Discovery and Biotechnological Exploitation of Glycoside-Phosphorylases
Abstract
1. Introduction
2. Glycoside-Phosphorylases
2.1. GP Catalytic Mechanism
2.2. Classification and Substrate Specificity
2.2.1. Retaining GPs
2.2.2. Inverting GPs
2.3. Tertiary and Quaternary Structures
2.4. Biotechnological Applications
2.5. Methods for Glycoside Phosphorylase Activity Detection
2.5.1. Chromogenic Assays
2.5.2. Chromatographic and Spectroscopic Methods for Carbohydrate Detection and Characterization
2.6. Enzyme Discovery by Functional (Meta)Genomics
2.6.1. Activity-Based Approaches
2.6.2. Sequence-Based Approaches
- COG: the protein belongs to COG2152: Predicted glycosyl hydrolase, GH43/DUF377 family (Carbohydrate transport and metabolism). The biochemical characterization of this enzyme [109], indicated that these results are false, this enzyme being a mannoside-phosphorylase of the GH130 family, which does not contain a GH43 module.
- Pfam: the protein contains the domain Glyco_hydro_130 (PF04041), described as ‘beta-1,4-mannooligosaccharide phosphorylase’. This is true, but the same result is found for the GH130 β-1,2-Mannosidase AAO78885.1 [194].
- Kegg: no result.
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- André, I.; Potocki-Véronèse, G.; Barbe, S.; Moulis, C.; Remaud-Siméon, M. CAZyme discovery and design for sweet dreams. Curr. Opin. Chem. Biol. 2014, 19, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Benkoulouche, M.; Fauré, R.; Remaud-Siméon, M.; Moulis, C.; André, I. Harnessing glycoenzyme engineering for synthesis of bioactive oligosaccharides. Interface Focus 2019, 9, 20180069. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; McArthur, J.B.; Chen, X. Strategies for chemoenzymatic synthesis of carbohydrates. Carbohydr. Res. 2019, 472, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Rudroff, F.; Mihovilovic, M.D.; Gröger, H.; Snajdrova, R.; Iding, H.; Bornscheuer, U.T. Opportunities and challenges for combining chemo- and biocatalysis. Nat. Catal. 2018, 1, 12–22. [Google Scholar] [CrossRef]
- Lairson, L.L.; Henrissat, B.; Davies, G.J.; Withers, S.G. Glycosyltransferases: Structures, Functions, and Mechanisms. Annu. Rev. Biochem. 2008, 77, 521–555. [Google Scholar] [CrossRef]
- Wong, C.-H. Practical synthesis of oligosaccharides based on glycosyltransferases and glycosylphosphites. In Modern Methods in Carbohydrate Synthesis; Khan, S.H., Ed.; CRC Press: Boca Raton, FL, USA, 1996; pp. 467–491. ISBN 978-3-7186-5785-8. [Google Scholar]
- Overkleeft, H.S.; Seeberger, P.H. Chemoenzymatic synthesis of glycans and glycoconjugates. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015. [Google Scholar]
- Fierfort, N.; Samain, E. Genetic engineering of Escherichia coli for the economical production of sialylated oligosaccharides. J. Biotechnol. 2008, 134, 261–265. [Google Scholar] [CrossRef]
- Samain, E.; Priem, B. Procede de Production D’oligosaccharides. European Patent EP1194584B1, 22 February 2006. [Google Scholar]
- McArthur, J.B.; Chen, X. Glycosyltransferase engineering for carbohydrate synthesis. Biochem. Soc. Trans. 2016, 44, 129–142. [Google Scholar] [CrossRef]
- Matsuzawa, T. The metagenome approach: A new resource for glycosidases. Trends Glycosci. Glycotechnol. 2019, 31, E15–E20. [Google Scholar] [CrossRef]
- Teze, D.; Hendrickx, J.; Czjzek, M.; Ropartz, D.; Sanejouand, Y.-H.; Tran, V.; Tellier, C.; Dion, M. Semi-rational approach for converting a GH1 β-glycosidase into a β-transglycosidase. Protein Eng. Des. Sel. 2014, 27, 13–19. [Google Scholar] [CrossRef]
- Daudé, D.; Vergès, A.; Cambon, E.; Emond, S.; Tranier, S.; André, I.; Remaud-Siméon, M. Neutral genetic drift-based engineering of a sucrose-utilizing enzyme toward glycodiversification. ACS Catal. 2019, 9, 1241–1252. [Google Scholar] [CrossRef]
- Zeuner, B.; Teze, D.; Muschiol, J.; Meyer, A.S. Synthesis of human milk oligosaccharides: Protein Engineering strategies for improved enzymatic transglycosylation. Molecules 2019, 24, 2033. [Google Scholar] [CrossRef] [PubMed]
- Tran, L.T.; Blay, V.; Luang, S.; Eurtivong, C.; Choknud, S.; González-Díaz, H.; Cairns, J.R.K. Engineering faster transglycosidases and their acceptor specificity. Green Chem. 2019, 21, 2823–2836. [Google Scholar] [CrossRef]
- Albert, M.; Repetschnigg, W.; Ortner, J.; Gomes, J.; Paul, B.J.; Illaszewicz, C.; Weber, H.; Steiner, W.; Dax, K. Simultaneous detection of different glycosidase activities by 19F NMR spectroscopy. Carbohydr. Res. 2000, 327, 395–400. [Google Scholar] [CrossRef]
- Okuyama, M.; Mori, H.; Watanabe, K.; Kimura, A.; Chiba, S. α-Glucosidase mutant catalyzes “α-Glycosynthase”-type reaction. Biosci. Biotechnol. Biochem. 2002, 66, 928–933. [Google Scholar] [CrossRef][Green Version]
- Higuchi, Y.; Eshima, Y.; Huang, Y.; Kinoshita, T.; Sumiyoshi, W.; Nakakita, S.-I.; Takegawa, K. Highly efficient transglycosylation of sialo-complex-type oligosaccharide using Coprinopsis cinerea endoglycosidase and sugar oxazoline. Biotechnol. Lett. 2017, 39, 157–162. [Google Scholar] [CrossRef]
- Cobucci-Ponzano, B.; Conte, F.; Bedini, E.; Corsaro, M.M.; Parrilli, M.; Sulzenbacher, G.; Lipski, A.; Dal Piaz, F.; Lepore, L.; Rossi, M.; et al. beta-Glycosyl azides as substrates for alpha-glycosynthases: Preparation of efficient Alpha-L-Fucosynthases. Chem. Biol. 2009, 16, 1097–1108. [Google Scholar] [CrossRef]
- Hayes, M.R.; Pietruszka, J. Synthesis of glycosides by glycosynthases. Molecules 2017, 22, 1434. [Google Scholar] [CrossRef]
- Cori, C.F.; Cori, G.T. Mechanism of formation of hexosemonophosphate in muscle and isolation of a new phosphate ester. Proc. Soc. Exp. Biol. Med. 1936, 34, 702–705. [Google Scholar] [CrossRef]
- Alexander, J.K. Characteristics of cellobiose phosphorylase. J. Bacteriol. 1961, 81, 903–910. [Google Scholar] [CrossRef]
- Koshland, D.E. Stereochemistry and the mechanism of enzymatic reactions. Biol. Rev. 1953, 28, 416–436. [Google Scholar] [CrossRef]
- Hamura, K.; Saburi, W.; Abe, S.; Morimoto, N.; Taguchi, H.; Mori, H.; Matsui, H. Enzymatic characteristics of cellobiose phosphorylase from Ruminococcus albus NE1 and kinetic mechanism of unusual substrate inhibition in reverse phosphorolysis. Biosci. Biotechnol. Biochem. 2012, 76, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Nihira, T.; Nakai, H.; Kitaoka, M. 3-O-α-D-glucopyranosyl-L-rhamnose phosphorylase from Clostridium phytofermentans. Carbohydr. Res. 2012, 350, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Wim, S.; Tom, D. Characterization of β-Galactoside phosphorylases with diverging acceptor specificities. Enzym. Microb. Technol. 2011, 49, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, Y.; Hatada, Y.; Akita, M.; Yoshida, M.; Nakamura, N.; Takada, M.; Nakakuki, T.; Ito, S.; Horikoshi, K. Maltose phosphorylase from a deep-sea Paenibacillus Sp.: Enzymatic properties and nucleotide and amino-acid sequences. Enzym. Microb. Technol. 2005, 37, 185–194. [Google Scholar] [CrossRef]
- Franceus, J.; Pinel, D.; Desmet, T. Glucosylglycerate phosphorylase, an enzyme with novel specificity involved in compatible solute metabolism. Appl. Environ. Microbiol. 2017, 83, e01434-17. [Google Scholar] [CrossRef]
- Liu, N.; Fosses, A.; Kampik, C.; Parsiegla, G.; Denis, Y.; Vita, N.; Fierobe, H.-P.; Perret, S. In Vitro and in vivo exploration of the cellobiose and cellodextrin phosphorylases panel in Ruminiclostridium Cellulolyticum: Implication for cellulose catabolism. Biotechnol. Biofuels 2019, 12, 208. [Google Scholar] [CrossRef]
- Macdonald, S.S.; Patel, A.; Larmour, V.L.C.; Morgan-Lang, C.; Hallam, S.J.; Mark, B.L.; Withers, S.G. Structural and mechanistic analysis of a β-glycoside phosphorylase identified by screening a metagenomic library. J. Biol. Chem. 2018, 293, 3451–3467. [Google Scholar] [CrossRef]
- Macdonald, S.S.; Blaukopf, M.; Withers, S.G. N-Acetylglucosaminidases from CAZy family GH3 are really glycoside phosphorylases, thereby explaining their use of histidine as an acid/base catalyst in place of glutamic Acid. J. Biol. Chem. 2015, 290, 4887–4895. [Google Scholar] [CrossRef]
- Van den Broek, L.A.M.; van Boxtel, E.L.; Kievit, R.P.; Verhoef, R.; Beldman, G.; Voragen, A.G.J. Physico-chemical and transglucosylation properties of recombinant sucrose phosphorylase from Bifidobacterium adolescentis DSM20083. Appl. Microbiol. Biotechnol. 2004, 65, 219–227. [Google Scholar] [CrossRef]
- Kullin, B.; Abratt, V.R.; Reid, S.J. A functional analysis of the Bifidobacterium longum cscA and scrP genes in sucrose utilization. Appl. Microbiol. Biotechnol. 2006, 72, 975–981. [Google Scholar] [CrossRef]
- Kim, M.; Kwon, T.; Joo Lee, H.; Heon Kim, K.; Kyun Chung, D.; Eog Ji, G.; Byeon, E.-S.; Lee, J.-H. Cloning and expression of sucrose phosphorylase gene from Bifidobacterium longum in E. coli and characterization of the recombinant enzyme. Biotechnol. Lett. 2003, 25, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Sun, X.; Li, W.; Li, T.; Li, S.; Kitaoka, M. Expression and Characterization of Recombinant Sucrose Phosphorylase. Protein J. 2018, 37, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Altermann, E.; Hutkins, R.; Cano, R.; Klaenhammer, T.R. Functional and comparative genomic analyses of an operon involved in fructooligosaccharide utilization by Lactobacillus acidophilus. Proc. Natl. Acad. Sci. USA 2003, 100, 8957–8962. [Google Scholar] [CrossRef] [PubMed]
- Goedl, C.; Schwarz, A.; Minani, A.; Nidetzky, B. Recombinant sucrose phosphorylase from Leuconostoc mesenteroides: Characterization, kinetic studies of transglucosylation, and application of immobilised enzyme for production of α-d-glucose 1-phosphate. J. Biotechnol. 2007, 129, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Moon, Y.-H.; Kim, N.; Kim, Y.-M.; Kang, H.-K.; Jung, J.-Y.; Abada, E.; Kang, S.-S.; Kim, D. Cloning and expression of the sucrose phosphorylase gene from Leuconostoc mesenteroides in Escherichia coli. Biotechnol. Lett. 2008, 30, 749–754. [Google Scholar] [CrossRef]
- Kawasaki, H.; Nakamura, N.; Ohmori, M.; Sakai, T. Cloning and expression in Escherichia coli of sucrose phosphorylase gene from Leuconostoc mesenteroides No. 165. Biosci. Biotechnol. Biochem. 1996, 60, 322–324. [Google Scholar] [CrossRef]
- Lee, J.-H.; Yoon, S.-H.; Nam, S.-H.; Moon, Y.-H.; Moon, Y.-Y.; Kim, D. Molecular cloning of a gene encoding the sucrose phosphorylase from Leuconostoc mesenteroides B-1149 and the expression in Escherichia coli. Enzym. Microb. Technol. 2006, 39, 612–620. [Google Scholar] [CrossRef]
- Teixeira, J.S.; Abdi, R.; Su, M.S.-W.; Schwab, C.; Gänzle, M.G. Functional characterization of sucrose phosphorylase and scrR, a regulator of sucrose metabolism in Lactobacillus reuteri. Food Microbiol. 2013, 36, 432–439. [Google Scholar] [CrossRef]
- Franceus, J.; Decuyper, L.; D’hooghe, M.; Desmet, T. Exploring the sequence diversity in glycoside hydrolase family 13_18 reveals a novel glucosylglycerol phosphorylase. Appl. Microbiol. Biotechnol. 2018, 102, 3183–3191. [Google Scholar] [CrossRef]
- Trethewey, R.N.; Fernie, A.R.; Bachmann, A.; Fleischer-Notter, H.; Geigenberger, P.; Willmitzer, L. Expression of a bacterial sucrose phosphorylase in potato tubers results in a glucose-independent induction of glycolysis: Sugar signalling in heterotrophic plant tissue. Plant Cell Environ. 2001, 24, 357–365. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Bruel, L.; Laville, E.; Nicoletti, C.; Navarro, D.; Henrissat, B.; Perrier, J.; Potocki-Veronese, G.; Giardina, T.; Lafond, M. Sucrose 6F-phosphate phosphorylase: A novel insight in the human gut microbiome. Microb. Genom. 2019, 5, e000253. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.R.; Mukasa, H.; Shimamura, A.; Ferretti, J.J. Streptococcus mutans gtfA gene specifies sucrose phosphorylase. Infect. Immun. 1988, 56, 2763–2765. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, M.; Tam, A.; Steinberg, D. Differential gene expression profiling of Streptococcus mutans cultured under biofilm and planktonic conditions. Microbiology 2007, 153, 1307–1317. [Google Scholar] [CrossRef] [PubMed]
- Goedl, C.; Nidetzky, B. Sucrose Phosphorylase Harbouring a Redesigned, Glycosyltransferase-Like Active Site Exhibits Retaining Glucosyl Transfer in the Absence of a Covalent Intermediate. ChemBioChem 2009, 10, 2333–2337. [Google Scholar] [CrossRef]
- Verhaeghe, T.; Aerts, D.; Diricks, M.; Soetaert, W.; Desmet, T. The quest for a thermostable sucrose phosphorylase reveals sucrose 6′-Phosphate phosphorylase as a novel specificity. Appl. Microbiol. Biotechnol. 2014, 98, 7027–7037. [Google Scholar] [CrossRef]
- Du, L.; Yang, H.; Huo, Y.; Wei, H.; Xu, Y.; Wei, Y.; Huang, R. A novel sucrose phosphorylase from the metagenomes of sucrose-rich environment: Isolation and characterization. World J. Microbiol. Biotechnol. 2012, 28, 2871–2878. [Google Scholar] [CrossRef]
- Franceus, J.; Capra, N.; Desmet, T.; Thunnissen, A.-M.W.H. Structural comparison of a promiscuous and a highly specific sucrose 6F-phosphate phosphorylase. Int. J. Mol. Sci. 2019, 20, 3906. [Google Scholar] [CrossRef]
- Nakai, H.; Baumann, M.J.; Petersen, B.O.; Westphal, Y.; Schols, H.; Dilokpimol, A.; Hachem, M.A.; Lahtinen, S.J.; Duus, J.Ø.; Svensson, B. The maltodextrin transport system and metabolism in Lactobacillus acidophilus NCFM and production of novel α-glucosides through reverse phosphorolysis by maltose phosphorylase: Maltose phosphorylase and reverse phosphorolysis. FEBS J. 2009, 276, 7353–7365. [Google Scholar] [CrossRef]
- Nihira, T.; Saito, Y.; Kitaoka, M.; Otsubo, K.; Nakai, H. Identification of Bacillus selenitireducens MLS10 maltose phosphorylase possessing synthetic ability for branched α-D-glucosyl trisaccharides. Carbohydr. Res. 2012, 360, 25–30. [Google Scholar] [CrossRef]
- Inoue, Y.; Yasutake, N.; Oshima, Y.; Yamamoto, Y.; Tomita, T.; Miyoshi, S.; Yatake, T. Cloning of the maltose phosphorylase gene from Bacillus sp. strain RK-1 and efficient production of the cloned gene and the trehalose phosphorylase gene from Bacillus stearothermophilus SK-1 in Bacillus subtilis. Biosci. Biotechnol. Biochem. 2002, 66, 2594–2599. [Google Scholar] [CrossRef]
- Ehrmann, M.A.; Vogel, R.F. Maltose metabolism of Lactobacillus sanfranciscensis: Cloning and heterologous expression of the key enzymes, maltose phosphorylase and phosphoglucomutase. FEMS Microbiol. Lett. 1998, 169, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, A.; Blancato, V.S.; Repizo, G.D.; Henry, C.; Pikis, A.; Bourand, A.; de Fátima Álvarez, M.; Immel, S.; Mechakra-Maza, A.; Hartke, A.; et al. Enterococcus faecalis utilizes maltose by connecting two incompatible metabolic routes via a novel maltose 6′-phosphate phosphatase (MapP): Novel mode of maltose utilization by Enterococcus faecalis. Mol. Microbiol. 2013, 88, 234–253. [Google Scholar] [CrossRef] [PubMed]
- Hüwel, S.; Haalck, L.; Conrath, N.; Spener, F. Maltose phosphorylase from Lactobacillus brevis: Purification, characterization, and application in a biosensor for ortho-phosphate. Enzym. Microb. Technol. 1997, 21, 413–420. [Google Scholar] [CrossRef]
- Gao, Y.; Saburi, W.; Taguchi, Y.; Mori, H. Biochemical characteristics of maltose phosphorylase MalE from Bacillus sp. AHU2001 and chemoenzymatic synthesis of oligosaccharides by the enzyme. Biosci. Biotechnol. Biochem. 2019, 83, 2097–2109. [Google Scholar] [CrossRef]
- Chaen, H.; Nakada, T.; Nishimoto, T.; Kuroda, N.; Fukuda, S.; Sugimoto, T.; Kurimoto, M.; Tsujisaka, Y. Purification and characterization of thermostable Trehalose Phosphorylase from Thermoanaerobium brockii. J. Appl. Glycosci. 1999, 46, 399–405. [Google Scholar] [CrossRef]
- Inoue, Y.; Ishii, K.; Tomita, T.; Yatake, T.; Fukui, F. Characterization of Trehalose Phosphorylase from Bacillus stearothermophilus SK-1 and nucleotide sequence of the corresponding gene. Biosci. Biotechnol. Biochem. 2002, 66, 1835–1843. [Google Scholar] [CrossRef]
- Van der Borght, J.; Chen, C.; Hoflack, L.; Van Renterghem, L.; Desmet, T.; Soetaert, W. Enzymatic properties and substrate specificity of the trehalose phosphorylase from Caldanaerobacter subterraneus. Appl. Environ. Microbiol. 2011, 77, 6939–6944. [Google Scholar] [CrossRef]
- De Beul, E.; Jongbloet, A.; Franceus, J.; Desmet, T. Discovery of a kojibiose hydrolase by analysis of specificity-determining correlated positions in glycoside hydrolase family 65. Molecules 2021, 26, 6321. [Google Scholar] [CrossRef]
- Andersson, U.; Levander, F.; Rådström, P. Trehalose-6-phosphate phosphorylase is part of a novel metabolic pathway for trehalose utilization in Lactococcus lactis. J. Biol. Chem. 2001, 276, 42707–42713. [Google Scholar] [CrossRef]
- Mukherjee, K.; Narindoshvili, T.; Raushel, F.M. Discovery of a Kojibiose Phosphorylase in Escherichia coli K-12. Biochemistry 2018, 57, 2857–2867. [Google Scholar] [CrossRef]
- Yamamoto, T.; Nishio-Kosaka, M.; Izawa, S.; Aga, H.; Nishimoto, T.; Chaen, H.; Fukuda, S. Enzymatic Properties of recombinant kojibiose phosphorylase from Caldicellulosiruptor saccharolyticus ATCC43494. Biosci. Biotechnol. Biochem. 2011, 75, 1208–1210. [Google Scholar] [CrossRef] [PubMed]
