. Pharm. Pharmacol. 2021, 73, 1161–1168. [Google Scholar] [CrossRef]
- Khatoon, F.; Ali, S.; Kumar, V.; Elasbali, A.M.; Alhassan, H.H.; Alharethi, S.H.; Islam, A.; Hassan, M.I. Pharmacological Features, Health Benefits and Clinical Implications of Honokiol. J. Biomol. Struct. Dyn. 2022, 1–23. [Google Scholar] [CrossRef]
- Luo, L.-X.; Li, Y.; Liu, Z.-Q.; Fan, X.-X.; Duan, F.-G.; Li, R.-Z.; Yao, X.-J.; Leung, E.L.-H.; Liu, L. Honokiol Induces Apoptosis, G1 Arrest, and Autophagy in KRAS Mutant Lung Cancer Cells. Front. Pharmacol. 2017, 8, 199. [Google Scholar] [CrossRef]
- Storder, J.; Renard, P.; Arnould, T. Update on the Role of Sirtuin 3 in Cell Differentiation: A Major Metabolic Target That Can Be Pharmacologically Controlled. Biochem. Pharmacol. 2019, 169, 113621. [Google Scholar] [CrossRef]
- Li, Y.H.; Choi, D.H.; Lee, E.H.; Seo, S.R.; Lee, S.; Cho, E.-H. Sirtuin 3 (SIRT3) Regulates α-Smooth Muscle Actin (α-SMA) Production through the Succinate Dehydrogenase-G Protein-Coupled Receptor 91 (GPR91) Pathway in Hepatic Stellate Cells. J. Biol. Chem. 2016, 291, 10277–10292. [Google Scholar] [CrossRef]
- Tresch, S. Strategies and Future Trends to Identify the Mode of Action of Phytotoxic Compounds. Plant Sci. 2013, 212, 60–71. [Google Scholar] [CrossRef] [PubMed]
- Milanović, M.; Đurić, L.; Milošević, N.; Milić, N. Comprehensive Insight into Triclosan—From Widespread Occurrence to Health Outcomes. Environ. Sci. Pollut. Res. 2021, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ito, S.; Nguyen, H.T.; Yamamoto, K.; Tanoue, R.; Kunisue, T.; Iwata, H. Effects of Prenatal Exposure to Triclosan on the Liver Transcriptome in Chicken Embryos. Toxicol. Appl. Pharmacol. 2018, 347, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Szychowski, K.A.; Skóra, B.; Wójtowicz, A.K. Involvement of Sirtuins (Sirt1 and Sirt3) and Aryl Hydrocarbon Receptor (AhR) in the Effects of Triclosan (TCS) on Production of Neurosteroids in Primary Mouse Cortical Neurons Cultures. Pestic. Biochem. Physiol. 2022, 184, 105131. [Google Scholar] [CrossRef]
- Bao, S.; He, C.; Ku, P.; Xie, M.; Lin, J.; Lu, S.; Nie, X. Effects of Triclosan on the RedoximiRs/Sirtuin/Nrf2/ARE Signaling Pathway in Mosquitofish (Gambusia Affinis). Aquat. Toxicol. 2021, 230, 105679. [Google Scholar] [CrossRef]
- Dykes, S.S.; Friday, E.; Pruitt, K.; Cardelli, J.A. The Histone Deacetylase Inhibitor Cambinol Prevents Acidic PHe-Induced Anterograde Lysosome Trafficking Independently of Sirtuin Activity. Biochem. Biophys. Rep. 2015, 3, 83–93. [Google Scholar] [CrossRef][Green Version]
- Botta, L.; Filippi, S.; Bizzarri, B.M.; Meschini, R.; Caputo, M.; Proietti-De-Santis, L.; Iside, C.; Nebbioso, A.; Gualandi, G.; Saladino, R. Oxidative Nucleophilic Substitution Selectively Produces Cambinol Derivatives with Antiproliferative Activity on Bladder Cancer Cell Lines. Bioorg. Med. Chem. Lett. 2019, 29, 78–82. [Google Scholar] [CrossRef]
- Lugrin, J.; Ciarlo, E.; Santos, A.; Grandmaison, G.; dos Santos, I.; le Roy, D.; Roger, T. The Sirtuin Inhibitor Cambinol Impairs MAPK Signaling, Inhibits Inflammatory and Innate Immune Responses and Protects from Septic Shock. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2013, 1833, 1498–1510. [Google Scholar] [CrossRef]
