Neuroprotection of Kaji-Ichigoside F1 via the BDNF/Akt/mTOR Signaling Pathways against NMDA-Induced Neurotoxicity
Abstract
1. Introduction
2. Results
2.1. The Neuroprotective Mechanism of KF1 in PC12 Cells
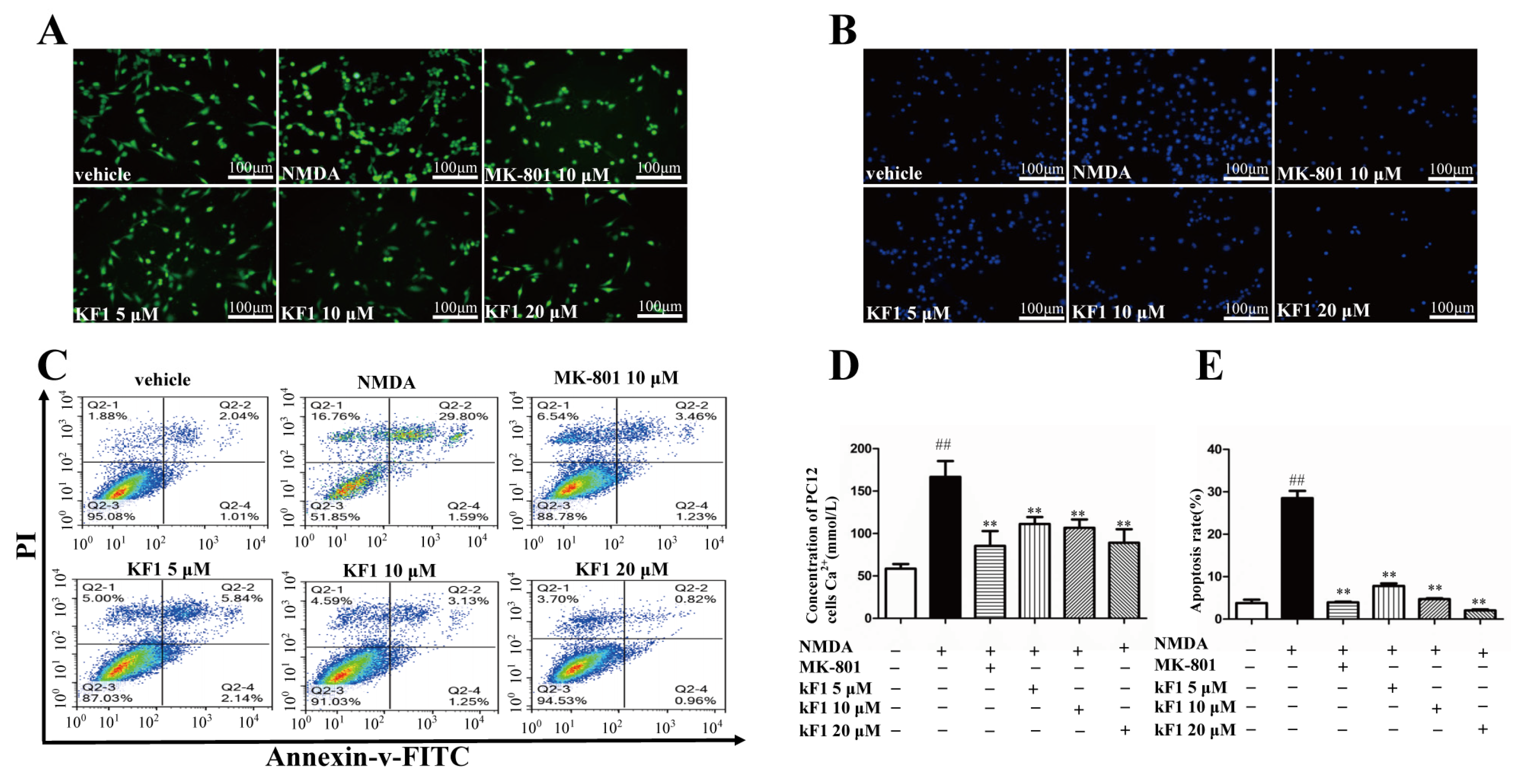
2.1.1. KF1 Alleviated NMDA-Induced Neurotoxicity in PC12 Cells
2.1.2. Effects of KF1 on NMDA-Induced Ca2+ Overload
2.1.3. Effects of KF1 on NMDA-Induced Apoptosis in PC12 Cells
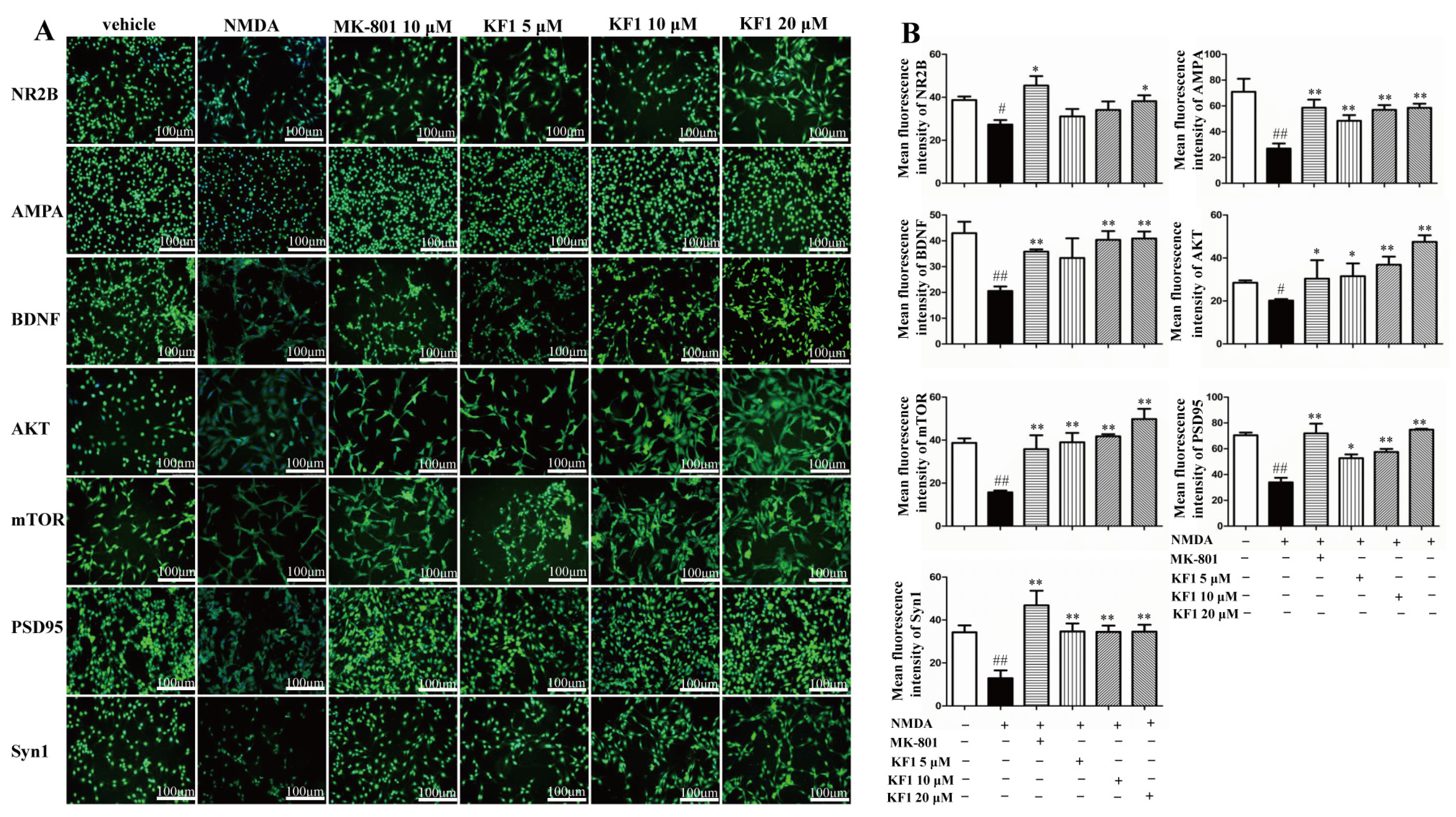
2.1.4. KF1 Effects on the Expression of NR2B, AMPA, BDNF, AKT, mTOR, PSD95, and Synapsin 1 (Syn1) Proteins in NMDA-Induced PC12 Cells by Immunofluorescence
2.2. In Vivo Analysis of the Mechanisms of Action of KF1
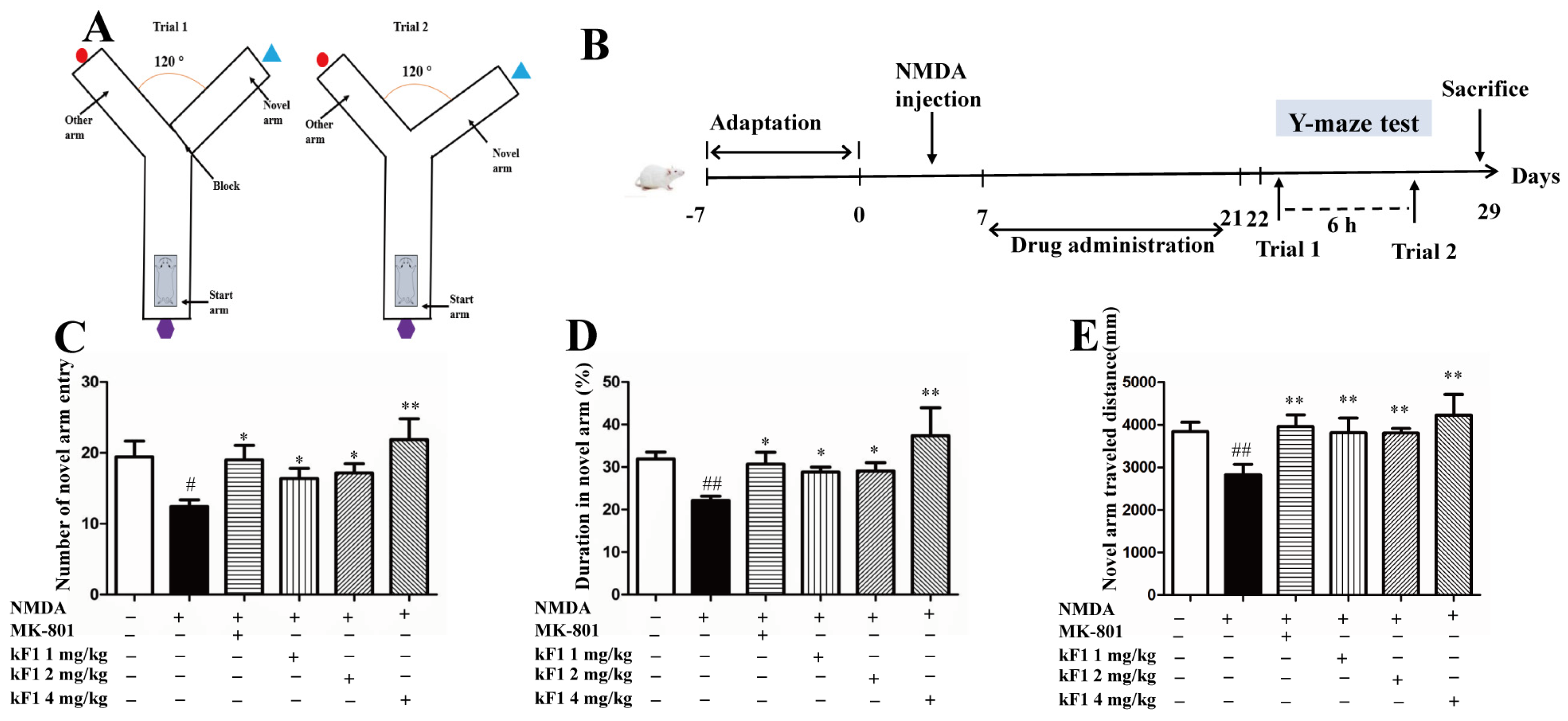
2.2.1. Effect of KF1 on Spatial Memory in the Y-Maze Test
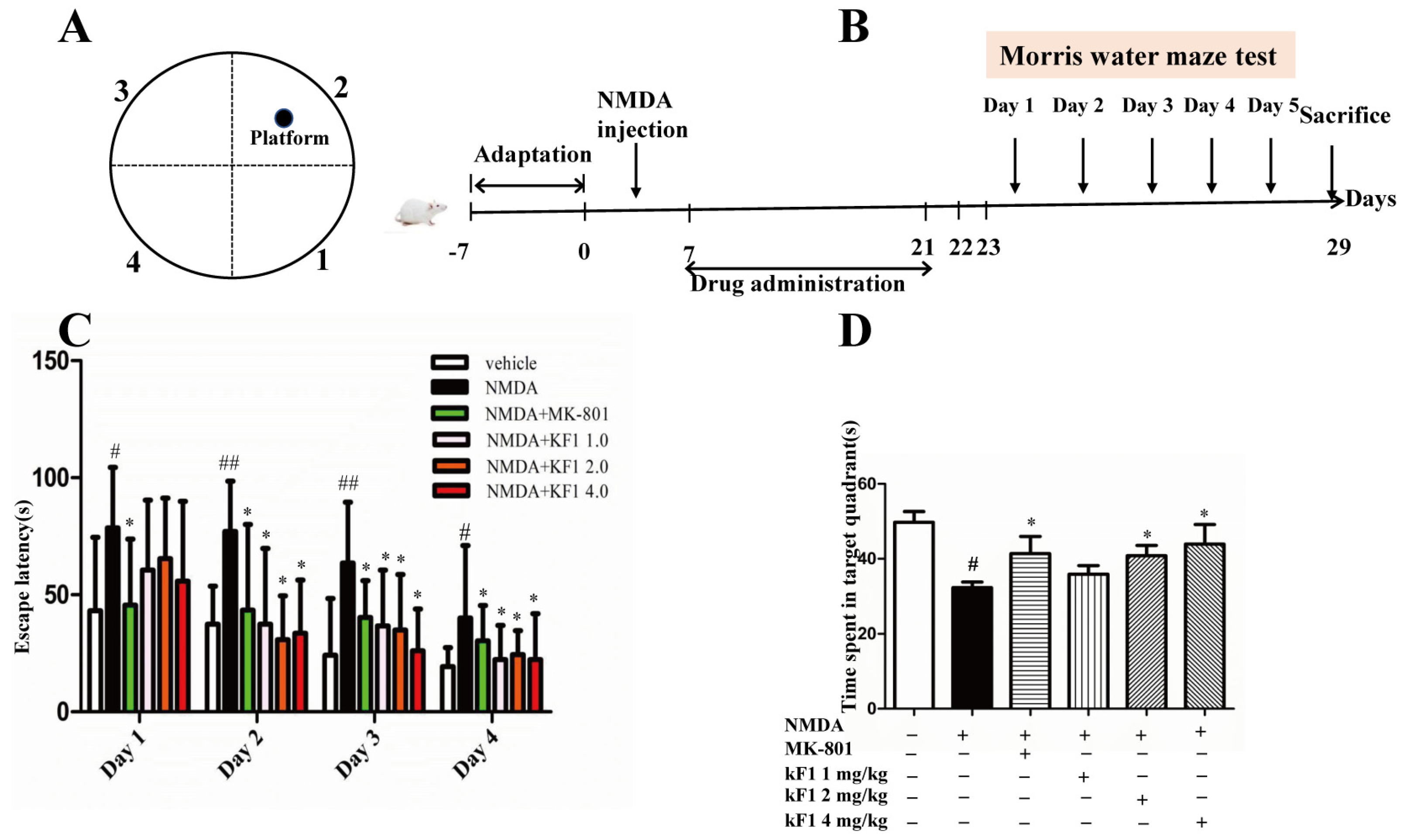
2.2.2. Effect of KF1 on the Morris Water Maze (MWM) Test
2.2.3. Effects of KF1 on Neurotransmitters and Monoamine Oxidase (MAO) Levels in the Hippocampus
2.2.4. Effects of KF1 on Ca2+ Concentration
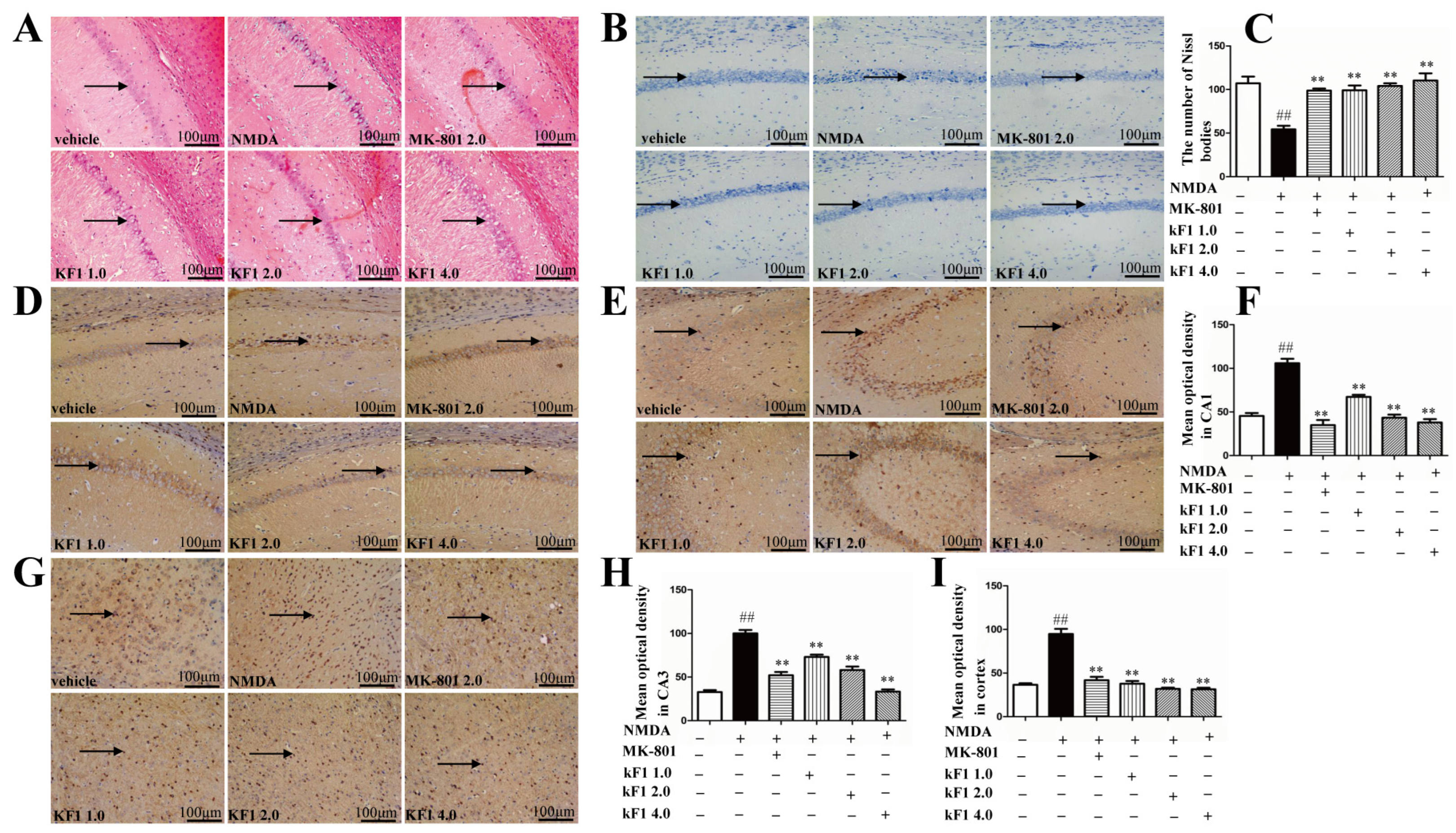
2.2.5. Histopathological Changes in the Hippocampus
2.2.6. Effects of KF1 on Hippocampal Neurons of NMDA-Induced Mice
2.2.7. Immunohistochemical Expression of NMDAR1 in the Hippocampus and Cortex
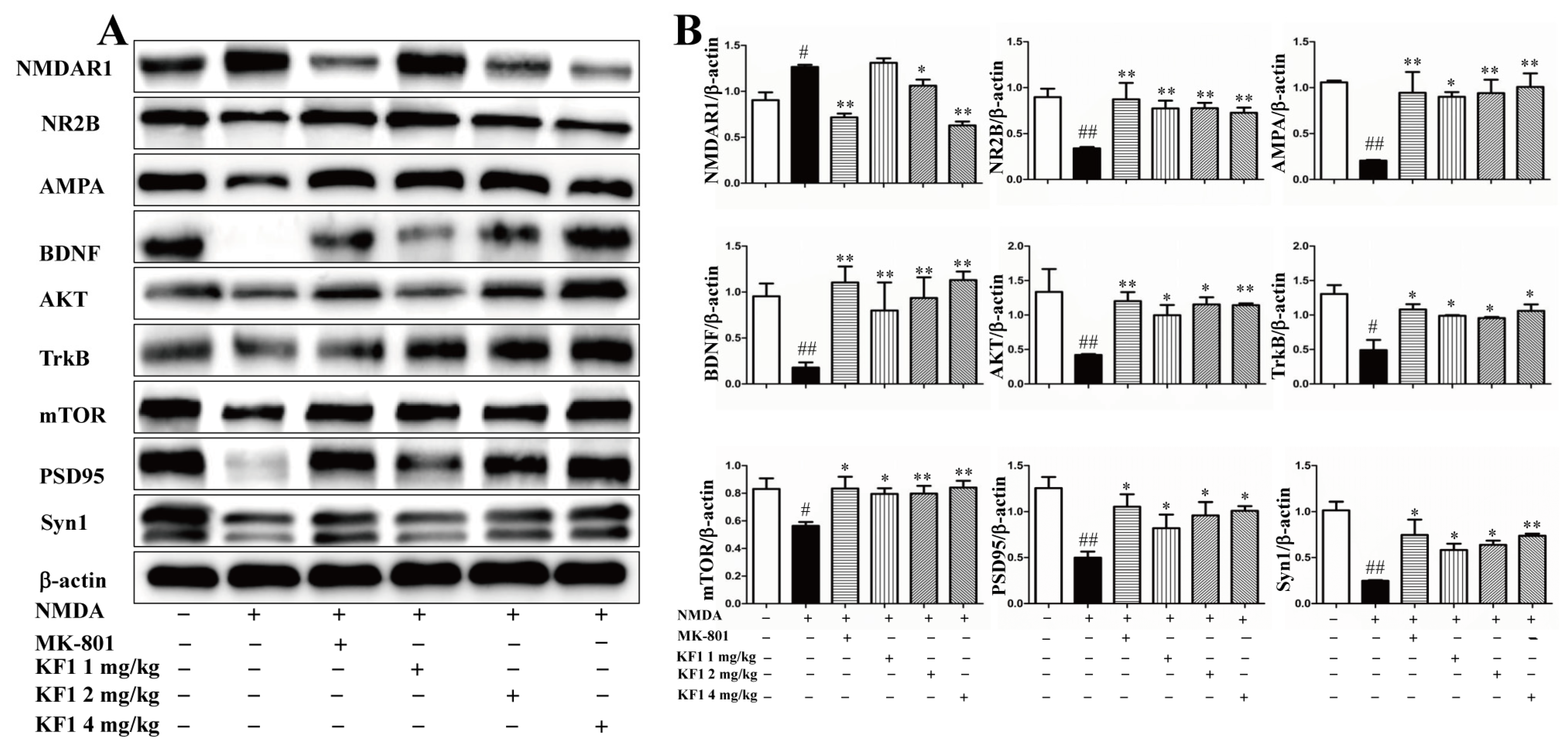
2.2.8. Western Blot Assay to Verify the Expression of NMDAR1, AMPA, BDNF, TrkB, AKT, mTOR, PSD95, and Syn1 In Vivo and In Vitro
3. Discussion
4. Materials and Methods
4.1. In Vitro Analysis of the Mechanisms of Action of KF1
4.1.1. Cell Management and Viability
4.1.2. LDH Release Assay
4.1.3. Measurement of Intracellular Ca2+ Concentration
4.1.4. Hoechst 33342 Staining
4.1.5. Annexin V-FITC/PI Flow Cytometry Assay
4.1.6. Fluorescence Staining
4.2. In Vivo Analysis of the Mechanisms of Action of KF1
4.2.1. Animals
4.2.2. Experimental Designs
4.2.3. Y-Maze Test
4.2.4. Morris Water Maze Test
4.2.5. Quantification of 5-HT, DA, MAO, and Ca2+ Concentration in the Hippocampus by ELISA
4.2.6. HE Staining of the Hippocampus
4.2.7. Nissl Staining of the Hippocampus
4.2.8. Immunohistochemistry Assay for NMDAR1 in the Hippocampus and Cortex
