Abstract
β-Enaminonitriles bearing 9-hydroxy-1H-benzo[f]chromene moiety was synthesized. The targeted compounds were evaluated for their anti-proliferative activity against three human tumor cell lines, PC-3, SKOV-3 and HeLa, and the active cytotoxic compounds were further evaluated against cancer cells, MCF-7/ADR, and two normal cell lines, HFL-1 and WI-38. Few compounds were assigned to be the most potent derivatives against PC-3, SKOV-3 and HeLa cell lines in comparison with Vinblastine and Doxorubicin. Several compounds possessed a relatively good potency against MCF-7/ADR cells as compared with Doxorubicin and were tested as a P-gp inhibitor. Moreover, the halogenated substituents, 2,4-F2, 2,3-Cl2, 2,5-Cl2 and 3,4-Cl2; have good potency against P-gp-mediated MDR in MCF-7/ADR as compared with Doxorubicin. Meanwhile, Rho123 accumulation assays revealed that few compounds effectively inhibited P-pg and efflux function. In addition, certain derivatives induced apoptosis and an accumulation of the treated MCF-7/ADR cells in the G1, S and G1/S phases.
1. Introduction
Among the most significant heterocyclics are the substituted chromenes and benzochromenes, owing to their antimicrobial [1,2,3,4,5], antiviral, anti-HIV, antileishmanial, anti-anaphylactic [6,7,8], anticancer [9,10,11,12,13,14,15,16], anti-inflammatory [17,18] and antioxidant [19] applications. β-enaminonitriles containing 4H-benzo[h]chromene systems have been used in cancer treatment approaches; therefore, they are described as prospective cancer pharmaceuticals’ targets. Examples include 2-amino/acetylamino substituents of 6-chloro/methoxy-4H-benzo[h]chromenes that exhibited cytotoxic effects against MCF-7, HCT-116 and HepG-2 [9,20,21], as well as 2-amino and 2,7-diamino derivatives that showed anti-proliferative and c-Src kinase inhibitory effects [14,22]. Additionally, 1H-benzo[f]chromene derivatives are advantageous for the creation of strong anticancer agents. For instance, 1H-benzo[f]chromenes with 8-bromo/methoxy and 1H-tetrazol-5-yl substituents block c-Src kinase inhibitory activity with anticancer characteristics [23,24]. Apoptosis and cell cycle arrest in human cancer cells are brought on by the 8/9-bromo substituents of 1H-benzo[f]chromenes, which inhibit topoisomerase I and II [9,10]. The 3-amino substituent of 1H-benzo[f]chromenes also exhibits anti-proliferative properties, and DNA binding also functions as a Bcl-2 protein inhibitor [25]. Meanwhile, fused benzochromenes are also valuable as effective anticancer agents; for instance, the 9-amino/benzylideneamino and 8-amino/imino derivatives of benzochromenopyrimidine and benzochromenopyrimidin-8-one, respectively, are found to be effective against caspase 3/7 activators and targets for cell cycle study [5,11]. Furthermore, the cell cycle was arrested in the 9-amino/methyl derivative of 7-(2,4/3,4-dimethoxyphenyl)benzochromenopyrimidine, responsible for apoptosis, caspase 3/7 activation, DNA breakage, cell invasion and migration [13,14].
Overexpression of ABC transporters such as P-glycoprotein (P-gp), multidrug resistance protein 1 (MDR1) and ATP-binding cassette subfamily B member 1 (ABCB1) is the main contributor to MDR [26,27]. On the other hand, MDR is contributing to chemotherapy failure [28]. The multidrug-resistant phenotype in cancer that mediates MDR also heavily relies on P-glycoprotein (P-gp/ABCB1) [29].
Based on our ongoing experiences to create effective anticancer benzochromene-based medicines, [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48], we have initiated the current study. Developing drugs with potential anti-proliferative activity against three cancer cell lines, PC-3, SKOV-3 and HeLa, while having no cytotoxic effect on normal cell lines HFL-1 and WI-38, was the main motivation behind our decision to design and synthesize β-enaminonitriles incorporating a 9-hydroxy-1H-benzo[f]chromene moiety. An additional objective is to target MCF-7/ADR cells; the active 9-hydroxy-1H-benzo[f]chromene derivatives were investigated as P-gp inhibitors utilizing various assays, including Western blot and Rho123 accumulation. The 9-hydroxy-1H-benzo[f]chromene derivatives 4i, 4e, 4d, 4a, 4g and 4c, respectively, have good potency against MCF-7/ADR cells based on screening results. Additionally, Rho123 accumulation experiments revealed that 4a, 4c and 4e efficiently inhibited P-pg and efflux function (Figure 1).
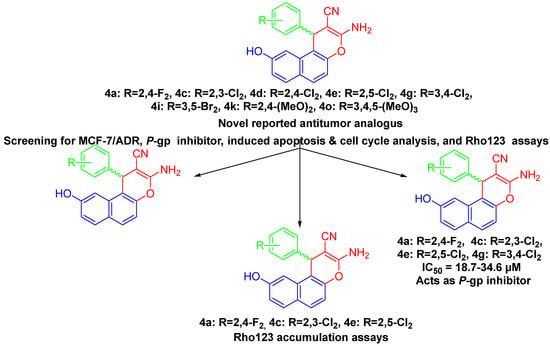
Figure 1.
Study of antitumor activities, MCF-7/ADR, P-gp inhibitor, apoptosis, cell cycle analysis, and Rh123 results.
These compounds were found to be effective against P-gp inhibitors. Additionally, compounds 4d and 4e caused apoptosis and an increase in MCF-7/ADR cells in the G1 phase, whereas compounds 4a, 4c, 4g and 4i did so in the S phase and G1/S phases, respectively (Figure 1). The type of substituents at the 9-position and the aryl group at the 1-position appears to be critical to the cytotoxic action; therefore, this was well thought out during the rational design of the 9-hydroxy-1H-benzo[f]chromene derivatives. Figure 2 compares the behaviors of the recently synthesized derivatives linked to a hydroxyl group at position 9 with those previously synthesized with unsubstituted naphthalene moiety: in first generation 1 and 2 [49] is a substituted naphthalene moiety linked to a bromine at position 9, second generation is 3–7 [10] and third generation with a methoxy group at position 9 is 8–12 [50]. The comparison revealed that the newly synthesized derivatives 4a, 4c, 4d, 4e, 4g, 4i, 4k and 4o significantly increased or paralleled the potency of the previously synthesized compounds 1–12 when used against cancer cell lines [10,49,50].
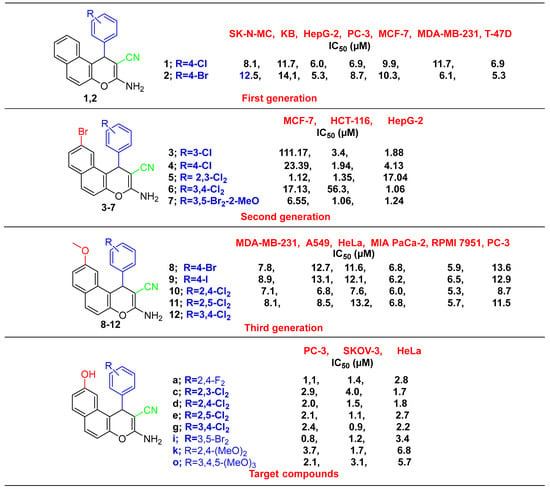
Figure 2.
Rationale for designing target benzochromene derivatives and previous studies compounds.
2. Results and Discussion
2.1. Chemistry
Ethanolic solutions of compounds (3-amino-1-aryl-9-hydroxy-1H-benzo[f]chromene-2-carbonitrile, 4a–p) were created by heating naphthalene-2,7-diol (1), malononitrile (2) and aromatic aldehydes (3a–p), and piperidine was kept under microwave irradiation for two minutes at 140 °C (Scheme 1). The best results were obtained using 400 W with a 2 min reaction period to produce the largest yield of compound 4. This reaction was repeated at different watt powers (200, 300, 400 W) and time intervals (1, 1.5, 2 min) while being monitored using TLC. For the synthesis of 4a–p, the optimum microwave irradiation conditions were mentioned in Table S1: Supplementary Materials.
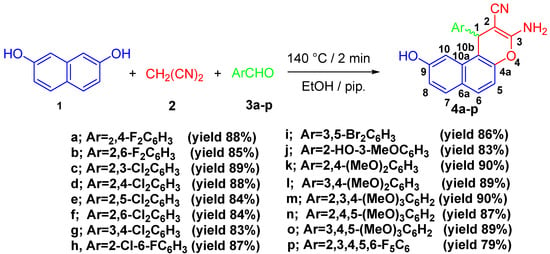
Scheme 1.
Microwave irradiation synthesis of 3-amino-1-aryl-9-hydroxy-1H-benzo[f]chromene-2-carbonitrile (4a–p).
2.2. Optical Activities
A Carl Zeiss polarimeter (Jena, Germany.) was used to gauge the target compound 4 which found “optical inactive” in the form of a racemic (±) mixture [51], refer to Scheme 1.
2.3. Spectroscopic Data
The structures and purities of the synthesized compounds 4a–p were confirmed by spectral analysis, i.e., IR, 1H NMR, 13C NMR and MS spectra. The IR spectra of 4a–p revealed the presence of peaks around υ 3490–3423, 3445–3408, 3353–3222, 3267–3177 cm −1 assigned to the amino and hydroxyl groups and for the CN groups in the region υ 2214–2175 cm.−1 The 1H NMR spectra of 4a–p showed the singlet signal of the OH, NH2 and CH protons around δ 10.77–9.82, 7.27–6.41 and δ 5.97–4.96 ppm, respectively. Furthermore, the 13 C NMR spectra of 4a–p showed the signals resonating at δ 38.47–26.51 ppm, attributable to the CH carbons. In addition, the MS spectra and the elemental analyses of 4a–p delivered absolute confirmation for their structures (see Supplementary Materials of the spectral data from Figure S1 to S55).
2.4. Biological Evaluation
2.4.1. In Vitro Cytotoxic Activity
Three human cancer cell lines, PC-3 (human prostate adenocarcinoma, metastatic), SKOV-3 (ovarian cancer cell line) and HeLa (cervical cancer cell line), were used to test the anti-proliferative activity of β-enaminonitrile (4a–p) using the MTT colorimetric assay [52] at concentrations ranging from 0–100 μM. The representative active cytotoxic substances 4a, 4c, 4d, 4e, 4g, 4i, 4k and 4o were also tested against two normal cell lines, human fetal lung (HFL-1) and human diploid fibroblasts (WI-38), as well as Adriamycin (ADR)-resistant human breast cancer cells (MCF-7/ADR). The reference cytotoxic agents, Vinblastine and Doxorubicin, were used in the tests. The results were expressed as growth inhibitory concentration (IC50 μM) values, which reveal the concentrations necessary for 50% inhibition of cell growth after 24 h of incubation compared to the untreated ones (Figure 3 and Figure 4 and Table 1).
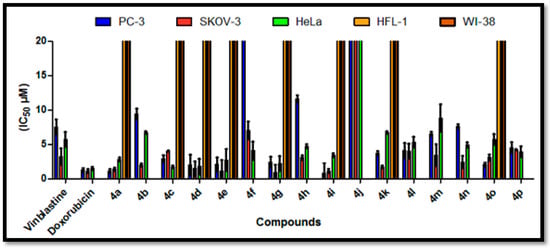
Figure 3.
