Zingerone Attenuates Carfilzomib-Induced Cardiotoxicity in Rats through Oxidative Stress and Inflammatory Cytokine Network
Abstract
1. Introduction
2. Results
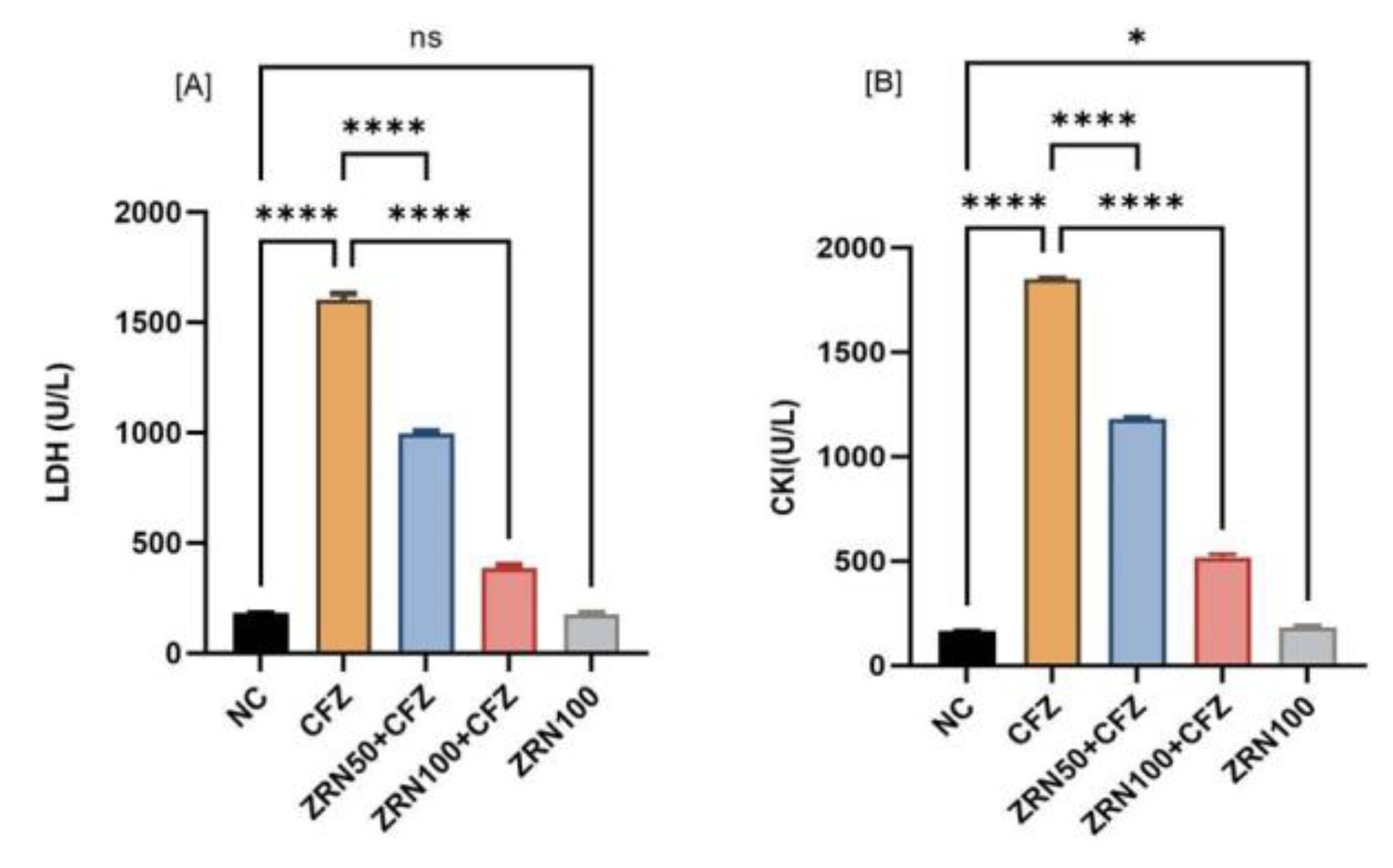
2.1. Impact of Zingerone and Carfilzomib on Cardiac Blood Marker
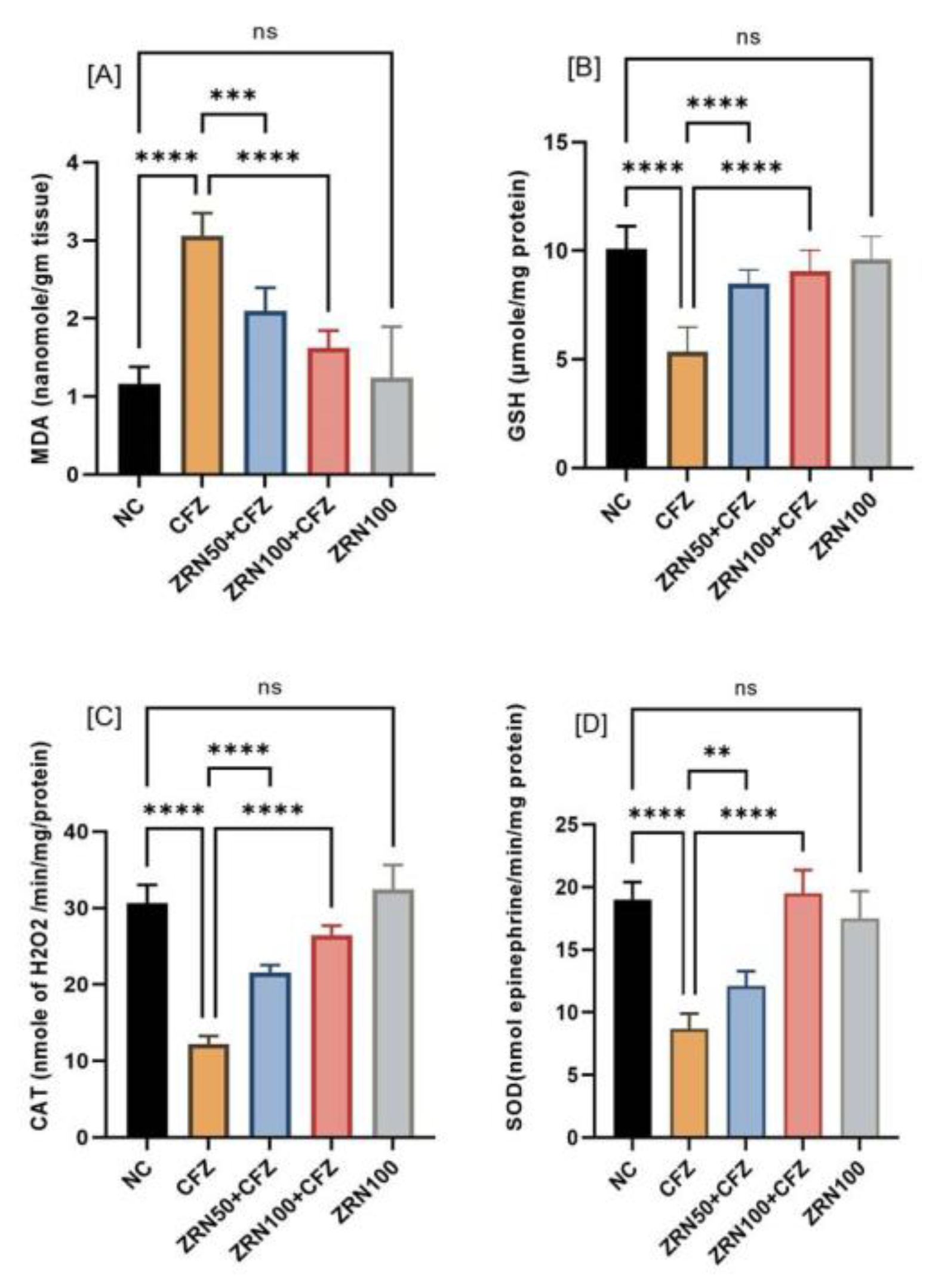
2.2. Impact of Zingerone and Carfilzomib on Oxidative Stress in Cardiac Tissue
2.3. Impact of Zingerone and Carfilzomib on Inflammatory Cytokine and Apoptotic Marker
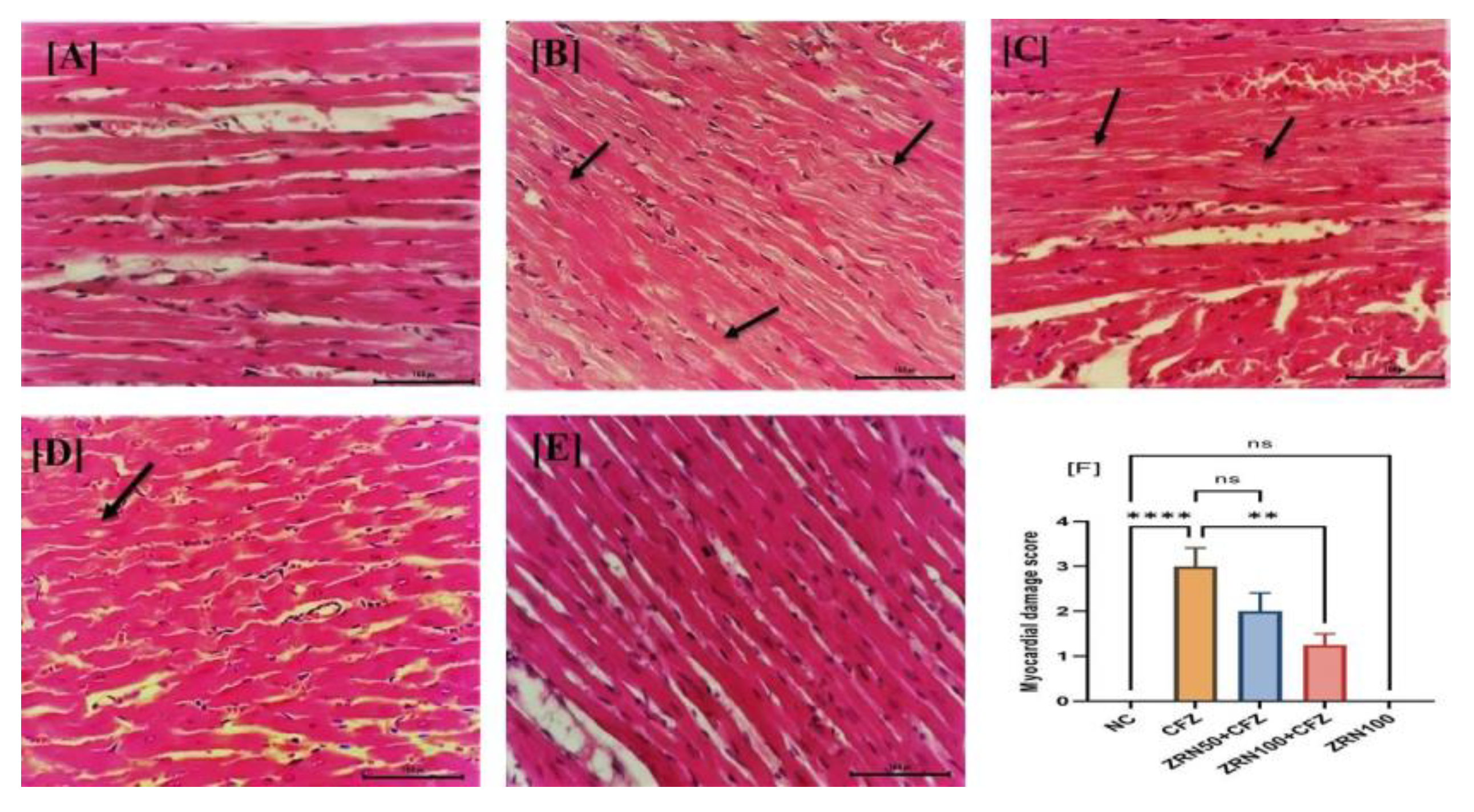
2.4. Impact of Zingerone and Carfilzomib on Cardiac Histology
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drugs and Chemicals
4.3. Experimental Scheme
4.4. Biochemical Analysis
4.5. Determination of Oxidative Stress in Myocardial Tissue
4.6. Determination of Inflammatory Cytokines in Myocardial Tissue
4.7. Histopathological Examination of Myocardial Tissue
4.8. Statistical Analysis:
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hajek, R.; Bryce, R.; Roman, S.; Klencke, B.; Ludwig, H. Design and rationale of FOCUS (PX-171-011): A randomized, open-label, phase 3 study of carfilzomib versus best supportive care regimen in patients with relapsed and refractory multiple myeloma (R/R MM). BMC Cancer 2012, 12, 415. [Google Scholar] [CrossRef]
- Herndon, T.M.; Deisseroth, A.; Kaminskas, E.; Kane, R.C.; Koti, K.M.; Rothmann, M.D.; Habtemariam, B.; Bullock, J.; Bray, J.D.; Hawes, J.; et al. U.S. Food and Drug Administration Approval: Carfilzomib for the Treatment of Multiple Myeloma. Clin. Cancer Res. 2013, 19, 4559–4563. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- Myung, J.; Kim, K.B.; Crews, C.M. The ubiquitin-proteasome pathway and proteasome inhibitors. Med. Res. Rev. 2001, 21, 245–273. [Google Scholar] [CrossRef]
- Khan, R.Z.; Badros, A. Role of carfilzomib in the treatment of multiple myeloma. Expert Rev. Hematol. 2012, 5, 361–372. [Google Scholar] [CrossRef]
