The Final Frontier of Sustainable Materials: Current Developments in Self-Healing Elastomers
Abstract
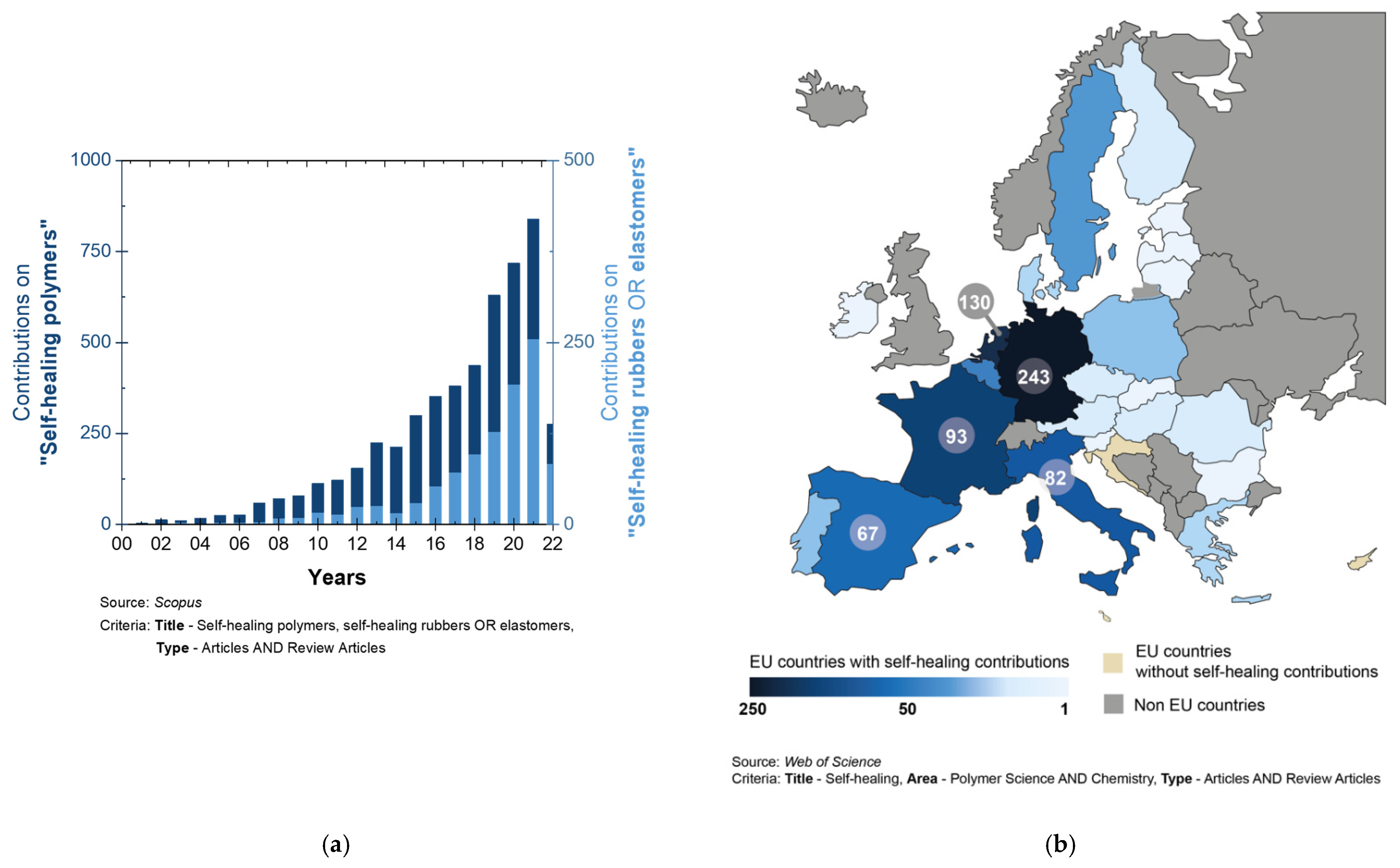
:1. Introduction
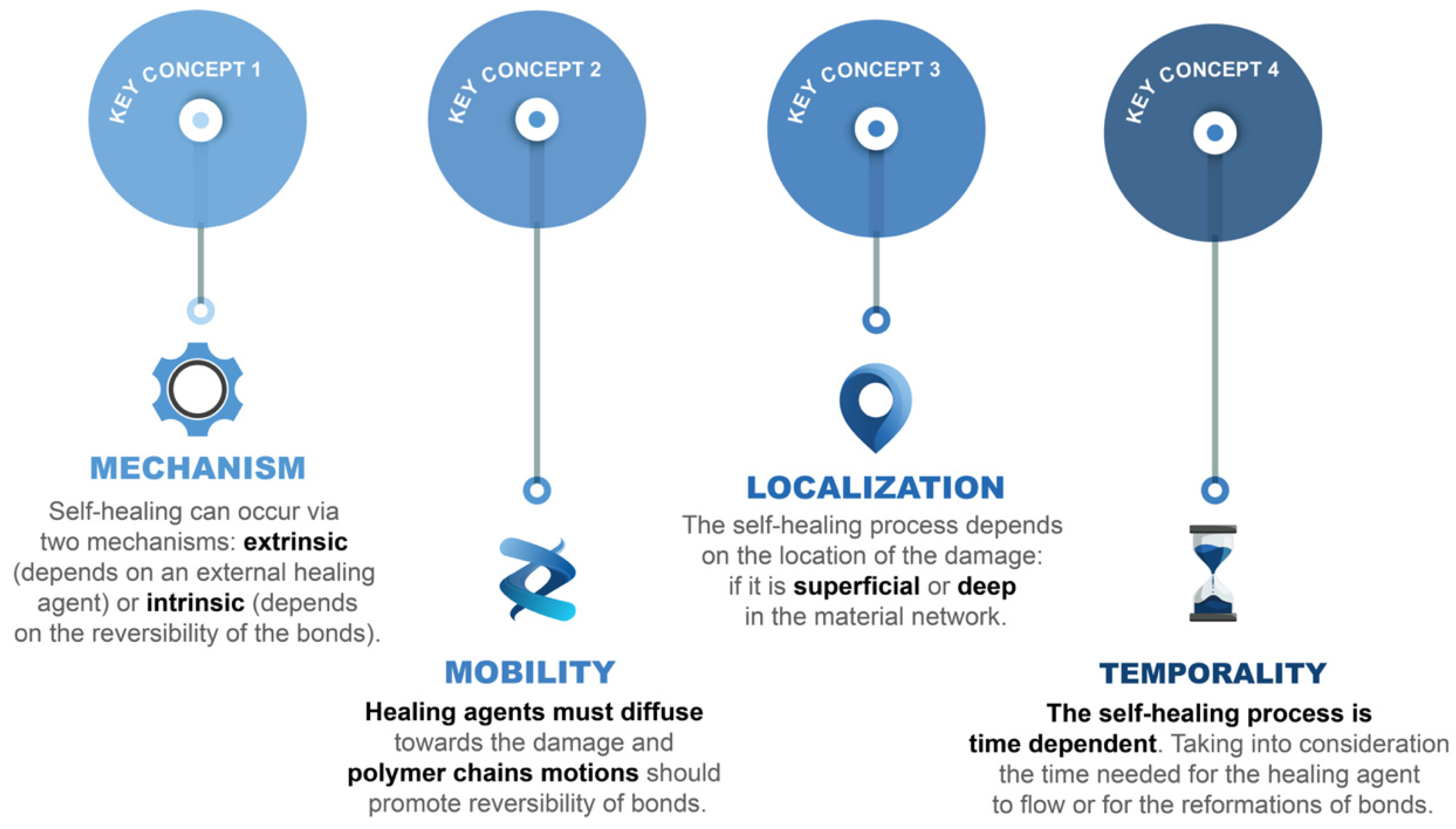
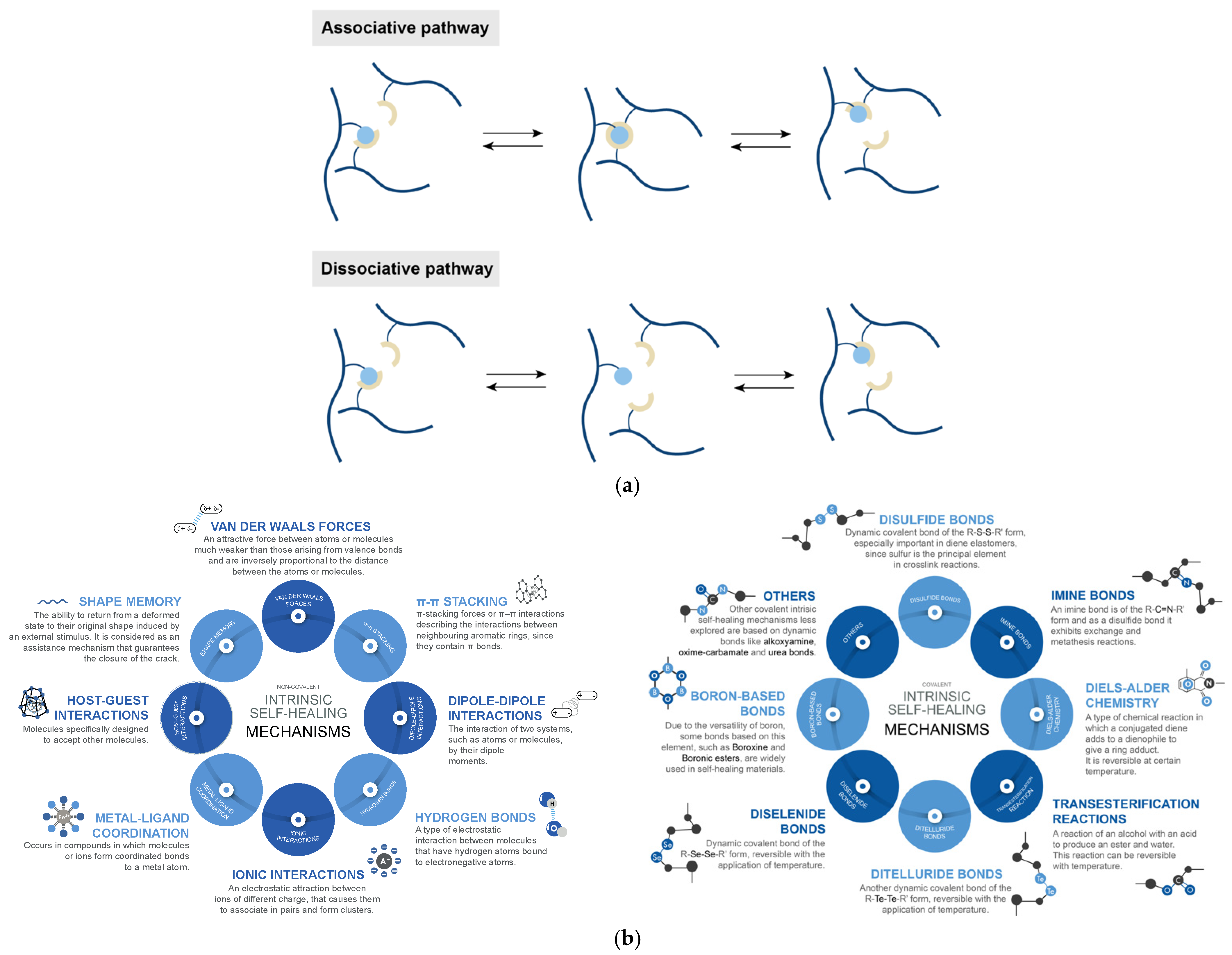
2. To Boldly Go Where No Material Has Gone before: Self-Healing Concepts
- Non-covalent intrinsic mechanisms, such as hydrogen bonds, ionic interactions, metal–ligand coordination, among others; and,
- Covalent intrinsic mechanisms, such as disulfide bond exchange (associative), Diels–Alder chemistry (dissociative), transesterification reactions (associative), bonds based on boron and imines chemistry (dissociative), among others.
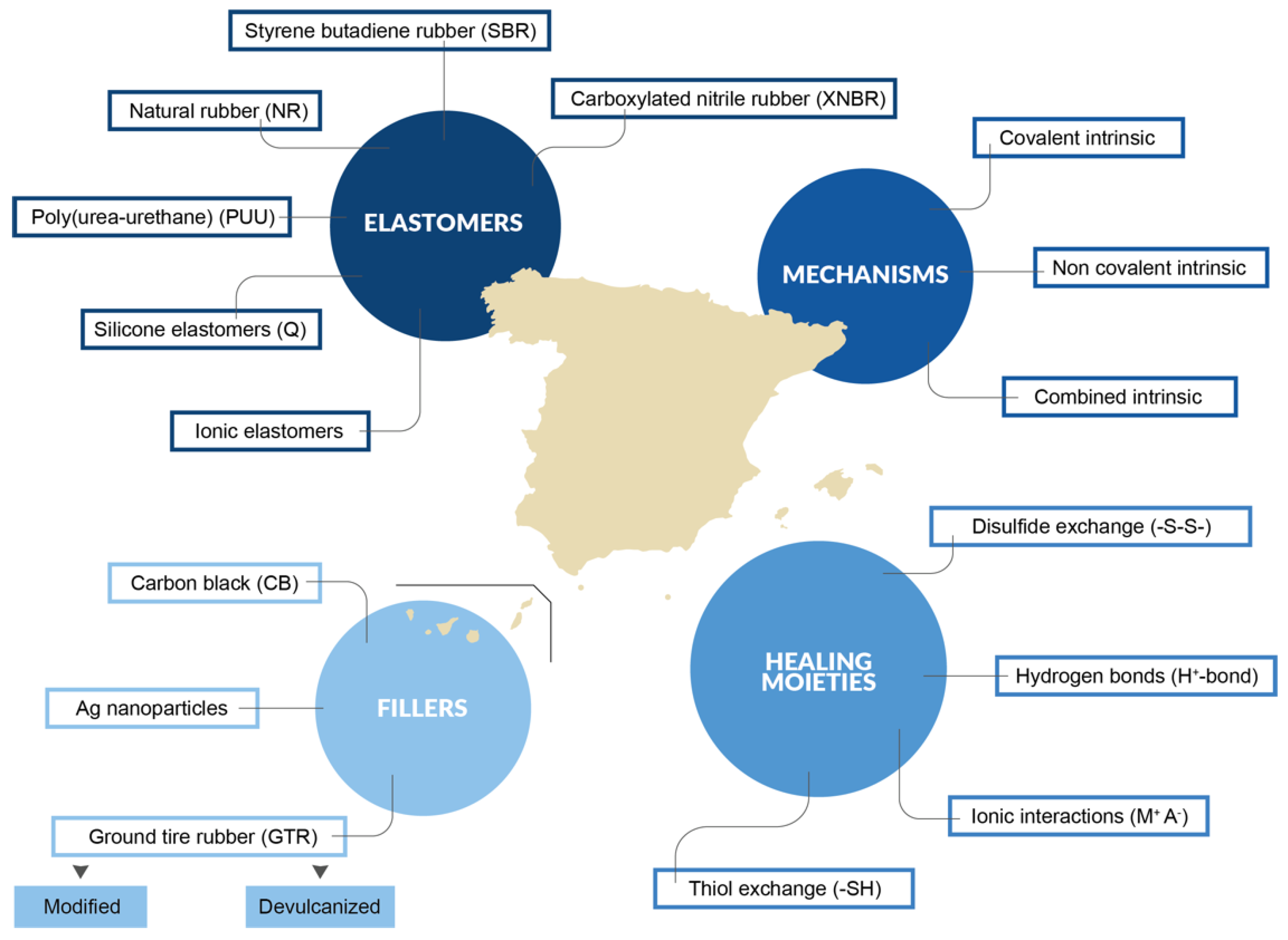
3. Current Developments in Self-Healing Elastomers
3.1. Self-Healing Natural Rubber
3.2. Self-Healing Synthetic Elastomers
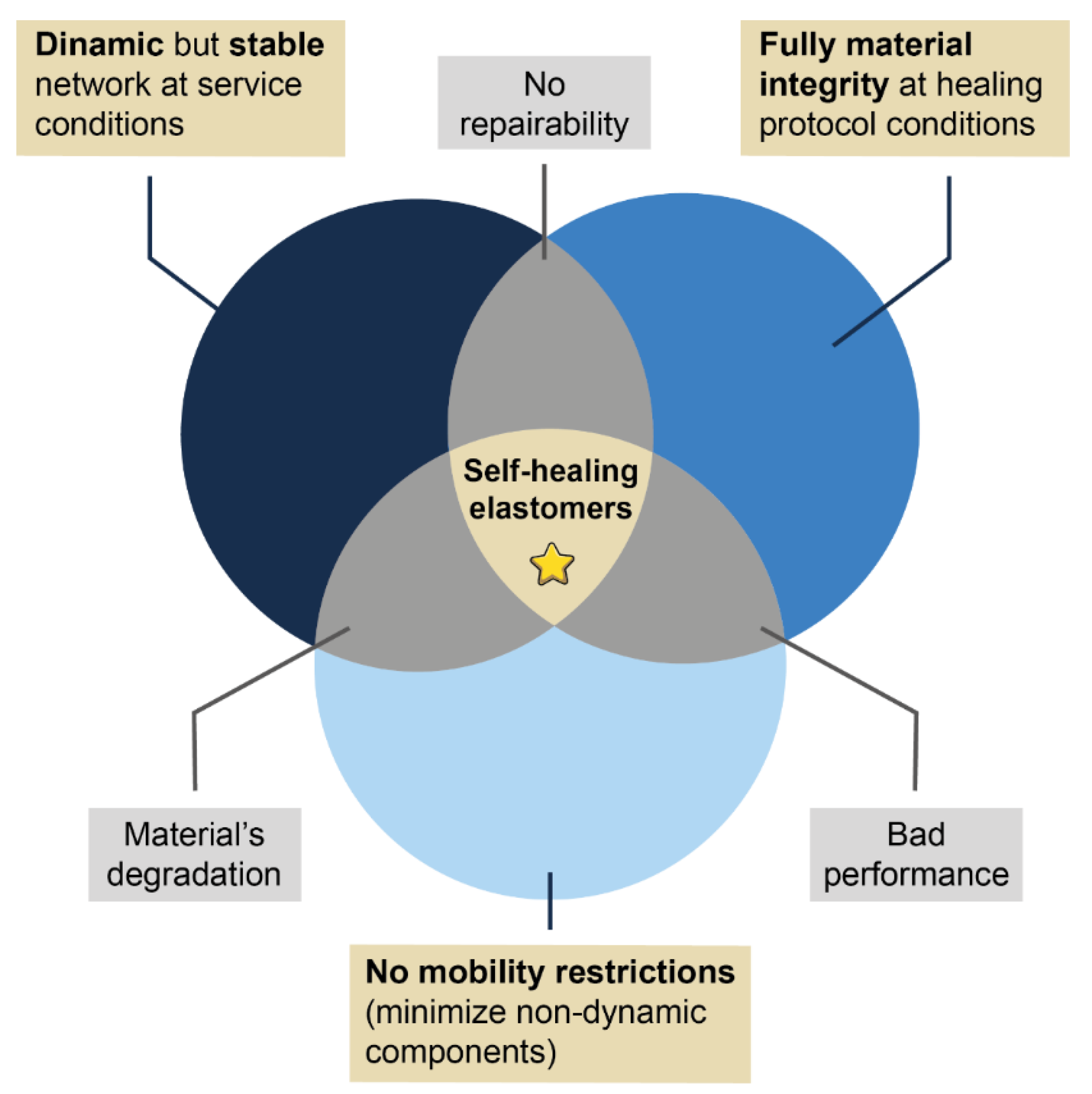
4. Challenges, Perspectives and Outlook
- The construction of a dynamic but stable and robust network at service temperatures to guarantee excellent mechanical performance;
- The minimization of components that can hinder the mobility necessary to achieve healing (e.g., secondary irreversible networks); and,
- The optimization of the appropriate conditions (temporality and external stimulus) for each repair mechanism. In turn, these conditions must be compatible with the material stability, to avoid its deterioration during the healing protocols.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wemyss, A.M.; Bowen, C.; Plesse, C.; Vancaeyzeele, C.; Nguyen, G.T.M.; Vidal, F.; Wan, C. Dynamic crosslinked rubbers for a green future: A material perspective. Mater. Sci. Eng. 2020, 141, 100561. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Z.; Wemyss, A.M.; Wan, C.; Liu, Y.; Song, P.; Wang, S. Effective thermal-oxidative reclamation of waste tire rubbers for producing high-performance rubber composites. ACS Sustain. Chem. Eng. 2020, 8, 9079–9087. [Google Scholar] [CrossRef]
- Zedler, Ł.; Przybysz-Romatowska, M.; Haponiuk, J.; Wang, S.; Formela, K. Modification of ground tire rubber—promising approach for development of green composites. J. Compos. Sci. 2020, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Zedler, Ł.; Colom, X.; Cañavate, J.; Saeb, M.R.; Haponiuk, J.T.; Formela, K. Investigating the impact of curing system on structure-property relationship of natural rubber modified with brewery by-product and ground tire rubber. Polymers 2020, 12, 545. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.A.; Zhang, Z.; Formela, K.; Wang, S. Thermo-oxidative exfoliation of carbon black from ground tire rubber as potential reinforcement in green tires. Compos. Sci. Technol. 2021, 214, 108991. [Google Scholar] [CrossRef]
- Formela, K.; Kurańska, M.; Barczewski, M. Recent advances in development of waste-based polymer materials: A review. Polymers 2022, 14, 1050. [Google Scholar] [CrossRef]
- Ghorai, S.; Bhunia, S.; Roy, M.; De, D. Mechanochemical devulcanization of natural rubber vulcanizate by dual function disulfide chemicals. Polym. Degrad. Stab. 2016, 129, 34–46. [Google Scholar] [CrossRef]
