Application of Droplet Digital PCR Technology in Muscular Dystrophies Research
Abstract
:1. Introduction
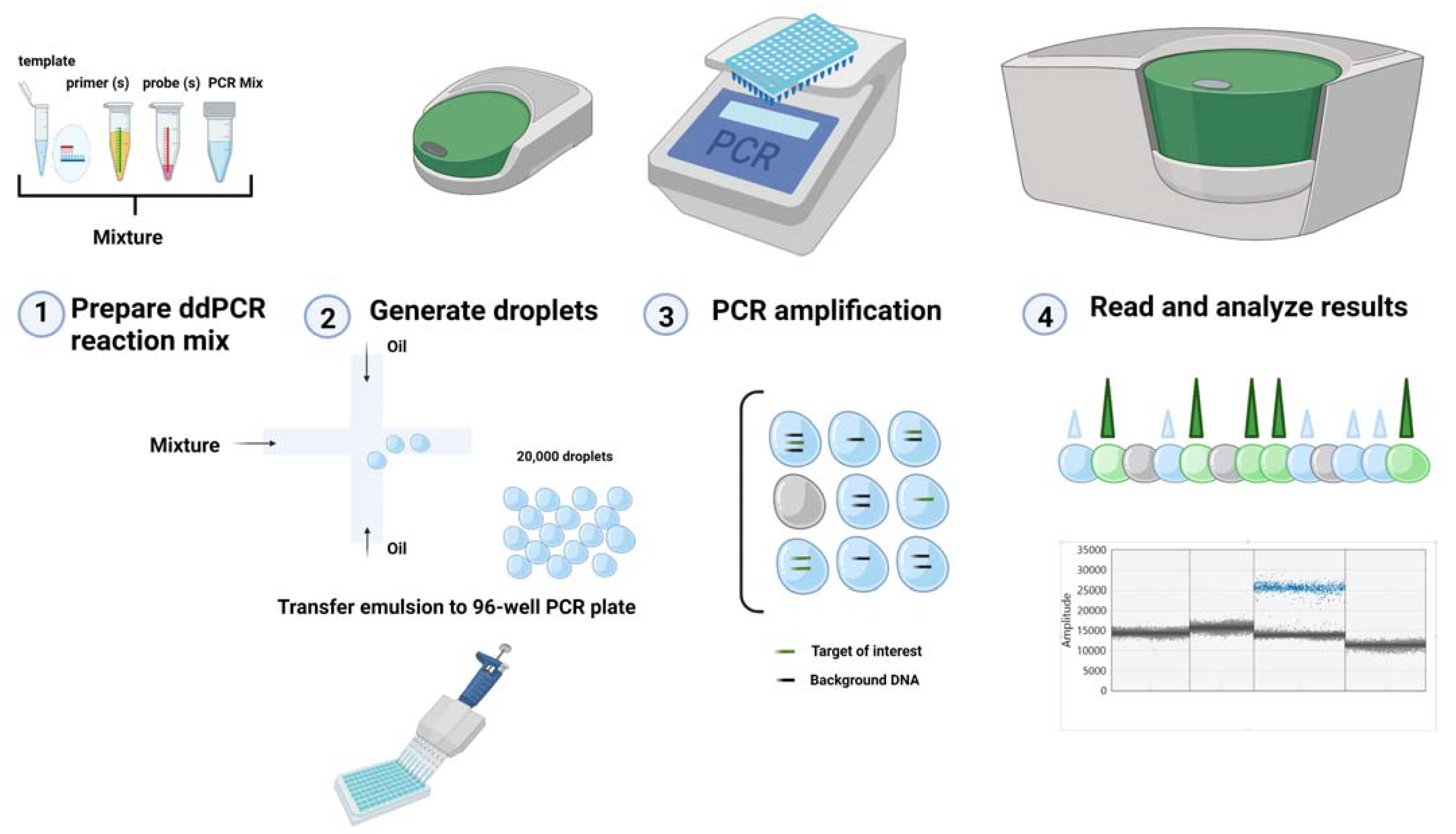
2. ddPCR Technology
3. Advantages and Disadvantages of ddPCR
4. Hallmarks of a Well-Designed ddPCR Assay
- (1)
- Nucleic acid samples concentration. As well as other molecular biology techniques, the quality, and the quantity of nucleic acid samples, may affect the result and are essential for the accuracy of the assay. No special requirements are necessary regarding the sample preparation. However, it should be noted that some methods of nucleic acid isolation may interfere with the generation of droplets [38] and, therefore, the method must be chosen which offers a good separation of positive and negative droplets as well as the best signal intensity. Qubit (Invitrogen, Waltham, USA) and Nanodrop (Thermo Scientific, Waltham, USA) measurement of nucleic acid samples concentration and purity is essential to achieve reliable results [39].
- (2)
- The design of primers and probes are among the most critical factors for the success of the experiment and should be carefully done to avoid self-annealing or cross-reactivity. Whether or not a design program is used, for primers and probes, the same rules as for qPCR analysis must be apply.
- (3)
- Assay optimization. Achieving accurate interpretable results requires a number of important factors to be considered when optimizing a ddPCR assay. The annealing temperature must be optimized for each target using a gradient PCR range between 55 and 65 °C, an interval in which most targets have an optimal temperature. A temperature is optimized when the largest separation between positive and negative droplets is achieved [37,41].
- (4)
- Controls. In ddPCR technology, the use of a reference gene is not mandatory because of the absolute quantification of the number of targets from a sample. Furthermore, the assays can be affected by technical problems associated with the reverse transcription step. Primer dimers and secondary structures are avoided, and the annealing temperature can be used for reaction optimization. An important aspect of the ddPCR assay is represented by the appropriate use of a specific set of controls that are important for method performance [46,47]:
- (i)
- negative controls—for monitoring a false-positive reaction, which may be a marker of contamination or a poor design of primers/probes, and for the determination of limit of detection (LoD);
- (ii)
- positive controls—useful to test for whether the template amplification occurs under the established reaction conditions;
- (iii)
- non-template controls (NTCs)—for control of contamination in all reagents [48]. Poor design optimization can lead to a bad assay performance.
