Solubility Enhancement of Dihydroquercetin via “Green” Phase Modification
Abstract
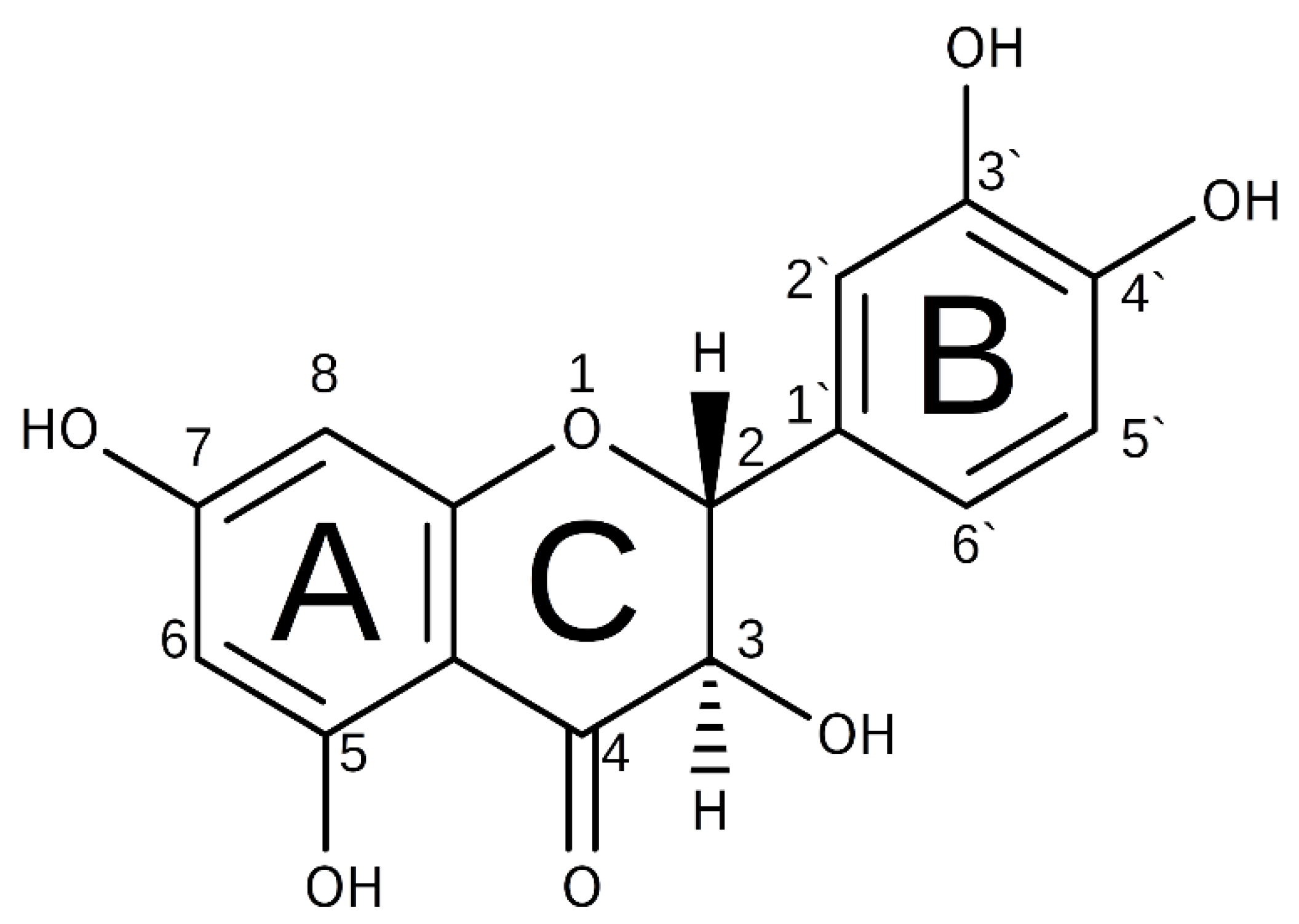
1. Introduction
2. Results
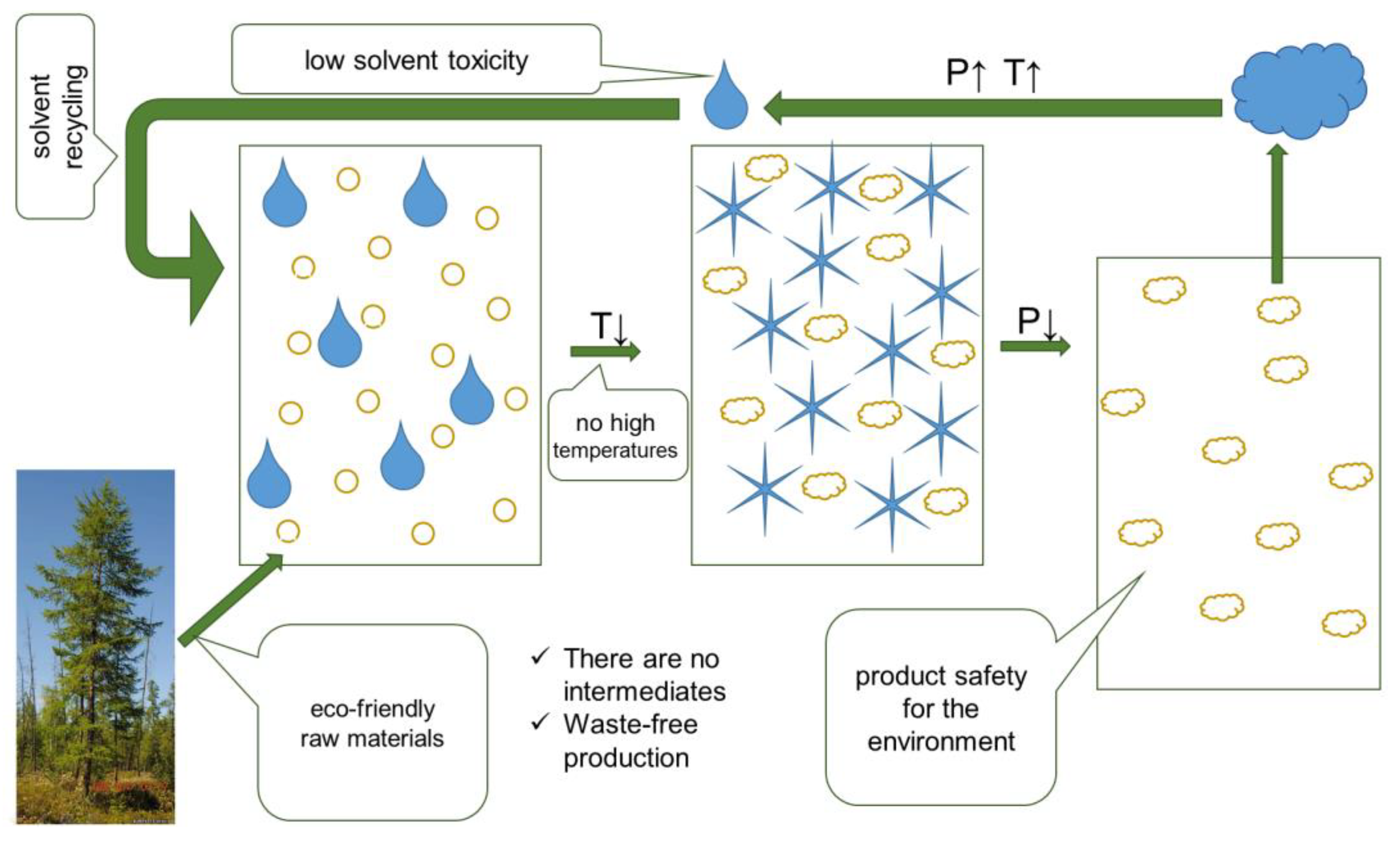
2.1. Design of “Green” Synthesis
2.2. Characterization of DHQ Lyophilizates
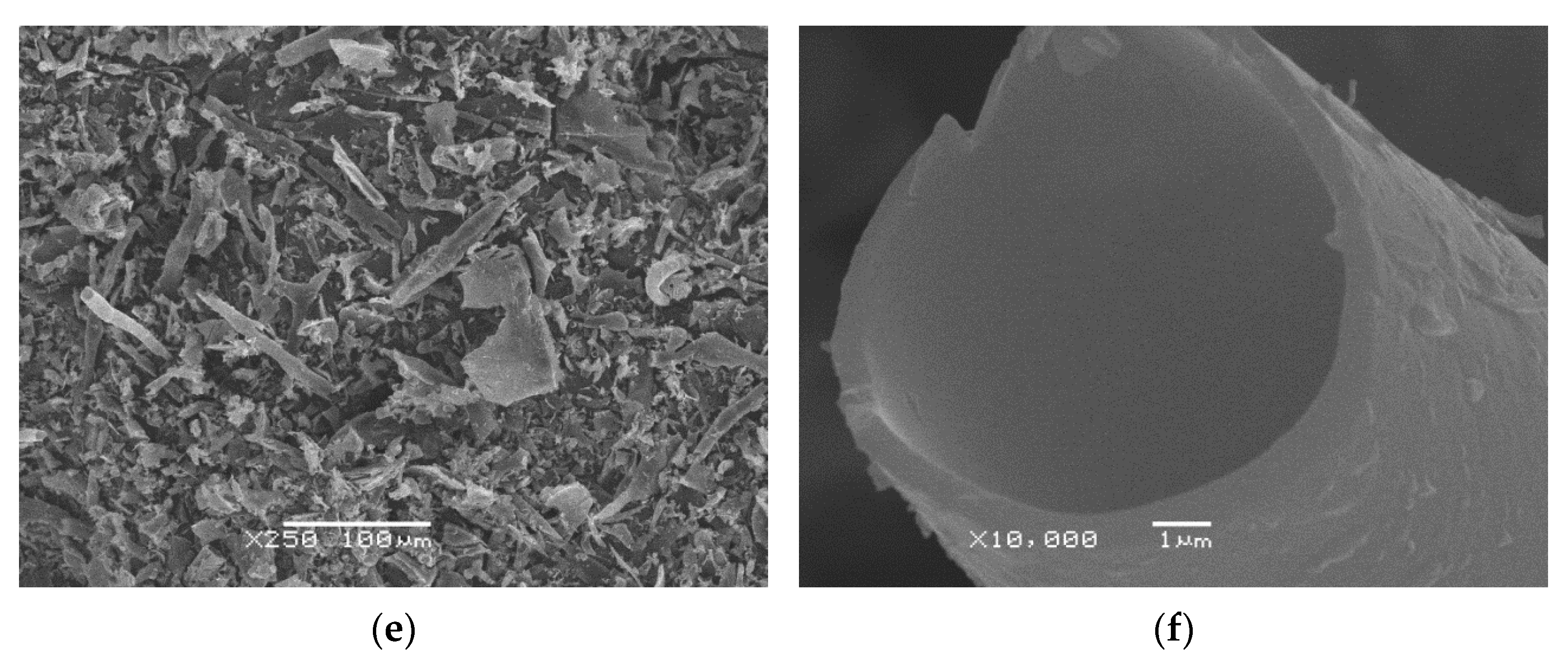
2.2.1. Morphology Analysis
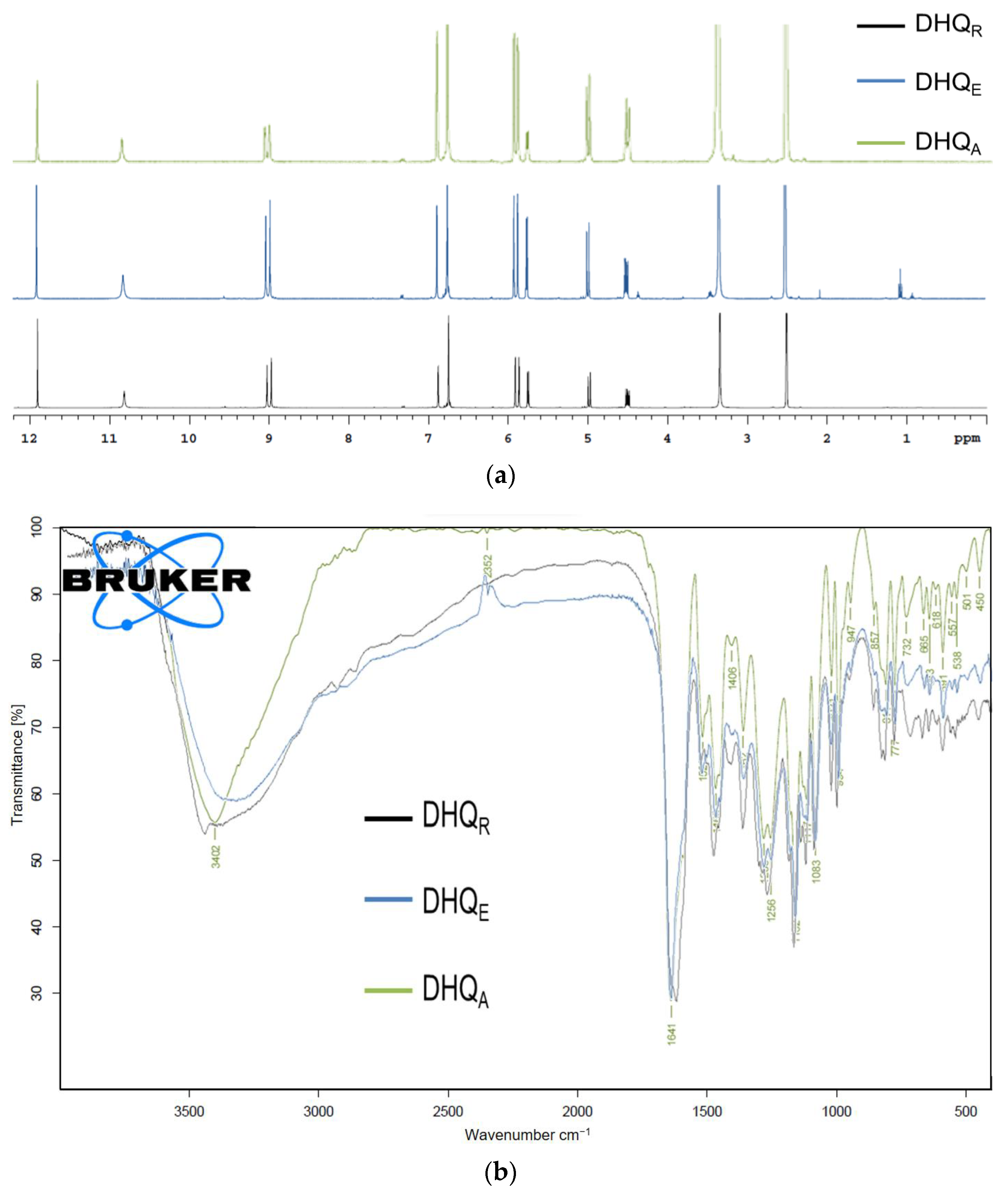
2.2.2. Spectral Analysis
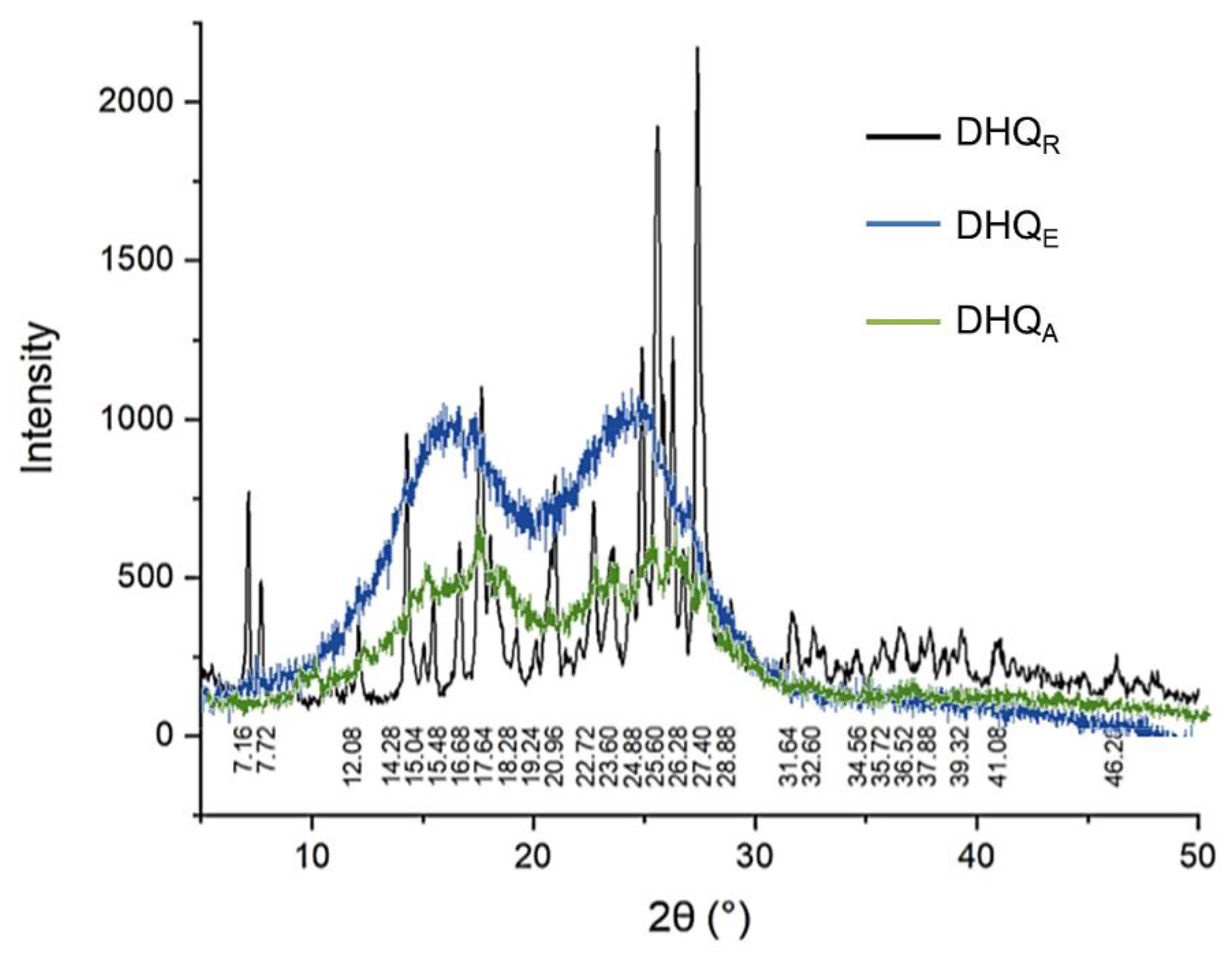
2.2.3. Phase Analysis
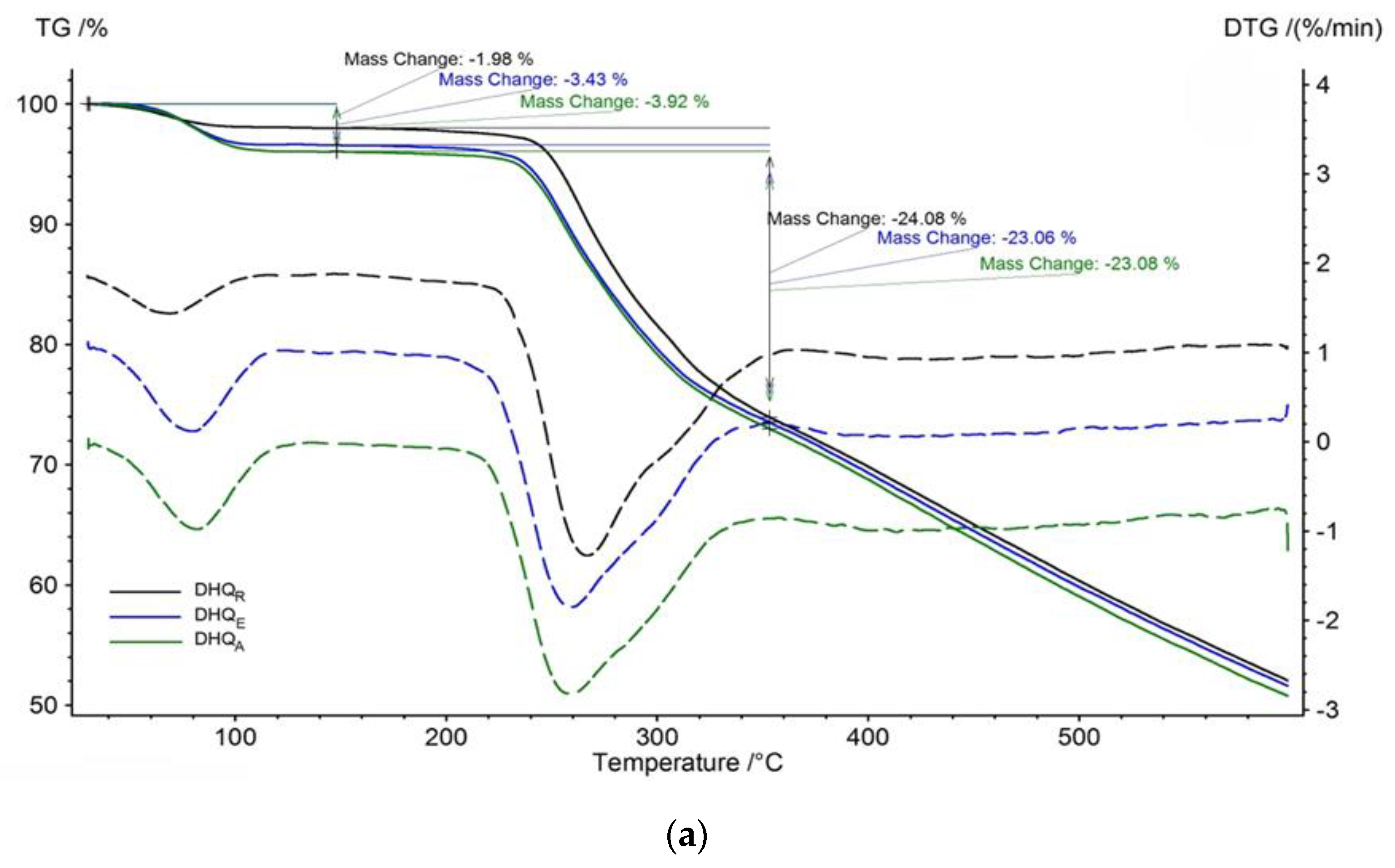
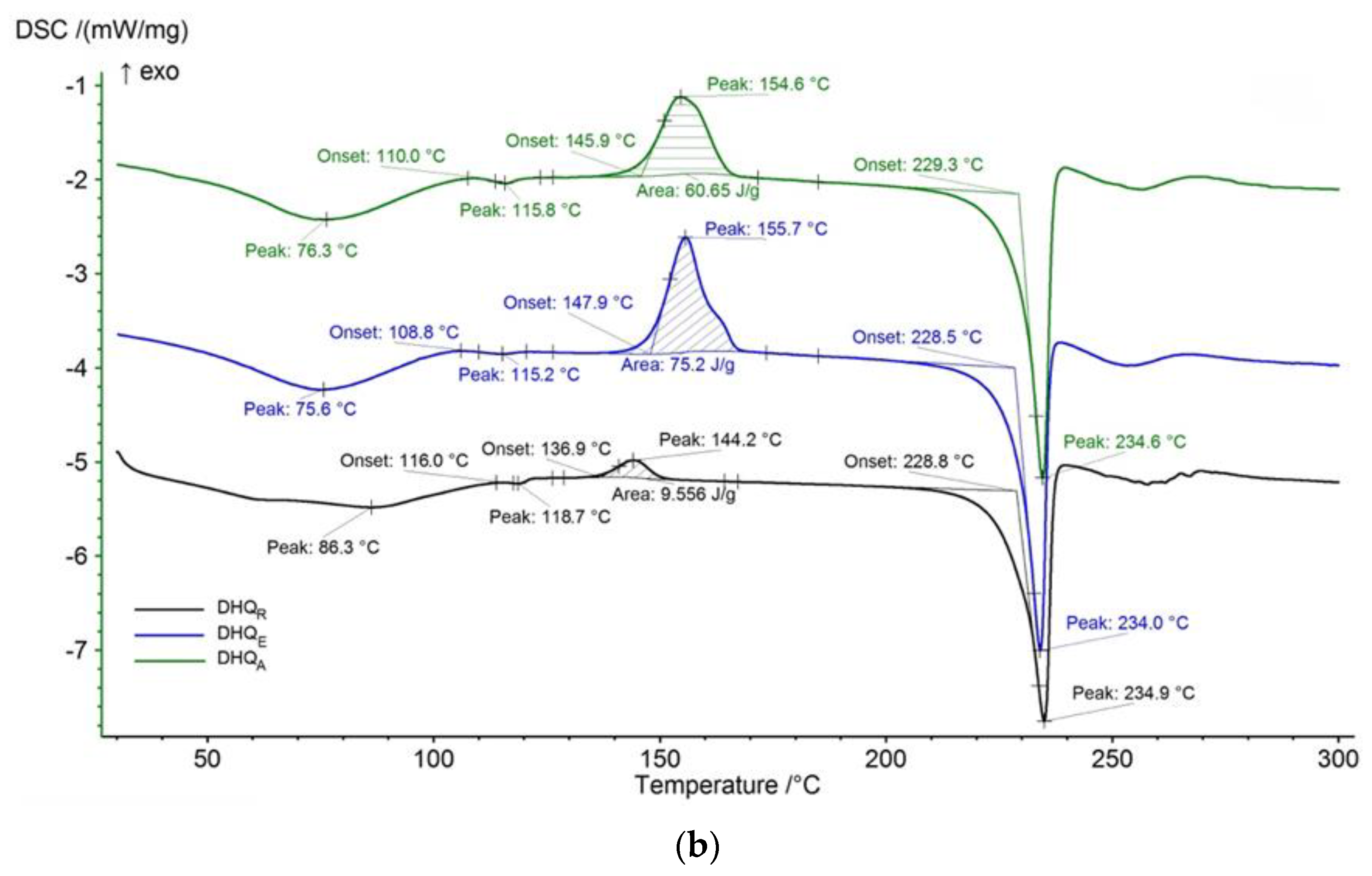
2.2.4. Functional Properties Analysis
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Lyophilizates Preparation
4.3. Assessment of Synthesis Eco-Friendliness
4.4. SEM
4.5. Mass-Spectrometry
4.6. NMR 1H Spectroscopy
4.7. IR Spectroscopy
4.8. XRPD
4.9. Thermal Analysis
4.10. Solubility
4.10.1. EP Method
4.10.2. Sedimentation Method
4.11. Antioxidant Capacity
4.12. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Horváth, I.T.; Anastas, P.T. Innovations and Green Chemistry. Chem. Rev. 2007, 107, 2169–2173. [Google Scholar] [CrossRef] [PubMed]
- Bethlendi, A.; Nagy, L.; Póra, A. Green Finance: The Neglected Consumer Demand. J. Sustain. Financ. Invest. 2022, 1–19. [Google Scholar] [CrossRef]
- Yoon, K.D.; Lee, J.Y.; Kim, T.Y.; Kang, H.; Ha, K.S.; Ham, T.H.; Ryu, S.N.; Kang, M.Y.; Kim, Y.H.; Kwon, Y.I. In Vitro and In Vivo Anti-Hyperglycemic Activities of Taxifolin and Its Derivatives Isolated from Pigmented Rice (Oryzae sativa L. cv. Superhongmi). J. Agric. Food Chem. 2020, 68, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Im, S.; Jeong, H.R.; Jung, Y.S.; Lee, I.; Kim, K.J.; Park, S.K.; Kim, D.O. Neuroprotective effects of korean red pine (Pinus densiflora) bark extract and its phenolics. J. Microbiol. Biotechnol. 