Phellinus baumii Polyphenol: A Potential Therapeutic Candidate against Lung Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Characterization of the Chemical Constituents of PBP
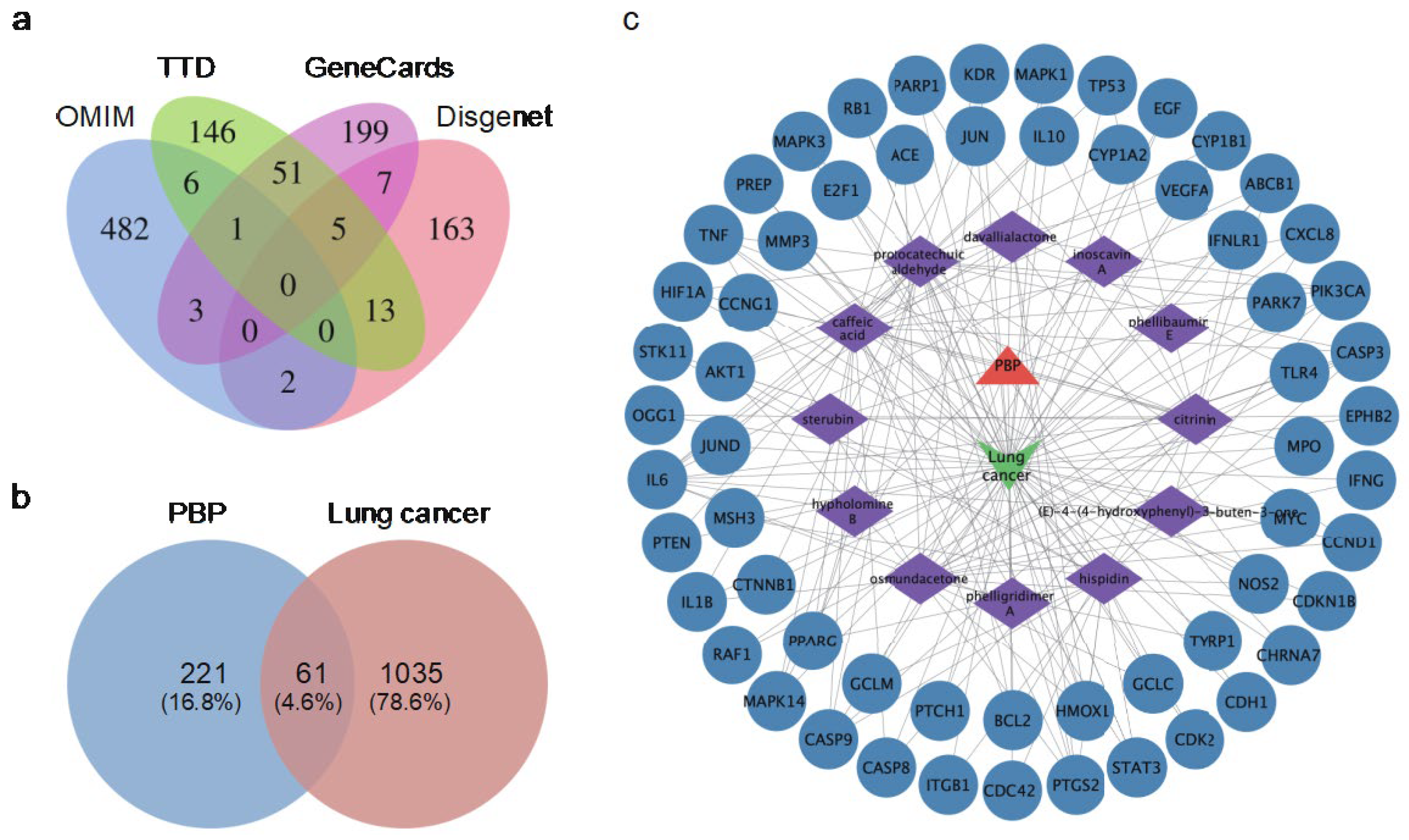
2.2. Identification of Potential Targets of PBP in Lung Cancer Treatment
2.3. Components-Disease-Targets Interaction Network
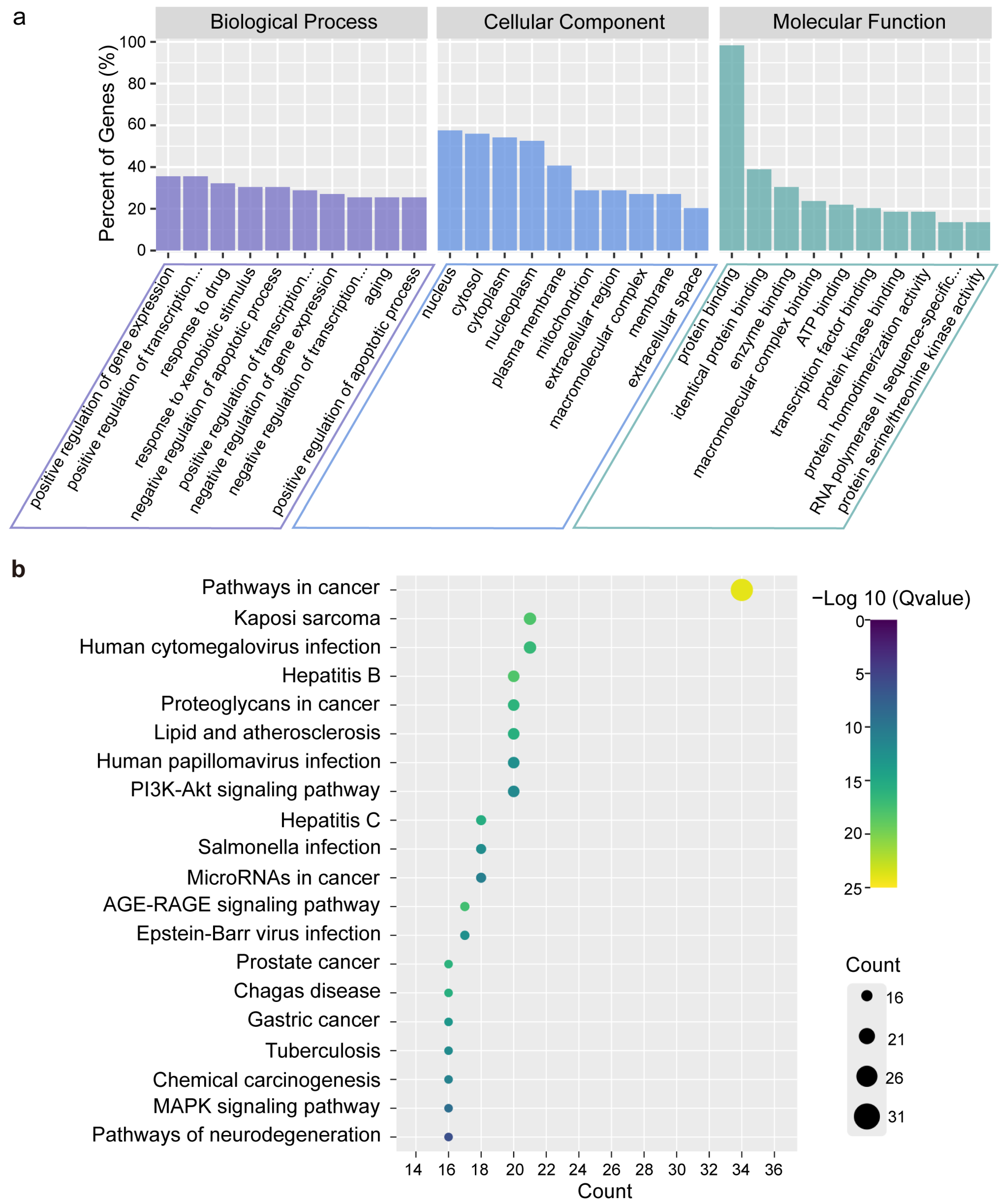
2.4. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis
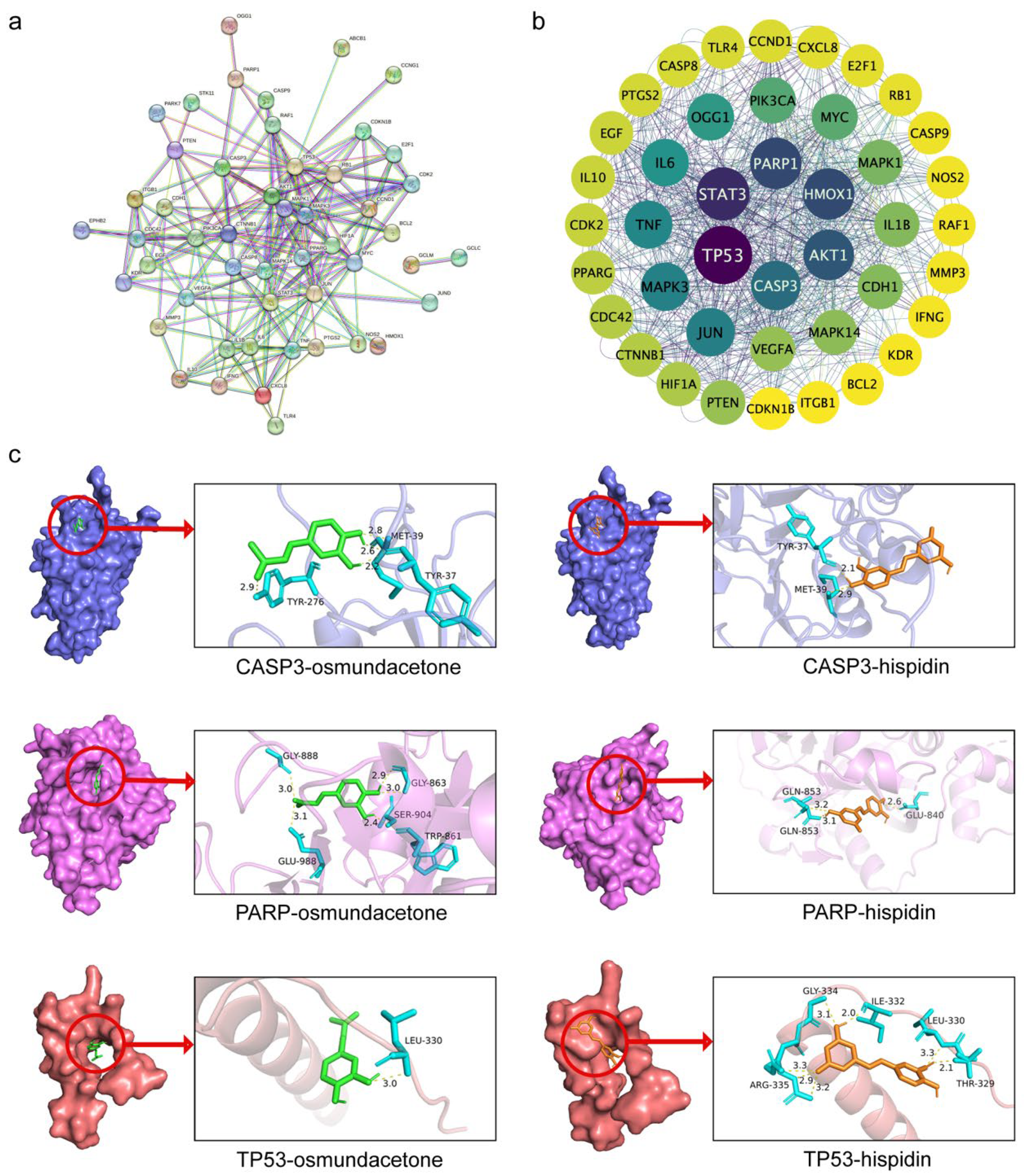
2.5. Protein-Protein Interaction (PPI) Network and Core Targets
2.6. Molecular Docking
2.7. PBP Significantly Inhibited the Viability of A549 Cells
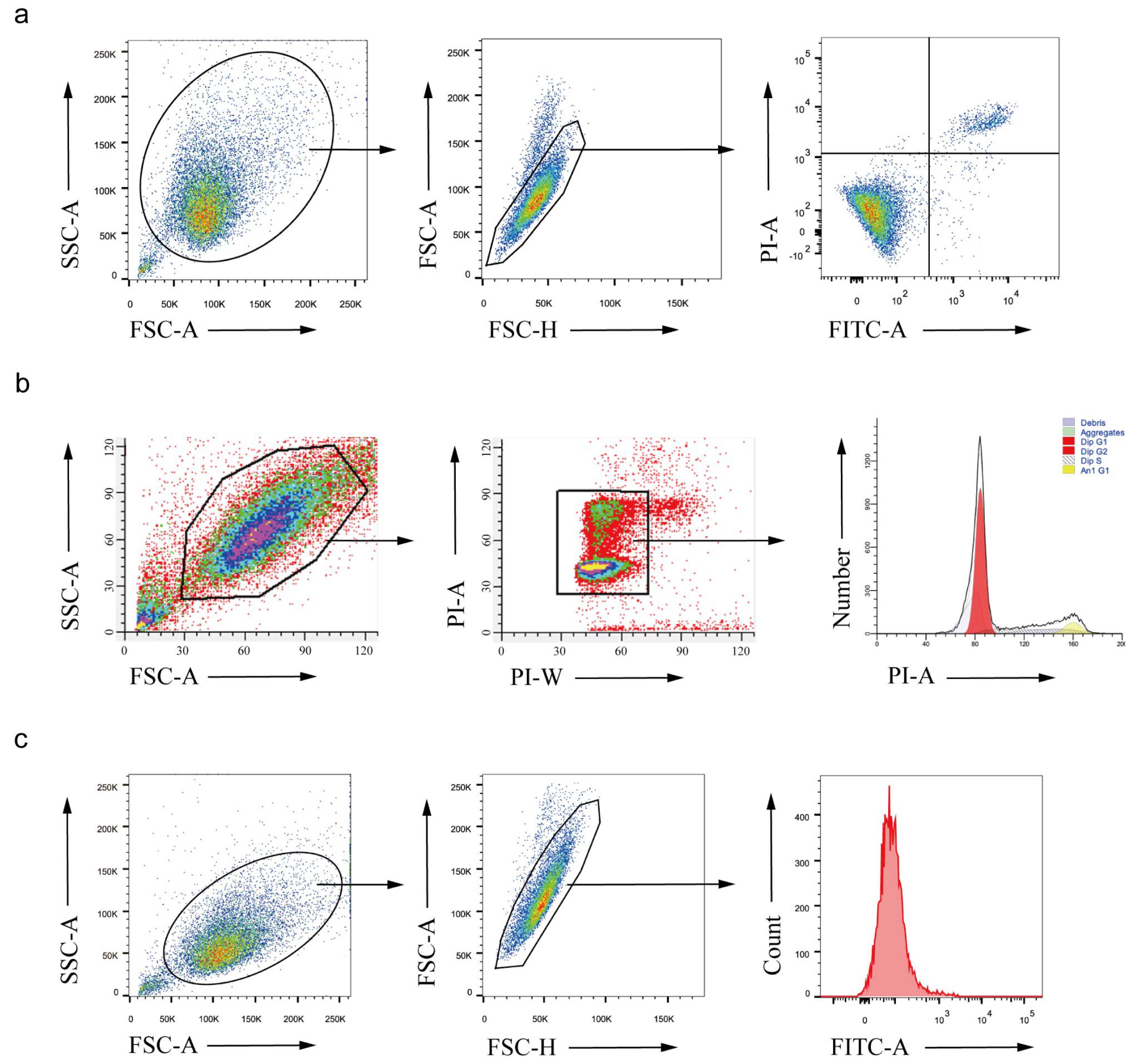
2.8. PBP Induced Apoptosis in A549 Cells
2.9. PBP Induced Mitochondrial Membrane Potential (MMP) Depolarization in A549 Cells
2.10. PBP Arrested the Cell Cycle of A549 Cells in S Phase
2.11. PBP Promoted Reactive Oxygen Species (ROS) Generation in A549 Cells
2.12. PBP Induced Mitochondrial Membrane Potential (MMP) Depolarization in A549 Cells
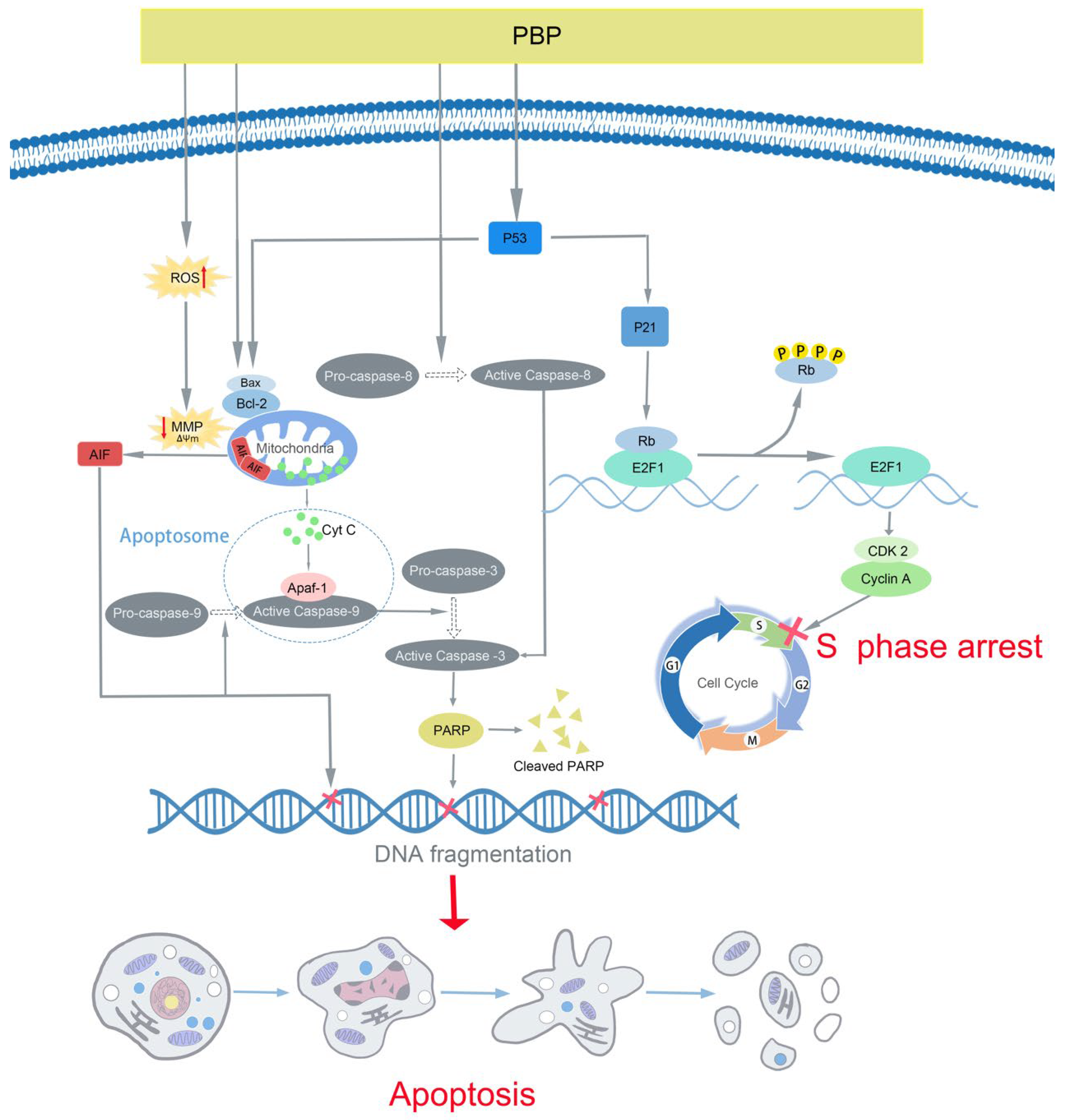
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of PBP
4.3. Identification of PBP Compounds by UPLC–ESI–QTOF–MS
4.4. Prediction of Potential Targets of PBP during Lung Cancer Treatment
4.5. Component-Disease-Target Interaction Network
4.6. GO and KEGG Enrichment Analysis
4.7. PPI Network and Core Targets
4.8. Molecular Docking
4.9. Cell Lines and Culture
4.10. Cell Viability Assay and Morphological Observations
4.11. Cell Apoptosis Assay
4.12. MMP Assay
4.13. Western Blot Assay
4.14. Cell Cycle Assay
4.15. ROS Generation Assay
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIF | Apoptosis Inducing Factor |
| Apaf-1 | Apoptotic Protease Activating Factor-1 |
| Bcl-2 | B-cell lymphoma-2 |
| BC | betweenness centrality |
| CC | closeness centrality |
| CCK-8 | Cell Counting Kit-8 |
| CDK2 | Cyclin Dependent Kinase 2 |
| CVD | cardiovascular diseases |
| DC | degree centrality |
| DMEM | dulbecco’s modified essential medium |
| DMSO | Di-methyl sulfoxide |
| E2F1 | E2F transcription factor 1 |
| FBS | fetal bovine serum |
| GO | Gene ontology |
| KEGG | kyoto encyclopedia of genes and genomes |
| MMP | mitochondrial membrane potential |
