Understanding Carbohydrate Metabolism and Insulin Resistance in Acute Intermittent Porphyria
Abstract
1. Introduction
2. Carbohydrate Metabolism in AIP
3. Role of Insulin in the Heme Synthesis Pathway
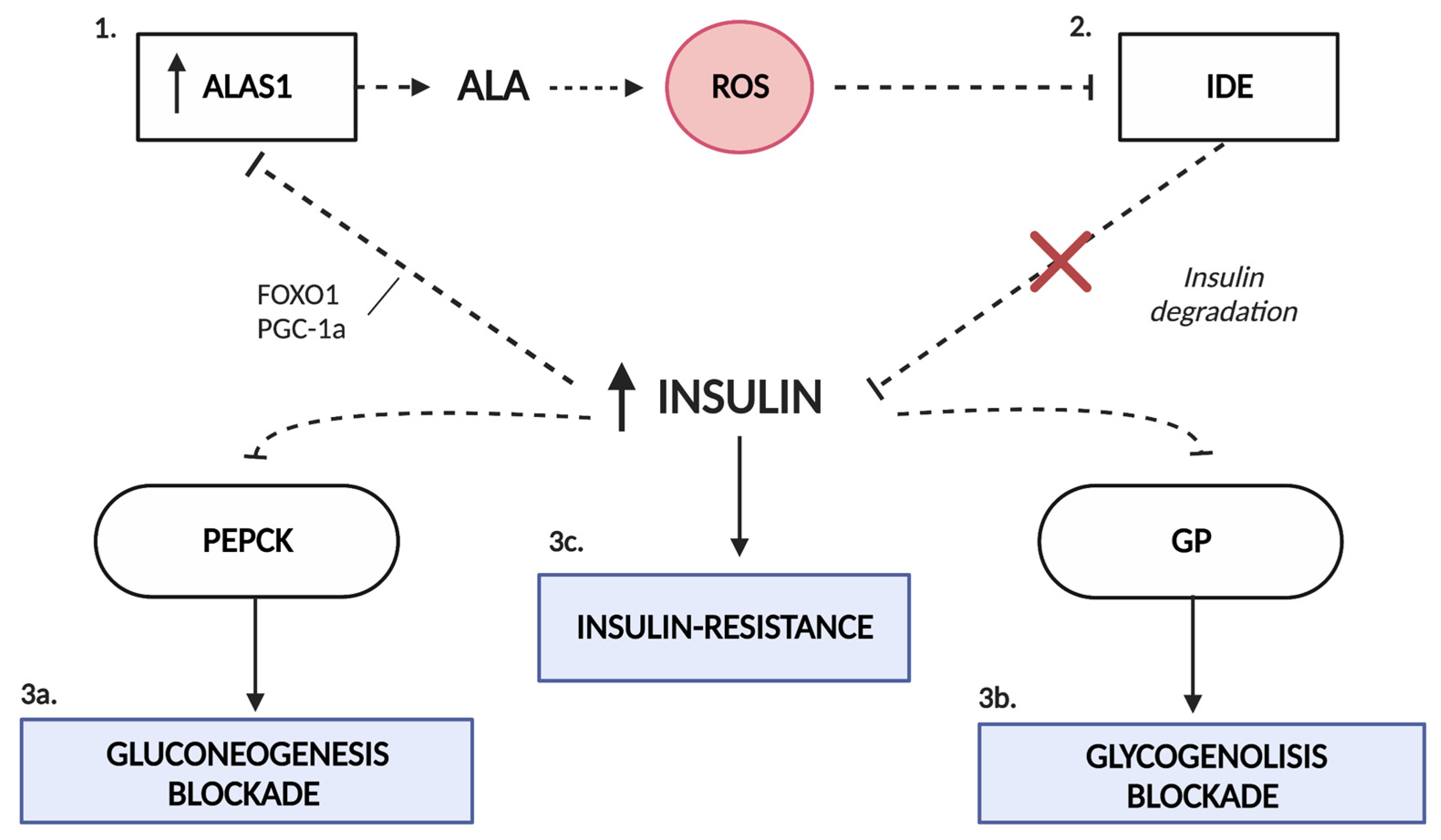
4. Insulin Resistance in AIP
5. Insulin as a Therapeutic Weapon
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anderson, K.; Bloomer, J.R.; Bonkovsky, H.L.; Kushner, J.P.; Pierach, C.A.; Pimstone, N.R.; Desnick, R.J. Recommendations for the Diagnosis and Treatment of the Acute Porphyrias. Ann. Intern. Med. 2005, 142, 439–450, Erratum in: Ann Intern Med. 2005, 143, 316. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.; Badminton, M.; Barth, J.; Rees, D.; Stewart, M.F. Best practice guidelines on clinical management of acute attacks of porphyria and their complications. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2013, 50, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Castelbón Fernández, F.J.; Solares Fernandez, I.; Arranz Canales, E.; Enríquez de Salamanca Lorente, R.; Morales Conejo, M. Protocol for Patients with Suspected Acute Porphyria [published online ahead of print, 2020 Mar 3]. Protocolo de actuación en pacientes con sospecha de porfiria aguda [published online ahead of print, 2020 Mar 3]. Rev. Clin. Esp. 2020, 220, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Desnick, R.J. The porphyrias: Advances in diagnosis and treatment. Blood 2012, 120, 4496–4504, Erratum in: Blood 2013, 122, 3090. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Li, H.; Du, Y.; Han, B. Congenital sideroblastic anemia: Advances in gene mutations and pathophysiology. Gene 2018, 668, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.; Harper, P.; Badminton, M.; Sandberg, S.; Deybach, J.-C. The incidence of inherited porphyrias in Europe. J. Inherit. Metab. Dis. 2012, 36, 849–857. [Google Scholar] [CrossRef]
- Homedan, C.; Laafi, J.; Schmitt, C.; Gueguen, N.; Lefebvre, T.; Karim, Z.; Desquiret-Dumas, V.; Wetterwald, C.; Deybach, J.-C.; Gouya, L.; et al. Acute intermittent porphyria causes hepatic mitochondrial energetic failure in a mouse model. Int. J. Biochem. Cell Biol. 2014, 51, 93–101. [Google Scholar] [CrossRef]
- Gomez-Gomez, A.; Aguilera, P.; Langohr, K.; Casals, G.; Pavon, C.; Marcos, J.; To-Figueras, J.; Pozo, O.J. Evaluation of Metabolic Changes in Acute Intermittent Porphyria Patients by Targeted Metabolomics. Int. J. Mol. Sci. 2022, 23, 3219. [Google Scholar] [CrossRef]
- Delaby, C.; To-Figueras, J.; Deybach, J.C.; Casamitjana, R.; Puy, H.; Herrero, C. Role of two nutritional hepatic markers (insulin-like growth factor 1 and transthyretin) in the clinical assessment and follow-up of acute intermittent porphyria patients. J. Intern. Med. 2009, 266, 277–285. [Google Scholar] [CrossRef]
