The Predictive Role of Extracellular NAPRT for the Detection of Advanced Fibrosis in Biopsy-Proven Non-Alcoholic Fatty Liver Disease
Abstract
1. Introduction
2. Results
2.1. Characteristics of the Study Cohort
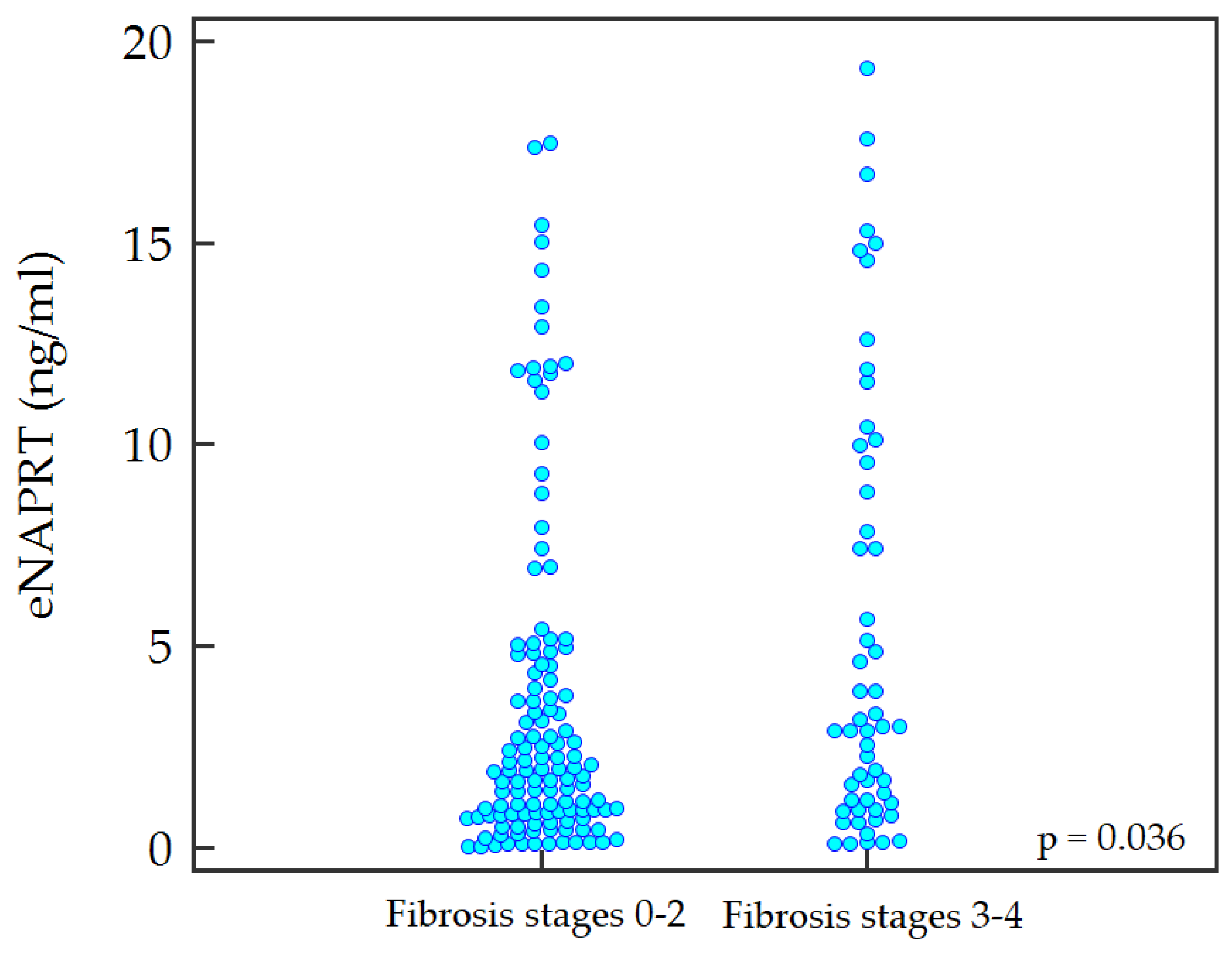
2.2. Predictive Ability of Non-Invasive Tests and eNAPRT for Advanced Fibrosis
2.3. Multiple Stepwise Regression Model for the Identification of Advanced Fibrosis
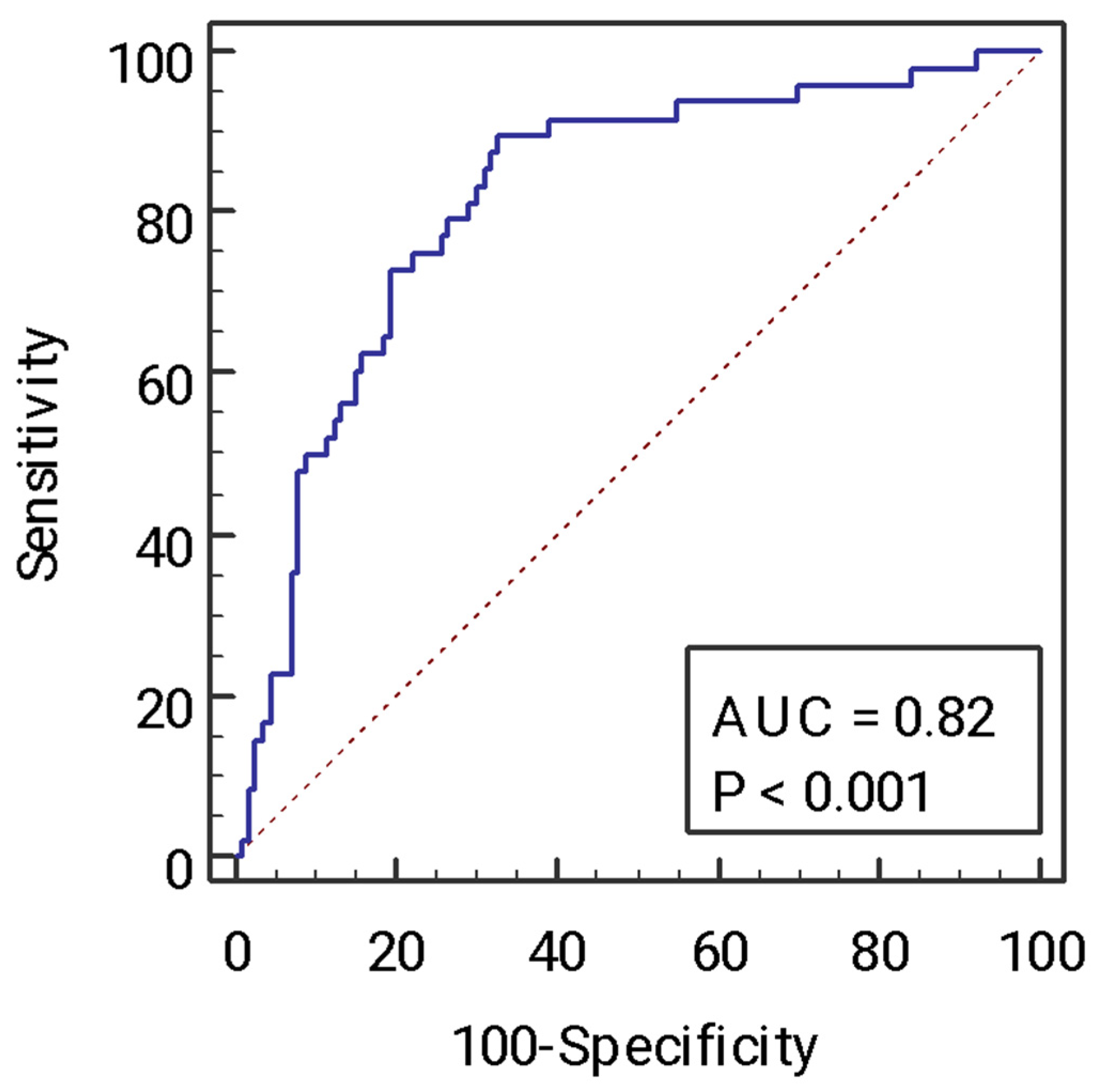
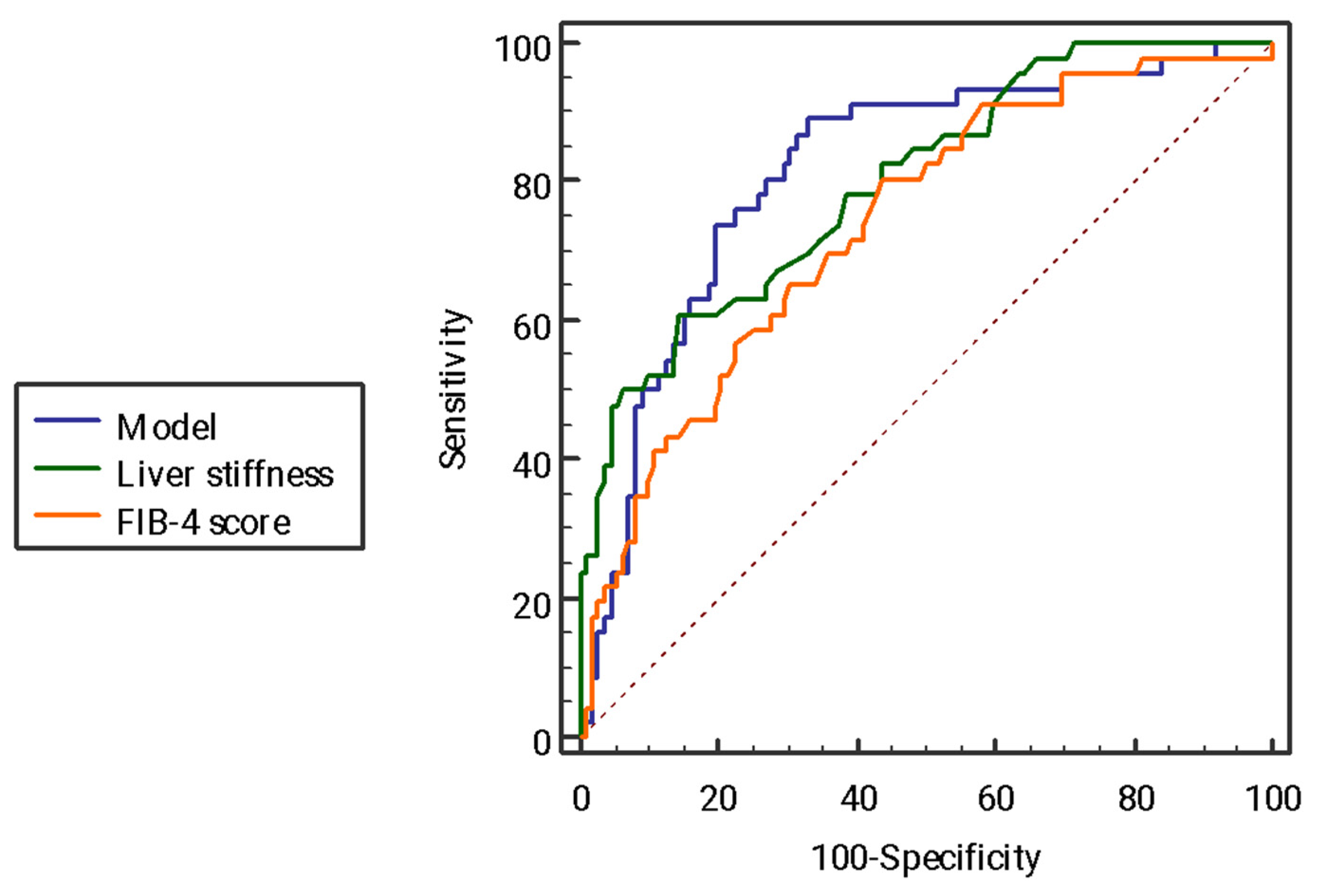
2.4. Receiving Operator Curve Analysis of the Model for the Identification of Advanced Fibrosis and Comparison with Other Non-Invasive Tests
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Analytical Determination
4.3. Histology
4.4. Non-Invasive Assessment of Liver Fibrosis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.M.; Koenig, A.B.; Abdelatif, D.; Fazel, Y.; Henry, L.; Wymer, M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016, 64, 73–84. [Google Scholar] [CrossRef]
- Armandi, A.; Rosso, C.; Caviglia, G.P.; Bugianesi, E. Insulin Resistance across the Spectrum of Nonalcoholic Fatty Liver Disease. Metabolites 2021, 11, 155. [Google Scholar] [CrossRef]
- Armandi, A.; Bugianesi, E. Natural history of NASH. Liver Int. 2021, 41 (Suppl. S1), 78–82. [Google Scholar] [CrossRef]
- Dulai, P.S.; Singh, S.; Patel, J.; Soni, M.; Prokop, L.J.; Younossi, Z.; Sebastiani, G.; Ekstedt, M.; Hagstrom, H.; Nasr, P.; et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: Systematic review and meta-analysis. Hepatology 2017, 65, 1557–1565. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; de Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015, 11, 535–546. [Google Scholar] [CrossRef]
- Travelli, C.; Aprile, S.; Mattoteia, D.; Colombo, G.; Clemente, N.; Scanziani, E.; Terrazzino, S.; Alisi, M.A.; Polenzani, L.; Grosa, G.; et al. Identification of potent triazolylpyridine nicotinamide phosphoribosyltransferase (NAMPT) inhibitors bearing a 1,2,3-triazole tail group. Eur. J. Med. Chem. 2019, 181, 111576. [Google Scholar] [CrossRef]
