Natural Taxanes: From Plant Composition to Human Pharmacology and Toxicity
Abstract
1. Introduction
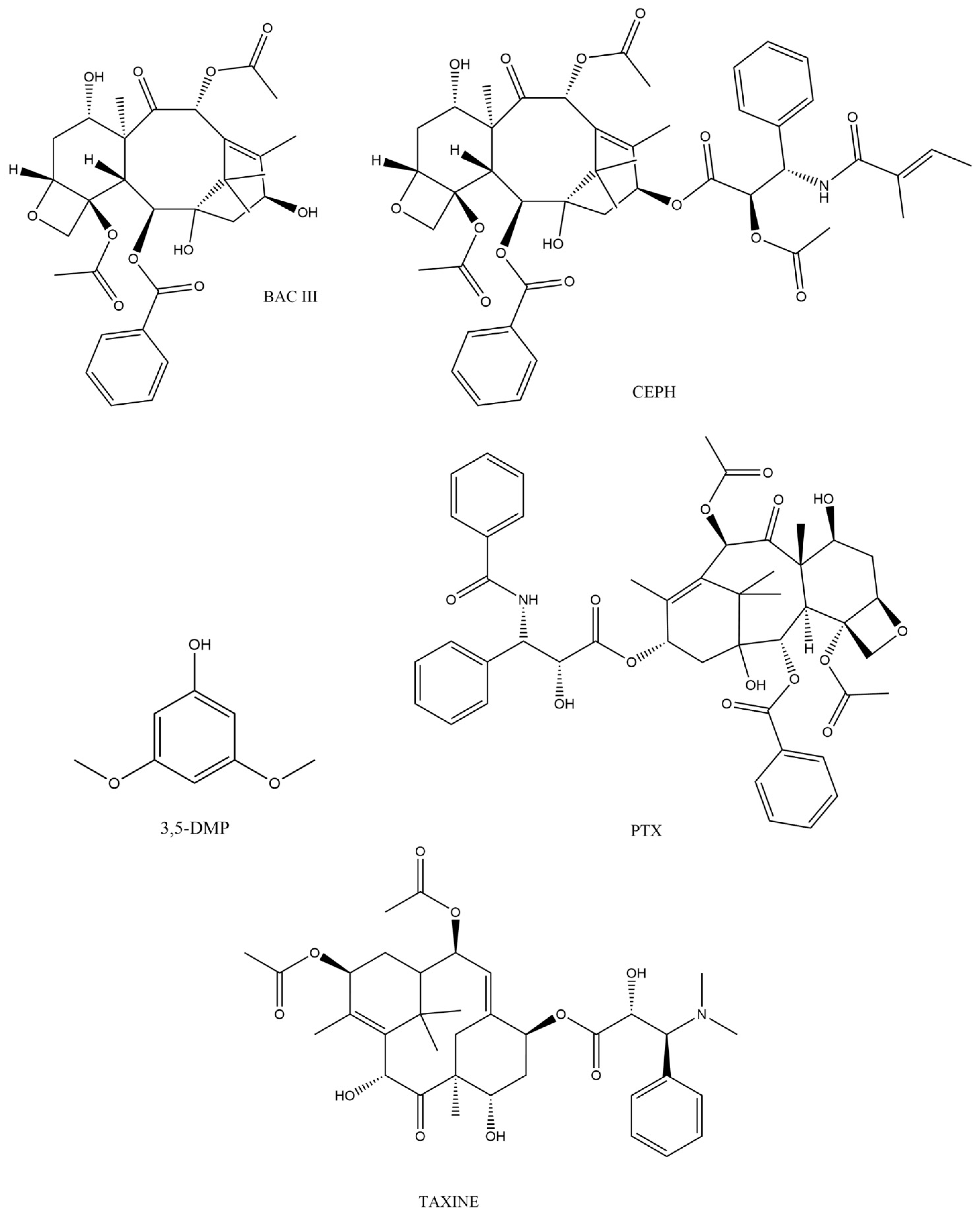
2. Taxus spp. and Biologically Active Compounds
2.1. Overall Distribution of Compounds
2.2. Physiochemical Properties of Biologically Active Taxanes
2.3. Method of Analysis in Taxus spp.
3. Pharmacology of Taxanes
3.1. Molecular Mechanism of Action
3.2. Human Pharmacokinetic Parameters of Taxanes
| PTX Formulation | Dose (mg/m2) | Cmax (ng/mL) | Tmax (h) | CL | AUC (h ng/mL) | Refs. |
|---|---|---|---|---|---|---|
| Cremophor EL® nab-paclitaxel | 175 (135–240) | median 4335 (2635–8160) | na | median 12 L/h/m2 | na | [79] |
| Micellar PTX | 260 | 8006 ± 1703 | 50 ± 17 | na | 13,484 ± 3491 (0–10 h) | [80,81] |
| nab-paclitaxel | 8073 ± 4124 | 46 ± 20 | na | 11,388 ± 3123 (0–10 h) | ||
| PTX Abraxane® | 260 | 12,295 ± 2370 12,771 ± 2065 | 0.5 0.5 | na | 12,587 ± 2932 (0-inf) 13,078 ± 2880 (0-inf) | [82] |
| Cremophore EL® | 250 | 725.9 ± 179.34 (steady state) | na | 256 ± 72 mL·min−1·m−2 | 17,336.2 ± 4355.4 | [86] |
| cisplatin + PTX cisplatin + PTX | 75 + 135 75 + 250 | 273.3 (102–2306); 691 (93.4–3074.4), | na | na | na | [87] |
| nab-PTX | 100 | 5482 ± 3967 | na | 37.16 | 5316 (3001) (0–48 h) | [88] |
3.3. Human Pharmacodynamic Parameters of Taxanes
3.3.1. Common Physiological and Unwanted Effects
3.3.2. Hypersensitivity Response and Anaphylaxis
3.3.3. Neurotoxicity
3.3.4. Gastrointestinal (GIT) Toxicity
3.3.5. Cardiovascular Toxicity
3.3.6. Haematological Toxicity, Myelosuppression
3.3.7. Respiratory System
3.3.8. Skin Toxicity
3.3.9. Sex Differences in Toxicity
3.3.10. Renal Toxicity
4. Toxicity of Taxanes
4.1. Clinical (Non-Lethal) Toxicity
4.2. Lethal Intoxications
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhuju, S.; Gauchan, D.P. Taxus wallichiana (Zucc.), an Endangered Anti-Cancerous Plant: A Review. Int. J. Res. 2018, 5, 10–21. [Google Scholar]
- Malik, S.; Cusidó, R.M.; Mirjalili, M.H.; Moyano, E.; Palazón, J.; Bonfill, M. Production of the anticancer drug taxol in Taxus baccata suspension cultures: A review. Process Biochem. 2011, 46, 23–34. [Google Scholar] [CrossRef]
- Glowniak, K.; Mroczek, T.; Zobel, A.M. Seasonal changes in the concentrations of four taxoids in Taxus baccata L. during the autumn-spring period. Phytomedicine 1999, 6, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Gallego-Jara, J.; Lozano-Terol, G.; Sola-Martínez, R.A.; Cánovas-Díaz, M.; de Diego Puente, T. A Compressive Review about Taxol®: History and Future Challenges. Molecules 2020, 25, 5986. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Tu, L.; Wang, G.; Qi, N.; Wu, W.; Zhang, W.; Feng, J. Multi-functional chitosan polymeric micelles as oral paclitaxel delivery systems for enhanced bioavailability and anti-tumor efficacy. Int. J. Pharm. 2020, 578, 119105. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhang, Y.; Luo, Z.; Zhan, R.; Xu, H.; Chen, W.; Huang, H. Synthesis, biological evaluation and low-toxic formulation development of glycosylated paclitaxel prodrugs. Molecules 2018, 23, 3211. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Huang, S.; Wang, H.; Chen, X.; Zhang, H.; Xu, Y.; Fan, W.; Pan, Y.; Wen, Q.; Lin, Z.; et al. Preparation and characterization of paclitaxel palmitate albumin nanoparticles with high loading efficacy: An in vitro and in vivo anti-tumor study in mouse models. Drug Deliv. 2021, 28, 1067. [Google Scholar] [CrossRef]
- Chen, C.; Shen, M.; Liao, H.; Guo, Q.; Fu, H.; Yu, J.; Duan, Y. A paclitaxel and microRNA-124 coloaded stepped cleavable nanosystem against triple negative breast cancer. J. Nanobiotechnol. 2021, 19, 55. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.S.; Ho, Y.; Lin, Y.W.; Naveen Raj, E.; Liu, K.K.; Chen, C.; Zhou, X.Z.; Lu, K.P.; Chao, J.I. Targeting EGFR of triple-negative breast cancer enhances the therapeutic efficacy of paclitaxel- and cetuximab-conjugated nanodiamond nanocomposite. Acta Biomater. 2019, 86, 395–405. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; He, M.; Liao, J.; Chen, N.; Li, Y.; Zhou, S.; Palmisano, M.; Yu, A.; Pai, M.P.; et al. Different Nanoformulations Alter the Tissue Distribution of Paclitaxel, Which Aligns with Reported Distinct Efficacy and Safety Profiles. Mol. Pharm. 2018, 15, 4505–4516. [Google Scholar] [CrossRef]
- Zhang, X.; Lv, J.; Wang, L.; Shao, H. Comparison of Pharmacokinetics and Biodistribution of 10-Deacetylbaccatin III after Oral Administration as Pure Compound or in Taxus chinensis Extract: A Pilot Study. Planta Med. 2016, 82, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Anand, U.; Pandey, S.K.; Ashby, C.R.; Assaraf, Y.G.; Chen, Z.S.; Dey, A. Therapeutic strategies to overcome taxane resistance in cancer. Drug Resist. Updates 2021, 55, 100754. [Google Scholar] [CrossRef] [PubMed]
- Nižnanský, L.; Nižnanská, Ž.; Kuruc, R.; Szórádová, A.; Šikuta, J.; Zummerová, A. Ayahuasca as a Decoction Applied to Human: Analytical Methods, Pharmacology and Potential Toxic Effects. J. Clin. Med. 2022, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.H.; Zhao, J.; Zhang, W.D.; Ma, B.L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: Focus on natural occurring nanoparticles. Pharmaceutics 2020, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Škubník, J.; Pavlíčková, V.; Ruml, T.; Rimpelová, S. Current Perspectives on Taxanes: Focus on Their Bioactivity, Delivery and Combination Therapy. Plants 2021, 10, 569. [Google Scholar] [CrossRef]
- Cai, D.; Jin, J.; Bi, H.; Zhong, G.; Zhou, M.; Guo, J.; Cai, Y.; Liang, M.; Gu, Q.; Hu, Z.; et al. Paclitaxel-Containing Extract Exerts Anti-Cancer Activity through Oral Administration in A549-Xenografted BALB/C Nude Mice: Synergistic Effect between Paclitaxel and Flavonoids or Lignoids. Evid.-Based Complement. Alternat. Med. 2022, 2022, 3648175. [Google Scholar] [CrossRef]
- Liu, Z.; Zheng, X.; Lv, J.; Zhou, X.; Wang, Q.; Wen, X.; Liu, H.; Jiang, J.; Wang, L. Pharmacokinetic synergy from the taxane extract of Taxus chinensis improves the bioavailability of paclitaxel. Phytomedicine 2015, 22, 573–578. [Google Scholar] [CrossRef]
- Bekhouche, M.; Benyammi, R.; Slaoui, M.K.; Krimat, S.; Paris, C.; Khelifi, L.; Morsli, A. Flavonoid profile and antioxidant properties of Algerian common yew (Taxus baccata L.). Clin. Phytosci. 2022, 8, 17. [Google Scholar] [CrossRef]
- Upreti, S.; Pandey, S.C.; Bisht, I.; Samant, M. Evaluation of the target-specific therapeutic potential of herbal compounds for the treatment of cancer. Mol. Divers. 2022, 26, 1823–1835. [Google Scholar] [CrossRef]
- Labossiere, A.W.; Thompson, D.F. Clinical Toxicology of Yew Poisoning. Ann. Pharmacother. 2018, 52, 591–599. [Google Scholar] [CrossRef]
- Jamloki, A.; Trivedi, V.L.; Nautiyal, M.C.; Semwal, P.; Cruz-Martins, N. Poisonous Plants of the Indian Himalaya: An Overview. Metabolites 2022, 12, 540. [Google Scholar] [CrossRef] [PubMed]
- Reijnen, G.; Bethlehem, C.; van Remmen, J.M.B.L.; Smit, H.J.M.; van Luin, M.; Reijnders, U.J.L. Post-mortem findings in 22 fatal Taxus baccata intoxications and a possible solution to its detection. J. Forensic Leg. Med. 2017, 52, 56–61. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Luo, X.; Zhang, C.; Xu, X.; Yu, C.; Jiang, Z.; Zhang, L.; Yuan, H.; Zheng, B.; Pi, E.; et al. Comparative metabolomic analysis reveals the variations in taxoids and flavonoids among three Taxus species. BMC Plant Biol. 2019, 19, 529. [Google Scholar] [CrossRef] [PubMed]
- Parmar, V.S.; Jha, A.; Bisht, K.S.; Taneja, P.; Singh, S.K.; Kumar, A.; Denmarkpp; Jain, R.; Olsen, C.E. Review Article Number 138: Constituents of the yew trees. Phytochemistry 1999, 50, 1267–1304. [Google Scholar] [CrossRef] [PubMed]
- Van Rozendaal, E.L.M.; Lelyveld, G.P.; van Beek, T.A. Screening of the needles of different yew species and cultivars for paclitaxel and related taxoids. Phytochemistry 2000, 53, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Seasonal Variation of Neutral and Basic Taxoid Contents in Shoots of European Yew (Taxus baccata). Available online: https://agris.fao.org/agris-search/search.do?recordID=US201302947451 (accessed on 3 November 2022).
