Progress in Laser Ablation and Biological Synthesis Processes: “Top-Down” and “Bottom-Up” Approaches for the Green Synthesis of Au/Ag Nanoparticles
Abstract
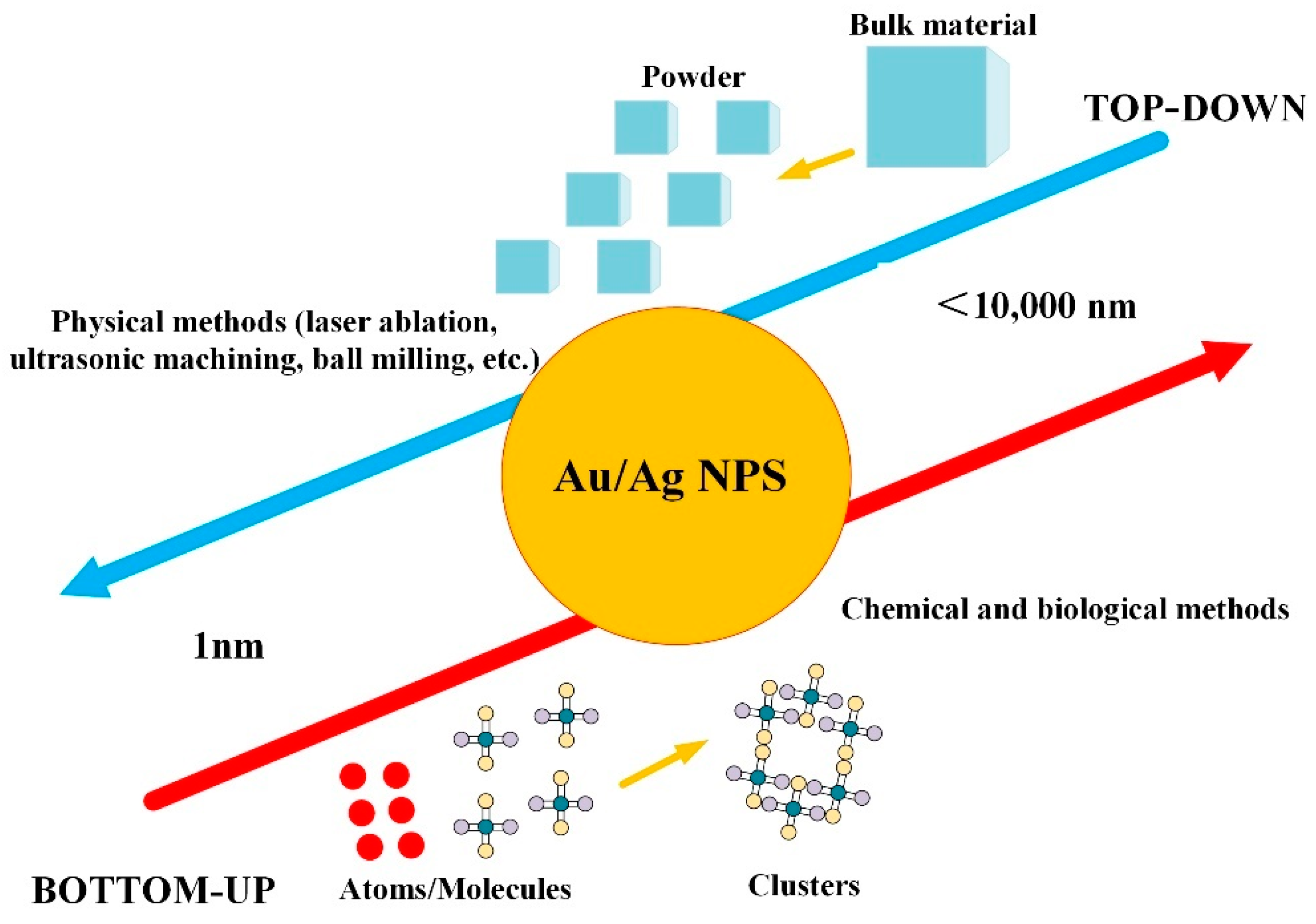
1. Introduction
2. Fundamentals of the Laser Ablation Method
2.1. Brief Introduction
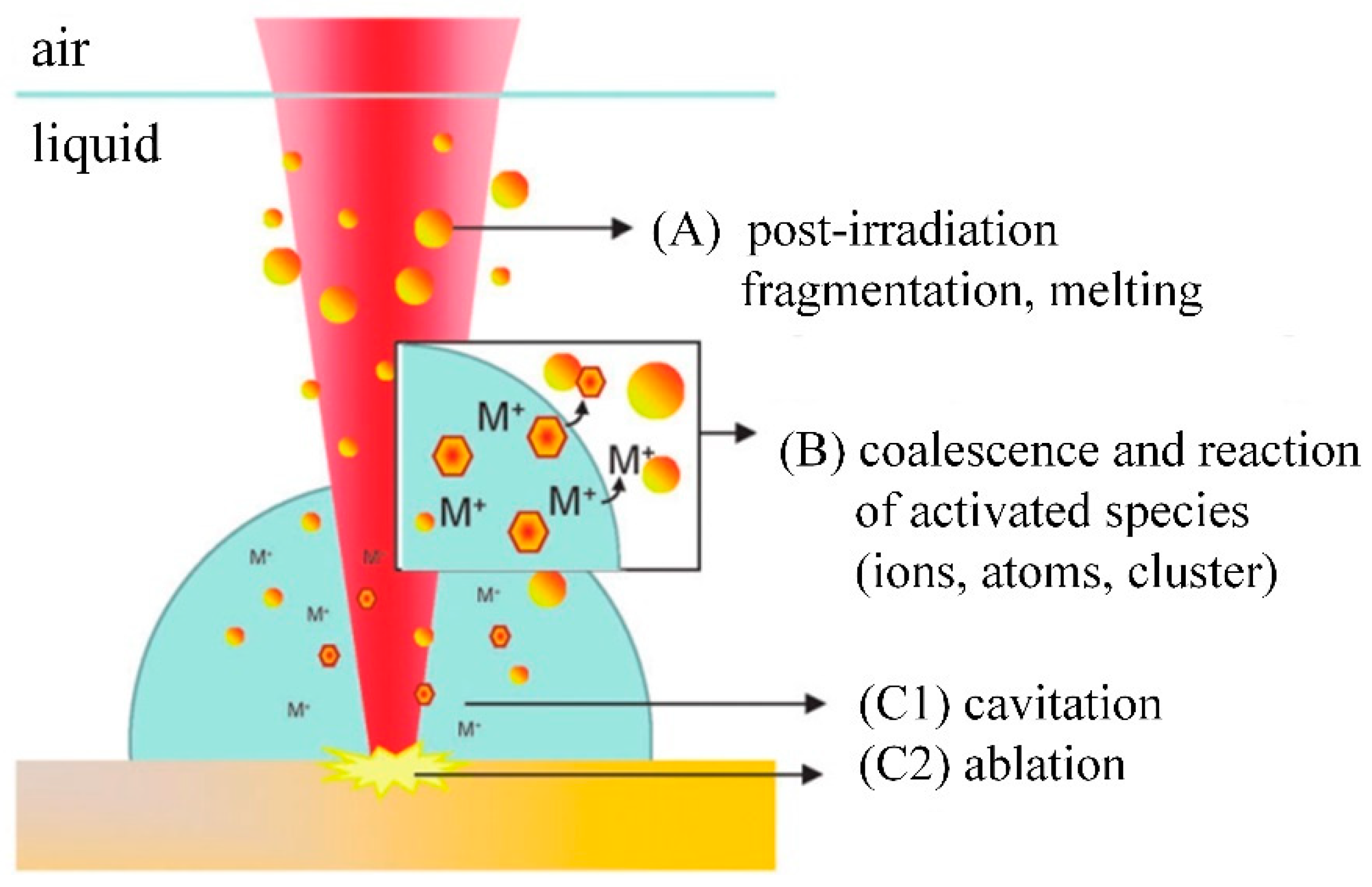
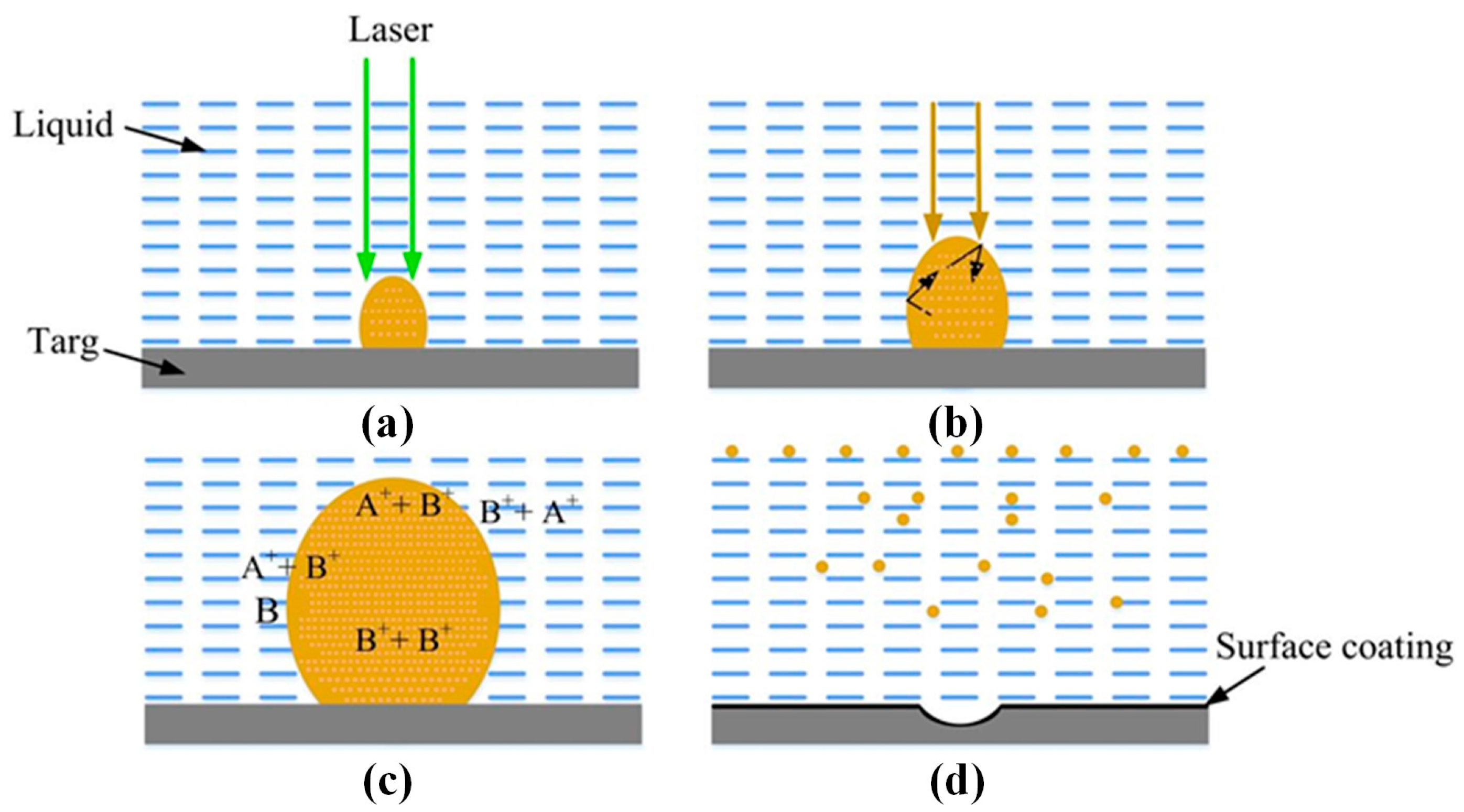
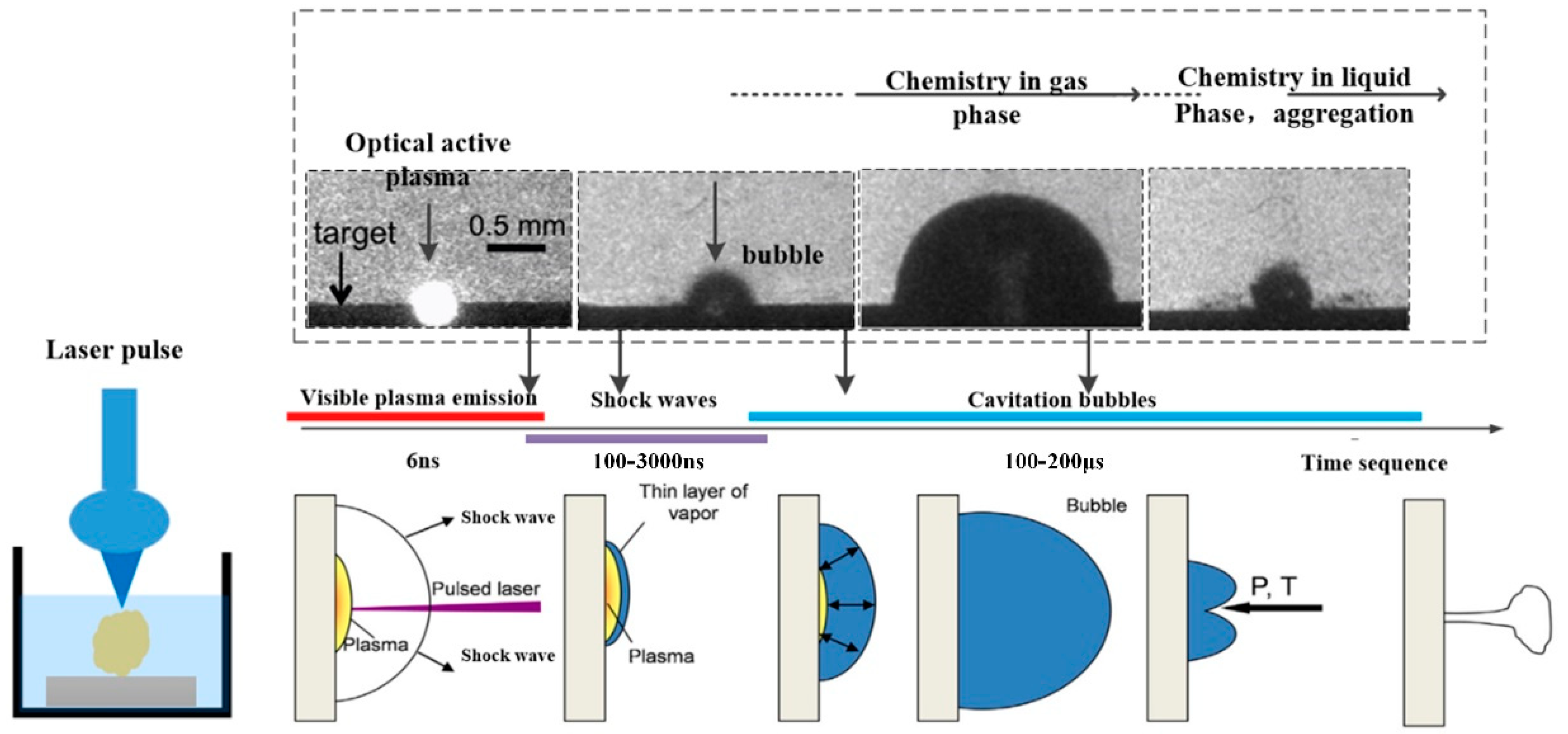
2.2. Laser Ablation Mechanism
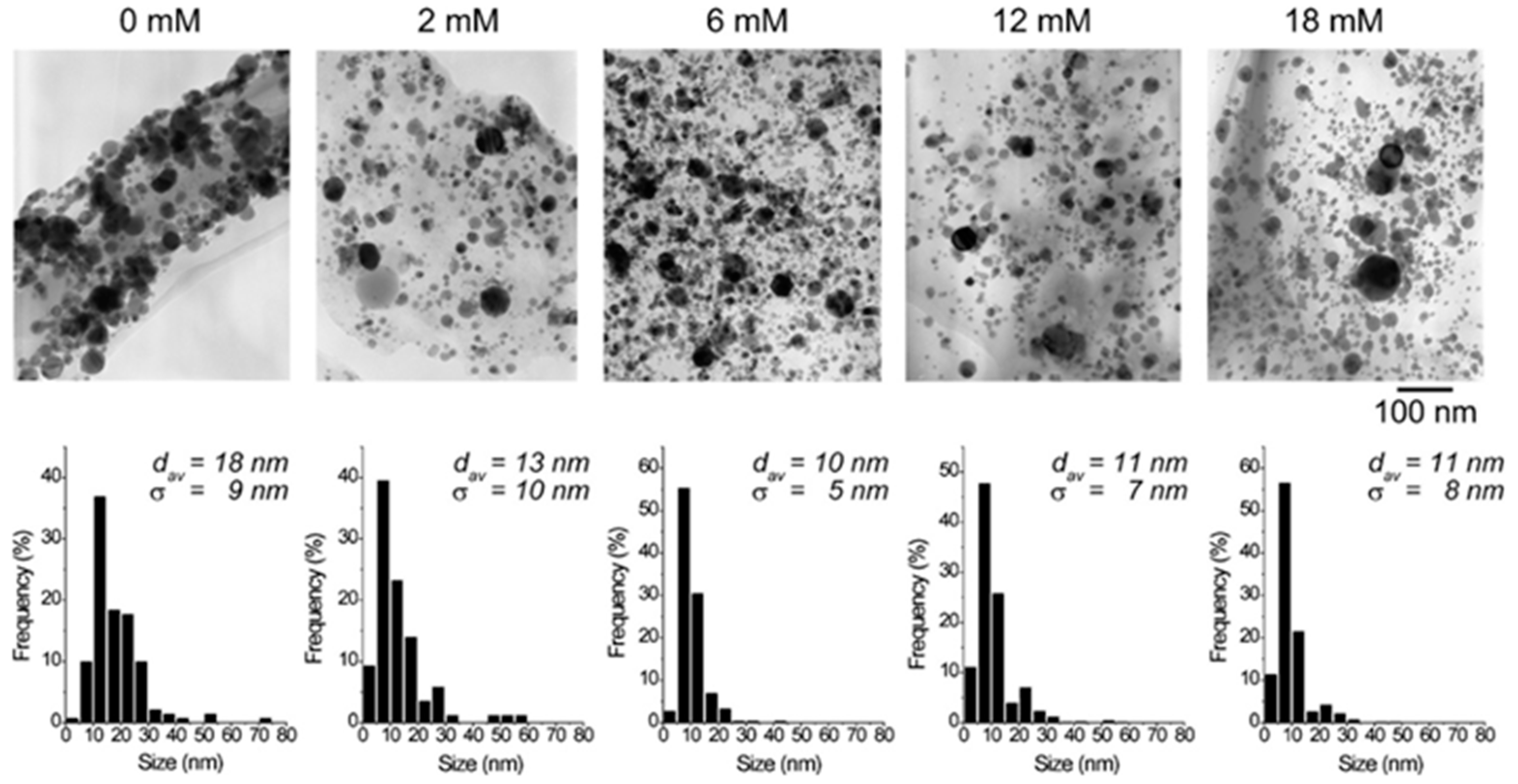
2.3. Size and Shape Control
2.4. Summary
3. Fundamentals of Biological Method
3.1. Brief Introduction
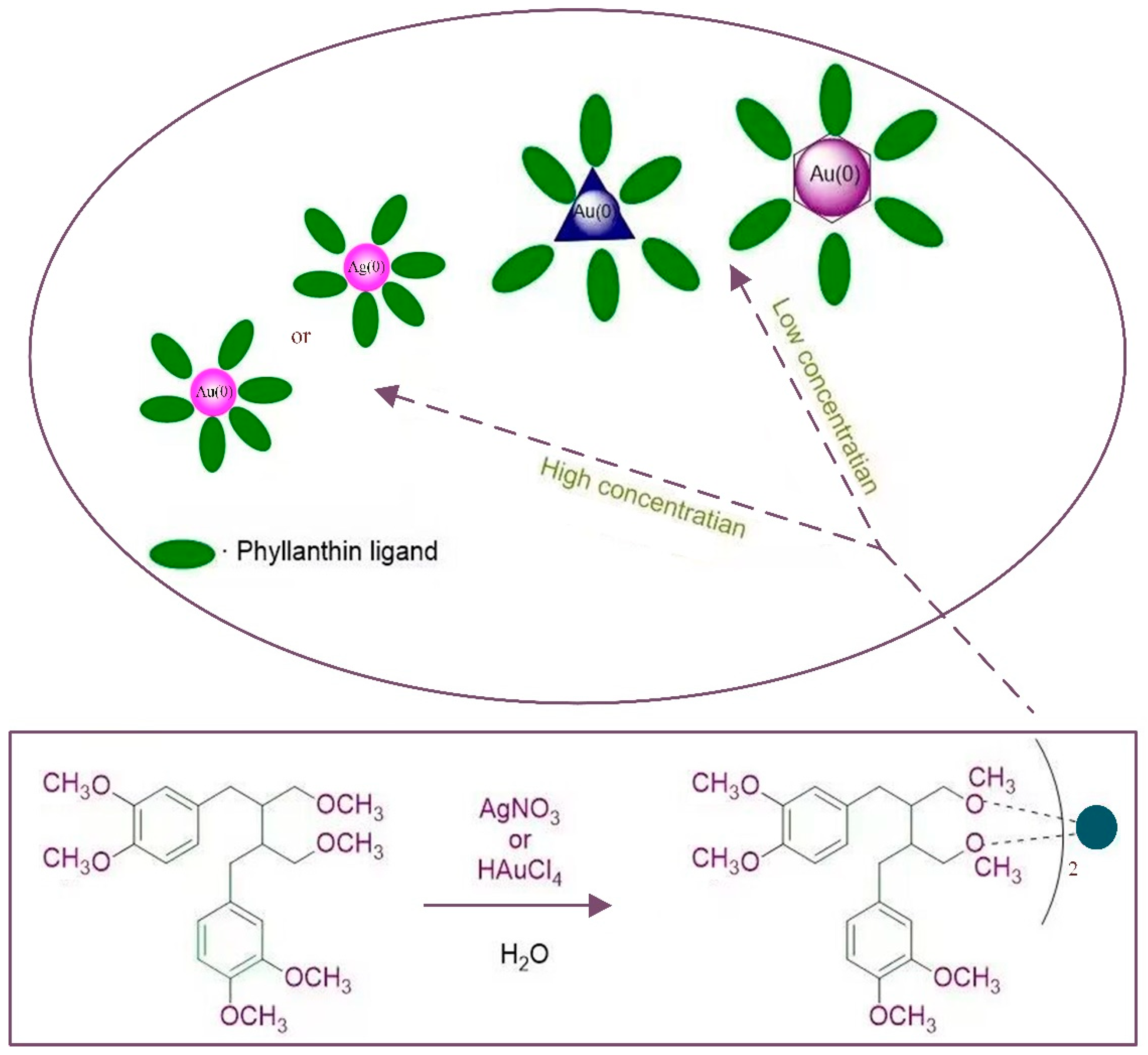
3.2. Synthesis Principle
3.3. Size and Shape Control
3.4. Summary
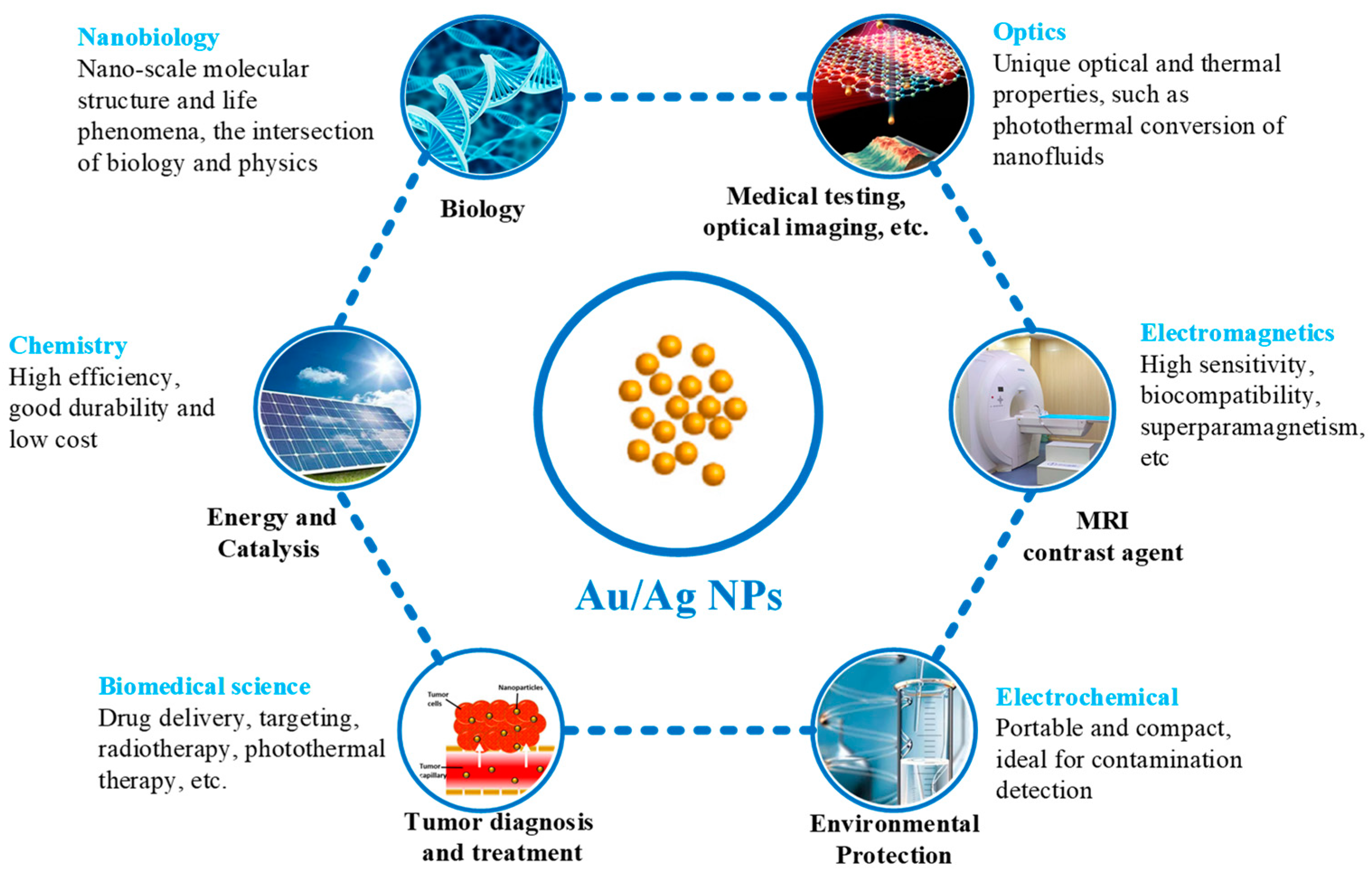
4. Applications
4.1. Biology
4.2. Tumor Diagnosis and Treatment
4.3. Others
5. Discussions
5.1. Similarity
5.2. Individuality
5.3. Complementarity
6. Outlooks
- (1)
- Laser synthesis and colloid processing are described as promising synthesis methods with unique properties. However, there is still a lack of effective preparation processes for size control and high productivity, especially with reliable processes that can achieve commercial-scale use. Preparation within some laboratories is also a challenging technical problem when obtaining high productivity. This shows that a preparation cost that is too high will limit the implementation of this process in practical applications. In order to solve this challenge, the core problem is to precisely clarify the LAL mechanism. Whether through experiment or in theory, breaking through its processing mechanism only technically answers the interaction between the laser and substrate, as well as the material ablation process when considering the behavior of bubble dynamics, but it can also be used for more economical means to prepare Au/Ag nanoparticle materials;
- (2)
- The biological method mainly involves plant-mediated synthesis, microbe-mediated synthesis, and algae-mediated synthesis, and the size and shape of the Au/Ag NPs prepared via different biosynthesis methods are different. Few existing studies have addressed the differences between these synthetic methods and how NPs can be prepared using different biosynthetic methods. In the future, the differences between the mechanisms of different biosynthetic methods should be further studied, and understanding how to choose suitable biosynthetic methods to prepare Au/Ag NPs for different application fields also needs attention. In addition, understanding how to rationally combine different biosynthetic methods to prepare Au/Ag NPs of a specific size and shape may also be interesting;
- (3)
- Whether using laser ablation or biosynthesis to create Au/Ag NPs, it is necessary to control their size distribution and shape and the production efficiency/cost of the prepared NPs, which involves process parameter optimization (single-objective or multiobjective optimization). At present, in the field of precision manufacturing, such as that within laser machining, electrical discharge machining, etc., the optimization of the process parameters has been extensively studied, and the optimization algorithm has also been integrated into this equipment. Therefore, for the laser ablation or biosynthesis of Au/Ag NPs, the optimization of the process parameters needs to focus on the synthesis process model. On the one hand, the establishment of these models depends on an in-depth study of their processing mechanisms. The connection between multiple inputs and outputs is established through the “white box” method via a mathematical formula [98]. On the other hand, for the complex synthesis process of Au/Ag NPs, this can also be realized via “black box” methods, such as those represented by regression models [99], neural networks [100,101], etc.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Molecules | Free Full-Text | The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Available online: https://www.mdpi.com/1420-3049/25/1/112 (accessed on 30 October 2022).
