Abstract
Root-zone restriction induces physiological stress on roots, thus limiting the vegetative and enhancing reproductive development, which promotes fruit quality and growth. Numerous bacterial-related growth-promoting, stress-mitigating, and disease-prevention activities have been described, but none in root-restricted cultivation. The study aimed to understand the activities of grapevine bacterial communities and plant-bacterial relationships to improve fruit quality. We used High-throughput sequencing, edaphic soil factors, and network analysis to explore the impact of restricted cultivation on the diversity, composition and network structure of bacterial communities of rhizosphere soil, roots, leaves, flowers and berries. The bacterial richness, diversity, and networking were indeed regulated by root-zone restriction at all phenological stages, with a peak at the veraison stage, yielding superior fruit quality compared to control plants. Moreover, it also handled the nutrient availability in treated plants, such as available nitrogen (AN) was 3.5, 5.7 and 0.9 folds scarcer at full bloom, veraison and maturity stages, respectively, compared to control plants. Biochemical indicators of the berry have proved that high-quality berry is yielded in association with the bacteria. Cyanobacteria were most abundant in the phyllosphere, Proteobacteria in the rhizosphere, and Firmicutes and Bacteroidetes in the endosphere. These bacterial phyla were most correlated and influenced by different soil factors in control and treated plants. Our findings are a comprehensive approach to the implications of root-zone restriction on the bacterial microbiota, which will assist in directing a more focused procedure to uncover the precise mechanism, which is still undiscovered.
1. Introduction
Grape is the world thirst most crucial crop after potato and tomato, holding the farmer’s gate value of USD 68 billion, with a production of 77.4 million tonnes (MT) from 7.1 million hectares (MH) of the area under grapevine cultivation [1]. As the world population will be 10 billion by 2057, agricultural land and cultivation resources must be sustainable for irrigation and land utilization to improve the quality of fruit as per the United Nations (UN) sustainable development goals (SDGs) 2&12. The need of the hour is to devise sustainable cultivation technologies of adequate land and water usage and ultimately yield better fruit quality. Root restriction is an innovative, stress-inducing technique in fruit tree cultivation based on fruit trees’ vegetative and reproductive growth, which can be regulated by restricting roots into a container [2,3], thus regulating the water and nutrition absorption capacity. This technique’s ideology aligns with the United Nations (UN) sustainable development goals (SDGs) number 2&12, which state zero hunger and sustainable management and efficient use of natural resources. Root restriction cultivation enables us to save resources on fertilization and irrigation. This method has been adopted in numerous vineyards in China due to its ecological and financial advantages, and it has shown promising results in the commercial production of grapes. Root restriction is a suitable method for high-density cropping. Another vital feature of root restriction cultivation is that it assisted in overcoming the low degree of sweetness in grapevines grown in southern China regions as a result of the hot, humid weather [4]. Root restriction is suitable for high-density, high-yield and high-efficiency cultivation [5]. Numerous studies on different fruit, such as grapes [2,6,7], nectarines [5], sweet cherry [8], chilli [9], apple [10] and tomato [11], have revealed the remarkable changes that can suppress vegetative growth and boosts the reproductive growth as well. The primary goal of implementing this strategy is to have the most delicate fruit quality possible, which is regulated by an increase in the size, colour, flavour, anthocyanin, and content of total soluble solids [3,4,12,13]. Grapevines grown with root-zone restriction displayed distinct growth behaviours as compared to standard cultivation. By the physiological stress applied, the primary roots were thinner and longer [14,15], while secondary and fibrous roots exhibited a high absorption capacity. The vine vitality, in particular, was severely reduced [16,17]. Meanwhile, root restriction controlled the distribution and partitioning of assimilates between vegetative and reproductive organs, resulting in better berry quality [2,4,12]. Phytohormones are also reported to be increased by root restriction, such as abscisic acid (ABA) and salicylic acid (SA) [4,18]. Root restriction causes high stress to peach tree growth as characterized by the photosynthesis efficiency, tree growth, fruit quality and yield [5]. The bacterial community structuring and diversity in root restriction is still a mystery, despite investigating these numerous physiological features.
Plants are aided by their associated microbes regarding host health, fruit quality, and adaptation to their fluctuating environmental conditions. Plants and bacteria form intricate and dynamic relationships that can vary from beneficial to commensal or harmful [19]. Their functionalities, such as soil quality, host productivity, and host health, are improved by the mineralization of soil organic matter, activation of plant defence systems even the synthesis of antibiotics against phytopathogens are all examples of direct or indirect processes [20,21,22]. Plant-associated bacteria populate as epiphytes and endophytes. The soil surrounding the plants acts as a microbial reservoir. Plant–microbe interactions are complex, with plant species and soil types influencing the soil microbial ecosystem and having a critical role in plant defence [23,24]. Bulgarelli [25] proposed that edaphic variable, substrate-driven community selection within the rhizosphere, and host genotype, shape the soil microbial architecture. Endophytic bacteria invade roots, leaves and eventually reproductive structures after rigorous filtering. It is now commonly known that the formation of a bacterial community in plants does not occur randomly but is governed by certain assembly principles [25,26,27]. Soil type is one of the elements influencing the organization of bacterial communities in plants [28,29]. Others are plant compartment [30], host genotype/species [31], plant immune system [27], plant trait variation/developmental stage [32,33], and residence time/season [34]. Although the bacterial microbiota is well studied in diverse environments and plant species, the influence of a stressful technique, root-zone restriction on the grapevine, is still unexplored. Here we hypothesized the role of the root-zone restriction technique in recruiting the plant-associated rhizospheric bacterial microbiota and its interaction with the soil edaphic factors. It increases the root formation, increasing the surface area and, consequently, critical bacterial taxa corresponding with plant organs and soil. It will assist us in comprehending the microbial terroir of grapevines grown in root-zone-restricted cultivation. Our primary focus was on how the root-zone restriction influences the bacterial community structures at different growth stages and its relationship with fruit quality.
2. Results
2.1. Biochemical Dynamics of Root Zone Restriction Approach on Grapevine Fruit
Comparing the significance of the biochemical parameters between treatment and control, other than Ph, the rest were statistically non-significant at the pre-veraison stage. TA and pH were significant, while anthocyanin and vitamin C depicted identical results at the veraison stage. All parameters were highly significant when compared between root-zone restriction and control at the maturity stage, as shown in Table 1.

Table 1.
Impact of treatment and control on fruit quality parameters.
2.2. Bacterial Taxonomic Diversity throughout the Grapevine Influenced by Root Zone Cultivation
The bacterial diversity of the Muscat Hamburg cultivar was determined in the rhizosphere soil, white roots, leaves, flower, and berries at three major phonological stages. They are full bloom, version, and maturity, comparing treatment (root-restricted plants) and control (non-root-restricted plants) by Illumina MiSeq sequencing of 16S rRNA genes.
A total of 1,600,033 V3–V4 amplicon sequence variants (ASVs) were documented from the rhizosphere, roots, leaves, flowers, and berry at full bloom, veraison, and maturity. The average length of sequences was 376 bp, while the majority were 407 bp, and samples were rarefied to 66,983 sequences each. Overall, 13,541 ASVs were identified after quality filtering from 72 samples (24 samples were run in 3 replicates) and approximately 600 ASVs per sample (Supplementary Materials Figure S1). Rhizosphere soil of control plants has 1982, while treated plants have 1770 ASVs. White roots had 927 and 1026 ASVs of control and treated plants, respectively. Leaves of control and treated plants have 1805 and 1997, respectively. Flower resided 893 and 589 ASVs in control and treated plants, respectively. Berry of control plants contained 1456, while 1085 were in treated plants. Sequencing statistics per sample and the number of observed ASVs (Supplementary Materials Table S1). The findings of a comparison of rarefaction curves (Supplementary Materials Figure S2) as a function of sampling depth and observed outs revealed that all curves are close to saturation. As a result, the richness of the samples has been meticulously documented or sequenced [35,36].
2.3. Impact on Bacterial Community Structure in Plant Organs across Three Growth Stages
Microbial community structure across the grapevine habitats is exposed at the phylum level (Figure 1). The relative abundance of Cyanobacteria was 40%, Proteobacteria 32%, Firmicutes 12%, and Bacteroidetes 4%, which were most abundant across all the treated and control plant organs at all growth stages. The highest relative abundance of Cyanobacteria was 94.03% in maturity leaf treatment (MLT) and the lowest in full bloom soil control (FBSC). Proteobacteria was profused in veraison soil treatment (VST) 90.42% and minimal abundant in MLT. Firmicutes were at 59.57% in the soil of control plants at the full bloom stage (FBSC), and 0.13% in the full bloom white roots treatment (FBWRT). Bacteriodiates were maximum classified at 14.04% in V_B_T (veraison, berry of treated plant) and were minimum in maturity white roots control (MWRC). Regarding the plant organs, rhizosphere soil had a maximum relative abundance of Proteobacteria with 90.42% at the veraison stage of treated plant (VST) and Cyanobacteria with 0.25% at the full bloom stage of control plants (FBSC). White roots were composed of 84.87% of Cyanobacteria at the maturity stage of control plants (MWRC) and a minimal abundance of Bacteroidetes with 0.12% at the maturity stage of control plants (MWRC). Leaves of the treated plant at maturity stage (MLT) had 94.03% of Cyanobacteria and 0.29% of Bacteroidetes at maturity stage (MLT). The flower of the treated plant at full bloom (FBBT) and berry of the treated plant at the veraison stage (VBT) was enriched with Proteobacteria at 59.17% and 64.46%, respectively. A minimal abundance of Firmicutes was observed in full bloom berry control (FBBC) at 4.25% and 2.66% at maturity berry control (MBC). For a detailed relative abundance of these phyla, see Supplementary Materials Table S2.
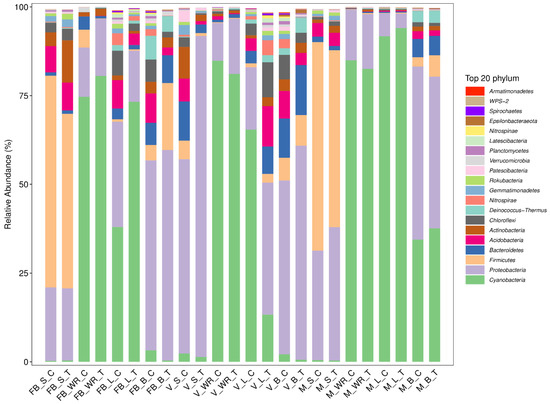
Figure 1.
Relative abundance (%) of the significant bacterial phyla existing in rhizosphere soil (S), white roots (WR), leaves (L) and berries (B) at three phonological stages as full bloom (FB), veraison (V), and maturity (M). Root-zone-restricted plants are denoted as treatment (T) while control plants as (C).
2.4. Richness and Diversity of Bacterial Communities Associated with Grapevine Habitats
Alpha diversity analysis was used to investigate the species richness and evenness of a bacterial ecosystem within the rhizosphere (soil and white roots) and phyllosphere (leaves, flower and berry). To validate our sample size, species accumulation curve and rank abundance curve were formulated (Supplementary Materials Figures S3 and S4). Alpha diversity was assessed by Chao1 and Shannon indices (Figure 2). The rhizosphere soil had Chao1 (p = 0.096) maximum at maturity soil treatment (MST), while Shannon (p = 0.0078) was at veraison soil control (VSC) (Figure 2a). White roots had Chao1 (p = 0.076) maximum at veraison white root control (VWRC) while Shannon (p = 0.21) at full bloom white root control (FBWRC) (Figure 2b). Shannon (p = 0.0078) and Chao1 (p = 0.015) were maximum at veraison leaf treatment (VLT) (Figure 2c). The flower was contemporary to the full bloom stage, so it has higher Chao1(p = 0.02) and Shannon (p = 0.031) at full bloom berry control (FBBC). Berries have higher Chao1 (p = 0.02) and Shannon (p = 0.031) at veraison berry control (VBC) (Figure 2d).