2018, 28, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Wang, Y.; Li, W.; Ma, C.; Liu, S. Homogenization-assisted cavitation hybrid rotation extraction and macroporous resin enrichment of dihydroquercetin from Larixgmelinii. J. Chromatogr. B 2017, 1070, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Voronin, K.S.; Fenin, A.A.; Zhevlakova, A.K.; Zavadskii, S.P.; Selivanova, I.A. Polyphenolic Profile of Larch Knotwood. Pharm. Chem. J. 2021, 55, 781–786. [Google Scholar] [CrossRef]
- Saito, S.; Tanaka, M.; Satoh-Asahara, N.; Carare, R.O.; Weller, R.O.; Ihara, M. Taxifolin is a novel therapeutic agent for Alzheimer’s disease and cerebral amyloid angiopathy. Preclinical small-molecule drug discovery. Alzheimer’s Dement. 2020, 16, e042762. [Google Scholar] [CrossRef]
- Plotnikov, M.B.; Plotnikov, D.M.; Aliev, O.I.; Maslov, M.Y.; Vasiliev, A.S.; Alifirova, V.M.; Tyukavkina, N.A. Hemorhe-ological and antioxidant effects of Ascovertin in patients with sclerosis of cerebral arteries. Clin. Hemorheol. Microcirc. 2004, 30, 449–452. [Google Scholar]
- Luo, H.; Jiang, B.-H.; King, S.; Chen, Y.C. Inhibition of Cell Growth and VEGF Expression in Ovarian Cancer Cells by Flavonoids. Nutr. Cancer 2008, 60, 800–809. [Google Scholar] [CrossRef]
- Rogovskiĭ, V.S.; Matiushin, A.I.; Shimanovskiĭ, N.L.; Semeĭkin, A.V.; Kukhareva, T.S.; Koroteev, A.M.; Koroteev, M.P.; Nifant’ev, E.E. Antiproliferative and antioxidant activity of new dihydroquercetin derivatives. Eksp. Kiln. Farmakol. 2010, 73, 39–42. (In Russian) [Google Scholar]
- Brusselmans, K.; Vrolix, R.; Verhoeven, G.; Swinnen, J.V. Induction of cancer cell apoptosis by flavonoids is associated with their ability to inhibit fatty acid synthase activity. J. Biol. Chem. 2005, 280, 5636–5645. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Sellner, M.; Neranjan, S.; Smieško, M.; Lill, M.A. Potential inhibitors for novel coronavirus protease identified by virtual screening of 606 million compounds. Int. J. Mol. Sci. 2020, 21, 3626. [Google Scholar] [CrossRef]
