Heme Oxygenase-1 Has a Greater Effect on Melanoma Stem Cell Properties Than the Expression of Melanoma-Initiating Cell Markers
Abstract
:1. Introduction
2. Results
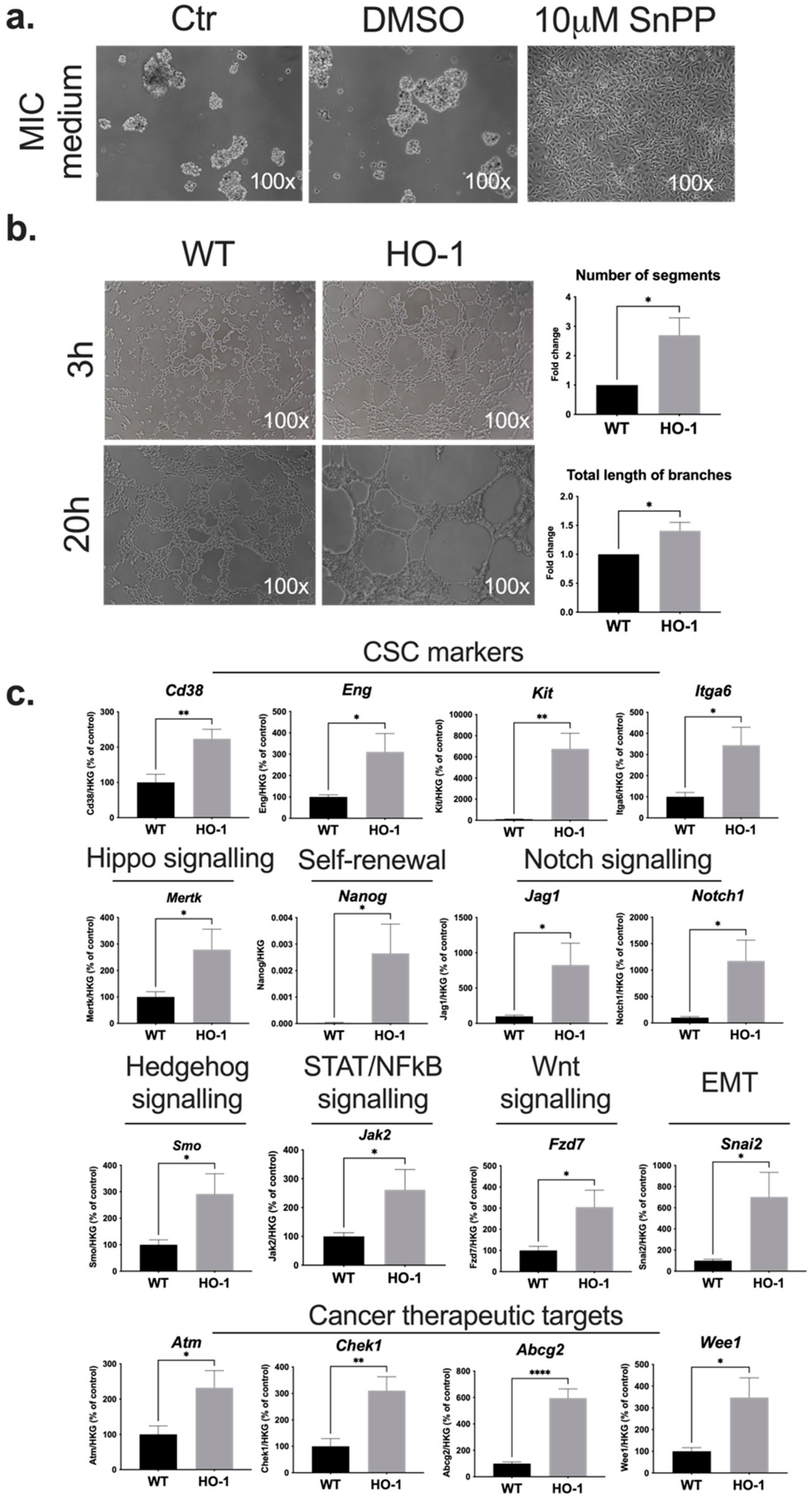
2.1. HO-1 Affects Non-Adherent Growth, Vasculogenic Mimicry, and Expression of CSC-Associated Genes in B16-F10 Melanoma Cells
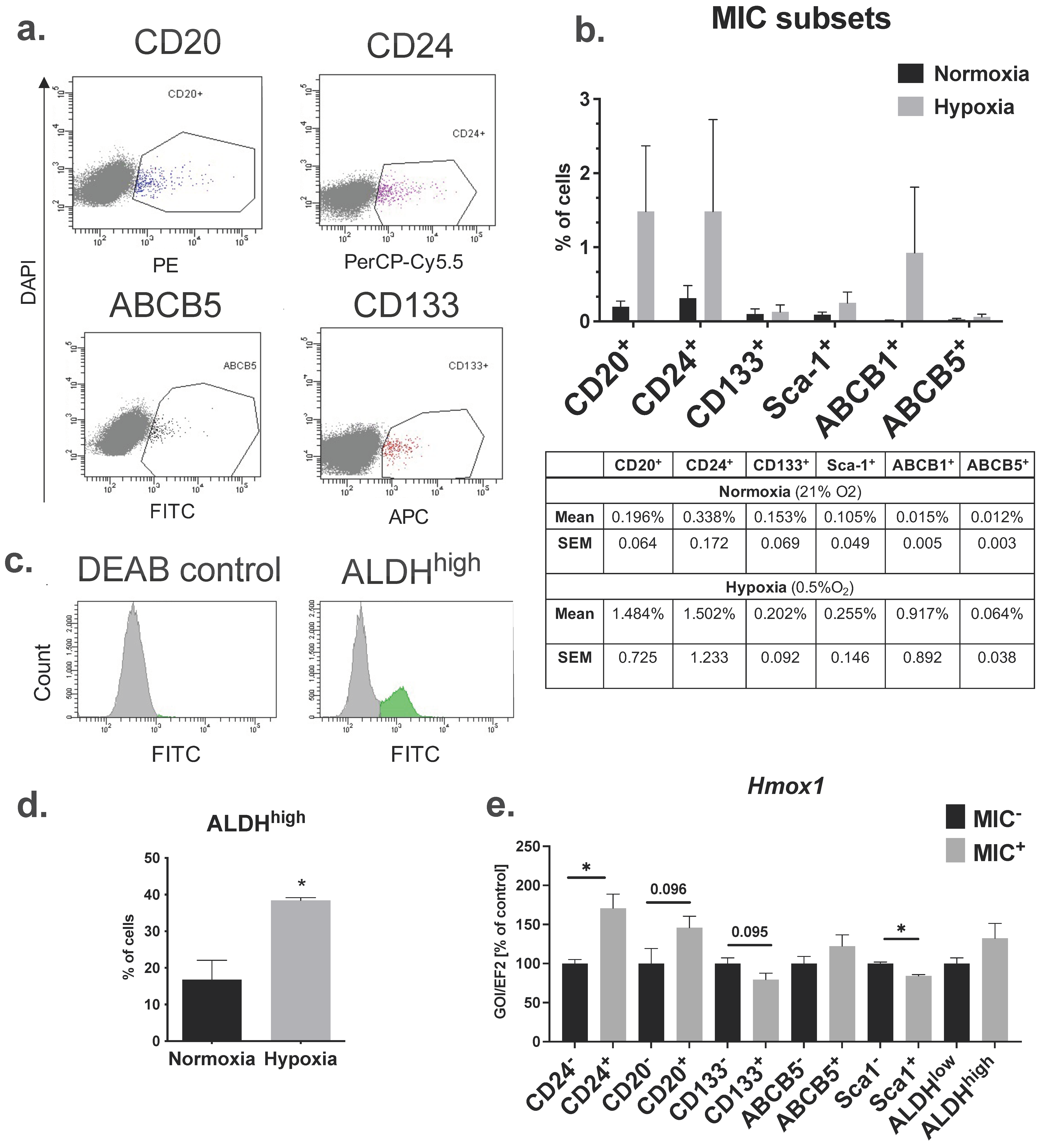
2.2. B16-F10 Cell Line Contains Cell Fractions That Express Cell Surface and Functional MIC Markers
2.3. Overexpression of HO-1 Decreases the Clonogenic Potential of Melanoma Cells In Vitro
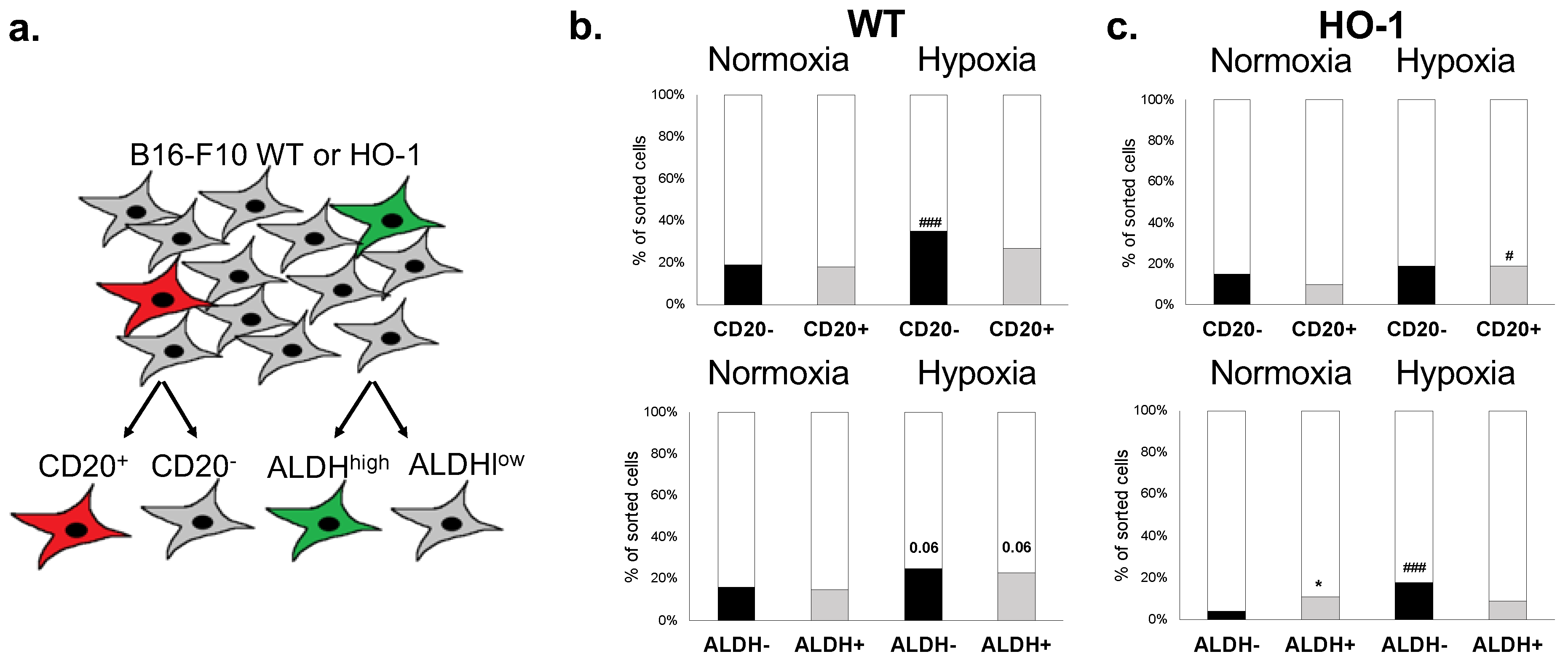
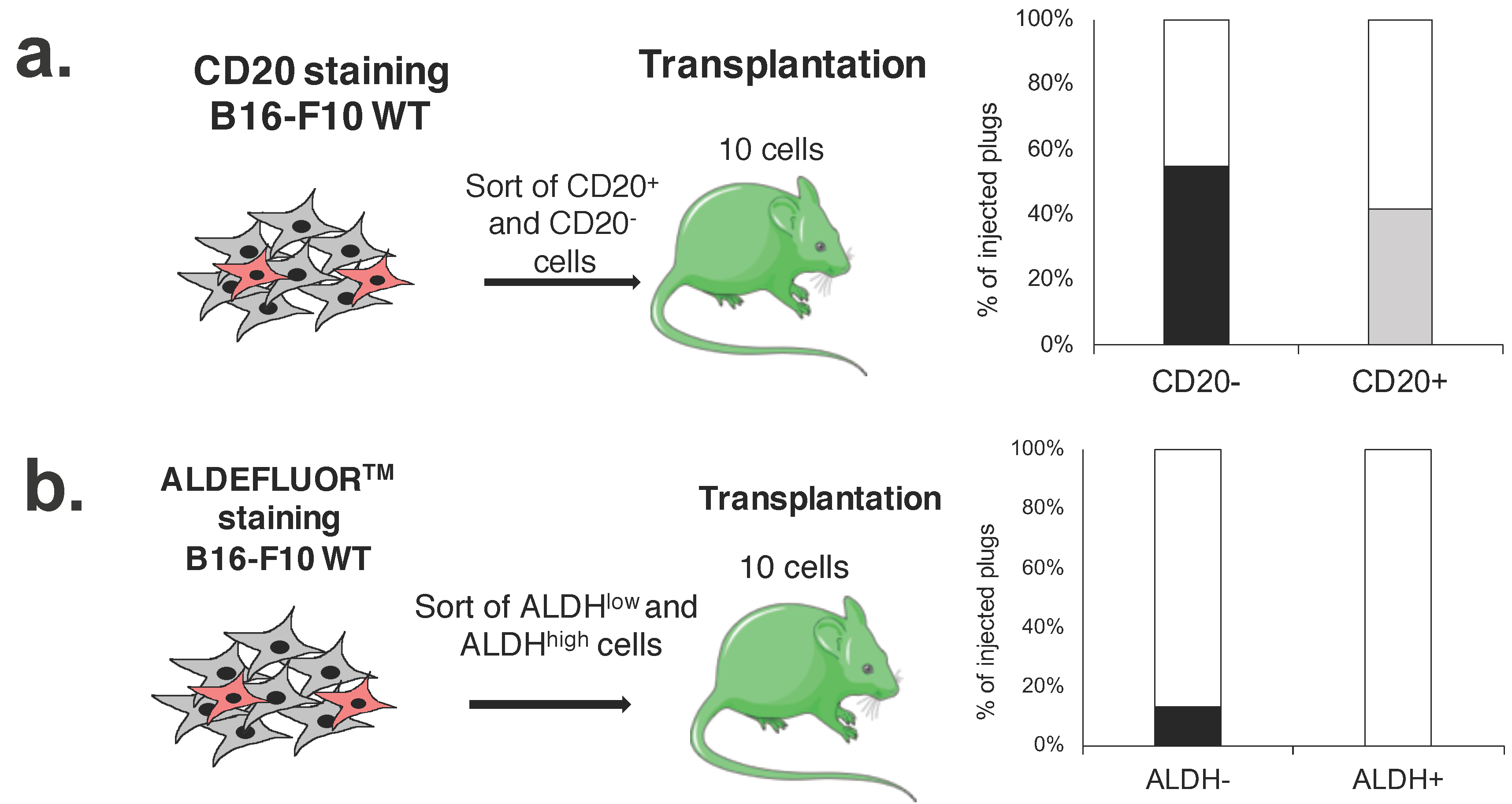
