Effects of Silver Nanoparticles on Physiological and Proteomic Responses of Tobacco (Nicotiana tabacum) Seedlings Are Coating-Dependent
Abstract
1. Introduction
2. Results
2.1. Synthesis, Characterization and Stability of AgNP-PVP and AgNP-CTAB in Nutrient Medium
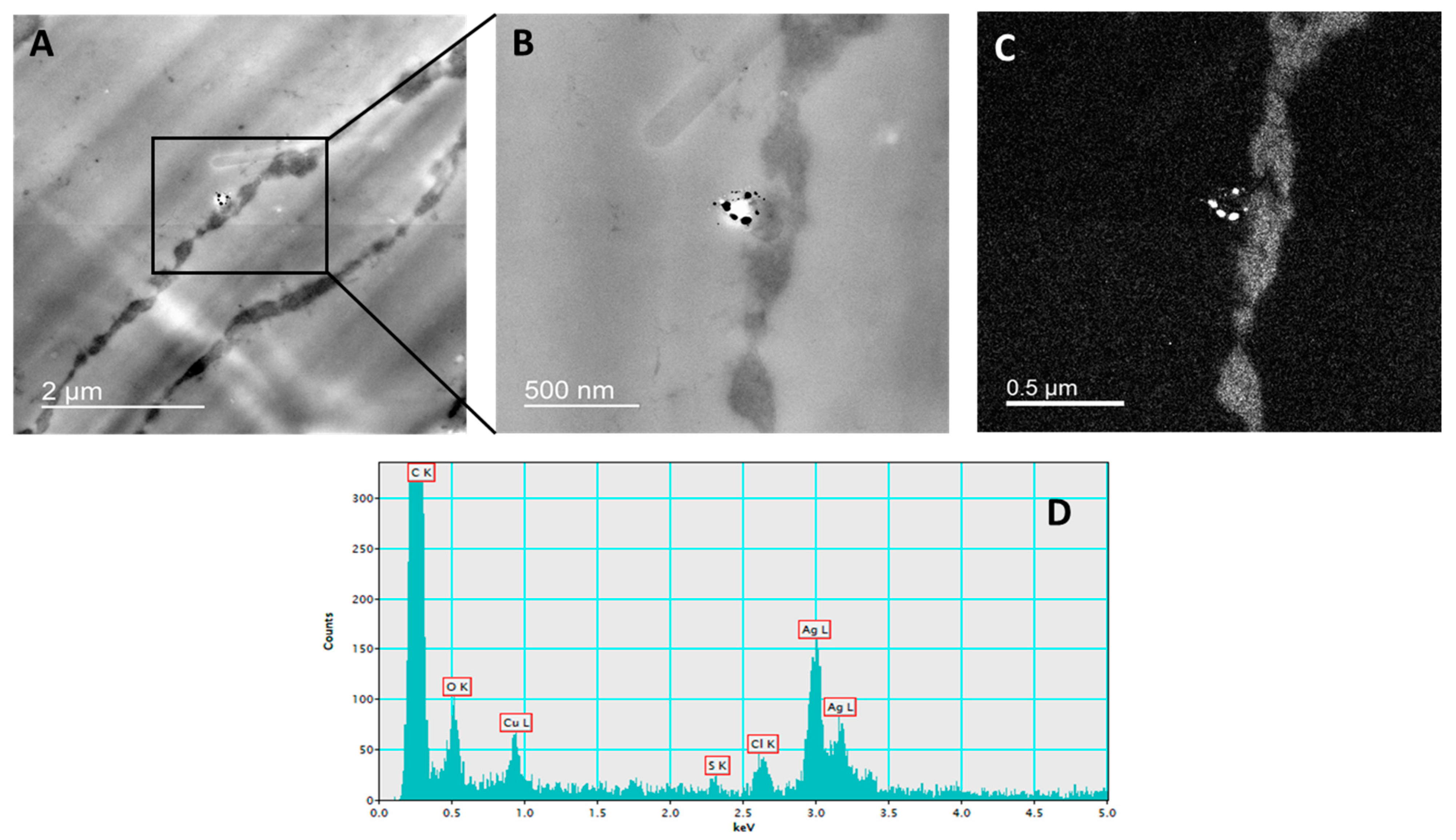
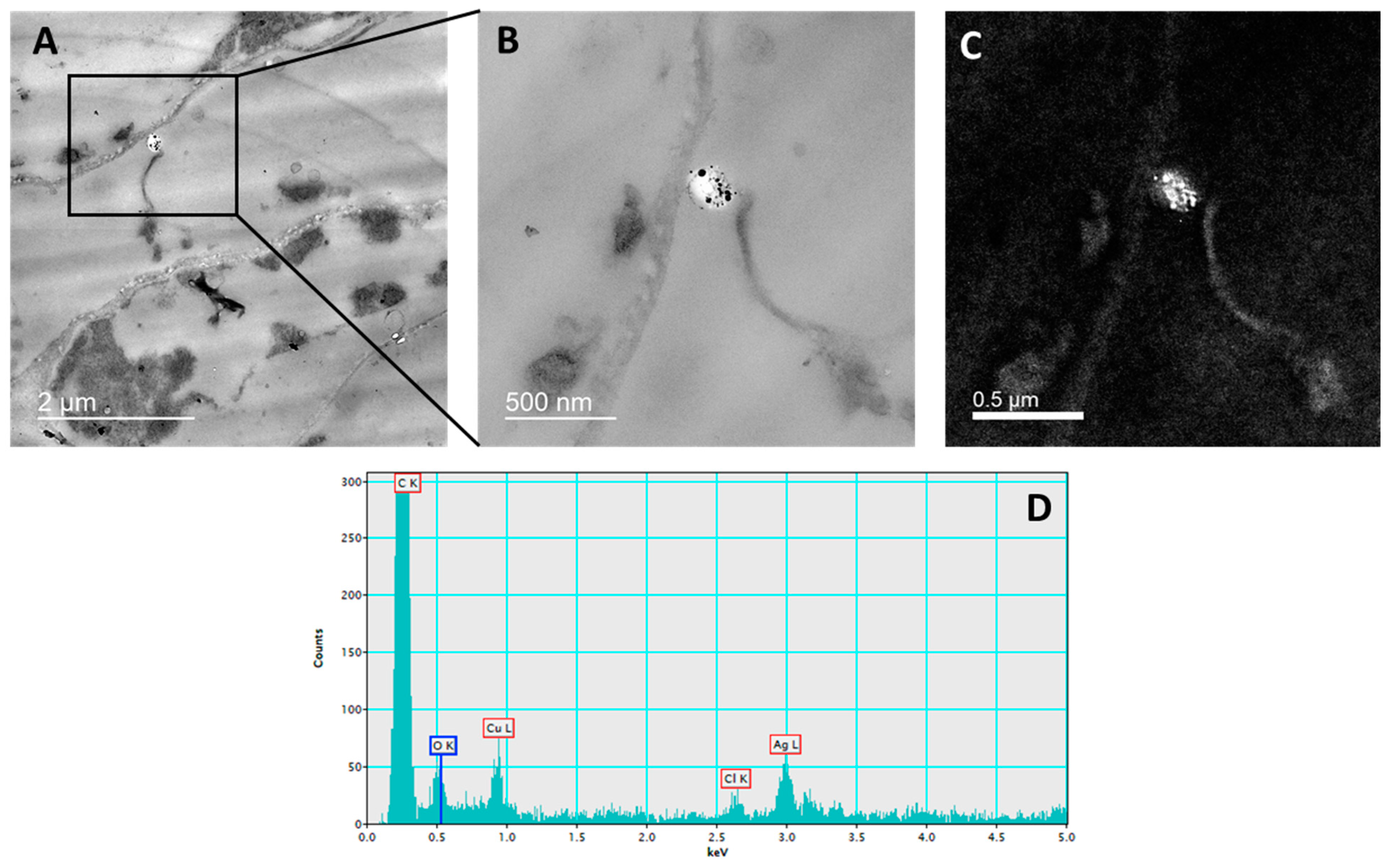
2.2. Silver Uptake and Localization
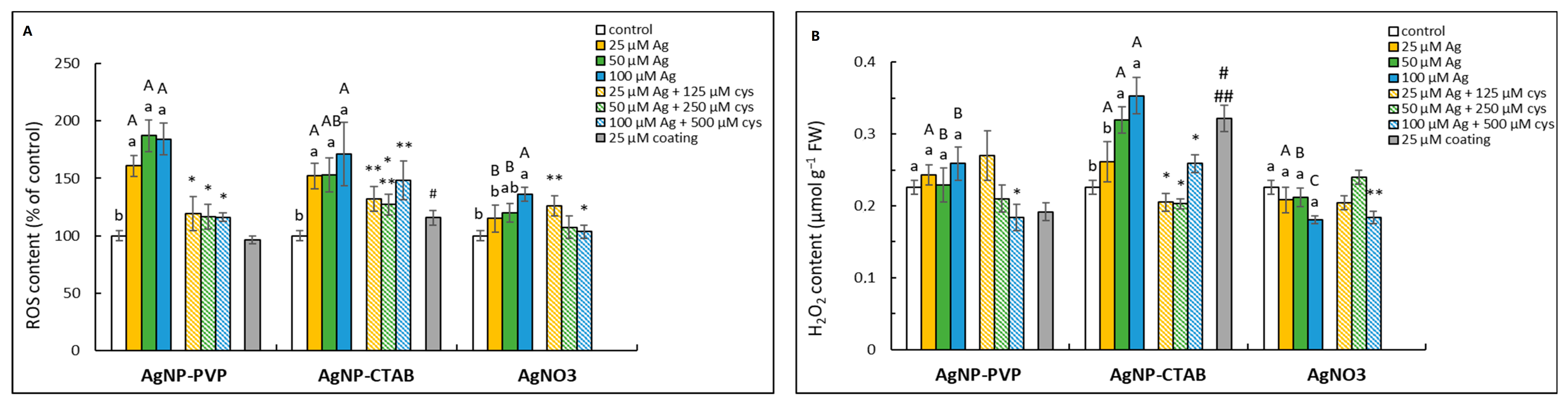
2.3. Induction of ROS Formation
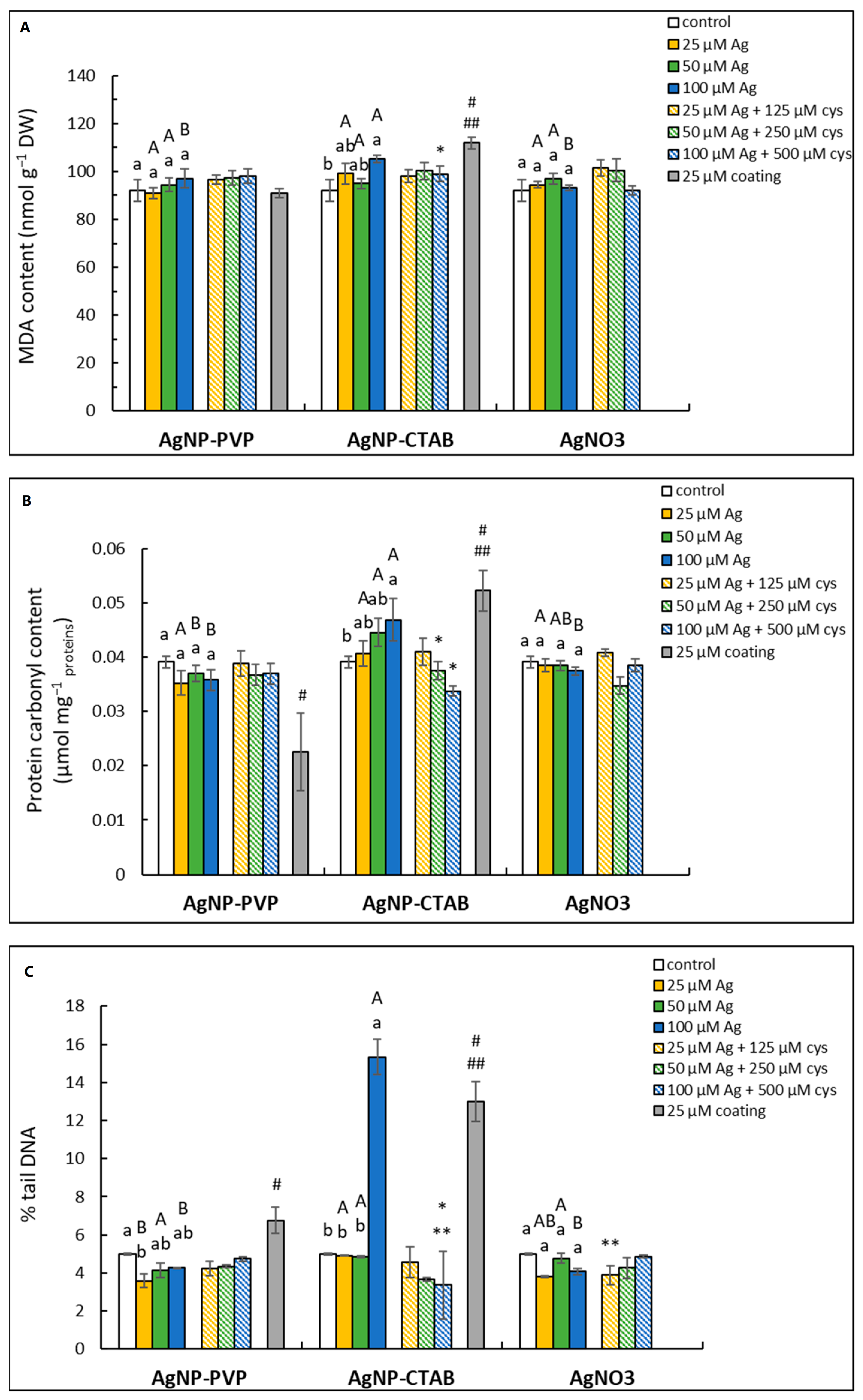
2.4. Oxidative Damage of Biomolecules
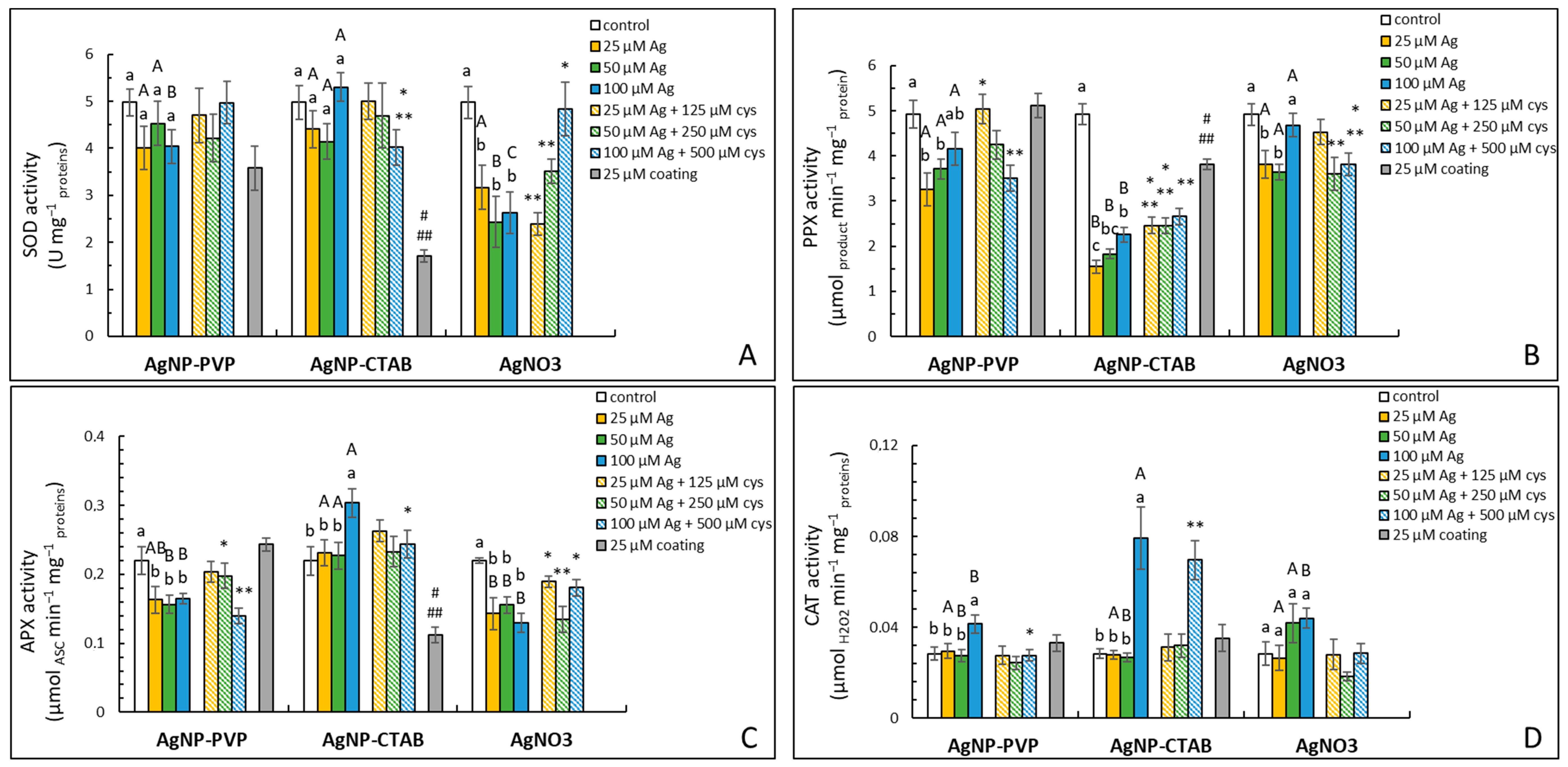
2.5. Activity of Antioxidative Enzymes
2.6. Nonenzymatic Antioxidants
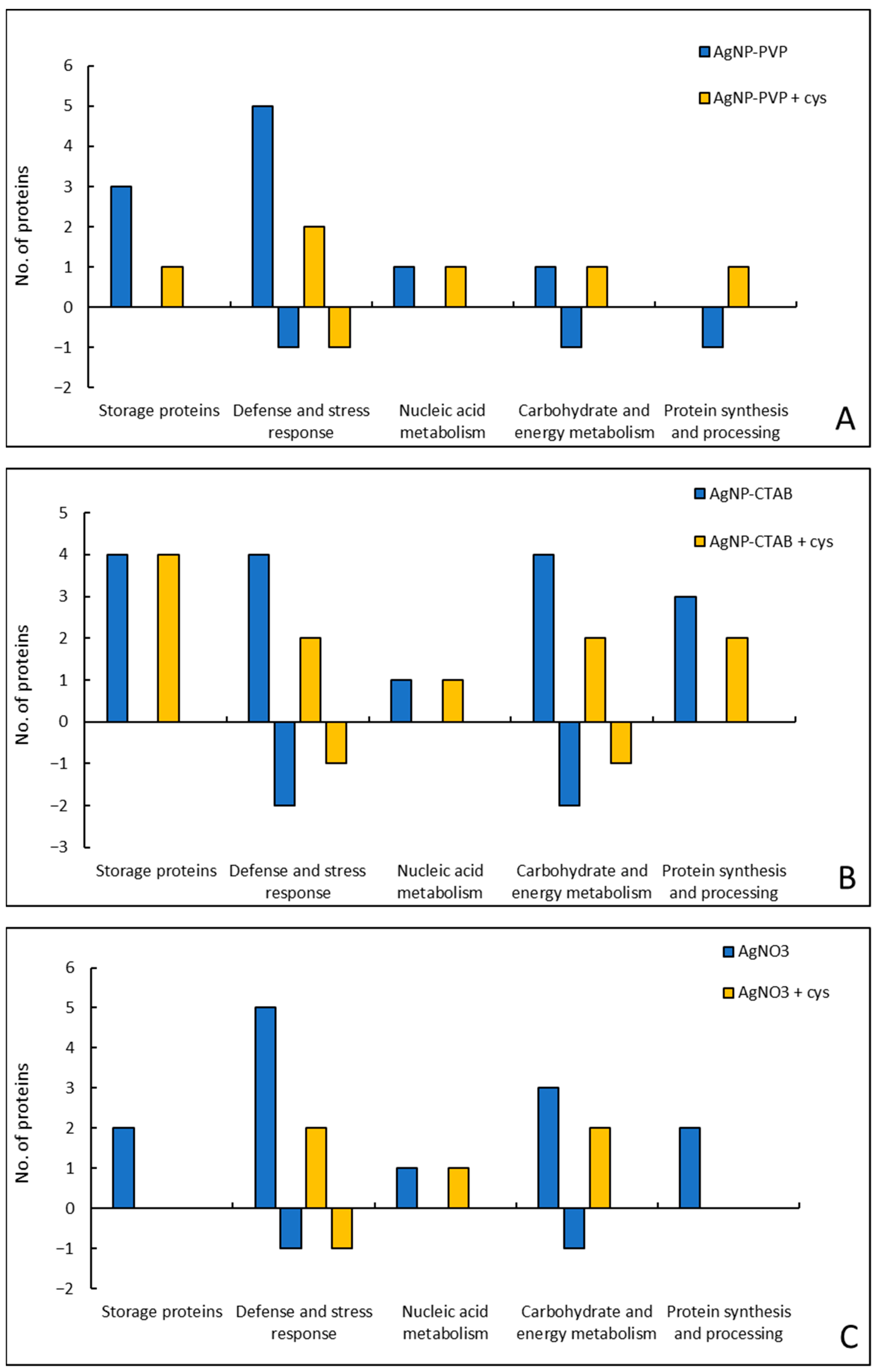
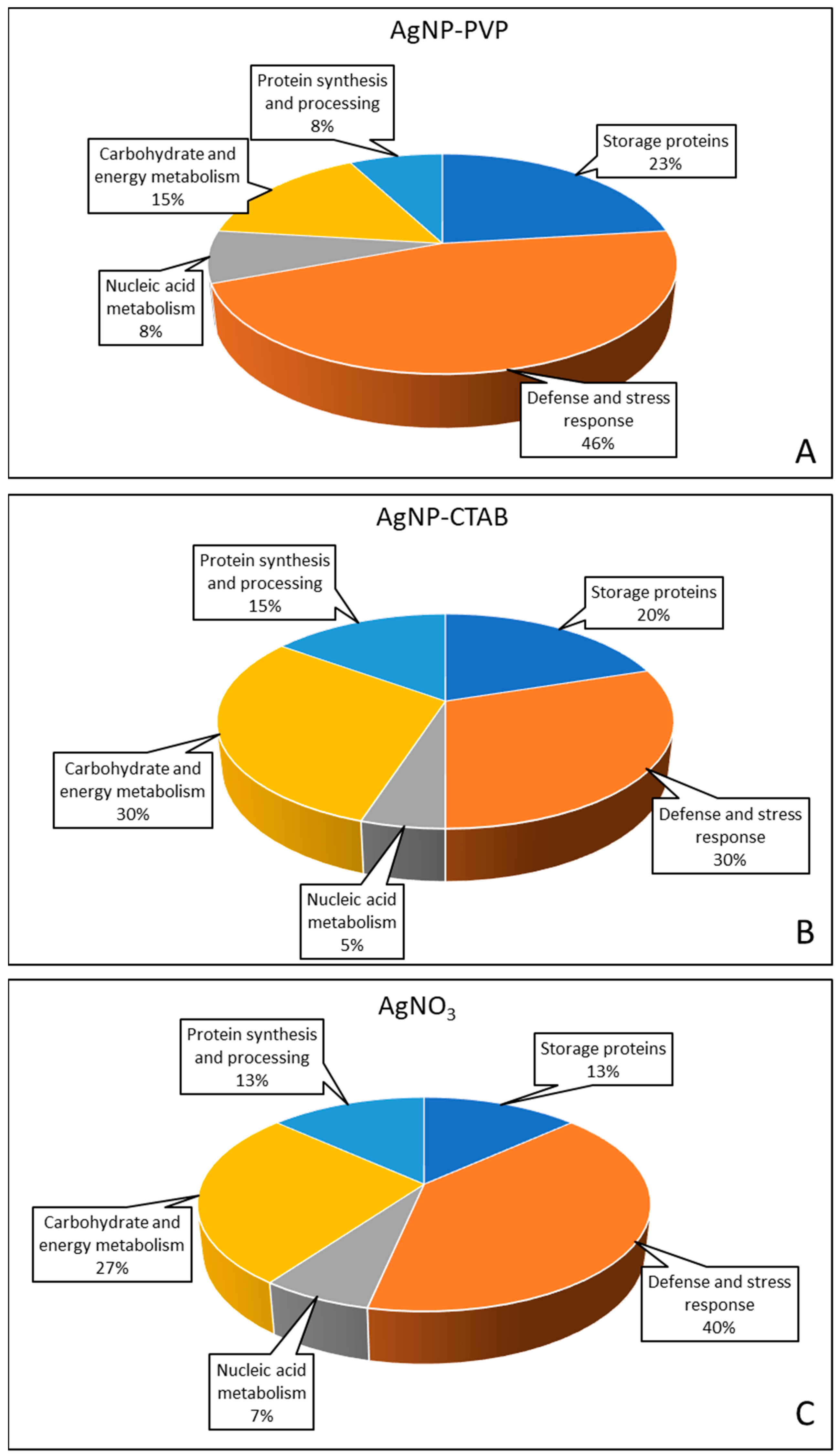
2.7. Changes in Proteome
3. Discussion
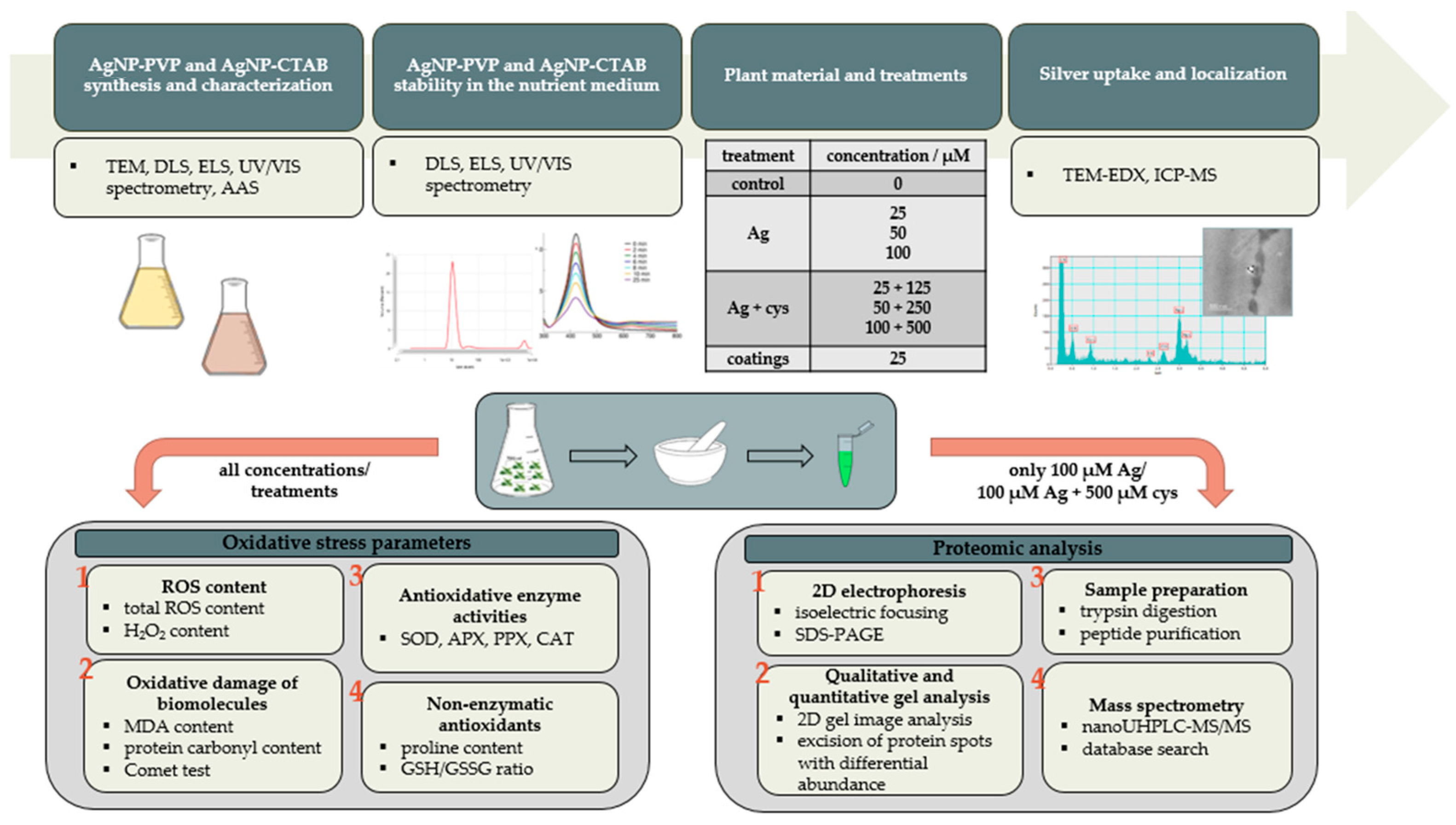
4. Materials and Methods
4.1. Materials
4.2. AgNP Synthesis and Characterization
4.3. Plant Material and Treatments
4.4. AgNP Stability in Liquid ½ MS Medium
4.5. Silver Uptake and Localization
4.6. Protein Extraction
4.7. ROS Content
4.8. MDA and Protein Carbonyl Content
4.9. Comet Assay
4.10. Antioxidative Enzyme Activities
4.11. Non-Enzymatic Antioxidants
4.12. Proteomic Analysis
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alotaibi, A.M.; Alsaleh, N.B.; Aljasham, A.T.; Tawfik, E.A.; Almutairi, M.M.; Assiri, M.A.; Alkholief, M.; Almutairi, M.M. Silver Nanoparticle-Based Combinations with Antimicrobial Agents against Antimicrobial-Resistant Clinical Isolates. Antibiotics 2022, 11, 1219. [Google Scholar] [CrossRef]
- Cosert, K.M.; Kim, S.; Jalilian, I.; Chang, M.; Gates, B.L.; Pinkerton, K.E.; Van Winkle, L.S.; Raghunathan, V.K.; Leonard, B.C.; Thomasy, S.M. Metallic Engineered Nanomaterials and Ocular Toxicity: A Current Perspective. Pharmaceutics 2022, 14, 981. [Google Scholar] [CrossRef] [PubMed]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.; Martínez, M.C.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms as an overview of its hazard for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Goshisht, M.K. Recent Advances and Mechanistic Insights into Antibacterial Activity, Antibiofilm Activity, and Cytotoxicity of Silver Nanoparticles. ACS Appl. Bio Mater. 2022, 5, 1391–1463. [Google Scholar] [CrossRef]
- Javaid, A.; Oloketuyi, S.F.; Khan, M.M.; Khan, F. Diversity of Bacterial Synthesis of Silver Nanoparticles. Bionanoscience 2018, 8, 43–59. [Google Scholar] [CrossRef]
- Feizi, S.; Cooksley, C.M.; Nepal, R.; Psaltis, A.J.; Wormald, P.-J.; Vreugde, S. Silver nanoparticles as a bioadjuvant of antibiotics against biofilm-mediated infections with methicillin-resistant Staphylococcus aureus and Pseudomonas aeruginosa in chronic rhinosinusitis patients. Pathology 2021, 54, 453–459. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Deng, L.; Qian, G.; Wang, Z.; Li, W.; Zhong, C. The antifungal activity and mechanism of silver nanoparticles against four pathogens causing kiwifruit post-harvest rot. Front. Microbiol. 2022, 13, 1–16. [Google Scholar] [CrossRef]
- Mussin, J.; Giusiano, G. Biogenic silver nanoparticles as antifungal agents. Front. Chem. 2022, 10, 1023542. [Google Scholar] [CrossRef]
- Ayipo, Y.O.; Bakare, A.A.; Badeggi, U.M.; Jimoh, A.A.; Lawal, A.; Mordi, M.N. Recent advances on therapeutic potentials of gold and silver nanobiomaterials for human viral diseases. Curr. Res. Chem. Biol. 2022, 2, 100021. [Google Scholar] [CrossRef]