- Vij, R.; Siegel, D.S.; Jagannath, S.; Jakubowiak, A.J.; Stewart, A.K.; McDonagh, K.; Bahlis, N.; Belch, A.; Kunkel, L.A.; Wear, S.; et al. An open-label, single-arm, phase 2 study of single-agent carfilzomib in patients with relapsed and/or refractory multiple myeloma who have been previously treated with bortezomib. Br. J. Haematol. 2012, 158, 739–748. [Google Scholar] [CrossRef]
- Xiao, Y.; Yin, J.; Wei, J.; Shang, Z. Incidence and Risk of Cardiotoxicity Associated with Bortezomib in the Treatment of Cancer: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e87671. [Google Scholar] [CrossRef]
- Waxman, A.J.; Clasen, S.C.; Garfall, A.L.; Carver, J.R.; Vogl, D.T.; O’Quinn, R.; Cohen, A.D.; Stadtmauer, E.A.; Ky, B.; Weiss, B.M. Carfilzomib-associated cardiovascular adverse events: A systematic review and meta-analysis. J. Clin. Oncol. 2017, 35, 8018. [Google Scholar] [CrossRef]
- Chari, A.; Hajje, D. Case series discussion of cardiac and vascular events following carfilzomib treatment: Possible mechanism, screening, and monitoring. BMC Cancer 2014, 14, 915. [Google Scholar] [CrossRef]
- Force, W.I.T. Report of the WHO/ISFC task force on the definition and classification of cardiomyopathies. Br. Heart J. 1980, 44, 672–673. [Google Scholar]
- Abelmann, W.H. Classification and natural history of primary myocardial disease. Prog. Cardiovasc. Dis. 1984, 27, 73–94. [Google Scholar] [CrossRef]
- Ahmad, B.; Rehman, M.U.; Amin, I.; Arif, A.; Rasool, S.; Bhat, S.A.; Afzal, I.; Hussain, I.; Bilal, S.; Mir, M. A Review on Pharmacological Properties of Zingerone (4-(4-Hydroxy-3-methoxyphenyl)-2-butanone). Sci. World J. 2015, 2015, 816364. [Google Scholar] [CrossRef]
- Vaibhav, K.; Shrivastava, P.; Tabassum, R.; Khan, A.; Javed, H.; Ahmed, E.; Islam, F.; Safhi, M.M.; Islam, F. Delayed administration of zingerone mitigates the behavioral and histological alteration via repression of oxidative stress and intrinsic programmed cell death in focal transient ischemic rats. Pharmacol. Biochem. Behav. 2013, 113, 53–62. [Google Scholar] [CrossRef]