IC50 values are expressed in (μM) of the target compounds 4a–p against PC-3, SKOV-3 and HeLa tumor cells and HFL-1 and WI-38 normal cell lines.
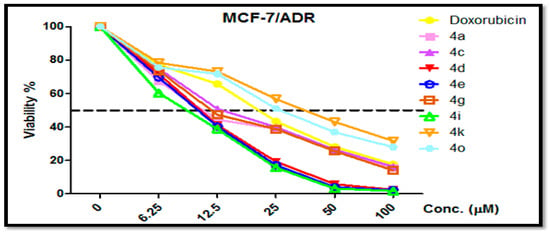
Figure 4.
Dose dependent cytotoxicity in MCF-7/ADR cells and the effect of varying concentrations of tested compounds 4a, 4c, 4d, 4e, 4g, 4i, 4k and 4o on cell growth of MCF-7/ADR cells following exposure for 24 h.

Table 1.
IC50 values of the target compounds 4a–p against PC-3, SKOV-3 and HeLa and the active compounds 4a, 4c, 4d, 4e, 4g, 4i, 4k and 4o against MCF-7/ADR, HFL-1 and WI-38 cell lines.
Table 1 indicated that the target compounds displayed excellent to modest anti-proliferative activity against PC-3, SKOV-3 and HeLa cell lines. In particular, compounds 4i and 4a (IC50 = 0.8 ± 0.1 and 1.1 ± 0.4 μM) exhibited the most potent counterparts against PC-3 cells as they were 9.4 and 6.8 times more active than Vinblastine (IC50 = 7.5 ± 1.3 μM) and 1.6 and 1.2 times more active than Doxorubicin (IC50 = 1.3 ± 0.3 μM), while compounds 4d, 4e, 4o, 4g, 4c, 4k, 4l, 4p and 4m with IC50 ranging from 2.0 to 6.5 μM had excellent anti-proliferative activities against PC-3 cells as compared to Vinblastine (IC50 = 7.5 ± 1.3 μM).
In addition, compounds 4g, 4e and 4i have excellent anti-proliferative activities against SKOV-3 cells (IC50 = 0.9 ± 0.3, 1.1 ± 0.4 and 1.1 ± 0.1 μM, respectively), which are better than the used reference drugs or equal, Vinblastine (IC50 = 3.2 ± 1.2 μM) and Doxorubicin (IC50 = 1.1 ± 0.2 μM), while compounds 4a, 4d, 4k, 4b, 4n, 4h and 4o with IC50 ranging from 1.4 to 3.1 μM showed anti-proliferative activity that was better than Vinblastine (IC50 = 3.2 ± 1.2 μM). Several compounds showed anti-proliferative activity that was less than Vinblastine (IC50 = 5.7 ± 1.1 μM), such as 4c, 4d, 4g, 4e, 4a, 4i, 4p, 4f, 4h, 4n and 4l with IC50 values ranging from 1.7–5.3 μM against HeLa cells. Additionally, compounds 4a, 4c, 4d, 4e, 4g, 4i, 4k and 4o were weakly active against two normal cell lines, HFL-1 and WI-38, with an IC50 ranging from 20.4 to 39.1 μM. Furthermore, compounds 4i, 4e, 4d, 4a, 4g and 4c possessed good potency against MCF-7/ADR cells with IC50 = 8.6 ± 0.7, 10.0 ± 0.3, 10.4 ± 0.3, 10.8 ± 0.4, 11.5 ± 0.4 and 12.4 μM, respectively, as compared with Doxorubicin (IC50 = 18.6 ± 0.3 μM), while compounds 4o and 4k were inactive against MCF-7/ADR cells with IC50 = 23.7 ± 0.4 and 36.8 ± 0.32. Finally, the other compounds had moderate-to-fair cytotoxic activities. In the near future, we will assess this in an in vivo model using MDA-MB-231 xenografts grown on the chorioallantoic membrane of fertilized chick eggs (CAM). Additionally, we will then study the drug likeness for the benzochromene derivatives.
2.4.2. P-Glycoprotein Expression and Function in MCF-7/ADR Cell Lysate
P-glycoprotein (P-gp)-mediated multidrug resistance (MDR) is a phenomenon in which cells become resistant to structurally and mechanistically unrelated drugs, resulting in low intracellular drug concentrations. It is one of the noteworthy problems in malignant tumor clinical therapeutics [53]. The most active compounds 4a, 4c–4e, 4g and 4i against MCF-7/ADR cells were tested as P-gp inhibitors using commercial ELISA kits (Human permeability glycoprotein ELISA Kit). The inhibition of P-gp content in MCF-7/ADR cell lysate using different compounds conc. as shown in Figure 5 and Table 2.
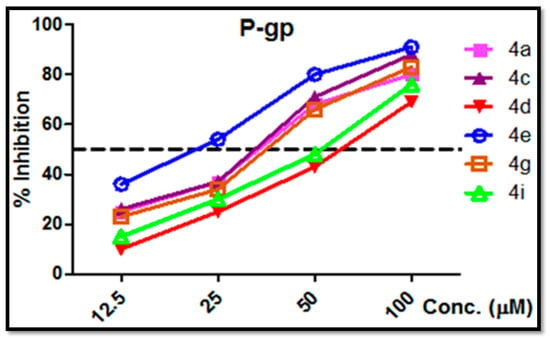
Figure 5.
Inhibition of P-gp content in the lysate of MCF-7/ADR cells uses varying conc. (12.5–100 µM) of tested compounds 4a, 4c, 4d, 4e, 4g and 4i following an exposure of 48 h as determined by ELISA.

Table 2.
IC50 values of the active compounds 4a, 4c–4e, 4g and 4i against P-gp content in MCF-7/ADR cells.
Table 2 indicated that compounds 4a, 4c, 4e and 4g that have 2,4-F2, 2,3-Cl2, 2,5-Cl2 and 3,4-Cl2 substituents have good potency against P-gp in MCF-7/ADR with IC50 ranging from 18.7 to 34.6 μM as compared with Doxorubicin (IC50 = 50.9 μM), as shown in Figure 5 and Table 2.
Despite having a high cytotoxic effect on MCF-7/ADR cells, chemicals 4d and 4i had an almost negligible inhibitory effect on the P-gp level (Figure 5); however, only 4a, 4c and 4e had the strong inhibitory effect needed to be highly effective in reversing the MDR in MCF-7/ADR in earlier studies [54,55]. Furthermore, the reversion of P-gp-mediated multidrug resistance could be achieved by the down-regulation of P-gp expression and/or P-gp efflux function inhibition [56]. Hence, the effect of the current synthesized drugs was tested for inhibitory potential of P-glycoprotein activity using a Rhodamine 123 Accumulation Assay (Rhodamine Competitive ELISA Kit).
The information presented in Table 3 shows that Rhodamine 123 has a (IC50) range of 14.6–28.7 µM for evaluating the P-gp functional inhibition of 4a, 4c and 4e in comparison with 14.3 µM referenced medication “Verapamil”. The compounds 4a, 4c and 4e reduced P-gp expression and its function as well, which had an impact on the recovery of sensitivity to MCF-7/ADR cells. Contrarily, the cytotoxic effect of 4d, 4g and 4i against MDR could be attributed to genetics, growth hormones and/or improved DNA repair capability [57].

Table 3.
P-gp inhibitory potential (IC50 values) based on P-gp content in MCF-7/ADR cell lysate and Rhodamine 123 accumulation assay.
2.4.3. Cell Cycle Arrest in Treated MCF-7/ADR Cancer Cells
Cancer cells undergo unscheduled cell divisions by the down-regulation of the four cell cycle stages (G1, S, G2 and M); therefore, the development of anticancer agents targeting cell cycle arrest represents an important therapeutic intervention [58,59]. P-gp breast cancer resistance proteins (BCRP) inhibit the action of anticancer drugs on cell cycle arrest by preventing intercellular drug accumulation [60]. By using flow cytometry and the FACS Calibers, it was possible to examine how the potent-produced compounds (4a, 4c, 4e, 4g and 4i) affected the regulation of cell cycle progression in MCF-7/ADR cells (Becton Dickinson). The distribution of cells along the G1 (2n), G2/M (4n) and S (2n to 4n) phases of the cycle was exhibited in the representative cell cycle distribution histogram of the stained DNA (Figure 6a). The MCF-7/ADR cancer cells were remedied with each derivative at its IC50 values for 24 h, a controlled experiment with no treatment given with ” blank trials”. The outcomes of the cell cycle progression give three different results showing compounds with cell cyclic arrest at the G1 phase (4d and 4e), while there were other compounds at the S phase (4a and 4c) and a final compound with cell cycle arrest at both of the G1/S phases (4g and 4i). Additionally, in comparison to the untreated controlled cells, these results were followed by a significant drop in percentage at the G2/M stages (Figure 6b). According to the cell cycle analysis, the tested derivatives considerably stopped the MCF-7/ADR cancer cells in the G1/S phase.
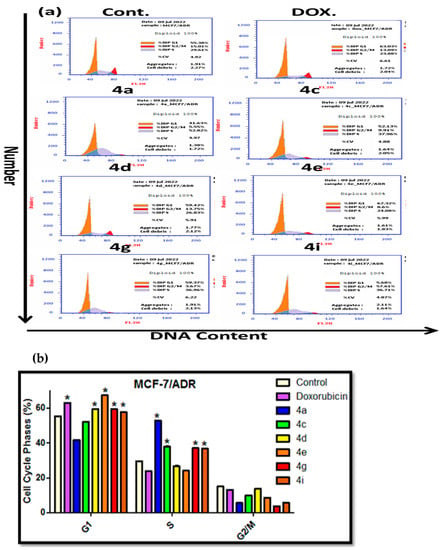
Figure 6.
Effects of compounds 4a, 4c–4e, 4g and 4i on the cell cycle phases of MCF-7/ADR cells. (a) Representative histograms of the DNA content distribution of cells were incubated with IC50 values for 24 h and stained with propidium iodide (PI). Their DNA content was analyzed using fluorescence flow cytometry. (b) The percentage of MCF-7/ADR cells in the G1, S and G2/M phases after incubation with tested compounds 4a, 4c–4e, 4g and 4i (IC50 value) for 24 h. The data are expressed as the mean ± SD of three independent experiments in triplicate. Significances are shown in comparison to control cells (* p < 0.001).
2.4.4. Apoptosis Induction in MCF-7/ADR Cancer Cells
One of the optional mechanisms regarding the lethal action of chemotherapeutic medicines is blocking cell cycle progression, triggering apoptosis with otherwise mutual effect [61]. Moreover, P-gp prevents the release of cytochrome c, which is controlled by the intrinsic mitochondrial pathway, hence inhibiting apoptosis [62]. The pivotal relation of the newly synthesized MCF-7/ADR “anticancer drugs” was further evaluated using the Annexin V/PI double staining flow cytometric test [63]. Figure 7a shows specimen dot plots of the double-stained MCF-7/ADR cells following treatment with the various substances under investigation. In comparison to Doxorubicin’s 30% total apoptosis rate, which was nearly identical to 4a, 4c and 4g, compounds 4d, 4e and 4i were leading with percentages of 41%, 31% and 38%, respectively. Only compounds 4a (8.5%) and 4g (11%) had greater effects on necrosis compared to doxorubicin (5%). Other examined substances caused both early and late apoptosis in all treated cells (Figure 7b). Therefore, MCF-7/ADR cytotoxicity is induced by apoptotic processes, apparently without negative effects on P-gp.