- Wang, X.; Huang, Y.; Mort, A.J.; Zeng, Y.; Tauer, C.G.; Cochran, K.D. Variation of taxane content in needles of Taxus x media cultivars with different growth characteristics. Z. Naturforsch. C. 2006, 61, 619–624. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Antoniewska, A.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Skoczeń-Słupska, R. Bioactive components, volatile profile and in vitro antioxidative properties of Taxus baccata L. Red arils. Molecules 2021, 26, 4474. [Google Scholar] [CrossRef]
- Hu, Z.; Chen, J.T.; Jiang, S.C.; Liu, Z.; Ge, S.B.; Zhang, Z. Chemical components and functions of Taxus chinensis extract. J. King Saud Univ.–Sci. 2020, 32, 1562–1568. [Google Scholar] [CrossRef]
- Wei, Q.; Yin, C.W. Chemical Composition of Essential Oils from the Stems of Taxus chinensis var. mairei. J. Essent. Oil Bear. Plants 2019, 22, 1144–1149. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, X.; Zheng, T.; Guo, X.; Chen, Q.; Tang, Z. Investigation of bioactivities of Taxus chinensis, Taxus cuspidata, and Taxus × media by gas chromatography-mass spectrometry. Open Life Sci. 2021, 16, 287–296. [Google Scholar] [CrossRef]
- Huong, L.T.; Thuong, N.T.H.; Chac, L.D.; Dai, D.N.; Ogunwande, I.A. Antimicrobial Activity and Chemical Constituents of Essential Oils from the Leaf and Wood of Taxus chinensis (Rehder & E.H. Wilson) Rehder (Taxaceae) from Vietnam. J. Biol. Act. Prod. Nat. 2020, 10, 8–17. [Google Scholar] [CrossRef]
- Bekhouche, M.; Benyammi, R.; Slaoui, M.K.; Khelifi, L.; Morsli, A. Free Radical Scavenging Activity and Detailed Flavonoid Profiling of Algerian Yew (Taxus baccata L.) by LC–ESI–MS/MS. Int. J. Pharm. Sci. Res. 2020, 12, 2613–2619. [Google Scholar]
- Shao, F.; Zhang, L.; Guo, J.; Liu, X.; Ma, W.; Wilson, I.W.; Qiu, D. A comparative metabolomics analysis of the components of heartwood and sapwood in Taxus chinensis (Pilger) Rehd. Sci. Rep. 2019, 9, 17647. [Google Scholar] [CrossRef] [PubMed]
- Kayan, B.; Gizir, A.M.; Kalderis, D. Ultrasonic-assisted extraction of 10-deacetylbaccatin III from Taxus baccata L.: Optimization using response surface methodology. J. Iran. Chem. Soc. 2020, 18, 37–45. [Google Scholar] [CrossRef]
- Dalmaris, E.; Avramidou, E.V.; Xanthopoulou, A.; Aravanopoulos, F.A. Dataset of Targeted Metabolite Analysis for Five Taxanes of Hellenic Taxus baccata L. Populations. Data 2020, 5, 22. [Google Scholar] [CrossRef]
- Dalmaris, E.; Sarrou, E.; Multari, S.; Xanthopoulou, A.; Avramidou, E.; Mansuero, D.; Martens, S.; Aravanopoulos, F. Targeted LC-MS/MS analysis for the quantification of taxanes: Assessment of chemodiversity in different European yew (Taxus baccata) populations from Greece. Planta Med. 2019, 85, 1467–1468. [Google Scholar] [CrossRef]
- Tabaszewska, M.; Rutkowska, J.; Skoczylas, Ł.; Słupski, J.; Antoniewska, A.; Smoleń, S.; Łukasiewicz, M.; Baranowski, D.; Duda, I.; Pietsch, J. Red arils of Taxus baccata L.—A new source of valuable fatty acids and nutrients. Molecules 2021, 26, 723. [Google Scholar] [CrossRef]
- Yu, C.; Zhan, X.; Zhang, C.; Xu, X.; Huang, J.; Feng, S.; Shen, C.; Wang, H. Comparative metabolomic analyses revealed the differential accumulation of taxoids, flavonoids and hormones among six Taxaceae trees. Sci. Hortic. 2021, 285, 110196. [Google Scholar] [CrossRef]
- Wang, Y.F.; Shi, Q.W.; Dong, M.; Kiyota, H.; Gu, Y.C.; Cong, B. Natural taxanes: Developments since 1828. Chem. Rev. 2011, 111, 7652–7709. [Google Scholar] [CrossRef]
- Jiang, P.; Zhang, Q.; Zhao, Y.; Xiong, J.; Wang, F.; Zhang, T.; Zhang, C. Extraction, Purification, and Biological Activities of Polysaccharides from Branches and Leaves of Taxus cuspidata S. et Z. Molecules 2019, 24, 2926. [Google Scholar] [CrossRef]
- Yu, C.; Luo, X.; Zhan, X.; Hao, J.; Zhang, L.; Song, Y.B.L.; Shen, C.; Dong, M. Comparative metabolomics reveals the metabolic variations between two endangered Taxus species (T. fuana and T. yunnanensis) in the Himalayas. BMC Plant Biol. 2018, 18, 197. [Google Scholar] [CrossRef] [PubMed]
- Mubeen, S.; Li, Z.L.; Huang, Q.M.; He, C.T.; Yang, Z.Y. Comparative Transcriptome Analysis Revealed the Tissue-Specific Accumulations of Taxanes among Three Experimental Lines of Taxus yunnanensis. J. Agric. Food Chem. 2018, 66, 10410–10420. [Google Scholar] [CrossRef] [PubMed]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Al-Mana, F.A.; Mahmoud, E.A.; Ali Zin El-Abedin, T.K.; Mattar, M.A.; Ekiert, H. Phenolic Compounds of Catalpa speciosa, Taxus cuspidate, and Magnolia acuminata have Antioxidant and Anticancer Activity. Molecules 2019, 24, 412. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, Y.; Meng, H.; Li, W.; Wang, S. Identification and Optimization of a Novel Taxanes Extraction Process from Taxus cuspidata Needles by High-Intensity Pulsed Electric Field. Molecules 2022, 27, 3010. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.P.; Li, J.; Pu, S.B. Chemical Constituents of Leaves of Taxus chinensis. Chem. Nat. Compd. 2018, 54, 841–845. [Google Scholar] [CrossRef]
- Schex, R.; Lieb, V.M.; Schäfer, C.; Schweiggert, R.; Steingass, C.B. Carotenoid profiles of red- and yellow-colored arils of cultivars of Taxus baccata L. and Taxus × media Rehder. Phytochemistry 2021, 186, 112741. [Google Scholar] [CrossRef]
- Siegle, L.; Pietsch, J. Taxus ingredients in the red arils of Taxus baccata L. determined by HPLC-MS/MS. Phytochem. Anal. 2018, 29, 446–451. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Z.; Li, W.; Tang, Y.; Meng, H.; Wang, S. Separation and Purification of Taxanes from Crude Taxus cuspidata Extract by Antisolvent Recrystallization Method. Separations 2022, 9, 304. [Google Scholar] [CrossRef]
- Adhikari, P.; Joshi, K.; Singh, M.; Pandey, A. Influence of altitude on secondary metabolites, antioxidants, and antimicrobial activities of Himalayan yew (Taxus wallichiana). Plant Biosyst.–Int. J. Deal. All Asp. Plant Biol. 2020, 156, 187–195. [Google Scholar] [CrossRef]
- Ghaffar, N.; Lee, L.S.; Choi, Y.J.; Perre, C.; Khan, B. Efficient Heated Ultrasound Assisted Extraction and Clean-Up Method for Quantifying Paclitaxel Concentrations in Taxus wallichiana. Int. J. Environ. Anal. Chem. 2019, 101, 549–560. [Google Scholar] [CrossRef]
- Fan, X.H.; Wang, L.T.; Chang, Y.H.; An, J.Y.; Zhu, Y.W.; Yang, Q.; Meng, D.; Fu, Y.J. Application of green and recyclable menthol-based hydrophobic deep eutectic solvents aqueous for the extraction of main taxanes from Taxus chinensis needles. J. Mol. Liq. 2021, 326, 114970. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, F.; Zhuang, W.; Shu, X.; Wang, Z. Metabolic variations of flavonoids in leaves of T. media and T. mairei obtained by UPLC-ESI-MS/MS. Molecules 2019, 24, 3323. [Google Scholar] [CrossRef] [PubMed]
- Nagy, M.; Grančai, D.; Mučaji, P. Farmacognosis, Biogenesis of Natural Substances, 1st ed.; Osveta: Martin, Slovakia, 2011; ISBN 978-80-8063-368-4. [Google Scholar]
- Lange, B.M.; Conner, C.F. Taxanes and taxoids of the genus Taxus—A comprehensive inventory of chemical diversity. Phytochemistry 2021, 190, 112829. [Google Scholar] [CrossRef] [PubMed]
- Jahodář, L. Farmaceuticky Významné Semenné Rastliny; Univerzita Karlova, Nakladatelství Karolinium: Prague, Czech Republic, 2022; ISBN 978-80-246-4952-8. [Google Scholar]
- Mazumder, K.; Aktar, A.; Roy, P.; Biswas, B.; Hossain, M.E.; Sarkar, K.K.; Bachar, S.C.; Ahmed, F.; Monjur-Al-hossain, A.S.M.; Fukase, K. A Review on Mechanistic Insight of Plant Derived Anticancer Bioactive Phytocompounds and Their Structure Activity Relationship. Molecules 2022, 27, 3036. [Google Scholar] [CrossRef]
- Hao, D.-C.; Ge, G.-B.; Wang, P.; Yang, L. Impact of Drug Metabolism/Pharmacokinetics and Their Relevance Upon Taxus-based Drug Development. Curr. Drug Metab. 2018, 19, 930–959. [Google Scholar] [CrossRef]
- Matesanz, R.; Rodríguez-Salarichs, J.; Pera, B.; Canales, Á.; Andreu, J.M.; Jiménez-Barbero, J.; Bras, W.; Nogales, A.; Fang, W.S.; Díaz, J.F. Modulation of microtubule interprotofilament interactions by modified taxanes. Biophys. J. 2011, 101, 2970–2980. [Google Scholar] [CrossRef]
- Alves, R.C.; Fernandes, R.P.; Eloy, J.O.; Salgado, H.R.N.; Chorilli, M. Characteristics, Properties and Analytical Methods of Paclitaxel: A Review. Crit. Rev. Anal. Chem. 2018, 48, 110–118. [Google Scholar] [CrossRef]
- Dörwald, F.Z. Lead Optimization for Medicinal Chemists; Wiley: Weinheim, Germany, 2012; ISBN 978-3-527-33226-7. [Google Scholar]
- Zou, L.; Zhang, Z.; Feng, J.; Ding, W.; Li, Y.; Liang, D.; Xie, T.; Li, F.; Li, Y.; Chen, J.; et al. Paclitaxel-Loaded TPGS2k/Gelatin-Grafted Cyclodextrin/Hyaluronic Acid-Grafted Cyclodextrin Nanoparticles for Oral Bioavailability and Targeting Enhancement. J. Pharm. Sci. 2022, 111, 1776–1784. [Google Scholar] [CrossRef]
- Uslu, B.; Lingeman, H.; Ozkan, S.A.; Palit, M.; Dogan-Topal, B. Analytical Method Development and Validation of Pharmaceutical Analysis Using Chromatographic Techniques. Chromatogr. Res. Int. 2012, 2012, 948129. [Google Scholar] [CrossRef]
- Siddiqui, M.R.; AlOthman, Z.A.; Rahman, N. Analytical techniques in pharmaceutical analysis: A review. Arab. J. Chem. 2017, 10, S1409–S1421. [Google Scholar] [CrossRef]
- Vázquez, M.F.B.; Comini, L.R.; Martini, R.E.; Montoya, S.C.N.; Bottini, S.; Cabrera, J.L. Ultrasonic-assisted extraction of anthraquinones from Heterophyllaea pustulata Hook f. (Rubiaceae) using ethanol–water mixtures. Ind. Crop. Prod. 2015, 69, 278–283. [Google Scholar] [CrossRef]
- Baloglu, E.; Kingston, D.G.I. The taxane diterpenoids. J. Nat. Prod. 1999, 62, 1448–1472. [Google Scholar] [CrossRef] [PubMed]
- Gai, Q.Y.; Jiao, J.; Wang, X.; Liu, J.; Fu, Y.J.; Lu, Y.; Wang, Z.Y.; Xu, X.J. Simultaneous determination of taxoids and flavonoids in twigs and leaves of three Taxus species by UHPLC-MS/MS. J. Pharm. Biomed. Anal. 2020, 189, 113456. [Google Scholar] [CrossRef] [PubMed]
- Knossow, M.; Campanacci, V.; Khodja, L.A.; Gigant, B. The Mechanism of Tubulin Assembly into Microtubules: Insights from Structural Studies. iScience 2020, 23, 101511. [Google Scholar] [CrossRef] [PubMed]
- Caplow, M.; Ruhlen, R.; Shanks, J.; Walker, R.A.; Salmon, E.D. Stabilization of Microtubules by Tubulin-GDP-Pi Subunits. Biochemistry 1989, 28, 8136–8141. [Google Scholar] [CrossRef]
- Jain, K.; Basu, J.; Roy, M.; Yadav, J.; Patil, S.; Athale, C.A. Polymerization kinetics of tubulin from mung seedlings modeled as a competition between nucleation and GTP-hydrolysis rates. Cytoskeleton 2021, 78, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Buey, R.M.; Díaz, J.F.; Andreu, J.M.; O’Brate, A.; Giannakakou, P.; Nicolaou, K.C.; Sasmal, P.K.; Ritzén, A.; Namoto, K. Interaction of epothilone analogs with the paclitaxel binding site: Relationship between binding affinity, microtubule stabilization, and cytotoxicity. Chem. Biol. 2004, 11, 225–236. [Google Scholar] [CrossRef]