- Nazarko, J.; Ejdys, J.; Gudanowska, A.E.; Halicka, K.; Kononiuk, A.; Magruk, A.; Nazarko, Ł. Roadmapping in Regional Technology Foresight: A Contribution to Nanotechnology Development Strategy. IEEE Trans. Eng. Manag. 2022, 69, 179–194. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Huang, H.; Xu, Y.; Luo, G.; Xie, Z.; Ming, W. Molecular Dynamics Study of Laser Interaction with Nanoparticles in Liquids and Its Potential Application. Nanomaterials 2022, 12, 1524. [Google Scholar] [CrossRef]
- Ming, W.; Jiang, Z.; Luo, G.; Xu, Y.; He, W.; Xie, Z.; Shen, D.; Li, L. Progress in Transparent Nano-Ceramics and Their Potential Applications. Nanomaterials 2022, 12, 1491. [Google Scholar] [CrossRef] [PubMed]
- Roduner, E. Size Matters: Why Nanomaterials Are Different. Chem. Soc. Rev. 2006, 35, 583–592. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Shape-Dependent Catalytic Activity of Platinum Nanoparticles in Colloidal Solution. Nano Lett. 2004, 4, 1343–1348. [Google Scholar] [CrossRef]
- Liu, R.; Duay, J.; Lee, S.B. Heterogeneous Nanostructured Electrode Materials for Electrochemical Energy Storage. Chem. Commun. 2011, 47, 1384–1404. [Google Scholar] [CrossRef]
- Lee, J.; Mahendra, S.; Alvarez, P.J. Nanomaterials in the Construction Industry: A Review of Their Applications and Environmental Health and Safety Considerations. ACS Nano 2010, 4, 3580–3590. [Google Scholar] [CrossRef]
- Banerjee, K.; Das, S.; Choudhury, P.; Ghosh, S.; Baral, R.; Choudhuri, S.K. A Novel Approach of Synthesizing and Evaluating the Anticancer Potential of Silver Oxide Nanoparticles in Vitro. Chemotherapy 2017, 62, 279–289. [Google Scholar] [CrossRef]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.; Kyzioł, A. Development of Noncytotoxic Chitosan–Gold Nanocomposites as Efficient Antibacterial Materials. ACS Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef]
- Ahmadi, T.S.; Wang, Z.L.; Green, T.C.; Henglein, A.; El-Sayed, M.A. Shape-Controlled Synthesis of Colloidal Platinum Nanoparticles. Science 1996, 272, 1924–1925. [Google Scholar] [CrossRef]
- Stasyuk, N.Y.; Smutok, O.V.; Zakalskiy, A.E.; Zakalska, O.M.; Gonchar, M.V. Methylamine-Sensitive Amperometric Biosensor Based on (His) 6-Tagged Hansenula Polymorpha Methylamine Oxidase Immobilized on the Gold Nanoparticles. BioMed Res. Int. 2014, 2014, 480498. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.-C.; Astruc, D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2004, 104, 293–346. [Google Scholar] [CrossRef] [PubMed]
- Kus-Liśkiewicz, M.; Fickers, P.; Ben Tahar, I. Biocompatibility and Cytotoxicity of Gold Nanoparticles: Recent Advances in Methodologies and Regulations. Int. J. Mol. Sci. 2021, 22, 10952. [Google Scholar] [CrossRef] [PubMed]
- Nicolae-Maranciuc, A.; Chicea, D.; Chicea, L.M. Ag Nanoparticles for Biomedical Applications—Synthesis and Characterization—A Review. Int. J. Mol. Sci. 2022, 23, 5778. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Jha, S.; Ramteke, S.; Jain, N.K. Pharmaceutical Aspects of Silver Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2018, 46, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, S.P.; Patil, S.M.; Mullani, S.B.; Delekar, S.D. Silver Nanoparticles as an Effective Disinfectant: A Review. Mater. Sci. Eng. C 2019, 97, 954–965. [Google Scholar] [CrossRef] [PubMed]
- Dengler, S.; Kübel, C.; Schwenke, A.; Ritt, G.; Eberle, B. Near- and off-Resonant Optical Limiting Properties of Gold–Silver Alloy Nanoparticles for Intense Nanosecond Laser Pulses. J. Opt. 2012, 14, 075203. [Google Scholar] [CrossRef]
- Zhu, Y.; Jiang, X.; Wang, H.; Wang, S.; Wang, H.; Sun, B.; Su, Y.; He, Y. A Poly Adenine-Mediated Assembly Strategy for Designing Surface-Enhanced Resonance Raman Scattering Substrates in Controllable Manners. Anal. Chem. 2015, 87, 6631–6638. [Google Scholar] [CrossRef]