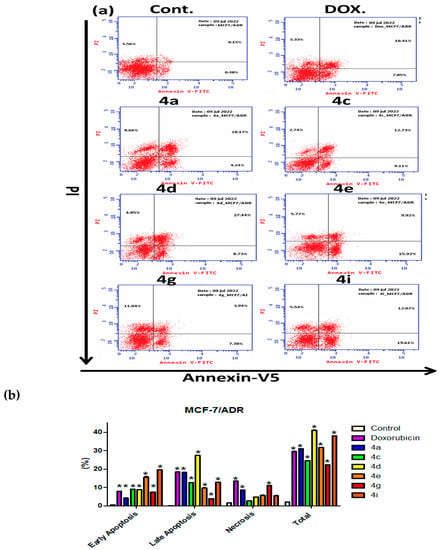
Figure 7.
The apoptosis percentage of MCF-7/ADR cells after incubation with tested compounds 4a, 4c–4e, 4g and 4i (IC50 value) for 24 h. (a) The dot plot of the Annexin V/PI-stained cells, treated with the indicated drugs. (b) The apoptosis percentage of MCF-7/ADR cells after incubation with tested compounds (IC50 value) for 24 h. The data are expressed as the mean ± SD of three independent experiments in triplicate. Significances are shown in comparison to control cells (* p < 0.001).
2.4.5. Structure Activity Relationship (SAR)
In a comparison of the cytotoxic activities of the two series, the disubstituents 4a–l and the polysubstituents 4m–p on the aryl groups linked to the 9-hydroxy-1H-benzo[f]-chromene scaffold at 1-postion against PC-3 cells; the activities of the first series were decreased in the order of 3,5-Br2 > 2,4-F2 > 2,4-Cl2 > 2,5-Cl2 > 3,4-Cl2 > 2,3-Cl2 > 2,4-(MeO)2 > 3,4-(MeO)2 > 2,6-F2 > 2,6-ClF > 2,6-Cl2 > 2,3-HO,MeO with IC50 ranging from 0.8 to 33.5 μM, and for the second series in the order of 3,4,5-(MeO)3 > C6F5 > 2,3,4-(MeO)3 > 2,4,5-(MeO)3 with IC50 ranging from 2.1 to 7.6 μM, implying that grafting a lipophilic bulky electron-withdrawing substituent such as halogens is more beneficial than a non-bulky electron-donating substituent such as methoxy for the activity. Furthermore, exploration of the impact of the substitution on the 1-position of the pendant phenyl group showed that the order of anti-proliferative activities of the disubstituents 4a–l against SKOV-3 cells were decreased in the order of 3,4-Cl2 > 2,5-Cl2 > 3,5-Br2 > 2,4-F2 > 2,4-Cl2 > 2,4-(MeO)2 > 2,6-F2 > 2,6-ClF > 2,3-Cl2 > 3,4-(MeO)2 > 2,6-Cl2 > 2,3-HO,MeO with IC50 ranging from 0.9 to 97.4 μM, and for the second series in the order of 2,4,5-(MeO)3 > 3,4,5-(MeO)3 > 2,3,4-(MeO)3 > C6F5 with IC50 ranging from 2.4 to 4.2 μM, hinting that grafting a lipophilic bulky electron-withdrawing substituent such as halogens has greatly enhanced the activity. Concerning the effect of the two series, the disubstituents 4a–l and the polysubstituents 4m–p against HeLa cells, the activities were decreased in the order of 2,3-Cl2 > 2,4-Cl2 > 3,4-Cl2 > 2,5-Cl2 > 2,4-F2 > 3,5-Br2 > 2,6-Cl2 > 2,6-ClF > 3,4-(MeO)2 > 2,4-(MeO)2 > 2,6-F2 > 2,3-HO,MeO with IC50 ranging from 1.7 to 50.4 μM, and for the second series the order of anti-proliferative activities was C6F5 > 2,4,5-(MeO)3 > 3,4,5-(MeO)3 > 2,3,4-(MeO)3 with IC50 ranging from 3.9 to 8.8 μM; this suggested that the prepared disubstituents 4a–l exert their biological activity more than the polysubstituents 4m–p. In addition, compounds 4i, 4o, 4d, 4a, 4g and 4c possessed good potency against MCF-7/ADR cells with IC50 ranging from 8.6–11.5 μM, as compared with Doxorubicin (IC50 = 18.6 μM), while compounds 4o and 4k were inactive against MCF-7/ADR cells with IC50 = 23.7 ± 0.4 and 36.8 ± 0.32 μM. Finally, compounds 4a, 4c, 4d, 4e, 4g, 4i, 4k and 4o had weak inhibitory activity against two normal cell lines, HFL-1 and WI-38, with IC50 ranging from 19.1 to 30.1 μM. In particular, compounds 4a, 4c, 4d, 4e, 4g, 4i, 4k and 4o were weakly active against these control normal cell lines, with strong selectivity in anti-proliferative effects for the cancer lines.
2.4.6. Molecular Docking
In order to confirm the link between the in vitro cytotoxicity results and the binding affinity of hybrids 4a–p, docking simulation experiments were carried out. More attention is devoted to P-glycoprotein (P-gp) which exists in cryo-EM structure; it contains the active site (PDB ID: 6FN1 [64]). The active site for 6FN1 was determined using CASTp to be Trp231, 1GLU874, MET875, GLN945 and MET948. The 3D loop structure of 6FN1 was created by the mGenTHERADER [65] within the docking framework, while the TS protein was created using the PyMol v:1.4 package. Nevertheless, the P-gp protein was validated using PROCHECK server [66], which also provided a 97.36 overall quality factor score on ERRAT server, to overview the statistics of nonbonding interactions between various atom types. When compared to the reference drug Zosuquidar, which blocked Glu874 and Trp231 with an RMSD of 1.20 Å, the toxicity behavior of 4a-p molecules was demonstrated in binding energy (BE) with a P-gp receptor. After being re-docked, the studied 4a-p hybrids attained a root mean square deviation (RMSD) of less than 2 Å (Table 4). To evaluate the binding affinity of the synthesized compounds, the pose with the lowest BE and RMSD was chosen. To further support the validity of the BE, the inhibitory constant (Ki) and ligand efficacy (LE) were calculated [67].

Table 4.
The binding affinity (kcal/mol) of 4a–p hybrids against P-gp receptor.
The most active compounds (4a, 4c, 4d, 4e and 4i) display promising binding scores (−6.30, −7.17, −7.10, −8.12 and −6.07 Kcal/mol), respectively, compared to Zosuquidar (−8.22 Kcal/mol). These BE values are supportive to the cytotoxicity data for tested compounds. All compounds were successful in blocking active binding sites in a manner similar to Zosuquidar. The docking statistical analysis implemented herein favors 30% of the tested compounds and can inhibit the subpocket P-gp protein by blocking the vital amino acid Glu 874 in the binding pocket (Figure 8).
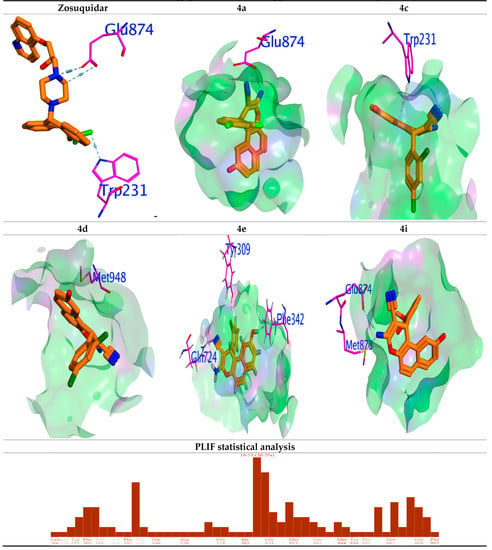
Figure 8.
Binding interaction of most active compounds 4a, 4c, 4d, 4e and 4i into P-gp, and PLIF statistical analysis histogram for compounds 4a–p and residues of P-gp protein.
The most active compound 4e bearing 2,5-Cl2 substituents (IC50 18.7 μM) showed the most binding affinity −8.12 Kcal/mol and inhibited the binding site by the formation of two strong H-bonding interactions with Tyr309, Gln7724 and Phe342 (Figure 8). The compound showed promising bioactive parameters LE, Ki and FQ (Table 4) but moderate activity was obtained for compounds 4a and 4c bearing 2,4-F2 and 2,3-Cl2 substituents.
The 2,4-F2 and 2,3-Cl2 rings are arranged with the main GLU874 and TRP231 in a perpendicular manner to be stabilized in the binding pocket, in addition to strong H-bonding interactions with the respective amino acids. For all compounds, the bioactivity metrics LE, Ki and FQ were found within the traditional range [68]. As per the docking outcomes, P-gp protein inhibition supports the in vitro findings.
3. Experimental Section
3.1. Materials and Equipment
All chemicals were purchased from Sigma-Aldrich Chemical Co. (Sigma-Aldrich Corp., St. Louis, MO, USA). All the melting points were measured with a Stuart Scientific Co. Ltd. Apparatus (Camberley, UK), which means they are uncorrected. The IR spectra were recorded on a KBr disc on a Jasco FT/IR 460 plus spectrophotometer (Jasco, Tokyo, Japan). The 1H/13C (500/125 MHz) NMR spectra were measured on a BRUKER AV 500 MHz spectrometer (BRUKER, Billerica, MA, USA) in DMSO-d6, as solvent, using tetramethylsilane (TMS) as an internal standard. The microwave apparatus utilized is Milestone Sr1, Microsynth (Milstone, Shelton, CT, USA.). The mass spectra were determined on a Shimadzu GC/MS-QP5050A spectrometer (Shimadzu, Kyoto, Japan). The elemental analysis was carried out at the Regional Center for Mycology and Biotechnology (RCMP), Al-Azhar University, Cairo, Egypt, and the results were within ± 0.25%. The reaction courses and products were routinely monitored using thin layer chromatography (TLC) on silica gel precoated F254 Merck plates (Merck, Billerica, MA, USA).
3.2. General Procedure for Synthesis of 1H-Benzo[f]chromene Derivatives (4a–p)
A reaction mixture of naphthalene-2,7-diol (1) (0.01 mol), malononitrile (2) (0.01 mol), different aromatic aldehydes (3a–p) and piperidine (0.5 mL) in an absolute ethanol solution (30 mL) was heated under microwave irradiation conditions for 2 min at 140 °C. After the reaction reached completion, the reaction mixture was cooled to room temperature and the precipitated solid was filtered off, washed with methanol and recrystallized from ethanol/benzene. The physical and spectral data of compounds (4a–p) are as follows:
3.2.1. 3-Amino-1-(2,4-Difluorophenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4a)
Orange crystals; yield 88%; m.p. 203–204 °C; IR (KBr) υ (cm−1): 3423, 3414, 3328, 3208 (NH2 and OH), 2193 (CN); 1H NMR δ: 9.96 (s, 1H, OH), 8.04 (t, 1H, J = 8.8 Hz, H-7), 8.00 (s, 1H, H-10), 7.80 (d, 1H, J = 8.8 Hz, H-6), 7.76 (d, 1H, J = 8.7 Hz, H-5), 7.24 (m, 1H, J = 5.5 Hz, H-8), 7.04 (bs, 2H, NH2), 7.06 (1H, d, J = 2.21 Hz, Ar, H-6), 6.98 (s, 1H, Ar, H-3), 6.89 (1H, d, J = 2.21 Hz, Ar, H-5), 5.29 (s, 1H, H-1); 13C NMR δ: 160.6 (C-3), 157.0 (C-9), 152.3(C-4a), 150.1, 148.1 (C-2,4, Ar, d, J = 250 Hz, C-F), 132.3 (C-10a), 130.8 (C-6, Ar), 130.1 (C-7), 130.0 (C-6), 125.6 (C-6a, C-1, Ar), 120.7 (C-10b), 117.7 (CN), 116.4 (C-5, Ar), 115.7 (C-8), 113.6 (C-5), 110.5 (C-3, Ar), 105.3 (C-10), 56.3 (C-2), 32.3 (C-1); MS m/z (%): 350 (M+, 100); Anal. Calcd for C20H12F2N2O2 (350.32): C, 68.57; H, 3.45; N, 8.00. Found: C, 68.64; H, 3.51; N, 8.06%.
