Abstract
Methomyl is one of the most important carbamates that has caused potential hazardous effects on both human beings and the environment. Here, we systematically investigated the hydrolysis mechanism of methomyl catalyzed by esterase PestE using molecular dynamics simulations (MD) and quantum mechanics/molecular mechanics (QM/MM) calculations. The hydrolysis mechanism involves two elementary steps: (Ⅰ) serine-initiated nucleophilic attack and (Ⅱ) C-O bond cleavage. Our work elicits the atomic level details of the hydrolysis mechanism and free energy profiles along the reaction pathway. The Boltzmann-weighted average potential barriers are 19.1 kcal/mol and 7.5 kcal/mol for steps Ⅰ and Ⅱ, respectively. We identified serine-initiated nucleophilic attack as the rate determining-step. The deep learning-based kcat prediction model indicated that the barrier of the rate-determining step is 15.4 kcal/mol, which is in good agreement with the calculated results using Boltzmann-weighted average method. We have elucidated the importance of the protein–substrate interactions and the roles of the key active site residues during the hydrolysis process through noncovalent interactions analysis and electrostatic potential (ESP) analysis. The results provide practical value for achieving efficient degradation of carbamates by hydrolases.
1. Introduction
Methomyl is a carbamate first synthesized by Du Pont Company in 1966, and has been applied to control invading organisms in agriculture around the world [1,2,3]. Methomyl is still extensively produced and employed nowadays, although it was forbidden in many countries [4]. According to Sapec Agro Portugal, the active ingredient concentration of methomyl can reach levels higher than 830 mg/L per hectare currently [5]. Because of high solubility (57.9 g/L, 20 °C) and low soil affinity, methomyl can easily cause contamination of groundwater and surface water in the natural environment [6]. Continuous exposure to methomyl further results in accumulation in human tissue, including blood, kidney, liver and brain [3,7,8,9]. Methomyl can cause various health hazards, such as hepatotoxicity, neurotoxicity, and cytotoxicity [10]. Therefore, it is important to investigate the environmental behavior of methomyl and further assess its persistence, ecological risk and adverse effects.
Catalytic reactions are widely used in the transformation of various organic maters [11,12,13,14]. Among catalytic reactions, hydrolysis is one of the most efficient catalytic reactions. Hydrolysis is considered to be a crucial degradation pathway for methomyl in the natural environment because it has a carboxylic ester bond in the molecular structure. The hydrolysis products (methomyl oxime) were observed to be the largest pesticide concentration (40 μg/L) among 90 pesticide transformation products in a nationally distributed synoptic assessment of contaminant exposures [15]. Therefore, the investigation of hydrolysis of methomyl is important and necessary for assessing their environmental persistence and hazard.
Esterase is a class of hydrolase family with the ability to hydrolyze ester group that is successfully applied to degrade environmental pollutants [16,17]. Xu and co-workers reported a strain named MDW-1, isolated from the enrichment of activated sludge, could transform methomyl to S-methyl-N-hydroxythioacetamidate [18]. Zhang and co-workers showed that two bacterial strains named MDW-2 and MDW-3, isolated from the enrichment, can convert methomyl to methomyl oxime and methylcarbamic acid, which can later be used as the carbon source for the growth of MDW-2 and MDW-3 [19]. Unfortunately, the crystal structures of these strains have not been resolved so far. However, Fan and co-workers reported that an extremely thermophilic esterase PestE, isolated from Pyrobaculum calidifontis VA1, could be used as the template for hydrolysis of the organic pesticides [20,21]. The core domain of PestE structure is a classical α/β-hydrolase fold structure with a cap structure at the C-terminal of the β-sheet. The catalytic active center of esterase PestE consists of the catalytic triad (Ser157, His284, and Asp254) and the oxyanion hole (Gly85 and Gly86) [21]. The structural diagram of secondary structure of esterase PestE and active amino acid residues was shown in Figure 1. Although the hydrolysis of methomyl has been experimentally studied in previous studies, the detailed binding interaction and roles of key amino acids during catalysis are still unknown. Due to the high rate of the enzyme–catalyzed reaction, the intermediates in the reaction process were rapidly decomposed and cannot be found in the environment, and these intermediates are indispensable for understanding the reaction mechanism.
