Disabled-2 (DAB2): A Key Regulator of Anti- and Pro-Tumorigenic Pathways
Abstract
1. Introduction
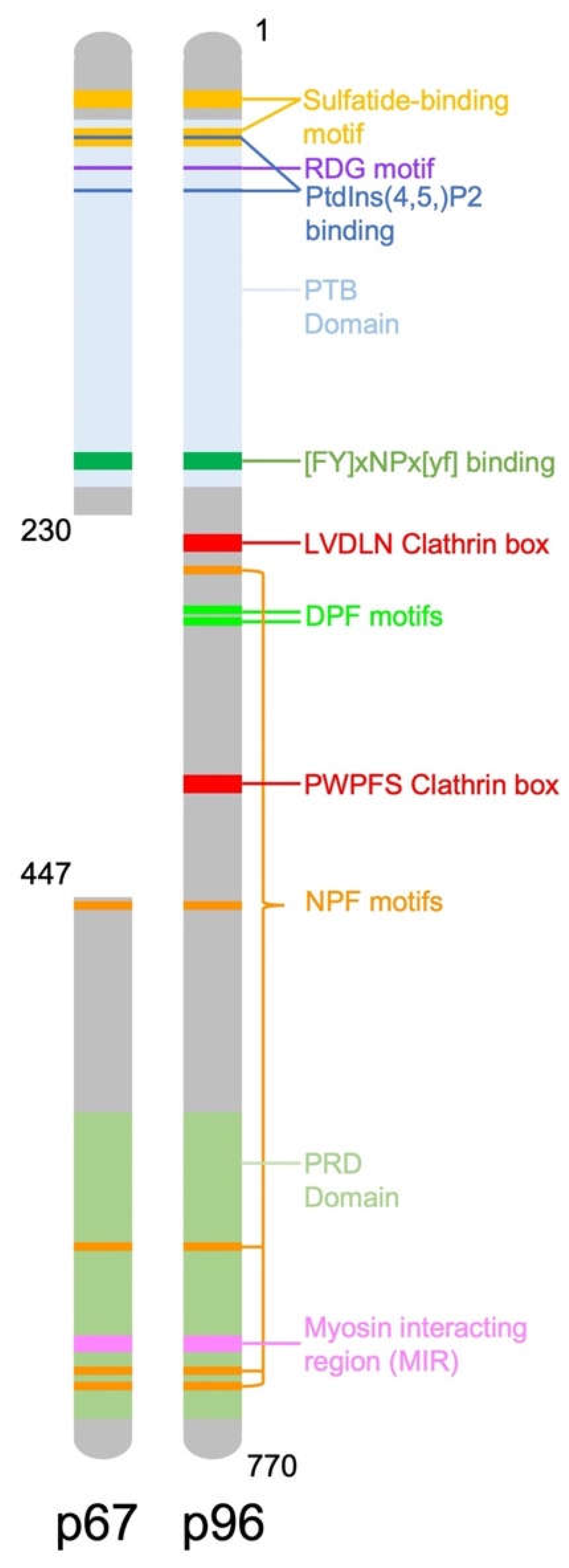
2. DAB2 Structure and Function
3. Role of DAB2 as a Tumour Suppressor
3.1. Expression of DAB2 in Cancer
| Cancer Type | DAB2 Expression | Ref |
|---|---|---|
| Breast | DAB2 p96 downregulated in cancer | [5] |
| DAB2 p67 low expression in normal and cancer tissue | [29] | |
| Ovarian | DAB2 downregulated in serous ovarian cancer | [25] |
| DAB2 maintained in mucinous ovarian cancer | [25] | |
| DAB2 downregulated in ovarian cancer | [6,26] | |
| DAB2 downregulated in serous, adenocarcinoma and mucinous cancer | [24] | |
| Choriocarcinoma | DAB2 increasingly downregulated from normal placental tissue, to partial mole, complete mole and choriocarcinoma | [39] |
| Urothelial Carcinoma of the Bladder (UCB) | DAB2 downregulated in UCB Decreased DAB2 expression associated with poor patient prognosis | [37] |
| Urothelial Carcinoma of the Bladder (UCB) | High DAB2 expression associated with poor patient prognosis | [38] |
| Lung | Low DAB2 gene and protein expression associated with significantly reduced PFS and OS | [40,41] |
| Low DAB2 associated with poor differentiation, higher tumour stage and lymph node metastasis | [40] | |
| DAB2 gene and protein expression downregulated in cancer | [7,30,40,41] | |
| Methylation of DAB2 promoter increased in cancer (93%) versus normal (35%) | [40] | |
| Oesophageal squamous cell carcinoma (ESCC) | DAB2 downregulated in cancer | [35,42] |
| Low DAB2 expression associated with poor patient prognosis | [35] | |
| Cervical | DAB2 downregulated in cancer | [31] |
| Gastric | DAB2 downregulated in cancer | [32] |
| DAB2 downregulated in metastatic vs. primary tumours | [43] | |
| Pancreatic | DAB2 upregulated in cancer | [34] |
| Prostate | DAB2 downregulated in cancer | [33] |
| Nasopharyngeal Carcinoma (NPC) | DAB2 downregulated in cancer | [44] |
3.2. Mechanisms for DAB2 Deregulation in Cancer
3.2.1. Methylation of DAB2 Promoter
3.2.2. Translational Regulation of DAB2 Expression
3.2.3. DAB2 Phosphorylation
3.2.4. MicroRNA Regulation of DAB2
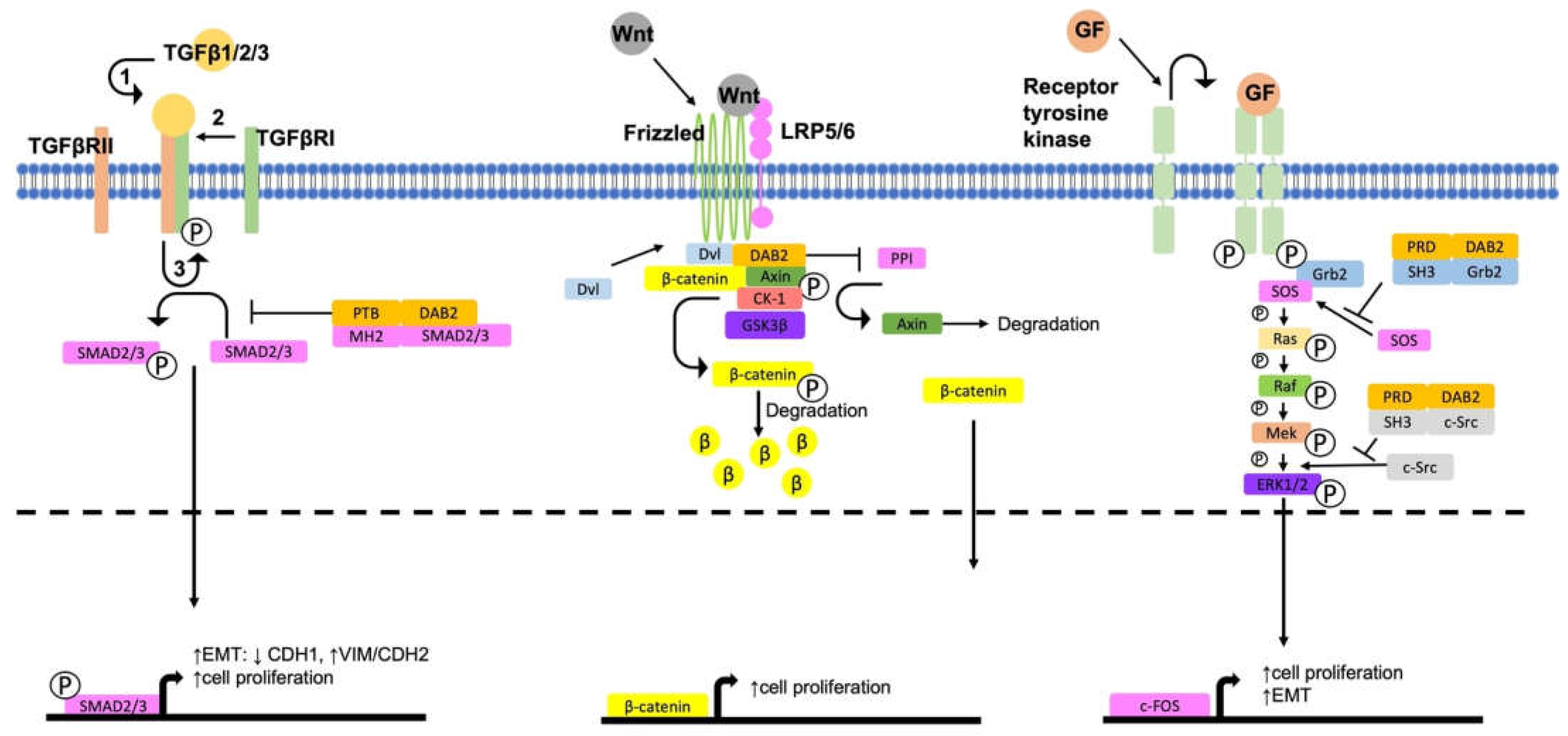
4. DAB2, a Negative Regulator of Pro-Tumorigenic Signalling Pathways
4.1. Activation of ERK/MAPK Signalling
4.2. Activation of Wnt/β-Catenin Signalling
4.3. Regulation of TGFβ Signalling Pathways
5. The Role of DAB2 as a Tumour Promoter
5.1. DAB2 and TGFβ Pro-Tumorigenic Signalling
5.2. DAB2 Promotes EMT and Metastasis
5.3. Tumour Associated Macrophages (TAMs)
5.4. Regulation of Angiogenesis
6. Targeting DAB2
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mok, S.C.; Wong, K.K.; Chan, R.K.; Lau, C.C.; Tsao, S.W.; Knapp, R.C.; Berkowitz, R.S. Molecular cloning of differentially expressed genes in human epithelial ovarian cancer. Gynecol. Oncol. 1994, 52, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.X.; Yi, T.; Tang, B.; Lambeth, J.D. Disabled-2 (DAB2) is an SH3 domain-binding partner of Grb2. Oncogene 1998, 16, 1561–1569. [Google Scholar] [CrossRef]
- Xu, X.X.; Yang, W.; Jackowski, S.; Rock, C.O. Cloning of a novel phosphoprotein regulated by colony-stimulating factor 1 shares a domain with the Drosophila disabled gene product. J. Biol. Chem. 1995, 270, 14184–14191. [Google Scholar] [CrossRef] [PubMed]
- Gertler, F.B.; Bennett, R.L.; Clark, M.J.; Hoffmann, F.M. Drosophila abl tyrosine kinase in embryonic CNS axons: A role in axonogenesis is revealed through dosage-sensitive interactions with disabled. Cell 1989, 58, 103–113. [Google Scholar] [CrossRef] [PubMed]
- Bagadi, S.A.; Prasad, C.P.; Srivastava, A.; Prashad, R.; Gupta, S.D.; Ralhan, R. Frequent loss of DAB2 protein and infrequent promoter hypermethylation in breast cancer. Breast Cancer Res. Treat. 2007, 104, 277–286. [Google Scholar] [CrossRef]
- Sheng, Z.; Sun, W.; Smith, E.; Cohen, C.; Sheng, Z.; Xu, X.X. Restoration of positioning control following Disabled-2 expression in ovarian and breast tumor cells. Oncogene 2000, 19, 4847–4854. [Google Scholar] [CrossRef]
- Xu, H.T.; Yang, L.H.; Li, Q.C.; Liu, S.L.; Liu, D.; Xie, X.M.; Wang, E.H. Disabled-2 and Axin are concurrently colocalized and underexpressed in lung cancers. Hum. Pathol. 2011, 42, 1491–1498. [Google Scholar] [CrossRef]
- Sheng, Z.; He, J.; Tuppen, J.A.; Sun, W.; Fazili, Z.; Smith, E.R.; Dong, F.B.; Xu, X.X. Structure, sequence, and promoter analysis of human disabled-2 gene (DAB2). Genomics 2000, 70, 381–386. [Google Scholar] [CrossRef]