4.2.9. Western Blot
4.3. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Y.; Li, B.; Ismail, N.; Smith, K.; Li, T.; Dai, R.; Deng, Y. Neurotoxicity and Underlying Mechanisms of Endogenous Neurotoxins. Int. J. Mol. Sci. 2021, 22, 12805. [Google Scholar] [CrossRef] [PubMed]
- Fan, P.; Wang, Y.; Xu, M.; Han, X.; Liu, Y. The Application of Brain Organoids in Assessing Neural Toxicity. Front. Mol. Neurosci. 2022, 15, 799397. [Google Scholar] [CrossRef] [PubMed]
- Yadav, A.; Tandon, A.; Seth, B.; Goyal, S.; Singh, S.J.; Tiwari, S.K.; Agarwal, S.; Nair, S.; Chaturvedi, R.K. Cypermethrin Impairs Hippocampal Neurogenesis and Cognitive Functions by Altering Neural Fate Decisions in the Rat Brain. Mol. Neurobiol. 2021, 58, 263–280. [Google Scholar] [CrossRef] [PubMed]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate receptor ion channels: Structure, regulation, and function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [PubMed]
- Simões, A.P.; Silva, C.G.; Marques, J.M.; Pochmann, D.; Porciúncula, L.O.; Ferreira, S.; Oses, J.P.; Beleza, R.O.; Real, J.I.; Köfalvi, A.; et al. Glutamate-induced and NMDA receptor-mediated neurodegeneration entails P2Y1 receptor activation. Cell Death Dis. 2018, 9, 297. [Google Scholar] [CrossRef]
- Liu, Y.; Wong, T.P.; Aarts, M.; Rooyakkers, A.; Liu, L.; Lai, T.W.; Wu, D.C.; Lu, J.; Tymianski, M.; Craig, A.M.; et al. NMDA receptor subunits have differential roles in mediating excitotoxic neuronal death both in vitro and in vivo. J. Neurosci. Off. J. Soc. Neurosci. 2007, 27, 2846–2857. [Google Scholar] [CrossRef]
- Liu, S.B.; Zhang, N.; Guo, Y.Y.; Zhao, R.; Shi, T.Y.; Feng, S.F.; Wang, S.Q.; Yang, Q.; Li, X.Q.; Wu, Y.M.; et al. G-protein-coupled receptor 30 mediates rapid neuroprotective effects of estrogen via depression of NR2B-containing NMDA receptors. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 4887–4900. [Google Scholar] [CrossRef]
- Paoletti, P.; Bellone, C.; Zhou, Q. NMDA receptor subunit diversity: Impact on receptor properties, synaptic plasticity and disease. Nat. Rev. Neurosci. 2013, 14, 383–400. [Google Scholar] [CrossRef]