- Huang, C.; Wen, T.; Shi, F.-J.; Zeng, X.-Y.; Jiao, Y.-J. Rapid Detection of IgM Antibodies against the SARS-CoV-2 Virus via Colloidal Gold Nanoparticle-Based Lateral-Flow Assay. ACS Omega 2020, 5, 12550–12556. [Google Scholar] [CrossRef]
- Shin, W.-K.; Cho, J.; Kannan, A.G.; Lee, Y.-S.; Kim, D.-W. Cross-Linked Composite Gel Polymer Electrolyte Using Mesoporous Methacrylate-Functionalized SiO2 Nanoparticles for Lithium-Ion Polymer Batteries. Sci. Rep. 2016, 6, 26332. [Google Scholar] [CrossRef]
- Zhang, D.; Gökce, B.; Barcikowski, S. Laser Synthesis and Processing of Colloids: Fundamentals and Applications. Chem. Rev. 2017, 117, 3990–4103. [Google Scholar] [CrossRef]
- Patil, P.P.; Phase, D.M.; Kulkarni, S.A.; Ghaisas, S.V.; Kulkarni, S.K.; Kanetkar, S.M.; Ogale, S.B.; Bhide, V.G. Pulsed-Laser–Induced Reactive Quenching at Liquid-Solid Interface: Aqueous Oxidation of Iron. Phys. Rev. Lett. 1987, 58, 238. [Google Scholar] [CrossRef] [PubMed]
- Ogale, S.B. Pulsed-Laser-Induced and Ion-Beam-Induced Surface Synthesis and Modification of Oxides, Nitrides and Carbides. Thin Solid Films 1988, 163, 215–227. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, D.; Zhang, Y.; Xue, T.; Huang, Y.; Zhang, G. Fabrication and Droplet Impact Performance of Superhydrophobic Ti6Al4V Surface by Laser Induced Plasma Micro-Machining. Appl. Surf. Sci. 2022, 605, 154661. [Google Scholar] [CrossRef]
- Gujba, A.K.; Medraj, M. Laser Peening Process and Its Impact on Materials Properties in Comparison with Shot Peening and Ultrasonic Impact Peening. Materials 2014, 7, 7925–7974. [Google Scholar] [CrossRef]
- Amendola, V.; Amans, D.; Ishikawa, Y.; Koshizaki, N.; Scirè, S.; Compagnini, G.; Reichenberger, S.; Barcikowski, S. Room-Temperature Laser Synthesis in Liquid of Oxide, Metal-Oxide Core-Shells, and Doped Oxide Nanoparticles. Chem. Eur. J. 2020, 26, 9206–9242. [Google Scholar] [CrossRef]
- Barcikowski, S.; Devesa, F.; Moldenhauer, K. Impact and Structure of Literature on Nanoparticle Generation by Laser Ablation in Liquids. J. Nanopart. Res. 2009, 11, 1883–1893. [Google Scholar] [CrossRef]
- Saitow, K.; Okamoto, Y.; Yano, Y.F. Fractal of Gold Nanoparticles Controlled by Ambient Dielectricity: Synthesis by Laser Ablation as a Function of Permittivity. J. Phys. Chem. C 2012, 116, 17252–17258. [Google Scholar] [CrossRef]
- Scaramuzza, S.; Zerbetto, M.; Amendola, V. Synthesis of Gold Nanoparticles in Liquid Environment by Laser Ablation with Geometrically Confined Configurations: Insights to Improve Size Control and Productivity. J. Phys. Chem. C 2016, 120, 9453–9463. [Google Scholar] [CrossRef]
- Neumeister, A.; Jakobi, J.; Rehbock, C.; Moysig, J.; Barcikowski, S. Monophasic Ligand-Free Alloy Nanoparticle Synthesis Determinants during Pulsed Laser Ablation of Bulk Alloy and Consolidated Microparticles in Water. Phys. Chem. Chem. Phys. 2014, 16, 23671–23678. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.W. Laser Ablation in Liquids: Applications in the Synthesis of Nanocrystals. Prog. Mater. Sci. 2007, 52, 648–698. [Google Scholar] [CrossRef]
- Menendez-Manjon, A.; Chichkov, B.N.; Barcikowski, S. Influence of Water Temperature on the Hydrodynamic Diameter of Gold Nanoparticles from Laser Ablation. J. Phys. Chem. C 2010, 114, 2499–2504. [Google Scholar] [CrossRef]
- Ageev, V.A. Investigation into Light Erosion of Metals in Liquids. Zhurnal Prikl. Spektrosk. 1975, 23, 42–46. [Google Scholar]
- Dell’Aglio, M.; Gaudiuso, R.; De Pascale, O.; De Giacomo, A. Mechanisms and Processes of Pulsed Laser Ablation in Liquids during Nanoparticle Production. Appl. Surf. Sci. 2015, 348, 4–9. [Google Scholar] [CrossRef]
- Zhang, Z.; Qiu, W.; Zhang, G.; Liu, D.; Wang, P. Progress in Applications of Shockwave Induced by Short Pulsed Laser on Surface Processing. Opt. Laser Technol. 2023, 157, 108760. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, Y.; Liu, D.; Zhang, Y.; Zhao, J.; Zhang, G. Bubble Behavior and Its Effect on Surface Integrity in Laser-Induced Plasma Micro-Machining Silicon Wafer. J. Manuf. Sci. Eng. 2022, 144, 091008. [Google Scholar] [CrossRef]