- He, Q.; Lu, J.; Liu, N.; Lu, W.; Li, Y.; Shang, C.; Li, X.; Hu, L.; Jiang, G. Antiviral Properties of Silver Nanoparticles against SARS-CoV-2: Effects of Surface Coating and Particle Size. Nanomaterials 2022, 12, 990. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yin, L.; Li, W.; Jiang, H.-S. Low Concentrations of Silver Nanoparticles Inhibit Spore Germination and Disturb Gender Differentiation of Ceratopteris thalictroides (L.) Brongn. Nanomaterials 2022, 12, 1730. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Martínez, G.; Merinero, M.; Pérez-Aranda, M.; Pérez-Soriano, E.M.; Ortiz, T.; Begines, B.; Alcudia, A. Environmental Impact of Nanoparticles’ Application as an Emerging Technology: A Review. Materials 2020, 14, 166. [Google Scholar] [CrossRef]
- Yu, S.; Yin, Y.; Liu, J. Silver nanoparticles in the environment. Environ. Sci. Process. Impacts 2013, 15, 78–92. [Google Scholar] [CrossRef]
- Sharma, V.K.; Siskova, K.M.; Zboril, R.; Gardea-Torresdey, J.L. Organic-coated silver nanoparticles in biological and environmental conditions: Fate, stability and toxicity. Adv. Colloid Interface Sci. 2014, 204, 15–34. [Google Scholar] [CrossRef]
- Biba, R.; Košpić, K.; Komazec, B.; Markulin, D.; Cvjetko, P.; Pavoković, D.; Peharec Štefanić, P.; Tkalec, M.; Balen, B. Surface Coating-Modulated Phytotoxic Responses of Silver Nanoparticles in Plants and Freshwater Green Algae. Nanomaterials 2022, 12, 24. [Google Scholar] [CrossRef]
- Lahuta, L.B.; Szablińska-Piernik, J.; Stałanowska, K.; Głowacka, K.; Horbowicz, M. The Size-Dependent Effects of Silver Nanoparticles on Germination, Early Seedling Development and Polar Metabolite Profile of Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2022, 23, 13255. [Google Scholar] [CrossRef]
- Reidy, B.; Haase, A.; Luch, A.; Dawson, K.A.; Lynch, I. Mechanisms of Silver Nanoparticle Release, Transformation and Toxicity: A Critical Review of Current Knowledge and Recommendations for Future Studies and Applications. Materials 2013, 6, 2295–2350. [Google Scholar] [CrossRef]
- Liang, L.; Tang, H.; Deng, Z.; Liu, Y.; Chen, X.; Wang, H. Ag nanoparticles inhibit the growth of the bryophyte, Physcomitrella patens. Ecotoxicol. Environ. Saf. 2018, 164, 739–748. [Google Scholar] [CrossRef]
- Barabanov, P.V.; Gerasimov, A.V.; Blinov, A.V.; Kravtsov, A.A.; Kravtsov, V.A. Influence of nanosilver on the efficiency of Pisum sativum crops germination. Ecotoxicol. Environ. Saf. 2018, 147, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Biba, R.; Matić, D.; Lyons, D.M.; Peharec Štefanić, P.; Cvjetko, P.; Tkalec, M.; Pavoković, D.; Letofsky-Papst, I.; Balen, B. Coating-Dependent Effects of Silver Nanoparticles on Tobacco Seed Germination and Early Growth. Int. J. Mol. Sci. 2020, 21, 3441. [Google Scholar] [CrossRef] [PubMed]
- Peharec Štefanić, P.; Košpić, K.; Lyons, D.; Jurković, L.; Balen, B.; Tkalec, M. Phytotoxicity of Silver Nanoparticles on Tobacco Plants: Evaluation of Coating Effects on Photosynthetic Performance and Chloroplast Ultrastructure. Nanomaterials 2021, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Košpić, K.; Biba, R.; Štefanić, P.P.; Cvjetko, P.; Tkalec, M.; Balen, B. Silver Nanoparticle Effects on Antioxidant Response in Tobacco Are Modulated by Surface Coating. Plants 2022, 11, 2402. [Google Scholar] [CrossRef]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J.J. Phytostimulation of Poplars and Arabidopsis Exposed to Silver Nanoparticles and Ag+ at Sublethal Concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; Prinsi, B.; Marsoni, M.; Espen, L.; Bracale, M. Morphological and Proteomic Responses of Eruca sativa Exposed to Silver Nanoparticles or Silver Nitrate. PLoS ONE 2013, 8, e68752. [Google Scholar] [CrossRef]
