Research on Graphene and Its Derivatives in Oral Disease Treatment
Abstract
:1. Introduction
2. Graphene-Based Materials
2.1. Graphene and Its Derivatives
2.2. Preparation of Graphene and Its Derivatives
2.3. Compatibility of Graphene-Based Materials
3. Improving the Physical and Chemical Performance of Dental Materials
4. Potential Application of Graphene-Based Materials in Oral Disease Treatment
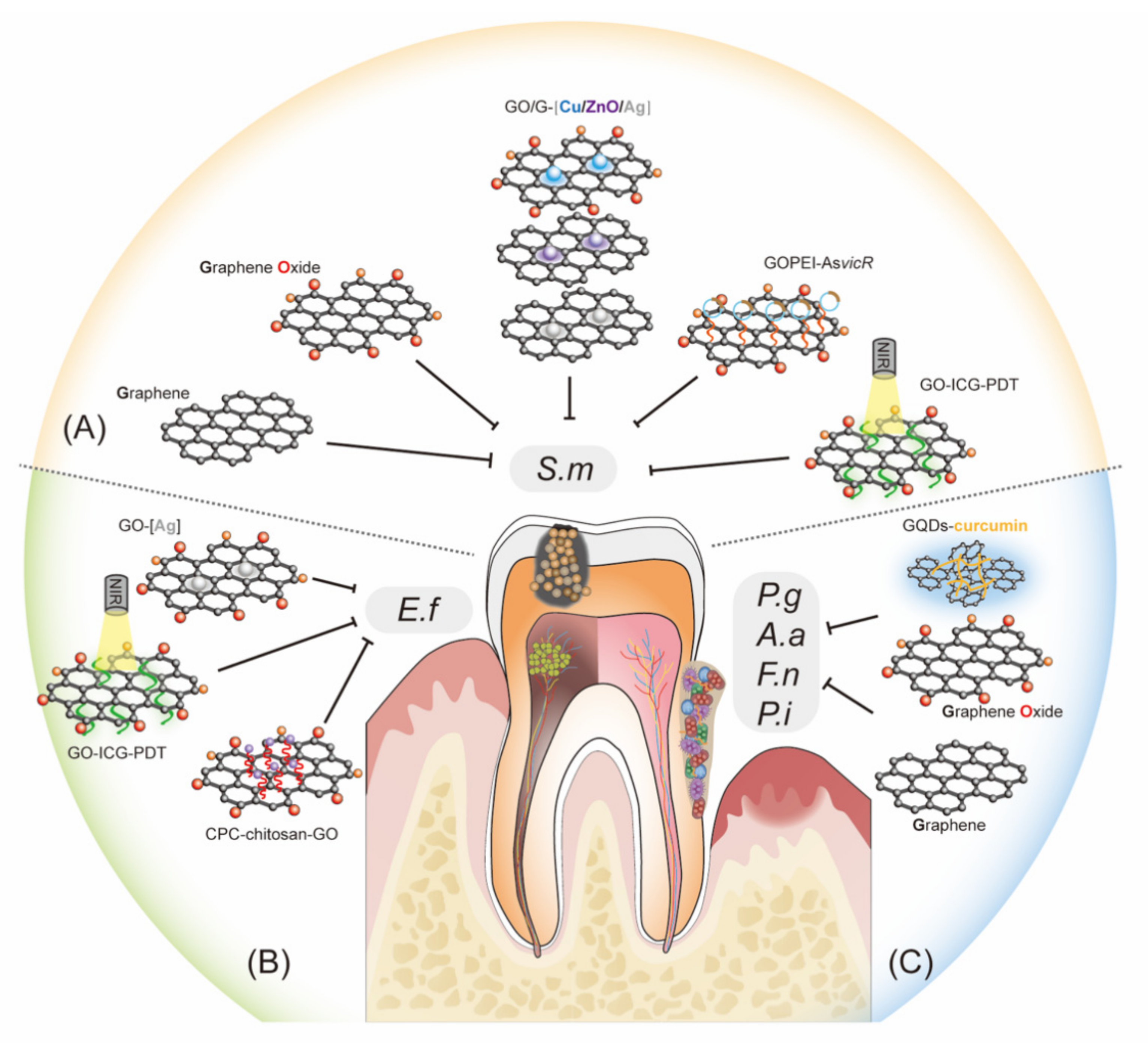
4.1. Inhibiting Cariogenic Bacteria and Preventing Demineralization of Teeth
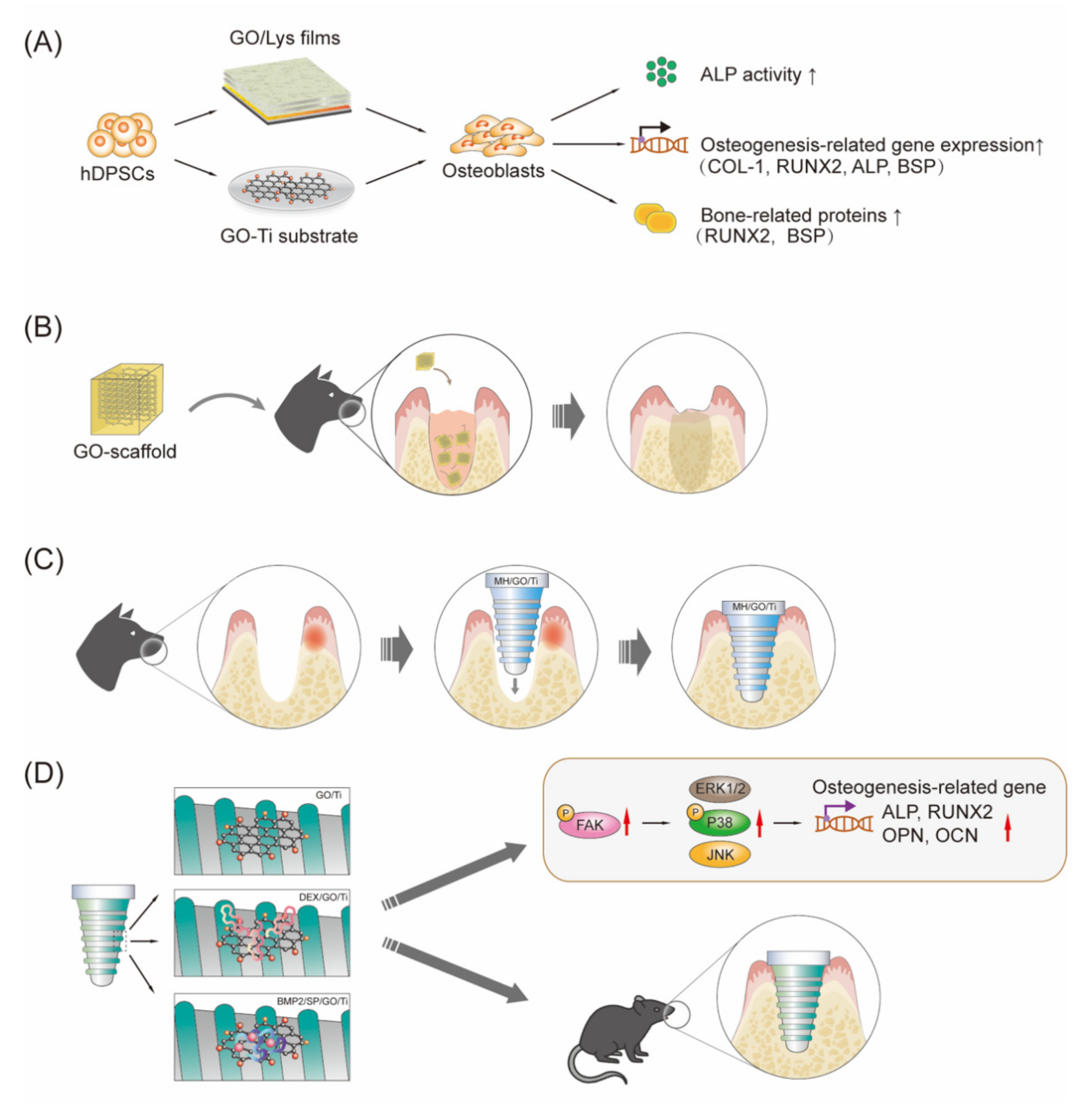
4.2. Control of Dental Pulp Infection and Promotion of hDPSC Differentiation
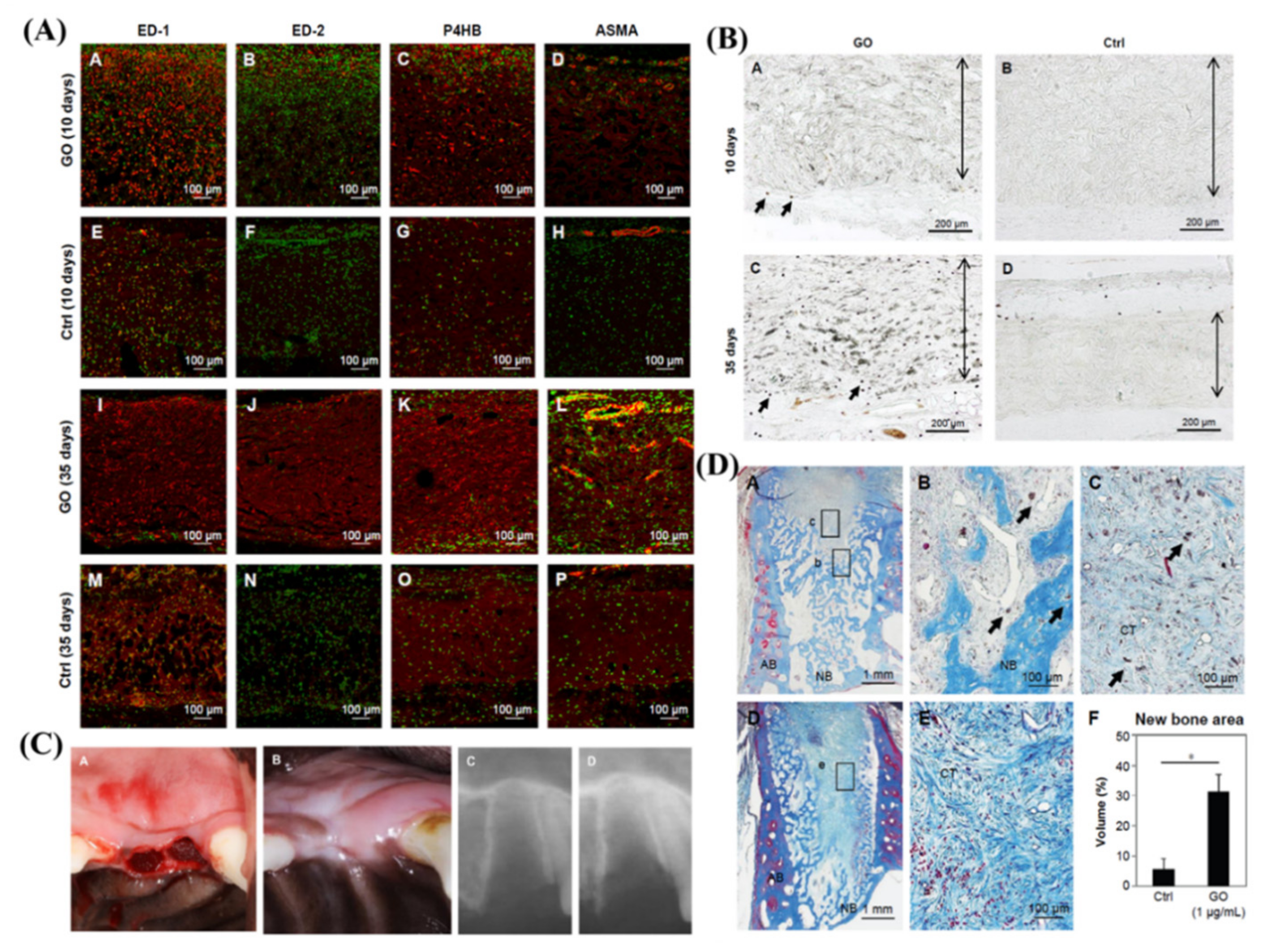
4.3. Suppressing Periodontal Bacteria and Facilitating Tissue Regeneration
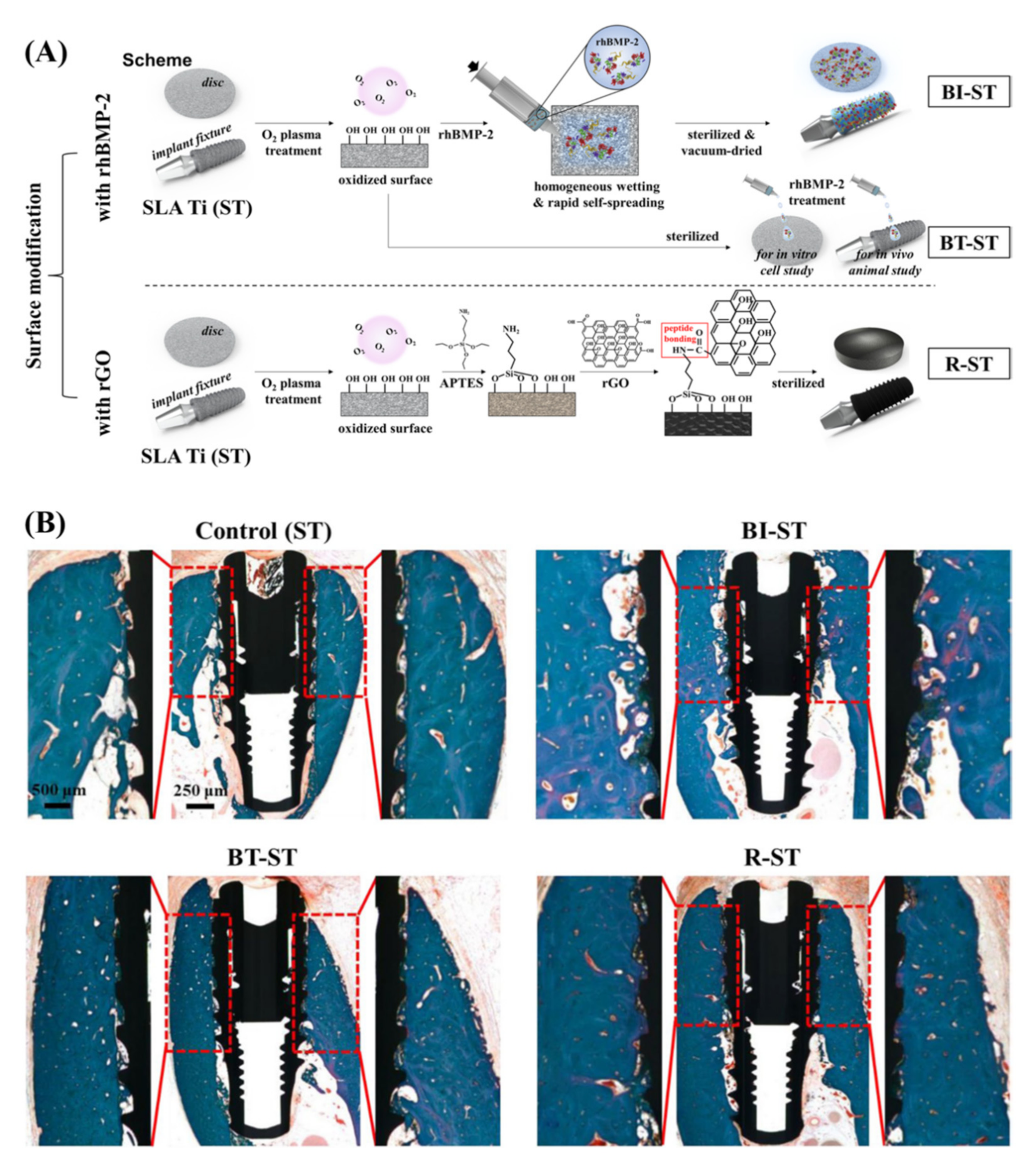
4.4. Implant Coating and Improving Osseointegration
5. Perspective and Summary
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diseases, G.B.D.; Injuries, C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the global burden of disease study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar]
- Moynihan, P.; Petersen, P.E. Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004, 7, 201–226. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontology 2020, 83, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Lamont, R.J.; Hajishengallis, G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol. Med. 2014, 21, 172–183. [Google Scholar] [CrossRef] [Green Version]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Tian, J.; Liu, C.; Zheng, X.; Jia, X.; Peng, X.; Yang, R.; Zhou, X.; Xu, X. Porphyromonas gingivalis Induces Insulin Resistance by Increasing BCAA Levels in Mice. J. Dent. Res. 2020, 99, 839–846. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Weir, M.D.; Melo, M.A.S.; Zhou, X.; Xu, H.H.K. Nanotechnology strategies for antibacterial and remineralizing composites and adhesives to tackle dental caries. Nanomed 2015, 10, 627–641. [Google Scholar] [CrossRef] [Green Version]
- Poole, S.F.; Pitondo-Silva, A.; Oliveira-Silva, M.; Moris, I.C.M.; Gomes, E.A. Influence of different ceramic materials and surface treatments on the adhesion of Prevotella intermedia. J. Mech. Behav. Biomed. Mater. 2020, 111, 104010. [Google Scholar] [CrossRef]
- Porter, J.R.; Ruckh, T.T.; Popat, K.C. Bone tissue engineering: A review in bone biomimetics and drug delivery strategies. Biotechnol. Prog. 2009, 25, 1539–1560. [Google Scholar] [CrossRef]
- Liao, C.Z.; Li, K.; Wong, H.M.; Tong, W.Y.; Yeung, K.W.K.; Tjong, S.C. Novel polypropylene biocomposites reinforced with carbon nanotubes and hydroxyapatite nanorods for bone replacements. Mater. Sci. Eng. C 2013, 33, 1380–1388. [Google Scholar] [CrossRef]
- Bottino, M.C.; Thomas, V.; Schmidt, G.; Vohra, Y.K.; Chu, T.-M.G.; Kowolik, M.J.; Janowski, G. Recent advances in the development of GTR/GBR membranes for periodontal regeneration—A materials perspective. Dent. Mater. 2012, 28, 703–721. [Google Scholar] [CrossRef] [PubMed]
- Ul Hassan, S.; Bilal, B.; Nazir, M.S.; Naqvi, S.A.R.; Ali, Z.; Nadeem, S.; Muhammad, N.; Palvasha, B.A.; Mohyuddin, A. Recent progress in materials development and biological properties of GTR membranes for periodontal regeneration. Chem. Biol. Drug Des. 2021, 98, 1007–1024. [Google Scholar] [CrossRef] [PubMed]
- Pjetursson, B.E.; Heimisdottir, K. Dental implants—are they better than natural teeth? Eur. J. Oral Sci. 2018, 126, 81–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alghamdi, H.S.; Jansen, J.A. The development and future of dental implants. Dent. Mater. J. 2020, 31, 167–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Mekawy, N.; Fouad, M.M.; El-Hawary, Y.M.; Al-Shahat, M.A.; El-Gendy, R. Scanning electron microscopy observations of osseointegration failures of dental implants that support mandibular overdentures. Implant Dent. 2013, 22, 645–649. [Google Scholar] [CrossRef]
- Li, M.; Li, H.; Pan, Q.; Gao, C.; Wang, Y.; Yang, S.; Zan, X.; Guan, Y. Graphene Oxide and Lysozyme Ultrathin Films with Strong Antibacterial and Enhanced Osteogenesis. Langmuir 2019, 35, 6752–6761. [Google Scholar] [CrossRef]