- Nihira, T.; Nakai, H.; Chiku, K.; Kitaoka, M. Discovery of nigerose phosphorylase from Clostridium phytofermentans. Appl. Microbiol. Biotechnol. 2012, 93, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Nihira, T.; Nishimoto, M.; Nakai, H.; Ohtsubo, K.; Kitaoka, M. Characterization of Two α-1,3-Glucoside Phosphorylases from Clostridium phytofermentans. J. Appl. Glycosci. 2014, 61, 59–66. [Google Scholar] [CrossRef]
- Nihira, T.; Saito, Y.; Ohtsubo, K.; Nakai, H.; Kitaoka, M. 2-O-α-D-Glucosylglycerol phosphorylase from Bacillus selenitireducens MLS10 possessing hydrolytic activity on β-D-Glucose 1-Phosphate. PLoS ONE 2014, 9, e86548. [Google Scholar] [CrossRef]
- Yernool, D.A.; McCarthy, J.K.; Eveleigh, D.E.; Bok, J.-D. Cloning and characterization of the glucooligosaccharide catabolic pathway β-Glucan glucohydrolase and cellobiose phosphorylase in the marine hyperthermophile Thermotoga neapolitana. J. Bacteriol. 2000, 182, 5172–5179. [Google Scholar] [CrossRef]
- Reichenbecher, M.; Lottspeich, F.; Bronnenmeier, K. Purification and properties of a cellobiose phosphorylase (CepA) and a cellodextrin phosphorylase (CepB) from the cellulolytic thermophile Clostridium Stercorarium. Eur. J. Biochem. 1997, 247, 262–267. [Google Scholar] [CrossRef]
- Rajashekhara, E.; Kitaoka, M.; Kim, Y.-K.; Hayashi, K. Characterization of a cellobiose phosphorylase from a Hyperthermophilic Eubacterium, Thermotoga maritima MSB8. Biosci. Biotechnol. Biochem. 2002, 66, 2578–2586. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Kitaoka, M.; Krishnareddy, M.; Mori, Y.; Hayashi, K. Kinetic studies of a recombinant cellobiose phosphorylase (CBP) of the Clostridium thermocellum YM4 strain expressed in Escherichia coli. J. Biochem. 2002, 132, 197–203. [Google Scholar] [CrossRef]
- Nidetzky, B.; Griessler, R.; Schwarz, A.; Splechtna, B. Cellobiose phosphorylase from Cellulomonas uda: Gene cloning and expression in Escherichia coli, and application of the recombinant enzyme in a ‘glycosynthase-type’ reaction. J. Mol. Catal. B Enzym. 2004, 29, 241–248. [Google Scholar] [CrossRef]
- Ha, S.-J.; Galazka, J.M.; Joong Oh, E.; Kordić, V.; Kim, H.; Jin, Y.-S.; Cate, J.H.D. Energetic benefits and rapid cellobiose fermentation by Saccharomyces cerevisiae expressing cellobiose phosphorylase and mutant cellodextrin transporters. Metab. Eng. 2013, 15, 134–143. [Google Scholar] [CrossRef]
- Nakai, H.; Hachem, M.A.; Petersen, B.O.; Westphal, Y.; Mannerstedt, K.; Baumann, M.J.; Dilokpimol, A.; Schols, H.A.; Duus, J.Ø.; Svensson, B. Efficient chemoenzymatic oligosaccharide synthesis by reverse phosphorolysis using cellobiose phosphorylase and cellodextrin phosphorylase from Clostridium thermocellum. Biochimie 2010, 92, 1818–1826. [Google Scholar] [CrossRef] [PubMed]
- Fosses, A.; Maté, M.; Franche, N.; Liu, N.; Denis, Y.; Borne, R.; de Philip, P.; Fierobe, H.-P.; Perret, S. A seven-gene cluster in Ruminiclostridium cellulolyticum is essential for signalization, uptake and catabolism of the degradation products of cellulose hydrolysis. Biotechnol. Biofuels 2017, 10, 250. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.K. Purification and specificity of cellobiose phosphorylase from Clostridium thermocellum. J. Biol. Chem. 1968, 243, 2899–2904. [Google Scholar] [CrossRef]
- Sasaki, T.; Tanaka, T.; Nakagawa, S.; Kainuma, K. Purification and properties of Cellvibrio gilvus cellobiose phosphorylase. Biochem. J. 1983, 209, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Dakhova, O.N.; Kurepina, N.E.; Zverlov, V.V.; Svetlichnyi, V.A.; Velikodvorskaya, G.A. Cloning and expression in Escherichia coli of Thermotoga neapolitana genes coding for enzymes of carbohydrate substrate degradation. Biochem. Biophys. Res. Commun. 1993, 194, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Macdonald, S.S.; Armstrong, Z.; Morgan-Lang, C.; Osowiecka, M.; Robinson, K.; Hallam, S.J.; Withers, S.G. Development and application of a high-throughput functional metagenomic screen for glycoside phosphorylases. Cell Chem. Biol. 2019, 26, 1001.e5–1012.e5. [Google Scholar] [CrossRef]
- Sawano, T.; Saburi, W.; Hamura, K.; Matsui, H.; Mori, H. Characterization of Ruminococcus albus cellodextrin phosphorylase and identification of a key phenylalanine residue for acceptor specificity and affinity to the phosphate group. FEBS J. 2013, 280, 4463–4473. [Google Scholar] [CrossRef]
- Iñón de Iannino, N.; Briones, G.; Tolmasky, M.; Ugalde, R.A. Molecular cloning and characterization of cgs, the Brucella abortus Cyclic β(1-2) glucan synthetase gene: Genetic complementation of Rhizobium meliloti ndvB and Agrobacterium tumefaciens chvB mutants. J. Bacteriol. 1998, 180, 4392–4400. [Google Scholar] [CrossRef]
- Kitaoka, M.; Matsuoka, Y.; Mori, K.; Nishimoto, M.; Hayashi, K. Characterization of a bacterial laminaribiose phosphorylase. Biosci. Biotechnol. Biochem. 2012, 76, 343–348. [Google Scholar] [CrossRef]
- Kuhaudomlarp, S.; Walpole, S.; Stevenson, C.E.M.; Nepogodiev, S.A.; Lawson, D.M.; Angulo, J.; Field, R.A. Unravelling the Specificity of Laminaribiose Phosphorylase from Paenibacillus sp. YM-1 towards Donor Substrates Glucose/Mannose 1-Phosphate by Using X-ray Crystallography and Saturation Transfer Difference NMR Spectroscopy. ChemBioChem 2019, 20, 181–192. [Google Scholar] [CrossRef]
- Park, J.K.; Keyhani, N.O.; Roseman, S. Chitin Catabolism In the marine bacterium Vibrio Furnissii identification, molecular cloning, and characterization of An,N′-Diacetylchitobiose phosphorylase. J. Biol. Chem. 2000, 275, 33077–33083. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Kitaoka, M.; Hayashi, K. Reaction mechanism of chitobiose phosphorylase from Vibrio proteolyticus: Identification of family 36 glycosyltransferase in Vibrio. Biochem. J. 2004, 377, 225–232. [Google Scholar] [CrossRef] [PubMed]
- De Doncker, M.; De Graeve, C.; Franceus, J.; Beerens, K.; Křen, V.; Pelantová, H.; Vercauteren, R.; Desmet, T. Exploration of GH94 sequence space for enzyme discovery reveals a novel Glucosylgalactose phosphorylase specificity. Chembiochem 2021, 22, 3319–3325. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Toyoizumi, H.; Abe, K.; Nakai, H.; Taguchi, H.; Kitaoka, M. 1,2-β-Oligoglucan Phosphorylase from Listeria innocua. PLoS ONE 2014, 9, e92353. [Google Scholar] [CrossRef]
- Nakajima, M.; Tanaka, N.; Furukawa, N.; Nihira, T.; Kodutsumi, Y.; Takahashi, Y.; Sugimoto, N.; Miyanaga, A.; Fushinobu, S.; Taguchi, H.; et al. Mechanistic insight into the substrate specificity of 1,2-β-oligoglucan phosphorylase from LachnoClostridium phytofermentans. Sci. Rep. 2017, 7, 42671. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Ikeuchi, Y.; Tsutsumi, N.; Kan, A.; Sumitani, J.-I.; Arai, M. Cloning, nucleotide sequence, and expression of the Clostridium thermocellum cellodextrin phosphorylase gene and its application to synthesis of cellulase inhibitors. J. Ferment. Bioeng. 1998, 85, 144–149. [Google Scholar] [CrossRef]
- Nihira, T.; Saito, Y.; Nishimoto, M.; Kitaoka, M.; Igarashi, K.; Ohtsubo, K.; Nakai, H. Discovery of cellobionic acid phosphorylase in cellulolytic bacteria and fungi. FEBS Lett. 2013, 587, 3556–3561. [Google Scholar] [CrossRef]
- Nam, Y.-W.; Nihira, T.; Arakawa, T.; Saito, Y.; Kitaoka, M.; Nakai, H.; Fushinobu, S. Crystal structure and substrate recognition of cellobionic acid phosphorylase, which plays a key role in oxidative cellulose degradation by microbes. J. Biol. Chem. 2015, 290, 18281–18292. [Google Scholar] [CrossRef]
- Nihira, T.; Saito, Y.; Kitaoka, M.; Nishimoto, M.; Otsubo, K.; Nakai, H. Characterization of a laminaribiose phosphorylase from Acholeplasma laidlawii PG-8A and production of 1,3-β-d-glucosyl disaccharides. Carbohydr. Res. 2012, 361, 49–54. [Google Scholar] [CrossRef]
- Nakajima, M.; Nishimoto, M.; Kitaoka, M. Characterization of d-galactosyl-β1→4-l-rhamnose phosphorylase from Opitutus terrae. Enzym. Microb. Technol. 2010, 46, 315–319. [Google Scholar] [CrossRef]
- Nakajima, M.; Nishimoto, M.; Kitaoka, M. Characterization of three β-Galactoside Phosphorylases from Clostridium phytofermentans. J. Biol. Chem. 2009, 284, 19220–19227. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Kitaoka, M. Identification of Lacto- N -Biose I Phosphorylase from Vibrio vulnificus CMCP6. Appl. Environ. Microbiol. 2008, 74, 6333–6337. [Google Scholar] [CrossRef] [PubMed]
- Pichler, M.J.; Yamada, C.; Shuoker, B.; Alvarez-Silva, C.; Gotoh, A.; Leth, M.L.; Schoof, E.; Katoh, T.; Sakanaka, M.; Katayama, T.; et al. Butyrate producing colonic Clostridiales metabolise human milk oligosaccharides and cross feed on mucin via conserved pathways. Nat. Commun. 2020, 11, 3285. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, M.; Tian, J.; Nishimoto, M. Novel putative galactose operon involving Lacto- N -Biose phosphorylase in Bifidobacterium longum. Appl. Environ. Microbiol. 2005, 71, 3158–3162. [Google Scholar] [CrossRef]
- Nishimoto, M.; Kitaoka, M. Identification of the putative proton donor residue of Lacto- N -biose phosphorylase (EC 2.4.1.211). Biosci. Biotechnol. Biochem. 2007, 71, 1587–1591. [Google Scholar] [CrossRef]
- Nakajima, M.; Nishimoto, M.; Kitaoka, M. Characterization of β-1,3-galactosyl-N-acetylhexosamine phosphorylase from Propionibacterium acnes. Appl. Microbiol. Biotechnol. 2009, 83, 109–115. [Google Scholar] [CrossRef]
- Tsuda, T.; Nihira, T.; Chiku, K.; Suzuki, E.; Arakawa, T.; Nishimoto, M.; Kitaoka, M.; Nakai, H.; Fushinobu, S. Characterization and crystal structure determination of β-1,2-mannobiose phosphorylase from Listeria innocua. FEBS Lett. 2015, 589, 3816–3821. [Google Scholar] [CrossRef]
- Chiku, K.; Nihira, T.; Suzuki, E.; Nishimoto, M.; Kitaoka, M.; Ohtsubo, K.; Nakai, H. Discovery of two β-1,2-Mannoside Phosphorylases showing different chain-length specificities from Thermoanaerobacter sp. X-514. PLoS ONE 2014, 9, e114882. [Google Scholar] [CrossRef]
- Awad, F.N.; Laborda, P.; Wang, M.; Lu, A.M.; Li, Q.; Cai, Z.P.; Liu, L.; Voglmeir, J. Discovery and biochemical characterization of a mannose phosphorylase catalyzing the synthesis of novel β-1,3-mannosides. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 3231–3237. [Google Scholar] [CrossRef]
- Saburi, W.; Tanaka, Y.; Muto, H.; Inoue, S.; Odaka, R.; Nishimoto, M.; Kitaoka, M.; Mori, H. Functional reassignment of Cellvibrio vulgaris EpiA to cellobiose 2-epimerase and an evaluation of the biochemical functions of the 4-O-β-d-mannosyl-d-glucose phosphorylase-like protein, UnkA. Biosci. Biotechnol. Biochem. 2015, 79, 969–977. [Google Scholar] [CrossRef]
- Kawahara, R.; Saburi, W.; Odaka, R.; Taguchi, H.; Ito, S.; Mori, H.; Matsui, H. Metabolic Mechanism of Mannan in a Ruminal Bacterium, Ruminococcus albus, Involving Two Mannoside Phosphorylases and Cellobiose 2-Epimerase. J. Biol. Chem. 2012, 287, 42389–42399. [Google Scholar] [CrossRef] [PubMed]
- Senoura, T.; Ito, S.; Taguchi, H.; Higa, M.; Hamada, S.; Matsui, H.; Ozawa, T.; Jin, S.; Watanabe, J.; Wasaki, J.; et al. New microbial mannan catabolic pathway that involves a novel mannosylglucose phosphorylase. Biochem. Biophys. Res. Commun. 2011, 408, 701–706. [Google Scholar] [CrossRef] [PubMed]
- La Rosa, S.L.; Leth, M.L.; Michalak, L.; Hansen, M.E.; Pudlo, N.A.; Glowacki, R.; Pereira, G.; Workman, C.T.; Arntzen, M.Ø.; Pope, P.B.; et al. The human gut Firmicute Roseburia intestinalis is a primary degrader of dietary β-mannans. Nat. Commun. 2019, 10, 905. [Google Scholar] [CrossRef] [PubMed]
- Jaito, N.; Saburi, W.; Odaka, R.; Kido, Y.; Hamura, K.; Nishimoto, M.; Kitaoka, M.; Matsui, H.; Mori, H. Characterization of a thermophilic 4-O-β-d-mannosyl-d-glucose phosphorylase from Rhodothermus marinus. Biosci. Biotechnol. Biochem. 2014, 78, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Nihira, T.; Suzuki, E.; Kitaoka, M.; Nishimoto, M.; Ohtsubo, K.; Nakai, H. Discovery of β-1,4-d-Mannosyl-N-acetyl-d-glucosamine phosphorylase involved in the metabolism of N-Glycans. J. Biol. Chem. 2013, 288, 27366–27374. [Google Scholar] [CrossRef]
- Ladevèze, S.; Tarquis, L.; Cecchini, D.A.; Bercovici, J.; André, I.; Topham, C.M.; Morel, S.; Laville, E.; Monsan, P.; Lombard, V.; et al. Role of glycoside phosphorylases in mannose foraging by human gut bacteria. J. Biol. Chem. 2013, 288, 32370–32383. [Google Scholar] [CrossRef]
- Grimaud, F.; Pizzut-Serin, S.; Tarquis, L.; Ladevèze, S.; Morel, S.; Putaux, J.-L.; Potocki-Veronese, G. In Vitro Synthesis and crystallization of β-1,4-Mannan. Biomacromolecules 2019, 20, 846–853. [Google Scholar] [CrossRef]
- Chekan, J.R.; Kwon, I.H.; Agarwal, V.; Dodd, D.; Revindran, V.; Mackie, R.I.; Cann, I.; Nair, S.K. Structural and biochemical basis for mannan utilization by Caldanaerobius polysaccharolyticus Strain ATCC BAA-17. J. Biol. Chem. 2014, 289, 34965–34977. [Google Scholar] [CrossRef]
- Li, A.; Laville, E.; Tarquis, L.; Lombard, V.; Ropartz, D.; Terrapon, N.; Henrissat, B.; Guieysse, D.; Esque, J.; Durand, J.; et al. Analysis of the diversity of the glycoside hydrolase family 130 in mammal gut microbiomes reveals a novel mannoside-phosphorylase function. Microb. Genom. 2020, 6, mgen000404. [Google Scholar] [CrossRef]
- Ollivier, S.; Tarquis, L.; Fanuel, M.; Li, A.; Durand, J.; Laville, E.; Potocki-Veronese, G.; Ropartz, D.; Rogniaux, H. Anomeric retention of carbohydrates in multistage cyclic ion mobility (IMSn): De novo structural elucidation of enzymatically produced Mannosides. Anal. Chem. 2021, 93, 6254–6261. [Google Scholar] [CrossRef]
- Kuhaudomlarp, S.; Stevenson, C.E.M.; Lawson, D.M.; Field, R.A. The structure of a GH149 β-(1→3) glucan phosphorylase reveals a new surface oligosaccharide binding site and additional domains that are absent in the disaccharide-specific GH94 glucose-β-(1→3)-glucose (Laminaribiose) phosphorylase. Proteins 2019, 87, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Kuhaudomlarp, S.; Patron, N.J.; Henrissat, B.; Rejzek, M.; Saalbach, G.; Field, R.A. Identification of Euglena gracilis β-1,3-glucan phosphorylase and establishment of a new glycoside hydrolase (GH) family GH149. J. Biol. Chem. 2018, 293, 2865–2876. [Google Scholar] [CrossRef] [PubMed]
- Kuhaudomlarp, S.; Pergolizzi, G.; Patron, N.J.; Henrissat, B.; Field, R.A. Unraveling the subtleties of β-(1→3)-glucan phosphorylase specificity in the GH94, GH149, and GH161 glycoside hydrolase families. J. Biol. Chem. 2019, 294, 6483–6493. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Kawashima, D.; Hashizume, A.; Hisamatsu, M.; Isono, N. Purification and characterization of 1,3-β-d-Glucan Phosphorylase from Ochromonas danica. Biosci. Biotechnol. Biochem. 2013, 77, 1949–1954. [Google Scholar] [CrossRef]
- Saito, K.; Kase, T.; Takahashi, E.; Takahashi, E.; Horinouchi, S. Purification and characterization of a trehalose synthase from the basidiomycete Grifola frondosa. Appl. Environ. Microbiol. 1998, 64, 4340–4345. [Google Scholar] [CrossRef]
- Han, S.-E.; Kwon, H.-B.; Lee, S.-B.; Yi, B.-Y.; Murayama, I.; Kitamoto, Y.; Byun, M.-O. Cloning and characterization of a gene encoding trehalose phosphorylase (TP) from Pleurotus sajor-caju. Protein Expr. Purif. 2003, 30, 194–202. [Google Scholar] [CrossRef]
- Eis, C.; Nidetzky, B. Characterization of trehalose phosphorylase from Schizophyllum commune. Biochem. J. 1999, 341 Pt 2, 385–393. [Google Scholar] [CrossRef]
- Goedl, C.; Griessler, R.; Schwarz, A.; Nidetzky, B. Structure–function relationships for Schizophyllum commune trehalose phosphorylase and their implications for the catalytic mechanism of family GT-4 glycosyltransferases. Biochem. J. 2006, 397, 491–500. [Google Scholar] [CrossRef]
- Strazzulli, A.; Cobucci-Ponzano, B.; Iacono, R.; Giglio, R.; Maurelli, L.; Curci, N.; Schiano-di-Cola, C.; Santangelo, A.; Contursi, P.; Lombard, V.; et al. Discovery of hyperstable carbohydrate-active enzymes through metagenomics of extreme environments. FEBS J. 2020, 287, 1116–1137. [Google Scholar] [CrossRef]
- Lee, H.-S.; Shockley, K.R.; Schut, G.J.; Conners, S.B.; Montero, C.I.; Johnson, M.R.; Chou, C.-J.; Bridger, S.L.; Wigner, N.; Brehm, S.D.; et al. Transcriptional and biochemical analysis of starch metabolism in the Hyperthermophilic archaeon Pyrococcus furiosus. J. Bacteriol. 2006, 188, 2115–2125. [Google Scholar] [CrossRef]
- Mizanur, R.M.; Griffin, A.K.K.; Pohl, N.L. Recombinant production and biochemical characterization of a hyperthermostable α-glucan/maltodextrin phosphorylase from Pyrococcus furiosus. Archaea 2008, 2, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Xavier, K.B.; Peist, R.; Kossmann, M.; Boos, W.; Santos, H. Maltose Metabolism in the Hyperthermophilic archaeon Thermococcus litoralis: Purification and characterization of key enzymes. J. Bacteriol. 1999, 181, 3358–3367. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C.M.; Rodriguez, M.; Johnson, C.M.; Martin, S.L.; Chu, T.M.; Wolfinger, R.D.; Hauser, L.J.; Land, M.L.; Klingeman, D.M.; Syed, M.H.; et al. Global transcriptome analysis of Clostridium thermocellum ATCC 27405 during growth on dilute acid pretreated Populus and switchgrass. Biotechnol. Biofuels 2013, 6, 179. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.C.; Rashid, A.M.; Stevenson, C.E.M.; Hetru, A.-C.; Gunning, A.P.; Rejzek, M.; Nepogodiev, S.A.; Bornemann, S.; Lawson, D.M.; Field, R.A. Sugar-coated sensor chip and nanoparticle surfaces for the in vitro enzymatic synthesis of starch-like materials. Chem. Sci. 2014, 5, 341–350. [Google Scholar] [CrossRef]
