Abstract
An association between the BRAFV600E mutation and the clinicopathological progression of papillary thyroid microcarcinoma (PTMC) has been suggested. We aimed to summarize the relevant literature and determine the predictive value of BRAFV600E mutation in predicting clinical outcomes and risk stratification in patients with PTMC. A systematic search using PubMed, Cochrane, and Embase up to February 2020 was performed. A total of 33 studies met the inclusion criteria, resulting in a pool of 8838 patients, of whom 5043 (57.1%) patients were positive for BRAFV600E mutation. Tumors with positive BRAFV600E mutation had a higher tendency for multifocality (RR = 1.09, 95%CI = 1.03–1.16), extrathyroidal extension (RR = 1.79, 95%CI = 1.37–2.32), and lymph node metastasis (RR = 1.43, 95%CI = 1.19–1.71). Patients with BRAFV600E mutation were at increased risk of disease recurrence (RR = 1.90, 95%CI = 1.43–2.53). PTMC in patients positive for the BRAFV600E mutation is more aggressive than wild-type BRAF PTMC. Since BRAF-mutated PTMC is generally more resistant to radioiodine treatment, patients with BRAFV600E-mutated PTMC may require earlier management, such as a minimally invasive ablative intervention. Conservative management by active surveillance may be suitable for patients with wild-type BRAFV600E PTMC.
1. Introduction
Papillary thyroid cancer (PTC) is the most common endocrine malignancy, with detection rates consistently increasing over the past four decades [1]. PTC is generally considered an indolent disease with patients experiencing an acceptable prognosis. Since tumor size is a known risk factor of PTC progression [2], it has been suggested that patients with papillary thyroid microcarcinoma (PTMC), defined as tumors less than or equal to 1.0 cm in diameter, have a better prognosis and may undergo less aggressive treatment. Considering the rather stable incidence-based mortality rates but increased incidence rates of detection, the American Thyroid Association (ATA) supports hemithyroidectomy and active surveillance as potential management options for patients with PTMC [3]. Since early detection and treatment is a long-standing notion in the field of oncology and delayed surgical intervention may increase mortality risk by as much as 94% in thyroid cancer patients [4], it is especially important to determine the tumor molecular characteristics that may predict clinical progression.
The BRAFV600E mutation is caused by substituting valine (V) with glutamic acid (E) at amino acid 600. BRAF mutation is common in patients with PTC, with prevalence rates as high as 51% [5]. BRAF mutation in thyroid cancer occurs only in PTC and PTC-derived anaplastic thyroid cancers, suggesting a role between the two [6]. Mutation of the BRAF oncogene has been associated with extrathyroidal invasion (ETE), lymph node metastasis (LNM), and decreased 10-year survival [6,7,8]. Despite this, a recent 2020 meta-analysis reporting on 11 studies (4674 patients) found that disease recurrence rates were similar between PTC patients with and without BRAF mutation (HR 1.16, 95%CI 0.78–1.71) [9].
Elucidating the clinical implications of BRAFV600E mutation will allow the thyroidology community to better optimize the extent of treatment that patients with PTMC may receive. This meta-analysis aimed to determine the prognostic value of BRAFV600E mutation in predicting clinical outcomes to allow better risk stratification in patients with PTMC.
2. Results
2.1. Literature Search
A total of 676 articles were obtained from the search query, of which 497 remained following duplicate-article deletion. Of these, 409 articles did not meet the initial inclusion criteria, and a total of 88 articles underwent full-text screening. Ultimately, 33 unique articles met the inclusion criteria and were included in this meta-analysis (Figure 1). The works were published between 2005 and 2021, with 11 articles being published within the last 5 years, suggesting interest in the role of BRAF mutation. The articles displayed broad geographic variability, including 8 from China, 8 from South Korea, and 7 from Italy.
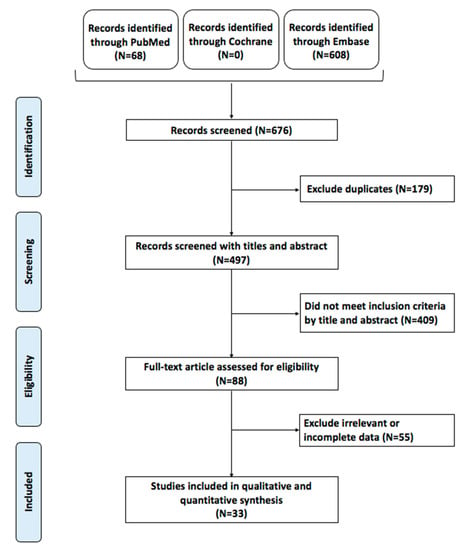
Figure 1.
Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow chart of the included studies.
2.2. Study Population
A total of 8838 patients were included in this meta-analysis. Of these, 5043 (57.1%) patients were positive for the BRAFV600E mutation. Testing for BRAFV600E gene mutation was typically done postoperatively on surgical pathology, while a few studies preoperatively confirmed mutation by fine needle aspiration (FNA) genetic analysis. Characteristics of all 33 included studies are shown in Table 1.

Table 1.
Characteristics of the articles included.
2.3. Demographic Characteristics
Overall, 16 studies classified their patient population into young (≤45 years of age) and older (>45 years) cohorts, accounting for 2804 patients. The AJCC recommends a cutoff of 45 years of age. There was no association between a younger (≤45 years of age) patient population and BRAFV600E mutation (OR = 0.92, 95%CI = 0.78–1.08, Figure 2A). Similarly, no association was found in patients older than 45 years of age and BRAFV600E mutation (OR = 1.10, 95%CI = 0.93–1.28, Figure 2B). BRAFV600E mutation was slightly more likely to present in females (OR = 0.82, 95%CI = 0.72–0.94, Figure 2C) than males (OR = 1.21, 95%CI = 1.07–1.38, Figure 2D).