- Biba, R.; Peharec Štefanić, P.; Cvjetko, P.; Tkalec, M.; Balen, B. Silver nanoparticles phytotoxicity mechanisms. In Silver Nanomaterials for Agri-Food Applications; Kamel, A.A.-E., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 317–356. ISBN 9780128235287. [Google Scholar]
- Belava, V.N.; Panyuta, O.O.; Yakovleva, G.M.; Pysmenna, Y.M.; Volkogon, M.V. The Effect of Silver and Copper Nanoparticles on the Wheat-Pseudocercosporella herpotrichoides Pathosystem. Nanoscale Res. Lett. 2017, 12, 250. [Google Scholar] [CrossRef]
- Saha, N.; Dutta Gupta, S. Low-dose toxicity of biogenic silver nanoparticles fabricated by Swertia chirata on root tips and flower buds of Allium cepa. J. Hazard. Mater. 2017, 330, 18–28. [Google Scholar] [CrossRef]
- Flores-López, L.Z.; Espinoza-Gómez, H.; Somanathan, R. Silver nanoparticles: Electron transfer, reactive oxygen species, oxidative stress, beneficial and toxicological effects. Mini review. J. Appl. Toxicol. 2019, 39, 16–26. [Google Scholar] [CrossRef]
- He, W.; Zhou, Y.-T.; Wamer, W.G.; Boudreau, M.D.; Yin, J.-J. Mechanisms of the pH dependent generation of hydroxyl radicals and oxygen induced by Ag nanoparticles. Biomaterials 2012, 33, 7547–7555. [Google Scholar] [CrossRef]
- Wang, B.; Yin, J.-J.; Zhou, X.; Kurash, I.; Chai, Z.; Zhao, Y.; Feng, W. Physicochemical Origin for Free Radical Generation of Iron Oxide Nanoparticles in Biomicroenvironment: Catalytic Activities Mediated by Surface Chemical States. J. Phys. Chem. C 2012, 117, 383–392. [Google Scholar] [CrossRef]
- Yu, Z.; Li, Q.; Wang, J.; Yu, Y.; Wang, Y.; Zhou, Q.; Li, P. Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field. Nanoscale Res. Lett. 2020, 15, 115. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Sun, S.; Scheckel, K.G.; Malysheva, A.; McKenna, B.A.; Menzies, N.W.; Zhao, F.J.; Kopittke, P.M. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. Nano 2017, 4, 448–460. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.S.; Yin, L.Y.; Ren, N.N.; Zhao, S.T.; Li, Z.; Zhi, Y.; Shao, H.; Li, W.; Gontero, B. Silver nanoparticles induced reactive oxygen species via photosynthetic energy transport imbalance in an aquatic plant. Nanotoxicology 2017, 11, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Tkalec, M.; Peharec Štefanić, P.; Balen, B. Phytotoxicity of silver nanoparticles and defence mechanisms. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2019; Volume 84, pp. 145–198. [Google Scholar] [CrossRef]
- Gondikas, A.P.; Morris, A.; Reinsch, B.C.; Marinakos, S.M.; Lowry, G.V.; Hsu-Kim, H. Cysteine-Induced Modifications of Zero-valent Silver Nanomaterials: Implications for Particle Surface Chemistry, Aggregation, Dissolution, and Silver Speciation. Environ. Sci. Technol. 2012, 46, 7037–7045. [Google Scholar] [CrossRef]
- Cvjetko, P.; Milošić, A.; Domijan, A.-M.; Vinković Vrček, I.; Tolić, S.; Peharec Štefanić, P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef]
- Jiang, H.-S.; Qiu, X.-N.; Li, G.-B.; Li, W.; Yin, L.-Y. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ. Toxicol. Chem. 2014, 33, 1398–1405. [Google Scholar] [CrossRef]