- Ugalde, J.E.; Lepek, V.; Uttaro, A.; Estrella, J.; Iglesias, A.; Ugalde, R.A. Gene organization and transcription analysis of the Agrobacterium tumefaciens glycogen (glg) operon: Two transcripts for the single phosphoglucomutase gene. J. Bacteriol. 1998, 180, 6557–6564. [Google Scholar] [CrossRef] [PubMed]
- Bhuiyan, S.H.; Rus’d, A.A.; Kitaoka, M.; Hayashi, K. Characterization of a hyperthermostable glycogen phosphorylase from Aquifex aeolicus expressed in Escherichia coli. J. Mol. Catal. B Enzym. 2003, 22, 173–180. [Google Scholar] [CrossRef]
- Kiel, J.A.K.W.; Boels, J.M.; Beldman, G.; Venema, G. Glycogen in Bacillus subtilis: Molecular characterization of an operon encoding enzymes involved in glycogen biosynthesis and degradation. Mol. Microbiol. 1994, 11, 203–218. [Google Scholar] [CrossRef]
- Chen, S.; Liu, J.; Pei, H.; Li, J.; Zhou, J.; Xiang, H. Molecular investigation of a novel thermostable glucan phosphorylase from Thermoanaerobacter tengcongensis. Enzym. Microb. Technol. 2007, 41, 390–396. [Google Scholar] [CrossRef]
- Griessler, R.; Psik, B.; Schwarz, A.; Nidetzky, B. Relationships between structure, function and stability for pyridoxal 5′-phosphate-dependent starch phosphorylase from Corynebacterium callunae as revealed by reversible cofactor dissociation studies: Cofactor dissociation studies of starch phosphorylase. Eur. J. Biochem. 2004, 271, 3319–3329. [Google Scholar] [CrossRef]
- Seibold, G.M.; Wurst, M.; Eikmanns, B.J. Roles of maltodextrin and glycogen phosphorylases in maltose utilization and glycogen metabolism in Corynebacterium glutamicum. Microbiology 2009, 155, 347–358. [Google Scholar] [CrossRef]
- Rybak, K.V.; Slivinskaya, E.A.; Voroshilova, E.B.; Kozlov, Y.I. Method for Producing an l-Amino Acid Using a Bacterium of the Enterobacteriaceae Family Having a Pathway of Glycogen Biosynthesis Disrupted. US Patent US-0275569, 17 January 2006. [Google Scholar]
- Palm, D.; Goerl, R.; Burger, K.J. Evolution of catalytic and regulatory sites in phosphorylases. Nature 1985, 313, 500–502. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.; Watson, K.A.; Schinzel, R.; Palm, D.; Johnson, L.N. Oligosaccharide substrate binding in Escherichia coli maltodextrin phosphorylase. Nat. Struct. Mol. Biol. 1997, 4, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Takata, H.; Takaha, T.; Okada, S.; Takagi, M.; Imanaka, T. Characterization of a gene cluster for glycogen biosynthesis and a heterotetrameric ADP-glucose pyrophosphorylase from Bacillus stearothermophilus. J. Bacteriol. 1997, 179, 4689–4698. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sato, Y.; Okamoto-Shibayama, K.; Azuma, T. The malQ gene is essential for starch metabolism in Streptococcus mutans. J. Oral Microbiol. 2013, 5, 21285. [Google Scholar] [CrossRef] [PubMed]
- Koksharova, O.A.; Brandt, U.; Cerff, R. The gap1 operon of the cyanobacterium Synechococcus PCC 7942 carries a gene encoding glycogen phosphorylase and is induced under anaerobic conditions. Mikrobiologiia 2004, 73, 388–392. [Google Scholar] [CrossRef]
- Bibel, M.; Brettl, C.; Gosslar, U.; Kriegshäuser, G.; Liebl, W. Isolation and analysis of genes for amylolytic enzymes of the hyperthermophilic bacterium Thermotoga maritima. FEMS Microbiol. Lett. 1998, 158, 9–15. [Google Scholar] [CrossRef]
- Takaha, T.; Yanase, M.; Takata, H.; Okada, S. Structure and properties of Thermus aquaticus. ALPHA.-Glucan phosphorylase expressed in Escherichia coli. J. Appl. Glycosci. 2001, 48, 71–78. [Google Scholar] [CrossRef]
- Alonso-Casajús, N.; Dauvillée, D.; Viale, A.M.; Muñoz, F.J.; Baroja-Fernández, E.; Morán-Zorzano, M.T.; Eydallin, G.; Ball, S.; Pozueta-Romero, J. Glycogen Phosphorylase, the Product of the glgP Gene, catalyzes glycogen breakdown by removing glucose units from the nonreducing ends in Escherichia coli. J. Bacteriol. 2006, 188, 5266–5272. [Google Scholar] [CrossRef]
- Tsujino, S.; Shanske, S.; Valberg, S.J.; Cardinet, G.H.; Smith, B.P.; DiMauro, S. Cloning of bovine muscle glycogen phosphorylase cDNA and identification of a mutation in cattle with myophosphorylase deficiency, an animal model for McArdle’s disease. Neuromuscul. Disord. 1996, 6, 19–26. [Google Scholar] [CrossRef]
- Dauvillée, D.; Chochois, V.; Steup, M.; Haebel, S.; Eckermann, N.; Ritte, G.; Ral, J.-P.; Colleoni, C.; Hicks, G.; Wattebled, F.; et al. Plastidial phosphorylase is required for normal starch synthesis in Chlamydomonas reinhardtii. Plant J. 2006, 48, 274–285. [Google Scholar] [CrossRef]
- Rogers, P.V.; Luo, S.; Sucic, J.F.; Rutherford, C.L. Characterization and cloning of glycogen phosphorylase 1 from Dictyostelium discoideum. Biochim. Biophys. Acta (BBA)-Gene Struct. Expr. 1992, 1129, 262–272. [Google Scholar] [CrossRef]
- Jones, T.H.D.; Wright, B.E. Partial purification and characterization of glycogen phosphorylase from Dictyostelium discoideum. J. Bacteriol. 1970, 104, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Tick, G.; Cserpán, I.; Dombrádi, V.; Mechler, B.M.; Török, I.; Kiss, I. Structural and functional characterization of the DrosophilaGlycogen phosphorylase gene. Biochem. Biophys. Res. Commun. 1999, 257, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Muller, M. Glycogen Phosphorylase Sequences from the Amitochondriate Protists, Trichomonas vaginalis, Mastigamoeba balamuthi, Entamoeba histolytica and Giardia intestinalis1. J. Eukaryot. Microbiol. 2003, 50, 366–372. [Google Scholar] [CrossRef]
- Takeyasu, K.; Kawase, T.; Yoshimura, S.H. Intermolecular Interaction between Na +/K +-ATPase α subunit and glycogen phosphorylase. Ann. N. Y. Acad. Sci. 2003, 986, 522–524. [Google Scholar] [CrossRef]
- Newgard, C.B.; Littman, D.R.; van Genderen, C.; Smith, M.; Fletterick, R.J. Human brain glycogen phosphorylase. Cloning, sequence analysis, chromosomal mapping, tissue expression, and comparison with the human liver and muscle isozymes. J. Biol. Chem. 1988, 263, 3850–3857. [Google Scholar] [CrossRef]
- Newgard, C.B.; Nakano, K.; Hwang, P.K.; Fletterick, R.J. Sequence analysis of the cDNA encoding human liver glycogen phosphorylase reveals tissue-specific codon usage. Proc. Natl. Acad. Sci. USA 1986, 83, 8132–8136. [Google Scholar] [CrossRef]
- Burke, J.; Hwang, P.; Anderson, L.; Lebo, R.; Gorin, F.; Fletterick, R. Intron/exon structure of the human gene for the muscle isozyme of glycogen phosphorylase. Proteins 1987, 2, 177–187. [Google Scholar] [CrossRef]
- Cuesta-Seijo, J.A.; Ruzanski, C.; Krucewicz, K.; Meier, S.; Hägglund, P.; Svensson, B.; Palcic, M.M. Functional and structural characterization of plastidic starch phosphorylase during barley endosperm development. PLoS ONE 2017, 12, e0175488. [Google Scholar] [CrossRef]
- Lin, C.-T.; Yeh, K.-W.; Lee, P.-D.; Su, J.-C. Primary structure of sweet potato starch phosphorylase deduced from its cDNA sequence. Plant Physiol. 1991, 95, 1250–1253. [Google Scholar] [CrossRef]
- Lu, Y.; Steichen, J.M.; Yao, J.; Sharkey, T.D. The role of cytosolic α-Glucan Phosphorylase in maltose metabolism and the comparison of amylomaltase in Arabidopsis and Escherichia coli. Plant Physiol. 2006, 142, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Schliselfeld, L.H.; Danon, M.J. Inverse relationship of skeletal muscle glycogen from wild-type and genetically modified mice to their phosphorylase a activity. Biochem. Biophys. Res. Commun. 2002, 290, 874–877. [Google Scholar] [CrossRef] [PubMed]
- McInerney, M.; Serrano Rodriguez, G.; Pawlina, W.; Hurt, C.B.; Fletcher, B.S.; Laipis, P.J.; Frost, S.C. Glycogen phosphorylase is activated in response to glucose deprivation but is not responsible for enhanced glucose transport activity in 3T3-L1 adipocytes. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2002, 1570, 53–62. [Google Scholar] [CrossRef]
- Titani, K.; Koide, A.; Hermann, J.; Ericsson, L.H.; Kumar, S.; Wade, R.D.; Walsh, K.A.; Neurath, H.; Fischer, E.H. Complete amino acid sequence of rabbit muscle glycogen phosphorylase. Proc. Natl. Acad. Sci. USA 1977, 74, 4762–4766. [Google Scholar] [CrossRef]
- Fukui, T.; Shimomura, S.; Nakano, K. Potato and rabbit muscle phosphorylases: Comparative studies on the structure, function and regulation of regulatory and nonregulatory enzymes. Mol. Cell. Biochem. 1982, 42, 129–144. [Google Scholar] [CrossRef]
- Hwang, S.-K.; Nishi, A.; Satoh, H.; Okita, T.W. Rice endosperm-specific plastidial α-glucan phosphorylase is important for synthesis of short-chain malto-oligosaccharides. Arch. Biochem. Biophys. 2010, 495, 82–92. [Google Scholar] [CrossRef]
- Jha, A.B.; Dubey, R.S. Carbohydrate metabolism in growing rice seedlings under arsenic toxicity. J. Plant Physiol. 2004, 161, 867–872. [Google Scholar] [CrossRef]
- Walker, K.R. Characterisation of the Ovine Model of McArdle’s Disease: Development of Therapeutic Strategies. Ph.D. Thesis, Murdoch University, Perth, Australia, 2006. [Google Scholar]
- Tan, P.; Allen, J.G.; Wilton, S.D.; Akkari, P.A.; Huxtable, C.R.; Laing, N.G. A splice-site mutation causing ovine McArdle’s disease. Neuromuscul. Disord. 1997, 7, 336–342. [Google Scholar] [CrossRef]
- Crerar, M.M.; Hudson, J.W.; Matthews, K.E.; David, E.S.; Golding, G.B. Studies on the expression and evolution of the glycogen phosphorylase gene family in the rat. Genome 1988, 30, 582–590. [Google Scholar] [CrossRef]
- Tan, A.W.H.; Nuttall, F.Q. Characteristics of the dephosphoryated form of phosphorylase purified from rat liver and measurement of its activity in crude liver preparations. Biochim. Biophys. Acta (BBA)-Enzymol. 1975, 410, 45–60. [Google Scholar] [CrossRef]
- Berndt, N.; Rösen, P. Isolation and partial characterization of two forms of rat heart glycogen phosphorylase. Arch. Biochem. Biophys. 1984, 228, 143–154. [Google Scholar] [CrossRef]
- Hwang, P.K.; Tugendreich, S.; Fletterick, R.J. Molecular analysis of GPH1, the gene encoding glycogen phosphorylase in Saccharomyces cerevisiae. Mol. Cell. Biol. 1989, 9, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Rath, V.L.; Hwang, P.K.; Fletterick, R.J. Purification and crystallization of glycogen phosphorylase from Saccharomyces cerevisiae. J. Mol. Biol. 1992, 225, 1027–1034. [Google Scholar] [CrossRef]
- Mori, H.; Tanizawa, K.; Fukui, T. Potato tuber type H phosphorylase isozyme. Molecular cloning, nucleotide sequence, and expression of a full-length cDNA in Escherichia coli. J. Biol. Chem. 1991, 266, 18446–18453. [Google Scholar] [CrossRef]
- Nakano, K.; Mori, H.; Fukui, T. Molecular cloning of cDNA encoding potato amyloplast a-Glucan phosphorylase and the structure of its transit peptide1. J. Biochem. 1989, 106, 691–695. [Google Scholar] [CrossRef]
- Sonnewald, U.; Basner, A.; Greve, B.; Steup, M. A second L-type isozyme of potato glucan phosphorylase: Cloning, antisense inhibition and expression analysis. Plant Mol Biol 1995, 27, 567–576. [Google Scholar] [CrossRef]
- Schupp, N.; Ziegler, P. The relation of starch phosphorylases to starch metabolism in wheat. Plant Cell Physiol. 2004, 45, 1471–1484. [Google Scholar] [CrossRef]
- Buchner, P.; Borisjuk, L.; Wobus, U. Glucan phosphorylases in Vicia faba L.: Cloning, structural analysis and expression patterns of cytosolic and plastidic forms in relation to starch. Planta 1996, 199, 64–73. [Google Scholar] [CrossRef]
- Sernee, M.F.; Ralton, J.E.; Nero, T.L.; Sobala, L.F.; Kloehn, J.; Vieira-Lara, M.A.; Cobbold, S.A.; Stanton, L.; Pires, D.E.V.; Hanssen, E.; et al. A family of dual-activity glycosyltransferase-phosphorylases mediates mannogen turnover and virulence in leishmania parasites. Cell Host Microbe 2019, 26, 385.e9–399.e9. [Google Scholar] [CrossRef]
- Puchart, V. Glycoside phosphorylases: Structure, catalytic properties and biotechnological potential. Biotechnol. Adv. 2015, 33, 261–276. [Google Scholar] [CrossRef]
- Teze, D.; Coines, J.; Raich, L.; Kalichuk, V.; Solleux, C.; Tellier, C.; André-Miral, C.; Svensson, B.; Rovira, C. A single point mutation converts GH84 O -GlcNAc Hydrolases into phosphorylases: Experimental and theoretical evidence. J. Am. Chem. Soc. 2020, 142, 2120–2124. [Google Scholar] [CrossRef] [PubMed]
- Mayer, C.; Vocadlo, D.J.; Mah, M.; Rupitz, K.; Stoll, D.; Warren, R.A.J.; Withers, S.G. Characterization of a β-N-acetylhexosaminidase and a β-N-acetylglucosaminidase/β-glucosidase from Cellulomonas fimi. FEBS J. 2006, 273, 2929–2941. [Google Scholar] [CrossRef] [PubMed]
- Reith, J.; Mayer, C. Peptidoglycan turnover and recycling in Gram-positive bacteria. Appl. Microbiol. Biotechnol. 2011, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ferjani, A.; Mustardy, L.; Sulpice, R.; Marin, K.; Suzuki, I.; Hagemann, M.; Murata, N. Glucosylglycerol, a compatible solute, sustains cell division under salt stress. Plant Physiol. 2003, 131, 1628–1637. [Google Scholar] [CrossRef]
- Franceus, J.; Desmet, T. Sucrose phosphorylase and related enzymes in glycoside hydrolase family 13: Discovery, application and engineering. Int. J. Mol. Sci. 2020, 21, 2526. [Google Scholar] [CrossRef]
- Kitamoto, Y.; Akashi, H.; Tanaka, H.; Mori, N. α-Glucose-1-phosphate formation by a novel trehalose phosphorylase from Flammulina velutipes. FEMS Microbiol. Lett. 1988, 55, 147–150. [Google Scholar] [CrossRef][Green Version]
- Cori, G.T.; Cori, C.F. Crystalline Muscle Phosphorylase Iv. Formation of Glycogen. J. Biol. Chem. 1943, 151, 57–63. [Google Scholar] [CrossRef]
- Lee, Y.P. Potato phosphorylase. I. Purification, physicochemical properties and catalytic activity. Biochim. Biophys. Acta 1960, 43, 18–24. [Google Scholar] [CrossRef]
- Maruta, K.; Mukai, K.; Yamashita, H.; Kubota, M.; Chaen, H.; Fukuda, S.; Kurimoto, M. Gene encoding a trehalose phosphorylase from Thermoanaerobacter brockii ATCC 35047. Biosci. Biotechnol. Biochem. 2002, 66, 1976–1980. [Google Scholar] [CrossRef]
- Yamamoto, T.; Maruta, K.; Mukai, K.; Yamashita, H.; Nishimoto, T.; Kubota, M.; Fukuda, S.; Kurimoto, M.; Tsujisaka, Y. Cloning and sequencing of kojibiose phosphorylase gene from Thermoanaerobacter brockii ATCC35047. J. Biosci. Bioeng. 2004, 98, 99–106. [Google Scholar] [CrossRef]
- Kiss, J.Z.; Vasconcelos, A.C.; Triemer, R.E. Structure of the Euglenoid storage carbohydrate, paramylon. Am. J. Bot. 1987, 74, 877–882. [Google Scholar] [CrossRef]
- Nakajima, M.; Nihira, T.; Nishimoto, M.; Kitaoka, M. Identification of galacto-N-biose phosphorylase from Clostridium perfringens ATCC13124. Appl. Microbiol. Biotechnol. 2008, 78, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Derensy-Dron, D.; Krzewinski, F.; Brassart, C.; Bouquelet, S. β-1,3-Galactosyl-N-acetylhexosamine phosphorylase from Bifidobacterium bifidum DSM 20082: Characterization, partial purification and relation to mucin degradation. Biotechnol. Appl. Biochem. 1999, 29, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Ladevèze, S. Functional and Structural Insights into Glycoside Hydrolase Family 130 Enzymes: Implications in Carbohydrate Foraging by Human Gut Bacteria. Ph.D. Thesis, INSA de Toulouse, Toulouse, France, 2015. [Google Scholar]
- Cuskin, F.; Lowe, E.C.; Temple, M.J.; Zhu, Y.; Cameron, E.A.; Pudlo, N.A.; Porter, N.T.; Urs, K.; Thompson, A.J.; Cartmell, A.; et al. Human gut Bacteroidetes can utilize yeast mannan through a selfish mechanism. Nature 2015, 517, 165–169. [Google Scholar] [CrossRef] [PubMed]
- L’vov, V.L.; Shashkov, A.S.; Knirel, Y.A.; Arifulina, A.E.; Senchenkova, S.N.; Yakovlev, A.V.; Dmitriev, B.A. Structure of the O-specific polysaccharide chain of Shigella boydii type 5 lipopolysaccharide: A repeated study. Carbohydr. Res. 1995, 279, 183–192. [Google Scholar] [CrossRef]
- Garron, M.-L.; Henrissat, B. The continuing expansion of CAZymes and their families. Curr Opin Chem Biol 2019, 53, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Drula, E.; Garron, M.-L.; Dogan, S.; Lombard, V.; Henrissat, B.; Terrapon, N. The carbohydrate-active enzyme database: Functions and literature. Nucleic Acids Res. 2022, 50, D571–D577. [Google Scholar] [CrossRef]
- Cuskin, F.; Baslé, A.; Ladevèze, S.; Day, A.M.; Gilbert, H.J.; Davies, G.J.; Potocki-Véronèse, G.; Lowe, E.C. The GH130 family of mannoside phosphorylases contains glycoside hydrolases that target β-1,2-mannosidic linkages in Candida mannan. J. Biol. Chem. 2015, 290, 25023–25033. [Google Scholar] [CrossRef]
- Ladevèze, S.; Cioci, G.; Roblin, P.; Mourey, L.; Tranier, S.; Potocki-Véronèse, G. Structural bases for N-glycan processing by mannoside phosphorylase. Acta Cryst. D 2015, 71, 1335–1346. [Google Scholar] [CrossRef]
