Abstract
Porphobilinogen deaminase (PBGD) haploinsufficiency (acute intermittent porphyria, AIP) is characterized by neurovisceral attacks associated with high production, accumulation and urinary excretion of heme precursors, δ-aminolevulinic acid (ALA) and porphobilinogen (PBG). The estimated clinical penetrance for AIP is extremely low (<1%), therefore it is likely that other factors may play an important role in the predisposition to developing attacks. Fasting is a known triggering factor. Given the increased prevalence of insulin resistance in patients and the large urinary loss of succinyl-CoA to produce ALA and PBG, we explore the impact of reduced availability of energy metabolites in the severity of AIP pathophysiology. Classic studies found clinical improvement in patients affected by AIP associated with the administration of glucose and concomitant insulin secretion, or after hyperinsulinemia associated with diabetes. Molecular studies have confirmed that glucose and insulin administration induces a repressive effect on hepatic ALA Synthase, the first and regulatory step of the heme pathway. More recently, the insulin-mimicking α-lipoic acid has been shown to improve glucose metabolism and mitochondrial dysfunction in a hepatocyte cell line transfected with interfering RNA targeting PBGD. In AIP mice, preventive treatment with an experimental fusion protein of insulin and apolipoprotein A-I improved the disease by promoting fat mobilization in adipose tissue, increasing the metabolite bioavailability for the TCA cycle and inducing mitochondrial biogenesis in the liver. In this review, we analyze the possible mechanisms underlying abnormal hepatocellular carbohydrate homeostasis in AIP.
1. Introduction
Acute intermittent porphyria (AIP, MIM 176000) is an autosomal dominant disease caused by a partial deficiency of the hepatic porphobilinogen deaminase (PBGD, EC 4.3.1.8), the third enzyme of the heme synthesis pathway. AIP is characterized by acute attacks of abdominal pain, nausea, vomiting and fatigue that can be triggered by endogenous or exogenous factors, such as a low carbohydrate diet or fasting, that up-regulate the expression of the first enzyme of the pathway, δ-aminolevulinate (ALA) synthase 1 (ALAS1, EC 2.3.1.27) [1,2]. This mechanism can cause an excessive accumulation of toxic substrates, ALA and porphobilinogen (PBG) [3,4,5].
Various studies have reflected a high prevalence of HMBS enzyme mutations, with figures of around 1 in 1700 individuals. Penetrance, however, is markedly low, less than 10% among families of AIP patients, and could be as low as <=1% depending on the degree of underdiagnosis [6]. Thus, not all carriers of a mutation in HMBS will go on to experience an acute attack of porphyria in the course of their lives, but the association with other causative or modifier genes, or other inducing factors will be necessary to trigger these attacks. Furthermore, a substantial number of asymptomatic patients maintain a high excretion of ALA and PBG, which suggests that the pathophysiology of acute events cannot only be associated with the accumulation of neurotoxic by-products. Homedan et al. [7] suggest that the removal of succinyl-CoA from the TCA, used to support the increased demand for heme synthesis in the context of an AIP attack, could have a profound, although reversible, impact on mitochondrial energy exchange. Several additional studies have addressed the metabolic aspect of acute porphyrias that could be associated with an energy misbalance due to sustained overproduction of heme-precursors in the liver [8]. Indeed, reduced serum accumulation of insulin-like growth factor 1 and transthyretin, associated with a state of under-nutrition and/or with hepatic inflammation due to the sustained accumulation of heme-precursors, have been proposed as biomarkers of morbidity/severity of the disease for the clinical follow-up of patients with AIP [9].
A balanced diet of proteins and fats and a carbohydrate intake of 45–60% of total energy intake is recommended in patients with acute porphyrias [10,11]. Carbohydrate loading is especially important when patients begin to have emerging porphyria symptoms.
The effect of diet on experimental porphyria was known even before the biochemical basis of porphyria was discovered. In 1961, Rose et al. [12] observed that the administration of carbohydrates reduced the urinary excretion of PBG in a murine model of porphyria induced by 2-allyl-2-isopropyl-acetamide (AIA), a porphyrinogenic drug. This finding was initially interpreted as a direct interaction between AIA and carbohydrates. However, in 1964, Tschudy [13] demonstrated that the administration of carbohydrates inhibits the induction of ALAS1. This finding explained why fasted animals had a greater overproduction of heme precursors than non-fasted animals in experimental models of porphyria [14]. Subsequently, it was found that the excretion of ALA and PBG is also modified by the amount of carbohydrates ingested in humans [15]. Since then, carbohydrate overload has been administered as a treatment during acute attacks of porphyria, although the exact mechanism by which carbohydrates exerted this effect was not described until 2005 [16].
2. Carbohydrate Metabolism in AIP
Glucose is an essential human nutrient and acts as the main energy supply. The liver has a primary role in glycemic control. It regulates the balance between glucose storage, through glycogenogenesis and its release, through glycogenolysis and gluconeogenesis [17].
