Transient Receptor Potential Channels and Itch
Abstract
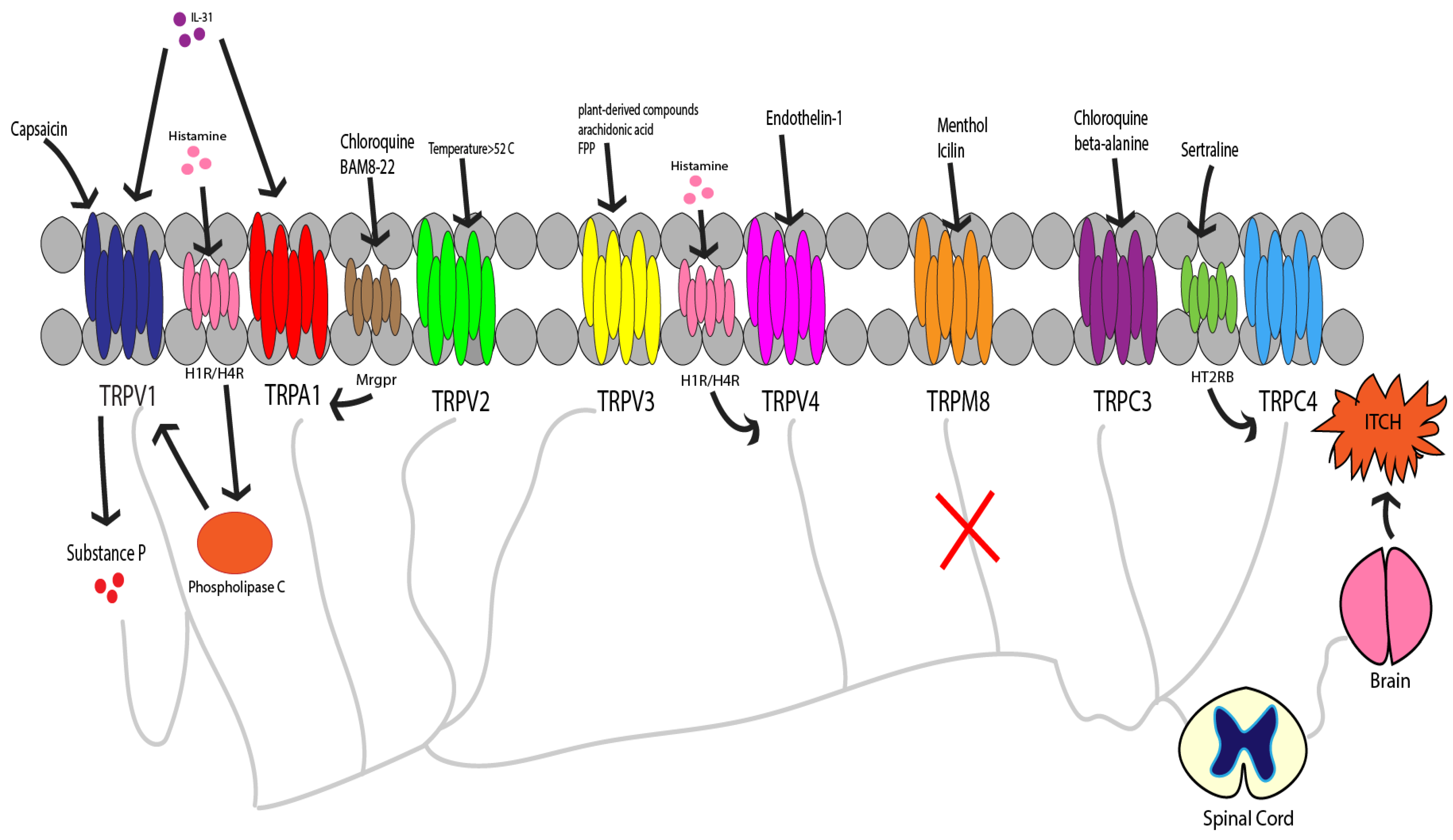
1. Introduction
2. TRP Ankyrin 1 (TRPA1)
3. TRP Vanilloid 1 (TRPV1)
4. TRP Vanilloid 2 (TRPV2)
5. TRP Vanilloid 3 (TRPV3)
6. TRP Vanilloid 4 (TRPV4)
7. TRP Melastatin 8 (TRPM8)
8. TRP Cannonical 3 and 4 (TRPC3, TRPC4)
9. Possible Ethnic Differences in TRP Channels and Itch
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yosipovitch, G.; Rosen, J.D.; Hashimoto, T. Itch: From mechanism to (novel) therapeutic approaches. J. Allergy Clin. Immunol. 2018, 142, 1375–1390. [Google Scholar] [CrossRef]
- Tsagareli, M.G.; Nozadze, I. An overview on transient receptor potential channels superfamily. Behav. Pharmacol. 2019, 31, 413–434. [Google Scholar] [CrossRef]
- Wilson, S.R.; Gerhold, K.A.; Bifolck-Fisher, A.; Liu, Q.; Patel, K.N.; Dong, X.; Bautista, D.M. TRPA1 is required for histamine-independent, Mas-related G protein–coupled receptor–mediated itch. Nat. Neurosci. 2011, 14, 595–602. [Google Scholar] [CrossRef]
- Valdes-Rodriguez, R.; B Kaushik, S.; Yosipovitch, G. Transient receptor potential channels and dermatological disorders. Curr. Top. Med. Chem. 2013, 13, 335–343. [Google Scholar] [CrossRef]
- Bíró, T.; Tóth, B.I.; Marincsák, R.; Dobrosi, N.; Géczy, T.; Paus, R. TRP channels as novel players in the pathogenesis and therapy of itch. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2007, 1772, 1004–1021. [Google Scholar] [CrossRef]
- Sun, S.; Dong, X. Trp channels and itch. Semin. Immunopathol. 2016, 38, 293–307. [Google Scholar] [CrossRef]
- Moore, C.; Gupta, R.; Jordt, S.-E.; Chen, Y.; Liedtke, W.B. Regulation of pain and itch by TRP channels. Neurosci. Bull. 2018, 34, 120–142. [Google Scholar] [CrossRef]
- Tseng, P.-Y.; Zheng, Q.; Li, Z.; Dong, X. MrgprX1 mediates neuronal excitability and itch through tetrodotoxin-resistant sodium channels. Itch 2019, 4, 1. [Google Scholar] [CrossRef]
- Feng, J.; Yang, P.; Mack, M.R.; Dryn, D.; Luo, J.; Gong, X.; Liu, S.; Oetjen, L.K.; Zholos, A.V.; Mei, Z. Sensory TRP channels contribute differentially to skin inflammation and persistent itch. Nat. Commun. 2017, 8, 980. [Google Scholar] [CrossRef]
- Liu, B.; Escalera, J.; Balakrishna, S.; Fan, L.; Caceres, A.I.; Robinson, E.; Sui, A.; McKay, M.C.; McAlexander, M.A.; Herrick, C.A. TRPA1 controls inflammation and pruritogen responses in allergic contact dermatitis. FASEB J. 2013, 27, 3549–3563. [Google Scholar] [CrossRef]
- Nattkemper, L.A.; Tey, H.L.; Valdes-Rodriguez, R.; Lee, H.; Mollanazar, N.K.; Albornoz, C.; Sanders, K.M.; Yosipovitch, G. The genetics of chronic itch: Gene expression in the skin of patients with atopic dermatitis and psoriasis with severe itch. J. Investig. Dermatol. 2018, 138, 1311–1317. [Google Scholar] [CrossRef] [PubMed]
