Mesenchymal Stem-Cell Remodeling of Adsorbed Type-I Collagen—The Effect of Collagen Oxidation
Abstract
:1. Introduction
2. Results
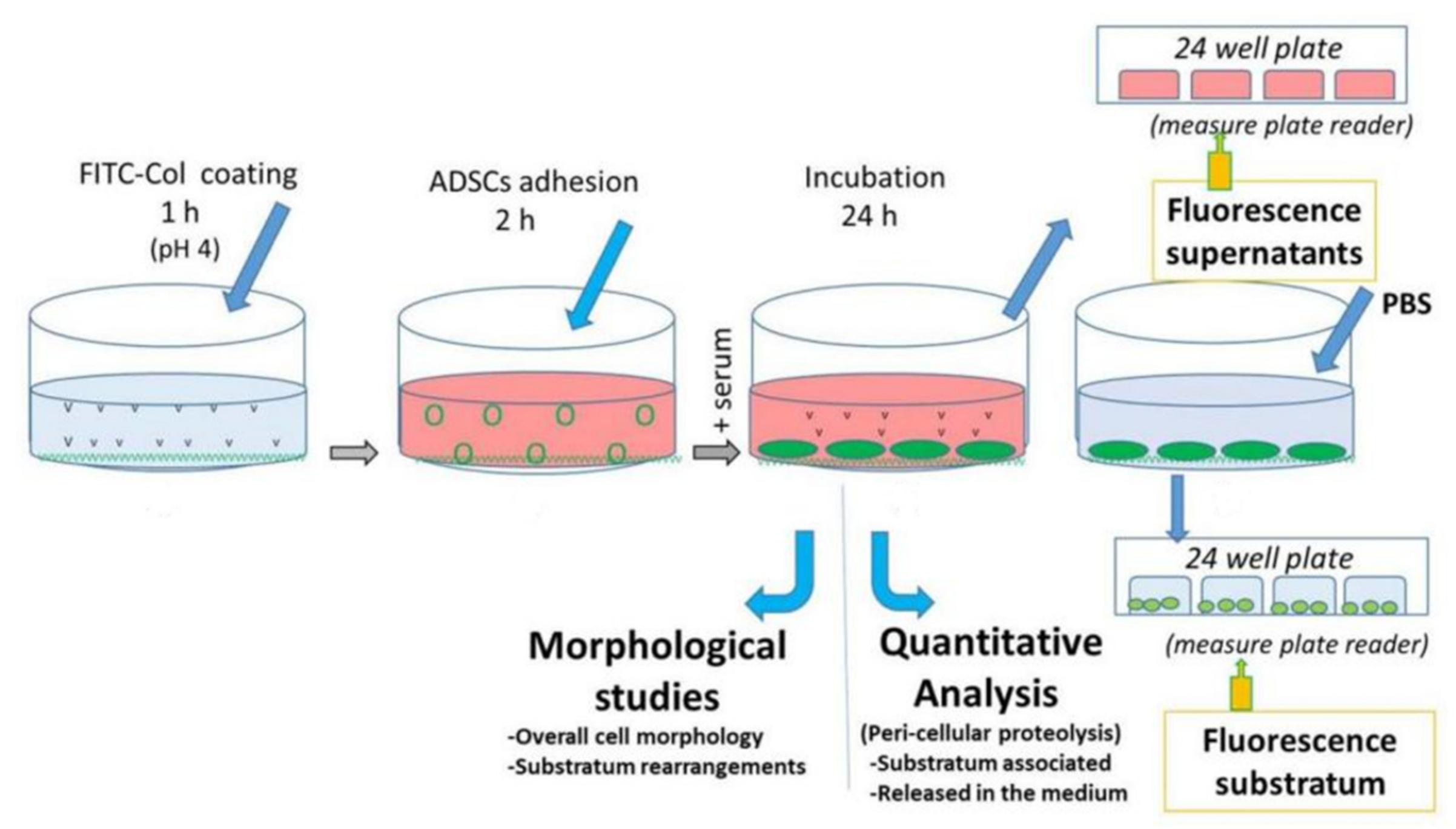
2.1. Overall Design of the Experiments
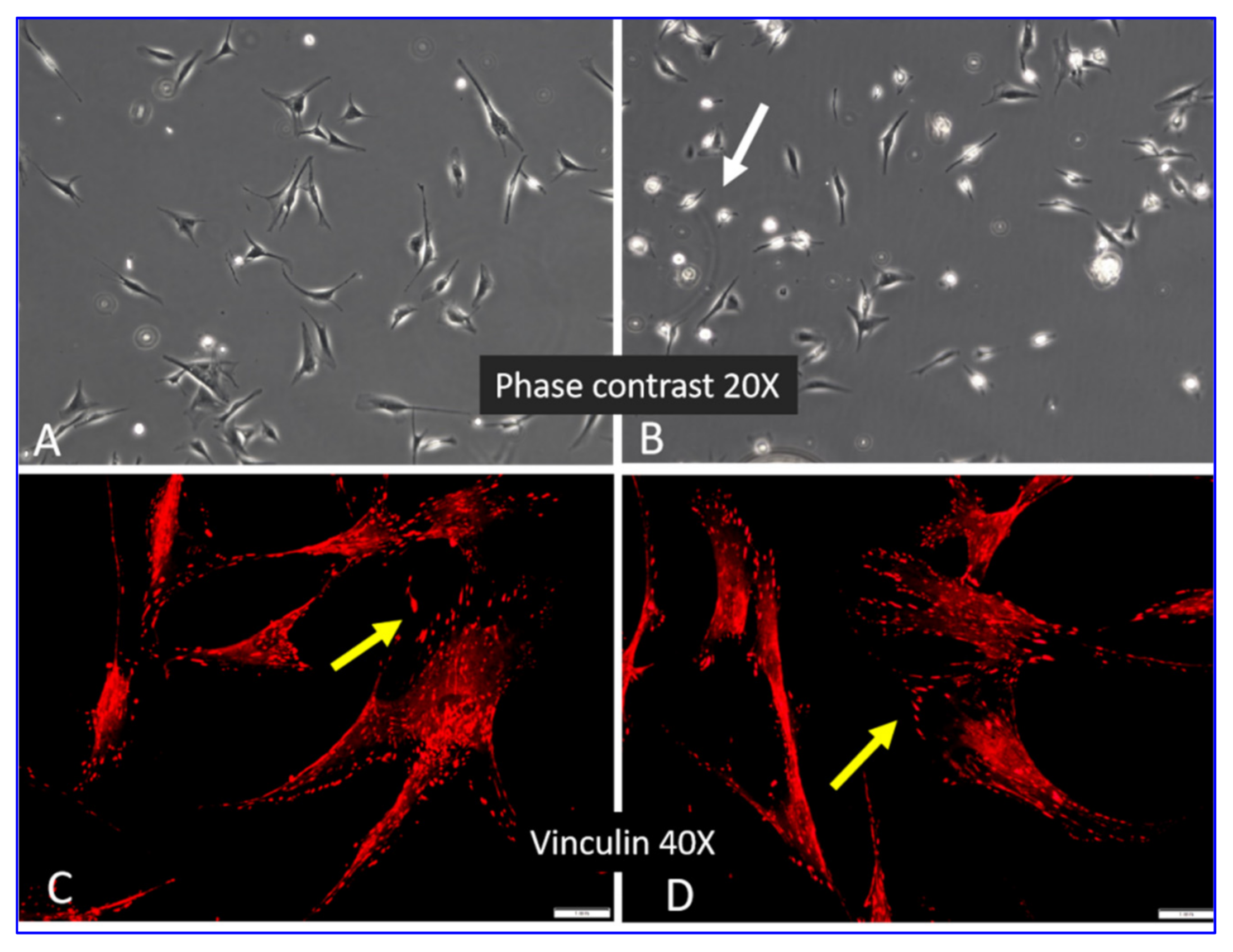
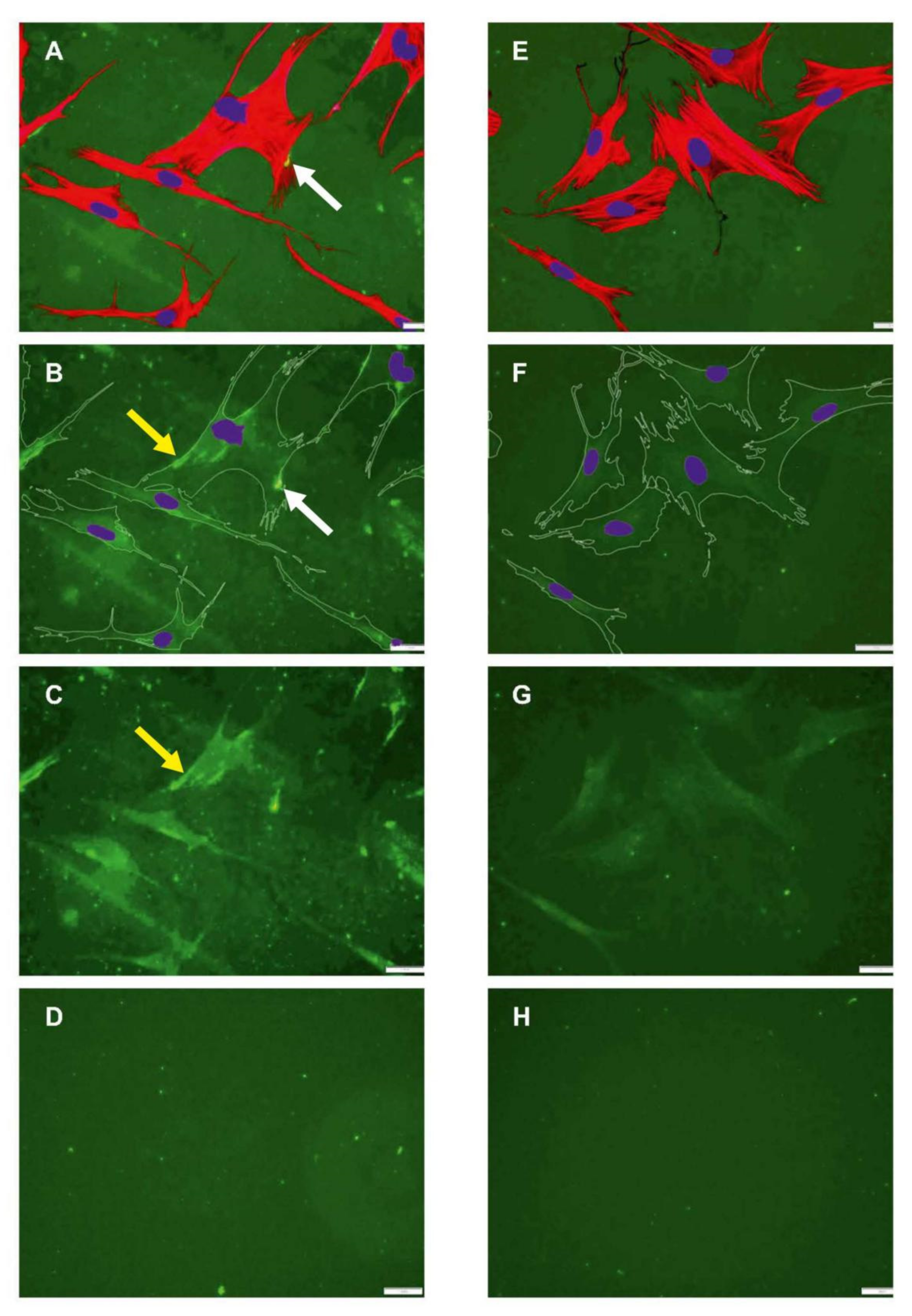
2.2. Morphology of ADMSCs Adhered to Col I or Col I-OXI
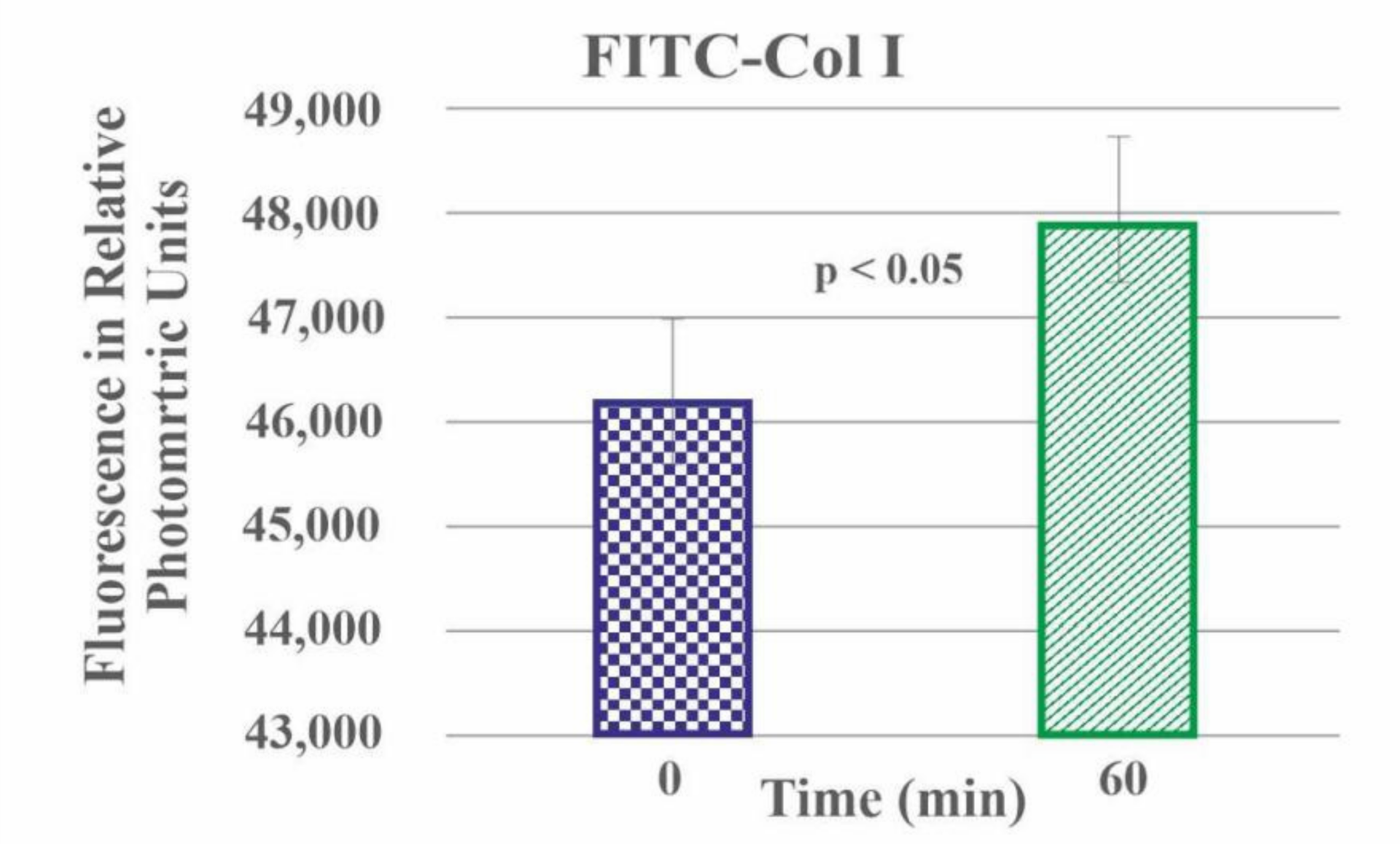
2.3. Quantitative Measurement of FITC-Col I Binding
2.4. Quantitative Measurement of Col I Absorption
2.5. Quantitative Measurement of Col I Degradation
2.6. De-Quenching of FITC-Col I by ADMSCs—Effect of Oxidation
2.7. De-Quenching of FITC-Col I by Added CH Collagenase in a Cell-Free System
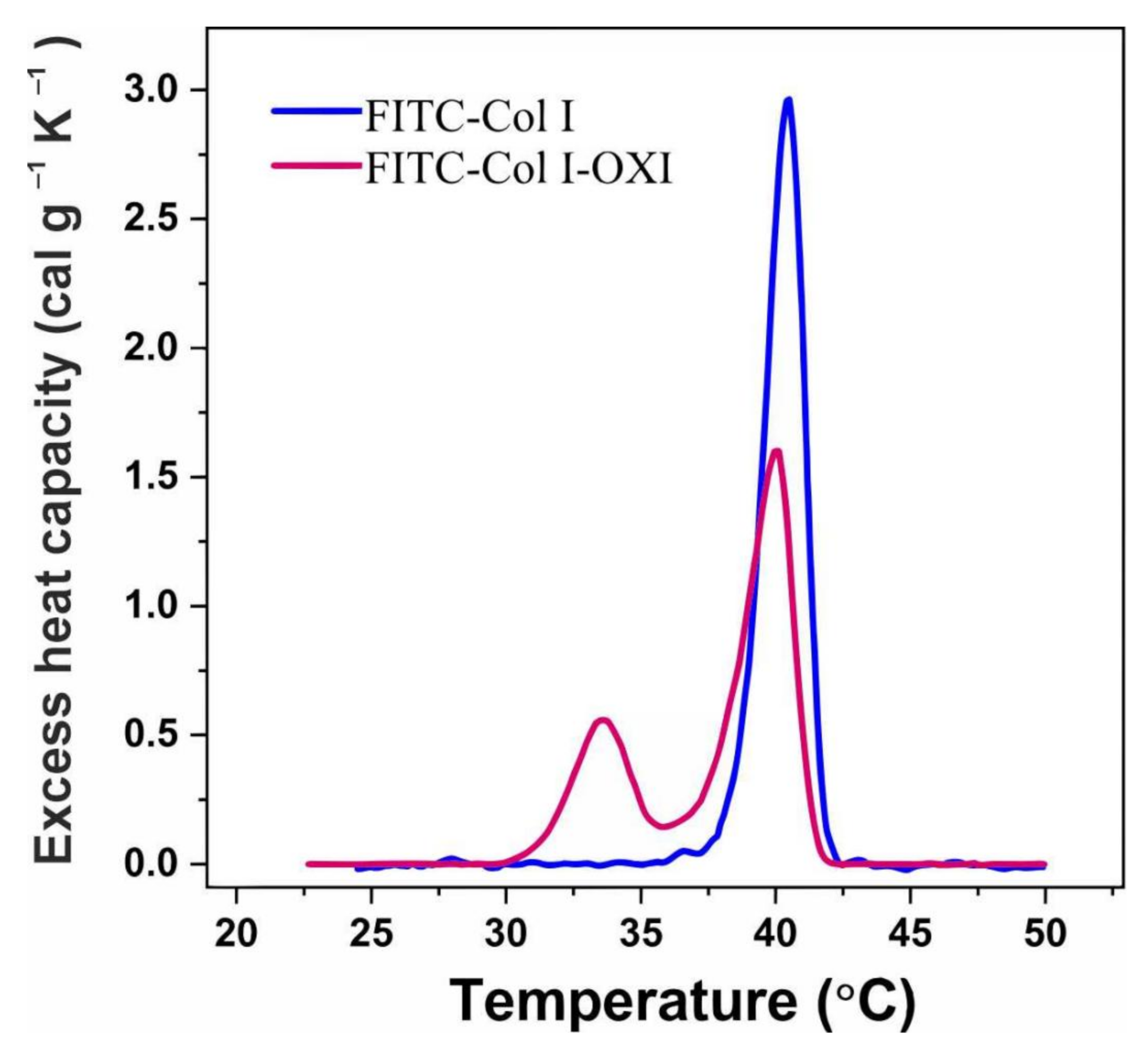
2.8. DSC Analysis of FITC-Col I–Effect of Oxidation
3. Discussion
4. Materials and Methods
4.1. Collagen Preparation
4.2. Fluorescent Labelling of Collagen
4.3. Collagen Oxidation
Calculation of the Adsorption Rate of FITC-Col I and FITC-Col I-OXI
4.4. Cells
4.5. Morphological Studies
4.5.1. Quantification of Cell-Associated Fibrils of FITC-Collagen
4.5.2. Quantitative Morphological Analysis of Raw Format Images via FibrilTool Plug-In for ImageJ
4.6. FITC-Collagen Degradation in a Cell-Free System
4.7. FITC-Collagen Degradation by ADMSCs
4.8. DSC Measurements
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Song, Y.H.; Shon, S.H.; Shan, M.R.; Stroock, A.D.; Fischbach, C. Adipose-derived stem cells increase angiogenesis through matrix metalloproteinase-dependent collagen remodeling. Integr. Biol. 2016, 8, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Han, S.; Li, Y.Y.; Chan, B.P. Protease inhibitors enhance extracellular collagen fibril deposition in human mesenchymal stem cells. Stem Cell Res. Ther. 2015, 6, 197. [Google Scholar] [CrossRef] [Green Version]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wade, R.J.; Burdick, J.A. Engineering ECM signals into biomaterials. Mater. Today 2012, 15, 454–459. [Google Scholar] [CrossRef]
- Frese, L.; Dijkman, P.E.; Hoerstrup, S.P. Adipose Tissue-Derived Stem Cells in Regenerative Medicine. Transfus. Med. Hemotherapy 2016, 43, 268–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karsdal, M.A.; Genovese, F.; Madsen, E.A.; Manon-Jensen, T.; Schuppan, D. Collagen and tissue turnover as a function of age: Implications for fibrosis. J. Hepatol. 2016, 64, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosman, F.T.; Stamenkovic, I. Functional structure and composition of the extracellular matrix. J. Pathol. 2003, 200, 423–428. [Google Scholar] [CrossRef]