- Andreu, J.M.; Barasoain, I. The-interaction of baccatin III with the Taxol binding site of microtubules determined by a homogeneous assay with fluorescent taxoid. Biochemistry 2001, 40, 11975–11984. [Google Scholar] [CrossRef]
- Matesanz, R.; Barasoain, I.; Yang, C.G.; Wang, L.; Li, X.; De Ines, C.; Coderch, C.; Gago, F.; Barbero, J.J.; Andreu, J.M.; et al. Optimization of Taxane Binding to Microtubules: Binding Affinity Dissection and Incremental Construction of a High-Affinity Analog of Paclitaxel. Chem. Biol. 2008, 15, 573–585. [Google Scholar] [CrossRef]
- Pineda, J.J.; Miller, M.A.; Song, Y.; Kuhn, H.; Mikula, H.; Tallapragada, N.; Weissleder, R.; Mitchison, T.J. Site occupancy calibration of taxane pharmacology in live cells and tissues. Proc. Natl. Acad. Sci. USA 2018, 115, E11406–E11414. [Google Scholar] [CrossRef]
- Kuh, H.-J.; Jang, S.H.; Wientjes, M.G.; Au, J.L.-S. Computational Model of Intracellular Pharmacokinetics of Paclitaxel. J. Pharmacol. Exp. Ther. 2000, 293, 761–770. [Google Scholar] [PubMed]
- Maloney, S.M.; Hoover, C.A.; Morejon-Lasso, L.V.; Prosperi, J.R. cancers Mechanisms of Taxane Resistance. Cancers 2020, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Posocco, B.; Buzzo, M.; Follegot, A.; Giodini, L.; Sorio, R.; Marangon, E.; Toffoli, G. A new high-performance liquid chromatography-tandem mass spectrometry method for the determination of paclitaxel and 6α-hydroxy-paclitaxel in human plasma: Development, validation and application in a clinical pharmacokinetic study. PLoS ONE 2018, 13, e0193500. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, J.; Zhang, J.; Wang, L.; Chan, J.; Wang, H.; Jin, Y.; Yu, L.; Grainger, D.W.; Ying, W. A cell-based pharmacokinetics assay for evaluating tubulin-binding drugs. Int. J. Med. Sci. 2014, 11, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Stage, T.B.; Bergmann, T.K.; Kroetz, D.L. Clinical Pharmacokinetics of Paclitaxel Monotherapy: An Updated Literature Review. Clin. Pharmacokinet. 2018, 57, 7–19. [Google Scholar] [CrossRef]
- Borgå, O.; Lilienberg, E.; Bjermo, H.; Hansson, F.; Heldring, N.; Dediu, R. Pharmacokinetics of Total and Unbound Paclitaxel After Administration of Paclitaxel Micellar or Nab-Paclitaxel: An Open, Randomized, Cross-Over, Explorative Study in Breast Cancer Patients. Adv. Ther. 2019, 36, 2825–2837. [Google Scholar] [CrossRef]
- Roger, O.B.; Helena, H.; Elsa, B.; Nina, L.; Loman, H.N. Maximum Tolerated Dose and Pharmacokinetics of Paclitaxel Micellar in Patients with Recurrent Malignant Solid Tumours: A Dose-Escalation Study. Adv. Ther. 2019, 36, 1150–1163. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Dai, X.; Zhong, D.; Ding, Y.; Chen, X. Bioequivalence of paclitaxel protein-bound particles in patients with breast cancer: Determining total and unbound paclitaxel in plasma by rapid equilibrium dialysis and liquid chromatography–tandem mass spectrometry. Drug Des. Devel. Ther. 2019, 13, 1739–1749. [Google Scholar] [CrossRef]
- Gardner, E.R.; Dahut, W.L.; Scripture, C.D.; Jones, J.; Aragon-Ching, J.B.; Desai, N.; Hawkins, M.J.; Sparreboom, A.; Figg, W.D. Randomized Crossover Pharmacokinetic Study of Solvent-Based Paclitaxel and nab-Paclitaxel. Clin. Cancer Res. 2008, 14, 4200–4205. [Google Scholar] [CrossRef]
- Desai, N.; Trieu, V.; Yao, Z.; Louie, L.; Ci, S.; Yang, A.; Tao, C.; De, T.; Beals, B.; Dykes, D.; et al. Increased Antitumor Activity, Intratumor Paclitaxel Concentrations, and Endothelial Cell Transport of Cremophor-Free, Albumin-Bound Paclitaxel, ABI-007, Compared with Cremophor-Based Paclitaxel. Clin. Cancer Res. 2006, 12, 1317–1324. [Google Scholar] [CrossRef]
- Van Eerden, R.A.G.; van Doorn, L.; de Man, F.M.; Heersche, N.; Doukas, M.; van den Bosch, T.P.P.; Oomen-de Hoop, E.; de Bruijn, P.; Bins, S.; Ibrahim, E.; et al. Tissue Type Differences in ABCB1 Expression and Paclitaxel Tissue Pharmacokinetics in Patients with Esophageal Cancer. Front. Pharmacol. 2021, 12, 759146. [Google Scholar] [CrossRef] [PubMed]
- Jamis-Dow, C.A.; Klecker, R.W.; Sarosy, G.; Reed, E.; Collins, J.M. Steady-state plasma concentrations and effects of taxol for a 250 mg/m2 dose in combination with granulocyte-colony stimulating factor in patients with ovarian cancer. Cancer Chemother. Pharmacol. 1993, 33, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Rowinsky, E.K.; Jiroutek, M.; Bonomi, P.; Johnson, D.; Baker, S.D. Paclitaxel steady-state plasma concentration as a determinant of disease outcome and toxicity in lung cancer patients treated with paclitaxel and cisplatin. Clin. Cancer Res. 1999, 5, 767–774. [Google Scholar] [PubMed]
- Hurria, A.; Blanchard, M.S.; Synold, T.W.; Mortimer, J.; Chung, C.T.; Luu, T.; Katheria, V.; Rotter, A.J.; Wong, C.; Choi, A.; et al. Age-Related Changes in Nanoparticle Albumin-Bound Paclitaxel Pharmacokinetics and Pharmacodynamics: Influence of Chronological Versus Functional Age. Oncologist 2015, 20, 37–44. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, B.; Chang, H.; Xiao, M.; Wu, Y.; Liu, Y. Paclitaxel suppresses proliferation and induces apoptosis through regulation of ROS and the AKT/MAPK signaling pathway in canine mammary gland tumor cells. Mol. Med. Rep. 2018, 17, 8289–8299. [Google Scholar] [CrossRef]
- Al-Mahayri, Z.N.; AlAhmad, M.M.; Ali, B.R. Current opinion on the pharmacogenomics of paclitaxel-induced toxicity. Expert Opin. Drug Metab. Toxicol. 2021, 17, 785–801. [Google Scholar] [CrossRef]
- Ngoi, N.Y.; Syn, N.L.; Goh, R.M.; Goh, B.C.; Huang, R.Y.J.; Soon, Y.Y.; James, E.; Cook, A.; Clamp, A.; Tan, D.S. Weekly versus tri-weekly paclitaxel with carboplatin for first-line treatment in women with epithelial ovarian cancer. Cochrane Database Syst. Rev. 2022, 2022. 12, 13. [Google Scholar] [CrossRef]
- Chan, J.K.; Brady, M.F.; Penson, R.T.; Huang, H.; Birrer, M.J.; Walker, J.L.; Disilvestro, P.A.; Rubin, S.C.; Martin, L.P.; Davidson, S.A.; et al. Weekly vs. Every-3-Week Paclitaxel and Carboplatin for Ovarian Cancer. N. Engl. J. Med. 2016, 381, 738–748. [Google Scholar] [CrossRef]
- Lu, H.; Zha, S.; Zhang, W.; Wang, Q.; Jiang, D.; Xu, X.; Zheng, X.; Qiu, M.; Shan, C. A systematic review and meta-analysis of nab-paclitaxel mono-chemotherapy for metastatic breast cancer. BMC Cancer 2021, 21, 830. [Google Scholar] [CrossRef]
- Chou, P.L.; Huang, Y.P.; Cheng, M.H.; Rau, K.M.; Fang, Y.P. Improvement of paclitaxel-associated adverse reactions (ADRs) via the use of nano-based drug delivery systems: A systematic review and network meta-analysis. Int. J. Nanomed. 2020, 15, 1731–1743. [Google Scholar] [CrossRef]
- Marschner, N.; Salat, C.; Söling, U.; Hansen, R.; Grebhardt, S.; Harde, J.; Nusch, A.; Potthoff, K. Final Effectiveness and Safety Results of NABUCCO: Real-World Data from a Noninterventional, Prospective, Multicenter Study in 697 Patients with Metastatic Breast Cancer Treated with nab-Paclitaxel. Clin. Breast Cancer 2018, 18, e1323–e1337. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhang, H.; Li, X.; Quan, X. Risk Factors for Chemotherapy-Induced Peripheral Neuropathy Caused by Nanoparticle Albumin-Bound Paclitaxel in Advanced Breast Cancer. Biomed Res. Int. 2022, 2022, 9430952. [Google Scholar] [CrossRef] [PubMed]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Moghadam, E.R.; Hashemi, F.; Entezari, M.; Hushmandi, K.; Mohammadinejad, R.; Najafi, M. Curcumin in cancer therapy: A novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020, 256, 117984. [Google Scholar] [CrossRef] [PubMed]
- Picard, M. Management of Hypersensitivity Reactions to Taxanes. Immunol. Allergy Clin. N. Am. 2017, 37, 679–693. [Google Scholar] [CrossRef] [PubMed]
- D’Errico, S.; Baldari, B.; Arcangeli, M.; Santurro, A.; Frati, P.; Fineschi, V. Mast cells activation and high blood tryptase levels due to paclitaxel administration. Is Cremophor EL the culprit? A case report. Medicine 2020, 99, e22814. [Google Scholar] [CrossRef]
- Pagani, M.; Bavbek, S.; Alvarez-Cuesta, E.; Berna Dursun, A.; Bonadonna, P.; Castells, M.; Cernadas, J.; Chiriac, A.; Sahar, H.; Madrigal-Burgaleta, R.; et al. Hypersensitivity reactions to chemotherapy: An EAACI Position Paper. Allergy 2022, 77, 388–403. [Google Scholar] [CrossRef]
- Van Eerden, R.A.G.; Mathijssen, R.H.J.; Koolen, S.L.W. Recent clinical developments of nanomediated drug delivery systems of taxanes for the treatment of cancer. Int. J. Nanomed. 2020, 15, 8151–8166. [Google Scholar] [CrossRef]
- Altundag, K.; Dede, D.S.; Purnak, T. Albumin-bound paclitaxel (ABI-007; Abraxane) in the management of basal-like breast carcinoma. J. Clin. Pathol. 2007, 60, 958. [Google Scholar] [CrossRef]
- Otani, I.M.; Lax, T.; Long, A.A.; Slawski, B.R.; Camargo, C.A.; Banerji, A. Utility of Risk Stratification for Paclitaxel Hypersensitivity Reactions. J. Allergy Clin. Immunol. Pract. 2018, 6, 1266–1273.e2. [Google Scholar] [CrossRef]
- Thangwonglers, T.; Santimaleeworagun, W.; Therasakvichya, S.; Saengsukkasemsak, N.; Pimsi, P. Characteristics of immediate hypersensitivity reaction to paclitaxel-based chemotherapy in gynecologic cancer patients. Asian Pacific J. Allergy Immunol. 2020. [Google Scholar] [CrossRef]
- Kim, J.S.; Suh, K.J.; Lee, D.W.; Woo, G.U.; Kim, M.; Kim, S.H.; Ryu, H.S.; Lee, K.H.; Kim, T.Y.; Han, S.W.; et al. A Real-world Efficacy of Nab-paclitaxel Monotherapy in Metastatic Breast Cancer. Cancer Res. Treat. 2022, 54, 488–496. [Google Scholar] [CrossRef] [PubMed]
- Gornstein, E.L.; Schwarz, T.L. Neurotoxic mechanisms of paclitaxel are local to the distal axon and independent of transport defects. Exp. Neurol. 2017, 288, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Dhawan, S.; Andrews, R.; Kumar, L.; Wadhwa, S.; Shukla, G. A Randomized Controlled Trial to Assess the Effectiveness of Muscle Strengthening and Balancing Exercises on Chemotherapy-Induced Peripheral Neuropathic Pain and Quality of Life among Cancer Patients. Cancer Nurs. 2020, 43, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Shigematsu, H.; Hirata, T.; Nishina, M.; Yasui, D.; Ozaki, S. Cryotherapy for the prevention of weekly paclitaxel-induced peripheral adverse events in breast cancer patients. Support. Care Cancer 2020, 28, 5005–5011. [Google Scholar] [CrossRef] [PubMed]
- Cario, E. Toll-like receptors in the pathogenesis of chemotherapy-induced gastrointestinal toxicity. Curr. Opin. Support. Palliat. Care 2016, 10, 157–164. [Google Scholar] [CrossRef]
- Mallick, P.; Basu, S.; Moorthy, B.; Ghose, R. Role of Toll-like receptor 4 in Drug-drug Interaction between Paclitaxel and Irinotecan in vitro. Toxicol. In Vitro 2017, 41, 75. [Google Scholar] [CrossRef]
- Moisan, A.; Michielin, F.; Jacob, W.; Kronenberg, S.; Wilson, S.; Avignon, B.; Gerard, R.; Benmansour, F.; McIntyre, C.; Meneses-Lorente, G.; et al. Mechanistic investigations of diarrhea toxicity induced by Anti-HER2/3 combination therapy. Mol. Cancer Ther. 2018, 17, 1464–1474. [Google Scholar] [CrossRef]