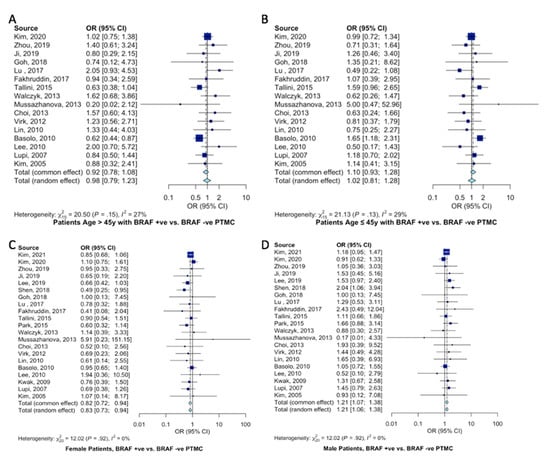
Figure 2.
Meta-analysis of demographic characteristics and association with BRAFV600E mutation. Forest plot for (A) older patients (>45 years) [11,12,15,19,20,21,22,26,27,29,30,34,35,36,41,42], (B) younger patients (≤45 years) [11,12,15,19,20,21,22,26,27,29,30,34,35,36,41,42], (C) female patients [10,11,12,15,16,18,19,20,21,22,23,26,27,29,30,34,35,36,37,41,42], and (D) male patients [10,11,12,15,16,18,19,20,21,22,23,26,27,29,30,34,35,36,37,41,42].
2.4. Pathological Features
Pooled estimates of pathological and clinical characteristics of PTMC patients according to the BRAFV600E gene mutation were analyzed. Tumors with positive BRAFV600E mutation were less likely to be <5 mm in size (RR = 0.79, 95%CI = 0.64–0.98, Figure 3A) and at increased odds of being ≥5mm in size (RR = 1.18, 95%CI = 1.04–1.34, Figure 3B). BRAFV600E mutant PTMCs were at 79% increased risk of displaying extrathyroidal extension (RR = 1.79, 95%CI = 1.37–2.32, Figure 3C) and 9% increased risk of displaying tumor multifocality (RR = 1.09, 95%CI = 1.03–1.16, Figure 3D).
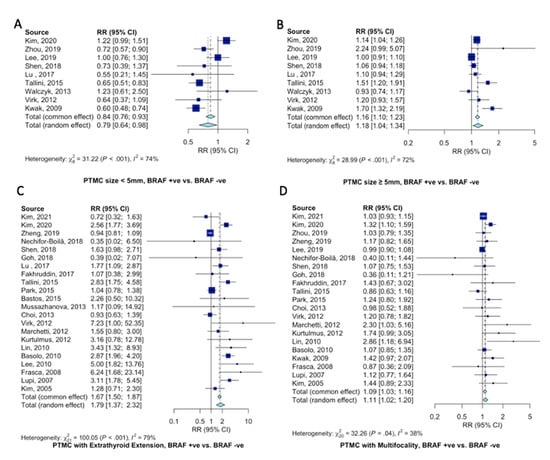
Figure 3.
Pooled analysis of clinicopathological characteristics in PTMC patients according to BRAFV600E gene mutation. Forest plots for (A) PTMC < 5mm [11,12,16,18,20,22,26,30,37], (B) PTMC ≥ 5mm [11,12,16,18,20,22,26,30,37], (C) extrathyroidal extension [10,11,13,17,18,19,20,21,22,23,24,27,29,30,32,33,34,35,36,39,41,42], and (D) multifocality [10,11,12,13,16,17,18,19,21,22,23,29,30,32,33,34,35,37,39,41,42].
Compared to patients with wild-type BRAFV600E PTMC, patients with BRAFV600E-mutant PTMC were at 43% increased risk of presenting with LNM (RR = 1.43, 95%CI = 1.19–1.71, Figure 4A). Specifically, BRAFV600E mutation increased the risk of central lymph node metastasis 36% (RR = 1.36, 95%CI = 1.08–1.71, Figure 4B). Seemingly, since PTMC typically spread through the central compartment, there only tended to be an increase in lateral LNM (RR = 1.28, 95%CI = 0.60–2.74, Figure 4C).
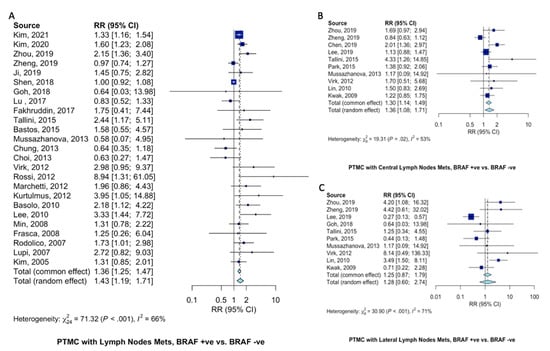
Figure 4.
Pooled analysis of lymph node metastasis in PTMC patients according to BRAFV600E mutation. Forest plots for (A) LNM [10,11,12,13,15,18,19,20,21,22,24,27,28,29,30,31,32,33,35,36,38,39,40,41,42], (B) central LNM [12,13,14,16,22,23,27,30,34,37], and (C) lateral LNM [12,13,16,19,22,23,27,30,34,37].