In response to low blood sugar and the resulting increase in glucagon, hepatocytes activate glycogen breakdown to release glucose into the bloodstream for uptake by other cells. The enzyme glycogen phosphorylase (GP, EC 2.4.1.1) plays a major role in the mobilization of glucose stored in tissues during glycogenolysis. This enzyme catalyzes the cleavage of glycogen to glucose-1-phosphate [18]. Insulin also inhibits this enzyme by promoting the phosphorylation of transcription factors and blocking its expression [19].
Gluconeogenesis is the process of glucose formation from non-glucidic substrates such as lactate and alanine. This metabolic pathway is essential for glucose production during prolonged fasting. A key step in gluconeogenesis is the formation of phosphoenolpyruvate from oxaloacetate, which is catalyzed by the enzyme phosphoenolpyruvate carboxykinase (PEPCK EC 4.1.1.32) [20]. The transcription of PEPCK is regulated by multiple factors, both dietary and hormonal: cyclic AMP, glucocorticoids and thyroid hormones increase PEPCK gene expression [21]. By contrast, insulin inhibits its transcription [22].
In 2005, Lelli et al. [23] described a blockade of the gluconeogenesis and glycogenolysis pathways in pharmacologically induced porphyric mice. This blockade seems to be a consequence of a decrease in hepatic expression of PEPCK and GP. This finding was corroborated by Collantes et al. [24], who described deficient induction of glycogenolysis in fasted AIP mice when compared with wild-type (WT) mice. By contrast, AIP mice activated the ketogenic pathway. Those PBGD-deficient mice also showed impaired glucose on a tolerance test, as well as serum hyperinsulinemia compared with the WT group. Notably, both parameters were normalized when hepatic PBGD deficiency was corrected through liver gene therapy. These data suggest that insulin resistance is associated with hepatic PBGD deficiency. Other authors [25,26] had previously suggested that the increase in the insulin/glucagon ratio is responsible for the abnormalities in oral tolerance to glucose in patients with porphyria
3. Role of Insulin in the Heme Synthesis Pathway
The counterregulatory hormones insulin and glucagon are responsible for the regulation of ALAS1 during periods of fasting and feeding through coactivator 1-alpha of peroxisome proliferator-activated receptor gamma (PGC-1α) [16] (Figure 1). PGC-1α is a main transcriptional coactivator for the control of mitochondrial biogenesis and hepatic gluconeogenesis [27]. This cofactor increases hepatic ALAS1 transcription through the interactions of the ALAS1 promoter with the transcription factor FOXO1 (forkhead box O1). In a dephosphorylated state, FOXO1 binds to PGC-1 and forms a transcriptional complex capable of inducing ALAS1 expression.
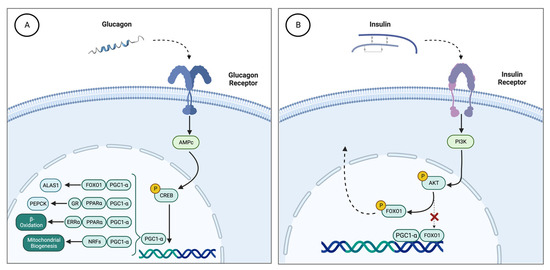
Figure 1.
(A) Effect of glucagon on hepatic ALAS1 through PGC1a. During fasting periods, there is an increase in serum glucagon. This hormone stimulates the cAMP and CREB pathways, which activate the expression of the PGC-1 gene. Then, PGC-1α recruits different transcription factor binding promoters such as PEPCK (PGC1α, GR and PPARα) to induce gluconeogenesis, GP (FOXO, HNF4α and PGC1α) to enhance glycogenolysis, induces the expression of NRFs that increase mitochondrial DNA transcription and replication, and stimulates the heme biosynthetic pathway through the activation of ALAS1 (FOXO1 and PGC1α). (B) Effect of insulin on PGC1α. Carbohydrate intake produces an increase in serum insulin levels, with the consequent activation of the PI3K pathway. PI3K regulates the phosphorylation of AKT, which in turn phosphorylates FOXO1. Phosphorylation of FOXO1 disrupts the transcriptional complex it forms with PGC1α, such that FOXO1p translocates out of the nucleus and PGC1α loses its ability to bind to the ALAS1 transcriptional regulatory sequence. ALAS1, δ-aminolevulinate synthase 1; AMPc, cyclic adenosine monophosphate; FOXO1, forkhead box O1; NRFs, nuclear respiratory factor 1; PEPCK, phosphoenolpyruvate carboxykinase; GP, glycogen phosphorylase; PI3K, phosphatidylinositol-3 kinase; PGC1α, proliferator-activated receptor γ coactivator 1α.
During fasting, glucagon induces PGC-1α through the stimulation of the cyclic AMP pathway. PGC-1α recruits transcription factors that bind the promoter of gluconeogenic genes, such as PEPCK, to stimulate beta-oxidation of fatty acids for energy supply during periods of prolonged fasting [27].
Therefore, the increase in serum glucagon stimulates the production of PGC-1α, which induces the synthesis of ALAS1. Through this mechanism, fasting causes activation of the heme biosynthetic pathway and can act as a trigger for an acute attack of porphyria [28] (Figure 1A).