- Morita, T.; McClain, S.P.; Batia, L.M.; Pellegrino, M.; Wilson, S.R.; Kienzler, M.A.; Lyman, K.; Olsen, A.S.B.; Wong, J.F.; Stucky, C.L. HTR7 mediates serotonergic acute and chronic itch. Neuron 2015, 87, 124–138. [Google Scholar] [CrossRef] [PubMed]
- Kittaka, H.; Tominaga, M. The molecular and cellular mechanisms of itch and the involvement of TRP channels in the peripheral sensory nervous system and skin. Allergol. Int. 2017, 66, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Bilsborough, J.; Leung, D.Y.; Maurer, M.; Howell, M.; Boguniewcz, M.; Yao, L.; Storey, H.; LeCiel, C.; Harder, B.; Gross, J.A. IL-31 is associated with cutaneous lymphocyte antigen–positive skin homing T cells in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2006, 117, 418–425. [Google Scholar] [CrossRef]
- Meng, J.; Li, Y.; Fischer, M.J.; Steinhoff, M.; Chen, W.; Wang, J. Th2 modulation of transient receptor potential channels: An unmet therapeutic intervention for atopic dermatitis. Front. Immunol. 2021, 12, 2590. [Google Scholar] [CrossRef]
- Shirolkar, P.; Mishra, S.K. Role of TRP ion channels in pruritus. Neurosci. Lett. 2022, 768, 136379. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, H. TRP channels as drug targets to relieve itch. Pharmaceuticals 2018, 11, 100. [Google Scholar] [CrossRef]
- Wilson, S.R.; Thé, L.; Batia, L.M.; Beattie, K.; Katibah, G.E.; McClain, S.P.; Pellegrino, M.; Estandian, D.M.; Bautista, D.M. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell 2013, 155, 285–295. [Google Scholar] [CrossRef]
- Dong, X.; Dong, X. Peripheral and central mechanisms of itch. Neuron 2018, 98, 482–494. [Google Scholar] [CrossRef]
- Mela, M.; Mancuso, A.; Burroughs, A. Pruritus in cholestatic and other liver diseases. Aliment. Pharmacol. Ther. 2003, 17, 857–870. [Google Scholar] [CrossRef]
- Kremer, A.E.; Martens, J.J.; Kulik, W.; Ruëff, F.; Kuiper, E.M.; van Buuren, H.R.; van Erpecum, K.J.; Kondrackiene, J.; Prieto, J.; Rust, C. Lysophosphatidic acid is a potential mediator of cholestatic pruritus. Gastroenterology 2010, 139, 1008–1018.e1. [Google Scholar] [CrossRef] [PubMed]
- Ruzicka, T.; Simmet, T.; Peskar, B.A.; Ring, J. Skin levels of arachidonic acid-derived inflammatory mediators and histamine in atopic dermatitis and psoriasis. J. Investig. Dermatol. 1986, 86, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Zeng, D.; Chen, C.; Zhou, W.; Ma, X.; Pu, X.; Zeng, Y.; Zhou, W.; Lv, F. TRPA1 deficiency alleviates inflammation of atopic dermatitis by reducing macrophage infiltration. Life Sci. 2021, 266, 118906. [Google Scholar] [CrossRef] [PubMed]
