Preparation, Properties, and Mechanism of Flame-Retardant Poly(vinyl alcohol) Aerogels Based on the Multi-Directional Freezing Method
Abstract
1. Introduction
2. Results and Discussion
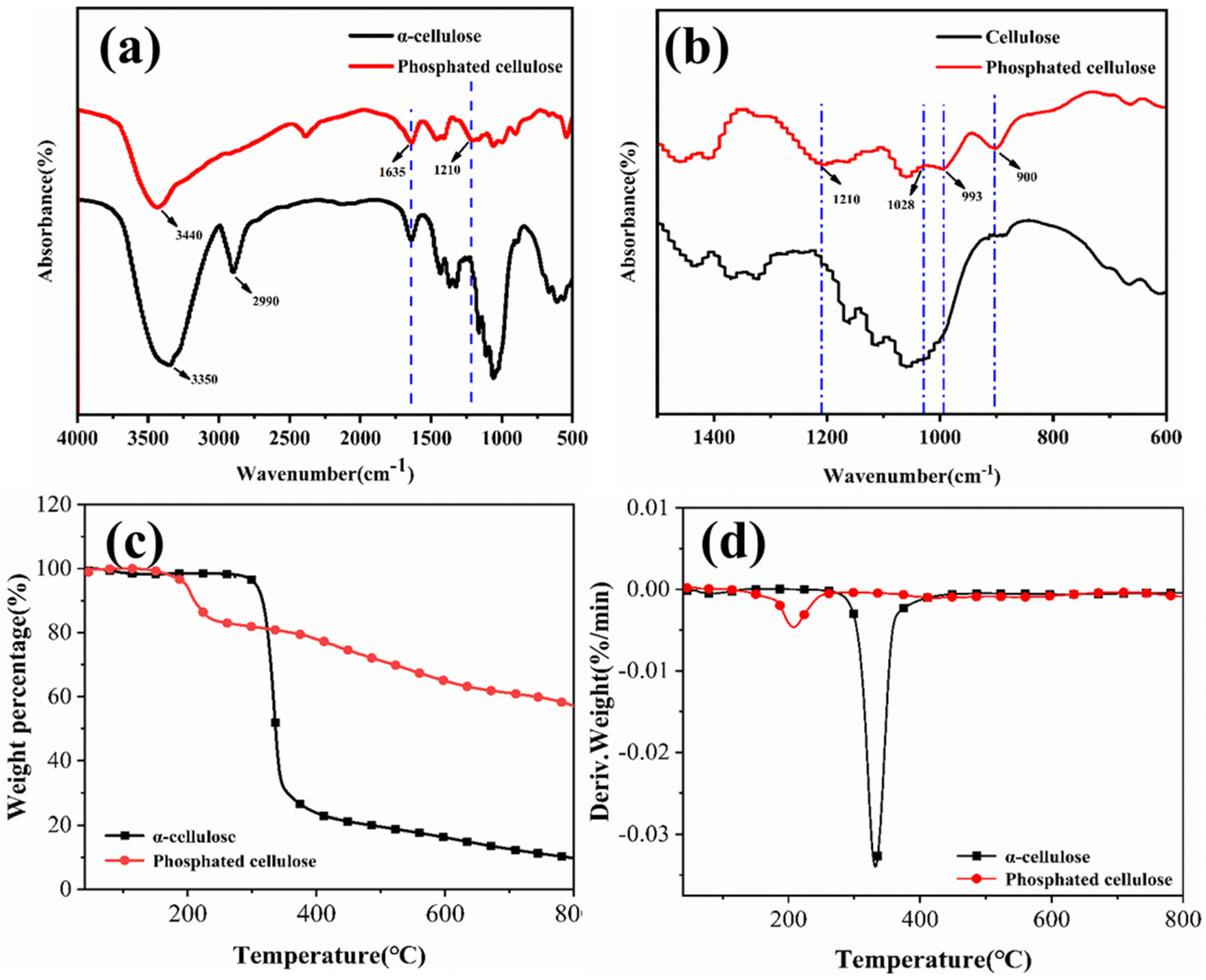
2.1. Structural and Morphological Analyses of PCF
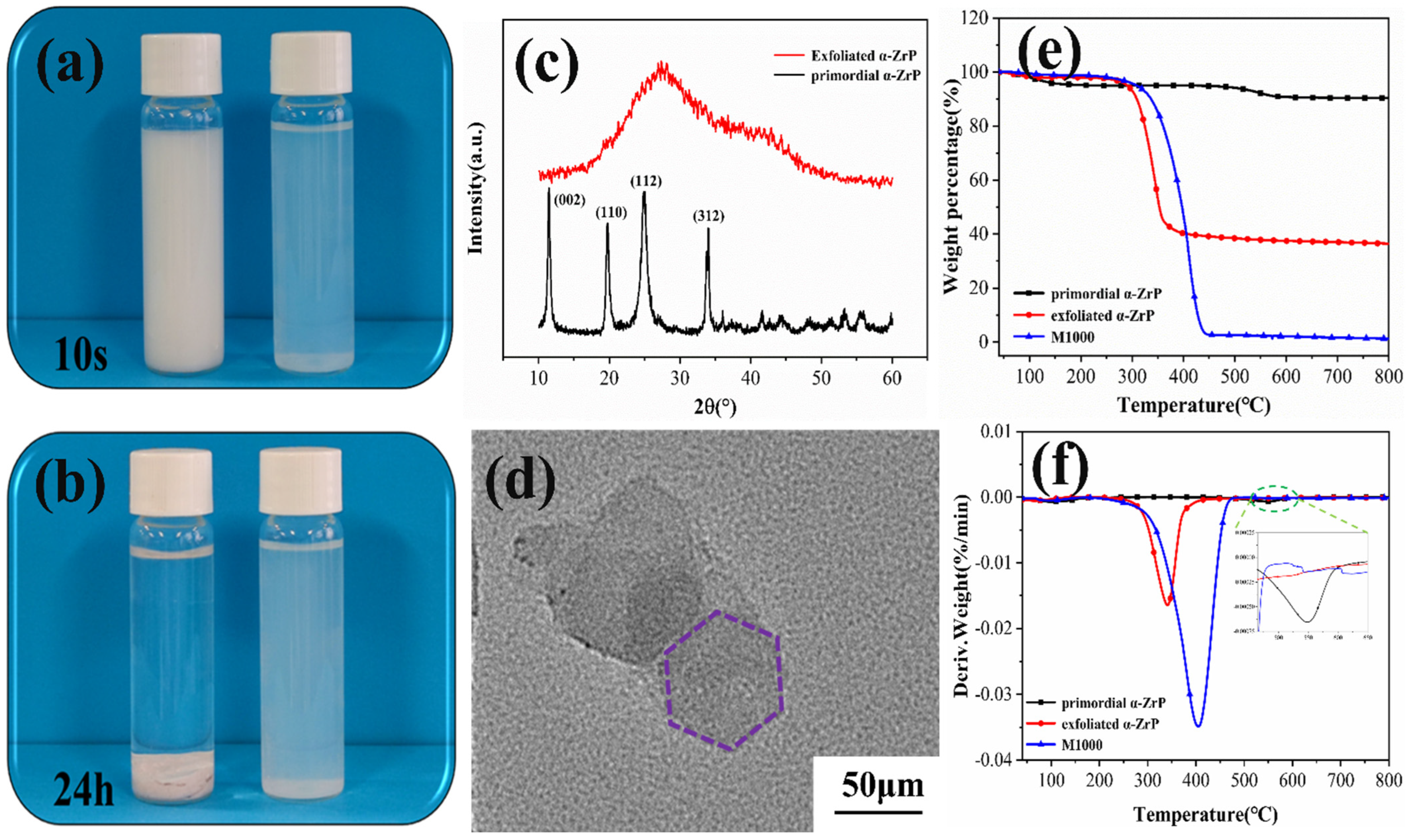
2.2. Chemical Structure and Morphological Analysis of α-ZrP
2.3. Discussion on the Unidirectional PVA Aerogels
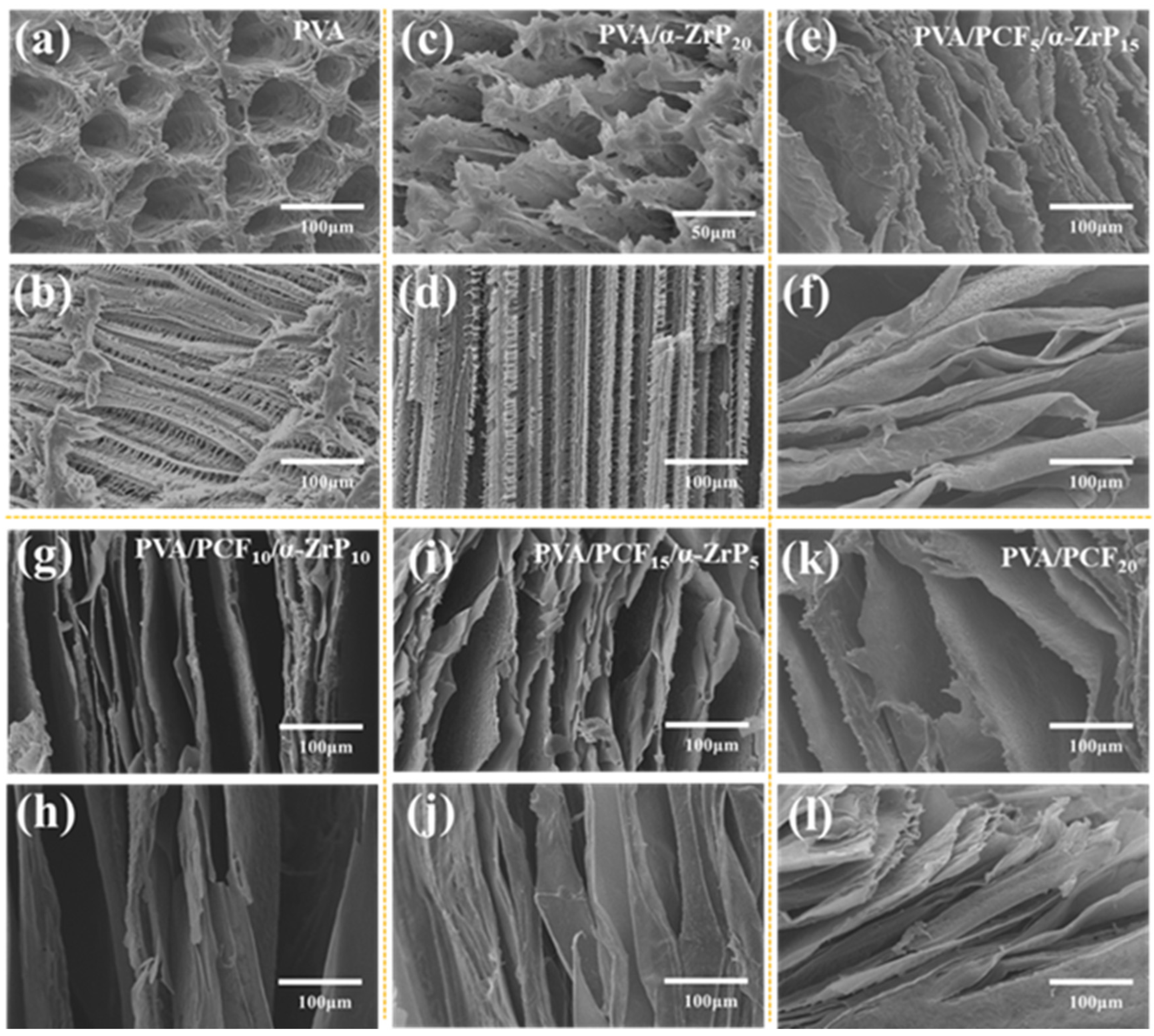
2.3.1. Formation and Structure of the Unidirectional PVA Aerogels
2.3.2. Mechanical Properties of the Unidirectional PVA Aerogels
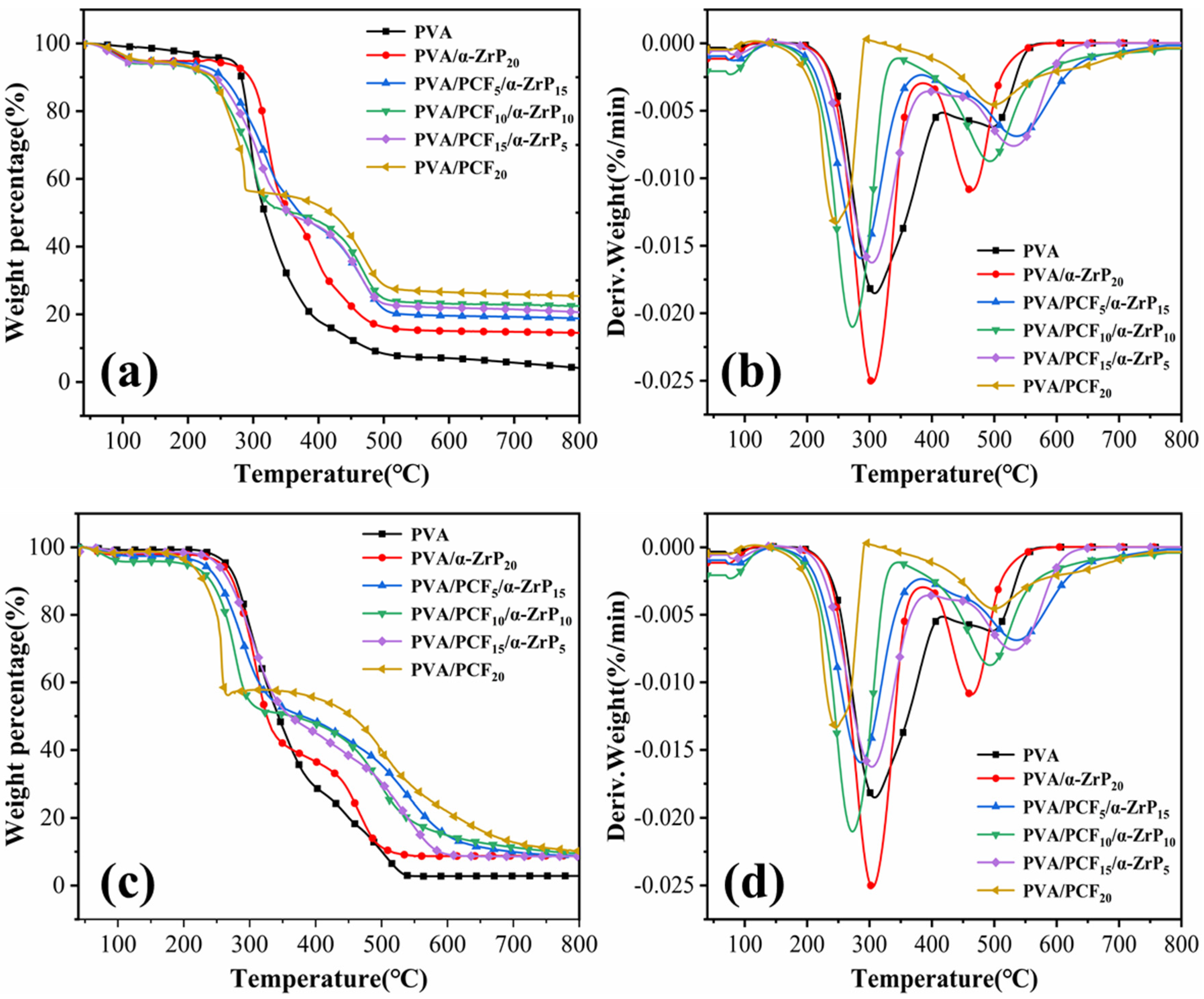
2.3.3. Thermal Stabilities of Unidirectional PVA Aerogels
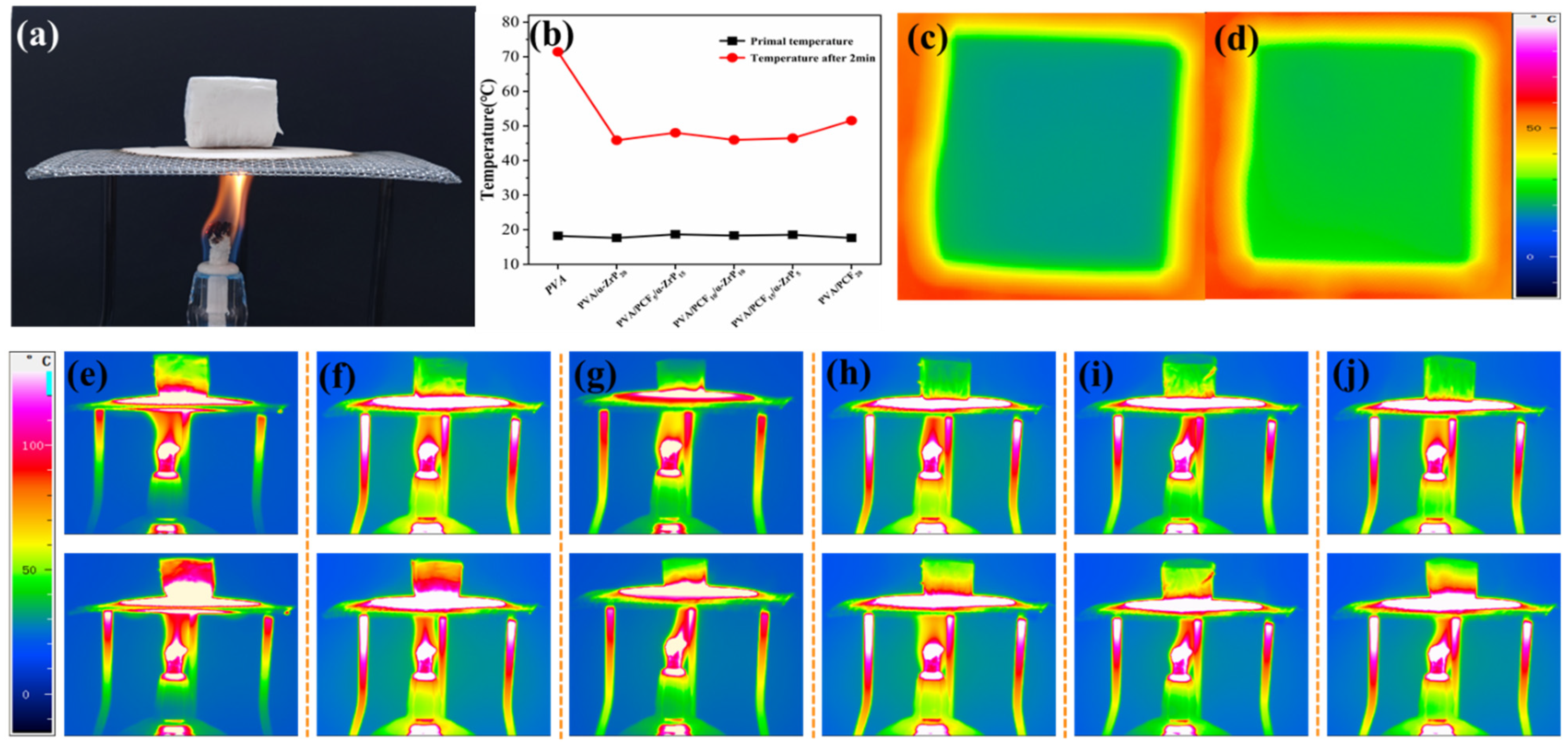
2.3.4. Thermal Insulation Properties of the Unidirectional PVA Aerogels
2.4. Discussion of the Multi-Directional PVA/PCF10/α-ZrP10-x Aerogels
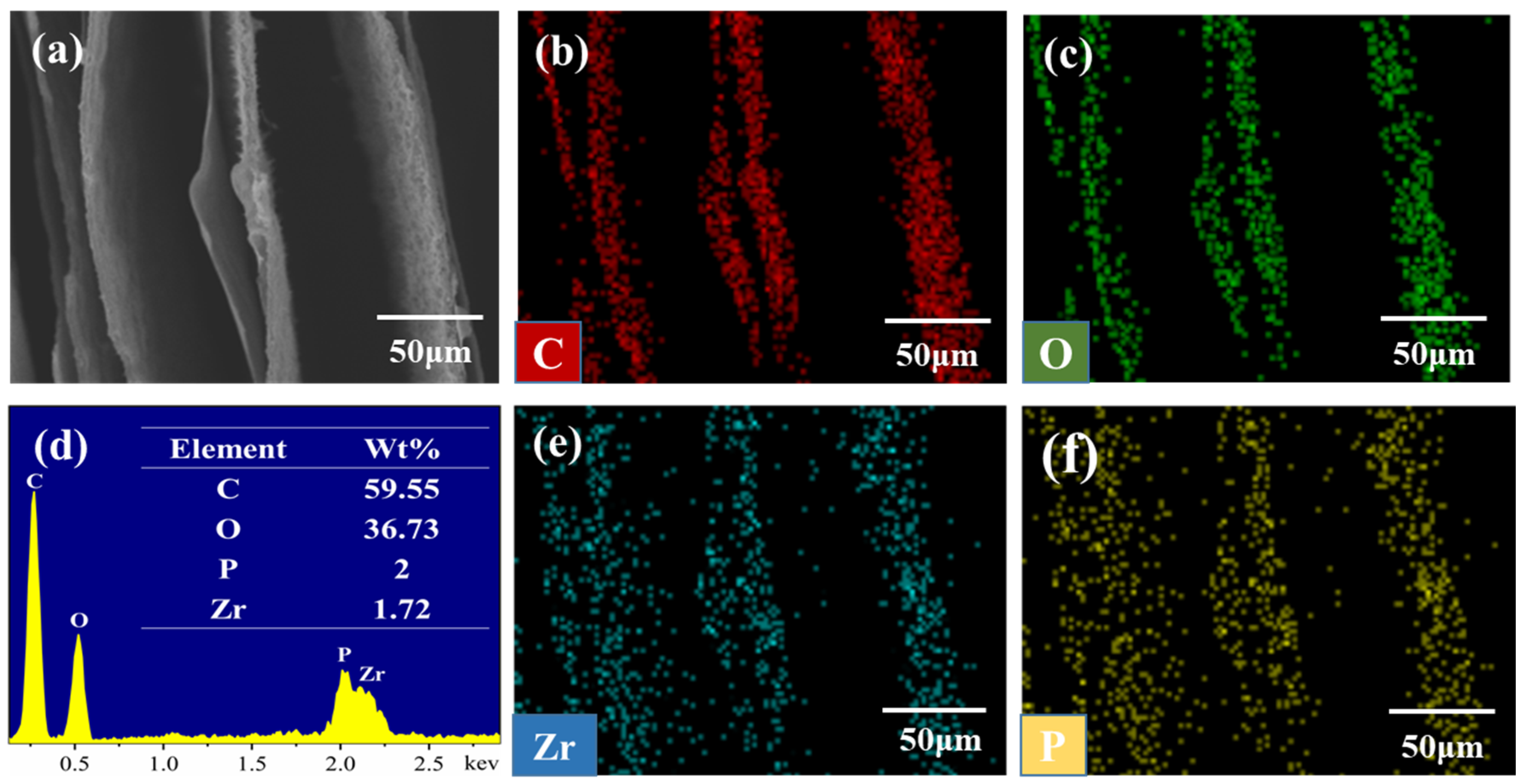
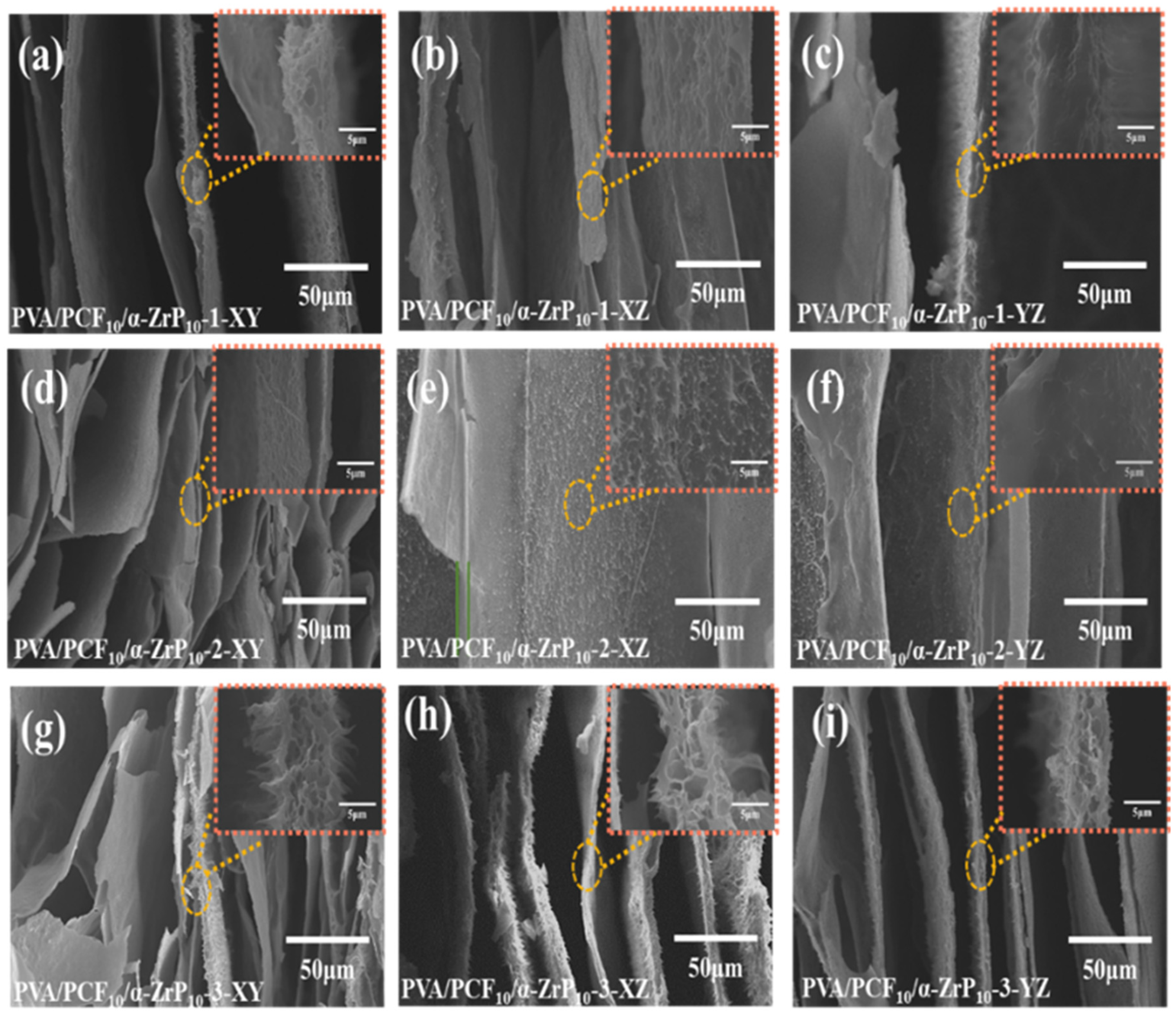
2.4.1. Formation and Structure of the Multi-Directional PVA/PCF10/α-ZrP10-x Aerogels
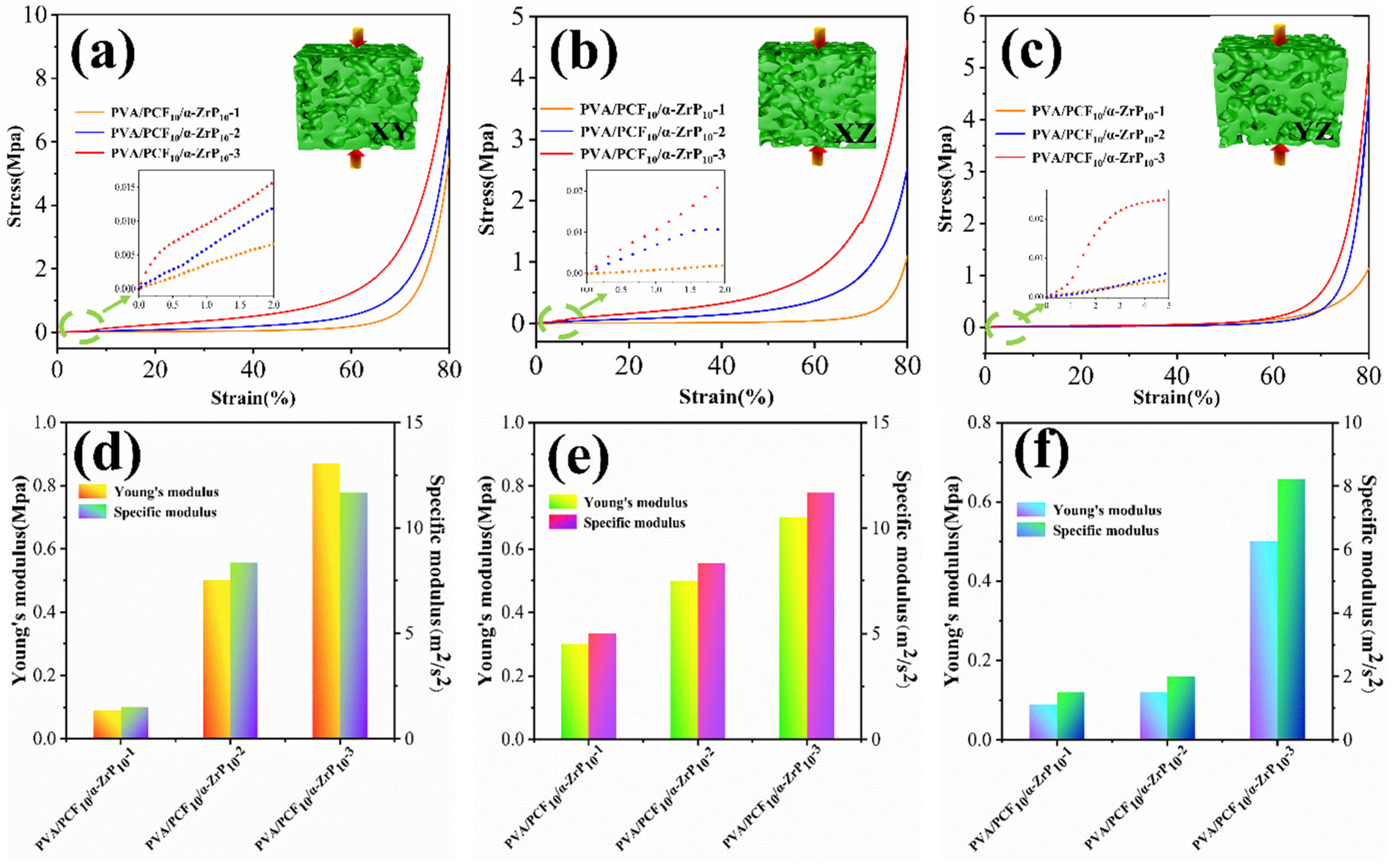
2.4.2. Mechanical Properties of Multi-Directional PVA/PCF10/α-ZrP10-x Aerogels
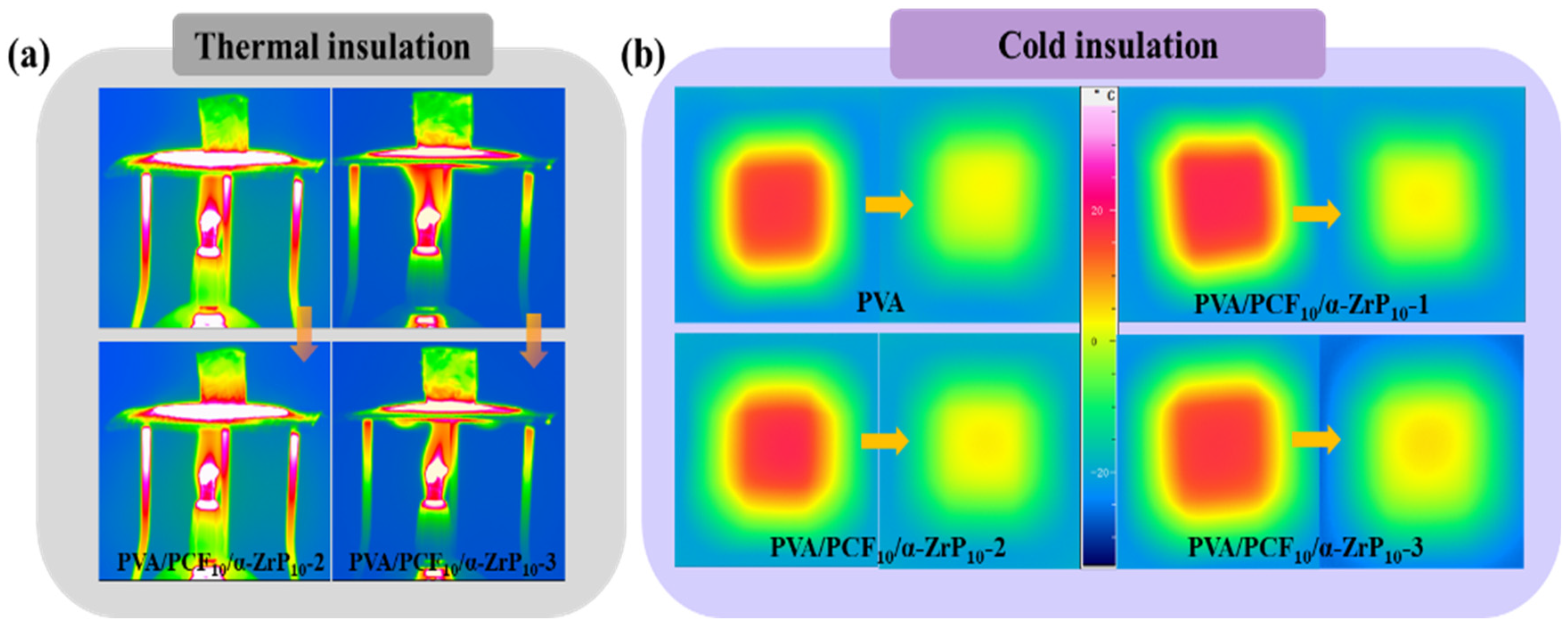
2.5. Thermal Insulation Properties of Aerogels
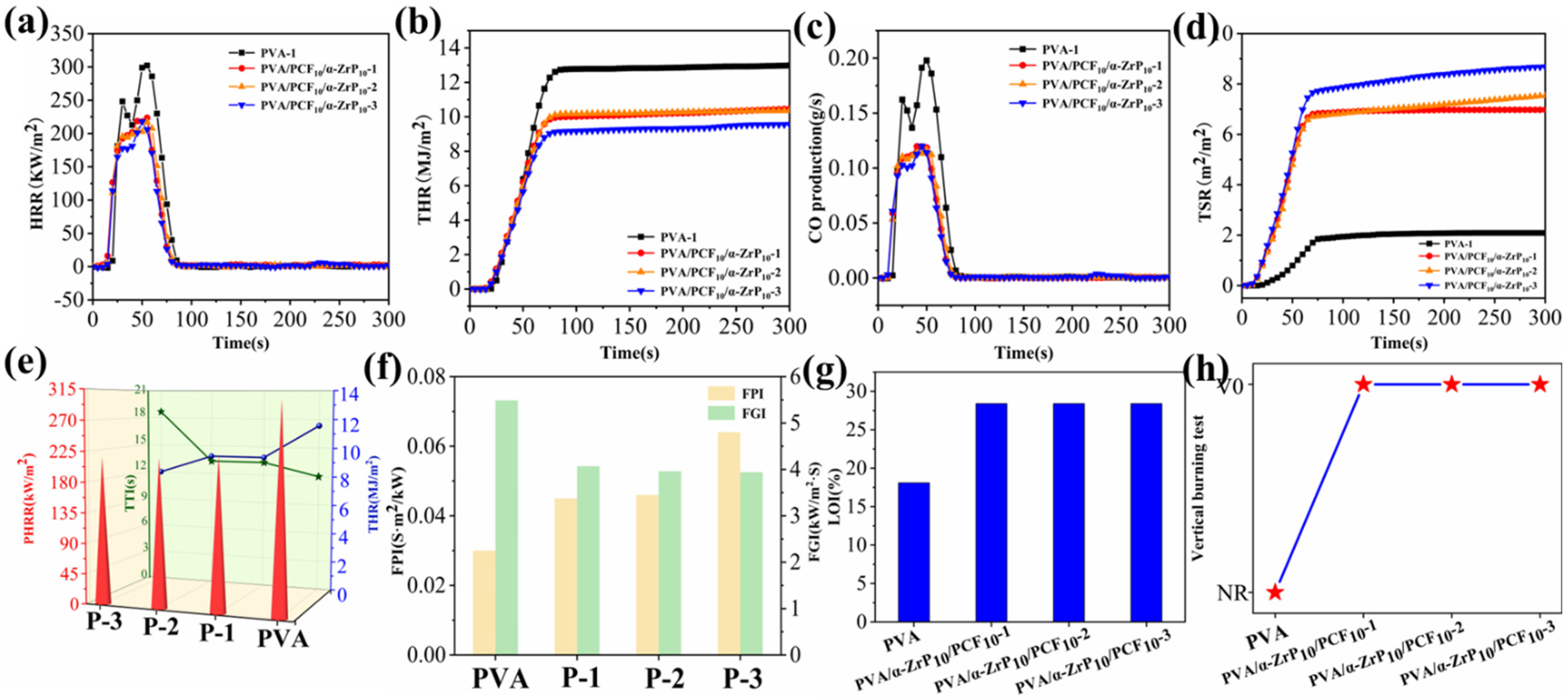
2.6. Combustion Behaviors of Aerogels
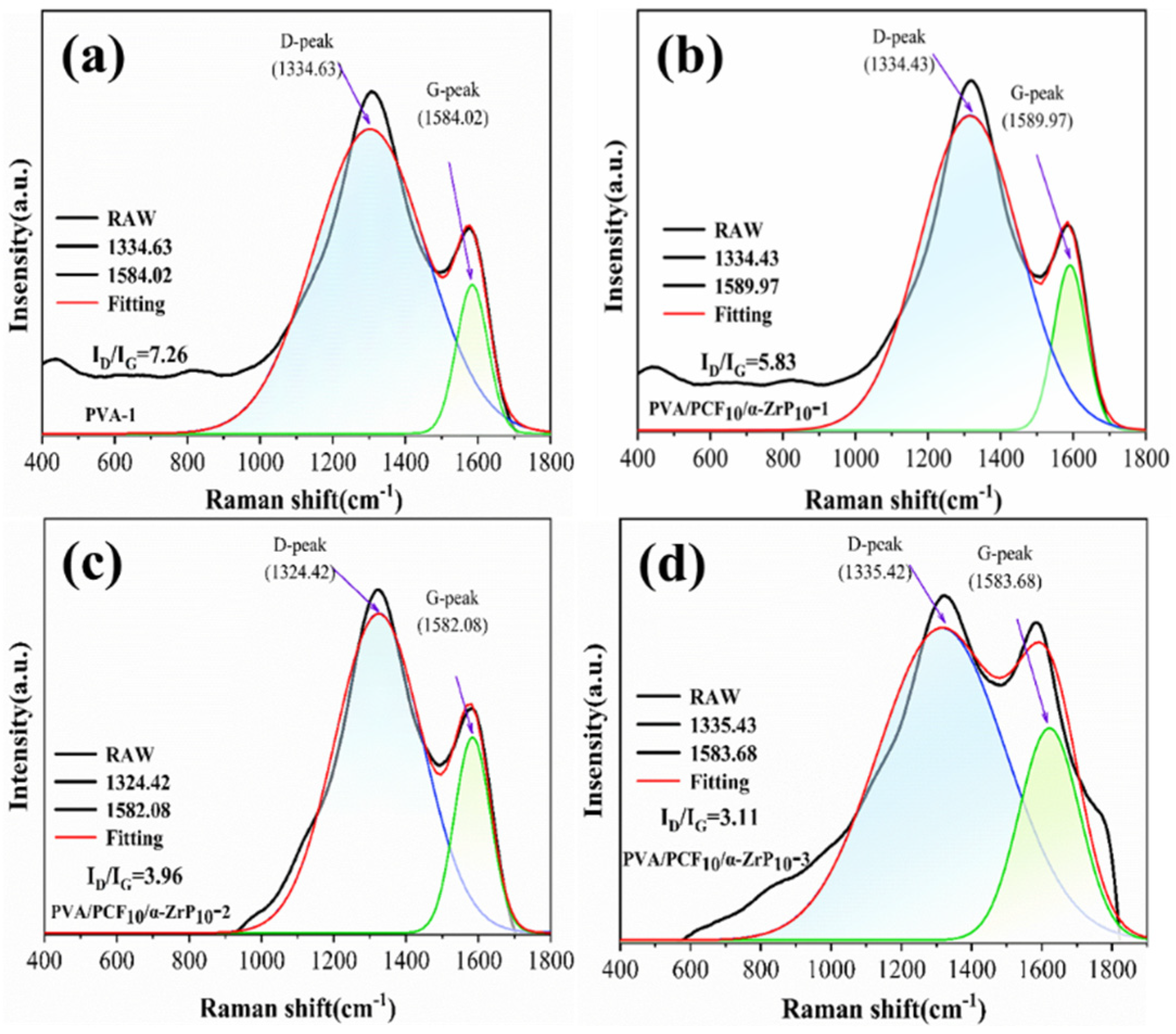
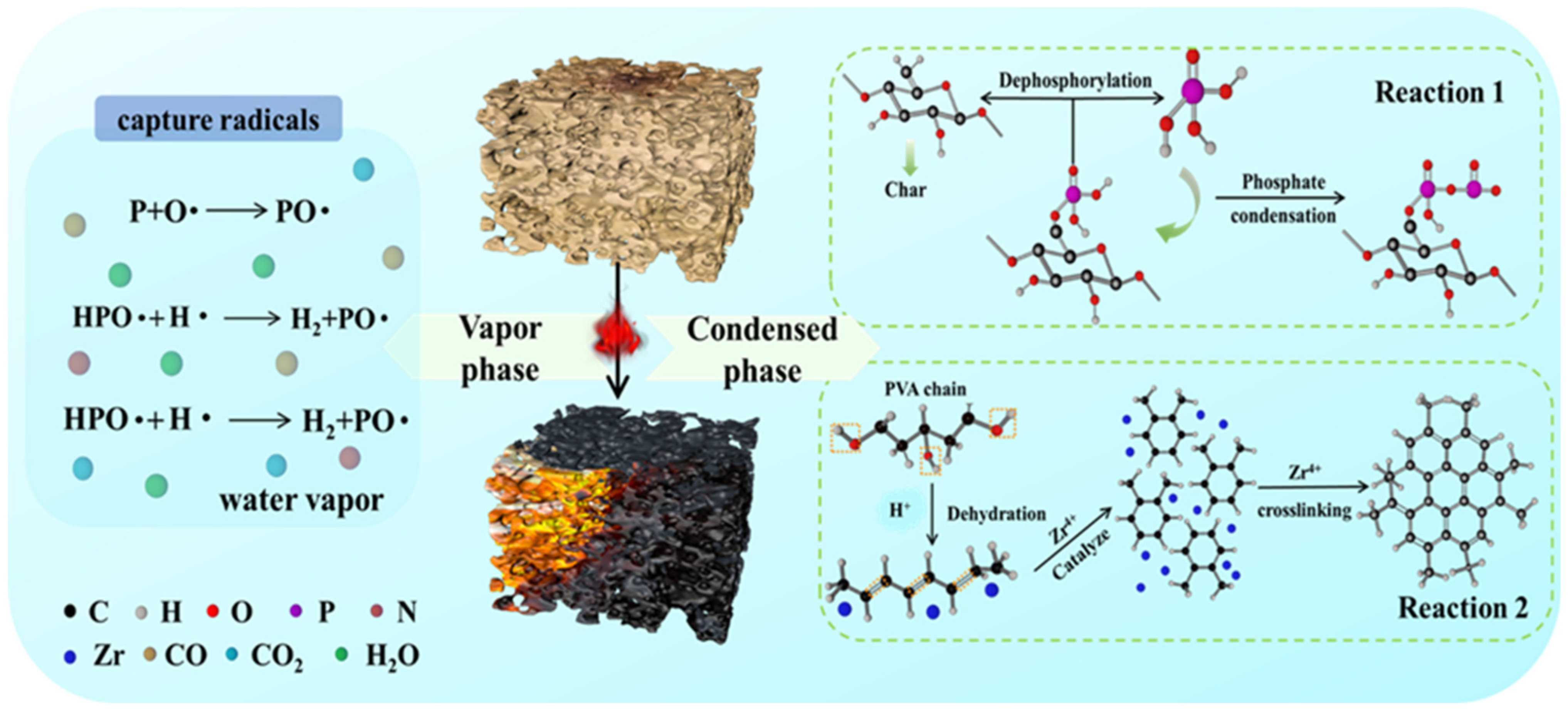
2.7. Mechanism of Flame Retardancy
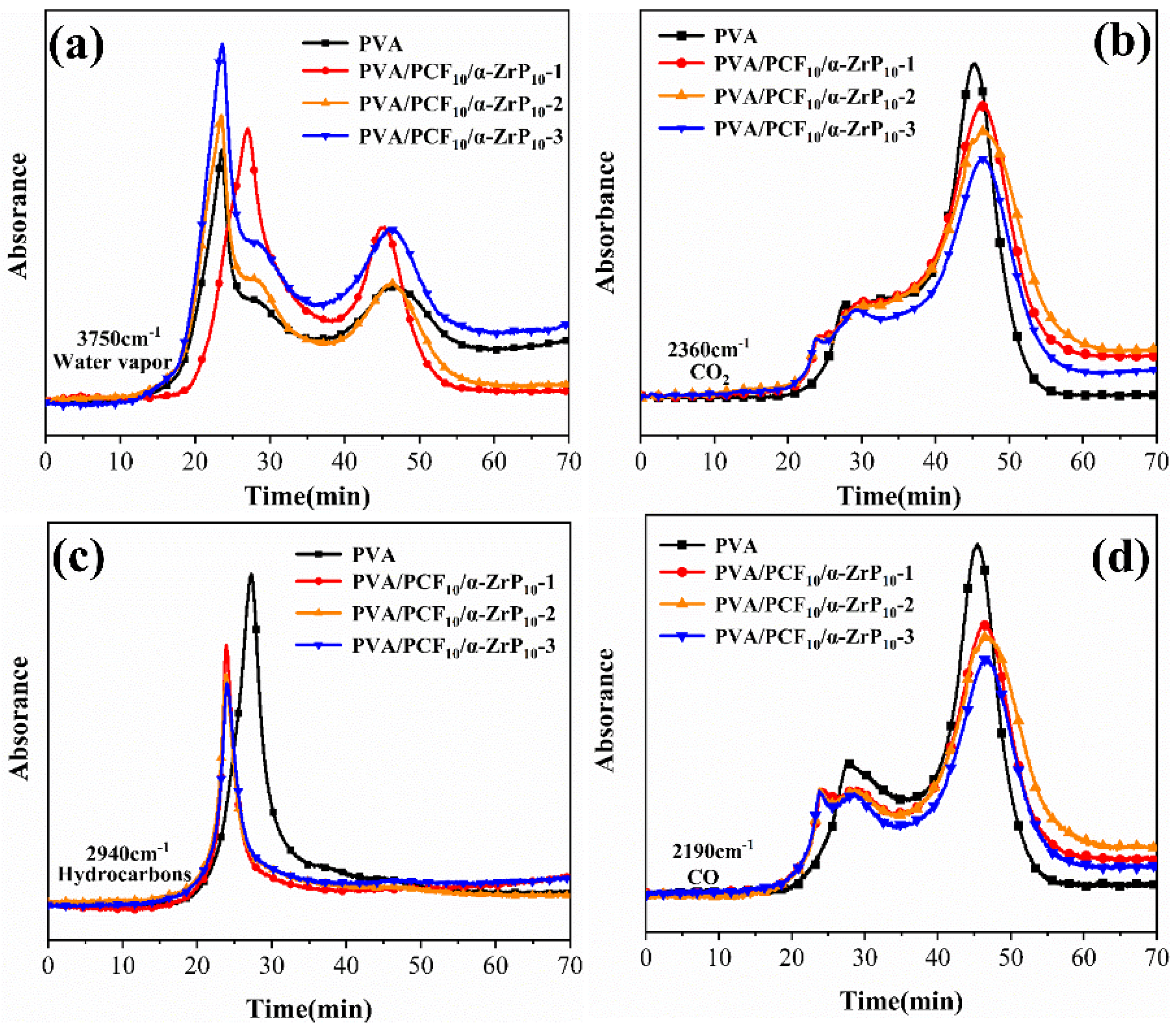
2.7.1. Vapor Phase Analysis
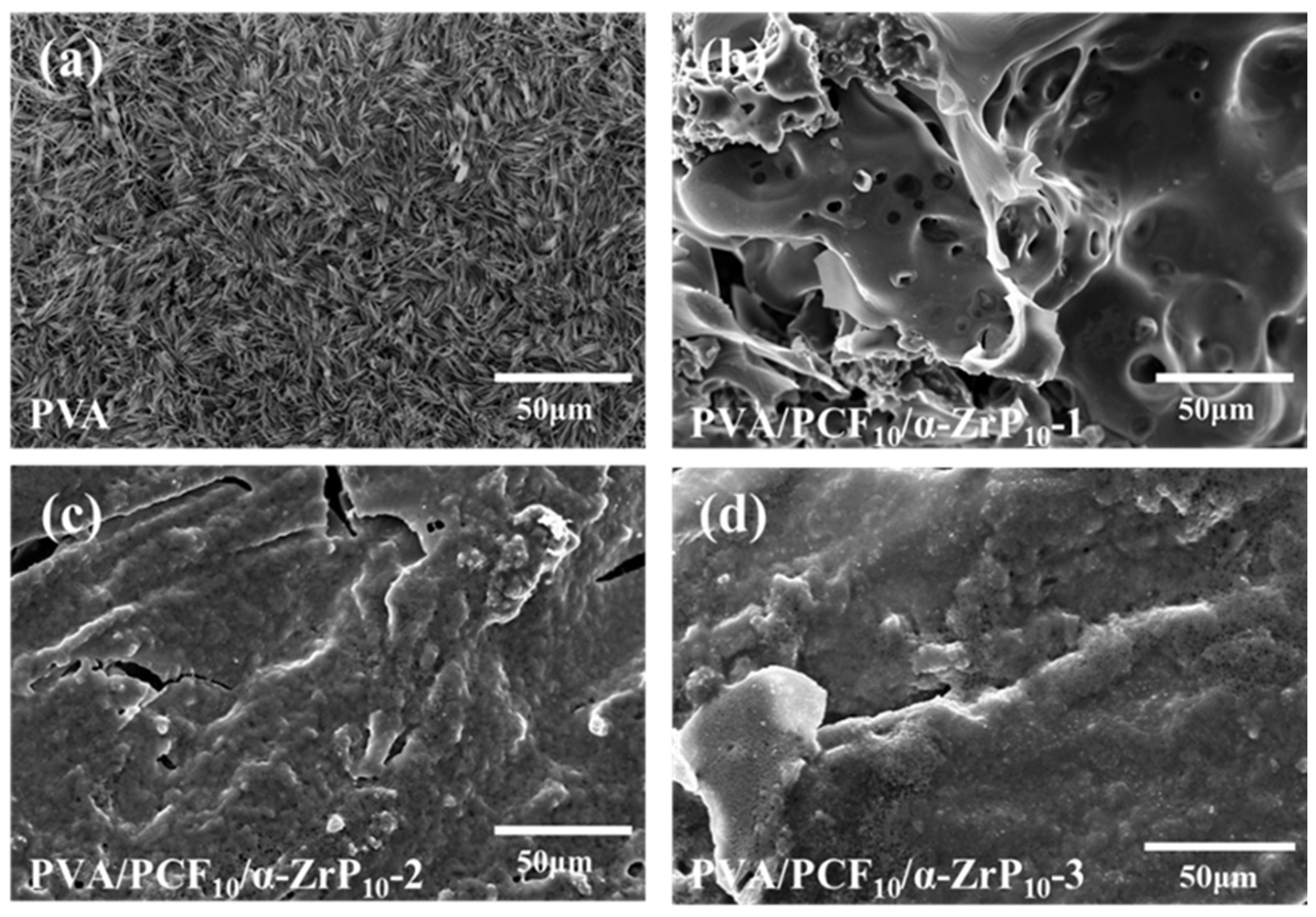
2.7.2. Condensed Phase Analysis
2.7.3. Proposed Mechanism of Flame Retardancy
3. Materials and Methods
3.1. Materials
3.2. Fabrication
3.2.1. Preparation of Phosphated Cellulose
3.2.2. Preparation and Exfoliation α-ZrP
3.2.3. Fabrication of the Unidirectional PVA Aerogel
3.2.4. Fabrication of the Multi-Directional PVA Aerogel
3.3. Measurements and Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zu, G.; Shen, J.; Zou, L.; Wang, W.; Lian, Y.; Zhang, Z.; Du, A. Nanoengineering Super Heat-Resistant, Strong Alumina Aerogels. Chem. Mater. 2013, 25, 4757–4764. [Google Scholar] [CrossRef]
