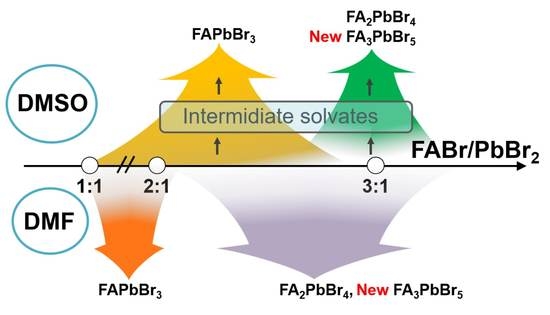
Crystallization Pathways of FABr-PbBr2-DMF and FABr-PbBr2-DMSO Systems: The Comprehensive Picture of Formamidinium-Based Low-Dimensional Perovskite-Related Phases and Intermediate Solvates
Abstract
1. Introduction
2. Results and Discussion
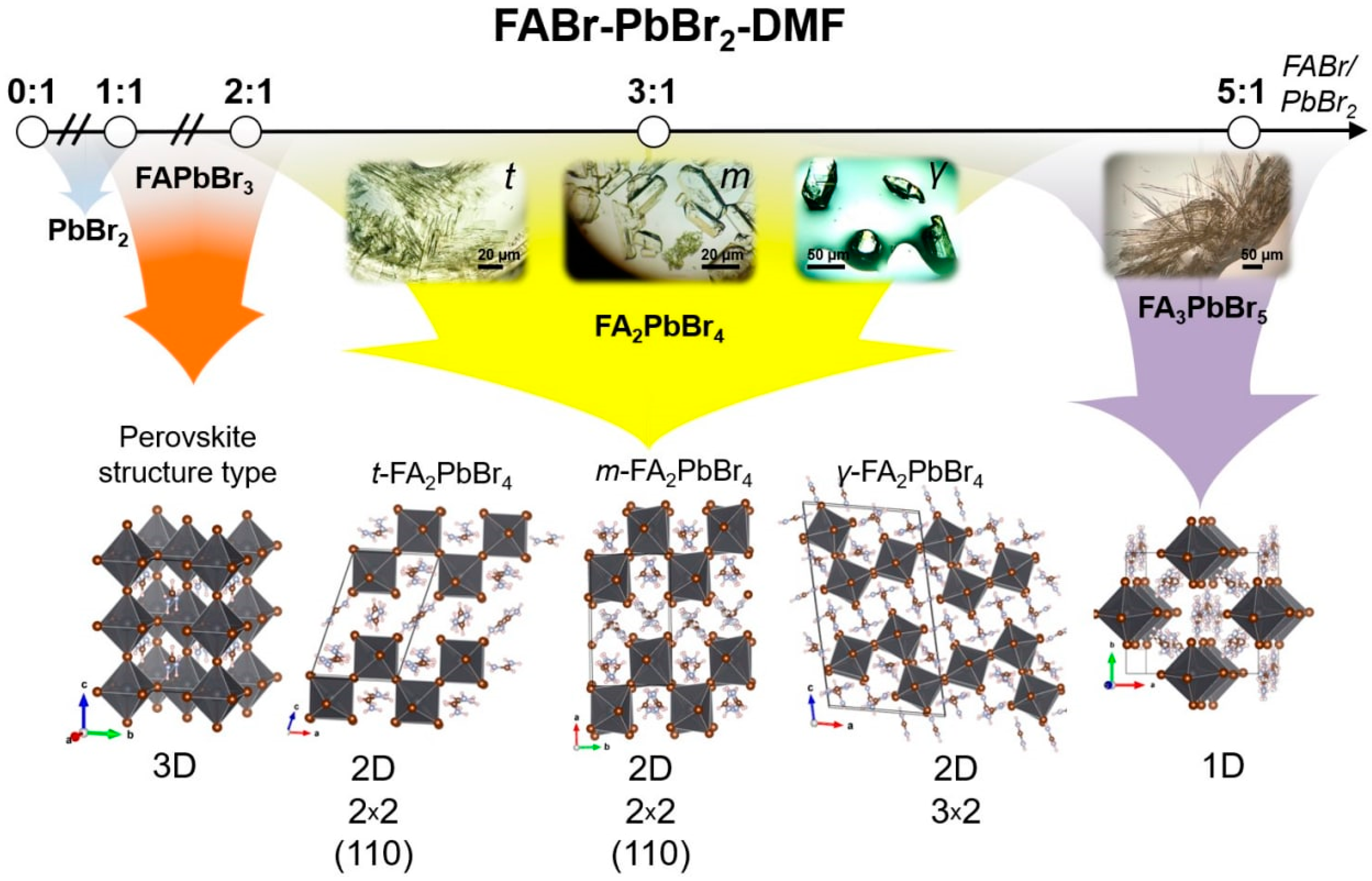
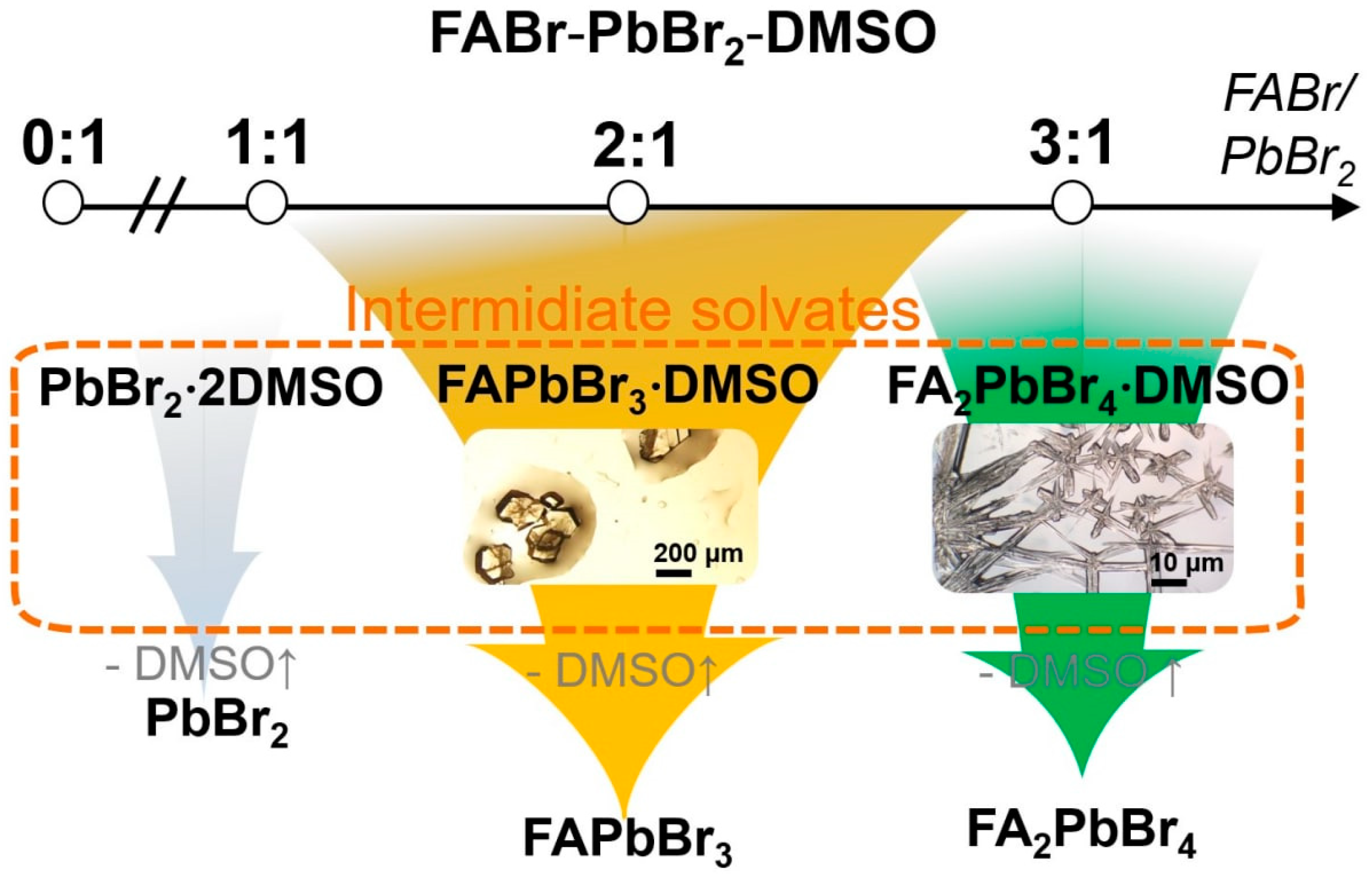
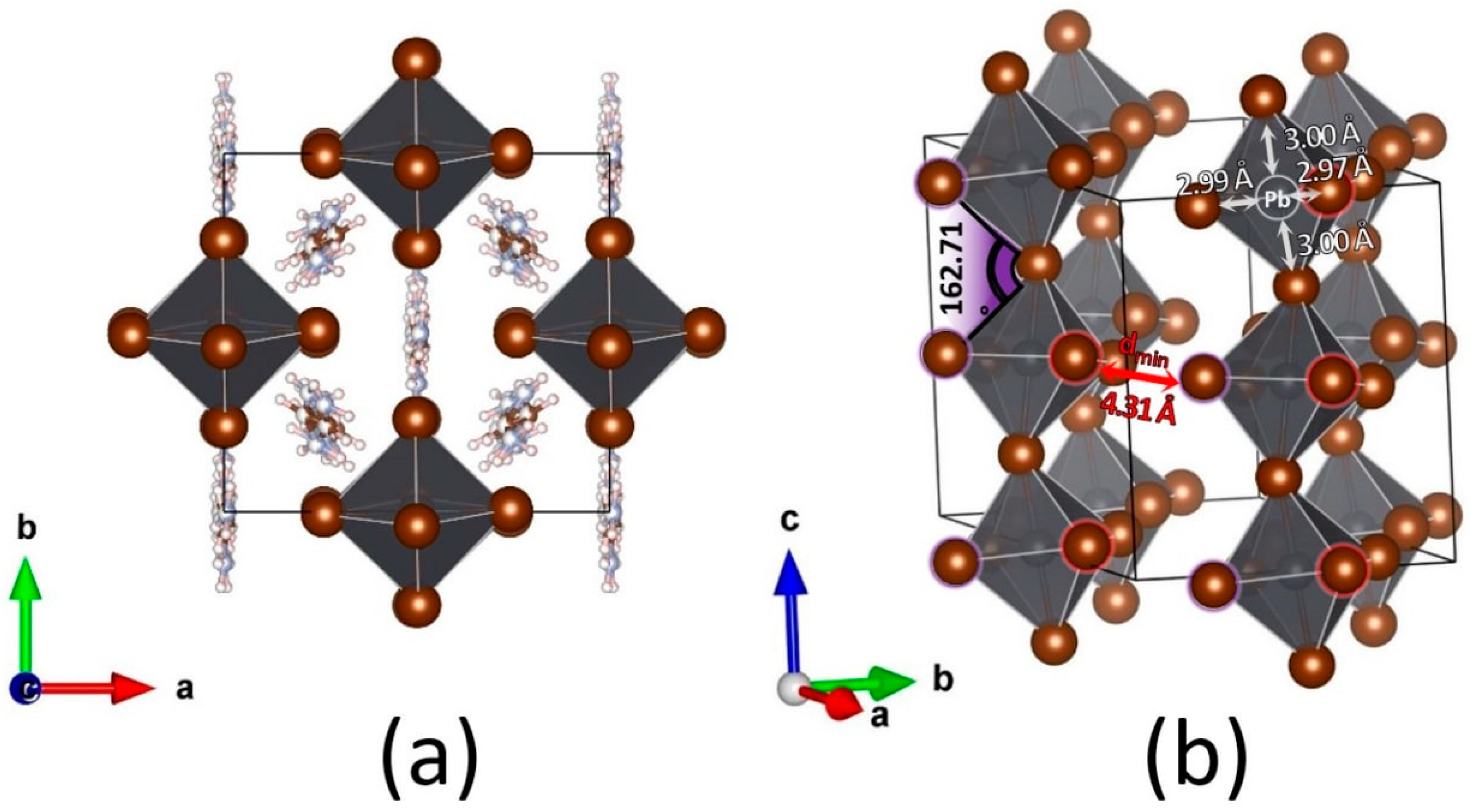
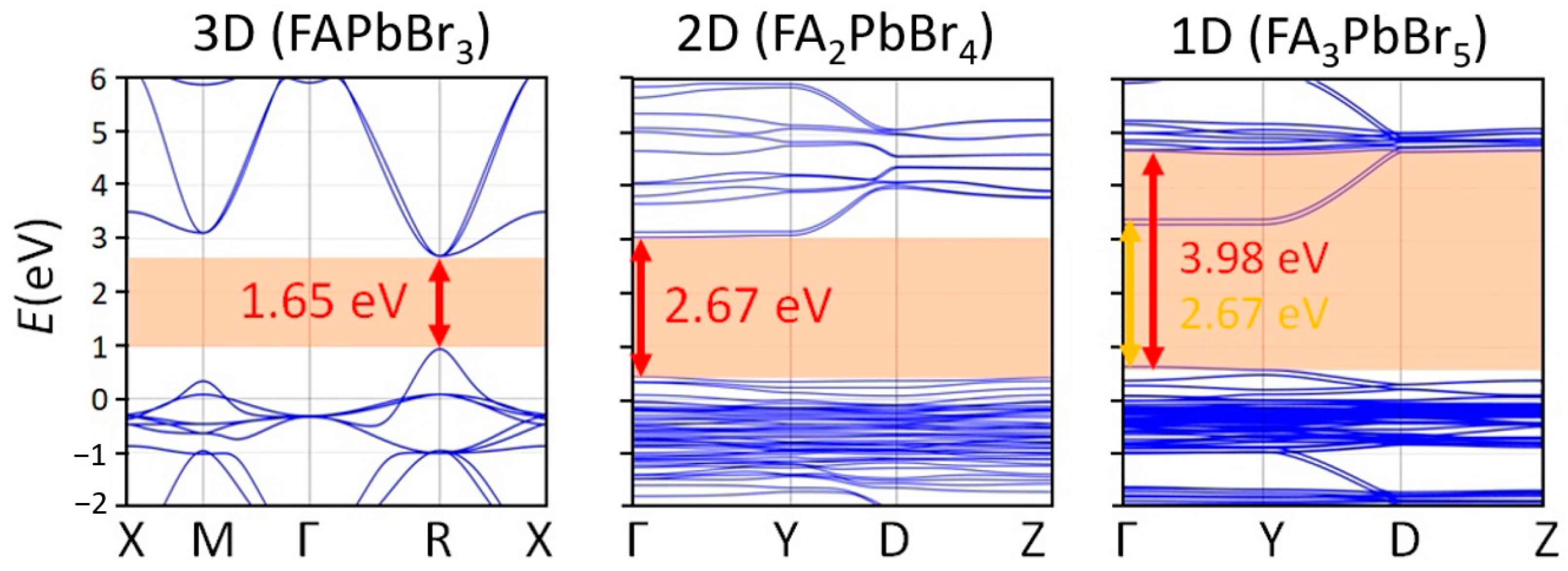
2.1. FABr-PbBr2-DMF/DMSO Systems: Equilibrium State
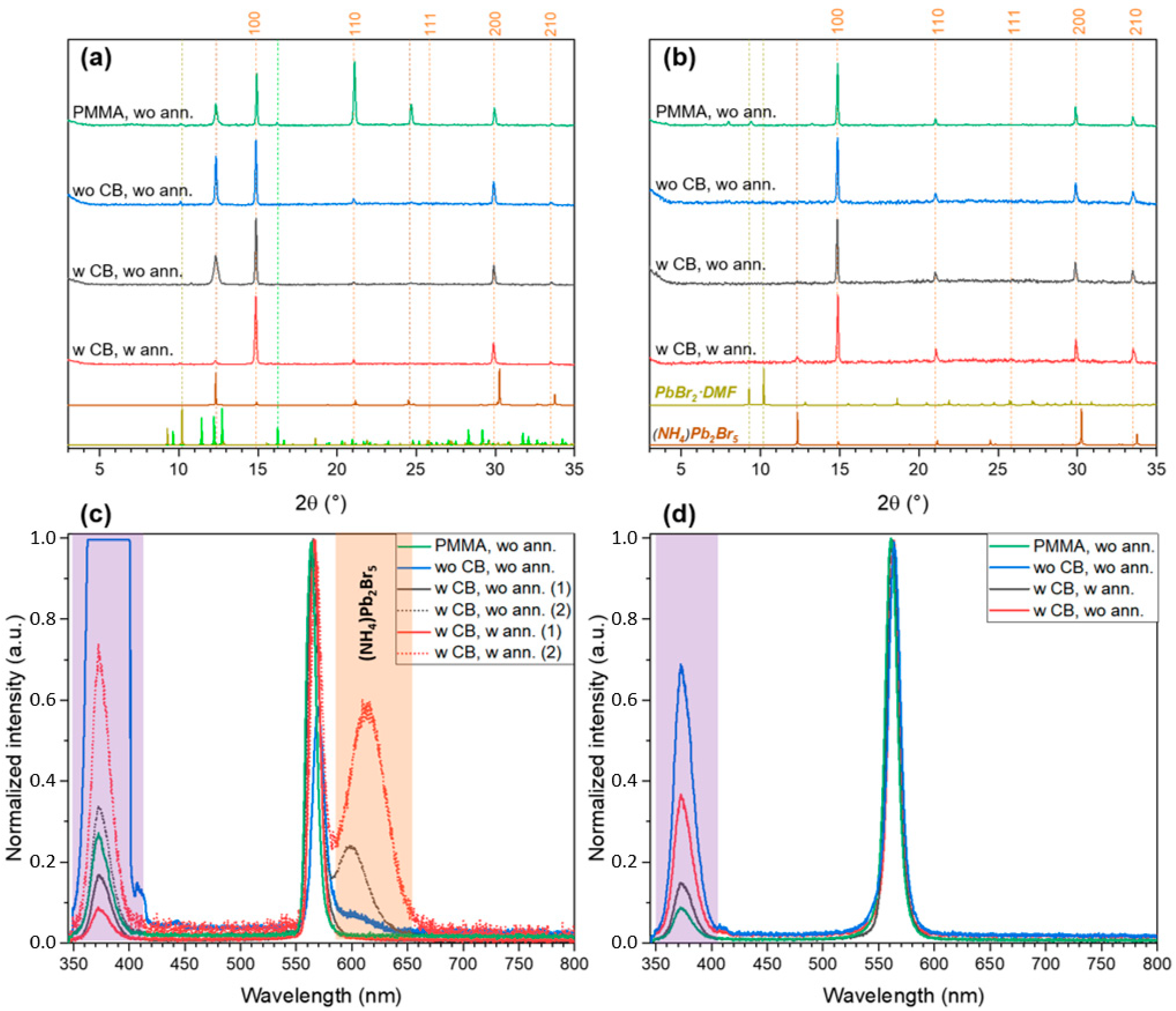
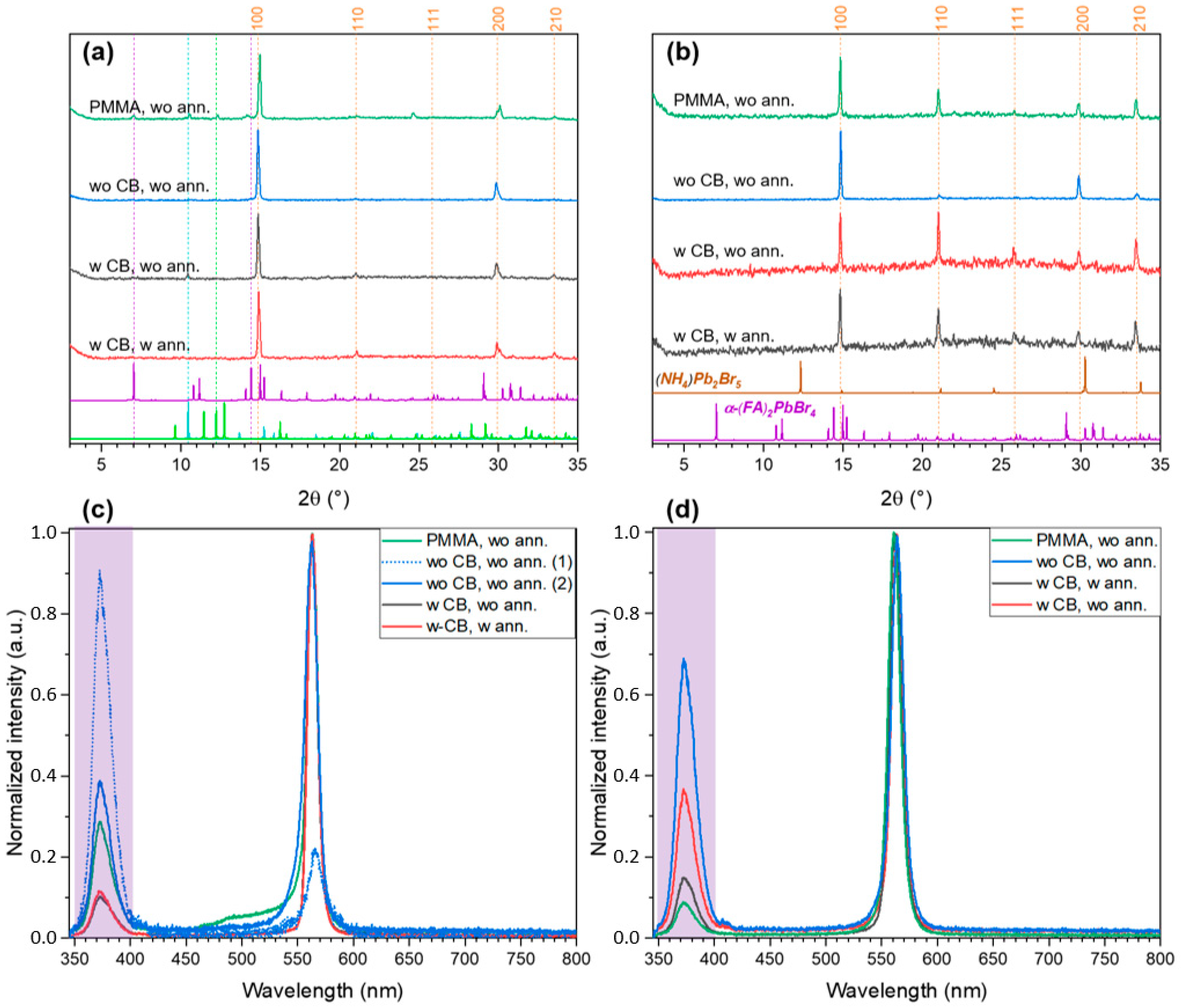
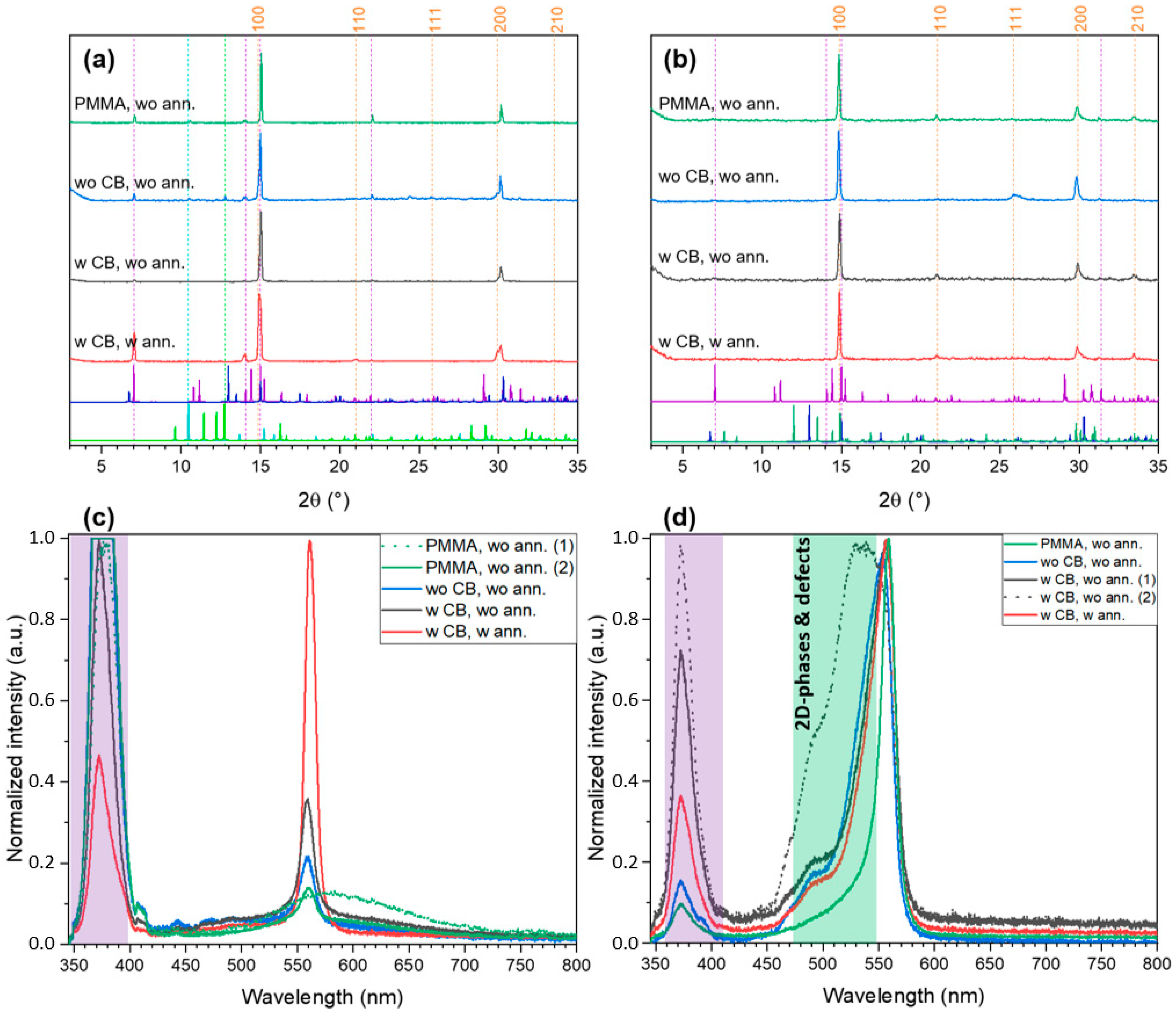
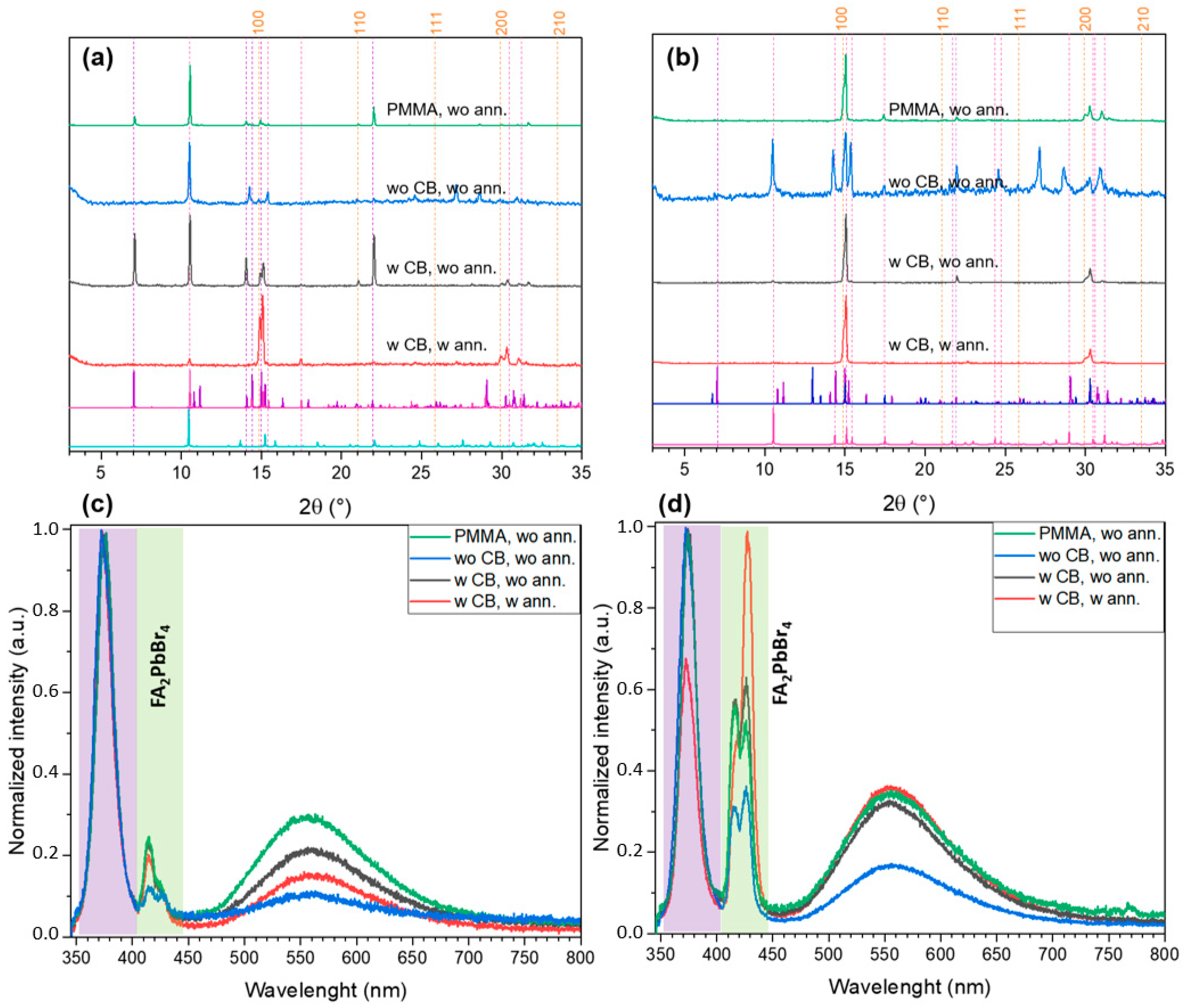
2.2. FABr-PbBr2-DMF/DMSO System: Thin Films
3. Materials and Methods
3.1. Materials
3.2. Single Crystal Growth
3.3. Single-Crystal X-ray Diffraction (XRD)
3.4. Powder X-ray Diffraction
3.5. Thin Films Preparation
3.6. Photoluminescence Measurements
3.7. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, Z.; Meng, W.; Wang, J.; Mitzi, D.B.; Yan, Y. Searching for promising new perovskite-based photovoltaic absorbers: The importance of electronic dimensionality. Mater. Horizons 2017, 4, 206–216. [Google Scholar] [CrossRef]
- Stranks, S.D.; Snaith, H.J. Metal-halide perovskites for photovoltaic and light-emitting devices. Nat. Nanotechnol. 2015, 10, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, X.; Deng, H.; Qiao, K.; Farooq, U.; Ishaq, M.; Yi, F. Low-Dimensional Halide Perovskites and Their Advanced Optoelectronic Applications. Nano-Micro Lett. 2017, 9, 2–26. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Liao, Y.; Wei, Q.; Wang, Z.; Xiang, B.; Ke, Y.; Liu, W.; Ning, Z. Highly stable hybrid perovskite light-emitting diodes based on Dion-Jacobson structure. Sci. Adv. 2019, 5, eaaw8072. [Google Scholar] [CrossRef] [PubMed]