- Chowdhury, S.; Sripathy, S.; Webster, A.A.; Park, A.; Lao, U.; Hsu, J.H.; Loe, T.; Bedalov, A.; Simon, J.A. Discovery of Selective SIRT2 Inhibitors as Therapeutic Agents in B-Cell Lymphoma and Other Malignancies. Molecules 2020, 25, 455. [Google Scholar] [CrossRef]
- Wawruszak, A.; Luszczki, J.; Okon, E.; Czerwonka, A.; Stepulak, A. Antagonistic Pharmacological Interaction between Sirtuin Inhibitor Cambinol and Paclitaxel in Triple-Negative Breast Cancer Cell Lines: An Isobolographic Analysis. Int. J. Mol. Sci. 2022, 23, 6458. [Google Scholar] [CrossRef]
- Hong, J.Y.; Lin, H. Sirtuin Modulators in Cellular and Animal Models of Human Diseases. Front. Pharmacol. 2021, 12, 735044. [Google Scholar] [CrossRef] [PubMed]
- Khalil, N.A.; Ahmed, E.M.; Zaher, A.F.; El-Zoghbi, M.S.; Sobh, E.A. Synthesis of Certain Benzothieno[3,2-d]Pyrimidine Derivatives as a Selective SIRT2 Inhibitors. Eur. J. Med. Chem. 2020, 187, 111926. [Google Scholar] [CrossRef]
- Nikseresht, S.; Khodagholi, F.; Ahmadiani, A. Protective Effects of Ex-527 on Cerebral Ischemia–Reperfusion Injury through Necroptosis Signaling Pathway Attenuation. J. Cell. Physiol. 2019, 234, 1816–1826. [Google Scholar] [CrossRef] [PubMed]
- Kundu, A.; Richa, S.; Dey, P.; Kim, K.S.; Son, J.Y.; Kim, H.R.; Lee, S.-Y.; Lee, B.-H.; Lee, K.Y.; Kacew, S.; et al. Protective Effect of EX-527 against High-Fat Diet-Induced Diabetic Nephropathy in Zucker Rats. Toxicol. Appl. Pharmacol. 2020, 390, 114899. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.H.; Wang, Z.; Xiao, H.; Jiao, J.; Wang, L.; Bhatti, T.R.; Hancock, W.W.; Beier, U.H. Targeting Sirtuin-1 Prolongs Murine Renal Allograft Survival and Function. Kidney Int. 2016, 89, 1016–1026. [Google Scholar] [CrossRef][Green Version]
- Velázquez-Ulloa, N.A.; Heres-Pulido, M.E.; Santos-Cruz, L.F.; Durán-Díaz, A.; Castañeda-Partida, L.; Browning, A.; Carmona-Alvarado, C.; Estrada-Guzmán, J.C.; Ferderer, G.; Garfias, M.; et al. Complex Interactions between Nicotine and Resveratrol in the Drosophila Melanogaster Wing Spot Test. Heliyon 2022, 8, e09744. [Google Scholar] [CrossRef]
- Li, R.; Zhan, W.; Ren, J.; Zhang, F.; Huang, X.; Ma, Y. Temporal Trends in Risk of Bisphenol A, Benzophenone-3 and Triclosan Exposure among U.S. Children and Adolescents Aged 6–19 Years: Findings from the National Health and Nutrition Examination Survey 2005–2016. Environ. Res. 2023, 216, 114474. [Google Scholar] [CrossRef]
- Li, S.; Feng, S.; van Schepdael, A.; Wang, X. Hollow Fiber Membrane-Protected Amino/Hydroxyl Bifunctional Microporous Organic Network Fiber for Solid-Phase Microextraction of Bisphenols A, F, S, and Triclosan in Breast Milk and Infant Formula. Food Chem. 2022, 390, 133217. [Google Scholar] [CrossRef]
- Beroukhim, G.; Kayani, J.; Taylor, H.S.; Pal, L. Implications of Triclosan for Female Fertility: Results from the National Health and Nutrition Examination Survey, 2013–2016. F S Rep. 2022, 3, 204–210. [Google Scholar] [CrossRef]