- Zhao, S.; Malfait, W.J.; Demilecamps, A.; Zhang, Y.; Brunner, S.; Huber, L.; Tingaut, P.; Rigacci, A.; Budtova, T.; Koebel, M.M. Strong, Thermally Superinsulating Biopolymer–Silica Aerogel Hybrids by Cogelation of Silicic Acid with Pectin. Angew. Chem. Int. Ed. 2015, 54, 14282–14286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Meng, D.; Ma, Z.; Zhang, Z.; Ning, H.; Wang, Y. Synthesis of a novel organic–inorganic hybrid flame retardant based on Ca(H2PO4)2 and hexachlorocyclotriphosphazene and its performance in polyvinyl alcohol. J. Appl. Polym. Sci. 2020, 138, 1–13. [Google Scholar]
- Hou, X.-B.; Mao, Y.-Q.; Zhang, R.-B.; Fang, D.-N. Super-flexible polyimide nanofiber cross-linked polyimide aerogel mem-branes for high efficient flexible thermal protection. Chem. Eng. J. 2021, 417, 129341–129349. [Google Scholar] [CrossRef]
- Troncoso, O.P.; Torres, F.G. Bacterial Cellulose—Graphene Based Nanocomposites. Int. J. Mol. Sci. 2020, 21, 6532. [Google Scholar] [CrossRef]
- Chen, K.; Zhang, H. Alginate/pectin aerogel microspheres for controlled release of proanthocyanidins. Int. J. Biol. Macromol. 2019, 136, 936–943. [Google Scholar] [CrossRef]
- Chen, J.; Shi, X.; Ren, L.; Wang, Y. Graphene oxide/PVA inorganic/organic interpenetrating hydrogels with excellent me-chanical properties and biocompatibility. Carbon 2017, 111, 18–27. [Google Scholar] [CrossRef]
- Chen, H.-B.; Wang, Y.-Z.; Schiraldi, D.A. Preparation and Flammability of Poly(vinyl alcohol) Composite Aerogels. ACS Appl. Mater. Interfaces 2014, 6, 6790–6796. [Google Scholar] [CrossRef]
- Shang, K.; Ye, D.-D.; Kang, A.-H.; Wang, Y.-T.; Liao, W.; Xu, S.-M.; Wang, Y.-Z. Robust andfire retardant borate-crosslinked poly (vinyl alcohol)/montmorillonite aerogel via melt-crosslink. Polymer 2017, 131, 111–119. [Google Scholar] [CrossRef]
- Wang, H.; Cao, M.; Zhao, H.-B.; Liu, J.-X.; Geng, C.-Z.; Wang, Y.-Z. Double-cross-linked aerogels towards ultrahigh mechanical properties and thermal insulation at extreme environment. Chem. Eng. J. 2020, 399, 125698. [Google Scholar] [CrossRef]