- Tamura, A.; Matsumoto, A.; Fukami, K.; Nishi, N.; Sakka, T. Simultaneous Observation of Nascent Plasma and Bubble Induced by Laser Ablation in Water with Various Pulse Durations. J. Appl. Phys. 2015, 117, 173304. [Google Scholar] [CrossRef]
- Henglein, A. Physicochemical Properties of Small Metal Particles in Solution: “Microelectrode” Reactions, Chemisorption, Composite Metal Particles, and the Atom-to-Metal Transition. J. Phys. Chem. 1993, 97, 5457–5471. [Google Scholar] [CrossRef]
- Mafuné, F.; Kohno, J.; Takeda, Y.; Kondow, T.; Sawabe, H. Structure and Stability of Silver Nanoparticles in Aqueous Solution Produced by Laser Ablation. J. Phys. Chem. B 2000, 104, 8333–8337. [Google Scholar] [CrossRef]
- Procházka, M.; Mojzeš, P.; Štěpánek, J.; Vlčková, B.; Turpin, P.-Y. Probing Applications of Laser-Ablated Ag Colloids in SERS Spectroscopy: Improvement of Ablation Procedure and SERS Spectral Testing. Anal. Chem. 1997, 69, 5103–5108. [Google Scholar] [CrossRef]
- Tsuji, T.; Thang, D.-H.; Okazaki, Y.; Nakanishi, M.; Tsuboi, Y.; Tsuji, M. Preparation of Silver Nanoparticles by Laser Ablation in Polyvinylpyrrolidone Solutions. Appl. Surf. Sci. 2008, 254, 5224–5230. [Google Scholar] [CrossRef]
- Tomko, J.; O’Malley, S.M.; Trout, C.; Naddeo, J.J.; Jimenez, R.; Griepenburg, J.C.; Soliman, W.; Bubb, D.M. Cavitation Bubble Dynamics and Nanoparticle Size Distributions in Laser Ablation in Liquids. Colloids Surf. A Physicochem. Eng. Asp. 2017, 522, 368–372. [Google Scholar] [CrossRef]
- Setoura, K.; Okada, Y.; Hashimoto, S. CW-Laser-Induced Morphological Changes of a Single Gold Nanoparticle on Glass: Observation of Surface Evaporation. Phys. Chem. Chem. Phys. 2014, 16, 26938–26945. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ye, Y.; Liu, J.; Wu, S.; Li, P.; Liang, C. Stability Evolution of Ultrafine Ag Nanoparticles Prepared by Laser Ablation in Liquids. J. Colloid Interface Sci. 2021, 585, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Maciulevičius, M.; Vinčiūnas, A.; Brikas, M.; Butsen, A.; Tarasenka, N.; Tarasenko, N.; Račiukaitis, G. On-Line Characterization of Gold Nanoparticles Generated by Laser Ablation in Liquids. Phys. Procedia 2013, 41, 531–538. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Tiemann, K.J.; Gamez, G.; Dokken, K.; Tehuacanero, S.; Jose-Yacaman, M. Gold Nanoparticles Obtained by Bio-Precipitation from Gold (III) Solutions. J. Nanopart. Res. 1999, 1, 397–404. [Google Scholar] [CrossRef]
- Kasthuri, J.; Kathiravan, K.; Rajendiran, N. Phyllanthin-Assisted Biosynthesis of Silver and Gold Nanoparticles: A Novel Biological Approach. J. Nanopart. Res. 2009, 11, 1075–1085. [Google Scholar] [CrossRef]
- Basavegowda, N.; Sobczak-Kupiec, A.; Fenn, R.I.; Dinakar, S. Bioreduction of Chloroaurate Ions Using Fruit Extract Punica Granatum (Pomegranate) for Synthesis of Highly Stable Gold Nanoparticles and Assessment of Its Antibacterial Activity. Micro Nano Lett. 2013, 8, 400–404. [Google Scholar] [CrossRef]
- Zhan, G.; Huang, J.; Lin, L.; Lin, W.; Emmanuel, K.; Li, Q. Synthesis of Gold Nanoparticles by Cacumen Platycladi Leaf Extract and Its Simulated Solution: Toward the Plant-Mediated Biosynthetic Mechanism. J. Nanopart. Res. 2011, 13, 4957–4968. [Google Scholar] [CrossRef]
- Narayanan, K.B.; Sakthivel, N. Coriander Leaf Mediated Biosynthesis of Gold Nanoparticles. Mater. Lett. 2008, 62, 4588–4590. [Google Scholar] [CrossRef]
- Phuoc, T.X.; Soong, Y.; Chyu, M.K. Synthesis of Ag-Deionized Water Nanofluids Using Multi-Beam Laser Ablation in Liquids. Opt. Lasers Eng. 2007, 45, 1099–1106. [Google Scholar] [CrossRef]
- Pahal, V.; Kumar, P.; Kumar, P.; Kumar, V. Phytofabrication of Gold and Bimetallic Gold-Silver Nanoparticles Using Water Extract of Wheatgrass (Triticum Aestivum), Their Characterization and Comparative Assessment of Antibacterial Potential. Plant Sci. Today 2022, 9, 345–356. [Google Scholar] [CrossRef]
