New Therapeutics for HCC: Does Tumor Immune Microenvironment Matter?
Abstract
1. Introduction
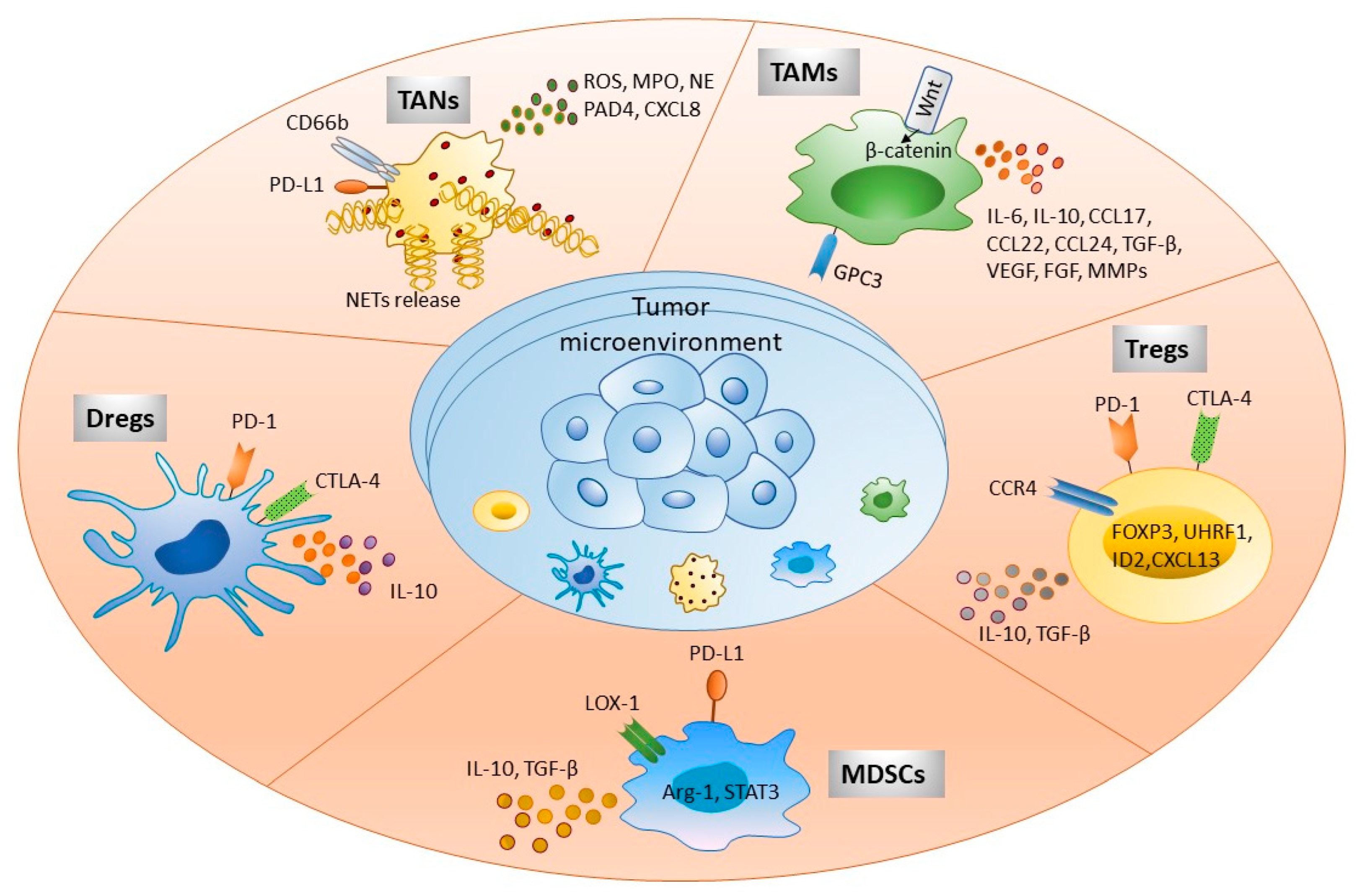
2. Cellular Component of the Hepatic Tumor Microenvironment
2.1. Tumor-Associated Neutrophils: New Felon in HCC Tumor Immune Microenvironment
2.2. Regulatory Dendritic Cells: Another Feather in the Cap of Immune Suppression during HCC
2.3. Tumor-Associated Macrophages: One of the Gritty Culprit
2.4. Myeloid-Derived Suppressor Cells: Frenemy of HCC
2.5. Regulatory T Cells: Persistent Suppressors of Anti-Tumor Immunity
3. Importance of Immune-Based Therapies in HCC
3.1. Use of Cell-Based Therapies
3.1.1. Adoptive Cell Therapy
3.1.2. CAR T-Cell Therapy
3.1.3. Tumor-Infiltrating Lymphocytes
3.1.4. Dendritic Cell Therapy
3.2. Use of Immune Checkpoint Inhibitors
3.3. Use of Immune-Related Nanoparticles
4. Challenges and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Prim. 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivas, A.N.; Kumar, D.P. Etiology of Hepatocellular Carcinoma: Special Focus on Fatty Liver Disease. Front. Oncol. 2020, 10, 601710. [Google Scholar] [CrossRef] [PubMed]
- Ajayi, F.; Jan, J.; Singal, A.G.; Rich, N.E. Racial and Sex Disparities in Hepatocellular Carcinoma in the USA. Curr. Hepatol. Rep. 2020, 19, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Vandenbulcke, H.; Moreno, C.; Colle, I.; Knebel, J.F.; Francque, S.; Sersté, T.; George, C.; de Galocsy, C.; Laleman, W.; Delwaide, J.; et al. Alcohol intake increases the risk of HCC in hepatitis C virus-related compensated cirrhosis: A prospective study. J. Hepatol. 2016, 65, 543–551. [Google Scholar] [CrossRef]
- Morgan, T.R.; Mandayam, S.; Jamal, M.M. Alcohol and hepatocellular carcinoma. Gastroenterology 2004, 127 (Suppl. S1), S87–S96. [Google Scholar] [CrossRef]
- Zhang, X.; Guan, L.; Tian, H.; Zeng, Z.; Chen, J.; Huang, D.; Sun, J.; Guo, J.; Cui, H.; Li, Y. Risk Factors and Prevention of Viral Hepatitis-Related Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 686962. [Google Scholar] [CrossRef]
- Rodríguez, M.; González-Diéguez, M.L.; Varela, M.; Cadahía, V.; Andrés-Vizán, S.M.; Mesa, A.; Castaño, A.; Alvarez-Navascués, C. Impact of Alcohol Abstinence on the Risk of Hepatocellular Carcinoma in Patients With Alcohol-Related Liver Cirrhosis. Am. J. Gastroenterol. 2021, 116, 2390–2398. [Google Scholar] [CrossRef]
- Tanaka, M.; Miyajima, A. Liver regeneration and fibrosis after inflammation. Inflamm. Regen. 2016, 36, 19. [Google Scholar] [CrossRef]
- Roehlen, N.; Crouchet, E.; Baumert, T.F. Liver Fibrosis: Mechanistic Concepts and Therapeutic Perspectives. Cells 2020, 9, 875. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Khanam, A.; Saleeb, P.G.; Kottilil, S. Pathophysiology and Treatment Options for Hepatic Fibrosis: Can It Be Completely Cured? Cells 2021, 10, 1097. [Google Scholar] [CrossRef]
- Yu, L.X.; Ling, Y.; Wang, H.Y. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis. Oncol. 2018, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Ho, D.W.-H.; Tsui, Y.-M.; Chan, L.-K.; Sze, K.M.-F.; Zhang, X.; Cheu, J.W.-S.; Chiu, Y.-T.; Lee, J.M.-F.; Chan, A.C.-Y.; Cheung, E.T.-Y.; et al. Single-cell RNA sequencing shows the immunosuppressive landscape and tumor heterogeneity of HBV-associated hepatocellular carcinoma. Nat. Commun. 2021, 12, 3684. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Greten, T.F.; Lai, C.W.; Li, G.; Staveley-O’Carroll, K.F. Targeted and Immune-Based Therapies for Hepatocellular Carcinoma. Gastroenterology 2019, 156, 510–524. [Google Scholar] [CrossRef]
- Hendrickson, P.G.; Olson, M.; Luetkens, T.; Weston, S.; Han, T.; Atanackovic, D.; Fine, G.C. The promise of adoptive cellular immunotherapies in hepatocellular carcinoma. Oncoimmunology 2020, 9, 1673129. [Google Scholar] [CrossRef]
- Zhang, T.; Merle, P.; Wang, H.; Zhao, H.; Kudo, M. Combination therapy for advanced hepatocellular carcinoma: Do we see the light at the end of the tunnel? Hepatobiliary Surg. Nutr. 2021, 10, 180–192. [Google Scholar] [CrossRef]
- Barry, A.E.; Baldeosingh, R.; Lamm, R.; Patel, K.; Zhang, K.; Dominguez, D.A.; Kirton, K.J.; Shah, A.P.; Dang, H. Hepatic Stellate Cells and Hepatocarcinogenesis. Front. Cell Dev. Biol. 2020, 8, 709. [Google Scholar] [CrossRef]