By contrast, postprandial carbohydrate overload stimulates insulin secretion, which induces phosphorylation of FOXO1 and disrupts the transcriptional complex with PGC1a (Figure 1B). Therefore, insulin signaling through PI3K downregulates the overexpression of hepatic ALAS1 after glucose loading therapy [29]. Therefore, insulin resistance due to receptor deficiency or abnormal PI3K signaling in the liver may condition the therapeutic effect of glucose in acute porphyria.
Some groups have described a differential behavior in AIP depending on their insulinemia. Storjord et al. found that stable patients have higher insulin levels than those with symptoms [30]. Likewise, fasting insulinemia is lower in those patients who present a high level of urinary PBG [31].
4. Insulin Resistance in AIP
In 1949, Sterling et al. [32] were the first to report the association between the development of diabetes and porphyria. They described three cases of diabetes mellitus in a series of eight patients with AIP, although the impact of this relationship was unknown at that moment. Subsequently, Sixel-Dietrich [33] compared the results of an oral glucose tolerance test in an AIP patient during an acute crisis versus an asymptomatic carrier of the disease. He found that in the AIP patient, parallel to the decrease in neurotoxic precursors, there was a marked increase in serum glucose and insulin levels. These findings contrasted with a glycemic curve and an insulin concentration within the normal range presented by the asymptomatic control. Subsequently, several human case series [34,35,36] and experimental murine models [37] have confirmed the association between abnormal oral glucose tolerance tests and insulin resistance.
However, the occurrence of insulin resistance is not homogeneous among individuals with acute porphyria. Between Norwegian patients with AIP and controls, no significant differences were observed in insulinemia or the homeostasis model assessment (HOMA) index, which is a method for quantifying insulin resistance and beta-cell function; however, those patients with periodontitis showed an increased prevalence of insulin resistance [38].
In a recent observational case-control study, we compared the prevalence of insulin resistance in 44 Spanish patients with AIP and 55 age-, gender- and BMI-matched control volunteers. We found a significantly higher prevalence of insulin resistance and HOMA index in patients with stable disease than in patients with active porphyria (Figure 2) [39]. There were no significant differences in overweight, sedentary lifestyle, or metabolic syndrome between AIP patients and the control group; thus, finding significantly more insulin-resistant patients in the AIP group cannot be related to the factors characteristically associated with insulin resistance.
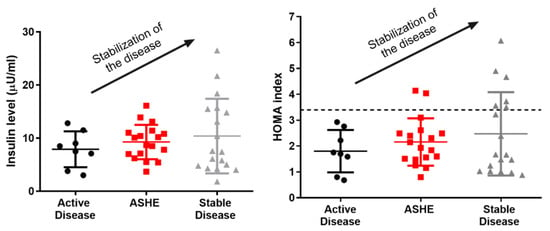
Figure 2.
Plasma insulin quantification and HOMA index in porphyric patients. It is clear that, as the insulinemia and the HOMA of the patients rise, their disease stabilizes. AD: active disease, ASHE: asymptomatic high excreters, HOMA: homeostasis model assessment, SD: stable disease.
To our knowledge, no molecular mechanism has been described that could explain the pancreatic hypersecretion of insulin in some patients with AIP. The most widely accepted model of the pathogenesis of type 2 diabetes postulates that a high-fat diet leads to obesity and insulin resistance [40]. According to this theory, excess energy metabolites cause a reduction in insulin receptor signaling pancreatic β cells to release more insulin. However, the physiological mechanism of this hyperstimulation is unclear since it often occurs before hyperglycemia. Subsequently, pancreatic β cells become exhausted, leading to the development of type 2 diabetes. This hypothesis suggests that hyperinsulinemia is a compensation state for systemic insulin resistance [41]. Nevertheless, multiple studies have shown that insulin hypersecretion precedes obesity and insulin resistance, which is not consistent with the notion of hyperinsulinemia being simply an adaptive response to insulin resistance [42,43].
Shanik et al. [44] describe different situations in which insulin appears to be an essential quantitative contributor to insulin resistance, such as in patients with insulinomas. They conclude that hyperinsulinemia may result from and be a driver of insulin resistance. Thus, it is also possible that the hyperinsulinemia observed in patients with AIP is responsible for the subsequent development of insulin resistance. The exact mechanism by which patients with porphyria, especially those who are asymptomatic, present elevated insulinemia is still unknown. Matkovic et al. suggested that the increase in insulin levels in porphyria may be partly due to a decrease in its degradation [45]. The high production of reactive oxygen species in AIP promotes oxidative stress, which seems to interfere with the activity of glutathione-insulin transhydrogenase (EC 1.8.4.2), the enzyme responsible for insulin degradation [46]. The sustained increase in insulinemia in these patients may be responsible for the subsequent development of insulin resistance and the blockade of the gluconeogenesis and glycogenolysis pathways by causing the inhibition of PEPCK and GP, respectively (Figure 3).