- Bylesjö, I.; Wikberg, A.; Andersson, C. Clinical aspects of acute intermittent porphyria in northern Sweden: A population-based study. Scand. J. Clin. Lab. Investig. 2009, 69, 612–618. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Alcala-Diaz, J.F.; Delgado-Lista, J.; Garcia-Rios, A.; Gomez-Delgado, F.; Marin-Hinojosa, C.; Rodriguez-Cantalejo, F.; Delgado-Casado, N.; Perez-Caballero, A.I.; Fuentes-Jimenez, F.J.; et al. Metabolic phenotypes of obesity influence triglyceride and inflammation homoeostasis. Eur. J. Clin. Investig. 2014, 44, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.A.; Hellman, E.S.; Tschudy, D.P. Effect of diet on induction of experimental porphyria. Metabolism 1961, 10, 514–521. [Google Scholar]
- Tschudy, D.P.; Welland, F.H.; Collins, A.; Hunter, G.W. The effect of carbohydrate feeding on the induction of δ-aminolevulinic acid synthetase. Metabolism 1964, 13, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Bonkowsky, H.L.; Sinclair, P.R.; Sinclair, J.F. Hepatic heme metabolism and its control. Yale J. Biol. Med. 1979, 52, 13–37. [Google Scholar]
- Welland, F.H.; Hellman, E.S.; Gaddis, E.M.; Collins, A.; Hunter, G.W.; Tschudy, D.P. Factors affecting the excretion of porphyrin precursors by patients with acute intermittent porphyria I. The effect of diet. Metabolism 1964, 13, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Lin, J.; Rhee, J.; Peyer, A.-K.; Chin, S.; Wu, P.-H.; Meyer, U.A.; Spiegelman, B.M. Nutritional Regulation of Hepatic Heme Biosynthesis and Porphyria through PGC-1α. Cell 2005, 122, 505–515. [Google Scholar] [CrossRef]
- Nordlie, R.C.; Foster, J.D.; Lange, A.J. Regulation of glucose production by the liver. Annu. Rev. Nutr. 1999, 19, 379–406. [Google Scholar] [CrossRef]
- Newgard, C.B.; Hwang, P.K.; Fletterick, R.J. The Family of Glycogen Phosphorylases: Structure and Functio. Crit. Rev. Biochem. Mol. Biol. 1989, 24, 69–99. [Google Scholar] [CrossRef]
- Agius, L. Role of glycogen phosphorylase in liver glycogen metabolism. Mol. Asp. Med. 2015, 46, 34–45. [Google Scholar] [CrossRef]
- She, P.; Shiota, M.; Shelton, K.D.; Chalkley, R.; Postic, C.; Magnuson, M.A. Phosphoenolpyruvate Carboxykinase Is Necessary for the Integration of Hepatic Energy Metabolism. Mol. Cell. Biol. 2000, 20, 6508–6517. [Google Scholar] [CrossRef]
- Hanson, R.W.; Reshef, L. Regulation of phosphoenolpyruvate carboxykinase (gtp) gene expression. Annu. Rev. Biochem. 1997, 66, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Cimbala, M.A.; Lamers, W.H.; Nelson, K.; Monahan, J.E.; Yoo-Warren, H.; Hanson, R.W. Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney. Effects of insulin and cyclic AMP. J. Biol. Chem. 1982, 257, 7629–7636. [Google Scholar] [CrossRef] [PubMed]
- Lelli, S.M.; De Viale, L.C.S.M.; Mazzetti, M.B. Response of glucose metabolism enzymes in an acute porphyria model: Role of reactive oxygen species. Toxicology 2005, 216, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Collantes, M.; Serrano-Mendioroz, I.; Benito, M.; Molinet-Dronda, F.; Delgado, M.; Vinaixa, M.; Sampedro, A.; de Salamanca, R.E.; Prieto, E.; Pozo, M.A.; et al. Glucose metabolism during fasting is altered in experimental porphobilinogen deaminase deficiency. Hum. Mol. Genet. 2016, 25, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.W.; Stephens, J.K.; Marks, G.S. Effect of varying the insulin to glucagon ratio on porphyrin biosynthesis in chick embryo liver cells. Mol. Pharmacol. 1978, 14, 717–721. [Google Scholar]