- Jaillon, S.; Ponzetta, A.; Di Mitri, D.; Santoni, A.; Bonecchi, R.; Mantovani, A. Neutrophil diversity and plasticity in tumour progression and therapy. Nat. Rev. Cancer 2020, 20, 485–503. [Google Scholar] [CrossRef]
- Mackey, J.B.G.; Coffelt, S.B.; Carlin, L.M. Neutrophil Maturity in Cancer. Front. Immunol. 2019, 10, 1912. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Gregory, A.D.; Houghton, A.M. Tumor-associated neutrophils: New targets for cancer therapy. Cancer Res. 2011, 71, 2411–2416. [Google Scholar] [CrossRef] [PubMed]
- Demers, M.; Krause, D.S.; Schatzberg, D.; Martinod, K.; Voorhees, J.R.; Fuchs, T.A.; Scadden, D.T.; Wagner, D.D. Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 13076–13081. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Li, Y.; Sun, R.; Hu, H.; Liu, Y.; Herrmann, M.; Zhao, Y.; Muñoz, L.E. Receptor-Mediated NETosis on Neutrophils. Front. Immunol. 2021, 12, 775267. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, A.S.; Slade, D.J.; Thompson, P.R.; Mowen, K.A. Activation of PAD4 in NET formation. Front. Immunol. 2012, 3, 360. [Google Scholar] [CrossRef]
- Van der Windt, D.J.; Sud, V.; Zhang, H.; Varley, P.R.; Goswami, J.; Yazdani, H.O.; Tohme, S.; Loughran, P.; O’Doherty, R.M.; Minervini, M.I.; et al. Neutrophil extracellular traps promote inflammation and development of hepatocellular carcinoma in nonalcoholic steatohepatitis. Hepatology 2018, 68, 1347–1360. [Google Scholar] [CrossRef]
- Yang, L.Y.; Luo, Q.; Lu, L.; Zhu, W.W.; Sun, H.T.; Wei, R.; Lin, Z.F.; Wang, X.Y.; Wang, C.Q.; Lu, M.; et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J. Hematol. Oncol. 2020, 13, 3. [Google Scholar] [CrossRef]
- Sun, R.; Xiong, Y.; Liu, H.; Gao, C.; Su, L.; Weng, J.; Yuan, X.; Zhang, D.; Feng, J. Tumor-associated neutrophils suppress antitumor immunity of NK cells through the PD-L1/PD-1 axis. Transl. Oncol. 2020, 13, 100825. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, H.; Deng, Y.; Tai, Y.; Zeng, K.; Zhang, Y.; Liu, W.; Zhang, Q.; Yang, Y. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018, 9, 422. [Google Scholar] [CrossRef]
- Song, M.; He, J.; Pan, Q.Z.; Yang, J.; Zhao, J.; Zhang, Y.J.; Huang, Y.; Tang, Y.; Wang, Q.; He, J.; et al. Cancer-Associated Fibroblast-Mediated Cellular Crosstalk Supports Hepatocellular Carcinoma Progression. Hepatology 2021, 73, 1717–1735. [Google Scholar] [CrossRef]
- Kuang, D.M.; Zhao, Q.; Wu, Y.; Peng, C.; Wang, J.; Xu, Z.; Yin, X.Y.; Zheng, L. Peritumoral neutrophils link inflammatory response to disease progression by fostering angiogenesis in hepatocellular carcinoma. J. Hepatol. 2011, 54, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-L.; Zhou, Z.-J.; Hu, Z.-Q.; Huang, X.-W.; Wang, Z.; Chen, E.-B.; Fan, J.; Cao, Y.; Dai, Z.; Zhou, J. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology 2016, 150, 1646–1658.e17. [Google Scholar] [CrossRef] [PubMed]
- Li, X.F.; Chen, D.P.; Ouyang, F.Z.; Chen, M.M.; Wu, Y.; Kuang, D.M.; Zheng, L. Increased autophagy sustains the survival and pro-tumourigenic effects of neutrophils in human hepatocellular carcinoma. J. Hepatol. 2015, 62, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ormandy, L.A.; Farber, A.; Cantz, T.; Petrykowska, S.; Wedemeyer, H.; Horning, M.; Lehner, F.; Manns, M.P.; Korangy, F.; Greten, T.F. Direct ex vivo analysis of dendritic cells in patients with hepatocellular carcinoma. World J. Gastroenterol. 2006, 12, 3275–3282. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Thompson, C.B. The Emerging Hallmarks of Cancer Metabolism. Cell Metab. 2016, 23, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Mellor, A.L. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Investig. 2007, 117, 1147–1154. [Google Scholar] [CrossRef]
- Ma, Y.; Shurin, G.V.; Gutkin, D.W.; Shurin, M.R. Tumor associated regulatory dendritic cells. Semin. Cancer Biol. 2012, 22, 298–306. [Google Scholar] [CrossRef]
- Ma, Y.; Shurin, G.V.; Peiyuan, Z.; Shurin, M.R. Dendritic cells in the cancer microenvironment. J. Cancer 2013, 4, 36–44. [Google Scholar] [CrossRef]
- Shurin, M.R.; Naiditch, H.; Zhong, H.; Shurin, G.V. Regulatory dendritic cells: New targets for cancer immunotherapy. Cancer Biol. Ther. 2011, 11, 988–992. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, C.; Sun, A.; Zheng, Y.; Wang, L.; Cao, X. Tumor-educated CD11bhighIalow regulatory dendritic cells suppress T cell response through arginase I. J. Immunol. 2009, 182, 6207–6216. [Google Scholar] [CrossRef]