- Qin, W.; Li, Y.; Ma, J.; Liang, Q.; Cui, X.; Jia, H.; Tang, B. Osseointegration and biosafety of graphene oxide wrapped porous CF/PEEK composites as implantable materials: The role of surface structure and chemistry. Dent. Mater. 2020, 36, 1289–1302. [Google Scholar] [CrossRef]
- Park, C.; Park, S.; Lee, D.; Choi, K.S.; Lim, H.P.; Kim, J. Graphene as an enabling strategy for dental implant and tissue regeneration. Tissue Eng. Regen. Med. 2017, 14, 481–493. [Google Scholar] [CrossRef]
- Kim, K.T.; Eo, M.Y.; Nguyen, T.T.H.; Kim, S.M. General review of titanium toxicity. Int. J. Implant Dent. 2019, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Mok, Z.H.; Proctor, G.; Thanou, M. Emerging nanomaterials for dental treatments. Emerg. Top. Life Sci. 2020, 4, 613–625. [Google Scholar]
- Tan, B.; Tang, Q.; Zhong, Y.; Wei, Y.; He, L.; Wu, Y.; Wu, J.; Liao, J. Biomaterial-based strategies for maxillofacial tumor therapy and bone defect regeneration. Int. J. Oral Sci. 2021, 13, 9. [Google Scholar] [CrossRef] [PubMed]
- Terrones, M.; Botello-Méndez, A.R.; Campos-Delgado, J.; López-Urías, F.; Vega-Cantu, Y.I.; Rodriguez-Macias, F.J.; Elías, A.L.; Munoz-Sandoval, E.; Cano-Márquez, A.G.; Charlier, J.-C.; et al. Graphene and graphite nanoribbons: Morphology, properties, synthesis, defects and applications. Nano Today 2010, 5, 351–372. [Google Scholar] [CrossRef]
- Dang, X.; Zhang, H.; Lin, L.; Li, P.; Ren, L.; Zhang, W.; Song, R. The anti-inflammatory and osteogenic activity of chitosan/polyvinyl alcohol/graphene oxide/astaxanthin nanofibers membranes in vitro study. J. Biomater. Appl. 2022, 36, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Kanaparthy, R.; Kanaparthy, A. The changing face of dentistry: Nanotechnology. Int. J. Nanomed. 2011, 6, 2799–2804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef]
- Peng, S.; Jin, Z.; Yao, Y.; Huang, X.; Zhang, D.; Niu, J.; Shi, J.; Zhang, Y.; Yu, G. Controllable p-to-n type conductance transition in top-gated graphene field effect transistor by interface trap engineering. Adv. Electron. Mater. 2020, 6, 2000496. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Novoselov, K.S. The rise of graphene. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef]
- Lee, C.; Wei, X.; Kysar Jeffrey, W.; Hone, J. Measurement of the Elastic Properties and intrinsic strength of monolayer graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal′ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Peng, S.; Zhang, J.; Zhi, J.; Zhang, D.Y.; Shi, J.Y.; Wei, S.H. Electric-field induced doping polarity conversion in top-gated transistor based on chemical vapor deposition of graphene engineering. Crystals 2022, 12, 2. [Google Scholar] [CrossRef]
- Peng, S.; Jin, Z.; Zhang, D.; Shi, J.; Niu, J.; Zhu, C.; Zhang, Y.; Yu, G. The effect of metal contact doping on the scaled graphene field effect transistor. Adv. Eng. Mater. 2021, 24, 2100935. [Google Scholar] [CrossRef]
- Hu, W.; Peng, C.; Luo, W.; Lv, M.; Li, X.; Li, D.; Huang, Q.; Fan, C. Graphene-based antibacterial paper. ACS Nano 2010, 4, 4317–4323. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, Y.-R.; Kim, Y.; Lim, K.T.; Seonwoo, H.; Park, S.; Hong, B.H.; Choung, P.-H.; Chung, T.D.; Choung, Y.-H.; et al. Graphene-incorporated chitosan substrata for adhesion and differentiation of human mesenchymal stem cells. J. Mater. Chem. B 2013, 1, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Srimaneepong, V.; Skallevold, H.E.; Khurshid, Z.; Zafar, M.S.; Rokaya, D.; Sapkota, J. Graphene for antimicrobial and coating application. Int. J. Mol. Sci. 2022, 23, 499. [Google Scholar] [CrossRef] [PubMed]
- Gholami, A.; Emadi, F.; Amini, A.; Shokripour, M.; Chashmpoosh, M.; Omidifar, N. Functionalization of graphene oxide nanosheets can reduce their cytotoxicity to dental pulp stem cells. J. Nanomater. 2020, 2020, 6942707. [Google Scholar] [CrossRef]
- Emadi, F.; Emadi, A.; Gholami, A. A Comprehensive insight toward pharmaceutical aspects of graphene nanosheets. Curr. Pharm. Biotechnol. 2020, 21, 1016–1027. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Z.; Huang, D.; Liu, Z.; Guo, X.; Zhong, H. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials 2011, 32, 8555–8561. [Google Scholar] [CrossRef]