- Fernandes, C.A.; Fievez, L.; Neyrinck, A.M.; Delzenne, N.M.; Bureau, F.; Vanbever, R. Sirtuin Inhibition Attenuates the Production of Inflammatory Cytokines in Lipopolysaccharide-Stimulated Macrophages. Biochem. Biophys. Res. Commun. 2012, 420, 857–861. [Google Scholar] [CrossRef]
- Morris, B.J. Sirtuins and Aging. In Sirtuin Biology in Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 49–77. [Google Scholar]
- Goggins, A.; Matten, G. The Sirtfood Diet; Simon and Schuster: New York, NY, USA, 2017; ISBN 1501163779 or 9781501163777. [Google Scholar]
- He, Y.; Huang, W.; Zhang, C.; Chen, L.; Xu, R.; Li, N.; Wang, F.; Han, L.; Yang, M.; Zhang, D. Energy Metabolism Disorders and Potential Therapeutic Drugs in Heart Failure. Acta Pharm. Sin. B 2021, 11, 1098–1116. [Google Scholar] [CrossRef] [PubMed]
- Zaganjor, E.; Yoon, H.; Spinelli, J.B.; Nunn, E.R.; Laurent, G.; Keskinidis, P.; Sivaloganathan, S.; Joshi, S.; Notarangelo, G.; Mulei, S.; et al. SIRT4 Is an Early Regulator of Branched-Chain Amino Acid Catabolism That Promotes Adipogenesis. Cell Rep. 2021, 36, 109345. [Google Scholar] [CrossRef] [PubMed]
- Yudoh, K.; Yui, N.; Terauchi, K.; Kobayashi, H.; Kumai, T.; Somemura, S. Sirtuins in Bone and Cartilage Biology. In Sirtuin Biology in Medicine; Elsevier: Amsterdam, The Netherlands, 2021; pp. 341–351. [Google Scholar]
- Moon, Y.J.; Zhang, Z.; Bang, I.H.; Kwon, O.K.; Yoon, S.-J.; Kim, J.R.; Lee, S.; Bae, E.J.; Park, B.-H. Sirtuin 6 in Preosteoclasts Suppresses Age- and Estrogen Deficiency-Related Bone Loss by Stabilizing Estrogen Receptor α. Cell Death Differ. 2019, 26, 2358–2370. [Google Scholar] [CrossRef]
- Dai, Y.; Lin, J.; Ren, J.; Zhu, B.; Wu, C.; Yu, L. NAD+ Metabolism in Peripheral Neuropathic Pain. Neurochem. Int. 2022, 161, 105435. [Google Scholar] [CrossRef]
- Govindarajulu, M.; Ramesh, S.; Neel, L.; Fabbrini, M.; Buabeid, M.; Fujihashi, A.; Dwyer, D.; Lynd, T.; Shah, K.; Mohanakumar, K.P.; et al. Nutraceutical Based SIRT3 Activators as Therapeutic Targets in Alzheimer’s Disease. Neurochem. Int. 2021, 144, 104958. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Tremblay, C.; Émond, V.; Lebbadi, M.; Salem, N.; Bennett, D.A.; Calon, F. Sirtuin 1 Reduction Parallels the Accumulation of Tau in Alzheimer Disease. J. Neuropathol. Exp. Neurol. 2009, 68, 48–58. [Google Scholar] [CrossRef]
- Mohamad Nasir, N.F.; Zainuddin, A.; Shamsuddin, S. Emerging Roles of Sirtuin 6 in Alzheimer’s Disease. J. Mol. Neurosci. 2018, 64, 157–161. [Google Scholar] [CrossRef]
- Kumar, V.; Kundu, S.; Singh, A.; Singh, S. Understanding the Role of Histone Deacetylase and Their Inhibitors in Neurodegenerative Disorders: Current Targets and Future Perspective. Curr. Neuropharmacol. 2022, 20, 158–178. [Google Scholar] [CrossRef]
- Yu, Z.; Fan, D.; Gui, B.; Shi, L.; Xuan, C.; Shan, L.; Wang, Q.; Shang, Y.; Wang, Y. Neurodegeneration-Associated TDP-43 Interacts with Fragile X Mental Retardation Protein (FMRP)/Staufen (STAU1) and Regulates SIRT1 Expression in Neuronal Cells. J. Biol. Chem. 2012, 287, 22560–22572. [Google Scholar] [CrossRef]