- Cheng, J.T.; Deng, Y.N.; Yi, H.M.; Wang, G.Y.; Fu, B.S.; Chen, W.J.; Liu, W.; Tai, Y.; Peng, Y.W.; Zhang, Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis 2016, 5, e198. [Google Scholar] [CrossRef] [PubMed]
- Grohmann, U.; Fallarino, F.; Puccetti, P. Tolerance, DCs and tryptophan: Much ado about IDO. Trends Immunol. 2003, 24, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Van Baren, N.; Van den Eynde, B.J. Tryptophan-degrading enzymes in tumoral immune resistance. Front. Immunol. 2015, 6, 34. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Z.; Yang, Y.; Jiang, Z.; Gu, Y.; Liu, Y.; Lin, C.; Pan, Z.; Yu, Y.; Jiang, M.; et al. Human CD14+ CTLA-4+ regulatory dendritic cells suppress T-cell response by cytotoxic T-lymphocyte antigen-4-dependent IL-10 and indoleamine-2,3-dioxygenase production in hepatocellular carcinoma. Hepatology 2014, 59, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Enk, A.H.; Jonuleit, H.; Saloga, J.; Knop, J. Dendritic cells as mediators of tumor-induced tolerance in metastatic melanoma. Int. J. Cancer 1997, 73, 309–316. [Google Scholar] [CrossRef]
- Popov, A.; Schultze, J.L. IDO-expressing regulatory dendritic cells in cancer and chronic infection. J. Mol. Med. 2008, 86, 145–160. [Google Scholar] [CrossRef]
- Kalantari, T.; Ciric, B.; Kamali-Sarvestani, E.; Rostami, A. Bone marrow dendritic cells deficient for CD40 and IL-23p19 are tolerogenic in vitro. Iran. J. Basic Med. Sci. 2020, 23, 287–292. [Google Scholar]
- Marguti, I.; Yamamoto, G.L.; da Costa, T.B.; Rizzo, L.V.; de Moraes, L.V. Expansion of CD4+ CD25+ Foxp3+ T cells by bone marrow-derived dendritic cells. Immunology 2009, 127, 50–61. [Google Scholar] [CrossRef]
- Lim, T.S.; Chew, V.; Sieow, J.L.; Goh, S.; Yeong, J.P.; Soon, A.L.; Ricciardi-Castagnoli, P. PD-1 expression on dendritic cells suppresses CD8(+) T cell function and antitumor immunity. Oncoimmunology 2016, 5, e1085146. [Google Scholar] [CrossRef]
- Wen, Y.; Lambrecht, J.; Ju, C.; Tacke, F. Hepatic macrophages in liver homeostasis and diseases-diversity, plasticity and therapeutic opportunities. Cell. Mol. Immunol. 2021, 18, 45–56. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S. The M1 and M2 paradigm of macrophage activation: Time for reassessment. F1000prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; Shi, X.; He, X.; Gao, Y. Macrophage Phenotype and Function in Liver Disorder. Front. Immunol. 2019, 10, 3112. [Google Scholar] [CrossRef]
- Wang, K.; Qiu, X.; Zhao, Y.; Wang, H.; Chen, L. The Wnt/β-catenin signaling pathway in the tumor microenvironment of hepatocellular carcinoma. Cancer Biol. Med. 2021, 19, 305–318. [Google Scholar] [CrossRef]
- Wan, S.; Kuo, N.; Kryczek, I.; Zou, W.; Welling, T.H. Myeloid cells in hepatocellular carcinoma. Hepatology 2015, 62, 1304–1312. [Google Scholar] [CrossRef]
- Arwert, E.N.; Harney, A.S.; Entenberg, D.; Wang, Y.; Sahai, E.; Pollard, J.W.; Condeelis, J.S. A Unidirectional Transition from Migratory to Perivascular Macrophage Is Required for Tumor Cell Intravasation. Cell Rep. 2018, 23, 1239–1248. [Google Scholar] [CrossRef]
- Capece, D.; Fischietti, M.; Verzella, D.; Gaggiano, A.; Cicciarelli, G.; Tessitore, A.; Zazzeroni, F.; Alesse, E. The inflammatory microenvironment in hepatocellular carcinoma: A pivotal role for tumor-associated macrophages. BioMed Res. Int. 2013, 2013, 187204. [Google Scholar] [CrossRef]
- Takai, H.; Ashihara, M.; Ishiguro, T.; Terashima, H.; Watanabe, T.; Kato, A.; Suzuki, M. Involvement of glypican-3 in the recruitment of M2-polarized tumor-associated macrophages in hepatocellular carcinoma. Cancer Biol. Ther. 2009, 8, 2329–2338. [Google Scholar] [CrossRef]
- Arvanitakis, K.; Koletsa, T.; Mitroulis, I.; Germanidis, G. Tumor-Associated Macrophages in Hepatocellular Carcinoma Pathogenesis, Prognosis and Therapy. Cancers 2022, 14, 226. [Google Scholar] [CrossRef]
- Gordon, S.; Plüddemann, A. Macrophage Clearance of Apoptotic Cells: A Critical Assessment. Front. Immunol. 2018, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. Rev. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Ostrand-Rosenberg, S.; Beury, D.W.; Parker, K.H.; Horn, L.A. Survival of the fittest: How myeloid-derived suppressor cells survive in the inhospitable tumor microenvironment. Cancer Immunol. Immunother. 2020, 69, 215–221. [Google Scholar] [CrossRef]