- Aoudia, K.; Azem, S.; Ait Hocine, N.; Gratton, M.; Pettarin, V.; Seghar, S. Recycling of waste tire rubber: Microwave devulcanization and incorporation in a thermoset resin. Waste Manag. 2017, 60, 471–481. [Google Scholar] [CrossRef]
- de Sousa, F.D.B.; Scuracchio, C.H.; Hu, G.-H.; Hoppe, S. Devulcanization of waste tire rubber by microwaves. Polym. Degrad. Stab. 2017, 138, 169–181. [Google Scholar] [CrossRef]
- Seghar, S.; Asaro, L.; Rolland-Monnet, M.; Aït Hocine, N. Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour. Conserv. Recycl. 2019, 144, 180–186. [Google Scholar] [CrossRef]
- Colom, X.; Cañavate, J.; Formela, K.; Shadman, A.; Saeb, M.R. Assessment of the devulcanization process of EPDM waste from roofing systems by combined thermomechanical/microwave procedures. Polym. Degrad. Stab. 2021, 183, 109450. [Google Scholar] [CrossRef]
- Wemyss, A.M.; Ellingford, C.; Morishita, Y.; Bowen, C.; Wan, C. Dynamic polymer networks: A new avenue towards sustainable and advanced soft machines. Angew. Chem. Int. Ed. 2021, 60, 13725–13736. [Google Scholar] [CrossRef] [PubMed]
- Bosnjak, N.; Silberstein, M.N. Pathways to tough yet soft materials. Science 2021, 374, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Roy, N.; Bruchmann, B.; Lehn, J.-M. DYNAMERS: Dynamic polymers as self-healing materials. Chem. Soc. Rev. 2015, 44, 3786–3807. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; De Alwis Watuthanthrige, N.; Wanasinghe, S.V.; Averick, S.; Konkolewicz, D. Complementary dynamic chemistries for multifunctional polymeric materials. Adv. Fun. Mater. 2022, 32, 2108431. [Google Scholar] [CrossRef]
- Xie, Z.; Hu, B.-L.; Li, R.-W.; Zhang, Q. Hydrogen bonding in self-healing elastomers. ACS Omega 2021, 6, 9319–9333. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, Y.; Li, S.; Zhu, M.; Hao, T.; Zhou, Z.; Nie, Y. Blending polar rubber with polyurethane to construct self-healing rubber with multiple hydrogen bond networks. Polymer 2022, 246, 124768. [Google Scholar] [CrossRef]
- Liu, J.; Xiao, C.; Tang, J.; Liu, Y.; Hua, J. Construction of a dual ionic network in natural rubber with high self-healing efficiency through anionic mechanism. Ind. Eng. Chem. Res. 2020, 59, 12755–12765. [Google Scholar] [CrossRef]
- Das, M.; Naskar, K. Development, characterization, and applications of a unique self-healable elastomer: Exploring a facile metal-ligand interaction. Polymer 2021, 237, 124373. [Google Scholar] [CrossRef]
- Huang, J.; Gong, Z.; Chen, Y. A stretchable elastomer with recyclability and shape memory assisted self-healing capabilities based on dynamic disulfide bonds. Polymer 2022, 242, 124569. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; Mal, A.; Shen, H.; Nutt, S.R.; Sheran, K.; Wudl, F. A thermally re-mendable cross-linked polymeric material. Science 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Chen, X.; Wudl, F.; Mal, A.K.; Shen, H.; Nutt, S.R. New thermally remendable highly cross-linked polymeric materials. Macromolecules 2003, 36, 1802–1807. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 3218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, H.; Zhu, Y.; Xiong, H.; Wu, Q.; Gu, S.; Liu, X.; Huang, G.; Wu, J. Electron-donating effect enabled simultaneous improvement on the mechanical and self-healing properties of bromobutyl rubber ionomers. ACS Appl. Mater. Interfaces 2020, 12, 53239–53246. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Huang, J.; Gong, Z.; Ding, J.; Chen, Y. Multiple cross-linked networks enhanced ENR-based composite with excellent self-healing properties. Polym. Adv. Technol. 2021, 32, 2856–2865. [Google Scholar] [CrossRef]
- Yeh, C.-M.; Lin, C.-H.; Han, T.-Y.; Xiao, Y.-T.; Chen, Y.-A.; Chou, H.-H. Disulfide bond and Diels–Alder reaction bond hybrid polymers with high stretchability, transparency, recyclability, and intrinsic dual healability for skin-like tactile sensing. J. Mater. Chem. A 2021, 9, 6109–6116. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-healable fluorinated copolymers governed by dipolar interactions. Adv. Sci. 2021, 8, 2101399. [Google Scholar] [CrossRef]
- Gao, X.; Fan, W.; Zhu, W.; Jiuwei, G.; Zhang, P.; Wang, C.; Wang, X.; Xia, H.; Wang, Z.; Huang, W. Tough and healable elastomers via dynamic integrated moiety comprising covalent and noncovalent interactions. Chem. Mater. 2022, 34, 2981–2988. [Google Scholar] [CrossRef]
- Yilmaz, D.; Lansade, D.; Lewandowski, S.; Perraud, S.; Llevot, A.; Carlotti, S. Combination of permanent hydrosilylation and reversible Diels–Alder reactions for self-healing poly(dimethylsiloxane) materials with enhanced ageing properties. Mater. Today Chem. 2022, 24, 100860. [Google Scholar] [CrossRef]
- Jing, T.; Heng, X.; Guifeng, X.; Li, L.; Li, P.; Guo, X. Rapid self-healing and tough polyurethane based on the synergy of multi-level hydrogen and disulfide bonds for healing propellant microcracks. Mater. Chem. Front. 2022. [Google Scholar] [CrossRef]
- Tee, B.C.K.; Wang, C.; Allen, R.; Bao, Z. An electrically and mechanically self-healing composite with pressure- and flexion-sensitive properties for electronic skin applications. Nat. Nanotechnol. 2012, 7, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Le, H.H.; Böhme, F.; Sallat, A.; Wießner, S.; auf der Landwehr, M.; Reuter, U.; Stöckelhuber, K.-W.; Heinrich, G.; Radusch, H.-J.; Das, A. Triggering the self-healing properties of modified bromobutyl rubber by intrinsically electrical heating. Macromol. Mater. Eng. 2017, 302, 1600385. [Google Scholar] [CrossRef]
- Cerdan, K.; Van Assche, G.; van Puyvelde, P.; Brancart, J. A novel approach for the closure of large damage in self-healing elastomers using magnetic particles. Polymer 2020, 204, 122819. [Google Scholar] [CrossRef]
- Zhang, Y.; Khanbareh, H.; Roscow, J.; Pan, M.; Bowen, C.; Wan, C. Self-healing of materials under high electrical stress. Matter 2020, 3, 989–1008. [Google Scholar] [CrossRef]