- Karsdal, M.A.; Nielsen, M.J.; Sand, J.M.; Henriksen, K.; Genovese, F.; Bay-Jensen, A.C.; Smith, V.; Adamkewicz, J.I.; Christiansen, C.; Leeming, D.J. Extracellular Matrix Remodeling: The Common Denominator in Connective Tissue Diseases Possibilities for Evaluation and Current Understanding of the Matrix as More Than a Passive Architecture, but a Key Player in Tissue Failure. Assay Drug Dev. Technol. 2013, 11, 70–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grutters, J.C.; Lammers, J.-W.J. Connective tissue diseases. In Clinical Respiratory Medicine; Spiro, S.G., Silvestri, G.A., Agusti, A., Eds.; Saunders: Philadelphia, PA, USA, 2012; pp. 653–666. [Google Scholar]
- Cipak, A.; Mrakovcic, L.; Ciz, M.; Lojek, A.; Mihaylova, B.; Goshev, I.; Jaganjac, M.; Cindric, M.; Sitic, S.; Margaritoni, M.; et al. Growth suppression of human breast carcinoma stem cells by lipid peroxidation product 4-hydroxy-2-nonenal and hydroxyl radical-modified collagen. Acta Biochim. Pol. 2010, 57, 165–171. [Google Scholar] [CrossRef]
- Lee, C.H.; Singla, A.; Lee, Y. Biomedical applications of collagen. Int. J. Pharm. 2001, 221, 1–22. [Google Scholar] [CrossRef]
- Sorushanova, A.; Delgado, L.M.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.M.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The Collagen Suprafamily: From Biosynthesis to Advanced Biomaterial Development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitinger, B. Transmembrane Collagen Receptors. In Annual Review of Cell and Developmental Biology; Schekman, R., Goldstein, L., Lehmann, R., Eds.; Annual Reviews: Palo Alto, CA, USA, 2011; Volume 27, pp. 265–290. [Google Scholar]
- Yamauchi, M.; Sricholpech, M. Lysine post-translational modifications of collagen. In Lysine-Based Post-Translational Modification of Proteins; Scott, I., Ed.; Portland Press: London, UK, 2012; Volume 52, pp. 113–133. [Google Scholar]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Bonomini, F.; Tengattini, S.; Fabiano, A.; Bianchi, R.; Rezzani, R. Atherosclerosis and oxidative stress. Histol. Histopathol. 2008, 23, 381–390. [Google Scholar] [PubMed]