- Albertsen, H.M.; Smith, S.A.; Melis, R.; Williams, B.; Holik, P.; Stevens, J.; White, R. Sequence, genomic structure, and chromosomal assignment of human DOC-2. Genomics 1996, 33, 207–213. [Google Scholar] [CrossRef]
- Mishra, S.K.; Keyel, P.A.; Hawryluk, M.J.; Agostinelli, N.R.; Watkins, S.C.; Traub, L.M. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J. 2002, 21, 4915–4926. [Google Scholar] [CrossRef]
- Morris, S.M.; Cooper, J.A. Disabled-2 colocalizes with the LDLR in clathrin-coated pits and interacts with AP-2. Traffic 2001, 2, 111–123. [Google Scholar] [CrossRef]
- Maurer, M.E.; Cooper, J.A. The adaptor protein DAB2 sorts LDL receptors into coated pits independently of AP-2 and ARH. J Cell Sci. 2006, 119, 4235–4246. [Google Scholar] [CrossRef]
- Yu, C.; Feng, W.; Wei, Z.Y.; Miyanoiri, Y.; Wen, W.Y.; Zhao, Y.X.; Zhang, M.J. Myosin VI Undergoes Cargo-Mediated Dimerization. Cell 2009, 138, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.; Vang, D.; Ritt, M.; Sivaramakrishnan, S. Dynamic multimerization of DAB2-Myosin VI complexes regulates cargo processivity while minimizing cortical actin reorganization. J. Biol. Chem. 2021, 296, 100232:1–100232:13. [Google Scholar] [CrossRef] [PubMed]
- Morris, S.M.; Arden, S.D.; Roberts, R.C.; Kendrick-Jones, J.; Cooper, J.A.; Luzio, J.P.; Buss, F. Myosin VI binds to and localises with DAB2, potentially linking receptor-mediated endocytosis and the actin cytoskeleton. Traffic 2002, 3, 331–341. [Google Scholar] [CrossRef]
- Fili, N.; Hari-Gupta, Y.; Aston, B.; dos Santos, A.; Gough, R.E.; Alamad, B.; Wang, L.; Martin-Fernandez, M.L.; Toseland, C.P. Competition between two high-and low-affinity protein-binding sites in myosin VI controls its cellular function. J. Biol. Chem. 2020, 295, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Da Paz, V.F.; Ghishan, F.K.; Kiela, P.R. Emerging Roles of Disabled Homolog 2 (DAB2) in Immune Regulation. Front. Immunol. 2020, 11, 580302:1–580302:11. [Google Scholar] [CrossRef]
- Rosenbauer, F.; Kallies, A.; Scheller, M.; Knobeloch, K.P.; Rock, C.O.; Schwieger, M.; Stocking, C.; Horak, I. Disabled-2 is transcriptionally regulated by ICSBP and augments macrophage spreading and adhesion. Embo J. 2002, 21, 211–220. [Google Scholar] [CrossRef]
- Adamson, S.E.; Griffiths, R.; Moravec, R.; Senthivinayagam, S.; Montgomery, G.; Chen, W.; Han, J.; Sharma, P.R.; Mullins, G.R.; Gorski, S.A.; et al. Disabled homolog 2 controls macrophage phenotypic polarization and adipose tissue inflammation. J. Clin. Investig. 2016, 126, 1311–1322. [Google Scholar] [CrossRef]
- Jokubaitis, V.G.; Gresle, M.M.; Kemper, D.A.; Doherty, W.; Perreau, V.M.; Cipriani, T.L.; Jonas, A.; Shaw, G.; Kuhlmann, T.; Kilpatrick, T.J.; et al. Endogenously regulated DAB2 worsens inflammatory injury in experimental autoimmune encephalomyelitis. Acta Neuropathol. Commun. 2013, 1, 32:1–32:14. [Google Scholar] [CrossRef]
- Figliuolo da Paz, V.; Jamwal, D.R.; Gurney, M.; Midura-Kiela, M.; Harrison, C.A.; Cox, C.; Wilson, J.M.; Ghishan, F.K.; Kiela, P.R. Rapid Downregulation of DAB2 by Toll-Like Receptor Activation Contributes to a Pro-Inflammatory Switch in Activated Dendritic Cells. Front. Immunol. 2019, 10, 304:1–304:18. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Wang, W.; Wang, D.; Ling, W. Quercetin protects against atherosclerosis by inhibiting dendritic cell activation. Mol. Nutr. Food Res. 2017, 61, 1700031:1–1700031:12. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Nguyen, H.; Friedline, R.H.; Malhotra, N.; Brehm, M.; Koyanagi, M.; Bix, M.; Cooper, J.A.; Chambers, C.A.; Kang, J. Cutting edge: DAB2 is a FOXP3 target gene required for regulatory T cell function. J. Immunol. 2009, 183, 4192–4196. [Google Scholar] [CrossRef] [PubMed]
- Fazili, Z.; Sun, W.; Mittelstaedt, S.; Cohen, C.; Xu, X.-X. Disabled-2 inactivation is an early step in ovarian tumorigenicity. Oncogene 1999, 18, 3104–3113. [Google Scholar] [CrossRef]
- Mok, S.C.; Chan, W.Y.; Wong, K.K.; Cheung, K.K.; Lau, C.C.; Ng, S.W.; Baldini, A.; Colitti, C.V.; Rock, C.O.; Berkowitz, R.S. DOC-2, a candidate tumor suppressor gene in human epithelial ovarian cancer. Oncogene 1998, 16, 2381–2387. [Google Scholar] [CrossRef]
- Yang, D.H.; Smith, E.R.; Cohen, C.; Wu, H.; Patriotis, C.; Godwin, A.K.; Hamilton, T.C.; Xu, X.X. Molecular events associated with dysplastic morphologic transformation and initiation of ovarian tumorigenicity. Cancer 2002, 94, 2380–2392. [Google Scholar] [CrossRef]