2.4. CD20 Expression and High ALDH Activity Do Not Affect the Clonogenic Potential of Murine Melanoma Cells
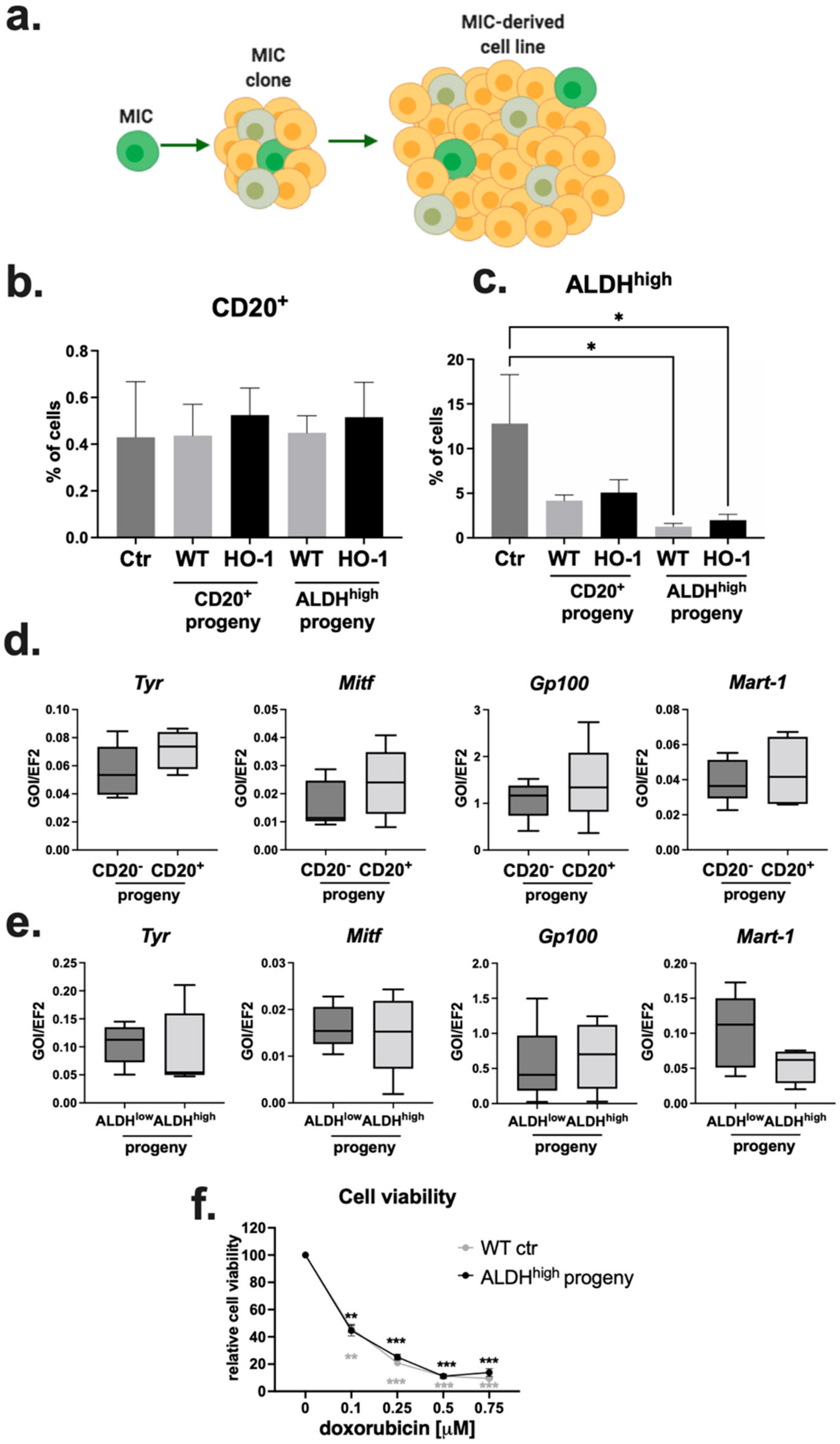
2.5. Progeny of MIC+ Cells Reconstitute the Heterogeneity of the Parental Cell Line
2.6. CD20+ and ALDHhigh Fractions Are Not Enriched in Tumorigenic Cells
2.7. Overexpression of HO-1 Enhances the Survival of Melanoma Cells but Decreases Their Self-Renewal and Tumorigenicity in Serial Transplantation Assay
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Generation of Stable B16-F10 Luc HO-1 Cell Line
4.3. Animals
4.4. Detection of Cell Surface Markers Using Flow Cytometry
4.5. ALDH Activity Assay
4.6. Quantitative RT Polymerase Chain Reaction (qRT-PCR)
4.7. AmpliGrid Pre-Amplification System for Gene Expression Analysis from a Limited Number of Cells
4.8. RT2 Profiler PCR Array
4.9. In Vitro Matrigel-Based Tube Formation Assay
4.10. MTT Assay
4.11. Clonogenicity Test and Obtaining Cell Lines from Single Clones
4.12. Soft Agar Assay
4.13. In Vivo Injection of Cells
4.14. In Vivo Serial Transplantations
4.15. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cancer Stat Facts: Melanoma of the Skin. Available online: https://seer.cancer.gov/statfacts/html/melan.html (accessed on 14 March 2022).