Patients with BRAFV600E-mutant PTMC at 61% (RR = 1.61, 95%CI = 1.14–2.28, Figure 5A) increased risk of presenting with advanced (TNM > 3) clinical stage. Risk of capsular invasion were similar (RR = 1.19, 95%CI = 0.90–1.57, Figure 5B) between PTMC patients with and without BRAFV600E mutation. Disease recurrence was almost twice as likely in patients with BRAFV600E-mutant PTMC (RR = 1.90, 95%CI = 1.43–2.53, Figure 5C) than those not harboring BRAFV600E mutation.
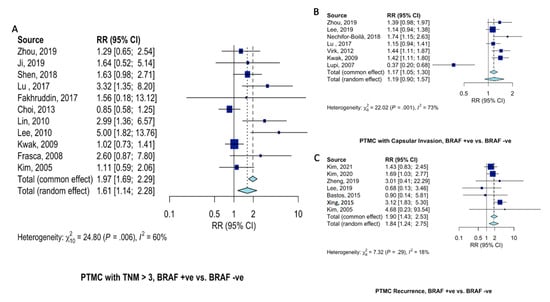
Figure 5.
Pooled analysis of clinical characteristics in PTMC patients according to BRAFV600E gene mutation. Forest plots for (A) advanced (TNM > 3) disease stage [12,15,18,20,21,29,34,36,37,39,42], (B) capsular invasion [12,16,17,20,30,37,41], and (C) recurrence [10,11,13,16,24,25,42].
3. Discussion
PTMC is the most common malignant tumor in patients aged 45 years and older [43]. Though most patients display excellent prognosis, a recent work of ours using the National Cancer Database (N = 5628 patients) found that 19% of patients with PTMC present with advanced features (defined as lymph node metastasis, extrathyroidal extension, or lymphovascular invasion) [44]. In around 45% of PTC, BRAF mutation is present [6]. Our results demonstrate that the BRAFV600E mutation was associated with larger PTMC’s (≥5mm but <10mm), multifocality, ETE, advanced stage, and higher recurrence rates. Considering that PTMC with BRAF mutation may not be as indolent a disease as conventionally thought, our work may assist surgeons and endocrinologists in appropriate treatment planning.
BRAFV600E mutation is prevalent in approximately 45% in PTC, though this value drops to around 30% in TNM I and II PTC [6]. Therefore, approximately one in three PTMC cases involve BRAF mutation [45]. This is consistent with our study findings, which found a significant relationship between tumor size ≥ 5mm and the BRAFV600E mutation. Accordingly, disease progression is known to correlate with tumor size [11], such that PTMC tumors ≥ 5 mm more often present with central lymph node metastasis than those < 5mm [46,47,48]. Therefore, patients with PTMC ≥ 5mm with confirmed BRAF mutation should be appropriately counseled and closely monitored for disease progression.
Numerous works have found BRAFV600E mutation to be associated with a worse initial presentation [49,50]. Our results found that tumors with BRAFV600E mutation were almost twice as likely (RR = 1.79) as those with BRAF wild-type PTMC to develop ETE. Interestingly, a recent 2020 work by Tallini et al. reported that PTMCs > 5mm in size were more frequently located in the peripheral region of the thyroid or were “subcapsular” [51]. The authors hypothesized that these tumors were influenced by the exterior microenvironment. Peripherally located PTMC has been associated with infiltrative growth, lymph node metastasis, and BRAFV600E mutation [30,52,53]. Our results also found that BRAFV600E mutated PTMC were approximately 40% more likely to develop lymph-node metastasis (RR = 1.43). This is consistent with several primary studies. Since the presence of clinicopathologic features such as LNM and ETE in BRAF-mutated PTMC allow synergistic aggressive behavior [8,25], patients with advanced PTMC disease are appropriately classified as “intermediate risk” as opposed to “low risk” by current ATA guideline recommendations [3].
Since patients with PTMC have an extremely low mortality rate, the bulk of patient clinical management lies in preventing and identifying disease recurrence [45]. Our analysis found that patients with BRAF-mutated PTMC are almost twice as likely as those with wild-type BRAF PTMC to develop disease recurrence (RR = 1.90). These findings are consistent with a recent multicenter international study that included 742 patients, which found that overall disease recurrence was 6.4% (32/502) in wild-type BRAF tumors but increased significantly to 10.8% (26/241) in BRAF-mutated PTC (p = 0.041, [11]). On multivariate analysis of low-risk PTMC, the authors found that BRAF mutation conferred six times the chance of disease recurrence (HR = 6.65, 95%CI = 1.80–24.65, [11]). Considering a reported negative predictive value of BRAF mutation of 98.7% with respect to disease recurrence in low-risk PTMC [11], BRAF mutation identification should be considered in patients seeking management by conservative methods such as active surveillance.