- Yin, L.; Colman, B.P.; McGill, B.M.; Wright, J.P.; Bernhardt, E.S. Effects of Silver Nanoparticle Exposure on Germination and Early Growth of Eleven Wetland Plants. PLoS ONE 2012, 7, e47674. [Google Scholar] [CrossRef]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of Metal and Metal Oxide Nanoparticles on Plant: A Critical Review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef]
- Anna, B.; Barbara, K.; Magdalena, O. How the surface properties affect the nanocytotoxicity of silver? Study of the influence of three types of nanosilver on two wheat varieties. Acta Physiol. Plant. 2018, 40, 31. [Google Scholar] [CrossRef]
- Akter, M.; Sikder, M.T.; Rahman, M.M.; Ullah, A.K.M.A.; Hossain, K.F.B.; Banik, S.; Hosokawa, T.; Saito, T.; Kurasaki, M. A systematic review on silver nanoparticles-induced cytotoxicity: Physicochemical properties and perspectives. J. Adv. Res. 2018, 9, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tolaymat, T.M.; El Badawy, A.M.; Genaidy, A.; Scheckel, K.G.; Luxton, T.P.; Suidan, M. An evidence-based environmental perspective of manufactured silver nanoparticle in syntheses and applications: A systematic review and critical appraisal of peer-reviewed scientific papers. Sci. Total. Environ. 2010, 408, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Matras, E.; Gorczyca, A.; Pociecha, E.; Przemieniecki, S.W.; Oćwieja, M. Phytotoxicity of Silver Nanoparticles with Different Surface Properties on Monocots and Dicots Model Plants. J. Soil Sci. Plant Nutr. 2022, 22, 1647–1664. [Google Scholar] [CrossRef]
- Deonarine, A.; Hsu-Kim, H. Precipitation of Mercuric Sulfide Nanoparticles in NOM-Containing Water: Implications for the Natural Environment. Environ. Sci. Technol. 2009, 43, 2368–2373. [Google Scholar] [CrossRef]
- Lubick, N. Nanosilver toxicity: Ions, nanoparticles—Or both? Environ. Sci. Technol. 2008, 42, 8617. [Google Scholar] [CrossRef] [PubMed]
- del Real, A.E.P.; Vidal, V.; Carrière, M.; Castillo-Michel, H.; Levard, C.; Chaurand, P.; Sarret, G. Silver Nanoparticles and Wheat Roots: A Complex Interplay. Environ. Sci. Technol. 2017, 51, 5774–5782. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-Q.; Qing, T.; Li, C.-C.; Li, F.; Ge, F.; Fei, J.-J.; Peijnenburg, W.J.G.M. Integration of subcellular partitioning and chemical forms to understand silver nanoparticles toxicity to lettuce (Lactuca sativa L.) under different exposure pathways. Chemosphere 2020, 258, 127349. [Google Scholar] [CrossRef]
- Rajeshwari, A.; Roy, B.; Chandrasekaran, N.; Mukherjee, A. Cytogenetic evaluation of gold nanorods using Allium cepa test. Plant Physiol. Biochem. 2016, 109, 209–219. [Google Scholar] [CrossRef]
- Peharec Štefanić, P.; Cvjetko, P.; Biba, R.; Domijan, A.-M.; Letofsky-Papst, I.; Tkalec, M.; Šikić, S.; Cindrić, M.; Balen, B. Physiological, ultrastructural and proteomic responses of tobacco seedlings exposed to silver nanoparticles and silver nitrate. Chemosphere 2018, 209, 640–653. [Google Scholar] [CrossRef]
- Tkalec, M.; Peharec Štefanić, P.; Cvjetko, P.; Šikić, S.; Pavlica, M.; Balen, B. The Effects of Cadmium-Zinc Interactions on Biochemical Responses in Tobacco Seedlings and Adult Plants. PLoS ONE 2014, 9, e87582. [Google Scholar] [CrossRef]
- Majsec, K.; Cvjetko, P.; Tolić, S.; Tkalec, M.; Balen, B.; Pavlica, M. Integrative approach gives new insights into combined Cd/Cu exposure in tobacco. Acta Physiol. Plant. 2016, 38, 142. [Google Scholar] [CrossRef]