- Wang, L.; Sánchez-Soto, M.; Maspoch, M.L. Polymer/clay aerogel composites with flame retardant agents: Mechanical, thermal and fire behavior. Mater. Des. 2013, 52, 609–614. [Google Scholar] [CrossRef]
- Wu, N.-J.; Niu, F.-K.; Lang, W.-C.; Xia, M.-F. Highly efficientflame-retardant and low-smoke-toxicity poly(vinyl alcohol)/alginate/ montmorillonite composite aerogels by two-step crosslinking strategy. Carbohydr. Polym. 2019, 221, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Hu, W.-Z.; Hu, Y. Polydopamine-Bridged Synthesis of Ternary h-BN@PDA@TiO2 as Nanoenhancers for Thermal Conductivity and Flame Retardant of Polyvinyl Alcohol. Front. Chem. 2020, 8, 587474–587485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Z.; Qi, X.; Zhang, W.; Wang, D.-Y. Size tailored bimetallic metal-organic framework (MOF) on graphene oxide with sandwich-like structure as functional nano-hybrids for improving fire safety of epoxy. Compos. Part B Eng. 2020, 188, 107881. [Google Scholar] [CrossRef]
- Tian, W.; Huang, L.-B.; Wang, D.-W. A general, rapid and sol-vent-free approach to fabricating nanostructured polymer surfaces. Sci. China-Technol. Sci. 2014, 57, 2328–2334. [Google Scholar] [CrossRef]
- Bobenko, N.-G.; Egorushkin, V.-E.; Melnikova, N.-V.; Belosludtseva, A.-A.; Barkalov, L.-D.; Ponomarev, A.-N. Low Tempera-ture Characteristics of Electronic Density of States in Epitaxial Graphene. J. Struct. Chem. 2018, 59, 853–859. [Google Scholar] [CrossRef]
- Lee, S.-W.; Cheng, Y.; Ryu, I.; Greer, J.R. Cold-temperature deformation of nano-sized tungsten and niobium as revealed by in-situ nano-mechanical experiments. Sci. China Technol. Sci. 2014, 57, 652–662. [Google Scholar] [CrossRef]
- Liu, B.; Zhao, H.; Wang, Y. Advanced Flame-Retardant Methods for Polymeric Materials. Adv. Mater. 2021, 34, 2107905. [Google Scholar] [CrossRef]
- Lu, H.; Wilkie, C.A.; Ding, M.; Song, L. Flammability performance of poly (vinyl alcohol) nano-composites with zirconium phosphate and layered silicates. Polym. Degrad. Stab. 2011, 96, 1219–1224. [Google Scholar] [CrossRef]