The management of PTMC has been long-debated, with the most recent ATA guidelines allowing consideration of active surveillance in an attempt to prevent overly aggressive intervention [3]. Active surveillance is the close monitoring of patients with PTC or PTMC with routine imaging screening for disease progression [54,55]. The potential for complications during thyroidectomy, such as recurrent laryngeal nerve paresis and hypoparathyroidism, make active surveillance an attractive treatment option (). The current ATA guidelines recommend surgery for patients with primary thyroid cancers but recommend considering conservative active surveillance in patients with very low-risk tumors (e.g., no clinical evidence of disease) or who are at high surgical risk (e.g., worrisome comorbid conditions). The guidelines recommended future studies to elucidate the role of BRAF mutation before its incorporation into risk stratification [3]. Considering the accordingly increased risk of tumor multifocality, ETE, capsular invasion, lymph node metastasis, and disease recurrence in BRAF mutated PTMC, patients with such tumors may be appropriately recommended an intervention of moderate aggressiveness, such as minimally invasive ablative techniques. Radiofrequency ablation (RFA) is one treatment option that has demonstrated impressive safety and efficacy profiles [56,57]. A 2021 study of 102 patients with PTMC found 100% resorption rates at the 5-year follow-up [58]. Befittingly, minimally invasive ablative technology is generally more efficacious in nodules of smaller sizes [59,60], making them an attractive option for patients with PTMC. Additionally, since BRAF mutation is thought to silence thyroid iodide-handling genes and make these carcinomas more resistant to radioiodine treatment, surgeons and endocrinologists should consider immediate management [6].
Our study is not without limitations. First, though the large sample size allowed for powerful analysis, all studies included a retrospective study design. The authors acknowledge this limitation and attempted to address it by evaluating the degree of bias. Additionally, while the studies represent a breadth of populations and allow for greater data generalizability, sub-group analyses for patient race were not possible as they were frequently not reported.
4. Materials and Methodology
4.1. Literature Search
This meta-analysis was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) guidelines. Three databases, including PubMed, Cochrane Library, and Embase were searched for primary peer-reviewed articles through November 2022. The following search terms were used: (thyroid) AND (microcarcinoma) AND (BRAFV600E). Reference lists from relevant review articles and included studies were also searched. Only articles reported in the English language were considered for inclusion. All articles that met the following inclusion criteria were considered for inclusion: studies that (a) were randomized controlled trials, observational design studies including cross-sectional, case-control, and/or cohort designs, (b) described PTMC, (c) considered patients with BRAFV600E mutation, and (d) analyzed potential prognostic factors in patients. Articles were excluded if they met the following criteria: (a) were review papers, conference papers, editorial letters, case reports, abstracts, or comments, (b) not reported in the English language, (c) did not report on PTMC patients with BRAFV600E mutation.
Results of the search query were screened for inclusion by two independent authors (A.S.A., A.E.), who screened first by title and abstract, and subsequently by full-text eligibility. A total of 33 articles met the final criteria, and their data were independently extracted into a pre-designed data sheet. Variables collected included the study first author, year of publication, the country where the study took place, study design, total sample size, age, gender, as well as clinicopathological features including BRAFV600E mutation status, tumor multifocality, LNM, ETE, tumor stage. Tumor staging included advanced stage >T2, recurrence rates, and tumor size with a cutoff value of 5mm where large tumors meant that the tumor was ≥ 5mm but less than 10mm. Discrepancies in screening or extraction were resolved by re-examination of the relevant study until consensus was achieved. Disagreements were resolved by discussion with a senior author.
4.2. Data Analysis
Data were analyzed using RStudio version 4.2.2 (meta and metafor package) (citation packages). Dichotomous values were used as input. Z-score (one-tail) at the optimum cutoff value was calculated if the data were reported as mean and standard deviation and the equivalent percentage of patients above and below the threshold was calculated. The Mantel–Haenszel method [61,62] was employed to calculate the common effect estimate and between-study heterogeneity statistic Q using the DerSimonian–Laird estimator [63]. Data were presented as risk ratio (RR) or odds ratio (OR) along with a 95% confidence interval (CI). Heterogeneity was examined by the chi-squared Q test and I2 statistic. The fixed or random-effects model was applied according to the presence or absence of heterogeneity. Publication bias was assessed using a funnel plot for precision and Egger’s regression test (Table S1 and Figure S1).
5. Conclusions
PTMC positive for the BRAFV600E mutation is more aggressive than wild-type BRAF PTMC. Since BRAF-mutated PTMC is generally more resistant to radioiodine treatment, patients with BRAFV600E-mutated PTMC may require earlier management, such as a minimally invasive ablative intervention. Conservative management by active surveillance may be suitable for patients with wild-type BRAFV600E PTMC.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms232415626/s1.
Author Contributions
Conceptualization: A.S.A., A.E., P.P.I., A.F., M.R.Y., E.T., E.K.; Methodology: all authors; Validation: M.H., M.A., M.S., E.T., E.K.; Formal Analysis: M.H., E.T.; Investigation: all authors. Data Curation: A.S.A., A.E., P.P.I., B.M.M.; Writing—Original Draft Preparation: A.S.A., A.E., P.P.I.; Writing—Review & Editing: All authors; Funding Acquisition: E.T.; All authors approved the final version to be published. All authors have read and agreed to the published version of the manuscript.
Funding
ThyCa: Thyroid Cancer Survivors’ Association, Inc. and administered by the American Thyroid Association, through grant number (THYROIDGRANT2021-0000000232) (to E.T.).
Institutional Review Board Statement
Not required.
Informed Consent Statement
Not required.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
All authors declare no conflict of interest.