- Robinson, J.T.; Tabakman, S.M.; Liang, Y.; Wang, H.; Casalongue, H.S.; Vinh, D.; Dai, H. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011, 133, 6825–6831. [Google Scholar] [CrossRef]
- Yang, K.; Hu, L.; Ma, X.; Ye, S.; Cheng, L.; Shi, X.; Li, C.; Li, Y.; Liu, Z. Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles. Adv. Mater. 2012, 24, 1868–1872. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nanographene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef] [Green Version]
- Rosa, V.; Xie, H.; Dubey, N.; Madanagopal, T.T.; Rajan, S.S.; Morin, J.L.P.; Islam, I.; Neto, A.H.C. Graphene oxide-based substrate: Physical and surface characterization, cytocompatibility and differentiation potential of dental pulp stem cells. Dent. Mater. 2016, 32, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Compton, O.C.; Nguyen, S.T. Graphene oxide, highly reduced graphene oxide, and graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Jiříčková, A.; Jankovský, O.; Sofer, Z.; Sedmidubský, D. Synthesis and applications of graphene oxide. Materials 2022, 15, 920. [Google Scholar] [CrossRef]
- Pei, S.; Cheng, H.-M. The reduction of graphene oxide. Carbon 2012, 50, 3210–3228. [Google Scholar] [CrossRef]
- Priyadarsini, S.; Mohanty, S.; Mukherjee, S.; Basu, S.; Mishra, M. Graphene and graphene oxide as nanomaterials for medicine and biology application. J. Nanostructure Chem. 2018, 8, 123–137. [Google Scholar] [CrossRef] [Green Version]
- La, W.-G.; Park, S.; Yoon, H.-H.; Jeong, G.-J.; Lee, T.-J.; Bhang, S.H.; Han, J.Y.; Char, K.; Kim, B.-S. Delivery of a therapeutic protein for bone regeneration from a substrate coated with graphene oxide. Small 2013, 9, 4051–4060. [Google Scholar] [CrossRef]
- Kim, B.-S.; La, W.-G.; Jin, M.; Park, S.; Yoon, H.-H.; Jeong, G.-J.; Bhang, S.H.; Park, H.; Char, K. Delivery of bone morphogenetic protein-2 and substance P using graphene oxide for bone regeneration. Int. J. Nanomed. 2014, 9, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Ren, N.; Li, J.; Qiu, J.; Yan, M.; Liu, H.; Ji, D.; Huang, J.; Yu, J.; Liu, H. Growth and accelerated differentiation of mesenchymal stem cells on graphene-oxide-coated titanate with dexamethasone on surface of titanium implants. Dent. Mater. 2017, 33, 525–535. [Google Scholar] [CrossRef]
- Lu, J.; Do, I.; Drzal, L.T.; Worden, R.M.; Lee, I. Nanometal-decorated exfoliated graphite nanoplatelet based glucose biosensors with high sensitivity and fast response. ACS Nano 2008, 2, 1825–1832. [Google Scholar] [CrossRef]
- Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S.H.M.; Li, H. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: From fabrication to applications. Materials 2022, 28, 1012. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, C.L.C.; Kessler, F.; Dubey, N.; Rosa, V.; Fechine, G.J.M. CVD graphene transfer procedure to the surface of stainless steel for stem cell proliferation. Surf. Coat. Technol. 2017, 311, 10–18. [Google Scholar] [CrossRef]
- Mousavi, S.M.; Yousefi, K.; Hashemi, S.A.; Afsa, M.; BahranI, S.; Gholami, A.; Ghahramani, Y.; Alizadeh, A.; Chiang, W.-H. Renewable carbon nanomaterials: Novel resources for dental tissue engineering. Nanomaterials 2021, 11, 2800. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.X.; Bets, K.V.; Algozeeb, W.A.; Stanford, M.G.; Kittrell, C.; Chen, W.; Salvatierra, R.V.; Ren, M.; McHugh, E.A.; Advincula, P.A.; et al. Gram-scale bottom-up flash graphene synthesis. Nature 2020, 577, 647–651. [Google Scholar] [CrossRef]
- De Silva, K.K.H.; Huang, H.H.; Joshi, R.K.; Yoshimura, M. Chemical reduction of graphene oxide using green reductants. Carbon 2017, 119, 190–199. [Google Scholar] [CrossRef]
- Agharkar, M.; Kochrekar, S.; Hidouri, S.; Azeez, M.A. Trends in green reduction of graphene oxides, issues and challenges: A review. Mater. Res. Bull. 2014, 59, 323–328. [Google Scholar] [CrossRef]
- Thakur, S.; Karak, N. Alternative methods and nature-based reagents for the reduction of graphene oxide: A review. Carbon 2015, 94, 224–242. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Tan, X.; Peng, R.; Liu, Z. Behavior and toxicity of graphene and its functionalized derivatives in biological systems. Small 2013, 9, 1492–1503. [Google Scholar] [CrossRef]