- Quan, L.N.; García de Arquer, F.P.; Sabatini, R.P.; Sargent, E.H. Perovskites for Light Emission. Adv. Mater. 2018, 30, 1801996. [Google Scholar] [CrossRef]
- Kim, Y.H.; Cho, H.; Lee, T.W. Metal halide perovskite light emitters. Proc. Natl. Acad. Sci. USA 2016, 113, 11694–11702. [Google Scholar] [CrossRef]
- Tan, Z.-K.; Moghaddam, R.S.; Lai, M.L.; Docampo, P.; Higler, R.; Deschler, F.; Price, M.; Sadhanala, A.; Pazos, L.M.; Credgington, D.; et al. Bright light-emitting diodes based on organometal halide perovskite. Nat. Nanotechnol. 2014, 9, 687–692. [Google Scholar] [CrossRef]
- Wei, Z.; Xing, J. The Rise of Perovskite Light-Emitting Diodes. J. Phys. Chem. Lett. 2019, 10, 3035–3042. [Google Scholar] [CrossRef]
- Zou, S.J.; Shen, Y.; Xie, F.M.; Chen, J.D.; Li, Y.Q.; Tang, J.X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Kovalenko, M.V. Monodisperse Formamidinium Lead Bromide Nanocrystals with Bright and Stable Green Photoluminescence. J. Am. Chem. Soc. 2016, 138, 14202–14205. [Google Scholar] [CrossRef]
- Chin, S.H.; Choi, J.W.; Hu, Z.; Mardegan, L.; Sessolo, M.; Bolink, H.J. Tunable luminescent lead bromide complexes. J. Mater. Chem. C 2020, 8, 15996–16000. [Google Scholar] [CrossRef]
- Mączka, M.; Ptak, M.; Gągor, A.; Stefańska, D.; Zarȩba, J.K.; Sieradzki, A. Methylhydrazinium Lead Bromide: Noncentrosymmetric Three-Dimensional Perovskite with Exceptionally Large Framework Distortion and Green Photoluminescence. Chem. Mater. 2020, 32, 1667–1673. [Google Scholar] [CrossRef]
- Fang, H.; Deng, W.; Zhang, X.; Xu, X.; Zhang, M.; Jie, J.; Zhang, X. Few-layer formamidinium lead bromide nanoplatelets for ultrapure-green and high-efficiency light-emitting diodes. Nano Res. 2019, 12, 171–176. [Google Scholar] [CrossRef]