3.2.2. 3-Amino-1-(2,6-Difluorophenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4b)
Colorless crystals; yield 85%; m.p. 193–194 °C; IR (KBr) υ (cm−1): 3457, 3445, 3322, 3226 (NH2 and OH), 2175 (CN); 1H NMR δ: 10.00 (s, 1H, OH), 7.78 (d, 1H, J = 8.9 Hz, H-7), 7.75 (d, 1H, J = 8.8 Hz, H-6), 7.32 (m, 1H, J = 4.9 Hz, H-5), 7.09 (bs, 2H, NH2), 7.06 (t, 1H, J = 9.0 Hz, Ar, H-4), 7.03 (d, 1H, J = 8.9 Hz, H-10), 6.99 (q, 2H, J = 3.7 Hz, Ar, H-3,5), 6.90 (d, 1H, J = 2.1 Hz, H-8), 5.45 (s, 1H, H-1); 13C NMR δ: 161.0 (C-3), 157.1 (C-9, C-4a), 150.1, 148.2 (C-2,6, Ar, d, J = 240 Hz, C-F), 132.5 (C-10a), 130.9 (C-7), 129.9 (C-6), 125.5 (C-6a), 120.7 (C-4, Ar), 120.3 (C-10b, C-1, Ar), 117.6 (CN), 113.5 (C-8), 112.6 (C-5), 111.4 (C-3,5, Ar), 104.5 (C-10), 54.1 (C-2), 28.6 (C-1); MS m/z (%): 350 (M+, 100); Anal. Calcd for C20H12F2N2O2 (350.32): C, 68.57; H, 3.45; N, 8.00. Found: C, 68.51; H, 3.40; N, 7.64%.
3.2.3. 3-Amino-1-(2,3-Dichlorophenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4c)
Yellow crystals; yield 89%; m.p. 296–297 °C; IR (KBr) υ (cm−1): 3438, 3421, 3320, 3208 (NH2 and OH), 2181 (CN); 1H NMR δ: 10.01 (s, 1H, OH), 7.83–6.96 (m, 10H, aromatic and NH2), 5.65 (s, 1H, H-1); 13C NMR δ: 161.0 (C-3), 157.1 (C-9), 148.2 (C-4a), 146.1 (C-1, Ar), 132.5 (C-10a), 130.9 (C-3, Ar), 130.3 (C-7), 129.9 (C-2, Ar), 129.4 (C-5, Ar), 129.4 (C-6), 125.7 (C-4, Ar), 124.6 (C-6, Ar), 121.4 (C-6a), 120.3 (C-10b), 117.8 (CN), 113.6 (C-8), 111.1 (C-5), 105.4 (C-10), 56.5 (C-2), 35.64 (C-1); MS m/z (%): 386 (M+ + 4, 10.10), 384 (M++2, 64.97), 382 (M+, 100); Anal. Calcd for C20H12Cl2N2O2 (383.23): C, 62.68; H, 3.16; N, 7.31. Found: C, 62.73; H, 3.20; N, 7.36%.
3.2.4. 3-Amino-1-(2,4-Dichlorophenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4d)
Colorless crystals; yield 88%; m.p. 221–222 °C; IR (KBr) υ (cm−1): 3463, 3441, 3339, 3197 (NH2 and OH), 2191 (CN); 1H NMR δ: 10.01 (s, 1H, OH), 7.82 (d, 1H, J = 8.8 Hz, H-7), 7.76 (d, 1H, J = 8.8 Hz, H-6), 7.66 (s, 1H, 10), 7.28 (d, 1H, J = 8.9 Hz, H-5), 7.08 (d, 1H, J = 8.9 Hz, H-8), 7.07 (bs, 2H, NH2), 7.01, 7.00 (dd, 2H, J = 8.7, 2.0 Hz, Ar H-5,6), 6.76 (s, 1H, Ar H-3), 5.50 (s, 1H, H-1); 13C NMR δ: 160.4 (C-3), 157.1 (C-9), 148.1 (C-4a), 142.1 (C-1, Ar), 132.7 (C-10a), 132.4 (C-2, Ar), 131.6 (C-4, Ar), 130.9 (C-6,3, Ar), 130.3 (C-7), 129.4 (C-6), 128.9 (C-5, Ar), 125.7 (C-6a), 120.3 (C-10b), 117.8 (CN), 113.6 (C-8), 112.9 (C-5), 105.5 (C-10), 56.1 (C-2), 31.2 (C-1); MS m/z (%): 386 (M+ + 4, 9.78), 384 (M+ + 2, 62.89), 382 (M+, 100); Anal. Calcd for C20H12Cl2N2O2 (383.23): C, 62.68; H, 3.16; N, 7.31. Found: C, 62.71; H, 3.19; N, 7.34%.
3.2.5. 3-Amino-1-(2,5-Dichlorophenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4e)
Pale yellow crystals; yield 84%; m.p. 293–294 °C; IR (KBr) υ (cm−1): 3461, 3423, 3342, 3206 (NH2 and OH), 2187 (CN); 1H NMR δ: 10.01 (s, 1H, OH), 7.84–6.77 (m, 10H, aromatic and NH2), 5.54 (s, 1H, H-1); 13C NMR δ: 160.5 (C-3), 157.2 (C-9), 148.2 (C-4a), 146.2 (C-1, Ar), 132.9 (C-10a), 132.4 (C-5, Ar), 132.0 (C-3, Ar), 130.9 (C-7), 130.7 (C-6, Ar), 130.4 (C-2, Ar), 129.1 (C-6), 125.7 (C-4, Ar), 122.2 (C-6a), 120.2 (C-10b), 117.8 (CN), 113.7 (C-8), 111.1 (C-5), 105.4 (C-10), 56.5 (C-2), 35.0 (C-1); MS m/z (%): 386 (M+ + 4, 9.98), 384 (M+ + 2, 65.9), 382 (M+, 100); Anal. Calcd for C20H12Cl2N2O2 (383.23): C, 62.68; H, 3.16; N, 7.31. Found: C, 62.62; H, 3.11; N, 7.26%.
3.2.6. 3-Amino-1-(2,6-Dichlorophenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4f)
Colorless crystals; yield 84%; m.p. 196–197 °C; IR (KBr) υ (cm−1): 3449, 3430, 3327, 3203 (NH2 and OH), 2190 (CN); 1H NMR δ: 9.88 (s, 1H, OH), 7.78 (d, 1H, J = 8.8 Hz, H-7), 7.74 (d, 1H, J = 8.7 Hz, H-6), 7.63 (dd, 1H, J = 7.9, 1.5 Hz, Ar H-3), 7.30 (t, 1H, J = 7.0 Hz, Ar H-4), 7.28 (dd, 1H, J = 7.0 Hz, Ar H-5), 7.05 (bs, 2H, NH2), 6.99 (d, 1H, J = 8.7, 2.2 Hz, H-8), 6.97 (s, 1H, 10), 6.72 (d, 1H, J = 2.5 Hz, H-5), 5.97 (s, 1H, H-1); 13C NMR δ: 160.9 (C-3), 156.9 (C-9), 148.8 (C-4a), 137.8 (C-1, Ar), 135.3, 135.20 (C-2,6, Ar), 132.8 (C-7), 131.3 (C-10a), 130.80 (C-4, Ar), 130.29 (C-6), 129.43 (C-3,5, Ar), 125.55 (C-6a), 120.12 (C-10b), 117.4 (CN), 113.4 (C-8), 111.1 (C-5), 105.7 (C-10), 53.2 (C-2), 35.6 (C-1); MS m/z (%): 386 (M+, 10.02) 384 (M+, 64.99) 382 (M+, 100); Anal. Calcd for C20H12Cl2N2O2 (383.23): C, 62.68; H, 3.16; N, 7.31. Found: C, 62.74; H, 3.22; N, 7.37%.
3.2.7. 3-Amino-1-(3,4-Dichlorophenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4g)
Colorless crystals; yield 83%; m.p. 275–276 °C; IR (KBr) υ (cm−1): 3477, 3408, 3335, 3267 (NH2 and OH), 2179 (CN); 1H NMR δ: 9.94 (s, 1H, OH), 7.81 (d, 1H, J = 8.9 Hz, H-7), 7.76 (d, 1H, J = 8.7 Hz, H-6), 7.54 (d, 1H, J = 8.4 Hz, H-5), 7.43 (d, 1H, J = 2.1 Hz, Ar, H-5), 7.09 (d, 1H, J = 8.8 Hz, H-10), 7.08 (bs, 2H, NH2) 7.06 (s, 1H, Ar, H-2), 6.99 (dd, 1H, J = 8.7, 2.3 Hz, Ar, H-6), 6.95 (d, 1H, J = 2.3 Hz, H-8), 5.17 (s, 1H, H-1); 13C NMR δ: 160.2 (C-3), 157.0 (C-9), 147.8 (C-4a), 147.1 (C-1, Ar), 132.4 (C-10a), 131.6 (C-3, Ar), 130.8 (C-4, Ar), 130.2 (C-7, C-5, Ar), 129.7 (C-2, Ar), 129.2 (C-6), 127.8 (C-6, Ar), 125.7 (C-6a), 120.7 (C-10b), 117.7 (CN), 113. (C-8), 112.9 (C-5), 106.1 (C-10), 57.4 (C-2), 37.8 (C-1); MS m/z (%): 386 (M+ + 4, 10.01), 384 (M+ + 2, 46.07), 382 (M+, 70) with base peak at 237; Anal. Calcd for C20H12Cl2N2O2 (383.23): C, 62.68; H, 3.16; N, 7.31. Found: C, 62.61; H, 3.11; N, 7.27%.
3.2.8. 3-Amino-1-(2-Chloro-6-Flourophenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4h)
Pale yellow crystals; yield 87%; m.p. 298–299 °C; IR (KBr) υ (cm−1): 3490, 3433, 3336, 3211 (NH2 and OH), 2187 (CN); 1H NMR δ: 9.93 (s, 1H, OH), 7.78 (d, 1H, J = 8.8 Hz, H-7), 7.75 (d, 1H, J = 8.8 Hz, H-6), 7.43 (s, 1H, Ar, H-3,5), 7.31 (d, 1H, J = 3.8 Hz, H-5), 7.07 (s, 1H, H-10), 7.01 (bs, 2H, NH2), 6.87 (s, 1H, Ar, H-4), 5.65 (s, 1H, H-1); 13C NMR δ: 162.9, 161.7 (C-6, Ar, d, J = 250 Hz, C-F), 160.9 (C-9), 157.0 (C-4a, C-3), 148.2 (C-2, Ar), 133.1 (C-10a), 132.7 (C-1, Ar), 130.8 (C-7), 130.1 (C-4, Ar), 130.0 (C-6), 126.1 (C-3, Ar), 125.5 (C-6a), 120.4 (C-10b), 117.5 (CN), 113.5 (C-8, C-5, Ar), 112.0 (C-5), 105.3 (C-10), 53.9 (C-2), 33.0 (C-1); MS m/z (%): 368 (M+ + 2, 65.96) 366 (M+, 100); Anal. Calcd for C20H12ClFN2O2 (366.77): C, 65.49; H, 3.30; N, 7.64. Found: C, 65.41; H, 3.23; N, 7.58%.
