How to Heal the Gut’s Brain: Regeneration of the Enteric Nervous System
Abstract
1. Introduction
2. Therapeutic Approaches to Treat ENS Disorders
3. What Constitutes Nervous System Regeneration?
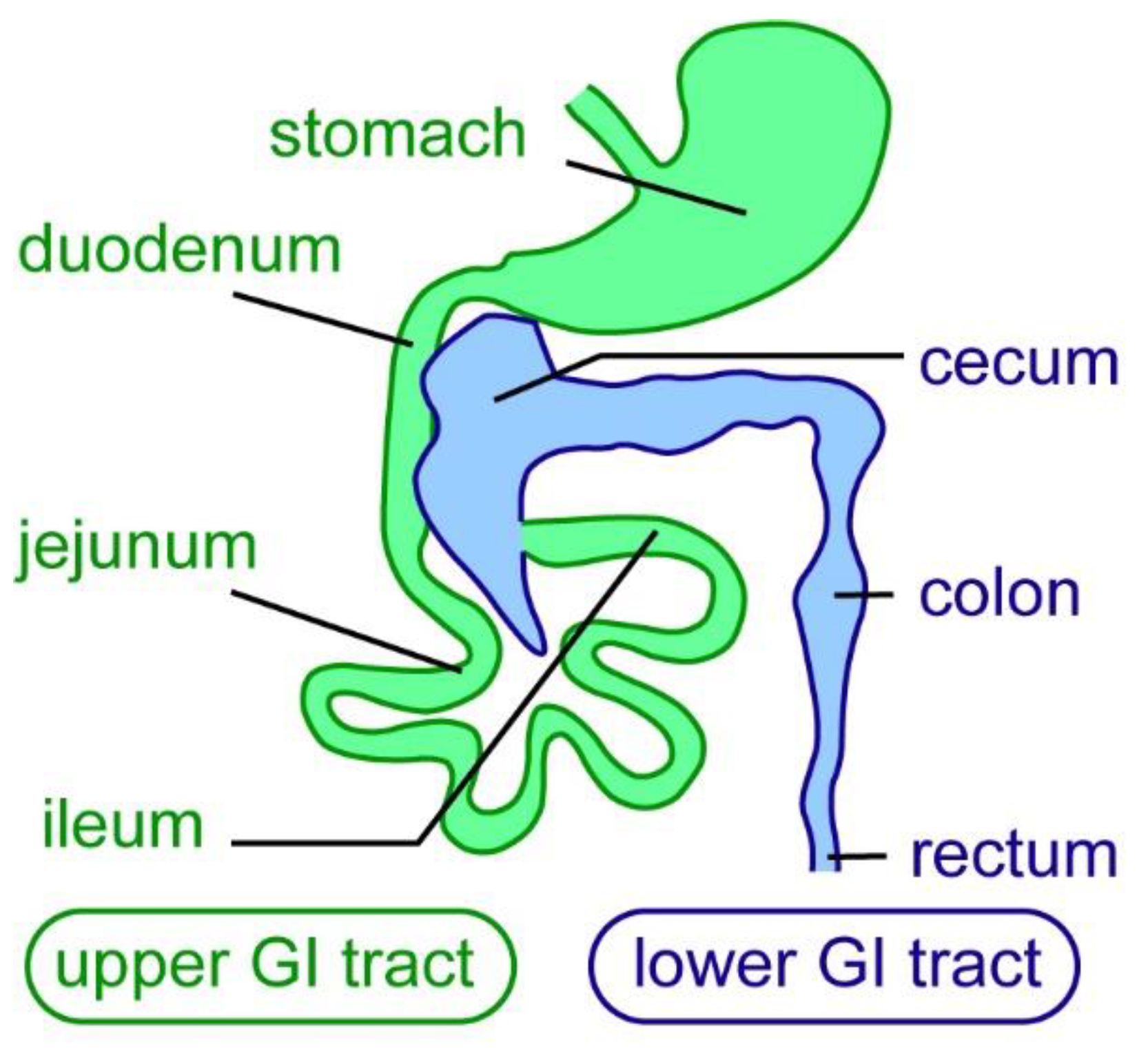
4. The Mammalian ENS Has Limited Regenerative Ability
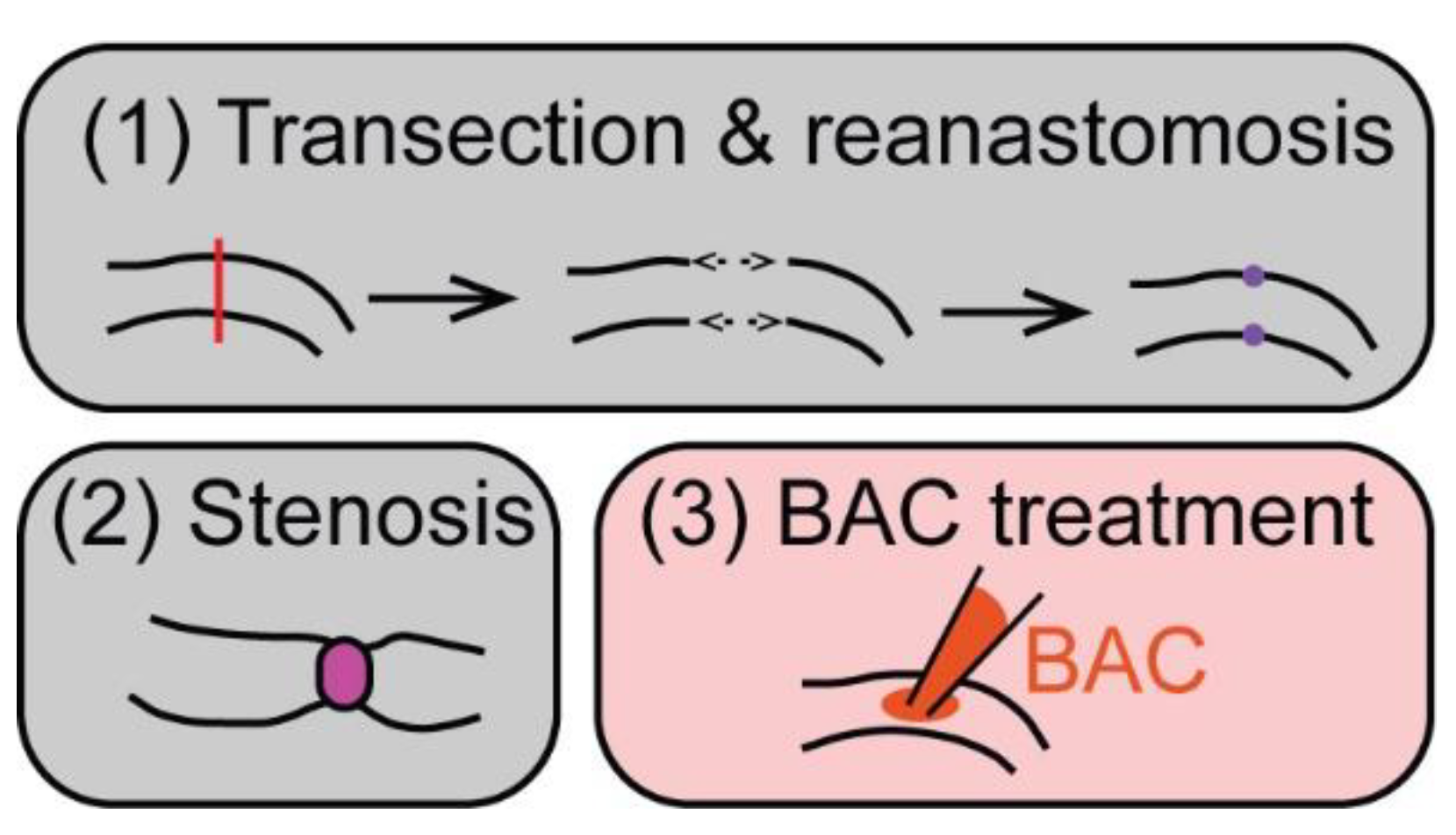
5. ENS Regeneration after Surgical/Mechanical Injury in Mammals
6. ENS Regeneration after Chemically Induced Injury in Mammals
7. Regeneration in Animal Genetic Models of Hirschsprung Disease
8. What Aspects Might Impact the Regenerative Process in Mammals?
8.1. Time Required for Full Regeneration
8.2. Impact of Inflammation
8.3. Ability of Mammalian Adult Neurogenesis in the ENS
9. The Zebrafish ENS Regenerates Neurons after Focal Ablation
10. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Ganz, J. Gut feelings: Studying enteric nervous system development, function, and disease in the zebrafish model system. Dev. Dyn. 2018, 247, 268–278. [Google Scholar] [CrossRef]
- Heanue, T.A.; Shepherd, I.T.; Burns, A.J. Enteric nervous system development in avian and zebrafish models. Dev. Biol. 2016, 417, 129–138. [Google Scholar] [CrossRef]
- Rao, M.; Gershon, M.D. Enteric nervous system development: What could possibly go wrong? Nat. Rev. Neurosci. 2018, 19, 552–565. [Google Scholar] [CrossRef] [PubMed]
- Belkind-Gerson, J.; Graeme-Cook, F.; Winter, H. Enteric nervous system disease and recovery, plasticity, and regeneration. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. The Enteric Nervous System; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Rolig, A.S.; Mittge, E.K.; Ganz, J.; Troll, J.V.; Melancon, E.; Wiles, T.J.; Alligood, K.; Stephens, W.Z.; Eisen, J.S.; Guillemin, K. The enteric nervous system promotes intestinal health by constraining microbiota composition. PLoS Biol. 2017, 15, e2000689. [Google Scholar] [CrossRef]
- Wiles, T.J.; Jemielita, M.; Baker, R.P.; Schlomann, B.H.; Logan, S.L.; Ganz, J.; Melancon, E.; Eisen, J.S.; Guillemin, K.; Parthasarathy, R. Host Gut Motility Promotes Competitive Exclusion within a Model Intestinal Microbiota. PLoS Biol. 2016, 14, e1002517. [Google Scholar] [CrossRef]
- Neunlist, M.; Van Landeghem, L.; Mahe, M.M.; Derkinderen, P.; des Varannes, S.B.; Rolli-Derkinderen, M. The digestive neuronal-glial-epithelial unit: A new actor in gut health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 90–100. [Google Scholar] [CrossRef]
- Nishiyama, K.; Sugiyama, M.; Mukai, T. Adhesion Properties of Lactic Acid Bacteria on Intestinal Mucin. Microorganisms 2016, 4, 34. [Google Scholar] [CrossRef]