| P-Rb | Phosphorylated Retinoblastoma protein |
| PARP | Poly ADP-Ribose Polymerase |
| PBP | Phellinus baumii polyphenol |
| PPI | Protein-protein interaction |
| PVDF | polyvinylidene difluoride |
| ROS | reactive oxygen species |
| SDS—PAGE | sulfate—polyacrylamide gel electrophoresis |
| TCMSP | traditional chinese medicine systems pharmacology database and analysis platform |
| TTD | treatment targets database |
| UPLC-ESI-QTOF-MS | Ultra Performance Liquid Chromatography-Electrospray Ionization-Quadrupole Time of Flight-Mass Spectrometry |
Appendix A

References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515. [Google Scholar] [CrossRef] [PubMed]
- Yi, J.; Qu, H.; Wu, Y.; Wang, Z.; Wang, L. Study on antitumor, antioxidant and immunoregulatory activities of the purified polyphenols from pinecone of Pinus koraiensis on tumor-bearing S180 mice in vivo. Int. J. Biol. Macromol. 2017, 94, 735–744. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Wang, X.; Lu, G.; Picinich, S.C. Cancer prevention by tea: Animal studies, molecular mechanisms and human relevance. Nat. Rev. Cancer 2009, 9, 429–439. [Google Scholar] [CrossRef]
- Sinha, D.; Sarkar, N.; Biswas, J.; Bishayee, A. Resveratrol for breast cancer prevention and therapy: Preclinical evidence and molecular mechanisms. Semin. Cancer Biol. 2016, 40–41, 209–232. [Google Scholar] [CrossRef]
- Zhang, Q.; Huang, H.; Zheng, F.; Liu, H.; Qiu, F.; Chen, Y.; Liang, C.L.; Dai, Z. Resveratrol exerts antitumor effects by downregulating CD8(+)CD122(+) Tregs in murine hepatocellular carcinoma. Oncoimmunology 2020, 9, 1829346. [Google Scholar] [CrossRef]
- He, P.; Zhang, Y.; Li, N. The phytochemistry and pharmacology of medicinal fungi of the genus Phellinus: A review. Food Funct. 2021, 12, 1856–1881. [Google Scholar] [CrossRef]
- Liu, M.M.; Zeng, P.; Li, X.T.; Shi, L.G. Antitumor and immunomodulation activities of polysaccharide from Phellinus baumii. Int. J. Biol. Macromol. 2016, 91, 1199–1205. [Google Scholar] [CrossRef]
- Li, G.; Kim, D.H.; Kim, T.D.; Park, B.J.; Park, H.D.; Park, J.I.; Na, M.K.; Kim, H.C.; Hong, N.D.; Lim, K.; et al. Protein-bound polysaccharide from Phellinus linteus induces G2/M phase arrest and apoptosis in SW480 human colon cancer cells. Cancer Lett. 2004, 216, 175–181. [Google Scholar] [CrossRef]