- Shankar, P.D.; Shobana, S.; Karuppusamy, I.; Pugazhendhi, A.; Ramkumar, V.S.; Arvindnarayan, S.; Kumar, G. A Review on the Biosynthesis of Metallic Nanoparticles (Gold and Silver) Using Bio-Components of Microalgae: Formation Mechanism and Applications. Enzym. Microb. Technol. 2016, 95, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Gericke, M.; Pinches, A. Biological Synthesis of Metal Nanoparticles. Hydrometallurgy 2006, 83, 132–140. [Google Scholar] [CrossRef]
- Basavaraja, S.; Balaji, S.D.; Lagashetty, A.; Rajasab, A.H.; Venkataraman, A. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Fusarium Semitectum. Mater. Res. Bull. 2008, 43, 1164–1170. [Google Scholar] [CrossRef]
- Bhainsa, K.C.; D’souza, S.F. Extracellular Biosynthesis of Silver Nanoparticles Using the Fungus Aspergillus Fumigatus. Colloids Surf. B Biointerfaces 2006, 47, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Senapati, S.; Mandal, D.; Ahmad, A.; Khan, M.I.; Kumar, R.; Sastry, M. Extracellular Synthesis of Gold Nanoparticles by the Fungus Fusarium Oxysporum. ChemBioChem 2002, 3, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Senapati, S.; Khan, M.I.; Kumar, R.; Ramani, R.; Srinivas, V.; Sastry, M. Intracellular Synthesis of Gold Nanoparticles by a Novel Alkalotolerant Actinomycete, Rhodococcus Species. Nanotechnology 2003, 14, 824. [Google Scholar] [CrossRef]
- Shedbalkar, U.; Singh, R.; Wadhwani, S.; Gaidhani, S.; Chopade, B.A. Microbial Synthesis of Gold Nanoparticles: Current Status and Future Prospects. Adv. Colloid Interface Sci. 2014, 209, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, H.; Chen, Z.-S.; Chen, G. Biosynthesis of Nanoparticles by Microorganisms and Their Applications. J. Nanomater. 2011, 2011, 270974. [Google Scholar] [CrossRef]
- Zuercher, A.W.; Fritsche, R.; Corthésy, B.; Mercenier, A. Food Products and Allergy Development, Prevention and Treatment. Curr. Opin. Biotechnol. 2006, 17, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Miyashita, K. The Carotenoid Fucoxanthin from Brown Seaweed Affects Obesity. Lipid Technol. 2009, 21, 186–190. [Google Scholar] [CrossRef]
- Mohamed, S.; Hashim, S.N.; Rahman, H.A. Seaweeds: A Sustainable Functional Food for Complementary and Alternative Therapy. Trends Food Sci. Technol. 2012, 23, 83–96. [Google Scholar] [CrossRef]
- Mahdavi, M.; Namvar, F.; Ahmad, M.B.; Mohamad, R. Green Biosynthesis and Characterization of Magnetic Iron Oxide (Fe3O4) Nanoparticles Using Seaweed (Sargassum Muticum) Aqueous Extract. Molecules 2013, 18, 5954–5964. [Google Scholar] [CrossRef]
- Hamida, R.S.; Ali, M.A.; Abdelmeguid, N.E.; Al-Zaban, M.I.; Baz, L.; Bin-Meferij, M.M. Lichens—A Potential Source for Nanoparticles Fabrication: A Review on Nanoparticles Biosynthesis and Their Prospective Applications. J. Fungi 2021, 7, 291. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Low, S.S.; Chew, K.W.; Ling, T.C.; Rinklebe, J.; Juan, J.C.; Ng, E.P.; Show, P.L. Prospects and Environmental Sustainability of Phyconanotechnology: A Review on Algae-Mediated Metal Nanoparticles Synthesis and Mechanism. Environ. Res. 2022, 212, 113140. [Google Scholar] [CrossRef]
- Venkatesan, J.; Manivasagan, P.; Kim, S.-K.; Kirthi, A.V.; Marimuthu, S.; Rahuman, A.A. Marine Algae-Mediated Synthesis of Gold Nanoparticles Using a Novel Ecklonia Cava. Bioprocess Biosyst. Eng. 2014, 37, 1591–1597. [Google Scholar] [CrossRef] [PubMed]
- Mata, Y.N.; Torres, E.; Blazquez, M.L.; Ballester, A.; González, F.; Munoz, J.A. Gold (III) Biosorption and Bioreduction with the Brown Alga Fucus Vesiculosus. J. Hazard. Mater. 2009, 166, 612–618. [Google Scholar] [CrossRef] [PubMed]
- Prasad, T.N.; Kambala, V.S.R.; Naidu, R. Phyconanotechnology: Synthesis of Silver Nanoparticles Using Brown Marine Algae Cystophora Moniliformis and Their Characterisation. J. Appl. Phycol. 2013, 25, 177–182. [Google Scholar] [CrossRef]