- Chen, E.; Abu-Sbeih, H.; Thirumurthi, S.; Mallepally, N.; Khurana, S.; Wei, D.; Altan, M.; Morris, V.K.; Tan, D.; Barcenas, C.H.; et al. Clinical characteristics of colitis induced by taxane-based chemotherapy. Ann. Gastroenterol. 2020, 33, 59–67. [Google Scholar] [CrossRef]
- Morelli, M.B.; Bongiovanni, C.; Da Pra, S.; Miano, C.; Sacchi, F.; Lauriola, M.; D’Uva, G. Cardiotoxicity of Anticancer Drugs: Molecular Mechanisms and Strategies for Cardioprotection. Front. Cardiovasc. Med. 2022, 9, 680. [Google Scholar] [CrossRef]
- Gergs, U.; Weisgut, J.; Griethe, K.; Mißlinger, N.; Kirchhefer, U.; Neumann, J. Human histamine H2 receptors can initiate cardiac arrhythmias in a transgenic mouse. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 1963. [Google Scholar] [CrossRef]
- Dermitzakis, E.V.; Kimiskidis, V.K.; Lazaridis, G.; Alexopoulou, Z.; Timotheadou, E.; Papanikolaou, A.; Romanidou, O.; Georgiadis, G.; Kalogeras, K.T.; Tsiptsios, I.; et al. The impact of paclitaxel and carboplatin chemotherapy on the autonomous nervous system of patients with ovarian cancer. BMC Neurol. 2016, 16, 190. [Google Scholar] [CrossRef] [PubMed]
- Florescu, M.; Cinteza, M.; Vinereanu, D. Chemotherapy-induced Cardiotoxicity. Maedica A J. Clin. Med. 2013, 8, 59–67. [Google Scholar]
- Batra, A.; Patel, B.; Addison, D.; Baldassarre, L.A.; Desai, N.; Weintraub, N.; Deswal, A.; Hussain, Z.; Brown, S.A.; Ganatra, S.; et al. Cardiovascular safety profile of taxanes and vinca alkaloids: 30 years FDA registry experience. Open Heart 2021, 8, e001849. [Google Scholar] [CrossRef]
- Bikiewicz, A.; Banach, M.; von Haehling, S.; Maciejewski, M.; Bielecka-Dabrowa, A. Adjuvant breast cancer treatments cardiotoxicity and modern methods of detection and prevention of cardiac complications. ESC Heart Fail. 2021, 8, 2397–2418. [Google Scholar] [CrossRef] [PubMed]
- Lanza, O.; Ferrera, A.; Reale, S.; Solfanelli, G.; Petrungaro, M.; Melato, G.T.; Volpe, M.; Battistoni, A. New Insights on the Toxicity on Heart and Vessels of Breast Cancer Therapies. Med. Sci. 2022, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Schlitt, A.; Jordan, K.; Vordermark, D.; Schwamborn, J.; Langer, T.; Thomssen, C. Cardiotoxicity and oncological treatments. Dtsch. Arztebl. Int. 2014, 111, 161–168. [Google Scholar] [CrossRef]
- Marupudi, N.I.; Han, J.E.; Li, K.W.; Renard, V.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar] [CrossRef]
- Mahtani, R.L.; Parisi, M.; Glück, S.; Ni, Q.; Park, S.; Pelletier, C.; Faria, C.; Braiteh, F. Comparative effectiveness of early-line nab-paclitaxel vs. paclitaxel in patients with metastatic breast cancer: A US community-based real-world analysis. Cancer Manag. Res. 2018, 10, 249–256. [Google Scholar] [CrossRef]
- Anoop, T.M.; Joseph, R.; Unnikrishnan, P.; Thomas, F.; Venugopal, M. Taxane-induced acute interstitial pneumonitis in patients with breast cancer and outcome of taxane rechallenge. Lung India 2022, 39, 158. [Google Scholar] [CrossRef]
- Fujimoto, D.; Kato, R.; Morimoto, T.; Shimizu, R.; Sato, Y.; Kogo, M.; Ito, J.; Teraoka, S.; Nagata, K.; Nakagawa, A.; et al. Characteristics and Prognostic Impact of Pneumonitis during Systemic Anti-Cancer Therapy in Patients with Advanced Non-Small-Cell Lung Cancer. PLoS ONE 2016, 11, e0168465. [Google Scholar] [CrossRef]
- Khaled, M.; Kondamudi, N.P. Type IV Hypersensitivity Reaction; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Fujimori, K.; Yokoyama, A.; Kurita, Y.; Uno, K.; Saijo, N. Paclitaxel-induced cell-mediated hypersensitivity pneumonitis. Diagnosis using leukocyte migration test, bronchoalveolar lavage and transbronchial lung biopsy. Oncology 1998, 55, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Njigha, E.; Vuky, J.; Paxton, J.; Palumbo, A. Use of Nanoparticle Albumin-Bound Paclitaxel in Patients with Breast Cancer and Paclitaxel-Induced Pneumonitis: Single-Center Case Series. J. Hematol. Oncol. Pharm. 2019, 9, 199–205. [Google Scholar]
- Liu, W.J.; Zhong, Z.J.; Cao, L.H.; Li, H.T.; Zhang, T.H.; Lin, W.Q. Paclitaxel-induced lung injury and its amelioration by parecoxib sodium. Sci. Rep. 2015, 5, 12977. [Google Scholar] [CrossRef] [PubMed]
- Matsuno, O. Drug-induced interstitial lung disease: Mechanisms and best diagnostic approaches. Respir. Res. 2012, 13, 39. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; McNally, D.; Tutschka, P.J.; Bilgrami, S. Paclitaxel-Induced Acute Bilateral Pneumonitis. Ann. Pharmacother. 2016, 31, 1471–1474. [Google Scholar] [CrossRef]
- McNeish, I.A.; Kanfer, E.J.; Haynes, R.; Giles, C.; Harland, S.J.; Driver, D.; Rustin, G.J.S.; Newlands, E.S.; Seckl, M.J. Paclitaxel-containing high-dose chemotherapy for relapsed or refractory testicular germ cell tumours. Br. J. Cancer 2004, 90, 1169. [Google Scholar] [CrossRef]
- Kashiwada, T.; Saito, Y.; Terasaki, Y.; Hisakane, K.; Takeuchi, S.; Sugano, T.; Miyanaga, A.; Noro, R.; Minegishi, Y.; Seike, M.; et al. Interstitial lung disease associated with nanoparticle albumin-bound paclitaxel treatment in patients with lung cancer. Jpn. J. Clin. Oncol. 2019, 49, 165–173. [Google Scholar] [CrossRef]
- Ardolino, L.; Lau, B.; Wilson, I.; Chen, J.; Borella, L.; Stone, E.; Lim, E. Case Report: Paclitaxel-Induced Pneumonitis in Early Breast Cancer: A Single Institution Experience and Review. Front. Oncol. 2021, 11, 2479. [Google Scholar] [CrossRef]
- Bielopolski, D.; Evron, E.; Moreh-Rahav, O.; Landes, M.; Stemmer, S.M.; Salamon, F. Paclitaxel-induced pneumonitis in patients with breast cancer: Case series and review of the literature. J. Chemother. 2017, 29, 113–117. [Google Scholar] [CrossRef]
- Marks, D.H.; Qureshi, A.; Friedman, A. Evaluation of Prevention Interventions for Taxane-Induced Dermatologic Adverse Events: A Systematic Review. JAMA Dermatol. 2018, 154, 1465–1472. [Google Scholar] [CrossRef]
- Lee, C.; Gianos, M.; Klaustermeyer, W.B. Diagnosis and management of hypersensitivity reactions related to common cancer chemotherapy agents. Ann. Allergy Asthma Immunol. 2009, 102, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fidalgo, J.A.; Navarro, C.E.; Garcia, C.S.; Perez-Leal, M.; Ferriols, J.P.; Cervantes, A.; Paya, J.M.; Gimeno, J.C. Mechanisms of skin toxicity of paclitaxel: An in vitro preclinical assessment. J. Clin. Oncol. 2020, 38, e15511. [Google Scholar] [CrossRef]
- Jennings, E.; Huang, S.; Lee, J.B.; Cha, J.; Hsu, S. Toxic Erythema of Chemotherapy Secondary to Gemcitabine and Paclitaxel. Dermatol. Online J. 2020, 26, 13030. [Google Scholar] [CrossRef]
- Su, M.H.; Chen, G.Y.; Lin, J.H.; Lee, H.H.; Chung, K.C.; Wang, P.H. Paclitaxel-related dermatological problems: Not only alopecia occurs. Taiwan. J. Obstet. Gynecol. 2019, 58, 877–879. [Google Scholar] [CrossRef] [PubMed]
- Sibaud, V.; Leboeuf, N.R.; Roche, H.; Belum, V.R.; Gladieff, L.; Deslandres, M.; Montastruc, M.; Eche, A.; Vigarios, E.; Dalenc, F.; et al. Dermatological adverse events with taxane chemotherapy. Eur. J. Dermatol. 2016, 26, 427–443. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Adderley, H.; Alameddine, M.; Armstrong, A.; Arundell, D.; Fox, R.; Harries, M.; Lim, J.; Salih, Z.; Tetlow, C.; et al. Permanent hair loss associated with taxane chemotherapy use in breast cancer: A retrospective survey at two tertiary UK cancer centres. Eur. J. Cancer Care 2021, 30, e13395. [Google Scholar] [CrossRef]
- Lugtenberg, R.T.; van den Hurk, C.J.G.; Smorenburg, C.H.; Mosch, L.; Houtsma, D.; Deursen, M.A.G.D.H.V.; Kaptein, A.A.; Gelderblom, H.; Kroep, J.R. Comparable effectiveness of 45- and 20-min post-infusion scalp cooling time in preventing paclitaxel-induced alopecia—A randomized controlled trial. Support. Care Cancer 2022, 30, 6641–6648. [Google Scholar] [CrossRef]
- Chmielewski, N.N.; Limoli, C.L. Sex Differences in Taxane Toxicities. Cancers 2022, 14, 3325. [Google Scholar] [CrossRef]
- Rabah, S.O. Acute Taxol nephrotoxicity: Histological and ultrastructural studies of mice kidney parenchyma. Saudi J. Biol. Sci. 2010, 17, 105–114. [Google Scholar] [CrossRef]
- Wilson, D.B.; Beck, T.M.; Gundlach, C.A. Paclitaxel formulation as a cause of ethanol intoxication. Ann. Pharmacother. 1997, 31, 873–875. [Google Scholar] [CrossRef]
- Perry, J.R.; Warner, E. Transient encephalopathy after paclitaxel (Taxol) infusion. Neurology 1996, 46, 1596–1599. [Google Scholar] [CrossRef] [PubMed]
- Fries, H.; Hitzschke, M.; Lordick, F. A Different Kind of Relapse: Ethanol as an Additive in Chemotherapy Formulations. Oncol. Res. Treat. 2019, 42, 350–353. [Google Scholar] [CrossRef]
- Mirza, A.; Mithal, N. Alcohol intoxication with the new formulation of docetaxel. Clin. Oncol. 2011, 23, 560–561. [Google Scholar] [CrossRef] [PubMed]
- Pilija, V.; Djurendic-Brenesel, M.; Miletic, S. Fatal poisoning by ingestion of Taxus baccata leaves. Forensic Sci. Int. 2018, 290, e1–e4. [Google Scholar] [CrossRef]
- Dahlqvist, M.; Venzin, R.; König, S.; Faber, K.; Weinmann, W.; Terbeck, S.; Ceschi, A.; Dünser, M.W. Haemodialysis in Taxus baccata poisoning: A case report. QJM Int. J. Med. 2012, 105, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Vardon Bounes, F.; Tardif, E.; Ruiz, S.; Gallart, J.C.; Conil, J.M.; Delmas, C. Suicide attempt with self-made Taxus baccata leaf capsules: Survival following the application of extracorporeal membrane oxygenation for ventricular arrythmia and refractory cardiogenic shock. Clin. Toxicol. 2017, 55, 925–928. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.; Badowski, D.; Koschny, R.; Skopp, G.; Brcic, A.; Szabo, G.B. Extracorporeal life support and digoxin-specific Fab fragments for successful management of Taxus baccata intoxication with low output and ventricular arrhythmia. Am. J. Emerg. Med. 2017, 35, 1987.e3–1987.e7. [Google Scholar] [CrossRef]
- Lott, C.; Truhlář, A.; Alfonzo, A.; Barelli, A.; González-Salvado, V.; Hinkelbein, J.; Nolan, J.P.; Paal, P.; Perkins, G.D.; Thies, K.C.; et al. European Resuscitation Council Guidelines 2021: Cardiac arrest in special circumstances. Resuscitation 2021, 161, 152–219. [Google Scholar] [CrossRef]
- Thooft, A.; Goubella, A.; Fagnoul, D.; Taccone, F.S.; Brimioulle, S.; Vincent, J.-L.; De Backer, D. Combination of veno-arterial extracorporeal membrane oxygenation and hypothermia for out-of-hospital cardiac arrest due to Taxus intoxication. CJEM 2014, 16, 504–507. [Google Scholar] [CrossRef]
- Vališ, M.; Kočí, J.; Tuček, D.; Lutonský, T.; Kopová, J.; Bartoń, P.; Vyšata, O.; Krajíčková, D.; Korábečný, J.; Masopust, J.; et al. Common yew intoxication: A case report. J. Med. Case Rep. 2014, 8, 2–4. [Google Scholar] [CrossRef]