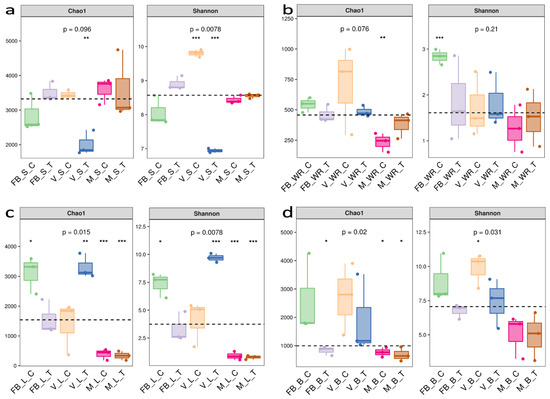
Figure 2.
Alpha diversity indices Chao1 and Shannon. (a) Rhizosphere soil, (b) white roots, (c) leaves and (d) berries at three growth stages with comparison to treatment and control.
Bacterial community structures were visualized by nonmetric multidimensional scaling (NMDS) based on the Bray–Curtis distance. Dissimilarities between the organs at different phonological stages were measured with orientation to control and treatment. The soil at the veraison stage of treatment and control plants (FBST and FBSC) showed a significant community shift (r2 = 0.87, p < 0.001, stress = 0.000862). In contrast, as indicated in the plot, all other groupings were centred on a single spot (Figure 3a). In Figure 3b, white roots were most distinct between treatment and control plants at full bloom, followed by maturity (r2 = 0.58, p < 0.001, stress = 0.0917). In Figure 3c, our treatment impacted the bacterial community structure only during the full bloom and veraison stages in the leaves of treated and control plants (r2 = 0.68, p < 0.001, stress = 0.0488). In Figure 3d, the root-zone restriction influenced the community composition in the berries at full bloom and veraison, although the communities at maturity were identical (r2 = 0.42, p < 0.005, stress = 0.0771). To comprehend the beta diversity of microbial communities established as a result of the NGS, we constructed a hierarchical clustering analysis known as UPGMA. Plant tissues were similar intra-group of treatment and control but at a distance in the case of the intergroup (Supplementary Materials Figure S5).
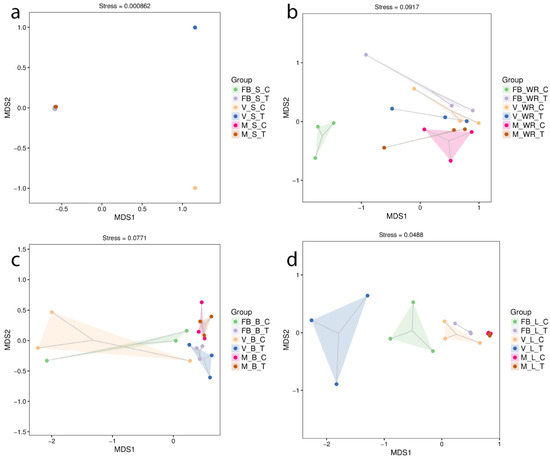
Figure 3.
Non-metric multidimensional scaling (NMDS) for evaluation of microbial communities in samples from treated and control plants at full bloom, veraison and maturity. (a) Rhizospheric soil, (b) white roots, (c) leaves, and (d) berries.
According to our hypothesis that microbes have a beneficial effect on fruit quality, we tried to silhouette a relation between the fruit quality parameters and the bacterial communities in the berries of treated and control plants at the veraison and maturity stage. Multivariate analysis as canonical correspondence analysis (CCA) revealed that Vitamin C (r2 = 0.81, p < 0.005), pH (r2 = 0.68, p < 0.005), and anthocyanin (r2 = 0.39, p < 0.005) were significantly correlated with the microbial communities of the berries at maturity stage in our treated plants. The variables along the CCA1 explained 18.2% of the variation, while 11.41% was along the CCA2 (Supplementary Materials Figure S6).
2.5. The Preponderant Influence of Soil Parameters on Bacterial Community Structuring
Soil chemical properties are in Table 2 and Supplementary Materials Figure S7. The relation of the soil chemical properties towards the structuring of the bacterial community was explored by constituting a redundancy analysis, which indicated that the soil nutrition, growth stage and root-zone restriction technique are the primary driver of the bacterial diversity in the soil. The two axes of the RDA plot explain 81.14% of the total variation caused by the soil factors with the Monte Carlo permutation p < 0.001 and the corresponding correlation factor r2 (Figure 4). The soil pH (r2 = 0.48) significantly affected the abundance of unclassified Enterobacteriaceae, whereas cec (r2 = 0.31) provoked the abundance of Enterobacter and Pseudomonas in VST. Zinc (r2 =0.62) turned out to be the major contributor to the diversity of Bacillus sp at MSC and MST. OM (r2 = 0.47) considerably regulated the bacterial community at FBST, and the most affected genus was Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium. AN (r2 =0.61), TN (r2 =0.38), AK (r2 = 0.59), TK (r2 = 0.0.41), AP (r2 = 0.34), TP (r2 = 0.24), Mg (r2 = 0.49), and Mn (r2 = 0.0.34) were responsible while Fe (r2 = 0.73) being most correlated with Sphingomonas at VSC in terms of shaping the bacterial population and Devosia, Pseudolabrys and Duganella were the most affected genera.

Table 2.
Change in soil properties at successional developmental stages of control and treated plants.
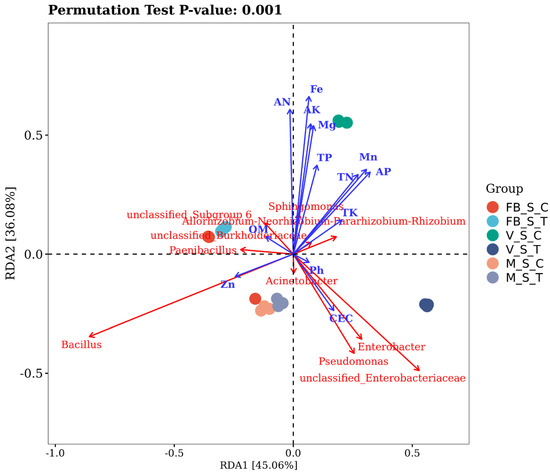
Figure 4.
The sampling groups depicted in the colour dots, soil parameters with blue arrows and the most affected are mentioned in red colour. The smaller the p-value, the greater the significant impact of the overall influencing factors on the composition of the soil microbiota.
The network analysis method was employed to investigate the connection between bacterial genera and the edaphic soil factors, along with the influence of the nutritional profile of the soil affecting the bacterial population density. The structuring was significantly different in both the control and treated plants. A correlation was also noticed between the soil edaphic factors, corresponding to their abundance and jointly venturing towards bacterial networking.
According to our sieving conditions of the constituted network r > 0.6 or r < −0.6 and p < 0.05, we observed 1072 (179 − ve, 893 + ve) effective interactions in control plants while 994 (463 − ve, 531 + ve) interactions were recorded in the treated plants. The analysis was performed on the genus (Supplementary Materials Figure S8) and phylum levels. The co-occurrence network was compartmentalized into clusters connecting the edaphic factors as the bacterial niches. According to the network diagram of control plants (Figure 5a), it was observed that there is a correlation among the soil elements, and they are mutually steering the community configuration. The correlation between AN and AK was r2 = 0.9; p > 0.0009, Mg and AN were r2 = 0.93; p > 0.0002, while Mg and AK were r2 = 0.83; p > 0.005. The maximum number of nodes were related to Firmicutes, Proteobacteria, Acidobacteria, Actinobacteria and Bacteroidetes. TK was reported to influence the genera under the Bacteroidetes and Dababacteria significantly. TP, Mn and Zn were correlated with Cyanobacteria. CEC, OM and AP governed Chloroflexi and Planctomycetes.
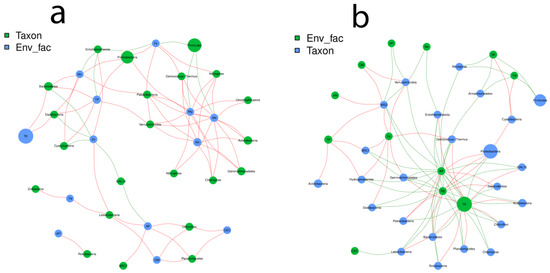
Figure 5.
Pearson correlation between the edaphic soil factors and bacterial population in the soil. (a) Control plants, (b) treated plants.
The relationship between the treatment and soil factors was different, as observed in the control plants. More nodes were connected and concentrated between TK, Mg, AP and Fe. According to Figure 5b, there was a significant negative correlation between AP and TK r2= −0.8; p > 0.004, Mg and AP r2= 0.866; p > 0.002 while Mg and TK were r2= −0.82; p > 0.005. Proteobacteria, Acidobacteria, Chloroflexi, Firmicutes, Bacteroidetes, Gemmatimonadetes, Deinococcus-Thermus and Rokubacteria were majorly influenced by TK, Mg and AP, respectively. Fe also retained a considerable number of nodes after the above-stated three elements. A highly positive correlation was identified between the genera belonging to Deinococcus-Thermus, Verrucomicrobia, Dadabacteria, BRC1, Hydrogenedentes, WS2 and Fe in the soil of treated plants.
2.6. Species Difference Analysis and Notable Species
We used the Venn diagram for community analysis to observe the core and unique ASVs among different plant organs. 155 core ASVs were shared between the soils (Supplementary Materials Figure S9a), most belonging to phylum Firmicutes (65%). Thirty-five were in white roots and seventy-eight in the leaves from Cyanobacteria (Supplementary Materials Figure S9b,c), with a relative abundance of 85% and 88%, respectively. While 157 were in the berries Proteobacteria (68%) (Supplementary Materials Figure S9d) of different stages independent of the treatment and growth.
The LEfSe (linear discriminant analysis effect size) analysis may directly perform difference analysis on all classification levels. At the same time, it emphasizes the discovery of distinct species differences between groups, i.e., biomarkers. The histogram of LDA values distribution shows the significantly different taxon. An LDA score of a minimum of 2 was used to identify diverse bacterial assemblages with statistical significance (p < 0.05). Our treatment (root restriction) showed more significant bacterial groups than control plants. Most V_S_T contains class Gammaproteobacteria, followed by an abundance of phylum Bacteroidetes in V_B_T. FB_B_T had class Clostridia in enormous numbers. Last but not least, M_L_T had mainly recruited the class Alphaproteobacteria (Supplementary Materials Figure S10a,b).
2.7. Metagenome Prediction
Using the bacterial 16S rRNA gene sequence and PICRUSt algorithm, bacterial community metabolic functions were estimated. The KEGG pathway hierarchy level 2 classified 45 metabolic pathway subfunctions into six groups. According to Supplementary Materials Figure S11, only the top 10 most abundant pathways are displayed. Carbohydrate metabolism was higher in soil and white roots, followed by amino acid and energy metabolism. The predicted microbial metabolism in leaves was the same as in white roots but with increased abundance. More metabolic pathways were predicted in the berries of treated plants, majorly at the maturity stage. Overall, three predicted metabolic pathways surpassed all the predicted ones, such as carbohydrate, amino acid and energy metabolisms.