- Watson, W.H.; Ritzenthaler, J.D.; Roman, J. Lung extracellular matrix and redox regulation. Redox Biol. 2016, 8, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Ramalingam, M.; Kim, S.J. Reactive oxygen/nitrogen species and their functional correlations in neurodegenerative diseases. J. Neural Transm. 2012, 119, 891–910. [Google Scholar] [CrossRef]
- Saremi, A.; Arora, R. Vitamin E and Cardiovascular Disease. Am. J. Ther. 2010, 17, E56–E65. [Google Scholar] [CrossRef]
- Daniil, Z.D.; Papageorgiou, E.; Koutsokera, A.; Kostikas, K.; Kiropoulos, T.; Papaioannou, A.I.; Gourgoulianis, K.I. Serum levels of oxidative stress as a marker of disease severity in idiopathic pulmonary fibrosis. Pulm. Pharmacol. Ther. 2008, 21, 26–31. [Google Scholar] [CrossRef]
- Reeg, S.; Grune, T. Protein Oxidation in Aging: Does It Play a Role in Aging Progression? Antioxid. Redox Signal. 2015, 23, 239–255. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, R.V.; Costa, A.M.A.; Grzeskowiak, L. Oxidative Stress and Tissue Repair: Mechanism, Biomarkers, and Therapeutics. Oxidative Med. Cell. Longev. 2021, 2021, 6204096. [Google Scholar] [CrossRef]
- Dichi, I.; Bregano, J.W.; Simao, A.N.C.; Cecchini, R. Role of Oxidative Stress in Chronic Diseases, 1st ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Q.; Fang, G.; Li, B.S.; Wu, D.B.; Guo, W.J.; Hong, S.S.; Hong, L. Collagen metabolic disorder induced by oxidative stress in human uterosacral ligament-derived fibroblasts: A possible pathophysiological mechanism in pelvic organ prolapse. Mol. Med. Rep. 2016, 13, 2999–3008. [Google Scholar] [CrossRef] [Green Version]
- Serras, F. The benefits of oxidative stress for tissue repair and regeneration. Fly 2016, 10, 128–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krahn, K.N.; Bouten, C.V.C.; van Tuijl, S.; van Zandvoort, M.; Merkx, M. Fluorescently labeled collagen binding proteins allow specific visualization of collagen in tissues and live cell culture. Anal. Biochem. 2006, 350, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Sigrid, A.L. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Maneva-Radicheva, L.; Ebert, U.; Dimoudis, N.; Altankov, G. Fibroblast remodeling of adsorbed collagen type IV is altered in contact with cancer cells. Histol. Histopathol. 2008, 23, 833–842. [Google Scholar]