- Baum, C.; Bohnen, S.; Sill, B.; Philipp, S.; Damerow, H.; Kluge, S.; Reichenspurner, H.; Blankenberg, S.; Söffker, G.; Barten, M.J.; et al. Prolonged resuscitation and cardiogenic shock after intoxication with European yew (Taxus baccata): Complete recovery after intermittent mechanical circulatory support. Int. J. Cardiol. 2015, 181, 176–178. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.R.; Sauer, J.M.; Hooser, S.B. Taxines: A review of the mechanism and toxicity of yew (Taxus spp.) alkaloids. Toxicon 2001, 39, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, J.; Rauber-Lüthy, C.; Kupferschmidt, H.; Kupper, J.; Kullak-Ublick, G.A.; Ceschi, A. Acute plant poisoning: Analysis of clinical features and circumstances of exposure. Clin. Toxicol. 2011, 49, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Czerwek, H.; Fischer, W. Tödlicher Vergiftungsfall mit Taxus baccata. Arch. Toxikol. 1960, 18, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Frohne, D.; Pribilla, O. Tödliche Vergiftung mit Taxus baccata. Arch. Toxikol. 1965, 21, 150–162. [Google Scholar] [CrossRef]
- Schulte, T. Tödliche Vergiftung mit Eibennadeln (Taxus baccata). Arch. Toxicol. 1975, 34, 153–158. [Google Scholar] [CrossRef]
- Yersin, B.; Frey, J.G.; Schaller, M.D.; Nicod, P.; Perret, C. Fatal cardiac arrhythmias and shock following yew leaves ingestion. Ann. Emerg. Med. 1987, 16, 1396–1397. [Google Scholar] [CrossRef]
- Sinn, L.E. Fatal Taxine Poisoning from Yew Leaf Ingestion. J. Forensic. Sci. 1991, 36, 599–601. [Google Scholar] [CrossRef]
- Van Ingen, G.; Visser, R.; Peltenburg, H.; van der Ark, A.M.; Voortman, M. Sudden unexpected death due to Taxus poisoning. A report of five cases, with review of the literature. Forensic Sci. Int. 1992, 56, 81–87. [Google Scholar] [CrossRef]
- Mufihoff, F.; Jacob, B.; Fowinkel, C.; Daldrup, T. Suicidal yew leave ingestion—Phloroglucindimethylether (3, 5-dimethoxyphenol) as a marker for poisoning from Taxus baccata. Int. J. Leg. Med. 1993, 106, 45–50. [Google Scholar]
- Beike, J.; Karger, B.; Meiners, T.; Brinkmann, B.; Köhler, H. LC-MS determination of Taxus alkaloids in biological specimens. Int. J. Leg. Med. 2003, 117, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Frommherz, L.; Kintz, P.; Kijewski, H.; Köhler, H.; Lehr, M.; Brinkmann, B.; Beike, J. Quantitative determination of taxine B in body fluids by LC-MS-MS. Int. J. Leg. Med. 2006, 120, 346–351. [Google Scholar] [CrossRef]
- Pietsch, J.; Schulz, K.; Schmidt, U.; Andresen, H.; Schwarze, B.; Dreßler, J. A comparative study of five fatal cases of Taxus poisoning. Int. J. Leg. Med. 2007, 121, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Roll, P.; Beham-Schmid, C.; Beham, A.; Kollroser, M.; Reichenpfader, B. Suicidal yew ingestion. Forensic Sci. Int. Suppl. Ser. 2009, 1, 20–21. [Google Scholar] [CrossRef]
- Froldi, R.; Croci, P.F.; Dell’Acqua, L.; Farè, F.; Tassoni, G.; Gambaro, V. Preliminary gas chromatography with mass spectrometry determination of 3,5-dimethoxyphenol in biological specimens as evidence of Taxus poisoning. J. Anal. Toxicol. 2010, 34, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Stríbrný, J.; Dogosi, M.; Snupárek, Z.; Toupalík, P.; Baláz, P.; Bartos, P. 3,5-Dimethoxyfenol—Marker Intoxication with Taxus baccata. Soud. Lek. 2010, 55, 36–39. [Google Scholar]
- Grobosch, T.; Schwarze, B.; Stoecklein, D.; Binscheck, T. Fatal poisoning with Taxus baccata. Quantification of paclitaxel (taxol A), 10-deacetyltaxol, baccatin III, 10-deacetylbaccatin III, cephalomannine (taxol B), and 3,5-dimethoxyphenol in body fluids by liquid chromatography-tandem mass spectrometry. J. Anal. Toxicol. 2012, 36, 36–43. [Google Scholar] [CrossRef]
- Grobosch, T.; Schwarze, B.; Felgenhauer, N.; Riesselmann, B.; Roscher, S.; Binscheck, T. Eight cases of fatal and non-fatal poisoning with Taxus baccata. Forensic Sci. Int. 2013, 227, 118–126. [Google Scholar] [CrossRef]
- Tranca, S.; Petrisor, C.L. A fatal case of Taxus poisoning. Clujul Med. 2013, 86, 279–281. [Google Scholar]
- Perju-Dumbravǎ, D.; Morar, S.; Chiroban, O.; Lechintan, E.; Cioca, A. Suicidal poisoning by ingestion of Taxus baccata leaves. Case report and literature review. Rom. J. Leg. Med. 2013, 21, 115–118. [Google Scholar] [CrossRef]
- Piskač, O.; Stříbrný, J.; Rakovcová, H.; Malý, M. Cardiotoxicity of yew. Cor Vasa 2015, 57, e234–e238. [Google Scholar] [CrossRef]
- Arens, A.M.; Anaebere, T.C.; Horng, H.; Olson, K. Fatal Taxus baccata ingestion with perimortem serum taxine B quantification. Clin. Toxicol. 2016, 54, 878–880. [Google Scholar] [CrossRef] [PubMed]
- Brooks-Lim, E.W.L.; Mérette, S.A.; Hawkins, B.J.; Maxwell, C.; Washbrook, A.; Shapiro, A.M. Fatal ingestion of Taxus baccata: English yew. J. Forensic Sci. 2022, 67, 820–826. [Google Scholar] [CrossRef]
- Alarfaj, M.; Goswami, A. Cardiotoxicity in yew berry poisoning. Am. J. Emerg. Med. 2021, 50, 812.e1–812.e4. [Google Scholar] [CrossRef] [PubMed]
| Taxus/Anatomical Parts | Analytes | Concentration of Analytes (µg/g) | Analysis Time (min) | Analytical Method | Citation |
|---|---|---|---|---|---|
| T. Baccata L. Red arils | Ascorbic acid Carotenoids Polyphenols Volatile compound profile Antioxidants Flavonoids | 607–1450 33–54.2 12.2–538 na na 85–211 | na 20–50 55 | UV-Vis HPLC-DAD GC-MS | [28] |
| T. chinesis Bark, sapwood, and heartwood | Ethers Alcohols Acids Flavonoids Ketones Phenols Esters Aldehydes Pyridines | na na na na na na na na na | na 35 | FTIR GC-MS | [29] |
| T. chinesis Stems | Fatty acids Phenols Volatile components Benzenes Acids Esters Ketones Alkanes | na na na na na na na na | 60 | GC-MS | [30] |
| T. chinensis, T. cuspidata T. media | Amino acids Saccharides Acids Alcohols Amines Unspecified compounds | na na na na na na | 40.5 | GC-MS | [31] |
| T. chinensis Leaf and wood essential oil hydrodistilled | Monoterpenes | Yields of the yellow oils 0.15 %, 0.11 % (v/w) | 52 | GC-MS | [32] |
| T. baccata Needles | Flavonoids | 166,000–220,100 | More than 100 | LC–ESI–MS/MS | [33] |
| T. chinensis Heartwood (HW) Sapwood (SW) | Lipids Organic acids and their derivatives Nucleotides and their derivatives Flavonoids Amino acids and their derivatives Alkaloids Phenylpropanoids Vitamins Terpenes Carbohydrates | na na na na na na na na na na na | na | LC-MS | [34] |
| T. baccata L. Needles | 10-DAB III | 21.1–113.2 | na | LC-MS/MS | [35] |
| T. baccata Needles | 10-DAB III BAC III 10-DAT PTX CEPH | na na na na na | cca 10 | UPLC-MS/MS LC-MS/MS | [36] |
| T. baccata Needles | PTX 10-DAB III 10-DAT BAC III CEPH | 17.8–29.7 162.3–703.4 14.7–17.5 trace 19.2–29.3 | na | UPLC-MS/MS | [37] |
| T. baccata L. | Flavonoids | 204,260 ± 6020 | cca 100 | HPLC–DAD–ESI–MS/MS | [18] |
| T. baccata L. Red arils | Fatty acids Proteins Lipids Carbohydrates Amino acids 10-DAB III BAC III CEPH Taxinine M Taxol A Macroelements Microelements | 7199–15,831 17,900–38,000 13,900–35,500 184,300–193,000 9167–15,831 3.9–19.8 2–6.3 0.05–0.18 0.02–0.13 0.02–0.10 8.6–8783.8 0.0013–25.37 | More than 45 GC-FID: more than 175 | LC-MS/MS GC-FID Macroelements, microelements, and trace metals ICP-OES | [38] |
| T. cuspidata T. mairei T. media Twigs | Secondary metabolites Amino acids Cofactors and vitamins Carbohydrates Lipids Nucleotides Energy-related metabolites Flavonoids PTX BAC III 10-DAT 10-DAB III | na na na na na na na T. cuspidata 1670 T. mairei 660 T. media 1220 T. cuspidata 800 T. mairei > 400 T. media > 200 T. cuspidata 800 T. mairei 200 T. media 800 T. cuspidata 800 T. mairei 1000 T. media 400 | cca. 15 for untargeted analysis | HPLC-MS/MS UPLC-MS/MS | [23] |
| T. media T. cuspidata T. mairei T. grandis Twigs | Terpenoids Amino acids Flavonoids Phenylpropanoids PTX 7-epi 10-DAT 10-DAB III BAC III 10-DAT CEPH 7-E-DAB | na na na na T. cuspidata < 100 T. media 300 T. mairei < 100 T. grandis 0 T. cuspidata < 70 T. media 60 T. mairei < 10 T. grandis 0 T. cuspidata < 50 T. media 150 T. mairei 150 T. grandis 0 T. cuspidata 5 T. media < 20 T. mairei 20 T. grandis 0 T. cuspidata < 250 T. media > 300 T. mairei 100 T. grandis 0 T. cuspidata < 100 T. media < 160 T. mairei 20 T. grandis 0 T. cuspidata 300 T. media < 200 T. mairei 250 T. grandis 0 | Untargeted metabolomic profiling: 15 Hormones: 8 | LC-QTOF LC-MS/MS UPLC-MS/MS | [39] |
| T. chinensis T. cuspidata T. media Twigs and leaves | 10-DAB III BAC III 7-xyl-10-DAT 10-DAT CEPH PTX 7-epi-PTX Flavonoids | T. chinesis 3–50 T. cuspidata cca 0–70 T. media 140–420 T chinesis 3.5–22.5 T. cuspidata 2–15 T. media 2–45 T. chinesis 420–1110 T. cuspidata 0–90 T. media >90 T. chinesis <72–430 T. cuspidata 144–288 T. media 216–288 T. chinesis <110–330 T. cuspidata 330–600 T. media 330–550 T. chinesis 15–90 T. cuspidata 60–95 T media <15–180 T. chinesis <1.5–3.7 T. cuspidata <3.7–7.4 T. media 1.5–22.2 T. chinesis 5400–10,800 T. cuspidata <5400–16,200 T. media 5400–10,800 | 5 | UHPLC-MS/MS | [40] |
| T. cuspidata Branches and leaves | Polysaccharides | 4.47 w/w % | na | Ion chromatograph UV-VIS | [41] |
| T. fuana T. yunnanensis Twig | Alkaloids Amino acids Hormones Lipids Terpenoids Phenylpropanoids Saccharides PTX 10-DAB III DAB III Flavonoids 10-DAT 7-epi-PTX 7-Epi 10-DAT CEPH | na na na na na na T. Fuana 27 T. yunnanensis 84 T. fuana 200 T. yunnanensis 500 T. fuana 10 T. yunnanensis 25 T. fuana 150,000 T. yunnanensis 115,000 T. fuana 15 T. yunnanensis 150 T. fuana max 10 T. yunnanensis max 15 T. fuana max 10 T. yunnanensis 250 T. fuana 50 T. yunnanensis 100 | 15 | UPLC-MS/MS | [42] |
| T. yunnanensis Needles | PTX 10-DAB III | na na | 15 | HPLC-PDA | [43] |
| T. cuspidata Bark | Caffeic acid Chlorogenic acid Gallic acid p-Hydroxybenzoic acid Hydroxycaffeic acid Protocatechuic acid | 30.5 ± 0.1 83 ± 2.2 20.4 ± 0.7 24.2 ± 1.6 239.8 ± 13 209.7 ± 5.6 | na | HPLC-DAD | [44] |
| T. cuspidata Stems and leaves | 10-DAB III BAC III 10-DAT CEPH PTX | 212–354 20–56.6 51–91 36–79 46–92 | 30 | HPLC-UV or DAD | [45] |
| T. chinensis Leaves | (E)-1-O-(p-coumaroyl)-3-methoxy-myo-inositol p-Hydroxybenzaldehyde p-Hydroxybenzoic acid Palmitic acid Protocatechuic acid Sciadopitysin Ginkgetin Sequoiaflavonoid Taxacine 10-DAB III 5-deacetyltaxachitriene B Makisterone C 7-β-xylosyl-10-decetyltaxol Taxiphyllin | na na na na na na na na na na na na na na na | na | NMR HR-MS TLC | [46] |
| T. baccata L. T. media Arils | Carotenoids Xanthophyll esters | T. media 20.33–58.78 T. baccata 17–19.55 | na | HPLC-DAD-ESI/APCI-MSn | [47] |
| T. baccata L. Red arils | Taxol A 10-DAB III BAC III CEPH Taxinine M 3,5-DMP | 0.07–5.5 5.6–38 1–28 0.04–7.2 0.04–2 0.04–0.82 | 15.5 | HPLC-MS/MS | [48] |
| T. cuspidata Needles | PTX 10-DAB III BAC III DXT DOT 7-EPT CEPH 10-DAT | na na na na na na na na | 40 | HPLC | [49] |
| T. wallichiana Needles | Total phenolic content Total flavanol content Total flavonoid content Total tannin content | 92,670 ± 680 na 84,660 ± 520 na | na | HPLC-PDA | [50] |
| T. Wallichiana Leaf, stem, bark and roots | Docetaxel PTX | na 9.31–36.46 | 35 20 | HPLC-UV QToF/MS | [51] |
| T. chinensis Needles | 10-DAB III BAC III 7-xyl-10-DAT 10-DAT CEPH 7-epi-10-DAT PTX | 434.75–626.44 45.51–120.48 351.44–546.95 104.05–236.49 42.74–105.69 94.45–166.44 85.79–162.75 | 25 | HPLC | [52] |
| T. media T. mairei Leaves | Flavonoid content | T. media 14,464 T. mairei 19,953 | 15 | UPLC-ESI-MS/MS | [53] |