- Kuraoka, M.; Amatya, V.J.; Kushitani, K.; Mawas, A.S.; Miyata, Y.; Okada, M.; Kishimoto, T.; Inai, K.; Nishisaka, T.; Sueda, T.; et al. Identification of DAB2 and Intelectin-1 as Novel Positive Immunohistochemical Markers of Epithelioid Mesothelioma by Transcriptome Microarray Analysis for Its Differentiation From Pulmonary Adenocarcinoma. Am. J. Surg. Pathol. 2017, 41, 1045–1052. [Google Scholar] [CrossRef]
- Naso, J.R.; Cheung, S.; Ionescu, D.N.; Churg, A. Utility of SOX6 and DAB2 for the Diagnosis of Malignant Mesothelioma. Am. J. Surg. Pathol. 2021, 45, 1245–1251. [Google Scholar] [CrossRef]
- Martin, J.C.; Herbert, B.S.; Hocevar, B.A. Disabled-2 downregulation promotes epithelial-to-mesenchymal transition. Br. J. Cancer 2010, 103, 1716–1723. [Google Scholar] [CrossRef]
- Ma, S.; Zhang, W.L.; Leckey, B.D., Jr.; Xu, H.T.; Yang, L.H.; Wang, E. X-ray irradiation induced Disabled-2 gene promoter de-methylation enhances radiosensitivity of non-small-cell lung carcinoma cells. J. Exp. Clin. Cancer Res. 2018, 37, 315:1–315:12. [Google Scholar] [CrossRef]
- Cheng, Y.; Guo, Y.; Zhang, Y.; You, K.; Li, Z.; Geng, L. MicroRNA-106b is involved in transforming growth factor beta1-induced cell migration by targeting disabled homolog 2 in cervical carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 11:1–11:11. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Huang Y Fau-Mok, S.C.; Mok Sc Fau-Zimmermann, A.; Zimmermann A Fau-Friess, H.; Friess H Fau-Büchler, M.W.; Büchler, M.W. Down-regulation of DOC-2 in colorectal cancer points to its role as a tumor suppressor in this malignancy. Dis. Colon. Rectum. 2002, 45, 1242–1248. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.-W.; Gao, Z.-Y.; Xiao, W.; Li, T.-Q.; Song, W.; Zheng, J.; Chen, H.; Chen, G.-H.; Zou, H.-Y. MiR-93 functions as a tumor promoter in prostate cancer by targeting disabled homolog 2 (DAB2) and an antitumor polysaccharide from green tea (Camellia sinensis) on their expression. Int. J. Biol. Macromol. 2019, 125, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Friess, H.; Kleeff, J.; Esposito, I.; Zhu, Z.; Liu, S.; Mok, S.C.; Zimmermann, A.; Büchler, M.W. Doc-2/hDAB2 expression is up-regulated in primary pancreatic cancer but reduced in metastasis. Lab. Investig. 2001, 81, 863–873. [Google Scholar] [CrossRef]
- Wang, W.L.; Chang, W.L.; Yang, H.B.; Wang, Y.C.; Chang, I.W.; Lee, C.T.; Chang, C.Y.; Lin, J.T.; Sheu, B.S. Low disabled-2 expression promotes tumor progression and determines poor survival and high recurrence of esophageal squamous cell carcinoma. Oncotarget 2016, 7, 71169–71181. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Niu, N.; Lawson, B.; Qi, L.; Zhang, J.; Li, T.; Zhang, H.; Liu, J. GATA6: A new predictor for prognosis in ovarian cancer. Hum. Pathol. 2019, 86, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Karam, J.A.; Shariat, S.F.; Huang, H.Y.; Pong, R.C.; Ashfaq, R.; Shapiro, E.; Lotan, Y.; Sagalowsky, A.I.; Wu, X.R.; Hsieh, J.T. Decreased DOC-2/DAB2 expression in urothelial carcinoma of the bladder. Clin. Cancer Res. 2007, 13, 4400–4406. [Google Scholar] [CrossRef][Green Version]
- Itami, Y.; Miyake, M.; Ohnishi, S.; Tatsumi, Y.; Gotoh, D.; Hori, S.; Morizawa, Y.; Iida, K.; Ohnishi, K.; Nakai, Y.; et al. Disabled Homolog 2 (DAB2) Protein in Tumor Microenvironment Correlates with Aggressive Phenotype in Human Urothelial Carcinoma of the Bladder. Diagnostics 2020, 10, 54. [Google Scholar] [CrossRef]
- Fulop, V.; Colitti, C.V.; Genest, D.; Berkowitz, R.S.; Yiu, G.K.; Ng, S.W.; Szepesi, J.; Mok, S.C. DOC-2/hDAB2, a candidate tumor suppressor gene involved in the development of gestational trophoblastic diseases. Oncogene 1998, 17, 419–424. [Google Scholar] [CrossRef]
- Xie, X.M.; Zhang, Z.Y.; Yang, L.H.; Yang, D.L.; Tang, N.; Zhao, H.Y.; Xu, H.T.; Li, Q.C.; Wang, E.H. Aberrant hypermethylation and reduced expression of disabled-2 promote the development of lung cancers. Int. J. Oncol. 2013, 43, 1636–1642. [Google Scholar] [CrossRef]
- Du, L.; Zhao, Z.; Ma, X.; Hsiao, T.H.; Chen, Y.; Young, E.; Suraokar, M.; Wistuba, I.; Minna, J.D.; Pertsemlidis, A. miR-93-directed downregulation of DAB2 defines a novel oncogenic pathway in lung cancer. Oncogene 2014, 33, 4307–4315. [Google Scholar] [CrossRef] [PubMed]
