TRP Channels: Recent Development in Translational Research and Potential Therapeutic Targets in Migraine
Abstract
1. Migraine and Other Headache Disorders
1.1. Headache Disorders
1.2. Migraine
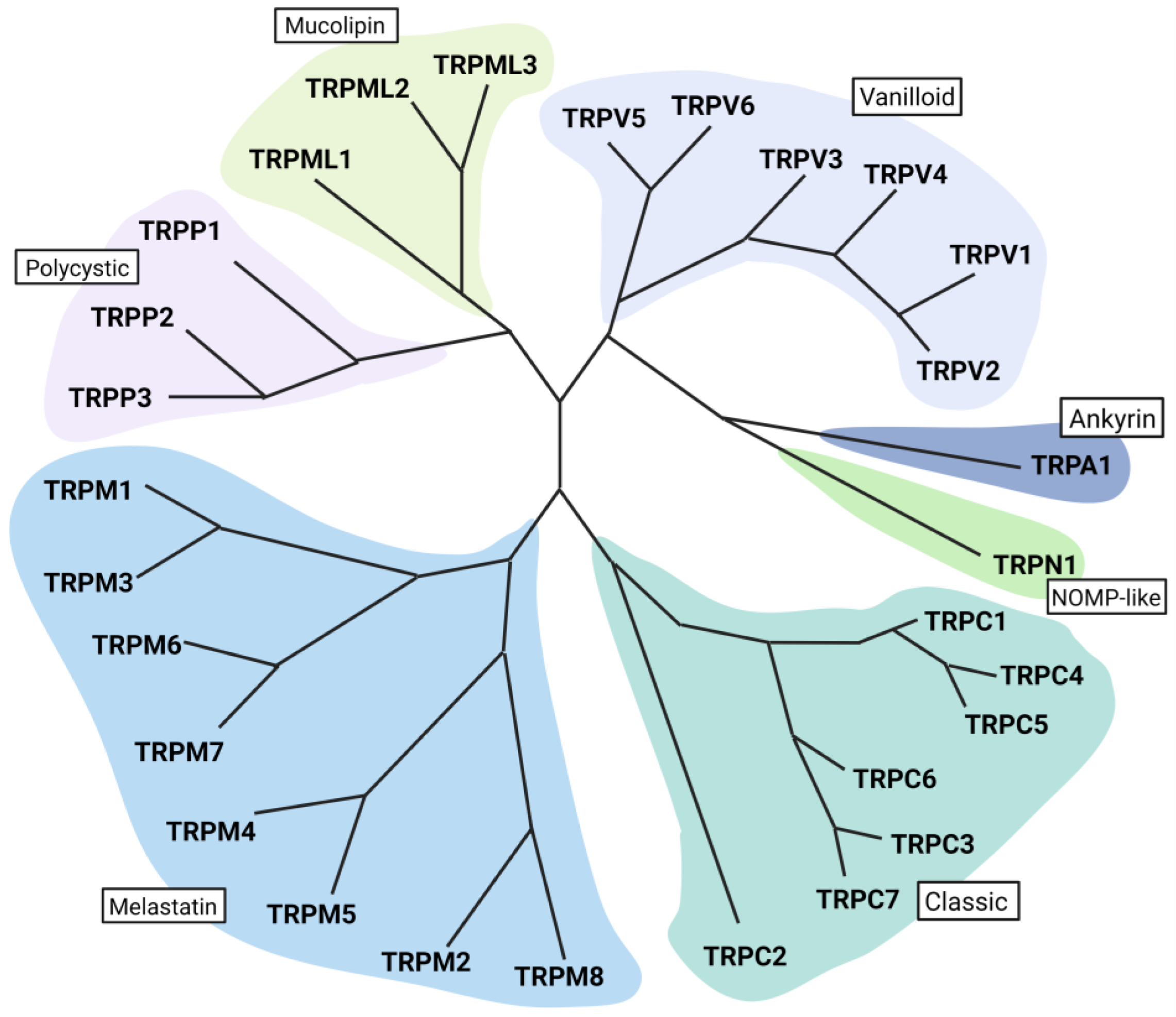
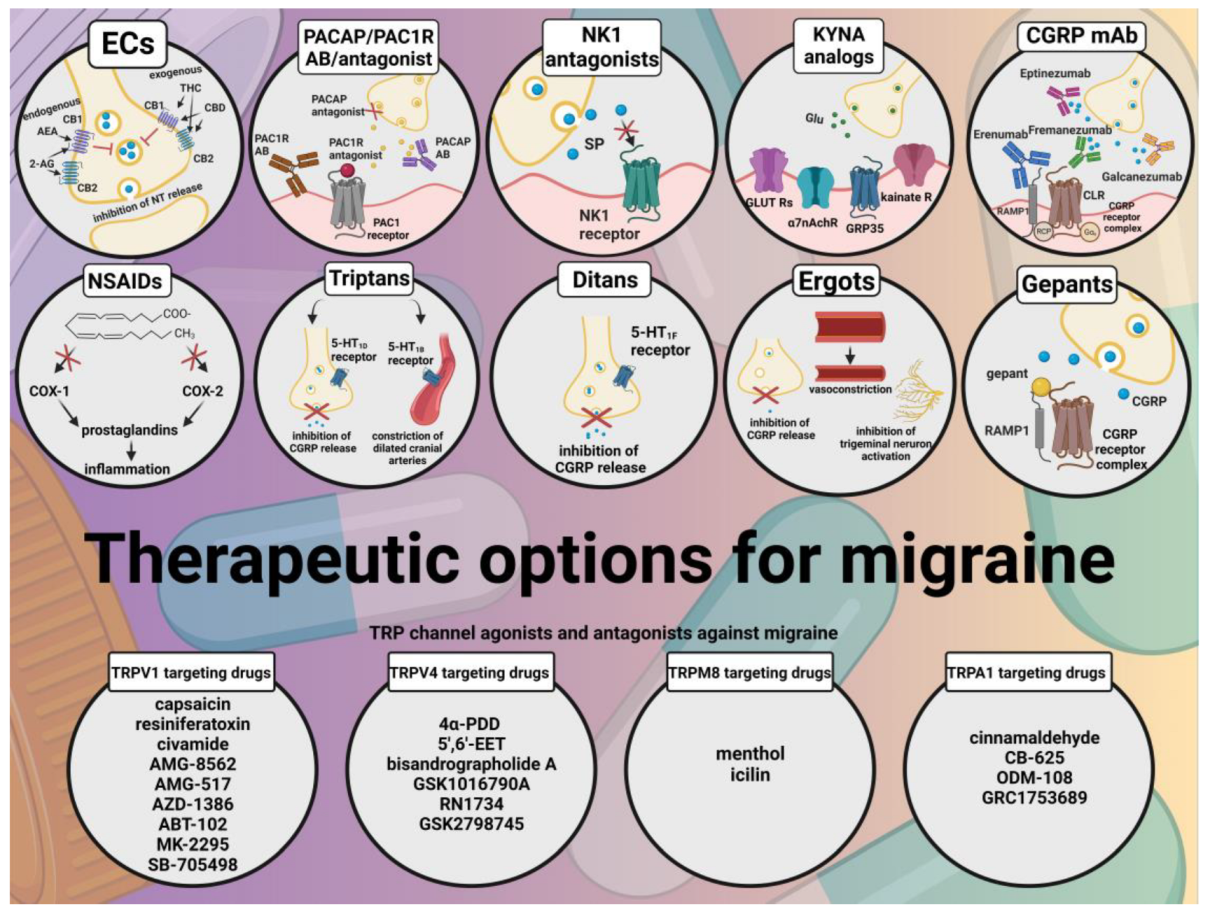
2. Transient Receptor Potential Channels
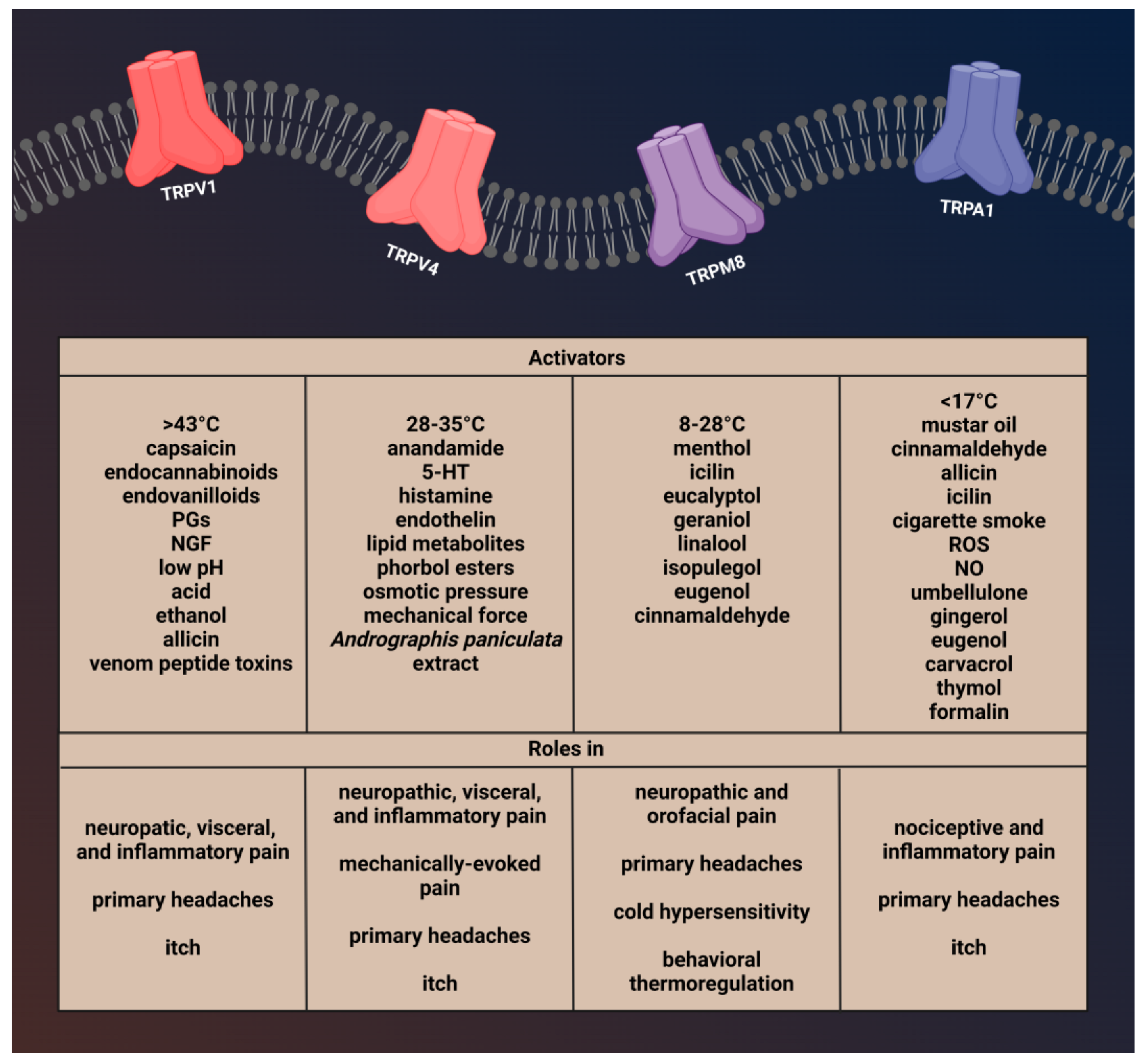
2.1. Transient Receptor Potential Vanilloid 1 (TRPV1)
2.1.1. Characterization of TRPV1 and Role in Pain and Headaches
2.1.2. Preclinical Data
2.1.3. Clinical Data
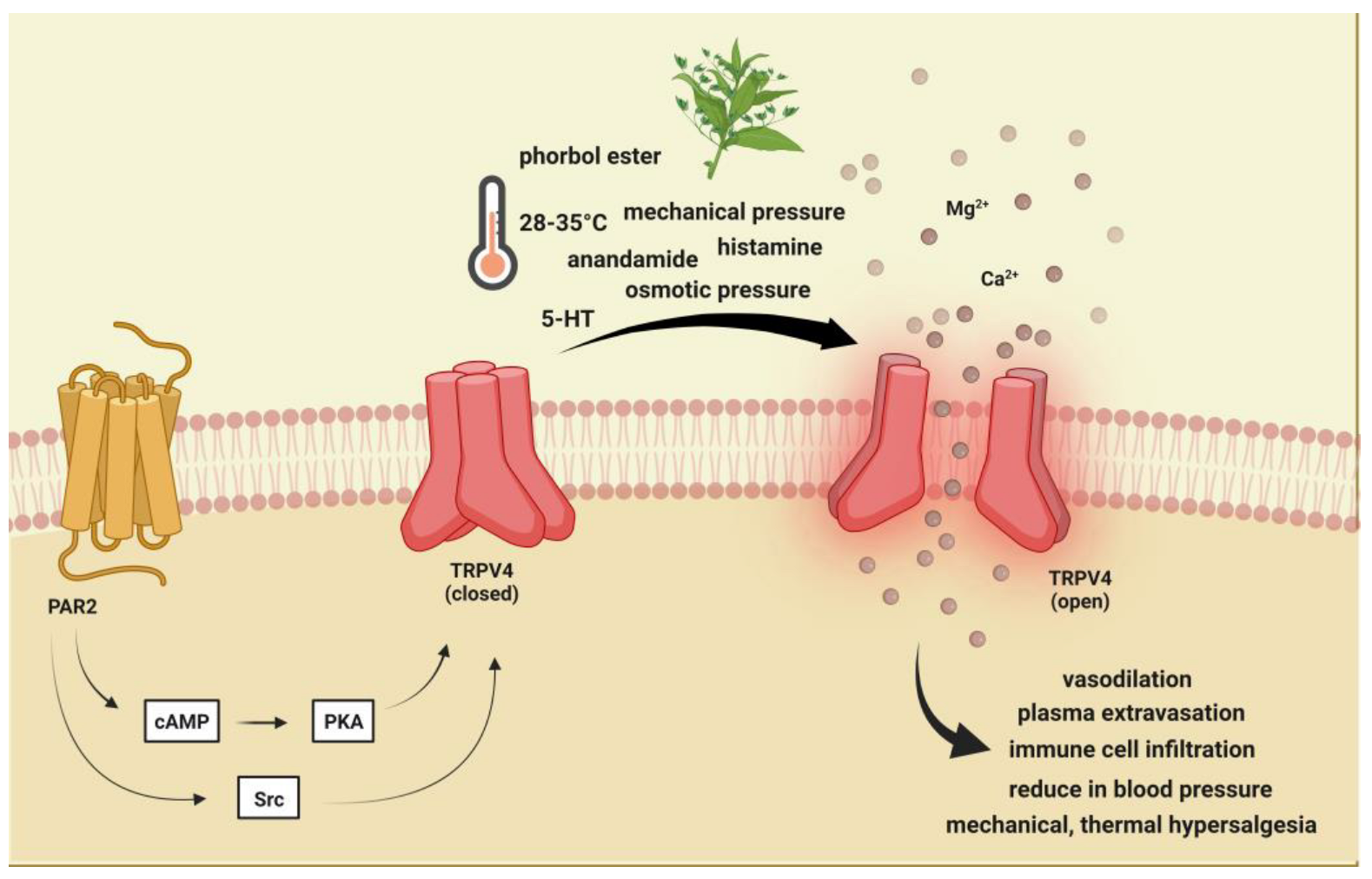
2.2. Transient Receptor Potential Vanilloid 4 (TRPV4)
2.2.1. Brief Description of TRPV4 and Its Role in Pain and Headache
2.2.2. Preclinical Data
2.2.3. Clinical Data
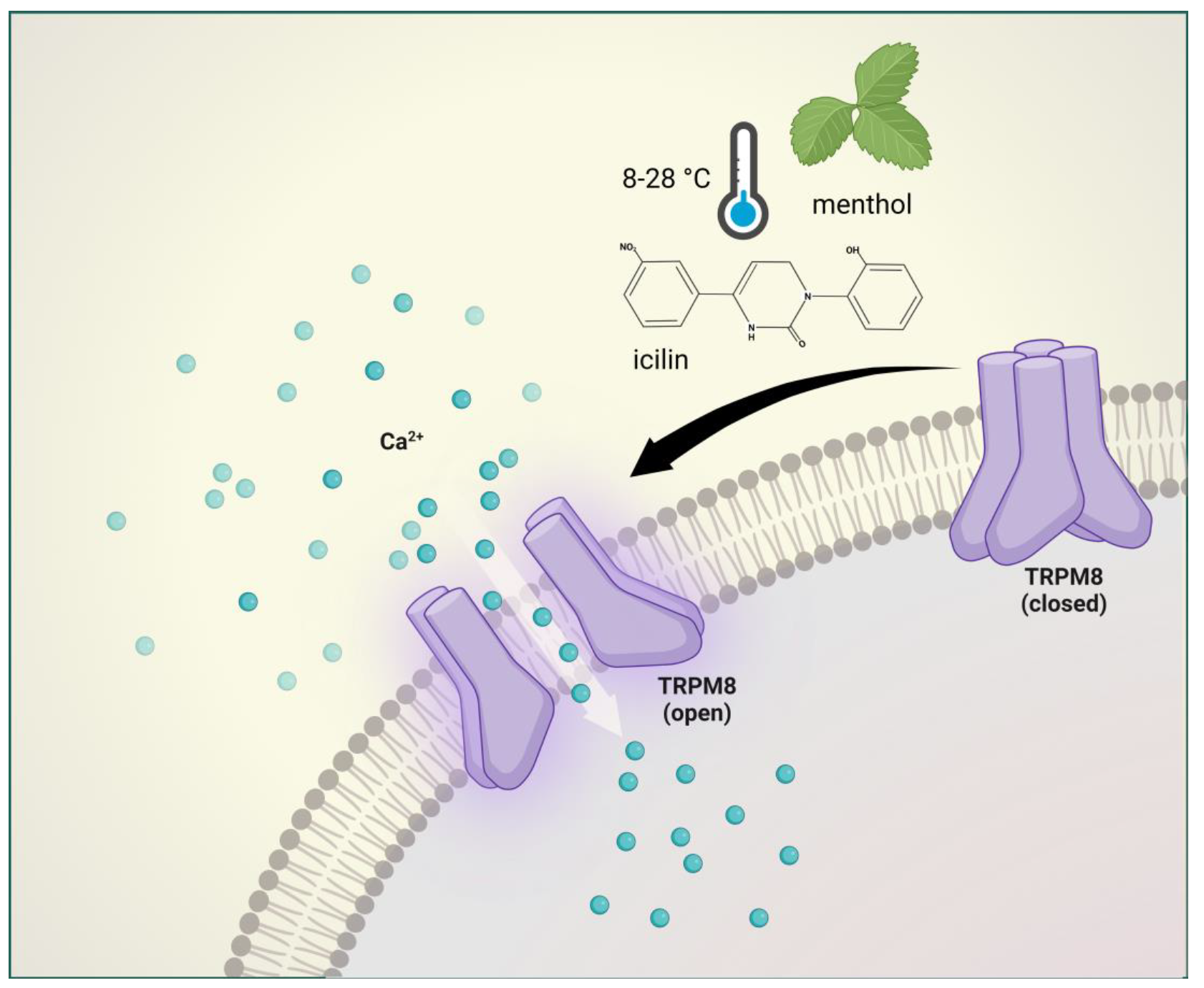
2.3. Transient Receptor Potential Melastatin 8 (TRPM8)
2.3.1. Brief Description of TRPM8 and Its Involvement in Pain and Headache
2.3.2. Preclinical Data
2.3.3. Clinical Data
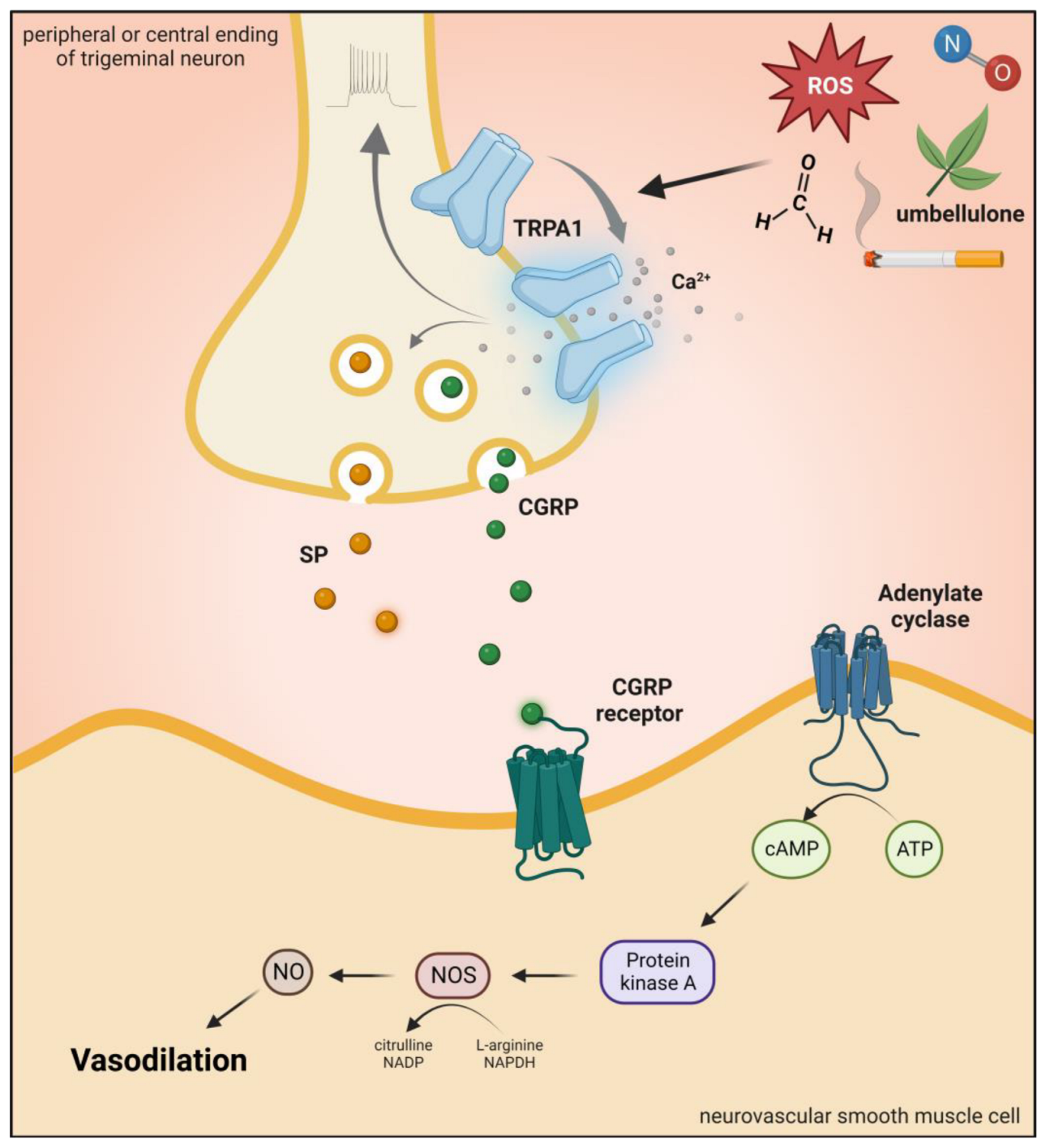
2.4. Transient Receptor Potential Ankyrin 1 (TRPA1)
2.4.1. Brief Description of TRPA1 Channel and Role in Pain and Headaches
2.4.2. Preclinical Data
2.4.3. Clinical Data
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4α-PDD | 4α-phorbol-12,13-didecanoate |
| 5-HT | serotonin |
| BBA | bisandrographolide A |
| CAMKII | calmodulin-dependent kinase II |
| CGRP | calcitonin gene-related peptide |
| COPD | chronic obstructive pulmonary disease |
| CSD | cortical spreading depression |
| DRG | dorsal root ganglion |
| GABA | gamma-aminobutyric acid |
| GWAS | genome-wide association studies |
| IASP | International Association for the Study of Pain |
| IL-10 | interleukin 10 |
| KYNA | kynurenic acid |
| NGF | nerve-growth factor |
| NI | neurogenic inflammation |
| NMDA | N-methyl-D-aspartate receptor |
| NO | nitrogen oxide |
| NSAIDs | nonsteroidal anti-inflammatory drugs |
| NTG | nitroglycerin |
| MOH | medication overuse headache |
| PACAP | pituitary adenylate cyclase-activating peptide |
| PAG | periaqueductal gray matter |
| PAR2 | protease-activated receptor 2 |
| PGs | prostaglandins |
| PKC | protein kinases C |
| PKA | protein kinases A |
| PNS | peripheral nervous system |
| ROS | reactive oxygen species |
| RTX | resiniferatoxin |
| SP | substance P |
| TG | trigeminal ganglion |
| TNF | tumor necrosis factor |
| TRP | transient receptor potential |
| TRPV1 | transient receptor potential vanilloid-1 receptor |
| TRPV4 | transient receptor potential vanilloid-4 receptor |
| TRPM8 | transient receptor potential melastatin 8 |
| TRPA1 | transient receptor potential ankyrin 1 |
| VG | vagal ganglia |
References
- Steiner, T.J.; Fontebasso, M. Headache. BMJ 2002, 325, 881–886. [Google Scholar] [CrossRef] [PubMed]
- Edvinsson, L.; Uddman, R. Neurobiology in primary headaches. Brain Res. Brain Res. Rev. 2005, 48, 438–456. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004, 24 (Suppl. 1), 9–160. [Google Scholar] [CrossRef]
- Ahmed, F. Headache disorders: Differentiating and managing the common subtypes. Br. J. Pain 2012, 6, 124–132. [Google Scholar] [CrossRef]
- May, A.; Schwedt, T.J.; Magis, D.; Pozo-Rosich, P.; Evers, S.; Wang, S.J. Cluster headache. Nat. Rev. Dis. Prim. 2018, 4, 18006. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.; May, A. Diagnosis, pathophysiology, and management of cluster headache. Lancet Neurol. 2018, 17, 75–83. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef]
- Villar-Martinez, M.D.; Goadsby, P.J. Pathophysiology and Therapy of Associated Features of Migraine. Cells 2022, 11, 2767. [Google Scholar] [CrossRef]
- GBD 2016 Headache Collaborators. Global, regional, and national burden of migraine and tension-type headache, 1990–2016, A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018, 17, 954–976. [Google Scholar] [CrossRef]
- Hainer, B.L.; Matheson, E.M. Approach to acute headache in adults. Am. Fam. Physician 2013, 87, 682–687. [Google Scholar]
- Parashar, R.; Bhalla, P.; Rai, N.K.; Pakhare, A.; Babbar, R. Migraine: Is it related to hormonal disturbances or stress? Int. J. Womens Health 2014, 6, 921–925. [Google Scholar] [CrossRef] [PubMed]
- Takayama, Y.; Derouiche, S.; Maruyama, K.; Tominaga, M. Emerging Perspectives on Pain Management by Modulation of TRP Channels and ANO1. Int. J. Mol. Sci. 2019, 20, 3411. [Google Scholar] [CrossRef] [PubMed]
- Lima, A.M.; Sapienza, G.B.; Giraud Vde, O.; Fragoso, Y.D. Odors as triggering and worsening factors for migraine in men. Arq. Neuropsiquiatr. 2011, 69, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, P.; Trevisani, M.; Manconi, M.; Gatti, R.; De Siena, G.; Zagli, G.; Benemei, S.; Capone, J.A.; Geppetti, P.; Pini, L.A. Ethanol causes neurogenic vasodilation by TRPV1 activation and CGRP release in the trigeminovascular system of the guinea pig. Cephalalgia 2008, 28, 9–17. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Fusco, B.M.; Barzoi, G.; Agrò, F. Repeated intranasal capsaicin applications to treat chronic migraine. Br. J. Anaesth. 2003, 90, 812. [Google Scholar] [CrossRef]
- Edvinsson, L. Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia 2011, 31, 737–747. [Google Scholar] [CrossRef]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef]
- Reducha, P.V.; Edvinsson, L.; Haanes, K.A. Could Experimental Inflammation Provide Better Understanding of Migraines? Cells 2022, 11, 2444. [Google Scholar] [CrossRef]
- Vikelis, M.; Mitsikostas, D.D. The role of glutamate and its receptors in migraine. CNS Neurol. Disord. Drug Targets 2007, 6, 251–257. [Google Scholar] [CrossRef]
- Fejes-Szabó, A.; Bohár, Z.; Vámos, E.; Nagy-Grócz, G.; Tar, L.; Veres, G.; Zádori, D.; Szentirmai, M.; Tajti, J.; Szatmári, I.; et al. Pre-treatment with new kynurenic acid amide dose-dependently prevents the nitroglycerine-induced neuronal activation and sensitization in cervical part of trigemino-cervical complex. J. Neural. Transm. 2014, 121, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Körtési, T.; Tuka, B.; Tajti, J.; Bagoly, T.; Fülöp, F.; Helyes, Z.; Vécsei, L. Kynurenic Acid Inhibits the Electrical Stimulation Induced Elevated Pituitary Adenylate Cyclase-Activating Polypeptide Expression in the TNC. Front. Neurol. 2018, 8, 745. [Google Scholar] [CrossRef]