- Gabella, G. On the plasticity of form and structure of enteric ganglia. J. Auton. Nerv. Syst. 1990, 30, S59–S66. [Google Scholar] [CrossRef]
- Saffrey, M.J. Cellular changes in the enteric nervous system during ageing. Dev. Biol. 2013, 382, 344–355. [Google Scholar] [CrossRef] [PubMed]
- Saffrey, M.J. Aging of the mammalian gastrointestinal tract: A complex organ system. Age 2014, 36, 9603. [Google Scholar] [CrossRef] [PubMed]
- Brosens, E.; Burns, A.J.; Brooks, A.S.; Matera, I.; Borrego, S.; Ceccherini, I.; Tam, P.K.; Garcia-Barcelo, M.M.; Thapar, N.; Benninga, M.A.; et al. Genetics of enteric neuropathies. Dev. Biol. 2016, 417, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Heuckeroth, R.O. Hirschsprung disease-integrating basic science and clinical medicine to improve outcomes. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 152–167. [Google Scholar] [CrossRef]
- Chalazonitis, A.; Rao, M. Enteric nervous system manifestations of neurodegenerative disease. Brain Res. 2018, 1693, 207–213. [Google Scholar] [CrossRef]
- Rao, M.; Gershon, M.D. The bowel and beyond: The enteric nervous system in neurological disorders. Nat. Rev. Gastroenterol. Hepatol. 2016, 13 Pt B, 517–528. [Google Scholar] [CrossRef]
- De Giorgio, R.; Bianco, F.; Latorre, R.; Caio, G.; Clavenzani, P.; Bonora, E. Enteric neuropathies: Yesterday, Today and Tomorrow. Adv. Exp. Med. Biol. 2016, 891, 123–133. [Google Scholar]
- De Giorgio, R.; Guerrini, S.; Barbara, G.; Stanghellini, V.; De Ponti, F.; Corinaldesi, R.; Moses, P.L.; Sharkey, K.A.; Mawe, G.M. Inflammatory neuropathies of the enteric nervous system. Gastroenterology 2004, 126, 1872–1883. [Google Scholar] [CrossRef]
- Burns, A.J.; Goldstein, A.M.; Newgreen, D.F.; Stamp, L.; Schafer, K.H.; Metzger, M.; Hotta, R.; Young, H.M.; Andrews, P.W.; Thapar, N.; et al. White paper on guidelines concerning enteric nervous system stem cell therapy for enteric neuropathies. Dev. Biol. 2016, 417, 229–251. [Google Scholar] [CrossRef]
- Goldstein, A.M.; Thapar, N.; Karunaratne, T.B.; De Giorgio, R. Clinical aspects of neurointestinal disease: Pathophysiology, diagnosis, and treatment. Dev. Biol. 2016, 417, 217–228. [Google Scholar] [CrossRef]
- Burns, A.J.; Thapar, N. Neural stem cell therapies for enteric nervous system disorders. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Bondurand, N.; Natarajan, D.; Thapar, N.; Atkins, C.; Pachnis, V. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development 2003, 130, 6387–6400. [Google Scholar] [CrossRef] [PubMed]