| Compound | Molecular Formula | Mw (g/mol) | Topological Polar Surface Area |
|---|---|---|---|
| Paclitaxel | C47H51NO14 | 853.98 | 221 Å2 |
| Cephalomannine | C45H53NO14 | 831.9 | 221 Å2 |
| Baccatine III | C31H38O11 | 586.6 | 166 Å2 |
| Brevifoliol | C31H40O9 | 556.6 | 140 Å2 |
| 9-Dihydro-13-acetylbaccatine III | C33H42O12 | 630.7 | 175 Å2 |
| Taxine A | C35H47NO10 | 641.7 | 160 Å2 |
| 2-Deacetyltaxine A | C33H45NO9 | 599.7 | 154 Å2 |
| 10-Deacetylbaccatine III | C29H36O10 | 544.6 | 160 Å2 |
| Taxine B | C33H45NO8 | 583.7 | 134 Å2 |
| Isotaxine B | C33H45NO8 | 583.7 | 134 Å2 |
| Taxinine M | C35H42O14 | 686.7 | na |
| 10-Deacetyltaxol | C45H49NO13 | 811.9 | 215 Å2 |
| 7-Epitaxol | C47H51NO14 | 853.9 | 221 Å2 |
| 7-Epi-10-deacetylbaccatine III | C29H36O10 | 544.6 | 160 Å2 |
| Anamnesis, Case History Clinical Findings | Autopsy Findings | Taxane Concentration | Analytical Method | Cause and Manner of Death | Ref. |
|---|---|---|---|---|---|
| 20-year-old female, prepared tea (decoction) from three spoons of yew needles, let it brew for 0.5 h, then drank the tea and ate the infused yew needles with bread. After 1 h dizziness, strong palpitations, ringing in the ears, burning in the whole body, vomiting (vomits contained needles), soon unconsciousness. On admission to the hospital, pallor, tachycardia, hypotension, later hypertension, then tachycardia again. 24 h after ingestion death. Medical history: psychological problems, suicidal thoughts | Isolated needles of Taxus leaves found in the stomach throughout the small intestine and a large number of Taxus needles Mild necrosis of the gastric mucosa with small haemorrhages Dilation of heart chambers Blood flow to internal organs Histology: myocardium interstitial oedema, signs of fatty degeneration in the liver and kidneys | na | na | Cause of death: Heart failure in taxus intoxication Manner of death: Suicide | [159] |
| 24-year-old male, a year after the death of his ex-fiancée, he suffered from depression, often expressing suicidal thoughts. He went to a ball, drank very little alcohol. When he was returning home, his current fiancée told him that she was probably expecting his child. About 4 h later, he was found at home lying in bed unconscious, breathing. On admission to the hospital, deep unconsciousness, gasping breathing, pale skin, cyanosis of the face, neck and upper chest, very wide unresponsive pupils (mydriasis), weak pulse, weak irregular heart activity, poor reflexes, unmeasurable blood pressure. Despite immediate treatment, respiratory and cardiac arrest in a short time → death. | In the stomach, approx. 200 mL of porridge mixed with vegetable matter (39 g of vegetable matter in total). In the duodenum, only a moderate amount of food (mass) mixed with isolated plant components. Slightly greenish mucus in the larynx, acute enlargement of the heart chambers, acute congestion and pulmonary oedema, severe acute congestion of the spleen, severe acute congestion of the liver, congestion and swelling of the kidneys, severe swelling of the brain Microscopic examination of stomach contents: found cut-up Taxus baccata presses | Taxine | UV Thin-layer chromatography | Cause of death: acute circulatory failure in case of poisoning by T. baccata Manner of death: Not reported | [160] |
| 28-year-old female, teacher, she knew the toxicity of yew, she ingested 4 to 5 handfuls of yew needles with suicidal intent. A yew tree was in the garden in front of her house. About 1 h after taking dizziness, nausea, no vomiting, diffuse abdominal pain. Taken to the hospital, in a drowsy state, tonic-clonic seizure in the reception room, then unconsciousness, tachycardia, wide pupils (mydriasis), weak, later no reflexes, respiratory arrest, unmeasurable blood pressure. Subsequently, intubation, artificial ventilation, intensive care. In a few minutes, asystole → followed by cardiac stimulation and resuscitation, after 50 min resuscitation ended, heart, circulation and breathing stopped → death. Performed gastric lavage with 10 litres of water, a large number of needles and food residues obtained during the lavage. | 2 to 3 cm long coniferous yew twigs found in the stomach Acute haemorrhagic gastroenteritis Acute congestion of the liver, kidneys and spleen, pulmonary and myocardial oedema, punctate subpleural haemorrhages, flaccid and dilated all cavities of the heart Histology: small necrosis of cardiomyocytes of the left ventricle of the heart, acute small hepatocellular necrosis in the liver, incipient diffuse fatty degeneration of the liver | na | na | Cause of death: Heart failure Manner of death: Suicide | [161] |
| 40-year-old female, she intentionally ate about 150 yew leaves (Taxus baccata). 2 h after taking it, she sought medical help due to vomiting and abdominal pain. Admitted to hospital. Symptoms: hypotension, followed by shock and respiratory arrest. Immediate resuscitation (external cardiac massage, intubation, artificial ventilation, heart rhythm disorder on EKG, right ventricular pacemaker inserted). Aspiration of gastric fluid performed immediately after intubation showed the presence of yew leaves (needles). After 3 h, ventricular fibrillation unresponsive to treatment, death 5 h after yew leaves ingestion. Medical history: chronic psychosis. | Autopsy not performed | na | na | Cause of death: Cardiogenic shock Manner of death: Suicide | [162] |
| 22-year-old male, 4th year university student of agriculture. Found on a winter morning lying on the “step” of a high bridge; he did not respond. A bag with washed clothes was next to the body. He was seen walking down the road a few minutes before. After an examination, the doctor at the emergency room declared the man dead; alcohol test negative. Medical history: loner, marijuana user. A bag of marijuana was found in his pocket. | Fresh green grass-like leaves found in jejunum—identified by agronomist as yew leaves Smell of fresh yew leaves detected during autopsy Cannabinoids present in urine at a concentration of more than 75 ng/mL | na | GC-FID | Cause of death: Yew poisoning Manner of death: Not reported | [163] |
| 19-year-old female, found dead. She had told her friend she was considering suicide. | Green particles found in the stomach, identified as parts of Taxus baccata Congestion of lungs, liver, kidneys Dilation (expansion) of heart chambers | na | na | Cause of death: Yew poisoning Manner of death: Suicide | [164] |
| 70-year-old female, admitted to the hospital after a suicide attempt by diazepam intoxication. After recovery, she was taken to a psychiatric hospital, where she was allowed to walk around the campus. After several days of severe abdominal pain, she admitted to the doctor that she had eaten parts of Taxus tree bark. Hypotension, bradycardia, cardiac arrest → resuscitation unsuccessful → death. | Autopsy not performed | na | na | Cause and manner of death: Not reported | [164] |
| 23-year-old female, hospitalized at psychiatry, found dead. | Green mass with plant fibres found in the stomach and duodenum—identified as parts of Taxus leaves. Congestion of organs Dilated heart chambers | na | na | Cause of death: Yew poisoning (intoxication) Manner of death: Not reported | [164] |
| 26-year-old female, psychiatric patient, found dead. She was known to hear voices urging her to commit suicide. | The stomach and duodenum contained a brown liquid in which green plant parts were present. These were identified as Taxus baccata leaf fragments. Congestion of organs Dilated heart chambers | na | na | Cause of death: Yew poisoning (intoxication) Manner of death: Not reported | [164] |
| 37-year-old female, prisoner, found dead in her prison cell bed. She was considering suicide. A small Taxus plant was found in her prison cell. | Green plant parts were found in the stomach and duodenum, which were identified as leaves and leaf parts of Taxus baccata | na | na | Cause of death: Yew poisoning (intoxication) Manner of death: Suicide | [164] |
| 19-year-old male, found dead by friends in a remote cellar after leaving home two days earlier. He was lying on the couch, partially undressed. An empty teapot with fragments of brown-green leaves on the sides was found on the floor. Boiled and pressed leaves with a teaspoon were piled on the carpet near the teapot. Cellar was with no signs of vomiting or diarrhoea. Medical history: depression | Fragments of greenish needle-like leaves found in the mouth, oesophagus, stomach and intestines (not in the anus) (30% of the stomach contents were made up of leaves, i.e., 150 out of 500 g) All organs were heavily blood-stained, bronchial epithelium was inflamed Histology: significant to severe congestion of organs, slight damage to brain neurons, massive desquamation of the alveolar epithelium in the lungs, small to medium vacuolar degeneration of the myocardium | 3,5-DMP stomach 20,000 ng/g cardiac blood 0.32 mg/kg, 1.31 g/kg ethanol | HPLC, UV, GC-MS, IR 1H-NMR | Cause of death: Acute cardio-circulatory failure Manner of death: Not reported | [165] |
| 43-year-old male, schizophrenic, undergoing treatment several times, attempted suicide by cutting his wrist in the past. He was prescribed antipsychotic perazine (Taxilan) for a long time. Due to frequent side effects, he was looking for an alternative medicine. For this purpose, he bought a small yew tree, made a decoction from its leaves and drank it. He later told the nurse that he was tolerating yew better than perazine, and that he intended to replace this antipsychotic drug with regular use of yew tea. It is not known when he drank the second yew tea. Then, he sat with the nurse, complaining of nausea and lack of blood circulation. He told her that he had taken Taxus baccata leaves but did not specify the amount. He started vomiting, the nurse carried him to bed. When she went to check on him after 3 h, she found him lying dead in bed. There was an empty tea strainer in the kitchen, which the nurse later cleaned up. | Acute organ congestion, massive brain oedema, haemorrhagic pulmonary oedema, stomach dilatation Histology: significant interstitial oedema and signs of myocardial hypoxia, mild fatty degeneration of the liver, massive dilatation of submucosal gastric vessels Smears of aqueous stomach contents were not successful in identifying particles typical for Taxus baccata | 11,000 ng taxine/g blood (sum of taxine B and isotaxine B) | LC-MS LC-MS/MS | Cause of death:Taxus baccata intoxication Manner of death: Not reported | [166] |
| 24-year-old male, ingested yew needles with the aim of committing suicide. | Many yew leaves present in the stomach; other autopsy findings not stated | Taxine B/isotaxine B Blood-105 ng/g Stomach content 2000 ng/g Urine 0 ng/mL | LC-MS/MS | Cause of death: Not reported Manner of death: Suicide | [167] |