- Franco, J.; Piacente, F.; Walter, M.; Fratta, S.; Ghanem, M.; Benzi, A.; Caffa, I.; Kurkin, A.V.; Altieri, A.; Herr, P.; et al. Structure-Based Identification and Biological Characterization of New NAPRT Inhibitors. Pharmaceuticals 2022, 15, 855. [Google Scholar] [CrossRef]
- Ghanem, M.S.; Caffa, I.; Del Rio, A.; Franco, J.; Parenti, M.D.; Monacelli, F.; Cea, M.; Khalifa, A.; Nahimana, A.; Duchosal, M.A.; et al. Identification of NAPRT Inhibitors with Anti-Cancer Properties by In Silico Drug Discovery. Pharmaceuticals 2022, 15, 848. [Google Scholar] [CrossRef]
- Audrito, V.; Messana, V.G.; Deaglio, S. NAMPT and NAPRT: Two Metabolic Enzymes With Key Roles in Inflammation. Front. Oncol. 2020, 10, 358. [Google Scholar] [CrossRef]
- Audrito, V.; Messana, V.G.; Brandimarte, L.; Deaglio, S. The Extracellular NADome Modulates Immune Responses. Front. Immunol. 2021, 12, 704779. [Google Scholar] [CrossRef]
- Managò, A.; Audrito, V.; Mazzola, F.; Sorci, L.; Gaudino, F.; Gizzi, K.; Vitale, N.; Incarnato, D.; Minazzato, G.; Ianniello, A.; et al. Extracellular nicotinate phosphoribosyltransferase binds Toll like receptor 4 and mediates inflammation. Nat. Commun. 2019, 10, 4116. [Google Scholar] [CrossRef]
- Schuppan, D.; Ashfaq-Khan, M.; Yang, A.T.; Kim, Y.O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018, 68–69, 435–451. [Google Scholar] [CrossRef]
- Rosso, C.; Kazankov, K.; Younes, R.; Esmaili, S.; Marietti, M.; Sacco, M.; Carli, F.; Gaggini, M.; Salomone, F.; Møller, H.J.; et al. Crosstalk between adipose tissue insulin resistance and liver macrophages in non-alcoholic fatty liver disease. J. Hepatol. 2019, 71, 1012–1021. [Google Scholar] [CrossRef]