- Ma, Y.; Xia, R.; Ma, X.; Judson-Torres, R.L.; Zeng, H. Mucosal Melanoma: Pathological Evolution, Pathway Dependency and Targeted Therapy. Front. Oncol. 2021, 11, 702287. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, N.; Della Corte, M.; Pelaia, C.; Piazzetta, G.; Lobello, N.; Del Duca, E.; Bennardo, L.; Nisticò, S.P. Primary Mucosal Melanoma Presenting with a Unilateral Nasal Obstruction of the Left Inferior Turbinate. Medicina 2021, 57, 359. [Google Scholar] [CrossRef] [PubMed]
- Kozar, I.; Margue, C.; Rothengatter, S.; Haan, C.; Kreis, S. Many ways to resistance: How melanoma cells evade targeted therapies. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Andor, N.; Graham, T.A.; Jansen, M.; Xia, L.C.; Aktipis, C.A.; Petritsch, C.; Ji, H.P.; Maley, C.C. Pan-cancer analysis of the extent and consequences of intratumor heterogeneity. Nat. Med. 2016, 22, 105–113. [Google Scholar] [CrossRef]
- Clarke, M.F.; Dick, J.E.; Dirks, P.B.; Eaves, C.J.; Jamieson, C.H.M.; Jones, D.L.; Visvader, J.; Weissman, I.L.; Wahl, G.M. Cancer Stem Cells—Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006, 66, 9339–9344. [Google Scholar] [CrossRef] [Green Version]
- Clarke, M.F.; Fuller, M.T. Stem Cells and Cancer: Two Faces of Eve. Cell 2006, 124, 1111–1115. [Google Scholar] [CrossRef] [Green Version]
- Afify, S.M.; Seno, M. Conversion of Stem Cells to Cancer Stem Cells: Undercurrent of Cancer Initiation. Cancers 2019, 11, 345. [Google Scholar] [CrossRef] [Green Version]
- Friedmann-Morvinski, D.; Verma, I.M. Dedifferentiation and reprogramming: Origins of cancer stem cells. EMBO Rep. 2014, 15, 244–253. [Google Scholar] [CrossRef] [Green Version]
- Fang, D.; Nguyen, T.K.; Leishear, K.; Finko, R.; Kulp, A.N.; Hotz, S.; Van Belle, P.A.; Xu, X.; Elder, D.E.; Herlyn, M. A Tumorigenic Subpopulation with Stem Cell Properties in Melanomas. Cancer Res. 2005, 65, 9328–9337. [Google Scholar] [CrossRef] [Green Version]
- Frank, N.Y.; Margaryan, A.; Huang, Y.; Schatton, T.; Waaga-Gasser, A.M.; Gasser, M.; Sayegh, M.H.; Sadee, W.; Frank, M. ABCB5-Mediated Doxorubicin Transport and Chemoresistance in Human Malignant Melanoma. Cancer Res. 2005, 65, 4320–4333. [Google Scholar] [CrossRef] [Green Version]
- Schatton, T.; Murphy, G.F.; Frank, N.Y.; Yamaura, K.; Waaga-Gasser, A.M.; Gasser, M.; Zhan, Q.; Jordan, S.; Duncan, L.M.; Weishaupt, C.; et al. Identification of cells initiating human melanomas. Nature 2008, 451, 345–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, B.J.; Saab, K.R.; Ma, J.; Schatton, T.; Pütz, P.; Zhan, Q.; Murphy, G.F.; Gasser, M.; Waaga-Gasser, A.M.; Frank, N.Y.; et al. ABCB5 Maintains Melanoma-Initiating Cells through a Proinflammatory Cytokine Signaling Circuit. Cancer Res. 2014, 74, 4196–4207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monzani, E.; Facchetti, F.; Galmozzi, E.; Corsini, E.; Benetti, A.; Cavazzin, C.; Gritti, A.; Piccinini, A.; Porro, D.; Santinami, M.; et al. Melanoma contains CD133 and ABCG2 positive cells with enhanced tumourigenic potential. Eur. J. Cancer 2007, 43, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Rappa, G.; Fodstad, O.; Lorico, A. The Stem Cell-Associated Antigen CD133 (Prominin-1) Is a Molecular Therapeutic Target for Metastatic Melanoma. Stem Cells 2008, 26, 3008–3017. [Google Scholar] [CrossRef] [Green Version]
- Boiko, A.D.; Razorenova, O.V.; van de Rijn, M.; Swetter, S.M.; Johnson, D.L.; Ly, D.P.; Butler, P.D.; Yang, G.P.; Joshua, B.; Kaplan, M.J.; et al. Human melanoma-initiating cells express neural crest nerve growth factor receptor CD271. Nat. Cell Biol. 2010, 466, 133–137. [Google Scholar] [CrossRef]
- Civenni, G.; Walter, A.; Kobert, N.; Mihic-Probst, D.; Zipser, M.; Belloni, B.; Seifert, B.; Moch, H.; Dummer, R.; Broek, M.V.D.; et al. Human CD271-Positive Melanoma Stem Cells Associated with Metastasis Establish Tumor Heterogeneity and Long-term Growth. Cancer Res. 2011, 71, 3098–3109. [Google Scholar] [CrossRef] [Green Version]
- Restivo, G.; Diener, J.; Cheng, P.; Kiowski, G.; Bonalli, M.; Biedermann, T.; Reichmann, E.; Levesque, M.P.; Dummer, R.; Sommer, L. The low affinity neurotrophin receptor CD271 regulates phenotype switching in melanoma. Nat. Commun. 2017, 8, 1988. [Google Scholar] [CrossRef] [Green Version]
- Radke, J.; Roßner, F.; Redmer, T. CD271 determines migratory properties of melanoma cells. Sci. Rep. 2017, 7, 9834. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Dallaglio, K.; Chen, Y.; Robinson, W.A.; Robinson, S.E.; McCarter, M.D.; Wang, J.; Gonzalez, R.; Thompson, D.C.; Norris, D.A.; et al. ALDH1A Isozymes are Markers of Human Melanoma Stem Cells and Potential Therapeutic Targets. Stem Cells 2012, 30, 2100–2113. [Google Scholar] [CrossRef] [Green Version]
- Wouters, J.; Stas, M.; Gremeaux, L.; Govaere, O.; Broeck, A.V.D.; Maes, H.; Agostinis, P.; Roskams, T.; Oord, J.J.V.D.; Vankelecom, H. The Human Melanoma Side Population Displays Molecular and Functional Characteristics of Enriched Chemoresistance and Tumorigenesis. PLoS ONE 2013, 8, e76550. [Google Scholar] [CrossRef] [Green Version]
- Roesch, A.; Fukunaga-Kalabis, M.; Schmidt, E.C.; Zabierowski, S.E.; Brafford, P.A.; Vultur, A.; Basu, D.; Gimotty, P.; Vogt, T.; Herlyn, M. A Temporarily Distinct Subpopulation of Slow-Cycling Melanoma Cells Is Required for Continuous Tumor Growth. Cell 2010, 141, 583–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puig, I.; Tenbaum, S.P.; Chicote, I.; Arqués, O.; Martínez-Quintanilla, J.; Cuesta-Borrás, E.; Ramírez, L.; Gonzalo, P.; Soto, A.; Aguilar, S.; et al. TET2 controls chemoresistant slow-cycling cancer cell survival and tumor recurrence. J. Clin. Investig. 2018, 128, 3887–3905. [Google Scholar] [CrossRef] [PubMed]