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J. Biol. Sci. 2018, 25, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef]
- Jiang, H.-S.; Li, M.; Chang, F.-Y.; Li, W.; Yin, L.-Y. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ. Toxicol. Chem. 2012, 31, 1880–1886. [Google Scholar] [CrossRef]
- Barbasz, A.; Kreczmer, B.; Oćwieja, M. Effects of exposure of callus cells of two wheat varieties to silver nanoparticles and silver salt (AgNO3). Acta Physiol. Plant. 2016, 38, 76. [Google Scholar] [CrossRef]
- Masi, A.; Ghisi, R.; Ferretti, M. Measuring low-molecular-weight thiols by detecting the fluorescence of their SBD-derivatives: Application to studies of diurnal and UV-B induced changes in Zea mays L. J. Plant Physiol. 2002, 159, 499–507. [Google Scholar] [CrossRef]
- Tausz, M.; Šircelj, H.; Grill, D. The glutathione system as a stress marker in plant ecophysiology: Is a stress-response concept valid? J. Exp. Bot. 2004, 55, 1955–1962. [Google Scholar] [CrossRef] [PubMed]
- Breiman, A. Plant Hsp90 and its co-chaperones. Curr. Protein Pept. Sci. 2014, 15, 232–244. [Google Scholar] [CrossRef]
- Peharec Štefanić, P.; Jarnević, M.; Cvjetko, P.; Biba, R.; Šikić, S.; Tkalec, M.; Cindrić, M.; Letofsky-Papst, I.; Balen, B. Comparative proteomic study of phytotoxic effects of silver nanoparticles and silver ions on tobacco plants. Environ. Sci. Pollut. Res. 2019, 26, 22529–22550. [Google Scholar] [CrossRef]
- Wu, C.-T.; Leubner-Metzger, G.; Meins, F.; Bradford, K.J. Class I β-1,3-Glucanase and Chitinase Are Expressed in the Micropylar Endosperm of Tomato Seeds Prior to Radicle Emergence. Plant Physiol. 2001, 126, 1299–1313. [Google Scholar] [CrossRef]
- Beata, P.; Ildiko, M.; Awaad, A.S.; Kaushik, G.; Govil, J.N. Plant defense against heavy metals: The involvement of pathogenesis-related (PR) proteins. In Recent Progress in Medicinal Plants. Vol 31: Mechanism and Action of Phytoconstituents; Awaad, A.S., Kaushik, G., Govil, J.N., Eds.; Studium Press: New Delhi, India, 2011; pp. 179–205. [Google Scholar]
- Perdomo, J.A.; Capó-Bauçà, S.; Carmo-Silva, E.; Galmés, J. Rubisco and Rubisco Activase Play an Important Role in the Biochemical Limitations of Photosynthesis in Rice, Wheat, and Maize under High Temperature and Water Deficit. Front. Plant Sci. 2017, 8, 490. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Pandey, P.; James, D.; Chandrasekhar, K.; Achary, V.M.M.; Kaul, T.; Tripathy, B.C.; Reddy, M.K. Enhancing C3 photosynthesis: An outlook on feasible interventions for crop improvement. Plant Biotechnol. J. 2014, 12, 1217–1230. [Google Scholar] [CrossRef] [PubMed]
- Galmés, J.; Medrano, H.; Flexas, J. Photosynthetic limitations in response to water stress and recovery in Mediterranean plants with different growth forms. New Phytol. 2007, 175, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Galazzi, R.M.; Lopes Júnior, C.A.; de Lima, T.B.; Gozzo, F.C.; Arruda, M.A.Z. Evaluation of some effects on plant metabolism through proteins and enzymes in transgenic and non-transgenic soybeans after cultivation with silver nanoparticles. J. Proteom. 2019, 191, 88–106. [Google Scholar] [CrossRef]
- Staneloni, R.J.; Rodriguez-Batiller, M.J.; Casal, J.J. Abscisic Acid, High-Light, and Oxidative Stress Down-Regulate a Photosynthetic Gene via a Promoter Motif Not Involved in Phytochrome-Mediated Transcriptional Regulation. Mol. Plant 2008, 1, 75–83. [Google Scholar] [CrossRef]