- Raj, U.; Varadwaj, P.K. Flavonoids as multi-target inhibitors for proteins associated with Ebola virus: In silico discovery using virtual screening and molecular docking studies. Interdiscip. Sci. 2016, 8, 132–141. [Google Scholar] [CrossRef]
- Li, J.; Dong, J.; Ouyang, J.; Cui, J.; Chen, Y.; Wang, F.; Wang, J. Synthesis, Characterization, Solubilization, Cytotoxicity and Antioxidant Activity of Aminomethylated Dihydroquercetin. Med. Chem. Commun. 2017, 8, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Shikov, A.N.; Pozharitskaya, O.N.; Miroshnyk, I.; Mirza, S.; Urakova, I.N.; Hirsjärvi, S.; Makarov, V.G.; Heinämäki, J.; Yliruusi, J.; Hiltunen, R. Nanodispersions of Taxifolin: Impact of Solid-State Properties on Dissolution Behavior. Int. J. Pharm. 2009, 377, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Fadeev, R.S.; Kaptsov, V.V.; Uminsky, A.A.; Akatov, V.S. Cytotoxic Effect of Dihydroquercetin and Its Derivatives in Liposomal Form and in the Form of Fat Nanoscale Emulsions. Biochem. Suppl. Ser. A 2011, 5, 45–50. [Google Scholar] [CrossRef]
- Selivanova, I.A.; Terekhov, R.P. Engineering of Dihydroquercetin Crystals. Pharm. Chem. J. 2020, 53, 1081–1085. [Google Scholar] [CrossRef]
- Terekhov, R.P.; Selivanova, I.A.; Tyukavkina, N.A.; Shylov, G.V.; Utenishev, A.N.; Porozov, Y.B. Taxifolin Tubes: Crystal Engineering and Characteristics. Acta Cryst. B 2019, 75, 175–182. [Google Scholar] [CrossRef]
- Terekhov, R.P.; Selivanova, I.A.; Tyukavkina, N.A.; Ilyasov, I.R.; Zhevlakova, A.K.; Dzuban, A.V.; Bogdanov, A.G.; Davidovich, G.N.; Shylov, G.V.; Utenishev, A.N.; et al. Assembling the Puzzle of Taxifolin Polymorphism. Molecules 2020, 25, 5437. [Google Scholar] [CrossRef]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2009, 39, 301–312. [Google Scholar] [CrossRef]
- Corver, J. The Evolution of Freeze-Drying. Innov. Pharm. Technol. 2009, 29, 66–70. [Google Scholar]
- Cameron, P. (Ed.) Good Pharmaceutical Freeze-Drying Practice, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013; ISBN 978-0-429-17115-4. [Google Scholar]