- McCann, C.J.; Thapar, N. Enteric neural stem cell therapies for enteric neuropathies. Neurogastroenterol. Motil. 2018, 30, e13369. [Google Scholar] [CrossRef] [PubMed]
- Fattahi, F.; Steinbeck, J.A.; Kriks, S.; Tchieu, J.; Zimmer, B.; Kishinevsky, S.; Zeltner, N.; Mica, Y.; El-Nachef, W.; Zhao, H.; et al. Deriving human ENS lineages for cell therapy and drug discovery in Hirschsprung disease. Nature 2016, 531, 105–109. [Google Scholar] [CrossRef]
- Barber, K.; Studer, L.; Fattahi, F. Derivation of enteric neuron lineages from human pluripotent stem cells. Nat. Protoc. 2019, 14, 1261–1279. [Google Scholar] [CrossRef]
- Alhawaj, A.F. Stem cell-based therapy for hirschsprung disease, do we have the guts to treat? Gene Ther. 2021. [Google Scholar] [CrossRef]
- Pan, W.; Goldstein, A.M.; Hotta, R. Opportunities for novel diagnostic and cell-based therapies for Hirschsprung disease. J. Pediatr. Surg. 2021. [Google Scholar] [CrossRef]
- McCann, C.J.; Borrelli, O.; Thapar, N. Stem cell therapy in severe pediatric motility disorders. Curr. Opin. Pharmacol. 2018, 43, 145–149. [Google Scholar] [CrossRef]
- Cigliola, V.; Becker, C.J.; Poss, K.D. Building bridges, not walls: Spinal cord regeneration in zebrafish. Dis. Model. Mech. 2020, 13, dmm044131. [Google Scholar] [CrossRef]
- Goldman, D. Muller glial cell reprogramming and retina regeneration. Nat. Rev. Neurosci. 2014, 15, 431–442. [Google Scholar] [CrossRef]
- Lahne, M.; Nagashima, M.; Hyde, D.R.; Hitchcock, P.F. Reprogramming Muller Glia to Regenerate Retinal Neurons. Annu. Rev. Vis. Sci. 2020, 6, 171–193. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, G.G.; Ransom, R.C.; Zielins, E.R.; Leavitt, T.; Flacco, J.S.; Hu, M.S.; Lee, A.S.; Longaker, M.T.; Wan, D.C. Stem Cells in Bone Regeneration. Stem Cell Rev. Rep. 2016, 12, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Steward, M.M.; Sridhar, A.; Meyer, J.S. Neural regeneration. Curr. Top. Microbiol. Immunol. 2013, 367, 163–191. [Google Scholar]
- Matsumoto, T.; Sarna, S.K.; Condon, R.E.; Cowles, V.E.; Frantzides, C. Differential sensitivities of morphine and motilin to initiate migrating motor complex in isolated intestinal segments. Regeneration of intrinsic nerves. Gastroenterology 1986, 90, 61–67. [Google Scholar] [CrossRef]
- Sarna, S.; Condon, R.E.; Cowles, V. Enteric mechanisms of initiation of migrating myoelectric complexes in dogs. Gastroenterology 1983, 84, 814–822. [Google Scholar] [CrossRef]
- Deloose, E.; Janssen, P.; Depoortere, I.; Tack, J. The migrating motor complex: Control mechanisms and its role in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 271–285. [Google Scholar] [CrossRef]