- Yan, D.; Yang, Q.; Shi, M.; Zhong, L.; Wu, C.; Meng, T.; Yin, H.; Zhou, J. Polyunsaturated fatty acids promote the expansion of myeloid-derived suppressor cells by activating the JAK/STAT3 pathway. Eur. J. Immunol. 2013, 43, 2943–2955. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- LaGory, E.L.; Giaccia, A.J. The ever-expanding role of HIF in tumour and stromal biology. Nat. Cell Biol. 2016, 18, 356–365. [Google Scholar] [CrossRef]
- Ma, C.; Zhang, Q.; Greten, T.F. MDSCs in liver cancer: A critical tumor-promoting player and a potential therapeutic target. Cell. Immunol. 2021, 361, 104295. [Google Scholar] [CrossRef]
- Nan, J.; Xing, Y.F.; Hu, B.; Tang, J.X.; Dong, H.M.; He, Y.M.; Ruan, D.Y.; Ye, Q.J.; Cai, J.R.; Ma, X.K.; et al. Endoplasmic reticulum stress induced LOX-1(+) CD15(+) polymorphonuclear myeloid-derived suppressor cells in hepatocellular carcinoma. Immunology 2018, 154, 144–155. [Google Scholar] [CrossRef]
- Qu, X.; Zhuang, G.; Yu, L.; Meng, G.; Ferrara, N. Induction of Bv8 expression by granulocyte colony-stimulating factor in CD11b+Gr1+ cells: Key role of Stat3 signaling. J. Biol. Chem. 2012, 287, 19574–19584. [Google Scholar] [CrossRef]
- Zhao, H.Q.; Li, W.M.; Lu, Z.Q.; Yao, Y.M. Roles of Tregs in development of hepatocellular carcinoma: A meta-analysis. World J. Gastroenterol. 2014, 20, 7971–7978. [Google Scholar] [CrossRef] [PubMed]
- Workman, C.J.; Szymczak-Workman, A.L.; Collison, L.W.; Pillai, M.R.; Vignali, D.A. The development and function of regulatory T cells. Cell. Mol. Life Sci. 2009, 66, 2603–2622. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Putnam, A.L.; Xu-Yu, Z.; Szot, G.L.; Lee, M.R.; Zhu, S.; Gottlieb, P.A.; Kapranov, P.; Gingeras, T.R.; Fazekas de St Groth, B.; et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006, 203, 1701–1711. [Google Scholar] [CrossRef] [PubMed]
- Sukiennicki, T.L.; Fowell, D.J. Distinct molecular program imposed on CD4+ T cell targets by CD4+CD25+ regulatory T cells. J. Immunol. 2006, 177, 6952–6961. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; You, M.; Fu, J.; Tian, M.; Zhong, X.; Du, C.; Hong, Z.; Zhu, Z.; Liu, J.; Markowitz, G.J.; et al. Intratumoral stem-like CCR4+ regulatory T cells orchestrate the immunosuppressive microenvironment in HCC associated with hepatitis B. J. Hepatol. 2022, 76, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zheng, L.; Yoo, J.K.; Guo, H.; Zhang, Y.; Guo, X.; Kang, B.; Hu, R.; Huang, J.Y.; Zhang, Q.; et al. Landscape of Infiltrating T Cells in Liver Cancer Revealed by Single-Cell Sequencing. Cell 2017, 169, 1342–1356.e16. [Google Scholar] [CrossRef]
- Weng, N.P.; Araki, Y.; Subedi, K. The molecular basis of the memory T cell response: Differential gene expression and its epigenetic regulation. Nat. Rev. Immunol. 2012, 12, 306–315. [Google Scholar] [CrossRef]
- Seidel, J.A.; Otsuka, A.; Kabashima, K. Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations. Front. Oncol. 2018, 8, 86. [Google Scholar] [CrossRef]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022, 20, 44. [Google Scholar] [CrossRef]
- Marshall, L.A.; Marubayashi, S.; Jorapur, A.; Jacobson, S.; Zibinsky, M.; Robles, O.; Hu, D.X.; Jackson, J.J.; Pookot, D.; Sanchez, J.; et al. Tumors establish resistance to immunotherapy by regulating T(reg) recruitment via CCR4. J. Immunother. Cancer 2020, 8, e000764. [Google Scholar] [CrossRef]
- Nakano, S.; Eso, Y.; Okada, H.; Takai, A.; Takahashi, K.; Seno, H. Recent Advances in Immunotherapy for Hepatocellular Carcinoma. Cancers 2020, 12, 775. [Google Scholar] [CrossRef]
- Sangro, B.; Sarobe, P.; Hervás-Stubbs, S.; Melero, I. Advances in immunotherapy for hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 525–543. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A.; Restifo, N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015, 348, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, H.; Rurik, J.G.; Epstein, J.A. CAR-based therapies: Opportunities for immuno-medicine beyond cancer. Nat. Metab. 2022, 4, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, X.; Zhou, W.-L.; Huang, Y.; Liang, X.; Jiang, L.; Yang, X.; Sun, J.; Li, Z.; Han, W.-D.; et al. Genetically engineered T cells for cancer immunotherapy. Signal Transduct. Target. Ther. 2019, 4, 35. [Google Scholar] [CrossRef]