- Coelho, N.M.; Llopis-Hernandez, V.; Salmeron-Sanchez, M.; Altankov, G. Dynamic Reorganization and Enzymatic Remodeling of Type IV Collagen at Cell-Biomaterial Interface. In Insights into Enzyme Mechanisms and Functions from Experimental and Computational Methods; Christov, C.Z., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 105, pp. 81–104. [Google Scholar]
- Komsa-Penkova, R.; Koynova, R.; Kostov, G.; Tenchov, B. Discrete reduction of type I collagen thermal stability upon oxidation. Biophys. Chem. 2000, 83, 185–195. [Google Scholar] [CrossRef]
- Jedeszko, C.; Sameni, M.; Olive, M.B.; Moin, K.; Sloane, B.F. Visualizing Protease Activity in Living Cells: From Two Dimensions to Four Dimensions. Curr. Protoc. Cell Biol. 2008, 39, 4–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doyle, A.D. Fluorescent Labeling of Rat-tail Collagen for 3D Fluorescence Imaging. Bio-Protocol 2018, 8, e2919. [Google Scholar] [CrossRef]
- Friedl, P.; Wolf, K. Proteolytic and non-proteolytic migration of tumour cells and leucocytes. In Proteases and the Regulation of Biological Processes; Saklatvala, J., Nagase, H., Salvesen, G., Eds.; Portland Press: London, UK, 2003; Volume 70, pp. 277–285. [Google Scholar]
- Tzoneva, R.; Groth, T.; Altankov, G.; Paul, D. Remodeling of fibrinogen by endothelial cells in dependence on fibronectin matrix assembly. Effect of substratum wettability. J. Mater. Sci.-Mater. Med. 2002, 13, 1235–1244. [Google Scholar] [CrossRef]
- Llopis-Hernandez, V.; Rico, P.; Moratal, D.; Altankov, G.; Salmeron-Sanchez, M. Role of Material-Driven Fibronectin Fibrillogenesis in Protein Remodeling. Bioresearch Open Access 2013, 2, 364–373. [Google Scholar] [CrossRef] [Green Version]
- Altankov, G.; Groth, T. Reorganization of substratum-bound fibronectin on hydrophilic and hydrophobic materials is related to biocompatibility. J. Mater. Sci. Mater. Med. 1994, 5, 732–737. [Google Scholar] [CrossRef]
- Toromanov, G.; Gugutkov, D.; Gustaysson, J.; Planell, J.; Salmeron-Sanchez, M.; Altankov, G. Dynamic Behavior of Vitronectin at the Cell-Material Interface. Acs Biomater. Sci. Eng. 2015, 1, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Coelho, N.M.; Salmeron-Sanchez, M.; Altankov, G. Fibroblasts remodeling of type IV collagen at a biomaterials interface. Biomater. Sci. 2013, 1, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Ricard-Blum, S. The Collagen Family. Cold Spring Harb. Perspect. Biol. 2011, 3, a004978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangtrongsup, S.; Kisiday, J.D. Modulating the oxidative environment during mesenchymal stem cells chondrogenesis with serum increases collagen accumulation in agarose culture. J. Orth. Res. 2018, 36, 506–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sart, S.; Song, L.Q.; Li, Y. Controlling Redox Status for Stem Cell Survival, Expansion, and Differentiation. Oxid. Med. Cell. Longev. 