2.8. Microbial Co-Relation Network Analysis
In all networks, we found a considerably greater amount of co-occurrence interactions as compared to mutual exclusions. Microbial associations of the rhizosphere soil of the control plant and treated plant show us the correlation between them. According to the Supplementary Materials Figure S12a) network association among our samples, the soil of control plants had 426 nodes (vertices) corresponding to 4603 edges. The treatment plant had 436 and 4333 nodes and edges, respectively (Supplementary Materials Table S3). Degree assortativity (Pearson correlation coefficient) for samples was 0.535 and 0.523 for treatment control plants, respectively. The ratio of edges per node was higher in the treated plants. The relation of the bacterial phyla is depicted in (Figure 6a). The top abundant phyla are, Proteobacteria, Firmicutes, Acidobacteria and Bacteroides. Their relative abundance percentage is mentioned above in the bacterial phyla distribution topic in the results portion; an important aspect is that our treatment surpasses the control in the richness of the top bacterial phyla. Networking topologies of the soil samples for phyla are 2007 edges and 242 nodes, diameter of (longest distance) 9.26 nodes. The average path length (length between two nodes) was 3.5, degree assortativity was 0.293, transitivity (clustering coefficient, which states how deeply nodes are embedded in their surroundings, and hence the degree to which they prefer to cluster together) was 0.66 and 0.29 was the modularity index. In white roots, 209 nodes and 494 edges existed in the control plants, while 194 nodes with 310 edges were present in the treated plants. The degree of assortativity was 0.84 and 0.94 for treated and control plants, respectively. Control plants tend to have a higher ratio of edges per node in the associated network of samples (Supplementary Materials Figure S12b). Cyanobacteria, Proteobacteria, Bacteroidetes and Firmicutes were the highest abundant phyla in microbial phyla networking (Figure 6b). Other than Proteobacteria, all three were more profused in control plants. Topologies demonstrate that 271 nodes with 1137 edges, the diameter was 11.82 nodes, 4.96 was the average path length, degree assortativity was 0.425, transitivity was 0.525 and 0.64 was the modularity index. Apropos the network association of sample groups (Supplementary Materials Figure S12c), leaves were expressive to contain 323 nodes and 1216 edges of control plants, contrasting to 325 nodes and 1316 edges of treated plants. The degree of assortativity was 1.05 and 1.15 for treated and control plants, respectively. A higher ratio of edges per node in the associated network of samples existed in treated plants. Cyanobacteria, Proteobacteria, Acidobacteria, and Chloroflexi, stood maximum abundant in the treated plants rendering microbial associations (Figure 6c). Two hundred and thirty-four nodes with nine hundred and fifty-one edges, the diameter was 8.59, the average path length stayed at 3.9, Degree assortativity was recorded at 0.458, Transitivity was 0.513, and 0.516 was the modularity index. The last plant organ under our contemplation is the flower/berry. The flower of the control plants had more nodes and edges than the treated plants. A total of 358 nodes correspond to 1124 edges, while 262 nodes with 554 edges in control and treated plants were present in the association between the samples, respectively. The degree of assortativity was 1.41 and 1.43 for treated and control plants, respectively. A higher ratio of edges per node in the associated network of samples existed in control plants (Supplementary Materials Figure S12d). Conferring to (Figure 6d) the associations between the microbial taxa at different growth stages observed in the flower/berries of control and treated plants, Proteobacteria, Cyanobacteria, Bacteriodates and Firmicutes all were responsible for fabricating the microbial architecture of the treated plants. Topologies depict that 247 nodes were consequential to 933 edges, average path length stayed at 3.9, degree assortativity was noted at 0.518, transitivity and modularity were 0.517 and 0.561, respectively. Each node’s Zi and Pi values were computed by using the modular analysis results to make a scatter plot. The Zi value represents within-module connection, whereas the Pi value represents among-module connectivity. In this network, the majority of nodes were in the peripherals (regarded as habitat specialists), three module hubs (generalists), and two were on the brink between peripherals and connectors (generalists). In contrast, no node was recorded in the network hub (super generalists) in the bacterial community network (Supplementary Materials Figure S13, Table S4, Text S2). These structural properties enable instant and easy comparisons across large datasets from various ecosystem types in order to understand how the basic aspects of a certain habitat type may influence the assembly of microbial communities. The most connected node taxonomy changed considerably across organs of treated and control plants. A dominant specie network with a grouping abundance pie chart was also compiled to figure out the species most abundant in each plant organ, with special emphasis on growth stage and treatment (Supplementary Materials Figure S14).
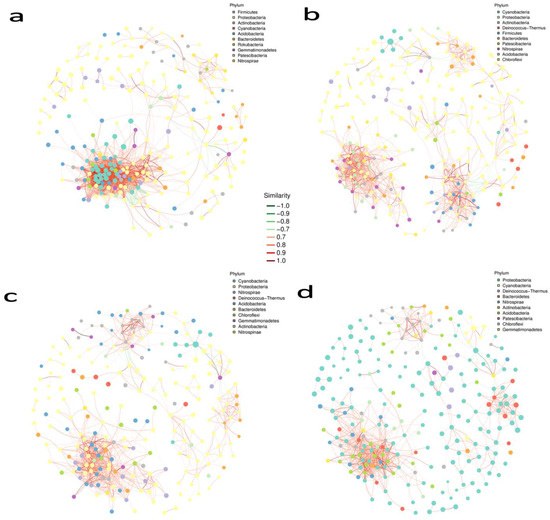
Figure 6.
Network analysis of grape rhizosphere and phyllosphere bacterial communities. ASVs with less than 10 total sequences and less than 5 samples were filtered out. Nodes denote different interacting phyla. The top 10 modules (phyla) with the most significant number of interacting nodes are shown with their respective colours. Node size is proportional to the abundance. Edges connect different nodes with red and green lines as positive and negative correlations, respectively. (a) Rhizosphere soil, (b) white roots, (c) leaves, (d) berry.
3. Discussion
Rhizosphere soil and plant parts uphold distinctive microbial communities, with sample type accounting for the most significant variance in microbial community structure of all parameters [37,38]. Numerous studies have reported their functionalities, mainly plant growth protentional, superior fruit quality and disease suppression [24,39,40,41]. The possible effects of plant phenological stages, composition of soil and above-ground plant organ microbial communities have been widely studied, primarily in annual crops and grapevines [20,42,43,44,45,46]. Nevertheless, no research has been reported before on the behaviour of grapevine-associated microbiota and the effect of soil properties on them under the stressful cultivation root-zone restriction, compared to control plants at three main phonological stages. Biotic and abiotic factors are an administrator of endophytic communities. The plant, including the host genotype and developmental stage, and the environment from which endophytes originated, plays a critical role in regulating plant function [47]. Our results provided a novel approach that the bacterial communities associated with plant organs considerably contrasted in richness and diversity, fruit quality is undoubtedly regulated by microbes, and soil properties have a distinct correlation with particular microbes under a stressful cultivation name root-zone restriction.
3.1. Root-Zone Restriction Influences the Bacterial Structuring across Successional Stages
Significant diversity was recorded among the soil samples of treatment and control plants in the veraison stage; the earlier was less diverse but highly rich than the latter. The most abundant phylum was Proteobacteria, with a rise of 60.5% in VST, with the subsequent class Gamma-proteobacteria presenting a 1.2-fold increase in VST as compared to VSC. The vital function associated with them is nitrogen fixation. Proteobacteria are well known for their part in the carbon metabolic cycle and the generation of secondary metabolites [48], encompassing spoilage and fermenting species [49]. The soil is predominated by proteobacteria [50], and they are also very stressed responsive in a constrained environment [26]. Less diversity in our treatment shows that root-zone restriction influences the soil diversity, and Proteobacteria was the dominant phyla among others. Typically, the rhizosphere is where root microbial diversity first emerges. It has been discovered that vineyard management approaches, local bio-geographical factors, and soil physicochemical properties all impact the make-up of root-associated populations [50,51,52]. The root exudates draw microorganisms from the soil around them and are carried further throughout the growing season [53]. Additionally, bacteria may develop an endophytic phenotype and spread from the inside of the roots to other plant tissues [54,55]. Here we found that Cyanobacteria was most abundant in the roots, showing a 1% increasing trend from full bloom to maturity in treated plants, while the opposite was observed in control. Followed by Proteobacteria. Steroidobacter spp., which was abundant in roots of treated plants at full bloom (7.12%) and version (3.1%) stage than in soil, may be extremely important to the physiology and growth of plants. Brassinosteroids may be crucial to the growth and physiology of plants since they have been shown to influence seed germination, stem and root elongation, vascular differentiation, fruit ripening and stress tolerance [56]. We can assume that under the effective signalling between the roots of grapevine and Cyanobacteria, it was more abundant in the roots. Cyanobacterial richness and diversity are severely affected by drought conditions [57]; perhaps in our results, due to appropriate irrigation, this trend was not observed. Factors driven by changes in host-plant genotype, structure and growth stage create a more distinct endophytic microbial population comprised solely of bacteria capable of passing into root tissues through the endodermis and pericycle stably [52,58]. Nevertheless, there was no momentous diversity between the control and treatment at maturity and veraison rather than full bloom (Figure 2b and Figure 3b) and Supplementary Materials Figure S5. We recorded a greater number of ASVs observed in the roots of the control plant than in the treated ones. This could be because root restriction alters the root architecture [2,59]; by peculiar signalling, only specific microbes are recruited by roots. Root exudates are also altered depending on the plant genotype, species, age, nutrition and stress [20,60,61]. Our treatment also depicts the specificity of microbes at particular stages in the roots by oozing specific types of root exudates, as reported by [62,63]. Endophytes observed in the leaves were more diverse at the veraison stage (Figure 2c). Other than Cyanobacteria and Proteobacteria, we recognized the abundance of Acidobacteria and Chloroflexi. Cyanobacteria were observed across all stages and were most abundant in the treated plant’s leaves at the maturity stage, with a 2.52% increased relative abundance compared to the control. However, it remained an unidentified chloroplast. Proteobacteria was less abundant in leaves at the veraison stage than in the soil of treated plants. It was least abundant at later stages. Acidobacteria and Chloroflexi were more abundant at earlier stages and least abundant till maturity (Figure 1). Root-zone restriction modifies the phyllosphere’s physiology and rhizosphere, thus shrinking the vegetative growth and escalating the reproductive growth [2]. This could be a reason for the high abundance of Cyanobacteria in the leaves, it migrates to the phyllosphere to support photosynthesis and to supply an adequate amount of nutrients for plants [64], and in return, we acquire a superior quality of fruit rather in a restricted environment [3]. The morphological features of leaves, their chemistry and physiology may alter depending on the grape species, as plant genetics may influence all of these aspects. This change may also result in different microbial community structure mixes [65]. Limited reporting exists about the presence of Cyanobacteria in the phyllosphere, grapes [65], Jingpai pear [66], and wheat [64]. While a majority of reporting is about the presence in the rhizosphere of Arabidopsis [32,67], potato [68] and wheat [69]. Flower and berries were abundant with Proteobacteria across all three stages, followed by peak abundance of Cyanobacteria in berries only with an 8.82% at maturity, Bacteriodates at version with 27.75%, and Firmicutes at full bloom stage with a 345% change in abundance of treated as compared to control plants. Berries were also recorded with maximum diversity at the veraison stage (Figure 2d). γ-proteobacteria, Bacteroides and Clostridia were the most abundant classes of Proteobacteria, Bacteroides and Firmicutes, respectively. Scanty bacterial numbers have been recorded from flowers and fruits belonging to these previously mentioned phyla [54]. Several documents reveal that different microbes associated with Proteobacteria are in charge of disease resistance, increasing plant biomass, nitrogen fixation, and growth enhancement [63,70,71]. It is relevant to have its share in wine making [72]. Allied microorganisms of plant and host collaborate vigorously, thus describing a stable holobiont where the partners cooperate to improve the overall holobiont’s fitness [73]. The functionality of the microbiome is not equal to the sum of its parts. Microbes form complex networks by mutual interaction, significantly influencing ecological processes and host adaptations [74]. Variability in bacterial community structure, particularly in the rhizosphere and phyllosphere, can be attributed to uneven colonization of the root system, variation in plant physiology (root-zone restriction), the development stage, root exudates and even miscellaneous occurrences. Microbial endophytes colonizing the plant roots is a well-known topic. The soil of the control plant was more diversified than the soil of the treated plant in the current investigation, where endophytes were studied. It confirmed most ASVs found in above and lower ground samples, indicating that soil is the main reservoir for plant-associated microorganisms, and root-zone restriction influences the recruitment.