- Knyihar-Csillik, E.; Mihaly, A.; Krisztin-Peva, B.; Robotka, H.; Szatmari, I.; Fulop, F.; Toldi, J.; Csillik, B.; Vecsei, L. The kynurenate analog SZR-72 prevents the nitroglycerol-induced increase of c-fos immunoreactivity in the rat caudal trigeminal nucleus: Comparative studies of the effects of SZR-72 and kynurenic acid. Neurosci. Res. 2008, 61, 429–432. [Google Scholar] [CrossRef] [PubMed]
- Vámos, E.; Fejes, A.; Koch, J.; Tajti, J.; Fülöp, F.; Toldi, J.; Párdutz, A.; Vécsei, L. Kynurenate derivative attenuates the nitroglycerin-induced CamKIIα and CGRP expression changes. Headache 2010, 50, 834–843. [Google Scholar] [CrossRef]
- Tuka, B.; Nyári, A.; Cseh, E.K.; Körtési, T.; Veréb, D.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; Tajti, J.; Vécsei, L. Clinical relevance of depressed kynurenine pathway in episodic migraine patients: Potential prognostic markers in the peripheral plasma during the interictal period. J. Headache Pain 2021, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Perugino, F.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered serum levels of kynurenine metabolites in patients affected by cluster headache. J. Headache Pain 2015, 17, 27. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, C.; Burstein, R. Sensitization of the trigeminovascular pathway: Perspective and implications to migraine pathophysiology. J. Clin. Neurol. 2012, 8, 89–99. [Google Scholar] [CrossRef]
- Edvinsson, L.; Haanes, K.A.; Warfvinge, K.; Krause, D.N. CGRP as the target of new migraine therapies-successful translation from bench to clinic. Nat. Rev. Neurol. 2018, 14, 338–350. [Google Scholar] [CrossRef]
- Premkumar, L.S.; Ahern, G.P. Induction of vanilloid receptor channel activity by protein kinase C. Nature 2000, 408, 985–990. [Google Scholar] [CrossRef]
- Bhave, G.; Hu, H.J.; Glauner, K.S.; Zhu, W.; Wang, H.; Brasier, D.J.; Oxford, G.S.; Gereau, R.W., 4th. Protein kinase C phosphorylation sensitizes but does not activate the capsaicin receptor transient receptor potential vanilloid 1 (TRPV1). Proc. Natl. Acad. Sci. USA 2003, 100, 12480–12485. [Google Scholar] [CrossRef]
- Planells-Cases, R.; Garcìa-Sanz, N.; Morenilla-Palao, C.; Ferrer-Montiel, A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflug. Arch. 2005, 451, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Goadsby, P.J.; Holland, P.R. An Update: Pathophysiology of Migraine. Neurol. Clin. 2019, 37, 651–671. [Google Scholar] [CrossRef] [PubMed]
- Pietrobon, D.; Moskowitz, M.A. Chaos and commotion in the wake of cortical spreading depression and spreading depolarizations. Nat. Rev. Neurosci. 2014, 15, 379–393. [Google Scholar] [CrossRef] [PubMed]
- Lauritzen, M. Cortical spreading depression in migraine. Cephalalgia 2001, 21, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Karatas, H.; Erdener, S.E.; Gursoy-Ozdemir, Y.; Lule, S.; Eren-Koçak, E.; Sen, Z.D.; Dalkara, T. Spreading depression triggers headache by activating neuronal Panx1 channels. Science 2013, 339, 1092–1095. [Google Scholar] [CrossRef] [PubMed]
- Schain, A.J.; Melo-Carrillo, A.; Stratton, J.; Strassman, A.M.; Burstein, R. CSD-Induced Arterial Dilatation and Plasma Protein Extravasation Are Unaffected by Fremanezumab: Implications for CGRP’s Role in Migraine with Aura. J. Neurosci. 2019, 39, 6001–6011. [Google Scholar] [CrossRef]
- May, A.; Burstein, R. Hypothalamic regulation of headache and migraine. Cephalalgia 2019, 39, 1710–1719. [Google Scholar] [CrossRef]
- Belvis, R.; Mas, N.; Aceituno, A. Migraine attack treatment: A tailor-made suit, not one size fits all. Recent Pat CNS Drug Discov. 2014, 9, 26–40. [Google Scholar] [CrossRef]
- Hoffmann, J.; Goadsby, P.J. Emerging targets in migraine. CNS Drugs 2014, 28, 11–17. [Google Scholar] [CrossRef]
- Ghiotto, N.; Sances, G.; Galli, F.; Tassorelli, C.; Guaschino, E.; Sandrini, G.; Nappi, G. Medication overuse headache and applicability of the ICHD-II diagnostic criteria: 1-year follow-up study (CARE I protocol). Cephalalgia 2009, 29, 233–243. [Google Scholar] [CrossRef]
- De Felice, M.; Ossipov, M.H.; Wang, R.; Lai, J.; Chichorro, J.; Meng, I.; Dodick, D.W.; Vanderah, T.W.; Dussor, G.; Porreca, F. Triptan-induced latent sensitization: A possible basis for medication overuse headache. Ann. Neurol. 2010, 67, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Vandervorst, F.; Van Deun, L.; Van Dycke, A.; Paemeleire, K.; Reuter, U.; Schoenen, J.; Versijpt, J. CGRP monoclonal antibodies in migraine: An efficacy and tolerability comparison with standard prophylactic drugs. J. Headache Pain 2021, 22, 128. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E.; Runnels, L.W.; Strübing, C. The TRP ion channel family. Nat. Rev. Neurosci. 2001, 2, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.J.; Sweet, T.B.; Clapham, D.E. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol. Rev. 2010, 62, 381–404. [Google Scholar] [CrossRef] [PubMed]
- Moran, M.M.; McAlexander, M.A.; Bíró, T.; Szallasi, A. Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 2011, 10, 601–620. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. TRP channels as cellular sensors. Nature 2003, 426, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Nilius, B. Transient receptor potential TRP channels as therapeutic drug targets: Next round! Curr. Top. Med. Chem. 2013, 13, 244–246. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, Y.; Szallasi, A. Transient receptor potential (TRP) channels: A clinical perspective. Br. J. Pharmacol. 2014, 171, 2474–2507. [Google Scholar] [CrossRef]
- Vennekens, R.; Menigoz, A.; Nilius, B. TRPs in the Brain. Rev. Physiol. Biochem. Pharmacol. 2012, 163, 27–64. [Google Scholar] [CrossRef]
- Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Nabissi, M. TRP channels: New potential therapeutic approaches in CNS neuropathies. CNS Neurol. Disord. Drug Targets 2013, 12, 274–293. [Google Scholar] [CrossRef]