- Tokui, K.; Sakanaka, M.; Kimura, S. Progressive reorganization of the myenteric plexus during one year following reanastomosis of the ileum of the guinea pig. Cell Tissue Res. 1994, 277, 259–272. [Google Scholar] [CrossRef]
- Karaosmanoglu, T.; Muftuoglu, S.; Dagdeviren, A.; Durgun, B.; Aygun, B.; Ors, U. Morphological changes in the myenteric plexus of rat ileum after transection and end-to-end anastomosis. J. Anat. 1996, 188 Pt 2, 323–331. [Google Scholar]
- Galligan, J.J.; Furness, J.B.; Costa, M. Migration of the myoelectric complex after interruption of the myenteric plexus: Intestinal transection and regeneration of enteric nerves in the guinea pig. Gastroenterology 1989, 97, 1135–1146. [Google Scholar] [CrossRef]
- Liu, M.T.; Kuan, Y.H.; Wang, J.; Hen, R.; Gershon, M.D. 5-HT4 receptor-mediated neuroprotection and neurogenesis in the enteric nervous system of adult mice. J. Neurosci. 2009, 29, 9683–9699. [Google Scholar] [CrossRef]
- Brookes, S.J.; Lam, T.C.; Lubowski, D.Z.; Costa, M.; King, D.W. Regeneration of nerve fibres across a colonic anastomosis in the guinea-pig. J. Gastroenterol. Hepatol. 1996, 11, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Matsuyoshi, H.; Kuniyasu, H.; Okumura, M.; Misawa, H.; Katsui, R.; Zhang, G.X.; Obata, K.; Takaki, M. A 5-HT(4)-receptor activation-induced neural plasticity enhances in vivo reconstructs of enteric nerve circuit insult. Neurogastroenterol. Motil. 2010, 22, 806–813 e226. [Google Scholar] [CrossRef] [PubMed]
- Katsui, R.; Kojima, Y.; Kuniyasu, H.; Shimizu, J.; Koyama, F.; Fujii, H.; Nakajima, Y.; Takaki, M. A new possibility for repairing the anal dysfunction by promoting regeneration of the reflex pathways in the enteric nervous system. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1084–G1093. [Google Scholar] [CrossRef] [PubMed]
- Benninghoff, V.A. Vermehrung und Vergrößerung von Nervenzellen bei Hypertrophie des Innervationsgebietes*. Z. Naturforschg. 1951, 6b, 38–41. [Google Scholar] [CrossRef][Green Version]
- Jew, J.Y.; Williams, T.H.; Gabella, G.; Zhang, M.Q. The intestine as a model for neuronal plasticity. Arch. Histol. Cytol. 1989, 52, 167–180. [Google Scholar] [CrossRef]
- Joseph, N.M.; He, S.; Quintana, E.; Kim, Y.G.; Nunez, G.; Morrison, S.J. Enteric glia are multipotent in culture but primarily form glia in the adult rodent gut. J. Clin. Investig. 2011, 121, 3398–3411. [Google Scholar] [CrossRef]