- Kido-Nakahara, M.; Buddenkotte, J.; Kempkes, C.; Ikoma, A.; Cevikbas, F.; Akiyama, T.; Nunes, F.; Seeliger, S.; Hasdemir, B.; Mess, C. Neural peptidase endothelin-converting enzyme 1 regulates endothelin 1–induced pruritus. J. Clin. Investig. 2014, 124, 2683–2695. [Google Scholar] [CrossRef] [PubMed]
- Koivisto, A.-P.; Belvisi, M.G.; Gaudet, R.; Szallasi, A. Advances in TRP channel drug discovery: From target validation to clinical studies. Nat. Rev. Drug Discov. 2022, 21, 41–59. [Google Scholar] [CrossRef]
- Papoiu, A.D.; Yosipovitch, G. Topical capsaicin. The fire of a ‘hot’medicine is reignited. Expert Opin. Pharmacother. 2010, 11, 1359–1371. [Google Scholar] [CrossRef]
- Wilzopolski, J.; Kietzmann, M.; Mishra, S.K.; Stark, H.; Bäumer, W.; Rossbach, K. TRPV1 and TRPA1 channels are both involved downstream of histamine-induced itch. Biomolecules 2021, 11, 1166. [Google Scholar] [CrossRef]
- Cevikbas, F.; Wang, X.; Akiyama, T.; Kempkes, C.; Savinko, T.; Antal, A.; Kukova, G.; Buhl, T.; Ikoma, A.; Buddenkotte, J. A sensory neuron–expressed IL-31 receptor mediates T helper cell–dependent itch: Involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 2014, 133, 448–460.e7. [Google Scholar] [CrossRef]
- Liu, B.; Tai, Y.; Achanta, S.; Kaelberer, M.M.; Caceres, A.I.; Shao, X.; Fang, J.; Jordt, S.-E. IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA 2016, 113, E7572–E7579. [Google Scholar] [CrossRef]
- Yun, J.-W.; Seo, J.A.; Jang, W.-H.; Koh, H.J.; BAE, I.-H.; Park, Y.-H. Antipruritic effects of TRPV1 antagonist in murine atopic dermatitis and itching models. J. Investig. Dermatol. 2011, 131, 1576–1579. [Google Scholar] [CrossRef]
- Ansari, A.; Weinstein, D.; Sami, N. Notalgia paresthetica: Treatment review and algorithmic approach. J. Dermatol. Treat. 2019, 31, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Metz, M.; Krause, K.; Maurer, M.; Magerl, M. Treatment of notalgia paraesthetica with an 8% capsaicin patch. Br. J. Dermatol. 2011, 165, 1359–1361. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Carvajal, A.; Fernández-Ballester, G.; Ferrer-Montiel, A. TRPV1 in chronic pruritus and pain: Soft modulation as a therapeutic strategy. Front. Mol. Neurosci. 2022, 15, 930964. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Gao, J.; Cao, X.; Chen, L.; Wang, H.; Ding, H. TRPV1 mediates itch-associated scratching and skin barrier dysfunction in DNFB-induced atopic dermatitis mice. Exp. Dermatol. 2022, 31, 398–405. [Google Scholar] [CrossRef]