- Shi, D.; Shi, Y.; Kaseb, A.O.; Qi, X.; Zhang, Y.; Chi, J.; Lu, Q.; Gao, H.; Jiang, H.; Wang, H.; et al. Chimeric Antigen Receptor-Glypican-3 T-Cell Therapy for Advanced Hepatocellular Carcinoma: Results of Phase I Trials. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 3979–3989. [Google Scholar] [CrossRef]
- Sun, L.; Gao, F.; Gao, Z.; Ao, L.; Li, N.; Ma, S.; Jia, M.; Li, N.; Lu, P.; Sun, B.; et al. Shed antigen-induced blocking effect on CAR-T cells targeting Glypican-3 in Hepatocellular Carcinoma. J. Immunother. Cancer 2021, 9, e001875. [Google Scholar] [CrossRef]
- Turshudzhyan, A.; Wu, G.Y. Persistently Rising Alpha-fetoprotein in the Diagnosis of Hepatocellular Carcinoma: A Review. J. Clin. Transl. Hepatol. 2022, 10, 159–163. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Xiang, J.; Long, L.; Green, S.; Yang, Z.; Zimdahl, B.; Lu, J.; Cheng, N.; Horan, L.H.; et al. Targeting Alpha-Fetoprotein (AFP)-MHC Complex with CAR T-Cell Therapy for Liver Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 478–488. [Google Scholar] [CrossRef]
- Jiang, W.; Li, T.; Guo, J.; Wang, J.; Jia, L.; Shi, X.; Yang, T.; Jiao, R.; Wei, X.; Feng, Z.; et al. Bispecific c-Met/PD-L1 CAR-T Cells Have Enhanced Therapeutic Effects on Hepatocellular Carcinoma. Front. Oncol. 2021, 11, 546586. [Google Scholar] [CrossRef]
- You, H.; Ding, W.; Dang, H.; Jiang, Y.; Rountree, C.B. c-Met represents a potential therapeutic target for personalized treatment in hepatocellular carcinoma. Hepatology 2011, 54, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Juneja, V.R.; McGuire, K.A.; Manguso, R.T.; LaFleur, M.W.; Collins, N.; Haining, W.N.; Freeman, G.J.; Sharpe, A.H. PD-L1 on tumor cells is sufficient for immune evasion in immunogenic tumors and inhibits CD8 T cell cytotoxicity. J. Exp. Med. 2017, 214, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jin, W.; Wang, S.; Ding, H. Progression on the Roles and Mechanisms of Tumor-Infiltrating T Lymphocytes in Patients With Hepatocellular Carcinoma. Front. Immunol. 2021, 12, 729705. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Sprengers, D.; Boor, P.P.C.; Doukas, M.; Schutz, H.; Mancham, S.; Pedroza-Gonzalez, A.; Polak, W.G.; de Jonge, J.; Gaspersz, M.; et al. Antibodies Against Immune Checkpoint Molecules Restore Functions of Tumor-Infiltrating T Cells in Hepatocellular Carcinomas. Gastroenterology 2017, 153, 1107–1119.e10. [Google Scholar] [CrossRef]
- Lee, J.H.; Lee, J.H.; Lim, Y.S.; Yeon, J.E.; Song, T.J.; Yu, S.J.; Gwak, G.Y.; Kim, K.M.; Kim, Y.J.; Lee, J.W.; et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology 2015, 148, 1383–1391.e6. [Google Scholar] [CrossRef]
- Sachdeva, M.; Chawla, Y.K.; Arora, S.K. Immunology of hepatocellular carcinoma. World J. Hepatol. 2015, 7, 2080–2090. [Google Scholar] [CrossRef]
- Sangro, B.; Palmer, D.; Melero, I. Immunotherapy of hepatocellular carcinoma. Hepatic Oncol. 2014, 1, 433–446. [Google Scholar] [CrossRef]
- Hao, X.; Sun, G.; Zhang, Y.; Kong, X.; Rong, D.; Song, J.; Tang, W.; Wang, X. Targeting Immune Cells in the Tumor Microenvironment of HCC: New Opportunities and Challenges. Front. Cell Dev. Biol. 2021, 9, 775462. [Google Scholar] [CrossRef]
- Tada, F.; Abe, M.; Hirooka, M.; Ikeda, Y.; Hiasa, Y.; Lee, Y.; Jung, N.C.; Lee, W.B.; Lee, H.S.; Bae, Y.S.; et al. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int. J. Oncol. 2012, 41, 1601–1609. [Google Scholar] [CrossRef]
- Sabado, R.L.; Balan, S.; Bhardwaj, N. Dendritic cell-based immunotherapy. Cell Res. 2017, 27, 74–95. [Google Scholar] [CrossRef]
- Cao, J.; Kong, F.H.; Liu, X.; Wang, X.B. Immunotherapy with dendritic cells and cytokine-induced killer cells for hepatocellular carcinoma: A meta-analysis. World J. Gastroenterol. 2019, 25, 3649–3663. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.Y.; Shi, Y.; Huang, D.H.; Yang, T.; Wang, J.H.; Yan, G.H.; Wang, H.Y.; Tang, X.J.; Xiao, C.Y.; Zhang, W.J.; et al. Cytokine-induced killer cells combined with dendritic cells inhibited liver cancer cells. Int. J. Clin. Exp. Med. 2015, 8, 5601–5610. [Google Scholar] [PubMed]