- Varanda, L.C.; Souza, C.G.; Moraes, D.A.; Neves, H.R.; Souza, J.B.; Silva, M.F.; Bini, R.A.; Albers, R.F.; Silva, T.L.; Beck, W. Size and Shape-Controlled Nanomaterials Based on Modified Polyol and Thermal Decomposition Approaches. A Brief Review. An. Da Acad. Bras. De Ciências 2019, 91. [Google Scholar] [CrossRef]
- Ankamwar, B. Size and Shape Effect on Biomedical Applications of Nanomaterials. In Biomedical Engineering: Technical Applications in Medicine; IntechOpen Limited: London, UK, 2012; pp. 93–114. [Google Scholar]
- Pinto, R.J.; Lucas, J.M.; Morais, M.P.; Santos, S.A.; Silvestre, A.J.; Marques, P.A.; Freire, C.S. Demystifying the Morphology and Size Control on the Biosynthesis of Gold Nanoparticles Using Eucalyptus Globulus Bark Extract. Ind. Crops Prod. 2017, 105, 83–92. [Google Scholar] [CrossRef]
- Kathiraven, T.; Sundaramanickam, A.; Shanmugam, N.; Balasubramanian, T. Green Synthesis of Silver Nanoparticles Using Marine Algae Caulerpa Racemosa and Their Antibacterial Activity against Some Human Pathogens. Appl. Nanosci. 2015, 5, 499–504. [Google Scholar] [CrossRef]
- Muddineti, O.S.; Ghosh, B.; Biswas, S. Current Trends in Using Polymer Coated Gold Nanoparticles for Cancer Therapy. Int. J. Pharm. 2015, 484, 252–267. [Google Scholar] [CrossRef]
- Rosi, N.L.; Mirkin, C.A. Nanostructures in Biodiagnostics. Chem. Rev. 2005, 105, 1547–1562. [Google Scholar] [CrossRef] [PubMed]
- Correard, F.; Maximova, K.; Estève, M.-A.; Villard, C.; Roy, M.; Al-Kattan, A.; Sentis, M.; Gingras, M.; Kabashin, A.V.; Braguer, D. Gold Nanoparticles Prepared by Laser Ablation in Aqueous Biocompatible Solutions: Assessment of Safety and Biological Identity for Nanomedicine Applications. Int. J. Nanomed. 2014, 9, 5415. [Google Scholar]
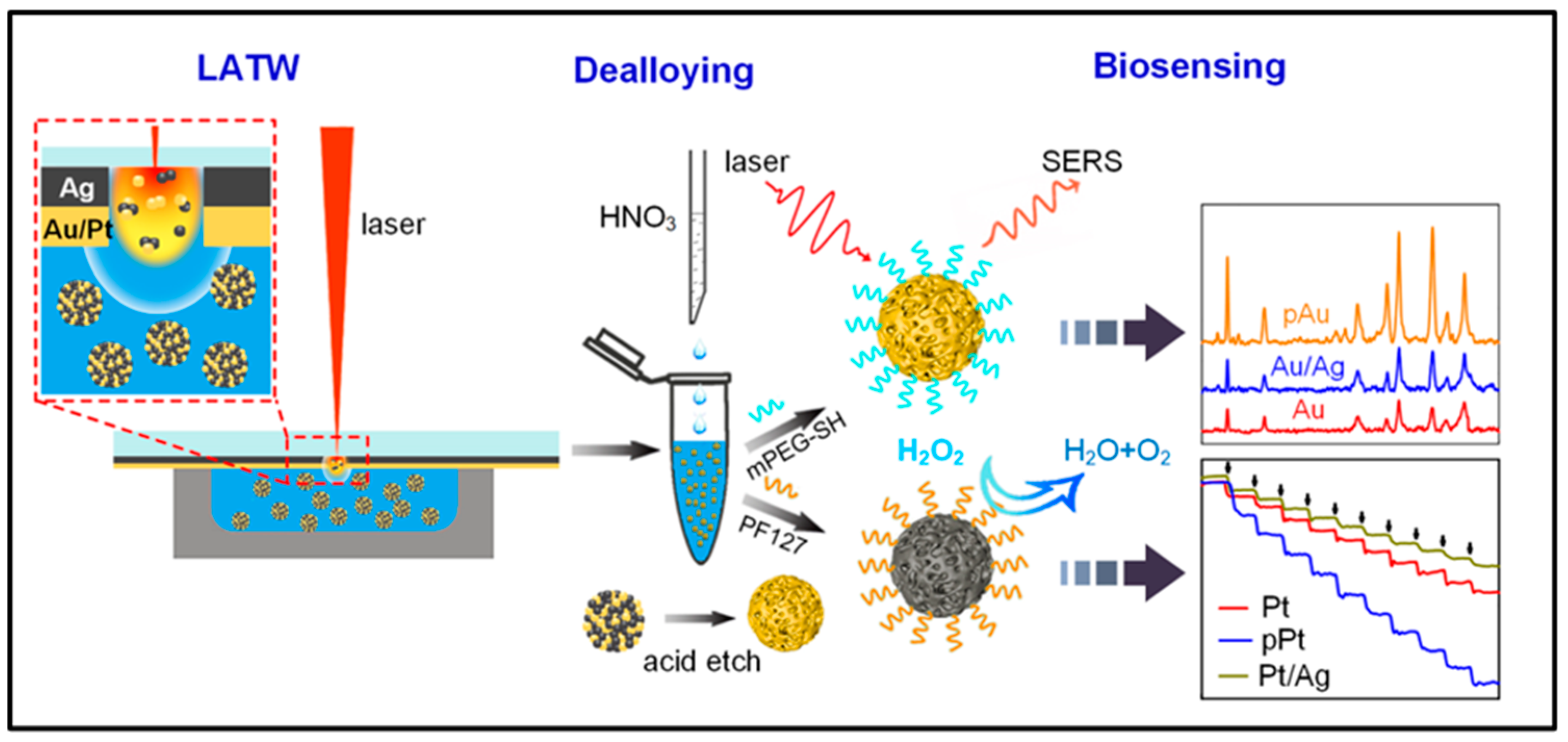
- Yang, Z.; Lin, Z.; Yang, J.; Wang, J.; Yue, J.; Liu, B.; Jiang, L. Fabrication of Porous Noble Metal Nanoparticles Based on Laser Ablation toward Water and Dealloying for Biosensing. Appl. Surf. Sci. 2022, 579, 152130. [Google Scholar] [CrossRef]
- Pandey, J.K.; Swarnkar, R.K.; Soumya, K.K.; Dwivedi, P.; Singh, M.K.; Sundaram, S.; Gopal, R. Silver Nanoparticles Synthesized by Pulsed Laser Ablation: As a Potent Antibacterial Agent for Human Enteropathogenic Gram-Positive and Gram-Negative Bacterial Strains. Appl. Biochem. Biotechnol. 2014, 174, 1021–1031. [Google Scholar] [CrossRef]
- Torrisi, L.; Torrisi, A. Gold Nanoparticles for Physics and Bio-Medicine Applications. Radiat. Eff. Defects Solids 2020, 175, 68–83. [Google Scholar] [CrossRef]
- Haque, S.; Patra, C.R. Chapter Eleven—Biosynthesized Nanoparticles (Gold, Silver and Platinum): Therapeutic Role in Angiogenesis. In Comprehensive Analytical Chemistry; Biosynthesized Nanomaterials; Verma, S.K., Das, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 94, pp. 471–505. [Google Scholar]
- Kumar, B.; Smita, K.; Angulo, Y.; Debut, A.; Cumbal, L. Honeybee Pollen Assisted Biosynthesis of Nanogold and Its Application as Catalyst in Reduction of 4-Nitrophenol. Heliyon 2022, 8, e10191. [Google Scholar] [CrossRef]
- Torrisi, L.; Restuccia, N. Laser-Generated Au Nanoparticles for Bio-Medical Applications. IRBM 2018, 39, 307–312. [Google Scholar] [CrossRef]
- Hashemi, S.F.; Tasharrofi, N.; Saber, M.M. Green Synthesis of Silver Nanoparticles Using Teucrium Polium Leaf Extract and Assessment of Their Antitumor Effects against MNK45 Human Gastric Cancer Cell Line. J. Mol. Struct. 2020, 1208, 127889. [Google Scholar] [CrossRef]
- Darvish, S.; Saeed Kahrizi, M.; Özbolat, G.; Khaleghi, F.; Mortezania, Z.; Sakhaei, D. Silver Nanoparticles: Biosynthesis and Cytotoxic Performance against Breast Cancer MCF-7 and MDA-MB-231 Cell Lines. Nanomed. Res. J. 2022, 7, 83–92. [Google Scholar]
- Soltani, L.; Darbemamieh, M. Biosynthesis of Silver Nanoparticles Using Hydroethanolic Extract of Cucurbita Pepo L. Fruit and Their Anti-Proliferative and Apoptotic Activity Against Breast Cancer Cell Line (MCF-7). Multidiscip. Cancer Investig. 2021, 5, 1–10. [Google Scholar] [CrossRef]