- Benemei, S.; Dussor, G. TRP Channels and Migraine: Recent Developments and New Therapeutic Opportunities. Pharmaceuticals 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Iannone, L.F.; De Logu, F.; Geppetti, P.; De Cesaris, F. The role of TRP ion channels in migraine and headache. Neurosci. Lett. 2022, 768, 136380. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Tate, S.; Woolf, C.J. Transient receptor potential channels: Targeting pain at the source. Nat. Rev. Drug Discov. 2009, 8, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Brederson, J.D.; Kym, P.R.; Szallasi, A. Targeting TRP channels for pain relief. Eur. J. Pharmacol. 2013, 716, 61–76. [Google Scholar] [CrossRef]
- Dussor, G.; Yan, J.; Xie, J.Y.; Ossipov, M.H.; Dodick, D.W.; Porreca, F. Targeting TRP channels for novel migraine therapeutics. ACS Chem. Neurosci. 2014, 5, 1085–1096. [Google Scholar] [CrossRef]
- Weyer, A.D.; Lehto, S.G. Development of TRPM8 Antagonists to Treat Chronic Pain and Migraine. Pharmaceuticals 2017, 10, 37. [Google Scholar] [CrossRef]
- Artero-Morales, M.; González-Rodríguez, S.; Ferrer-Montiel, A. TRP Channels as Potential Targets for Sex-Related Differences in Migraine Pain. Front. Mol. Biosci. 2018, 5, 73. [Google Scholar] [CrossRef]
- Cabañero, D.; Villalba-Riquelme, E.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. ThermoTRP channels in pain sexual dimorphism: New insights for drug intervention. Pharmacol. Ther. 2022, 240, 108297. [Google Scholar] [CrossRef]
- Dux, M.; Sántha, P.; Jancsó, G. The role of chemosensitive afferent nerves and TRP ion channels in the pathomechanism of headaches. Pflug. Arch. 2012, 464, 239–248. [Google Scholar] [CrossRef]
- Benemei, S.; De Cesaris, F.; Fusi, C.; Rossi, E.; Lupi, C.; Geppetti, P. TRPA1 and other TRP channels in migraine. J. Headache Pain 2013, 14, 71. [Google Scholar] [CrossRef]
- Geppetti, P.; Benemei, S.; De Cesaris, F. CGRP receptors and TRP channels in migraine. J. Headache Pain 2015, 16 (Suppl. 1), A21. [Google Scholar] [CrossRef]
- Koivisto, A.; Jalava, N.; Bratty, R.; Pertovaara, A. TRPA1 Antagonists for Pain Relief. Pharmaceuticals 2018, 11, 117. [Google Scholar] [CrossRef]
- Caterina, M.J.; Schumacher, M.A.; Tominaga, M.; Rosen, T.A.; Levine, J.D.; Julius, D. The capsaicin receptor: A heat-activated ion channel in the pain pathway. Nature 1997, 389, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Jancsó, N.; Jancsó-Gábor, A.; Szolcsányi, J. Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. Chemother. 1967, 31, 138–151. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Appendino, G.; Nilius, B. Pharmacology of vanilloid transient receptor potential cation channels. Mol. Pharmacol. 2009, 75, 1262–1279. [Google Scholar] [CrossRef]
- Jordt, S.E.; Tominaga, M.; Julius, D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. USA 2000, 97, 8134–8139. [Google Scholar] [CrossRef] [PubMed]
- Saloman, J.L.; Chung, M.K.; Ro, J.Y. P2X3 and TRPV1 functionally interact and mediate sensitization of trigeminal sensory neurons. Neuroscience 2013, 232, 226–238. [Google Scholar] [CrossRef]
- Mezey, E.; Tóth, Z.E.; Cortright, D.N.; Arzubi, M.K.; Krause, J.E.; Elde, R.; Guo, A.; Blumberg, P.M.; Szallasi, A. Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. USA 2000, 97, 3655–3660. [Google Scholar] [CrossRef]
- Cristino, L.; de Petrocellis, L.; Pryce, G.; Baker, D.; Guglielmotti, V.; Di Marzo, V. Immunohistochemical localization of cannabinoid type 1 and vanilloid transient receptor potential vanilloid type 1 receptors in the mouse brain. Neuroscience 2006, 139, 1405–1415. [Google Scholar] [CrossRef]
- Cavanaugh, D.J.; Chesler, A.T.; Jackson, A.C.; Sigal, Y.M.; Yamanaka, H.; Grant, R.; O’Donnell, D.; Nicoll, R.A.; Shah, N.M.; Julius, D.; et al. Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 2011, 31, 5067–5077. [Google Scholar] [CrossRef]
- Menigoz, A.; Boudes, M. The expression pattern of TRPV1 in brain. J. Neurosci. 2011, 31, 13025–13027. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Saloman, J.L.; Weiland, G.; Auh, Q.S.; Chung, M.K.; Ro, J.Y. Functional interactions between NMDA receptors and TRPV1 in trigeminal sensory neurons mediate mechanical hyperalgesia in the rat masseter muscle. Pain 2012, 153, 1514–1524. [Google Scholar] [CrossRef] [PubMed]
- Serra, G.P.; Guillaumin, A.; Dumas, S.; Vlcek, B.; Wallén-Mackenzie, Å. Midbrain Dopamine Neurons Defined by TrpV1 Modulate Psychomotor Behavior. Front. Neural Circuits 2021, 15, 726893. [Google Scholar] [CrossRef] [PubMed]
- Grueter, B.A.; Brasnjo, G.; Malenka, R.C. Postsynaptic TRPV1 triggers cell type-specific long-term depression in the nucleus accumbens. Nat. Neurosci. 2010, 13, 1519–1525. [Google Scholar] [CrossRef]
- Szallasi, A.; Blumberg, P.M. Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 1989, 30, 515–520. [Google Scholar] [CrossRef]
- Meents, J.E.; Neeb, L.; Reuter, U. TRPV1 in migraine pathophysiology. Trends Mol. Med. 2010, 16, 153–159. [Google Scholar] [CrossRef]
- Miyamoto, T.; Dubin, A.E.; Petrus, M.J.; Patapoutian, A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS ONE 2009, 4, e7596. [Google Scholar] [CrossRef]
- Julius, D. TRP channels and pain. Annu. Rev. Cell Dev. Biol. 2013, 29, 355–384. [Google Scholar] [CrossRef]
- Gouin, O.; L’Herondelle, K.; Lebonvallet, N.; Le Gall-Ianotto, C.; Sakka, M.; Buhé, V.; Plée-Gautier, E.; Carré, J.L.; Lefeuvre, L.; Misery, L.; et al. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: Pro-inflammatory response induced by their activation and their sensitization. Protein Cell 2017, 8, 644–661. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Numazaki, M.; Tominaga, T.; Toyooka, H.; Tominaga, M. Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J. Biol. Chem. 2002, 277, 13375–13378. [Google Scholar] [CrossRef] [PubMed]