- Alihosseini, F.; Ghaffari, S.; Dabirsiaghi, A.R.; Haghighat, S. Freeze-Drying of Ampicillin Solid Lipid Nanoparticles Using Mannitol as Cryoprotectant. Braz. J. Pharm. Sci. 2015, 51, 797–802. [Google Scholar] [CrossRef]
- Borisova, A.; De Bruyn, M.; Budarin, V.L.; Shuttleworth, P.S.; Dodson, J.R.; Segatto, M.L.; Clark, J.H. A Sustainable Freeze-Drying Route to Porous Polysaccharides with Tailored Hierarchical Meso- and Macroporosity. Macromol. Rapid Commun. 2015, 36, 774–779. [Google Scholar] [CrossRef]
- Ohtsuka, K.; Kondo, I.; Okada, T. Method of Separating and Purifying Spent Solvent Generated in Nuclear Fuel Cycle. U.S. Patent 5,523,515, 4 June 1996. [Google Scholar]
- Terekhov, R.P.; Selivanova, I.A.; Zhevlakova, A.K.; Porozov, Y.B.; Dzuban, A.V. Analysis of dihydroquercetin physical modification via in vitro and in silico methods. Biomed. Khim. 2019, 65, 152–158. (In Russian) [Google Scholar] [CrossRef][Green Version]
- Ilyasov, I.; Beloborodov, V.; Antonov, D.; Dubrovskaya, A.; Terekhov, R.; Zhevlakova, A.; Saydasheva, A.; Evteev, V.; Selivanova, I. Flavonoids with Glutathione Antioxidant Synergy: Influence of Free Radicals Inflow. Antioxidants 2020, 9, 695. [Google Scholar] [CrossRef] [PubMed]
- Tarahovsky, Y.; Kim, Y.; Ivanitsky, G. Taxifolin Fibers as Biomedical Nanomaterial. Dokl. Biochem. Biophys. 2008, 422, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Northanmshire, UK, 1998; ISBN 978-0-19-850234-0. [Google Scholar]
- Jimenez-Gonzalez, C.; Ponder, C.S.; Broxterman, Q.B.; Manley, J.B. Using the Right Green Yardstick: Why Process Mass In-tensity Is Used in the Pharmaceutical Industry to Drive More Sustainable Processes. Org. Process. Res. Dev. 2011, 15, 912–917. [Google Scholar] [CrossRef]
- Trost, B. The Atom Economy—A Search for Synthetic Efficiency. Science 1991, 254, 1471–1477. [Google Scholar] [CrossRef]