3.2. Improved Fruit Quality
Regardless of this root-zone restriction that recruits particular microbes and shows less diversity, we always get superior quality yield, which instigates us to ponder on the microbe-mediated phytohormone production in a restricted or stressful environment. According to several studies, microbes generate minute levels of plant hormones to support plant growth and stress tolerance in various stressful environments, including salt, heat, drought, and metal toxicity [75,76,77]. According to Supplementary Materials Figure S6, we recurrently have a higher quantity of TSS in the berry of root-restricted grapevines [12]. Moreover, this could also be due to the bacterial strains most abundant in the berries at the maturity stage, B. subtilis and Arthrobacter sp, as described by [78], that the total soluble sugars, wheat biomass, and salt content all were increased. ABA (abscisic acid) synthesis by B. licheniformis and P. fluorescens boosts grapevine development during water stress, as observed by [79]. Hence, the statistical relation confirms this association between microbes and bacterial abundance, yielding better fruit quality.
3.3. The Bacterial Population at Predisposal of Soil Elements in Root-Restricted Cultivation
Microbial niches originate from the proximity of the primary anchoring organ named roots, translocated throughout the plant and perform multiple functionalities related to plant development, fruit quality and disease resistance [54,80,81,82,83]. Soil microbial diversity is theorized to be driven by numerous biotic and abiotic factors [84], such as climate, soil properties, topography and land usage [85,86,87]. Thus, we have hypothesized that soil properties might get altered, consequently influencing the microbial community structuring under the stressful cultivation called root-zone restriction. Meanwhile, root restriction modifies plant physiology, nutrient uptake and other numerous functionalities. The soil edaphic factors in that restricted container must influence the microbial diversity, which was also compared to a control plant. Following the first section of our hypothesis, most edaphic soil factors varied significantly concerning the cultivation technique and the growth stages.
Another excellent point is that according to the Tukey’s-LSD pairwise mean comparison, the TN curtailed homogenous groupings (non-significant), while AN possessed heterogenous groupings (significant) at the full bloom and maturity stage in both the cultivations. In the value-wise comparison from (Table 2), it is evident that the amount of available nitrogen stood pretty low in the treated plant, which is aligned with our previous studies; root restriction limits the nitrogen availability in the grapevines [16,17]. Our finding also contrasts with [88], which state that nitrogen addition improved the relative abundance of Firmicutes, Actinobacteria and Acidobacteria across the N-addition gradient. However, the root-zone restriction limits the nitrogen availability, so the relative abundance of the prior mentioned bacteria possesses a diminishing trend, according to (Figure 1). Rda in (Figure 4) showed a similar trend that the effect of AN and TN on the bacterial community was not on the treated plants; instead, very low was recorded in the case of control plants. This coincides with the findings of (Liu et al., 2020) that TP, NH4+-N, and NO3−-N effects on the distribution of bacterial phyla were insignificant. Zn was positively and significantly correlated with the Bacillus spp., majorly in the soil at MSC and MST. This could be because Zn uptake is conducted by a rhizophagy cycle and could be affected by the atrocities caused by the root-zone restriction to the soil texture or structure. Microbes have siderophores and other methods for efficiently sequestering micronutrients, and many are motile and move around in soils to obtain nutrients. Zinc solubilization potential in B. altitudinis and B. safensis was reported by [89]. Metals also stick to microbe cell walls because the cell walls of bacteria have a net negative charge, whereas metals have a positive charge and may be absorbed by the roots [90,91].
3.4. Correlation between Soil and Bacterial Niches
Root-zone restriction and control plants demonstrated a high correlation between the edaphic soil factors and the soil bacterial populations. (Figure 5a,b). The gathered results imply that the existence of a relationship between the soil factors correlates with and influences the microbial existence in the rhizosphere soil. This perspective of edaphic soil characteristics and taxonomically different microorganisms will likely improve our understanding of microbial interaction. It was reported that soil edaphic factors regulate the abundant and rare microbial community structuring in the soil [92,93]. In this study, the majority of soil microbiota were persuaded by edaphic soil factors, which strengthens our belief that connected microbiota must possess a diverse ecological role which would benefit the plant’s overall growth and development. The phyla proteobacteria (Figure 5a) were most abundant in the treated plant’s soil and were significantly negatively correlated with TK and positively correlated with AP. This could be because KSBs (potassium-solubilizing bacteria) are from phylum Proteobacteria, Pseudomonas, and Bacillus spp., which are also enriched in our soil of treated plants [94,95]. However, if the solubilization is increased, the total phosphorus is decreased, as they are reported for their PGP activities in wheat and maize crops [96,97]. AP was also positively with Proteobacteria and negatively correlated with Deinococcus-Thermus, denoting the fact that phosphate-solubilizing bacteria are reported from both of them, and their diversity is being affected by AP in treated plants; perhaps their PGP potential is still intact [98,99,100]. Mg positively correlates with Proteobacteria and can alter bacterial diversity.
Mg is an essential constituent of chlorophyll and acts as a micronutrient. The increased Mg addition raises microbe diversification, making the microbial community more stable, which has a substantial advantage in the fight against Huanglongbing (HLB) [101]. In (Figure 5b) the control plants, we noticed an altogether different response of the soil elements towards the bacterial structuring and also, those bacteria which were negatively correlated with these elements in treatment were showing a positive correlation in the control plants and vice versa. This might be due to regular cultivation without any external stress. The majority of nodes were concentrated in MG, AN and AK. Magnesium was positively correlated with the Dienococus-Thermus phylum in control, while it was negative in treated plants. This may be because magnesium increases the production of the hydrolytic enzyme-producing bacteria, and this activity may be hindered by root-zone restriction [102]. A similar phylum remained possessed with a positive correlation with Fe in both the cultivation techniques because Fe-rich sources affect the diversity of Dienococus-Thermus, as reported by [103]. Another excellent and multifaceted bacteria, Cyanobacteria, constitute a diverse collection of soil bacteria that promote the development of their hosts through multiple direct and indirect methods, including enhanced nutrient availability, absorption, and augmentation in the plant [104]. They stood at the predisposal of different soil elements with a significantly positive correlation in treatment and control plants. TP and Mn; TN and AK were in control and treated, respectively. Cyanobacteria can be associated with nitrogen fixation in treated plants only as it stood correlated with TN. This also seconds the finding of another reported study that 50% less application of N fertilizer, at par values of grain yield with the control that received 100% recommended dose of N fertilizer, can be possible with cyanobacterial inoculation [105]. Wheat co-culture with Cyanobacteria has been shown to increase root dry weight and chlorophyll levels [106]; however, increased chlorophyll is reported in root-zone-restricted grapevines [16]. Here lies a future expedition to carve out the affinity between the cyanobacteria and root-zone restriction.
3.5. Root Restriction Regulates the Bacterial Networking in the Grapevine Habitats
The plant host and its associated microbes cooperate eagerly, thus, founding a stable holobiont in which the microbial cohorts thrive together to progress the overall fitness [73]. Microorganisms do not endure in isolation but rather create intricate network associations. Such networks are essential for understanding microbiome formation and response to environmental influences [107,108,109]. Meanwhile, a complex network is established among the microbial species, which significantly influences ecological processes and host adaptations since the functional capability of a microbiome is not equivalent to the sum of its constituents [74]. The network analysis highlighted notable species that might play a vital role in community stability. Even though we know very little about many of the species in the networks, we may highlight specific interactions as proof of the concept that the networks reflect genuine microbial connections and that the root-zone restriction causes its effect on the bacterial interaction. We found out that the results at node and network level topological features were different among all the plant organs of control and treated plants. In the soil of the treated plant, a more complex network was observed as compared to the control plants since more nodes and edges and the most significant number of positive interactions were also observed in it; this could be due to the reason that under the restricted container in a stressful environment soil microbes might start acting in a complex way. More positive interactions and correlations with soil microbes ultimately yield healthy grapes.
Moreover, weak network complexity was observed compared to the control plants in other grapevine habitats, such as white roots, leaves, flowers and berries. Nevertheless, the evidence supporting that weaker associations may have a diminishing or common effect on plant growth and development is also rare. This could also be validated; the decrease in network complexity may be ascribed. Increased resource availability caused by the decline process may diminish the obligation for strong cooperative and trophic connections among the functional groups of soil biota [109,110]. Regardless of the weak complexities in treated plants, we still achieve higher yields than control plants, which can be evident in increased fruit quality, chlorophyll content, and better root structuring. However, the mechanism is still poorly understood. Keystone species vital for community stability, ecological function, and plant health can be discovered through network analysis [74]. Previous research revealed that when keystone species were eliminated, the composition and function of communities altered [111]. Such taxa have a significant role in microbial ecological functions [112]. Here we have detected that most of the keystone ASVs were from Proteobacteria, Firmicutes, Cyanobacteria and Actinobacteria, respectively, as also stated by [112,113]. All of the mentioned phyla are known to contain the genera responsible for promising growth and disease suppression effects on plants [25,48,64]. Due to their critical roles in sustaining the bacterial ecosystem and network complexity, identifying the keystone taxa is a significant step that could guide toward more targeted research in plant and soil microbiome organization and engineering [114,115].
4. Materials and Methods
4.1. Plant Material and Sample Collection
Three-year-old grapevine cultivar ‘Muscat Hamburg’ (Vitis vinifera L.) planted in the vineyard at the School of Agriculture and Biology of Shanghai Jiao Tong University (Shanghai, China 31°11′ N, 121°29′ E) was sampled. Each grapevine was sampled for rhizosphere soil, roots, leaves, flower, and berries, during the growing season of 2019 at full bloom (May), veraison (June), and maturity stages (August), as previously reported by [83]. Grapevine nutrition and management were managed as reported by [3,12]. All samples were immediately placed in sterile bags, maintained in liquid nitrogen during field collection, and then transferred to −80 °C until DNA isolation.
4.2. Biochemical Characterization of Grapevine
For titratable acidity (TA), 10 mL of supernatant was diluted up to 50 mL with distilled water and titrated against 0.1 N NaOH, using 2–3 drops of phenolphthalein as an indicator and expressed as a percentage (g tartaric acid/100 mL juice).
The anthocyanin concentration in berry skins was measured at three growth stages; Berry skin (50 mg) was crushed with liquid nitrogen, homogenized in 1% (v/v) hydrochloric acid in methanol and shaken at 4 °C overnight. Concentrations were measured by the absorbance of the extract at 530 nm with an ultraviolet-visible (UV–vis) spectrophotometer [116].
Ascorbic acid was determined on juice samples diluted 1:10 (v/v) with oxalic acid and following the indophenol dye titration method [117].
Fruit juice pH was measured with a pH meter (Mettler Toledo S400, Columbus, OH, USA). The data were evaluated as a factorial experiment with three replications using a totally randomized design. Fisher LSD was used as a post hoc test to calculate the difference between the means at p < 0.05 with the SPSS.
4.3. Soil Physiochemical Characterization
As mentioned above, rhizosphere soil sampled from the plants was air-dried, sieved to 2 mm, and ground to a fine powder by an electrical grinder. Soil pH (pH) was measured using a glass electrode (Mettler Toledo Instruments, Shanghai, China) with a soil/water ratio of 1:2.5 (w/v). Soil organic matter (OM) was measured by the reduction of potassium dichromate (K2Cr2O7). Cation exchange capacity (CEC) was determined using a sodium acetate solution and flame photometer at a 767 nm wavelength [118]. The Kjeldahl technique was used to estimate total nitrogen (TN); total phosphorus (TP) and total potassium (TK) was digested with HNO3+ HCLO4 and then measured using inductively coupled plasma-optical emission spectrophotometry (ICP-OES Optima6000). Soil available nitrogen (AN) was determined using acid digestion followed by the alkali distillation method [119]. The molybdenum blue method (Olsen and Sommers, 1982) was incorporated to measure available phosphorus (AP) and available potassium (AK) levels by using sodium bicarbonate and ammonium acetate and then quantified using flame photometry (Cany Precision Instrument Co., Ltd. Shanghai, China). Micronutrients (Fe, Mn, Zn) were extracted using Diethylenetriamine penta-acetic acid-calcium chloride-triethanolamine (DTPA-CaCl2-TEA, pH 7.3) (Lindsay and Norvell, 1978) and evaluated using an atomic absorption spectrophotometer (PerkinElmer AAnalyst 800). Two-way ANOVA measured the difference between the soil properties of control and treatment plants, and Tukey’s LSD was later performed as a post hoc (p < 0.05) to compute the pairwise mean comparison between the soil sampling groups by using SPSS software.