- Vyklický, L.; Vlachová, V.; Vitásková, Z.; Dittert, I.; Kabát, M.; Orkand, R.K. Temperature coefficient of membrane currents induced by noxious heat in sensory neurones in the rat. J. Physiol. 1999, 517, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.B.; Gray, J.; Gunthorpe, M.J.; Hatcher, J.P.; Davey, P.T.; Overend, P.; Harries, M.H.; Latcham, J.; Clapham, C.; Atkinson, K.; et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 2000, 405, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Jancso, N.; Jancso, G.; Takats, I. Pain and inflammation induced by nicotine, acetylcholine and structurally related compounds and their prevention by desensitizing agents. Acta Physiol. Acad. Sci. Hung. 1961, 19, 113–132. [Google Scholar]
- Amaya, F.; Oh-hashi, K.; Naruse, Y.; Iijima, N.; Ueda, M.; Shimosato, G.; Tominaga, M.; Tanaka, Y.; Tanaka, M. Local inflammation increases vanilloid receptor 1 expression within distinct subgroups of DRG neurons. Brain Res. 2003, 963, 190–196. [Google Scholar] [CrossRef]
- Farajdokht, F.; Mohaddes, G.; Shanehbandi, D.; Karimi, P.; Babri, S. Ghrelin attenuated hyperalgesia induced by chronic nitroglycerin: CGRP and TRPV1 as targets for migraine management. Cephalalgia 2018, 38, 1716–1730. [Google Scholar] [CrossRef] [PubMed]
- Spekker, E.; Laborc, K.F.; Bohár, Z.; Nagy-Grócz, G.; Fejes-Szabó, A.; Szűcs, M.; Vécsei, L.; Párdutz, Á. Effect of dural inflammatory soup application on activation and sensitization markers in the caudal trigeminal nucleus of the rat and the modulatory effects of sumatriptan and kynurenic acid. J. Headache Pain 2021, 22, 17. [Google Scholar] [CrossRef]
- Fattori, V.; Hohmann, M.S.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current Understanding of Its Mechanisms and Therapy of Pain and Other Pre-Clinical and Clinical Uses. Molecules 2016, 21, 844. [Google Scholar] [CrossRef]
- Mason, L.; Moore, R.A.; Derry, S.; Edwards, J.E.; McQuay, H.J. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ 2004, 328, 991. [Google Scholar] [CrossRef]
- Cianchetti, C. Capsaicin jelly against migraine pain. Int. J. Clin. Pract. 2010, 64, 457–459. [Google Scholar] [CrossRef]
- Sicuteri, F.; Fusco, B.M.; Marabini, S.; Campagnolo, V.; Maggi, C.A.; Geppetti, P.; Fanciullacci, M. Beneficial effect of capsaicin application to the nasal mucosa in cluster headache. Clin. J. Pain 1989, 5, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Marks, D.R.; Rapoport, A.; Padla, D.; Weeks, R.; Rosum, R.; Sheftell, F.; Arrowsmith, F. A double-blind placebo-controlled trial of intranasal capsaicin for cluster headache. Cephalalgia 1993, 13, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Griglio, A.; Aprile, S.; Seiti, F.; Travelli, C.; Pattarino, F.; Grosa, G.; Sorba, G.; Genazzani, A.A.; Gonzalez-Rodriguez, S.; et al. Targeting Transient Receptor Potential Vanilloid 1 (TRPV1) Channel Softly: The Discovery of Passerini Adducts as a Topical Treatment for Inflammatory Skin Disorders. J. Med. Chem. 2018, 61, 4436–4455. [Google Scholar] [CrossRef] [PubMed]
- Hergenhahn, M.; Kusumoto, S.; Hecker, E. On the active principles of the spurge family (Euphorbiaceae). V. Extremely skin-irritant and moderately tumor-promoting diterpene esters from Euphorbia resinifera Berg. J. Cancer Res. Clin. Oncol. 1984, 108, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Karai, L.; Brown, D.C.; Mannes, A.J.; Connelly, S.T.; Brown, J.; Gandal, M.; Wellisch, O.M.; Neubert, J.K.; Olah, Z.; Iadarola, M.J. Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J. Clin. Invest. 2004, 113, 1344–1352. [Google Scholar] [CrossRef]
- Payne, C.K.; Mosbaugh, P.G.; Forrest, J.B.; Evans, R.J.; Whitmore, K.E.; Antoci, J.P.; Perez-Marrero, R.; Jacoby, K.; Diokno, A.C.; O’Reilly, K.J.; et al. ICOS RTX Study Group (Resiniferatoxin Treatment for Interstitial Cystitis). Intravesical resiniferatoxin for the treatment of interstitial cystitis: A randomized, double-blind, placebo controlled trial. J. Urol. 2005, 173, 1590–1594. [Google Scholar] [CrossRef]
- Heiss, J.; Iadarola, M.; Cantor, F.; Oughourli, A.; Smith, R.; Mannes, A. A Phase I study of the intrathecal administration of resiniferatoxin for treating severe refractory pain associated with advanced cancer. J. Pain 2014, 15, S67. [Google Scholar] [CrossRef]
- Holzer, P. Capsaicin: Cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 1991, 43, 143–201. [Google Scholar]
- Saper, J.R.; Klapper, J.; Mathew, N.T.; Rapoport, A.; Phillips, S.B.; Bernstein, J.E. Intranasal civamide for the treatment of episodic cluster headaches. Arch. Neurol. 2002, 59, 990–994. [Google Scholar] [CrossRef] [PubMed]
- Diamond, S.; Freitag, F.; Phillips, S.B.; Bernstein, J.E.; Saper, J.R. Intranasal civamide for the acute treatment of migraine headache. Cephalalgia 2000, 20, 597–602. [Google Scholar] [CrossRef]
- Lehto, S.G.; Tamir, R.; Deng, H.; Klionsky, L.; Kuang, R.; Le, A.; Lee, D.; Louis, J.C.; Magal, E.; Manning, B.H.; et al. Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J. Pharmacol. Exp. Ther. 2008, 326, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Gunthorpe, M.J.; Hannan, S.L.; Smart, D.; Jerman, J.C.; Arpino, S.; Smith, G.D.; Brough, S.; Wright, J.; Egerton, J.; Lappin, S.C.; et al. Characterization of SB-705498, a potent and selective vanilloid receptor-1 (VR1/TRPV1) antagonist that inhibits the capsaicin-, acid-, and heat-mediated activation of the receptor. J. Pharmacol. Exp. Ther. 2007, 321, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Chizh, B.A.; O’Donnell, M.B.; Napolitano, A.; Wang, J.; Brooke, A.C.; Aylott, M.C.; Bullman, J.N.; Gray, E.J.; Lai, R.Y.; Williams, P.M.; et al. The effects of the TRPV1 antagonist SB-705498 on TRPV1 receptor-mediated activity and inflammatory hyperalgesia in humans. Pain 2007, 132, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Aghazadeh Tabrizi, M.; Baraldi, P.G.; Baraldi, S.; Gessi, S.; Merighi, S.; Borea, P.A. Medicinal Chemistry, Pharmacology, and Clinical Implications of TRPV1 Receptor Antagonists. Med. Res. Rev. 2017, 37, 936–983. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Wang, Y.; Liu, L.; Qiao, Z.; Yan, L. A patent review of transient receptor potential vanilloid type 1 modulators (2014-present). Expert Opin. Ther. Pat. 2021, 31, 169–187. [Google Scholar] [CrossRef]