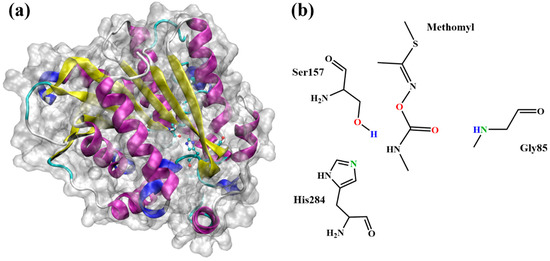
Figure 1.
(a) Structural diagram of secondary structure of esterase PestE. (b) The chemical structure of methomyl and a representation of QM region.
Quantum mechanics/molecular mechanics (QM/MM) method is a computational method that combines the accuracy of quantum mechanics with the speed advantage of molecular mechanics. In the QM/MM approach, a small part of the system is modeled quantum-mechanically, while the protein and solvent are treated by molecular mechanics [22]. QM/MM method can provide atomic-level details of enzyme catalysis mechanisms, and it is increasingly applied in computational enzyme chemistry. In the present study, the hydrolysis mechanism of esterase PestE towards methomyl was explored by using the molecular dynamics (MD) simulations and quantum mechanics/molecular mechanics (QM/MM) method. Two elementary steps are investigated in our simulation: (Ⅰ) serine-initiated nucleophilic attack and (Ⅱ) C-O bond cleavage. In addition, the reduced density gradient (RDC) function and electrostatic potential (ESP) analysis were performed to investigate the protein–substrate interactions and the roles of the key active site residues during hydrolysis process.
2. Results and Discussion
2.1. Molecular Dynamics
MD simulations were conducted to investigate the configuration changes of esterase PestE and the substrate. The stability of the system can be evaluated by the root-mean-square deviation (RMSD) and cluster analysis with TTclust [23]. As shown in Figure S1a, the RMSD among conformations maintains within 0.16 Å during the dynamic process, illustrating that the system is well-consistent. Same results can be obtained in Figure S2. Figure S1b shows the dendrogram of the system, which can be used to choose the appropriate clusterization level and describe the relationship among conformations in each cluster. Figure S1c presents the ratio of different clusters during the dynamic process. The ratio of the fifth cluster is the largest, which indicates that it is relatively stable. Figure S1d presents the 2D projection plot of the relative distances among clusters. The maximum and minimum relative distances among clusters are 0.77 and 0.74, respectively. The maximum and minimum RMSD values among clusters are 1.27 Å and 0.78 Å, respectively. According to the results of cluster analysis, dynamic trajectories can be classified thoroughly, which enables us to select reliable conformations for QM/MM simulations.
2.2. Reaction Mechanism and Energy Profile
As shown in Figure 2a, the hydrolysis mechanism of esterase PestE towards methomyl involves two concerted steps. First, the proton (H1) of Ser157 transfers to His284 while the oxygen atom (O1) acts as a nucleophile to attack the carbonyl carbon (C1) of the substrate. Subsequently, the C1-O3 bond of the substrate breaks while the lone pair of the oxygen atom (O3) attracts the hydrogen atom (H1) from His284. We selected five conformations from the MD trajectories for QM/MM calculations. The rate constant of an enzyme-catalyzed reaction generally exhibits a wide range of fluctuations. It is hypothesized that the snapshots obtained by MD simulations correspond to the local rate constant [24,25]. Hence, the Boltzmann-weighted average method was used to calculate the average energy barrier, which can be described according to the following equation:
where ΔE is the average energy barrier, R is the gas constant, T is the temperature, n is the number of snapshots, ΔEi is the energy barrier of path i.
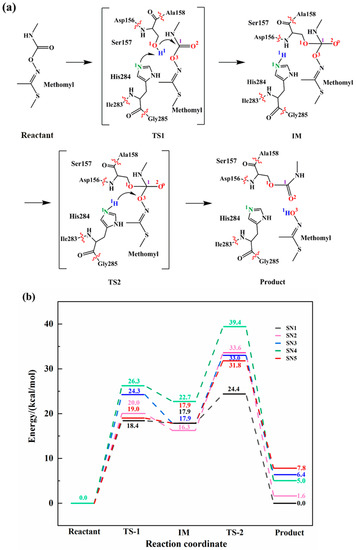
Figure 2.
Reaction mechanism and energy profile of enzymatic catalysis reaction. (a) The proposed mechanism of the biodegradation of methomyl; (b) Energy profiles of 5 snapshots with different color.