- An, H.J.; Lee, Y.; Liu, L.; Lee, S.; Lee, J.D.; Yi, Y. Physical and Chemical Stability of Formulations Loaded with Taxifolin Tetra-Octanoate. Chem. Pharm. Bull. 2019, 67, 985–991. [Google Scholar] [CrossRef]
- Zhang, W.; Cue, B.W. (Eds.) Green Techniques for Organic Synthesis and Medicinal Chemistry, 2nd ed.; John Wiley & Sons: New York, NY, USA, 2012; ISBN 978-0-470-71151-4. [Google Scholar]
- Kuznetsov, B.N.; Sudakova, I.G.; Garyntseva, N.V.; Levdansky, V.A.; Ivanchenko, N.M.; Pestunov, A.V.; Djakovitch, L.; Pinel, C. Green biorefinery of larch wood biomass to obtain the bioactive compounds, functional polymers and nanoporous materials. Wood Sci. Technol. 2018, 52, 1377–1394. [Google Scholar] [CrossRef]
- Higueras, P.L.; Sáez-Martínez, F.J.; Lefebvre, G.; Moilleron, R. Contaminated Sites, Waste Management, and Green Chemistry: New Challenges from Monitoring to Remediation. Environ. Sci. Pollut. Res. 2019, 26, 3095–3099. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Gu, F.L.; Zhan, Q.; Palvannan, T.; Mohd Yusoff, A.R. Flavonoids Mediated ‘Green’ Nanomaterials: A Novel Nanomedicine System to Treat Various Diseases—Current Trends and Future Perspective. Mater. Lett. 2018, 210, 26–30. [Google Scholar] [CrossRef]
- Schauss, A.G.; Tselyico, S.S.; Kuznetsova, V.A.; Yegorova, I. Toxicological and genotoxicity assessment of a dihydroquercetin-rich Dahurian larch tree (Larix gmelinii Rupr) extract (Lavitol). Int. J. Toxicol. 2015, 34, 162–181. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Cao, W.; Xia, M.; Pan, S.; Xu, X. Study of structure and permeability relationship of flavonoids in caco-2 cells. Nutrients 2017, 9, 1301. [Google Scholar] [CrossRef] [PubMed]
- Shkarenkov, A.A.; Beloshapko, A.A.; Kuznetsov, Y.B.; Borovkova, M.V.; Anikanova, V.V.; Krepkova, L.V. Preclinical toxicological study of Diquertin. Probl. Biol. Med. Pharm. Chem. 1998, 3, 36–39. (In Russian) [Google Scholar]
- FDA. Q3C—Tables and List Guidance for Industry; Federal Register: Washington, DC, USA, 1997.