- Carreño, O.; Corominas, R.; Fernández-Morales, J.; Camiña, M.; Sobrido, M.J.; Fernández-Fernández, J.M.; Pozo-Rosich, P.; Cormand, B.; Macaya, A. SNP variants within the vanilloid TRPV1 and TRPV3 receptor genes are associated with migraine in the Spanish population. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2012, 159, 94–103. [Google Scholar] [CrossRef]
- Yakubova, A.; Davidyuk, Y.; Tohka, J.; Khayrutdinova, O.; Kudryavtsev, I.; Nurkhametova, D.; Kamshilin, A.; Giniatullin, R.; Rizvanov, A. Searching for Predictors of Migraine Chronification: A Pilot Study of 1911A>G Polymorphism of TRPV1 Gene in Episodic Versus Chronic Migraine. J. Mol. Neurosci. 2021, 71, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W. Molecular mechanisms of TRPV4-mediated neural signaling. Ann. N. Y. Acad. Sci. 2008, 1144, 42–52. [Google Scholar] [CrossRef]
- White, J.P.; Cibelli, M.; Urban, L.; Nilius, B.; McGeown, J.G.; Nagy, I. TRPV4: Molecular Conductor of a Diverse Orchestra. Physiol. Rev. 2016, 96, 911–973. [Google Scholar] [CrossRef]
- Guarino, B.D.; Paruchuri, S.; Thodeti, C.K. The role of TRPV4 channels in ocular function and pathologies. Exp. Eye Res. 2020, 201, 108257. [Google Scholar] [CrossRef]
- Martínez-Rendón, J.; Sánchez-Guzmán, E.; Rueda, A.; González, J.; Gulias-Cañizo, R.; Aquino-Jarquín, G.; Castro-Muñozledo, F.; García-Villegas, R. TRPV4 Regulates Tight Junctions and Affects Differentiation in a Cell Culture Model of the Corneal Epithelium. J. Cell Physiol. 2017, 232, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- D’Aldebert, E.; Cenac, N.; Rousset, P.; Martin, L.; Rolland, C.; Chapman, K.; Selves, J.; Alric, L.; Vinel, J.P.; Vergnolle, N. Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology 2011, 140, 275–285. [Google Scholar] [CrossRef] [PubMed]
- Filosa, J.A.; Yao, X.; Rath, G. TRPV4 and the regulation of vascular tone. J. Cardiovasc. Pharmacol. 2013, 61, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Strassman, A.M.; Raymond, S.A.; Burstein, R. Sensitization of meningeal sensory neurons and the origin of headaches. Nature 1996, 384, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Levy, D.; Strassman, A.M. Mechanical response properties of A and C primary afferent neurons innervating the rat intracranial dura. J. Neurophysiol. 2002, 88, 3021–3031. [Google Scholar] [CrossRef]
- Shibata, M.; Tang, C. Implications of Transient Receptor Potential Cation Channels in Migraine Pathophysiology. Neurosci. Bull. 2021, 37, 103–116. [Google Scholar] [CrossRef]
- Vergnolle, N.; Cenac, N.; Altier, C.; Cellars, L.; Chapman, K.; Zamponi, G.W.; Materazzi, S.; Nassini, R.; Liedtke, W.; Cattaruzza, F.; et al. A role for transient receptor potential vanilloid 4 in tonicity-induced neurogenic inflammation. Br. J. Pharmacol. 2010, 159, 1161–1173. [Google Scholar] [CrossRef]
- Alessandri-Haber, N.; Yeh, J.J.; Boyd, A.E.; Parada, C.A.; Chen, X.; Reichling, D.B.; Levine, J.D. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron 2003, 39, 497–511. [Google Scholar] [CrossRef]
- Feng, X.; Takayama, Y.; Ohno, N.; Kanda, H.; Dai, Y.; Sokabe, T.; Tominaga, M. Increased TRPV4 expression in non-myelinating Schwann cells is associated with demyelination after sciatic nerve injury. Commun. Biol. 2020, 3, 716. [Google Scholar] [CrossRef]
- Chen, Y.; Williams, S.H.; McNulty, A.L.; Hong, J.H.; Lee, S.H.; Rothfusz, N.E.; Parekh, P.K.; Moore, C.; Gereau, R.W., 4th; Taylor, A.B.; et al. Temporomandibular joint pain: A critical role for Trpv4 in the trigeminal ganglion. Pain 2013, 154, 1295–1304. [Google Scholar] [CrossRef]
- Zhang, X.C.; Levy, D. Modulation of meningeal nociceptors mechanosensitivity by peripheral proteinase-activated receptor-2: The role of mast cells. Cephalalgia 2008, 28, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Cenac, N.; Altier, C.; Motta, J.P.; d’Aldebert, E.; Galeano, S.; Zamponi, G.W.; Vergnolle, N. Potentiation of TRPV4 signalling by histamine and serotonin: An important mechanism for visceral hypersensitivity. Gut 2010, 59, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Liedtke, W.; Friedman, J.M. Abnormal osmotic regulation in trpv4-/-mice. Proc. Natl. Acad. Sci. USA 2003, 100, 13698–13703. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, M.; Mizuno, A.; Kodaira, K.; Imai, M. Impaired pressure sensation in mice lacking TRPV4. J. Biol. Chem. 2003, 278, 22664–22668. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Kanju, P.; Fang, Q.; Lee, S.H.; Parekh, P.K.; Lee, W.; Moore, C.; Brenner, D.; Gereau, R.W., 4th; Wang, F.; et al. TRPV4 is necessary for trigeminal irritant pain and functions as a cellular formalin receptor. Pain 2014, 155, 2662–2672. [Google Scholar] [CrossRef] [PubMed]
- Alessandri-Haber, N.; Dina, O.A.; Yeh, J.J.; Parada, C.A.; Reichling, D.B.; Levine, J.D. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J. Neurosci. 2004, 24, 4444–4452, Erratum in: J. Neurosci. 2004, 24, 5457. [Google Scholar] [CrossRef] [PubMed]
- Poole, D.P.; Amadesi, S.; Veldhuis, N.A.; Abogadie, F.C.; Lieu, T.; Darby, W.; Liedtke, W.; Lew, M.J.; McIntyre, P.; Bunnett, N.W. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J. Biol. Chem. 2013, 288, 5790–5802. [Google Scholar] [CrossRef]
- Grace, M.S.; Lieu, T.; Darby, B.; Abogadie, F.C.; Veldhuis, N.; Bunnett, N.W.; McIntyre, P. The tyrosine kinase inhibitor bafetinib inhibits PAR2-induced activation of TRPV4 channels in vitro and pain in vivo. Br. J. Pharmacol. 2014, 171, 3881–3894. [Google Scholar] [CrossRef]
- Watanabe, H.; Davis, J.B.; Smart, D.; Jerman, J.C.; Smith, G.D.; Hayes, P.; Vriens, J.; Cairns, W.; Wissenbach, U.; Prenen, J.; et al. Activation of TRPV4 channels (hVRL-2/mTRP12) by phorbol derivatives. J. Biol. Chem. 2002, 277, 13569–13577. [Google Scholar] [CrossRef]
- Nilius, B.; Vriens, J.; Prenen, J.; Droogmans, G.; Voets, T. TRPV4 calcium entry channel: A paradigm for gating diversity. Am. J. Physiol. Cell Physiol. 2004, 286, C195–C205. [Google Scholar] [CrossRef]