- Guo, H.; Han, Y.; Zhao, W.; Yang, J.; Zhang, L. Universally autonomous self-healing elastomer with high stretchability. Nat. Commun. 2020, 11, 2037. [Google Scholar] [CrossRef] [PubMed]
- White, S.R.; Sottos, N.R.; Geubelle, P.H.; Moore, J.S.; Kessler, M.R.; Sriram, S.R.; Brown, E.N.; Viswanathan, S. Autonomic healing of polymer composites. Nature 2001, 409, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zheng, Q.; Ashour, A.; Han, B. Self-healing cement concrete composites for resilient infrastructures: A review. Compos. Part B 2020, 189, 107892. [Google Scholar] [CrossRef]
- Xu, S.; García, A.; Su, J.; Liu, Q.; Tabaković, A.; Schlangen, E. Self-healing asphalt review: From idea to practice. Adv. Mater. Interfaces 2018, 5, 1800536. [Google Scholar] [CrossRef]
- van Dijk, N.; van der Zwaag, S. Self-healing phenomena in metals. Adv. Mater. Interfaces 2018, 5, 1800226. [Google Scholar] [CrossRef]
- Wang, S.; Urban, M.W. Self-healing polymers. Nat. Rev. Mater. 2020, 5, 562–583. [Google Scholar] [CrossRef]
- Thakur, V.K.; Kessler, M.R. Self-healing polymer nanocomposite materials: A review. Polymer 2015, 69, 369–383. [Google Scholar] [CrossRef] [Green Version]
- Kanu, N.J.; Gupta, E.; Vates, U.K.; Singh, G.K. Self-healing composites: A state-of-the-art review. Compos. Part A 2019, 121, 474–486. [Google Scholar] [CrossRef]
- Huang, J.; Wróblewska, A.A.; Steinkoenig, J.; Maes, S.; Du Prez, F.E. Assembling lipoic acid and nanoclay into nacre-mimetic nanocomposites. Macromolecules 2021, 54, 4658–4668. [Google Scholar] [CrossRef]
- Yang, Y.; Urban, M.W. Self-healing polymeric materials. Chem. Soc. Rev. 2013, 42, 7446–7467. [Google Scholar] [CrossRef] [PubMed]
- Blaiszik, B.J.; Kramer, S.L.B.; Olugebefola, S.C.; Moore, J.S.; Sottos, N.R.; White, S.R. Self-healing polymers and composites. Annu. Rev. Mater. Res. 2010, 40, 179–211. [Google Scholar] [CrossRef]
- Binder, W.H. Self-Healing Polymers: From Principles to Applications; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014. [Google Scholar]
- Bekas, D.G.; Tsirka, K.; Baltzis, D.; Paipetis, A.S. Self-healing materials: A review of advances in materials, evaluation, characterization, and monitoring techniques. Compos. Part B 2016, 87, 92–119. [Google Scholar] [CrossRef]
- Hernández Santana, M.; den Brabander, M.; García, S.; van der Zwaag, S. Routes to make natural rubber heal: A review. Polym. Rev. 2018, 58, 585–609. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Verdejo, R.; Lopez-Manchado, M.A.; Hernandez Santana, M. Evolution of self-healing elastomers, from extrinsic to combined intrinsic mechanisms: A review. Mater. Horiz. 2020, 7, 2882–2902. [Google Scholar] [CrossRef]
- Araujo-Morera, J.; López-Manchado, M.A.; Verdejo, R.; Hernández Santana, M. Unravelling the effect of healing conditions and vulcanizing additives on the healing performance of rubber networks. Polymer 2022, 238, 124399. [Google Scholar] [CrossRef]
- Behera, P.K.; Mohanty, S.; Gupta, V.K. Self-healing elastomers based on conjugated diolefins: A review. Polym. Chem. 2021, 12, 1598–1621. [Google Scholar] [CrossRef]
