The Role of Biomaterials in Peripheral Nerve and Spinal Cord Injury: A Review
Abstract
1. Introduction
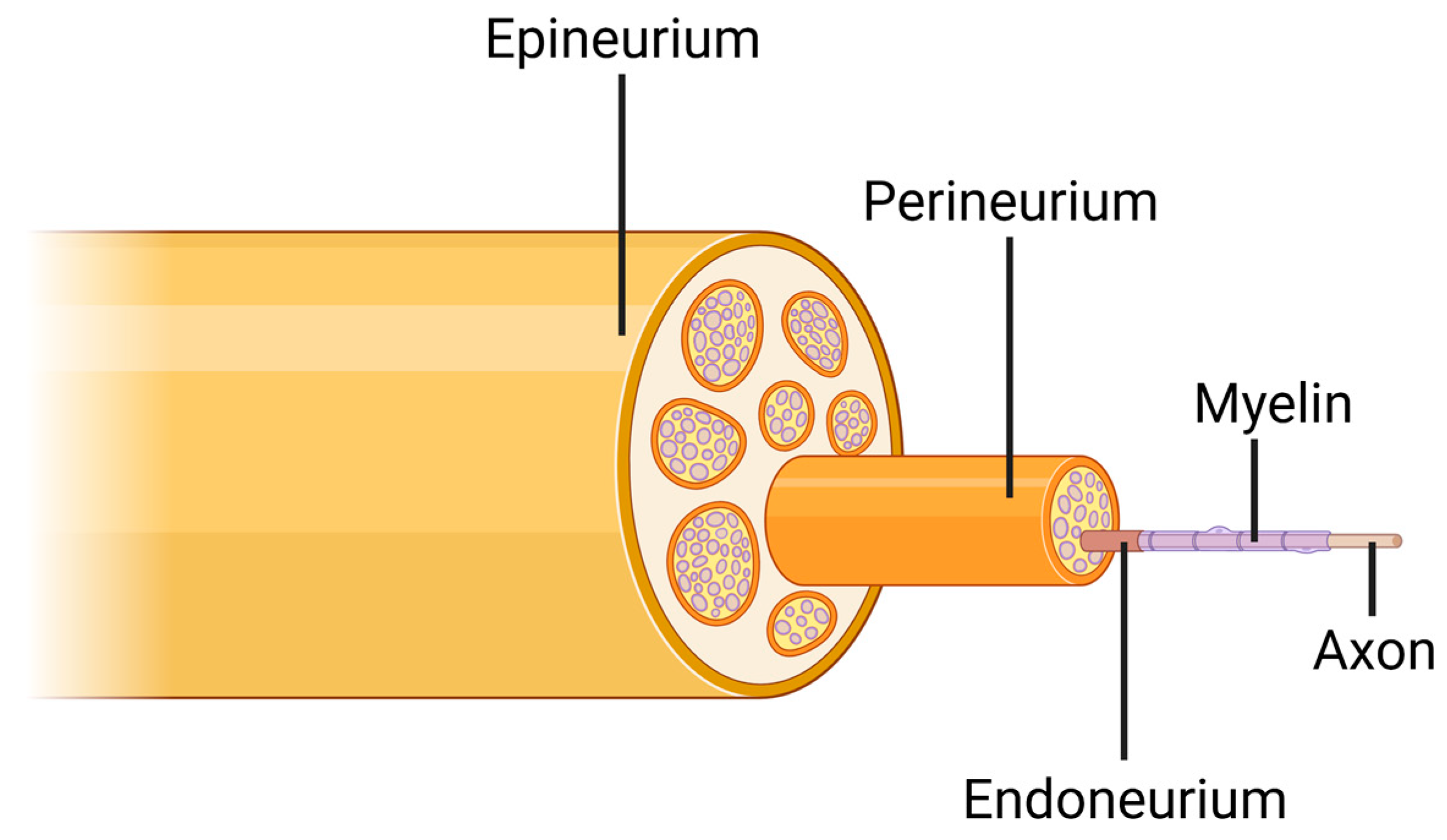
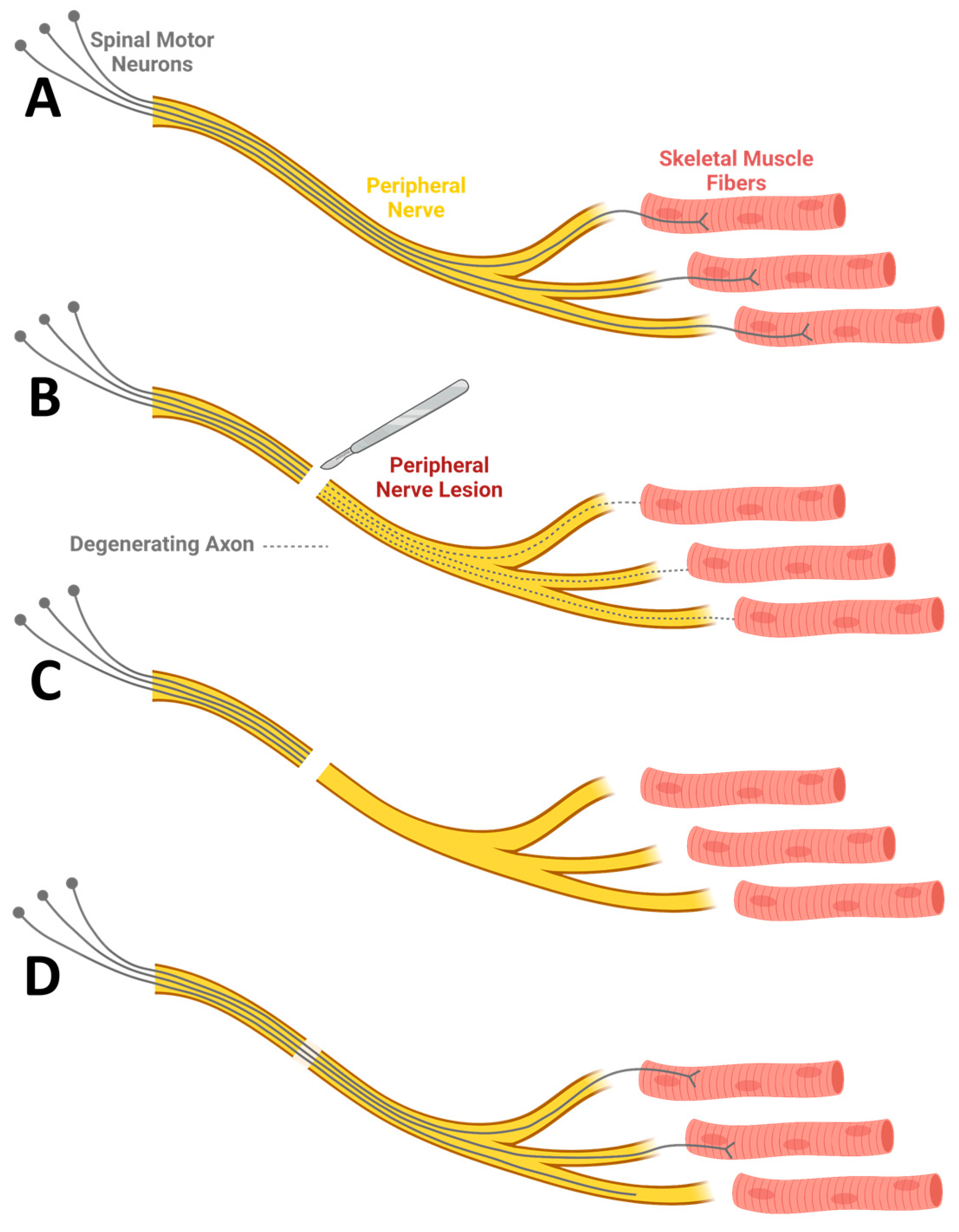
2. Peripheral Nerve Injury
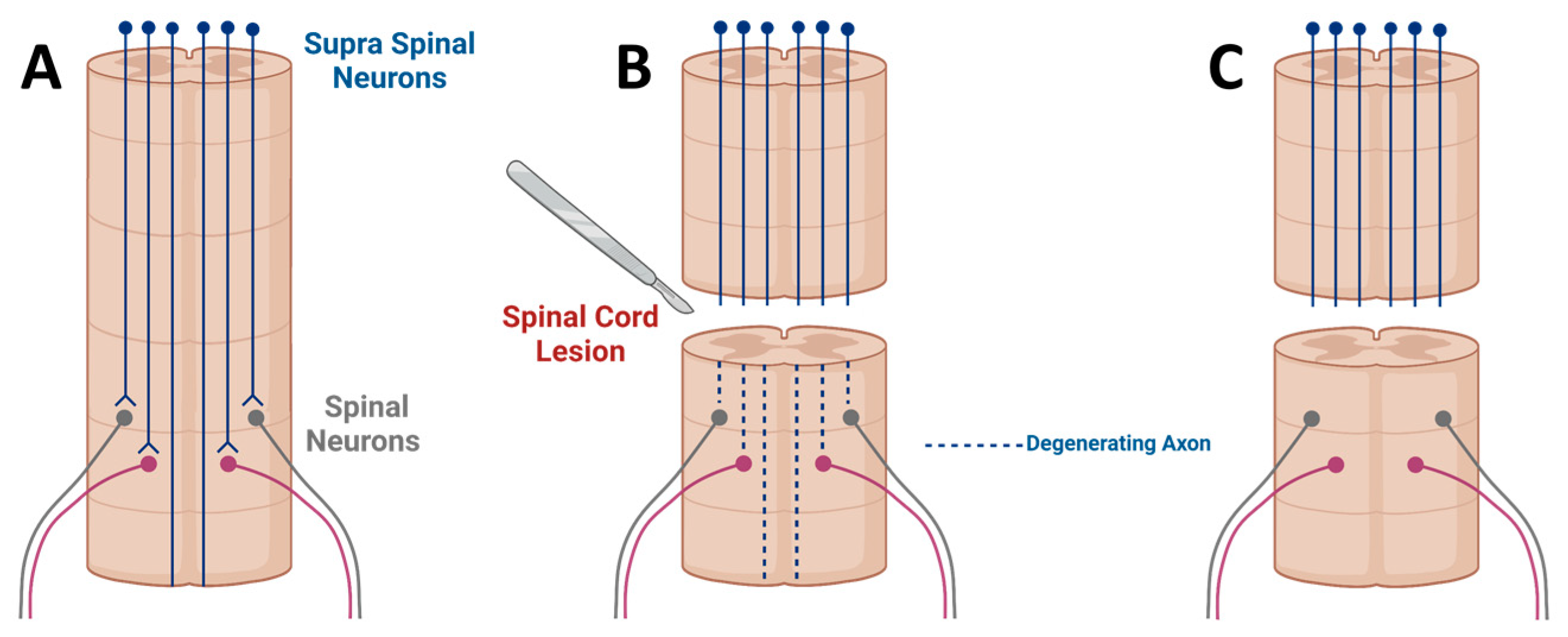
3. Spinal Cord Injury
4. Axonal Interaction with Biomaterials
5. Surface Modification
6. Biodegradation
7. Foreign Body Response
8. Topography-Mediated Axonal Guidance
- (1)
- Most importantly, in the absence of guidance, the number of axons that will reach their pre-injury targets will be very limited [98]. For example, with lack of guidance following peripheral nerve injuries, axons will regenerate; however, only a small fraction of them, if any, will reach the denervated muscle fibers.
- (2)
- In case axons do regenerate a long distance into their denervated targets, disoriented growth may lead them to inappropriate targets [74]. For example, motor axons may regenerate into sensory regions.
- (3)
- Disoriented growth from the end of a severed nerve will generate bulb-like structures known as neuromas, which consist of many disoriented axons and, via mechanisms not clearly understood, cause severe neuropathic pain [106].