References
- Davies, L.; Welch, H.G. Increasing Incidence of Thyroid Cancer in the United States, 1973-2002. JAMA 2006, 295, 2164–2167. [Google Scholar] [CrossRef]
- Sun, W.; Lan, X.; Zhang, H.; Dong, W.; Wang, Z.; He, L.; Zhang, T.; Liu, S. Risk Factors for Central Lymph Node Metastasis in CN0 Papillary Thyroid Carcinoma: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0139021. [Google Scholar] [CrossRef]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed]
- Fligor, S.C.; Lopez, B.; Uppal, N.; Lubitz, C.C.; James, B.C. Time to Surgery and Thyroid Cancer Survival in the United States. Ann. Surg. Oncol. 2021, 28, 3556–3565. [Google Scholar] [CrossRef] [PubMed]
- Kebebew, E.; Weng, J.; Bauer, J.; Ranvier, G.; Clark, O.H.; Duh, Q.-Y.; Shibru, D.; Bastian, B.; Griffin, A. The Prevalence and Prognostic Value of BRAF Mutation in Thyroid Cancer. Ann. Surg. 2007, 246, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF Mutation in Papillary Thyroid Cancer: Pathogenic Role, Molecular Bases, and Clinical Implications. Endocr. Rev. 2007, 28, 742–762. [Google Scholar] [CrossRef] [PubMed]
- Elisei, R.; Ugolini, C.; Viola, D.; Lupi, C.; Biagini, A.; Giannini, R.; Romei, C.; Miccoli, P.; Pinchera, A.; Basolo, F. BRAFV600E Mutation and Outcome of Patients with Papillary Thyroid Carcinoma: A 15-Year Median Follow-Up Study. J. Clin. Endocrinol. Metab. 2008, 93, 3943–3949. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Viola, D.; Elisei, R.; Bendlova, B.; Yip, L.; Mian, C.; Vianello, F.; Tuttle, R.M.; et al. Association Between BRAF V600E Mutation and Mortality in Patients With Papillary Thyroid Cancer. JAMA 2013, 309, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kwon, H. The Impact of BRAF Mutation on the Recurrence of Papillary Thyroid Carcinoma: A Meta-Analysis. Cancers 2020, 12, 2056. [Google Scholar] [CrossRef]
- Kim, J.Y.; Kim, K.J.; Bae, J.H.; Kim, J.H.; Kim, N.H.; Kim, H.Y.; Kim, H.Y.; Baek, S.-K.; Kim, S.G.; Jung, K.Y. Null Association between BRAF V600E Mutation and Tumor Recurrence in Patients with Papillary Thyroid Microcarcinoma in South Korea. Int. J. Thyroidol. 2021, 14, 135–142. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, S.G.; Tan, J.; Shen, X.; Viola, D.; Elisei, R.; Puxeddu, E.; Fugazzola, L.; Colombo, C.; Jarząb, B.; et al. BRAF V600E status may facilitate decision-making on active surveillance of low-risk papillary thyroid microcarcinoma. Eur. J. Cancer 2019, 124, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Li, J.; Wang, Y.; Xue, S.; Zhang, Y. Association of BRAF gene and TSHR with cervical lymph node metastasis of papillary thyroid microcarcinoma. Oncol. Lett. 2018, 17, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Xiangqian, Z.; Chen, P.; Ming, G.; Jingtai, Z.; Xiukun, H.; Jingzhu, Z.; Xi, W.; Jiadong, C.; Dapeng, L.; Biyun, Q. Risk factors for cervical lymph node metastasis in papillary thyroid microcarcinoma: A study of 1,587 patients. Cancer Biol. Med. 2019, 16, 121–130. [Google Scholar] [CrossRef]
- Chen, B.-D.; Zhang, Z.; Wang, K.-K.; Shang, M.-Y.; Zhao, S.-S.; Ding, W.-B.; Du, R.; Yu, Z.; Xu, X.-M. A multivariable model of BRAFV600E and ultrasonographic features for predicting the risk of central lymph node metastasis in cN0 papillary thyroid microcarcinoma. Cancer Manag. Res. 2019, 11, 7211–7217. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.; Xie, H.; Wei, B.; Shen, H.; Liu, A.; Gao, Y.; Wang, L. Relationship between BRAF V600E gene mutation and the clinical and pathologic characteristics of papillary thyroid microcarcinoma. Int. J. Clin. Exp. Pathol. 2019, 12, 3492–3499. [Google Scholar]
- Lee, S.M.; Lee, C.R.; Kang, S.-W.; Lee, J.; Jeong, J.J.; Nam, K.-H.; Chung, W.Y.; Park, C.S. Association between BRAFV600E Mutations and Clinicopathological Features of Papillary Thyroid Microcarcinoma (PTMC). J. Endocr. Surg. 2019, 19, 76–84. [Google Scholar] [CrossRef]
- Nechifor-Boilă, A.C.; Szász, E.A.; Descotes, F.; Berger, N.; Zahan, A.E.; Loghin, A.; Ceteraş, D.M.; Borda, A. Morphological features predictive for BRAF(V600E) mutation in papillary thyroid microcarcinomas. Romanian J. Morphol. Embryol. 2018, 59, 747–753. [Google Scholar]