| 33-year-old female, a glass with red yew fruits was found near the dead body. Yew plant material was ingested; it is not known whether she ate any berries or drank a decoction of them. | No plant material was found in the body; other autopsy findings not stated | Taxine B/isotaxine B Blood-174 ng/g Stomach content 50,000 ng/g Urine 3000 ng/mL | LC-MS/MS | Cause of death: Not reported Manner of death: Not reported | [167] |
| 23-year-old female, long-term psychiatric treatment, found dead in her apartment. Information materials about toxic plants found in the apartment. Previous suicide attempts. The police investigation found that the woman sought medical help 2 weeks before her death due to symptoms similar to yew poisoning—dizziness and arrhythmia. However, she refused hospitalisation. | Approx.. 200 g of greenish-brown plant particles, identified as whole leaves and fragments of Taxus baccata leaves, were found in the duodenum and large intestine Advanced decomposition of the body | Cardiac blood (ng/mL) 47 Femoral blood (ng/mL) na Urine (ng/mL) 8700 Brain (ng/g) < 30 Liver (ng/g) 161 Kidney (ng/g) 275 duodenum (ng/g) 7800 | HPLC-PDA HPLC-UV | Cause of death: Taxus intoxication (poisoning) Manner of death: Suicide | [168] |
| 20-year-old male, found dead in the park inappropriately dressed for the season. | Approx. 150 g of green leaves found in the stomach and duodenum, identified as parts of T. baccata Significantly dilated pupils (mydriasis), blood flow to the lungs and brain, dilated heart chambers | Cardiac blood (ng/mL) 97 Femoral blood (ng/mL) 29 Urine na Brain (ng/g) 35 Liver (ng/g) 512 Kidney (ng/g) 382 Stomach content (ng/g) 13,400 | HPLC-PDA HPLC-UV | Cause of death: Taxus intoxication (poisoning) Manner of death: Not reported | [168] |
| 26-year-old male, found dead in an upstairs room of the house, lying dressed on the bed, his face resting on a pillow. In the room found a blender containing the remains of green porridge and a bowl with green porridge stuck to it. | Greenish plant material was found in the stomach, in which fragments of yew needles were identified Marked swelling of the brain, acute organ congestion | Cardiac blood (ng/mL) 528 Femoral blood na Urine na Brain na Liver (ng/g) 918 Kidney (ng/g) 418 Stomach content (ng/g) 118,000 | HPLC-PDA HPLC-UV | Cause of death: Taxus intoxication (poisoning) Manner of death: Not reported | [168] |
| 23-year-old male, student, found dead on the stairs of the convention centre around lunchtime Before his death, he felt sick, dizzy. He was last seen drinking tea 2 h before his death. The owner of the apartment found a small plastic bag with yew leaves in his backpack | 200 mL of stomach contents contained green particles Perfusion of organs (brain, liver, spleen, kidneys) | Cardiac blood (ng/mL) 110 Femoral blood (ng/mL) 217 Urine na Brain na Bile (ng/g) 175 Kidney (ng/g) na Stomach content (ng/g) 1400 | HPLC-PDA HPLC-UV | Cause of death: Yew intoxication (poisoning) Manner of death: Not reported | [168] |
| 16-year-old female, found dead in the bathroom of her parents’ apartment. Several hours before death registered dizziness, nausea, abdominal pain, unconsciousness. Medical history: mental disorders, depression | A large number of fragments of Taxus baccata leaves found in the mouth, oesophagus, stomach and duodenum, as well as in the trachea, wide dilated pupils (mydriasis), pulmonary oedema, acute congestion of organs (liver, spleen and kidneys) | Cardiac blood (ng/mL) 31 Femoral blood na Urine (ng/mL) 2700 Brain na Liver na Kidney na Stomach content (ng/g) 600 | HPLC-PDA HPLC-UV | Cause of death: Taxus baccata poisoning Manner of death: Suicide | [168] |
| 41-year-old male, found dead lying on the ground near the parking lot where his car was parked. His hands were clenched in spasms. History and cause of death unknown, suspected epileptic seizure | In the stomach and small intestine (not in the large intestine) found fragments of greenish needle-like leaves (chopped leaves) identified as yew. Autopsy and histological findings in agreement with the literature Examined bone marrow—oedematous, slightly hypocellular, with irregular distribution of hematopoietic cells | 3,5-dimethoxyphenol quality | GC-MS | Cause of death: Yew poisoning (intoxication) Manner of death: Not reported | [169] |
| 30-year-old male; found dead in his bed; near the bed found a light brown vegetable matter in a plastic bag—it looked the same as dried or partially macerated coniferous leaves | Plant material found in the stomach similar to that found in the plastic bag near the bed—botanically identified as Taxus baccata leaf fragments All organs were markedly congested Non-specific morphological findings | 3,5-DMP Blood 146 ng/mL Urine 56 ng/mL Bile 50 ng/mL Gastric content 360 ng/g | GC-MS | Cause of death: Fatal cardiac arrythmia Manner of death: Not reported | [170] |
| 28-year-old male, found dead in the basement of the family home. He ingested a decoction of yew mixed with sodium hydroxide | Acute catarrhal inflammation of the oesophagus with mucosal sloughing, acute superficial gastritis crushed plant material in the stomach and duodenum—needles and small twigs of T. baccata (3.6 g in dry state), blood effusions under the pleura, epicardium and in the soft coverings of the skull, brain swelling, haemorrhagic pulmonary oedema, congestion of internal organs, liquid blood | The presence of 3,5-dimethoxyphenol and 11-nor-A9-tetrahy-drocanabi-nol-carboxylic acid was detected in blood and urine. The presence of 3,5-dimethoxyphenol was further demonstrated in gastric and duodenal contents. In the gastric contents and urine, substances of the same nature as those contained in yew were detected by TLC. | TLC | Cause of death: T. baccata poisoning Manner of death: Suicide | [171] |
| 20-year-old male, found dead in the area of the psychiatric hospital | Presence of a large number of green needles in the stomach, less in the small intestine—identified as yew Dark red-purple post-mortem spots, (dilated pupils)—mydriasis, conjunctival congestion signs of suffocation with dilatation of the right ventricle of the heart Histology: myocardial interstitial oedema, rarely increased eosinophilia of cardiomyocytes | The presence of taxine B, isotaxine B and other yew substances was proven in the blood and stomach contents. The presence of 3,5-dimethoxyphenol was proven in the blood and stomach contents. | LC/MS GC/MS | Cause of death: Taxus baccata poisoning Manner of death: Suicide | [171] |
| 43-year-old female, intentionally ingested an unknown amount of Taxus needles. Dizziness, impaired consciousness, dysrhythmias, circulatory failure, asystole, death. | Not reported | na | na | Cause of death: Not reported Manner of death: Suicide | [158] |
| 22-year-old male, a professional gardener with a history of drug abuse (cannabis), brought to the hospital for detoxification. After 3 weeks, he was transferred to another hospital, where he announced his intention to commit suicide using poisonous plants. In the following days, he collected twigs from the hospital garden (which were later identified as yew twigs) and brought them to his room. He ingested an unknown amount of yew leaves. On the morning of the day of death, severe nausea, heaviness without vomiting, hypotension. The evening shortly before his death, he was found unresponsive in bed, with breathing difficulties. | Vegetables and small green needle-like particles found in the stomach Flat green parts of plant materials found on the tongue and in the oesophagus—needle-like yew leaves Congestion and cyanosis of internal organs Brain and lung oedema Small haemorrhages in the epicardium Expansion (dilatation) of the atria and the right heart ventricle | PTX Stomach content 20 ng/mL Urine < 0.5 ng/mL Cardiac blood < 0.5 ng/mL Femoral blood < 0.5 ng/mL Bile 24 ng/mL Brain < 0.5 ng/g 10-DAT Stomach content 36 ng/mL Urine < 0.5 ng/mL Cardiac blood < 0.5 ng/mL Femoral blood < 0.5 ng/mL Bile 4900 ng/mL Brain < 0.5 ng/g BAC III Stomach content 4.5 ng/mL Urine 19 ng/mL Cardiac blood < 0.5 ng/mL Femoral blood < 0.5 ng/mL Bile 6.2 ng/mL Brain < 0.5 ng/g 10-DAB III Stomach content 132 ng/mL Urine 200 ng/mL Cardiac blood 12 ng/mL Femoral blood 7.3 ng/mL Bile 290 ng/mL Brain < 0.5 ng/g CEPH Stomach content 23 ng/mL Urine 1 ng/mL Cardiac blood < 0.5 ng/mL Femoral blood < 0.5 ng/mL Bile 37 ng/mL Brain < 0.5 ng/g 3,5-DMP Stomach content 150 ng/mL Urine 7250 ng/mL Cardiac blood 110 ng/mL Femoral blood 60 ng/mL Bile 250 ng/mL Brain < 2 ng/g | HPLC-MS LC-MS/MS | Cause of death: Circulatory arrest Manner of death: Suicide | [172] |
| 38-year-old male, found dead in bed. Small fragments of greenish needle-like leaves found at the site (on the table in the bowl, next to the bed, on the blanket, on the chin and mouth of the deceased man). Scissors on the table, which he probably used to cut the leaves into small pieces before eating. According to the police report, the green leaves were identified as Christmas tree needles. | Fragments of green needle-like leaves found on the chin, mouth and stomach Non-specific autopsy findings | PTX Stomach content 82 ng/mL Bile 800 ng/mL Urine, cardiac blood, femoral blood < 0.1 ng/mL 10-DAT Stomach content 39.1 ng/mL Bile 325 ng/mL Urine, cardiac blood, femoral blood BAC III Stomach content 14.7 ng/mL Bile 292 ng/mL Urine 64.5 ng/mL Cardiac blood 8.25, femoral blood 8.5 ng/mL 10-DAB III Stomach content 69.5 ng/mL Bile 1690 ng/mL Urine 74 ng/mL Cardiac blood 19.9 ng/mL Femoral blood 19.8 ng/mL CEPH Stomach content 45.7 ng/mL Bile 482 ng/mL Urine, cardiac blood, femoral blood, <0.1 ng/mL 3,5-DMP Stomach content 423 ng/mL Bile 138 ng/mL Urine 5750 ng/mL Cardiac blood 820 ng/mL Femoral blood 283 ng/mL | Cause of death: Taxine intoxication Manner of death: Suicide | [173] | |
| 43-year-old male, for suicidal reasons he ate the leaves of the common yew (T. baccata). Symptoms: severe hypokalaemia, ventricular arrhythmia, hemodynamic instability, respiratory insufficiency, acid-base imbalance disorder, hepatic dysfunction, renal failure, coma, and 12 h after ingestion of T. baccata, despite intensive medical care and resuscitation, death. Stomach lavage performed in the hospital with evacuation of T. baccata leaves | Congestion and pulmonary oedema Brain oedema Enlarged liver Histology: acute dystrophic changes in the liver; dilation of blood vessels in the myocardium | na | na | Cause of death: Not reported Manner of death: Suicide | [174] |