9. Drug Release
10. Clinical Translation
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, B.S.; Baez, C.E.; Atala, A. Biomaterials for tissue engineering. World J. Urol. 2000, 18, 2–9. [Google Scholar] [CrossRef]
- Naderi, H.; Matin, M.M.; Bahrami, A.R. Review paper: Critical issues in tissue engineering: Biomaterials, cell sources, angiogenesis, and drug delivery systems. J. Biomater. Appl. 2011, 26, 383–417. [Google Scholar] [CrossRef] [PubMed]
- Carriel, V.; Alaminos, M.; Garzón, I.; Campos, A.; Cornelissen, M. Tissue engineering of the peripheral nervous system. Expert Rev. Neurother. 2014, 14, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Boni, R.; Ali, A.; Shavandi, A.; Clarkson, A.N. Current and novel polymeric biomaterials for neural tissue engineering. J. Biomed. Sci. 2018, 25, 90. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Velumian, A.A.; Fehlings, M.G. The Role of Excitotoxicity in Secondary Mechanisms of Spinal Cord Injury: A Review with an Emphasis on the Implications for White Matter Degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Kadoya, K.; Tuszynski, M.H. Axonal growth and connectivity from neural stem cell grafts in models of spinal cord injury. Curr. Opin. Neurobiol. 2014, 27, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Piao, X.; Bonaldo, P. Role of macrophages in Wallerian degeneration and axonal regeneration after peripheral nerve injury. Acta Neuropathol. 2015, 130, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Nielson, J.L.; Sears-Kraxberger, I.; Strong, M.K.; Wong, J.K.; Willenberg, R.; Steward, O. Unexpected survival of neurons of origin of the pyramidal tract after spinal cord injury. J. Neurosci. 2010, 30, 11516–11528. [Google Scholar] [CrossRef]
- Catala, M.; Kubis, N. Gross anatomy and development of the peripheral nervous system. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2013; Volume 115, pp. 29–41. [Google Scholar]
- Grinberg, Y.; Schiefer, M.A.; Tyler, D.J.; Gustafson, K.J. Fascicular perineurium thickness, size, and position affect model predictions of neural excitation. IEEE Trans. Neural Syst. Rehabil. Eng. 2008, 16, 572–581. [Google Scholar] [CrossRef]
- Khan, H.; Perera, N. Peripheral nerve injury: An update. Orthop. Trauma 2020, 34, 168–173. [Google Scholar] [CrossRef]
- Pfister, B.J.; Gordon, T.; Loverde, J.R.; Kochar, A.S.; Mackinnon, S.E.; Cullen, D.K. Biomedical engineering strategies for peripheral nerve repair: Surgical applications, state of the art, and future challenges. Crit. Rev. Biomed. Eng. 2011, 39, 81–124. [Google Scholar] [CrossRef]
- Chandran, V.; Coppola, G.; Nawabi, H.; Omura, T.; Versano, R.; Huebner, E.A.; Zhang, A.; Costigan, M.; Yekkirala, A.; Barrett, L.; et al. A Systems-Level Analysis of the Peripheral Nerve Intrinsic Axonal Growth Program. Neuron 2016, 89, 956–970. [Google Scholar] [CrossRef]
- Bradke, F.; Fawcett, J.W.; Spira, M.E. Assembly of a new growth cone after axotomy: The precursor to axon regeneration. Nat. Rev. Neurosci. 2012, 13, 183–193. [Google Scholar] [CrossRef]
- Clements, M.P.; Byrne, E.; Camarillo Guerrero, L.F.; Cattin, A.L.; Zakka, L.; Ashraf, A.; Burden, J.J.; Khadayate, S.; Lloyd, A.C.; Marguerat, S.; et al. The Wound Microenvironment Reprograms Schwann Cells to Invasive Mesenchymal-like Cells to Drive Peripheral Nerve Regeneration. Neuron 2017, 96, 98–114.e7. [Google Scholar] [CrossRef]
- Dahlin, L.B. Techniques of peripheral nerve repair. Scand. J. Surg. 2008, 97, 310–316. [Google Scholar] [CrossRef]
- Bassilios Habre, S.; Bond, G.; Jing, X.L.; Kostopoulos, E.; Wallace, R.D.; Konofaos, P. The Surgical Management of Nerve Gaps: Present and Future. Ann. Plast. Surg. 2018, 80, 252–261. [Google Scholar] [CrossRef]
- Hundepool, C.A.; Bulstra, L.F.; Kotsougiani, D.; Friedrich, P.F.; Hovius, S.E.R.; Bishop, A.T.; Shin, A.Y. Comparable functional motor outcomes after repair of peripheral nerve injury with an elastase-processed allograft in a rat sciatic nerve model. Microsurgery 2018, 38, 772–779. [Google Scholar] [CrossRef]
- Lee, B.B.; Cripps, R.A.; Fitzharris, M.; Wing, P.C. The global map for traumatic spinal cord injury epidemiology: Update 2011, global incidence rate. Spinal Cord 2014, 52, 110–116. [Google Scholar] [CrossRef]
- Sekhon, L.H.; Fehlings, M.G. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine 2001, 26, S2–S12. [Google Scholar] [CrossRef]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From basics to clinical: A comprehensive review on spinal cord injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef]
- Kerschensteiner, M.; Schwab, M.E.; Lichtman, J.W.; Misgeld, T. In vivo imaging of axonal degeneration and regeneration in the injured spinal cord. Nat. Med. 2005, 11, 572–577. [Google Scholar] [CrossRef]
- Fehlings, M.G.; Sekhon, L.H.S.; Tator, C. The role and timing of decompression in acute spinal cord injury: What do we know? What should we do? Spine 2001, 26, S101–S110. [Google Scholar] [CrossRef]
- Bracken, M.B.; Holford, T.R. Neurological and functional status 1 year after acute spinal cord injury: Estimates of functional recovery in National Acute Spinal Cord Injury Study II from results modeled in National Acute Spinal Cord Injury Study III. J. Neurosurg. Spine 2002, 96, 259–266. [Google Scholar] [CrossRef]
- Tuszynski, M.H.H.; Steward, O. Concepts and Methods for the Study of Axonal Regeneration in the CNS. Neuron 2012, 74, 777–791. [Google Scholar] [CrossRef]
- Courtine, G.; Song, B.; Roy, R.R.; Zhong, H.; Herrmann, J.E.; Ao, Y.; Qi, J.; Edgerton, V.R.; Sofroniew, M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008, 14, 69–74. [Google Scholar] [CrossRef]
- Ankeny, D.P.; McTigue, D.M.; Jakeman, L.B. Bone marrow transplants provide tissue protection and directional guidance for axons after contusive spinal cord injury in rats. Exp. Neurol. 2004, 190, 17–31. [Google Scholar] [CrossRef]