- Gabella, G. Size of neurons and glial cells in the intramural ganglia of the hypertrophic intestine of the guinea-pig. J. Neurocytol. 1984, 13, 73–84. [Google Scholar] [CrossRef]
- Corvetti, G.; Fornaro, M.; Geuna, S.; Poncino, A.; Giacobini-Robecchi, M.G. Unscheduled DNA synthesis in rat adult myenteric neurons: An immunohistochemical study. Neuroreport 2001, 12, 2165–2168. [Google Scholar] [CrossRef]
- Laranjeira, C.; Sandgren, K.; Kessaris, N.; Richardson, W.; Potocnik, A.; Vanden Berghe, P.; Pachnis, V. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J. Clin. Investig. 2011, 121, 3412–3424. [Google Scholar] [CrossRef]
- Hanani, M.; Ledder, O.; Yutkin, V.; Abu-Dalu, R.; Huang, T.Y.; Hartig, W.; Vannucchi, M.G.; Faussone-Pellegrini, M.S. Regeneration of myenteric plexus in the mouse colon after experimental denervation with benzalkonium chloride. J. Comp. Neurol. 2003, 462, 315–327. [Google Scholar] [CrossRef]
- Ramalho, F.S.; Santos, G.C.; Ramalho, L.N.; Kajiwara, J.K.; Zucoloto, S. Myenteric neuron number after acute and chronic denervation of the proximal jejunum induced by benzalkonium chloride. Neurosci. Lett. 1993, 163, 74–76. [Google Scholar] [CrossRef]
- Luck, M.S.; Dahl, J.L.; Boyeson, M.G.; Bass, P. Neuroplasticity in the smooth muscle of the myenterically and extrinsically denervated rat jejunum. Cell Tissue Res. 1993, 271, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Belkind-Gerson, J.; Hotta, R.; Nagy, N.; Thomas, A.R.; Graham, H.; Cheng, L.; Solorzano, J.; Nguyen, D.; Kamionek, M.; Dietrich, J.; et al. Colitis induces enteric neurogenesis through a 5-HT4-dependent mechanism. Inflamm. Bowel Dis. 2015, 21, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.S., Jr.; Parr, E.J.; Sharkey, K.A. Effects of inflammation on cell proliferation in the myenteric plexus of the guinea-pig ileum. Cell Tissue Res. 1997, 289, 455–461. [Google Scholar] [CrossRef]
- Belkind-Gerson, J.; Graham, H.K.; Reynolds, J.; Hotta, R.; Nagy, N.; Cheng, L.; Kamionek, M.; Shi, H.N.; Aherne, C.M.; Goldstein, A.M. Colitis promotes neuronal differentiation of Sox2+ and PLP1+ enteric cells. Sci. Rep. 2017, 7, 2525. [Google Scholar] [CrossRef]
- Stead, R.H.; Kosecka-Janiszewska, U.; Oestreicher, A.B.; Dixon, M.F.; Bienenstock, J. Remodeling of B-50 (GAP-43)- and NSE-immunoreactive mucosal nerves in the intestines of rats infected with Nippostrongylus brasiliensis. J. Neurosci. 1991, 11, 3809–3821. [Google Scholar] [CrossRef]
- Ohno, M.; Nikaido, M.; Horiuchi, N.; Kawakami, K.; Hatta, K. The enteric nervous system in zebrafish larvae can regenerate via migration into the ablated area and proliferation of neural crest-derived cells. Development 2021, 148, dev195339. [Google Scholar]