- Sun, Y.; Huo, J.; Zhong, S.; Zhu, J.; Li, Y.; Li, X. Chemical structure and anti-inflammatory activity of a branched polysaccharide isolated from Phellinus baumii. Carbohydr. Polym. 2021, 268, 118214. [Google Scholar] [CrossRef]
- Feng, H.; Zhang, S.; Wan, J.M.; Gui, L.; Ruan, M.; Li, N.; Zhang, H.; Liu, Z.; Wang, H. Polysaccharides extracted from Phellinus linteus ameliorate high-fat high-fructose diet induced insulin resistance in mice. Carbohydr. Polym. 2018, 200, 144–153. [Google Scholar] [CrossRef]
- Wang, G.J.; Tsai, T.H.; Chang, T.T.; Chou, C.J.; Lin, L.C. Lanostanes from Phellinus igniarius and their iNOS inhibitory activities. Planta Med. 2009, 75, 1602–1607. [Google Scholar] [CrossRef]
- Jang, H.J.; Yang, K.S. Inhibition of nitric oxide production in RAW 264.7 macrophages by diterpenoids from Phellinus pini. Arch. Pharm. Res. 2011, 34, 913–917. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, R.; Zhang, J.; Bu, Q.; Wang, W.; Liu, Y.; Li, Q.; Guo, Y.; Zhang, L.; Yang, Y. The integration of metabolome and proteome reveals bioactive polyphenols and hispidin in ARTP mutagenized Phellinus baumii. Sci. Rep. 2019, 9, 16172. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, S.; Geng, Y.; Tian, B.; Cai, M.; Guan, R.; Li, Y.; Ye, B.; Sun, P. Anti-Inflammatory Properties In Vitro and Hypoglycaemic Effects of Phenolics from Cultivated Fruit Body of Phellinus baumii in Type 2 Diabetic Mice. Molecules 2021, 26, 2285. [Google Scholar] [CrossRef]
- Zheng, S.; Deng, S.; Huang, Y.; Huang, M.; Zhao, P.; Ma, X.; Wen, Y.; Wang, Q.; Yang, X. Anti-diabetic activity of a polyphenol-rich extract from Phellinus igniarius in KK-Ay mice with spontaneous type 2 diabetes mellitus. Food Funct. 2018, 9, 614–623. [Google Scholar] [CrossRef]
- Nogales, C.; Mamdouh, Z.M.; List, M.; Kiel, C.; Casas, A.I.; Schmidt, H. Network pharmacology: Curing causal mechanisms instead of treating symptoms. Trends Pharmacol. Sci. 2022, 43, 136–150. [Google Scholar] [CrossRef]
- Wu, C.S.; Lin, Z.M.; Wang, L.N.; Guo, D.X.; Wang, S.Q.; Liu, Y.Q.; Yuan, H.Q.; Lou, H.X. Phenolic compounds with NF-kappaB inhibitory effects from the fungus Phellinus baumii. Bioorg. Med. Chem. Lett. 2011, 21, 3261–3267. [Google Scholar] [CrossRef]