- Nakamoto, Y.; Mizukoshi, E.; Kitahara, M.; Arihara, F.; Sakai, Y.; Kakinoki, K.; Fujita, Y.; Marukawa, Y.; Arai, K.; Yamashita, T.; et al. Prolonged recurrence-free survival following OK432-stimulated dendritic cell transfer into hepatocellular carcinoma during transarterial embolization. Clin. Exp. Immunol. 2011, 163, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Song, Z.; Xiao, J.; Liu, X.; Luo, Y.; Yang, Z.; Luo, R.; Li, A. Blocking the PD-1/PD-L1 axis in dendritic cell-stimulated Cytokine-Induced Killer Cells with pembrolizumab enhances their therapeutic effects against hepatocellular carcinoma. J. Cancer 2019, 10, 2578–2587. [Google Scholar] [CrossRef]
- Bray, S.M.; Vujanovic, L.; Butterfield, L.H. Dendritic cell-based vaccines positively impact natural killer and regulatory T cells in hepatocellular carcinoma patients. Clin. Dev. Immunol. 2011, 2011, 249281. [Google Scholar] [CrossRef]
- Zhou, J.; Ma, P.; Li, J.; Song, W. Comparative analysis of cytotoxic T lymphocyte response induced by dendritic cells pulsed with recombinant adeno-associated virus carrying α-fetoprotein gene or cancer cell lysate. Mol. Med. Rep. 2015, 11, 3174–3180. [Google Scholar] [CrossRef][Green Version]
- Tian, H.; Li, W. Dendritic cell-derived exosomes for cancer immunotherapy: Hope and challenges. Ann. Transl. Med. 2017, 5, 221. [Google Scholar] [CrossRef]
- Lu, Z.; Zuo, B.; Jing, R.; Gao, X.; Rao, Q.; Liu, Z.; Qi, H.; Guo, H.; Yin, H. Dendritic cell-derived exosomes elicit tumor regression in autochthonous hepatocellular carcinoma mouse models. J. Hepatol. 2017, 67, 739–748. [Google Scholar] [CrossRef]
- Zhang, Q.; Vignali, D.A. Co-stimulatory and Co-inhibitory Pathways in Autoimmunity. Immunity 2016, 44, 1034–1051. [Google Scholar] [CrossRef]
- Okoye, I.S.; Houghton, M.; Tyrrell, L.; Barakat, K.; Elahi, S. Coinhibitory Receptor Expression and Immune Checkpoint Blockade: Maintaining a Balance in CD8(+) T Cell Responses to Chronic Viral Infections and Cancer. Front. Immunol. 2017, 8, 1215. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, J.; Deng, X.; Xiong, F.; Ge, J.; Xiang, B.; Wu, X.; Ma, J.; Zhou, M.; Li, X.; et al. Role of the tumor microenvironment in PD-L1/PD-1-mediated tumor immune escape. Mol. Cancer 2019, 18, 10. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways: Similarities, Differences, and Implications of Their Inhibition. Am. J. Clin. Oncol. 2016, 39, 98. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lu, Y.; Qin, S. Atezolizumab and bevacizumab for hepatocellular carcinoma: Mechanism, pharmacokinetics and future treatment strategies. Future Oncol. 2021, 17, 2243–2256. [Google Scholar] [CrossRef]
- Wong, J.S.L.; Kwok, G.G.W.; Tang, V.; Li, B.C.W.; Leung, R.; Chiu, J.; Ma, K.W.; She, W.H.; Tsang, J.; Lo, C.M.; et al. Ipilimumab and nivolumab/pembrolizumab in advanced hepatocellular carcinoma refractory to prior immune checkpoint inhibitors. J. Immunother. Cancer 2021, 9, e001945. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Kim, C.; Heery, C.R.; Guha, U.; Gulley, J.L. Nivolumab, anti-programmed death-1 (PD-1) monoclonal antibody immunotherapy: Role in advanced cancers. Hum. Vaccines Immunother. 2016, 12, 2219–2231. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Kang, Y.K.; Kim, T.Y.; El-Khoueiry, A.B.; Santoro, A.; Sangro, B.; Melero, I.; Kudo, M.; Hou, M.M.; Matilla, A.; et al. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial. JAMA Oncol. 2020, 6, e204564. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Park, J.W.; Finn, R.S.; Cheng, A.L.; Mathurin, P.; Edeline, J.; Kudo, M.; Harding, J.J.; Merle, P.; Rosmorduc, O.; et al. Nivolumab versus sorafenib in advanced hepatocellular carcinoma (CheckMate 459): A randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2022, 23, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Onuma, A.E.; Zhang, H.; Huang, H.; Williams, T.M.; Noonan, A.; Tsung, A. Immune Checkpoint Inhibitors in Hepatocellular Cancer: Current Understanding on Mechanisms of Resistance and Biomarkers of Response to Treatment. Gene Expr. 2020, 20, 53–65. [Google Scholar] [CrossRef]
- Bian, J.; Lin, J.; Long, J.; Yang, X.; Yang, X.; Lu, X.; Sang, X.; Zhao, H. T lymphocytes in hepatocellular carcinoma immune microenvironment: Insights into human immunology and immunotherapy. Am. J. Cancer Res. 2020, 10, 4585–4606. [Google Scholar]