3.2.9. 3-Amino-1-(3,5-Dibromophenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4i)
Colorless crystals; yield 86%; m.p. 283–284 °C; IR (KBr) υ (cm−1): 3463, 3418, 3347, 3196 (NH2 and OH), 2191 (CN); 1H NMR δ: 9.93 (s, 1H, OH), 7.91 (s, 1H, Ar, H-4), 7.83 (d, 1H, J = 8.8 Hz, H-7), 7.78 (t, 1H, J = 1.7 Hz, H-6), 7.68 (d, 1H, J = 8.8 Hz, H-5), 7.30 (s, 2H, Ar, H-2,6), 7.11 (bs, 2H, NH2), 7.09 (d, 1H, J = 8.9 Hz, H-10), 6.96 (d, 1H, J = 2.2 Hz, H-8), 5.18 (s, 1H, H-1); 13C NMR δ: 160.3 (C-3), 157.1 (C-9), 150.6 (C-4a), 147.7 (C-1, Ar), 133.4 (C-10a), 132.3 (C-4, Ar), 130.8 (C-2,6, Ar), 130.3 (C-7), 129.3 (C-6), 125.7 (C-6a), 123.8, 123.3 (C-3,5, Ar), 120.6 (C-10b), 117.8 (CN), 113.7 (C-8), 112.6 (C-5), 106.0 (C-10), 57.3 (C-2), 37.9 (C-1); MS m/z (%): 474 (M+ + 4, 35.07), 472 (M+ + 2, 76.97), 470 (M+, 40) with base peak at 237 (100); Anal. Calcd for C20H12Br2N2O2 (472.13): C, 50.88; H, 2.56; N, 5.93. Found: C, 50.81; H, 2.50; N, 5.87%.
3.2.10. 3-Amino-1-(2-Hydroxy-3-Methoxyphenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4j)
Pale yellow crystals; yield 83%; m.p. 269–270 °C; IR (KBr) υ (cm−1): 3445, 3421, 3353, 3203 (NH2 and OH), 2214 (CN); 1H NMR δ: 13.54 (s, 1H, HO-2, Ar), 10.77 (s, 1H, HO-9), 7.87–6.85 (m, 10H, aromatic and NH2), 5.60 (s, 1H, H-1), 3.86 (s, 3H, OMe); 13C NMR δ: 164.6 (C-3), 163.8 (C-3, Ar), 161.3 (C-2, Ar), 150.8 (C-9), 149.0 (C-4a), 147.6 (C-10a), 139.6 (C-7), 135.3 (C-6), 130.8 (C-6a), 124.9 (C-5, Ar), 121.4 (C-6, Ar), 121.0 (C-10b), 120.5 (C-1, Ar), 118.6 (CN), 118.4 (C-4, Ar), 115.2 (C-8), 111.8 (C-5), 97.7 (C-10), 56.2 (C-2), 56.2 (Me), 35.9 (C-1); MS m/z (%): 360 (M+, 15.97) with a base peak at 238 (100); Anal. Calcd for C21H16N2O4 (360.36): C, 69.99; H, 4.48; N, 7.77. Found: C, 69.92; H, 4.42; N, 7.72%.
3.2.11. 3-Amino-1-(2,4-Dimethoxyphenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4k)
Colorless crystals; yield 90%; m.p. 242–243 °C; IR (KBr) υ (cm−1): 3453, 3418, 3220, 3196 (NH2 and OH), 2212 (CN); 1H NMR δ: 9.83 (s, 1H, OH), 7.74 (d, 1H, j = 8.9 Hz, H-7), 7.72 (d, 1H, j = 8.8 Hz, H-6), 7.04 (d, 1H, j = 8.9 Hz, H-5), 6.97 (dd, 1H, J = 8.7, 2.3 Hz, Hz, Ar H-6), 6.92 (d, 1H, j = 2.5 Hz, H-10), 6.79 (s, 2H, NH2), 6.64 (d, 1H, J = 8.1 Hz, Ar, H-5), 6.59 (d, 1H, J = 2.5 Hz, Ar, H-8), 6.35 (dd, 1H, J = 8.5, 2.4 Hz, Ar, H-3), 5.30 (s, 1H, H-1), 3.88 (s, 3H, OMe), 3.69 (s, 3H, OMe); 13C NMR δ: 160.6 (C-3), 159.6 (C-2 Ar), 157.2 (C-4 Ar), 156.7 (C-9), 148.1 (C-4a), 132.7 (C-10a), 130.5 (C-6, Ar), 129.3 (C-7), 129.2 (C-6), 126.4 (C-6a), 125.6 (C-10b), 121.1 (C-8), 117.5 (CN), 114.7 (C-5), 114.4 (C-1, Ar), 106.0 (C-5, Ar), 105.9 (C-10), 99.0 (C-3, Ar), 57.8 (Me), 56.4 (Me), 55.6 (C-2), 32.7 (C-1); MS m/z (%): 374 (M+ + 1, 73) with base peak at 238; Anal. Calcd for C22H18N2O4 (374.39): C, 70.58; H, 4.85; N, 7.48. Found: C, 70.51; H, 4.79; N, 7.42%.
3.2.12. 3-Amino-1-(3,4-Dimethoxyphenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4l)
Colorless crystals; yield 89%; m.p. 268–269 °C; IR (KBr) υ (cm−1): 3468, 3435, 3227, 3199 (NH2 and OH), 2184 (CN); 1H NMR δ: 9.87 (s, 1H, OH), 7.78 (d, 1H, J = 8.9 Hz, H-7), 7.74 (d, 1H, J = 8.8 Hz, H-6), 7.07 (d, 1H, J = 8.8 Hz, H-5), 7.02 (d, 1H, J = 2.4 Hz, H-10), 6.98 (dd, 1H, J = 8.8, 2.3 Hz, Ar, H-5), 6.90 (s, 2H, NH2), 6.82 (d, 1H, J = 8.4 Hz, H-8), 6.81 (d, 1H, J = 2.0 Hz, Ar, H-2), 6.54 (dd, 1H, J = 8.3, 2.1 Hz, Ar, H-6), 4.96 (s, 1H, H-1), 3.69 (s, 3H, OMe), 3.67 (s, 3H, OMe); 13C NMR δ: 160.0 (C-3), 156.7 (C-2, Ar), 149.1 (C-9), 147.9 (C-3, Ar), 147.7 (C-4 Ar), 138.6 (C-4a), 132.7 (C-1, Ar), 130.6 (C-10a), 129.6 (C-7), 125.7 (C-6), 121.2 (C-6a), 119.3 (C-10b, C-6, Ar), 117.5 (CN), 114.3 (C-8), 113.6 (C-5), 112.5 (C-2, Ar), 111.3 (C-5, Ar), 106.3 (C-10), 58.6 (Me), 55.9 (C-2), 38.5 (C-1); MS m/z (%): 375 (M+ + 1, 100); Anal. Calcd for C22H18N2O4 (374.39): C, 70.58; H, 4.85; N, 7.48. Found: C, 70.64; H, 4.89; N, 7.55%.
3.2.13. 3-Amino-1-(2,3,4-Trimethoxyphenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4m)
Pale yellow crystals; yield 90%; m.p. 265–266 °C; IR (KBr) υ (cm−1): 3438, 3414, 3222, 3197 (NH2 and OH), 2203 (CN); 1H NMR δ: 9.82 (s, 1H, OH), 7.74 (d, 1H, j = 8.9 Hz, H-7), 7.72 (d, 1H, j = 8.7 Hz, H-6), 7.05 (s, 1H, H-10), 7.04 (d, 1H, j = 6.5 Hz, H-5), 6.98 (d, 1H, j = 8.5 Hz, H-8), 6.88 (s, 2H, NH2), 6.67, 6.66 (dd, 2H, Ar H-5,6), 5.18 (s, 1H, H-1), 3.78 (s, 3H, OMe), 3.74 (s, 3H, OMe), 3.70 (s, 3H, OMe); 13C NMR δ: 160.6 (C-3), 156.7 (C-9), 152.6 (C-4a), 150.8 (C-4, Ar), 147.8 (C-2, Ar), 141.7 (C-3 Ar), 132.7 (C-10a), 131.2 (C-7), 130.5 (C-6), 129.3 (C-6, Ar), 125.6 (C-6a), 123.6 (C-10b), 121.5 (C-5, Ar), 117.4 (CN), 114.8 (C-8), 113.7 (C-5), 108.5 (C-1, Ar), 106.0 (C-10, Ar), 61.6 (Me), 60.7 (Me), 57.7 (Me), 56.5 (C-2), 33.6 (C-1); MS m/z (%): 405 (M+ + 1, 100); Anal. Calcd for C23H20N2O5 (404.14): C, 68.31; H, 4.98; N, 6.93. Found: C, 68.38; H, 5.05; N, 6.99%.
3.2.14. 3-Amino-1-(2,4,5-Trimethoxyphenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4n)
Colorless crystals; yield 87%; m.p. 235–236 °C; IR (KBr) υ (cm−1): 3452, 3419, 3325, 3227, 3197 (NH2 and OH), 2192 (CN); 1H NMR δ: 9.82 (s, 1H, OH), 7.74 (d, 1H, J = 8.9 Hz, H-7), 7.72 (d, 1H, J = 8.6 Hz, H-6), 7.05 (d, 1H, J = 8.8 Hz, H-5), 6.98 (d, 1H, J = 6.0 Hz, H-8), 6.82 (s, 2H, NH2), 6.71 (s, 1H, H-10), 6.36 (s, 1H, Ar, H-3,6), 5.30 (s, 1H, H-1), 3.84 (s, 3H, OMe), 3.76 (s, 3H, OMe), 3.48 (s, 3H, OMe); 13C NMR δ: 160.6 (C-3), 156.7 (C-2, Ar), 150.9 (C-9), 149.1 (C-4, Ar), 148.0 (C-4a), 143.3 (C-5, Ar), 132.7 (C-10a), 130.5 (C-7), 129.3 (C-6), 125.6 (C-6a), 125.3 (C-6, Ar), 121.1 (C-10b), 117.5 (CN), 114.6 (C-8), 113.6 (C-5), 106.0 (C-10), 99.2 (C-3, Ar), 57.6 (Me), 57.3 (Me), 56.9 (Me), 56.2 (C-2), 31.2 (C-1); MS m/z (%): 405 (M+ + 1, 100); Anal. Calcd for C23H20N2O5 (404.14): C, 68.31; H, 4.98; N, 6.93. Found: C, 68.37; H, 5.05; N, 6.99%.