- Yun, J.-W.; Seo, J.A.; Jeong, Y.S.; Bae, I.-H.; Jang, W.-H.; Lee, J.; Kim, S.-Y.; Shin, S.-S.; Woo, B.-Y.; Lee, K.-W. TRPV1 antagonist can suppress the atopic dermatitis-like symptoms by accelerating skin barrier recovery. J. Dermatol. Sci. 2011, 62, 8–15. [Google Scholar] [CrossRef]
- Lee, Y.; Won, C.H.; Jung, K.; Nam, H.J.; Choi, G.; Park, Y.H.; Park, M.; Kim, B. Efficacy and safety of PAC-14028 cream–a novel, topical, nonsteroidal, selective TRPV 1 antagonist in patients with mild-to-moderate atopic dermatitis: A phase II b randomized trial. Br. J. Dermatol. 2019, 180, 1030–1038. [Google Scholar] [CrossRef]
- Park, C.W.; Kim, B.J.; Lee, Y.W.; Won, C.; Park, C.O.; Chung, B.Y.; Lee, D.H.; Jung, K.; Nam, H.-J.; Choi, G. Asivatrep, a TRPV1 antagonist, for the topical treatment of atopic dermatitis: Phase 3, randomized, vehicle-controlled study (CAPTAIN-AD). J. Allergy Clin. Immunol. 2022, 149, 1340–1347.e4. [Google Scholar] [CrossRef]
- Singh, A.; Hildebrand, M.; Garcia, E.; Snutch, T. The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br. J. Pharmacol. 2010, 160, 1464–1475. [Google Scholar] [CrossRef]
- Zhao, J.; Munanairi, A.; Liu, X.-Y.; Zhang, J.; Hu, L.; Hu, M.; Bu, D.; Liu, L.; Xie, Z.; Kim, B.S. PAR2 mediates itch via TRPV3 signaling in keratinocytes. J. Investig. Dermatol. 2020, 140, 1524–1532. [Google Scholar] [CrossRef]
- Larkin, C.; Chen, W.; Szabó, I.L.; Shan, C.; Dajnoki, Z.; Szegedi, A.; Buhl, T.; Fan, Y.; O’Neill, S.; Walls, D. Novel insights into the TRPV3-mediated itch in atopic dermatitis. J. Allergy Clin. Immunol. 2021, 147, 1110–1114.e1115. [Google Scholar] [CrossRef]
- Sun, X.-Y.; Sun, L.-L.; Qi, H.; Gao, Q.; Wang, G.-X.; Wei, N.-N.; Wang, K. Antipruritic effect of natural coumarin osthole through selective inhibition of thermosensitive TRPV3 channel in the skin. Mol. Pharmacol. 2018, 94, 1164–1173. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Qi, H.; Wu, H.; Qu, Y.; Wang, K. Anti-pruritic and anti-inflammatory effects of natural verbascoside through selective inhibition of temperature-sensitive Ca2+-permeable TRPV3 channel. J. Dermatol. Sci. 2020, 97, 229–231. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Luo, A.; Kamau, P.M.; Takomthong, P.; Hu, J.; Boonyarat, C.; Luo, L.; Lai, R. A plant-derived TRPV3 inhibitor suppresses pain and itch. Br. J. Pharmacol. 2021, 178, 1669–1683. [Google Scholar] [CrossRef] [PubMed]
- Um, J.Y.; Kim, H.B.; Kim, J.C.; Park, J.S.; Lee, S.Y.; Chung, B.Y.; Park, C.W.; Kim, H.O. TRPV3 and Itch: The Role of TRPV3 in Chronic Pruritus according to Clinical and Experimental Evidence. Int. J. Mol. Sci. 2022, 23, 14962. [Google Scholar] [CrossRef]