- Huang, Y.; Wang, T.; Yang, J.; Wu, X.; Fan, W.; Chen, J. Current Strategies for the Treatment of Hepatocellular Carcinoma by Modulating the Tumor Microenvironment via Nano-Delivery Systems: A Review. Int. J. Nanomed. 2022, 17, 2335–2352. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Dey, R.; Chen, Y. Nanomaterials for cancer therapy: Current progress and perspectives. J. Hematol. Oncol. 2021, 14, 85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ramjiawan, R.R.; Reiberger, T.; Ng, M.R.; Hato, T.; Huang, Y.; Ochiai, H.; Kitahara, S.; Unan, E.C.; Reddy, T.P.; et al. CXCR4 inhibition in tumor microenvironment facilitates anti-programmed death receptor-1 immunotherapy in sorafenib-treated hepatocellular carcinoma in mice. Hepatology 2015, 61, 1591–1602. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.Y.; Lin Ts, T.; Sung, Y.C.; Liu, Y.C.; Chiang, W.H.; Chang, C.C.; Liu, J.Y.; Chen, Y. CXCR4-targeted lipid-coated PLGA nanoparticles deliver sorafenib and overcome acquired drug resistance in liver cancer. Biomaterials 2015, 67, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tiruthani, K.; Li, S.; Hu, M.; Zhong, G.; Tang, Y.; Roy, S.; Zhang, L.; Tan, J.; Liao, C.; et al. mRNA Delivery of a Bispecific Single-Domain Antibody to Polarize Tumor-Associated Macrophages and Synergize Immunotherapy against Liver Malignancies. Adv. Mater. 2021, 33, e2007603. [Google Scholar] [CrossRef]
- Dai, X.; Ruan, J.; Guo, Y.; Sun, Z.; Liu, J.; Bao, X.; Zhang, H.; Li, Q.; Ye, C.; Wang, X.; et al. Enhanced radiotherapy efficacy and induced anti-tumor immunity in HCC by improving hypoxia microenvironment using oxygen microcapsules. Chem. Eng. J. 2021, 422, 130109. [Google Scholar] [CrossRef]
- Li, J.; Lin, W.; Chen, H.; Xu, Z.; Ye, Y.; Chen, M. Dual-target IL-12-containing nanoparticles enhance T cell functions for cancer immunotherapy. Cell. Immunol. 2020, 349, 104042. [Google Scholar] [CrossRef]
- Lai, I.; Swaminathan, S.; Baylot, V.; Mosley, A.; Dhanasekaran, R.; Gabay, M.; Felsher, D.W. Lipid nanoparticles that deliver IL-12 messenger RNA suppress tumorigenesis in MYC oncogene-driven hepatocellular carcinoma. J. Immunother. Cancer 2018, 6, 125. [Google Scholar] [CrossRef]
- Yang, X.; Lai, C.; Liu, A.; Hou, X.; Tang, Z.; Mo, F.; Yin, S.; Lu, X. Anti-Tumor Activity of Mannose-CpG-Oligodeoxynucleotides-Conjugated and Hepatoma Lysate-Loaded Nanoliposomes for Targeting Dendritic Cells In Vivo. J. Biomed. Nanotechnol. 2019, 15, 1018–1032. [Google Scholar] [CrossRef]
- Kim, K.M.; Sinn, D.H.; Jung, S.H.; Gwak, G.Y.; Paik, Y.H.; Choi, M.S.; Lee, J.H.; Koh, K.C.; Paik, S.W. The recommended treatment algorithms of the BCLC and HKLC staging systems: Does following these always improve survival rates for HCC patients? Liver Int. Off. J. Int. Assoc. Study Liver 2016, 36, 1490–1497. [Google Scholar] [CrossRef]
- Lee, Y.R.; Park, S.Y.; Tak, W.Y. Treatment Outcomes and Prognostic Factors of Acute Variceal Bleeding in Patients with Hepatocellular Carcinoma. Gut Liver 2020, 14, 500–508. [Google Scholar] [CrossRef]
- Giannini, E.G.; Cucchetti, A.; Erroi, V.; Garuti, F.; Odaldi, F.; Trevisani, F. Surveillance for early diagnosis of hepatocellular carcinoma: How best to do it? World J. Gastroenterol. 2013, 19, 8808–8821. [Google Scholar] [CrossRef] [PubMed]
- Raoul, J.L.; Adhoute, X.; Penaranda, G.; Perrier, H.; Castellani, P.; Oules, V.; Bourlière, M. Sorafenib: Experience and Better Manage-ment of Side Effects Improve Overall Survival in Hepatocellular Carcinoma Patients: A Real-Life Retrospective Analysis. Liver Cancer 2019, 8, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Nguang, S.H.; Wu, C.K.; Liang, C.M.; Tai, W.C.; Yang, S.C.; Ku, M.K.; Yuan, L.T.; Wang, J.W.; Tseng, K.L.; Hung, T.H.; et al. Treatment and Cost of Hepatocellular Carcinoma: A Population-Based Cohort Study in Taiwan. Int. J. Environ. Res. Public Health 2018, 15, 2655. [Google Scholar] [CrossRef]
| Cell Types | Patients Included (n) | Target | Phase | Trial Number |
|---|---|---|---|---|
| Anti-GPC3 CAR T-cells | 38 | Glypican-3 (GPC3 positive HCC) | I | NCT05003895 |
| ET140202 genetically modified autologous T cells to carry a TCR mimic | 2 | Tumor-specific intracellular antigens | I and II | NCT03998033 |
| Genetically modified autologous AFPc332 T cells | 45 | AFP-expressing tumors | I | NCT03132792 |