4.4. Genomic Extraction, 16S rDNA Library Preparation and MiSeq Sequencing
The Mag-bind Soil DNA Kit (D5635-02) (Omega Bio-Tek, Norcross, GA, USA) was used for genomic DNA extraction rendering to the manufacturer’s instructions. The quantity and quality of extracted DNA were measured by a NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) at 260 nm/280 nm and 260 nm/230 nm and agarose gel electrophoresis, respectively. The bacterial 16S rRNA gene’s V3-V4 region was amplified by using PCR and gene-specific primers 338F (5′-GCACCTAACTCCTACGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′). Sample-specific 7 bp barcodes were incorporated into the primers for multiplex sequencing. The PCR amplicons were purified using the AxyPrep DNA Gel Extraction Kit (Axygen, San Francisco, CA, USA, AP-GX-250) and quantified on a microplate reader (BioTek, Winooski, VT, USA, FLx800) with the Quant-iT Pico Green dsDNA Assay Kit (Invitrogen, Waltham, MA, USA, P7589), pooled together in equal amounts. Pair-end 2 × 250 bp sequencing was performed using the Illumina MiSeq platform with MiSeq Reagent Kit v3 (600-cycles-PE) (Illumina, San Diego, CA, USA, MS-102-3003). Illumina MiSeq sequencing was performed at Shanghai Personal Biotechnology Co., Ltd. (Shanghai, China). Detailed PCR conditions and library protocols can be seen in Text S1 in the Supplementary Material.
4.5. Computational, Bioinformatics and Statistical Approaches for MiSeq Analysis
Majorly the analysis was performed with QIIME 2 2019.4 [120]. Prior to statistical analysis, non-bacterial sequences of chloroplast and mitochondrial origin were removed. To summarise, raw sequence data were demultiplexed using the demux plugin, and primers were cut using the cutadapt plugin [121]. Using the DADA2 plugin, the sequences were quality filtered, denoised, combined, and chimaera eliminated [122]. The preceding procedures are examined independently for each library. All libraries, the ASVs feature sequence, and the ASV table are merged after denoising, and singletons ASVs are deleted. Non-singleton amplicon sequence variations (ASVs) were aligned using mafft [123]. The feature-classifier plugin, classify-sklearn naïve Bayes taxonomy classifier, was used to assign taxonomy to ASVs [124] against the Greengenes 16S rRNA gene database (release 13.8) with 99% OTUs reference sequences [125]. A phylogeny was constructed with fasttree2 using mafft-aligned ASVs [126].
4.6. Diversity and Statistical Analysis
The Shannon diversity index and the Chao1 richness estimate were used to compute the alpha diversity. A box plot was constructed as a graphical representation of the alpha indices. Kruskal–Wallis rank-sum test was applied to calculate the significant effect between the groups, and the Dunn test was used as a post hoc test to verify the significance of the difference. Beta diversity estimation was based on the Bray–Curtis distance matrix [127] to examine the community dissimilarity via non-metric multidimensional scaling (NMDS) [128] and hierarchical clustering analysis (UPGMA) by using the vegan package and Uclust function in the R package. Permutational multivariate analysis of variance (PERMANOVA) was carried out using the adonis function in the vegan package [129].
To examine compositional variations in the microbial community residing in the berries across the seasonal succession and to establish associations with root-zone restriction and fruit quality parameters, we conducted a multivariate analysis called canonical correspondence analysis (CCA), and the corresponding correlation factor was used to assign the r2 value by using R package ‘vegan’ (v 3.5.2). Redundancy analyses (RDA) were used to examine categorical environmental factors to predict the temporal fluctuation of microbial community structure. Monte Carlo permutation tests (p = 0.001) were used to evaluate significant associations between environmental factors and genera distributions. The correlation factor r2 was assigned by the corresponding correlation calculation method by ‘envfit’ function under the ‘vegan’ package in R (v3.5.2).
To highlight the common and unique ASVs among samples or groups, a petal map (Venn diagram) was generated using the R script (Venn Diagram package). It is predicated on the existence of ASVs in different samples/groups, independent of relative abundance. Using the default parameters for LEfSe (linear discriminant analysis effect size), the ggplot2 program was used to find differentially abundant taxa between groups [130]. It combines the nonparametric Kruskal–Wallis and Wilcoxon rank-sum tests (p-value 0.05, LDA > 2) with the effect size of linear discriminant analysis (LDA).
4.7. Prediction of Metabolic Functions
PICRUSt2 (Phylogenetic Investigation of Communities by Reconstruction of Unobserved States) is a tool that forecasts the metabolic abundance based on the 16SrRNA gene sequence data and microbial reference gene in the sample [131]. PICRUSt predicts the 16S rRNA gene using three spectrum databases: KEGG, COG (Clusters of Orthologous Groups), and Rfam. Metabolism, Genetic Information Processing, Environmental Information Processing, Cellular Processes, Organismal Systems, and Human Diseases are the six categories in which metabolic pathways are classified.
4.8. Bacterial Network Analysis
Statistical correlations underpin bacterial community co-occurrence patterns and molecular ecological networks. Distinct networks were constructed for ASVs of each plant organ and rhizosphere soil at three phonological stages. Before network construction, MENA was used in conjunction with random matrix theory (RMT)-based algorithms to automatically find the suitable similarity threshold or the minimal strength of the link between each pair of nodes [132]. Another network analysis was shown to compute the consequence of edaphic soil factors on the bacterial community structuring in grapevines under control and treatment plants. To measure the co-occurrence, Spearman’s correlation factor (r > 0.6 or r < −0.6) was incorporated into the abundance profile of each Asv and the soil factor, which was analysed by R. Furthermore, the significant relationship at p < 0.05 between soil and bacterial abundance was visualized in CYTOSCAPE (Version 3.7.2).
Indices of different nodes, modules, and interactions were used to derive network topological features, which were then shown, such as average node connectedness, average path length, diameter, cumulative degree distribution, clustering coefficient, and modularity. Properties were calculated to compare the differences between bacterial communities related to the rhizosphere soil and organs (white roots, leaves, and berries) of control and treated plants. Within each network related to specific soil and organ, to define the connection distribution of individual ASVs, the Z value (i.e., Zi: within-module connectivity) and p-value (Pi: among-module connectivity) was utilized [133]. These individual ASVs may be classified into four groups based on the criteria: peripherals (Zi 2.5, Pi 0.62), connections (Zi 2.5, Pi > 0.62), module hubs (Zi > 2.5, Pi 0.62), and network hubs (Zi > 2.5, Pi > 0.62) [133]. ASV abundance data in all samples of this project are used by default. ASV with less than ten total sequences and less than five samples are filtered out. The sparCC algorithm is used to construct a correlation matrix by using random matrix theory, and Igraph is used to construct the association network.
5. Conclusions
The root-zone restriction has ramifications for managing the bacterial consortiums during the successional developmental phases of grapevines in various habitats. Different communities were engaged by the restricted plants at the veraison stage, being the most significant in terms of richness and diversity among the control and treated plants which may point out that recruitment and selection of bacterial community are at the top. Our results also suggested that soil edaphic factors act accordingly and are delimited by restricted cultivation in terms of correlation and influencing bacterial community structuring. Relatively complex and stable networking was observed in the control plants, but restriction nullifies this concept by yielding better utilization of resources in terms of superior fruit quality. Root-zone restriction and soil factors regulated the profusion of keystone taxa. Our study provides the first example of the relationship between root-zone restriction, associated microbes, soil factors, and fruit quality. It will help us to decipher more underlying mechanisms of microbial stability, exitance and community stability in a confined ecosystem.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232415628/s1. Figure S1: Taxonomic annotation of the bacterial community. longer the columns at the genus and species levels and the shorter at the phylum level, indicates a higher resolution of the annotation. Figure S2: Alpha refraction curves of all the samples showing the observed OTU index. The flatness of the curve reflects the impact of sequencing depth on the diversity of the observed samples. The flatter the curve is, it indicates that the sequencing results are sufficient to reflect the diversity contained in the current samples. Figure S3: Species accumulation curve reflect the rate of increase in new species observed as the sample size continues to expand over the course of sampling the population. Species accumula-tion curves are similar to rarefaction curves and are used to measure and predict the increase in species richness in a community with the expansion of the sample size. Figure S4: Rank Abundance Curve shows the ASV ranks in each sample. The flatness of the broken line reflects the evenness of community composition. The gentler the broken line, the smaller the abundance difference between ASVs in the community, and the higher the evenness of the community composition. The steeper the broken line, the lower the evenness. Figure S5: Unweighted pair-group method with arith-metic means (UPGMA) cluster analysis. The samples are clustered according to their similarity with each other on the basis of bray Curtis dissimilarity index. The shorter the branch length between the samples, the more similar the two samples are. Figure S6: Canonical Correspondence analysis showing the correlation between the microbial communities of the sampling groups and the fruit quality indexes. Figure S7: Graphical representation of the edaphic soil factors of the vineyard rhizosphere soil, showing the radical effects of root-zone restriction on the soil, at three phenological stages as Full bloom, veraison and maturity. Figure S8: Network analysis on the basis of the Spearman Correlation between the edaphic factors and the bacterial community at genus level. Figure S9: Flower petal diagram showing the unique and shared ASVs in the sampling Groups. Rhizophore soil (a), White roots (b), leaves (c), berries (d). Figure S10: Lefse Clado-gram showing the bio-markers identified int eh bacterial community at different sampling stages (a). LDA score histo-gram showing the score of the identified microbial taxa, with a minimum lda score to quality was 2. Figure S11: Top 10 most abundant KEGG metabolic pathways int eh sampling groups are shown. Rhizophore soil (a), White roots (b), leaves (c), berries (d). Figure S12: Sub-network association with the sampling groups pie chart shown in the node. The pie-chart shows the contribution in terms of dominant taxa of sampling groups in a particular module. The Similarity index shows that the positive interactions are denoted by red lines while the negative with the green color. Rhizophore soil (a), White roots (b), leaves (c), berries (d). Figure S13: ZI-Pi scatter plot showing the network division in four major parts, peripherals, connectors, module hubs and network hubs. Rhizophore soil (a), White roots (b), leaves (c), berries (d). Figure S14: Subnetwork of dominant species with grouped abundance pie chart. The node (node) represents the ASV in the sample, and the size of the node is proportional to its abundance in (log2(CPM/n). The form of a pie chart shows the relative abundance ratio of the node in different samples (groups); the edge between nodes indicates that there is a cor-relation between the two connected nodes, the red line indicates a positive correlation, and the green line indicates a negative correlation. Text S1: PCR conditions and the library preparation protocols. Text S2: Network topological prop-erties. Table S1: Statistic of sequencing volume per sample. Table S2: Relative abundance of the phylum. Table S3: Sub-network topologies list. Table S4: Node topologies list.
Author Contributions
Conceptualization, M.S.Z., S.W., W.X., L.W. and S.S.; methodology, M.S.Z., Y.S., J.L. and X.L.; formal analysis, M.S.Z., M.H., Y.S., J.L., X.L. and D.G.; writing—original draft preparation, M.S.Z. and M.H; writing—review and editing, M.S.Z., M.H., W.X. and S.W.; supervision, W.X., S.W. and S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The authors would like to thank Shanghai municipal key task projects of “Prospering Agriculture by Science and Technology Plan” (Hunongke-tuizi2019-2-3-4), National Natural Science Foundation of China (Grant No. 31972383), Special Funds of Modern Industrial Technology System for Agriculture (CARS-29-zp-7) and China Scholarship Council.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on the request from the corresponding author.