5. Applications of ddPCR in Muscular Dystrophy Research
5.1. Absolute Quantification
5.2. Copy Number Variation
5.3. Gene Expression and miRNA Quantification
5.4. Non-Invasive Prenatal Diagnosis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dalkilic, I.; Kunkel, L.M. Muscular dystrophies: Genes to pathogenesis. Curr. Opin. Genet. Dev. 2003, 13, 231–238. [Google Scholar] [CrossRef]
- Mercuri, E.; Muntoni, F. Muscular Dystrophy. Curr Opin Pediatr. 2013, 25, 701–707. Available online: https://journals.lww.com/00008480-201312000-00012 (accessed on 16 March 2020). [CrossRef]
- Gaina, G.; Budisteanu, M.; Manole, E.; Ionica, E. Clinical and Molecular Diagnosis in Muscular Dystrophies. In Muscular Dystrophies; InTechOpen: London, UK, 2019; Available online: https://www.intechopen.com/books/muscular-dystrophies/clinical-and-molecular-diagnosis-in-muscular-dystrophies (accessed on 17 January 2022).
- Hoffman, E.P.; Brown, R.H.; Kunkel, L.M. Dystrophin: The protein product of the duchenne muscular dystrophy locus. Cell 1987, 51, 919–928. [Google Scholar] [CrossRef]
- Davies, K.E.; Nowak, K.J. Molecular Mechanisms of Muscular Dystrophies: Old and New Players. Nat. Rev. Mol. Cell Biol. 2006, 7, 762–773. Available online: http://www.nature.com/articles/nrm2024 (accessed on 13 February 2021). [CrossRef]
- Emery, A.E. The muscular Dystrophies. Lancet 2002, 359, 687–695. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0140673602078157 (accessed on 21 February 2022). [CrossRef]
- Gussoni, E.; Soneoka, Y.; Strickland, C.D.; Buzney, E.A.; Khan, M.K.; Flint, A.F.; Kunkel, L.M.; Mulligan, R.C. Dystrophin Expression in the mdx Mouse Restored by Stem Cell Transplantation. Nature 1999, 401, 390–394. Available online: http://www.nature.com/articles/43919 (accessed on 18 May 2020). [CrossRef]
- Partridge, T.A.; Morgan, J.E.; Coulton, G.R.; Hoffman, E.P.; Kunkel, L.M. Conversion of mdx Myofibres from Dystrophin-Negative to -Positive by Injection of Normal Myoblasts. Nature 1989, 337, 176–179. Available online: http://www.nature.com/articles/337176a0 (accessed on 25 March 2020). [CrossRef] [PubMed]
- Roy, P.; Rau, F.; Ochala, J.; Messéant, J.; Fraysse, B.; Lainé, J.; Agbulut, O.; Butler-Browne, G.; Furling, D.; Ferry, A. Dystrophin Restoration Therapy Improves both the Reduced Excitability and the Force Drop Induced by Lengthening Contractions in Dystrophic mdx Skeletal Muscle. Skelet. Muscle 2016, 6, 23. Available online: http://skeletalmusclejournal.biomedcentral.com/articles/10.1186/s13395-016-0096-4 (accessed on 23 October 2021). [CrossRef] [PubMed] [Green Version]
- Aartsma-Rus, A.; van Ommen, G.-J.B. Antisense-mediated exon skipping: A versatile tool with therapeutic and research applications. RNA 2007, 13, 1609–1624. Available online: http://www.ncbi.nlm.nih.gov/pubmed/17684229 (accessed on 11 October 2021). [CrossRef] [PubMed] [Green Version]
- Moretti, A.; Fonteyne, L.; Giesert, F.; Hoppmann, P.; Meier, A.B.; Bozoglu, T.; Baehr, A.; Schneider, C.M.; Sinnecker, D.; Klett, K.; et al. Somatic Gene Editing Ameliorates Skeletal and Cardiac Muscle Failure in Pig and Human Models of Duchenne Muscular Dystrophy. Nat. Med. 2020, 26, 207–214. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31988462 (accessed on 27 February 2022). [CrossRef] [PubMed]
- Nakamura, A. Mutation-Based Therapeutic Strategies for Duchenne Muscular Dystrophy: From Genetic Diagnosis to Therapy. J. Pers. Med. 2019, 9, 16. Available online: https://www.mdpi.com/2075-4426/9/1/16 (accessed on 21 February 2022). [CrossRef] [PubMed] [Green Version]
- Aartsma-Rus, A.; Ginjaar, I.B.; Bushby, K. The Importance of Genetic Diagnosis for Duchenne Muscular Dystrophy. J. Med. Genet. 2016, 53, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of Fetal DNA in Maternal Plasma and Serum. Lancet 1997, 350, 485–487. Available online: http://www.ncbi.nlm.nih.gov/pubmed/9274585 (accessed on 19 February 2022). [CrossRef]
- Wood, M.F.; Ms, S.C.H.; Ms, L.P.H.; Naylor, E.W.; Abdel-Hamid, H.Z.; Barmada, M.M.; Dobrowolski, S.F.; Stickler, D.E.; Clemens, P.R. Parental Attitudes toward Newborn Screening for Duchenne/Becker Muscular Dystrophy and Spinal Muscular Atrophy. Muscle Nerve 2014, 49, 822–828. [Google Scholar] [CrossRef] [Green Version]
- Sykes, P.J.; Neoh, S.H.; Brisco, M.J.; Hughes, E.; Condon, J.; Morley, A.A. Quantitation of Targets for PCR by Use of Limiting Dilution. Biotechniques 1992, 13, 444–449. Available online: http://www.ncbi.nlm.nih.gov/pubmed/1389177 (accessed on 17 November 2021). [PubMed]
- Jeffreys, A.J.; Neumann, R.; Wilson, V. Repeat unit sequence variation in minisatellites: A Novel Source of DNA Polymorphism for Studying Variation and Mutation by Single Molecule Analysis. Cell 1990, 60, 473–485. Available online: https://linkinghub.elsevier.com/retrieve/pii/0092867490905989 (accessed on 17 September 2020). [CrossRef]
- Vogelstein, B.; Kinzler, K.W. Digital PCR. Proc. Natl. Acad. Sci. USA 1999, 96, 9236–9241. [Google Scholar] [CrossRef] [Green Version]
- Verheul, R.C.; Van Deutekom, J.C.T.; Datson, N.A. Digital Droplet PCR for the Absolute Quantification of Exon Skipping Induced by Antisense Oligonucleotides in (Pre-)Clinical Development for Duchenne Muscular Dystrophy. PLoS ONE 2016, 11, e0162467. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.-L.; Chemello, F.; Li, H.; Rodriguez-Caycedo, C.; Sanchez-Ortiz, E.; Mireault, A.A.; McAnally, J.R.; Shelton, J.M.; Zhang, Y.; Bassel-Duby, R.; et al. Correction of Three Prominent Mutations in Mouse and Human Models of Duchenne Muscular Dystrophy by Single-Cut Genome Editing. Mol. Ther. 2020, 28, 2044–2055. [Google Scholar] [CrossRef]
- Cao, L.; Cui, X.; Hu, J.; Li, Z.; Choi, J.R.; Yang, Q.; Lin, M.; Hui, L.Y.; Xu, F. Advances in digital polymerase chain reaction (dPCR) and its emerging biomedical applications. Biosens. Bioelectron. 2017, 90, 459–474. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.bio-rad.com/webroot/web/pdf/lsr/literature/Bulletin_6407.pdf (accessed on 11 February 2022).