- Ikram, R.; Shamsuddin, S.A.A.; Mohamed Jan, B.; Abdul Qadir, M.; Kenanakis, G.; Stylianakis, M.M.; Anastasiadis, S.H. Impact of graphene derivatives as artificial extracellular matrices on mesenchymal stem cells. Molecules 2022, 27, 379. [Google Scholar] [CrossRef]
- Lammel, T.; Boisseaux, P.; Fernández-Cruz, M.-L.; Navas, J.M. Internalization and cytotoxicity of graphene oxide and carboxyl graphene nanoplatelets in the human hepatocellular carcinoma cell line Hep G2. Part. Fiber Toxicol. 2013, 10, 27. [Google Scholar] [CrossRef] [Green Version]
- Duan, G.; Zhang, Y.; Luan, B.; Weber, J.K.; Zhou, R.; Yang, Z.; Zhao, L.; Xu, J.; Luo, J.; Zhou, R. Graphene-induced pore formation on cell membranes. Sci. Rep. 2017, 7, 42767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, P.-P.; Sun, T.; Junaid, M.; Yang, L.; Ma, Y.-B.; Cui, Z.-S.; Wei, D.-P.; Shi, H.-F.; Pei, D.-S. Nanotoxicity of different sizes of graphene (G) and graphene oxide (GO) in vitro and in vivo. Environ. Pollut. 2019, 247, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Yang, S.-T.; Liu, J.-H.; Dong, E.; Wang, Y.; Cao, A.; Liu, Y.; Wang, H. In vitro toxicity evaluation of graphene oxide on A549 cells. Toxicol. Lett. 2011, 200, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Stone, V.; Donaldson, K. Signs of stress. Nat. Nanotechnol. 2006, 1, 23–24. [Google Scholar] [CrossRef]
- Pinto, A.M.; Moreira, J.A.; Magalhães, F.D.; Gonçalves, I.C. Polymer surface adsorption as a strategy to improve the biocompatibility of graphene nanoplatelets. Colloids Surf. B Biointerfaces 2016, 146, 818–824. [Google Scholar] [CrossRef]
- Liao, K.-H.; Lin, Y.-S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef]
- Mohammadrezaei, D.; Golzar, H.; Rezai Rad, M.; Omidi, M.; Rashedi, H.; Yazdian, F.; Tayebi, L. In vitro effect of graphene structures as an osteoinductive factor in bone tissue engineering: A systematic review. J. Biomed. Mater. Res. Part A 2018, 106, 2284–2343. [Google Scholar] [CrossRef] [Green Version]
- Bacali, C.; Baldea, I.; Moldovan, M.; Carpa, R.; Olteanu, D.E.; Filip, G.A.; Nastase, V.; Lascu, L.; Badea, M.; Constantiniuc, M.; et al. Flexural strength, biocompatibility, and antimicrobial activity of a polymethyl methacrylate denture resin enhanced with graphene and silver nanoparticles. Clin. Oral Investig. 2019, 24, 2713–2725. [Google Scholar] [CrossRef] [PubMed]
- Olteanu, E.D.; Filip, A.; Socaci, C.; Biris, A.R.; Filip, X.; Coros, M.; Rosu, M.C.; Pogacean, F.; Alb, C.; Baldea, I.; et al. Cytotoxicity assessment of graphene-based nanomaterials on human dental follicle stem cells. Colloids Surf. B Biointerfaces 2015, 136, 791–798. [Google Scholar] [CrossRef]
- Dreanca, A.; Sarosi, C.; Parvu, A.E.; Blidaru, M.; Enacrachi, G.; Purdoiu, R.; Nagy, A.; Sevastre, B.; Oros, N.A.; Marcus, I.; et al. Systemic and Local Biocompatibility Assessment of Graphene Composite Dental Materials in Experimental Mandibular Bone Defect. Materials 2020, 13, 2511. [Google Scholar] [CrossRef]
- Mansouri, N.; Al-Sarawi, S.; Losic, D.; Mazumdar, J.; Clark, J.; Gronthos, S.; Doig, R.O. Biodegradable and biocompatible graphene-based scaffolds for functional neural tissue engineering: A strategy approach using dental pulp stem cells and biomaterials. Biotechnol. Bioeng. 2021, 118, 4217–4230. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Ruan, J.; Song, H.; Zhang, J.; Wo, Y.; Guo, S.; Cui, D. Biocompatibility of Graphene Oxide. Nanoscale Res. Lett. 2010, 6, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Wan, J.; Zhang, S.; Zhang, Y.; Lee, S.-T.; Liu, Z. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano 2011, 5, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Gollavelli, G.; Ling, Y.-C. Multifunctional graphene as an in vitro and in vivo imaging probe. Biomaterials 2012, 33, 2532–2545. [Google Scholar] [CrossRef]
- Eltahlah, D.; Lynch, C.D.; Chadwick, B.L.; Blum, I.R.; Wilson, N.H. An update on the reasons for placement and replacement of direct restorations. J. Dent. 2018, 72, 1–7. [Google Scholar] [CrossRef]
- Wong, H.M.; Zhang, Y.Y.; Li, Q.L. An enamel-inspired bioactive material with multiscale structure and antibacterial adhesion property. Bioact. Mater. 2021, 7, 491–503. [Google Scholar] [CrossRef]