- Dias, J.L.; Lanza, M.; Ferreira, S.R.S. Cocrystallization: A Tool to Modulate Physicochemical and Biological Properties of Food-Relevant Polyphenols. Trends Food Sci. Technol. 2021, 110, 13–27. [Google Scholar] [CrossRef]
- Huang, R.; Qi, W.; Su, R.; Zhao, J.; He, Z. Solvent and Surface Controlled Self-Assembly of Diphenylalanine Peptide: From Microtubes to Nanofibers. Soft Matter 2011, 7, 6418–6421. [Google Scholar] [CrossRef]
- Huang, R.; Su, R.; Qi, W.; Zhao, J.; He, Z. Hierarchical, Interface-Induced Self-Assembly of Diphenylalanine: Formation of Peptide Nanofibers and Microvesicles. Nanotechnology 2011, 22, 245609. [Google Scholar] [CrossRef]
- Zu, Y.; Wu, W.; Zhao, X.; Li, Y.; Zhong, C.; Zhang, Y. The High Water Solubility of Inclusion Complex of Taxifolin-γ-CD Prepared and Characterized by the Emulsion Solvent Evaporation and the Freeze Drying Combination Method. Int. J. Pharm. 2014, 477, 148–158. [Google Scholar] [CrossRef]
- Vert, M.; Doi, Y.; Hellwich, K.-H.; Hess, M.; Hodge, P.; Kubisa, P.; Rinaudo, M.; Schué, F. Terminology for biorelated polymers and applications (IUPAC Recommendations 2012). Pure Appl. Chem. 2012, 84, 377–410. [Google Scholar] [CrossRef]
- Darrat, Y.; Naumenko, E.; Cavallaro, G.; Lazzara, G.; Lvov, Y.; Fakhrullin, R. Tubular Nanocontainers for Drug Delivery. In Materials Nanoarchitectonics; Agira, K., Ebara, M., Eds.; John Wiley & Sons: New York, NY, USA, 2018; pp. 85–108. ISBN 978-3-527-80831-1. [Google Scholar]
- Polkovnikova, Y.A.; Kovaleva, N.A. Modern Research in the Field of Microencapsulation (Review). Drug Dev. Registr. 2021, 10, 50–61. [Google Scholar] [CrossRef]
- Stenger Moura, F.C.; dos Santos Machado, C.L.; Reisdorfer Paula, F.; Garcia Couto, A.; Ricci, M.; Cechinel-Filho, V.; Bonomini, T.J.; Sandjo, L.P.; Bellé Bresolin, T.M. Taxifolin Stability: In Silico Prediction and in Vitro Degradation with HPLC-UV/UPLC–ESI-MS Monitoring. J. Pharm. Anal. 2021, 11, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Mabry, T.; Markham, K.R.; Thomas, M.B. The Systematic Identification of Flavonoids; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-88458-0. [Google Scholar]