- Wei, J.-X.; Zhao, C.-X.; Li, Y.-C.; Li, Y.-T.; Sun, Z.-M.; Xiang, D.; Li, H. A simple and green strategy for preparing poly(vinyl alcohol)/phosphate cellulose aerogel with enhanced flame-retardant properties. Polym. Eng. Sci. 2020, 61, 693–750. [Google Scholar]
- Wegst, U.-G.; Bai, H.; Saiz, E.; Tomsia, A.-P.; Ritchie, R.-O. Bioinspired structural materisls. Nat. Mater. 2015, 14, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Zeng, F.; Men, X.; Chen, M.; Liu, B.; Han, Q.; Huang, S.; Zhao, H.; Wang, Y. Molecular-micron multiscale toughening and flame retarding for polyurethane foams. Chem. Eng. J. 2023, 454, 140023. [Google Scholar] [CrossRef]
- Si, Y.; Wang, X.; Dou, L.; Yu, J.; Ding, B. Ultralight and fire-resistant ceramic nanofibrous aerogels with temperature-invariant supere-lasticity. Sci. Adv. 2018, 4, 8925–8934. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-W.; Cao, M.; Zhang, Y.-Y.; Wang, Y.-Z.; Zhao, H.-B. Multifunctional protective aerogel with superelasticity over −196 to 500 °C. Nano Res. 2022, 15, 7797–7805. [Google Scholar] [CrossRef]
- Cao, M.; Li, S.-L.; Cheng, J.-B.; Zhang, A.-N.; Wang, Y.-Z.; Zhao, H.-B. Fully bio-based, low fire-hazard and superelastic aerogel without hazardous cross-linkers for excellent thermal insulation and oil clean-up absorption. J. Hazard. Mater. 2020, 403, 123977. [Google Scholar] [CrossRef]
- Francis, N.L.; Hunger, P.M.; Donius, A.E.; Riblett, B.W.; Zavaliangos, A.; Wegst, U.G.K.; Wheatley, M.A. An ice-templated, linearly aligned chitosan-alginate scaffold for neural tissue engineering. J. Biomed. Mater. Res. Part A 2013, 101, 3493–3503. [Google Scholar] [CrossRef]
- Mao, L.-B.; Gao, H.-L.; Yao, H.-B.; Liu, L.; Cölfen, H.; Liu, G.; Chen, S.-M.; Li, S.-K.; Yan, Y.-X.; Liu, Y.-Y.; et al. Synthetic nacre by predesigned matrix-directed mineralization. Science 2016, 354, 107–110. [Google Scholar] [CrossRef]