| 43-year-old male, found unconscious at home by his family. Taken to hospital: coma (GCS 3 points), tachycardia, acute respiratory and hepatic failure, hypokalaemia, hypotension, mixed acidosis. Gastric lavage, administered activated charcoal, supportive hemodynamic treatment. After 4 h of admission, unresponsive cardiac arrest → death. Medical history: about 10 years of repeated depressive disorders treated irregularly, recently without treatment, he gradually stopped working, became interested in computers and suicide methods, and consulted information on the Internet about the yew plant and its toxic effects. A farewell letter and an empty plastic bag with the remains of green leaves were found near the body. A police investigation revealed that several yew bushes were found near the house. | In the stomach approx. 450 mL of dark liquid—partially digested food fragments and green-brown plant particles—botanically identified as whole leaves and fragments of yew leaves Non-specific symptoms of intoxication: swelling of the brain, blood flow to internal organs, pleural, pericardial and peritoneal effusions, hepatomegaly (2600 g) Histology: dystrophic changes of the liver and kidneys | na | na | Cause of death: Heart failure Fatal cardiac arrhythmia Manner of death: Suicide | [175] |
| 25-year-old male, drank a decoction of red yew with suicidal intent. Shortly after consuming the concoction, he reported it to his girlfriend, who called for medical help. When admitted to the hospital, about 1 h after taking it, conscious, with stable blood pressure, sinus tachycardia. Gastric lavage in the emergency room and in the hospital ward. Acidosis, liver and kidney functions and mineralogram were normal. After about 0.5 h ventricular fibrillation, cardiogenic shock, unconsciousness. Intubation, artificial pulmonary ventilation, repeated cardiopulmonary resuscitation, established temporary cardiostimulation. Hypokalaemia, acidosis, haemodialysis treatment without effect, cardiogenic shock, asystole → death approx. 6 h after administration. | Yew needles found in the small intestine. cerebral blood flow Congestion of the lungs with haemorrhages in the pulmonary alveoli Dilatation (enlargement) of the right atrium and heart chamber | na | na | Cause of death: Cardiac arrest due to red yew intoxication Manner of death: Suicide | [176] |
| 22-year-old female, after leaving the exhibition in the botanical garden she collapsed, tonic-clonic convulsions, no pulse, not breathing (apnoea). Laic cardiopulmonary resuscitation was started, medical help was called, transport to the hospital. Symptoms: hypotension, bradycardia, restoration of circulation, plant material found in the mouth, green plant material aspirated from the stomach with a nasogastric tube; a bag of plant material, identified as T. baccata was found in the woman’s purse. Despite the intensive care of heart rhythm disorders, after approx. 1.5 h death since admission to hospital. | Not reported | Serum 3,5-DMP 86.9 ng/mL Gastric content 73,200 ng/mL taxine B serum 80,900 ng/mL Gastric content 40.5 mg/mL | HPLC-MS | Cause of death: Not reported Manner of death: Not reported . | [177] |
| 19-year-old female, admitted to a closed department of a psychiatric hospital due to a suicide attempt. Found dead in bed in the morning. A document about the toxic effects of yew was found in her notebook. Inspection of the apartment—found several yew needles, 1 litre of tea from yew leaves, a teacup. No traces of yew leaves were found on the body, mouth and pharynx. | Autopsy not performed. | BAC III 10-DAB III 10-DAT Taxine B Isotaxine B PTX CEPH 3,5 DMP | LC-MS LC-MS/MS GC-MS HPLC-PDA | Cause of death: Not reported Manner of death: Based on the document in her notebook, yew leaves and a half-empty teacup, it was concluded that it was suicide by means of yew tea. | [22] |
| 30-year-old female, found dead at home lying in bed. Green plant material in a plastic bag was found near the bed. | A mass of dark green needle-like leaves found in the stomach, similar to those found in the plastic bag—identified as Taxus baccata leaves Acute congestion of the organs, significant pulmonary oedema | 3,5-DMP from Blood Kidney Bile Brain | GC-MS | Cause of death: Yew intoxication Manner of death: Suicide | [149] |
| 40-year-old female, had a video call with a friend, she suddenly developed breathing difficulty. The friend suggested calling an ambulance, the woman was unable to respond, the friend called her mother, who called an ambulance. Rescuers tried resuscitation, but without success. Numerous branches of plant material found at the scene of death, as well found in the stomach at autopsy (as if she had cooked/consumed it). Medical history: during her life, she had a tendency to collect natural herbs and edible plant material from forests. | Numerous partially digested plant leaves in the shape of needles and small twigs found in the stomach contents Significant pulmonary oedema Hepatosplenomegaly Obesity (BMI 38.8 kg/m2) Hepatic steatosis | MAT 1 MAT 2 Taxine B Isotaxine B MHDAT1 MHDAT 2 TAT MHTAT Blood | LC-MS/MS | Cause of death: Acute cardiorespiratory arrest. Manner of death: Not reported | [178] |
| 20-year-old female, brought to the emergency department by a friend. She stated that after reading information on the Internet, she consumed a large amount of yew leaves as a suicide attempt. Symptoms: 4 h after ingestion, nausea, flushing, hypotension, tachycardia, generalized tonic-clonic seizure, cardiac arrest. After resuscitation, restoration of spontaneous circulation, intubation, then arrhythmia. A small amount of green fluid and leaves present in the gastric lavage. Activated charcoal was administered, a transvenous pacemaker was introduced for heart rhythm disturbances, and she was transferred to the coronary unit, subsequently arrhythmias—tachycardia unresponsive to anti-arrhythmics. After consultation with the family, invasive medical measures were stopped and resuscitation ended—death shortly thereafter. Medical history: anxiety, bipolar disorder, obsessive-compulsive disorder, self-harm since age 12. | Not reported | na | na | Cause of death: Not reported Manner of death: Suicide | [179] |
| 35-year-old male, called 911 himself because he felt sick—his head was spinning. When medical help arrived, he was found in the car in the driver’s seat, with slightly impaired consciousness (somnolence), vomited. He stated that he had ingested ground needles mixed into yogurt. He repeatedly passed out on the spot, had apnoeic phases and convulsions, vomited contents with ground green needles, aggressive, resisted examination Transferred to the hospital for emergency admission—impaired consciousness, bradycardia, asystolic pauses, unmeasurable blood pressure and pulse, post-defecation, vomiting, acral cyanosis, pronounced mydriasis, vomiting and full of ground needles in the mouth. Resuscitation started—intubation, inserted nasogastric tube, gastric lavage—brown content with ground needles came out, external cardiostimulation, blood and urine collection for toxicological examination. Despite 185 min of resuscitation on EKG asystole, death was declared. Toxicology results: traces of 3,5-dimethoxyphenol (GC-MS)—a yew metabolite—were proven in the urine sample. In the car, on the back seat 2 grinders containing ground (crushed) green yew needles, on the driver’s seat and in front of the seat on the floor ground (crushed) green plant—yew, in the vehicle two plastic bags filled with green needles and yew twigs. Next to the car, a sweatshirt soiled by a green plant. Medical history: according to his brother, he was hospitalized in psychiatry a year earlier, ID no. without treatment. | A green mushy mass with flat green needles present in the small intestine—approx. 35 g in total a coating of black liquid on the tongue, in the pharynx and in the oesophagus Thin-mushy black matter in the stomach and duodenum Congestion of the gastric mucosa Pronounced mydriasis Significant blood flow to internal organs. Liquid blood Enlarged right atrium and heart chamber Marked swelling of the brain Swelling of the lungs Petechiae under the pleura Slightly underweight (64 kg) Signs after resuscitation Histology: significant swelling of the brain and dystrophic changes of nerve cells, swelling and acute emphysema of the lungs, dispersed acute ischemic changes in the myocardium with signs of acute blood circulation failure, dystrophic changes up to necrosis of individual liver cells; dystrophic changes in kidney tubule cells | Urine: qualitatively 3,5-dimetoxyphenol —a yew metabolite Caffeine Nicotine | GC/MS | Cause of death: Cardiac failure due to acute intoxication with T. baccata Manner of death: Most likely accidental intoxication | Our case I 2022 |
| 24-year-old female, found in the afternoon lying dead in the hallway of the rented apartment, in front of the toilet. A jug filled with coniferous branches, with a cooking pot and a strainer, was found in the kitchen. The previous evening, she wrote an e-mail to her friend with the text “I made mistakes, I love you”. In her notebook, the keywords “Suicide” and “Yew” were found in the history of the Internet browser. | Significant mydriasis (0.7 cm) Congestion of internal organs Swelling of the brain and lungs | Kidney: qualitatively present taxine Tea from the place: qualitative taxine Yew needles: qualitative taxine | TLC | Cause of death: Acute yew intoxication Manner of death: Suicide | Our case II 2022 |
| 39-year-old male, found dead lying in a meadow under the forest. Near the body, a plastic bag containing T. baccata needles. Medical history: psychiatric illness 2 months before his death, he had already consumed yew. | Green needles of T. baccata plant in oesophagus and stomach contents Marked swelling of the lungs Brain swelling Petechiae in the soft coverings of the skull Mydriasis | Stomach content and urine: 3,5- dimetoxyphenol | GC-MS, LC-MS Q-TOF | Cause of death: heart failure due to T. baccata intoxication Manner of death: Suicide | Our case III |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nižnanský, Ľ.; Osinová, D.; Kuruc, R.; Hengerics Szabó, A.; Szórádová, A.; Masár, M.; Nižnanská, Ž. Natural Taxanes: From Plant Composition to Human Pharmacology and Toxicity. Int. J. Mol. Sci. 2022, 23, 15619. https://doi.org/10.3390/ijms232415619
Nižnanský Ľ, Osinová D, Kuruc R, Hengerics Szabó A, Szórádová A, Masár M, Nižnanská Ž. Natural Taxanes: From Plant Composition to Human Pharmacology and Toxicity. International Journal of Molecular Sciences. 2022; 23(24):15619. https://doi.org/10.3390/ijms232415619
Chicago/Turabian StyleNižnanský, Ľuboš, Denisa Osinová, Roman Kuruc, Alexandra Hengerics Szabó, Andrea Szórádová, Marián Masár, and Žofia Nižnanská. 2022. "Natural Taxanes: From Plant Composition to Human Pharmacology and Toxicity" International Journal of Molecular Sciences 23, no. 24: 15619. https://doi.org/10.3390/ijms232415619
APA StyleNižnanský, Ľ., Osinová, D., Kuruc, R., Hengerics Szabó, A., Szórádová, A., Masár, M., & Nižnanská, Ž. (2022). Natural Taxanes: From Plant Composition to Human Pharmacology and Toxicity. International Journal of Molecular Sciences, 23(24), 15619. https://doi.org/10.3390/ijms232415619