- Anupam, K.; Tusharkant, C.; Gupta, S.D.; Ranju, R. Loss of disabled-2 expression is an early event in esophageal squamous tumorigenesis. World J. Gastroenterol. 2006, 12, 6041–6045. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Dong, S.; Liu, Y.; Ma, F.; Fang, J.; Zhang, W.; Shao, S.; Shen, H.; Jin, J. DAB2 suppresses gastric cancer migration by regulating the Wnt/beta-catenin and Hippo-YAP signaling pathways. Transl. Cancer Res. 2020, 9, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.H.; Ng, D.C.; Chau, S.L.; So, K.K.; Leung, P.P.; Lee, T.L.; Lung, R.W.; Chan, M.W.; Chan, A.W.; Lo, K.W.; et al. Putative tumour-suppressor gene DAB2 is frequently down regulated by promoter hypermethylation in nasopharyngeal carcinoma. BMC Cancer 2010, 10, 253. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, A.; Smith, P.; Kalna, G.; Lo Nigro, C.; Orange, C.; O’Brien, D.I.; Shah, R.; Syed, N.; Spender, L.C.; Herrera, B.; et al. Epigenetic downregulation of human disabled homolog 2 switches TGF-beta from a tumor suppressor to a tumor promoter. J. Clin. Investig. 2010, 120, 2842–2857. [Google Scholar] [CrossRef]
- Paluszczak, J.; Kiwerska, K.; Mielcarek-Kuchta, D. Frequent methylation of DAB2, a Wnt pathway antagonist, in oral and oropharyngeal squamous cell carcinomas. Pathol. Res. Pract. 2018, 214, 314–317. [Google Scholar] [CrossRef]
- Calvisi, D.F.; Ladu, S.; Gorden, A.; Farina, M.; Lee, J.S.; Conner, E.A.; Schroeder, I.; Factor, V.M.; Thorgeirsson, S.S. Mechanistic and prognostic significance of aberrant methylation in the molecular pathogenesis of human hepatocellular carcinoma. J. Clin. Investig. 2007, 117, 2713–2722. [Google Scholar] [CrossRef]
- Li, C.; Chen J Fau-Chen, T.; Chen T Fau-Xu, Z.; Xu Z Fau-Xu, C.; Xu C Fau-Ding, C.; Ding C Fau-Wang, Y.; Wang Y Fau-Lei, Z.; Lei Z Fau-Zhang, H.-T.; Zhang Ht Fau-Zhao, J.; Zhao, J. Aberrant Hypermethylation at Sites -86 to 226 of DAB2 Gene in Non-Small Cell Lung Cancer. Am. J. Med. Sci. 2015, 349, 425–431. [Google Scholar] [CrossRef]
- Chaudhury, A.; Hussey Gs Fau-Ray, P.S.; Ray Ps Fau-Jin, G.; Jin G Fau-Fox, P.L.; Fox Pl Fau-Howe, P.H.; Howe, P.H. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of DAB2 and ILEI. Nat. Cell Biol. 2010, 13, 286–293. [Google Scholar] [CrossRef]
- Zhou, J.; Hernandez, G.; Tu, S.W.; Scholes, J.; Chen, H.; Tseng, C.P.; Hsieh, J.T. Synergistic induction of DOC-2/DAB2 gene expression in transitional cell carcinoma in the presence of GATA6 and histone deacetylase inhibitor. Cancer Res. 2005, 65, 6089–6096. [Google Scholar] [CrossRef]
- Cai, K.Q.; Caslini, C.; Capo-chichi, C.D.; Slater, C.; Smith, E.R.; Wu, H.; Klein-Szanto, A.J.; Godwin, A.K.; Xu, X.X. Loss of GATA4 and GATA6 expression specifies ovarian cancer histological subtypes and precedes neoplastic transformation of ovarian surface epithelia. PLoS ONE 2009, 4, e6454. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.P.; Ely, B.D.; Pong, R.C.; Wang, Z.; Zhou, J.; Hsieh, J.T. The role of DOC-2/DAB2 protein phosphorylation in the inhibition of AP-1 activity. An underlying mechanism of its tumor-suppressive function in prostate cancer. J. Biol. Chem. 1999, 274, 31981–31986. [Google Scholar] [CrossRef]
- Shapira, K.E.; Hirschhorn, T.; Barzilay, L.; Smorodinsky, N.I.; Henis, Y.I.; Ehrlich, M. DAB2 inhibits the cholesterol-dependent activation of JNK by TGF-beta. Mol. Biol. Cell 2014, 25, 1620–1628. [Google Scholar] [CrossRef] [PubMed]
- Garces de Los Fayos Alonso, I.; Liang, H.C.; Turner, S.D.; Lagger, S.; Merkel, O.; Kenner, L. The Role of Activator Protein-1 (AP-1) Family Members in CD30-Positive Lymphomas. Cancers 2018, 10, 93. [Google Scholar] [CrossRef]
- He, J.; Xu, J.; Xu, X.X.; Hall, R.A. Cell cycle-dependent phosphorylation of Disabled-2 by cdc2. Oncogene 2003, 22, 4524–4530. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chetrit, D.; Barzilay, L.; Horn, G.; Bielik, T.; Smorodinsky, N.I.; Ehrlich, M. Negative regulation of the endocytic adaptor disabled-2 (DAB2) in mitosis. J. Biol. Chem. 2011, 286, 5392–5403. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiao, R.; Wang, X.; Khadka, B.; Fang, Z.; Yu, M.; Zhang, L.; Wu, J.; Liu, J. MicroRNA93 knockdown inhibits acute myeloid leukemia cell growth via inactivating the PI3K/AKT pathway by upregulating DAB2. Int. J. Oncol. 2021, 59. [Google Scholar] [CrossRef]
- Huang, Y.; Hong, W.; Wei, X. The molecular mechanisms and therapeutic strategies of EMT in tumor progression and metastasis. J. Hematol. Oncol. 2022, 15, 129:1–129:27. [Google Scholar] [CrossRef]