- Tjukavkina, N.A.; Naumov, V.V.; Kolesnik, Y.A.; Rulenko, I.A. Diquertin—A new bioflavonoid product obtained from plant raw materials. In Proceedings of the Polyphenols Communications 18th International Conference, Bordeaux, France, 15–18 July 1996; Groupe Polyphénols: Bordeaux, France, 1996; Volume 1, pp. 101–102. [Google Scholar]
- Stenger Moura, F.C.; Pinna, N.; Vivani, R.; Nunes, G.E.; Schoubben, A.; Bellé Bresolin, T.M.; Bechold, I.H.; Ricci, M. Exploring Taxifolin Polymorphs: Insights on Hydrate and Anhydrous Forms. Pharmaceutics 2021, 13, 1328. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines. European Pharmacopoeia 10.0; Council of Europe: Strasbourg, France, 2020. [Google Scholar]
- Zu, S.; Yang, L.; Huang, J.; Ma, C.; Wang, W.; Zhao, C.; Zu, Y. Micronization of Taxifolin by Supercritical Antisolvent Process and Evaluation of Radical Scavenging Activity. Int. J. Mol. Sci. 2012, 13, 8869–8881. [Google Scholar] [CrossRef] [PubMed]
- Terekhov, R.P.; Selivanova, I.A.; Anurova, M.N.; Zhevlakova, A.K.; Nikitin, I.D.; Cong, Z.; Ma, S.; Yang, F.; Dong, Z.; Liao, Y. Comparative Study of Wound-Healing Activity of Dihydroquercetin Pseudopolymorphic Modifications. Bull. Exp. Biol. Med. 2021, 170, 444–447. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A. Three ABTS•+ Radical Cation-Based Approaches for the Evaluation of Antioxidant Activity: Fast- and Slow-Reacting Antioxidant Behavior. Chem. Pap. 2018, 72, 1917–1925. [Google Scholar] [CrossRef]

| Solvent | Water: Organic Solvent Ratio | Yield, % | PMI | %AE, % |
|---|---|---|---|---|
| Ethanol | 85:15 | none * | - | - |
| 90:10 | none * | - | - | |
| 95:5 | 93.4 ± 1.1 | 1.07 | 100 | |
| Acetonitrile | 85:15 | 97.0 ± 1.9 | 1.03 | 100 |
| 90:10 | 95.6 ± 1.4 | 1.05 | 100 | |
| 95:5 | 91.7 ± 1.1 | 1.09 | 100 |