- Sprogøe, D.; van den Broek, L.A.M.; Mirza, O.; Kastrup, J.S.; Voragen, A.G.J.; Gajhede, M.; Skov, L.K. Crystal structure of sucrose phosphorylase from Bifidobacterium adolescentis. Biochemistry 2004, 43, 1156–1162. [Google Scholar] [CrossRef]
- Egloff, M.-P.; Uppenberg, J.; Haalck, L.; van Tilbeurgh, H. Crystal structure of maltose phosphorylase from Lactobacillus brevis. Structure 2001, 9, 689–697. [Google Scholar] [CrossRef]
- Touhara, K.K.; Nihira, T.; Kitaoka, M.; Nakai, H.; Fushinobu, S. Structural basis for reversible phosphorolysis and hydrolysis reactions of 2-O-α-Glucosylglycerol Phosphorylase. J. Biol. Chem. 2014, 289, 18067–18075. [Google Scholar] [CrossRef] [PubMed]
- Okada, S.; Yamamoto, T.; Watanabe, H.; Nishimoto, T.; Chaen, H.; Fukuda, S.; Wakagi, T.; Fushinobu, S. Structural and mutational analysis of substrate recognition in kojibiose phosphorylase. FEBS J. 2014, 281, 778–786. [Google Scholar] [CrossRef]
- Van Hoorebeke, A.; Stout, J.; Van der Meeren, R.; Kyndt, J.; Van Beeumen, J.; Savvides, S.N. Crystallization and X-ray diffraction studies of inverting trehalose phosphorylase from Thermoanaerobacter sp. Acta Cryst. F Struct. Biol. Cryst. Commun. 2010, 66, 442–447. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.C.; Pergolizzi, G.; Stevenson, C.E.M.; Lawson, D.M.; Nepogodiev, S.A.; Field, R.A. Cellodextrin phosphorylase from RuminiClostridium thermocellum: X-ray crystal structure and substrate specificity analysis. Carbohydr. Res. 2017, 451, 118–132. [Google Scholar] [CrossRef] [PubMed]
- Bianchetti, C.M.; Elsen, N.L.; Fox, B.G.; Phillips, G.N. Structure of cellobiose phosphorylase from Clostridium thermocellum in complex with phosphate. Acta Cryst. F Struct. Biol. Cryst. Commun. 2011, 67, 1345–1349. [Google Scholar] [CrossRef]
- Hidaka, M.; Kitaoka, M.; Hayashi, K.; Wakagi, T.; Shoun, H.; Fushinobu, S. Structural dissection of the reaction mechanism of cellobiose phosphorylase. Biochem. J. 2006, 398, 37–43. [Google Scholar] [CrossRef]
- Fushinobu, S.; Hidaka, M.; Hayashi, A.M.; Wakagi, T.; Shoun, H.; Kitaoka, M. Interactions between glycoside hydrolase family 94 cellobiose phosphorylase and glucosidase inhibitors. J. Appl. Glycosci. 2011, 58, 91–97. [Google Scholar] [CrossRef]
- Hidaka, M.; Honda, Y.; Kitaoka, M.; Nirasawa, S.; Hayashi, K.; Wakagi, T.; Shoun, H.; Fushinobu, S. Chitobiose Phosphorylase from Vibrio proteolyticus, a member of glycosyl transferase family 36, has a Clan GH-L-like (α/α)6 barrel fold. Structure 2004, 12, 937–947. [Google Scholar] [CrossRef]
- Hidaka, M.; Nishimoto, M.; Kitaoka, M.; Wakagi, T.; Shoun, H.; Fushinobu, S. The crystal structure of Galacto-N-biose/Lacto-N-biose I Phosphorylase. J. Biol. Chem. 2009, 284, 7273–7283. [Google Scholar] [CrossRef]
- Koyama, Y.; Hidaka, M.; Nishimoto, M.; Kitaoka, M. Directed evolution to enhance thermostability of galacto-N-biose/lacto-N-biose I phosphorylase. Protein Eng. Des. Sel. 2013, 26, 755–761. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nakae, S.; Ito, S.; Higa, M.; Senoura, T.; Wasaki, J.; Hijikata, A.; Shionyu, M.; Ito, S.; Shirai, T. Structure of novel enzyme in mannan biodegradation process 4-O-β-d-Mannosyl-d-Glucose phosphorylase MGP. J. Mol. Biol. 2013, 425, 4468–4478. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Saburi, W.; Odaka, R.; Kato, K.; Sakurai, N.; Komoda, K.; Nishimoto, M.; Kitaoka, M.; Mori, H.; Yao, M. Structural insights into the difference in substrate recognition of two mannoside phosphorylases from two GH130 subfamilies. FEBS Lett. 2016, 590, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Chang, Z.; Yang, J.; Liu, W.; Yang, Y.; Chen, C.-C.; Zhang, L.; Huang, J.-W.; Sun, Y.; Guo, R.-T. Structural investigation of a thermostable 1,2-β-mannobiose phosphorylase from Thermoanaerobacter sp. X-514. Biochem. Biophys. Res. Commun. 2021, 579, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.A. Phosphorylase recognition and phosphorolysis of its oligosaccharide substrate: Answers to a long outstanding question. EMBO J. 1999, 18, 4619–4632. [Google Scholar] [CrossRef]
- Geremia, S.; Campagnolo, M.; Schinzel, R.; Johnson, L.N. Enzymatic catalysis in crystals of Escherichia coli maltodextrin phosphorylase. J. Mol. Biol. 2002, 322, 413–423. [Google Scholar] [CrossRef]
- Campagnolo, M.; Campa, C.; Zorzi, R.D.; Wuerges, J.; Geremia, S. X-ray studies on ternary complexes of maltodextrin phosphorylase. Arch. Biochem. Biophys. 2008, 471, 11–19. [Google Scholar] [CrossRef]
- Mathieu, C.; de la Sierra-Gallay, I.L.; Duval, R.; Xu, X.; Cocaign, A.; Léger, T.; Woffendin, G.; Camadro, J.-M.; Etchebest, C.; Haouz, A.; et al. Insights into brain glycogen metabolism. J. Biol. Chem. 2016, 291, 18072–18083. [Google Scholar] [CrossRef]
- Rath, V.L.; Ammirati, M.; Danley, D.E.; Ekstrom, J.L.; Gibbs, E.M.; Hynes, T.R.; Mathiowetz, A.M.; McPherson, R.K.; Olson, T.V.; Treadway, J.L.; et al. Human liver glycogen phosphorylase inhibitors bind at a new allosteric site. Chem. Biol. 2000, 7, 677–682. [Google Scholar] [CrossRef]
- Rath, V.L.; Ammirati, M.; LeMotte, P.K.; Fennell, K.F.; Mansour, M.N.; Danley, D.E.; Hynes, T.R.; Schulte, G.K.; Wasilko, D.J.; Pandit, J. Activation of human liver glycogen phosphorylase by alteration of the secondary structure and packing of the catalytic core. Mol. Cell. 2000, 6, 139–148. [Google Scholar] [CrossRef]
- Ekstrom, J.L.; Pauly, T.A.; Carty, M.D.; Soeller, W.C.; Culp, J.; Danley, D.E.; Hoover, D.J.; Treadway, J.L.; Gibbs, E.M.; Fletterick, R.J.; et al. Structure-Activity analysis of the purine binding site of human liver glycogen phosphorylase. Chem. Biol. 2002, 9, 915–924. [Google Scholar] [CrossRef]
- Wright, S.W.; Rath, V.L.; Genereux, P.E.; Hageman, D.L.; Levy, C.B.; McClure, L.D.; McCoid, S.C.; McPherson, R.K.; Schelhorn, T.M.; Wilder, D.E.; et al. 5-Chloroindoloyl glycine amide inhibitors of glycogen phosphorylase: Synthesis, In Vitro, in vivo, and X-ray crystallographic characterization. Bioorganic Med. Chem. Lett. 2005, 15, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Klabunde, T.; Wendt, K.U.; Kadereit, D.; Brachvogel, V.; Burger, H.-J.; Herling, A.W.; Oikonomakos, N.G.; Kosmopoulou, M.N.; Schmoll, D.; Sarubbi, E.; et al. Acyl Ureas as human liver glycogen phosphorylase inhibitors for the treatment of Type 2 Diabetes. J. Med. Chem. 2005, 48, 6178–6193. [Google Scholar] [CrossRef] [PubMed]
- Pautsch, A.; Stadler, N.; Wissdorf, O.; Langkopf, E.; Moreth, W.; Streicher, R. Molecular recognition of the protein phosphatase 1 glycogen targeting subunit by glycogen phosphorylase. J. Biol. Chem. 2008, 283, 8913–8918. [Google Scholar] [CrossRef]
- Onda, K.; Suzuki, T.; Shiraki, R.; Yonetoku, Y.; Negoro, K.; Momose, K.; Katayama, N.; Orita, M.; Yamaguchi, T.; Ohta, M.; et al. Synthesis of 5-chloro-N-aryl-1H-indole-2-carboxamide derivatives as inhibitors of human liver glycogen phosphorylase a. Bioorganic Med. Chem. 2008, 16, 5452–5464. [Google Scholar] [CrossRef]
- Anderka, O.; Loenze, P.; Klabunde, T.; Dreyer, M.K.; Defossa, E.; Wendt, K.U.; Schmoll, D. Thermodynamic characterization of allosteric glycogen phosphorylase inhibitors. Biochemistry 2008, 47, 4683–4691. [Google Scholar] [CrossRef]
- Thomson, S.A.; Banker, P.; Bickett, D.M.; Boucheron, J.A.; Carter, H.L.; Clancy, D.C.; Cooper, J.P.; Dickerson, S.H.; Garrido, D.M.; Nolte, R.T.; et al. Anthranilimide based glycogen phosphorylase inhibitors for the treatment of type 2 diabetes. Part 3: X-ray crystallographic characterization, core and urea optimization and in vivo efficacy. Bioorganic Med. Chem. Lett. 2009, 19, 1177–1182. [Google Scholar] [CrossRef]
- Lukacs, C.M.; Oikonomakos, N.G.; Crowther, R.L.; Hong, L.-N.; Kammlott, R.U.; Levin, W.; Li, S.; Liu, C.-M.; Lucas-McGady, D.; Pietranico, S.; et al. The crystal structure of human muscle glycogen phosphorylase a with bound glucose and AMP: An intermediate conformation with T-state and R-state features. Proteins 2006, 63, 1123–1126. [Google Scholar] [CrossRef]
- Whittamore, P.R.O.; Addie, M.S.; Bennett, S.N.L.; Birch, A.M.; Butters, M.; Godfrey, L.; Kenny, P.W.; Morley, A.D.; Murray, P.M.; Oikonomakos, N.G.; et al. Novel thienopyrrole glycogen phosphorylase inhibitors: Synthesis, In Vitro SAR and crystallographic studies. Bioorganic Med. Chem. Lett. 2006, 16, 5567–5571. [Google Scholar] [CrossRef]
- Lin, K.; Rath, V.L.; Dai, S.C.; Fletterick, R.J.; Hwang, P.K. A protein phosphorylation switch at the conserved allosteric site in GP. Science 1996, 273, 1539–1541. [Google Scholar] [CrossRef]
- Bhaumik, P.; Dhepe, P.L. Conversion of Biomass into Sugars. In Biomass Sugars for Non-Fuel Applications; RSC Publishing: London, UK, 2015; pp. 1–53. [Google Scholar]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Dashtban, M.; Schraft, H.; Qin, W. Fungal bioconversion of lignocellulosic residues; opportunities & perspectives. Int. J. Biol. Sci. 2009, 5, 578–595. [Google Scholar] [CrossRef] [PubMed]
- Chen, R. Enzym. Microb. Technol. for synthesis of bioactive oligosaccharides: An update. Appl. Microbiol. Biotechnol. 2018, 102, 3017–3026. [Google Scholar] [CrossRef] [PubMed]
- Anderson, L.A.; Islam, M.A.; Prather, K.L.J. Synthetic biology strategies for improving microbial synthesis of “green” biopolymers. J. Biol. Chem. 2018, 293, 5053–5061. [Google Scholar] [CrossRef] [PubMed]
- Raimo, A. Carbohydrate Chemistry: Fundamentals and Applications; World Scientific Publishing Company: Singapore, 2018; ISBN 978-981-322-366-0. [Google Scholar]
- Blow, N. Glycobiology: A spoonful of sugar. Nature 2009, 457, 617–620. [Google Scholar] [CrossRef] [PubMed]
- Panza, M.; Pistorio, S.G.; Stine, K.J.; Demchenko, A.V. Automated chemical oligosaccharide synthesis: Novel approach to traditional challenges. Chem. Rev. 2018, 118, 8105–8150. [Google Scholar] [CrossRef]
- Eller, S.; Collot, M.; Yin, J.; Hahm, H.S.; Seeberger, P.H. Automated solid-phase synthesis of chondroitin sulfate glycosaminoglycans. Angew. Chem. Int. Ed. Engl. 2013, 52, 5858–5861. [Google Scholar] [CrossRef]
- Walvoort, M.T.C.; van den Elst, H.; Plante, O.J.; Kröck, L.; Seeberger, P.H.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D.C. Automated solid-phase synthesis of β-mannuronic acid alginates. Angew. Chem. Int. Ed. Engl. 2012, 51, 4393–4396. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Pedersen, C.M. Catalytic Glycosylations in oligosaccharide synthesis. Chem. Rev. 2018, 118, 8285–8358. [Google Scholar] [CrossRef]
- Pergolizzi, G.; Kuhaudomlarp, S.; Kalita, E.; Field, R.A. Glycan Phosphorylases in multi-enzyme synthetic processes. Protein Pept. Lett. 2017, 24, 696–709. [Google Scholar] [CrossRef]
- Nishimoto, M.; Kitaoka, M. Practical preparation of lacto-N-biose I, a candidate for the bifidus factor in human milk. Biosci. Biotechnol. Biochem. 2007, 71, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, M. Large scale production of lacto-N-biose I, a building block of type I human milk oligosaccharides, using sugar phosphorylases. Biosci. Biotechnol. Biochem. 2020, 84, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Goedl, C.; Sawangwan, T.; Wildberger, P.; Nidetzky, B. Sucrose phosphorylase: A powerful transglucosylation catalyst for synthesis of α-d-glucosides as industrial fine chemicals. Biocatal. Biotransformation 2010, 28, 10–21. [Google Scholar] [CrossRef]
- De Bruyn, F.; De Paepe, B.; Maertens, J.; Beauprez, J.; De Cocker, P.; Mincke, S.; Stevens, C.; De Mey, M. Development of an in vivo glucosylation platform by coupling production to growth: Production of phenolic glucosides by a glycosyltransferase of Vitis vinifera. Biotechnol. Bioeng. 2015, 112, 1594–1603. [Google Scholar] [CrossRef]
- Samain, E.; Lancelon-Pin, C.; Férigo, F.; Moreau, V.; Chanzy, H.; Heyraud, A.; Driguez, H. Phosphorolytic synthesis of cellodextrins. Carbohydr. Res. 1995, 271, 217–226. [Google Scholar] [CrossRef]
- Hiraishi, M.; Igarashi, K.; Kimura, S.; Wada, M.; Kitaoka, M.; Samejima, M. Synthesis of highly ordered cellulose II In Vitro using cellodextrin phosphorylase. Carbohydr. Res. 2009, 344, 2468–2473. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Gonzalez-Martinez, A.; Gonzalez-Lopez, J.; Martin-Ramos, D.; Martinez-Toledo, M.V.; Rivadeneyra, M.A. Precipitation of phosphate minerals by microorganisms isolated from a fixed-biofilm reactor used for the treatment of domestic wastewater. Int. J. Environ. Res. Public Health 2014, 11, 3689–3704. [Google Scholar] [CrossRef]
- Liu, J.; Yin, X.; Li, Z.; Wu, X.; Zheng, Z.; Fang, J.; Gu, G.; Wang, P.G.; Liu, X. Facile Enzymatic Synthesis of Diverse Naturally-Occurring β-d-Mannopyranosides catalyzed by glycoside phosphorylases. ACS Catal. 2021, 11, 2763–2768. [Google Scholar] [CrossRef]
- Britton, J.; Majumdar, S.; Weiss, G.A. Continuous flow biocatalysis. Chem. Soc. Rev. 2018, 47, 5891–5918. [Google Scholar] [CrossRef]
- Zhong, C.; Duić, B.; Bolivar, J.M.; Nidetzky, B. Three-Enzyme phosphorylase cascade immobilized on solid support for biocatalytic synthesis of cello–oligosaccharides. ChemCatChem 2020, 12, 1350–1358. [Google Scholar] [CrossRef]
- Yu, H.; Chen, X. One-pot multienzyme (OPME) systems for chemoenzymatic synthesis of carbohydrates. Org. Biomol. Chem. 2016, 14, 2809–2818. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Yang, J.; Li, Y.; Zhang, T.; Li, J.; Ren, C.; Men, Y.; Chen, P.; You, C.; Sun, Y.; et al. Artificially designed routes for the conversion of starch to value-added mannosyl compounds through coupling In Vitro and in vivo Metab. Eng. strategies. Metab. Eng. 2020, 61, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Maertens, J.; Beauprez, J.; Mey, M.D. Metabolically Engineered Organisms for the Production of Added Value Bio-Products. Patent WO2012007481A3, 19 January 2012. [Google Scholar]
- De Groeve, M.R.M.; De Baere, M.; Hoflack, L.; Desmet, T.; Vandamme, E.J.; Soetaert, W. Creating lactose phosphorylase enzymes by directed evolution of cellobiose phosphorylase. Protein Eng. Des. Sel. 2009, 22, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Groeve, M.R.M.D.; Remmery, L.; Hoorebeke, A.V.; Stout, J.; Desmet, T.; Savvides, S.N.; Soetaert, W. Construction of cellobiose phosphorylase variants with broadened acceptor specificity towards anomerically substituted glucosides. Biotechnol. Bioeng. 2010, 107, 413–420. [Google Scholar] [CrossRef]
- Kraus, M.; Grimm, C.; Seibel, J. Switching enzyme specificity from phosphate to resveratrol glucosylation. Chem. Commun. 2017, 53, 12181–12184. [Google Scholar] [CrossRef]
- Waites, M.J.; Morgan, N.L.; Rockey, J.S.; Higton, G. Industrial Microbiology: An Introduction; John Wiley & Sons: Hoboken, NJ, USA, 2009; ISBN 978-1-4443-1158-7. [Google Scholar]
- Fiske, C.H.; Subbarow, Y. The colorimetric determination of phosphorus. J. Biol. Chem. 1925, 66, 375–400. [Google Scholar] [CrossRef]
- Drueckes, P.; Schinzel, R.; Palm, D. Photometric microtiter assay of inorganic phosphate in the presence of acid-labile organic phosphates. Anal. Biochem. 1995, 230, 173–177. [Google Scholar] [CrossRef]
- Cogan, E.B.; Birrell, G.B.; Griffith, O.H. A robotics-based automated assay for inorganic and organic phosphates. Anal. Biochem. 1999, 271, 29–35. [Google Scholar] [CrossRef]
- Gawronski, J.D.; Benson, D.R. Microtiter assay for glutamine synthetase biosynthetic activity using inorganic phosphate detection. Anal. Biochem. 2004, 327, 114–118. [Google Scholar] [CrossRef]
- De Groeve, M.R.M.; Tran, G.H.; Van Hoorebeke, A.; Stout, J.; Desmet, T.; Savvides, S.N.; Soetaert, W. Development and application of a screening assay for glycoside phosphorylases. Anal. Biochem. 2010, 401, 162–167. [Google Scholar] [CrossRef]
- Tedokon, M.; Suzuki, K.; Kayamori, Y.; Fujita, S.; Katayama, Y. Enzymatic assay of inorganic phosphate with use of sucrose phosphorylase and phosphoglucomutase. Clin. Chem. 1992, 38, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Serrano, A.; Löffelhardt, W. Identification of two different glyceraldehyde-3-phosphate dehydrogenases (phosphorylating) in the photosynthetic protist Cyanophora paradoxa. Arch. Microbiol. 1994, 162, 14–19. [Google Scholar] [CrossRef]
- Suárez, A.S.G.; Stefan, A.; Lemma, S.; Conte, E.; Hochkoeppler, A. Continuous enzyme-coupled assay of phosphate- or pyrophosphate-releasing enzymes. BioTechniques 2012, 53, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, M.N.; Stecher, G.; Sultana, T.; Abel, G.; Popp, M.; Bonn, G.K. Determination of carbohydrates in medicinal plants-comparison between TLC, mf-MELDI-MS and GC-MS. Phytochem. Anal. 2011, 22, 296–302. [Google Scholar] [CrossRef]
- Harvey, D.J. Derivatization of carbohydrates for analysis by chromatography; electrophoresis and mass spectrometry. J. Chromatogr. B 2011, 879, 1196–1225. [Google Scholar] [CrossRef]
- Corradini, C.; Cavazza, A.; Bignardi, C. High-Performance Anion-Exchange Chromatography Coupled with Pulsed Electrochemical Detection as a Powerful Tool to Evaluate Carbohydrates of Food Interest: Principles and Applications. Available online: https://www.hindawi.com/journals/ijcc/2012/487564/ (accessed on 21 April 2020).