- Beattie, M.S.; Bresnahan, J.C.; Komon, J.; Tovar, C.A.; Van Meter, M.; Anderson, D.K.; Faden, A.I.; Hsu, C.Y.; Noble, L.J.; Salzman, S.; et al. Endogenous Repair after Spinal Cord Contusion Injuries in the Rat. Exp. Neurol. 1997, 148, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.L.; Letourneau, P.C.; Palm, S.L.; McCarthy, J.; Furcht, L.T. Neurite extension by peripheral and central nervous system neurons in response to substratum-bound fibronectin and laminin. Dev. Biol. 1983, 98, 212–220. [Google Scholar] [CrossRef]
- Barton, M.J.; St John, J.; Clarke, M.; Wright, A.; Ekberg, J. The glia response after peripheral nerve injury: A comparison between Schwann cells and olfactory ensheathing cells and their uses for neural regenerative therapies. Int. J. Mol. Sci. 2017, 18, 287. [Google Scholar] [CrossRef]
- Tuszynski, M.H.; Peterson, D.A.; Ray, J.; Baird, A.; Nakahara, Y.; Gages, F.H. Fibroblasts Genetically Modified to Produce Nerve Growth Factor Induce Robust Neuritic Ingrowth after Grafting to the Spinal Cord. Exp. Neurol. 1994, 126, 1–14. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Dissecting spinal cord regeneration. Nature 2018, 557, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.D.; Romero, M.I.; Lush, M.E.; Lu, Q.R.; Henkemeyer, M.; Parada, L.F. Ephrin-B3 is a myelin-based inhibitor of neurite outgrowth. Proc. Natl. Acad. Sci. USA 2005, 102, 10694–10699. [Google Scholar] [CrossRef]
- Messersmith, E.K.; Leonardo, E.D.; Shatz, C.J.; Tessier-Lavigne, M.; Goodman, C.S.; Kolodkin, A.L. Sernaphorin III can function as a selective chemorepellent to pattern sensory projections in the spinal cord. Neuron 1995, 14, 949–959. [Google Scholar] [CrossRef]
- Bregman, B.S.; Kunkel-Bagden, E.; Schnell, L.; Dai, H.N.; Gao, D.; Schwab, M.E. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature 1995, 378, 498–501. [Google Scholar] [CrossRef]
- Simonen, M.; Pedersen, V.; Weinmann, O.; Schnell, L.; Buss, A.; Ledermann, B.; Christ, F.; Sansig, G.; van der Putten, H.; Schwab, M.E. Systemic Deletion of the Myelin-Associated Outgrowth Inhibitor Nogo-A Improves Regenerative and Plastic Responses after Spinal Cord Injury. Neuron 2003, 38, 201–211. [Google Scholar] [CrossRef]
- Zheng, B.; Ho, C.; Li, S.; Keirstead, H.; Steward, O.; Tessier-Lavigne, M. Lack of Enhanced Spinal Regeneration in Nogo-Deficient Mice. Neuron 2003, 38, 213–224. [Google Scholar] [CrossRef]
- Levine, J.M. Increased expression of the NG2 chondroitin-sulfate proteoglycan after brain injury. J. Neurosci. 1994, 14, 4716–4730. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Moon, L.D.F.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.W.; McMahon, S.B. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef]
- Rolls, A.; Shechter, R.; Schwartz, M. The bright side of the glial scar in CNS repair. Nat. Rev. Neurosci. 2009, 10, 235–241. [Google Scholar] [CrossRef]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte scar formation aids central nervous system axon regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef]
- Kawaja, M.D.; Gage, F.H. Reactive astrocytes are substrates for the growth of adult CNS axons in the presence of elevated levels of nerve growth factor. Neuron 1991, 7, 1019–1030. [Google Scholar] [CrossRef]
- Huang, Z.; Wang, W.; Ma, J.; Li, B.; Chen, J.; Yang, H.; Saijilafu. mTOR signaling pathway differently regulates central and peripheral axon regeneration. Acta Biochim. Biophys. Sin. 2017, 49, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Lu, Y.; Lee, J.K.; Samara, R.; Willenberg, R.; Sears-Kraxberger, I.; Tedeschi, A.; Park, K.K.; Jin, D.; Cai, B.; et al. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat. Neurosci. 2010, 13, 1075–1081. [Google Scholar] [CrossRef]
- Jin, D.; Liu, Y.; Sun, F.; Wang, X.; Liu, X.; He, Z. Restoration of skilled locomotion by sprouting corticospinal axons induced by co-deletion of PTEN and SOCS3. Nat. Commun. 2015, 6, 8074. [Google Scholar] [CrossRef]
- Lu, P.; Wang, Y.; Graham, L.; McHale, K.; Gao, M.; Wu, D.; Brock, J.; Blesch, A.; Rosenzweig, E.S.; Havton, L.A.; et al. Long-distance growth and connectivity of neural stem cells after severe spinal cord injury. Cell 2012, 150, 1264–1273. [Google Scholar] [CrossRef]
- Poplawski, G.H.D.; Lie, R.; Hunt, M.; Kumamaru, H.; Kawaguchi, R.; Lu, P.; Schäfer, M.K.E.; Woodruff, G.; Robinson, J.; Canete, P.; et al. Adult rat myelin enhances axonal outgrowth from neural stem cells. Sci. Transl. Med. 2018, 10, eaal2563. [Google Scholar] [CrossRef]
- Turnley, A.M.; Bartlett, P.F. MAG and MOG enhance neurite outgrowth of embryonic mouse spinal cord neurons. Neuroreport 1998, 9, 1987–1990. [Google Scholar] [CrossRef]
- Myers, J.P.; Santiago-Medina, M.; Gomez, T.M. Regulation of axonal outgrowth and pathfinding by integrin-ecm interactions. Dev. Neurobiol. 2011, 71, 901–923. [Google Scholar] [CrossRef]
- Nieuwenhuis, B.; Haenzi, B.; Andrews, M.R.; Verhaagen, J.; Fawcett, J.W. Integrins promote axonal regeneration after injury of the nervous system. Biol. Rev. 2018, 93, 1339–1362. [Google Scholar] [CrossRef]
- Venstrom’ And, X.A.; Reichardt, L.F. Extracellular Matrix 2: Role of extracellular matrix molecules and their receptors in the nervous system. FASEB J. 1993, 7, 996–1003. [Google Scholar] [CrossRef]
- Zhang, C.; Morozova, A.Y.; Abakumov, M.A.; Gubsky, I.L.; Douglas, P.; Feng, S.; Bryukhovetskiy, A.S.; Chekhonin, V.P. Precise Delivery Into Chronic Spinal Cord Injury Syringomyelic Cysts with Magnetic Nanoparticles MRI Visualization. Med. Sci. Monit. 2015, 21, 3179–3185. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Zhang, L.; Li, K.; Fan, H.; Fan, Y.; Liang, J.; Zhang, X. Collagen hydrogel as an immunomodulatory scaffold in cartilage tissue engineering. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Masand, S.N.; Chen, J.; Perron, I.J.; Hammerling, B.C.; Loers, G.; Schachner, M.; Shreiber, D.I. The effect of glycomimetic functionalized collagen on peripheral nerve repair. Biomaterials 2012, 33, 8353–8362. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Stabenfeldt, S.E.; Garcia, A.J.; LaPlaca, M.C. Thermoreversible laminin-functionalized hydrogel for neural tissue engineering. J. Biomed. Mater. Res. Part A 2006, 77A, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Fan, Y.; Zhang, H.; Zhang, Q.; Zhao, Y.; Xiao, Z.; Liu, W.; Chen, B.; Gao, L.; Sun, Z.; et al. Small molecules combined with collagen hydrogel direct neurogenesis and migration of neural stem cells after spinal cord injury. Biomaterials 2021, 269, 120479. [Google Scholar] [CrossRef]