- Perego, M.; Maurer, M.; Wang, J.X.; Shaffer, S.; Müller, A.C.; Parapatics, K.; Li, L.; Hristova, D.; Shin, S.; Keeney, F.; et al. A slow-cycling subpopulation of melanoma cells with highly invasive properties. Oncogene 2017, 37, 302–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana, E.; Shackleton, M.; Sabel, M.S.; Fullen, D.R.; Johnson, T.M.; Morrison, S.J. Efficient tumour formation by single human melanoma cells. Nature 2008, 456, 593–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quintana, E.; Shackleton, M.; Foster, H.R.; Fullen, D.R.; Sabel, M.S.; Johnson, T.M.; Morrison, S.J. Phenotypic Heterogeneity among Tumorigenic Melanoma Cells from Patients that Is Reversible and Not Hierarchically Organized. Cancer Cell 2010, 18, 510–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grasso, C.; Anaka, M.; Hofmann, O.; Sompallae, R.; Broadley, K.; Hide, W.; Berridge, M.V.; Cebon, J.; Behren, A.; McConnell, M.J. Iterative sorting reveals CD133+ and CD133− melanoma cells as phenotypically distinct populations. BMC Cancer 2016, 16, 726. [Google Scholar] [CrossRef] [Green Version]
- Boyle, S.E.; Fedele, C.G.; Corbin, V.; Wybacz, E.; Szeto, P.; Lewin, J.; Young, R.J.; Wong, A.; Fuller, R.; Spillane, J.; et al. CD271 Expression on Patient Melanoma Cells Is Unstable and Unlinked to Tumorigenicity. Cancer Res. 2016, 76, 3965–3977. [Google Scholar] [CrossRef] [Green Version]
- Prasmickaite, L.; Engesæter, B.; Skrbo, N.; Hellenes, T.; Kristian, A.; Oliver, N.K.; Suo, Z.; Mælandsmo, G.M. Aldehyde Dehydrogenase (ALDH) Activity Does Not Select for Cells with Enhanced Aggressive Properties in Malignant Melanoma. PLoS ONE 2010, 5, e10731. [Google Scholar] [CrossRef]
- Girouard, S.D.; Murphy, G.F. Melanoma stem cells: Not rare, but well done. Lab. Investig. 2011, 91, 647–664. [Google Scholar] [CrossRef] [Green Version]
- Dulak, J.; Jozkowicz, A. Heme Oxygenase-1 in Tumor Biology and Therapy. Curr. Drug Targets 2010, 11, 1551–1570. [Google Scholar] [CrossRef]
- Goodman, A.I.; Choudhury, M.; Da Silva, J.-L.; Schwartzman, M.L.; Abraham, N.G. Overexpression of the Heme Oxygenase Gene in Renal Cell Carcinoma. Exp. Biol. Med. 1997, 214, 54–75. [Google Scholar] [CrossRef]
- Maines, M.D.; Abrahamsson, P.-A. Expression of heme oxygenase-1 (HSP32) in human prostate: Normal, hyperplastic, and tumor tissue distribution. Urology 1996, 47, 727–733. [Google Scholar] [CrossRef]
- Miyake, M.; Ishii, M.; Kawashima, K.; Kodama, T.; Sugano, K.; Fujimoto, K.; Hirao, Y. siRNA-mediated Knockdown of the Heme Synthesis and Degradation Pathways: Modulation of Treatment Effect of 5-Aminolevulinic Acid-based Photodynamic Therapy in Urothelial Cancer Cell Lines. Photochem. Photobiol. 2009, 85, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Mayerhofer, M.; Florian, S.; Krauth, M.-T.; Aichberger, K.J.; Bilban, M.; Marculescu, R.; Printz, D.; Fritsch, G.; Wagner, O.; Selzer, E.; et al. Identification of Heme Oxygenase-1 As a Novel BCR/ABL-Dependent Survival Factor in Chronic Myeloid Leukemia. Cancer Res. 2004, 64, 3148–3154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciesla, M.; Marona, P.; Kozakowska, M.; Jez, M.; Seczynska, M.; Loboda, A.; Bukowska-Strakova, K.; Szade, A.; Walawender, M.; Kusior, M.; et al. Heme Oxygenase-1 Controls an HDAC4-miR-206 Pathway of Oxidative Stress in Rhabdomyosarcoma. Cancer Res. 2016, 76, 5707–5718. [Google Scholar] [CrossRef] [Green Version]
- Podkalicka, P.; Mucha, O.; Józkowicz, A.; Dulak, J.; Łoboda, A. Heme oxygenase inhibition in cancers: Possible tools and targets. Contemp. Oncol. 2018, 22, 23–32. [Google Scholar] [CrossRef]
- Okamoto, I.; Krögler, J.; Endler, G.; Kaufmann, S.; Mustafa, S.; Exner, M.; Mannhalter, C.; Wagner, O.; Pehamberger, H. A microsatellite polymorphism in theheme oxygenase-1 gene promoter is associated with risk for melanoma. Int. J. Cancer 2006, 119, 1312–1315. [Google Scholar] [CrossRef]
- Was, H.; Cichoń, T.; Smolarczyk, R.; Rudnicka, D.; Stopa, M.; Chevalier, C.; Leger, J.J.; Lackowska, B.; Grochot-Przeczek, A.; Bojkowska, K.; et al. Overexpression of Heme Oxygenase-1 in Murine Melanoma: Increased Proliferation and Viability of Tumor Cells, Decreased Survival of Mice. Am. J. Pathol. 2006, 169, 2181–2198. [Google Scholar] [CrossRef] [Green Version]
- Barbagallo, I.; Parenti, R.; Zappalà, A.; Vanella, L.; Tibullo, D.; Pepe, F.; Onni, T.; Volti, G.L. Combined inhibition of Hsp90 and heme oxygenase-1 induces apoptosis and endoplasmic reticulum stress in melanoma. Acta Histochem. 2015, 117, 705–711. [Google Scholar] [CrossRef]
- Nowis, D.; Legat, M.; Grzela, T.; Niderla, J.; Wilczek, E.; Wilczynski, G.; Głodkowska, E.; Mrówka, P.; Issat, T.; Dulak, J.; et al. Heme oxygenase-1 protects tumor cells against photodynamic therapy-mediated cytotoxicity. Oncogene 2006, 25, 3365–3374. [Google Scholar] [CrossRef] [Green Version]