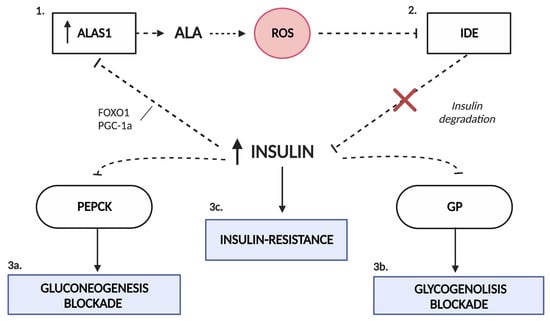
Figure 3.
Interaction of carbohydrate metabolism and the heme biosynthetic pathway. The state of oxidative stress produced by the excess of ALA in porphyria (1) favors the dysfunction of the enzyme glutathione-insulin transhydrogenase, responsible for the degradation of insulin (2). Hyperinsulinemia alters the pathways of gluconeogenesis (3a) and glycogenolysis (3b) due to the inhibition that insulin exerts on PEPCK and GP enzymes. This increase in serum insulin favors the inhibition of ALAS1 by the disruption of the FOXO1-PGC1α complex (1). In addition, it is likely that it promotes the development of insulin resistance in porphyric patients (3c). ALAS1, δ-aminolevulinate synthase 1; FOXO1, forkhead box O1; GP, glycogen phosphorylase; IDE, insulin-degrading enzyme; PEPCK, phosphoenolpyruvate carboxykinase; PGC1α, proliferator-activated receptor γ coactivator 1α; ROS, reactive oxygen species.
Although the appearance of insulin resistance is not fully explained, our results suggest that the increase in insulinemia could be associated with the stabilization of porphyria, from both a clinical and biochemical point of view. This fact is supported by the known repressive effect of insulin on the transcription of PGC1α, a cofactor with the ability to repress ALAS1 transcription. Thus, insulin administration might be an innovative therapeutic tool for treating acute crises of porphyria
5. Insulin as a Therapeutic Weapon
One study already reported in 1970 showed how some patients experienced a clinical improvement when small amounts of insulin were administered together with carbohydrates during crises [47]. Experimental studies conducted by Handschin et al. [16] also found that the combination of glucose and insulin causes more potent inhibition of ALAS1 than administering glucose alone. On the other hand, although Oliveri et al. [48] did not find a reduction in the levels of PGC-1α with insulin administration, they observed that this treatment reduced the expression of ALAS1.
However, a possible drawback of using this drug could be the iatrogenic induction of hypoglycemia [39]. This could stimulate glucagon secretion; therefore, the effect obtained would be the induction of hepatic ALAS1. A proof-of-concept study with a recombinant fusion protein insulin coupled to apolipoprotein A-I (Ins-ApoAI) apolipoprotein A-I (Apo) confirmed a slow but prolonged mechanism of action, which diminishes the risk of hypoglycemia [49].
The Ins-ApoAI protein has a natural tropism for the liver [50,51] and a long biological half-life in the serum. We reported the repressive effect of the co-administration of glucose and Ins-ApoAI to counteract the direct induction of the hepatic ALAS1 gene modulated by extended fasting [39]. Furthermore, the administration of exogenous insulin tends to restore the expression of PEPCK and GP enzymes, regulators of the gluconeogenesis and glycogenolysis pathways, which suggests normalization of glucose homeostasis. In muscles, the recombinant Ins-ApoAI protein was also able to increase insulin sensitivity and enhance direct glucose uptake via AMP-activated protein kinase [52]. Finally, preventive treatment with insulin-ApoAI would also enable the beta-oxidation of fatty acids to be activated, as an important source of energy supply for the rest of the organs and of metabolites in the form of Acetyl-CoA that would be incorporated into the hepatocyte TCA cycle [39].
More recently, the administration of glucose and the insulin-mimicking α-lipoic [53] acid improved glucose metabolism and mitochondrial dysfunction in a hepatocyte cell line where the expression of PBGD was interfered with using a short interfering RNA [53]. The authors reported the restoration of the cross-talk between cytosolic glycolysis and mitochondrial respiration and, therefore, hepatocellular homeostasis.
However, we found that prophylactic administration of the recombinant Ins-ApoAI protein and glucose was not sufficient to achieve biochemical protection against a severe attack induced after recurrent phenobarbital administration [39]. The hepatic transcriptome of AIP mice revealed that phenobarbital also induced dysregulation of the gene involved in mitochondrial biogenesis (PGC1α) and oxidative phosphorylation, whose regulation closely depends on peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PPARGC1A, encoding the PGC1-transcription factor) [28]. Notably, prophylactic administration of insulin-ApoAI was associated with behavioral improvements, probably related to the protective effect associated with the ApoAI-moiety promoting mitochondrial biogenesis at the hepatic level, as previously described by Song P. et al. [54]. Thus, further studies are needed to confirm whether new insulins or insulin-mimicking substances can improve insulin resistance, promoting glucose availability in other organs by restoring hepatic gluconeogenesis and glycogenolysis, and increasing mitochondrial dynamics and oxidative phosphorylation activity to increase energy production in hepatocytes.