2015, 2015, 105135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, S.C.; Yuan, Q.J.; Yin, T.; You, J.; Gu, Z.P.; Xiong, S.B.; Hu, Y. Self-assembly of collagen-based biomaterials: Preparation, characterizations and biomedical applications. J. Mater. Chem. B 2018, 6, 2650–2676. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.W.; Liu, B.Y.; Liu, G.; Yang, F.; Cao, F.; Dou, X.J.; Yu, W.T.; Wang, B.J.; Zheng, G.S.; Cheng, L.L.; et al. Mesenchymal stem cell-loaded porous tantalum integrated with biomimetic 3D collagen-based scaffold to repair large osteochondral defects in goats. Stem Cell. Res. Ther. 2019, 10, 1–17. [Google Scholar] [CrossRef]
- Chosa, N.; Ishisaki, A. Two novel mechanisms for maintenance of stemness in mesenchymal stem cells: SCRG1/BST1 axis and cell–cell adhesion through N-cadherin. Jpn. Dent. Sci. Rev. 2018, 54, 37–44. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef]
- Kim, K.S.; Choi, H.W.; Yoon, H.E.; Kim, I.Y. Reactive Oxygen Species Generated by NADPH Oxidase 2 and 4 Are Required for Chondrogenic Differentiation. J. Biol. Chem. 2010, 285, 40294–40302. [Google Scholar] [CrossRef] [Green Version]
- Somoza, R.A.; Welter, J.F.; Correa, D.; Caplan, A.I. Chondrogenic Differentiation of Mesenchymal Stem Cells: Challenges and Unfulfilled Expectations. Tissue Eng. Part B-Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef] [Green Version]
- Abraham, L.C.; Zuena, E.; Perez-Ramirez, B.; Kaplan, D.L. Guide to collagen characterization for biomaterial studies. J. Biomed. Mater Res. B Appl. Biomater. 2008, 87, 264–285. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.M.; Desai, L.P. Reciprocal regulation of TGF-beta and reactive oxygen species: A perverse cycle for fibrosis. Redox Biol. 2015, 6, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Rosin, N.L.; Sopel, M.J.; Falkenham, A.; Lee, T.D.G.; Legare, J.F. Disruption of Collagen Homeostasis Can Reverse Established Age-Related Myocardial Fibrosis. Am. J. Pathol. 2015, 185, 631–642. [Google Scholar] [CrossRef]