The reaction energy barriers of five snapshots calculated from QM/MM were plotted in Figure 2b. We calculated the Boltzmann-weighted average barriers of five snapshots to avoid mistakes caused by certain factors [16]. In the first step, the H1 of Ser157 transfers to His284, while the O1 on the Ser157 attacks the substrate. The Boltzmann-weighted average barrier of this step is 19.1 kcal/mol, thus it is the rate-determining step of the reaction. We have also carried out the deep learning-based kcat prediction model [15] to predict energy barrier. The results indicated that the barrier of the rate-determining step of the reaction is 15.4 kcal/mol, which is in good agreement with the calculated results using Boltzmann-weighted average method. In the second step, the C1-O3 bond of the acylase intermediate cleaves, while the proton H1 from His284 transfers to O3 of the substrate of which the Boltzmann-weighted average barrier is 7.5 kcal/mol.
2.3. Reaction Structure Details
In order to figure out the details of the catalytic reaction process, we focused on the analysis of changes in structures (bond length, bond angle, dihedral angle) of the intermediates and transition states. As shown in Figure 3, the key structure change details of the snapshot along the lowest energy barrier pathway were provided during the enzyme-catalytic reaction process. The key structure change details of other snapshots are described in Table S1. In the first step, we optimized the reactant and found that the distance between Ser157 and substrate (C1-O1) is 2.57 Å. Subsequently, after a transition state, the distance of C1-O1 decreases to 1.51 Å, while the angle (O1-C1-O3) changes from 84.77° to 103.39° and the angle (O1-C1-O2) changes from 99.31° to 111.34°, suggesting that the enzyme-substrate complex has formed. Meanwhile, Ser157 is deprotonated by His284 and the distance between H1 and N1 changes from 1.85 Å to 1.07 Å. In the second step, the distance between C1 and O3 increases from 1.43 Å to 2.67 Å, indicating that the C1-O3 bond of the substrate breaks. In addition, the angle (O1-C1-O3) of the substrate changes from 103.39° to 76.11°, and the dihedral angle (O1-C1-O3-H1) increases from 26.68° to 37.27°. All changes above are consistent with the changes in distance. While the C1-O3 bond of the substrate is breaking, the length of H1-O3 decreases from 3.25 Å to 1.00 Å, which indicates a hydroxylated product is forming. During the whole process, the hydrogen bond among the key amino acid residues Ser157, His284 and Gly85 around the substrate has the ability to stabilize the substrate and promote the catalytic reaction. Specifically, the N-H group of Gly85 and the O2 atom of substrate maintain at approximately 2 Å, invariably performing as a strong hydrogen bond.
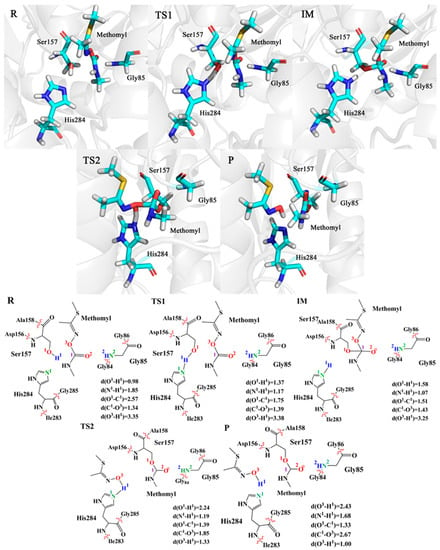
Figure 3.
Optimized structures and key bond distances for the R, TS1, IM, TS2 and P. The unit of distance is Å.
2.4. Noncovalent Interactions
Noncovalent interactions dominate chemical interactions between a protein and a drug, or a catalyst and its substrate, and even several chemical reactions [26,27]. Johnson and co-workers designed a visual method for studying noncovalent interactions [26], which is based on the electron density and its derivatives in real space. Reduced density gradient (RDG) is a dimensionless quantity derived from electron density ρ(r) and its first derivative,
The nature and strength of noncovalent interactions shown in the molecules can be comprehended by mapping ρ(r) against sign (λ2) [28]. The sign of λ2 is used to distinguish repulsive (λ2 > 0, unbonded) and attractive (λ2 < 0, bonded) interactions. The ρ(r)sign(λ2) peaks reveal the strength of noncovalent interactions. With the help of Multiwfn 3.7 [29] and VMD 1.9.3 [30], the color-filled RDG iso-surfaces and the scatter plots of weak interactions between methomyl and key amino acid residues were drawn for the structure of R, TS1 and TS2.