- Xu, Y.-H.; Liu, R.; Yan, L.; Liu, Z.-Q.; Jiang, S.-C.; Shen, Y.-Y.; Wang, X.-F.; Zhang, D.-P. Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis. J. Exp. Bot. 2012, 63, 1095–1106. [Google Scholar] [CrossRef]
- Liu, R.; Xu, Y.-H.; Jiang, S.-C.; Lu, K.; Lu, Y.-F.; Feng, X.-J.; Wu, Z.; Liang, S.; Yu, Y.-T.; Wang, X.-F.; et al. Light-harvesting chlorophyll a/b-binding proteins, positively involved in abscisic acid signalling, require a transcription repressor, WRKY40, to balance their function. J. Exp. Bot. 2013, 64, 5443–5456. [Google Scholar] [CrossRef]
- Vannini, C.; Domingo, G.; Onelli, E.; De Mattia, F.; Bruni, I.; Marsoni, M.; Bracale, M. Phytotoxic and genotoxic effects of silver nanoparticles exposure on germinating wheat seedlings. J. Plant Physiol. 2014, 171, 1142–1148. [Google Scholar] [CrossRef]
- Jansson, S. The light-harvesting chlorophyll ab-binding proteins. Biochim. Biophys. Acta 1994, 1184, 1–19. [Google Scholar] [CrossRef]
- Zhang, E.; Brewer, J.M.; Minor, W.; Carreira, L.A.; Lebioda, L. Mechanism of Enolase: The Crystal Structure of Asymmetric Dimer Enolase−2-Phospho-d-glycerate/Enolase−Phosphoenolpyruvate at 2.0 Å Resolution. Biochemistry 1997, 36, 12526–12534. [Google Scholar] [CrossRef]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Schober, Y.; Römpp, A.; Ghassempour, A.; Spengler, B. Proteomics study of silver nanoparticles toxicity on Oryza sativa L. Ecotoxicol. Environ. Saf. 2014, 108, 335–339. [Google Scholar] [CrossRef] [PubMed]
- Jhanzab, H.M.; Razzaq, A.; Bibi, Y.; Yasmeen, F.; Yamaguchi, H.; Hitachi, K.; Tsuchida, K.; Komatsu, S. Proteomic Analysis of the Effect of Inorganic and Organic Chemicals on Silver Nanoparticles in Wheat. Int. J. Mol. Sci. 2019, 20, 825. [Google Scholar] [CrossRef] [PubMed]
- Müntz, K. Deposition of storage proteins. Plant Mol. Biol. 1998, 38, 77–99. [Google Scholar] [CrossRef] [PubMed]
- de Souza Cândido, E.; Pinto, M.F.S.; Pelegrini, P.B.; Lima, T.B.; Silva, O.N.; Pogue, R.; Grossi-De-Sá, M.F.; Franco, O.L. Plant storage proteins with antimicrobial activity: Novel insights into plant defense mechanisms. FASEB J. 2011, 25, 3290–3305. [Google Scholar] [CrossRef]
- Chen, C.-E.; Yeh, K.-C.; Wu, S.-H.; Wang, H.-I.; Yeh, H.-H. A Vicilin-Like Seed Storage Protein, PAP85, Is Involved in Tobacco Mosaic Virus Replication. J. Virol. 2013, 87, 6888–6900. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Mustafa, G.; Nishiuchi, T.; Komatsu, S. Comparative Analysis of the Effect of Inorganic and Organic Chemicals with Silver Nanoparticles on Soybean under Flooding Stress. Int. J. Mol. Sci. 2020, 21, 1300. [Google Scholar] [CrossRef]
- Xu, R.; Song, F.; Zheng, Z. OsBISAMT1, a gene encoding S-adenosyl-L-methionine:salicylic acid carboxyl methyltransferase, is differentially expressed in rice defense responses. Mol. Biol. Rep. 2006, 33, 223–231. [Google Scholar] [CrossRef]
- Köllner, T.G.; Lenk, C.; Zhao, N.; Seidl-Adams, I.; Gershenzon, J.; Chen, F.; Degenhardt, J. Herbivore-Induced SABATH Methyltransferases of Maize That Methylate Anthranilic Acid Using S-Adenosyl-l-Methionine. Plant Physiol. 2010, 153, 1795–1807. [Google Scholar] [CrossRef]