- Shen, G.; Kou, Y.; Liu, B.; Huang, R.; Kuang, A. The BRAFV600E Mutation in Papillary Thyroid Microcarcinoma with Intermediate-Risk to High-Risk Features: Does the Mutation Have an Effect on Clinical Response to Radioiodine Therapy? Nucl. Med. Commun. 2019, 40, 8. [Google Scholar] [CrossRef]
- Goh, X.; Lum, J.; Yang, S.P.; Chionh, S.B.; Koay, E.; Chiu, L.; Parameswaran, R.; Ngiam, K.Y.; Loh, T.K.S.; Nga, M.E.; et al. BRAF mutation in papillary thyroid cancer-Prevalence and clinical correlation in a South-East Asian cohort. Clin. Otolaryngol. 2018, 44, 114–123. [Google Scholar] [CrossRef]
- Lu, H.-Z.; Qiu, T.; Ying, J.-M.; Lyn, N. Association between BRAFV600E mutation and the clinicopathological features of solitary papillary thyroid microcarcinoma. Oncol. Lett. 2017, 13, 1595–1600. [Google Scholar] [CrossRef]
- Fakhruddin, N.; Jabbour, M.; Novy, M.; Tamim, H.; Bahmad, H.; Farhat, F.; Zaatari, G.; Aridi, T.; Kriegshauser, G.; Oberkanins, C.; et al. BRAF and NRAS Mutations in Papillary Thyroid Carcinoma and Concordance in BRAF Mutations Between Primary and Corresponding Lymph Node Metastases. Sci. Rep. 2017, 7, 4666. [Google Scholar] [CrossRef] [PubMed]
- Tallini, G.; De Biase, D.; Durante, C.; Acquaviva, G.; Bisceglia, M.; Bruno, R.; Reggiani, M.L.B.; Casadei, G.P.; Costante, G.; Cremonini, N.; et al. BRAF V600E and risk stratification of thyroid microcarcinoma: A multicenter pathological and clinical study. Mod. Pathol. 2015, 28, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Park, V.; Kim, E.-K.; Lee, H.S.; Moon, H.J.; Yoon, J.H.; Kwak, J.Y. Real-Time PCR Cycle Threshold Values for the BRAFV600E Mutation in Papillary Thyroid Microcarcinoma May Be Associated With Central Lymph Node Metastasis. Medicine 2015, 94, e1149. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.U.; Oler, G.; Nozima, B.H.N.; Moysés, R.A.; Cerutti, J.M. BRAF V600E and decreased NIS and TPO expression are associated with aggressiveness of a subgroup of papillary thyroid microcarcinoma. Eur. J. Endocrinol. 2015, 173, 525–540. [Google Scholar] [CrossRef]
- Xing, M.; Alzahrani, A.S.; Carson, K.A.; Shong, Y.K.; Kim, T.Y.; Viola, D.; Elisei, R.; Bendlová, B.; Yip, L.; Mian, C.; et al. Association Between BRAF V600E Mutation and Recurrence of Papillary Thyroid Cancer. J. Clin. Oncol. 2015, 33, 42–50. [Google Scholar] [CrossRef]
- Walczyk, A.; Kowalska, A.; Kowalik, A.; Sygut, J.; Wypiórkiewicz, E.; Chodurska, R.; Pięciak, L.; Góźdź, S. The BRAFV600E mutation in papillary thyroid microcarcinoma: Does the mutation have an impact on clinical outcome? Clin. Endocrinol. 2014, 80, 899–904. [Google Scholar] [CrossRef]
- Mussazhanova, Z.; Matsuda, K.; Naruke, Y.; Mitsutake, N.; Stanojevic, B.; Rougounovitch, T.; Saenko, V.; Suzuki, K.; Nishihara, E.; Hirokawa, M.; et al. Significance of p53-binding protein 1 (53BP1) expression in thyroid papillary microcarcinoma: Association withBRAFV600Emutation status. Histopathology 2013, 63, 726–734. [Google Scholar] [CrossRef]
- Chung, S.Y.; Lee, J.S.; Lee, H.; Park, S.H.; Kim, S.J.; Ryu, H.S. Cytomorphological Factors and BRAF Mutation Predicting Risk of Lymph Node Metastasis in Preoperative Liquid-Based Fine Needle Aspirations of Papillary Thyroid Carcinoma. Acta Cytol. 2013, 57, 252–258. [Google Scholar] [CrossRef]
- Choi, S.Y.; Park, H.; Kang, M.K.; Lee, D.K.; Lee, K.D.; Lee, H.S.; Kim, S.W.; Lee, E.N.; Hong, J.C. The relationship between the BRAFV600E mutation in papillary thyroid microcarcinoma and clinicopathologic factors. World J. Surg. Oncol. 2013, 11, 291. [Google Scholar] [CrossRef] [PubMed]
- Virk, R.K.; Van Dyke, A.L.; Finkelstein, A.; Prasad, A.; Gibson, J.; Hui, P.; Theoharis, C.G.; Carling, T.; A Roman, S.; A Sosa, J.; et al. BRAFV600E mutation in papillary thyroid microcarcinoma: A genotype–phenotype correlation. Mod. Pathol. 2012, 26, 62–70. [Google Scholar] [CrossRef]