- Fan, J.; Hu, L.; Yue, Z.; Liao, D.; Guo, F.; Ke, H.; Jiang, D.; Yang, Y.; Lei, X. Structural basis of TRPV3 inhibition by an antagonist. Nat. Chem. Biol. 2022; 1–10, Online ahead of print. [Google Scholar]
- Ju, T.; Vander Does, A.; Mohsin, N.; Yosipovitch, G. Lichen Simplex Chronicus Itch: An Update. Acta Derm. Venereol. 2022, 102, adv00796. [Google Scholar] [CrossRef]
- Zhang, Q.; Henry, G.; Chen, Y. Emerging Role of Transient Receptor Potential Vanilloid 4 (TRPV4) Ion Channel in Acute and Chronic Itch. Int. J. Mol. Sci. 2021, 22, 7591. [Google Scholar] [CrossRef]
- Zhang, Q.; Dias, F.; Fang, Q.; Henry, G.; Wang, Z.; Suttle, A.; Chen, Y. Involvement of Sensory Neurone-TRPV4 in Acute and Chronic Itch Behaviours. Acta Derm. Venereol. 2022, 102, adv00651. [Google Scholar] [CrossRef]
- Luo, J.; Feng, J.; Yu, G.; Yang, P.; Mack, M.R.; Du, J.; Yu, W.; Qian, A.; Zhang, Y.; Liu, S. Transient receptor potential vanilloid 4–expressing macrophages and keratinocytes contribute differentially to allergic and nonallergic chronic itch. J. Allergy Clin. Immunol. 2018, 141, 608–619.e7. [Google Scholar] [CrossRef]
- Yan, J.; Ye, F.; Ju, Y.; Wang, D.; Chen, J.; Zhang, X.; Yin, Z.; Wang, C.; Yang, Y.; Zhu, C. Cimifugin relieves pruritus in psoriasis by inhibiting TRPV4. Cell Calcium 2021, 97, 102429. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Z.-L.; Yeo, M.; Zhang, Q.-J.; López-Romero, A.E.; Ding, H.-P.; Zhang, X.; Zeng, Q.; Morales-Lázaro, S.L.; Moore, C. Epithelia-sensory neuron cross talk underlies cholestatic itch induced by lysophosphatidylcholine. Gastroenterology 2021, 161, 301–317.e16. [Google Scholar] [CrossRef]
- Liu, Y.; Mikrani, R.; He, Y.; Baig, M.M.F.A.; Abbas, M.; Naveed, M.; Tang, M.; Zhang, Q.; Li, C.; Zhou, X. TRPM8 channels: A review of distribution and clinical role. Eur. J. Pharmacol. 2020, 882, 173312. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jordt, S.-E. Cooling the Itch via TRPM8. J. Investig. Dermatol. 2018, 138, 1254–1256. [Google Scholar] [CrossRef] [PubMed]
- Palkar, R.; Ongun, S.; Catich, E.; Li, N.; Borad, N.; Sarkisian, A.; McKemy, D.D. Cooling relief of acute and chronic itch requires TRPM8 channels and neurons. J. Investig. Dermatol. 2018, 138, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Kardon, A.P.; Polgár, E.; Hachisuka, J.; Snyder, L.M.; Cameron, D.; Savage, S.; Cai, X.; Karnup, S.; Fan, C.R.; Hemenway, G.M. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron 2014, 82, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Ständer, S.; Augustin, M.; Roggenkamp, D.; Blome, C.; Heitkemper, T.; Worthmann, A.; Neufang, G. Novel TRPM 8 agonist cooling compound against chronic itch: Results from a randomized, double-blind, controlled, pilot study in dry skin. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.J.; Kim, J.C.; Wei, E.T.; Selescu, T.; Chung, B.Y.; Park, C.W.; Kim, H.O. A randomized, vehicle-controlled clinical trial of a synthetic TRPM8 agonist (Cryosim-1) gel for itch. J. Am. Acad. Dermatol. 2021, 84, 869–871. [Google Scholar] [CrossRef]
- Kang, S.; Choi, M.; Wei, E.; Selescu, T.; Lee, S.; Kim, J.; Chung, B.; Park, C.; Kim, H. TRPM8 agonist (cryosim-1) gel for scalp itch: A randomised, vehicle-controlled clinical trial. J. Eur. Acad. Dermatol. Venereol. 2022, 36, e588–e589. [Google Scholar] [CrossRef]
- Miller, M.; Shi, J.; Zhu, Y.; Kustov, M.; Tian, J.-b.; Stevens, A.; Wu, M.; Xu, J.; Long, S.; Yang, P. Identification of ML204, a novel potent antagonist that selectively modulates native TRPC4/C5 ion channels. J. Biol. Chem. 2011, 286, 33436–33446. [Google Scholar] [CrossRef]
- Dong, P.; Guo, C.; Huang, S.; Ma, M.; Liu, Q.; Luo, W. TRPC3 is dispensable for β-alanine triggered acute itch. Sci. Rep. 2017, 7, 13869. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Narang, C.; Limjunyawong, N.; Jamaldeen, H.; Yu, S.; Patiram, S.; Nie, H.; Caterina, M.J.; Dong, X. Sensory neuron expressed TRPC3 mediates acute and chronic itch. Pain 2021, 164, 98–110. [Google Scholar] [CrossRef]
- Wang, H.; Papoiu, A.; Coghill, R.; Patel, T.; Wang, N.; Yosipovitch, G. Ethnic differences in pain, itch and thermal detection in response to topical capsaicin: African Americans display a notably limited hyperalgesia and neurogenic inflammation. Br. J. Dermatol. 2010, 162, 1023–1029. [Google Scholar] [CrossRef] [PubMed]
- Nickerson, A.P.; Corbin, L.J.; Timpson, N.J.; Phillips, K.; Pickering, A.E.; Dunham, J.P. Evaluating the association of TRPA1 gene polymorphisms with pain sensitivity: A protocol for an adaptive recall by genotype study. BMC Med. Genom. 2022, 15, 9. [Google Scholar] [CrossRef] [PubMed]
| TRP Channel | Activating Compounds | Associated Pathways/Diseases |
|---|---|---|
| TRPA1 | Chloroquine, cowhage, allyl isothiocyanate, cinnamaldehyde, allicin, carvacrol, LTB4, bradykinin, arachidonic acid, prostaglandins, TSLP, 5-HT, bile acids, LPA, IL-31, IL-13, BAM8-22, hydrogen peroxide, tBHP, endothelin | Mrgpr-associated nonhistaminergic pruritus, PAR-mediated nonhistaminergic pruritus; AD, allergic contact dermatitis, cholestatic pruritus, psoriasis |
| TRPV1 | Capsaicin, histamine, ATP, lipoxygenase products, prostaglandins, imiquimod | Histaminergic pruritus, PAR-mediated nonhistaminergic pruritus, IL-31, and IL-33 mediated itch pathways; AD, psoriasis, prurigo nodularis |