- Kim, B.N.; Ahn, D.H.; Kang, N.; Yeo, C.D.; Kim, Y.K.; Lee, K.Y.; Kim, T.J.; Lee, S.H.; Park, M.S.; Yim, H.W.; et al. TGF-beta induced EMT and stemness characteristics are associated with epigenetic regulation in lung cancer. Sci. Rep. 2020, 10, 10597:1–10597:11. [Google Scholar] [CrossRef]
- Piao, J.L.; You, K.; Guo, Y.L.; Zhang, Y.Y.; Li, Z.J.; Geng, L. Substrate stiffness affects epithelial-mesenchymal transition of cervical cancer cells through miR-106b and its target protein DAB2. Int. J. Oncol. 2017, 50, 2033–2042. [Google Scholar] [CrossRef]
- Sun, C.; Yao, X.; Jiang, Q.; Sun, X. miR-106b targets DAB2 to promote hepatocellular carcinoma cell proliferation and metastasis. Oncol. Lett. 2018, 16, 3063–3069. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, M.; Trabulo, S.M.; Vallespinos, M.; Raj, D.; Kheir, T.B.; Lin, M.L.; Begum, J.; Baker, A.M.; Amgheib, A.; Saif, J.; et al. The miR-25-93-106b cluster regulates tumor metastasis and immune evasion via modulation of CXCL12 and PD-L1. Oncotarget 2017, 8, 21609–21625. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.L.; Iwanaga, R.; Drasin, D.J.; Micalizzi, D.S.; Vartuli, R.L.; Tan, A.C.; Ford, H.L. The miR-106b-25 cluster targets Smad7, activates TGF-beta signaling, and induces EMT and tumor initiating cell characteristics downstream of Six1 in human breast cancer. Oncogene 2012, 31, 5162–5171. [Google Scholar] [CrossRef] [PubMed]
- Min, K.; Kim, J.Y.; Lee, S.K. Epstein-Barr virus miR-BART1-3p suppresses apoptosis and promotes migration of gastric carcinoma cells by targeting DAB2. Int. J. Biol. Sci. 2020, 16, 694–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Huang, P.; Li, Q.; Wang, D.; Xu, C.X. miR-134-5p Promotes Stage I Lung Adenocarcinoma Metastasis and Chemoresistance by Targeting DAB2. Mol. Nucleic Acids 2019, 18, 627–637. [Google Scholar] [CrossRef]
- Tian, X.; Zhang, Z. miR-191/DAB2 axis regulates the tumorigenicity of estrogen receptor-positive breast cancer. IUBMB Life 2018, 70, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Vuong, N.H.; Salah Salah, O.; Vanderhyden, B.C. 17beta-Estradiol sensitizes ovarian surface epithelium to transformation by suppressing Disabled-2 expression. Sci. Rep. 2017, 7, 16702. [Google Scholar] [CrossRef]
- Wang, B.W.; Fang, W.J.; Shyu, K.G. MicroRNA-145 regulates disabled-2 and Wnt3a expression in cardiomyocytes under hyperglycaemia. Eur. J. Clin. Investig 2018, 48, e12867. [Google Scholar] [CrossRef]
- Lin, C.M.; Fang, W.J.; Wang, B.W.; Pan, C.M.; Chua, S.K.; Hou, S.W.; Shyu, K.G. (-)-Epigallocatechin Gallate Promotes MicroRNA 145 Expression against Myocardial Hypoxic Injury through DAB2/Wnt3a/β-catenin. Am. J. Chin. Med. 2020, 48, 341–356. [Google Scholar] [CrossRef]
- Lu, M.; Xu, L.; Wang, M.; Guo, T.; Luo, F.; Su, N.; Yi, S.; Chen, T. miR149 promotes the myocardial differentiation of mouse bone marrow stem cells by targeting DAB2. Mol. Med. Rep. 2018, 17, 8502–8509. [Google Scholar] [CrossRef]
- Dongre, A.; Weinberg, R.A. New insights into the mechanisms of epithelial–mesenchymal transition and implications for cancer. Nat. Rev. Mol. Cell Biol. 2019, 20, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Chao, A.; Lin, C.Y.; Lee, Y.S.; Tsai, C.L.; Wei, P.C.; Hsueh, S.; Wu, T.I.; Tsai, C.N.; Wang, C.J.; Chao, A.S.; et al. Regulation of ovarian cancer progression by microRNA-187 through targeting Disabled homolog-2. Oncogene 2012, 31, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Y.; Zhang, Y.L.; Jiang, L.; Zhang, M.M.; Chen, Z.W.; Liu, D.; Huang, Q.H. Disabled homolog 2 is required for migration and invasion of prostate cancer cells. Front. Med. 2015, 9, 312–321. [Google Scholar] [CrossRef]
- Wang, S.C.; Makino, K.; Xia, W.Y.; Kim, J.S.; Im, S.A.; Peng, H.; Mok, S.C.; Singletary, S.E.; Hung, M.C. DOC-2/hDab-2 inhibits ILK activity and induces anoikis in breast cancer cells through an Akt-independent pathway. Oncogene 2001, 20, 6960–6964. [Google Scholar] [CrossRef]
- Prunier, C.; Howe, P.H. Disabled-2 (DAB2) is required for transforming growth factor beta-induced epithelial to mesenchymal transition (EMT). J. Biol. Chem. 2005, 280, 17540–17548. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Buse, M.; Busuioc, C.; Drula, R.; Gulei, D.; Raduly, L.; Rusu, A.; Irimie, A.; Atanasov, A.G.; Slaby, O.; et al. A Comprehensive Review on MAPK: A Promising Therapeutic Target in Cancer. Cancers 2019, 11, 1618. [Google Scholar] [CrossRef] [PubMed]