- Cecarini, V.; Gee, J.; Fioretti, E.; Amici, M.; Angeletti, M.; Eleuteri, A.M.; Keller, J.N. Protein oxidation and cellular homeostasis: Emphasis on metabolism. Biochim. et Biophys. Acta-Mol. Cell Res. 2007, 1773, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Quan, T. Oxidative Stress and Human Skin Connective Tissue Aging. Cosmetics 2016, 3, 28. [Google Scholar] [CrossRef]
- Nowotny, K.; Grune, T. Degradation of oxidized and glycoxidized collagen: Role of collagen cross-linking. Arch. Biochem. Biophys. 2014, 542, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Menges, D.A.; Ternullo, D.L.; TanWilson, A.L.; Gal, S. Continuous assay of proteases using a microtiter plate fluorescence reader. Anal. Biochem. 1997, 254, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Eckhard, U.; Huesgen, P.F.; Brandstetter, H.; Overall, C.M. Proteomic protease specificity profiling of clostridial collagenases reveals their intrinsic nature as dedicated degraders of collagen. J. Proteomics 2014, 100, 102–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osberger, T.J.; Rogness, D.C.; Kohrt, J.T.; Stepan, A.F.; White, M.C. Oxidative diversification of amino acids and peptides by small-molecule iron catalysis. Nature 2016, 537, 214–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bochi, G.V.; Torbitz, V.D.; de Campos, L.P.; Sangoi, M.B.; Fernandes, N.F.; Gomes, P.; Moretto, M.B.; Barbisan, F.; da Cruz, I.B.; Moresco, R.N. In Vitro Oxidation of Collagen Promotes the Formation of Advanced Oxidation Protein Products and the Activation of Human Neutrophils. Inflammation 2016, 39, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Davies, M.J. The oxidative environment and protein damage. Biochim. et Biophys. Acta-Proteins Proteom. 2005, 1703, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Stadtman, E.R. Metal ion-catalyzed oxidation of proteins: Biochemical mechanism and biological consequences. Free Radic. Biol. Med. 1990, 9, 315–325. [Google Scholar] [CrossRef] [Green Version]
- Puma, P.L.; Hubbard, A.L. Transcytosis: Crossing Cellular Barriers. Physiol. Rev. 2003, 83, 871–932. [Google Scholar] [CrossRef]
- He, F.F.; Gong, Y.; Li, Z.Q.; Wu, L.; Jiang, H.J.; Su, H.; Zhang, C.; Wang, Y.M. A New Pathogenesis of Albuminuria: Role of Transcytosis. Cell Physiol. Biochem. 2018, 47, 1274–1286. [Google Scholar] [CrossRef] [Green Version]