- Dong, Y.; Qiu, P.; Zhu, R.; Zhao, L.; Zhang, P.; Wang, Y.; Li, C.; Chai, K.; Shou, D.; Zhao, H. A Combined Phytochemistry and Network Pharmacology Approach to Reveal the Potential Antitumor Effective Substances and Mechanism of Phellinus igniarius. Front. Pharmacol. 2019, 10, 266. [Google Scholar] [CrossRef]
- Jung, J.Y.; Lee, I.K.; Seok, S.J.; Lee, H.J.; Kim, Y.H.; Yun, B.S. Antioxidant polyphenols from the mycelial culture of the medicinal fungi Inonotus xeranticus and Phellinus linteus. J. Appl. Microbiol. 2008, 104, 1824–1832. [Google Scholar] [CrossRef]
- Suabjakyong, P.; Saiki, R.; Van Griensven, L.J.; Higashi, K.; Nishimura, K.; Igarashi, K.; Toida, T. Polyphenol extract from Phellinus igniarius protects against acrolein toxicity in vitro and provides protection in a mouse stroke model. PLoS ONE 2015, 10, e0122733. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.J.; Mo, S.Y.; Li, S.; Yang, Y.C.; Shi, J.G. Phelligridimer A, a highly oxygenated and unsaturated 26-membered macrocyclic metabolite with antioxidant activity from the fungus Phellinus igniarius. Org. Lett. 2005, 7, 4733–4736. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kang, Y.H.; Jung, J.Y.; Kang, I.J.; Han, S.N.; Chung, J.S.; Shin, H.K.; Lim, S.S. Inhibitory constituents of aldose reductase in the fruiting body of Phellinus linteus. Biol. Pharm. Bull. 2008, 31, 765–768. [Google Scholar] [CrossRef]
- Ding, Y.Y.; Liu, F.; Shi, C.; Zhang, Y.; Li, N. Chemical constituents from Phellinus igniarius and their anti-tumor activity in vitro. Zhongguo Zhong Yao Za Zhi 2016, 41, 3042–3048. [Google Scholar] [CrossRef]
- Wang, Y.; Shang, X.Y.; Wang, S.J.; Mo, S.Y.; Li, S.; Yang, Y.C.; Ye, F.; Shi, J.G.; He, L. Structures, biogenesis, and biological activities of pyrano [4,3-c]isochromen-4-one derivatives from the Fungus Phellinus igniarius. J. Nat. Prod. 2007, 70, 296–299. [Google Scholar] [CrossRef]
- Lee, I.K.; Yun, B.S. Styrylpyrone-class compounds from medicinal fungi Phellinus and Inonotus spp., and their medicinal importance. J. Antibiot. 2011, 64, 349–359. [Google Scholar] [CrossRef]