- Führmann, T.; Anandakumaran, P.N.; Shoichet, M.S. Combinatorial Therapies After Spinal Cord Injury: How Can Biomaterials Help? Adv. Healthc. Mater. 2017, 6, 1601130. [Google Scholar] [CrossRef]
- Tummino, M.L.; Magnacca, G.; Cimino, D.; Laurenti, E.; Nisticò, R. The innovation comes from the sea: Chitosan and alginate hybrid gels and films as sustainable materials for wastewater remediation. Int. J. Mol. Sci. 2020, 21, 550. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef]
- Rowley, J.A.; Mooney, D.J. Alginate type and RGD density control myoblast phenotype. J. Biomed. Mater. Res. 2002, 60, 217–223. [Google Scholar] [CrossRef]
- Shahriari, D.; Koffler, J.Y.; Tuszynski, M.H.; Campana, W.M.; Sakamoto, J.S. Hierarchically Ordered Porous and High Volume poly caprolactone (PCL) Microchannel Scaffolds Enhanced Axon Growth in Transected Spinal Cords. Tissue Eng. Part A 2017, 23, 415–425. [Google Scholar] [CrossRef]
- Kaplan, B.; Merdler, U.; Szklanny, A.A.; Redenski, I.; Guo, S.; Bar-Mucha, Z.; Michael, N.; Levenberg, S. Rapid prototyping fabrication of soft and oriented polyester scaffolds for axonal guidance. Biomaterials 2020, 251, 120062. [Google Scholar] [CrossRef]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef]
- Edri, R.; Gal, I.; Noor, N.; Harel, T.; Fleischer, S.; Adadi, N.; Green, O.; Shabat, D.; Heller, L.; Shapira, A.; et al. Personalized Hydrogels for Engineering Diverse Fully Autologous Tissue Implants. Adv. Mater. 2019, 31, 1803895. [Google Scholar] [CrossRef]
- Lu, P.; Woodruff, G.; Wang, Y.; Graham, L.; Hunt, M.; Wu, D.; Boehle, E.; Ahmad, R.; Poplawski, G.; Brock, J.; et al. Long-Distance Axonal Growth from Human Induced Pluripotent Stem Cells after Spinal Cord Injury. Neuron 2014, 83, 789–796. [Google Scholar] [CrossRef]
- Kadoya, K.; Lu, P.; Nguyen, K.; Lee-Kubli, C.; Kumamaru, H.; Yao, L.; Knackert, J.; Poplawski, G.; Dulin, J.N.; Strobl, H.; et al. Spinal cord reconstitution with homologous neural grafts enables robust corticospinal regeneration. Nat. Med. 2016, 22, 479–487. [Google Scholar] [CrossRef]
- Lu, P.; Graham, L.; Wang, Y.; Wu, D.; Tuszynski, M. Promotion of Survival and Differentiation of Neural Stem Cells with Fibrin and Growth Factor Cocktails after Severe Spinal Cord Injury. J. Vis. Exp. JoVE 2014, 89, 50641. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Haggerty, A.E.; Yan, J.; Lan, M.; Seu, M.; Yang, M.; Marlow, M.M.; Maldonado-Lasunción, I.; Cho, B.; et al. The effect of a nanofiber-hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials 2020, 245, 119978. [Google Scholar] [CrossRef]
- Saltzman, E.B.; Villa, J.C.; Doty, S.B.; Feinberg, J.H.; Lee, S.K.; Wolfe, S.W. A Comparison Between Two Collagen Nerve Conduits and Nerve Autograft: A Rat Model of Motor Nerve Regeneration. J. Hand Surg. 2019, 44, 700.e1–700.e9. [Google Scholar] [CrossRef]
- Shapira, Y.; Tolmasov, M.; Nissan, M.; Reider, E.; Koren, A.; Biron, T.; Bitan, Y.; Livnat, M.; Ronchi, G.; Geuna, S.; et al. Comparison of results between chitosan hollow tube and autologous nerve graft in reconstruction of peripheral nerve defect: An experimental study. Microsurgery 2016, 36, 664–671. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, X.; Zhang, Z.; Luo, J.; Cai, Z.; Wang, Y.; Li, Y. Repairing Transected Peripheral Nerve Using a Biomimetic Nerve Guidance Conduit Containing Intraluminal Sponge Fillers. Adv. Healthc. Mater. 2019, 8, 1900913. [Google Scholar] [CrossRef]
- Whitlock, E.L.; Tuffaha, S.H.; Luciano, J.P.; Yan, Y.; Hunter, D.A.; Magill, C.K.; Moore, A.M.; Tong, A.Y.; Mackinnon, S.E.; Borschel, G.H. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 2009, 39, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yu, Y.; Zhang, L.; Zheng, F.; Yin, Y.; Gao, Y.; Li, K.; Xu, J.; Wen, J.; Chen, H.; et al. Sustainable release of nerve growth factor for peripheral nerve regeneration using nerve conduits laden with Bioconjugated hyaluronic acid-chitosan hydrogel. Compos. Part B Eng. 2022, 230, 109509. [Google Scholar] [CrossRef]
- Stokols, S.; Sakamoto, J.; Breckon, C.; Holt, T.; Weiss, J.; Tuszynski, M.H. Templated Agarose Scaffolds Support Linear Axonal Regeneration. Tissue Eng. 2006, 12, 2777–2787. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Redenski, I.; Landau, S.; Szklanny, A.; Merdler, U.; Levenberg, S. Prevascularized Scaffolds Bearing Human Dental Pulp Stem Cells for Treating Complete Spinal Cord Injury. Adv. Healthc. Mater. 2020, 9, 2000974. [Google Scholar] [CrossRef]
- Ganz, J.; Shor, E.; Guo, S.; Sheinin, A.; Arie, I.; Michaelevski, I.; Pitaru, S.; Offen, D.; Levenberg, S. Implantation of 3D Constructs Embedded with Oral Mucosa-Derived Cells Induces Functional Recovery in Rats with Complete Spinal Cord Transection. Front. Neurosci. 2017, 11, 589. [Google Scholar] [CrossRef]
- Whitaker, R.; Hernaez-Estrada, B.; Hernandez, R.M.; Santos-Vizcaino, E.; Spiller, K.L. Immunomodulatory Biomaterials for Tissue Repair. Chem. Rev. 2021, 121, 11305–11335. [Google Scholar] [CrossRef]
- Amani, H.; Arzaghi, H.; Bayandori, M.; Dezfuli, A.S.; Pazoki-Toroudi, H.; Shafiee, A.; Moradi, L. Controlling Cell Behavior through the Design of Biomaterial Surfaces: A Focus on Surface Modification Techniques. Adv. Mater. Interfaces 2019, 6, 1900572. [Google Scholar] [CrossRef]
- Manchineella, S.; Thrivikraman, G.; Basu, B.; Govindaraju, T. Surface-functionalized silk fibroin films as a platform to guide neuron-like differentiation of human mesenchymal stem cells. ACS Appl. Mater. Interfaces 2016, 8, 22849–22859. [Google Scholar] [CrossRef]