- Hellerqvist, C.G.; Lindberg, B.; Svensson, S.; Holme, T.; Lindberg, A.A. Structural studies on the O-specific side-chains of the cell-wall lipopolysaccharide from Salmonella typhimurium 395 ms. Carbohydr. Res. 1968, 8, 43–55. [Google Scholar] [CrossRef]
- Ciucanu, I. Per-O-methylation reaction for structural analysis of carbohydrates by mass spectrometry. Anal. Chim. Acta 2006, 576, 147–155. [Google Scholar] [CrossRef]
- Irague, R.; Massou, S.; Moulis, C.; Saurel, O.; Milon, A.; Monsan, P.; Remaud-Siméon, M.; Portais, J.-C.; Potocki-Véronèse, G. NMR-Based structural Glycomics for high-throughput screening of carbohydrate-active enzyme specificity. Anal. Chem. 2011, 83, 1202–1206. [Google Scholar] [CrossRef]
- Handelsman, J. Metagenomics: Application of genomics to uncultured microorganisms. Microbiol. Mol. Biol. Rev. 2004, 68, 669–685. [Google Scholar] [CrossRef]
- Han, L.; Costello, C.E. Mass spectrometry of glycans. Biochemistry 2013, 78, 710–720. [Google Scholar] [CrossRef]
- Ropartz, D.; Fanuel, M.; Ujma, J.; Palmer, M.; Giles, K.; Rogniaux, H. Structure determination of large isomeric oligosaccharides of natural origin through multipass and multistage cyclic traveling-wave ion mobility mass spectrometry. Anal. Chem. 2019, 91, 12030–12037. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.-T.; Liew, C.Y.; Hsu, C.; Huang, S.-P.; Weng, W.-C.; Kuo, Y.-H.; Ni, C.-K. Automatic full glycan structural determination through logically derived sequence tandem mass spectrometry. ChemBioChem 2019, 20, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wuertz, S. Bacteria and archaea on Earth and their abundance in biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Van de Guchte, M.; Blottière, H.M.; Doré, J. Humans as holobionts: Implications for prevention and therapy. Microbiome 2018, 6, 81. [Google Scholar] [CrossRef]
- Handelsman, J.; Rondon, M.R.; Brady, S.F.; Clardy, J.; Goodman, R.M. Molecular biological access to the chemistry of unknown soil microbes: A new frontier for natural products. Chem. Biol. 1998, 5, R245–R249. [Google Scholar] [CrossRef]
- Pace, N.R.; Stahl, D.A.; Lane, D.J.; Olsen, G.J. The analysis of natural microbial populations by ribosomal RNA Sequences. In Advances in Microbial Ecology; Marshall, K.C., Ed.; Advances in Microbial Ecology; Springer US: Boston, MA, USA, 1986; pp. 1–55. ISBN 978-1-4757-0611-6. [Google Scholar]
- Healy, F.G.; Ray, R.M.; Aldrich, H.C.; Wilkie, A.C.; Ingram, L.O.; Shanmugam, K.T. Direct isolation of functional genes encoding cellulases from the microbial consortia in a thermophilic, anaerobic digester maintained on lignocellulose. Appl. Microbiol. Biotechnol. 1995, 43, 667–674. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Tierney, B.T.; Yang, Z.; Luber, J.M.; Beaudin, M.; Wibowo, M.C.; Baek, C.; Mehlenbacher, E.; Patel, C.J.; Kostic, A.D. The Landscape of genetic content in the gut and oral human microbiome. Cell Host Microbe 2019, 26, 283.e8–295.e8. [Google Scholar] [CrossRef]
- Xiao, L.; Feng, Q.; Liang, S.; Sonne, S.B.; Xia, Z.; Qiu, X.; Li, X.; Long, H.; Zhang, J.; Zhang, D.; et al. A catalog of the mouse gut metagenome. Nat. Biotechnol. 2015, 33, 1103–1108. [Google Scholar] [CrossRef]
- Lesker, T.R.; Durairaj, A.C.; Gálvez, E.J.C.; Lagkouvardos, I.; Baines, J.F.; Clavel, T.; Sczyrba, A.; McHardy, A.C.; Strowig, T. An integrated metagenome catalog reveals new insights into the murine gut microbiome. Cell Rep. 2020, 30, 2909.e6–2922.e6. [Google Scholar] [CrossRef]
- Xiao, L.; Estellé, J.; Kiilerich, P.; Ramayo-Caldas, Y.; Xia, Z.; Feng, Q.; Liang, S.; Pedersen, A.Ø.; Kjeldsen, N.J.; Liu, C.; et al. A reference gene catalogue of the pig gut microbiome. Nat. Microbiol. 2016, 1, 16161. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhong, H.; Ramayo-Caldas, Y.; Terrapon, N.; Lombard, V.; Potocki-Veronese, G.; Estellé, J.; Popova, M.; Yang, Z.; Zhang, H.; et al. A catalog of microbial genes from the bovine rumen unveils a specialized and diverse biomass-degrading environment. GigaScience 2020, 9, giaa057. [Google Scholar] [CrossRef] [PubMed]
- Bailly, J.; Fraissinet-Tachet, L.; Verner, M.-C.; Debaud, J.-C.; Lemaire, M.; Wésolowski-Louvel, M.; Marmeisse, R. Soil eukaryotic functional diversity, a metatranscriptomic approach. ISME J. 2007, 1, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Tasse, L.; Bercovici, J.; Pizzut-Serin, S.; Robe, P.; Tap, J.; Klopp, C.; Cantarel, B.L.; Coutinho, P.M.; Henrissat, B.; Leclerc, M.; et al. Functional metagenomics to mine the human gut microbiome for dietary fiber catabolic enzymes. Genome Res. 2010, 20, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.N.; Charles, T.C. Strong spurious transcription likely contributes to DNA insert bias in typical metagenomic clone libraries. Microbiome 2015, 3, 22. [Google Scholar] [CrossRef]
- Taupp, M.; Mewis, K.; Hallam, S.J. The art and design of functional metagenomic screens. Curr. Opin. Biotechnol. 2011, 22, 465–472. [Google Scholar] [CrossRef]
- Lewin, A.; Lale, R.; Wentzel, A. Expression Platforms for Functional Metagenomics: Emerging Technology Options Beyond Escherichia coli. In Functional Metagenomics: Tools and Applications; Charles, T.C., Liles, M.R., Sessitsch, A., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; ISBN 978-3-319-61508-0. [Google Scholar]
- Lam, K.N.; Martens, E.C.; Charles, T.C. Developing a Bacteroides system for function-based screening of DNA from the human gut microbiome. mSystems 2018, 3, e00195-17. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Laville, E.; Cecchini, D.; Blottière, H.M.; Leclerc, M.; Doré, J.; Potocki-Veronese, G. Human gut metagenomics: Success and limits of the activity-based approaches. In Functional Metagenomics: Tools and Applications; Charles, T.C., Liles, M.R., Sessitsch, A., Eds.; Springer International Publishing: Cham, The Netherlands, 2017; pp. 161–178. ISBN 978-3-319-61510-3. [Google Scholar]
- Ufarté, L.; Potocki-Veronese, G.; Laville, É. Discovery of new protein families and functions: New challenges in functional metagenomics for biotechnologies and microbial ecology. Front. Microbiol. 2015, 6, 563. [Google Scholar] [CrossRef]
- Colin, P.-Y.; Kintses, B.; Gielen, F.; Miton, C.M.; Fischer, G.; Mohamed, M.F.; Hyvönen, M.; Morgavi, D.P.; Janssen, D.B.; Hollfelder, F. Ultrahigh-throughput discovery of promiscuous enzymes by picodroplet functional metagenomics. Nat. Commun. 2015, 6, 10008. [Google Scholar] [CrossRef]
- Cottrell, M.T.; Yu, L.; Kirchman, D.L. Sequence and expression analyses of Cytophaga-like hydrolases in a Western arctic metagenomic library and the Sargasso Sea. Appl. Environ. Microbiol. 2005, 71, 8506–8513. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Tatusov, R.L.; Koonin, E.V.; Lipman, D.J. A genomic perspective on protein families. Science 1997, 278, 631–637. [Google Scholar] [CrossRef] [PubMed]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam protein families database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Coggill, P.; Finn, R.D.; Bateman, A. Identifying Protein Domains with the Pfam Database. Curr. Protoc. Bioinform. 2008, 23, 2.5.1–2.5.17. [Google Scholar] [CrossRef] [PubMed]
- Markowitz, V.M.; Ivanova, N.N.; Szeto, E.; Palaniappan, K.; Chu, K.; Dalevi, D.; Chen, I.-M.A.; Grechkin, Y.; Dubchak, I.; Anderson, I.; et al. IMG/M: A data management and analysis system for metagenomes. Nucleic Acids Res 2008, 36, D534–D538. [Google Scholar] [CrossRef] [PubMed]
- Meyer, F.; Paarmann, D.; D’Souza, M.; Olson, R.; Glass, E.; Kubal, M.; Paczian, T.; Rodriguez, A.; Stevens, R.; Wilke, A.; et al. The metagenomics RAST server–a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinform. 2008, 9, 386. [Google Scholar] [CrossRef]
- Lapébie, P.; Lombard, V.; Drula, E.; Terrapon, N.; Henrissat, B. Bacteroidetes use thousands of enzyme combinations to break down glycans. Nat. Commun. 2019, 10, 1–7. [Google Scholar] [CrossRef]
- Lobb, B.; Doxey, A.C. Novel function discovery through sequence and structural data mining. Curr. Opin. Struct. Biol. 2016, 38, 53–61. [Google Scholar] [CrossRef]
- Gerlt, J.A.; Bouvier, J.T.; Davidson, D.B.; Imker, H.J.; Sadkhin, B.; Slater, D.R.; Whalen, K.L. Enzyme Function Initiative-Enzyme Similarity Tool (EFI-EST): A Web Tool for Generating Protein Sequence Similarity Networks. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2015, 1854, 1019–1037. [Google Scholar] [CrossRef]
- Atkinson, H.J.; Morris, J.H.; Ferrin, T.E.; Babbitt, P.C. Using sequence similarity networks for visualization of relationships across diverse protein superfamilies. PLoS ONE 2009, 4, e4345. [Google Scholar] [CrossRef]
- Levin, B.J.; Huang, Y.Y.; Peck, S.C.; Wei, Y.; Campo, A.M.; Marks, J.A.; Franzosa, E.A.; Huttenhower, C.; Balskus, E.P. A prominent glycyl radical enzyme in human gut microbiomes metabolizes trans-4-hydroxy-l-proline. Science 2017, 355, eaai8386. [Google Scholar] [CrossRef] [PubMed]
- Viborg, A.H.; Terrapon, N.; Lombard, V.; Michel, G.; Czjzek, M.; Henrissat, B.; Brumer, H. A Subfamily Roadmap for Functional Glycogenomics of the Evolutionarily Diverse Glycoside Hydrolase Family 16 (GH16). J. Biol. Chem. 2019, 294, 15973–15986. [Google Scholar] [CrossRef] [PubMed]
- Romero, P.A.; Tran, T.M.; Abate, A.R. Dissecting enzyme function with microfluidic-based deep mutational scanning. Proc. Natl. Acad. Sci. USA 2015, 112, 7159–7164. [Google Scholar] [CrossRef] [PubMed]
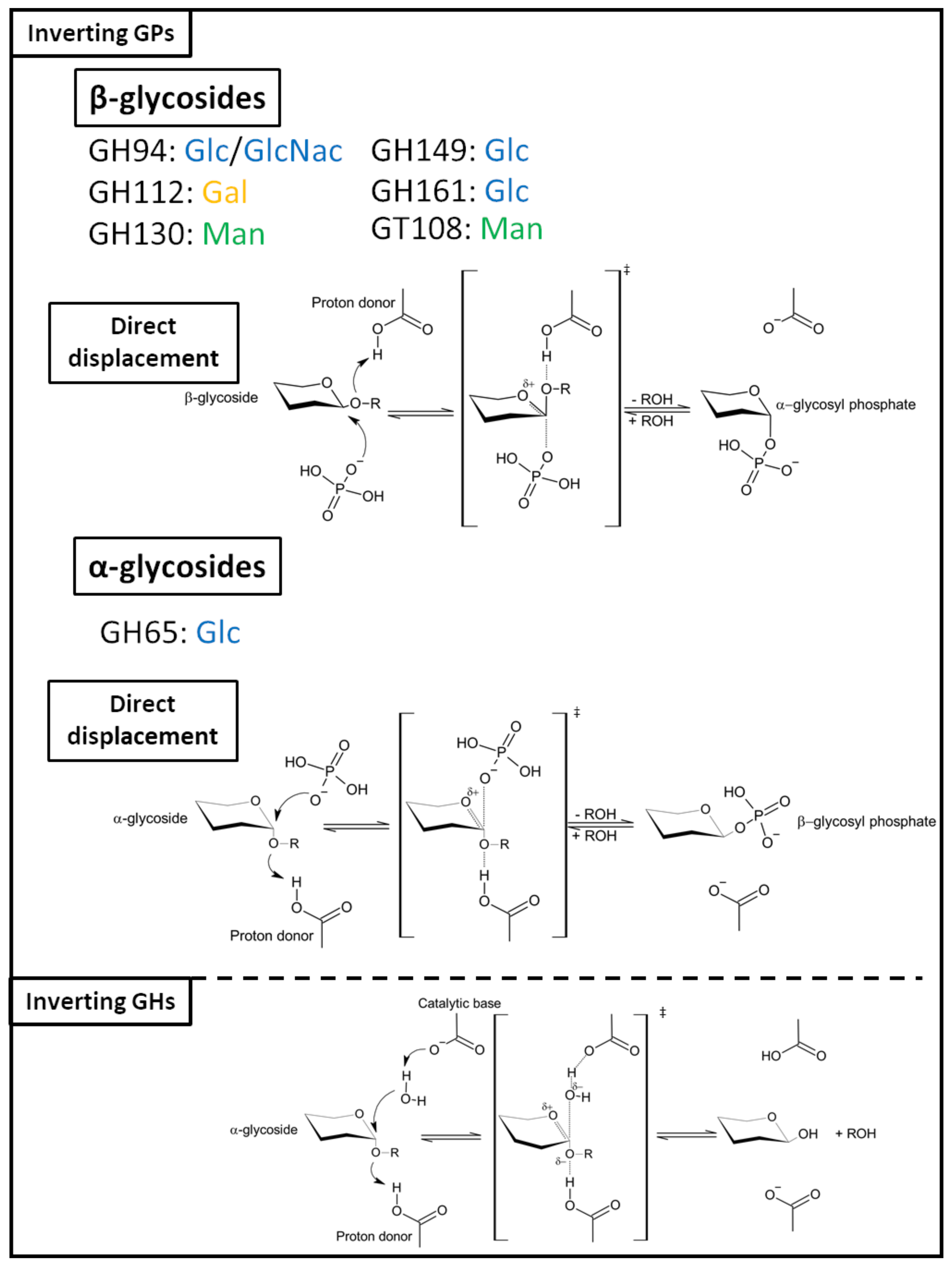

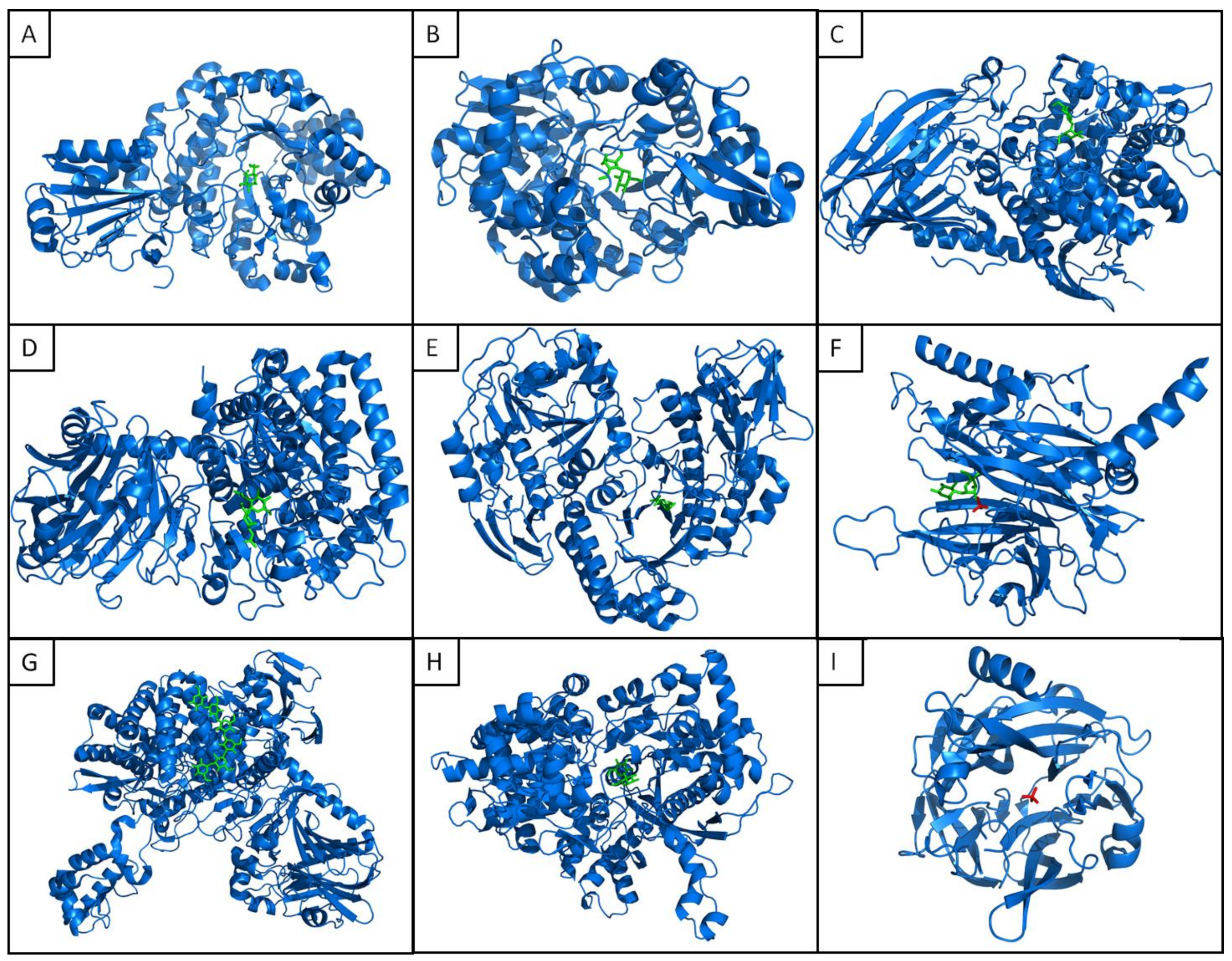
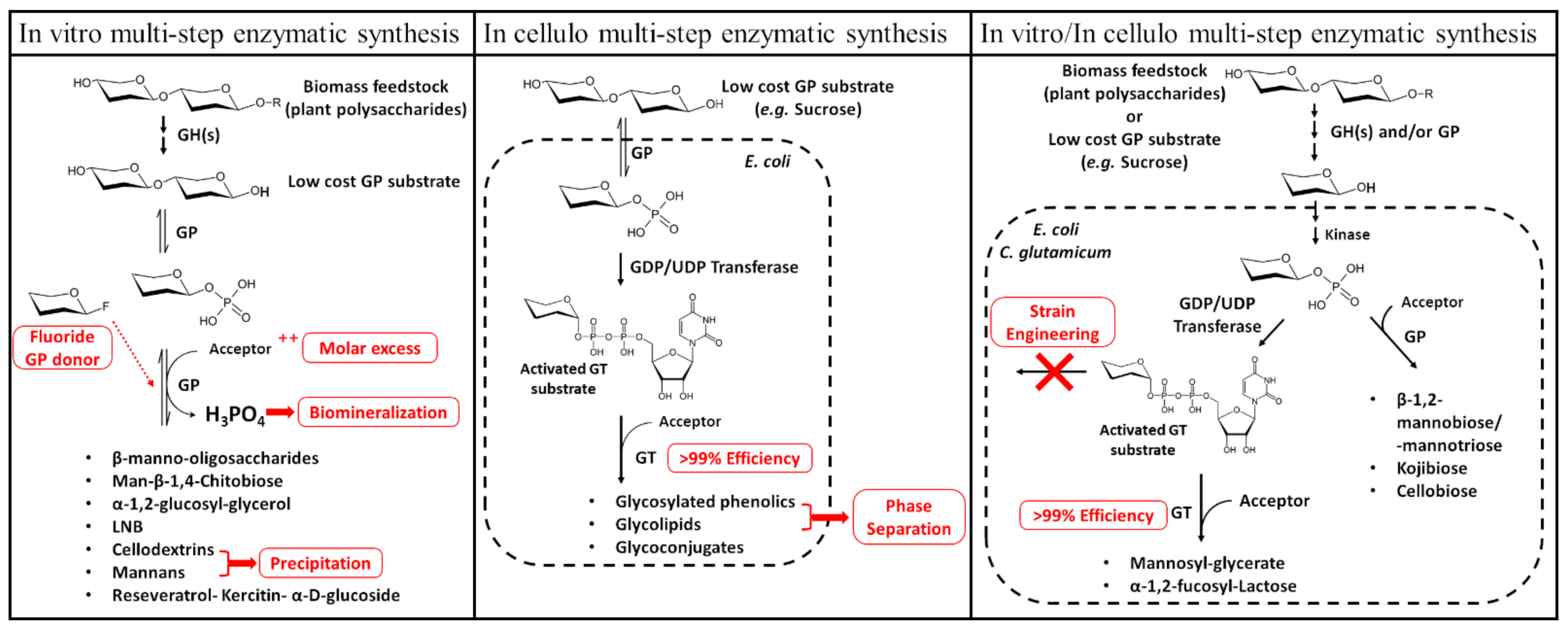
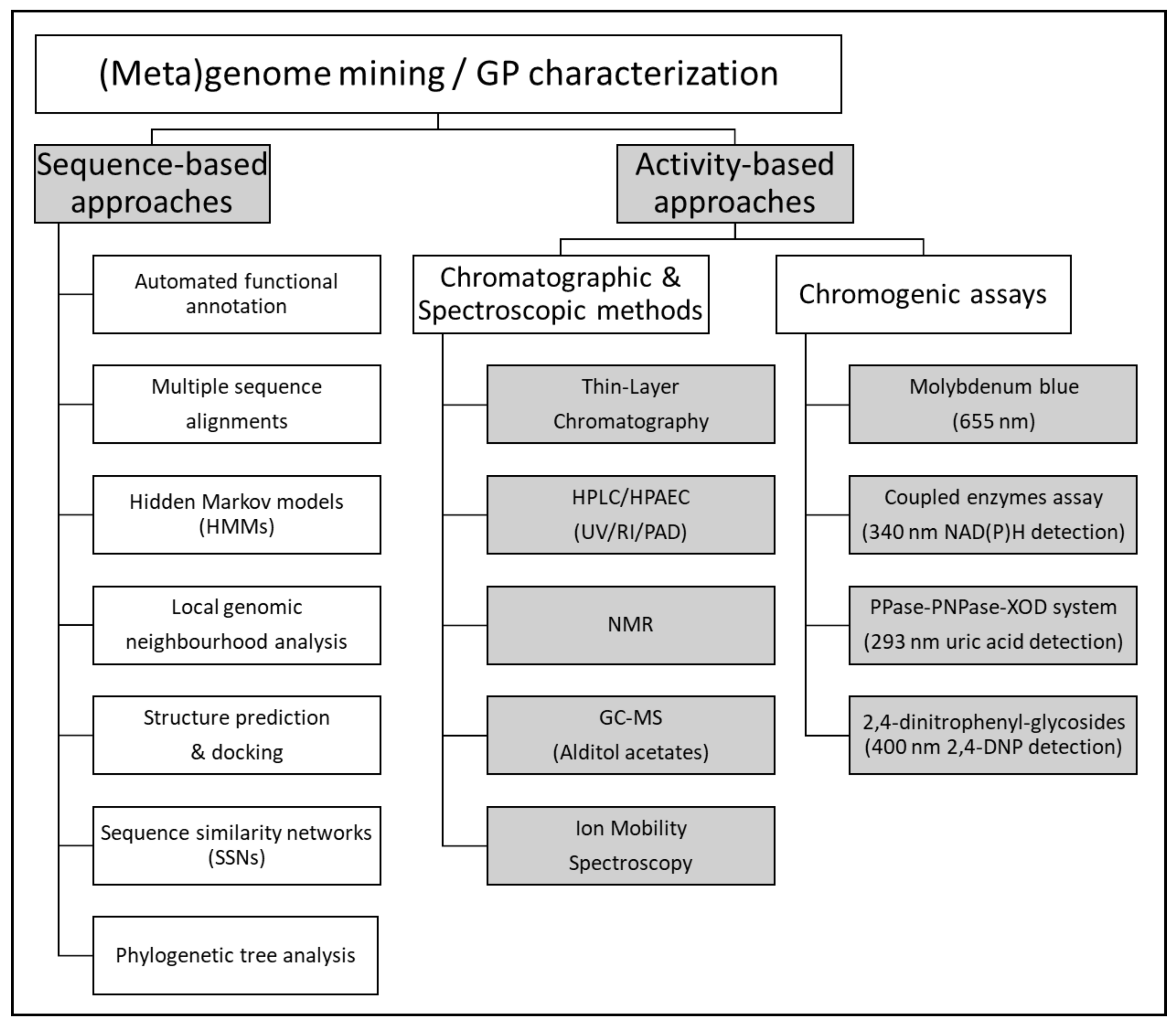
| Family | Mechanism | GenBank Uniprot | Protein Name | Organism | PDB | Reverse-Phosphorolysis Activity | Reference (DOI) | ||
|---|---|---|---|---|---|---|---|---|---|
| Donor | Acceptor | Product | |||||||
| GH3 | Retaining | AUG44408.1 | β-glucoside phosphorylase (BglP) | uncultured bacterium | 5VQD, 5VQE | β-GlcNac1P β-Glc1P | p-NP/DNP | p-Nitrophenyl-N-acetyl-β-d-glucosaminide p-nitrophenyl-β-d-glucopyranoside 2,4-Dinitrophenyl-β- d-glucopyranoside | [30] |
| GH3 | Retaining | AAQ05801.1 | Bifunctional N-acetyl-β-glucosaminidase/β-glucosidase (Nag3) | Cellulomonas fimi ATCC 484 | β-Glc1P | DNP | 2,4-Dinitrophenyl-β-d-glucopyranoside | [31] | |
| GH13_18 | Retaining | AAO33821.1 | Sucrose phosphorylase (SucP;SP;BaSP) | Bifidobacterium adolescentis DSM 20083 | 1R7A, 2GDU, 2GDV, 5C8B, 5M9X, 5MAN, 5MB2, 6FME | α-Glc1P | d-fructose | Sucrose: (α-d-glucopyranosyl-(1→2)-β-d-fructofuranoside) | [32] |
| GH13_18 | Retaining | AAN24362.1 | Sucrose phosphorylase (Spl;BL0536) | Bifidobacterium longum NCC2705 | α-Glc1P | d-fructose | Sucrose | [33] | |
| GH13_18 | Retaining | AAO84039.1 | Sucrose phosphorylase (SplP) | Bifidobacterium longum SJ32 | α-Glc1P | d fructose | Sucrose | [34] | |
| GH13_18 | Retaining | BAF62433.1 | Sucrose phosphorylase (Spl) | Bifidobacterium longum subsp. longum JCM 1217 | α-Glc1P | d-fructose | Sucrose | [35] | |
| GH13_18 | Retaining | AAC74391.2 | Glucosylglycerate phosphorylase (YcjM) | Escherichia coli str. K-12 substr. MG1655 | α-Glc1P | d glycerate | Glucosyl-glycerate | [28] | |
| GH13_18 | Retaining | AAO21868.1 | Sucrose phosphorylase (LaSP;GtfA2;LBA1437) | Lactobacillus acidophilus NCFM | α-Glc1P | d fructose | Sucrose | [36] | |
| GH13_18 | Retaining | BAA14344.1 | Sucrose phosphorylase (LmSPase) | Leuconostoc mesenteroides ATCC 12291 | α-Glc1P | d-fructose | Sucrose | [37] | |
| GH13_18 | Retaining | ABS59292.1 | Sucrose phosphorylase (742sp) | Leuconostoc mesenteroides B-742 | α-Glc1P | d-fructose | Sucrose | [38] | |
| GH13_18 | Retaining | 2207198A | Sucrose phosphorylase | Leuconostoc mesenteroides NO. 165 | α-Glc1P | d-fructose | Sucrose | [39] | |
| GH13_18 | Retaining | AAX33736.1 | Sucrose phosphorylase (LmSP1) | Leuconostoc mesenteroides NRRL B-1149 | α-Glc1P | d-fructose | Sucrose | [40] | |
| GH13_18 | Retaining | AGK37834.1 | Sucrose phosphorylase (ScrP) | Limosilactobacillus reuteri LTH5448 | α-Glc1P | d-fructose | Sucrose | [41] | |
| GH13_18 | Retaining | ADP98617.1 | Glucosylglycerol phosphorylase (HP15_2853) | Marinobacter adhaerens HP15 | α-Glc1P | d-glycerol | Glucosyl-glycerol: (α-d-glucopyranosyl-(1→2)-glycerol) | [42] | |
| GH13_18 | Retaining | ADH62582.1 | Glucosylglycerate phosphorylase (MSGGaP;Mesil_0665) | Meiothermus silvanus DSM 9946 | α-Glc1P | d-glycerate | Glucosyl-glycerate: (α-d-glucopyranosyl-(1→2)-glycerate) | [28] | |
| GH13_18 | Retaining | AAD40317.1 | Sucrose phosphorylase | Pelomonas saccharophila IAM 14368 | α-Glc1P | d-fructose | Sucrose | [43] | |
| GH13_18 | Retaining | CCA61958.1 | Sucrose 6(F)-phosphate phosphorylase (RUGNEv3_61221;SucP) | Ruminococcus gnavus E1 | α-Glc1P | d-fructose-6-P | Sucrose-6-phosphate | [44] | |
| GH13_18 | Retaining | AEJ61152.1 | Glucosylglycerate phosphorylase (Spith_0877) | Spirochaeta thermophila DSM 6578 | α-Glc1P | d-glycerate | Glucosyl-glycerate | [28] | |
| GH13_18 | Retaining | CAA30846.1 | Sucrose phosphorylase (GftA;SmSP) | Streptococcus mutans INGBRITT/GS5 | α-Glc1P | d-fructose | Sucrose | [45] | |