- McGivern, N.; El-Helali, A.; Mullan, P.; McNeish, I.A.; Paul Harkin, D.; Kennedy, R.D.; McCabe, N. Activation of MAPK signalling results in resistance to saracatinib (AZD0530) in ovarian cancer. Oncotarget 2018, 9, 4722–4736. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, G.; Li, J.; Liu, S.; Jin, Q.; Zhang, Z.; Qi, F.; Zhang, J.; Xu, J. Loss of PR55alpha promotes proliferation and metastasis by activating MAPK/AKT signaling in hepatocellular carcinoma. Cancer Cell Int. 2021, 21, 107. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: Interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [Google Scholar] [CrossRef]
- Zhou, J.; Scholes, J.; Hsieh, J.T. Characterization of a novel negative regulator (DOC-2/DAB2) of c-Src in normal prostatic epithelium and cancer. J. Biol. Chem. 2003, 278, 6936–6941. [Google Scholar] [CrossRef]
- Zhou, J.; Hsieh, J.T. The inhibitory role of DOC-2/DAB2 in growth factor receptor-mediated signal cascade. DOC-2/DAB2-mediated inhibition of ERK phosphorylation via binding to Grb2. J. Biol. Chem. 2001, 276, 27793–27798. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.R.; Smedberg, J.L.; Rula, M.E.; Hamilton, T.C.; Xu, X.X. Disassociation of MAPK activation and c-Fos expression in F9 embryonic carcinoma cells following retinoic acid-induced endoderm differentiation. J. Biol. Chem. 2001, 276, 32094–32100. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, T.T.; Zhang, S.H.; Wang, J.K.; Chen, Y.Y.; Zhao, H.Z.; Yang, Y.X.; Shi, S.L.; Chen, Q.; Liu, K.C. The Wnt signaling pathway in tumorigenesis, pharmacological targets, and drug development for cancer therapy. Biomark. Res. 2021, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X. Targeting the Wnt/beta-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Hofsteen, P.; Robitaille Aaron, M.; Chapman Daniel, P.; Moon Randall, T.; Murry Charles, E. Quantitative proteomics identify DAB2 as a cardiac developmental regulator that inhibits WNT/β-catenin signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 1002–1007. [Google Scholar] [CrossRef]
- Hocevar, B.A.; Mou, F.; Rennolds, J.L.; Morris, S.M.; Cooper, J.A.; Howe, P.H. Regulation of the Wnt signaling pathway by disabled-2 (DAB2). EMBO J. 2003, 22, 3084–3094. [Google Scholar] [CrossRef]
- Li, L.; Yuan, H.; Weaver, C.D.; Mao, J.; Farr, G.H., 3rd; Sussman, D.J.; Jonkers, J.; Kimelman, D.; Wu, D. Axin and Frat1 interact with dvl and GSK, bridging Dvl to GSK in Wnt-mediated regulation of LEF-1. EMBO J. 1999, 18, 4233–4240. [Google Scholar] [CrossRef]
- Kim, S.E.; Huang, H.; Zhao, M.; Zhang, X.; Zhang, A.; Semonov, M.V.; MacDonald, B.T.; Zhang, X.; Garcia Abreu, J.; Peng, L.; et al. Wnt stabilization of beta-catenin reveals principles for morphogen receptor-scaffold assemblies. Science 2013, 340, 867–870. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, W.; Howe, P.H. DAB2 stabilizes Axin and attenuates Wnt/beta-catenin signaling by preventing protein phosphatase 1 (PP1)-Axin interactions. Oncogene 2009, 28, 2999–3007. [Google Scholar] [CrossRef]
- Jiang, Y.; He X Fau-Howe, P.H.; Howe, P.H. Disabled-2 (DAB2) inhibits Wnt/β-catenin signalling by binding LRP6 and promoting its internalization through clathrin. EMBO J. 2012, 31, 2336–2349. [Google Scholar] [CrossRef]
- Yamamoto, H.; Komekado, H.; Kikuchi, A. Caveolin Is Necessary for Wnt-3a-Dependent Internalization of LRP6 and Accumulation of β-Catenin. Dev. Cell 2006, 11, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Alam, A.; Pant, R.; Chattopadhyay, S. Wnt Signaling and Its Significance Within the Tumor Microenvironment: Novel Therapeutic Insights. Front. Immunol. 2019, 10, 2872. [Google Scholar] [CrossRef] [PubMed]
- French, R.; Feng, Y.; Pauklin, S. Targeting TGFbeta Signalling in Cancer: Toward Context-Specific Strategies. Trends Cancer 2020, 6, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer-A Betrayal Within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef]
- Janda, E.; Lehmann, K.; Killisch, I.; Jechlinger, M.; Herzig, M.; Downward, J.; Beug, H.; Grünert, S. Ras and TGF[beta] cooperatively regulate epithelial cell plasticity and metastasis: Dissection of Ras signaling pathways. J. Cell Biol. 2002, 156, 299–313. [Google Scholar] [CrossRef]
- Hocevar, B.A. Loss of Disabled-2 Expression in Pancreatic Cancer Progression. Sci. Rep. 2019, 9, 7532:1–7532:11. [Google Scholar] [CrossRef]