- Chen, W.S.; Guo, L.Y.; Tang, C.C.; Tsai, C.K.; Huang, H.H.; Chin, T.Y.; Yang, M.L.; Chen-Yang, Y.W. The effect of laminin surface modification of electrospun silica nanofiber substrate on neuronal tissue engineering. Nanomaterials 2018, 8, 165. [Google Scholar] [CrossRef]
- De Luca, A.C.; Terenghi, G.; Downes, S. Chemical surface modification of poly-ε-caprolactone improves Schwann cell proliferation for peripheral nerve repair. J. Tissue Eng. Regen. Med. 2014, 8, 153–163. [Google Scholar] [CrossRef]
- He, L.; Tang, S.; Prabhakaran, M.P.; Liao, S.; Tian, L.; Zhang, Y.; Xue, W.; Ramakrishna, S. Surface Modification of PLLA Nano-scaffolds with Laminin Multilayer by LbL Assembly for Enhancing Neurite Outgrowth. Macromol. Biosci. 2013, 13, 1601–1609. [Google Scholar] [CrossRef]
- Novikova, L.N.; Pettersson, J.; Brohlin, M.; Wiberg, M.; Novikov, L.N. Biodegradable poly-β-hydroxybutyrate scaffold seeded with Schwann cells to promote spinal cord repair. Biomaterials 2008, 29, 1198–1206. [Google Scholar] [CrossRef]
- Hersel, U.; Dahmen, C.; Kessler, H. RGD modified polymers: Biomaterials for stimulated cell adhesion and beyond. Biomaterials 2003, 24, 4385–4415. [Google Scholar] [CrossRef]
- Lee, D.J.; Fontaine, A.; Meng, X.; Park, D. Biomimetic Nerve Guidance Conduit Containing Intraluminal Microchannels with Aligned Nanofibers Markedly Facilitates in Nerve Regeneration. ACS Biomater. Sci. Eng. 2016, 2, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Wang, K.; Ma, T.; Huang, L.; Xia, B.; Zhu, S.; Yang, Y.; Liu, Z.; Quan, X.; Luo, K.; et al. Noncovalent Bonding of RGD and YIGSR to an Electrospun Poly(ε-Caprolactone) Conduit through Peptide Self-Assembly to Synergistically Promote Sciatic Nerve Regeneration in Rats. Adv. Healthc. Mater. 2017, 6, 1600860. [Google Scholar] [CrossRef]
- Sever-Bahcekapili, M.; Yilmaz, C.; Demirel, A.; Kilinc, M.C.; Dogan, I.; Caglar, Y.S.; Guler, M.O.; Tekinay, A.B. Neuroactive Peptide Nanofibers for Regeneration of Spinal Cord after Injury. Macromol. Biosci. 2021, 21, 2000234. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, L.; Zhang, W. Control of scaffold degradation in tissue engineering: A review. Tissue Eng. Part B Rev. 2014, 20, 492–502. [Google Scholar] [CrossRef]
- Iqbal, N.; Khan, A.S.; Asif, A.; Yar, M.; Haycock, J.W.; Rehman, I.U. Recent concepts in biodegradable polymers for tissue engineering paradigms: A critical review. Int. Mater. Rev. 2019, 64, 91–126. [Google Scholar] [CrossRef]
- Godavitarne, C.; Robertson, A.; Peters, J.; Rogers, B. Biodegradable materials. Orthop. Trauma 2017, 31, 316–320. [Google Scholar] [CrossRef]
- Koffler, J.; Zhu, W.; Qu, X.; Platoshyn, O.; Dulin, J.N.; Brock, J.; Graham, L.; Lu, P.; Sakamoto, J.; Marsala, M.; et al. Biomimetic 3D-printed scaffolds for spinal cord injury repair. Nat. Med. 2019, 25, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Shahriari, D.; Koffler, J.; Lynam, D.A.; Tuszynski, M.H.; Sakamoto, J.S. Characterizing the degradation of alginate hydrogel for use in multilumen scaffolds for spinal cord repair. J. Biomed. Mater. Res. Part A 2016, 104, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Vert, M. Aliphatic Polyesters: Great Degradable Polymers That Cannot Do Everything†. Biomacromolecules 2004, 6, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Levenberg, S.; Rouwkema, J.; Macdonald, M.; Garfein, E.S.; Kohane, D.S.; Darland, D.C.; Marini, R.; van Blitterswijk, C.A.; Mulligan, R.C.; D’Amore, P.A.; et al. Engineering vascularized skeletal muscle tissue. Nat. Biotechnol. 2005, 23, 879–884. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, K.M.; Koffler, J.; Shahriari, D.; Galvan, A.; Tuszynski, M.H.; Sakamoto, J. Microstructure and in vivo characterization of multi-channel nerve guidance scaffolds. Biomed. Mater. 2018, 13, 044104. [Google Scholar] [CrossRef]
- Pawar, K.; Cummings, B.J.; Thomas, A.; Shea, L.D.; Levine, A.; Pfaff, S.; Anderson, A.J. Biomaterial bridges enable regeneration and re-entry of corticospinal tract axons into the caudal spinal cord after SCI: Association with recovery of forelimb function. Biomaterials 2015, 65, 1–12. [Google Scholar] [CrossRef]
- Morais, J.M.; Papadimitrakopoulos, F.; Burgess, D.J. Biomaterials/tissue interactions: Possible solutions to overcome foreign body response. AAPS J. 2010, 12, 188–196. [Google Scholar] [CrossRef]
- Gros, T.; Sakamoto, J.S.; Blesch, A.; Havton, L.A.; Tuszynski, M.H. Regeneration of long-tract axons through sites of spinal cord injury using templated agarose scaffolds. Biomaterials 2010, 31, 6719–6729. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Tang, J.; Gu, Y.; Liu, L.; Liu, X.; Deng, L.; Martins, C.; Sarmento, B.; Cui, W.; Chen, L. Bioinspired Hydrogel Electrospun Fibers for Spinal Cord Regeneration. Adv. Funct. Mater. 2019, 29, 1806899. [Google Scholar] [CrossRef]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.-Z.Z.; Walsh, P.J.; Monat, J.R.; Meng, F.; Park, S.H.; Dutton, J.R.; Parr, A.M.; et al. 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds. Adv. Funct. Mater. 2018, 28, 1801850. [Google Scholar] [CrossRef]
- Johnson, B.N.; Lancaster, K.Z.; Zhen, G.; He, J.; Gupta, M.K.; Kong, Y.L.; Engel, E.A.; Krick, K.D.; Ju, A.; Meng, F.; et al. 3D Printed Anatomical Nerve Regeneration Pathways. Adv. Funct. Mater. 2015, 25, 6205–6217. [Google Scholar] [CrossRef]
- Swartzlander, M.D.; Barnes, C.A.; Blakney, A.K.; Kaar, J.L.; Kyriakides, T.R.; Bryant, S.J. Linking the foreign body response and protein adsorption to PEG-based hydrogels using proteomics. Biomaterials 2015, 41, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.R.E.; Pasterkamp, R.J.; van den Berg, L.H. Axon guidance proteins: Novel therapeutic targets for ALS? Prog. Neurobiol. 2009, 88, 286–301. [Google Scholar] [CrossRef]