3.2.15. 3-Amino-1-(3,4,5-Trimethoxyphenyl)-9-Hydroxy-1H-Benzo[f]chromene-2-Carbonitrile (4o)
Colorless crystals; yield 89%; m.p. 252–253 °C; IR (KBr) υ (cm−1): 3461, 3427, 3294, 3177 (NH2 and OH), 2204 (CN); 1H NMR δ: 9.90 (s, 1H, OH), 7.80 (d, 1H, j = 8.9 Hz, H-7), 7.77 (d, 1H, j = 8.7 Hz, H-6), 7.09 (d, 1H, j = 8.8 Hz, H-5), 7.05 (d, 1H, j = 8.5 Hz, H-8), 7.00 (s, 1H, H-10), 6.94 (dd, 2H, J = 8.7, 2.3 Hz, Ar, H-2,6,), 6.41 (s, 2H, NH2), 4.98 (s, 1H, H-1), 3.66 (s, 6H, 2OMe), 3.60 (s, 3H, OMe); 13C NMR δ: 160.2 (C-3), 156.8 (C-3,5 Ar), 153.4 (C-9), 147.7 (C-4a), 141.8 (C-4, Ar), 136.6 (C-1, Ar), 132.7 (C-10a), 130.6 (C-7), 129.8 (C-6), 125.7 (C-6a), 121.1 (C-10b), 117.6 (CN), 114.0 (C-8), 113.7 (C-5, Ar), 106.2 (C-2,6, Ar), 104.6 (C-10), 60.4 (Me), 58.3 (Me), 56.2 (C-2), 39.1 (C-1); MS m/z (%): 405 (M+ + 1, 100); Anal. Calcd for C23H20N2O5 (404.14): C, 68.31; H, 4.98; N, 6.93. Found: C, 68.26; H, 4.93; N, 6.89%.
3.2.16. 3-Amino-9-Hydroxy-1-(Perfluorophenyl)-1H-Benzo[f]chromene-2-Carbonitrile (4p)
Yellow crystals; yield 79%; m.p. 299–300 °C; IR (KBr) υ (cm−1): 3485, 3421, 3353, 3203 (NH2 and OH), 2192 (CN); 1H NMR δ: 10.10 (s, 1H, OH), 7.82 (d, 1H, J = 8.9 Hz, H-7), 7.79 (d, 1H, j = 8.8, Hz, H-6), 7.27 (s, 2H, NH2), 7.05 (d, 1H, j = 8.8 Hz, H-5), 7.00 (q, 1H, J = 3.66 Hz, H-10), 6.82 (d, 1H, j = 2.2, H-8), 5.57 (s, 1H, H-1); 13C NMR δ: 161.1 (C-3), 157.4 (C-9), 157.3 (C-4a), 148.3 (C-3,5 Ar), 132.3 (C-4, Ar), 132.2 (C-2,6 Ar), 131.1 (C-10a), 130.6 (C-7), 130.3 (C-6), 125.5 (C-6a), 120.3 (C-10b), 117.9 (CN), 113.5 (C-8), 110.6 (C-5), 109.9 (C-1, Ar), 104.2 (C-10), 53.3 (C-2), 29.8 (C-1); MS m/z (%): 405 (M+, 100) with a base peak at 375 (100); Anal. Calcd for C20H9F5N2O2 (404.29): C, 59.42; H, 2.24; N, 6.93. Found: C, 59.49; H, 2.30; N, 6.99%.
3.3. Biological Screening
3.3.1. Cell Culture
The tumor cell lines MCF-7, HepG-2, PC-3 MCF-7/ADR, HFL-1 and (WI-38) were obtained from the American Type Culture Collection (ATCC, Rockville, MD, USA). The cells were grown on RPMI-1640 medium supplemented with 10% inactivated fetal calf serum and 50 µg/mL gentamycin. The cells were maintained at 37 οC in a humidified atmosphere with 5% CO2 and were subcultured two to three times a week.
3.3.2. Cytotoxicity Evaluation using Viability Assay
The cytotoxic activity was appraised using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) colorimetric assay for the antitumor cell lines, as reported previously [52], and the ELISA assay for MCF-7/ADR [69].
3.3.3. In Vitro Analysis of P-gp Content
The content of P-gp in the MCF-7/ADR cell lysates after incubation with varying conc. (12.5–100 µM) of tested compounds 4a, 4c, 4d, 4e, 4g and 4i following exposure for 48 hr was determined using a commercial human P-gp (Permeability Glycoprotein) ELISA Kit (MBS2506188, MyBioSource Inc., San Diego, CA, USA). Absorption was recorded at 450 nm with a Spectramax Gemini fluorescence microplate reader (Molecular Devices, Sunnyvale, CA, USA) [70].
3.3.4. Rhodamine 123 Accumulation Assay
P-gp activity was determined by measuring intracellular accumulation of Rhodamine 123 in MCF-7/ADR cells in the absence or presence of compounds 4a, 4c, 4d, 4e, 4g and 4i, according to the commercial Rhodamine Competitive ELISA Kit (AKR-5142, Cell Biolabs Inc., San Diego, CA, USA) which provides a convenient method for the detection of total rhodamine in extracts from cells [71]. Briefly, resistant cancer cells were harvested, washed twice and counted. After dilution to 1 × 106 cells/mL in each well of 6 well plates, 1 mL of fresh media containing different concentrations of compounds 4a, 4c, 4d, 4e, 4g and 4i were added and incubated at 37°C for 4 h in an atmosphere containing 5% CO2. Subsequently, 5.25 μM of Rho123 was added to each well and the wells were incubated for another 30 min at 37 °C. Finally, cells were washed and lysed as described before, and intracellular quantification levels of Rhodamine 123 were further analyzed according to the ELISA protocol kit. Absorbance at 450 nm of each well was measured using a Spectramax Gemini fluorescence microplate reader (Molecular Devices, Sunnyvale, CA, USA). The total content of Rhodamine in each sample was determined by comparison with a Rhodamine standard curve.
3.4. Molecular Docking
We have used a program similar to that used by Jaguar et al. [72] to generate all possible tautomeric and stereo-isomeric stats for the structures. Crystal structures of P-glycoprotein were taken from the protein data bank and bonded with 5-florouracil as the reference drug. All ligands were imported into Ligprep module and redocked into the appropriate binding sites using Glide’s module. The Glide tool was applied to perform the molecular docking, then a grid for protein was charged using the default aspects of force field. The (SP) scoring function was produced to study the binding affinity, and then charged with a Charm force field. The low root square devotion RMSD score was utilized to obtain the other poses. To draw, the Schrodinger builder was applied.
4. Conclusions
In summary, we have synthesized di/polysubstituted 1H-benzo[f]chromene derivatives and evaluated their cytotoxic activities against three cancer cell lines (PC-3, SKOV-3 and HeLa). Several target compounds showed cytotoxic activity against PC-3, SKOV-3 and HeLa cancer cell lines. In particular, compounds 4a, 4c, 4d, 4e, 4g, 4i, 4k and 4o displayed the highest activity against PC-3, SKOV-3 and HeLa, with IC50 values ranging from 0.8–3.7, 0.9–3.1 and 1.7–5.7 μM, respectively. In addition, compounds 4i, 4o, 4d, 4a, 4g and 4c, respectively, possessed good potency against MCF-7/ADR cells with IC50 ranging from 8.6–11.5 μM and a weak inhibitory activity against two normal cell lines, HFL-1 and WI-38, with IC50 = 19.1–30.1 μM. The active compounds 4a, 4c, 4d, 4e, 4g and 4i against MCF-7/ADR lines were tested as P-gp expression and function inhibitors, and compounds 4a, 4c, 4e and 4g were found to have good inhibitory potency against P-gp levels in MCF-7/ADR lysate with IC50 ranging from 18.7 to 34.6 μM as compared with Doxorubicin (IC50 = 50.9 μM). Rho123 accumulation assays showed that compounds 4a, 4c and 4e effectively inhibited P-pg expression and efflux function, while compounds 4d and 4e induced apoptosis and showed cell cyclic arrest at G1 phase, while other compounds showed this at S phase (4a and 4c) and finally showed compounds with both cell cycle arrest at G1/S phases (4g and 4i).
The introduction of halogen atoms in 1-position on the pendant phenyl group plays a more important role in enhancing antitumor activities than methoxy groups, according to structure activity relationships (SARs).
Supplementary Materials
The following spectral data supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms24010049/s1,
Author Contributions
Conceptualization, A.M.E.-A., F.F.A. and T.H.A.; methodology, A.M.F., M.A.A.E.-N. and H.K.A.E.-M.; software, R.A.E.-E. and M.B.I.M.; validation, A.M. and F.F.A.; formal analysis, R.A.E.-E.; investigation, A.A.E.; resources, A.M.F.; data curation, A.M.; writing—original draft preparation, A.M.E.-A.; writing—review and editing, T.H.A.; supervision, A.M.E.-A.; project administration, A.M.E.-A.; funding acquisition, A.M.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Science Research at King Khalid University, Saudi Arabia, through the General Research Project under Grant Number (RGP.1/245/43).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to express gratitude to the Deanship of Science Research at King Khalid University, Saudi Arabia, for funding this work through the General Research Project under Grant Number (RGP.1/245/43).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fouda, A.M.; Hassan, A.H.; Eliwa, E.M.; Ahmed, H.E.A.; Al-Dies, A.M.; Omar, A.M.; Nassar, H.S.; Halawa, A.H.; Aljuhani, N.; El-Agrody, A.M. Targeted potent antimicrobial benzochromene-based analogues: Synthesis, computational studies, and inhibitory effect against 14α-Demethylase and DNA Gyrase. Bioorg. Chem. 2020, 105, 104387. [Google Scholar] [CrossRef] [PubMed]
- Okasha, R.M.; Albalawi, F.F.; Afifi, T.H.; Fouda, A.M.; Al-Dies, A.-A.M.; El-Agrody, A.M. Structural Characterization and Antimicrobial Activities of 7H-Benzo[h]chromeno[2,3-d]pyrimidine and 14H-Benzo[h]chromeno[3,2-e][1,2,4]triazolo[1,5-c] pyrimidine Derivatives. Molecules 2016, 21, 1450. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.M.; Abd EL-Wahab, A.H.; El-Agrody, A.M.; Bedair, A.H.; Eid, F.A.; Khafagy, M.M.; Abd-EL-Rehem, K.A. Synthesis and characterization of new diiodocoumarin derivatives with promising antimicrobial activities. Beilstein J. Org. Chem. 2011, 7, 1688–1696. [Google Scholar] [CrossRef]
- Raj, V.; Lee, J. 2H/4H-Chromenes-A Versatile Biologically Attractive Scaffold. Front. Chem. 2020, 8, 623. [Google Scholar] [CrossRef] [PubMed]
- El-Agrody, A.; Sabry, N.; Motlaq, S. Synthesis of some new 2-substituted 12H-chromeno [3, 2-e][1, 2, 4] triazolo [1, 5-c] pyrimidine, 3-ethoxycarbonyl-12H-chromeno[3,2-e]-[1,2, 4]triazolo[1,5-c]pyrimidine-2-one,ethyl 2-formylamino \acetylamino-4H-chromene-3-carboxylate and some of their antimicrobial activities. J. Chem. Res. 2011, 35, 77–83. [Google Scholar] [CrossRef]