- Mashkoor, F.; Lee, S.J.; Yi, H.; Noh, S.M.; Jeong, C. Self-healing materials for electronics applications. Int. J. Mol. Sci. 2022, 23, 622. [Google Scholar] [CrossRef] [PubMed]
- Terryn, S.; Langenbach, J.; Roels, E.; Brancart, J.; Bakkali-Hassani, C.; Poutrel, Q.-A.; Georgopoulou, A.; George Thuruthel, T.; Safaei, A.; Ferrentino, P.; et al. A review on self-healing polymers for soft robotics. Mater. Today 2021, 47, 187–205. [Google Scholar] [CrossRef]
- Cordier, P.; Tournilhac, F.; Soulie-Ziakovic, C.; Leibler, L. Self-healing and thermoreversible rubber from supramolecular assembly. Nature 2008, 451, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Sattar, M.A.; Patnaik, A. Design principles of interfacial dynamic bonds in self-healing materials: What are the parameters? Chem.-Asian J. 2020, 15, 4215–4240. [Google Scholar] [CrossRef]
- Kohjiya, S.; Ikeda, Y. Chemistry, Manufacture, and Applications of Natural Rubber; Woodhead Publishing: Cambridge, UK, 2014. [Google Scholar]
- Men, X.; Wang, F.; Chen, G.-Q.; Zhang, H.-B.; Xian, M. Biosynthesis of natural rubber: Current state and perspectives. Int. J. Mol. Sci. 2019, 20, 50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toki, S.; Fujimaki, T.; Okuyama, M. Strain-induced crystallization of natural rubber as detected real-time by wide-angle X-ray diffraction technique. Polymer 2000, 41, 5423–5429. [Google Scholar] [CrossRef]
- Amnuaypornsri, S.; Sakdapipanich, J.; Toki, S.; Hsiao, B.S.; Ichikawa, N.; Tanaka, Y. Strain-induced crystallization of natural rubber: Effect of proteins and phospholipids. Rubber Chem. Technol. 2008, 81, 753–766. [Google Scholar] [CrossRef]
- Carretero–González, J.; Ezquerra, T.A.; Amnuaypornsri, S.; Toki, S.; Verdejo, R.; Sanz, A.; Sakdapipanich, J.; Hsiao, B.S.; López–Manchado, M.A. Molecular dynamics of natural rubber as revealed by dielectric spectroscopy: The role of natural cross–linking. Soft Matter 2010, 6, 3636–3642. [Google Scholar] [CrossRef] [Green Version]
- Amnuaypornsri, S.; Toki, S.; Hsiao, B.S.; Sakdapipanich, J. The effects of endlinking network and entanglement to stress–strain relation and strain-induced crystallization of un-vulcanized and vulcanized natural rubber. Polymer 2012, 53, 3325–3330. [Google Scholar] [CrossRef]
- Toki, S.; Che, J.; Rong, L.; Hsiao, B.S.; Amnuaypornsri, S.; Nimpaiboon, A.; Sakdapipanich, J. Entanglements and networks to strain-induced crystallization and stress–strain relations in natural rubber and synthetic polyisoprene at various temperatures. Macromolecules 2013, 46, 5238–5248. [Google Scholar] [CrossRef]
- Toki, S. 5-The effect of strain-induced crystallization (SIC) on the physical properties of natural rubber (NR). In Chemistry, Manufacture, and Applications of Natural Rubber; Kohjiya, S., Ikeda, Y., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 135–167. [Google Scholar]