- Kumar, L.; Chhibber, S.; Harjai, K. Hepatoprotective effect of zingerone (4-(4-hydroxy-3-methoxyphenyl) butan-2-one) in lipopolysaccharide induced liver injury mouse model through down regulation of inflammatory mediators. Int. J. Pharmacogn. Phytochem. Res. 2014, 6, 308–314. [Google Scholar]
- Hemalatha, K.L.; Stanley, P.; Prince, M. Antihyperlipidaemic, antihypertrophic and reducing effects of Zingerone on experimentally iso-proterenol induced myocardial infracted rats. J. Biochem. Mol. Toxicol. 2015, 29, 182–188. [Google Scholar] [CrossRef]
- Soliman, A.F.; Anees, L.M.; Ibrahim, D.M. Cardioprotective effect of zingerone against oxidative stress, inflammation, and apoptosis induced by cisplatin or gamma radiation in rats. Naunyn Schmiedebergs Arch. Pharmacol. 2018, 391, 819–832. [Google Scholar] [CrossRef]
- Yeh, E.T.H.; Tong, A.T.; Lenihan, D.J.; Yusuf, S.W.; Swafford, J.; Champion, C.; Durand, J.-B.; Gibbs, H.; Zafarmand, A.A.; Ewer, M.S. Review: Current perspective: Cardiovascular complications of cancer therapy diagnosis, pathogenesis, and management. Circulation 2004, 109, 3122–3131. [Google Scholar] [CrossRef]
- Sheppard, R.J.; Berger, J.; Sebag, I.A. Cardiotoxicity of cancer therapeutics: Current issues in screening, prevention, and therapy. Front. Pharm. 2013, 4, 19. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Li, J.S.; Chen, L.H.; Peng, W.W.; Cai, B.C. Simultaneous determination of five gingerols in raw and processed ginger by HPLC. Chin. Pharm. J. 2012, 47, 471–474. [Google Scholar]
- Wu, P.; Oren, O.; Gertz, M.A.; Yang, E.H. Proteasome Inhibitor-Related Cardiotoxicity: Mechanisms, Diagnosis, and Management. Curr. Oncol. Rep. 2020, 8, 66. [Google Scholar] [CrossRef]