- Rossi, E.D.; Martini, M.; Bd, S.C.; Lombardi, C.P.; Pontecorvi, A.; Vellone, V.G.; Zannoni, G.F.; Larocca, L.M.; Fadda, G. BRAF(V600E) mutation analysis on liquid-based cytology-processed aspiration biopsies predicts bilaterality and lymph node involvement in papillary thyroid microcarcinoma. Cancer Cytopathol. 2012, 121, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, I.; Iervasi, G.; Mazzanti, C.M.; Lessi, F.; Tomei, S.; Naccarato, A.G.; Aretini, P.; Alberti, B.; Di Coscio, G.; Bevilacqua, G.; et al. Detection of the BRAFV600E Mutation in Fine Needle Aspiration Cytology of Thyroid Papillary Microcarcinoma Cells Selected by Manual Macrodissection: An Easy Tool to Improve the Preoperative Diagnosis. Thyroid 2012, 22, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Kurtulmus, N.; Duren, M.; Ince, U.; Yakicier, M.C.; Peker, O.; Aydın, O.; Altiok, E.; Giray, S.; Azizlerli, H. BRAFV600E mutation in Turkish patients with papillary thyroid cancer: Strong correlation with indicators of tumor aggressiveness. Endocrine 2012, 42, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.-L.; Wang, O.-C.; Zhang, X.-H.; Dai, X.-X.; Hu, X.-Q.; Qu, J.-M. The BRAF Mutation Is Predictive of Aggressive Clinicopathological Characteristics in Papillary Thyroid Microcarcinoma. Ann. Surg. Oncol. 2010, 17, 3294–3300. [Google Scholar] [CrossRef] [PubMed]
- Basolo, F.; Torregrossa, L.; Giannini, R.; Miccoli, M.; Lupi, C.; Sensi, E.; Berti, P.; Elisei, R.; Vitti, P.; Baggiani, A. Correlation between the BRAF V600E Mutation and Tumor Invasiveness in Papillary Thyroid Carcinomas Smaller than 20 Millimeters: Analysis of 1060 Cases. J. Clin. Endocrinol. Metab. 2010, 95, 4197–4205. [Google Scholar] [CrossRef]
- Lee, X.; Gao, M.; Ji, Y.; Yü, Y.; Feng, Y.; Li, Y.; Zhang, Y.; Cheng, W.; Zhao, W. Analysis of Differential BRAFV600E Mutational Status in High Aggressive Papillary Thyroid Microcarcinoma. Ann. Surg. Oncol. 2008, 16, 240–245. [Google Scholar] [CrossRef]
- Kwak, J.Y.; Kim, E.-K.; Chung, W.Y.; Moon, H.J.; Kim, M.J.; Choi, J.R. Association of BRAFV600E Mutation with Poor Clinical Prognostic Factors and US Features in Korean Patients with Papillary Thyroid Microcarcinoma. Radiology 2009, 253, 854–860. [Google Scholar] [CrossRef]
- Min, H.S.; Choe, G.; Kim, S.-W.; Park, Y.J.; Park, D.J.; Youn, Y.-K.; Park, S.H.; Cho, B.Y.; Park, S.Y. S100A4 expression is associated with lymph node metastasis in papillary microcarcinoma of the thyroid. Mod. Pathol. 2008, 21, 748–755. [Google Scholar] [CrossRef][Green Version]
- Frasca, F.; Nucera, C.; Pellegriti, G.; Gangemi, P.; Attard, M.; Stella, M.; Loda, M.; Vella, V.; Giordano, C.; Trimarchi, F.; et al. BRAF(V600E) mutation and the biology of papillary thyroid cancer. Endocrine-Related Cancer 2008, 15, 191–205. [Google Scholar] [CrossRef]
- Rodolico, V.; Cabibi, D.; Pizzolanti, G.; Richiusa, P.; Gebbia, N.; Martorana, A.; Russo, A.; Amato, M.C.; Galluzzo, A.; Giordano, C. BRAFV600E mutation and p27kip1 expression in papillary carcinomas of the thyroid ≤1 cm and their paired lymph node metastases. Cancer 2007, 110, 1218–1226. [Google Scholar] [CrossRef]
- Lupi, C.; Giannini, R.; Ugolini, C.; Proietti, A.; Berti, P.; Minuto, M.; Materazzi, G.; Elisei, R.; Santoro, M.; Miccoli, P.; et al. Association of BRAF V600E Mutation with Poor Clinicopathological Outcomes in 500 Consecutive Cases of Papillary Thyroid Carcinoma. J. Clin. Endocrinol. Metab. 2007, 92, 4085–4090. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, W.B.; Song, J.Y.; Rhee, Y.S.; Gong, G.; Cho, Y.M.; Kim, S.Y.; Kim, S.C.; Hong, S.J. The BRAFV600E mutation is not associated with poor prognostic factors in Korean patients with conventional papillary thyroid microcarcinoma. Clin. Endocrinol. 2005, 63, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzini, L.; Baroli, A.; Marzoli, L.; Guglielmi, R.; Papini, E. Cancer recurrence in papillary thyroid microcarcinoma: A multivariate analysis on 231 patients with a 12-year follow-up. Minerva Endocrinol. 2013, 38. [Google Scholar]
- Al-Qurayshi, Z.; Nilubol, N.; Tufano, R.P.; Kandil, E. Wolf in Sheep’s Clothing: Papillary Thyroid Microcarcinoma in the US. J. Am. Coll. Surg. 2020, 230, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Xing, M. BRAF Mutation in Papillary Thyroid Microcarcinoma: The Promise of Better Risk Management. Ann. Surg. Oncol. 2009, 16, 801–803. [Google Scholar] [CrossRef]
- Lim, Y.C.; Choi, E.C.; Yoon, Y.-H.; Kim, E.-H.; Koo, B.S. Central lymph node metastases in unilateral papillary thyroid microcarcinoma. Br. J. Surg. 2009, 96, 253–257. [Google Scholar] [CrossRef]