- Tanasi, P.; Hernández Santana, M.; Carretero-González, J.; Verdejo, R.; López-Manchado, M.A. Thermo-reversible crosslinked natural rubber: A Diels-Alder route for reuse and self-healing properties in elastomers. Polymer 2019, 175, 15–24. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Hernández Santana, M.; Verdejo, R.; López-Manchado, M.A. Design of rubber composites with autonomous self-healing capability. ACS Omega 2020, 5, 1902–1910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández, M.; Bernal, M.M.; Grande, A.M.; Zhong, N.; van der Zwaag, S.; García, S.J. Effect of graphene content on the restoration of mechanical, electrical, and thermal functionalities of a self-healing natural rubber. Smart Mater. Struct. 2017, 26, 085010. [Google Scholar] [CrossRef]
- Hernández, M.; Grande, A.M.; Dierkes, W.; Bijleveld, J.; van der Zwaag, S.; García, S.J. Turning vulcanized natural rubber into a self-healing polymer: Effect of the disulfide/polysulfide ratio. ACS Sustainable Chem. Eng. 2016, 4, 5776–5784. [Google Scholar] [CrossRef]
- Hernández, M.; Grande, A.M.; van der Zwaag, S.; Garcia, S.J. Monitoring network and interfacial healing processes by broadband dielectric spectroscopy: A case study on natural rubber. ACS Appl. Mater. Interfaces 2016, 8, 10647–10656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, C.S.L.; Gelling, I.R.; Newell, R. Epoxidized natural rubber. Rubber Chem. Technol. 1985, 58, 67–85. [Google Scholar] [CrossRef]
- Arroyo, M.; López-Manchado, M.A.; Valentín, J.L.; Carretero, J. Morphology/behaviour relationship of nanocomposites based on natural rubber/epoxidized natural rubber blends. Compos. Sci. Technol. 2007, 67, 1330–1339. [Google Scholar] [CrossRef]
- Sengloyluan, K.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Silica-reinforced tire tread compounds compatibilized by using epoxidized natural rubber. Eur. Polym. J. 2014, 51, 69–79. [Google Scholar] [CrossRef]
- Cao, L.; Yuan, D.; Xu, C.; Chen, Y. Biobased, self-healable, high strength rubber with tunicate cellulose nanocrystals. Nanoscale 2017, 9, 15696–15706. [Google Scholar] [CrossRef]
- Huang, J.; Cao, L.; Yuan, D.; Chen, Y. Design of novel self-healing thermoplastic vulcanizates utilizing thermal/magnetic/light-triggered shape memory effects. ACS Appl. Mater. Interfaces 2018, 10, 40996–41002. [Google Scholar] [CrossRef]
- Nie, J.; Mou, W.; Ding, J.; Chen, Y. Bio-based epoxidized natural rubber/chitin nanocrystals composites: Self-healing and enhanced mechanical properties. Compos. Part B 2019, 172, 152–160. [Google Scholar] [CrossRef]
- Xu, C.; Nie, J.; Wu, W.; Zheng, Z.; Chen, Y. Self-healable, recyclable, and strengthened epoxidized natural rubber/carboxymethyl chitosan biobased composites with hydrogen bonding supramolecular hybrid networks. ACS Sustain. Chem. Eng. 2019, 7, 15778–15789. [Google Scholar] [CrossRef]
- Nie, J.; Fan, J.; Gong, Z.; Xu, C.; Chen, Y. Frame-structured and self-healing ENR-based nanocomposites for strain sensors. Eur. Polym. J. 2021, 154, 110569. [Google Scholar] [CrossRef]