- Paidari, S.; Ibrahim, S.A. Potential Application of Gold Nanoparticles in Food Packaging: A Mini Review. Gold Bull. 2021, 54, 31–36. [Google Scholar] [CrossRef]
- Kurtjak, M.; Vukomanović, M.; Suvorov, D. Antibacterial Nanocomposite of Functionalized Nanogold and Gallium-Doped Hydroxyapatite. Mater. Lett. 2017, 193, 126–129. [Google Scholar] [CrossRef]
- Nie, B.; Luo, Y.; Shi, J.; Gao, L.; Duan, G. Bowl-like Pore Array Made of Hollow Au/Ag Alloy Nanoparticles for SERS Detection of Melamine in Solid Milk Powder. Sens. Actuators B Chem. 2019, 301, 127087. [Google Scholar] [CrossRef]
- Demirezen Yılmaz, D.; Aksu Demirezen, D.; Mıhçıokur, H. Colorimetric Detection of Mercury Ion Using Chlorophyll Functionalized Green Silver Nanoparticles in Aqueous Medium. Surf. Interfaces 2021, 22, 100840. [Google Scholar] [CrossRef]
- Sharma, R. Synthesis of Terminalia Bellirica Fruit Extract Mediated Silver Nanoparticles and Application in Photocatalytic Degradation of Wastewater from Textile Industries. Mater. Today Proc. 2021, 44, 1995–1998. [Google Scholar] [CrossRef]
- Paturi, U.M.R.; Kumar, G.N.; Vamshi, V.S. Silver Nanoparticle-Based Tween 80 Green Cutting Fluid (AgNP-GCF) Assisted MQL Machining—An Attempt towards Eco-Friendly Machining. Clean. Eng. Technol. 2020, 1, 100025. [Google Scholar] [CrossRef]
- Anvar, A.; Haghighat Kajavi, S.; Ahari, H.; Sharifan, A.; Motallebi, A.; Kakoolaki, S.; Paidari, S. Evaluation of the Antibacterial Effects of Ag-Tio2 Nanoparticles and Optimization of Its Migration to Sturgeon Caviar (Beluga). Iran. J. Fish. Sci. 2019, 18, 954–967. [Google Scholar]
- Ming, W.; Zhang, S.; Zhang, G.; Du, J.; Ma, J.; He, W.; Cao, C.; Liu, K. Progress in Modeling of Electrical Discharge Machining Process. Int. J. Heat Mass Transf. 2022, 187, 122563. [Google Scholar] [CrossRef]
- Ming, W.; Cao, C.; Shen, F.; Zhang, Z.; Liu, K.; Du, J.; Jia, H. Numerical and Experimental Study on WEDM of BN-AlN-TiB2 Composite Ceramics Based on a Fusion FEM Model. J. Manuf. Process. 2022, 76, 138–154. [Google Scholar] [CrossRef]
- Ming, W.; Guo, X.; Xu, Y.; Zhang, G.; Jiang, Z.; Li, Y.; Li, X. Progress in Non-Traditional Machining of Amorphous Alloys. Ceram. Int. 2022, 49, 1585–1604. [Google Scholar] [CrossRef]
- Yao, Q.; Feng, Y.; Fung, V.; Yu, Y.; Jiang, D.; Yang, J.; Xie, J. Precise Control of Alloying Sites of Bimetallic Nanoclusters via Surface Motif Exchange Reaction. Nat. Commun. 2017, 8, 1555. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, W.; Wang, C.; Zheng, Z. Optimization and Modeling for One-Step Synthesis Process of Ag–Cu Nano-Particles Using DOE Methodology. J. Mater. Sci. Mater. Electron. 2016, 27, 4265–4274. [Google Scholar] [CrossRef]
- Dehghani, S.; Peighambardoust, S.H.; Peighambardoust, S.J.; Fasihnia, S.H.; Khosrowshahi, N.K.; Gullón, B.; Lorenzo, J.M. Optimization of the Amount of ZnO, CuO, and Ag Nanoparticles on Antibacterial Properties of Low-Density Polyethylene (LDPE) Films Using the Response Surface Method. Food Anal. Methods 2021, 14, 98–107. [Google Scholar] [CrossRef]
- Shabanzadeh, P.; Yusof, R.; Shameli, K. Neural Network Modelling for Prediction Size of Silver Nanoparticles in Montmorillonite/Starch Synthesis by Chemical Reduction Method. Dig. J. Nanomater. Biostruct. 2014, 9, 1699–1711. [Google Scholar]



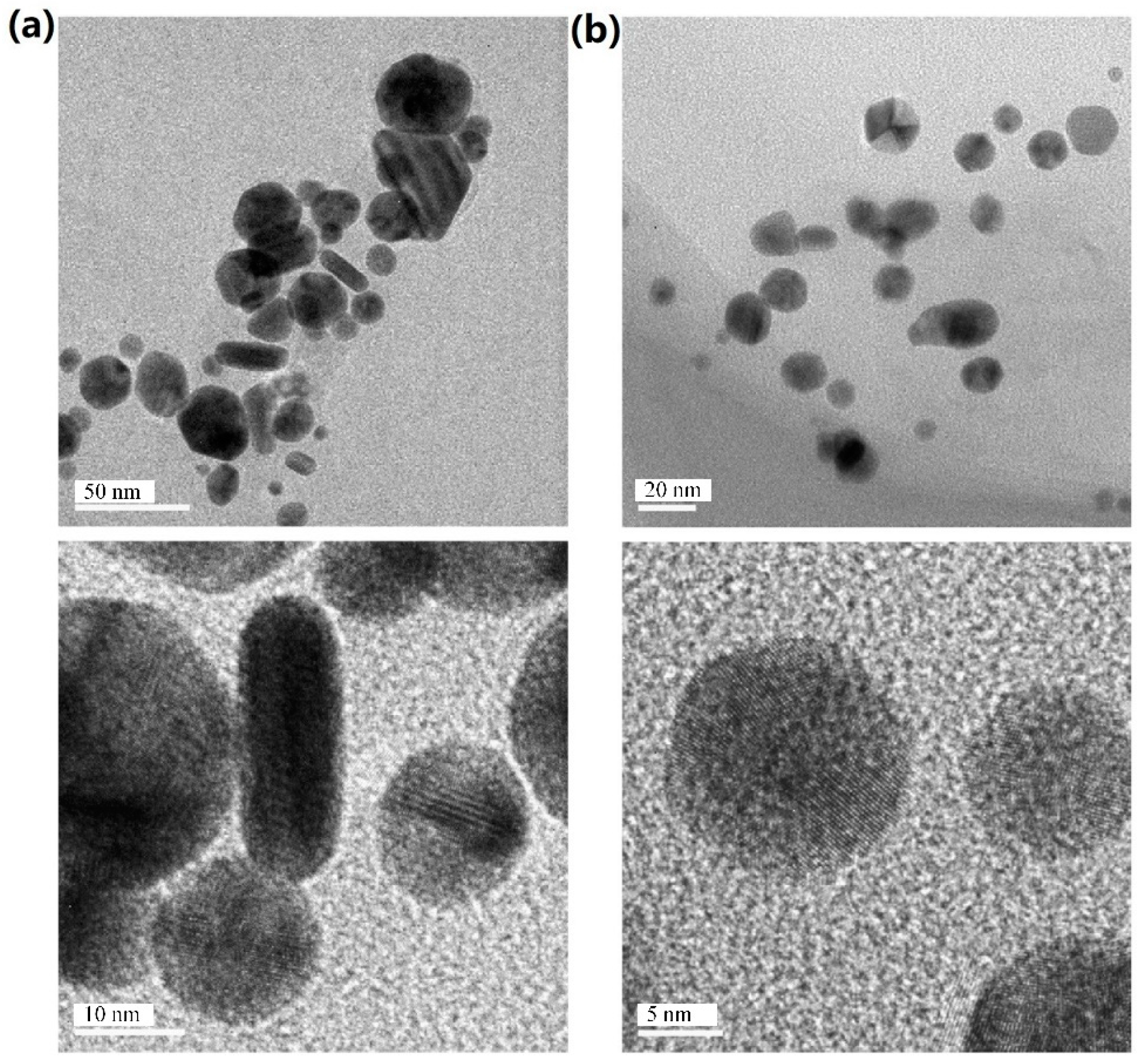


| NPs | Methods | Size (nm) | Shape | TEM Images | References |
|---|---|---|---|---|---|
| Ag | LAL | 100–200 nm | Spherical |  | [16] |
| Ag | LAL | 5–140 nm | Spherical |  | [42] |
| Ag | LAL. | 1.66 ± 0.37 nm | Catenary | 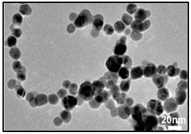 | [46] |
| Au | LAL | 20–40 nm | Spherical |  | [34] |
| Au | LAL | 20 nm | Spherical |  | [43] |
| Au | LAL | 20–30 nm | Spherical |  | [47] |
| Au | LAL (nanosecond pulsed) | 20 nm | Fractal structures |  | [30] |