- Cieplak, P.; Strongin, A.Y. Matrix metalloproteinases—From the cleavage data to the prediction tools and beyond. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 1952–1963. [Google Scholar] [CrossRef] [PubMed]
- Laronha, H.; Caldeira, J. Structure and Function of Human Matrix Metalloproteinases. Cells 2020, 9, 1076. [Google Scholar] [CrossRef] [PubMed]
- Sipila, K.H.; Drushinin, K.; Rappu, P.; Jokinen, J.; Salminen, T.A.; Salo, A.M.; Kapyla, J.; Myllyharju, J.; Heino, J. Proline hydroxylation in collagen supports integrin binding by two distinct mechanisms. J. Biol. Chem. 2018, 293, 7645–7658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komsa-Penkova, R.; Koynova, R.; Kostov, G.; Tenchov, B.G. Thermal stability of calf skin collagen type I in salt solutions. Biochim. Biophys. Acta-Protein Struct. Mol. 1996, 1297, 171–181. [Google Scholar] [CrossRef]
- Milev, S. DSC and Protein Stability: What Does The Enthalpy Change Mean? Available online: https://www.materials-talks.com/blog/2020/08/25/dsc-and-protein-stability-what-does-the-enthalpy-change-mean/ (accessed on 25 August 2020).
- Johnson, C.M. Differential scanning calorimetry as a tool for protein folding and stability. Arch. Biochem. Biophys. 2013, 531, 100–109. [Google Scholar] [CrossRef]
- Chandrakasan, G.; Torchia, D.; Aa Piez, K.A. Preparation of intact monomeric collagen from rat tail tendon and skin and structure of nonhelical ends in solution. J. Biol. Chem. 1976, 251, 6062–6067. [Google Scholar] [CrossRef]
- Rajan, N.; Habermehl, J.; Coté, M.F.; Doillon, C.J.; Mantovani, D. Preparation of ready-to-use, storable and reconstituted type I collagen from rat tail tendon for tissue engineering applications. Nat. Protoc. 2007, 1, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Komsa-Penkova, R.; Spirova, R.; Bechev, B. Modification of Lowry’s method for collagen concentration measurement. J. Biochem. Biophys. Methods 1996, 32, 33–43. [Google Scholar] [CrossRef]
- Kuznetsova, N.; Leikin, S. Does the triple helical domain of type I collagen encode molecular recognition and fiber assembly while telopeptides serve as catalytic domains? Effect of proteolytic cleavage on fibrillogenesis and on collagen-collagen interaction in fibers. J. Biol. Chem. 1999, 274, 36083–36088. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.sigmaaldrich.com/deepweb/assets/sigmaaldrich/product/documents/222/544/f7250pis.pdf (accessed on 23 January 2022).