- Ezaki, B.; Higashi, A.; Nanba, N.; Nishiuchi, T. An S-adenosyl Methionine Synthetase (SAMS) Gene from Andropogon virginicus L. Confers Aluminum Stress Tolerance and Facilitates Epigenetic Gene Regulation in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1627. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Michalski, R.; Thiebaut, D.; Michałowski, B.; Ayhan, M.M.; Hardy, M.; Ouari, O.; Rostkowski, M.; Smulik-Izydorczyk, R.; Artelska, A.; Marcinek, A. Oxidation of ethidium-based probes by biological radicals: Mechanism, kinetics and implications for the detection of superoxide. Sci. Rep. 2020, 10, 18626. [Google Scholar] [CrossRef]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts: I. kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Levine, R.L.; Williams, J.A.; Stadtman, E.R.; Shacter, E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994, 233, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Salbitani, G.; Bottone, C.; Carfagna, S. Determination of Reduced and Total Glutathione Content in Extremophilic Microalga Galdieria phlegrea. Bio-Protocol 2017, 7, e2372. [Google Scholar] [CrossRef]
- Pavoković, D.; Križnik, B.; Krsnik-Rasol, M. Evaluation of Protein Extraction Methods for Proteomic Analysis of Non-Model Recalcitrant Plant Tissues. Croat. Chem. Acta 2012, 85, 177–183. [Google Scholar] [CrossRef]
- Peharec Štefanić, P.; Cindrić, M.; Balen, B. Proteomic Analysis of Non-model Plant Tissues Using Phenol Extraction, Two-Dimensional Electrophoresis, and MALDI Mass Spectrometry. Methods Mol. Biol. 2018, 1815, 351–370. [Google Scholar] [CrossRef] [PubMed]

| Ag Content (μg g−1 DW) | ||||
|---|---|---|---|---|
| Treatment | Concentrations (µM) | AgNP-PVP | AgNP-CTAB | AgNO3 |
| Control | 0 | 0 ± 0 d | 0 ± 0 d | 0 ± 0 d |
| 25 | 39.6 ± 1.0 c,A | 33.3 ± 6.8 c,A | 32.3 ± 6.9 c,A | |
| Ag | 50 | 64.7 ± 6.7 b,A | 58.2 ± 4.2 b,A | 56.2 ± 4.0 b,A |
| 100 | 102.3 ± 7.4 a,A | 96.6 ± 3.4 a,A | 91.7 ± 1.5 a,A | |
| 25 + 125 | 24.3 ± 2.6 *,# | 20.7 ± 1.1 # | 9.7 ± 1.8 *,# | |
| Ag + cys | 50 + 250 | 42.7 ± 1.5 *,# | 34.9 ± 7.0 # | 17.9 ± 6.1 *,# |
| 100 + 500 | 81.1 ± 5.4 # | 58.0 ± 4.5 *,# | 50.1 ± 7.6 *,# | |
| Protein Spot | Protein Name | MW (kDa) | pI | Molecular Function | Biological Process | Cellular Compartment | Differential Expression | ||
|---|---|---|---|---|---|---|---|---|---|
| AgNP-PVP | AgNP-CTAB | AgNO3 | |||||||
| Storage proteins | (−cys/+cys) | ||||||||
| 2, 12, 25 | legumin A-like | 53.6 | 8.4 | nutrient reservoir activity | storage protein | vacuole | ↑/= | ↑/↑ | =/= |
| 9 | vicilin-like antimicrobial peptides 2-3 | 94.6 | 7.1 | nutrient reservoir activity | storage protein | vacuole | ↑/↑ | ↑/↑ | ↑/= |
| 14, 21 | 11S globulin subunit beta-like | 56.7 | 8.3 | nutrient reservoir activity | storage protein | vacuole | ↑/= | ↑/↑ | ↑/= |
| 15, 20, 22 | 11S globulin seed storage protein 2-like | 55.6 | 7.6 | nutrient reservoir activity | storage protein | vacuole | =/= | ↑/↑ | =/= |
| Defense and stress response | |||||||||
| 1 | stromal 70 kDa heat shock-related protein, chloroplastic | 75.3 | 5.4 | unfolded protein binding | stress response, protein folding | chloroplast | ↑/↑ | =/= | ↑/↑ |
| 3 | glutathione S-transferase L3-like isoform X2 | 27.0 | 4.9 | transferase activity | glutathione metabolic process | cytoplasm | ↑/= | ↑/= | ↑/= |
| 18 | glutathione S-transferase L3-like isoform X1 | 27.3 | 5.1 | transferase activity | glutathione metabolic process | cytoplasm | ↑/= | ↑/↑ | ↑/= |
| 5 | hsp70-Hsp90 organizing protein 2-like | 55.5 | 6.0 | Hsp90 protein binding | stress response | cytoplasm, nucleus | ↑/= | ↓/= | =/= |