- Marks, G.S.; Stephens, J.K.; Fischer, P.W.F.; Morgan, R.O. Hormonal effects on the regulation of hepatic heme biosynthesis. Mol. Cell. Biochem. 1979, 25, 111–123. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC-1α): Transcriptional Coactivator and Metabolic Regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef]
- Chen, B.; Wang, M.; Gan, L.; Zhang, B.; Desnick, R.J.; Yasuda, M. Characterization of the hepatic transcriptome following phenobarbital induction in mice with AIP. Mol. Genet. Metab. 2019, 128, 382–390. [Google Scholar] [CrossRef]
- Scassa, M.E.; Guberman, A.S.; Varone, C.L.; Cánepa, E.T. Phosphatidylinositol 3-Kinase and Ras/Mitogen-Activated Protein Kinase Signaling Pathways Are Required for the Regulation of 5-Aminolevulinate Synthase Gene Expression by Insulin. Exp. Cell Res. 2001, 271, 201–213. [Google Scholar] [CrossRef]
- Storjord, E.; Dahl, A.J.; Landsem, A.; Fure, H.; Ludviksen, J.K.; Goldbeck-Wood, S.; Karlsen, O.B.; Berg, K.S.; Mollnes, E.T.; Nielsen, E.W.; et al. Systemic inflammation in acute intermittent porphyria: A case–control study. Clin. Exp. Immunol. 2016, 187, 466–479. [Google Scholar] [CrossRef][Green Version]
- Storjord, E.; Dahl, J.A.; Landsem, A.; Ludviksen, J.K.; Karlsen, M.B.; Karlsen, B.O.; Brekke, O.-L. Lifestyle factors including diet and biochemical biomarkers in acute intermittent porphyria: Results from a case-control study in northern Norway. Mol. Genet. Metab. 2018, 128, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Hedger, R.W.; Wehrmacher, W.H.; French, A.V. Porphyria syndrome associated with diabetic nephrosclerosis and erythropoietin. Compr. Ther. 2006, 32, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sixel-Dietrich, F.; Verspohl, F.; Doss, M. Hyperinsulinemia in Acute Intermittent Porphyria. Horm. Metab. Res. 1985, 17, 375–376. [Google Scholar] [CrossRef]
- Yalouris, A.G.; A Raptis, S. Effect of diabetes on porphyric attacks. BMJ 1987, 295, 1237–1238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andersson, C.; Bylesjo, I.; Lithner, F. Effects of diabetes mellitus on patients with acute intermittent porphyria. J. Intern. Med. 1999, 245, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Lithner, F. Diabetisk metabolism skydd vid svår akut intermittent porfyri [Diabetic metabolism protective in severe acute intermittent porphyria]. Lakartidningen 2001, 98, 5874–5876. [Google Scholar] [PubMed]
- Bitar, M.; Weiner, M. Diabetes-induced Metabolic Alterations in Heme Synthesis and Degradation and Various Heme-containing Enzymes in Female Rats. Diabetes 1984, 33, 37–44. [Google Scholar] [CrossRef]