- Li, T.; Song, J.-W.; Zhao, X.-P.; Yang, Z.; Pastel, G.; Xu, S.-M.; Jia, C.; Dai, J.-Q.; Chen, C.-J.; Gong, A.; et al. Anisotropic, lightweight, strong, and super thermally insulating nano-wood with naturally aligned nanocellulose. Sci. Adv. 2018, 4, 3724–3733. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.; Wu, H. Preparation and properties of poly(vinyl alcohol)/exfoliated alpha-zirconium phosphate nano-com-posite films. Polym. Test. 2009, 28, 371–377. [Google Scholar] [CrossRef]
- Ding, J.; Zhao, C.-X.; Zhao, L.; Li, Y.-T.; Xiang, D. Synergistic effect of α-ZrP and graphene oxide nanofillers on the gas barrier properties of PVA films. J. Appl. Polym. Sci. 2018, 135, 46455–46464. [Google Scholar] [CrossRef]
- Mosby, B.M.; Díaz, A.; Bakhmutov, V.; Clearfield, A. Surface Functionalization of Zirconium Phosphate Nanoplatelets for the Design of Polymer Fillers. ACS Appl. Mater. Interfaces 2013, 6, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Wang, K.; Xu, C.-A.; Li, Y.; Jiao, E.; Chen, B.; Meng, H.; Cui, X.; Shi, J.; Wu, K. Simultaneous enhancement in thermal conductivity and flame retardancy of flexible film by introducing covalent bond connection. Chem. Eng. J. 2021, 421, 129729. [Google Scholar] [CrossRef]
- Freitas, F.-S.D.; Mattos, G.-C.; Mendes, L.-C. Investigation on miscibility, thermal, crystallographic diffraction and dy-namic-mechanical properties of poly(vinylpyrrolidone)/zirconium phosphate nanocomposites. J. Therm. Anal. Calorim. 2020, 145, 319–329. [Google Scholar] [CrossRef]
- Guo, D.; Wang, Q.; Bai, S.-B. Poly(vinyl alcohol)/melamine phosphate composites prepared through thermal processing: Thermal stability and flame retardancy. Polym. Adv. Technol. 2013, 24, 339–347. [Google Scholar] [CrossRef]
- Wu, G.-M.; Schartel, B.; Yu, D.; Kleemeier, M.; Hartwig, A. Synergistic fire retardancy in layered-silicate nanocomposite com-bined with low-melting phenysiloxane glass. J. Fire Sci. 2011, 30, 69–87. [Google Scholar] [CrossRef]
- Qiang, X.H.; Guo, X.; Su, H.X.; Zhao, H.; Yang, C.W.; Huang, D.J. In situ nanoarchitectonics of magnesium hydroxide particles for property regulation of carboxymethyl cellulose/poly(vinyl alcohol) aerogels. RSC Adv. 2021, 11, 35197–35205. [Google Scholar] [CrossRef]