- Hindson, C.M.; Chevillet, J.R.; Briggs, H.A.; Gallichotte, E.N.; Ruf, I.K.; Hindson, B.J.; Vessella, R.L.; Tewari, M. Absolute Quantification by Droplet Digital PCR Versus Analog Real-Time PCR. Nat. Methods 2013, 10, 1003–1005. Available online: http://www.nature.com/articles/nmeth.2633 (accessed on 11 October 2021). [CrossRef] [PubMed]
- Kreutz, J.E.; Munson, T.; Huynh, T.; Shen, F.; Du, W.; Ismagilov, R.F. Theoretical Design and Analysis of Multivolume Digital Assays with Wide Dynamic Range Validated Experimentally with Microfluidic Digital PCR. Anal. Chem. 2011, 83, 8158–8168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Majumdar, N.; Wessel, T.; Marks, J. Digital PCR Modeling for Maximal Sensitivity, Dynamic Range and Measurement Precision. Margis, R., Ed. PLoS ONE 2015, 10, e0118833. [Google Scholar] [CrossRef] [PubMed]
- Turner, S.A.; Tsongalis, G.J. Automaction of the Molecular Diagnostic Laboratory. In Diagnostic Molecular Pathology; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 35–46. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128008867000042 (accessed on 18 December 2021).
- Day, E.; Dear, P.H.; McCaughan, F. Digital PCR strategies in the development and analysis of molecular biomarkers for personalized medicine. Methods 2013, 59, 101–107. [Google Scholar] [CrossRef]
- Campomenosi, P.; Gini, E.; Noonan, D.M.; Poli, A.; D’Antona, P.; Rotolo, N.; Dominioni, L.; Imperatori, A.S. A Comparison between Quantitative PCR and Droplet Digital PCR Technologies for Circulating microRNA Quantification in Human Lung Cancer. BMC Biotechnol. 2016, 16, 60. Available online: http://bmcbiotechnol.biomedcentral.com/articles/10.1186/s12896-016-0292-7 (accessed on 11 January 2022). [CrossRef] [Green Version]
- Quan, P.L.; Sauzade, M.; Brouzes, E. DPCR: A Technology Review. Sensors 2018, 18, 1271. [Google Scholar] [CrossRef] [Green Version]
- Kuhlmann, K.; Cieselski, M.; Schumann, J. Relative versus absolute RNA quantification: A Comparative Analysis Based on the Example of Endothelial Expression of Vasoactive Receptors. Biol. Proced. Online 2021, 23, 6. Available online: https://biologicalproceduresonline.biomedcentral.com/articles/10.1186/s12575-021-00144-w (accessed on 17 February 2022). [CrossRef] [PubMed]
- Mao, X.; Liu, C.; Tong, H.; Chen, Y.; Liu, K. Principles of digital PCR and its applications in current obstetrical and gynecological diseases. Am. J. Transl. Res. 2019, 11, 7209–7222. [Google Scholar]
- Taylor, S.C.; Laperriere, G.; Germain, H. Droplet Digital PCR Versus qPCR for Gene Expression Analysis with Low Abundant Targets: From Variable Nonsense to Publication Quality Data. Sci. Rep. 2017, 7, 2409. Available online: http://www.ncbi.nlm.nih.gov/pubmed/28546538 (accessed on 11 January 2022). [CrossRef] [PubMed] [Green Version]
- Taylor, S.C.; Carbonneau, J.; Shelton, D.N.; Boivin, G. Optimization of Droplet Digital PCR from RNA and DNA extracts with direct comparison to RT-qPCR: Clinical implications for quantification of Oseltamivir-resistant subpopulations. J. Virol. Methods 2015, 224, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Olmedillas-López, S.; García-Arranz, M.; García-Olmo, D. Current and Emerging Applications of Droplet Digital PCR in Oncology. Mol. Diagn. Ther. 2017, 21, 493–510. [Google Scholar] [CrossRef]
- Available online: https://geneticeducation.co.in/real-time-pcr-principle-procedure-advantages-limitations-and-applications (accessed on 17 February 2022).
- Kim, T.G.; Jeong, S.-Y.; Cho, K.-S. Comparison of droplet digital PCR and quantitative real-time PCR for examining population dynamics of bacteria in soil. Appl. Microbiol. Biotechnol. 2014, 98, 6105–6113. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, L.; Iwobi, A.; Busch, U.; Pecoraro, S. Optimization of digital droplet polymerase chain reaction for quantification of genetically modified organisms. Biomol. Detect. Quantif. 2016, 7, 9–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pecoraro, S.; Berben, G.; Burns, M.; Corbisier, P.; De Giacomo, M.; De Loose, M.; Dagand, E.; Dobnik, D.; Eriksson, R.; Holst-Jensen, A.; et al. Overview and Recommendations for the Application of Digital PCR European Network of GMO Laboratories (ENGL); Publications Office of the European Union Location: Luxembourg, 2019; pp. 1–60. Available online: https://ec.europa.eu/jrc (accessed on 28 February 2022).