- Saraiva, L.; Fresco, P.; Pinto, E.; Sousa, E.; Pinto, M.; Goncalves, J. Inhibition of alpha, betaI, delta, eta, and zeta protein kinase C isoforms by xanthonolignoids. J. Enzyme Inhib. Med. Chem. 2003, 18, 357–370. [Google Scholar] [CrossRef]
- Fischer, W.; Currais, A.; Liang, Z.; Pinto, A.; Maher, P. Old age-associated phenotypic screening for Alzheimer’s disease drug candidates identifies sterubin as a potent neuroprotective compound from Yerba santa. Redox Biol. 2019, 21, 101089. [Google Scholar] [CrossRef]
- He, W.; Wang, J.; Jin, Q.; Zhang, J.; Liu, Y.; Jin, Z.; Wang, H.; Hu, L.; Zhu, L.; Shen, M.; et al. Design, green synthesis, antioxidant activity screening, and evaluation of protective effect on cerebral ischemia reperfusion injury of novel monoenone monocarbonyl curcumin analogs. Bioorg. Chem. 2021, 114, 105080. [Google Scholar] [CrossRef]
- de Carvalho, J.T.G.; Da Silva Baldivia, D.; de Castro, D.T.H.; Dos Santos, H.F.; Dos Santos, C.M.; Oliveira, A.S.; Alfredo, T.M.; Vilharva, K.N.; de Picoli Souza, K.; Dos Santos, E.L. The immunoregulatory function of polyphenols: Implications in cancer immunity. J. Nutr. Biochem. 2020, 85, 108428. [Google Scholar] [CrossRef]
- Elenkov, I.J.; Hrvacic, B.; Markovic, S.; Mesic, M.; Klonkay, A.C.; Lerman, L.; Sucic, A.F.; Vujasinovic, I.; Bosnjak, B.; Brajsa, K.; et al. Synthesis and Anti-inflammatory Activity of Novel Furochromenes. Croat. Chem. Acta 2013, 86, 253–264. [Google Scholar] [CrossRef]
- Tsujimoto, Y. Role of Bcl-2 family proteins in apoptosis: Apoptosomes or mitochondria? Genes Cells 1998, 3, 697–707. [Google Scholar] [CrossRef]
- Daugas, E.; Susin, S.A.; Zamzami, N.; Ferri, K.F.; Irinopoulou, T.; Larochette, N.; Prévost, M.C.; Leber, B.; Andrews, D.; Penninger, J.; et al. Mitochondrio-nuclear translocation of AIF in apoptosis and necrosis. FASEB J. 2000, 14, 729–739. [Google Scholar] [CrossRef]
- Circu, M.L.; Aw, T.Y. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Tie, F.; Li, G.; Hu, N.; Li, J.; Wang, Z.; Wang, H. Oligostilbenes extracts from Iris lactea Pall. var. chinensis (Fisch.) Koidz improve lipid metabolism in HFD/STZ-induced diabetic mice and inhibit adipogenesis in 3T3-L1 cells. Biomed. Pharmacother. 2020, 131, 110800. [Google Scholar] [CrossRef]