- Vazquez-Carretero, M.D.; Garcia-Miranda, P.; Balda, M.S.; Matter, K.; Ilundain, A.A.; Peral, M.J. Proper E-cadherin membrane location in colon requires DAB2 and it modifies by inflammation and cancer. J. Cell Physiol. 2021, 236, 1083–1093. [Google Scholar] [CrossRef]
- Yakymovych, I.; Yakymovych, M.; Heldin, C.H. Intracellular trafficking of transforming growth factor beta receptors. Acta Biochim. Biophys. Sin. 2018, 50, 3–11. [Google Scholar] [CrossRef]
- Penheiter, S.G.; Singh Rd Fau-Repellin, C.E.; Repellin Ce Fau-Wilkes, M.C.; Wilkes Mc Fau-Edens, M.; Edens M Fau-Howe, P.H.; Howe Ph Fau-Pagano, R.E.; Pagano Re Fau-Leof, E.B.; Leof, E.B. Type II transforming growth factor-beta receptor recycling is dependent upon the clathrin adaptor protein DAB2. Mol. Biol. Cell 2010, 21, 4009–4019. [Google Scholar] [CrossRef]
- Hocevar, B.A.; Smine, A.; Xu, X.X.; Howe, P.H. The adaptor molecule Disabled-2 links the transforming growth factor beta receptors to the Smad pathway. EMBO J. 2001, 20, 2789–2801. [Google Scholar] [CrossRef]
- Teckchandani, A.; Toida, N.; Goodchild, J.; Henderson, C.; Watts, J.; Wollscheid, B.; Cooper, J.A. Quantitative proteomics identifies a DAB2/integrin module regulating cell migration. J. Cell Biol. 2009, 186, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Chao, W.T.; Kunz, J. Focal adhesion disassembly requires clathrin-dependent endocytosis of integrins. FEBS Lett. 2009, 583, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- van der Heijden, A.G.; Mengual, L.; Lozano, J.J.; Ingelmo-Torres, M.; Ribal, M.J.; Fernandez, P.L.; Oosterwijk, E.; Schalken, J.A.; Alcaraz, A.; Witjes, J.A. A five-gene expression signature to predict progression in T1G3 bladder cancer. Eur. J. Cancer 2016, 64, 127–136. [Google Scholar] [CrossRef]
- Marigo, I.; Trovato, R.; Hofer, F.; Ingangi, V.; Desantis, G.; Leone, K.; De Sanctis, F.; Ugel, S.; Cane, S.; Simonelli, A.; et al. Disabled Homolog 2 Controls Prometastatic Activity of Tumor-Associated Macrophages. Cancer Discov. 2020, 10, 1758–1773. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Marchesi, F.; Garlanda, C. Macrophages as tools and targets in cancer therapy. Nat. Rev. Drug Discov. 2022, 21, 799–820. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Song, J.; Hao, J.; Zhao, H.; Du, X.; Li, E.; Kuang, Y.; Yang, F.; Wang, W.; Deng, J.; et al. M2 macrophages promote NSCLC metastasis by upregulating CRYAB. Cell Death Dis. 2019, 10, 377:1–377:11. [Google Scholar] [CrossRef]
- Cai, H.; Zhang, Y.C.; Wang, J.; Gu, J.Y. Defects in Macrophage Reprogramming in Cancer Therapy: The Negative Impact of PD-L1/PD-1. Front. Immunol. 2021, 12. [Google Scholar] [CrossRef]
- Jin, M.Z.; Jin, W.L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct.Target 2020, 5, 166:1–166:16. [Google Scholar] [CrossRef]
- Kim, H.J.; Ji, Y.R.; Lee, Y.M. Crosstalk between angiogenesis and immune regulation in the tumor microenvironment. Arch. Pharmacal. Res. 2022, 45, 401–416. [Google Scholar] [CrossRef]
- Cheong, S.M.; Choi H Fau-Hong, B.S.; Hong Bs Fau-Gho, Y.S.; Gho Ys Fau-Han, J.-K.; Han, J.K. DAB2 is pivotal for endothelial cell migration by mediating VEGF expression in cancer cells. Exp. Cell Res. 2012, 318, 550–557. [Google Scholar] [CrossRef]
- Sawamiphak, S.; Seidel, S.; Essmann, C.L.; Wilkinson, G.A.; Pitulescu, M.E.; Acker, T.; Acker-Palmer, A. Ephrin-B2 regulates VEGFR2 function in developmental and tumour angiogenesis. Nature 2010, 465, 487–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Nakayama, M.; Pitulescu, M.E.; Schmidt, T.S.; Bochenek, M.L.; Sakakibara, A.; Adams, S.; Davy, A.; Deutsch, U.; Luthi, U.; et al. Ephrin-B2 controls VEGF-induced angiogenesis and lymphangiogenesis. Nature 2010, 465, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Nakayama, A.; van Lessen, M.; Yamamoto, H.; Hoffmann, S.; Drexler, H.C.; Itoh, N.; Hirose, T.; Breier, G.; Vestweber, D.; et al. Spatial regulation of VEGF receptor endocytosis in angiogenesis. Nat. Cell Biol. 2013, 15, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Jia-jun, W.; Wei-jie, Y. Phosphorylation of DAB2 is involved in inhibited VEGF-VEGFR-2 signaling induced by downregulation of syndecan-1 in glomerular endothelial cell. Cell Biol. Int. 2020, 44, 894–904. [Google Scholar] [CrossRef]
- Lao, Y.; Li, Y.; Hou, Y.; Chen, H.; Qiu, B.; Lin, W.; Sun, A.; Wei, H.; Jiang, Y.; He, F. Proteomic Analysis Reveals DAB2 Mediated Receptor Endocytosis Promotes Liver Sinusoidal Endothelial Cell Dedifferentiation. Sci. Rep. 2017, 7, 13456. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | Observation | Role | Ref |