- Huang, Y.J.; Zhou, T.; He, S.; Xiao, H.; Dai, H.M.; Yuan, B.H.; Chen, X.F. Flame-retardant polyvinyl alcohol/cellulose nanofibers hybrid carbon aerogel by freeze drying with ultra-low phosphorus. Appl. Surf. Sci. 2019, 497, 143775–143783. [Google Scholar] [CrossRef]
- Guo, W.W.; Liu, J.J.; Hu, Y. Multi-functional hydroxyapatite/polyvinyl alcohol composite aerogels with self-cleaning, superior fire resistance and low thermal conductivity. Compos. Sci. Technol. 2018, 158, 128–136. [Google Scholar] [CrossRef]
- Li, H.; Sun, Z.; Zhao, C.; Li, Y.; Xiang, D.; Wu, Y.; Wei, J.; Que, Y. Polybenzoxazine Resins with Cellulose Phosphide: Preparation, Flame Retardancy and Mechanisms. Polymers 2021, 13, 4288. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, C.; Guo, C.; Li, Y.; Li, S.; Sun, L.; Li, H.; Xiang, D. Polybenzoxazine Resins with Polyphosphazene Microspheres: Synthesis, Flame Retardancy, Mechanisms, and Applications. ACS Omega 2019, 4, 20275–20284. [Google Scholar] [CrossRef]
- Ai, F.-Y.; Liu, X.-D.; Bai, W.-B.; Lin, C.-Y.; Xie, R.-R.; Jian, R.-K. From herbicide to flame retardant: The lamellar-like phos-pho-rus-bridged amitrole toward high fire safety epoxy resin with light smoke and low toxicity. Chemosphere 2022, 291, 132704–132711. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, D.; Zhang, Y.; Cai, W.; Yao, C.; Hu, Y.; Hu, W. Construction of multifunctional boron nitride nanosheet to-wards reducing toxic volatiles (CO and HCN) generation and fire hazard of thermoplastic polyurethane. J. Haz-Ardous Mater. 2019, 362, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Ghanadpour, M.; Wicklein, B.; Carosio, F.; Wågberg, L. All-natural and highly flame-resistant freeze-cast foams based on phosphorylated cellulose nanofibrils. Nanoscale 2018, 10, 4085–4095. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Mao, Y.; Wang, D.; Fu, S. B-N-P-linked covalent organic frameworks for efficient flame retarding and toxic smoke suppression of polyacrylonitrile composite fiber. Chem. Eng. J. 2021, 430, 133120. [Google Scholar] [CrossRef]