6. Conclusions
Heme synthesis is fully integrated into the metabolism network because it uses glycine and succinyl-CoA from the TCA cycle. Hence, stimulation of heme biosynthesis during an acute attack requires TCA cycle replenishment, driving pyruvate and acetyl-CoA from the degradation of glucose, fatty acid β-oxidation, and ketogenic and glucogenic amino acids. All these data suggest the close integration of heme biosynthesis into carbohydrate, lipid and protein metabolism.
However, several studies have addressed the higher prevalence of insulin resistance and other alterations in glucose metabolism in patients with porphyria compared to healthy individuals. This alteration can be related to various causes such as the dysfunction of glutathione-insulin transhydrogenase and the chronic carbohydrate overload to which patients with AIP are exposed [39]. However, it seems that ALA itself can control glucose metabolism since several cohort studies have demonstrated the potential of ALA as a treatment for individuals with prediabetes and type-2 diabetes mellitus [55,56], and there is in vivo proof that ALA deficiency attenuates mitochondrial function and causes IGT and IR [57] even in porphyric mice subjected to the same diet as the wild type [24].
In addition, fasting secondary to nausea and vomiting and major losses of hepatic succinyl-CoA during an acute attack, as well as impaired glucose metabolism, reduce the availability of energy metabolites and could play a role in modulating the severity of porphyria attacks.
The intravenous administration of an experimental insulin-ApoAI protein or oral supplementation with a molecule that mimics insulin can improve glucose therapy by the repressive effect of insulin on the transcription of hepatic ALAS1, by increasing the energy supply to replenish the hepatocyte TCA cycle, as well as by enhancing mitochondrial respiration.
Author Contributions
Conceptualization and design of the work, I.S., D.J., K.M.C., R.E.d.S. and A.F.; writing—original draft preparation, I.S. and A.F.; review and editing, I.S. and A.F. assisted by D.J., K.M.C., M.M.-C., J.E. and R.E.d.S.; supervision: J.E., A.F. and R.E.d.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by grants from the Spanish Institute of Health Carlos III (FIS) established by the European Union (ERDF/ESF, “A way to make Europe”/”Investing in your future” (grant numbers PI18/00860 and PI21/00546)), the Spanish Fundación Mutua Madrileña de Investigación Médica, and the Spanish Fundación FEDER para la investigación de enfermedades raras. The financial sponsors had no role in the analysis or in the development of the study’s conclusions. The researchers are solely responsible for the content and the decision to submit the manuscript for publication.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Anderson, K.; Bloomer, J.R.; Bonkovsky, H.L.; Kushner, J.P.; Pierach, C.A.; Pimstone, N.R.; Desnick, R.J. Recommendations for the Diagnosis and Treatment of the Acute Porphyrias. Ann. Intern. Med. 2005, 142, 439–450, Erratum in: Ann Intern Med. 2005, 143, 316. [Google Scholar] [CrossRef] [PubMed]
- Stein, P.; Badminton, M.; Barth, J.; Rees, D.; Stewart, M.F. Best practice guidelines on clinical management of acute attacks of porphyria and their complications. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2013, 50, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Castelbón Fernández, F.J.; Solares Fernandez, I.; Arranz Canales, E.; Enríquez de Salamanca Lorente, R.; Morales Conejo, M. Protocol for Patients with Suspected Acute Porphyria [published online ahead of print, 2020 Mar 3]. Protocolo de actuación en pacientes con sospecha de porfiria aguda [published online ahead of print, 2020 Mar 3]. Rev. Clin. Esp. 2020, 220, 592–596. [Google Scholar] [CrossRef] [PubMed]
- Balwani, M.; Desnick, R.J. The porphyrias: Advances in diagnosis and treatment. Blood 2012, 120, 4496–4504, Erratum in: Blood 2013, 122, 3090. [Google Scholar] [CrossRef] [PubMed]
- Long, Z.; Li, H.; Du, Y.; Han, B. Congenital sideroblastic anemia: Advances in gene mutations and pathophysiology. Gene 2018, 668, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Elder, G.; Harper, P.; Badminton, M.; Sandberg, S.; Deybach, J.-C. The incidence of inherited porphyrias in Europe. J. Inherit. Metab. Dis. 2012, 36, 849–857. [Google Scholar] [CrossRef]