- El-Nachef, W.N.; Bronner, M.E. De novo enteric neurogenesis in post-embryonic zebrafish from Schwann cell precursors rather than resident cell types. Development 2020, 147, dev186619. [Google Scholar] [CrossRef]
- Sato, A.; Yamamoto, M.; Imamura, K.; Kashiki, Y.; Kunieda, T.; Sakata, K. Pathophysiology of aganglionic colon and anorectum: An experimental study on aganglionosis produced by a new method in the rat. J. Pediatr. Surg. 1978, 13, 399–435. [Google Scholar] [CrossRef]
- Fox, D.A.; Epstein, M.L.; Bass, P. Surfactants selectively ablate enteric neurons of the rat jejunum. J. Pharmacol. Exp. Ther. 1983, 227, 538–544. [Google Scholar]
- Soret, R.; Schneider, S.; Bernas, G.; Christophers, B.; Souchkova, O.; Charrier, B.; Righini-Grunder, F.; Aspirot, A.; Landry, M.; Kembel, S.W.; et al. Glial Cell-Derived Neurotrophic Factor Induces Enteric Neurogenesis and Improves Colon Structure and Function in Mouse Models of Hirschsprung Disease. Gastroenterology 2020, 159, 1824–1838 e17. [Google Scholar] [CrossRef] [PubMed]
- Kyritsis, N.; Kizil, C.; Brand, M. Neuroinflammation and central nervous system regeneration in vertebrates. Trends Cell Biol. 2014, 24, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Bosak, V.; Murata, K.; Bludau, O.; Brand, M. Role of the immune response in initiating central nervous system regeneration in vertebrates: Learning from the fish. Int. J. Dev. Biol. 2018, 62, 403–417. [Google Scholar] [CrossRef]
- Nagashima, M.; Hitchcock, P.F. Inflammation Regulates the Multi-Step Process of Retinal Regeneration in Zebrafish. Cells 2021, 10, 783. [Google Scholar] [CrossRef]
- Tsarouchas, T.M.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.; et al. Dynamic control of proinflammatory cytokines Il-1beta and Tnf-alpha by macrophages in zebrafish spinal cord regeneration. Nat. Commun. 2018, 9, 4670. [Google Scholar] [CrossRef] [PubMed]
- Linden, D.R.; Couvrette, J.M.; Ciolino, A.; McQuoid, C.; Blaszyk, H.; Sharkey, K.A.; Mawe, G.M. Indiscriminate loss of myenteric neurones in the TNBS-inflamed guinea-pig distal colon. Neurogastroenterol. Motil. 2005, 17, 751–760. [Google Scholar] [CrossRef] [PubMed]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; De Ponti, F.; De Giorgio, R. Enteric neuroplasticity evoked by inflammation. Auton. Neurosci. 2006, 126–127, 264–272. [Google Scholar] [CrossRef]
- Kaslin, J.; Ganz, J.; Brand, M. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 101–122. [Google Scholar] [CrossRef]