- Wang, X.; Liu, R.; Zhang, W.; Hyink, D.P.; Das, G.C.; Das, B.; Li, Z.; Wang, A.; Yuan, W.; Klotman, P.E.; et al. Role of SIRT1 in HIV-Associated Kidney Disease. Am. J. Physiol.-Ren. Physiol. 2020, 319, F335–F344. [Google Scholar] [CrossRef]
- Palomer, X.; Aguilar-Recarte, D.; García, R.; Nistal, J.F.; Vázquez-Carrera, M. Sirtuins: To Be or Not To Be in Diabetic Cardiomyopathy. Trends Mol. Med. 2021, 27, 554–571. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Pan, L.; Li, Y.; Zou, X.; Liu, B.; Jiang, B.; Zhang, C. Deacetylation-Activated Construction of Single Quantum Dot-Based Nanosensor for Sirtuin 1 Assay. Talanta 2021, 224, 121918. [Google Scholar] [CrossRef] [PubMed]
- Kratz, E.M.; Kokot, I.; Dymicka-Piekarska, V.; Piwowar, A. Sirtuins—The New Important Players in Women’s Gynecological Health. Antioxidants 2021, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, C.; Monteiro-Reis, S.; Almeida-Rios, D.; Vieira, R.; Castro, A.; Moutinho, M.; Rodrigues, M.; Graça, I.; Lopes, J.M.; Jerónimo, C. Assessing Sirtuin Expression in Endometrial Carcinoma and Non-Neoplastic Endometrium. Oncotarget 2016, 7, 1144–1154. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Yang, L.; Yang, X.; Xiong, Y.; Fu, W.; Li, J.; Yin, S. Sirtuin 7 Is Essential for the Survival and Synthesis of Oestrogen in Yak (Bos Grunniens) Cumulus Granulosa Cells. Reprod. Domest. Anim. 2022, 1–10. [Google Scholar] [CrossRef]
- Xu, D.; He, H.; Jiang, X.; Hua, R.; Chen, H.; Yang, L.; Cheng, J.; Duan, J.; Li, Q. SIRT2 Plays a Novel Role on Progesterone, Estradiol and Testosterone Synthesis via PPARs/LXRα Pathways in Bovine Ovarian Granular Cells. J. Steroid Biochem. Mol. Biol. 2019, 185, 27–38. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, J.; Wang, F.; Pan, M.; Cui, L.; Li, M.; Qu, F. Mitochondrial and Glucose Metabolic Dysfunctions in Granulosa Cells Induce Impaired Oocytes of Polycystic Ovary Syndrome through Sirtuin 3. Free Radic. Biol. Med. 2022, 187, 1–16. [Google Scholar] [CrossRef]
- Porcu, M.; Chiarugi, A. The Emerging Therapeutic Potential of Sirtuin-Interacting Drugs: From Cell Death to Lifespan Extension. Trends Pharmacol. Sci. 2005, 26, 94–103. [Google Scholar] [CrossRef]
- Fernando, K.K.M.; Wijayasinghe, Y.S. Sirtuins as Potential Therapeutic Targets for Mitigating Neuroinflammation Associated With Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 372. [Google Scholar] [CrossRef]
- Mourits, V.P.; Helder, L.S.; Matzaraki, V.; Koeken, V.A.C.M.; Groh, L.; de Bree, L.C.J.; Moorlag, S.J.C.F.M.; van der Heijden, C.D.C.C.; Keating, S.T.; van Puffelen, J.H.; et al. The Role of Sirtuin 1 on the Induction of Trained Immunity. Cell. Immunol. 2021, 366, 104393. [Google Scholar] [CrossRef]