- Vossen, R.H.A.M.; White, S.J. Quantitative DNA Analysis Using Droplet Digital PCR. Bacteriophages 2016, 1492, 167–177. [Google Scholar] [CrossRef]
- Basu, A.S. Digital Assays Part I: Partitioning Statistics and Digital PCR. SLAS Technol. Transl. Life Sci. Innov. 2017, 22, 369–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lievens, A.; Jacchia, S.; Kagkli, D.; Savini, C.; Querci, M. Measuring Digital PCR Quality: Performance Parameters and Their Optimization. Chan, K.Y.K., Ed. PLoS ONE 2016, 11, e0153317. [Google Scholar] [CrossRef] [Green Version]
- McDermott, G.P.; Do, D.; Litterst, C.M.; Maar, D.; Hindson, C.M.; Steenblock, E.R.; Legler, T.C.; Jouvenot, Y.; Marrs, S.H.; Bemis, A.; et al. Multiplexed Target Detection Using DNA-Binding Dye Chemistry in Droplet Digital PCR. Anal. Chem. 2013, 85, 11619–11627. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Li, M.; Berger, S.; Meilak, M.; Rientjes, J.; Currie, P.D. Effect of Ataluren on dystrophin mutations. J. Cell. Mol. Med. 2020, 24, 6680–6689. [Google Scholar] [CrossRef]
- Zhong, Q.; Bhattacharya, S.; Kotsopoulos, S.; Olson, J.; Taly, V.; Griffiths, A.D.; Link, D.R.; Larson, J.W. Multiplex digital PCR: Breaking the one target per color barrier of quantitative PCR. Lab Chip 2011, 11, 2167–2174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurbich, T.A.; Ilinsky, V.V. Classify CNV: A tool for clinical annotation of copy-number variants. Sci. Rep. 2020, 10, 20375. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, L.B.; Coleman, V.A.; Hindson, C.M.; Herrmann, J.; Hindson, B.J.; Bhat, S.; Emslie, K.R. Evaluation of a Droplet Digital Polymerase Chain Reaction Format for DNA Copy Number Quantification. Anal. Chem. 2012, 84, 1003–1011. [Google Scholar] [CrossRef]
- Huggett, J.F.; Cowen, S.; Foy, C.A. Considerations for Digital PCR as an Accurate Molecular Diagnostic Tool. Clin. Chem. 2015, 61, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Kokkoris, V.; Vukicevich, E.; Richards, A.; Thomsen, C.; Hart, M.M. Challenges Using Droplet Digital PCR for Environmental Samples. Appl. Microbiol. 2021, 1, 74–88. Available online: https://www.mdpi.com/2673-8007/1/1/7 (accessed on 18 February 2022). [CrossRef]
- Cao, Z.; Wu, W.; Wei, H.; Gao, C.; Zhang, L.; Wu, C.; Hou, L. Using Droplet Digital PCR in the Detection of Mycobacterium Tuberculosis DNA in FFPE Samples. Int. J. Infect. Dis. 2020, 99, 77–83. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1201971220305890 (accessed on 18 February 2022). [CrossRef]
- Rotondo, J.C.; Oton-Gonzalez, L.; Mazziotta, C.; Lanzillotti, C.; Iaquinta, M.R.; Tognon, M.; Martini, F. Simultaneous Detection and Viral DNA Load Quantification of Different Human Papillomavirus Types in Clinical Specimens by the High Analytical Droplet Digital PCR Method. Front. Microbiol. 2020, 11, 2818. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2020.591452/full (accessed on 19 February 2022). [CrossRef]
- Li, H.; Bai, R.; Zhao, Z.; Tao, L.; Ma, M.; Ji, Z.; Jian, M.; Ding, Z.; Dai, X.; Bao, F.; et al. Application of Droplet Digital PCR to Detect the Pathogens of Infectious Diseases. Biosci. Rep. 2018, 38, BSR20181170. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30341241 (accessed on 18 February 2022). [CrossRef] [Green Version]
- Tedim, A.P.; Almansa, R.; Domínguez-Gil, M.; González-Rivera, M.; Micheloud, D.; Ryan, P.; Méndez, R.; Blanca-López, N.; Pérez-García, F.; Bustamante, E.; et al. Comparison of Real-Time and Droplet Digital PCR to Detect and Quantify SARS-CoV-2 RNA in Plasma. Eur. J. Clin. Investig. 2021, 51, e13501. [Google Scholar] [CrossRef]
- Hosen, I.; Forey, N.; Durand, G.; Voegele, C.; Bilici, S.; Avogbe, P.H.; Delhomme, T.M.; Foll, M.; Manel, A.; Vian, E.; et al. Development of Sensitive Droplet Digital PCR Assays for Detecting Urinary TERT Promoter Mutations as Non-Invasive Biomarkers for Detection of Urothelial Cancer. Cancers 2020, 12, 3541. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33260905 (accessed on 17 February 2022). [CrossRef]
- Antoury, L.; Hu, N.; Balaj, L.; Das, S.; Georghiou, S.; Darras, B.; Clark, T.; Breakefield, X.O.; Wheeler, T.M. Analysis of Extracellular mRNA in Human Urine Reveals Splice Variant Biomarkers of Muscular Dystrophies. Nat. Commun. 2018, 9, 3906. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30254196 (accessed on 11 November 2021). [CrossRef] [Green Version]
- Koutsoulidou, A.; Phylactou, L.A. Circulating Biomarkers in Muscular Dystrophies: Disease and Therapy Monitoring. Mol. Ther. Methods Clin. Dev. 2020, 18, 230–239. [Google Scholar] [CrossRef]
- Lancíková, V.; Hricová, A. Digital Absolute Gene Expression Analysis of Essential Starch-Related Genes in a Radiation Developed Amaranthus Cruentus L. Variety in Comparison with Real-Time PCR. Plants 2020, 9, 966. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32751665 (accessed on 21 February 2022). [CrossRef] [PubMed]
- Abdelrazig, A.O.; Siriyod, N.; Suwannarat, S.; Rijiravanich, P.; Surareungchai, W. Development of Species-Specific Primers and Highly Sensitive Duplex ddPCR Assay for the Identification and Detection of Chili Anthracnose. Eur. J. Plant Pathol. 2021, 162, 609–619. Available online: https://link.springer.com/10.1007/s10658-021-02424-3 (accessed on 11 February 2022). [CrossRef]
- Basanisi, M.G.; La Bella, G.; Nobili, G.; Coppola, R.; Damato, A.M.; Cafiero, M.A.; La Salandra, G. Application of the Novel Droplet Digital PCR Technology for Identification of Meat Species. Int. J. Food Sci. Technol. 2020, 55, 1145–1150. Available online: https://onlinelibrary.wiley.com/doi/10.1111/ijfs.14486 (accessed on 18 February 2022). [CrossRef] [Green Version]
- NoSolid Biosciences Solid Biosciences Announces Clinical Hold On SGT-001 Phase I/II Clinical Trial for Duchenne Muscular Dystrophy. Available online: https://investors.solidbio.com/news-releases/news-release-details/solid-biosciences-announces-clinical-hold-sgt-001-phase-iii (accessed on 19 December 2020).