|---|---|---|---|
| Cell proliferation | |||
| Acute Myeloid Leukaemia | Knockdown of miR-93 enhances DAB2 expression and inhibits cell proliferation in THP-1 cells in vitro and in vivo | TS * | [57] |
| Hepatocellular carcinoma (HCC) | miR-106b knockdown of DAB2 enhances Hep3B cell proliferation in vitro | TP ** | [61] |
| Lung adenocarcinoma | Knockdown of DAB2 inhibits A549 and H1299 cell growth and overexpression of DAB2 enhance A549 and H1299 cell growth | TP | [65] |
| Breast cancer | Oestrogen enhances miR-191 and silences DAB2 expression and promotes cell proliferation in ER positive breast cancer | TS | [66] |
| Head and Neck and Vulval Squamous cell carcinoma (SCC) | TGFβ inhibits cell proliferation in cell lines (HN30, H376, H413, Procotor, UMSCV1A, UMSCV1B and UMSCV7) that have high levels of DAB2 | TS | [45] |
| Urothelial Carcinoma of the Bladder (UCB) | Downregulation of DAB2 decreases the proliferation of UM-UC3, J82 and T24 cells | TP | [38] |
| Ovarian cancer | miR-187 in SKOV-3 cells suppressed DAB2 expression and enhanced cell proliferation | TS | [72] |
| Migration | |||
| Hepatocellular carcinoma (HCC) | miR-106b knockdown of DAB2 enhances Hep3B cell migration | TS | [61] |
| Head and Neck and Vulval SCC | TGFβ inhibits cell motility in cell lines (HN30, H413, UMSCV1A, UMSCV1B and UMSCV7) that express high levels of DAB2 | TS | [45] |
| Lung adenocarcinoma | Silencing DAB2 enhances cell migration in A549 and H1299 cells in vitro and overexpression of DAB2 reduced cell migration in A549 and H1299 cells | TS | [65] |
| Prostate cancer | DAB2 overexpression enhanced LNCaP cell migration and DAB2 knockdown by shRNA inhibited PC3 cell migration | TP | [73] |
| Urothelial UCB | Downregulation of DAB2 decrease the migration of UM-UC3 and T24 cells | TP | [38] |
| Ovarian cancer | miR-187 suppressed DAB2 expression and inhibited cell migration in SKOV-3 cells | TP | [72] |
| Gastric cancer | Downregulation of DAB2 promote SGC cell migration via Wnt/β-catenin and Hippo-YAP signalling pathways | TS | [43] |
| Invasion | |||
| Prostate cancer | DAB2 expression enhanced LNCaP cell invasion and DAB2 knockdown inhibited PC3 cell invasion | TP | [73] |
| Urothelial UCB | Downregulation of DAB2 decreased cell invasion of J82 and T24 cells | TP | [38] |
| Apoptosis | |||
| Breast Cancer | DAB2 promotes anoikis in SK-BR-3 and MDA-MB-453 cells | TS | [74] |
| Normal murine mammary gland (NMuMG) | Down regulation of DAB2 enhance TGFβ induced apoptosis | TP | [75] |
| Breast cancer | DAB2 sensitises SK-BR-3 and MDA-MB-453 cells to apoptosis by inhibiting the activity of integrin-linked kinas (ILK) | TS | [74] |
| In vivo tumour growth and metastasis | |||
| Lung adenocarcinoma | DAB2 is a target for miR-134-5p. Overexpression of miR-134-5p increased A549 cells tumour growth in mouse model | TS | [65] |
| Prostate cancer | DAB2 knockdown inhibits PC3 cells tumour growth and metastasis in the mouse model | TP | [73] |
| Urothelial UCB | Reduced tumour growth and invasion in xenograft tumours of UM-UC-3 cells treated with DAB2 targeting siRNA | TP | [38] |
| Ovarian | DAB2 overexpression reduces SKOV3 tumour formation in nude mice | TS | [25] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Price, Z.K.; Lokman, N.A.; Yoshihara, M.; Kajiyama, H.; Oehler, M.K.; Ricciardelli, C. Disabled-2 (DAB2): A Key Regulator of Anti- and Pro-Tumorigenic Pathways. Int. J. Mol. Sci. 2023, 24, 696. https://doi.org/10.3390/ijms24010696
Price ZK, Lokman NA, Yoshihara M, Kajiyama H, Oehler MK, Ricciardelli C. Disabled-2 (DAB2): A Key Regulator of Anti- and Pro-Tumorigenic Pathways. International Journal of Molecular Sciences. 2023; 24(1):696. https://doi.org/10.3390/ijms24010696
Chicago/Turabian StylePrice, Zoe K., Noor A. Lokman, Masato Yoshihara, Hiroaki Kajiyama, Martin K. Oehler, and Carmela Ricciardelli. 2023. "Disabled-2 (DAB2): A Key Regulator of Anti- and Pro-Tumorigenic Pathways" International Journal of Molecular Sciences 24, no. 1: 696. https://doi.org/10.3390/ijms24010696
APA StylePrice, Z. K., Lokman, N. A., Yoshihara, M., Kajiyama, H., Oehler, M. K., & Ricciardelli, C. (2023). Disabled-2 (DAB2): A Key Regulator of Anti- and Pro-Tumorigenic Pathways. International Journal of Molecular Sciences, 24(1), 696. https://doi.org/10.3390/ijms24010696