Acknowledgments
Generous financial support from the Chinese Scholarship Council, P.R. China, for Muhammad Salman Zahid’s doctoral program is highly appreciated. Analytical Center, Building C, School of Agriculture and Biology, Shanghai Jiao Tong University, Shanghai, China, for allowing us to conduct the analytical experiments related to this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Alston, J.M.; Sambucci, O. Grapes in the World Economy. In The Grape Genome; Cantu, D., Walker, M.A., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–24. ISBN 978-3-030-18601-2. [Google Scholar]
- Wang, S.; Okamoto, G.; Hirano, K.; Lu, J.; Zhang, C. Effects of Restricted Rooting Volume on Vine Growth and Berry Development of Kyoho Grapevines. Am. J. Enol. Vitic. 2001, 52, 248–253. [Google Scholar] [CrossRef]
- Xie, Z.; Forney, C.; Xu, W.; Wang, S. Effects of Root Restriction on Ultrastructure of Phloem Tissues in Grape Berry. HortScience 2009, 44, 1334–1339. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Liu, B.; Wang, R.; Yan, Y.; Li, G.; Wang, L.; Ma, C.; Xu, W.; Zhao, L.; et al. Effects of Root Restriction on Phytohormone Levels in Different Growth Stages and Grapevine Organs. Sci. Rep. 2022, 12, 1323. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, C.; Wang, F.; Niu, R.; Zhu, W.; Wu, X. Root Restriction Effects of Nectarines Grown in a Non-Arable Land Greenhouse. Sci. Hortic. 2019, 250, 399–404. [Google Scholar] [CrossRef]
- Xie, Z.S.; Li, B.; Forney, C.F.; Xu, W.P.; Wang, S.P. Changes in Sugar Content and Relative Enzyme Activity in Grape Berry in Response to Root Restriction. Sci. Hortic. 2009, 123, 39–45. [Google Scholar] [CrossRef]
- Jiu, S.; Xu, Y.; Wang, J.; Haider, M.S.; Xu, J.; Wang, L.; Wang, S.; Li, J.; Liu, X.; Sun, W.; et al. Molecular Mechanisms Underlying the Action of Strigolactones Involved in Grapevine Root Development by Interacting with Other Phytohormone Signaling. Sci. Hortic. 2022, 293, 110709. [Google Scholar] [CrossRef]
- White, M.; Tustin, D.; Foote, K.; Campbell, J. GROWTH OF YOUNG SWEET CHERRY TREES IN RESPONSE TO ROOT RESTRICTION USING ROOT CONTROL BAGS. Acta Hortic. 2001, 557, 391–398. [Google Scholar] [CrossRef]
- Zakaria, N.I.; Ismail, M.R.; Awang, Y.; Megat Wahab, P.E.; Berahim, Z. Effect of Root Restriction on the Growth, Photosynthesis Rate, and Source and Sink Relationship of Chilli (Capsicum annuum L.) Grown in Soilless Culture. Biomed Res. Int. 2020, 2020, 2706937. [Google Scholar] [CrossRef]
- Hooijdonk, B.; Woolley, D.; Warrington, I.; Tustin, S. Architectural Development of “Royal Gala” Apple Scions in Response to Rootstock, Root Restriction, and Benzylaminopurine. Acta Hortic. 2006, 727, 561–567. [Google Scholar] [CrossRef]
- Ayarna, A.W.; Tsukagoshi, S.; Nkansah, G.O. Effect of Root Restriction on the Performance of Three-Truss Cultivated Tomato in the Low-Node Pinching Order at High-Density Cultivation System. Horticulturae 2021, 7, 60. [Google Scholar] [CrossRef]
- Wang, B.; He, J.; Duan, C.; Yu, X.; Zhu, L.; Xie, Z.; Zhang, C.; Xu, W.; Wang, S. Root Restriction Affects Anthocyanin Accumulation and Composition in Berry Skin of “Kyoho” Grape (Vitis vinifera L. × Vitis labrusca L.) during Ripening. Sci. Hortic. 2012, 137, 20–28. [Google Scholar] [CrossRef]
- Li, D.; Liu, B.; Wang, Z.; Li, X.; Sun, S.; Ma, C.; Wang, L.; Wang, S. Sugar Accumulation May Be Regulated by a Transcriptional Cascade of ABA-VvGRIP55-VvMYB15-VvSWEET15 in Grape Berries under Root Restriction. Plant Sci. 2022, 322, 111288. [Google Scholar] [CrossRef]
- Li, H.; Gao, Z.; Chen, Q.; Li, Q.; Luo, M.; Wang, J.; Hu, L.; Zahid, M.S.; Wang, L.; Zhao, L.; et al. Grapevine ABA Receptor VvPYL1 Regulates Root Hair Development in Transgenic Arabidopsis. Plant Physiol. Biochem. 2020, 149, 190–200. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, Q.; Liu, J.; Dou, F.; Wang, H.; Song, Y.; Ren, Y.; He, J.; Wang, L.; Zhang, C.; et al. Identification of Grape MiRNA Revealed Vvi-MiR164b Involved in Auxin Induced Root Development. Sci. Hortic. 2022, 295, 110804. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, S.; Yang, T.; Zhang, C.; Xu, W. Vine Growth and Nitrogen Metabolism of “Fujiminori” Grapevines in Response to Root Restriction. Sci. Hortic. 2006, 107, 143–149. [Google Scholar] [CrossRef]
- Yu, X.; Wang, B.; Zhang, C.; Xu, W.; He, J.; Zhu, L.; Wang, S. Effect of Root Restriction on Nitrogen Levels and Glutamine Synthetase Activity in “Kyoho” Grapevines. Sci. Hortic. 2012, 137, 156–163. [Google Scholar] [CrossRef]
- Li, D.; Pang, Y.; Li, H.; Guo, D.; Wang, R.; Ma, C.; Xu, W.; Wang, L.; Wang, S. Comparative Analysis of the Gene Expression Profile under Two Cultivation Methods Reveals the Critical Role of ABA in Grape Quality Promotion. Sci. Hortic. 2021, 281, 109924. [Google Scholar] [CrossRef]
- Orozco-Mosqueda, M.D.C.; Santoyo, G. Plant-Microbial Endophytes Interactions: Scrutinizing Their Beneficial Mechanisms from Genomic Explorations. Curr. Plant Biol. 2021, 25, 100189. [Google Scholar] [CrossRef]
- Compant, S.; Clément, C.; Sessitsch, A. Plant Growth-Promoting Bacteria in the Rhizo- and Endosphere of Plants: Their Role, Colonization, Mechanisms Involved and Prospects for Utilization. Soil Biol. Biochem. 2010, 42, 669–678. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Compant, S.; Cambon, M.C.; Vacher, C.; Mitter, B.; Samad, A.; Sessitsch, A. The Plant Endosphere World—Bacterial Life within Plants. Environ. Microbiol. 2021, 23, 1812–1829. [Google Scholar] [CrossRef]
- Garbeva, P.; van Veen, J.A.; van Elsas, J.D. MICROBIAL DIVERSITY IN SOIL: Selection of Microbial Populations by Plant and Soil Type and Implications for Disease Suppressiveness. Annu. Rev. Phytopathol. 2004, 42, 243–270. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; Domínguez-Begines, J.; Villa-Sanabria, E.; García, L.V.; Muñoz-Pajares, A.J. Tree Decline and Mortality Following Pathogen Invasion Alters the Diversity, Composition and Network Structure of the Soil Microbiome. Soil Biol. Biochem. 2022, 166, 108560. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Schlaeppi, K.; Spaepen, S.; van Themaat, E.V.L.; Schulze-Lefert, P. Structure and Functions of the Bacterial Microbiota of Plants. Annu. Rev. Plant Biol. 2013, 64, 807–838. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial Life in the Phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Reinhold-Hurek, B.; Bünger, W.; Burbano, C.S.; Sabale, M.; Hurek, T. Roots Shaping Their Microbiome: Global Hotspots for Microbial Activity. Annu. Rev. Phytopathol. 2015, 53, 403–424. [Google Scholar] [CrossRef]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Vieira, S.; Sikorski, J.; Dietz, S.; Herz, K.; Schrumpf, M.; Bruelheide, H.; Scheel, D.; Friedrich, M.W.; Overmann, J. Drivers of the Composition of Active Rhizosphere Bacterial Communities in Temperate Grasslands. ISME J. 2020, 14, 463–475. [Google Scholar] [CrossRef]
- Bodenhausen, N.; Horton, M.W.; Bergelson, J. Bacterial Communities Associated with the Leaves and the Roots of Arabidopsis Thaliana. PLoS ONE 2013, 8, e56329. [Google Scholar] [CrossRef]
- Tkacz, A.; Cheema, J.; Chandra, G.; Grant, A.; Poole, P.S. Stability and Succession of the Rhizosphere Microbiota Depends upon Plant Type and Soil Composition. ISME J. 2015, 9, 2349–2359. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere Microbiome Assemblage Is Affected by Plant Development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Vink, S.N.; Chrysargyris, A.; Tzortzakis, N.; Salles, J.F. Bacterial Community Dynamics Varies with Soil Management and Irrigation Practices in Grapevines (Vitis vinifera L.). Appl. Soil Ecol. 2021, 158, 103807. [Google Scholar] [CrossRef]
- Dombrowski, N.; Schlaeppi, K.; Agler, M.T.; Hacquard, S.; Kemen, E.; Garrido-Oter, R.; Wunder, J.; Coupland, G.; Schulze-Lefert, P. Root Microbiota Dynamics of Perennial Arabis Alpina Are Dependent on Soil Residence Time but Independent of Flowering Time. ISME J. 2017, 11, 43–55. [Google Scholar] [CrossRef]
- Heck, K.L.; van Belle, G.; Simberloff, D. Explicit Calculation of the Rarefaction Diversity Measurement and the Determination of Sufficient Sample Size. Ecology 1975, 56, 1459–1461. [Google Scholar] [CrossRef]
- Kemp, P.F.; Aller, J.Y. Bacterial Diversity in Aquatic and Other Environments: What 16S RDNA Libraries Can Tell Us. FEMS Microbiol. Ecol. 2004, 47, 161–177. [Google Scholar] [CrossRef]
- Chen, S.; Waghmode, T.R.; Sun, R.; Kuramae, E.E.; Hu, C.; Liu, B. Root-Associated Microbiomes of Wheat under the Combined Effect of Plant Development and Nitrogen Fertilization. Microbiome 2019, 7, 136. [Google Scholar] [CrossRef]
- Gobbi, A.; Acedo, A.; Imam, N.; Santini, R.G.; Ortiz-Álvarez, R.; Ellegaard-Jensen, L.; Belda, I.; Hansen, L.H. A Global Microbiome Survey of Vineyard Soils Highlights the Microbial Dimension of Viticultural Terroirs. Commun. Biol. 2022, 5, 241. [Google Scholar] [CrossRef]
- Akköprü, A.; Akat, Ş.; Özaktan, H.; Gül, A.; Akbaba, M. The Long-Term Colonization Dynamics of Endophytic Bacteria in Cucumber Plants, and Their Effects on Yield, Fruit Quality and Angular Leaf Spot Disease. Sci. Hortic. 2021, 282, 110005. [Google Scholar] [CrossRef]
- Rho, H.; Van Epps, V.; Kim, S.H.; Doty, S.L. Endophytes Increased Fruit Quality with Higher Soluble Sugar Production in Honeycrisp Apple (Malus pumila). Microorganisms 2020, 8, 699. [Google Scholar] [CrossRef] [PubMed]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates: From Plant to Rhizosphere and Beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Bakker, M.G.; Sugiyama, A.; Manter, D.K.; Vivanco, J.M. Root Exudation of Phytochemicals in Arabidopsis Follows Specific Patterns That Are Developmentally Programmed and Correlate with Soil Microbial Functions. PLoS ONE 2013, 8, e55731. [Google Scholar] [CrossRef]