- Vriens, J.; Owsianik, G.; Janssens, A.; Voets, T.; Nilius, B. Determinants of 4 alpha-phorbol sensitivity in transmembrane domains 3 and 4 of the cation channel TRPV4. J. Biol. Chem. 2007, 282, 12796–12803. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Edelmayer, R.M.; Yan, J.; Dussor, G. Activation of TRPV4 on dural afferents produces headache-related behavior in a preclinical rat model. Cephalalgia 2011, 31, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Vriens, J.; Watanabe, H.; Janssens, A.; Droogmans, G.; Voets, T.; Nilius, B. Cell swelling, heat, and chemical agonists use distinct pathways for the activation of the cation channel TRPV4. Proc. Natl. Acad. Sci. USA 2004, 101, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, J.; Lorenzo, I.M.; Andrade, Y.N.; Garcia-Elias, A.; Serra, S.A.; Fernández-Fernández, J.M.; Valverde, M.A. IP3 sensitizes TRPV4 channel to the mechano- and osmotransducing messenger 5’-6’-epoxyeicosatrienoic acid. J. Cell Biol. 2008, 181, 143–155. [Google Scholar] [CrossRef]
- Berna-Erro, A.; Izquierdo-Serra, M.; Sepúlveda, R.V.; Rubio-Moscardo, F.; Doñate-Macián, P.; Serra, S.A.; Carrillo-Garcia, J.; Perálvarez-Marín, A.; González-Nilo, F.; Fernández-Fernández, J.M.; et al. Structural determinants of 5’,6’-epoxyeicosatrienoic acid binding to and activation of TRPV4 channel. Sci. Rep. 2017, 7, 10522. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.L.; Maloney, K.N.; Pothen, R.G.; Clardy, J.; Clapham, D.E. Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J. Biol. Chem. 2006, 281, 29897–29904. [Google Scholar] [CrossRef]
- Baratchi, S.; Keov, P.; Darby, W.G.; Lai, A.; Khoshmanesh, K.; Thurgood, P.; Vahidi, P.; Ejendal, K.; McIntyre, P. The TRPV4 Agonist GSK1016790A Regulates the Membrane Expression of TRPV4 Channels. Front. Pharmacol. 2019, 10, 6. [Google Scholar] [CrossRef]
- Tabuchi, K.; Suzuki, M.; Mizuno, A.; Hara, A. Hearing impairment in TRPV4 knockout mice. Neurosci. Lett. 2005, 382, 304–308. [Google Scholar] [CrossRef]
- Gevaert, T.; Vriens, J.; Segal, A.; Everaerts, W.; Roskams, T.; Talavera, K.; Owsianik, G.; Liedtke, W.; Daelemans, D.; Dewachter, I.; et al. Deletion of the transient receptor potential cation channel TRPV4 impairs murine bladder voiding. J. Clin. Invest. 2007, 117, 3453–3462. [Google Scholar] [CrossRef]
- Cortright, D.N.; Krause, J.E.; Broom, D.C. TRP channels and pain. Biochim. Biophys. Acta 2007, 1772, 978–988. [Google Scholar] [CrossRef]
- Lawhorn, B.G.; Brnardic, E.J.; Behm, D.J. TRPV4 antagonists: A patent review (2015–2020). Expert Opin. Ther. Pat. 2021, 31, 773–784. [Google Scholar] [CrossRef] [PubMed]
- McKemy, D.D.; Neuhausser, W.M.; Julius, D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 2002, 416, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Mickle, A.D.; Shepherd, A.J.; Mohapatra, D.P. Sensory TRP channels: The key transducers of nociception and pain. Prog. Mol. Biol. Transl. Sci. 2015, 131, 73–118. [Google Scholar] [CrossRef] [PubMed]
- Dhaka, A.; Earley, T.J.; Watson, J.; Patapoutian, A. Visualizing cold spots: TRPM8-expressing sensory neurons and their projections. J. Neurosci. 2008, 28, 566–575. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Jordt, S.E.; Nikai, T.; Tsuruda, P.R.; Read, A.J.; Poblete, J.; Yamoah, E.N.; Basbaum, A.I.; Julius, D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell 2006, 124, 1269–1282. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Fukuoka, T.; Obata, K.; Yamanaka, H.; Dai, Y.; Tokunaga, A.; Noguchi, K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005, 493, 596–606. [Google Scholar] [CrossRef]
- Ordás, P.; Hernández-Ortego, P.; Vara, H.; Fernández-Peña, C.; Reimúndez, A.; Morenilla-Palao, C.; Guadaño-Ferraz, A.; Gomis, A.; Hoon, M.; Viana, F.; et al. Expression of the cold thermoreceptor TRPM8 in rodent brain thermoregulatory circuits. J. Comp. Neurol. 2021, 529, 234–256. [Google Scholar] [CrossRef]
- Khalil, M.; Alliger, K.; Weidinger, C.; Yerinde, C.; Wirtz, S.; Becker, C.; Engel, M.A. Functional Role of Transient Receptor Potential Channels in Immune Cells and Epithelia. Front. Immunol. 2018, 9, 174. [Google Scholar] [CrossRef]
- Gauchan, P.; Andoh, T.; Kato, A.; Kuraishi, Y. Involvement of increased expression of transient receptor potential melastatin 8 in oxaliplatin-induced cold allodynia in mice. Neurosci. Lett. 2009, 458, 93–95. [Google Scholar] [CrossRef]
- Ramachandran, R.; Hyun, E.; Zhao, L.; Lapointe, T.K.; Chapman, K.; Hirota, C.L.; Ghosh, S.; McKemy, D.D.; Vergnolle, N.; Beck, P.L.; et al. TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc. Natl. Acad. Sci. USA 2013, 110, 7476–7481. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Yu, X.; Yan, X.J.; Lei, F.; Chai, Y.S.; Jiang, J.F.; Yuan, Z.Y.; Xing, D.M.; Du, L.J. TRPM8 in the negative regulation of TNFα expression during cold stress. Sci. Rep. 2017, 7, 45155. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Kim, B.; McKemy, D.D. Transient receptor potential melastatin 8 is required for nitroglycerin- and calcitonin gene-related peptide-induced migraine-like pain behaviors in mice. Pain 2022, 163, 2380–2389. [Google Scholar] [CrossRef] [PubMed]
- Colburn, R.W.; Lubin, M.L.; Stone, D.J., Jr.; Wang, Y.; Lawrence, D.; D’Andrea, M.R.; Brandt, M.R.; Liu, Y.; Flores, C.M.; Qin, N. Attenuated cold sensitivity in TRPM8 null mice. Neuron 2007, 54, 379–386. [Google Scholar] [CrossRef]
- Bettella, F.; Stefansson, H.; Olesen, J. Replication and meta-analysis of common variants identifies a genome-wide significant locus in migraine. Eur. J. Neurol. 2013, 20, 765–772. [Google Scholar] [CrossRef]
- Chasman, D.I.; Anttila, V.; Buring, J.E.; Ridker, P.M.; Schürks, M.; Kurth, T. International Headache Genetics Consortium. Selectivity in genetic association with sub-classified migraine in women. PLoS Genet. 2014, 10, e1004366, Erratum in: PLoS Genet. 2015, 11, e1005330. [Google Scholar] [CrossRef] [PubMed]
- Dussor, G.; Cao, Y.Q. TRPM8 and Migraine. Headache 2016, 56, 1406–1417. [Google Scholar] [CrossRef]
- Burstein, R.; Cutrer, M.F.; Yarnitsky, D. The development of cutaneous allodynia during a migraine attack clinical evi-dence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain 2000, 123 Pt 8, 1703–1709. [Google Scholar] [CrossRef]