- Hua, R.; Wang, G.-Z.; Shen, Q.-W.; Yang, Y.-P.; Wang, M.; Wu, M.; Shao, Y.-K.; He, M.; Zang, Y.; Yao, Q.-Y.; et al. Sleeve Gastrectomy Ameliorated High-Fat Diet (HFD)-Induced Non-Alcoholic Fatty Liver Disease and Upregulated the Nicotinamide Adenine Dinucleotide +/ Sirtuin-1 Pathway in Mice. Asian J. Surg. 2021, 44, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Shen, S.; Ding, S.; Wang, L. Suppression of MicroRNA-323-3p Restrains Vascular Endothelial Cell Apoptosis via Promoting Sirtuin-1 Expression in Coronary Heart Disease. Life Sci. 2021, 270, 119065. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wei, G.; Li, D.; Fan, Y.; Zeng, Y.; Qian, Z.; Jia, Z.; Tang, Y.; Shi, Y.; Wu, H.; et al. Sirtuin 1 Alleviates Microglia-Induced Inflammation by Modulating the PGC-1α/Nrf2 Pathway after Traumatic Brain Injury in Male Rats. Brain Res. Bull. 2022, 185, 28–38. [Google Scholar] [CrossRef]
- Chao, C.-C.; Huang, C.-L.; Cheng, J.-J.; Chiou, C.-T.; Lee, I.-J.; Yang, Y.-C.; Hsu, T.-H.; Yei, C.-E.; Lin, P.-Y.; Chen, J.-J.; et al. SRT1720 as an SIRT1 Activator for Alleviating Paraquat-Induced Models of Parkinson’s Disease. Redox Biol. 2022, 58, 102534. [Google Scholar] [CrossRef]
- Hao, L.; Park, J.; Jang, H.-Y.; Bae, E.J.; Park, B.-H. Inhibiting Protein Kinase Activity of Pyruvate Kinase M2 by SIRT2 Deacetylase Attenuates Psoriasis. J. Investig. Dermatol. 2021, 141, 355–363.e6. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wang, B.; Yang, W.; Jiang, Q.; Loor, J.J.; Ouyang, L.; Tang, H.; Chang, R.; Peng, T.; Xu, C. Sirtuin 3 Relieves Inflammatory Responses Elicited by Lipopolysaccharide via the PGC1α-NFκB Pathway in Bovine Mammary Epithelial Cells. J. Dairy Sci. 2022. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, C.; Yang, Q.; Wang, Y.; Zhao, W.; Li, L.; Ren, X.; Zhao, J.; Zang, W.; Cao, J. Spinal Sirtuin 3 Contributes to Electroacupuncture Analgesia in Mice with Chronic Constriction Injury–Induced Neuropathic Pain. Neuromodulation Technol. Neural Interface 2022. [Google Scholar] [CrossRef]
- Zhang, Q.; Ren, J.; Wang, F.; Li, M.; Pan, M.; Zhang, H.; Qu, F. Chinese Herbal Medicine Alleviates the Pathogenesis of Polycystic Ovary Syndrome by Improving Oxidative Stress and Glucose Metabolism via Mitochondrial Sirtuin 3 Signaling. Phytomedicine 2022, 154556. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, L.; Hua, F.; Zhang, C.; Zhang, C.; Mi, X.; Qin, N.; Wang, J.; Zhu, A.; Qin, Z.; et al. FOXM1-Activated SIRT4 Inhibits NF-ΚB Signaling and NLRP3 Inflammasome to Alleviate Kidney Injury and Podocyte Pyroptosis in Diabetic Nephropathy. Exp. Cell Res. 2021, 408, 112863. [Google Scholar] [CrossRef]
- Zheng, D.; Zeng, Q.; He, D.; He, Y.; Yang, J. SIRT5 Alleviates Hepatic Ischemia and Reperfusion Injury by Diminishing Oxidative Stress and Inflammation via Elevating SOD1 and IDH2 Expression. Exp. Cell Res. 2022, 419, 113319. [Google Scholar] [CrossRef]
- Hu, T.; Shukla, S.K.; Vernucci, E.; He, C.; Wang, D.; King, R.J.; Jha, K.; Siddhanta, K.; Mullen, N.J.; Attri, K.S.; et al. Metabolic Rewiring by Loss of Sirt5 Promotes Kras-Induced Pancreatic Cancer Progression. Gastroenterology 2021, 161, 1584–1600. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Shang, J.; Gao, C.; Guan, X.; Chen, Y.; Zhu, L.; Zhang, L.; Zhang, C.; Zhang, J.; Pang, T. A Novel SIRT6 Activator Ameliorates Neuroinflammation and Ischemic Brain Injury via EZH2/FOXC1 Axis. Acta Pharm. Sin. B 2021, 11, 708–726. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wei, X.; Chen, X.; Wang, Q.; Zhang, J.; Kalvakolanu, D.V.; Guo, B.; Zhang, L. GRIM-19 Inhibits Proliferation and Induces Apoptosis in a P53-Dependent Manner in Colorectal Cancer Cells through the SIRT7/PCAF/MDM2 Axis. Exp. Cell Res. 2021, 407, 112799. [Google Scholar] [CrossRef]