- Sugiyama, A.; Ueda, Y.; Zushi, T.; Takase, H.; Yazaki, K. Changes in the Bacterial Community of Soybean Rhizospheres during Growth in the Field. PLoS ONE 2014, 9, e100709. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Van Der Lelie, D.; Zarraonaindia, I. Microbial Terroir for Wine Grapes. Proc. Natl. Acad. Sci. USA 2014, 111, 5–6. [Google Scholar] [CrossRef]
- Compant, S.; Kaplan, H.; Sessitsch, A.; Nowak, J.; Ait Barka, E.; Clément, C. Endophytic Colonization of Vitis vinifera L. by Burkholderia Phytofirmans Strain PsJN: From the Rhizosphere to Inflorescence Tissues. FEMS Microbiol. Ecol. 2008, 63, 84–93. [Google Scholar] [CrossRef]
- Van Overbeek, L.; Van Elsas, J.D. Effects of Plant Genotype and Growth Stage on the Structure of Bacterial Communities Associated with Potato (Solanum tuberosum L.). FEMS Microbiol. Ecol. 2008, 64, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef]
- Jenkins, S.N.; Waite, I.S.; Blackburn, A.; Husband, R.; Rushton, S.P.; Manning, D.C.; O’Donnell, A.G. Actinobacterial Community Dynamics in Long Term Managed Grasslands. Antonie Van Leeuwenhoek 2009, 95, 319–334. [Google Scholar] [CrossRef]
- Barata, A.; Malfeito-Ferreira, M.; Loureiro, V. The Microbial Ecology of Wine Grape Berries. Int. J. Food Microbiol. 2012, 153, 243–259. [Google Scholar] [CrossRef]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S.; et al. The Soil Microbiome Influences Grapevine-Associated Microbiota. MBio 2015, 6, e02527-14. [Google Scholar] [CrossRef]
- Marasco, R.; Rolli, E.; Fusi, M.; Cherif, A.; Abou-Hadid, A.; El-Bahairy, U.; Borin, S.; Sorlini, C.; Daffonchio, D. Plant Growth Promotion Potential Is Equally Represented in Diverse Grapevine Root-Associated Bacterial Communities from Different Biopedoclimatic Environments. Biomed Res. Int. 2013, 2013, 491091. [Google Scholar] [CrossRef]
- Samad, A.; Trognitz, F.; Compant, S.; Antonielli, L.; Sessitsch, A. Shared and Host-Specific Microbiome Diversity and Functioning of Grapevine and Accompanying Weed Plants. Environ. Microbiol. 2017, 19, 1407–1424. [Google Scholar] [CrossRef] [PubMed]
- Burns, K.N.; Kluepfel, D.A.; Strauss, S.L.; Bokulich, N.A.; Cantu, D.; Steenwerth, K.L. Vineyard Soil Bacterial Diversity and Composition Revealed by 16S RRNA Genes: Differentiation by Geographic Features. Soil Biol. Biochem. 2015, 91, 232–247. [Google Scholar] [CrossRef]
- Compant, S.; Mitter, B.; Colli-Mull, J.G.; Gangl, H.; Sessitsch, A. Endophytes of Grapevine Flowers, Berries, and Seeds: Identification of Cultivable Bacteria, Comparison with Other Plant Parts, and Visualization of Niches of Colonization. Microb. Ecol. 2011, 62, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Lareen, A.; Burton, F.; Schäfer, P. Plant Root-Microbe Communication in Shaping Root Microbiomes. Plant Mol. Biol. 2016, 90, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Fahrbach, M.; Kuever, J.; Remesch, M.; Huber, B.E.; Kämpfer, P.; Dott, W.; Hollender, J. Steroidobacter Denitrificans Gen. Nov., Sp. Nov., a Steroidal Hormone-Degrading Gammaproteobacterium. Int. J. Syst. Evol. Microbiol. 2008, 58, 2215–2223. [Google Scholar] [CrossRef]
- Holguin, J.; Collins, S.L.; McLaren, J.R. Belowground Responses to Altered Precipitation Regimes in Two Semi-Arid Grasslands. Soil Biol. Biochem. 2022, 171, 108725. [Google Scholar] [CrossRef]
- Compant, S.; Van Der Heijden, M.G.A.; Sessitsch, A. Climate Change Effects on Beneficial Plant-Microorganism Interactions. FEMS Microbiol. Ecol. 2010, 73, 197–214. [Google Scholar] [CrossRef]
- Li, H.; Gao, Z.; Zahid, M.S.; Li, D.; Javed, H.U.; Wang, L.; Song, S.; Zhao, L.; Xu, W.; Zhang, C.; et al. Small RNA Sequencing Analysis of MiRNA Expression Reveals Novel Insihts into Root Formation under Root Restriction Cultivation in Grapevine (Vitis vinifera L.). Int. J. Mol. Sci. 2020, 21, 3513. [Google Scholar] [CrossRef]
- Bever, J.D.; Platt, T.G.; Morton, E.R. Microbial Population and Community Dynamics on Plant Roots and Their Feedbacks on Plant Communities. Annu. Rev. Microbiol. 2012, 66, 265–283. [Google Scholar] [CrossRef]
- Pérez-Jaramillo, J.E.; Mendes, R.; Raaijmakers, J.M. Impact of Plant Domestication on Rhizosphere Microbiome Assembly and Functions. Plant Mol. Biol. 2016, 90, 635–644. [Google Scholar] [CrossRef]
- Grayston, S.J.; Wang, S.; Campbell, C.D.; Edwards, A.C. Selective Influence of Plant Species on Microbial Diversity in the Rhizosphere. Soil Biol. Biochem. 1998, 30, 369–378. [Google Scholar] [CrossRef]
- Berendsen, R.L.; Pieterse, C.M.J.; Bakker, P.A.H.M. The Rhizosphere Microbiome and Plant Health. Trends Plant Sci. 2012, 17, 478–486. [Google Scholar] [CrossRef] [PubMed]
- Gantar, M.; Kerby, N.W.; Rowell, P.; Obreht, Z.; Scrimgeour, C. Colonization of Wheat (Triticum vulgare L.) by Ng-Fixing Cyanobacteria. New Phytol. 1991, 118, 485–492. [Google Scholar] [CrossRef]
- Singh, P.; Santoni, S.; Weber, A.; This, P.; Péros, J.P. Understanding the Phyllosphere Microbiome Assemblage in Grape Species (Vitaceae) with Amplicon Sequence Data Structures. Sci. Rep. 2019, 9, 14294. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Dong, W.; Yan, D.H. Endophytic Bacterial Communities of Jingbai Pear Trees in North China Analyzed with Illumina Sequencing of 16S RDNA. Arch. Microbiol. 2019, 201, 199–208. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; del Rio, T.G.; et al. Defining the Core Arabidopsis thaliana Root Microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef]
- Weinert, N.; Piceno, Y.; Ding, G.C.; Meincke, R.; Heuer, H.; Berg, G.; Schloter, M.; Andersen, G.; Smalla, K. PhyloChip Hybridization Uncovered an Enormous Bacterial Diversity in the Rhizosphere of Different Potato Cultivars: Many Common and Few Cultivar-Dependent Taxa. FEMS Microbiol. Ecol. 2011, 75, 497–506. [Google Scholar] [CrossRef]
- Zhong, Y.; Yan, W.; Shangguan, Z. Impact of Long-Term N Additions upon Coupling between Soil Microbial Community Structure and Activity, and Nutrient-Use Efficiencies. Soil Biol. Biochem. 2015, 91, 151–159. [Google Scholar] [CrossRef]
- Vepštaitė-Monstavičė, I.; Lukša, J.; Stanevičienė, R.; Strazdaitė-Žielienė, Ž.; Yurchenko, V.; Serva, S.; Servienė, E. Distribution of Apple and Blackcurrant Microbiota in Lithuania and the Czech Republic. Microbiol. Res. 2018, 206, 1–8. [Google Scholar] [CrossRef]
- Enya, J.; Shinohara, H.; Yoshida, S.; Tsukiboshi, T.; Negishi, H.; Suyama, K.; Tsushima, S. Culturable Leaf-Associated Bacteria on Tomato Plants and Their Potential as Biological Control Agents. Microb. Ecol. 2007, 53, 524–536. [Google Scholar] [CrossRef]
- Stefanini, I.; Cavalieri, D. Metagenomic Approaches to Investigate the Contribution of the Vineyard Environment to the Quality of Wine Fermentation: Potentials and Difficulties. Front. Microbiol. 2018, 9, 991. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, E.; Zilber-Rosenberg, I. Microbes Drive Evolution of Animals and Plants: The Hologenome Concept. MBio 2016, 7, e01395-15. [Google Scholar] [CrossRef] [PubMed]
- Layeghifard, M.; Hwang, D.M.; Guttman, D.S. Disentangling Interactions in the Microbiome: A Network Perspective. Trends Microbiol. 2017, 25, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Sgroy, V.; Cassán, F.; Masciarelli, O.; Del Papa, M.F.; Lagares, A.; Luna, V. Isolation and Characterization of Endophytic Plant Growth-Promoting (PGPB) or Stress Homeostasis-Regulating (PSHB) Bacteria Associated to the Halophyte Prosopis Strombulifera. Appl. Microbiol. Biotechnol. 2009, 85, 371–381. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Kucharova, Z.; Davranov, K.; Berg, G.; Makarova, N.; Azarova, T.; Chebotar, V.; Tikhonovich, I.; Kamilova, F.; Validov, S.Z.; et al. Bacteria Able to Control Foot and Root Rot and to Promote Growth of Cucumber in Salinated Soils. Biol. Fertil. Soils 2011, 47, 197–205. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Jabborova, D.; Räsänen, L.A.; Liao, H. Coordination between Bradyrhizobium and Pseudomonas Alleviates Salt Stress in Soybean through Altering Root System Architecture. J. Plant Interact. 2017, 12, 100–107. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, J.S.; Saxena, A.K.; Singh, D.P. Impact of PGPR Inoculation on Growth and Antioxidant Status of Wheat under Saline Conditions. Plant Biol. 2012, 14, 605–611. [Google Scholar] [CrossRef]
- Salomon, M.V.; Bottini, R.; de Souza Filho, G.A.; Cohen, A.C.; Moreno, D.; Gil, M.; Piccoli, P. Bacteria Isolated from Roots and Rhizosphere of Vitis vinifera Retard Water Losses, Induce Abscisic Acid Accumulation and Synthesis of Defense-Related Terpenes in In Vitro Cultured Grapevine. Physiol. Plant. 2014, 151, 359–374. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, P.; Chen, D.; Howell, K. From the Vineyard to the Winery: How Microbial Ecology Drives Regional Distinctiveness of Wine. Front. Microbiol. 2019, 10, 2679. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Collins, T.; Masarweh, C.; Allen, G.; Heymann, H.; Ebeler, S.E.; Mills, D.A. Fermentation Behavior Suggest Microbial Contribution to Regional. MBio 2016, 7, e00631-16. [Google Scholar] [CrossRef]
- Knight, S.; Goddard, M.R. Quantifying Separation and Similarity in a Saccharomyces Cerevisiae Metapopulation. ISME J. 2015, 9, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.S.; Li, D.; Javed, H.U.; Sabir, I.A.; Wang, L.; Jiu, S.; Song, S.; Ma, C.; Wang, D.; Zhang, C.; et al. Comparative Fungal Diversity and Dynamics in Plant Compartments at Different Developmental Stages under Root-Zone Restricted Grapevines. BMC Microbiol. 2021, 21, 317. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N.; Jackson, R.B. The Diversity and Biogeography of Soil Bacterial Communities. Proc. Natl. Acad. Sci. USA 2006, 103, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.G.; Steenwerth, K.L.; Mills, D.A.; Cantu, D.; Bokulich, N.A. Sources and Assembly of Microbial Communities in Vineyards as a Functional Component of Winegrowing. Front. Microbiol. 2021, 12, 836. [Google Scholar] [CrossRef]