Figure 4(a1,b1,c1) show the scatter plots of weak interactions between the substrate and three key residues. The abscissa and ordinate represent the value of ρ(r)sign(λ2) and RDG function, respectively. The interaction regions are labeled blue, green, and red for attractive, van der Waals (vdW), and repulsive interactions, respectively. Figure 4(a2,b2,c2) display the color-filled RDG iso-surfaces, and blue regions represent for attractive interactions, the green regions for Vander Waals interactions and the red regions for repulsive interactions. As shown in Figure 4(a1), the peak value ranges from −0.04 to −0.03 a.u., indicating that the attraction between O1 and C1, which promotes the occurrence of the nucleophilic attack reaction. Comparing Figure 4(b1) with Figure 4(b2), we can find that the interactions of proton (H1) transfer and nucleophilic attack both exhibit a large red-blue annular region, illustrating that they happen at the same time. In addition, the steric effect between Ser157 and His284 is significantly enhanced, we conclude that the system is developing to a steady state while the oxygen (O1) of Ser157 combines with the substrate. It is worth mentioning that the phenomenon concluded from Figure 4(b1,b2) can also correspond to Figure 4(a1,a2). In addition, comparing Figure 4(b2) with Figure 4(c2), we can conclude that the interactions between C1 and O3 become stronger, displaying a larger red-blue annular region, which indicates that the bond between C1 and O3 will break. Meanwhile, H1 and O3 appear a large red-blue annular region, which implies that the second reaction is also a concerted reaction. Noted that the hydrogen bond interaction between substrate and Gly85 exists invariably, which stabilizes the substrate and contributes to the catalytic reaction process. It can be seen that the noncovalent interaction of attraction and repulsion play a central part in the catalytic reaction.
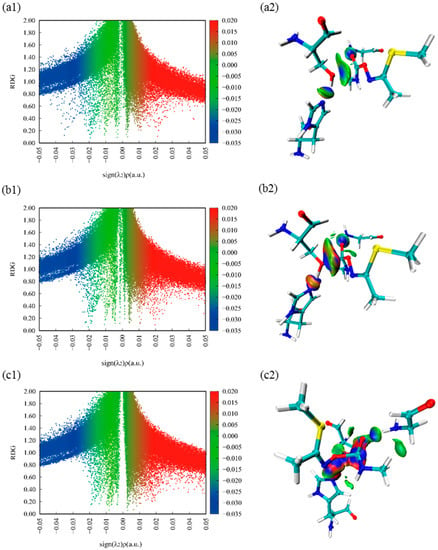
Figure 4.
Color-mapped reduced density gradient (RDG) iso-surface graphs and scatter plots of methomyl and three critical amino acids. Species shown: (a1), (b1), (c1) show the color-mapped reduced density gradient (RDG) scatter plots of methomyl and three critical amino acids of R, TS1, TS2, respectively. (a2), (b2), (c2) show the color-mapped reduced density gradient (RDG) iso-surface graphs of methomyl and three critical amino acids of R, TS1, TS2, respectively.
2.5. Electrostatic Potential
Electrostatic potential (ESP) is a concept in wave function analysis, which is of great significance to investigate electrostatic interactions between molecules and to predict reaction sites and molecular properties [29,31,32]. For the propose of illustrating the electrostatic interactions among molecules and figuring out reactive sites, the Electrostatic potential (ESP) was calculated by Mutiwfn 3.7 [29] and figured by VMD 1.9.3 [30].
The ESP distribution on the van der Waals (vdW) surface and extreme points of TS1 is exhibited in Figure 5a, noting that the blue and golden spheres, respectively, refer to the surface local minimum and maximum points of ESP. In addition, the positive extreme points represent that the electrostatic potential in this area is dominated by the nuclear charge, and the negative extreme points represent that the contribution of electrons is greater. According to Figure 5a, the significant ESP minimum points on the surface contain the hydroxyl hydrogen, methyl group and hydrogen bonds, and the significant ESP maximum points are attributed to the hydroxyl oxygen and nitrogen lone pair electrons. Interestingly, the ESP absolute value surrounding O3 (−60.9 kcal/mol) is large, which provides a new idea that O3 will undergo a proton transferring process, and we also verified this idea through QM/MM calculations. The vdW surface areas in different ESP ranges are shown in Figure 5b. According to Figure 5b, we can find that the values of ESP between −15.7 and 9.7 kcal/mol account for 51.1% of the surface areas, and the positive and negative ESP take up approximately 45% and 55% of the total areas, respectively.