- Study to Evaluate the Safety and Tolerability of pf-06939926 Gene Therapy in Duchenne Muscular Dystrophy. Available online: https://clinicaltrials.gov/ct2/show/NCT03362502 (accessed on 17 February 2022).
- A Gene Transfer Therapy Study to Evaluate the Safety of SRP-9001 in Participants with Duchenne Muscular Dystrophy (DMD). Available online: https://clinicaltrials.gov/ct2/show/NCT03375164 (accessed on 17 February 2022).
- Monaco, A.P.; Bertelson, C.J.; Liechti-Gallati, S.; Moser, H.; Kunkel, L.M. An Explanation for the Phenotypic Differences between Patients Bearing Partial Deletions of the DMD Locus. Genomics 1988, 2, 90–95. Available online: https://linkinghub.elsevier.com/retrieve/pii/0888754388901139 (accessed on 30 September 2021). [CrossRef]
- Lim, K.R.Q.; Nguyen, Q.; Yokota, T. Genotype–Phenotype Correlations in Duchenne and Becker Muscular Dystrophy Patients from the Canadian Neuromuscular Disease Registry. J. Pers. Med. 2020, 10, 241. Available online: https://www.mdpi.com/2075-4426/10/4/241 (accessed on 17 January 2022). [CrossRef]
- Godfrey, C.; Muses, S.; McClorey, G.; Wells, K.E.; Coursindel, T.; Terry, R.L.; Betts, C.; Hammond, S.; O’Donovan, L.; Hildyard, J.; et al. How much Dystrophin is Enough: The Physiological Consequences of Different Levels of Dystrophin in the mdx Mouse. Hum. Mol. Genet. 2015, 24, 4225–4237. [Google Scholar] [CrossRef] [Green Version]
- Neri, M.; Torelli, S.; Brown, S.; Ugo, I.; Sabatelli, P.; Merlini, L.; Spitali, P.; Rimessi, P.; Gualandi, F.; Sewry, C.; et al. Dystrophin Levels as Low as 30% Are Sufficient to Avoid Muscular Dystrophy in the Human. Neuromuscul. Disord. 2007, 17, 913–918. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0960896607006773 (accessed on 18 November 2021). [CrossRef]
- Turczynski, S.; Titeux, M.; Pironon, N.; Hovnanian, A. Antisense-Mediated Exon Skipping to Reframe Transcripts. Adv. Struct. Saf. Stud. 2012, 867, 221–238. Available online: http://link.springer.com/10.1007/978-1-61779-767-5_15 (accessed on 18 February 2022).
- Hammond, S.M.; Aartsma-Rus, A.; Alves, S.; Borgos, S.E.; Buijsen, R.A.M.; Collin, R.W.J.; Covello, G.; Denti, M.A.; Desviat, L.R.; Echevarría, L.; et al. Delivery of oligonucleotide-based therapeutics: Challenges and opportunities. EMBO Mol. Med. 2021, 13, e13243. [Google Scholar] [CrossRef]
- Veltrop, M.; van Vliet, L.; Hulsker, M.; Claassens, J.; Brouwers, C.; Breukel, C.; Van Der Kaa, J.; Linssen, M.M.; Dunnen, J.T.D.; Verbeek, S.; et al. A Dystrophic DUCHENNE Mouse Model for Testing Human Antisense Oligonucleotides. PLoS ONE 2018, 13, e0193289. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29466448 (accessed on 11 February 2022). [CrossRef]
- Yavas, A.; Weij, R.; van Putten, M.; Kourkouta, E.; Beekman, C.; Puoliväli, J.; Bragge, T.; Ahtoniemi, T.; Knijnenburg, J.; Hoogenboom, M.E.; et al. Detailed Genetic and Functional Analysis of the hDMDdel52/mdx Mouse Model. PLoS ONE 2020, 15, e0244215. [Google Scholar] [CrossRef]
- Hiller, M.; Spitali, P.; Datson, N.; Aartsma-Rus, A. Exon 51 Skipping Quantification by Digital Droplet PCR in del52hDMD/mdx Mice. Methods Mol. Biol. 2018, 1828, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, B.; Shah, S.N.; Lu, P.; Lu, Q. Saponins Enhance Exon Skipping of 2′-O-Methyl Phosphorothioate Oligonucleotide In Vitro and In Vivo. Drug Des. Dev. Ther. 2018, 12, 3705–3715. Available online: https://www.dovepress.com/saponins-enhance-exon-skipping-of-2prime-o-methyl-phosphorothioate-oli-peer-reviewed-article-DDDT (accessed on 28 February 2022). [CrossRef] [PubMed] [Green Version]
- van Putten, M.; Tanganyika-de Winter, C.; Bosgra, S.; Aartsma-Rus, A. Nonclinical Exon Skipping Studies with 2′-O-Methyl Phosphorothioate Antisense Oligonucleotides in mdx and mdx-utrn-/- Mice Inspired by Clinical Trial Results. Nucleic Acid Ther. 2019, 29, 92–103. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30672725 (accessed on 28 January 2022). [CrossRef] [PubMed] [Green Version]
- McDonald, C.M.; Shieh, P.B.; Abdel-Hamid, H.Z.; Connolly, A.M.; Ciafaloni, E.; Wagner, K.R.; Goemans, N.; Mercuri, E.; Khan, N.; Koenig, E.; et al. Open-Label Evaluation of Eteplirsen in Patients with Duchenne Muscular Dystrophy Amenable to Exon 51 Skipping: PROMOVI Trial. J. Neuromuscul. Dis. 2021, 8, 989–1001. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34120909 (accessed on 28 February 2022). [CrossRef] [PubMed]