- Fierer, N. Microbial Diversity Across Space and Time. In Accessing Uncultivated Microorganisms; Wiley: Hoboken, NJ, USA, 2008; pp. 95–116. ISBN 9781683671466. [Google Scholar] [CrossRef]
- Holland, T.C.; Bowen, P.A.; Bogdanoff, C.P.; Lowery, T.D.; Shaposhnikova, O.; Smith, S.; Hart, M.M. Evaluating the Diversity of Soil Microbial Communities in Vineyards Relative to Adjacent Native Ecosystems. Appl. Soil Ecol. 2016, 100, 91–103. [Google Scholar] [CrossRef]
- Li, J.G.; Shen, M.C.; Hou, J.F.; Li, L.; Wu, J.X.; Dong, Y.H. Effect of Different Levels of Nitrogen on Rhizosphere Bacterial Community Structure in Intensive Monoculture of Greenhouse Lettuce. Sci. Rep. 2016, 6, 25305. [Google Scholar] [CrossRef] [PubMed]
- de Melo, L.H.V.; Rocha, F.Y.O.; Vidal, M.S.; Gitahy, P.D.M.; Arruda, G.M.; Barreto, C.P.; Alves, P.B.; Ramos, E.T.D.A.; Rossi, C.N.; Schwab, S.; et al. Diversity and Biotechnological Potential of Endophytic Bacillus Species Originating from the Stem Apoplast Fluid of Sugarcane Plants. Appl. Soil Ecol. 2021, 166, 103985. [Google Scholar] [CrossRef]
- White, J.F.; Kingsley, K.L.; Zhang, Q.; Verma, R.; Obi, N.; Dvinskikh, S.; Elmore, M.T.; Verma, S.K.; Gond, S.K.; Kowalski, K.P. Review: Endophytic Microbes and Their Potential Applications in Crop Management. Pest Manag. Sci. 2019, 75, 2558–2565. [Google Scholar] [CrossRef]
- Johnstone, T.C.; Nolan, E.M. Beyond Iron: Non-Classical Biological Functions of Bacterial Siderophores. Dalt. Trans. 2015, 44, 6320–6339. [Google Scholar] [CrossRef]
- Xia, Q.; Rufty, T.; Shi, W. Soil Microbial Diversity and Composition: Links to Soil Texture and Associated Properties. Soil Biol. Biochem. 2020, 149, 107953. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, Y.; Zhang, F. Abundant and Rare Bacteria Possess Different Diversity and Function in Crop Monoculture and Rotation Systems across Regional Farmland. Soil Biol. Biochem. 2022, 171, 108742. [Google Scholar] [CrossRef]
- Qi, D.; Wieneke, X.; Tao, J.; Zhou, X.; Desilva, U. Soil PH Is the Primary Factor Correlating with Soil Microbiome in Karst Rocky Desertification Regions in the Wushan County, Chongqing, China. Front. Microbiol. 2018, 9, 1027. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Verma, J.P.; Meena, R.S. Potassium Solubilizing Microorganisms for Sustainable Agriculture. In Potassium Solubilizing Microorganisms for Sustainable Agriculture; Springer: Cham, Switzerland, 2016; pp. 1–331. [Google Scholar] [CrossRef]
- Leaungvutiviroj, C.; Ruangphisarn, P.; Hansanimitkul, P.; Shinkawa, H.; Sasaki, K. Development of a New Biofertilizer with a High Capacity for N2 Fixation, Phosphate and Potassium Solubilization and Auxin Production. Biosci. Biotechnol. Biochem. 2010, 74, 1098–1101. [Google Scholar] [CrossRef]
- Sheng, X.F.; Zhao, F.; He, L.Y.; Qiu, G.; Chen, L. Isolation and Characterization of Silicate Mineral-Solubilizing Bacillus Globisporus Q12 from the Surfaces of Weathered Feldspar. Can. J. Microbiol. 2008, 54, 1064–1068. [Google Scholar] [CrossRef] [PubMed]
- Hameeda, B.; Harini, G.; Rupela, O.P.; Wani, S.P.; Reddy, G. Growth Promotion of Maize by Phosphate-Solubilizing Bacteria Isolated from Composts and Macrofauna. Microbiol. Res. 2008, 163, 234–242. [Google Scholar] [CrossRef]
- Chung, H.; Park, M.; Madhaiyan, M.; Seshadri, S.; Song, J.; Cho, H.; Sa, T. Isolation and Characterization of Phosphate Solubilizing Bacteria from the Rhizosphere of Crop Plants of Korea. Soil Biol. Biochem. 2005, 37, 1970–1974. [Google Scholar] [CrossRef]
- Wan, W.; Qin, Y.; Wu, H.; Zuo, W.; He, H.; Tan, J.; Wang, Y.; He, D. Isolation and Characterization of Phosphorus Solubilizing Bacteria With Multiple Phosphorus Sources Utilizing Capability and Their Potential for Lead Immobilization in Soil. Front. Microbiol. 2020, 11, 752. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, Y.; Hu, C.; Zhan, T.; Zhang, S.; Cai, M.; Zhao, X. Soil Applied Ca, Mg and B Altered Phyllosphere and Rhizosphere Bacterial Microbiome and Reduced Huanglongbing Incidence in Gannan Navel Orange. Sci. Total Environ. 2021, 791, 148046. [Google Scholar] [CrossRef]
- Mehetre, G.; Shah, M.; Dastager, S.G.; Dharne, M.S. Untapped Bacterial Diversity and Metabolic Potential within Unkeshwar Hot Springs, India. Arch. Microbiol. 2018, 200, 753–770. [Google Scholar] [CrossRef]
- Kochetkova, T.V.; Toshchakov, S.V.; Zayulina, K.S.; Elcheninov, A.G.; Zavarzina, D.G.; Lavrushin, V.Y.; Bonch-Osmolovskaya, E.A.; Kublanov, I. V Hot in Cold: Microbial Life in the Hottest Springs in Permafrost. Microorganisms 2020, 8, 1308. [Google Scholar] [CrossRef]
- Khan, A.G. Role of Soil Microbes in the Rhizospheres of Plants Growing on Trace Metal Contaminated Soils in Phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, R.; Bidyarani, N.; Babu, S.; Hossain, F.; Shivay, Y.S.; Nain, L. Cyanobacterial Inoculation Elicits Plant Defense Response and Enhanced Zn Mobilization in Maize Hybrids. Cogent Food Agric. 2015, 1, 998507. [Google Scholar] [CrossRef]
- Obreht, Z.; Kerby, N.W.; Gantar, M.; Rowell, P. Effects of Root-Associated N2-Fixing Cyanobacteria on the Growth and Nitrogen Content of Wheat (Triticum vulgare L.) Seedlings. Biol. Fertil. Soils 1993, 15, 68–72. [Google Scholar] [CrossRef]
- Edwards, J.; Johnson, C.; Santos-medellín, C.; Lurie, E.; Kumar, N. Structure, Variation, and Assembly of the Root-Associated Microbiomes of Rice. Proc. Natl. Acad. Sci. USA 2015, 112, E911–E920. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, K.S.; Geisen, S.; Morriën, E.; Snoek, B.L.; van der Putten, W.H. Network Analyses Can Advance Above-Belowground Ecology. Trends Plant Sci. 2018, 23, 759–768. [Google Scholar] [CrossRef]
- Morriën, E.; Hannula, S.E.; Snoek, L.B.; Helmsing, N.R.; Zweers, H.; de Hollander, M.; Soto, R.L.; Bouffaud, M.-L.; Buée, M.; Dimmers, W.; et al. Soil Networks Become More Connected and Take up More Carbon as Nature Restoration Progresses. Nat. Commun. 2017, 8, 14349. [Google Scholar] [CrossRef]
- Banerjee, S.; Walder, F.; Büchi, L.; Meyer, M.; Held, A.Y.; Gattinger, A.; Keller, T.; Charles, R.; van der Heijden, M.G.A. Agricultural Intensification Reduces Microbial Network Complexity and the Abundance of Keystone Taxa in Roots. ISME J. 2019, 13, 1722–1736. [Google Scholar] [CrossRef]
- Banerjee, S.; Schlaeppi, K.; van der Heijden, M.G.A. Keystone Taxa as Drivers of Microbiome Structure and Functioning. Nat. Rev. Microbiol. 2018, 16, 567–576. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, J.; Li, Z.; Xin, X.; Guo, Z.; Wang, D.; Li, D. Long-Term Phosphorus Deficiency Decreased Bacterial-Fungal Network Complexity and Efficiency across Three Soil Types in China as Revealed by Network Analysis. Appl. Soil Ecol. 2020, 148, 103506. [Google Scholar] [CrossRef]
- Zhao, D.; Shen, F.; Zeng, J.; Huang, R.; Yu, Z.; Wu, Q.L. Science of the Total Environment Network Analysis Reveals Seasonal Variation of Co-Occurrence Correlations between Cyanobacteria and Other Bacterioplankton. Sci. Total Environ. 2016, 573, 817–825. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.; Meng, D.; Qin, C.; Liu, X.; Liang, Y.; Xiao, Y.; Liu, Z. Integrated Network Analysis Reveals the Importance of Microbial Interactions for Maize Growth. Appl. Microbiol. Biotechnol. 2018, 102, 3805–3818. [Google Scholar] [CrossRef] [PubMed]
- Lurie, S.; Ovadia, R.; Nissim-Levi, A.; Oren-Shamir, M.; Kaplunov, T.; Zutahy, Y.; Weksler, H.; Lichter, A. Abscisic Acid Improves Colour Development in “Crimson Seedless” Grapes in the Vineyard and on Detached Berries. J. Hortic. Sci. Biotechnol. 2009, 84, 639–644. [Google Scholar] [CrossRef]
- Ruck, J.A. Chemical Methods for Analysis of Fruit and Vegetable Products. In Chemical Methods for Analysis of Fruit and Vegetable Products; Canada Department of Agriculture: Ottawa, ON, Canada, 1969; p. 1154. [Google Scholar] [CrossRef]
- Chapman, H.D. Cation-exchange Capacity. Methods Soil Anal. Part 2 Chem. Microbiol. Prop. 1965, 9, 891–901. [Google Scholar]
- Estefan, G.; Sommer, R.; Ryan, J. Methods of Soil, Plant, and Water Analysis. A Man. West Asia North Africa Reg. 2013, 3, 65–119. [Google Scholar]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet 2011, 17, 10. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Kazutaka, K.; Misakwa, K.; Kei-ichi, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Gregory Caporaso, J. Optimizing Taxonomic Classification of Marker-Gene Amplicon Sequences with QIIME 2’s Q2-Feature-Classifier Plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- McDonald, D.; Price, M.N.; Goodrich, J.; Nawrocki, E.P.; Desantis, T.Z.; Probst, A.; Andersen, G.L.; Knight, R.; Hugenholtz, P. An Improved Greengenes Taxonomy with Explicit Ranks for Ecological and Evolutionary Analyses of Bacteria and Archaea. ISME J. 2012, 6, 610–618. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2—Approximately Maximum-Likelihood Trees for Large Alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef] [PubMed]
- Bray, J.R.; Curtis, J.T. An Ordination of the Upland Forest Communities of Southern Wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Legendre, P.; Legendre, L. Numerical Ecology, 3rd ed.; Elsevier: Amsterdam, the Netherlands, 2012; p. 1006. ISBN 0444538690. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Wagner, H. Vegan: Community Ecology Package. 2019. R Package Vegan, Vers. 2.2-1. World Agrocentre 2015, 3, 7–81. [Google Scholar]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Douglas, G.M.; Maffei, V.J.; Zaneveld, J.R.; Yurgel, S.N.; Brown, J.R.; Taylor, C.M.; Huttenhower, C.; Langille, M.G.I. PICRUSt2 for Prediction of Metagenome Functions. Nat. Biotechnol. 2020, 38, 685–688. [Google Scholar] [CrossRef]
- Deng, Y.; Jiang, Y.H.; Yang, Y.; He, Z.; Luo, F.; Zhou, J. Molecular Ecological Network Analyses. BMC Bioinform. 2012, 13, 113. [Google Scholar] [CrossRef]
- Guimerà, R.; Nunes Amaral, L.A. Functional Cartography of Complex Metabolic Networks—Supplementary Material. Nature 2005, 433, 895–900. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).