Mathematical Model for Evaluation of Tumor Response in Targeted Radionuclide Therapy with 211At Using Implanted Mouse Tumor
Abstract
1. Introduction
2. Results
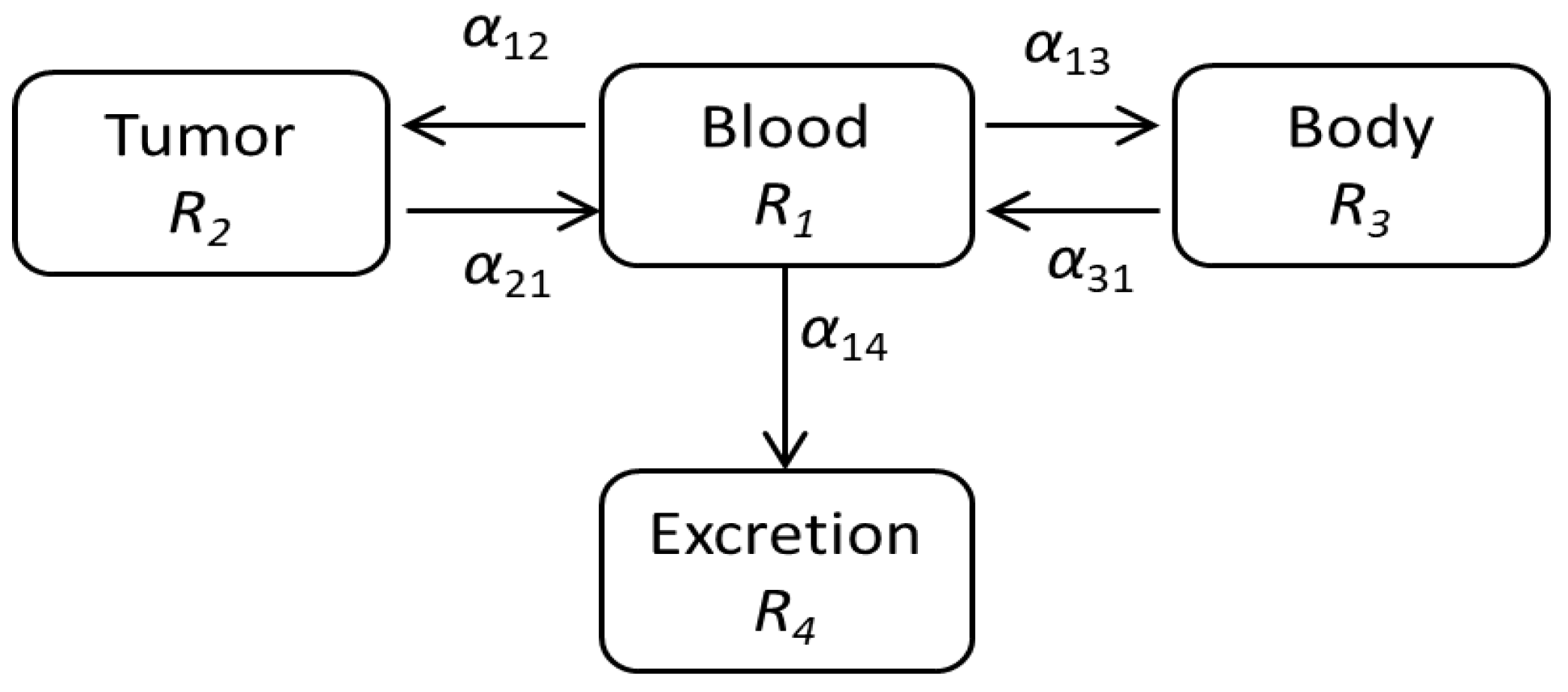
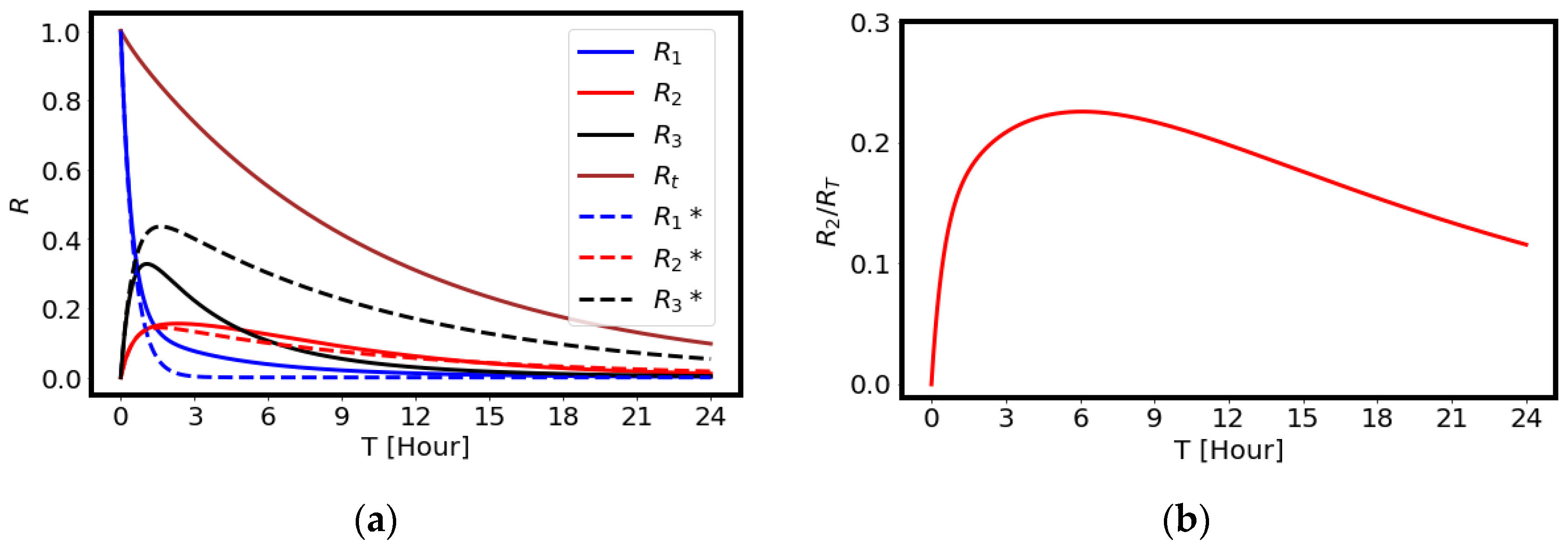
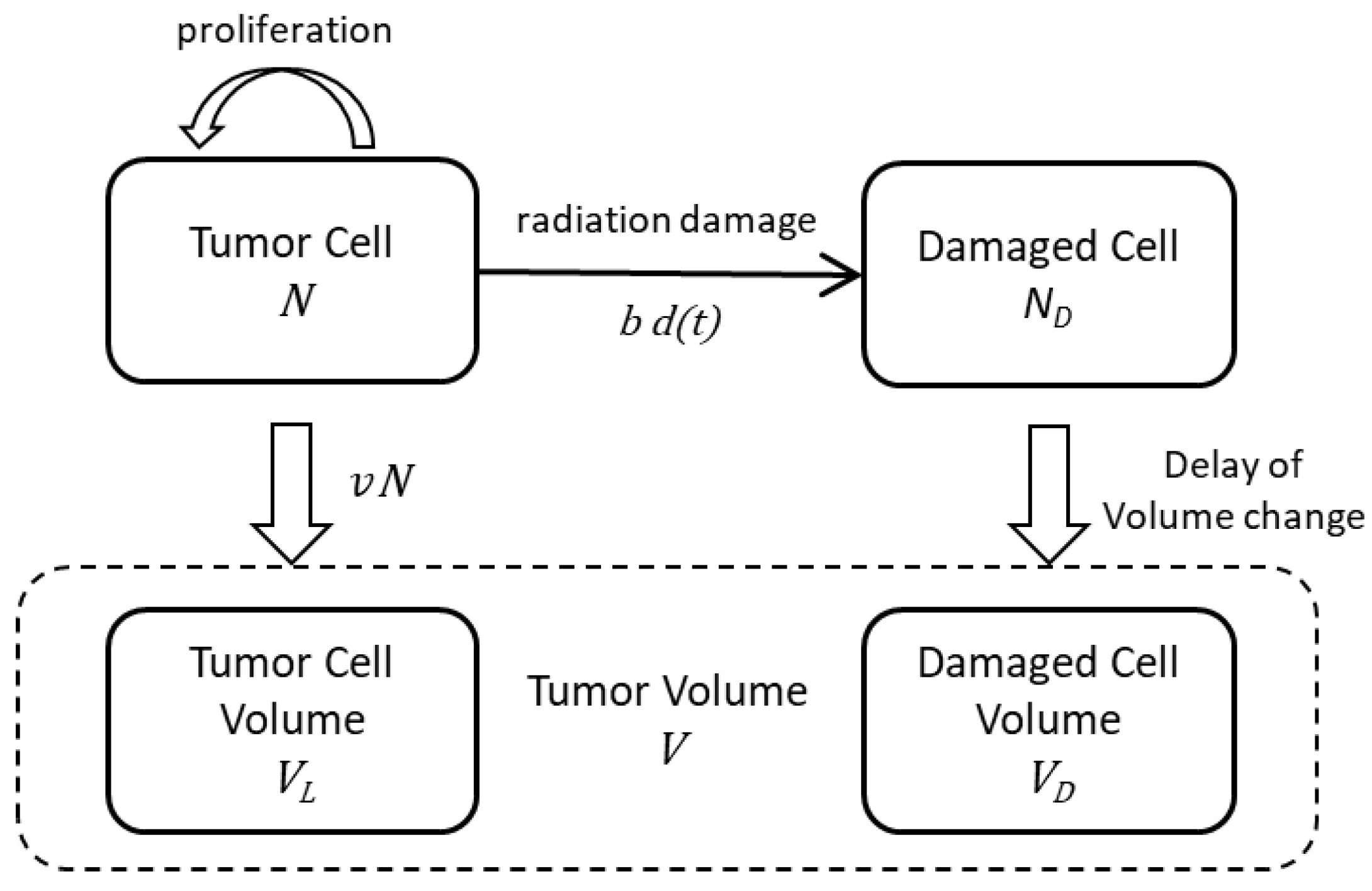
2.1. Modeling of the Drug Delivery
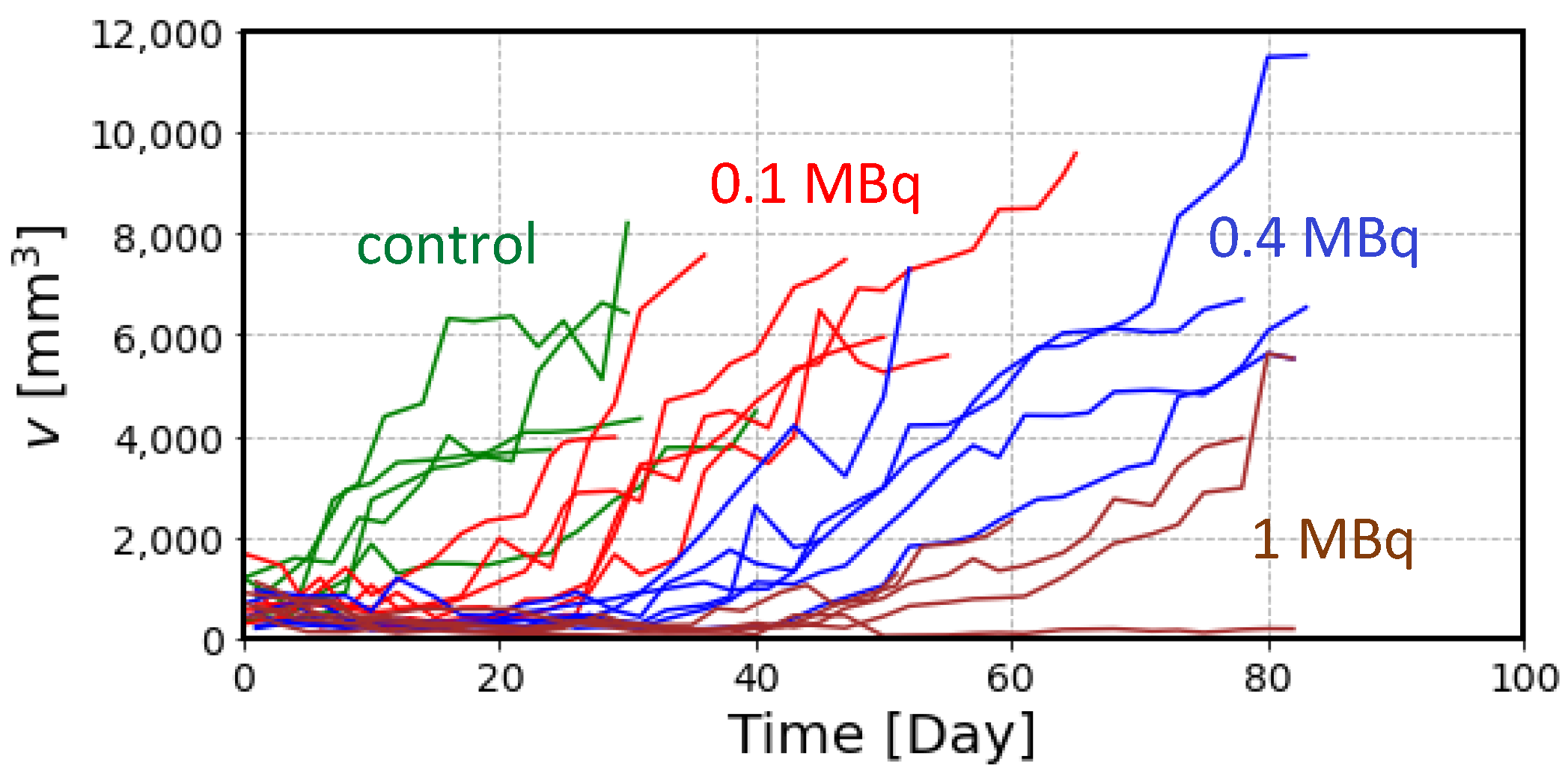
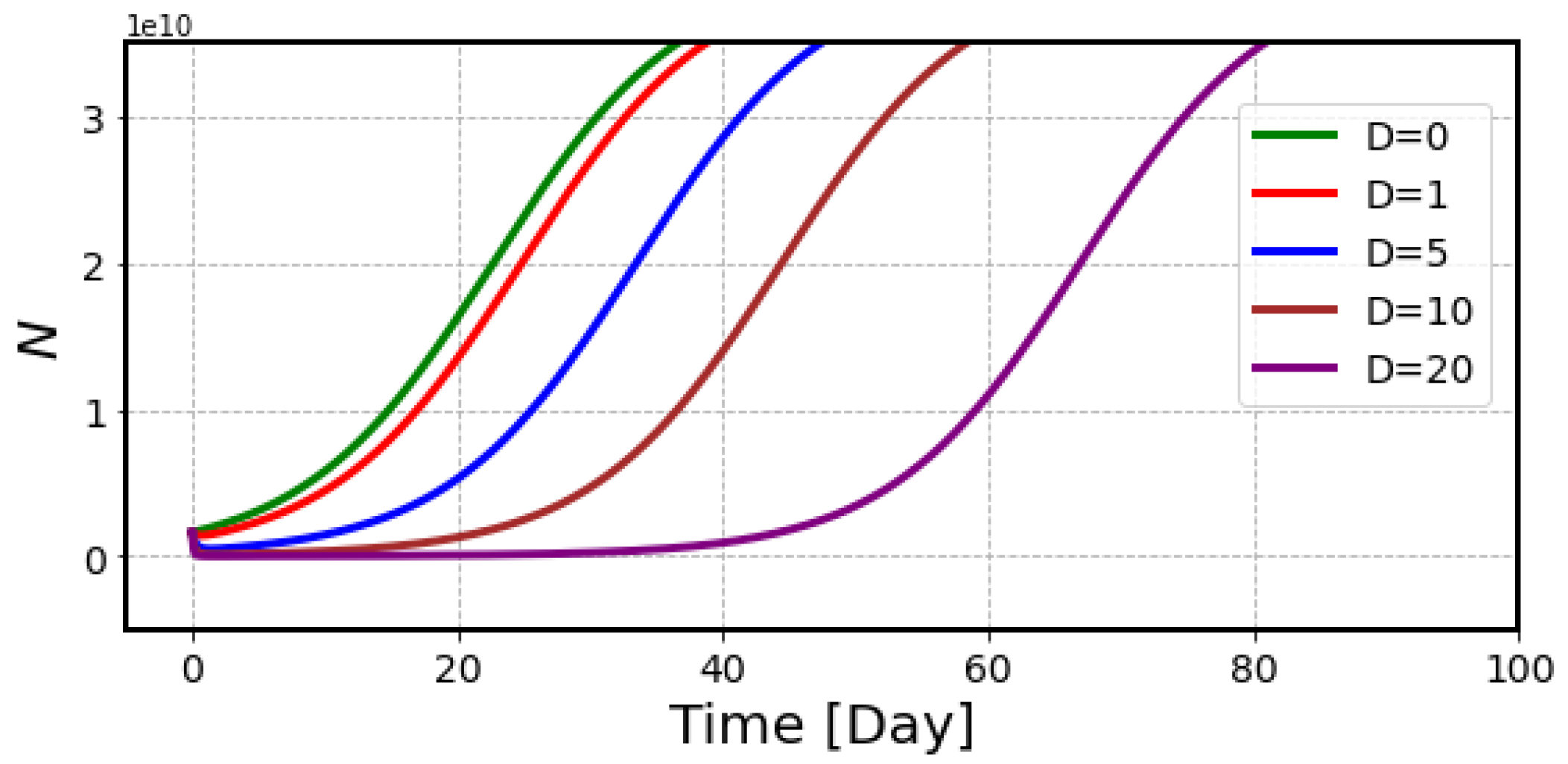
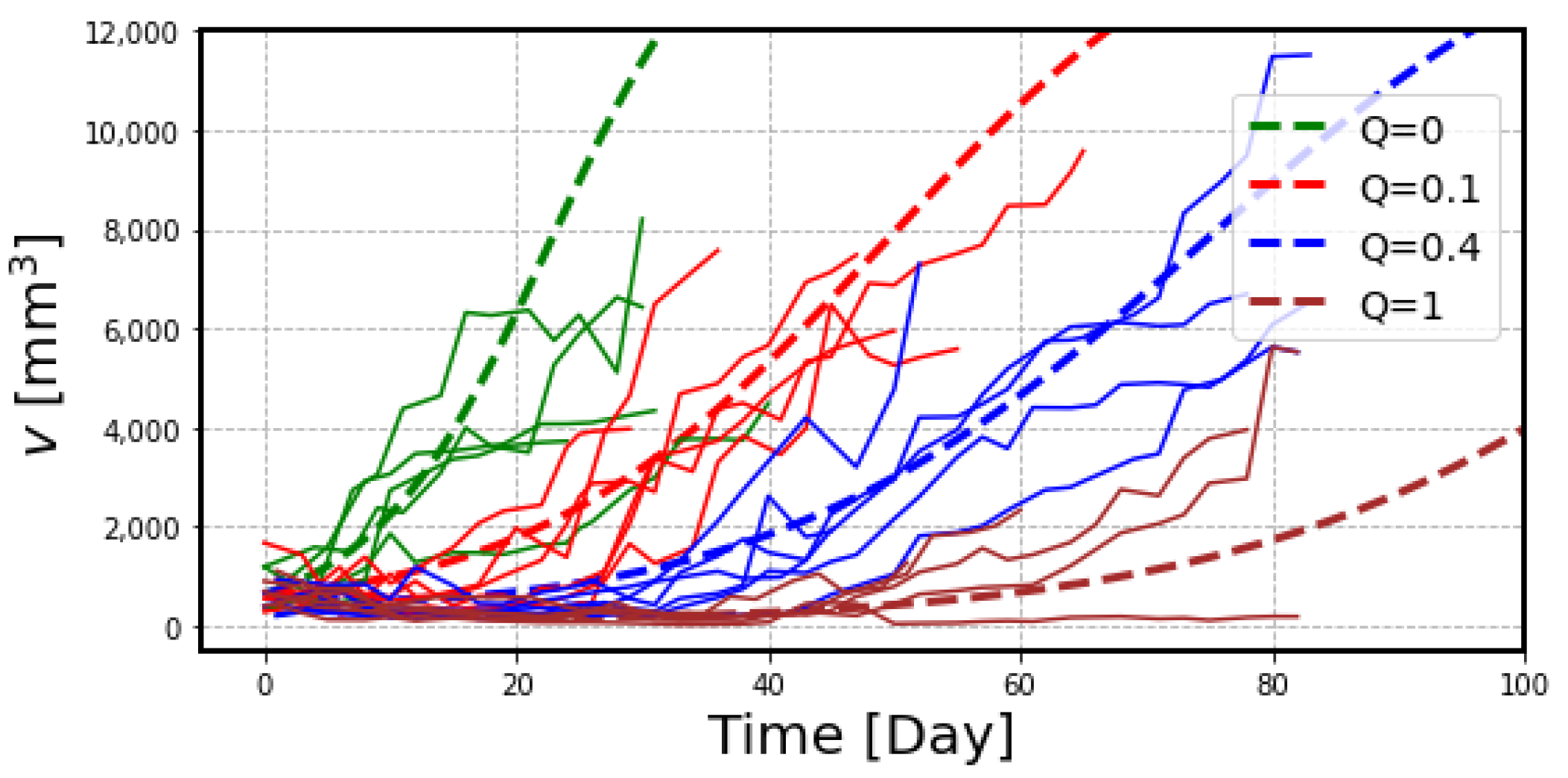
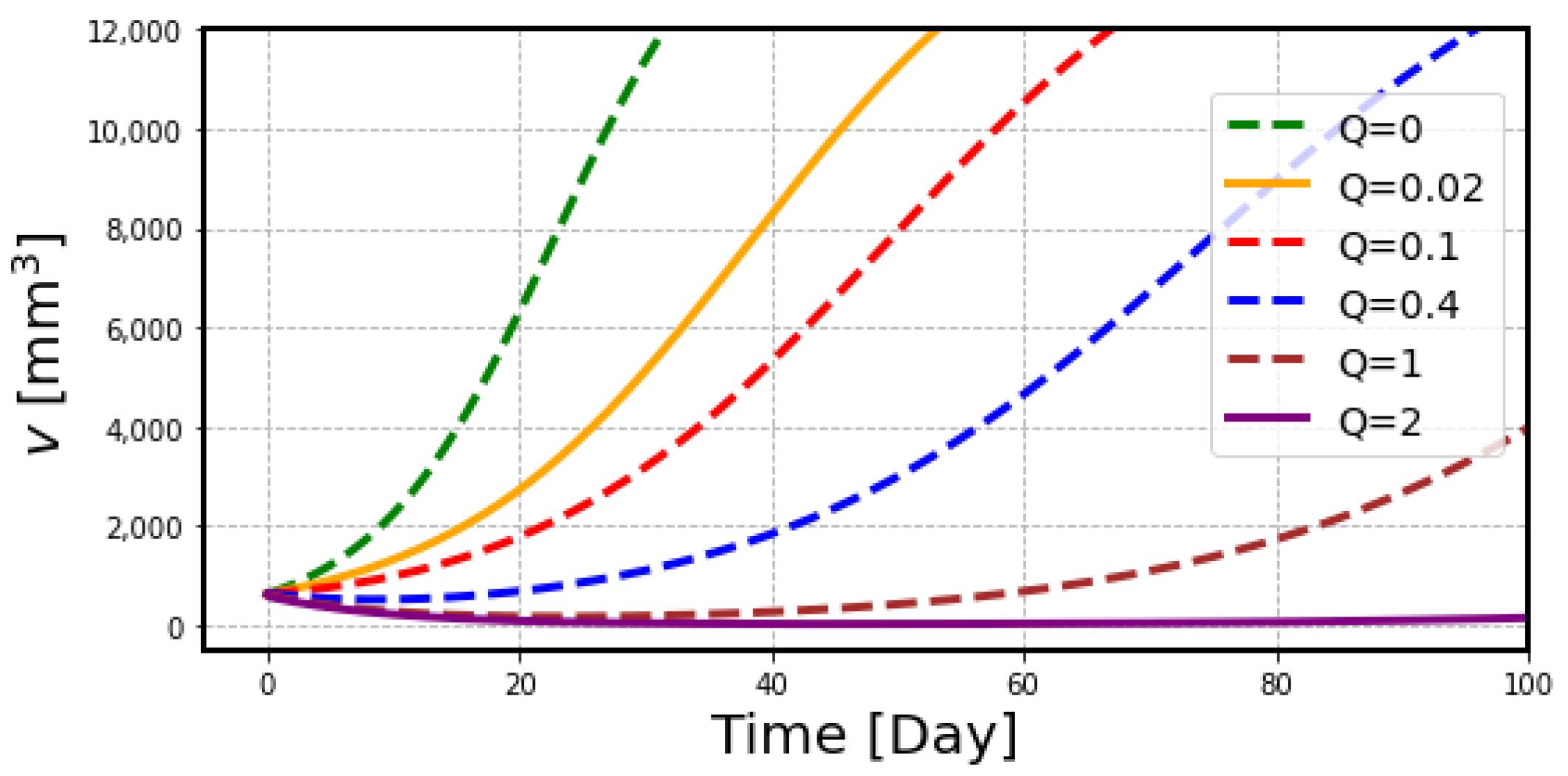
2.2. The Effects of Radiation on Tumor Volume
3. Discussion
4. Materials and Methods
4.1. Animal Experiment
4.2. Estimation of the Drug Delivery
4.3. Estimation of Radiation Effects on Tumor Volume
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, C.; Nilsson, S.; Heinrich, D.; Helle, S.I.; O’Sullivan, J.M.; Fosså, S.D.; Chodacki, A.; Wiechno, P.; Logue, J.; Seke, M.; et