| GH13_18 | Retaining | AAN58596.1 | Sucrose phosphorylase (GtfA;SMU.881) | Streptococcus mutans UA159 | α-Glc1P | d-fructose | Sucrose | [46] | |
| GH13_18 | Retaining | CAA80424.1* | Sucrose phosphorylase | Agrobacterium vitis | α-Glc1P | d-fructose | Sucrose | [47] | |
| GH13_18 | Retaining | ADL69407.1 | Sucrose 6(F)-phosphate phosphorylase (SPP;TtSPP;Tthe_1921) | Thermoanaerobacterium thermosaccharolyticum DSM 571 | 6S9V | α-Glc1P | d-fructose-6-P | Sucrose-6-phosphate | [48] |
| GH13_18 | Retaining | ACJ83248.1 | Sucrose phosphorylase (Unspase) | Uncultured bacterium | α-Glc1P | d-fructose | Sucrose | [49] | |
| GH13_18 | Retaining | BAN03569.1 | Sucrose 6(F)-phosphate phosphorylase (YM304_32550) | Ilumatobacter coccineus YM16-304 | 6S9U | α-Glc1P | d-fructose-6-P | Sucrose-6-phosphate | [50] |
| GH65 | Inverting | AAV43670.1 | Maltose phosphorylase (MalP;LBA1870) | Lactobacillus acidophilus NCFM | β-Glc1P | d-glucose | Maltose: (α-d-glucosyl-(1→4)-d-glucose) | [51] | |
| GH65 | Inverting | ADH99560.1 | Maltose phosphorylase (Bsel_2056) | Bacillus selenitireducens MLS10 | β-Glc1P | d-glucose | Maltose | [52] | |
| GH65 | Inverting | BAC54904.1 | Maltose phosphorylase (MPase) | Bacillus sp. RK-1 | β-Glc1P | d-glucose | Maltose | [53] | |
| GH65 | Inverting | BAD97810.1 | Maltose phosphorylase (MapA) | Paenibacillus sp. SH-55 | β-Glc1P | d-glucose | Maltose | [27] | |
| GH65 | Inverting | CAA11905.1 | Maltose phosphorylase | Fructilactobacillus sanfranciscensis DSM 20451(T) | β-Glc1P | d-glucose | Maltose | [54] | |
| GH65 | Inverting | AAO80764.1 | Maltose phosphorylase (MalP;EF0957 | Enterococcus faecalis V583 | β-Glc1P | d-glucose | Maltose | [55] | |
| GH65 | Inverting | Q7SIE1 | Maltose phosphorylase | Levilactobacillus brevis ATCC 8287 | 1H54 | β-Glc1P | d-glucose | Maltose | [56] |
| GH65 | Inverting | BAQ19546.1 | Maltose phosphorylase (MalE) | Bacillus sp. AHU2001 | β-Glc1P | d-glucose | Maltose | [57] | |
| GH65 | Inverting | BAB97299.1 | Trehalose phosphorylase (TreP) | Thermoanaerobacter brockii ATCC 35047 | Preliminary data PMID: 20383018 | β-Glc1P | d-glucose | Trehalose: (α-d-glucosyl-(1→1)-d-glucose) | [58] |
| GH65 | Inverting | BAC20640.1 | Trehalose phosphorylase (TPase) | Geobacillus stearothermophilus SK-1 | β-Glc1P | d-glucose | Trehalose | [59] | |
| GH65 | Inverting | KKC30106.1 | Trehalose phosphorylase (TP) | Caldanaerobacter subterraneus subsp. Pacificus DSM 12653 | β-Glc1P | d-glucose | Trehalose | [60] | |
| GH65 | Inverting | ADG89586.1 | Trehalose phosphorylase (TbGP; Tbis_2887) | Thermobispora bispora DSM 43833 | β-Glc1P | d-glucose | Trehalose | [61] | |
| GH65 | Inverting | AAK04526.1 | Trehalose-6-phosphate phosphorylase (TrePP;YeeA;L39593; LL0428) | Lactococcus lactis subsp. lactis Il1403 | β-Glc1P | d-glucose-6-phosphate | Trehalose-6-phosphate: (α-d-glucosyl-(1→1)-d-glucopyranoside-6-phosphate) | [62] | |
| GH65 | Inverting | APF18594.1 | Trehalose-6-phosphate phosphorylase (CaGP;Cabys_1845) | Caldithrix abyssi DSM 13497 LF13 | β-Glc1P | d-glucose-6-phosphate | Trehalose-6-phosphate | [61] | |
| GH65 | Inverting | AAE30762.1 | Kojibiose phosphorylase (KojP;KPase) | Thermoanaerobacter brockii ATCC 35047 | β-Glc1P | d-glucose | Kojibiose: (α-d-glucosyl-(1→2)-d-glucose) | [58] | |
| GH65 | Inverting | AAC74398.1 | Kojibiose phosphorylase (YcjT) | Escherichia coli str. K-12 substr. MG1655 | β-Glc1P | d-glucose | Kojibiose | [63] | |
| GH65 | Inverting | ABP66077.1 | Kojibiose phosphorylase (CsKP;Csac_0439) | Caldicellulosiruptor saccharolyticus DSM 8903 | 3WIQ, 3WIR | β-Glc1P | d-glucose | Kojibiose | [64] |
| GH65 | Inverting | ACL68803.1 | Kojibiose phosphorylase (HoGP; Hore_00420) | Halothermothrix orenii H 168 | β-Glc1P | d-glucose | Kojibiose | [61] | |
| GH65 | Inverting | ABX42243.1 | Nigerose phosphorylase (Cphy_1874) | Lachnoclostridium phytofermentans ISDg | β-Glc1P | d-glucose | Nigerose: (α-d-glucosyl-(1→3)-d-glucose) | [65] | |
| GH65 | Inverting | ABX43667.1 | Nigerose phosphorylase (Cphy_3313) | Lachnoclostridium phytofermentans ISDg | β-Glc1P | d-glucose | Nigerose | [66] | |
| GH65 | Inverting | ADQ05832.1 | 1,3-α-oligoglucan phosphorylase (ChGP;Calhy_0070) | Caldicellulosiruptor hydrothermalis 108 | β-Glc1P | Maltose Kojibiose Nigerose | α-1,3-oligoglucan | [61] | |
| GH65 | Inverting | ADC90669.1 | Trisaccharide α-glucan phosphorylase (MiGP;HMPREF0868_0806) | Mageeibacillus indolicus UPII9-5 | β-Glc1P | Isomaltose Isomaltulose Maltose Kojibiose Nigerose | α-1,3-oligoglucan | [61] | |
| GH65 | Inverting | ABX43668.1 | α-1,3-oligoglucan phosphorylase (Cphy_3314) | Lachnoclostridium phytofermentans ISDg | β-Glc1P | d-glucose | α-1,3-oligoglucan | [66] | |
| GH65 | Inverting | ABX41399.1 | 3-O-α-glucopyranosyl-L-rhamnose phosphorylase (Cphy_1019) | Lachnoclostridium phytofermentans ISDg | β-Glc1P | l-rhamnose | α-d-glucopyranosyl-(1,3)-l-rhamnose | [25] | |
| GH65 | Inverting | ADI00307.1 | α-1,2-glucosylglycerol phosphorylase (Bsel_2816) | Bacillus selenitireducens MLS10 | 4KTP, 4KTR | β-Glc1P | d-glycerol | (α-d-glucosyl-(1→2)-glycerol) | [67] |
| GH94 | Inverting | AAB95491.2 | Cellobiose phosphorylase (CbpA) | Thermotoga neapolitana | α-Glc1P | d-glucose | Cellobiose: (β-d-glucopyranosyl-(1,4)-d-glucopyranose) | [68] | |
| GH94 | Inverting | AAC45510.1 | Cellobiose phosphorylase (CepA) | Thermoclostridium stercorarium NCIB 11754 | α-Glc1P | d-glucose | Cellobiose | [69] | |
| GH94 | Inverting | AAD36910.1 | Cellobiose phosphorylase (CepA;TM1848;Tmari_1863 | Thermotoga maritima MSB8 | α-Glc1P | d-glucose | Cellobiose | [70] | |
| GH94 | Inverting | AAL67138.1 | Cellobiose phosphorylase (Cbp) | Acetivibrio thermocellus YM4 | 3QDE | α-Glc1P | d-glucose | Cellobiose | [71] |
| GH94 | Inverting | AAQ20920.1 | Cellobiose phosphorylase (CbP) | Cellulomonas uda DSM 20108 | 3RRS, 3RSY, 3S4A, 3S4B, 3S4C, 3S4D | α-Glc1P | d-glucose | Cellobiose | [72] |
| GH94 | Inverting | ABD80580.1 | Cellobiose phosphorylase 94A (Cbp;SdCBP;Sde1318, Cep94A) | Saccharophagus degradans 2-40 | α-Glc1P | d-glucose | Cellobiose | [73] | |
| GH94 | Inverting | ABN51514.1 | Cellobiose phosphorylase (Cbp;CtCBP;Cthe_0275) | Acetivibrio thermocellus ATCC 27405 | α-Glc1P | d-glucose | Cellobiose | [74] | |
| GH94 | Inverting | ACL76454.1 | Cellobiose phosphorylase (CbpA;Ccel_2109) | Ruminiclostridium cellulolyticum H10 | α-Glc1P | d-glucose | Cellobiose | [75] | |
| GH94 | Inverting | ADU20744.1 | Cellobiose phosphorylase (CBP;RaCBP;Rumal_0187) | Ruminococcus albus 7 (DSM 20455) | α-Glc1P | d-glucose | Cellobiose | [24] | |
| GH94 | Inverting | BAA25846.1 | Cellobiose-phosphorylase (Cbp) | Acetivibrio thermocellus YM4 | α-Glc1P | d-glucose | Cellobiose | [76] | |
| GH94 | Inverting | BAA28631.1 | Cellobiose phosphorylase (Cbp;CgCBP) | Cellulomonas gilvus ATCC13127 | 2CQS, 2CQT, 3ACS, 3ACT, 3AFJ, 3QFY, 3QFZ, 3QG0 | α-Glc1P | d-glucose | Cellobiose | [77] |
| GH94 | Inverting | CAB16926.1* | Cellobiose phosphorylase (CepA) | Thermotoga neapolitana Z2706-MC24 | α-Glc1P | d-glucose | Cellobiose | [78] | |
| GH94 | Inverting | AAC45511.1 | Cellodextrin phosphorylase (CepB; CsCdP) | Thermoclostridium stercorarium DSM8532 | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [69] | |
| GH94 | Inverting | ACL76454.1 | Cellobiose phosphorylase (CbpA;Ccel_2109) | Ruminiclostridium cellulolyticum H10 | α-Glc1P | d-glucose | Cellobiose | [29] | |
| GH94 | Inverting | QCO92836.1 | Cellobiose phosphorylase (GH94_G) | Uncultured bacterium | α-Glc1P | d-glucose | Cellobiose | [79] | |
| GH94 | Inverting | QCO92799.1 | Cellobiose phosphorylase (GH94_E) | Uncultured bacterium | α-Glc1P | d-glucose | Cellobiose | [79] | |
| GH94 | Inverting | ADU22883.1 | Cellodextrin phosphorylase (CDP;RaCDP;Rumal_2403) | Ruminococcus albus 7 (DSM 20455) | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [80] | |
| GH94 | Inverting | ACD71661.1 | Cyclic β-1,2-glucan synthetase (Cgs) | Brucella abortus S19 | α-Glc1P | [(1→2)-β-d-glucosyl]n | [(1→2)-β-d-glucosyl]n+1 | [81] | |
| GH94 | Inverting | ACL75793.1 | Cellodextrin phosphorylase (CdpA; Ccel_1439) | Ruminiclostridium cellulolyticum H10 | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [29] | |
| GH94 | Inverting | ACL77700.1 | Cellodextrin phosphorylase (CdpC; Ccel_3412) | Ruminiclostridium cellulolyticum H10 | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [29] | |
| GH94 | Inverting | ACL76688.1 | Cellodextrin phosphorylase (CdpB; Ccel_2354) | Ruminiclostridium cellulolyticum H10 | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [29] | |
| GH94 | Inverting | QCO92991.1 | Cellodextrin phosphorylase (GH94_A) | Uncultured bacterium | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [29] | |
| GH94 | Inverting | QCO92946.1 | Cellodextrin phosphorylase (GH94_D) | Uncultured bacterium | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [29] | |
| GH94 | Inverting | BAJ10826.1 | Laminaribiose phosphorylase (LbpA) | Paenibacillus sp. YM1 | 6GGY, 6GH2, 6GH3 | α-Glc1P | d-glucose | Laminaribiose: (β-d-glucopyranosyl-(1,3)-d-glucopyranose) | [82,83] |
| GH94 | Inverting | AAG23740.1 | Diacetylchitobiose phosphorylase (ChbP) | Vibrio furnissii | α-GlcNAc-1P | N-acetyl-d-glucosamine | N,N’-diacetylchitobiose: (N-acetyl-d-glucosaminyl-(1,4)-N-acetyl-d-glucosamine) | [84] | |
| GH94 | Inverting | BAC87867.1 | Chitobiose phosphorylase (ChbP;VpChBP) | Vibrio proteolyticus | 1V7V, 1V7W, 1V7X | α-GlcNAc-1P | N-acetyl-d-glucosamine | N,N’-diacetylchitobiose | [85] |
| GH94 | Inverting | AFH48717.1 | Chitobiose phosphorylase (IaGP;IALB_1006) | Ignavibacterium album JCM 16511 | α-GlcNAc-1P | N-acetyl-d-glucosamine | N,N’-diacetylchitobiose | [86] | |
| GH94 | Inverting | CAC97070.1 | β-1,2-oligoglucan phosphorylase (LiSOGP;Lin1839) | Listeria innocua Clip11262 | α-Glc1P | [(1→2)-β-d-glucosyl]n | [(1→2)-β-d-glucosyl]n+1 | [87] | |
| GH94 | Inverting | ABX41081.1 | β-1,2-oligoglucan phosphorylase (LpSOGP;Cphy_0694) | Lachnoclostridium phytofermentans ISDg | 5H3Z, 5H40, 5H41, 5H42 | α-Glc1P | [(1→2)-β-d-glucosyl]n | [(1→2)-β-d-glucosyl]n+1 | [88] |
| GH94 | Inverting | QCO92990.1 | Laminaribiose phosphorylase (GH94_B) | Uncultured bacterium | α-Glc1P | d-glucose | Laminaribiose | [79] | |
| GH94 | Inverting | QCO92945.1 | Laminaribiose phosphorylase (GH94_C) | Uncultured bacterium | α-Glc1P | d-glucose | Laminaribiose | [79] | |
| GH94 | Inverting | BAB71818.1 | Cellodextrin phosphorylase (Cdp-ym4) | Acetivibrio thermocellus YM4 | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [71] | |
| GH94 | Inverting | ABN54185.1 | Cellodextrin-phosphorylase (Cdp;CtCDP;Cthe_2989) | Acetivibrio thermocellus ATCC 27405 | 5NZ7, 5NZ8 | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [89] |
| GH94 | Inverting | ADZ85667.1* | Cellodextrin phosphorylase (Cdp;ClCDP;Clole_3989) | Clostridium lentocellum | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [73] | |
| GH94 | Inverting | AAM43298.1 | Cellobionic acid phosphorylase (CelAP;NdvB;XCC4077) | Xanthomonas campestris pv. campestris str. ATCC 33913 | α-Glc1P | d-gluconate | β-d-glucopyranosyl-(1,4)-d-gluconate | [90] | |
| GH94 | Inverting | ABD80168.1 | Cellobionic acid phosphorylase 94B (Cep94B;CBAP; SdCBAP;Sde_0906) | Saccharophagus degradans 2-40 | 4ZLE, 4ZLF, 4ZLG, 4ZLI | α-Glc1P | d-gluconate | β-d-glucopyranosyl-(1,4)-d-gluconate | [91] |
| GH94 | Inverting | EAA28929.1 | Cellobionic acid phosphorylase (CelAP;NdvB; NCU09425) | Neurospora crassa OR74A | α-Glc1P | d-gluconate | β-d-glucopyranosyl-(1,4)-d-gluconate | [90] | |
| GH94 | Inverting | AAC45511.1 | Cellodextrin phosphorylase (CepB;CsCdP) | Thermoclostridium stercorarium DSM8532 | α-Glc1P | [(1→4)-β-d-glucosyl]n | [(1→4)-β-d-glucosyl]n+1 | [69] | |
| GH94 | Inverting | AHC20114.1 | Glucosylgalactose Phosphorylase (GGalP) | Paenibacillus polymyxa CR1 | α-Glc1P | d-galactose | β-d-glucopyranosyl-(1,4)-d-galactose | [86] | |
| GH94 | Inverting | ABX81345.1 | Laminaribiose phosphorylase (ACL_0729;ACL0729) | Acholeplasma laidlawii PG-8A | α-Glc1P | d-glucose | Laminaribiose | [92] | |
| GH112 | Inverting | ACB74662.1 | D-galactosyl-β-1,4-L-rhamnose phosphorylase (GalRhaP;Oter_1377) | Opitutus terrae PB90-1 | α-Gal1P | l-rhamnose | (β-d-galactosyl-(1,4)-l-rhamnose) | [93] | |
| GH112 | Inverting | ABX42289.1 | D-galactosyl-β-1,4-L-rhamnose phosphorylase (Cphy_1920) | Lachnoclostridium phytofermentans ISDg | α-Gal1P | l-rhamnose | (β-d-galactosyl-(1,4)-l-rhamnose) | [94] | |
| GH112 | Inverting | AAO07997.1 | β-1,3-galactosyl-N-acetylhexosamine phosphorylase (GalGlyNAcP;VV2_1091) | Vibrio vulnificus CMCP6 | α-Gal1P | N-acetyl-d-glucosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-glucosamine) | [95] | |
| GH112 | Inverting | ABX40964.1 | β-1,3-galactosyl-N-acetylhexosamine phosphorylase (Cphy_0577) | Lachnoclostridium phytofermentans ISDg | α-Gal1P | N-acetyl-d-glucosamine N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-glucosamine) (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [94] | |