- Heo, Y.A. Golodirsen: First Approval. Drugs 2020, 80, 329–333. Available online: http://www.ncbi.nlm.nih.gov/pubmed/32026421 (accessed on 24 January 2022). [CrossRef]
- Anwar, S.; Yokota, T. Golodirsen for Duchenne Muscular Dystrophy. Drugs Today 2020, 56, 491–504. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33025945 (accessed on 24 February 2022). [CrossRef]
- Dhillon, S. Viltolarsen: First Approval. Drugs 2020, 80, 1027–1031. Available online: https://link.springer.com/10.1007/s40265-020-01339-3 (accessed on 30 January 2022). [CrossRef]
- Wagner, K.R.; Kuntz, N.L.; Koenig, E.; East, L.; Upadhyay, S.; Han, B.; Shieh, P.B. Safety, Tolerability, and Pharmacokinetics of Casimersen in Patients with DUCHENNE Muscular Dystrophy Amenable to Exon 45 Skipping: A Randomized, Double-Blind, Placebo-Controlled, Dose-Titration Trial. Muscle Nerve 2021, 64, 285–292. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34105177 (accessed on 27 February 2022). [CrossRef]
- Shirley, M. Casimersen: First Approval. Drugs 2021, 81, 875–879. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33861387 (accessed on 21 February 2022). [CrossRef]
- Hiller, M.; Falzarano, M.S.; Garcia-Jimenez, I.; Sardone, V.; Verheul, R.C.; Popplewell, L.; Anthony, K.; Ruiz-Del-Yerro, E.; Osman, H.; Goeman, J.J.; et al. A Multicenter Comparison of Quantification Methods for Antisense Oligonucleotide-Induced DMD Exon 51 Skipping in Duchenne Muscular Dystrophy Cell Cultures. PLoS ONE 2018, 13, e0204485. Available online: http://www.ncbi.nlm.nih.gov/pubmed/30278058 (accessed on 21 February 2022). [CrossRef] [PubMed] [Green Version]
- Novak, J.S.; Spathis, R.; Dang, U.J.; Fiorillo, A.A.; Hindupur, R.; Tully, C.B.; Mázala, D.A.; Canessa, E.; Brown, K.J.; Partridge, T.A.; et al. Interrogation of Dystrophin and Dystroglycan Complex Protein Turnover After Exon Skipping Therapy. J. Neuromuscul. Dis. 2021, 8, S383–S402. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34569969 (accessed on 24 February 2022). [CrossRef] [PubMed]
- Michael, E.; Sofou, K.; Wahlgren, L.; Kroksmark, A.-K.; Tulinius, M. Long Term Treatment with Ataluren—The Swedish Experience. BMC Musculoskelet. Disord. 2021, 22, 837. Available online: https://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-021-04700-z (accessed on 21 February 2022). [CrossRef] [PubMed]
- Available online: https://www.ema.europa.eu/en/documents/product-information/translarna-epar-product-information_en.pdf (accessed on 21 February 2022).
- Doudna, J.A.; Charpentier, E. The new frontier of genome engineering with CRISPR-Cas9. Genome Ed. Science 2014, 346, 1258096. [Google Scholar] [CrossRef]
- Soblechero-Martín, P.; Albiasu-Arteta, E.; Anton-Martinez, A.; de la Puente-Ovejero, L.; Garcia-Jimenez, I.; González-Iglesias, G.; Larrañaga-Aiestaran, I.; López-Martínez, A.; Poyatos-García, J.; Ruiz-Del-Yerro, E.; et al. Duchenne Muscular Dystrophy Cell Culture Models Created by CRISPR/Cas9 Gene Editing and Their Application in Drug Screening. Sci. Rep. 2021, 11, 18188. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34521928 (accessed on 13 December 2021). [CrossRef]
- Lostal, W.; Roudaut, C.; Faivre, M.; Charton, K.; Suel, L.; Bourg, N.; Best, H.; Smith, J.E.; Gohlke, J.; Corre, G.; et al. Titin splicing regulates cardiotoxicity associated with calpain 3 gene therapy for limb-girdle muscular dystrophy type 2A. Sci. Transl. Med. 2019, 11, eaat6072. [Google Scholar] [CrossRef] [PubMed]
- Pirooznia, M.; Goes, F.S.; Zandi, P.P. Whole-genome CNV Analysis: Advances in Computational Approaches. Front. Genet. 2015, 6, 138. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25918519 (accessed on 11 November 2021). [CrossRef] [Green Version]
- Iafrate, A.J.; Feuk, L.; Rivera, M.N.; Listewnik, M.L.; Donahoe, P.K.; Qi, Y.; Scherer, S.; Lee, C. Detection of LARGE-scale Variation in the Human Genome. Nat. Genet. 2004, 36, 949–951. Available online: http://www.ncbi.nlm.nih.gov/pubmed/15286789 (accessed on 11 November 2021). [CrossRef] [PubMed] [Green Version]
- Bell, A.D.; Usher, C.L.; McCarroll, S.A. Analyzing Copy Number Variation with Droplet Digital PCR. Recent Results Cancer Res. 2018, 1768, 143–160. Available online: http://www.ncbi.nlm.nih.gov/pubmed/29717442 (accessed on 17 December 2021).