al. for the ALSYMPCA Investigators. Alpha emitter radium-223 and survival in metastatic prostate cancer. N. Engl. J. Med. 2013, 369, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Kratochwil, C.; Bruchertseifer, F.; Giesel, F.L.; Weis, M.; Verburg, F.A.; Mottaghy, F.; Kopka, K.; Apostolidis, C.; Haberkorn, U.; Morgestern, A. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J. Nucl. Med. 2016, 57, 1941–1944. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.J.; Giaccia, A.J. Radiobiology for the Radiologist, 8th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2019; pp. 398–416. [Google Scholar]
- Xu, X.G.; Bednartz, T.; Paganetti, H. A review of dosimetry studies on external-beam radiation treatment with respect to second cancer induction. Phys. Med. Biol. 2008, 53, R193–R241. [Google Scholar] [CrossRef] [PubMed]
- Caudell, J.J.; Torres-Roca, J.F.; Gillies, R.J.; Enderling, H.; Kim, S.; Rishi, A.; Moros, E.G.; Harrison, L.B. The future of personalised radiotherapy for head and neck cancer. Lancet Oncol. 2017, 18, e266–e273. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, Y.; Tsujii, H.; Hopewell, J.W.; Lopez, P.O.; Cosset, J.-M.; Paganetti, H.; Montelius, A.; Schrdt, D.; Jones, B.; Nakamura, T. Radiological protection in ion beam radiotherapy. ICRP Publication 127. Ann. ICRP 2014, 42, 5–113. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Kaneda-Nakashima, K.; Liu, Y.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Shimosegawa, E.; Fukuda, M.; Shinohara, A.; Hatazawa, J. Enhancement of 211At uptake via the