- Al-Harbi, N.O. Carfilzomib-induced cardiotoxicity mitigated by dexrazoxane through inhibition of hypertrophic gene expression and oxidative stress in rats. Toxicol. Mech. Methods 2016, 26, 95–189. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R.; Deng, Z. Separation procedures for naturally occurring antioxidant phytochemicals. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2004, 812, 85–99. [Google Scholar] [CrossRef]
- Castellani, P.; Balza, E.; Rubartelli, A. Inflammation, DAMPs tumor development, and progression: A vicious circle orchestrated by redox signaling. Antioxid. Redox Signal. 2014, 20, 1086–1097. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.T.; Staal, F.J.T.; Gitler, C.; Herzenberg, L.A.; Herzenberg, L.A. Separation of oxidant-initiated and redox-regulated steps in the NF-κB signal transduction pathway. Proc. Natl. Acad. Sci. USA 1994, 91, 11527–11531. [Google Scholar] [CrossRef] [PubMed]
- Flohé, L.; Brigelius-Flohé, R.; Saliou, C.; Traber, M.G.; Packer, L. Redox regulation of NF-κB activation. Free Radic. Biol. Med. 1997, 22, 1115–1126. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in NLRP3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.S.; Accardi, C.J.; Ziegler, T.R.; Blanco, R.A.; Ritzenthaler, J.D.; Rojas, M.; Roman, J.; Jones, D.P. Cysteine Redox Potential Determines Pro-Inflammatory IL-1β Levels. PLoS ONE 2009, 4, e5017. [Google Scholar] [CrossRef]
- Go, Y.M.; Jones, D.P. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation 2005, 111, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.F.; Alshahrani, S.; Alamira, E.A.; Alhazmi, M.A.; Anwer, T.; Khan, G.; Khan, A.; Tanweer, K.T.; Moni, S.S. Nephroprotective effects of 4-4(hydroxyl-3 methoxyphenyl)-2-butane against sodium tellurite induced acute kidney dysfunction by attenuating oxidative stress and inflammatory cytokines in rats. Arab. J. Chem. 2022, 15, 103857. [Google Scholar] [CrossRef]