- Homedan, C.; Laafi, J.; Schmitt, C.; Gueguen, N.; Lefebvre, T.; Karim, Z.; Desquiret-Dumas, V.; Wetterwald, C.; Deybach, J.-C.; Gouya, L.; et al. Acute intermittent porphyria causes hepatic mitochondrial energetic failure in a mouse model. Int. J. Biochem. Cell Biol. 2014, 51, 93–101. [Google Scholar] [CrossRef]
- Gomez-Gomez, A.; Aguilera, P.; Langohr, K.; Casals, G.; Pavon, C.; Marcos, J.; To-Figueras, J.; Pozo, O.J. Evaluation of Metabolic Changes in Acute Intermittent Porphyria Patients by Targeted Metabolomics. Int. J. Mol. Sci. 2022, 23, 3219. [Google Scholar] [CrossRef]
- Delaby, C.; To-Figueras, J.; Deybach, J.C.; Casamitjana, R.; Puy, H.; Herrero, C. Role of two nutritional hepatic markers (insulin-like growth factor 1 and transthyretin) in the clinical assessment and follow-up of acute intermittent porphyria patients. J. Intern. Med. 2009, 266, 277–285. [Google Scholar] [CrossRef]
- Bylesjö, I.; Wikberg, A.; Andersson, C. Clinical aspects of acute intermittent porphyria in northern Sweden: A population-based study. Scand. J. Clin. Lab. Investig. 2009, 69, 612–618. [Google Scholar] [CrossRef]
- Perez-Martinez, P.; Alcala-Diaz, J.F.; Delgado-Lista, J.; Garcia-Rios, A.; Gomez-Delgado, F.; Marin-Hinojosa, C.; Rodriguez-Cantalejo, F.; Delgado-Casado, N.; Perez-Caballero, A.I.; Fuentes-Jimenez, F.J.; et al. Metabolic phenotypes of obesity influence triglyceride and inflammation homoeostasis. Eur. J. Clin. Investig. 2014, 44, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Rose, J.A.; Hellman, E.S.; Tschudy, D.P. Effect of diet on induction of experimental porphyria. Metabolism 1961, 10, 514–521. [Google Scholar]
- Tschudy, D.P.; Welland, F.H.; Collins, A.; Hunter, G.W. The effect of carbohydrate feeding on the induction of δ-aminolevulinic acid synthetase. Metabolism 1964, 13, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Bonkowsky, H.L.; Sinclair, P.R.; Sinclair, J.F. Hepatic heme metabolism and its control. Yale J. Biol. Med. 1979, 52, 13–37. [Google Scholar]
- Welland, F.H.; Hellman, E.S.; Gaddis, E.M.; Collins, A.; Hunter, G.W.; Tschudy, D.P. Factors affecting the excretion of porphyrin precursors by patients with acute intermittent porphyria I. The effect of diet. Metabolism 1964, 13, 232–250. [Google Scholar] [CrossRef] [PubMed]
- Handschin, C.; Lin, J.; Rhee, J.; Peyer, A.-K.; Chin, S.; Wu, P.-H.; Meyer, U.A.; Spiegelman, B.M. Nutritional Regulation of Hepatic Heme Biosynthesis and Porphyria through PGC-1α. Cell 2005, 122, 505–515. [Google Scholar] [CrossRef]
- Nordlie, R.C.; Foster, J.D.; Lange, A.J. Regulation of glucose production by the liver. Annu. Rev. Nutr. 1999, 19, 379–406. [Google Scholar] [CrossRef]
- Newgard, C.B.; Hwang, P.K.; Fletterick, R.J. The Family of Glycogen Phosphorylases: Structure and Functio. Crit. Rev. Biochem. Mol. Biol. 1989, 24, 69–99. [Google Scholar] [CrossRef]
- Agius, L. Role of glycogen phosphorylase in liver glycogen metabolism. Mol. Asp. Med. 2015, 46, 34–45. [Google Scholar] [CrossRef]
- She, P.; Shiota, M.; Shelton, K.D.; Chalkley, R.; Postic, C.; Magnuson, M.A. Phosphoenolpyruvate Carboxykinase Is Necessary for the Integration of Hepatic Energy Metabolism. Mol. Cell. Biol. 2000, 20, 6508–6517. [Google Scholar] [CrossRef]
- Hanson, R.W.; Reshef, L. Regulation of phosphoenolpyruvate carboxykinase (gtp) gene expression. Annu. Rev. Biochem. 1997, 66, 581–611. [Google Scholar] [CrossRef] [PubMed]
- Cimbala, M.A.; Lamers, W.H.; Nelson, K.; Monahan, J.E.; Yoo-Warren, H.; Hanson, R.W. Rapid changes in the concentration of phosphoenolpyruvate carboxykinase mRNA in rat liver and kidney. Effects of insulin and cyclic AMP. J. Biol. Chem. 1982, 257, 7629–7636. [Google Scholar] [CrossRef] [PubMed]
- Lelli, S.M.; De Viale, L.C.S.M.; Mazzetti, M.B. Response of glucose metabolism enzymes in an acute porphyria model: Role of reactive oxygen species. Toxicology 2005, 216, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Collantes, M.; Serrano-Mendioroz, I.; Benito, M.; Molinet-Dronda, F.; Delgado, M.; Vinaixa, M.; Sampedro, A.; de Salamanca, R.E.; Prieto, E.; Pozo, M.A.; et al. Glucose metabolism during fasting is altered in experimental porphobilinogen deaminase deficiency. Hum. Mol. Genet. 2016, 25, 1318–1327. [Google Scholar] [CrossRef] [PubMed]