- Lin, K.; Xing, J.; Quan, L.N.; de Arquer, F.P.G.; Gong, X.; Lu, J.; Xie, L.; Zhao, W.; Zhang, D.; Yan, C.; et al. Perovskite light-emitting diodes with external quantum efficiency exceeding 20 per cent. Nature 2018, 562, 245–248. [Google Scholar] [CrossRef]
- Shen, Y.; Cheng, L.P.; Li, Y.Q.; Li, W.; Chen, J.D.; Lee, S.T.; Tang, J.X. High-Efficiency Perovskite Light-Emitting Diodes with Synergetic Outcoupling Enhancement. Adv. Mater. 2019, 31, 1901517. [Google Scholar] [CrossRef]
- Petrov, A.A.; Sokolova, I.P.; Belich, N.A.; Peters, G.S.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Khrustalev, V.N.; Petrov, A.V.; Grätzel, M.; Goodilin, E.A.; et al. Crystal Structure of DMF-Intermediate Phases Uncovers the Link Between CH3NH3PbI3 Morphology and Precursor Stoichiometry. J. Phys. Chem. C 2017, 121, 20739–20743. [Google Scholar] [CrossRef]
- Petrov, A.A.; Marchenko, E.I.; Fateev, S.A.; Yumao, L.; Goodilin, E.A.; Tarasov, A.B. Solvate phases crystallizing from hybrid halide perovskite solutions: Chemical classification and structural relations. Mendeleev Commun. 2022, 32, 311–314. [Google Scholar] [CrossRef]
- Petrov, A.A.; Fateev, S.A.; Khrustalev, V.N.; Li, Y.; Dorovatovskii, P.V.; Zubavichus, Y.V.; Goodilin, E.A.; Tarasov, A.B. Formamidinium Haloplumbate Intermediates: The Missing Link in a Chain of Hybrid Perovskites Crystallization. Chem. Mater. 2020, 32, 7739–7745. [Google Scholar] [CrossRef]
- Fateev, S.A.; Petrov, A.A.; Marchenko, E.I.; Zubavichus, Y.V.; Khrustalev, V.N.; Petrov, A.V.; Aksenov, S.M.; Goodilin, E.A.; Tarasov, A.B. FA2PbBr4: Synthesis, Structure, and Unusual Optical Properties of Two Polymorphs of Formamidinium-Based Layered (110) Hybrid Perovskite. Chem. Mater. 2021, 33, 1900–1907. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Elliott, C.; McNulty, J.A.; Cordes, D.B.; Slawin, A.M.Z.; Lightfoot, P. New Variants of (110)-Oriented Layered Lead Bromide Perovskites, Templated by Formamidinium or Pyrazolium. Eur. J. Inorg. Chem. 2021, 2021, 3404–3411. [Google Scholar] [CrossRef]
- Zhang, L.; Yuan, F.; Jiao, B.; Dong, H.; Li, J.; Wu, Z. Exploiting a Multiphase Pure Formamidinium Lead Perovskite for Efficient Green-Light-Emitting Diodes. ACS Appl. Mater. Interfaces 2021, 13, 23067–23073. [Google Scholar] [CrossRef]
- Minh, D.N.; Nguyen, L.A.T.; Trinh, C.T.; Oh, C.; Eom, S.; Vu, T.V.; Choi, J.; Sim, J.H.; Lee, K.G.; Kim, J.; et al. Low-Dimensional Single-Cation Formamidinium Lead Halide Perovskites (FAm+2PbmBr3m+2): From Synthesis to Rewritable Phase-Change Memory Film. Adv. Funct. Mater. 2021, 31, 2011093. [Google Scholar] [CrossRef]
- Kanwat, A.; Ghosh, B.; Ng, S.E.; Rana, P.J.S.; Lekina, Y.; Hooper, T.J.N.; Yantara, N.; Kovalev, M.; Chaudhary, B.; Kajal, P.; et al. Reversible Photochromism in ⟨110⟩ Oriented Layered Halide Perovskite. ACS Nano 2022, 16, 2942–2952. [Google Scholar] [CrossRef]
- Hu, C.; Shivarudraiah, S.B.; Sung, H.H.Y.; Williams, I.D.; Halpert, J.E.; Yang, S. Discovery of a New Intermediate Enables One-Step Deposition of High-Quality Perovskite Films via Solvent Engineering. Sol. RRL 2021, 5, 2000712. [Google Scholar] [CrossRef]
- Marchenko, E.I.; Korolev, V.V.; Mitrofanov, A.; Fateev, S.A.; Goodilin, E.A.; Tarasov, A.B. Layer shift factor in layered hybrid perovskites—New univocal quantitative descriptor of composition-structure-property relationships. Chem. Mater. 2021, 33, 1213–1217. [Google Scholar] [CrossRef]