- Yang, K.-E.; Jang, H.-J.; Hwang, I.-H.; Hong, E.M.; Lee, M.-G.; Lee, S.; Jang, I.-S.; Choi, J.-S. Stereoisomer-Specific Ginsenoside 20(S)-Rg3 Reverses Replicative Senescence of Human Diploid Fibroblasts via Akt-MTOR-Sirtuin Signaling. J. Ginseng Res. 2020, 44, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Okada, N.; Oshima, K.; Iwasaki, Y.; Maruko, A.; Matsumura, K.; Iioka, E.; Vu, T.-D.; Fujitsuka, N.; Nishi, A.; Sugiyama, A.; et al. Intron Retention as a New Pre-Symptomatic Marker of Aging and Its Recovery to the Normal State by a Traditional Japanese Multi-Herbal Medicine. Gene 2021, 794, 145752. [Google Scholar] [CrossRef] [PubMed]
- Morris, B.J.; Willcox, B.J.; Donlon, T.A. Genetic and Epigenetic Regulation of Human Aging and Longevity. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2019, 1865, 1718–1744. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Diao, D.; Shi, Z.; Zhu, X.; Gao, Y.; Gao, S.; Liu, X.; Wu, Y.; Rudolph, K.L.; Liu, G.; et al. SIRT6 Controls Hematopoietic Stem Cell Homeostasis through Epigenetic Regulation of Wnt Signaling. Cell Stem Cell 2016, 18, 495–507. [Google Scholar] [CrossRef]
- Hadar, A.; Milanesi, E.; Walczak, M.; Puzianowska-Kuźnicka, M.; Kuźnicki, J.; Squassina, A.; Niola, P.; Chillotti, C.; Attems, J.; Gozes, I.; et al. SIRT1, MiR-132 and MiR-212 Link Human Longevity to Alzheimer’s Disease. Sci. Rep. 2018, 8, 8465. [Google Scholar] [CrossRef]
- Donlon, T.A.; Morris, B.J.; Chen, R.; Masaki, K.H.; Allsopp, R.C.; Willcox, D.C.; Tiirikainen, M.; Willcox, B.J. Analysis of Polymorphisms in 59 Potential Candidate Genes for Association With Human Longevity. J. Gerontol. Ser. A 2018, 73, 1459–1464. [Google Scholar] [CrossRef]
- Giblin, W.; Lombard, D.B. Sirtuins, Healthspan, and Longevity in Mammals. In Handbook of the Biology of Aging; Elsevier: Amsterdam, The Netherlands, 2016; pp. 83–132. [Google Scholar]
- Lasigliè, D. Sirtuins and the Prevention of Immunosenescence. In Vitamins and Hormones; Academic Press: Cambridge, MA, USA, 2021; pp. 221–264. [Google Scholar]
- Apparao, Y.; Phan, C.W.; Kuppusamy, U.R.; Sabaratnam, V. Ergothioneine and Its Prospects as an Anti-Ageing Compound. Exp. Gerontol. 2022, 111982. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Giovane, A.; Casale, R.; Vitiello, M.; Marfella, R.; Paolisso, G.; Balestrieri, M.L. Ergothioneine Oxidation in the Protection against High-Glucose Induced Endothelial Senescence: Involvement of SIRT1 and SIRT6. Free Radic. Biol. Med. 2016, 96, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.D.; Vaccarezza, M. Nicotinamide Adenine Dinucleotide and the Sirtuins Caution: Pro-cancer Functions. Aging Med. 2021, 4, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Liberale, L.; Akhmedov, A.; Vlachogiannis, N.I.; Bonetti, N.; Nageswaran, V.; Miranda, M.X.; Montecucco, F.; Beer, J.; Lüscher, T.F.; Stamatelopoulos, K.; et al. Sirtuin 5 Promotes Arterial Thrombosis through Endothelial Plasminogen Activator Inhibitor-1. Atherosclerosis 2020, 315, e78–e79. [Google Scholar] [CrossRef]