| ECT204 autologous T cell therapy | 12 | - | I and II | NCT04864054 |
| Autologous HBV-specific T cell receptor engineered T cells | 36 | - | I | NCT05339321 |
| Expanded activated lymphocytes | 430 | - | II | NCT05213637 |
| TCR-redirected T cells Therapy | 10 | - | I | NCT03899415 |
| TACE combined with PD-1 knockout engineered T cells | 10 | - | I | NCT04417764 |
| New antigen reactive cells (NRT) in combination with radiotherapy | 40 | - | IB/II | NCT03199807 |
| Autologous gamma delta T cells | 8 | - | Early phase 1 | NCT04518774 |
| B7H3 CAR T-cells | 5 | B7H3+ cancer cells | I1 | NCT05323201 |
| Adoptive transfer of specific HCC antigens CD8+ T cells | 18 | - | I | NCT03175705 |
| Adoptive transfer of iNKT cells | 18 | - | I | NCT03175679 |
| Neoantigen-based dendritic cell vaccine | 24 | - | I | NCT03674073 |
| DC-CIK | 60 | - | II | NCT02487017 |
| DC vaccine | 18 | - | I | NCT01974661 |
| DCs coactivated by HBV-specific antigen peptides and HepG2 cell lysate | 70 | - | I and II | NCT03086564 |
| Cyclophosphamide and multiple signals-loaded dendritic cell vaccine | 600 | - | II | NCT04317248 |
| Drug | Patients Included (n) | Target | Phase | Trial Number |
|---|---|---|---|---|
| Monotherapy | ||||
| Anti-PD-1 antibody Pembrolizumab | 30 | PD-1 | II | NCT03419481 |
| Nivolumab | 743 | PD-1 | III | NCT02576509 |
| Nivolumab | 659 | PD-1 | I | NCT01658878 |
| Camrelizumab | 20 | PD-1 | I | NCT04564313 |
| Combination therapy with two immune checkpoint inhibitors | ||||
| TSR022 and TSR042 | 42 | TIM3 and PD-1 | II | NCT03680508 |
| Nivolumab plus Ipilimumab | 40 | PD-1 and CTLA-4 | NCT03510871 | |
| Nivolumab with and without Relatlimab | 20 | PD-1 and LAG-3 | I | NCT04658147 |
| Durvalumab plus Tremelimumab | 30 | PDL-1 and CTLA-4 | II | NCT03638141 |
| Combination therapy of immune checkpoint inhibitors with tyrosine kinase inhibitors | ||||
| Telipril plus Apatinib in combination with SBRT | 20 | PD-1 and tyrosine kinase | II | NCT04165174 |
| Camrelizumab and Rivoceranib | 482 | PD-1 and tyrosine kinase | III | NCT04639180 |
| Toripalimab (JS001) plus Lenvatinib | 519 | PD-1 and tyrosine kinase | III | NCT04523493 |
| Sintilimab plus Anlotinib | 20 | Tyrosine kinase and PD-1 | II | NCT04052152 |
| Camrelizumab plus Rivoceranib | 674 | PD-1 and tyrosine kinase | III | NCT04639180 |
| Pembrolizumab plus Regorafenib | 95 | PD-1 and multiple kinases | II | NCT04696055 |
| Combination of immune checkpoint inhibitors with VEGF/VEGFR inhibitors | ||||
| Atezolizumab plus Bevacizumab | 434 | PD-L1 and VEGF | III | NCT4803994 |
| Atezolizumab plus Bevacizumab | 45 | PD-L1 and VEGF | II | NCT044954339 |
| TQB2450 and Anlotinib | 70 | PDL-1 and VEGFR | II | NCT05311319 |
| HX008 in combination with Bevacizumab or Lenvatinib | 72 | PD-1, VEGFR, and VEGF | II | NCT04741165 |
| AK104 with and without Lenvatinib | 75 | Bispecific for PD-1 and CTLA-4, VEGFR | II | NCT04728321 |
| KN046 combined with Lenvatinib | 55 | Bispecific for PD-L1 and CTLA-4, VEGFR | II | NCT04542837 |
| Combination of immune checkpoint inhibitors with chemokine inhibitor | ||||
| Nivolumab combined with BMS-986253 | 74 | PD-1 and IL-8 | II | NCT04050462 |
| Combination of immune checkpoint inhibitors with non-invasive/minimally invasive procedure | ||||
| Sintilimab and TACE | 41 | PD-1 | II | NCT04842565 |
| Pembrolizumab plus RFA | 30 | PD-1 | II | NCT03753659 |
| Anti-PD-1 antibody and RT | 39 | PD-1 | II | NCT04193696 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khanam, A.; Kottilil, S. New Therapeutics for HCC: Does Tumor Immune Microenvironment Matter? Int. J. Mol. Sci. 2023, 24, 437. https://doi.org/10.3390/ijms24010437
Khanam A, Kottilil S. New Therapeutics for HCC: Does Tumor Immune Microenvironment Matter? International Journal of Molecular Sciences. 2023; 24(1):437. https://doi.org/10.3390/ijms24010437
Chicago/Turabian StyleKhanam, Arshi, and Shyam Kottilil. 2023. "New Therapeutics for HCC: Does Tumor Immune Microenvironment Matter?" International Journal of Molecular Sciences 24, no. 1: 437. https://doi.org/10.3390/ijms24010437
APA StyleKhanam, A., & Kottilil, S. (2023). New Therapeutics for HCC: Does Tumor Immune Microenvironment Matter? International Journal of Molecular Sciences, 24(1), 437. https://doi.org/10.3390/ijms24010437