- Borhani Haghighi, A.; Motazedian, S.; Rezaii, R.; Mohammadi, F.; Salarian, L.; Pourmokhtari, M.; Khodaei, S.; Vossoughi, M.; Miri, R. Cutaneous application of menthol 10% solution as an abortive treatment of migraine without aura: A randomised, double-blind, placebo-controlled, crossed-over study. Int. J. Clin. Pract. 2010, 64, 451–456. [Google Scholar] [CrossRef]
- Prince, P.B.; Rapoport, A.M.; Sheftell, F.D.; Tepper, S.J.; Bigal, M.E. The effect of weather on headache. Headache 2004, 44, 596–602. [Google Scholar] [CrossRef]
- Burgos-Vega, C.C.; Ahn, D.D.; Bischoff, C.; Wang, W.; Horne, D.; Wang, J.; Gavva, N.; Dussor, G. Meningeal transient receptor potential channel M8 activation causes cutaneous facial and hindpaw allodynia in a preclinical rodent model of headache. Cephalalgia 2016, 36, 185–193. [Google Scholar] [CrossRef]
- Kayama, Y.; Shibata, M.; Takizawa, T.; Ibata, K.; Shimizu, T.; Ebine, T.; Toriumi, H.; Yuzaki, M.; Suzuki, N. Functional interactions between transient receptor potential M8 and transient receptor potential V1 in the trigeminal system: Relevance to migraine pathophysiology. Cephalalgia 2018, 38, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Alarcón-Alarcón, D.; Cabañero, D.; de Andrés-López, J.; Nikolaeva-Koleva, M.; Giorgi, S.; Fernández-Ballester, G.; Fernández-Carvajal, A.; Ferrer-Montiel, A. TRPM8 contributes to sex dimorphism by promoting recovery of normal sensitivity in a mouse model of chronic migraine. Nat. Commun. 2022, 13, 6304. [Google Scholar] [CrossRef] [PubMed]
- Eccles, R. Menthol and related cooling compounds. J. Pharm. Pharmacol. 1994, 46, 618–630. [Google Scholar] [CrossRef] [PubMed]
- Lasanen, R.; Julkunen, P.; Airaksinen, O.; Töyräs, J. Menthol concentration in topical cold gel does not have significant effect on skin cooling. Ski. Res. Technol. 2016, 22, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.H.; Gazerani, P.; Arendt-Nielsen, L. High-Concentration L-Menthol Exhibits Counter-Irritancy to Neurogenic Inflammation, Thermal and Mechanical Hyperalgesia Caused by Trans-cinnamaldehyde. J. Pain 2016, 17, 919–929. [Google Scholar] [CrossRef] [PubMed]
- Colvin, L.A.; Johnson, P.R.; Mitchell, R.; Fleetwood-Walker, S.M.; Fallon, M. From bench to bedside: A case of rapid reversal of bortezomib-induced neuropathic pain by the TRPM8 activator, menthol. J. Clin. Oncol. 2008, 26, 4519–4520. [Google Scholar] [CrossRef]
- Willis, D.N.; Liu, B.; Ha, M.A.; Jordt, S.E.; Morris, J.B. Menthol attenuates respiratory irritation responses to multiple cigarette smoke irritants. FASEB J. 2011, 25, 4434–4444. [Google Scholar] [CrossRef]
- Nilius, B.; Owsianik, G.; Voets, T.; Peters, J.A. Transient receptor potential cation channels in disease. Physiol. Rev. 2007, 87, 165–217. [Google Scholar] [CrossRef]
- Jordt, S.E.; Bautista, D.M.; Chuang, H.H.; McKemy, D.D.; Zygmunt, P.M.; Högestätt, E.D.; Meng, I.D.; Julius, D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 2004, 427, 260–265. [Google Scholar] [CrossRef]
- Bautista, D.M.; Movahed, P.; Hinman, A.; Axelsson, H.E.; Sterner, O.; Högestätt, E.D.; Julius, D.; Jordt, S.E.; Zygmunt, P.M. Pungent products from garlic activate the sensory ion channel TRPA1. Proc. Natl. Acad. Sci. USA 2005, 102, 12248–12252. [Google Scholar] [CrossRef]
- Bandell, M.; Story, G.M.; Hwang, S.W.; Viswanath, V.; Eid, S.R.; Petrus, M.J.; Earley, T.J.; Patapoutian, A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 2004, 41, 849–857. [Google Scholar] [CrossRef]
- Xu, H.; Delling, M.; Jun, J.C.; Clapham, D.E. Oregano, thyme and clove-derived flavors and skin sensitizers activate specific TRP channels. Nat. Neurosci. 2006, 9, 628–635. [Google Scholar] [CrossRef]
- Lee, S.P.; Buber, M.T.; Yang, Q.; Cerne, R.; Cortés, R.Y.; Sprous, D.G.; Bryant, R.W. Thymol and related alkyl phenols activate the hTRPA1 channel. Br. J. Pharmacol. 2008, 153, 1739–1749. [Google Scholar] [CrossRef]
- Story, G.M.; Peier, A.M.; Reeve, A.J.; Eid, S.R.; Mosbacher, J.; Hricik, T.R.; Earley, T.J.; Hergarden, A.C.; Andersson, D.A.; Hwang, S.W.; et al. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 2003, 112, 819–829. [Google Scholar] [CrossRef]
- Latorre, R. Perspectives on TRP channel structure and the TRPA1 puzzle. J. Gen. Physiol. 2009, 133, 227–229. [Google Scholar] [CrossRef]
- Duric, V.; McCarson, K.E. Neurokinin-1 (NK-1) receptor and brain-derived neurotrophic factor (BDNF) gene expression is differentially modulated in the rat spinal dorsal horn and hippocampus during inflammatory pain. Mol. Pain 2007, 3, 32. [Google Scholar] [CrossRef]
- Taylor-Clark, T.E.; Undem, B.J.; Macglashan, D.W., Jr.; Ghatta, S.; Carr, M.J.; McAlexander, M.A. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1). Mol. Pharmacol. 2008, 73, 274–281. [Google Scholar] [CrossRef]
- Messlinger, K.; Hanesch, U.; Kurosawa, M.; Pawlak, M.; Schmidt, R.F. Calcitonin gene related peptide released from dural nerve fibers mediates increase of meningeal blood flow in the rat. Can. J. Physiol. Pharmacol. 1995, 73, 1020–1024. [Google Scholar] [CrossRef]
- Edelmayer, R.M.; Le, L.N.; Yan, J.; Wei, X.; Nassini, R.; Materazzi, S.; Preti, D.; Appendino, G.; Geppetti, P.; Dodick, D.W.; et al. Activation of TRPA1 on dural afferents: A potential mechanism of headache pain. Pain 2012, 153, 1949–1958. [Google Scholar] [CrossRef]
- Kelman, L. The triggers or precipitants of the acute migraine attack. Cephalalgia 2007, 27, 394–402. [Google Scholar] [CrossRef]
- McNamara, C.R.; Mandel-Brehm, J.; Bautista, D.M.; Siemens, J.; Deranian, K.L.; Zhao, M.; Hayward, N.J.; Chong, J.A.; Julius, D.; Moran, M.M.; et al. TRPA1 mediates formalin-induced pain. Proc. Natl. Acad. Sci. USA 2007, 104, 13525–13530. [Google Scholar] [CrossRef] [PubMed]
- Benemei, S.; Fusi, C.; Trevisan, G.; Geppetti, P. The TRPA1 channel in migraine mechanism and treatment. Br. J. Pharmacol. 2014, 171, 2552–2567. [Google Scholar] [CrossRef] [PubMed]
- Bautista, D.M.; Siemens, J.; Glazer, J.M.; Tsuruda, P.R.; Basbaum, A.I.; Stucky, C.L.; Jordt, S.E.; Julius, D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 2007, 448, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Geppetti, P.; Nassini, R.; Materazzi, S.; Benemei, S. The concept of neurogenic inflammation. BJU Int. 2008, 101 (Suppl. 3), 2–6. [Google Scholar] [CrossRef]