- Yikmis, M.; Steinbüchel, A. Historical and recent achievements in the field of microbial degradation of natural and synthetic rubber. Appl. Environ. Microbiol. 2012, 78, 4543–4551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández Santana, M.; Huete, M.; Lameda, P.; Araujo, J.; Verdejo, R.; López-Manchado, M.A. Design of a new generation of sustainable SBR compounds with good trade-off between mechanical properties and self-healing ability. Eur. Polym. J. 2018, 106, 273–283. [Google Scholar] [CrossRef]
- Utrera-Barrios, S.; Araujo-Morera, J.; Pulido de Los Reyes, L.; Verdugo Manzanares, R.; Verdejo, R.; López-Manchado, M.Á.; Hernández Santana, M. An effective and sustainable approach for achieving self-healing in nitrile rubber. Eur. Polym. J. 2020, 139, 110032. [Google Scholar] [CrossRef]
- Martin, R.; Rekondo, A.; Ruiz de Luzuriaga, A.; Cabañero, G.; Grande, H.J.; Odriozola, I. The processability of a poly(urea-urethane) elastomer reversibly crosslinked with aromatic disulfide bridges. J. Mater. Chem. A 2014, 2, 5710–5715. [Google Scholar] [CrossRef]
- Rekondo, A.; Martin, R.; Ruiz de Luzuriaga, A.; Cabañero, G.; Grande, H.J.; Odriozola, I. Catalyst-free room-temperature self-healing elastomers based on aromatic disulfide metathesis. Mater. Horiz. 2014, 1, 237–240. [Google Scholar] [CrossRef]
- O’Harra, K.; Sadaba, N.; Irigoyen, M.; Ruipérez, F.; Aguirresarobe, R.; Sardon, H.; Bara, J. Nearly perfect 3D structures obtained by assembly of printed parts of polyamide ionene self-healing elastomer. ACS Appl. Polym. Mater. 2020, 2, 4352–4359. [Google Scholar] [CrossRef]
- Aboudzadeh, A.; Fernandez, M.; Muñoz, M.E.; Santamaría, A.; Mecerreyes, D. Ionic supramolecular networks fully based on chemicals coming from renewable sources. Macromol. Rapid Commun. 2014, 35, 460–465. [Google Scholar] [CrossRef]
- Araujo-Morera, J.; Hernández Santana, M.; Verdejo, R.; López-Manchado, M.A. Giving a second opportunity to tire waste: An alternative path for the development of sustainable self-healing styrene–butadiene rubber compounds overcoming the magic triangle of tires. Polymers 2019, 11, 2122. [Google Scholar] [CrossRef] [Green Version]
- Alonso Pastor, L.E.; Núñez Carrero, K.C.; Araujo-Morera, J.; Hernández Santana, M.; Pastor, J.M. Setting relationships between structure and devulcanization of ground tire rubber and their effect on self-healing elastomers. Polymers 2022, 14, 11. [Google Scholar] [CrossRef]
- Martín, R.; Rekondo, A.; Echeberria, J.; Cabañero, G.; Grande, H.J.; Odriozola, I. Room temperature self-healing power of silicone elastomers having silver nanoparticles as crosslinkers. Chem. Commun. 2012, 48, 8255–8257. [Google Scholar] [CrossRef] [PubMed]
- Utrera-Barrios, S.; Verdugo Manzanares, R.; Araujo-Morera, J.; González, S.; Verdejo, R.; López-Manchado, M.Á.; Hernández Santana, M. Understanding the molecular dynamics of dual crosslinked networks by dielectric spectroscopy. Polymers 2021, 13, 3234. [Google Scholar] [CrossRef] [PubMed]
- Aguirresarobe, R.H.; Nevejans, S.; Reck, B.; Irusta, L.; Sardon, H.; Asua, J.M.; Ballard, N. Healable and self-healing polyurethanes using dynamic chemistry. Prog. Polym. Sci. 2021, 114, 101362. [Google Scholar] [CrossRef]