- Guo, Y.-Y.; McNulty, J.A.; Mica, N.A.; Samuel, I.D.W.; Slawin, A.M.Z.; Bühl, M.; Lightfoot, P. Structure-directing effects in (110)-layered hybrid perovskites containing two distinct organic moieties. Chem. Commun. 2019, 55, 9935–9938. [Google Scholar] [CrossRef]
- Baranyi, A.D.; Onyszchuk, M.; Page, Y.L.; Donnay, G. The crystal and molecular structure of lead(II)bromide-bis-dimethylsulphoxide, PbBr2•2[(CH3)2SO]. Can. J. Chem. 1977, 55, 849–855. [Google Scholar] [CrossRef]
- Katan, C.; Mercier, N.; Even, J. Quantum and Dielectric Confinement Effects in Lower-Dimensional Hybrid Perovskite Semiconductors. Chem. Rev. 2019, 119, 3140–3192. [Google Scholar] [CrossRef]
- Marchenko, E.I.; Fateev, S.A.; Goodilin, E.A.; Tarasov, A.B. Band Gap and Topology of 1D Perovskite-Derived Hybrid Lead Halide Structures. Crystals 2022, 12, 657. [Google Scholar] [CrossRef]
- Villars, P.; Cenzual, K.; Daams, J.; Gladyshevskii, R.; Shcherban, O.; Dubenskyy, V.; Kuprysyuk, V.; Savysyuk, I.; Zaremba, R. Structure Types. Part 10: Space Groups (140) I4/mcm – (136) P42/mnm; Villars, P., Cenzual, K., Eds.; Landolt-Börnstein—Group III Condensed Matter; Springer: Berlin/Heidelberg, Germany, 2011; Volume 43A10, ISBN 978-3-642-19661-4. [Google Scholar]
- Liu, X.; Xu, M.; Hao, Y.; Fu, J.; Wang, F.; Zhang, B.; Bennett, S.; Sellin, P.; Jie, W.; Xu, Y. Solution-Grown Formamidinium Hybrid Perovskite (FAPbBr3) Single Crystals for α-Particle and γ-Ray Detection at Room Temperature. ACS Appl. Mater. Interfaces 2021, 13, 15383–15390. [Google Scholar] [CrossRef]
- Almatarneh, M.H.; Flinn, C.G.; Poirier, R.A. Ab initio study of the decomposition of formamidine. Can. J. Chem. 2005, 83, 2082–2090. [Google Scholar] [CrossRef]
- Pustovarov, V.A.; Ogorodnikov, I.N.; Bastrikova, N.S.; Smirnov, A.A.; Isaenko, L.I.; Eliseev, A.P. Low-temperature time-resolved spectroscopy of APb2X5 crystals (A ≡ K, Rb; X ≡ Cl, Br). Opt. Spectrosc. 2006, 101, 234–244. [Google Scholar] [CrossRef]