- Yang, X.; Sun, X.; Wu, J.; Ma, J.; Si, P.; Yin, L.; Zhang, Y.; Yan, L.J.; Zhang, C. Regulation of the SIRT1 signaling pathway in NMDA-induced Excitotoxicity. Toxicol. Lett. 2020, 322, 66–76. [Google Scholar] [CrossRef]
- Zhang, S.; Xue, R.; Geng, Y.; Wang, H.; Li, W. Fisetin Prevents HT22 Cells From High Glucose-Induced Neurotoxicity via PI3K/Akt/CREB Signaling Pathway. Front. Neurosci. 2020, 14, 241. [Google Scholar] [CrossRef]
- Fang, Y.C.; Hsieh, J.Y.; Vidyanti, A.N.; Yang, C.H.; Jan, J.S.; Chang, K.W.; Hu, C.J.; Tu, Y.K. HDACi protects against vascular cognitive impairment from CCH injury via induction of BDNF-related AMPA receptor activation. J. Cell. Mol. Med. 2021, 25, 7418–7425. [Google Scholar] [CrossRef] [PubMed]
- Jelen, L.A.; Young, A.H.; Stone, J.M. Ketamine: A tale of two enantiomers. J. Psychopharmacol. 2021, 35, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Ji, J.; Zhang, S.; Yuan, M.; Zhang, M.; Tang, L.; Wang, P.; Liu, Y.; Xu, C.; Luo, P.; Gao, X. Fermented Rosa Roxburghii Tratt Juice Alleviates High-Fat Diet-Induced Hyperlipidemia in Rats by Modulating Gut Microbiota and Metabolites. Front. Pharmacol. 2022, 13, 883629. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, T.P.; Li, L.Z.; Luo, M.Y.; Jia, W.Y.; Zeng, Y.; Jiang, Y.L.; Tao, G.C. Multigene Phylogeny, Diversity and Antimicrobial Potential of Endophytic Sordariomycetes From Rosa roxburghii. Front. Microbiol. 2021, 12, 755919. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Cai, X.; Zhang, W.; Li, Y.; Qiu, P.; Lu, D.; He, X. Flavonoids of Rosa roxburghii Tratt exhibit radioprotection and anti-apoptosis properties via the Bcl-2(Ca(2+))/Caspase-3/PARP-1 pathway. Apoptosis Int. J. Program. Cell Death 2016, 21, 1125–1143. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.S.; Chen, F.J.; Li, L.Q.; Wang, Y.; Yang, L.S.; Li, Q.J.; Li, L.L.; Pan, X.; Yang, Y. An Active Extract of Rosa Roxburghii Tratt with Antidepressant Properties and Application. CN112618614A, 2021. [Google Scholar]