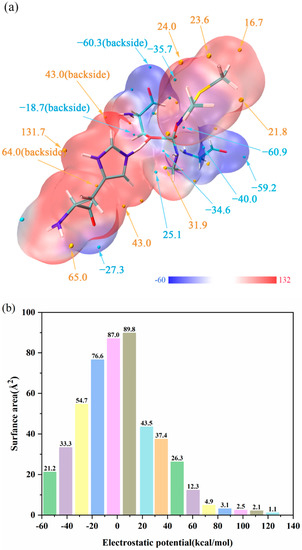
Figure 5.
(a) ESP distribution on molecular vdW surface of TS1. The unit is kcal/mol, and important surface local minima and maxima of ESP are represented as blue and golden spheres, respectively. (b) Surface area in each ESP range on the vdW surface of the system.
3. Computational Methods
3.1. System Preparation and MD Simulations
The initial structure studied was selected from the crystal structure of esterase PestE (PDB code: 3ZWQ, resolution: 2.0 Å, http://www.rcsb.org/ (accessed on 17 October 2020)) [21]. Methomyl was docked into the catalytic active center of esterase PestE via Autodock Vina and AutoDock Tools [33,34]. In the docking simulation, the grid box size was set 25 × 25 × 25 Å centered around the Ser 157 of esterase PestE. Nine docking models were obtained, and the best binding conformation was selected for the subsequent MD simulations. The topology and parameter of methomyl was generated by Swissparam [35]. The protonation states of the amino acid residues were considered by PROKA program on account of the pKa values [36]. The enzyme–substrate complex structure was optimized by the HBULD facility of CHARMM package [37,38]. The structure was dissolved in a TIP3P water sphere model with a diameter of 65 Å [39]. In order to maintain electrical neutrality, two sodium ions were stochastically added to the structure. The structure contains 14123 atoms. Firstly, the structure was heated from 0 K to 298.15 K in 50 ps, then it was equilibrated in 500 ps with a 1 fs time step, followed by a 30 ns stochastic boundary molecular dynamic (SBMD) simulation using canonical ensemble NVT at 298.15 K [2,37,40,41,42]. In the SBMD simulations, Langevin dynamics and Leap-frog algorithm of CHARMM package were implemented. Cluster analysis on 3000 conformations obtained by MD simulations was performed via TTClust [23], and the conformations were selected from the stable clusters for QM/MM calculations.
3.2. QM/MM Methodology
The QM/MM calculations were performed by the ChemShell 3.6.0 platform [43] that combines the quantum chemistry program Turbomole 7.2 [44] with the molecular mechanics module DL-POLY [45]. The electrostatic field embedding and charge shift model were used during QM/MM calculations [46,47]. The QM region (61 atoms) contains the substrate methomyl, Gly85, Ser157, His284. The geometry optimizations of intermediates and transition states were performed at the M06-2X/6-31G(d,p) level, and single-point energies were calculated at the M06-2X/6-311G(d,p) level. The CHARMM27 force field was used for the MM region. During the QM/MM calculation, the atoms within 20 Å of the substrate were allowed to move.
4. Conclusions
Using MD and QM/MM approaches, we have elucidated the hydrolysis mechanism and the structural details of esterase PestE reacting with environmental pollutant methomyl. The hydrolysis process involves two steps. In the first step, the H1 of Ser157 transfers to His284, while the O1 on the Ser157 attacks the substrate. The Boltzmann-weighted average barrier of this step is 19.1 kcal/mol, and it is the rate-determining step of the reaction. In the second step, the C1-O3 bond of the acylase intermediate cleaves while the proton H1 from His284 transfers to the O3 of the substrate, the Boltzmann-weighted average barrier of this step is 7.5 kcal/mol. Through noncovalent interactions analysis and electrostatic potential (ESP) analysis, we have confirmed the importance of the protein–substrate interactions and the roles of key active site residues during the hydrolysis process. The active site residues His284 and Ser157 participate in the proton transfer and nucleophilic during the catalysis process. Another active residue Gly85 stabilizes the substrate and enhances the enzymatic efficiency. We believe that these results are useful to interpret crystal structures and kinetic experiments of methomyl hydrolysis. Further, the atomic level details obtained in this study can facilitate designing a rational enzyme engineering strategy to efficiently degrade pesticides, thus generating benefits for environmental sustainability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24010433/s1. Table S1. Key bond distances (in Å) at the M06-2X//6-31G(d,p)//CHARMM27 level during the whole reactions in 4 snapshots. Table S2. The force field of Methomyl generated by SwissParam. Figure S1. TTcluster was applied to RMSD and cluster analysis of 30 ns MD simulations performed with CHARMM. (a) The heatmap of the possible cluster number of the system is saved by the distance matrix; (b) Clustering dendrogram of five clusters of the system; (c) The distribution of clusters in dynamic simulation; (d) 2D projection plot of the relative distances among five clusters based on the RMSD of representative frames. Figure S2. Root-mean-square deviations (RMSD) of the backbone for molecular dynamic simulation involved in the system.