- Tutantsev, A.S.; Marchenko, E.I.; Udalova, N.N.; Fateev, S.A.; Goodilin, E.A.; Tarasov, A.B. Structural Disorder in Layered Hybrid Halide Perovskites: Types of Stacking Faults, Influence on Optical Properties and Their Suppression by Crystallization Engineering. Nanomaterials 2021, 11, 3333. [Google Scholar] [CrossRef]
- Nazarenko, O.; Kotyrba, M.R.; Yakunin, S.; Aebli, M.; Rainò, G.; Benin, B.M.; Wörle, M.; Kovalenko, M.V. Guanidinium-Formamidinium Lead Iodide: A Layered Perovskite-Related Compound with Red Luminescence at Room Temperature. J. Am. Chem. Soc. 2018, 140, 3850–3853. [Google Scholar] [CrossRef]
- Fateev, S.A.; Khrustalev, V.N.; Simonova, A.V.; Belikova, D.E.; Goodilin, E.A.; Tarasov, A.B. Acetamidinium-Methylammonium-Based Layered Hybrid Halide Perovskite [CH3C(NH2)2][CH3NH3]PbI4: Synthesis, Structure, and Optical Properties. Russ. J. Inorg. Chem. 2022, 67, 997–1003. [Google Scholar] [CrossRef]
- Soe, C.M.M.; Stoumpos, C.C.; Kepenekian, M.; Traoré, B.; Tsai, H.; Nie, W.; Wang, B.; Katan, C.; Seshadri, R.; Mohite, A.D.; et al. New Type of 2D Perovskites with Alternating Cations in the Interlayer Space, (C(NH2)3)(CH3NH3)nPbnI3n+1: Structure, Properties, and Photovoltaic Performance. J. Am. Chem. Soc. 2017, 139, 16297–16309. [Google Scholar] [CrossRef]
- Bruker, S. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2013. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D.J. SADABS 2016/2. J. Appl. Cryst. 2016, 48, 3–10. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Evans, P. Scaling and assessment of data quality. Acta Crystallogr. 2006, D62, 72–82. [Google Scholar] [CrossRef]