- Tampa, M.; Sarbu, M.-I.; Matei, C.; Mitran, C.-I.; Mitran, M.-I.; Caruntu, C.; Constantin, C.; Neagu, M.; Georgescu, S.-R. Photodynamic therapy: A hot topic in dermato-oncology (Review). Oncol. Lett. 2019, 17, 4085–4093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivanov, V.N.; Hei, T.K. Regulation of apoptosis in human melanoma and neuroblastoma cells by statins, sodium arsenite and TRAIL: A role of combined treatment versus monotherapy. Apoptosis 2011, 16, 1268–1284. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, A.L.; Ottonello, S.; Loi, G.; Cossu, I.; Piras, S.; Spagnolo, F.; Queirolo, P.; Marinari, U.M.; Moretta, L.; Pronzato, M.A.; et al. HO-1 downregulation favors BRAF V600 melanoma cell death induced by Vemurafenib/PLX4032 and increases NK recognition. Int. J. Cancer 2019, 146, 1950–1962. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Wu, Y.; Bian, C.; Nisar, M.F.; Wang, M.; Hu, X.; Diao, Q.; Nian, W.; Wang, E.; Xu, W.; et al. Heme oxygenase 1 facilitates cell proliferation via the B-Raf-ERK signaling pathway in melanoma. Cell Commun. Signal. 2019, 17, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Was, H.; Cichon, T.; Smolarczyk, R.; Lackowska, B.; Mazur-Bialy, A.; Mazur, M.; Szade, A.; Dominik, P.; Mazan, M.; Kotlinowski, J.; et al. Effect of Heme Oxygenase-1 on Melanoma Development in Mice—Role of Tumor-Infiltrating Immune Cells. Antioxidants 2020, 9, 1223. [Google Scholar] [CrossRef]
- Franco, S.S.; Szczesna, K.; Iliou, M.S.; Al-Qahtani, M.; Mobasheri, A.; Kobolák, J.; Dinnyés, A. In vitro models of cancer stem cells and clinical applications. BMC Cancer 2016, 16, 23–49. [Google Scholar] [CrossRef] [Green Version]
- Stecca, B.; Santini, R.; Pandolfi, S.; Penachioni, J.Y. Culture and Isolation of Melanoma-Initiating Cells. Curr. Protoc. Stem Cell Biol. 2013, 24, 3.6.1–3.6.12. [Google Scholar] [CrossRef]
- Frank, N.Y.; Schatton, T.; Kim, S.; Zhan, Q.; Wilson, B.J.; Ma, J.; Saab, K.R.; Osherov, V.; Widlund, H.; Gasser, M.; et al. VEGFR-1 Expressed by Malignant Melanoma-Initiating Cells Is Required for Tumor Growth. Cancer Res. 2011, 71, 1474–1485. [Google Scholar] [CrossRef] [Green Version]
- Iii, R.A.F.; Faibish, M.; Shao, R. A Matrigel-Based Tube Formation Assay to Assess the Vasculogenic Activity of Tumor Cells. J. Vis. Exp. 2011, e3040. [Google Scholar] [CrossRef]
- Widmer, D.S.; Hoek, K.S.; Cheng, P.; Eichhoff, O.M.; Biedermann, T.; Raaijmakers, M.I.; Hemmi, S.; Dummer, R.; Levesque, M.P. Hypoxia Contributes to Melanoma Heterogeneity by Triggering HIF1α-Dependent Phenotype Switching. J. Investig. Dermatol. 2013, 133, 2436–2443. [Google Scholar] [CrossRef] [Green Version]
- Cheli, Y.; Guiliano, S.; Botton, T.; Rocchi, S.; Hofman, V.; Hofman, P.; Bahadoran, P.; Bertolotto, C.; Ballotti, R. Mitf is the key molecular switch between mouse or human melanoma initiating cells and their differentiated progeny. Oncogene 2011, 30, 2307–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, D.W.; Palle, K. Aldehyde dehydrogenases in cancer stem cells: Potential as therapeutic targets. Ann. Transl. Med. 2016, 4, 518. [Google Scholar] [CrossRef] [PubMed]
- Alison, M.R.; Lim, S.M.L.; Nicholson, L.J. Cancer stem cells: Problems for therapy? J. Pathol. 2010, 223, 148–162. [Google Scholar] [CrossRef]
- Kuch, V.; Schreiber, C.; Thiele, W.; Umansky, V.; Sleeman, J.P. Tumor-initiating properties of breast cancer and melanoma cells in vivo are not invariably reflected by spheroid formation in vitro, but can be increased by long-term culturing as adherent monolayers. Int. J. Cancer 2012, 132, E94–E105. [Google Scholar] [CrossRef]
- Zhao, F.; He, X.; Sun, J.; Wu, D.; Pan, M.; Li, M.; Wu, S.; Zhang, R.; Yan, C.; Dou, J. Cancer stem cell vaccine expressing ESAT-6-gpi and IL-21 inhibits melanoma growth and metastases. Am. J. Transl. Res. 2015, 7, 1870–1882. [Google Scholar]
- Klimkiewicz, K.; Weglarczyk, K.; Collet, G.; Paprocka, M.; Guichard, A.; Sarna, M.; Jozkowicz, A.; Dulak, J.; Sarna, T.; Grillon, C.; et al. A 3D model of tumour angiogenic microenvironment to monitor hypoxia effects on cell interactions and cancer stem cell selection. Cancer Lett. 2017, 396, 10–20. [Google Scholar] [CrossRef]
- Sztiller-Sikorska, M.; Hartman, M.; Talar, B.; Jakubowska, J.; Zalesna, I.; Czyz, M. Phenotypic diversity of patient-derived melanoma populations in stem cell medium. Lab. Investig. 2015, 95, 672–683. [Google Scholar] [CrossRef] [Green Version]
- Opdenaker, L.M.; Modarai, S.; Boman, B.M. The Proportion of ALDEFLUOR-Positive Cancer Stem Cells Changes with Cell Culture Density Due to the Expression of Different ALDH Isoforms. Cancer Stud. Mol. Med. Open J. 2015, 2, 87–95. [Google Scholar] [CrossRef]
- Held, M.A.; Curley, D.P.; Dankort, D.; McMahon, M.; Muthusamy, V.; Bosenberg, M.W. Characterization of Melanoma Cells Capable of Propagating Tumors from a Single Cell. Cancer Res. 2010, 70, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Prasmickaite, L.; Skrbo, N.; Høifødt, H.K.; Suo, Z.; Engebråten, O.; Gullestad, H.P.; Aamdal, S.; Fodstad, Ø.; Maelandsmo, G.M.; Mælandsmo, G.M. Human malignant melanoma harbours a large fraction of highly clonogenic cells that do not express markers associated with cancer stem cells. Pigment Cell Melanoma Res. 2010, 23, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-H.; Yoon, H.-J.; Cha, Y.-N.; Surh, Y.-J. Role of heme oxygenase-1 and its reaction product, carbon monoxide, in manifestation of breast cancer stem cell-like properties: Notch-1 as a putative target. Free Radic. Res. 2018, 52, 1336–1347. [Google Scholar] [CrossRef] [PubMed]
- Jasmer, K.J.; Hou, J.; Mannino, P.; Cheng, J.; Hannink, M. Heme oxygenase promotes B-Raf-dependent melanosphere formation. Pigment Cell Melanoma Res. 2020, 33, 850–868. [Google Scholar] [CrossRef] [PubMed]