Author Contributions
Conceptualization, Z.W., Q.Z. and G.W.; methodology, Z.W.; resources, Q.Z. and G.W.; writing—original draft preparation, Z.W.; writing—review and editing, Q.Z. and G.W.; visualization, Z.W., Q.Z. and G.W. supervision, W.W. and Q.W.; funding acquisition, Q.Z. All authors have read and agree to the published version of the manuscript.
Funding
This research was funded by National Natural Science Foundation of China grant number 22236004.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The work was financially supported by the National Natural Science Foundation of China (project No. 22236004).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Tomašević, A.; Petrović, S.; Mijin, D. Photochemical processes for removal of carbamate pesticides from water. Adv. Technol. 2019, 8, 72–81. [Google Scholar] [CrossRef]
- Omeiri, M.; Khnayzer, R.; Yusef, H.; Tokajian, S.; Salloum, T.; Mokh, S. Bacillus spp. isolated from soil in Lebanon can simultaneously degrade methomyl in contaminated soils and enhance plant growth. Biocatal. Agric. Biotechnol. 2022, 39, 102280. [Google Scholar] [CrossRef]
- Seleem, A.A. Teratogenicity and neurotoxicity effects induced by methomyl insecticide on the developmental stages of Bufo arabicus. Neurotoxicol. Teratol. 2019, 72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Boucaud-Maitre, D.; Rambourg, M.O.; Sinno-Tellier, S.; Puskarczyk, E.; Pineau, X.; Kammerer, M.; Bloch, J.; Langrand, J. Human exposure to banned pesticides reported to the French Poison Control Centers: 2012–2016. Environ. Toxicol. Pharmacol. 2019, 69, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Queirós, L.; Martins, A.C.; Krum, B.N.; Ke, T.; Aschner, M.; Pereira, J.L.; Gonçalves, F.J.M.; Milne, G.L.; Pereira, P. Assessing the neurotoxicity of the carbamate methomyl in Caenorhabditis elegans with a multi-level approach. Toxicology 2021, 451, 152684. [Google Scholar] [CrossRef]
- Strathmann, T.J.; Stone, A.T. Reduction of the Carbamate Pesticides Oxamyl and Methomyl by Dissolved FeII and CuI. Environ. Sci. Technol. 2001, 35, 2461–2469. [Google Scholar] [CrossRef]
- Hoizey, G.; Canas, F.; Binet, L.; Kaltenbach, M.L.; Jeunehomme, G.; Bernard, M.H.; Lamiable, D. Thiodicarb and methomyl tissue distribution in a fatal multiple compounds poisoning. J. Forensic Sci. 2008, 53, 499–502. [Google Scholar] [CrossRef]
- Driskell, W.J.; Groce, D.F.; Hill, R.H., Jr.; Birky, M.M. Methomyl in the Blood of a Pilot Who Crashed during Aerial Spraying. J. Anal. Toxicol. 1991, 15, 339–340. [Google Scholar] [CrossRef]
- Lee, B.K.; Jeung, K.W.; Lee, H.Y.; Jung, Y.H. Mortality rate and pattern following carbamate methomyl poisoning. Comparison with organophosphate poisoning of comparable toxicity. Clin. Toxicol. 2011, 49, 828–833. [Google Scholar] [CrossRef]
- Seleem, A.A. Induction of hyperpigmentation and heat shock protein 70 response to the toxicity of methomyl insecticide during the organ development of the Arabian toad, Bufo arabicus (Heyden,1827). J. Histotechnol. 2019, 42, 104–115. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Sustainable Inhibitors for Corrosion Mitigation in Aggressive Corrosive Media: A Comprehensive Study. J. Bio- Tribo-Corros. 2021, 7, 67. [Google Scholar] [CrossRef]
- Thakur, A.; Kaya, S.; Kumar, A. Recent Innovations in Nano Container-Based Self-Healing Coatings in the Construction Industry. Curr. Nanosci. 2022, 18, 203–216. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A. Encapsulated nanoparticles in organic polymers for corrosion inhibition. In Corrosion Protection at the Nanoscale; Elsevier: Amsterdam, The Netherlands, 2020; pp. 345–362. [Google Scholar]