- Gehrke, N.; Schattenberg, J.M. Metabolic Inflammation-A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidities in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020, 158, 1929–1947.e6. [Google Scholar] [CrossRef]
- Schuppan, D.; Surabattula, R.; Wang, X.Y. Determinants of fibrosis progression and regression in NASH. J. Hepatol. 2018, 68, 238–250. [Google Scholar] [CrossRef]
- Mózes, F.E.; Lee, J.A.; Selvaraj, E.A.; Jayaswal, A.N.A.; Trauner, M.; Boursier, J.; Fournier, C.; Staufer, K.; E Stauber, R.; Bugianesi, E.; et al. Diagnostic accuracy of non-invasive tests for advanced fibrosis in patients with NAFLD: An individual patient data meta-analysis. Gut 2022, 71, 1006–1019. [Google Scholar] [CrossRef]
- Sun, Z.; Lei, H.; Zhang, Z. Pre-B cell colony enhancing factor (PBEF), a cytokine with multiple physiological functions. Cytokine Growth Factor Rev. 2013, 24, 433–442. [Google Scholar] [CrossRef]
- Van den Bergh, R.; Morin, S.; Sass, H.J.; Grzesiek, S.; Vekemans, M.; Florence, E.; Thanh Thi Tran, H.; Imiru, R.G.; Heyndrickx, L.; Vanham, G.; et al. Monocytes contribute to differential immune pressure on R5 versus X4 HIV through the adipocytokine visfatin/NAMPT. PLoS ONE 2012, 7, e35074. [Google Scholar] [CrossRef]
- Camp, S.M.; Ceco, E.; Evenoski, C.L.; Danilov, S.M.; Zhou, T.; Chiang, E.T.; Moreno-Vinasco, L.; Mapes, B.; Zhao, J.; Gursoy, G.; et al. Unique Toll-Like Receptor 4 Activation by NAMPT/PBEF Induces NFκB Signaling and Inflammatory Lung Injury. Sci. Rep. 2015, 5, 13135. [Google Scholar] [CrossRef]
- Colombo, G.; Travelli, C.; Porta, C.; Genazzani, A.A. Extracellular nicotinamide phosphoribosyltransferase boosts IFNγ-induced macrophage polarization independently of TLR4. iScience 2022, 25, 104147. [Google Scholar] [CrossRef]
- Aller, R.; De Luis, D.A.; Izaola, O.; Sagrado, M.G.; Conde-Vicente, R.; Velasco, M.C.; Alvarez, T.; Pacheco, D.; González, J.M. Influence of visfatin on histopathological changes of non-alcoholic fatty liver disease. Dig. Dis. Sci. 2009, 54, 1772–1777. [Google Scholar] [CrossRef]