- Storjord, E.; Airila-Månsson, S.; Karlsen, K.; Madsen, M.; Dahl, J.A.; Landsem, A.; Fure, H.; Ludviksen, J.K.; Fjøse, J.; Dickey, A.K.; et al. Dental and Periodontal Health in Acute Intermittent Porphyria. Life 2022, 12, 1270. [Google Scholar] [CrossRef]
- Solares, I.; Izquierdo-Sánchez, L.; Morales-Conejo, M.; Jericó, D.; Castelbón, F.; Córdoba, K.; Sampedro, A.; Lumbreras, C.; Moreno-Aliaga, M.; de Salamanca, R.E.; et al. High Prevalence of Insulin Resistance in Asymptomatic Patients with Acute Intermittent Porphyria and Liver-Targeted Insulin as a Novel Therapeutic Approach. Biomedicines 2021, 9, 255. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.-Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia Drives Diet-Induced Obesity Independently of Brain Insulin Production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Dankner, R.; Chetrit, A.; Shanik, M.H.; Raz, I.; Roth, J. Basal-State Hyperinsulinemia in Healthy Normoglycemic Adults Is Predictive of Type 2 Diabetes Over a 24-Year Follow-Up: A preliminary report. Diabetes Care 2009, 32, 1464–1466. [Google Scholar] [CrossRef] [PubMed]
- Morita, I.; Tanimoto, K.; Akiyama, N.; Naya, N.; Fujieda, K.; Iwasaki, T.; Yukioka, H. Chronic hyperinsulinemia contributes to insulin resistance under dietary restriction in association with altered lipid metabolism in Zucker diabetic fatty rats. Am. J. Physiol. Metab. 2017, 312, E264–E272. [Google Scholar] [CrossRef] [PubMed]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin Resistance and Hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31 (Suppl. 2), S262–S268. [Google Scholar] [CrossRef]
- Matkovic, L.B.; D’Andrea, F.; Fornes, D.; de Viale, L.C.S.M.; Mazzetti, M.B. How porphyrinogenic drugs modeling acute porphyria impair the hormonal status that regulates glucose metabolism. Their relevance in the onset of this disease. Toxicology 2011, 290, 22–30. [Google Scholar] [CrossRef]
- Monteiro, H.P.; Abdalla, D.S.; Augusto, O.; Bechara, E.J. Free radical generation during δ-Aminolevulinic acid autoxidation: Induction by hemoglobin and connections with porphyrinpathies. Arch. Biochem. Biophys. 1989, 271, 206–216. [Google Scholar] [CrossRef]
- A Stein, J.; Tschudy, D.P. Acute intermittent porphyria. A clinical and biochemical study of 46 patients. Medicine 1970, 49, 1–16. [Google Scholar] [CrossRef]
- Oliveri, L.M.; Davio, C.; Batlle, A.M.D.C.; Gerez, E.N. ALAS1 gene expression is down-regulated by Akt-mediated phosphorylation and nuclear exclusion of FOXO1 by vanadate in diabetic mice. Biochem. J. 2012, 442, 303–310. [Google Scholar] [CrossRef]
- Rajpal, G.; Liu, M.; Zhang, Y.; Arvan, P. Single-Chain Insulins as Receptor Agonists. Mol. Endocrinol. 2009, 23, 679–688. [Google Scholar] [CrossRef]
- Drew, B.G.; Rye, K.-A.; Duffy, S.J.; Barter, P.; Kingwell, B.A. The emerging role of HDL in glucose metabolism. Nat. Rev. Endocrinol. 2012, 8, 237–245. [Google Scholar] [CrossRef]