- Chadwick, B.; Treasure, E.; Dummer, P.; Dunstan, F.; Gilmour, A.; Jones, R.; Phillips, C.; Stevens, J.; Rees, J.; Richmond, S. Challenges with studies investigating longevity of dental restorations—a critique of a systematic review. J. Dent. 2001, 29, 155–161. [Google Scholar] [CrossRef]
- Fan, C.; Chu, L.; Rawls, H.R.; Norling, B.K.; Cardenas, H.L.; Whang, K. Development of an antimicrobial resin—A pilot study. Dent. Mater. 2011, 27, 322–328. [Google Scholar] [CrossRef]
- Malik, S.; Ruddock, F.M.; Dowling, A.H.; Byrne, K.; Schmitt, W.; Khalakhan, I.; Nemoto, Y.; Guo, H.; Shrestha, L.K.; Ariga, K.; et al. Graphene composites with dental and biomedical applicability. Beilstein J. Nanotechnol. 2018, 9, 801–808. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Yan, Z.; Duan, Y.; Zhang, J.; Liu, B. Improvement of the mechanical, tribological and antibacterial properties of glass ionomer cements by fluorinated graphene. Dent. Mater. 2018, 34, e115–e127. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, Q.; Peng, J.; Yang, X.; Yu, D.; Zhao, W. Antibacterial and mechanical properties of reduced graphene-silver nanoparticle nanocomposite modified glass ionomer cements. J. Dent. 2020, 96, 103332. [Google Scholar] [CrossRef]
- Dubey, N.; Rajan, S.S.; Bello, Y.D.; Min, K.-S.; Rosa, V. Graphene nanosheets to improve physico-mechanical properties of bioactive calcium silicate cements. Materials 2017, 10, 606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.; Jin, H.; Zhang, H.; Wu, L.; Chen, G.; Shao, H.; Wang, S.; He, X.; Zheng, S.; Cao, C.Y.; et al. Synergistic effects of graphene quantum dots and carbodiimide in promoting resin–dentin bond durability. Dent. Mater. 2021, 37, 1498–1510. [Google Scholar] [CrossRef]
- Bacali, C.; Badea, M.; Moldovan, M.; Sarosi, C.; Nastase, V.; Baldea, I.; Chiorean, R.S.; Constantiniuc, M. The influence of graphene in improvement of physico-mechanical properties in PMMA denture base resins. Materials 2019, 12, 2335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azevedo, L.; Antonaya-Martin, J.L.; Molinero-Mourelle, P.; Del Río-Highsmith, J. Improving PMMA resin using graphene oxide for a definitive prosthodontic rehabilitation—A clinical report. J. Clin. Exp. Dent. 2019, 11, e670–e674. [Google Scholar] [CrossRef] [PubMed]
- Paz, E.; Ballesteros, Y.; Abenojar, J.; del Real, J.C.; Dunne, N.J. Graphene oxide and graphene reinforced PMMA bone cements: Evaluation of thermal properties and biocompatibility. Materials 2019, 12, 3146. [Google Scholar] [CrossRef] [Green Version]
- Alshahrani, A.; Bin-Shuwaish, M.S.; Al-Hamdan, R.S.; Almohareb, T.; Maawadh, A.M.; Al Deeb, M.; Alhenaki, A.M.; Abduljabbar, T.; Vohra, F. Graphene oxide nanofiller based experimental dentine adhesive. A SEM/EDX, Micro-Raman and microtensile bond strength analysis. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020966936. [Google Scholar]
- Khan, A.A.; Al-Khureif, A.A.; Saadaldin, S.A.; Mohamed, B.A.; Musaibah, A.S.O.; Divakar, D.D.; Eldwakhly, E. Graphene oxide-based experimental silane primers enhance shear bond strength between resin composite and zirconia. Eur. J. Oral Sci. 2019, 127, 570–576. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Li, M.; Liu, Q.; Jia, Z.J.; Xu, X.C.; Cheng, Y.; Zheng, Y.F. Electrophoretic deposition of graphene oxide reinforced chitosan-hydroxyapatite nanocomposite coatings on Ti substrate. J. Mater. Sci.-Mater. Med. 2016, 27, 48. [Google Scholar] [CrossRef]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral Biofilms: Pathogens, matrix, and polymicrobial Interactions in Microenvironments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef]
- Klein, M.I.; Hwang, G.; Santos, P.H.S.; Campanella, O.H.; Koo, H. Streptococcus mutans-derived extracellular matrix in cariogenic oral biofilms. Front. Cell Infect. Microbiol. 2015, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Autio-Gold, J. The role of chlorhexidine in caries prevention. Oper. Dent. 2008, 33, 710–716. [Google Scholar] [CrossRef] [PubMed]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossù, M.; Ierardo, G.; Polimeni, A. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15, 89. [Google Scholar] [CrossRef] [PubMed]