- Foroumadi, A.; Emami, S.; Sorkhi, M.; Nakhjiri, M.; Nazarian, Z.; Heydari, S.; Ardestani, S.K.; Poorrajab, F.; Shafiee, A. Chromene-Based Synthetic Chalcones as Potent Antileishmanial Agents: Synthesis and Biological Activity. Chem. Biol. Drug Des. 2010, 75, 590–596. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, E.; Azimi, S.C. Li (OHCH2CH2NH2)(CF3OAC): A novel and homogeneous acidic ionic liquid catalyst for efficient synthesis of 2-amino-4H-chromene derivatives. Iran. J. Catal. 2014, 4, 281–288. [Google Scholar]
- Denish, C.; Hetal, K.; Nilesh, K. Synthesis, characterization & anti-HIV activity of 4-Hydroxy-3-(5-methylisoxazol-3-yl) pyrano (3, 2-C) chromene-2, 5-dione. AJBPR 2012, 2, 126–130. [Google Scholar]
- Fouda, A.M.; Okasha, R.M.; Alblewi, F.F.; Mora, A.; Afifi, T.H.; El-Agrody, A.M. A proficient microwave synthesis with structure elucidation and the exploitation of the biological behavior of the newly halogenated 3-amino-1H-benzo[f]- chromene molecules, targeting dual inhibition of topoisomerase II and microtubules. Bioorg. Chem. 2020, 95, 103549. [Google Scholar] [CrossRef]
- Fouda, A.M.; Assiri, M.A.; Mora, A.; Ali, T.E.; Afifi, T.H.; El-Agrody, A.M. Microwave synthesis of novel halogenated β-enaminonitriles linked 9-bromo-1H-benzo[f]chromene moieties: Induces cell cycle arrest and apoptosis in human cancer cells via dual inhibition of topoisomerase I and II. Bioorg. Chem. 2019, 93, 103289. [Google Scholar] [CrossRef]
- Alblewi, F.F.; Okasha, R.M.; Eskandrani, A.A.; Afifi, T.H.; Mohamed, H.M.; Halawa, A.H.; Fouda, A.M.; Al-Dies, A.-A.M.; Mora, A.; El-Agrody, A.M. Design and Synthesis of Novel Heterocyclic-Based 4H-benzo[h]chromene Moieties: Targeting antitumor caspase 3/7 activities and cell cycle analysis. Molecules 2019, 24, 1060. [Google Scholar] [CrossRef] [PubMed]
- Halawa, A.H.; Elaasser, M.M.; El Kerdawy, A.M.; El-Hady, A.; Ahmed, M.; Emam, H.A.; El-Agrody, A.M. Anticancer activities, molecular docking and structure–activity relationship of novel synthesized 4H-chromene, and 5H-chromeno[2, 3-d]pyrimidine candidates. Med. Chem. Res. 2017, 26, 2624–2638. [Google Scholar] [CrossRef]
- Alblewi, F.F.; Okasha, R.M.; Hritani, Z.M.; Mohamed, H.M.; El-Nassag, M.A.; Halawa, A.H.; Mora, A.; Fouda, A.M.; Assiri, M.A.; Al-Dies, A.-A.M. Antiproliferative effect, cell cycle arrest and apoptosis generation of novel synthesized anticancer heterocyclic derivatives based 4H-benzo[h]chromene. Bioorg. Chem. 2019, 87, 560–571. [Google Scholar] [CrossRef] [PubMed]
- El-Agrody, A.M.; Abd El-Mawgoud, H.K.; Fouda, A.M.; Khattab, E.S. Synthesis, in-vitro cytotoxicity of 4H-benzo[h]chromene derivatives and structure–activity relationships of 4-aryl group and 3-, 7-positions. Chem. Pap. 2016, 70, 1279–1292. [Google Scholar] [CrossRef]
- El-Agrody, A.; Khattab, S.A.; Fouda, A.M. Synthesis, structure-activity relationship (SAR) studies on some 4-Aryl-4Hchromenes and relationship between lipophilicity and antitumor activity. Lett. Drug Des. Discov. 2014, 11, 1167–1176. [Google Scholar] [CrossRef]
- Costa, M.; Dias, T.A.; Brito, A.; Proença, F. Biological importance of structurally diversified chromenes. Eur. J. Med. Chem. 2016, 123, 487–507. [Google Scholar] [CrossRef] [PubMed]
- Indulatha, V.; Gopal, N.; Jayakar, B. Anti-inflammatory activity of newly synthesised N-[4′-Oxo-2′-(substituted aryl/heteryl)-thiazolidin-3′-yl]-3-carboxamido-2H-chromen-2-one derivatives. Int. J. ChemTech Res. 2011, 3, 1930–1937. [Google Scholar]
- Elenkov, I.J.; Hrvačić, B.; Marković, S.; Mesić, M.; Čempuh Klonkay, A.; Lerman, L.; Filipović Sučić, A.; Vujasinović, I.; Bošnjak, B.; Brajša, K. Synthesis and anti-inflammatory activity of novel furochromenes. Croat. Chem. Acta 2013, 86, 253–264. [Google Scholar] [CrossRef]
- Gregor, W.; Grabner, G.; Adelwohrer, C.; Rosenau, T.; Gille, L. Antioxidant Properties of Natural and Synthetic Chromanol Derivatives: Study by Fast Kinetics and Electron Spin Resonance Spectroscopy. J. Org. Chem. 2005, 70, 3472–3483. [Google Scholar] [CrossRef]
- Mohamed, H.M.; Fouda, A.M.; Khattab, E.S.; El-Agrody, A.M.; Afifi, T.H. Synthesis, in-vitro cytotoxicity of 1H-benzo[f]chromene derivatives and structure–activity relationships of the 1-aryl group and 9-position. Z. Für Nat. C 2017, 72, 161–171. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Fouda, A.M.; Khattab, E.S.A. Halogenated 2-amino-4H-benzo [h] chromene derivatives as antitumor agents and the relationship between lipophilicity and antitumor activity. Med. Chem. Res. 2017, 26, 691–700. [Google Scholar] [CrossRef]
- Ahmed, H.E.; El-Nassag, M.A.; Hassan, A.H.; Mohamed, H.M.; Halawa, A.H.; Okasha, R.M.; Ihmaid, S.; Abd El-Gilil, S.M.; Khattab, E.S.; Fouda, A.M. Developing lipophilic aromatic halogenated fused systems with specific ring orientations, leading to potent anticancer analogs and targeting the c-Src Kinase enzyme. J. Mol. Struct. 2019, 1186, 212–223. [Google Scholar] [CrossRef]
- Ahmed, H.E.; El-Nassag, M.A.; Hassan, A.H.; Okasha, R.M.; Ihmaid, S.; Fouda, A.M.; Afifi, T.H.; Aljuhani, A.; El-Agrody, A.M. Introducing novel potent anticancer agents of 1H-benzo[f]chromene scaffolds, targeting c-Src kinase enzyme with MDA-MB-231 cell line anti-invasion effect. J. Enzym. Inhib. Med. Chem. 2018, 33, 1074–1088. [Google Scholar] [CrossRef] [PubMed]
- Gorle, S.; Maddila, S.; Maddila, S.N.; Naicker, K.; Singh, M.; Singh, P.; Jonnalagadda, S.B. Synthesis, molecular docking study and in vitro anticancer activity of tetrazole linked benzochromene derivatives. Anti-Cancer Agents Med. Chem. 2017, 17, 464–470. [Google Scholar] [CrossRef]
- Ahagh, M.H.; Dehghan, G.; Mehdipour, M.; Teimuri-Mofrad, R.; Payami, E.; Sheibani, N.; Ghaffari, M.; Asadi, M. Synthesis, characterization, anti-proliferative properties and DNA binding of benzochromene derivatives: Increased Bax/Bcl-2 ratio and caspase-dependent apoptosis in colorectal cancer cell line. Bioorg. Chem. 2019, 93, 103329. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Assaraf, Y.G.; Zhao, K.; Xu, X.; Xie, J.; Yang, D.-H.; Chen, Z.-S. Overcoming ABC transporter-mediated multidrug resistance: Molecular mechanisms and novel therapeutic drug strategies. Drug Resist. Updat. 2016, 27, 14–29. [Google Scholar] [CrossRef]
- Verhalen, B.; Dastvan, R.; Thangapandian, S.; Peskova, Y.; Koteiche, H.A.; Nakamoto, R.K.; Tajkhorshid, E.; Mchaourab, H.S. Energy transduction and alternating access of the mammalian ABC transporter P-glycoprotein. Nature 2017, 543, 738–741. [Google Scholar] [CrossRef]
- Saraswathy, M.; Gong, S. Different strategies to overcome multidrug resistance in cancer. Biotechnol. Adv. 2013, 31, 1397–1407. [Google Scholar] [CrossRef]
- Callaghan, R.; Luk, F.; Bebawy, M. Inhibition of the multidrug resistance P-glycoprotein: Time for a change of strategy? Drug Metab. Dispos. 2014, 42, 623–631. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Al-Ghamdi, A.M. Synthesis of certain novel 4H-pyrano [3, 2-h] quinoline derivatives. Arkivoc 2011, 11, 134–146. [Google Scholar] [CrossRef]
- Halawa, A.H.; Fouda, A.M.; Al-Dies, A.-A.M.; El-Agrody, A.M. Synthesis, Biological Evaluation and Molecular Docking Studies of 4-Hbenzo[h]chromenes, 7H-benzo[h]chromeno[2, 3-d]pyrimidines as Antitumor Agents. Lett. Drug Des. Discov. 2016, 13, 77–88. [Google Scholar] [CrossRef]
- Al-Sehemi, A.G.; Irfan, A.; El-Agrody, A.M. Synthesis, characterization and DFT study of 4H-benzo[h]chromene derivatives. J. Mol. Struct. 2012, 1018, 171–175. [Google Scholar] [CrossRef]
- Sayed, A.Z.; El-Hady, N.A.; El-Agrody, A.M. Condensation of α-cyanocinnamonitriles with 6-bromo-2-naphthol: Synthesis of pyrano[2, 3-d]pyrimidine and pyrano[3, 2-e][1, 2, 4]triazolo [2, 3-c]pyrimidine derivatives. J. Chem. Res. 2000, 2000, 164–166. [Google Scholar] [CrossRef]
- El-Agrody, A.; El-Latif, M.A.; Fakery, A.; Bedair, A. Heteroaromatization with 4-hydroxycoumarin Part I: Synthesis of some new pyranocoumarins and coumarinopyranopyrimidines. J. Chem. Res. 2000, 2000, 26–27. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Al-Dies, A.-A.M.; Fouda, A.M. Microwave assisted synthesis of 2-amino-6-methoxy-4H-benzo[h]- chromene derivatives. Eur. J. Chem. 2014, 5, 133–137. [Google Scholar] [CrossRef]
- El-Wahab, A.H.A.; Mohamed, H.M.; El-Agrody, A.M.; El-Nassag, M.A.; Bedair, A.H. Synthesis and biological screening of 4-benzyl-2 H-phthalazine derivatives. Pharmaceuticals 2011, 4, 1158–1170. [Google Scholar] [CrossRef]
- Abd-El-Aziz, A.S.; Shipman, P.O.; Neeland, E.G.; Corkery, T.C.; Mohammed, S.; Harvey, P.D.; Mohamed, H.M.; Bedair, A.H.; El-Agrody, A.M.; Aguiar, P.M. Benzo [f]-and Benzo [h] Coumarin-Containing Poly (methyl methacrylate) s and Poly (methyl methacrylate) s with Pendant Coumarin-Containing Azo Dyes. Macromol. Chem. Phys. 2008, 209, 84–103. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Khattab, E.S.A.; Fouda, A.M.; Al-Ghamdi, A.M. Synthesis and antitumor activities of certain novel 2-amino-9-(4-halostyryl)-4H-pyrano[3,2-h]quinoline derivatives. Med. Chem. Res. 2012, 21, 4200–4213. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Ali, F.M.; Eid, F.A.; El-Nassag, M.A.; El-Sherbeny, G.; Bedair, A.H. Synthesis and antimicrobial activity of thioxopyrimidines and related derivatives. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 839–864. [Google Scholar] [CrossRef]