- Formoso, E.; Asua, J.M.; Matxain, J.M.; Ruipérez, F. The role of non-covalent interactions in the self-healing mechanism of disulfide-based polymers. Phys. Chem. Chem. Phys. 2017, 19, 18461–18470. [Google Scholar] [CrossRef] [PubMed]
- Irigoyen, M.; Fernández, A.; Ruiz, A.; Ruipérez, F.; Matxain, J.M. Diselenide bonds as an alternative to outperform the efficiency of disulfides in self-healing materials. J. Org. Chem. 2019, 84, 4200–4210. [Google Scholar] [CrossRef]
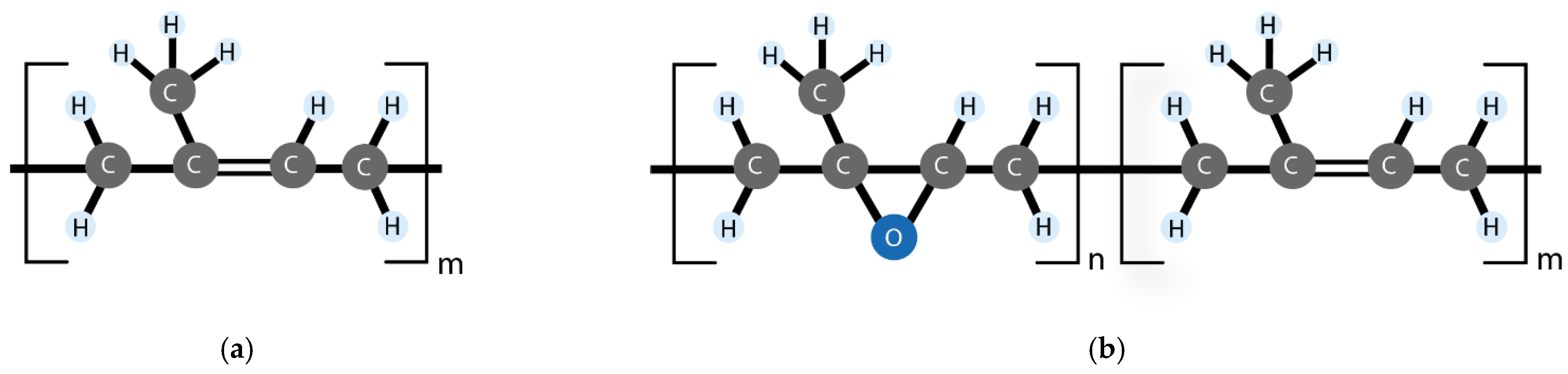
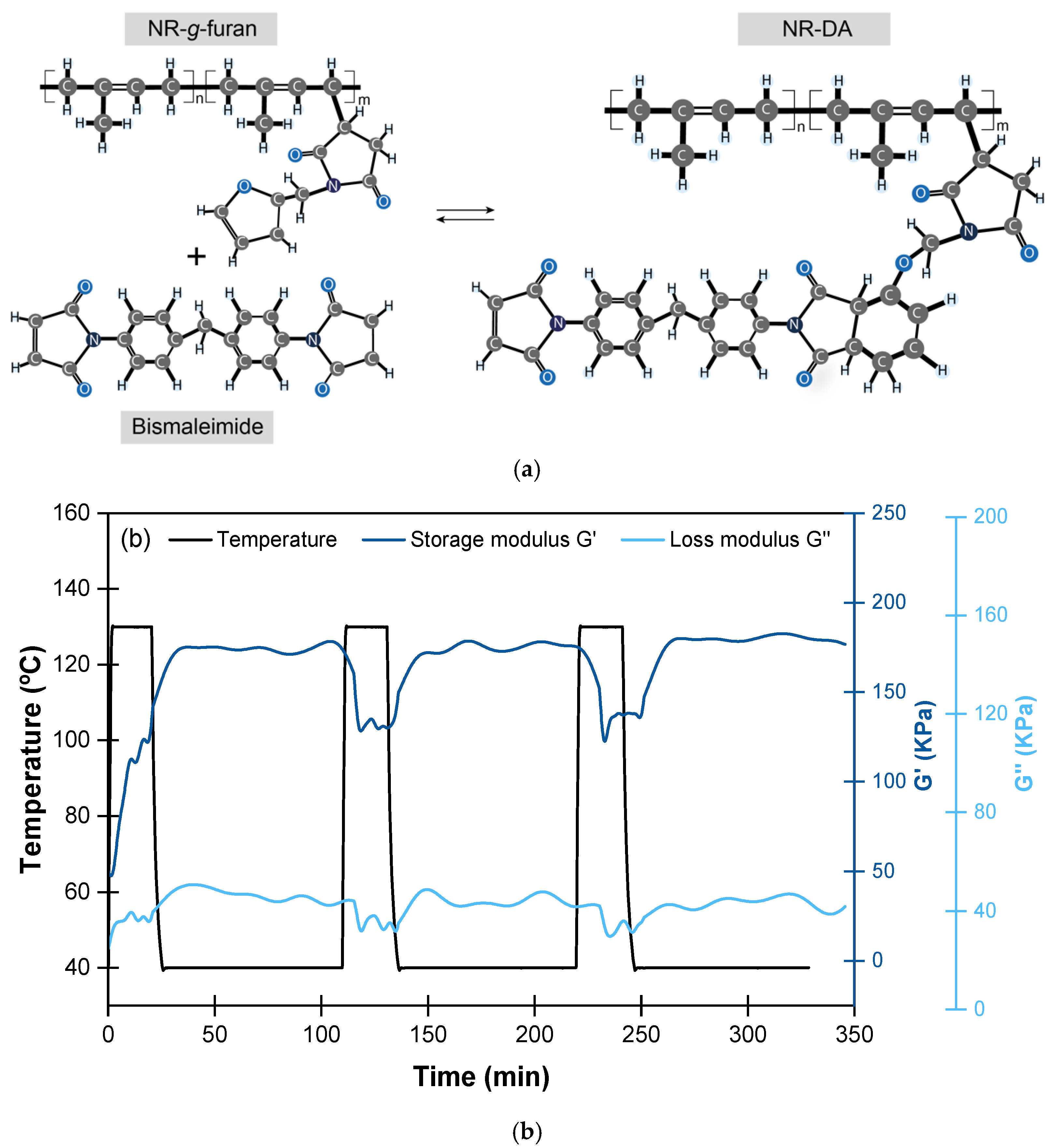



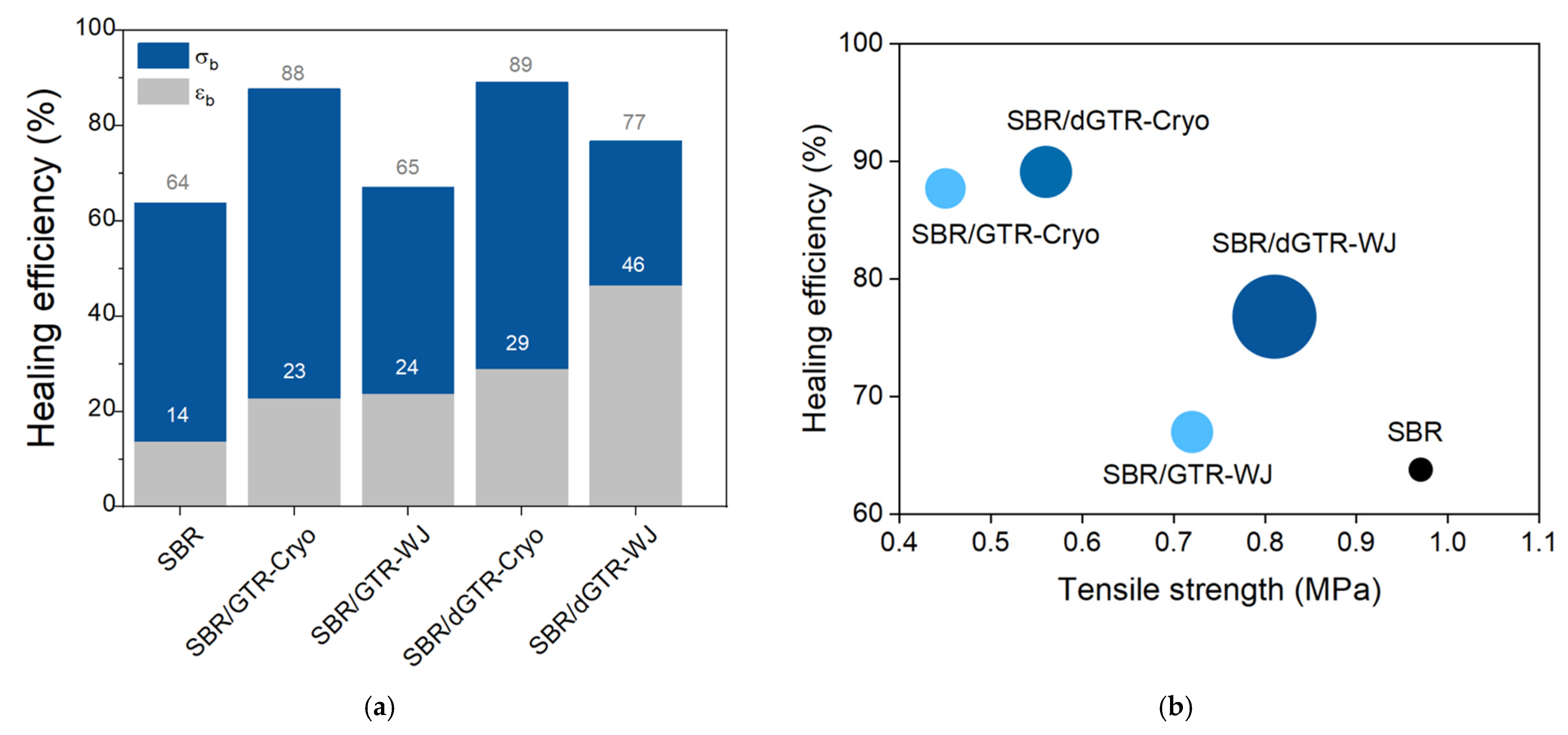
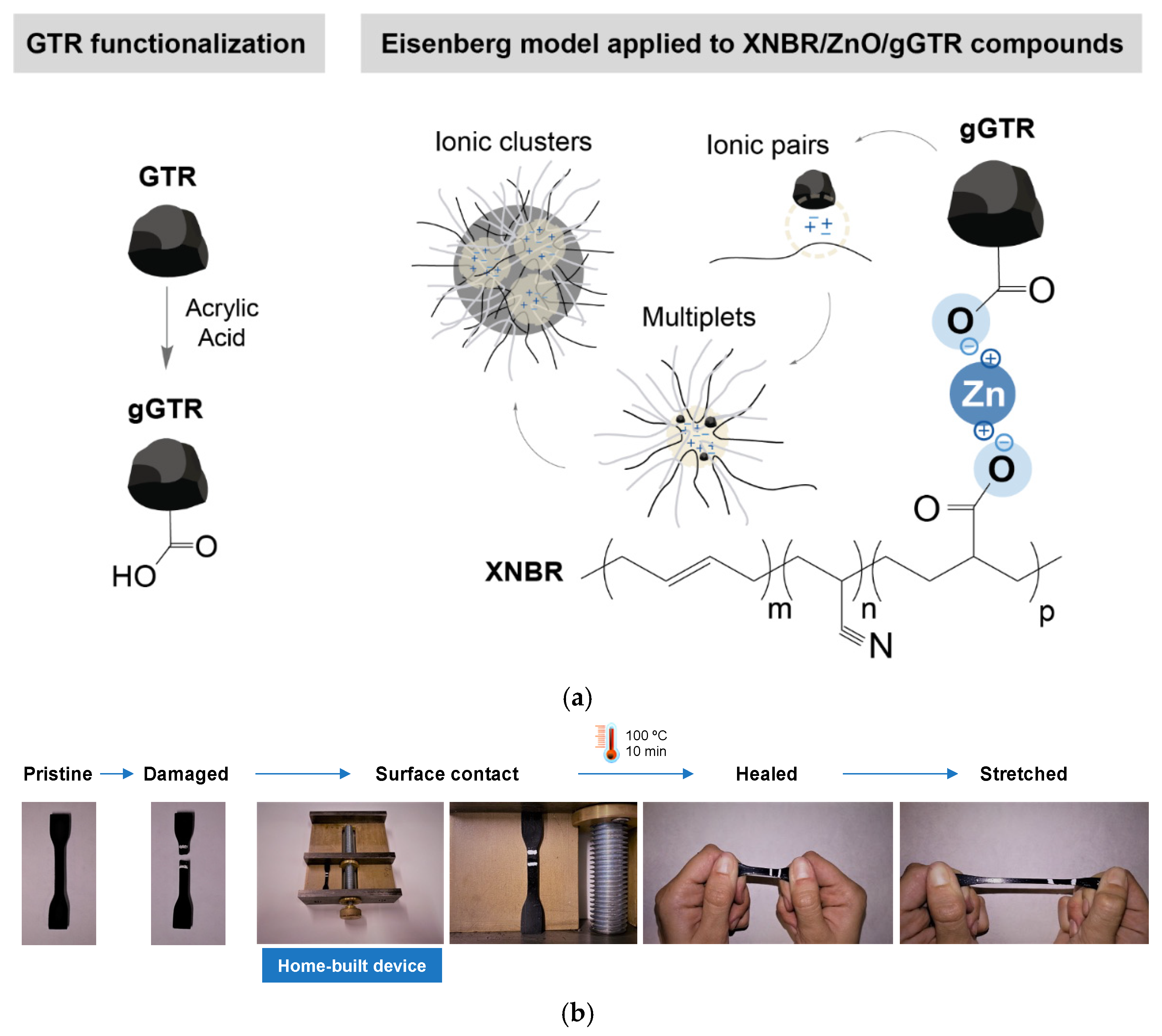
| Matrix | Mechanism | Healing Moieties | Filler | Reference |
|---|---|---|---|---|
| NR | Covalent intrinsic | Diels–Alder chemistry | Unfilled | [64] |
| ENR | Non-covalent intrinsic | Hydrogen bonds | Unfilled | [65] |
| NR | Covalent intrinsic | Disulfide exchange | Graphene oxide | [66] |
| ENR | Combined intrinsic | Hydrogen bonds + Transesterification reactions | Graphene oxide | [65] |
| Matrix | Mechanism | Healing Moieties | Filler | Reference |
|---|---|---|---|---|
| SBR | Covalent intrinsic | Disulfide exchange | Unfilled | [50,78] |
| XNBR | Non-covalent intrinsic | Ionic interactions | Unfilled | [79] |
| PUU | Covalent intrinsic | Disulfide exchange | Unfilled | [80,81] |
| Polyamide ionene | Combined intrinsic | Ionic interactions + Hydrogen bonds + π-π stacking | Unfilled | [82] |
| Ionic elastomer | Non-covalent intrinsic | Ionic interactions | Unfilled | [83] |
| SBR | Covalent intrinsic | Disulfide exchange | GTR 1 | [78,84] |
| SBR | Covalent intrinsic | Disulfide exchange | dGTR 2 | [85] |
| XNBR | Non-covalent intrinsic | Ionic interactions | GTR | [79] |
| Silicone elastomer | Covalent intrinsic | Thiol exchange | Ag nanoparticles | [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Utrera-Barrios, S.; Verdejo, R.; López-Manchado, M.Á.; Santana, M.H. The Final Frontier of Sustainable Materials: Current Developments in Self-Healing Elastomers. Int. J. Mol. Sci. 2022, 23, 4757. https://doi.org/10.3390/ijms23094757
Utrera-Barrios S, Verdejo R, López-Manchado MÁ, Santana MH. The Final Frontier of Sustainable Materials: Current Developments in Self-Healing Elastomers. International Journal of Molecular Sciences. 2022; 23(9):4757. https://doi.org/10.3390/ijms23094757
Chicago/Turabian StyleUtrera-Barrios, Saul, Raquel Verdejo, Miguel Ángel López-Manchado, and Marianella Hernández Santana. 2022. "The Final Frontier of Sustainable Materials: Current Developments in Self-Healing Elastomers" International Journal of Molecular Sciences 23, no. 9: 4757. https://doi.org/10.3390/ijms23094757
APA StyleUtrera-Barrios, S., Verdejo, R., López-Manchado, M. Á., & Santana, M. H. (2022). The Final Frontier of Sustainable Materials: Current Developments in Self-Healing Elastomers. International Journal of Molecular Sciences, 23(9), 4757. https://doi.org/10.3390/ijms23094757