- Thapar, A.; Cooper, M. Copy Number Variation: What Is It and What Has It Told Us About Child Psychiatric Disorders? J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 772–774. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23880486 (accessed on 13 December 2021). [CrossRef] [Green Version]
- Zhang, F.; Gu, W.; Hurles, M.E.; Lupski, J.R. Copy Number Variation in Human Health, Disease, and Evolution. Annu. Rev. Genom. Hum. Genet. 2009, 10, 451–481. Available online: http://www.ncbi.nlm.nih.gov/pubmed/19715442 (accessed on 11 November 2021). [CrossRef] [Green Version]
- Lauer, S.; Gresham, D. An Evolving View of Copy Number Variants. Curr. Genet. 2019, 65, 1287–1295. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31076843 (accessed on 7 December 2021). [CrossRef] [PubMed]
- Piluso, G.; Dionisi, M.; Del Vecchio Blanco, F.; Torella, A.; Aurino, S.; Savarese, M.; Giugliano, T.; Bertini, E.; Terracciano, A.; Vainzof, M.; et al. Motor Chip: A Comparative Genomic Hybridization Microarray for Copy-Number Mutations in 245 Neuromuscular Disorders. Clin. Chem. 2011, 57, 1584–1596. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21896784 (accessed on 17 November 2021). [CrossRef] [Green Version]
- Wojciechowska, M.; Sobczak, K.; Kozlowski, P.; Sedehizadeh, S.; Wojtkowiak-Szlachcic, A.; Czubak, K.; Markus, R.; Lusakowska, A.; Kaminska, A.; Brook, J.D. Quantitative Methods to Monitor RNA Biomarkers in Myotonic Dystrophy. Sci. Rep. 2018, 8, 5885. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.K.; Lee, Y.S.; Sivaprasad, U.; Malhotra, A.; Dutta, A. Muscle-Specific microRNA miR-206 Promotes Muscle Differentiation. J. Cell Biol. 2006, 174, 677–687. Available online: http://www.ncbi.nlm.nih.gov/pubmed/16923828 (accessed on 11 October 2021). [CrossRef]
- Koutsoulidou, A.; Mastroyiannopoulos, N.P.; Furling, D.; Uney, J.B.; Phylactou, L.A. Expression of miR-1, miR-133a, miR-133b and miR-206 Increases during Development of Human Skeletal Muscle. BMC Dev. Biol. 2011, 11, 34. Available online: http://www.ncbi.nlm.nih.gov/pubmed/21645416 (accessed on 17 November 2021). [CrossRef] [Green Version]
- Zaharieva, I.T.; Calissano, M.; Scoto, M.; Preston, M.; Cirak, S.; Feng, L.; Collins, J.; Kole, R.; Guglieri, M.; Straub, V.; et al. Dystromirs as Serum Biomarkers for Monitoring the Disease Severity in Duchenne Muscular Dystrophy. PLoS ONE 2013, 8, e80263. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24282529 (accessed on 11 February 2022). [CrossRef] [Green Version]
- Ma, G.; Wang, Y.; Li, Y.; Cui, L.; Zhao, Y.; Zhao, B.; Li, K. MiR-206, a Key Modulator of Skeletal Muscle Development and Disease. Int. J. Biol. Sci. 2015, 11, 345–352. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25678853 (accessed on 11 February 2022). [CrossRef]
- Vignier, N.; Amor, F.; Fogel, P.; Duvallet, A.; Poupiot, J.; Charrier, S.; Arock, M.; Montus, M.; Nelson, I.; Richard, I.; et al. Distinctive Serum miRNA Profile in Mouse Models of Striated Muscular Pathologies. PLoS ONE 2013, 8, e55281. Available online: http://www.ncbi.nlm.nih.gov/pubmed/23418438 (accessed on 21 January 2022). [CrossRef] [PubMed]
- Horak, M.; Novak, J.; Bienertova-Vasku, J. Muscle-Specific microRNAs in Skeletal Muscle Development. Dev. Biol. 2016, 410, 1–13. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0012160615303638 (accessed on 21 January 2022). [CrossRef]
- Aartsma-Rus, A.; Ferlini, A.; Vroom, E. Biomarkers and Surrogate Endpoints in Duchenne: Meeting Report. Neuromuscul. Disord. 2014, 24, 743–745. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24951452 (accessed on 9 February 2022). [CrossRef] [PubMed]
- Llano-Diez, M.; Ortez, C.I.; Gay, J.A.; Álvarez-Cabado, L.; Jou, C.; Medina, J.; Nascimento, A.; Jimenez-Mallebrera, C. Digital PCR Quantification of miR-30c and miR-181a as Serum Biomarkers for Duchenne Muscular Dystrophy. Neuromuscul. Disord. 2017, 27, 15–23. [Google Scholar] [CrossRef]
- Trifunov, S.; Natera-de Benito, D.; Exposito Escudero, J.M.; Ortez, C.; Medina, J.; Cuadras, D.; Badosa, C.; Carrera, L.; Nascimento, A.; Jimenez-Mallebrera, C. Longitudinal Study of Three microRNAs in Duchenne Muscular Dystrophy and Becker Muscular Dystrophy. Front. Neurol. 2020, 11, 304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marozzo, R.; Pegoraro, V.; Angelini, C. MiRNAs, Myostatin, and Muscle MRI Imaging as Biomarkers of Clinical Features in Becker Muscular Dystrophy. Diagnostics 2020, 10, 713. Available online: https://www.mdpi.com/2075-4418/10/9/713 (accessed on 15 February 2022). [CrossRef]
- Saad, N.Y.; Al-Kharsan, M.; Garwick-Coppens, S.E.; Chermahini, G.A.; Harper, M.A.; Palo, A.; Boudreau, R.L.; Harper, S.Q. Human miRNA miR-675 Inhibits DUX4 Expression and may Be Exploited as a Potential Treatment for Facioscapulohumeral Muscular Dystrophy. Nat. Commun. 2021, 12, 7128. Available online: https://www.nature.com/articles/s41467-021-27430-1 (accessed on 24 February 2022). [CrossRef] [PubMed]
- Haslett, J.N.; Sanoudou, D.; Kho, A.T.; Han, M.; Bennett, R.R.; Kohane, I.S.; Beggs, A.H.; Kunkel, L.M. Gene EXPRESSION profiling of Duchenne Muscular Dystrophy Skeletal Muscle. Neurogenetics 2003, 4, 163–171. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12698323 (accessed on 8 February 2022). [CrossRef]