- Sarfraz, A.; Rasul, A.; Sarfraz, I.; Shah, M.A.; Hussain, G.; Shafiq, N.; Masood, M.; Adem, S.; Sarker, S.D.; Li, X. Hispolon: A natural polyphenol and emerging cancer killer by multiple cellular signaling pathways. Environ. Res. 2020, 190, 110017. [Google Scholar] [CrossRef]
- Lim, J.H.; Lee, Y.M.; Park, S.R.; Kim, D.H.; Lim, B.O. Anticancer activity of hispidin via reactive oxygen species-mediated apoptosis in colon cancer cells. Anticancer Res. 2014, 34, 4087–4093. [Google Scholar]
- Chandimali, N.; Huynh, D.L.; Jin, W.Y.; Kwon, T. Combination Effects of Hispidin and Gemcitabine via Inhibition of Stemness in Pancreatic Cancer Stem Cells. Anticancer Res. 2018, 38, 3967–3975. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Kondo, S. Apoptosis by antitumor agents and other factors in relation to cell cycle checkpoints. J. Radiat Res. 1995, 36, 56–62. [Google Scholar] [CrossRef]
- Ingham, M.; Schwartz, G.K. Cell-Cycle Therapeutics Come of Age. J. Clin. Oncol. 2017, 35, 2949–2959. [Google Scholar] [CrossRef]
- Han, Y.; Ishibashi, S.; Iglesias-Gonzalez, J.; Chen, Y.; Love, N.R.; Amaya, E. Ca(2+)-Induced Mitochondrial ROS Regulate the Early Embryonic Cell Cycle. Cell. Rep. 2018, 22, 218–231. [Google Scholar] [CrossRef]
- Wang, Z.; Yin, F.; Xu, J.; Zhang, T.; Wang, G.; Mao, M.; Wang, Z.; Sun, W.; Han, J.; Yang, M.; et al. CYT997(Lexibulin) induces apoptosis and autophagy through the activation of mutually reinforced ER stress and ROS in osteosarcoma. J. Exp. Clin. Cancer. Res. 2019, 38, 44. [Google Scholar] [CrossRef]
- Fang, J.; Gao, S.; Islam, R.; Teramoto, Y.; Maeda, H. Extracts of Phellinus linteus, Bamboo (Sasa senanensis) Leaf and Chaga Mushroom (Inonotus obliquus) Exhibit Antitumor Activity through Activating Innate Immunity. Nutrients 2020, 12, 2279. [Google Scholar] [CrossRef]
- Bae, J.S.; Jang, K.H.; Yim, H.; Jin, H.K. Polysaccharides isolated from Phellinus gilvus inhibit melanoma growth in mice. Cancer Lett. 2005, 218, 43–52. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Guo, A.C.; Lo, E.J.; Marcu, A.; Grant, J.R.; Sajed, T.; Johnson, D.; Li, C.; Sayeeda, Z.; et al. DrugBank 5.0: A major update to the DrugBank database for 2018. Nucleic Acids Res. 2018, 46, D1074–D1082. [Google Scholar] [CrossRef]