- Fontes, J.A.; Rose, N.R.; Čiháková, D. The varying faces of IL-6: From cardiac protection to cardiac failure. Cytokine 2015, 74, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wollert, K.C.; Taga, T.; Saito, M.; Narazaki, M.; Kishimoto, T.; Glembotski, C.C.; Vernallis, A.B.; Heath, J.K.; Pennica, D.; Wood, W.I.; et al. Cardiotrophin-1 activates a distinct form of cardiac muscle cell hypertrophy: Assembly of sarcomeric units in series VIA gp130/leukemia inhibitory factor receptor-dependent pathways. J. Biol. Chem. 1996, 271, 9535–9545. [Google Scholar] [CrossRef] [PubMed]
- Terrell, A.M.; Crisostomo, P.R.; Wairiuko, G.M.; Wang, M.; Morrell, E.D. Meldrum DR. Jak/STAT/SOCS signaling circuits and associated cytokine-mediated inflammation and hypertrophy in the heart. Shock 2006, 26, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Zheng, R.; Hu, S.; Ma, Y.; Choudhry, M.A.; Messina, J.L.; Rue, L.W., III; Bland, K.I.; Chaudry, I.H. Mechanism of cardiac depression after trauma-hemorrhage: Increased cardiomyocyte IL-6 and effect of sex steroids on IL-6 regulation and cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, 2183–2191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, Z.; Bing, O.H.; Long, X.; Robinson, K.G.; Lakatta, E.G. Increased cardiomyocyte apoptosis during the transition to heart failure in the spontaneously hypertensive rat. Am. J. Physiol. 1997, 272, 2313–2319. [Google Scholar] [CrossRef]
- El-Sawalhi, M.M.; Ahmed, L.A. Exploring the protective role of apocynin, a specific NADPH oxidase inhibitor, in cisplatin induced cardiotoxicity in rats. Chem. Biol. Interact. 2014, 207, 58–66. [Google Scholar] [CrossRef]
- Connell, B.J.; Saleh, M.C.; Khan, B.V.; Saleh, T.M. Apocynin may limit total cell death following cerebral ischemia and reperfusion by enhancing apoptosis. Food Chem. Toxicol. 2011, 49, 3063–3069. [Google Scholar] [CrossRef]