- Almeida, C.F.; Martins, P.C.; Vainzof, M. Comparative Transcriptome Analysis of Muscular Dystrophy Models Largemyd, Dmdmdx/Largemyd and Dmdmdx: What Makes Them Different? Eur. J. Hum. Genet. 2016, 24, 1301–1309. Available online: http://www.nature.com/articles/ejhg201616 (accessed on 8 February 2022). [CrossRef] [PubMed] [Green Version]
- Camerino, G.M.; Cannone, M.; Giustino, A.; Massari, A.M.; Capogrosso, R.F.; Cozzoli, A.; De Luca, A. Gene Expression in mdx Mouse Muscle in Relation to Age and Exercise: Aberrant Mechanical–Metabolic Coupling and Implications for Pre-Clinical Studies in Duchenne Muscular Dystrophy. Hum. Mol. Genet. 2014, 23, 5720–5732. Available online: http://www.ncbi.nlm.nih.gov/pubmed/24916377 (accessed on 18 January 2022). [CrossRef] [PubMed] [Green Version]
- Zhang, R.; Lv, L.; Ban, W.; Dang, X.; Zhang, C. Identification of Hub Genes in Duchenne Muscular Dystrophy: Evidence from Bioinformatic Analysis. J. Comput. Biol. 2020, 27, 1–8. Available online: http://www.ncbi.nlm.nih.gov/pubmed/31390219 (accessed on 18 January 2022). [CrossRef] [PubMed]
- Xu, X.; Hao, Y.; Wu, J.; Zhao, J.; Xiong, S. Assessment of Weighted Gene Co-Expression Network Analysis to Explore Key Pathways and Novel Biomarkers in Muscular Dystrophy. Pharm. Pers. Med. 2021, 14, 431–444. Available online: http://www.ncbi.nlm.nih.gov/pubmed/33883925 (accessed on 17 February 2022). [CrossRef]
- Wang, J.; Fan, Q.; Yu, T.; Zhang, Y. Identifying the Hub Genes for Duchenne Muscular Dystrophy and Becker Muscular Dystrophy by Weighted Correlation Network Analysis. BMC Genom. Data 2021, 22, 57. Available online: http://www.ncbi.nlm.nih.gov/pubmed/34922439 (accessed on 17 February 2022). [CrossRef]
- Howard, Z.M.; Lowe, J.; Blatnik, A.J.; Roberts, D.; Burghes, A.H.M.; Bansal, S.S.; Rafael-Fortney, J.A. Early Inflammation in Muscular Dystrophy Differs between Limb and Respiratory Muscles and Increases with Dystrophic Severity. Am. J. Pathol. 2021, 191, 730–747. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0002944021000389 (accessed on 19 February 2022). [CrossRef]
- D’Aversa, E.; Breveglieri, G.; Pellegatti, P.; Guerra, G.; Gambari, R.; Borgatti, M. Non-Invasive Fetal Sex Diagnosis in Plasma of Early Weeks Pregnants Using Droplet Digital PCR. Mol. Med. 2018, 24, 14. Available online: https://molmed.biomedcentral.com/articles/10.1186/s10020-018-0016-7 (accessed on 17 November 2021). [CrossRef] [Green Version]
- Zhou, Y.; Zhu, Z.; Gao, Y.; Yuan, Y.; Guo, Y.; Zhou, L.; Liao, K.; Wang, J.; Du, B.; Hou, Y.; et al. Effects of Maternal and Fetal Characteristics on Cell-Free Fetal DNA Fraction in Maternal Plasma. Reprod. Sci. 2015, 22, 1429–1435. Available online: http://www.ncbi.nlm.nih.gov/pubmed/25963912 (accessed on 16 November 2021). [CrossRef]
- Kubota, A.; Ishiura, H.; Porto, K.J.L.; Tanaka, M.; Mitsui, J.; Unuma, A.; Maki, H.; Komuro, I.; Tsuji, S.; Shimizu, J.; et al. DMD Exon 2 Duplication due to a Complex Genomic Rearrangement Is Associated with a Somatic Mosaicism. Neuromuscul. Disord. 2021, 32, 263–269. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0960896621007379 (accessed on 17 February 2022). [CrossRef]
- Jin, P.; Gao, X.; Wang, M.; Qian, Y.; Yang, J.; Yang, Y.; Xu, Y.; Xu, Y.; Dong, M. Case Report: Identification of Maternal Low-Level Mosaicism in the Dystrophin Gene by Droplet Digital Polymerase Chain Reaction. Front. Genet. 2021, 12, 824. Available online: https://www.frontiersin.org/articles/10.3389/fgene.2021.686993/full (accessed on 19 January 2022). [CrossRef]
| Strengths | Similarities | Differences | ||
|---|---|---|---|---|
| qPCR | ddPCR | ddPCR/qPCR | qPCR | ddPCR |
| Gold standard technique for target DNA quantitation and gene expression analysis | High precision quantification at low input copy number sequence in a complex background | Both methods have multiplex capability | Relative measurement | Absolute measurement |
| Economic costs | High sensitivity | Both methods are easy to use. | Standard curves needed | No need for calibration or standard curves |
| Rapid test results | Independent analysis and data processing of samples | Quantification of the amount of target in a certain sample | No sample partitioning | The sample is partitioned into a large number of individual reactions |
| High tolerance to PCR inhibitor | The same components used in the reaction (PCR Master Mix, primers, fluorescent probes (Taqman probs FAM and HEX/VIC) | Real time PCR data acquisition | End point data collection | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambrescu, I.; Popa, A.; Manole, E.; Ceafalan, L.C.; Gaina, G. Application of Droplet Digital PCR Technology in Muscular Dystrophies Research. Int. J. Mol. Sci. 2022, 23, 4802. https://doi.org/10.3390/ijms23094802
Lambrescu I, Popa A, Manole E, Ceafalan LC, Gaina G. Application of Droplet Digital PCR Technology in Muscular Dystrophies Research. International Journal of Molecular Sciences. 2022; 23(9):4802. https://doi.org/10.3390/ijms23094802
Chicago/Turabian StyleLambrescu, Ioana, Alexandra Popa, Emilia Manole, Laura Cristina Ceafalan, and Gisela Gaina. 2022. "Application of Droplet Digital PCR Technology in Muscular Dystrophies Research" International Journal of Molecular Sciences 23, no. 9: 4802. https://doi.org/10.3390/ijms23094802
APA StyleLambrescu, I., Popa, A., Manole, E., Ceafalan, L. C., & Gaina, G. (2022). Application of Droplet Digital PCR Technology in Muscular Dystrophies Research. International Journal of Molecular Sciences, 23(9), 4802. https://doi.org/10.3390/ijms23094802