- Dent, E.W.; Gertler, F.B. Cytoskeletal Dynamics and Review Transport in Growth Cone Motility and Axon Guidance. Neuron 2003, 40, 209–227. [Google Scholar] [CrossRef]
- Stein, E.; Zou, Y.; Poo, M.; Tessier-Lavigne, M. Binding of DCC by netrin-1 to mediate axon guidance independent of adenosine A2B receptor activation. Science 2001, 291, 1976–1982. [Google Scholar] [CrossRef]
- Foltán, R.; Klíma, K.; Špačková, J.; Šedý, J. Mechanism of traumatic neuroma development. Med. Hypotheses 2008, 71, 572–576. [Google Scholar] [CrossRef]
- Hoffman-Kim, D.; Mitchel, J.A.; Bellamkonda, R.V. Topography, Cell Response, and Nerve Regeneration. Annu. Rev. Biomed. Eng. 2010, 12, 203–231. [Google Scholar] [CrossRef]
- Wang, M.; Zhai, P.; Chen, X.; Schreyer, D.J.; Sun, X.; Cui, F. Bioengineered scaffolds for spinal cord repair. Tissue Eng. Part B Rev. 2011, 17, 177–194. [Google Scholar] [CrossRef]
- Gao, M.; Lu, P.; Lynam, D.; Bednark, B.; Campana, W.M.; Sakamoto, J.; Tuszynski, M. BDNF gene delivery within and beyond templated agarose multi-channel guidance scaffolds enhances peripheral nerve regeneration. J. Neural Eng. 2016, 13, 066011. [Google Scholar] [CrossRef]
- Stokols, S.; Tuszynski, M.H. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials 2006, 27, 443–451. [Google Scholar] [CrossRef]
- Cipitria, A.; Skelton, A.; Dargaville, T.R.; Dalton, P.D.; Hutmacher, D.W. Design, fabrication and characterization of PCL electrospun scaffolds—A review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bashur, C.A.; Goldstein, A.S.; Schmidt, C.E. Polypyrrole-coated electrospun PLGA nanofibers for neural tissue applications. Biomaterials 2009, 30, 4325–4335. [Google Scholar] [CrossRef] [PubMed]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of Collagen Nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Liu, S.; Yang, Y.; Gao, S.; Li, W.; Cao, J.; Wan, Y.; Huang, Z.; Fan, G.; Chen, Q.; et al. Aligned microfiber-induced macrophage polarization to guide schwann-cell-enabled peripheral nerve regeneration. Biomaterials 2021, 272, 120767. [Google Scholar] [CrossRef]
- Bulstra, L.F.; Hundepool, C.A.; Friedrich, P.F.; Bishop, A.T.; Hovius, S.E.R.; Shin, A.Y. Functional Outcome after Reconstruction of a Long Nerve Gap in Rabbits Using Optimized Decellularized Nerve Allografts. Plast. Reconstr. Surg. 2020, 145, 1442–1450. [Google Scholar] [CrossRef]
- Rao, Z.; Lin, T.; Qiu, S.; Zhou, J.; Liu, S.; Chen, S.; Wang, T.; Liu, X.; Zhu, Q.; Bai, Y.; et al. Decellularized nerve matrix hydrogel scaffolds with longitudinally oriented and size-tunable microchannels for peripheral nerve regeneration. Mater. Sci. Eng. C 2021, 120, 111791. [Google Scholar] [CrossRef]
- Xue, W.; Shi, W.; Kong, Y.; Kuss, M.; Duan, B. Anisotropic scaffolds for peripheral nerve and spinal cord regeneration. Bioact. Mater. 2021, 6, 4141–4160. [Google Scholar] [CrossRef]
- Tetzlaff, W.; Okon, E.B.; Karimi-Abdolrezaee, S.; Hill, C.E.; Sparling, J.S.; Plemel, J.R.; Plunet, W.T.; Tsai, E.C.; Baptiste, D.; Smithson, L.J.; et al. A systematic review of cellular transplantation therapies for spinal cord injury. J. Neurotrauma 2011, 28, 1611–1682. [Google Scholar] [CrossRef]
- Lukovic, D.; Manzano, V.M.; Stojkovic, M.; Bhattacharya, S.S.; Erceg, S. Concise Review: Human Pluripotent Stem Cells in the Treatment of Spinal Cord Injury. Stem Cells 2012, 30, 1787–1792. [Google Scholar] [CrossRef]
- Coutts, M.; Keirstead, H.S. Stem cells for the treatment of spinal cord injury. Exp. Neurol. 2008, 209, 368–377. [Google Scholar] [CrossRef]
- Wright, K.T.; El Masri, W.; Osman, A.; Chowdhury, J.; Johnson, W.E.B. Concise Review: Bone Marrow for the Treatment of Spinal Cord Injury: Mechanisms and Clinical Applications. Stem Cells 2011, 29, 169–178. [Google Scholar] [CrossRef]
- Zhang, R.C.; Du, W.Q.; Zhang, J.Y.; Yu, S.X.; Lu, F.Z.; DIng, H.M.; Cheng, Y.B.; Ren, C.; Geng, D.Q. Mesenchymal stem cell treatment for peripheral nerve injury: A narrative review. Neural Regen. Res. 2021, 16, 2170–2176. [Google Scholar] [CrossRef]
- Wang, C.; Lu, C.F.; Peng, J.; Hu, C.D.; Wang, Y. Roles of neural stem cells in the repair of peripheral nerve injury. Neural Regen. Res. 2017, 12, 2106–2112. [Google Scholar] [CrossRef]
- Yi, S.; Zhang, Y.; Gu, X.; Huang, L.; Zhang, K.; Qian, T.; Gu, X. Application of stem cells in peripheral nerve regeneration. Burn. Trauma 2020, 8, tkaa002. [Google Scholar] [CrossRef]
- Lopes, C.D.F.; Gonçalves, N.P.; Gomes, C.P.; Saraiva, M.J.; Pêgo, A.P. BDNF gene delivery mediated by neuron-targeted nanoparticles is neuroprotective in peripheral nerve injury. Biomaterials 2017, 121, 83–96. [Google Scholar] [CrossRef]
- Han, Q.; Sun, W.; Lin, H.; Zhao, W.; Gao, Y.; Zhao, Y.; Chen, B.; Xiao, Z.; Hu, W.; Li, Y.; et al. Linear ordered collagen scaffolds loaded with collagen-binding brain-derived neurotrophic factor improve the recovery of spinal cord injury in rats. Tissue Eng. Part A 2009, 15, 2927–2935. [Google Scholar] [CrossRef]
- Robinson, J.; Lu, P. Optimization of trophic support for neural stem cell grafts in sites of spinal cord injury. Exp. Neurol. 2017, 291, 87–97. [Google Scholar] [CrossRef]
- Raspa, A.; Carminati, L.; Pugliese, R.; Fontana, F.; Gelain, F. Self-assembling peptide hydrogels for the stabilization and sustained release of active Chondroitinase ABC in vitro and in spinal cord injuries. J. Control. Release 2021, 330, 1208–1219. [Google Scholar] [CrossRef]
- Francis, N.L.; Hunger, P.M.; Donius, A.E.; Wegst, U.G.K.; Wheatley, M.A. Strategies for neurotrophin-3 and chondroitinase ABC release from freeze-cast chitosan-alginate nerve-guidance scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, B.D.; McWilliams, A.D.; Whitener, G.B.; Messer, T.M. Early Clinical Experience With Collagen Nerve Tubes in Digital Nerve Repair. J. Hand Surg. Am. 2008, 33, 1081–1087. [Google Scholar] [CrossRef]
- Saeki, M.; Tanaka, K.; Imatani, J.; Okamoto, H.; Watanabe, K.; Nakamura, T.; Gotani, H.; Ohi, H.; Nakamura, R.; Hirata, H. Efficacy and safety of novel collagen conduits filled with collagen filaments to treat patients with peripheral nerve injury: A multicenter, controlled, open-label clinical trial. Injury 2018, 49, 766–774. [Google Scholar] [CrossRef]