- Kukla, M.; Ciupińska-Kajor, M.; Kajor, M.; Wyleżoł, M.; Żwirska-Korczala, K.; Hartleb, M.; Berdowska, A.; Mazur, W. Liver visfatin expression in morbidly obese patients with nonalcoholic fatty liver disease undergoing bariatric surgery. Pol. J. Pathol. 2010, 61, 147–153. [Google Scholar]
- Ismaiel, A.; Leucuta, D.C.; Popa, S.L.; Dumitrascu, D.L. Serum Visfatin Levels in Nonalcoholic Fatty Liver Disease and Liver Fibrosis: Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 3029. [Google Scholar] [CrossRef]
- Li, X.-Q.; Lei, J.; Mao, L.-H.; Wang, Q.-L.; Xu, F.; Ran, T.; Zhou, Z.-H.; He, S. NAMPT and NAPRT, Key Enzymes in NAD Salvage Synthesis Pathway, Are of Negative Prognostic Value in Colorectal Cancer. Front. Oncol. 2019, 9, 736. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Sterling, R.K.; Lissen, E.; Clumeck, N.; Sola, R.; Correa, M.C.; Montaner, J.; Sulkowski, M.S.; Torriani, F.J.; Dieterich, D.T.; Thomas, D.L.; et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology 2006, 43, 1317–1325. [Google Scholar] [CrossRef]
| Parameter (n = 180) | |
|---|---|
| Male sex, n (%) | 114 (63.3) |
| Age (years), median [IQR] | 47.0 [38.0–58.0] |
| BMI (kg/m2), median [IQR] | 29.2 [26.3–32.9] |
| Type 2 diabetes, n (%) | 50 (27.8) |
| AST (IU/L), median [IQR] | 31.0 [21.5–43.5] |
| ALT (IU/L), median [IQR] | 48.0 [33.0–76.0] |
| GGT (IU/L), median [IQR] | 47.0 [28.0–97.5] |
| Total bilirubin (mg/dL), median [IQR] | 0.7 [0.5–0.9] |
| Platelet count (109/L), median [IQR] | 230.5 [196.5–274.0] |
| Glucose (mg/dL), median [IQR] | 93.0 [85.5–109.5] |
| Triglycerides (mg/dL), median [IQR] | 124.0 [91.0–175.5] |
| Total cholesterol (mg/dL), median [IQR] | 185.5 [162.0–211.0] |
| HDL cholesterol (mg/dL), median [IQR] | 47.0 [39.0–55.0] |