- Hubbard, L.E.; Kolpin, D.W.; Givens, C.E.; Blackwell, B.R.; Bradley, P.M.; Gray, J.L.; Lane, R.F.; Masoner, J.R.; McCleskey, R.B.; Romanok, K.M.; et al. Food, Beverage, and Feedstock Processing Facility Wastewater: A Unique and Underappreciated Source of Contaminants to U.S. Streams. Environ. Sci. Technol. 2022, 56, 1028–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhu, L.; Chen, J.; Wang, W.; Zhang, R.; Li, Y.; Zhang, Q.; Wang, W. Degradation mechanism for Zearalenone ring-cleavage by Zearalenone hydrolase RmZHD: A QM/MM study. Sci. Total Environ. 2020, 709, 135897. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Yue, Y.; Chen, J.; Zhou, J.; Li, Y.; Zhang, Q. Biodegradation mechanism of polycaprolactone by a novel esterase MGS0156: A QM/MM approach. Environ. Sci. Process. Impacts 2020, 22, 2332–2344. [Google Scholar] [CrossRef]
- Xu, J.-L.; Wu, J.; Wang, Z.-C.; Wang, K.; Li, M.-Y.; Jiang, J.-D.; He, J.; Li, S.-P. Isolation and Characterization of a Methomyl-Degrading Paracoccus sp. mdw-1. Pedosphere 2009, 19, 238–243. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, Z.; Jin, W.; Wang, X.; Zhang, Y.; Zhu, S.; Yu, X.; Hu, G.; Hong, Q. Degradation of methomyl by the combination of Aminobacter sp. MDW-2 and Afipia sp. MDW-3. Lett. Appl. Microbiol. 2017, 64, 289–296. [Google Scholar] [CrossRef]
- Fan, S.; Li, K.; Yan, Y.; Wang, J.; Wang, J.; Qiao, C.; Yang, T.; Jia, Y.; Zhao, B. A novel chlorpyrifos hydrolase CPD from Paracoccus sp. TRP: Molecular cloning, characterization and catalytic mechanism. Electron. J. Biotechnol. 2018, 31, 10–16. [Google Scholar] [CrossRef]
- Palm, G.J.; Fernandez-Alvaro, E.; Bogdanovic, X.; Bartsch, S.; Sczodrok, J.; Singh, R.K.; Böttcher, D.; Atomi, H.; Bornscheuer, U.T.; Hinrichs, W. The crystal structure of an esterase from the hyperthermophilic microorganism Pyrobaculum calidifontis VA1 explains its enantioselectivity. Appl. Microbiol. Biotechnol. 2011, 91, 1061–1072. [Google Scholar] [CrossRef]
- Dos Santos, A.M.; Oliveira, A.R.S.; da Costa, C.H.S.; Kenny, P.W.; Montanari, C.A.; Varela, J.D.J.G.; Lameira, J. Assessment of Reversibility for Covalent Cysteine Protease Inhibitors Using Quantum Mechanics/Molecular Mechanics Free Energy Surfaces. J. Chem. Inf. Model. 2022, 62, 4083–4094. [Google Scholar] [CrossRef] [PubMed]
- Tubiana, T.; Carvaillo, J.-C.; Boulard, Y.; Bressanelli, S. TTClust: A Versatile Molecular Simulation Trajectory Clustering Program with Graphical Summaries. J. Chem. Inf. Model. 2018, 58, 2178–2182. [Google Scholar] [CrossRef] [PubMed]
- Lonsdale, R.; Harvey, J.N.; Mulholland, A.J. Compound I Reactivity Defines Alkene Oxidation Selectivity in Cytochrome P450cam. J. Phys. Chem. B 2010, 114, 1156–1162. [Google Scholar] [CrossRef] [PubMed]
- Rommel, J.B.; Kästner, J. The Fragmentation–Recombination Mechanism of the Enzyme Glutamate Mutase Studied by QM/MM Simulations. J. Am. Chem. Soc. 2011, 133, 10195–10203. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Kaya, S.; Abousalem, A.S.; Kumar, A. Experimental, DFT and MC simulation analysis of Vicia sativa weed aerial extract as sustainable and eco-benign corrosion inhibitor for mild steel in acidic environment. Sustain. Chem. Pharm. 2022, 29, 100785. [Google Scholar] [CrossRef]