| Methods | NPs | Species | Size (nm) | Shape | References |
|---|---|---|---|---|---|
| Plants-mediated synthesis | Au/Ag | Phylloxacin extract | 20–100 | Spherical-, triangular-, hexagonal- and rod-shaped with irregular contours | [49] |
| Ag | Sterile geranium leaves extract | 20–40 | Rods, flat sheets and triangular | [46] | |
| Au | Pomegranate fruit extract | 10–50 | Spherical shape | [50] | |
| Au-Ag | Water leaf extract of wheat | 5–30 | Spherical to elliptical shapes | [53] | |
| Microbes-mediated synthesis | Au | V. luteoalbum and Isolate 6–3 | A few to approximately 100 | Spherical shape | [56] |
| Ag | Fungus “Fusarium hemitectum” | 10–60 | Spherical shape | [57] | |
| Au | Fungal enzymes | 8–40 | Spherical and triangular shapes | [59] | |
| Au | A novel alkalotolerant actinomycete | 5–15 | Spherical shape | [60] | |
| Algae-mediated synthesis | Au | A novel Ecklonia cava | Average particle size of 30 ± 0.25 nm | Spherical and triangular shapes | [68] |
| Ag | Australian brown alga Nostoc algae | 75–2000 | Spherical and polydispersed shapes | [71] | |
| Au | Clover | 5–35 | Spherical and triangular shapes | [60] | |
| Ag | Amaranth | 5–25 | Spherical and triangular shapes | [75] |
| Year | NPs | Application Fields | Remarks | References |
|---|---|---|---|---|
| 2021 | Au | Food packaging | Gold nanoparticles can be used in the nano packaging industry. | [88] |
| 2017 | Au | Antibacterial nanocomposite material | Gold nanoparticles have non-toxic antibacterial properties against Escherichia coli and Staphylococcus aureus. | [89] |
| 2020 | Au/Ag | SERS detection of melamine | The Au/Ag BPHAN array exhibited a strong SERS performance. | [90] |
| 2021 | Ag | Sewage detection | The recovery of this method was between 94–97%. | [91] |
| 2021 | Ag | Wastewater degradation | The synthesis method of the green nanoparticles can be used for antibacterial and catalytic action. | [92] |
| 2020 | Ag | Environmentally friendly cutting fluid | MQL machining assisted by 0.6 wt% AgNP-GCF is 55% more effective in reducing surface roughness. | [93] |
| 2019 | Ag | Food packaging | The growth of fungi and bacteria is reduced. | [94] |
| Methods | Advantages | Disadvantages | Remarks |
|---|---|---|---|
| Laser ablation process | Low cost for mass production | The initial investment cost is high, and the process involves many parameters. | An important development direction of mass production in the future |
| Biosynthetic method | Low initial investment cost | It is difficult to control the size and shape of Au/Ag NP. | The mechanism of the process needs to be further studied to improve the controllability of Au/Ag NPs preparation. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Li, L.; Huang, H.; He, W.; Ming, W. Progress in Laser Ablation and Biological Synthesis Processes: “Top-Down” and “Bottom-Up” Approaches for the Green Synthesis of Au/Ag Nanoparticles. Int. J. Mol. Sci. 2022, 23, 14658. https://doi.org/10.3390/ijms232314658
Jiang Z, Li L, Huang H, He W, Ming W. Progress in Laser Ablation and Biological Synthesis Processes: “Top-Down” and “Bottom-Up” Approaches for the Green Synthesis of Au/Ag Nanoparticles. International Journal of Molecular Sciences. 2022; 23(23):14658. https://doi.org/10.3390/ijms232314658
Chicago/Turabian StyleJiang, Zhiwen, Liwei Li, Hao Huang, Wenbin He, and Wuyi Ming. 2022. "Progress in Laser Ablation and Biological Synthesis Processes: “Top-Down” and “Bottom-Up” Approaches for the Green Synthesis of Au/Ag Nanoparticles" International Journal of Molecular Sciences 23, no. 23: 14658. https://doi.org/10.3390/ijms232314658
APA StyleJiang, Z., Li, L., Huang, H., He, W., & Ming, W. (2022). Progress in Laser Ablation and Biological Synthesis Processes: “Top-Down” and “Bottom-Up” Approaches for the Green Synthesis of Au/Ag Nanoparticles. International Journal of Molecular Sciences, 23(23), 14658. https://doi.org/10.3390/ijms232314658