- Nasim, I.; Rajesh Kumar, S.; Vishnupriya, V.; Jabin, Z. Cytotoxicity and antimicrobial analysis of silver and graphene oxide bio nanoparticles. Bioinformation 2020, 16, 831–836. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.; Kim, H.S.; Alam, K.; Ji, M.K.; Cho, H.S.; Lim, H.P. Direct-deposited graphene oxide on dental implants for antimicrobial activities and osteogenesis. Int. J. Nanomed. 2021, 16, 5745–5754. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Zhu, X.; Qi, Z.; Wang, C.; Mao, X.; Zhu, C.; He, Z.; Li, M.; Tang, Z. Killing dental pathogens using antibacterial graphene oxide. ACS Appl. Mater. Interfaces 2015, 7, 5605–5611. [Google Scholar] [CrossRef]
- Akram, Z.; Aati, S.; Clode, P.; Saunders, M.; Ngo, H.; Fawzy, A.S. Formulation of nanographene doped with nano silver modified dentin bonding agents with enhanced interfacial stability and antibiofilm properties. Dent. Mater. 2021, 38, 347–362. [Google Scholar] [CrossRef]
- Mao, M.; Zhang, W.; Huang, Z.; Huang, J.; Wang, J.; Li, W.; Gu, S. Graphene oxide-copper nanocomposites suppress cariogenic Streptococcus mutans biofilm formation. Int. J. Nanomed. 2021, 16, 7727–7739. [Google Scholar] [CrossRef]
- Kulshrestha, S.; Khan, S.; Meena, R.; Singh, B.R.; Khan, A.U. A graphene/zinc oxide nanocomposite film protects dental implant surfaces against cariogenic Streptococcus mutans. Biofouling 2014, 30, 1281–1294. [Google Scholar] [CrossRef]
- Zanni, E.; Chandraiahgari, C.R.; De Bellis, G.; Montereali, M.R.; Armiento, G.; Ballirano, P.; Polimeni, A.; Sarto, M.S.; Uccelletti, D. Zinc oxide nanorods-decorated graphene nanoplatelets: A promising antimicrobial agent against the cariogenic bacterium Streptococcus mutans. Nanomaterials 2016, 6, 179. [Google Scholar] [CrossRef]
- Dou, C.; Ding, N.; Luo, F.; Hou, T.; Cao, Z.; Bai, Y.; Liu, C.; Xu, J.; Dong, S. Graphene-Based MicroRNA Transfection blocks preosteoclast fusion to increase bone formation and vascularization. Adv. Sci. 2017, 5, 1700578. [Google Scholar] [CrossRef]
- Yin, D.; Li, Y.; Lin, H.; Guo, B.; Du, Y.; Li, X.; Jia, H.; Zhao, X.; Tang, J.; Zhang, L. Functional graphene oxide as a plasmid-based Stat3 siRNA carrier inhibits mouse malignant melanoma growth in vivo. Nanotechnology 2013, 24, 105102. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Stipp, R.N.; Chen, T.; Wu, S.Z.; Hu, T.; Duncan, M.J. Activity of Streptococcus mutans VicR is modulated by antisense RNA. J. Dent. Res. 2018, 97, 1477–1484. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Zhang, H.; Lei, L. Nanographene oxide with antisense vicR RNA reduced exopolysaccharide synthesis and biofilm aggregation for Streptococcus mutans. Dent. Mater. J. 2020, 39, 278–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gholibegloo, E.; Karbasi, A.; Pourhajibagher, M.; Chiniforush, N.; Ramazani, A.; Akbari, T.; Bahador, A.; Khoobi, M. Carnosine-graphene oxide conjugates decorated with hydroxyapatite as promising nanocarrier for ICG loading with enhanced antibacterial effects in photodynamic therapy against Streptococcus mutans. J. Photochem. Photobiol. B 2018, 181, 14–22. [Google Scholar] [CrossRef]
- Lee, S.M.; Yoo, K.H.; Yoon, S.Y.; Kim, I.R.; Park, B.S.; Son, W.S.; Ko, C.-C.; Son, S.-A.; Kim, Y.-I. Enamel anti-demineralization effect of orthodontic adhesive containing bioactive glass and graphene oxide: An in vitro study. Materials 2018, 11, 1728. [Google Scholar] [CrossRef] [Green Version]
- Nam, H.-J.; Kim, Y.-M.; Kwon, Y.H.; Kim, I.-R.; Park, B.-S.; Son, W.-S.; Lee, S.-M.; Kim, Y.-I. Enamel surface remineralization effect by fluorinated graphite and bioactive glass-containing orthodontic bonding resin. Materials 2019, 12, 1308. [Google Scholar] [CrossRef] [Green Version]
- Wu, R.; Zhao, Q.; Lu, S.; Fu, Y.; Yu, D.; Zhao, W. Inhibitory effect of reduced graphene oxide-silver nanocomposite on progression of artificial enamel caries. J. Appl. Oral Sci. 2018, 27, e20180042. [Google Scholar] [CrossRef] [Green Version]