- Battye, T.G.G.; Kontogiannis, L.; Johnson, O.; Powell, H.R.; Leslie, A.G.W. iMOSFLM: A new graphical interface for diffraction-image processing with MOSFLM. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 271–281. [Google Scholar] [CrossRef]
- Winn, M.D.; Ballard, C.C.; Cowtan, K.D.; Dodson, E.J.; Emsley, P.; Evans, P.R.; Keegan, R.M.; Krissinel, E.B.; Leslie, A.G.W.; McCoy, A.; et al. Overview of the CCP 4 suite and current developments. Acta Crystallogr. Sect. D Biol. Crystallogr. 2011, 67, 235–242. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baroni, S.; Bonini, N.; Calandra, M.; Car, R.; Cavazzoni, C.; Ceresoli, D.; Chiarotti, G.L.; Cococcioni, M.; Dabo, I.; et al. QUANTUM ESPRESSO: A modular and open-source software project for quantum simulations of materials. J. Phys. Condens. Matter 2009, 21, 395502. [Google Scholar] [CrossRef]
- Giannozzi, P.; Baseggio, O.; Bonfà, P.; Brunato, D.; Car, R.; Carnimeo, I.; Cavazzoni, C.; De Gironcoli, S.; Delugas, P.; Ferrari Ruffino, F.; et al. Quantum ESPRESSO toward the exascale. J. Chem. Phys. 2020, 152, 154105. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Lin, J.S.; Qteish, A.; Payne, M.C.; Heine, V. Optimized and transferable nonlocal separable ab initio pseudopotentials. Phys. Rev. B 1993, 47, 4174–4180. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
| Phase | FA3PbBr5 |
|---|---|
| Appearance | needle-shaped crystals |
| Crystal system | monoclinic |
| Space group | C2/c |
| Unit cell parameters | a = 12.466(3) Å, b = 11.465(2) Å, c = 11.868(3) Å, α = 90°, β = 99.029(6)°, γ = 90° |
| Cell volume, Å3 | 1675.2(7) |
| Z | 4 |
| Density (calculated), g/cm3 | 2.943 |
| Reflections collected | 12281 |
| Independent reflections | 3040 |
| data/restraints/parameters | 3040/41/72 |
| goodness-of-fit | 1.013 |