- Jue, C.; Lin, C.; Zhisheng, Z.; Yayun, Q.; Feng, J.; Min, Z.; Haibo, W.; Youyang, S.; Hisamitsu, T.; Shintaro, I.; et al. Notch1 promotes vasculogenic mimicry in hepatocellular carcinoma by inducing EMT signaling. Oncotarget 2016, 8, 2501–2513. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Zhao, H.; Wang, F.; Wang, Y.; He, Y.; Wang, T.; Zhang, K.; Yang, H.; Zhou, Z.; Shi, H.; et al. A novel humanized Frizzled-7-targeting antibody enhances antitumor effects of Bevacizumab against triple-negative breast cancer via blocking Wnt/β-catenin signaling pathway. J. Exp. Clin. Cancer Res. 2021, 40, 30. [Google Scholar] [CrossRef]
- Hu, A.; Huang, J.-J.; Jin, X.-J.; Li, J.-P.; Tang, Y.-J.; Huang, X.-F.; Cui, H.-J.; Xu, W.-H.; Sun, G.-B. Curcumin Suppresses Invasiveness and Vasculogenic Mimicry of Squamous Cell Carcinoma of the Larynx through the Inhibition of JAK-2/STAT-3 Signaling Pathway. Am. J. Cancer Res. 2015, 5, 278–288. [Google Scholar]
- Shin, C.H.; Ryu, S.; Kim, H.H. hnRNPK-regulated PTOV1-AS1 modulates heme oxygenase-1 expression via miR-1207-5p. BMB Rep. 2017, 50, 220–225. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.-S.; Hsu, Y.-C.; Lee, J.-J.; Chen, M.-J.; Huang, S.-Y.; Cheng, S.-P. Heme Oxygenase-1 Inhibitors Induce Cell Cycle Arrest and Suppress Tumor Growth in Thyroid Cancer Cells. Int. J. Mol. Sci. 2018, 19, 2502. [Google Scholar] [CrossRef] [Green Version]
- Podkalicka, P.; Mucha, O.; Kruczek, S.; Biela, A.; Andrysiak, K.; Stępniewski, J.; Mikulski, M.; Gałęzowski, M.; Sitarz, K.; Brzózka, K.; et al. Synthetically Lethal Interactions of Heme Oxygenase-1 and Fumarate Hydratase Genes. Biomolecules 2020, 10, 143. [Google Scholar] [CrossRef] [Green Version]
- Mucha, O.; Podkalicka, P.; Mikulski, M.; Barwacz, S.; Andrysiak, K.; Biela, A.; Mieczkowski, M.; Kachamakova-Trojanowska, N.; Ryszawy, D.; Białas, A.; et al. Development and characterization of a new inhibitor of heme oxygenase activity for cancer treatment. Arch. Biochem. Biophys. 2019, 671, 130–142. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Z.; Yang, S.; Chen, H.; Wang, D.; Li, J. All-trans retinoic acid-encapsulated, CD20 antibody-conjugated poly(lactic-co-glycolic acid) nanoparticles effectively target and eliminate melanoma-initiating cells in vitro. OncoTargets Ther. 2018, 11, 6177–6187. [Google Scholar] [CrossRef] [Green Version]
- Flahaut, M.; Jauquier, N.; Chevalier, N.; Nardou, K.; Balmas Bourloud, K.; Joseph, J.-M.; Barras, D.; Widmann, C.; Gross, N.; Renella, R.; et al. Aldehyde dehydrogenase activity plays a Key role in the aggressive phenotype of neuroblastoma. BMC Cancer 2016, 16, 781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, C.; Sullivan, J.P.; Girard, L.; Augustyn, A.; Yenerall, P.; Rodriguez-Canales, J.; Liu, H.; Behrens, C.; Shay, J.W.; Wistuba, I.I.; et al. Essential Role of Aldehyde Dehydrogenase 1A3 for the Maintenance of Non–Small Cell Lung Cancer Stem Cells Is Associated with the STAT3 Pathway. Clin. Cancer Res. 2014, 20, 4154–4166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awad, O.; Yustein, J.T.; Shah, P.; Gul, N.; Katuri, V.; O′Neill, A.; Kong, Y.; Brown, M.L.; Toretsky, J.A.; Loeb, D.M. High ALDH Activity Identifies Chemotherapy-Resistant Ewing′s Sarcoma Stem Cells That Retain Sensitivity to EWS-FLI1 Inhibition. PLoS ONE 2010, 5, e13943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, N.; Couts, K.L.; Luo, Y.; Fujita, M. Understanding melanoma stem cells. Melanoma Manag. 2015, 2, 179–188. [Google Scholar] [CrossRef]
- Huang, S.-D.; Yuan, Y.; Tang, H.; Liu, X.-H.; Fu, C.-G.; Cheng, H.-Z.; Bi, J.-W.; Yu, Y.-W.; Gong, D.-J.; Zhang, W.; et al. Tumor Cells Positive and Negative for the Common Cancer Stem Cell Markers Are Capable of Initiating Tumor Growth and Generating Both Progenies. PLoS ONE 2013, 8, e54579. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, P.; Kopecky, C.; Hombach, A.; Zigrino, P.; Mauch, C.; Abken, H. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc. Natl. Acad. Sci. USA 2011, 108, 2474–2479. [Google Scholar] [CrossRef] [Green Version]
- Schlaak, M.; Schmidt, P.; Bangard, C.; Kurschat, P.; Mauch, C.; Abken, H. Regression of metastatic melanoma by targeting cancer stem cells. Oncotarget 2012, 3, 22–30. [Google Scholar] [CrossRef] [Green Version]
- Pinc, A.; Somasundaram, R.; Wagner, C.; Hörmann, M.; Karanikas, G.; Jalili, A.; Bauer, W.; Brunner, P.; Grabmeier-Pfistershammer, K.; Gschaider, M.; et al. Targeting CD20 in Melanoma Patients at High Risk of Disease Recurrence. Mol. Ther. 2012, 20, 1056–1062. [Google Scholar] [CrossRef] [Green Version]
- Boonyaratanakornkit, J.B.; Yue, L.; Strachan, L.R.; Scalapino, K.J.; LeBoit, P.E.; Lu, Y.; Leong, S.P.; Smith, J.E.; Ghadially, R. Selection of Tumorigenic Melanoma Cells Using ALDH. J. Investig. Dermatol. 2010, 130, 2799–2808. [Google Scholar] [CrossRef] [Green Version]
- Moroishi, T.; Hayashi, T.; Pan, W.-W.; Fujita, Y.; Holt, M.V.; Qin, J.; Carson, D.A.; Guan, K.-L. The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell 2016, 167, 1525–1539.e17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulesza, D.W.; Przanowski, P.; Kaminska, B. Knockdown of STAT3 targets a subpopulation of invasive melanoma stem-like cells. Cell Biol. Int. 2019, 43, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Donizy, P.; Pagacz, K.; Marczuk, J.; Fendler, W.; Maciejczyk, A.; Halon, A.; Matkowski, R. Upregulation of FOXP1 is a new independent unfavorable prognosticator and a specific predictor of lymphatic dissemination in cutaneous melanoma patients. OncoTargets Ther. 2018, 11, 1413–1422. [Google Scholar] [CrossRef] [Green Version]