- Son, S.A.; Kim, D.H.; Yoo, K.H.; Yoon, S.Y.; Kim, Y.I. Mesoporous bioactive glass combined with graphene oxide quantum dot as a new material for a new treatment option for dentin hypersensitivity. Nanomaterials 2020, 10, 621. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Weir, M.D.; Lei, L.; Liu, J.; Xu, H.H.K. Novel nanographene oxide-calcium phosphate cement inhibits Enterococcus faecalis biofilm and supports dental pulp stem cells. J. Orthop. Surg. Res. 2021, 16, 580. [Google Scholar] [CrossRef]
- Ioannidis, K.; Niazi, S.; Mylonas, P.; Mannocci, F.; Deb, S. The synthesis of nano silver-graphene oxide system and its efficacy against endodontic biofilms using a novel tooth model. Dent. Mater. 2019, 35, 1614–1629. [Google Scholar] [CrossRef] [PubMed]
- Akbari, T.; Pourhajibagher, M.; Hosseini, F.; Chiniforush, N.; Gholibegloo, E.; Khoobi, M.; Shahabi, S.; Bahador, A. The effect of indocyanine green loaded on a novel nanographene oxide for high performance of photodynamic therapy against Enterococcus faecalis. Photodiagnosis Photodyn. Ther. 2017, 20, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, M.; Akamine, A. The application of tissue engineering to regeneration of pulp and dentin in endodontics. J. Endod. 2005, 31, 711–718. [Google Scholar] [CrossRef] [Green Version]
- Nakashima, M.; Iohara, K.; Murakami, M.; Nakamura, H.; Sato, Y.; Ariji, Y.; Matsushita, K. Pulp regeneration by transplantation of dental pulp stem cells in pulpitis: A pilot clinical study. Stem Cell Res. Ther. 2017, 8, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, N.; Yin, S.; Lu, Y.; Zhang, W.; Jiang, X. Graphene oxide-coated porous titanium for pulp sealing: An antibacterial and dentino-inductive restorative material. J. Mater. Chem. B 2020, 8, 5606–5619. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Kim, I.-R.; Kim, Y.; Kim, D.-H.; Park, S.-B.; Park, B.-S.; Bae, M.-K.; Kim, Y.-I. The effect of mesoporous bioactive glass nanoparticles/graphene oxide composites on the differentiation and mineralization of human dental pulp stem cells. Nanomaterials 2020, 10, 620. [Google Scholar] [CrossRef] [Green Version]
- Di Carlo, R.; Zara, S.; Ventrella, A.; Siani, G.; Da Ros, T.; Iezzi, G.; Cataldi, A.; Fontana, A. Covalent decoration of cortical membranes with graphene oxide as a substrate for dental pulp stem cells. Nanomaterials 2019, 9, 604. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Qiao, S.; Zhang, X.; Li, Y.; Zhang, Y.; Wei, S.; Shi, J.; Lai, H. Graphene-reinforced titanium enhances soft tissue seal. Front. Bioeng. Biotechnol. 2021, 9, 665305. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Parker, S.; Chiniforush, N.; Bahador, A. Photoexcitation triggering via semiconductor graphene quantum dots by photochemical doping with curcumin versus perio-pathogens mixed biofilms. Photodiagnosis Photodyn. Ther. 2019, 28, 125–131. [Google Scholar] [CrossRef]
- Qin, W.; Wang, C.; Jiang, C.; Sun, J.; Yu, C.; Jiao, T. Graphene oxide enables the reosteogenesis of previously contaminated titanium in vitro. J. Dent. Res. 2020, 99, 922–929. [Google Scholar] [CrossRef]
- Zhou, Q.; Yang, P.; Li, X.; Liu, H.; Ge, S. Bioactivity of periodontal ligament stem cells on sodium titanate coated with graphene oxide. Sci. Rep. 2016, 6, 19343. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lozano, F.J.; Garcia-Bernal, D.; Aznar-Cervantes, S.; Ros-Roca, M.A.; Alguero, M.C.; Atucha, N.M.; Lozano-García, A.A.; Moraleda, J.M.; Cenis, J.L. Effects of composite films of silk fibroin and graphene oxide on the proliferation, cell viability and mesenchymal phenotype of periodontal ligament stem cells. J. Mater. Sci.-Mater. Med. 2014, 25, 2731–2741. [Google Scholar] [CrossRef] [PubMed]
- Miyaji, H.; Kato, A.; Takita, H.; Iwanaga, T.; Momose, T.; Ogawa, K.; Murakami, S.; Sugaya, T.; Kawanami, M.; Nishida, E. Graphene oxide scaffold accelerates cellular proliferative response and alveolar bone healing of tooth extraction socket. Int. J. Nanomed. 2016, 11, 2265–2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qian, W.; Qiu, J.; Liu, X. Minocycline hydrochloride-loaded graphene oxide films on implant abutments for peri-implantitis treatment in beagle dogs. J. Periodontol. 