- Imam, F.; Al-Harbi, N.O.; Al-Harbia, M.M.; Korashy, H.M.; Ansari, M.A.; Sayed-Ahmed, M.M.; Nagi, M.N.; Iqbal, M.; Khalid Anwer, M.; Kazmi, I.; et al. Rutin Attenuates Carfilzomib-Induced Cardiotoxicity Through Inhibition of NF-κB, Hypertrophic Gene Expression and Oxidative Stress. Cardiovasc. Toxicol. 2017, 17, 58–66. [Google Scholar] [CrossRef]
- Alam, M.F.; Safhi, M.M.; Anwer, T.; Siddiqui, R.; Khan, G.; Moni, S.S. Therapeutic potential of Vanillylacetone against CCl4 induced hepatotoxicity by suppressing the serum marker, oxidative stress, inflammatory cytokines and apoptosis in Swiss albino mice. Exp. Mol. Pathol. 2018, 105, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Utley, H.G.; Bernheim, F.; Hochstein, P. Effect of sulfhydryl reagents on peroxidation in microsomes. Arch. Biochem. Biophys. 1967, 118, 29–32. [Google Scholar] [CrossRef]
- Jollow, D.J. Bromobenzene-induced liver necrosis: Protective role of glutathione and evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 1974, 11, 151–169. [Google Scholar] [CrossRef]
- Claiborne, A. Catalase activity. In Handbook of Methods for Oxygen Radical Research; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 283–284. [Google Scholar]
- Stevens, M.; Obrosova, I.; Cao, X.; van Huysen, C.; Greene, D.A. Effects of DL-alpa-lipoic acid on peripheral nerve conduction, blood flow, energy metabolism and oxidative, blood flow, energy metabolism and oxidative stress in experimental diabetic neuropathy. Diabetes 2000, 49, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef] [PubMed]

| Cardiac Marker | Normal Control (NC) | Carfilzomib (CFZ) | Zingerone 50 mg + Carfilzomib (ZRN50 + CFZ) | Zingerone 100 + Cariflzomib (ZRN100 + CFZ) | Zingerone 100 mg (ZRN100) |
|---|---|---|---|---|---|
| Na (mmol/L) | 144 ± 1.41 | 142.80 ± 1.33 ns | 143.20 ± 1.17 ns | 136.60 ± 6.71 * | 144.40 ± 1.36 ns |