- Obata, K.; Katsura, H.; Mizushima, T.; Yamanaka, H.; Kobayashi, K.; Dai, Y.; Fukuoka, T.; Tokunaga, A.; Tominaga, M.; Noguchi, K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J. Clin. Invest. 2005, 115, 2393–2401. [Google Scholar] [CrossRef]
- Petrus, M.; Peier, A.M.; Bandell, M.; Hwang, S.W.; Huynh, T.; Olney, N.; Jegla, T.; Patapoutian, A. A role of TRPA1 in mechanical hyperalgesia is revealed by pharmacological inhibition. Mol. Pain 2007, 3, 40. [Google Scholar] [CrossRef]
- Eid, S.R.; Crown, E.D.; Moore, E.L.; Liang, H.A.; Choong, K.C.; Dima, S.; Henze, D.A.; Kane, S.A.; Urban, M.O. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol. Pain 2008, 4, 48. [Google Scholar] [CrossRef]
- Dunham, J.P.; Kelly, S.; Donaldson, L.F. Inflammation reduces mechanical thresholds in a population of transient receptor potential channel A1-expressing nociceptors in the rat. Eur. J. Neurosci. 2008, 27, 3151–3160. [Google Scholar] [CrossRef]
- Jiang, L.; Ma, D.; Grubb, B.D.; Wang, M. ROS/TRPA1/CGRP signaling mediates cortical spreading depression. J. Headache Pain 2019, 20, 25. [Google Scholar] [CrossRef]
- Nassini, R.; Materazzi, S.; Vriens, J.; Prenen, J.; Benemei, S.; De Siena, G.; la Marca, G.; Andrè, E.; Preti, D.; Avonto, C.; et al. The ‘headache tree’ via umbellulone and TRPA1 activates the trigeminovascular system. Brain 2012, 135 Pt 2, 376–390. [Google Scholar] [CrossRef]
- Kunkler, P.E.; Ballard, C.J.; Oxford, G.S.; Hurley, J.H. TRPA1 receptors mediate environmental irritant-induced meningeal vasodilatation. Pain 2011, 152, 38–44. [Google Scholar] [CrossRef]
- Benemei, S.; Appendino, G.; Geppetti, P. Pleasant natural scent with unpleasant effects: Cluster headache-like attacks triggered by Umbellularia californica. Cephalalgia 2010, 30, 744–746. [Google Scholar] [CrossRef]
- Teicher, C.; De Col, R.; Messlinger, K. Hydrogen Sulfide Mediating both Excitatory and Inhibitory Effects in a Rat Model of Meningeal Nociception and Headache Generation. Front. Neurol. 2017, 8, 336. [Google Scholar] [CrossRef]
- Kosugi, M.; Nakatsuka, T.; Fujita, T.; Kuroda, Y.; Kumamoto, E. Activation of TRPA1 channel facilitates excitatory synaptic transmission in substantia gelatinosa neurons of the adult rat spinal cord. J. Neurosci. 2007, 27, 4443–4451. [Google Scholar] [CrossRef]
- Wrigley, P.J.; Jeong, H.J.; Vaughan, C.W. Primary afferents with TRPM8 and TRPA1 profiles target distinct subpopulations of rat superficial dorsal horn neurones. Br. J. Pharmacol. 2009, 157, 371–380. [Google Scholar] [CrossRef]
- Trevisani, M.; Siemens, J.; Materazzi, S.; Bautista, D.M.; Nassini, R.; Campi, B.; Imamachi, N.; Andrè, E.; Patacchini, R.; Cottrell, G.S.; et al. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc. Natl. Acad. Sci. USA 2007, 104, 13519–13524. [Google Scholar] [CrossRef]
- Pozsgai, G.; Bodkin, J.V.; Graepel, R.; Bevan, S.; Andersson, D.A.; Brain, S.D. Evidence for the pathophysiological relevance of TRPA1 receptors in the cardiovascular system in vivo. Cardiovasc. Res. 2010, 87, 760–768. [Google Scholar] [CrossRef]
- Koivisto, A.; Hukkanen, M.; Saarnilehto, M.; Chapman, H.; Kuokkanen, K.; Wei, H.; Viisanen, H.; Akerman, K.E.; Lindstedt, K.; Pertovaara, A. Inhibiting TRPA1 ion channel reduces loss of cutaneous nerve fiber function in diabetic animals: Sustained activation of the TRPA1 channel contributes to the pathogenesis of peripheral diabetic neuropathy. Pharmacol. Res. 2012, 65, 149–158. [Google Scholar] [CrossRef]
- Wei, H.; Koivisto, A.; Saarnilehto, M.; Chapman, H.; Kuokkanen, K.; Hao, B.; Huang, J.L.; Wang, Y.X.; Pertovaara, A. Spinal transient receptor potential ankyrin 1 channel contributes to central pain hypersensitivity in various pathophysiological conditions in the rat. Pain 2011, 152, 582–591. [Google Scholar] [CrossRef]
- Andersson, D.A.; Filipović, M.R.; Gentry, C.; Eberhardt, M.; Vastani, N.; Leffler, A.; Reeh, P.; Bevan, S. Streptozotocin Stimulates the Ion Channel TRPA1 Directly: INVOLVEMENT OF PEROXYNITRITE. J. Biol. Chem. 2015, 290, 15185–15196. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Alenmyr, L.; Killander, D.; Lewis, S.E.; Andersson, A.; Bucher, B.; Galzi, J.L.; Sterner, O.; Bevan, S.; et al. TRPA1 mediates spinal antinociception induced by acetaminophen and the cannabinoid Δ(9)-tetrahydrocannabiorcol. Nat. Commun. 2011, 2, 551. [Google Scholar] [CrossRef] [PubMed]
- Preti, D.; Szallasi, A.; Patacchini, R. TRP channels as therapeutic targets in airway disorders: A patent review. Expert Opin. Ther. Pat. 2012, 22, 663–695. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.S.; Baxter, M.; Dubuis, E.; Birrell, M.A.; Belvisi, M.G. Transient receptor potential (TRP) channels in the airway: Role in airway disease. Br. J. Pharmacol. 2014, 171, 2593–2607. [Google Scholar] [CrossRef]
- Namer, B.; Seifert, F.; Handwerker, H.O.; Maihöfner, C. TRPA1 and TRPM8 activation in humans: Effects of cinnamaldehyde and menthol. Neuroreport 2005, 16, 955–959. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spekker, E.; Körtési, T.; Vécsei, L. TRP Channels: Recent Development in Translational Research and Potential Therapeutic Targets in Migraine. Int. J. Mol. Sci. 2023, 24, 700. https://doi.org/10.3390/ijms24010700
Spekker E, Körtési T, Vécsei L. TRP Channels: Recent Development in Translational Research and Potential Therapeutic Targets in Migraine. International Journal of Molecular Sciences. 2023; 24(1):700. https://doi.org/10.3390/ijms24010700
Chicago/Turabian StyleSpekker, Eleonóra, Tamás Körtési, and László Vécsei. 2023. "TRP Channels: Recent Development in Translational Research and Potential Therapeutic Targets in Migraine" International Journal of Molecular Sciences 24, no. 1: 700. https://doi.org/10.3390/ijms24010700
APA StyleSpekker, E., Körtési, T., & Vécsei, L. (2023). TRP Channels: Recent Development in Translational Research and Potential Therapeutic Targets in Migraine. International Journal of Molecular Sciences, 24(1), 700. https://doi.org/10.3390/ijms24010700