- Gandhimathi, S.; Balakrishnan, C.; Theetharappan, M.; Neelakantan, M.A.; Venkataraman, R. Noncovalent interactions from electron density topology and solvent effects on spectral properties of Schiff bases. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 175, 134–144. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. WIREs Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Thakur, A.; Kaya, S.; Abousalem, A.S.; Sharma, S.; Ganjoo, R.; Assad, H.; Kumar, A. Computational and experimental studies on the corrosion inhibition performance of an aerial extract of Cnicus Benedictus weed on the acidic corrosion of mild steel. Process Saf. Environ. Prot. 2022, 161, 801–818. [Google Scholar] [CrossRef]
- Gaillard, T. Evaluation of AutoDock and AutoDock Vina on the CASF-2013 Benchmark. J. Chem. Inf. Model. 2018, 58, 1697–1706. [Google Scholar] [CrossRef] [PubMed]
- Goodsell, D.S.; Morris, G.M.; Olson, A.J. Automated docking of flexible ligands: Applications of autodock. J. Mol. Recognit. 1996, 9, 1–5. [Google Scholar] [CrossRef]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam: A fast force field generation tool for small organic molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.H.M.; Søndergaard, C.R.; Rostkowski, M.; Jensen, J.H. PROPKA3: Consistent Treatment of Internal and Surface Residues in Empirical pKa Predictions. J. Chem. Theory Comput. 2011, 7, 525–537. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A program for macromolecular energy, minimization, and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Karplus, M. Molecular Dynamics Simulations of Biomolecules. Acc. Chem. Res. 2002, 35, 321–323. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Bashir, S.; Thakur, A.; Lgaz, H.; Chung, I.-M.; Kumar, A. Corrosion inhibition efficiency of bronopol on aluminium in 0.5 M HCl solution: Insights from experimental and quantum chemical studies. Surf. Interfaces 2020, 20, 100542. [Google Scholar] [CrossRef]
- Bashir, S.; Thakur, A.; Lgaz, H.; Chung, I.-M.; Kumar, A. Corrosion Inhibition Performance of Acarbose on Mild Steel Corrosion in Acidic Medium: An Experimental and Computational Study. Arab. J. Sci. Eng. 2020, 45, 4773–4783. [Google Scholar] [CrossRef]
- Parveen, G.; Bashir, S.; Thakur, A.; Saha, S.K.; Banerjee, P.; Kumar, A. Experimental and computational studies of imidazolium based ionic liquid 1-methyl-3-propylimidazolium iodide on mild steel corrosion in acidic solution. Mater. Res. Express 2019, 7, 016510. [Google Scholar] [CrossRef]
- Metz, S.; Kästner, J.; Sokol, A.A.; Keal, T.W.; Sherwood, P. ChemShell—A modular software package for QM/MM simulations. WIREs Comput. Mol. Sci. 2014, 4, 101–110. [Google Scholar] [CrossRef]
- Ahlrichs, R.; Bär, M.; Häser, M.; Horn, H.; Kölmel, C. Electronic structure calculations on workstation computers: The program system turbomole. Chem. Phys. Lett. 1989, 162, 165–169. [Google Scholar] [CrossRef]
- Smith, W.; Forester, T.R. DL_POLY_2.0: A general-purpose parallel molecular dynamics simulation package. J. Mol. Graph. 1996, 14, 136–141. [Google Scholar] [CrossRef]
- Bakowies, D.; Thiel, W. Hybrid Models for Combined Quantum Mechanical and Molecular Mechanical Approaches. J. Phys. Chem. 1996, 100, 10580–10594. [Google Scholar] [CrossRef]
- de Vries, A.H.; Sherwood, P.; Collins, S.J.; Rigby, A.M.; Rigutto, M.; Kramer, G.J. Zeolite Structure and Reactivity by Combined Quantum-Chemical−Classical Calculations. J. Phys. Chem. B 1999, 103, 6133–6141. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).