| K (mmol/L) | 4.34 ± 0.33 | 6 ± 1.18 ** (38.24%) | 5 ± 0.36 ns | 4.32 ± 0.73 ** (28%) | 4.80 ± 0.40 ns |
| TGL (mmol/L) | 1.25 ± 0.11 | 3.85 ± 0.78 *** (208%) | 2.25 ± 0.11 *** (41.55%) | 1.79 ± 0.19 *** (50.90%) | 1.21 ± 0.12 ns |
| AST (U/L) | 27.72 ± 2.31 | 196 ± 6 *** (607.07%) | 94 ± 6.72*** (52.04%) | 57.80 ± 3.71 *** (70.51%) | 35.80 ± 2.75 * (29.14%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alam, M.F.; Hijri, S.I.; Alshahrani, S.; Alqahtani, S.S.; Jali, A.M.; Ahmed, R.A.; Adawi, M.M.; Algassmi, S.M.; Shaheen, E.S.; Moni, S.S.; et al. Zingerone Attenuates Carfilzomib-Induced Cardiotoxicity in Rats through Oxidative Stress and Inflammatory Cytokine Network. Int. J. Mol. Sci. 2022, 23, 15617. https://doi.org/10.3390/ijms232415617
Alam MF, Hijri SI, Alshahrani S, Alqahtani SS, Jali AM, Ahmed RA, Adawi MM, Algassmi SM, Shaheen ES, Moni SS, et al. Zingerone Attenuates Carfilzomib-Induced Cardiotoxicity in Rats through Oxidative Stress and Inflammatory Cytokine Network. International Journal of Molecular Sciences. 2022; 23(24):15617. https://doi.org/10.3390/ijms232415617
Chicago/Turabian StyleAlam, Mohammad Firoz, Sami I. Hijri, Saeed Alshahrani, Saad S. Alqahtani, Abdulmajeed M. Jali, Rayan A. Ahmed, Mansour M. Adawi, Sameeh M. Algassmi, Emad Sayed Shaheen, Sivakumar S. Moni, and et al. 2022. "Zingerone Attenuates Carfilzomib-Induced Cardiotoxicity in Rats through Oxidative Stress and Inflammatory Cytokine Network" International Journal of Molecular Sciences 23, no. 24: 15617. https://doi.org/10.3390/ijms232415617
APA StyleAlam, M. F., Hijri, S. I., Alshahrani, S., Alqahtani, S. S., Jali, A. M., Ahmed, R. A., Adawi, M. M., Algassmi, S. M., Shaheen, E. S., Moni, S. S., & Anwer, T. (2022). Zingerone Attenuates Carfilzomib-Induced Cardiotoxicity in Rats through Oxidative Stress and Inflammatory Cytokine Network. International Journal of Molecular Sciences, 23(24), 15617. https://doi.org/10.3390/ijms232415617