| GH112 | Inverting | ABX43387.1 | β-1,3-galactosyl-N-acetylhexosamine phosphorylase (Cphy_3030) | Lachnoclostridium phytofermentans ISDg | α-Gal1P | N-acetyl-d-glucosamine N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-glucosamine) (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [94] | |
| GH112 | Inverting | AEN95946.1 | β-1,3-galactosyl-N-acetylhexosamine phosphorylase (RhGLnbp112;RHOM_04120) | Roseburia hominis A2-183 | α-Gal1P | N-acetyl-d-glucosamine N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-glucosamine) (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [96] | |
| GH112 | Inverting | EEG94248.1 | β-1,3-galactosyl-N-acetylhexosamine phosphorylase (RiGLnbp112; ROSEINA2194_01886; GnpA) | Roseburia inulinivorans DSM 16841 | α-Gal1P | N-acetyl-d-glucosamine N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-glucosamine) (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [96] | |
| GH112 | Inverting | BAD80751.1 | Galacto-N-biose/lacto-N-biose phosphorylase (LnpA1;LnbP;GLNBP; BLLJ_1623) | Bifidobacterium longum subsp. longum JCM 1217 | 2ZUS, 2ZUT, 2ZUU, 2ZUV, 2ZUW, 3WFZ | α-Gal1P | N-acetyl-d-glucosamine N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-glucosamine) (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [97] |
| GH112 | Inverting | BAD80752.1 | Lacto-N-biose phosphorylase (LnpA1;LnbP) | Bifidobacterium bifidum JCM 1254 | α-Gal1P | N-acetyl-d-glucosamine N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-glucosamine) (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [98] | |
| GH112 | Inverting | ACV29689.1 | β-1,3-galactosyl-N-acetylhexosamine phosphorylase/galacto-N-biose/lacto-N-biose I phosphorylase (GLNBP;Apre_1669) | Anaerococcus prevotii DSM 20548 | α-Gal1P | N-acetyl-d-glucosamine N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-glucosamine) (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [26] | |
| GH112 | Inverting | ZP_05748149.1 | β-1,3-galactosyl-N-acetylhexosamine phosphorylase/galacto-N-biose phosphorylase (GNBP;HMPREF0357_1319) | Erysipelothrix rhusiopathiae ATCC 19414 | α-Gal1P | N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [26] | |
| GH112 | Inverting | ACZ00636.1 | β-1,3-galactosyl-N-acetylhexosamine phosphorylase/galacto-N-biose phosphorylase (GNBP;Smon_0146) | Streptobacillus moniliformis DSM 12112 | α-Gal1P | N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [26] | |
| GH112 | Inverting | ABG83511.1 | Galacto-N-biose phosphorylase (CPF_0553) | Clostridium perfringens ATCC 13124 | α-Gal1P | N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [95] | |
| GH112 | Inverting | BAH10636.1 | β-1,3-galactosyl-N-acetylhexosamine phosphorylase (GnpA) | Cutibacterium acnes JCM 6425 | α-Gal1P | N-acetyl-d-galactosamine | (β-d-galactosyl-(1,3)-N-acetyl-d-galactosamine) | [99] | |
| GH130 | Inverting | CAC96089.1 | β-1,2-mannobiose phosphorylase (Lin0857) | Listeria innocua Clip11262 | 5B0P, 5B0Q, 5B0R, 5B0S | α-Man1P | d-mannose | β-1,2-mannobiose | [100] |
| GH130 | Inverting | ABY93074.1 | β-1,2-mannobiose phosphorylase (Teth514_1789) | Thermoanaerobacter sp. X514 | 7FIP, 7FIQ, 7FIR, 7FIS | α-Man1P | d-mannose | β-1,2-mannobiose | [101] |
| GH130 | Inverting | ABY93073.1 | β-1,2-oligomannan phosphorylase (Teth514_1788) | Thermoanaerobacter sp. X514 | α-Man1P | (β-1,2-d-mannose)n | (β-1,2-d-mannose)n+1 | [101] | |
| GH130 | Inverting | CAZ94304.1 | β-1,3-mannooligosaccharide phosphorylase (zobellia_231) | Zobellia galactanivorans DsijT | α-Man1P | (β-1,3-d-mannose)n | (β-1,3-d-mannose)n+1 | [102] | |
| GH130 | Inverting | AAS19693.1 | β-1,4-mannosylglucose phosphorylase (UnkA, CvMGP) | Cellvibrio vulgaris NCIMB8633 | α-Man1P | d-glucose | β-mannopyranosyl-(1→4)-d-glucopyranose | [103] | |
| GH130 | Inverting | ADU21379.1 | β-1,4-mannosylglucose phosphorylase (RaMP1; RaMGP; Rumal_0852) | Ruminococcus albus 7 (DSM 20455) | 5AY9, 5AYC | α-Man1P | d-glucose | β-mannopyranosyl-(1→4)-d-glucopyranose | [104] |
| GH130 | Inverting | CAH06518.1 | β-1,4-mannosylglucose phosphorylase (MGP; BF0772) | Bacteroides fragilis NCTC 9343 | 3WAS, 3WAT, 3WAU, 4KMI | α-Man1P | d-glucose | β-mannopyranosyl-(1→4)-d-glucopyranose | [105] |
| GH130 | Inverting | VCV21228.1 | β-1,4-mannosylglucose phosphorylase (RIL182_01099; ROSINTL182_07685) | Roseburia intestinalis L1-82 | α-Man1P | d-glucose | β-mannopyranosyl-(1→4)-d-glucopyranose | [106] | |
| GH130 | Inverting | ACY49318.1 | β-1,4-mannosylglucose phosphorylase (MGP; RmMGP; Rmar_2440) | Rhodothermus marinus R-10T (ATCC43812) | α-Man1P | d-glucose | β-mannopyranosyl-(1→4)-d-glucopyranose | [107] | |
| GH130 | Inverting | AAO76140.1 | β-1,4-mannosyl-N-acetyl-glucosamine phosphorylase (BT1033) | Bacteroides thetaiotaomicron VPI-5482 | α-Man1P | N-acetyl-d-glucosamine | β-mannopyranosyl-(1→4)-N-acetyl-d-glucosamine | [108] | |
| GH130 | Inverting | ADD61463.1 | β-1,4-mannopyranosyl-[N-glycan] phosphorylase β-1,4-mannopyranosyl-chitobiose phosphorylase (Uhgb_MP) | Uncultured bacterium | 4UDG, 4UDI, 4UDJ, 4UDK | α-Man1P | β-N-acetyl-d-glucosaminyl-(1,4)-N-acetyl-d-glucosamine | β-1,4-mannopyranosyl-chitobiose (β-mannopyranosyl-(1→4)-N-acetyl-β-d-glucosaminyl-(1→4)-N-acetyl-d-glucosamine) | [109] |
| GH130 | Inverting | ADU20661.1 | β-1,4-mannooligosaccharide phosphorylase (MOP; RaMP2; Rumal_0099) | Ruminococcus albus 7 (DSM 20455) | 5AYD, 5AYE | α-Man1P | (β-1,4-d-mannose)n | (β-1,4-d-mannose)n+1 | [104] |
| GH130 | Inverting | VCV21229.1 | β-1,4-mannooligosaccharide phosphorylase (RIL182_01100; ROSINTL182_05474) | Roseburia intestinalis L1-82 | α-Man1P | (β-1,4-d-mannose)n | (β-1,4-d-mannose)n+1 | [106] | |
| GH130 | Inverting | AAD36300.1 | β-1,4-mannooligosaccharide phosphorylase (TM1225) | Thermotoga maritima MSB8 | 1VKD | α-Man1P | (β-1,4-d-mannose)n | (β-1,4-d-mannose)n+1 | [110] |
| GH130 | Inverting | WP_026485574.1 | β-1,4-mannooligosaccharide phosphorylase (CalpoDRAFT_0075) | Caldanaerobius polysaccharolyticus ATCC BAA-17 | α-Man1P | (β-1,4-d-mannose)n | (β-1,4-d-mannose)n+1 | [111] | |
| GH130 | Inverting | WP_026486530.1 | β-1,4-mannooligosaccharide phosphorylase (CalpoDRAFT_1209) | Caldanaerobius polysaccharolyticus ATCC BAA-17 | α-Man1P | (β-1,4-d-mannose)n | (β-1,4-d-mannose)n+1 | [111] | |
| GH130 | Inverting | CAH1385115.1 | β-1,4-mannosyl-glucuronic acid phosphorylase | Uncultured bacterium | α-Man1P | d-glucuronic acid | β-mannopyranosyl-(1→4)-d-glucuronic acid | [112] | |
| GH130 | Inverting | CAH1385116.1 | β-1,4-mannooligosaccharide phosphorylase | Uncultured bacterium | α-Man1P | d-mannose β-1,4-mannobiose | β-1,4-mannobiose β-1,4-mannotriose | [112] | |
| GH130 | Inverting | CAH1385117.1 | β-1,3-mannosyl-glucose phosphorylase β-1,3-mannooligosaccharide phosphorylase | Uncultured bacterium | α-Man1P | d-glucose d-mannose | β-mannopyranosyl-(1→3)-d-glucopyranose β-1,3-mannobiose | [112] | |
| GH130 | Inverting | CAH1385118.1 | Mixed-linkage mannoside synthase Uhgb_MS | Uncultured bacterium | α-Man1P | d-mannose α-1,2-mannobiose | β-1,2-mannobiose α-1,2-mannotriose β-d-mannopyranosyl-(1→2)-α-1,2-mannotriose | [113] | |
| GH149 | Inverting | β-1,3-oligoglucan phosphorylase (Pro_7066) | Uncultured bacterium | 6HQ6, 6HQ8, 6HQ6 | α-Glc1P | [(1→3)-β-d-glucosyl]n | [(1→3)-β-d-glucosyl]n+1 | [114] | |
| GH149 | Inverting | AUO30192.1 | β-1,3-glucan phosphorylase (EgP1) | Euglena gracilis | α-Glc1P | [(1→3)-β-d-glucosyl]n | [(1→3)-β-d-glucosyl]n+1 | [115] | |
| GH149 | Inverting | QCO92814.1 | β-1,3-glucan phosphorylase (GH149_H) | Uncultured bacterium | α-Glc1P | [(1→3)-β-d-glucosyl]n | [(1→3)-β-d-glucosyl]n+1 | [79] | |
| GH161 | Inverting | WP_019688419.1 | β-1,3-glucan phosphorylase (PPT_RS0121460; PapP) | Paenibacillus polymyxa ATCC 842 | α-Glc1P | [(1→3)-β-d-glucosyl]n | [(1→3)-β-d-glucosyl]n+1 | [116] | |
| GH161 | Inverting | ACJ76363.1 | β-1,3-glucan phosphorylase (THA_1941; TaCDP) | Thermosipho africanus TCF52B | α-Glc1P | [(1→3)-β-d-glucosyl]n | [(1→3)-β-d-glucosyl]n+1 | [116] | |
| GH161 | Inverting | BAU78234.1 | β-1,3-glucan phosphorylase (OdBGP) | Ochromonas danica NIES-2142 | α-Glc1P | [(1→3)-β-d-glucosyl]n | [(1→3)-β-d-glucosyl]n+1 | [117] | |
| GT4 | Retaining | BAA31350.1 | Trehalose synthase (Tsase) | Grifola frondosa | α-Glc1P | d-glucose | Trehalose: (α-d-glucosyl-(1,1)-d-glucose) | [118] | |
| GT4 | Retaining | AAF22230.1 | Threalose phosphorylase (PsTP) | Lentinus sajor-caju ASI2070 (Pleurotus sajor-caju) | α-Glc1P | d-glucose | trehalose | [119] | |
| GT4 | Retaining | ABC84380.1 | Threalose phosphorylase | Schizophyllum commune BT2115 | α-Glc1P | d-glucose | trehalose | [120,121] | |
| GT35 | Retaining | ABP51432.1 | Glucan/maltodextrin phosphorylase (GlgP; PyglgP; Pars_1881) | Pyrobaculum arsenaticum DSM 13514 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [122] | |
| GT35 | Retaining | AAL81659.1 | α-glucan/maltodextrin phosphorylase (PF1535) | Pyrococcus furiosus DSM 3638 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [123,124] | |
| GT35 | Retaining | AAD28735.1 | Maltodextrin phosphorylase (MalP) | Thermococcus litoralis | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [125] | |
| GT35 | Retaining | ABN51595.1 | α-glucan phosphorylase (α-GP; Cthe_0357) | Acetivibrio thermocellus ATCC 27405 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [126] | |
| GT35 | Retaining | CAB61943.1 | α-glucan phosphorylase (PHS2; AtPHS2; At3g46970) | Arbidopsis thaliana | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [127] | |
| GT35 | Retaining | AAD03471.1 | Glycogen phosphorylase (GlgP) | Agrobacterium tumefaciens A348 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [128] | |
| GT35 | Retaining | AAC06896.1 | Glycogen phosphorylase (GlgP; Aq_717) | Aquifex aeolicus VF5 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [129] | |
| GT35 | Retaining | AAC00218.1 | Glycogen phosphorylase (GlgP; BSU30940) | Bacillus subtilis subsp. subtilis str. 168 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [130] | |
| GT35 | Retaining | AAM24997.1 | α-glucan phosphorylase (GlgP; TTE1805) | Caldanaerobacter subterraneus subsp. tengcongensis MB4 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [131] | |
| GT35 | Retaining | AAM52219.1 | Glycogen phosphorylase | Corynebacterium callunae | 2C4M | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [132] |
| GT35 | Retaining | BAB98701.1 | Maltodextrin/glycogen phosphorylase (MalP; GlgP1; cg1479) | Corynebacterium glutamicum ATCC 13032 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [133] | |
| GT35 | Retaining | BAB99480.1 | Glycogen phosphorylase (GlgP; GlgP2; cg2289) | Corynebacterium glutamicum ATCC 13032 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [133] | |
| GT35 | Retaining | AAC76453.1 | Glycogen phosphorylase (GlgP; b3428) | Escherichia coli str. K-12 substr. MG1655 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [134] | |
| GT35 | Retaining | AAC76442.1 | Maltodextrin phosphorylase (MalP; b3417) | Escherichia coli str. K-12 substr. MG1655 | 1AHP, 1E4O, 1L5V, 1L5W, 1L6I, 1QM5, 2ASV, 2AV6, 2AW3, 2AZD, 2ECP | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [135] [136] |
| GT35 | Retaining | BAA19592.1 | Glycogen phosphorylase (GlgP) | Geobacillus stearothermophilus TRBE14 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [137] | |
| GT35 | Retaining | AAN59210.1 | Maltodextrin phosphorylase (GlgP; SMU.1564) | Streptococcus mutans UA159 | 4L22 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [138] |
| GT35 | Retaining | AAL26558.1 | Glycogen phosphorylase (Glg) | Synechococcus elongatus PCC 7942 = FACHB-805 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [139] | |
| GT35 | Retaining | AGL50099.1 | α-glucan phosphorylase (AgpA; Tmari_1175) | Thermotoga maritima MSB8 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [140] | |
| GT35 | Retaining | BAB11741.1 | α-glucan phosphorylase (GlgP) | Thermus aquaticus | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [141] | |
| GT35 | Retaining | CAC93400.1 | Glycogen phosphorylase (glgP, YPO3938) | Yersinia pestis CO92 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [142] | |
| GT35 | Retaining | AAB46846.1 | Glycogen phosphorylase (myophosphorylase) | Bos taurus | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [143] | |
| GT35 | Retaining | ABB88567.1 | Plastidial starch phosphorylase (PhoB; Sta4) | Chlamydomonas reinhardtii 137C | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [144] | |
| GT35 | Retaining | CAA44069.1 | Glycogen phosphorylase 1 (GlpV; GP1) | Dictyostelium discoideum | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [145] | |
| GT35 | Retaining | AAA33211.1 | Glycogen phosphorylase 2 (GlpD; GP2) | Dictyostelium discoideum | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [146] | |
| GT35 | Retaining | AAD46887.1 | glycogen phosphorylase (GlyP; GLYP; CG7254; Dmel_CG7254) | Drosophila melanogaster | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [147] | |