| TRPV2 | Increased temperature, physical stimuli | Mast cell degranulation, PKA-mediated inflammatory cascade |
| TRPV3 | Plant-derived compounds, arachidonic acid, farnesyl pyrophosphate | PAR-mediated nonhistaminergic pruritus, IL-31-mediated BNP synthesis; Olmsted syndrome, AD, psoriasis |
| TRPV4 | Histamine, endothelin-1, 5-HT, Lysophosphatidylcholine (LPC) | Histaminergic pruritus; dry skin pruritus, allergic contact dermatitis, psoriasis, chronic idiopathic pruritus |
| TRPM8 | Menthol, icilin | Histaminergic and nonhistaminergic pruritus, B5-I neuron-associated spinal interneuron circuit; dry skin pruritus, AD, urticaria, scalp pruritus |
| TRPC3 | Chloroquine, beta-alanine | Nonhistaminergic pruritus; Contact dermatitis |
| TRPC4 | Sertraline | Serotonin receptor HTR2B-associated itch |
| TRP Channel | Antagonists | Notes |
|---|---|---|
| TRPA1 | GRC 17536, HC-030031, A-967079 | Efficacy tested in diabetic neuropathy models, AD models, contact dermatitis models, LTB4-induced itch |
| TRPV1 | Asivatrep, PAC-14028 | Efficacy tested in AD models, AD patients |
| TRPV2 | SKF96365 | Inhibits mast cell degranulation secondary to channel activation |
| TRPV3 | coumarin osthole, verbascoside, citrusinine-II, dyclonine, trpvicin, KM001 * | KM001 undergoing trial for lichen simplex chronicus |
| TRPV4 | HC067047 | Efficacy tested in dry skin pruritus models |
| TRPC3 | None reported | |
| TRPC4 | ML204, M084, HC-070 | Act in non-selective manner |
| TRP Channel | Antagonists | Notes |
| TRPM8 | (1R,2S,5R)-N-(2-(2 pyridinyl) ethyl)-2-ispropyl-5-methylcyclohexancarboxamide, menthoxypropanediol, Cryosim-1, Icilin | Efficacy tested in dry skin pruritus, AD, urticaria, scalp pruritus |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, O.; Soares, G.B.; Yosipovitch, G. Transient Receptor Potential Channels and Itch. Int. J. Mol. Sci. 2023, 24, 420. https://doi.org/10.3390/ijms24010420
Mahmoud O, Soares GB, Yosipovitch G. Transient Receptor Potential Channels and Itch. International Journal of Molecular Sciences. 2023; 24(1):420. https://doi.org/10.3390/ijms24010420
Chicago/Turabian StyleMahmoud, Omar, Georgia Biazus Soares, and Gil Yosipovitch. 2023. "Transient Receptor Potential Channels and Itch" International Journal of Molecular Sciences 24, no. 1: 420. https://doi.org/10.3390/ijms24010420
APA StyleMahmoud, O., Soares, G. B., & Yosipovitch, G. (2023). Transient Receptor Potential Channels and Itch. International Journal of Molecular Sciences, 24(1), 420. https://doi.org/10.3390/ijms24010420