- Xu, Y.; Zeng, Q.; Sun, B.; Wei, S.; Wang, Q.; Zhang, A. Assessing the Role of Nrf2/GPX4-Mediated Oxidative Stress in Arsenic-Induced Liver Damage and the Potential Application Value of Rosa roxburghii Tratt [Rosaceae]. Oxidative Med. Cell. Longev. 2022, 2022, 9865606. [Google Scholar] [CrossRef] [PubMed]
- Wang, I.F.; Wang, Y.; Yang, Y.H.; Huang, G.J.; Tsai, K.J.; Shen, C.J. Activation of a hippocampal CREB-pCREB-miRNA-MEF2 axis modulates individual variation of spatial learning and memory capability. Cell Rep. 2021, 36, 109477. [Google Scholar] [CrossRef]
- Tong, X.; Li, X.; Ayaz, M.; Ullah, F.; Sadiq, A.; Ovais, M.; Shahid, M.; Khayrullin, M.; Hazrat, A. Neuroprotective Studies on Polygonum hydropiper L. Essential Oils Using Transgenic Animal Models. Front. Pharmacol. 2020, 11, 580069. [Google Scholar] [CrossRef]
- Fachim, H.A.; Pereira, A.C.; Iyomasa-Pilon, M.M.; Rosa, M.L. Differential Expression of AMPA Subunits Induced by NMDA Intrahippocampal Injection in Rats. Front. Neurosci. 2016, 10, 32. [Google Scholar] [CrossRef]
- Yang, X.; Si, P.; Qin, H.; Yin, L.; Yan, L.J.; Zhang, C. The Neuroprotective Effects of SIRT1 on NMDA-Induced Excitotoxicity. Oxidative Med. Cell. Longev. 2017, 2017, 2823454. [Google Scholar] [CrossRef]
- Matsumoto, M.; Takada, M. Distinct representations of cognitive and motivational signals in midbrain dopamine neurons. Neuron 2013, 79, 1011–1024. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.L.; Bao, Y.N.; Fan, J.F.; Ma, B.; Li, S.S.; Zhao, W.L.; Yu, B.Y.; Liu, J.H. Blockade of spinal dopamine D1/D2 receptor suppresses activation of NMDA receptor through Gαq and Src kinase to attenuate chronic bone cancer pain. J. Adv. Res. 2021, 28, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Robinson, B.L.; Dumas, M.; Cuevas, E.; Gu, Q.; Paule, M.G.; Ali, S.F.; Kanungo, J. Distinct effects of ketamine and acetyl L-carnitine on the dopamine system in zebrafish. Neurotoxicology Teratol. 2016, 54, 52–60. [Google Scholar] [CrossRef]
- Simić, G.; Kostović, I.; Winblad, B.; Bogdanović, N. Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J. Comp. Neurol. 1997, 379, 482–494. [Google Scholar] [CrossRef]