- Abbaszadegan, M.R.; Bagheri, V.; Razavi, M.S.; Momtazi, A.A.; Sahebkar, A.; Gholamin, M. Isolation, identification, and characterization of cancer stem cells: A review. J. Cell. Physiol. 2017, 232, 2008–2018. [Google Scholar] [CrossRef] [PubMed]
- Barikbin, R.; Berkhout, L.; Bolik, J.; Schmidt-Arras, D.; Ernst, T.; Ittrich, H.; Adam, G.; Parplys, A.; Casar, C.; Krech, T.; et al. Early heme oxygenase 1 induction delays tumour initiation and enhances DNA damage repair in liver macrophages of Mdr2−/− mice. Sci. Rep. 2018, 8, 16238. [Google Scholar] [CrossRef]
- Was, H.; Sokolowska, M.; Sierpniowska, A.; Dominik, P.; Skrzypek, K.; Lackowska, B.; Pratnicki, A.; Grochot-Przeczek, A.; Taha, H.; Kotlinowski, J.; et al. Effects of heme oxygenase-1 on induction and development of chemically induced squamous cell carcinoma in mice. Free Radic. Biol. Med. 2011, 51, 1717–1726. [Google Scholar] [CrossRef] [Green Version]
- Florczyk-Soluch, U.; Józefczuk, E.; Stępniewski, J.; Bukowska-Strakova, K.; Mendel, M.; Viscardi, M.; Nowak, W.N.; Józkowicz, A.; Dulak, J. Various roles of heme oxygenase-1 in response of bone marrow macrophages to RANKL and in the early stage of osteoclastogenesis. Sci. Rep. 2018, 8, 10797. [Google Scholar] [CrossRef] [Green Version]
- Szade, K.; Zukowska, M.; Szade, A.; Collet, G.; Kloska, D.; Kieda, C.; Jozkowicz, A.; Dulak, J. Spheroid-plug model as a tool to study tumor development, angiogenesis, and heterogeneity in vivo. Tumor Biol. 2015, 37, 2481–2496. [Google Scholar] [CrossRef] [Green Version]
- Foresti, R.; Clark, J.E.; Green, C.J.; Motterlini, R. Thiol Compounds Interact with Nitric Oxide in Regulating Heme Oxygenase-1 Induction in Endothelial Cells. J. Biol. Chem. 1997, 272, 18411–18417. [Google Scholar]
- Mucha, O.; Podkalicka, P.; Czarnek, M.; Biela, A.; Mieczkowski, M.; Kachamakova-Trojanowskaa, N.; Stepniewski, J.; Jozkowicz, A.; Dulak, J.; Loboda, A. Pharmacological versus Genetic Inhibition of Heme Oxygenase-1—The Comparison of Metalloporphyrins, ShRNA and CRISPR/Cas9 System. Acta Biochim. Pol. 2018, 65, 277–286. [Google Scholar] [CrossRef] [Green Version]


| Antibody | Fluorochrome, Clone, Company | Dilution |
|---|---|---|
| ABCB1 | Mouse monoclonal [JSB-1] to p-glycoprotein, Abcam (Cambridge, UK) | 1:100 |
| ABCB5 | Rabbit polyclonal, Bioss (Woburn, MA, USA) | 1:100 |
| Sca-1 | PE/Cy7 Anti-mouse Ly6A/E, clone E13-161.7 BioLegend (San Diego, CA, USA) | 1:100 |
| CD20 | PE anti-mouse CD20, clone SA275A11, BioLegend (San Diego, CA, USA) | 1:100 |
| CD133 | APC anti-mouse CD133, clone 315-2C11, BioLegend (San Diego, CA, USA) | 1:100 |
| CD24 | PerCP-Cy5.5 Rat Anti-Mouse CD24, clone M1/69, BD Pharminogen (San Diego, CA, USA) | 1:100 |
| Goat anti-rabbit IgG | Alexa Fluor 488, A-11008, Invitrogen (Waltham, MA, USA) (ABCB5 staining) | 1:100 |
| Goat anti-mouse IgG | Alexa Fluor 488, A28175, Invitrogen (Waltham, MA, USA) (ABCB1 staining) | 1:100 |
| Primer | Sequence | Length of Product | Tm |
|---|---|---|---|
| Ef2 For | 5′ GACATCACCAAGGGTGTGCAG 3′ | 214 bp | 60 °C |
| Ef2 Rev | 5′ TCAGCACACTGGCATAGAGGC 3′ | ||
| Tyr For | 5′ GCCCAGCATCCTTCTTCTCC 3′ | 101 bp | 55 °C |
| Tyr Rev | 5′ TAGTGGTCCCTCAGGTGTTC 3′ | ||
| Gp100 For | 5′ ACCACTATGGGTGTCCAGAGA 3′ | 108 bp | 60 °C |
| Gp100 Rev | 5′ GACACCAAGCCAGTCCTGAT 3′ | ||
| Mitf For Mitf Rev | 5′ AGAGCAGGGCAGAGAGTGAGT 3′ 5′ CAGGAGTTGCTGATGGTAAGG 3′ | 238 bp | 65 °C |
| Hmox1 For Hmox1 Rev | 5′ GTGGAGACGCTTTACATAGTGC 3′ 5′ CTTTCAGAAGGGTCAGGTGTCC 3′ | 250 bp | 60 °C |
| Mart-1 For Mart-1 Rev | 5′ CAGTACCAGCAGCCGATAAGCA 3′ | 166 bp | 55 °C |
| 5′ GGGAAGGTGTCCTGTGCTGAGT 3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusienicka, A.; Bukowska-Strakova, K.; Cieśla, M.; Nowak, W.N.; Bronisz-Budzyńska, I.; Seretny, A.; Żukowska, M.; Jeż, M.; Krutyhołowa, R.; Taha, H.; et al. Heme Oxygenase-1 Has a Greater Effect on Melanoma Stem Cell Properties Than the Expression of Melanoma-Initiating Cell Markers. Int. J. Mol. Sci. 2022, 23, 3596. https://doi.org/10.3390/ijms23073596
Kusienicka A, Bukowska-Strakova K, Cieśla M, Nowak WN, Bronisz-Budzyńska I, Seretny A, Żukowska M, Jeż M, Krutyhołowa R, Taha H, et al. Heme Oxygenase-1 Has a Greater Effect on Melanoma Stem Cell Properties Than the Expression of Melanoma-Initiating Cell Markers. International Journal of Molecular Sciences. 2022; 23(7):3596. https://doi.org/10.3390/ijms23073596
Chicago/Turabian StyleKusienicka, Anna, Karolina Bukowska-Strakova, Maciej Cieśla, Witold Norbert Nowak, Iwona Bronisz-Budzyńska, Agnieszka Seretny, Monika Żukowska, Mateusz Jeż, Rościsław Krutyhołowa, Hevidar Taha, and et al. 2022. "Heme Oxygenase-1 Has a Greater Effect on Melanoma Stem Cell Properties Than the Expression of Melanoma-Initiating Cell Markers" International Journal of Molecular Sciences 23, no. 7: 3596. https://doi.org/10.3390/ijms23073596
APA StyleKusienicka, A., Bukowska-Strakova, K., Cieśla, M., Nowak, W. N., Bronisz-Budzyńska, I., Seretny, A., Żukowska, M., Jeż, M., Krutyhołowa, R., Taha, H., Kachamakova-Trojanowska, N., Waś, H., Kieda, C., & Józkowicz, A. (2022). Heme Oxygenase-1 Has a Greater Effect on Melanoma Stem Cell Properties Than the Expression of Melanoma-Initiating Cell Markers. International Journal of Molecular Sciences, 23(7), 3596. https://doi.org/10.3390/ijms23073596