- Fischer, P.W.; Stephens, J.K.; Marks, G.S. Effect of varying the insulin to glucagon ratio on porphyrin biosynthesis in chick embryo liver cells. Mol. Pharmacol. 1978, 14, 717–721. [Google Scholar]
- Marks, G.S.; Stephens, J.K.; Fischer, P.W.F.; Morgan, R.O. Hormonal effects on the regulation of hepatic heme biosynthesis. Mol. Cell. Biochem. 1979, 25, 111–123. [Google Scholar] [CrossRef]
- Puigserver, P.; Spiegelman, B.M. Peroxisome Proliferator-Activated Receptor-γ Coactivator 1α (PGC-1α): Transcriptional Coactivator and Metabolic Regulator. Endocr. Rev. 2003, 24, 78–90. [Google Scholar] [CrossRef]
- Chen, B.; Wang, M.; Gan, L.; Zhang, B.; Desnick, R.J.; Yasuda, M. Characterization of the hepatic transcriptome following phenobarbital induction in mice with AIP. Mol. Genet. Metab. 2019, 128, 382–390. [Google Scholar] [CrossRef]
- Scassa, M.E.; Guberman, A.S.; Varone, C.L.; Cánepa, E.T. Phosphatidylinositol 3-Kinase and Ras/Mitogen-Activated Protein Kinase Signaling Pathways Are Required for the Regulation of 5-Aminolevulinate Synthase Gene Expression by Insulin. Exp. Cell Res. 2001, 271, 201–213. [Google Scholar] [CrossRef]
- Storjord, E.; Dahl, A.J.; Landsem, A.; Fure, H.; Ludviksen, J.K.; Goldbeck-Wood, S.; Karlsen, O.B.; Berg, K.S.; Mollnes, E.T.; Nielsen, E.W.; et al. Systemic inflammation in acute intermittent porphyria: A case–control study. Clin. Exp. Immunol. 2016, 187, 466–479. [Google Scholar] [CrossRef][Green Version]
- Storjord, E.; Dahl, J.A.; Landsem, A.; Ludviksen, J.K.; Karlsen, M.B.; Karlsen, B.O.; Brekke, O.-L. Lifestyle factors including diet and biochemical biomarkers in acute intermittent porphyria: Results from a case-control study in northern Norway. Mol. Genet. Metab. 2018, 128, 254–270. [Google Scholar] [CrossRef] [PubMed]
- Hedger, R.W.; Wehrmacher, W.H.; French, A.V. Porphyria syndrome associated with diabetic nephrosclerosis and erythropoietin. Compr. Ther. 2006, 32, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Sixel-Dietrich, F.; Verspohl, F.; Doss, M. Hyperinsulinemia in Acute Intermittent Porphyria. Horm. Metab. Res. 1985, 17, 375–376. [Google Scholar] [CrossRef]
- Yalouris, A.G.; A Raptis, S. Effect of diabetes on porphyric attacks. BMJ 1987, 295, 1237–1238. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Andersson, C.; Bylesjo, I.; Lithner, F. Effects of diabetes mellitus on patients with acute intermittent porphyria. J. Intern. Med. 1999, 245, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Lithner, F. Diabetisk metabolism skydd vid svår akut intermittent porfyri [Diabetic metabolism protective in severe acute intermittent porphyria]. Lakartidningen 2001, 98, 5874–5876. [Google Scholar] [PubMed]
- Bitar, M.; Weiner, M. Diabetes-induced Metabolic Alterations in Heme Synthesis and Degradation and Various Heme-containing Enzymes in Female Rats. Diabetes 1984, 33, 37–44. [Google Scholar] [CrossRef]
- Storjord, E.; Airila-Månsson, S.; Karlsen, K.; Madsen, M.; Dahl, J.A.; Landsem, A.; Fure, H.; Ludviksen, J.K.; Fjøse, J.; Dickey, A.K.; et al. Dental and Periodontal Health in Acute Intermittent Porphyria. Life 2022, 12, 1270. [Google Scholar] [CrossRef]
- Solares, I.; Izquierdo-Sánchez, L.; Morales-Conejo, M.; Jericó, D.; Castelbón, F.; Córdoba, K.; Sampedro, A.; Lumbreras, C.; Moreno-Aliaga, M.; de Salamanca, R.E.; et al. High Prevalence of Insulin Resistance in Asymptomatic Patients with Acute Intermittent Porphyria and Liver-Targeted Insulin as a Novel Therapeutic Approach. Biomedicines 2021, 9, 255. [Google Scholar] [CrossRef]
- Zaccardi, F.; Webb, D.R.; Yates, T.; Davies, M.J. Pathophysiology of type 1 and type 2 diabetes mellitus: A 90-year perspective. Postgrad. Med. J. 2016, 92, 63–69. [Google Scholar] [CrossRef]