sodium iodide symporter by the addition of ascorbic acid in targeted a-therapy of thyroid cancer. J. Nucl. Med. 2019, 60, 1301–1307. [Google Scholar] [CrossRef] [PubMed]
- European Society of Radiology (ESR). Summary of the European Directive 2013/59/Euratom: Essentials for health professionals in radiology. Insights Imaging 2015, 6, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Yonekura, Y.; Mattson, S.; Flux, G.; Bolch, W.E.; Dauer, L.T.; Fisher, D.R.; Palm, S.; Hosono, M.; Doruff, M.; Divgi, C.; et al. Radiological protection in therapy with radiopharmaceuticals. ICRP Publication 140. Ann. ICRP 2019, 48, 1–332. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.R.; Mangesius, J.; Skvortsova, I.I.; Ganswindt, U. The role of cancer stem cells in radiation resistance. Front. Oncol. 2020, 10, 164. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.A.; Kim, S.; Pilon-Thomas, S.; Enderling, H. Mathematical modeling of radiotherapy and its impact on tumor interactions with the immune system. Neoplasia 2022, 28, 100796. [Google Scholar] [CrossRef] [PubMed]
- Bando, M.; Tsunoyama, Y.; Suzuki, K.; Toki, H. WAM to SeeSaw model for cancer therapy –overcoming LQM difficulties–. Int. J. Radiat. Biol. 2021, 97, 228–239. [Google Scholar] [CrossRef] [PubMed]
- Spetz, J.; Rudqvist, N.; Forssell-Aronsson, E. Biodistribution and dosimetry of free 211At, 125I- and 131I- in rats. Cancer Biother. Radiopharm. 2013, 28, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Watabe, T.; Liu, Y.; Kaneda-Nakashima, K.; Sato, T.