- Kakefuda, K.; Fujita, Y.; Oyagi, A.; Hyakkoku, K.; Kojima, T.; Umemura, K.; Tsuruma, K.; Shimazawa, M.; Ito, M.; Nozawa, Y.; et al. Sirtuin 1 Overexpression Mice Show a Reference Memory Deficit, but Not Neuroprotection. Biochem. Biophys. Res. Commun. 2009, 387, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Teasley, H.E.; Beesley, A.; Kim, T.H.; Risinger, J.; Young, S.L.; Jeong, J.-W.; Schammel, D.P.; Lessey, B.A.; Elder, J.W.; Puls, L. Differential Expression of KRAS and SIRT1 in Ovarian Cancers with and Without Endometriosis. Reprod. Sci. 2020, 27, 145–151. [Google Scholar] [CrossRef]
- He, S.; Jia, Q.; Zhou, L.; Wang, Z.; Li, M. SIRT5 Is Involved in the Proliferation and Metastasis of Breast Cancer by Promoting Aerobic Glycolysis. Pathol. Res. Pract. 2022, 235, 153943. [Google Scholar] [CrossRef]
- Pande, S.; Raisuddin, S. The Underexplored Dimensions of Nutritional Hormesis. Curr. Nutr. Rep. 2022, 11, 386–394. [Google Scholar] [CrossRef]
- Visioli, F.; Ingram, A.; Beckman, J.S.; Magnusson, K.R.; Hagen, T.M. Strategies to Protect against Age-Related Mitochondrial Decay: Do Natural Products and Their Derivatives Help? Free Radic. Biol. Med. 2022, 178, 330–346. [Google Scholar] [CrossRef]
- Gaul, D.S.; Calatayud, N.; Pahla, J.; Bonetti, N.R.; Wang, Y.-J.; Weber, J.; Ambrosini, S.; Liberale, L.; Costantino, S.; Mohammed, S.A.; et al. Endothelial SIRT6 Deficiency Promotes Arterial Thrombosis in Mice. J. Mol. Cell. Cardiol. 2023, 174, 56–62. [Google Scholar] [CrossRef]
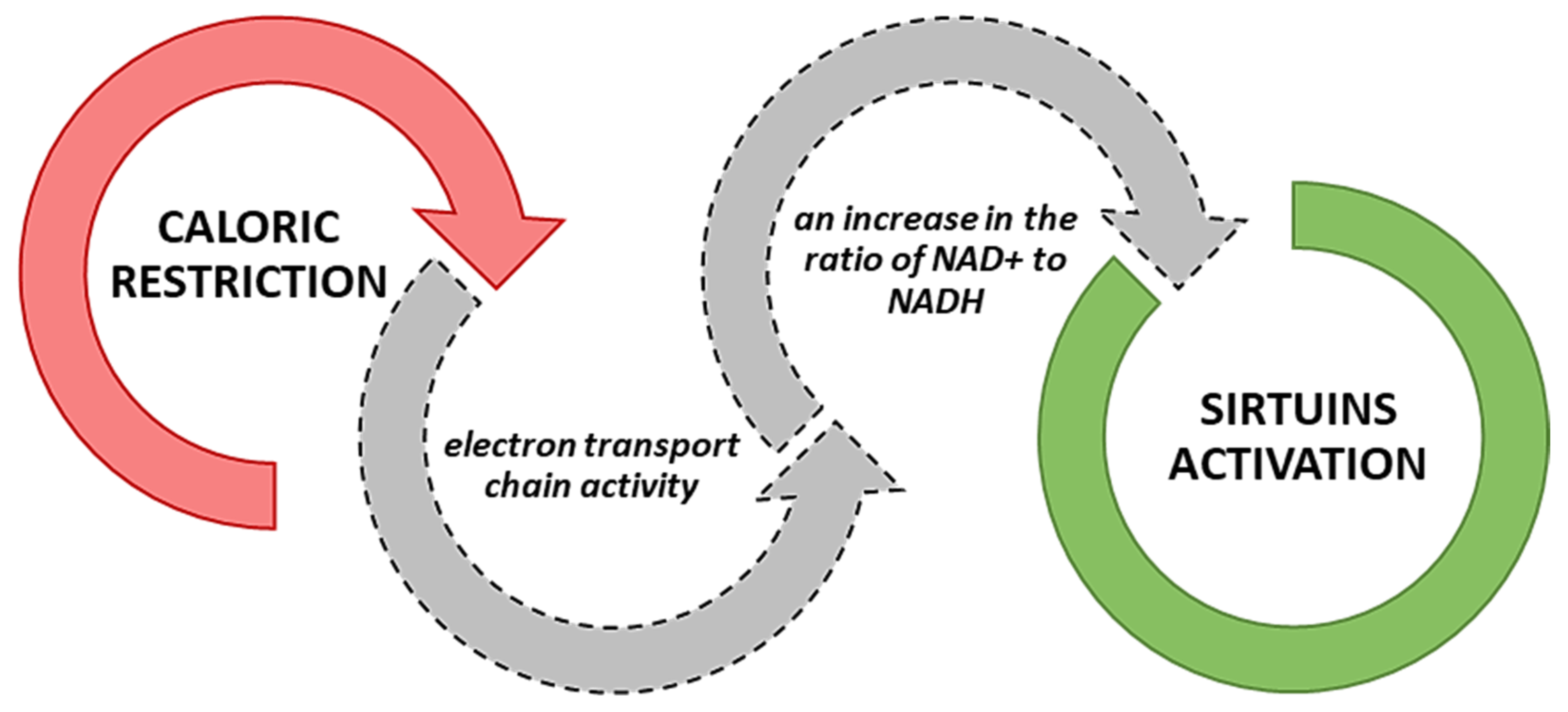
| Sirtuin | Organism | Modulator | Effect | Reference |
|---|---|---|---|---|
| ACTIVATORS | ||||
| Sirtuin 1 | Human and Drosophila melanogaster | ginsenosides (Panax ginseng Meyer extract) |
| [156] |
| Mammalian cells (Mus musculus) | resveratrol |
| [157] | |
| cucurbitacin E glucoside (CuE) |
| [158] | ||
| quercetin |
| [159] | ||
| Rattus norvegicus | resveratrol |
| [160] | |
| Humans | 1,4-dihydropyridine (DHP) |
| [161] | |
| Sirtuin 2 | Drosophila melanogaster | food nitrites |
| [162] |
| Human (HepG2 cells) | resveratrol |
| [163] | |
| Sirtuin 3 | Rattus rattus | theacrine (1,3,7,9-tetramethyluric acid) |
| [164] |
| Mus musculus | dexmedetomidine |
| [165] | |
| Human | oroxylin A |
| [166] | |
| Retama monosperma (L.) Boiss extract |
| [167] | ||
| Sirtuin 4 | Mus musculus | resveratrol |
| [168] |
| Sirtuin 5 | Mus musculus | dioscin |
| [169] |
| resveratrol |
| [170] | ||
| Sirtuin 6 | Mus musculus | glicyryzine |
| [171] |
| UBCS039 |
| [172] | ||
| Gami-Yukmijihwang-Tang extract |
| [173] | ||
| Sirtuin 7 | Bombyx mori | resveratrol |
| [174] |
| INHIBITORS | ||||
| Sirtuin 1 | Sprague Dawley rats | EX-527 |
| [43] |
| Rattus rattus |
| [175] | ||
| [176] | |||
| Bone marrow cells-Mus musculus | sirtinol |
| [177] | |
| Anser domesticus | nicotinamide |
| [178] | |