- Cai, W.; Wang, J.; Pan, Y.; Guo, W.; Mu, X.; Feng, X.; Yuan, B.; Wang, X.; Hu, Y. Mussel-inspired functionalization of elec-tro-chemically exfoliated graphene: Based on self-polymerization of dopamine and its suppression effect on the fire hazards and smoke toxicity of thermoplastic polyurethane. J. Hazard. Mater. 2018, 352, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Alongi, J.; Carosio, F.; Malucelli, G. Current emerging techniques to impart flame retardancy to fabrics: An overview. Polym. Degrad. Stab. 2014, 106, 138–149. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, K.K.R.; Hu, W.; Hu, Y. Air-Stable Polyphosphazene-Functionalized Few-Layer Black Phosphorene for Flame Retardancy of Epoxy Resins. Small 2019, 15, e1805175. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, M.; Hu, Z.; Liang, L.; Shi, J.; Huang, X.; Lu, M.; Wu, K. Casein phosphopeptide-biofunctionalized graphene oxide nanoplatelets based cellulose green nanocomposites with simultaneous high thermal conductivity and excellent flame re-tardancy. Chem. Eng. J. 2020, 382, 122733–122746. [Google Scholar] [CrossRef]
- Zhong, L.; Zhao, K.Y.; Tang, Q.-L.; Zhang, K.-X.; Deng, W.-H.; Zhang, L.-W.; Wang, R.; Chen, J.; Deng, J.-J.; Liao, W.; et al. Highly efficient flame-retardant and transparent epoxy resin. Polym. Adv. Technol. 2021, 32, 2940–2952. [Google Scholar]
- Luo, Y.; Xie, D.-L.; Chen, Y.-F.; Han, T.; Chen, R.-J.; Sheng, X.-X.; Mei, Y. Synergistic effect of ammonium polyphosphate and a-zirconium phosphate inflame-retardant poly(vinyl alcohol) aerogels. Polym. Degrad. Stab. 2019, 170, 109019–109031. [Google Scholar] [CrossRef]
- Song, R.; Jiang, Z.; Yu, H.; Liu, J.; Zhang, Z.; Wang, Q.; Tang, T. Strengthening Carbon Deposition of Polyolefin Using Combined Catalyst as a General Method for Improving Fire Retardancy. Macromol. Rapid Commun. 2008, 29, 789–793. [Google Scholar] [CrossRef]