- Grandel, H.; Brand, M. Comparative aspects of adult neural stem cell activity in vertebrates. Dev. Genes Evol. 2013, 223, 131–147. [Google Scholar] [CrossRef]
- Pham, T.D.; Gershon, M.D.; Rothman, T.P. Time of origin of neurons in the murine enteric nervous system: Sequence in relation to phenotype. J. Comp. Neurol. 1991, 314, 789–798. [Google Scholar] [CrossRef]
- Virtanen, H.; Garton, D.; Andressoo, J.O. Myenteric neurons do not replicate in small intestine under normal, physiological conditions in adult mouse. Cell. Mol. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.; Micci, M.A.; Leser, J.; Shin, C.; Tang, S.C.; Fu, Y.Y.; Liu, L.; Li, Q.; Saha, M.; Li, C.; et al. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc. Natl. Acad. Sci. USA 2017, 114, E3709–E3718. [Google Scholar] [CrossRef] [PubMed]
- McCallum, S.; Obata, Y.; Fourli, E.; Boeing, S.; Peddie, C.J.; Xu, Q.; Horswell, S.; Kelsh, R.; Collinson, L.; Wilkinson, D.; et al. Enteric glia as a source of neural progenitors in adult zebrafish. Elife 2020, 9, e56086. [Google Scholar] [CrossRef] [PubMed]
- Ganz, J.; Baker, R.P.; Hamilton, M.K.; Melancon, E.; Diba, P.; Eisen, J.S.; Parthasarathy, R. Image velocimetry and spectral analysis enable quantitative characterization of larval zebrafish gut motility. Neurogastroenterol. Motil. 2018, 30, e13351. [Google Scholar] [CrossRef]
- Field, H.A.; Kelley, K.A.; Martell, L.; Goldstein, A.M.; Serluca, F.C. Analysis of gastrointestinal physiology using a novel intestinal transit assay in zebrafish. Neurogastroenterol. Motil. 2009, 21, 304–312. [Google Scholar] [CrossRef]
- Boesmans, W.; Lasrado, R.; Vanden Berghe, P.; Pachnis, V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 2015, 63, 229–241. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rueckert, H.; Ganz, J. How to Heal the Gut’s Brain: Regeneration of the Enteric Nervous System. Int. J. Mol. Sci. 2022, 23, 4799. https://doi.org/10.3390/ijms23094799
Rueckert H, Ganz J. How to Heal the Gut’s Brain: Regeneration of the Enteric Nervous System. International Journal of Molecular Sciences. 2022; 23(9):4799. https://doi.org/10.3390/ijms23094799
Chicago/Turabian StyleRueckert, Helen, and Julia Ganz. 2022. "How to Heal the Gut’s Brain: Regeneration of the Enteric Nervous System" International Journal of Molecular Sciences 23, no. 9: 4799. https://doi.org/10.3390/ijms23094799
APA StyleRueckert, H., & Ganz, J. (2022). How to Heal the Gut’s Brain: Regeneration of the Enteric Nervous System. International Journal of Molecular Sciences, 23(9), 4799. https://doi.org/10.3390/ijms23094799