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Shimosegawa, E.; Wang, Y.; Haba, H.; et al. Comparison of the therapeutic effects of [211At]NaAt and [131I]NaI in an NIS-expressing thyroid cancer mouse model. Int. J. Mol. Sci. 2022, 23, 9434. [Google Scholar] [CrossRef] [PubMed]
- Sgouros, G.; Roeske, J.C.; McDevitt, M.R.; Palm, S.; Allen, B.J.; Fisher, D.R.; Brill, A.B.; Song, H.; Howell, R.W.; Akabani, G. MIRD Pamphlet No. 22 (Abridged): Radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J. Nucl. Med. 2010, 51, 311–328. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, R.F.; Howell, R.W.; Song, H.; Baechler, S.; Sgouros, G. Redefining relative biological effectiveness in the context of the EQDX formalism: Implications for alpha-particle emitter therapy. Radiat. Res. 2014, 181, 90–98. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Mean | Standard Deviation |
|---|---|---|
| λ (/day) | 0.14 | 0.08 |
| V0 (mm3) | 577 | 372 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yonekura, Y.; Toki, H.; Watabe, T.; Kaneda-Nakashima, K.; Shirakami, Y.; Ooe, K.; Toyoshima, A.; Nakajima, H.; Tomiyama, N.; Bando, M. Mathematical Model for Evaluation of Tumor Response in Targeted Radionuclide Therapy with 211At Using Implanted Mouse Tumor. Int. J. Mol. Sci. 2022, 23, 15966. https://doi.org/10.3390/ijms232415966
Yonekura Y, Toki H, Watabe T, Kaneda-Nakashima K, Shirakami Y, Ooe K, Toyoshima A, Nakajima H, Tomiyama N, Bando M. Mathematical Model for Evaluation of Tumor Response in Targeted Radionuclide Therapy with 211At Using Implanted Mouse Tumor. International Journal of Molecular Sciences. 2022; 23(24):15966. https://doi.org/10.3390/ijms232415966
Chicago/Turabian StyleYonekura, Yoshiharu, Hiroshi Toki, Tadashi Watabe, Kazuko Kaneda-Nakashima, Yoshifumi Shirakami, Kazuhiro Ooe, Atsushi Toyoshima, Hiroo Nakajima, Noriyuki Tomiyama, and Masako Bando. 2022. "Mathematical Model for Evaluation of Tumor Response in Targeted Radionuclide Therapy with 211At Using Implanted Mouse Tumor" International Journal of Molecular Sciences 23, no. 24: 15966. https://doi.org/10.3390/ijms232415966
APA StyleYonekura, Y., Toki, H., Watabe, T., Kaneda-Nakashima, K., Shirakami, Y., Ooe, K., Toyoshima, A., Nakajima, H., Tomiyama, N., & Bando, M. (2022). Mathematical Model for Evaluation of Tumor Response in Targeted Radionuclide Therapy with 211At Using Implanted Mouse Tumor. International Journal of Molecular Sciences, 23(24), 15966. https://doi.org/10.3390/ijms232415966