- Rebhan, M.; Chalifa-Caspi, V.; Prilusky, J.; Lancet, D. GeneCards: Integrating information about genes, proteins and diseases. Trends Genet. 1997, 13, 163. [Google Scholar] [CrossRef]
- Hamosh, A.; Amberger, J.S.; Bocchini, C.; Scott, A.F.; Rasmussen, S.A. Online Mendelian Inheritance in Man (OMIM®): Victor McKusick’s magnum opus. Am. J. Med. Genet. A 2021, 185, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Piñero, J.; Saüch, J.; Sanz, F.; Furlong, L.I. The DisGeNET cytoscape app: Exploring and visualizing disease genomics data. Comput. Struct. Biotechnol. J. 2021, 19, 2960–2967. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Li, F.; Zhou, Y.; Zhang, Y.; Wang, Z.; Zhang, R.; Zhu, J.; Ren, Y.; Tan, Y.; et al. Therapeutic target database 2020: Enriched resource for facilitating research and early development of targeted therapeutics. Nucleic Acids Res. 2020, 48, D1031–D1041. [Google Scholar] [CrossRef] [PubMed]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Gable, A.L.; Nastou, K.C.; Lyon, D.; Kirsch, R.; Pyysalo, S.; Doncheva, N.T.; Legeay, M.; Fang, T.; Bork, P.; et al. The STRING database in 2021: Customizable protein-protein networks, and functional characterization of user-uploaded gene/measurement sets. Nucleic Acids Res. 2021, 49, D605–D612. [Google Scholar] [CrossRef]
- Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Weissig, H.; Shindyalov, I.N.; Bourne, P.E. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
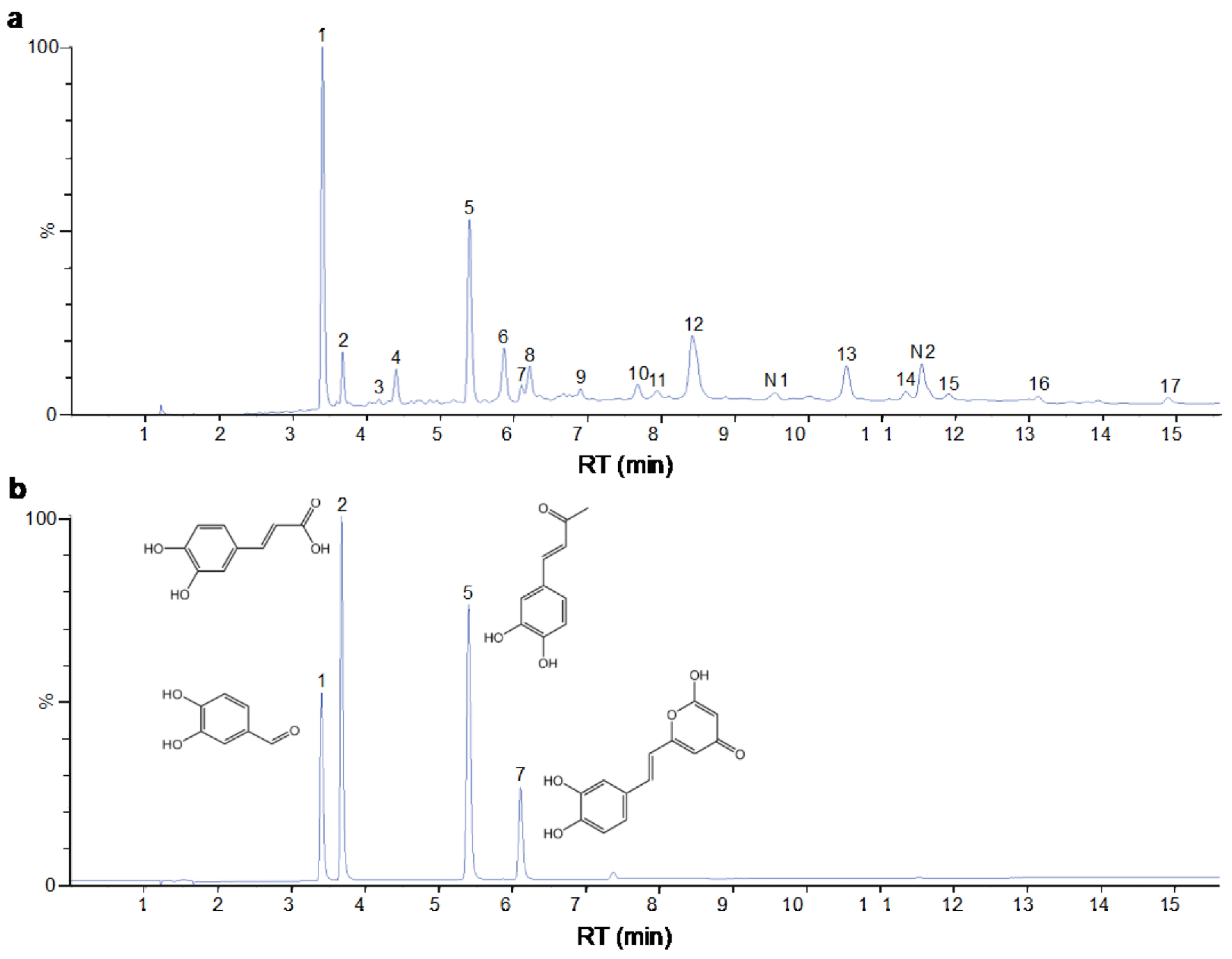



| Peak No. | Formula | Identification | 2D Structure | Peak No. | Formula | Identification | 2D Structure |
|---|---|---|---|---|---|---|---|
| 1 | C7H6O3 | protocatechuic aldehyde |  | 10 | C52H32O20 | phelligridimer A | 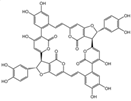 |
| 2 | C9H8O4 | caffeic acid | 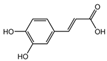 | 11 | C10H10O2 | (E)-4-(4-hydroxyphenyl)-3-buten-2-one | 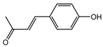 |
| 3 | C24H20O8 | kielcorin | 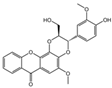 | 12 | C25H20O9 | davallialactone |  |
| 4 | C22H16O9 | phellibaumin B | 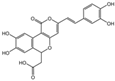 | 13 | C13H14O5 | citrinin | 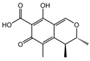 |
| 5 | C10H10O3 | osmundacetone | 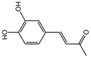 | 14 | C24H20O9 | phellibaumin E | 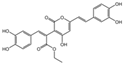 |