| Sample | Term by EP | Solubility, mg/mL | |
|---|---|---|---|
| By EP Method | By Sedimentation Method | ||
| DHQR | very slightly soluble | [0.1–1.0] | 0.70 ± 0.02 |
| DHQE | soluble | [100.0–30.0] | 3.09 ± 0.09 |
| DHQA | slightly soluble | [1.0–10.0] | 2.14 ± 0.06 |
| Sample | Antioxidant Capacity | |
|---|---|---|
| ΔA | n-Value | |
| DHQR | 0.511 ± 0.017 | 7.30 ± 0.24 |
| DHQE | 0.504 ± 0.026 | 7.19 ± 0.37 |
| DHQA | 0.499 ± 0.014 | 7.13 ± 0.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terekhov, R.P.; Ilyasov, I.R.; Beloborodov, V.L.; Zhevlakova, A.K.; Pankov, D.I.; Dzuban, A.V.; Bogdanov, A.G.; Davidovich, G.N.; Shilov, G.V.; Utenyshev, A.N.; et al. Solubility Enhancement of Dihydroquercetin via “Green” Phase Modification. Int. J. Mol. Sci. 2022, 23, 15965. https://doi.org/10.3390/ijms232415965
Terekhov RP, Ilyasov IR, Beloborodov VL, Zhevlakova AK, Pankov DI, Dzuban AV, Bogdanov AG, Davidovich GN, Shilov GV, Utenyshev AN, et al. Solubility Enhancement of Dihydroquercetin via “Green” Phase Modification. International Journal of Molecular Sciences. 2022; 23(24):15965. https://doi.org/10.3390/ijms232415965
Chicago/Turabian StyleTerekhov, Roman P., Igor R. Ilyasov, Vladimir L. Beloborodov, Anastasiya K. Zhevlakova, Denis I. Pankov, Alexander V. Dzuban, Anatoliy G. Bogdanov, Georgiy N. Davidovich, Gennadii V. Shilov, Andrey N. Utenyshev, and et al. 2022. "Solubility Enhancement of Dihydroquercetin via “Green” Phase Modification" International Journal of Molecular Sciences 23, no. 24: 15965. https://doi.org/10.3390/ijms232415965
APA StyleTerekhov, R. P., Ilyasov, I. R., Beloborodov, V. L., Zhevlakova, A. K., Pankov, D. I., Dzuban, A. V., Bogdanov, A. G., Davidovich, G. N., Shilov, G. V., Utenyshev, A. N., Saverina, E. A., & Selivanova, I. A. (2022). Solubility Enhancement of Dihydroquercetin via “Green” Phase Modification. International Journal of Molecular Sciences, 23(24), 15965. https://doi.org/10.3390/ijms232415965