| Sirtuin 1&2 | hepatocellular carcinoma cells | EX-527 & cambiol |
| [179] |
| colorectal carcinoma HCT 116 (CCL-247) cell line | BZD9L1 |
| [180] | |
| Sirtuin 1–3 | murine Neuroblastoma cell line | arekoline (Areca catechu extract) |
| [181] |
| Sirtuin 2 | MCF-7 human breast cancer cell line | thiadiazole derivatives |
| [154] |
| Sirtuin 3 | mass spectrometry of SIRT3 | ampicillin trihydrate |
| [37] |
| Sirtuin 4 | Human | nicotinamide |
| [182] |
| Sirtuin 5 | fluorogenic peptide substrates and human sirtuins | TW-37 |
| [183] |
| molecular docking | 4-((4-(4-acetamidophenyl)thiazol-2-yl)amino)-2-hydroxybenzoic acid |
| [184] | |
| Sirtuin 6 | articular chondrocytes from human | EX-527 |
| [185] |
| Sirtuin | Disease | Model | Effect | Reference |
|---|---|---|---|---|
| Sirtuin 1 | Non-alcoholic fatty liver disease (NAFLD) | C57BL/6 mice |
| [257] |
| Coronary heart disease (CHD) | Rat cell lines |
| [258] | |
| Traumatic brain injury (TBI) | Male rats |
| [259] | |
| Parkinson’s disease | Male C57BL/6JNarl mice |
| [260] | |
| Sirtuin 2 | Non-alcoholic fatty liver disease (NAFLD) | Mice |
| [52] |
| Psoriasis | Mice and Human embryonic kidney 93T cell line |
| [261] | |
| Sirtuin 3 | inflammatory responses elicited by lipopolysaccharide | Bovine mammary epithelial cells |
| [262] |
| Chronic Constriction Injury–Induced Neuropathic Pain | Mice |
| [263] | |
| Polycystic ovary syndrome (PCOS) | Sprague-Dawley rats |
| [264] | |
| Sirtuin 4 | Doxorubicin-Induced Cardiotoxicity (DIC) | Male C57BL/6 mice |
| [68] |
| Diabetic nephropathy |
| [265] | ||
| Sirtuin 5 | hepatic ischemia and reperfusion injury | Male C57BL/6J mice |
| [266] |
| Pancreatic Cancer | PDAC cell lines, genetically engineered mice models, and human tissue samples |
| [267] | |
| Sirtuin 6 | type 1 diabetes | C57BL/6 mice |
| [74] |
| neuroinflammation and ischemic brain injury | Cell culture from C57BL/6 mice and human blood cells |
| [268] | |
| Sirtuin 7 | Chronic kidney disease (CKD) with hypertensive | Mice |
| [82] |
| colorectal cancer | Human CRC cell lines HCT-8, HCT-116, DLD1, SW620 and HT15 |
| [269] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziętara, P.; Dziewięcka, M.; Augustyniak, M. Why Is Longevity Still a Scientific Mystery? Sirtuins—Past, Present and Future. Int. J. Mol. Sci. 2023, 24, 728. https://doi.org/10.3390/ijms24010728
Ziętara P, Dziewięcka M, Augustyniak M. Why Is Longevity Still a Scientific Mystery? Sirtuins—Past, Present and Future. International Journal of Molecular Sciences. 2023; 24(1):728. https://doi.org/10.3390/ijms24010728
Chicago/Turabian StyleZiętara, Patrycja, Marta Dziewięcka, and Maria Augustyniak. 2023. "Why Is Longevity Still a Scientific Mystery? Sirtuins—Past, Present and Future" International Journal of Molecular Sciences 24, no. 1: 728. https://doi.org/10.3390/ijms24010728
APA StyleZiętara, P., Dziewięcka, M., & Augustyniak, M. (2023). Why Is Longevity Still a Scientific Mystery? Sirtuins—Past, Present and Future. International Journal of Molecular Sciences, 24(1), 728. https://doi.org/10.3390/ijms24010728