- Xu, Y.H.; Wang, X.X.; Wang, M.J.; Liu, Y.Y.; Xue, Z.; Chen, J.X. Influence of progestational stress on BDNF and NMDARs in the hippocampus of male offspring and amelioration by Chaihu Shugan San. Biomed. Pharmacother. Biomed. Pharmacother. 2021, 135, 111204. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Bai, Y.; Cheng, Q.; Yu, S.; Cheng, M.; Wu, Y.; Zhang, X.; Liang, X.; Gu, X. Bidentatide, a Novel Plant Peptide Derived from Achyranthes bidentata Blume: Isolation, Characterization, and Neuroprotection through Inhibition of NR2B-Containing NMDA Receptors. Int. J. Mol. Sci. 2021, 22, 7977. [Google Scholar] [CrossRef]
- García, F.; Lobos, P.; Ponce, A.; Cataldo, K.; Meza, D.; Farías, P.; Estay, C.; Oyarzun-Ampuero, F.; Herrera-Molina, R.; Paula-Lima, A.; et al. Astaxanthin Counteracts Excitotoxicity and Reduces the Ensuing Increases in Calcium Levels and Mitochondrial Reactive Oxygen Species Generation. Mar. Drugs 2020, 18, 335. [Google Scholar] [CrossRef]
- Ehinger, R.; Kuret, A.; Matt, L.; Frank, N.; Wild, K.; Kabagema-Bilan, C.; Bischof, H.; Malli, R.; Ruth, P.; Bausch, A.E.; et al. Slack K(+) channels attenuate NMDA-induced excitotoxic brain damage and neuronal cell death. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2021, 35, e21568. [Google Scholar] [CrossRef]
- Moya-Alvarado, G.; Guerra, M.V.; Tiburcio, R.; Bravo, E.; Bronfman, F.C. The Rab11-regulated endocytic pathway and BDNF/TrkB signaling: Roles in plasticity changes and neurodegenerative diseases. Neurobiol. Dis. 2022, 171, 105796. [Google Scholar] [CrossRef]
- Koponen, E.; Võikar, V.; Riekki, R.; Saarelainen, T.; Rauramaa, T.; Rauvala, H.; Taira, T.; Castrén, E. Transgenic mice overexpressing the full-length neurotrophin receptor trkB exhibit increased activation of the trkB-PLCgamma pathway, reduced anxiety, and facilitated learning. Mol. Cell. Neurosci. 2004, 26, 166–181. [Google Scholar] [CrossRef] [PubMed]
- Qiu, L.L.; Pan, W.; Luo, D.; Zhang, G.F.; Zhou, Z.Q.; Sun, X.Y.; Yang, J.J.; Ji, M.H. Dysregulation of BDNF/TrkB signaling mediated by NMDAR/Ca(2+)/calpain might contribute to postoperative cognitive dysfunction in aging mice. J. Neuroinflamm. 2020, 17, 23. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhu, Q.Q.; Niu, M.L.; Li, N.; Ren, B.Q.; Yu, T.B.; Zhou, Z.S.; Guo, J.D.; Zhou, Y. Ghrelin infusion into the basolateral amygdala suppresses CTA memory formation in rats via the PI3K/Akt/mTOR and PLC/PKC signaling pathways. Acta Pharmacol. Sin. 2022, 43, 2242–2252. [Google Scholar] [CrossRef]