| 6 | C26H18O10 | hypholomine B | 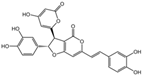 | 15 | C21H14O9 | 3-(4,6-Dihydroxy-2-oxochromen-3-yl)-8-hydroxy-2-methoxy-2,3-dihydrofuro [3,2-c] chromen-4-one | 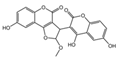 |
| 7 | C13H10O5 | hispidin |  | 16 | C33H20O13 | phelligridin I | 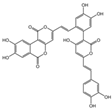 |
| 8 | C23H18O8 | interfungin B | 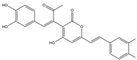 | 17 | C25H18O9 | inoscavin A | 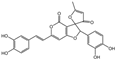 |
| Component | Degree | Binding Energy (CASP3) | Binding Energy (PARP) | Binding Energy (TP53) |
|---|---|---|---|---|
| protocatechuic aldehyde | 26 | - 1 | - | - |
| caffeic acid | 25 | - | - | - |
| osmundacetone | 22 | −6.4 kcal/mol | −7.2 kcal/mol | −5.3 kcal/mol |
| hispidin | 20 | −6.8 kcal/mol | −7.1 kcal/mol | −6.2 kcal/mol |
| citrinin | 15 | −6.3 kcal/mol | −6.4 kcal/mol | −6.4 kcal/mol |
| davallialactone | 11 | −8.4 kcal/mol | −9.4 kcal/mol | −7.1 kcal/mol |
| Tumor Cell Lines | A549 | HepG2 | T24 | Hela | HCT116 |
|---|---|---|---|---|---|
| IC50 (μg/mL) | 49.1 ± 0.5 | 140.1 ± 9.6 | 105.0 ± 10.7 | 96.1 ± 4.9 | 54.2 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Cui, S.; Dan, C.; Li, W.; Xie, H.; Li, C.; Shi, L. Phellinus baumii Polyphenol: A Potential Therapeutic Candidate against Lung Cancer Cells. Int. J. Mol. Sci. 2022, 23, 16141. https://doi.org/10.3390/ijms232416141
Liu X, Cui S, Dan C, Li W, Xie H, Li C, Shi L. Phellinus baumii Polyphenol: A Potential Therapeutic Candidate against Lung Cancer Cells. International Journal of Molecular Sciences. 2022; 23(24):16141. https://doi.org/10.3390/ijms232416141
Chicago/Turabian StyleLiu, Xue, Shiyao Cui, Caiyun Dan, Wenle Li, Hongqing Xie, Conghui Li, and Liangen Shi. 2022. "Phellinus baumii Polyphenol: A Potential Therapeutic Candidate against Lung Cancer Cells" International Journal of Molecular Sciences 23, no. 24: 16141. https://doi.org/10.3390/ijms232416141
APA StyleLiu, X., Cui, S., Dan, C., Li, W., Xie, H., Li, C., & Shi, L. (2022). Phellinus baumii Polyphenol: A Potential Therapeutic Candidate against Lung Cancer Cells. International Journal of Molecular Sciences, 23(24), 16141. https://doi.org/10.3390/ijms232416141