- Halawa, A.H.; El-Gilil, A.; Shimaa, M.; Bedair, A.H.; Eliwa, E.M.; Frese, M.; Sewald, N.; Shaaban, M.; El-Agrody, A.M. Synthesis of diverse amide linked bis-indoles and indole derivatives bearing coumarin-based moiety: Cytotoxicity and molecular docking investigations. Med. Chem. Res. 2018, 27, 796–806. [Google Scholar] [CrossRef]
- Eliwa, E.M.; Abdel-Razek, A.S.; Frese, M.; Wibberg, D.; Halawa, A.H.; El-Agrody, A.M.; Bedair, A.H.; Kalinowski, J.; Sewald, N.; Shaaban, M. New bioactive compounds from the marine-derived actinomycete Nocardiopsis lucentensis sp. ASMR2. Z. Für Nat. B 2017, 72, 351–360. [Google Scholar] [CrossRef]
- Al-Dies, A.-A.; Amr, A.-G.; El-Agrody, A.M.; Chia, T.S.; Fun, H.-K. 2-Amino-4-(4-fluorophenyl)-6-methoxy-4H-benzo[h]- chromene-3-carbonitrile. Acta Crystallogr. Sect. E Struct. Rep. Online 2012, 68, o1934–o1935. [Google Scholar] [CrossRef] [PubMed]
- Bedair, A.H.; Ali, F.M.; El-Agrody, A.M.; Eid, F.A.; El-Nassag, M.A.; El-Sherbeny, G. Preparation of 4-aminophenylacetic acid derivatives with promising antimicrobial activity. Acta Pharm. 2006, 56, 273–284. [Google Scholar] [PubMed]
- El-Agrody, A.M.; Hassan, S.M. Activated Nitriles in Heterocyclic Synthesis: Synthesis of Several New 2-Substituted Pyrano-1, 2, 4-triazolopyrimidine Derivatives. ChemInform 1995, 26. [Google Scholar] [CrossRef]
- El-Agrody, A. Activated nitriles in heterocyclic synthesis: Synthesis of several new naphtho[2,1-b]pyran-3-one derivatives. J. Chem. Res. Synop. 1994, 50–51. [Google Scholar] [CrossRef]
- Omar, A.M.; Bajorath, J.; Ihmaid, S.; Mohamed, H.M.; El-Agrody, A.M.; Mora, A.; El-Araby, M.E.; Ahmed, H.E. Novel molecular discovery of promising amidine-based thiazole analogues as potent dual Matrix Metalloproteinase-2 and 9 inhibitors: Anticancer activity data with prominent cell cycle arrest and DNA fragmentation analysis effects. Bioorg. Chem. 2020, 101, 103992. [Google Scholar] [CrossRef]
- Halawa, A.H.; Elgammal, W.E.; Hassan, S.M.; Hassan, A.H.; Nassar, H.S.; Ebrahim, H.Y.; Mehany, A.B.; El-Agrody, A.M. Synthesis, anticancer evaluation and molecular docking studies of new heterocycles linked to sulfonamide moiety as novel human topoisomerase types I and II poisons. Bioorg. Chem. 2020, 98, 103725. [Google Scholar] [CrossRef]
- Elgaafary, M.; Fouda, A.M.; Mohamed, H.M.; Hamed, A.; El-Mawgoud, H.K.; Jin, L.; Ulrich, J.; Simmet, T.; Syrovets, T.; El-Agrody, A.M. Synthesis of β-Enaminonitrile-Linked 8-Methoxy-1H-Benzo[f]Chromene Moieties and Analysis of Their Antitumor Mechanisms. Front. Chem. 2021, 9, 759149. [Google Scholar] [CrossRef]
- Kheirollahi, A.; Pordeli, M.; Safavi, M.; Mashkouri, S.; Naimi-Jamal, M.R.; Ardestani, S.K. Cytotoxic and apoptotic effects of synthetic benzochromene derivatives on human cancer cell lines. Naunyn-Schmiedeberg’s Arch Pharm. 2014, 387, 1199–1208. [Google Scholar] [CrossRef]
- Elgaafary, M.; Lehner, J.; Fouda, A.M.; Hamed, A.; Ulrich, J.; Simmet, T.; Syrovets, T.; El-Agrody, A.M. Synthesis and evaluation of antitumor activity of 9-methoxy-1H-benzo[f]chromene derivatives. Bioorg. Chem. 2021, 116, 105402. [Google Scholar] [CrossRef]
- Fouda, A.M.; El-Nassag, M.A.; Elhenawy, A.A.; Shati, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Alam, M.M.; El-Agrody, A.M. Synthesis of 1, 4-dihydropyrano[2,3-c]pyrazole derivatives and exploring molecular and cytotoxic properties based on DFT and molecular docking studies. J. Mol. Struct. 2022, 1249, 131555. [Google Scholar] [CrossRef]
- Demirci, F.; Başer, K.H.C. Bioassay Techniques for Drug Development By Atta-ur-Rahman, M. Iqbal Choudhary (HEJRIC, University of Karachi, Pakistan), William, J. Thomsen (Areana Pharmaceuticals, San Diego, CA). Harwood Academic Publishers, Amsterdam, The Netherlands. 2001. xii+ 223 pp. 15.5 × 23.5 cm. $79.00. ISBN 90-5823-051-1. J. Nat. Prod. 2002, 65, 1086–1087. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or evade multidrug resistance P-glycoprotein in cancer treatment: Miniperspective. J. Med. Chem. 2017, 61, 5108–5121. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-S.; Yang, X.; Zhao, D.-S.; Cai, Y.; Huang, Z.; Wu, R.; Wang, S.-J.; Liu, G.-J.; Wang, J.; Bao, X.-Z. Design, synthesis and bioactivity study on 5-phenylfuran derivatives as potent reversal agents against P-glycoprotein-mediated multidrug resistance in MCF-7/ADR cell. Eur. J. Med. Chem. 2021, 216, 113336. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, Q.; Wang, B.; Yuan, S.; Wang, X.; Li, K. Quercetin reversed MDR in breast cancer cells through down-regulating P-gp expression and eliminating cancer stem cells mediated by YB-1 nuclear translocation. Phytother. Res. 2018, 32, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Luqmani, Y. Mechanisms of drug resistance in cancer chemotherapy. Med. Princ. Pract. 2005, 14, 35–48. [Google Scholar] [CrossRef]
- Dallavalle, S.; Dobričić, V.; Lazzarato, L.; Gazzano, E.; Machuqueiro, M.; Pajeva, I.; Tsakovska, I.; Zidar, N.; Fruttero, R. Improvement of conventional anti-cancer drugs as new tools against multidrug resistant tumors. Drug Resist. Updat. 2020, 50, 100682. [Google Scholar] [CrossRef]
- Schwartz, G.K.; Shah, M.A. Targeting the cell cycle: A new approach to cancer therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef]
- Williams, G.H.; Stoeber, K. The cell cycle and cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef]
- De, U.; Chun, P.; Choi, W.S.; Lee, B.M.; Kim, N.D.; Moon, H.R.; Jung, J.H.; Kim, H.S. A novel anthracene derivative, MHY412, induces apoptosis in doxorubicin-resistant MCF-7/Adr human breast cancer cells through cell cycle arrest and downregulation of P-glycoprotein expression. Int. J. Oncol. 2014, 44, 167–176. [Google Scholar] [CrossRef][Green Version]
- Kim, R. Recent advances in understanding the cell death pathways activated by anticancer therapy. Cancer 2005, 103, 1551–1560. [Google Scholar] [CrossRef] [PubMed]
- Tainton, K.M.; Smyth, M.J.; Jackson, J.T.; Tanner, J.E.; Cerruti, L.; Jane, S.M.; Darcy, P.K.; Johnstone, R.W. Mutational analysis of P-glycoprotein: Suppression of caspase activation in the absence of ATP-dependent drug efflux. Cell Death Differ. 2004, 11, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar] [PubMed]
- Alam, A.; Küng, R.; Kowal, J.; McLeod, R.A.; Tremp, N.; Broude, E.V.; Roninson, I.B.; Stahlberg, H.; Locher, K.P. Structure of a zosuquidar and UIC2-bound human-mouse chimeric ABCB1. Proc. Natl. Acad. Sci. USA 2018, 115, E1973–E1982. [Google Scholar] [CrossRef]
- Almalki, A.S.A.; Nazreen, S.; Malebari, A.M.; Ali, N.M.; Elhenawy, A.A.; Alghamdi, A.A.A.; Ahmad, A.; Alfaifi, S.Y.M.; Alsharif, M.A.; Alam, M.M. Synthesis and Biological Evaluation of 1,2,3-Triazole Tethered Thymol-1,3,4-Oxadiazole Derivatives as Anticancer and Antimicrobial Agents. Pharmaceuticals 2021, 14, 866. [Google Scholar] [CrossRef]
- El Gaafary, M.; Syrovets, T.; Mohamed, H.M.; Elhenawy, A.A.; El-Agrody, A.M.; El-Galil, E.; Amr, A.; Ghabbour, H.A.; Almehizia, A.A. Synthesis, Cytotoxic Activity, Crystal Structure, DFT Studies and Molecular Docking of 3-Amino-1-(2,5-dichlorophenyl)-8-methoxy-1H-benzo[f]chromene-2-carbonitrile. Crystals 2021, 11, 184. [Google Scholar] [CrossRef]
- El-Agrody, A.M.; Fouda, A.M.; Mohamed, H.M.; Alshahrani, M.Y.; Ghabbour, H.A.; Amr, A.E.-G.E.; Okasha, R.M.; Naglah, A.M.; Almehizia, A.A.; Elhenawy, A.A. The Crystal Structure of 2-Amino-4-(2,3-Dichlorophenyl)-6-Methoxy-4H-Benzo[h]chromene-3-Carbonitrile: Antitumor and Tyrosine Kinase Receptor Inhibition Mechanism Studies. Crystals 2022, 12, 737. [Google Scholar] [CrossRef]
- Alam, M.M.; Nazreen, S.; Almalki, A.S.A.; Elhenawy, A.A.; Alsenani, N.I.; Elbehairi, S.E.I.; Malebari, A.M.; Alfaifi, M.Y.; Alsharif, M.A.; Alfaifi, S.Y.M. Naproxen Based 1,3,4-Oxadiazole Derivatives as EGFR Inhibitors: Design, Synthesis, Anticancer, and Computational Studies. Pharmaceuticals 2021, 14, 870. [Google Scholar] [CrossRef]
- Chaiyarit, S.; Thongboonkerd, V. Comparative analyses of cell disruption methods for mitochondrial isolation in high-throughput proteomics study. Anal. Biochem. 2009, 394, 249–258. [Google Scholar] [CrossRef]
- Shchulkin, A.V.; Abalenikhina, Y.V.; Erokhina, P.D.; Chernykh, I.V.; Yakusheva, E.N. The Role of P-Glycoprotein in Decreasing Cell Membranes Permeability during Oxidative Stress. Biochemistry 2021, 86, 197–206. [Google Scholar] [CrossRef]
- Jouan, E.; Le Vée, M.; Mayati, A.; Denizot, C.; Parmentier, Y.; Fardel, O. Evaluation of P-Glycoprotein Inhibitory Potential Using a Rhodamine 123 Accumulation Assay. Pharmaceutics 2016, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Bochevarov, A.D.; Harder, E.; Hughes, T.F.; Greenwood, J.R.; Braden, D.A.; Philipp, D.M.; Rinaldo, D.; Halls, M.D.; Zhang, J.; Friesner, R.A. Jaguar: A high-performance quantum chemistry software program with strengths in life and materials sciences. Int. J. Quantum Chem. 2013, 113, 2110–2142. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).