- Smith, K.R.; Jones, K.A.; Kopeikina, K.J.; Burette, A.C.; Copits, B.A.; Yoon, S.; Forrest, M.P.; Fawcett-Patel, J.M.; Hanley, J.G.; Weinberg, R.J.; et al. Cadherin-10 Maintains Excitatory/Inhibitory Ratio through Interactions with Synaptic Proteins. J. Neurosci. Off. J. Soc. Neurosci. 2017, 37, 11127–11139. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Jin, Y.; Li, Q.; Sun, X.; Zhu, H.; Cui, H. MiR-93-5p targeting PTEN regulates the NMDA-induced autophagy of retinal ganglion cells via AKT/mTOR pathway in glaucoma. Biomed. Pharmacother. = Biomed. Pharmacother. 2018, 100, 1–7. [Google Scholar] [CrossRef]
- Heras-Sandoval, D.; Pérez-Rojas, J.M.; Hernández-Damián, J.; Pedraza-Chaverri, J. The role of PI3K/AKT/mTOR pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 2014, 26, 2694–2701. [Google Scholar] [CrossRef]
- Ni, L.; Xu, Y.; Dong, S.; Kong, Y.; Wang, H.; Lu, G.; Wang, Y.; Li, Q.; Li, C.; Du, Z.; et al. The potential role of the HCN1 ion channel and BDNF-mTOR signaling pathways and synaptic transmission in the alleviation of PTSD. Transl. Psychiatry 2020, 10, 101. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Zhong, X.M.; Li, Z.Y.; Huang, Z. Paeoniflorin protects against NMDA-induced neurotoxicity in PC12 cells via Ca2+ antagonism. Phytother. Res. PTR 2011, 25, 681–685. [Google Scholar] [CrossRef]
- Huang, J.; Li, L.-S.; Yang, D.-L.; Gong, Q.-H.; Deng, J.; Huang, X.-N.J.E.-B.C.; Medicine, A. Inhibitory effect of ginsenoside Rg1 on vascular smooth muscle cell proliferation induced by PDGF-BB is involved in nitric oxide formation. Evid.-Based Complement. Altern. Med. 2012, 2012, 314395. [Google Scholar] [CrossRef]
- Wang, Y.W.; Dong, H.Z.; Tan, Y.X.; Bao, X.; Su, Y.M.; Li, X.; Jiang, F.; Liang, J.; Huang, Z.C.; Ren, Y.L.; et al. HIF-1α-regulated lncRNA-TUG1 promotes mitochondrial dysfunction and pyroptosis by directly binding to FUS in myocardial infarction. Cell Death Discov. 2022, 8, 178. [Google Scholar] [CrossRef]