| Liver stiffness (kPa), median [IQR] | 7.4 [5.8–10.0] |
| FIB-4 score, median [IQR] | 0.9 [0.6–1.3] |
| eNAMPT (ng/mL), median [IQR] | 4.1 [1.8–8.2] |
| eNAPRT (ng/mL), median [IQR] | 2.1 [0.9–5.1] |
| Parameter (n = 180) | |
|---|---|
| Steatosis, n (%) | |
| - Grade 1 - Grade 2 - Grade 3 | 87 (48.3) 64 (35.6) 29 (16.1) |
| Lobular inflammation, n (%) | |
| - Grade 0 - Grade 1 - Grade 2 | 33 (18.3) 130 (72.2) 17 (9.4) |
| Ballooning, n (%) | |
| - Grade 0 - Grade 1 - Grade 2 | 27 (15.0) 96 (53.3) 57 (31.7) |
| Fibrosis, n (%) | |
| - Stage 0 - Stage 1 - Stage 2 - Stage 3 - Stafe 4 | 46 (25.6) 52 (28.9) 27 (15.0) 35 (19.4) 20 (11.1) |
| Advanced fibrosis (stage 3–4), n (%) | 55 (30.6) |
| NASH, n (%) | 140 (77.8) |
| Parameter | OR | 95% Confidence Interval | p Value |
|---|---|---|---|
| eNAPRT (ng/mL) | 1.08 | 1.01–1.15 | 0.016 |
| Liver stiffness (kPa) | 1.33 | 1.20–1.49 | <0.0001 |
| FIB-4 score | 2.40 | 1.44–3.97 | <0.0001 |
| Parameter | AUC | Cut-off (Youden Index) | Se (%) | Sp (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|
| eNAPRT (ng/mL) | 0.60 | 2.76 | 56.4 | 64.0 | 40.8 | 76.9 |
| Liver stiffness (kPa) | 0.79 | 9.4 | 63.5 | 86.2 | 66.0 | 84.8 |
| FIB-4 score | 0.73 | 0.86 | 80.0 | 56.8 | 44.9 | 86.6 |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Parameter | OR | 95% Confidence Interval | p Value | OR | 95% Confidence Interval | p Value |
| eNAPRT > 2.76 ng/mL | 2.24 | 1.16–4.33 | 0.015 | 2.73 | 1.20–6.16 | 0.015 |
| Age (years) | 1.05 | 1.02–1.09 | 0.0001 | 1.03 | 1.01–1.07 | 0.049 |
| Type 2 diabetes | 4.28 | 2.13–8.58 | <0.0001 | 3.49 | 1.48–8.21 | 0.004 |
| Male sex | 2.65 | 1.38–5.10 | 0.003 | 2.84 | 1.63–9.05 | 0.002 |
| ALT (IU/L) | 1.01 | 1.00–1.05 | 0.050 | 1.01 | 1.00–1.02 | 0.021 |
| AST (IU/L) | 1.01 | 0.99–1.02 | 0.052 | - | ||
| BMI (kg/m2) | 1.08 | 1.01–1.16 | 0.018 | - | ||
| GGT (IU/L) | 1.00 | 0.99–1.00 | 0.891 | - | ||