- Mehran, A.E.; Templeman, N.M.; Brigidi, G.S.; Lim, G.E.; Chu, K.-Y.; Hu, X.; Botezelli, J.D.; Asadi, A.; Hoffman, B.G.; Kieffer, T.J.; et al. Hyperinsulinemia Drives Diet-Induced Obesity Independently of Brain Insulin Production. Cell Metab. 2012, 16, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Dankner, R.; Chetrit, A.; Shanik, M.H.; Raz, I.; Roth, J. Basal-State Hyperinsulinemia in Healthy Normoglycemic Adults Is Predictive of Type 2 Diabetes Over a 24-Year Follow-Up: A preliminary report. Diabetes Care 2009, 32, 1464–1466. [Google Scholar] [CrossRef] [PubMed]
- Morita, I.; Tanimoto, K.; Akiyama, N.; Naya, N.; Fujieda, K.; Iwasaki, T.; Yukioka, H. Chronic hyperinsulinemia contributes to insulin resistance under dietary restriction in association with altered lipid metabolism in Zucker diabetic fatty rats. Am. J. Physiol. Metab. 2017, 312, E264–E272. [Google Scholar] [CrossRef] [PubMed]
- Shanik, M.H.; Xu, Y.; Skrha, J.; Dankner, R.; Zick, Y.; Roth, J. Insulin Resistance and Hyperinsulinemia: Is hyperinsulinemia the cart or the horse? Diabetes Care 2008, 31 (Suppl. 2), S262–S268. [Google Scholar] [CrossRef]
- Matkovic, L.B.; D’Andrea, F.; Fornes, D.; de Viale, L.C.S.M.; Mazzetti, M.B. How porphyrinogenic drugs modeling acute porphyria impair the hormonal status that regulates glucose metabolism. Their relevance in the onset of this disease. Toxicology 2011, 290, 22–30. [Google Scholar] [CrossRef]
- Monteiro, H.P.; Abdalla, D.S.; Augusto, O.; Bechara, E.J. Free radical generation during δ-Aminolevulinic acid autoxidation: Induction by hemoglobin and connections with porphyrinpathies. Arch. Biochem. Biophys. 1989, 271, 206–216. [Google Scholar] [CrossRef]
- A Stein, J.; Tschudy, D.P. Acute intermittent porphyria. A clinical and biochemical study of 46 patients. Medicine 1970, 49, 1–16. [Google Scholar] [CrossRef]
- Oliveri, L.M.; Davio, C.; Batlle, A.M.D.C.; Gerez, E.N. ALAS1 gene expression is down-regulated by Akt-mediated phosphorylation and nuclear exclusion of FOXO1 by vanadate in diabetic mice. Biochem. J. 2012, 442, 303–310. [Google Scholar] [CrossRef]
- Rajpal, G.; Liu, M.; Zhang, Y.; Arvan, P. Single-Chain Insulins as Receptor Agonists. Mol. Endocrinol. 2009, 23, 679–688. [Google Scholar] [CrossRef]
- Drew, B.G.; Rye, K.-A.; Duffy, S.J.; Barter, P.; Kingwell, B.A. The emerging role of HDL in glucose metabolism. Nat. Rev. Endocrinol. 2012, 8, 237–245. [Google Scholar] [CrossRef]
- Kim, S.I.; Shin, D.; Choi, T.H.; Lee, J.C.; Cheon, G.-J.; Kim, K.-Y.; Park, M.; Kim, M. Systemic and Specific Delivery of Small Interfering RNAs to the Liver Mediated by Apolipoprotein A-I. Mol. Ther. 2007, 15, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.J.; Sun, Y.; Ong, K.L.; Li, Y.; Tang, S.; Barter, P.J.; Rye, K.-A. Apolipoprotein A-I Protects Against Pregnancy-Induced Insulin Resistance in Rats. Arter. Thromb. Vasc. Biol. 2019, 39, 1160–1171. [Google Scholar] [CrossRef] [PubMed]
- Longo, M.; Paolini, E.; Meroni, M.; Duca, L.; Motta, I.; Fracanzani, A.L.; Di Pierro, E.; Dongiovanni, P. α-Lipoic Acid Improves Hepatic Metabolic Dysfunctions in Acute Intermittent Porphyria: A Proof-of-Concept Study. Diagnostics 2021, 11, 1628. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Kwon, Y.; Yea, K.; Moon, H.-Y.; Yoon, J.H.; Ghim, J.; Hyun, H.; Kim, D.; Koh, A.; Berggren, P.-O.; et al. Apolipoprotein a1 increases mitochondrial biogenesis through AMP-activated protein kinase. Cell. Signal. 2015, 27, 1873–1881. [Google Scholar] [CrossRef]
- Higashikawa, F.; Noda, M.; Awaya, T.; Tanaka, T.; Sugiyama, M. 5-aminolevulinic acid, a precursor of heme, reduces both fasting and postprandial glucose levels in mildly hyperglycemic subjects. Nutrition 2013, 29, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, B.L.; Rodriguez, B.L.; Rodriguez, B.L.; Curb, J.D.; Curb, J.D.; Curb, J.D.; Davis, J.; Davis, J.; Davis, J.; Shintani, T.; et al. Use of the Dietary Supplement 5-Aminiolevulinic Acid (5-ALA) and Its Relationship with Glucose Levels and Hemoglobin A1C among Individuals with Prediabetes. Clin. Transl. Sci. 2012, 5, 314–320. [Google Scholar] [CrossRef]
- Saitoh, S.; Okano, S.; Nohara, H.; Nakano, H.; Shirasawa, N.; Naito, A.; Yamamoto, M.; Kelly, V.P.; Takahashi, K.; Tanaka, T.; et al. 5-aminolevulinic acid (ALA) deficiency causes impaired glucose tolerance and insulin resistance coincident with an attenuation of mitochondrial function in aged mice. PLoS ONE 2018, 13, e0189593. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).