- Li, X.; Qin, X.; Tian, J.; Gao, X.; Wu, X.; Du, G.; Zhou, Y. Liquiritin protects PC12 cells from corticosterone-induced neurotoxicity via regulation of metabolic disorders, attenuation ERK1/2-NF-κB pathway, activation Nrf2-Keap1 pathway, and inhibition mitochondrial apoptosis pathway. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2020, 146, 111801. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Zhou, Y.; Liang, D.; He, H.; Liu, X.; Zhu, R.; Zhang, M.; Luo, X.; Wang, Y.; Huang, G. Exosomes Secreted From Bone Marrow Mesenchymal Stem Cells Attenuate Oxygen-Glucose Deprivation/Reoxygenation-Induced Pyroptosis in PC12 Cells by Promoting AMPK-Dependent Autophagic Flux. Front. Cell. Neurosci. 2020, 14, 182. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res. Ther. 2020, 11, 97. [Google Scholar] [CrossRef]
- Zeng, M.; Feng, A.; Li, M.; Liu, M.; Guo, P.; Zhang, Y.; Zhang, Q.; Zhang, B.; Cao, B.; Jia, J.; et al. Corallodiscus flabellata B. L. Burtt extract and isonuomioside A ameliorate Aβ(25-35)-induced brain injury by inhibiting apoptosis, oxidative stress, and autophagy via the NMDAR2B/CamK Ⅱ/PKG pathway. Phytomed. Int. J. Phytother. Phytopharm. 2022, 101, 154114. [Google Scholar] [CrossRef]
- Boeck, C.R.; Ganzella, M.; Lottermann, A.; Vendite, D. NMDA preconditioning protects against seizures and hippocampal neurotoxicity induced by quinolinic acid in mice. Epilepsia 2004, 45, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.K.; Kim, H.G.; Kim, K.L. A novel trimeric peptide, Neuropep-1-stimulating brain-derived neurotrophic factor expression in rat brain improves spatial learning and memory as measured by the Y-maze and Morris water maze. J. Neurochem. 2011, 116, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.J.; Liu, B.; Wu, Q.; Liu, J.; Xu, Y.Y.; Zhou, S.Y.; Shi, J.S. Icariin Delays Brain Aging in Senescence-Accelerated Mouse Prone 8 (SAMP8) Model via Inhibiting Autophagy. J. Pharmacol. Exp. Ther. 2019, 369, 121–128. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, F.; Zhou, J.; Chang, X.; Li, L.; Hu, H.; Wang, Z.; Xiao, W. Anti-migraine and anti-depression activities of Tianshu capsule by mediating Monoamine oxidase. Biomed. Pharmacother. Biomed. Pharmacother. 2018, 100, 275–281. [Google Scholar] [CrossRef]