| Total cholesterol (mg/dL) | 0.99 | 0.99–1.00 | 0.658 | - | ||
| HDL cholesterol (mg/dL) | 1.01 | 0.98–1.04 | 0.257 | - | ||
| Triglycerides (mg/dL) | 0.99 | 0.99–1.00 | 0.361 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armandi, A.; Colombo, G.; Rosso, C.; Caviglia, G.P.; Olivero, A.; Abate, M.L.; Guariglia, M.; Perez Diaz del Campo, N.; Castelnuovo, G.; Ribaldone, D.G.; et al. The Predictive Role of Extracellular NAPRT for the Detection of Advanced Fibrosis in Biopsy-Proven Non-Alcoholic Fatty Liver Disease. Int. J. Mol. Sci. 2023, 24, 1172. https://doi.org/10.3390/ijms24021172
Armandi A, Colombo G, Rosso C, Caviglia GP, Olivero A, Abate ML, Guariglia M, Perez Diaz del Campo N, Castelnuovo G, Ribaldone DG, et al. The Predictive Role of Extracellular NAPRT for the Detection of Advanced Fibrosis in Biopsy-Proven Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences. 2023; 24(2):1172. https://doi.org/10.3390/ijms24021172
Chicago/Turabian StyleArmandi, Angelo, Giorgia Colombo, Chiara Rosso, Gian Paolo Caviglia, Antonella Olivero, Maria Lorena Abate, Marta Guariglia, Nuria Perez Diaz del Campo, Gabriele Castelnuovo, Davide Giuseppe Ribaldone, and et al. 2023. "The Predictive Role of Extracellular NAPRT for the Detection of Advanced Fibrosis in Biopsy-Proven Non-Alcoholic Fatty Liver Disease" International Journal of Molecular Sciences 24, no. 2: 1172. https://doi.org/10.3390/ijms24021172
APA StyleArmandi, A., Colombo, G., Rosso, C., Caviglia, G. P., Olivero, A., Abate, M. L., Guariglia, M., Perez Diaz del Campo, N., Castelnuovo, G., Ribaldone, D. G., Saracco, G. M., Genazzani, A. A., & Bugianesi, E. (2023). The Predictive Role of Extracellular NAPRT for the Detection of Advanced Fibrosis in Biopsy-Proven Non-Alcoholic Fatty Liver Disease. International Journal of Molecular Sciences, 24(2), 1172. https://doi.org/10.3390/ijms24021172