| 13 | pathogenesis-related protein 1C-like | 20.5 | 5.7 | pathogenesis-related protein | plant defense respose | extracellular | ↓/↓ | ↓/↓ | ↓/↓ |
| 16 | cytosolic ascorbate peroxidase | 27.4 | 5.7 | peroxidase activity | response to oxidative stress | cytoplasm | ↑/↑ | ↑/= | ↑/↑ |
| 17 | L-ascorbate peroxidase 1, cytosolic | 27.5 | 5.9 | peroxidase activity | response to oxidative stress | cytoplasm | =/= | ↑/↑ | ↑/= |
| Nucleic acid metabolism | |||||||||
| 4 | S-adenosylmethionine synthase | 42.6 | 6.1 | transferase activity | one-carbon metabolism | cytoplasm | ↑/↑ | ↑/↑ | ↑/↑ |
| Carbohydrate and energy metabolism | |||||||||
| 7 | phosphopyruvate hydratase | 47.8 | 5.6 | lyase | glycolysis | cytoplasm | =/= | ↑/↑ | ↑/↑ |
| 8 | ribulose bisphosphate carboxylase/oxygenase large chain | 52.9 | 6.9 | oxidoreductase | photosynthesis, photorespiration | chloroplast | =/= | ↓/= | ↓/= |
| 10 | 3-ketoacyl-CoA thiolase 2, peroxisomal-like | 48.8 | 7.6 | acyltransferase | fatty acid beta-oxidation | peroxisome | =/= | ↑/↑ | =/= |
| 11 | ribulose bisphosphate carboxylase/oxygenase activase 2, chloroplastic | 48.3 | 7.7 | ATPase activity, Rubisco activator activity | photosynthesis | chloroplast | ↓/= | ↓/↓ | =/= |
| 19 | dihydrolipoyllysine-residue succinyltransferase | 51.1 | 8.7 | transferase activity | tricarboxylic acid cycle | mitochondrion | ↑/↑ | ↑/= | ↑/↑ |
| 23 | chlorophyll a-b binding protein, chloroplastic | 29.3 | 8.7 | chlorophyll binding | photosynthesis | chloroplast | =/= | ↑/= | ↑/= |
| Protein synthesis and processing | |||||||||
| 6 | chaperonin 60 subunit beta 2, chloroplastic-like | 63.2 | 5.7 | chaperone | protein folding | chloroplast | =/= | ↑/↑ | =/= |
| 24 | proteasome subunit alpha type | 26.0 | 4.8 | endopeptidase activity | protein catabolic process | cytoplasm | =/↑ | ↑/↑ | ↑/= |
| 26 | protein disulfide-isomerase | 27.5 | 8.4 | isomerase activity | protein folding | endoplasmic reticulum | ↓/= | ↑/= | ↑/= |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biba, R.; Cvjetko, P.; Tkalec, M.; Košpić, K.; Štefanić, P.P.; Šikić, S.; Domijan, A.-M.; Balen, B. Effects of Silver Nanoparticles on Physiological and Proteomic Responses of Tobacco (Nicotiana tabacum) Seedlings Are Coating-Dependent. Int. J. Mol. Sci. 2022, 23, 15923. https://doi.org/10.3390/ijms232415923
Biba R, Cvjetko P, Tkalec M, Košpić K, Štefanić PP, Šikić S, Domijan A-M, Balen B. Effects of Silver Nanoparticles on Physiological and Proteomic Responses of Tobacco (Nicotiana tabacum) Seedlings Are Coating-Dependent. International Journal of Molecular Sciences. 2022; 23(24):15923. https://doi.org/10.3390/ijms232415923
Chicago/Turabian StyleBiba, Renata, Petra Cvjetko, Mirta Tkalec, Karla Košpić, Petra Peharec Štefanić, Sandra Šikić, Ana-Marija Domijan, and Biljana Balen. 2022. "Effects of Silver Nanoparticles on Physiological and Proteomic Responses of Tobacco (Nicotiana tabacum) Seedlings Are Coating-Dependent" International Journal of Molecular Sciences 23, no. 24: 15923. https://doi.org/10.3390/ijms232415923
APA StyleBiba, R., Cvjetko, P., Tkalec, M., Košpić, K., Štefanić, P. P., Šikić, S., Domijan, A.-M., & Balen, B. (2022). Effects of Silver Nanoparticles on Physiological and Proteomic Responses of Tobacco (Nicotiana tabacum) Seedlings Are Coating-Dependent. International Journal of Molecular Sciences, 23(24), 15923. https://doi.org/10.3390/ijms232415923