| GT35 | Retaining | AAN17338.1 | Glycogen phosphorylase-2 | Entamoeba histolytica | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [148] | |
| GT35 | Retaining | AAL23578.1 | Glycogen phosphorylase | Entamoeba histolytica | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [148] | |
| GT35 | Retaining | AAP33020.1 | Glycogen phosphorylase | Gallus gallus | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [149] | |
| GT35 | Retaining | AAK69600.1 | Glycogen phosphorylase | Giardia intestinalis ATCC30957 | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [148] | |
| GT35 | Retaining | AAB60395.1 | Glycogen phosphorylase (brain) (bGP) | Homo sapiens | 5IKO, 5IKP | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [150] |
| GT35 | Retaining | CAA75517.1 | Glycogen phosphorylase (liver) | Homo sapiens | 1EM6, 1EXV, 1FA9, 1FC0, 1L5Q, 1L5R, 1L5S, 1L7X, 1XOI, 2ATI, 2QLL, 2ZB2, 3CEH, 3CEJ, 3CEM, 3DD1, 3DDS, 3DDW | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [151] |
| GT35 | Retaining | AAC17451.1 | Glycogen phosphorylase (muscle) (PigM) | Homo sapiens | 1Z8D | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [152] |
| GT35 | Retaining | BAK00834.1 | Plastidial α-1,4-glucan phosphorylase (Pho1; HvPho1) | Hordeum vulgare subsp. Vulgare | 5LR8, 5LRA, 5LRB | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [153] |
| GT35 | Retaining | AAA63271.1 | α-glucan phosphorylase L | Ipomoea batatas | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [154] | |
| GT35 | Retaining | AAK01137.1 | Starch phosphorylase (fragment) | Ipomoea batatas | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [155] | |
| GT35 | Retaining | AAL23577.1 | Glycogen phosphorylase | Mastigamoeba balamuthi | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [148] | |
| GT35 | Retaining | AAD30476.1 | Glycogen phosphorylase (muscle) (PygM) | Mus musculus | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [156] | |
| GT35 | Retaining | AAG00588.1 | Glycogen phosphorylase | Mus musculus | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [157] | |
| GT35 | Retaining | ACJ76617.1 | Glycogen phosphorylase (muscle) (PygM) | Oryctolagus cuniculus | Dozens available—2GJ4[A] best solution | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [158,159] |
| GT35 | Retaining | AAK15695.1 | α-1,4-glucan phosphorylase L | Oryza sativa | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [160] | |
| GT35 | Retaining | BAB92854.1 | α-1,4-glucan phosphorylase (Os01g0851700) | Oryza sativa Japonica Group | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [161] | |
| GT35 | Retaining | AAV87308.1 | brain glycogen phosphorylase (PYGB) | Ovis aries | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [162] | |
| GT35 | Retaining | AAB68800.1 | glycogen phosphorylase (muscle) | Ovis aries | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [163] | |
| GT35 | Retaining | AAA41252.1 | glycogen phosphorylase (Brain) | Rattus norvegicus | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [164] | |
| GT35 | Retaining | AAH70901.1 | glycogen phosphorylase (liver) | Rattus norvegicus | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [165] | |
| GT35 | Retaining | AAA41253.1 | glycogen phosphorylase (muscle) | Rattus norvegicus | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [166] | |
| GT35 | Retaining | AAB68057.1 | Glycogen phosphorylase (Gph1; YPR160w) | Saccharomyces cerevisiae S288c | 1YGP | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [167,168] |
| GT35 | Retaining | AAA33809.1 | α-glucan phosphorylase H | Solanum tuberosum | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [169] | |
| GT35 | Retaining | BAA00407.1 | α-glucan phosphorylase L1 | Solanum tuberosum | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [170] | |
| GT35 | Retaining | CAA52036.1 | α-glucan phosphorylase L2 | Solanum tuberosum | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [171] | |
| GT35 | Retaining | CAA59464.1 | α-glucan phosphorylase | Spinacia oleracea | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [159] | |
| GT35 | Retaining | AAL23579.1 | glycogen phosphorylase | Trichomonas vaginalis | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [148] | |
| GT35 | Retaining | AAF82787.1 | α-glucan phosphorylase | Triticum aestivum | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [172] | |
| GT35 | Retaining | CAA84494.1 | α-glucan phosphorylase (Pho2;VfPho2) | Vicia faba var. minor | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [173] | |
| GT35 | Retaining | CAA85354.1 | α-glucan phosphorylase L | Vicia faba var. minor | α-Glc1P | [(1→4)-α-d-glucosyl]n | [(1→4)-α-d-glucosyl]n+1 | [173] | |
| GT108 | Inverting | CBZ24448.1 | MTP3 (LMXM_10_1250) | Leishmania mexicana MHOM/GT/2001/U1103 | α-Man1P | (β-1,2-d-mannose)n | (β-1,2-d-mannose)n+1 | [174] | |
| GT108 | Inverting | CBZ24451.1 | MTP6 (LMXM_10_1280) | Leishmania mexicana MHOM/GT/2001/U1103 | α-Man1P | (β-1,2-d-mannose)n | (β-1,2-d-mannose)n+1 | [174] | |
| GT108 | Inverting | CBZ24449.1 | MTP4 (LMXM_10_1260) | Leishmania mexicana MHOM/GT/2001/U1103 | 6Q50 | α-Man1P | (β-1,2-d-mannose)n | (β-1,2-d-mannose)n+1 | [174] |
| GT108 | Inverting | CBZ24452.1 | MTP7 (LMXM_10_1290) | Leishmania mexicana MHOM/GT/2001/U1103 | α-Man1P | (β-1,2-d-mannose)n | (β-1,2-d-mannose)n+1 | [174] | |
| CAZy Family | GenBank/ UniProtKB Accession | Organism Name | PDB Accession Numbers | Protein Name | Fold | Quaternary Structure | Reference |
|---|---|---|---|---|---|---|---|
| GH3 | AUG44408.1 | Uncultured bacterium | 5VQD[A], 5VQE[A] | β-glucoside phosphorylase (BglP) | (β/α)8 | Monomer | [30] |
| GH13_18 | AAO33821.1 | Bifidobacterium adolescentis DSM 20083 | 1R7A[A,B], 2GDU[A,B], 2GDV[A,B], 5C8B[B], 5M9X[B], 5MAN[B], 5MB2[B], 6FME[A,B] | Sucrose phosphorylase (SucP; SP; BaSP) | (β/α)8 | Dimer | [196] |
| GH13_18 | BAN03569.1 | Ilumatobacter coccineus YM16-304 | 6S9U[A] | Sucrose 6(F)-phosphate phosphorylase (YM304_32550) | (β/α)8 | Monomer | [50] |
| GH13_18 | ADL69407.1 | Thermoanaerobacterium thermosaccharolyticum DSM 571 | 6S9V[A,B] | Sucrose 6(F)-phosphate phosphorylase (SPP; TtSPP; Tthe_1921) | (β/α)8 | Monomer | [50] |
| GH65 | Q7SIE1 | Levilactobacillus brevis ATCC 8287 | 1H54[A] | Maltose phosphorylase | (α/α)6 | Dimer | [197] |
| GH65 | ADI00307.1 | Bacillus selenitireducens MLS10 | 4KTP[A,B], 4KTR[A,B,C,D,E,F,G,H] | 2-O-α-glucopyranosylglycerol: phosphate β-glucosyltransferase/ 2-O-α-glucosylglycerol phosphorylase (GGP; Bsel_2816) | (α/α)6 | Dimer | [198] |
| GH65 | ABP66077.1 | Caldicellulosiruptor saccharolyticus DSM 8903 | 3WIQ[A], 3WIR[A,B,C,D] | Kojibiose phosphorylase (CsKP; Csac_0439 | (α/α)6 | Dimer | [199] |
| GH65 | / | Uncultured bacterium | 6W0P[B,C,D], 6W0P[A] | Kojibiose phosphorylase | (α/α)6 | Dimer | Unpublished |
| GH65 | BAB97299.1 | Thermoanaerobacter brockii ATCC 35047 | Trehalose phosphorylase (TreP) | (α/α)6 | Dimer | [200] | |
| GH94 | ABN54185.1 | Acetivibrio thermocellus ATCC 27405 | 5NZ7[A,B], 5NZ8[A,B] | Cellodextrin-phosphorylase (Cdp; CtCDP; Cthe_2989) | (α/α)6 | Dimer | [201] |
| GH94 | AAL67138.1 | Acetivibrio thermocellus YM4 | 3QDE[A,B] | Cellobiose phosphorylase (Cbp) | (α/α)6 | Dimer | [202] |
| GH94 | BAA28631.1 | Cellulomonas gilvus ATCC13127 | 2CQS[A,B], 2CQT[A,B], 3ACS[A,B], 3ACT[A,B], 3AFJ[A,B], 3QFY[A,B], 3QFZ[A,B], 3QG0[A,B] | Cellobiose phosphorylase (Cbp; CgCBP) | (α/α)6 | Dimer | [203,204] |
| GH94 | AAQ20920.1 | Cellulomonas uda DSM 20108 | 3RRS[A,B], 3RSY[A,B], 3S4A[A,B], 3S4B[A,B], 3S4C[A], 3S4D[A] | Cellobiose phosphorylase (CbP) | (α/α)6 | Dimer | Unpublished |
| GH94 | ABX41081.1 | Lachnoclostridium phytofermentans ISDg | 5H3Z[A,B], 5H40[A,B], 5H41[A,B], 5H42[A,B] | β-1,2-oligoglucan phosphorylase (LpSOGP; Cphy_0694) | (α/α)6 | Monomer | [88] |
| GH94 | BAJ10826.1 | Paenibacillus sp. YM1 | 6GGY[A,B], 6GH2[A,B], 6GH3[A,B] | Laminaribiose phosphorylase (LbpA) | (α/α)6 | Dimer | [116] |
| GH94 | ABD80168.1 | Saccharophagus degradans 2-40 | 4ZLE[A], 4ZLF[A], 4ZLG[A], 4ZLI[A] | Cellobionic acid phosphorylase 94B (Cep94B; CBAP; SdCBAP; Sde_0906) | (α/α)6 | Dimer | [91] |
| GH94 | BAC87867.1 | Vibrio proteolyticus | 1V7V[A], 1V7W[A], 1V7X[A] | Chitobiose phosphorylase (ChbP; VpChBP) | (α/α)6 | Dimer | [205] |
| GH112 | BAD80751.1 | Bifidobacterium longum subsp. longum JCM 1217 | 2ZUS[A,B,C,D], 2ZUT[A,B,C,D], 2ZUU[A,B,C,D], 2ZUV[A,B], 2ZUW[A,B,C,D], 3WFZ[A,B,C,D] | Galacto-N-biose phosphorylase/Lacto-N-biose phosphorylase (LnpA1; LnbP; GLNBP; BLLJ_1623) | (β/α)8 | Dimer | [206,207] |
| GH130 | CAH06518.1 | Bacteroides fragilis NCTC 9343 | 3WAS[A,B], 3WAT[A,B], 3WAU[A,B], 4KMI[A,B | β-1,4-mannosylglucose phosphorylase (MGP; BF0772) | 5-fold β-propeller | Hexamer | [208] |
| GH130 | CAC96089.1 | Listeria innocua Clip11262 | 5B0P[A,B], 5B0Q[A,B], 5B0R[A,B], 5B0S[A,B] | β-1,2-mannobiose phosphorylase (Lin0857) | 5-fold β-propeller | Dimer | [100] |
| GH130 | ADU21379.1 | Ruminococcus albus 7 (DSM 20455) | 5AY9[A], 5AYC[A] | β-1,4-mannosylglucose phosphorylase (RaMP1; RaMGP; Rumal_0852) | 5-fold β-propeller | Trimer | [209] |
| GH130 | ADU20661.1 | Ruminococcus albus 7 (DSM 20455) | 5AYD[A,B,C,D,E,F], 5AYE[A,B,C,D,E,F] | β-1,4-mannooligosaccharide phosphorylase (MOP; RaMP2; Rumal_0099) | 5-fold β-propeller | Hexamer | [209] |
| GH130 | ABY93074.1 | Thermoanaerobacter sp. X514 | 7FIP, 7FIQ, 7FIR, 7FIS | β-1,2-mannobiose phosphorylase (Teth514_1789) | 5-fold β-propeller | Monomer | [210] |
| GH130 | AAD36300.1 | Thermotoga maritima MSB8 | 1VKD[A,B,C,D,E,F] | β-1,4-mannooligosaccharide phosphorylase (TM1225) | 5-fold β-propeller | Dimer | Unpublished |
| GH130 | ADD61463.1 | Uncultured bacterium | 4UDG[A,B,C,D,E,F], 4UDI[A,B,C,D,E,F], 4UDJ[A,B,C,D,E,F], 4UDK[A,B,C,D,E,F] | β-1,4-mannopyranosyl-[N-glycan] phosphorylase/β-1,4-mannopyranosyl-chitobiose phosphorylase (UhgbMP; Uhgb_MP) | 5-fold β-propeller | Hexamer | [195] |
| GH149 | / | Uncultured bacterium | 6HQ6[B], 6HQ8[A,B], 6HQ6[A] | β-1,3-oligoglucan phosphorylase (Pro_7066) | (α/α)6 | Dimer | [114] |
| GT35 | AAM52219.1 | Corynebacterium callunae | 2C4M[A,B,C,D] | Glycogen phosphorylase | GT-B | Dimer | Unpublished |
| GT35 | AAC76442.1 | Escherichia coli str. K-12 substr. MG1655 | 1AHP[A], 1E4O[A], 1L5V[A], 1L5W[A], 1L6I[A], 1QM5[A], 2ASV[A,B], 2AV6[A,B], 2AW3[A,B], 2AZD[A,B], 2ECP[A | Maltodextrin phosphorylase (MalP; b3417) | GT-B | Dimer | [136,211,212,213] |
| GT35 | AAN59210.1 | Streptococcus mutans UA159 | 4L22[A] | Maltodextrin phosphorylase (GlgP; SMU.1564) | GT-B | Monomer | Unpublished |
| GT35 | CAB61943.1 | Arabidopsis thaliana | 4BQE[A,B], 4BQF[A,B], 4BQI[A,B] | α-glucan phosphorylase (PHS2; AtPHS2; At3g46970) | GT-B | Dimer | [127] |
| GT35 | AAB60395.1 | Homo sapiens | 5IKO[A], 5IKP[A] | Glycogen phosphorylase (brain) (bGP) | GT-B | Dimer | [214] |
| GT35 | CAA75517.1 | Homo sapiens | 1EM6[A,B], 1EXV[A,B], 1FA9[A], 1FC0[A,B], 1L5Q[A,B], 1L5R[A,B], 1L5S[A,B], 1L7X[A,B], 1XOI[A,B], 2ATI[A,B], 2QLL[A], 2ZB2[A,B], 3CEH[A,B], 3CEJ[A,B], 3CEM[A,B], 3DD1[A,B], 3DDS[A,B], 3DDW[A,B] | Glycogen phosphorylase (liver) | GT-B | Dimer | [215,216,217,218,219,220,221,222,223] |
| GT35 | AAC17451.1 | Homo sapiens | 1Z8D[A] | Glycogen phosphorylase (muscle) (PigM) | GT-B | Dimer | [224] |
| GT35 | BAK00834.1 | Hordeum vulgare subsp. Vulgare | 5LR8[A,B], 5LRA[A,B] | Plastidial α-1,4-glucan phosphorylase (Pho1; HvPho1) | GT-B | Dimer | [153] |
| GT35 | ACJ76617.1 | Oryctolagus cuniculus | Dozens available-2GJ4[A] best solution | Glycogen phosphorylase (muscle) (PygM) | GT-B | Dimer | [225] |
| GT35 | AAB68057.1 | Saccharomyces cerevisiae S288c | 1YGP[A,B] | Glycogen phosphorylase (Gph1; YPR160w) | GT-B | Dimer | [226] |
| GT108 | CBZ24449.1 | Leishmania mexicana MHOM/GT/2001/U1103 | 6Q50[A] | MTP4 (LMXM_10_1260) | 5-fold β-propeller | Monomer | [174] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, A.; Benkoulouche, M.; Ladeveze, S.; Durand, J.; Cioci, G.; Laville, E.; Potocki-Veronese, G. Discovery and Biotechnological Exploitation of Glycoside-Phosphorylases. Int. J. Mol. Sci. 2022, 23, 3043. https://doi.org/10.3390/ijms23063043
Li A, Benkoulouche M, Ladeveze S, Durand J, Cioci G, Laville E, Potocki-Veronese G. Discovery and Biotechnological Exploitation of Glycoside-Phosphorylases. International Journal of Molecular Sciences. 2022; 23(6):3043. https://doi.org/10.3390/ijms23063043
Chicago/Turabian StyleLi, Ao, Mounir Benkoulouche, Simon Ladeveze, Julien Durand, Gianluca Cioci, Elisabeth Laville, and Gabrielle Potocki-Veronese. 2022. "Discovery and Biotechnological Exploitation of Glycoside-Phosphorylases" International Journal of Molecular Sciences 23, no. 6: 3043. https://doi.org/10.3390/ijms23063043
APA StyleLi, A., Benkoulouche, M., Ladeveze, S., Durand, J., Cioci, G., Laville, E., & Potocki-Veronese, G. (2022). Discovery and Biotechnological Exploitation of Glycoside-Phosphorylases. International Journal of Molecular Sciences, 23(6), 3043. https://doi.org/10.3390/ijms23063043