- Wada, N.; Duh, Q.-Y.; Sugino, K.; Iwasaki, H.; Kameyama, K.; Mimura, T.; Ito, K.; Takami, H.; Takanashi, Y. Lymph Node Metastasis from 259 Papillary Thyroid Microcarcinomas: Frequency, Pattern of Occurrence and Recurrence, and Optimal Strategy for Neck Dissection. Ann. Surg. 2003, 237, 399. [Google Scholar] [CrossRef]
- Miccoli, P.; Minuto, M.N.; Ugolini, C.; Panicucci, E.; Berti, P.; Massi, M.; Basolo, F. Intrathyroidal Differentiated Thyroid Carcinoma: Tumor Size-Based Surgical Concepts. World J. Surg. 2007, 31, 888–894. [Google Scholar] [CrossRef]
- Lee, D.Y.; Hwang, S.M.; An, J.H.; Son, K.R.; Baek, S.-K.; Kim, S.G.; Chae, Y.S.; Jung, K.-Y. Predicting Extrathyroidal Extension in Patients With Papillary Thyroid Microcarcinoma According to a BRAF Mutation. Clin. Exp. Otorhinolaryngol. 2017, 10, 174–181. [Google Scholar] [CrossRef]
- Issa, P.P.; Omar, M.; Buti, Y.; Issa, C.P.; Chabot, B.; Carnabatu, C.J.; Munshi, R.; Hussein, M.; Aboueisha, M.; Shama, M.; et al. Hashimoto’s Thyroiditis Minimizes Lymph Node Metastasis in BRAF Mutant Papillary Thyroid Carcinomas. Biomedicines 2022, 10, 2051. [Google Scholar] [CrossRef]
- Tallini, G.; De Leo, A.; Repaci, A.; de Biase, D.; Reggiani, M.L.B.; Di Nanni, D.; Ambrosi, F.; Di Gioia, C.; Grani, G.; Rhoden, K.J.; et al. Does the Site of Origin of the Microcarcinoma with Respect to the Thyroid Surface Matter? A Multicenter Pathologic and Clinical Study for Risk Stratification. Cancers 2020, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Niemeier, L.A.; Akatsu, H.K.; Song, C.; Carty, S.E.; Hodak, S.P.; Yip, L.; Ferris, R.L.; Tseng, G.C.; Seethala, R.R.; Lebeau, S.O.; et al. A combined molecular-pathologic score improves risk stratification of thyroid papillary microcarcinoma. Cancer 2011, 118, 2069–2077. [Google Scholar] [CrossRef] [PubMed]
- Apostol, D.C.; Giuşcă, S.E.; Căruntu, I.-D.; Lozneanu, L.; Andriescu, E.C.; Moscalu, M. Relationships between clinicopathological prognostic factors in papillary thyroid microcarcinoma: A refined analysis based on 428 cases. Int. J. Clin. Exp. Pathol. 2017, 10, 8944–8956. [Google Scholar]
- Tuttle, R.M.; Fagin, J.A.; Minkowitz, G.; Wong, R.J.; Roman, B.; Patel, S.; Untch, B.; Ganly, I.; Shaha, A.R.; Shah, J.P.; et al. Natural History and Tumor Volume Kinetics of Papillary Thyroid Cancers During Active Surveillance. JAMA Otolaryngol. Neck Surg. 2017, 143, 1015–1020. [Google Scholar] [CrossRef]
- Ito, Y.; Miyauchi, A.; Oda, H. Low-risk papillary microcarcinoma of the thyroid: A review of active surveillance trials. Eur. J. Surg. Oncol. (EJSO) 2017, 44, 307–315. [Google Scholar] [CrossRef]
- Kandil, E.; Omar, M.; Aboueisha, M.; Attia, A.S.; Ali, K.M.; Abu Alhuda, R.F.; Issa, P.P.; Wolfe, S.; Omari, S.; Buti, Y.; et al. Efficacy and Safety of Radiofrequency Ablation of Thyroid Nodules: A Multi-institutional Prospective Cohort Study. Ann. Surg. 2022. [Google Scholar] [CrossRef]
- Kandil, E.; Omar, M.; Attia, A.S.; Shihabi, A.; Shaear, M.; Metz, T.; Issa, P.P.; Russell, J.O.; Tufano, R.P. Radiofrequency ablation as a novel modality in the USA for treating toxic thyroid nodules: Case series and literature review. Gland Surg. 2022, 11, 1574–1583. [Google Scholar] [CrossRef]
- Zhu, Y.; Che, Y.; Gao, S.; Ren, S.; Tong, M.; Wang, L.; Yang, F. Long-term follow-up results of PTMC treated by ultrasound-guided radiofrequency ablation: A retrospective study. Int. J. Hyperth. 2021, 38, 1225–1232. [Google Scholar] [CrossRef]
- Valcavi, R.; Riganti, F.; Bertani, A.; Formisano, D.; Pacella, C.M. Percutaneous Laser Ablation of Cold Benign Thyroid Nodules: A 3-Year Follow-Up Study in 122 Patients. Thyroid 2010, 20, 1253–1261. [Google Scholar] [CrossRef]
- Cesareo, R.; Naciu, A.; Iozzino, M.; Pasqualini, V.; Simeoni, C.; Casini, A.; Campagna, G.; Manfrini, S.; Tabacco, G.; Palermo, A. Nodule Size as Predictive Factor of Efficacy of Radiofrequency Ablation in Treating Autonomously Functioning Thyroid Nodules. Int. J. Hyperthermia 2018, 34, 617–623. [Google Scholar] [CrossRef]
- Greenland, S.; Robins, J.M. Estimation of a Common Effect Parameter from Sparse Follow-Up Data. Biometrics 1985, 41, 55. [Google Scholar] [CrossRef] [PubMed]
- Robins, J. A new approach to causal inference in mortality studies with a sustained exposure period—Application to control of the healthy worker survivor effect. Math. Model. 1986, 7, 1393–1512. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).