| final R indices [I > 2σ(I)] | Robs = 0.0571, wRobs = 0.1347 |
| R indices [all data] | Rall = 0.0931, wRall = 0.1553 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fateev, S.A.; Marchenko, E.I.; Shatilova, A.S.; Khrustalev, V.N.; Goodilin, E.A.; Tarasov, A.B. Crystallization Pathways of FABr-PbBr2-DMF and FABr-PbBr2-DMSO Systems: The Comprehensive Picture of Formamidinium-Based Low-Dimensional Perovskite-Related Phases and Intermediate Solvates. Int. J. Mol. Sci. 2022, 23, 15344. https://doi.org/10.3390/ijms232315344
Fateev SA, Marchenko EI, Shatilova AS, Khrustalev VN, Goodilin EA, Tarasov AB. Crystallization Pathways of FABr-PbBr2-DMF and FABr-PbBr2-DMSO Systems: The Comprehensive Picture of Formamidinium-Based Low-Dimensional Perovskite-Related Phases and Intermediate Solvates. International Journal of Molecular Sciences. 2022; 23(23):15344. https://doi.org/10.3390/ijms232315344
Chicago/Turabian StyleFateev, Sergey A., Ekaterina I. Marchenko, Alexandra S. Shatilova, Victor N. Khrustalev, Eugene A. Goodilin, and Alexey B. Tarasov. 2022. "Crystallization Pathways of FABr-PbBr2-DMF and FABr-PbBr2-DMSO Systems: The Comprehensive Picture of Formamidinium-Based Low-Dimensional Perovskite-Related Phases and Intermediate Solvates" International Journal of Molecular Sciences 23, no. 23: 15344. https://doi.org/10.3390/ijms232315344
APA StyleFateev, S. A., Marchenko, E. I., Shatilova, A. S., Khrustalev, V. N., Goodilin, E. A., & Tarasov, A. B. (2022). Crystallization Pathways of FABr-PbBr2-DMF and FABr-PbBr2-DMSO Systems: The Comprehensive Picture of Formamidinium-Based Low-Dimensional Perovskite-Related Phases and Intermediate Solvates. International Journal of Molecular Sciences, 23(23), 15344. https://doi.org/10.3390/ijms232315344