- Dana, X.M.-S.; Gabrielle, C.-C.; Valentin, I.-P. Phosphorylation of polysaccharides: New results on synthesis and charac-teri-sation of phosphorylated cellulose. React. Funct. Polym. 2006, 66, 1240–1249. [Google Scholar]
- Wong, M.; Ishige, R.; White, K.L.; Li, P.; Kim, D.; Krishnamoorti, R.; Gunther, R.; Higuchi, T.; Jinnai, H.; Takahara, A.; et al. Large-scale self-assembled zirconium phosphate smectic layers via a simple spray-coating process. Nat. Commun. 2014, 5, 3589. [Google Scholar] [CrossRef] [PubMed]

| Sample | SE | |||
|---|---|---|---|---|
| Tdonset | Char Residues | |||
| N2 | Air | N2 | Air | |
| PVA/PCF5/α-ZrP15 | 1.14 | 1.02 | 1.009 | 2.03 |
| PVA/PCF10/α-ZrP10 | 1.39 | 1.13 | 1.13 | 2.33 |
| PVA/PC15/α-ZrP5 | 1.06 | 1.03 | 1.14 | 1.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, J.; Zhao, C.; Hou, Z.; Li, Y.; Li, H.; Xiang, D.; Wu, Y.; Que, Y. Preparation, Properties, and Mechanism of Flame-Retardant Poly(vinyl alcohol) Aerogels Based on the Multi-Directional Freezing Method. Int. J. Mol. Sci. 2022, 23, 15919. https://doi.org/10.3390/ijms232415919
Wei J, Zhao C, Hou Z, Li Y, Li H, Xiang D, Wu Y, Que Y. Preparation, Properties, and Mechanism of Flame-Retardant Poly(vinyl alcohol) Aerogels Based on the Multi-Directional Freezing Method. International Journal of Molecular Sciences. 2022; 23(24):15919. https://doi.org/10.3390/ijms232415919
Chicago/Turabian StyleWei, Jixuan, Chunxia Zhao, Zhaorun Hou, Yuntao Li, Hui Li, Dong Xiang, Yuanpeng Wu, and Yusheng Que. 2022. "Preparation, Properties, and Mechanism of Flame-Retardant Poly(vinyl alcohol) Aerogels Based on the Multi-Directional Freezing Method" International Journal of Molecular Sciences 23, no. 24: 15919. https://doi.org/10.3390/ijms232415919
APA StyleWei, J., Zhao, C., Hou, Z., Li, Y., Li, H., Xiang, D., Wu, Y., & Que, Y. (2022). Preparation, Properties, and Mechanism of Flame-Retardant Poly(vinyl alcohol) Aerogels Based on the Multi-Directional Freezing Method. International Journal of Molecular Sciences, 23(24), 15919. https://doi.org/10.3390/ijms232415919