- Kim, S.I.; Shin, D.; Choi, T.H.; Lee, J.C.; Cheon, G.-J.; Kim, K.-Y.; Park, M.; Kim, M. Systemic and Specific Delivery of Small Interfering RNAs to the Liver Mediated by Apolipoprotein A-I. Mol. Ther. 2007, 15, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.J.; Sun, Y.; Ong, K.L.; Li, Y.; Tang, S.; Barter, P.J.; Rye, K.-A. Apolipoprotein A-I Protects Against Pregnancy-Induced Insulin Resistance in Rats. Arter. Thromb. Vasc. Biol. 2019, 39, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Paolini, E.; Meroni, M.; Duca, L.; Motta, I.; Fracanzani, A.L.; Di Pierro, E.; Dongiovanni, P. α-Lipoic Acid Improves Hepatic Metabolic Dysfunctions in Acute Intermittent Porphyria: A Proof-of-Concept Study. Diagnostics 2021, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Kwon, Y.; Yea, K.; Moon, H.-Y.; Yoon, J.H.; Ghim, J.; Hyun, H.; Kim, D.; Koh, A.; Berggren, P.-O.; et al. Apolipoprotein a1 increases mitochondrial biogenesis through AMP-activated protein kinase. Cell. Signal. 2015, 27, 1873–1881. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Tanaka, T.; Sugiyama, M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition 2013, 29, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.L.; Rodriguez, B.L.; Rodriguez, B.L.; Curb, J.D.; Curb, J.D.; Curb, J.D.; Davis, J.; Davis, J.; Davis, J.; Shintani, T.; et al. Use of the Dietary Supplement 5-Aminiolevulinic Acid (5-ALA) and Its Relationship with Glucose Levels and Hemoglobin A1C among Individuals with Prediabetes. Clin. Transl. Sci. 2012, 5, 314–320. [Google Scholar] [CrossRef]
- Saitoh, S.; Okano, S.; Nohara, H.; Nakano, H.; Shirasawa, N.; Naito, A.; Yamamoto, M.; Kelly, V.P.; Takahashi, K.; Tanaka, T.; et al. 5-aminolevulinic acid (ALA) deficiency causes impaired glucose tolerance and insulin resistance coincident with an attenuation of mitochondrial function in aged mice. PLoS ONE 2018, 13, e0189593. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solares, I.; Jericó, D.; Córdoba, K.M.; Morales-Conejo, M.; Ena, J.; Enríquez de Salamanca, R.; Fontanellas, A. Understanding Carbohydrate Metabolism and Insulin Resistance in Acute Intermittent Porphyria. Int. J. Mol. Sci. 2023, 24, 51. https://doi.org/10.3390/ijms24010051
Solares I, Jericó D, Córdoba KM, Morales-Conejo M, Ena J, Enríquez de Salamanca R, Fontanellas A. Understanding Carbohydrate Metabolism and Insulin Resistance in Acute Intermittent Porphyria. International Journal of Molecular Sciences. 2023; 24(1):51. https://doi.org/10.3390/ijms24010051
Chicago/Turabian StyleSolares, Isabel, Daniel Jericó, Karol M. Córdoba, Montserrat Morales-Conejo, Javier Ena, Rafael Enríquez de Salamanca, and Antonio Fontanellas. 2023. "Understanding Carbohydrate Metabolism and Insulin Resistance in Acute Intermittent Porphyria" International Journal of Molecular Sciences 24, no. 1: 51. https://doi.org/10.3390/ijms24010051
APA StyleSolares, I., Jericó, D., Córdoba, K. M., Morales-Conejo, M., Ena, J., Enríquez de Salamanca, R., & Fontanellas, A. (2023). Understanding Carbohydrate Metabolism and Insulin Resistance in Acute Intermittent Porphyria. International Journal of Molecular Sciences, 24(1), 51. https://doi.org/10.3390/ijms24010051