- Weber Robert, A.; Breidenbach, W.C.; Brown, R.E.; Jabaley, M.E.; Mass, D.P. A randomized prospective study of polyglycolic acid conduits for digital nerve reconstruction in humans. Plast. Reconstr. Surg. 2000, 106, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhu, Q.; Chai, Y.; Ding, X.; Tang, J.; Gu, L.; Xiang, J.; Yang, Y.; Zhu, J.; Liu, X. Safety and efficacy evaluation of a human acellular nerve graft as a digital nerve scaffold: A prospective, multicentre controlled clinical trial. J. Tissue Eng. Regen. Med. 2015, 9, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Rbia, N.; Bulstra, L.F.; Saffari, T.M.; Hovius, S.E.R.; Shin, A.Y. Collagen Nerve Conduits and Processed Nerve Allografts for the Reconstruction of Digital Nerve Gaps: A Single-Institution Case Series and Review of the Literature. World Neurosurg. 2019, 127, e1176–e1184. [Google Scholar] [CrossRef] [PubMed]
- Haug, A.; Bartels, A.; Kotas, J.; Kunesch, E. Sensory recovery 1 year after bridging digital nerve defects with collagen tubes. J. Hand Surg. 2013, 38, 90–97. [Google Scholar] [CrossRef]
- Kusuhara, H.; Hirase, Y.; Isogai, N.; Sueyoshi, Y. A clinical multi-center registry study on digital nerve repair using a biodegradable nerve conduit of PGA with external and internal collagen scaffolding. Microsurgery 2019, 39, 395–399. [Google Scholar] [CrossRef]
- Navissano, M.; Malan, F.; Carnino, R.; Battiston, B. Neurotube® for facial nerve repair. Microsurgery 2005, 25, 268–271. [Google Scholar] [CrossRef]
- Gu, J.; Hu, W.; Deng, A.; Zhao, Q.; Lu, S.; Gu, X. Surgical repair of a 30 mm long human median nerve defect in the distal forearm by implantation of a chitosan–PGA nerve guidance conduit. J. Tissue Eng. Regen. Med. 2012, 6, 163–168. [Google Scholar] [CrossRef]
- Theodore, N.; Hlubek, R.; Danielson, J.; Neff, K.; Vaickus, L.; Ulich, T.R.; Ropper, A.E. First human implantation of a bioresorbable polymer scaffold for acute traumatic spinal cord injury: A clinical pilot study for safety and feasibility. Neurosurgery 2016, 79, E305–E312. [Google Scholar] [CrossRef]
- Xiao, Z.; Tang, F.; Tang, J.; Yang, H.; Zhao, Y.; Chen, B.; Han, S.; Wang, N.; Li, X.; Cheng, S.; et al. One-year clinical study of NeuroRegen scaffold implantation following scar resection in complete chronic spinal cord injury patients. Sci. China Life Sci. 2016, 59, 647–655. [Google Scholar] [CrossRef]
- Zhao, Y.; Tang, F.; Xiao, Z.; Han, G.; Wang, N.; Yin, N.; Chen, B.; Jiang, X.; Yun, C.; Han, W.; et al. Clinical study of neuroregen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 2017, 26, 891–900. [Google Scholar] [CrossRef]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien. Med. Wochenschr. 2018, 169, 240–251. [Google Scholar] [CrossRef]
- Li, X.; Liu, D.; Xiao, Z.; Zhao, Y.; Han, S.; Chen, B.; Dai, J. Scaffold-facilitated locomotor improvement post complete spinal cord injury: Motor axon regeneration versus endogenous neuronal relay formation. Biomaterials 2019, 197, 20–31. [Google Scholar] [CrossRef]
- Delaere, P.R.; Van Raemdonck, D. Commentary: The sobering truth about tracheal regeneration. J. Thorac. Cardiovasc. Surg. 2020, 159, 2537–2539. [Google Scholar] [CrossRef]
- Bretzner, F.; Gilbert, F.; Baylis, F.; Brownstone, R.M. Target Populations for First-In-Human Embryonic Stem Cell Research in Spinal Cord Injury. Cell Stem Cell 2011, 8, 468–475. [Google Scholar] [CrossRef]


| Type of Injury | Gap Size | Biomaterial Implant | Number of Patients Treated | Outcome | Reference |
|---|---|---|---|---|---|
| Digital nerve injury | 10–20 mm | Collagen | 12 | Sensory improvements | [130] |
| Sensory injury below the wrist | ≤30 mm | Collagen | 49 | Equivalent to autologous graft | [131] |
| Sensory injury below the wrist | ≤30 mm | PGA | 46 | Equivalent to standard repair or superior in some subgroups | [132] |
| Digital nerve injury | 10–50 mm | Human acellular nerve graft | 72 | Non-inferior to autologous grafts | [133] |
| Digital nerve injury | <25 mm | Collagen/Acellular graft | 19 (Collagen) 18 (Acellular) | Similar outcome between collagen and acellular graft | [134] |
| Digital nerve injury | ≤26 mm | Collagen | 35 | Good functional outcome in majority of cases | [135] |
| Digital nerve injury | 1–50 mm | PGA with collagen scaffolding | 20 | Meaningful recovery in 90% or repairs | [136] |
| Facial nerve injury | 10–30 mm | PGA | 7 | Some muscle recovery in the majoroty of patients | [137] |
| Median nerve injury in distal forearm | 30 mm | Chitosan-PGA | 1 | Significant motor, sensory and electrophysiological improvements | [138] |
| T11 Spinal cord injury | 10 mm | PLGA conjugated to Poly L lysine | 1 | Partial motor, sensory and autonomous recovery | [139] |
| C6-T12 Spinal cord injury | 5–45 mm | Collagen | 5 | Partial autonomous recovery | [140] |
| C6-T10 spinal cord injury | 13–50 mm | Collagen | 8 | Mild motor, sensory and autonomic recovery | [141] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaplan, B.; Levenberg, S. The Role of Biomaterials in Peripheral Nerve and Spinal Cord Injury: A Review. Int. J. Mol. Sci. 2022, 23, 1244. https://doi.org/10.3390/ijms23031244
Kaplan B, Levenberg S. The Role of Biomaterials in Peripheral Nerve and Spinal Cord Injury: A Review. International Journal of Molecular Sciences. 2022; 23(3):1244. https://doi.org/10.3390/ijms23031244
Chicago/Turabian StyleKaplan, Ben, and Shulamit Levenberg. 2022. "The Role of Biomaterials in Peripheral Nerve and Spinal Cord Injury: A Review" International Journal of Molecular Sciences 23, no. 3: 1244. https://doi.org/10.3390/ijms23031244
APA StyleKaplan, B., & Levenberg, S. (2022). The Role of Biomaterials in Peripheral Nerve and Spinal Cord Injury: A Review. International Journal of Molecular Sciences, 23(3), 1244. https://doi.org/10.3390/ijms23031244