- Boudaoud, A.; Burian, A.; Borowska-Wykręt, D.; Uyttewaal, M.; Wrzalik, R.; Kwiatkowska, D.; Hamant, O. FibrilTool, an ImageJ plug-in to quantify fibrillar structures in raw microscopy images. Nat. Protoc. 2014, 9, 457–463. [Google Scholar] [CrossRef] [PubMed]

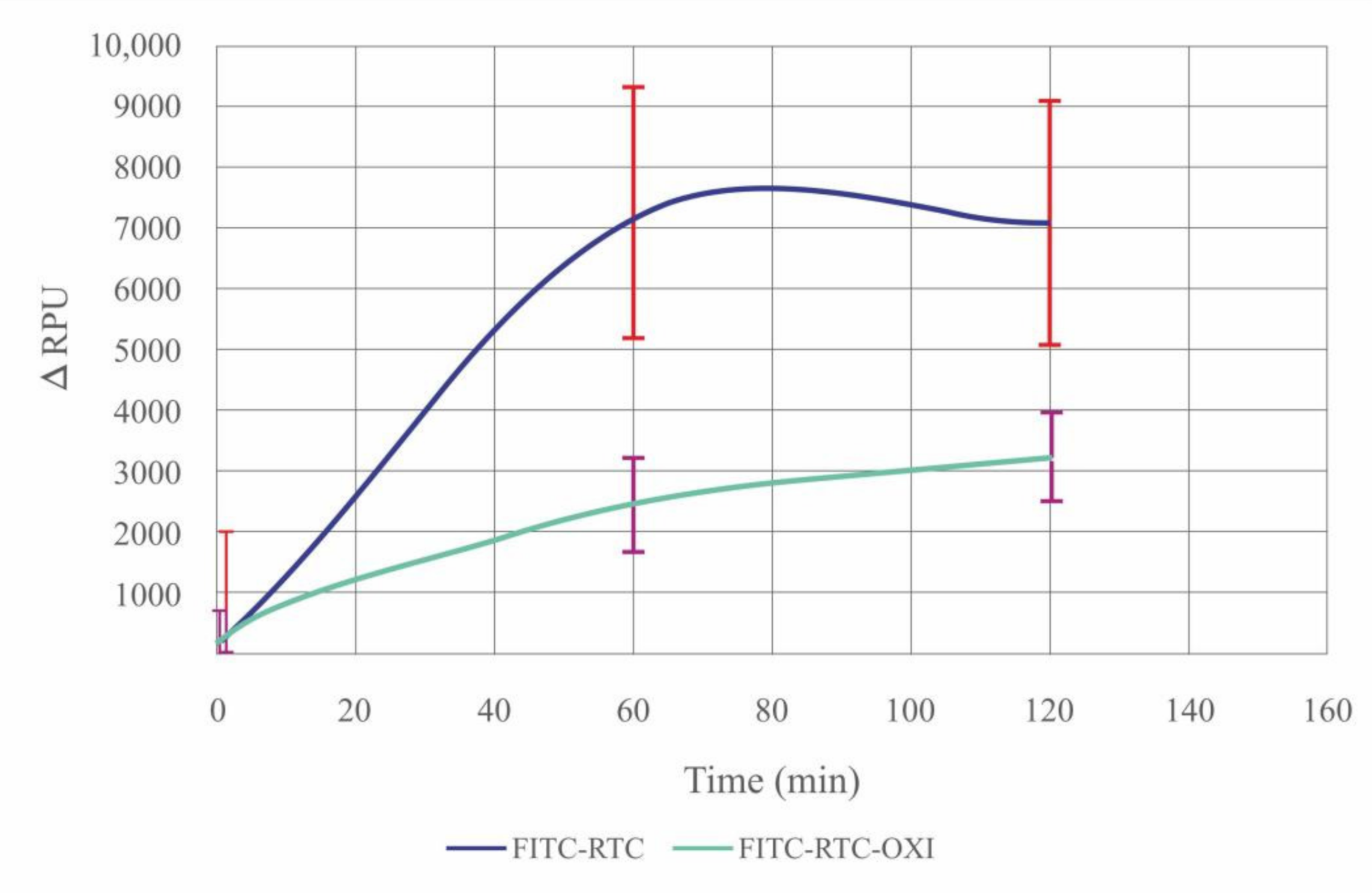
| Samples | FITC-Col I | FITC-Col I-OXI |
|---|---|---|
| Chi-Square | 12.000 | 4.000 |
| Sig. (p) | 0.002 | 0.135 |
| Collagen | TM-pre (°C) | TM-main (°C) | ∆Htotal (cal/g) | Δ TM-main½ (°C) |
|---|---|---|---|---|
| FITC-Col-I | - | 40.5 | 5.58 | 1.72 |
| FITC-Col I-OXI | 33.6 | 40.1 | 5.50 | 2.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komsa-Penkova, R.; Stavreva, G.; Belemezova, K.; Kyurkchiev, S.; Todinova, S.; Altankov, G. Mesenchymal Stem-Cell Remodeling of Adsorbed Type-I Collagen—The Effect of Collagen Oxidation. Int. J. Mol. Sci. 2022, 23, 3058. https://doi.org/10.3390/ijms23063058
Komsa-Penkova R, Stavreva G, Belemezova K, Kyurkchiev S, Todinova S, Altankov G. Mesenchymal Stem-Cell Remodeling of Adsorbed Type-I Collagen—The Effect of Collagen Oxidation. International Journal of Molecular Sciences. 2022; 23(6):3058. https://doi.org/10.3390/ijms23063058
Chicago/Turabian StyleKomsa-Penkova, Regina, Galya Stavreva, Kalina Belemezova, Stanimir Kyurkchiev, Svetla Todinova, and George Altankov. 2022. "Mesenchymal Stem-Cell Remodeling of Adsorbed Type-I Collagen—The Effect of Collagen Oxidation" International Journal of Molecular Sciences 23, no. 6: 3058. https://doi.org/10.3390/ijms23063058
APA StyleKomsa-Penkova, R., Stavreva, G., Belemezova, K., Kyurkchiev, S., Todinova, S., & Altankov, G. (2022). Mesenchymal Stem-Cell Remodeling of Adsorbed Type-I Collagen—The Effect of Collagen Oxidation. International Journal of Molecular Sciences, 23(6), 3058. https://doi.org/10.3390/ijms23063058