2020, 91, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Vera-Sanchez, M.; Aznar-Cervantes, S.; Jover, E.; Garcia-Bernal, D.; Onate-Sanchez, R.E.; Hernandez-Romero, D.; Moraleda, J.M.; Collado-González, M.; Rodríguez-Lozano, F.J.; Cenis, J.L. Silk-fibroin and graphene oxide composites promote human periodontal ligament stem cell spontaneous differentiation into osteo/cementoblast-like cells. Stem Cells Dev. 2016, 25, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, Z. Involvement of FAK/P38 signaling pathways in mediating the enhanced osteogenesis induced by nanographene oxide modification on titanium implant surface. Int. J. Nanomed. 2020, 15, 4659–4676. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, R.; Di Crescenzo, A.; Pilato, S.; Ventrella, A.; Piattelli, A.; Recinella, L.; Moraleda, J.M.; Collado-González, M.; Rodríguez-Lozano, F.J.; Cenis, J.L. Osteoblastic differentiation on graphene oxide-functionalized titanium surfaces: An in vitro study. Nanomaterials 2020, 10, 654. [Google Scholar] [CrossRef] [Green Version]
- Agarwalla, S.V.; Ellepola, K.; Silikas, N.; Castro Neto, A.H.; Seneviratne, C.J.; Rosa, V. Persistent inhibition of Candida albicans biofilm and hyphae growth on titanium by graphene nanocoating. Dent. Mater. 2021, 37, 370–377. [Google Scholar] [CrossRef]
- Jung, H.S.; Lee, T.; Kwon, I.K.; Kim, H.S.; Hahn, S.K.; Lee, C.S. Surface modification of multipass caliber-rolled Ti alloy with dexamethasone-loaded graphene for dental applications. ACS Appl. Mater. Interfaces 2015, 7, 9598–9607. [Google Scholar] [CrossRef]
- Shin, Y.C.; Bae, J.H.; Lee, J.H.; Raja, I.S.; Kang, M.S.; Kim, B.; Hong, S.W.; Huh, J.B.; Han, D.W. Enhanced osseointegration of dental implants with reduced graphene oxide coating. Biomater. Res. 2022, 26, 11. [Google Scholar] [CrossRef]

| Main Subject | Form of Graphene Materials | Method | Material Type | Role and Advantages | Ref |
|---|---|---|---|---|---|
| Dental Materials (Restorative Dentistry) | Graphene–Ag nanoparticles (G–AgNp) Graphene oxide (GO) | Adding G–AgNp to a PMMA auto-polymerizing resin GO sheets were infused into primer | PMMA auto-polymerizing resin Primer | Antibacterial activity, minimal toxicity, improved flexural properties. Enhance shear bond strength | [68] [88] |
| Endodontics | Graphene Oxide (GO) Graphene oxide (GO) | Nano-graphene oxide with antisense vicR RNA plasmid (GO–PEI–ASvicR). Graphene oxide (GO) adhesive | Plasmid Adhesive resin | Antibacterial (S. mutans) Shows comparable bond strength and durability of resin dentine bond. | [104] [87] |
| Periodontics | Graphene | Graphene Quantum Dot coupled with curcumin (GQD–Cur) | Photosensitizing agents | Downregulation of the biofilm genes expression | [119] |
| Implantology | Graphene oxide (GO) Graphene Reduced graphene oxide | Graphene oxide (GO) deposition (on a zirconia surface) Mg alloy with graphene nanoparticles (Gr) Reduced graphene oxide (rGO)-coated sandblasted | Direct-deposited graphene oxide on dental implants Coated on dental implants Coated on dental implants | Inhibited the attachment of S. mutans and stimulated proliferation and differentiation of osteoblasts. High cytocompatibility and superior osteogenic properties Accelerate the healing rate with the high potential of osseointegration. | [95] [88] [130] |
| Tissue engineering | Graphene oxide (GO) | GO dental materials | A rat model of a non-critical mandibular defect. | Bone regeneration and biocompatibility | [70] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Tan, D.; Chen, X.; Liao, J.; Wu, L. Research on Graphene and Its Derivatives in Oral Disease Treatment. Int. J. Mol. Sci. 2022, 23, 4737. https://doi.org/10.3390/ijms23094737
Liu C, Tan D, Chen X, Liao J, Wu L. Research on Graphene and Its Derivatives in Oral Disease Treatment. International Journal of Molecular Sciences. 2022; 23(9):4737. https://doi.org/10.3390/ijms23094737
Chicago/Turabian StyleLiu, Chengcheng, Dan Tan, Xiaoli Chen, Jinfeng Liao, and Leng Wu. 2022. "Research on Graphene and Its Derivatives in Oral Disease Treatment" International Journal of Molecular Sciences 23, no. 9: 4737. https://doi.org/10.3390/ijms23094737
APA StyleLiu, C., Tan, D., Chen, X., Liao, J., & Wu, L. (2022). Research on Graphene and Its Derivatives in Oral Disease Treatment. International Journal of Molecular Sciences, 23(9), 4737. https://doi.org/10.3390/ijms23094737