- Chen, J.X.; Li, W.; Zhao, X.; Yang, J.X. Effects of the Chinese traditional prescription Xiaoyaosan decoction on chronic immobilization stress-induced changes in behavior and brain BDNF, TrkB, and NT-3 in rats. Cell. Mol. Neurobiol. 2008, 28, 745–755. [Google Scholar] [CrossRef]
- Song, A.Q.; Gao, B.; Fan, J.J.; Zhu, Y.J.; Zhou, J.; Wang, Y.L.; Xu, L.Z.; Wu, W.N. NLRP1 inflammasome contributes to chronic stress-induced depressive-like behaviors in mice. J. Neuroinflamm. 2020, 17, 178. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, F.; Wang, L.; Jin, F.; Li, L.; Wang, T.; Gao, M.; Li, L.; Wang, Y.; Lou, Z.; Yang, J.; et al. Neuroprotection of Kaji-Ichigoside F1 via the BDNF/Akt/mTOR Signaling Pathways against NMDA-Induced Neurotoxicity. Int. J. Mol. Sci. 2022, 23, 16150. https://doi.org/10.3390/ijms232416150
Chen F, Wang L, Jin F, Li L, Wang T, Gao M, Li L, Wang Y, Lou Z, Yang J, et al. Neuroprotection of Kaji-Ichigoside F1 via the BDNF/Akt/mTOR Signaling Pathways against NMDA-Induced Neurotoxicity. International Journal of Molecular Sciences. 2022; 23(24):16150. https://doi.org/10.3390/ijms232416150
Chicago/Turabian StyleChen, Faju, Li Wang, Fengli Jin, Liangqun Li, Tao Wang, Ming Gao, Lilang Li, Yu Wang, Zhongsheng Lou, Juan Yang, and et al. 2022. "Neuroprotection of Kaji-Ichigoside F1 via the BDNF/Akt/mTOR Signaling Pathways against NMDA-Induced Neurotoxicity" International Journal of Molecular Sciences 23, no. 24: 16150. https://doi.org/10.3390/ijms232416150
APA StyleChen, F., Wang, L., Jin, F., Li, L., Wang, T., Gao, M., Li, L., Wang, Y., Lou, Z., Yang, J., Li, Q., & Yang, X. (2022). Neuroprotection of Kaji-Ichigoside F1 via the BDNF/Akt/mTOR Signaling Pathways against NMDA-Induced Neurotoxicity. International Journal of Molecular Sciences, 23(24), 16150. https://doi.org/10.3390/ijms232416150





