Probiotics and Commensal Gut Microbiota as the Effective Alternative Therapy for Multiple Sclerosis Patients Treatment
Abstract
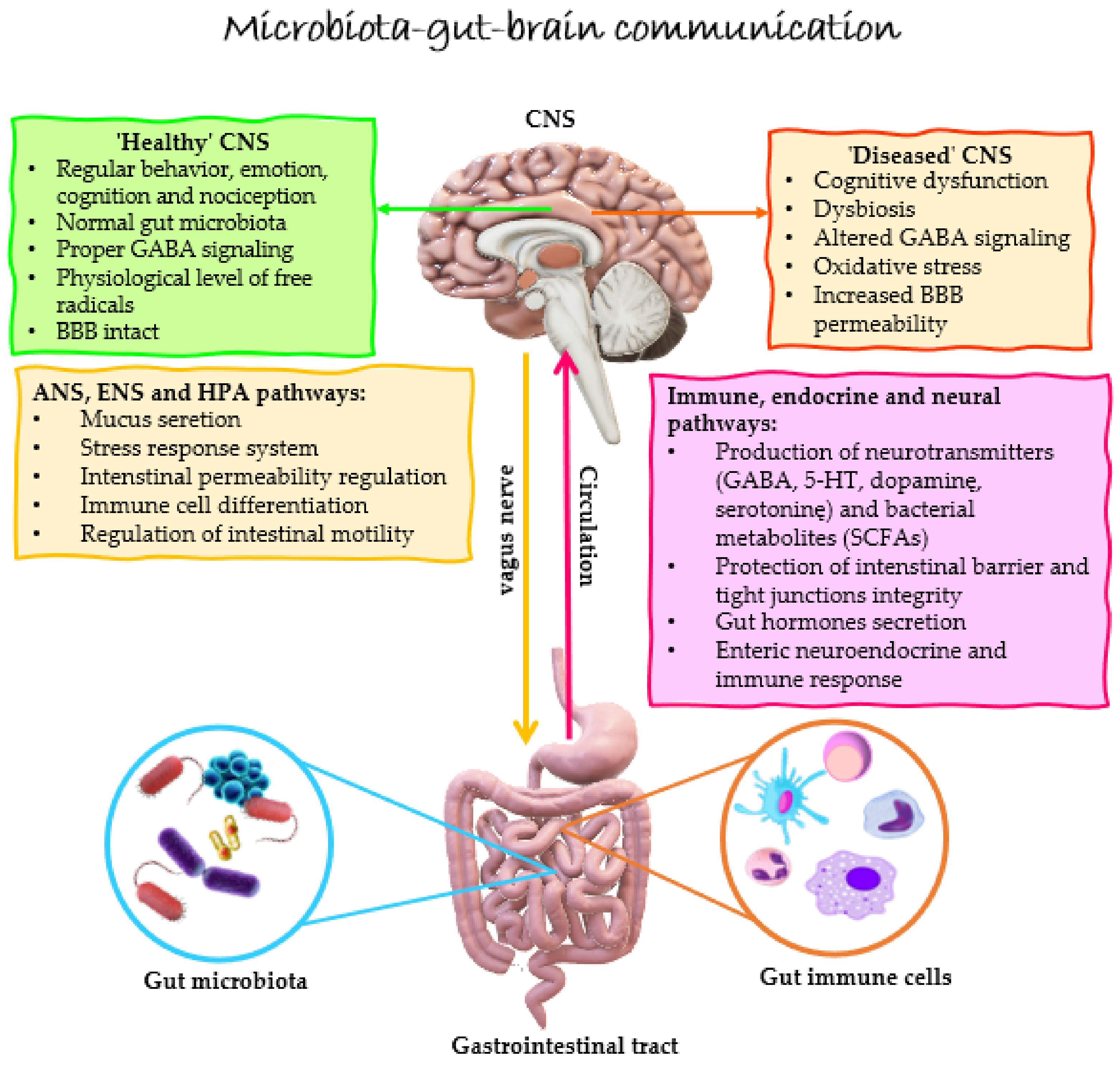
1. Introduction
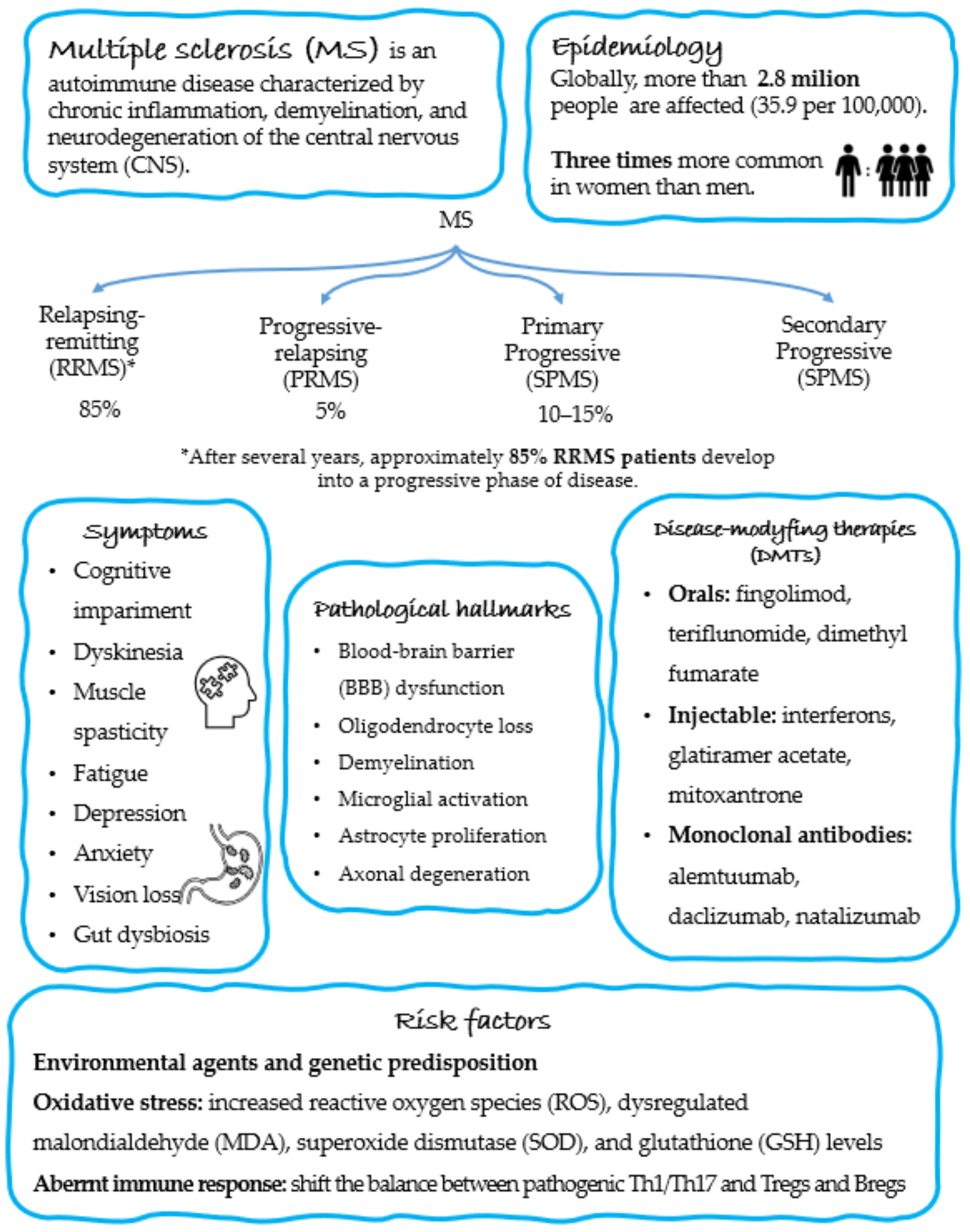
2. Epidemiology and Pathogenesis of MS
3. Gut Dysbiosis in MS
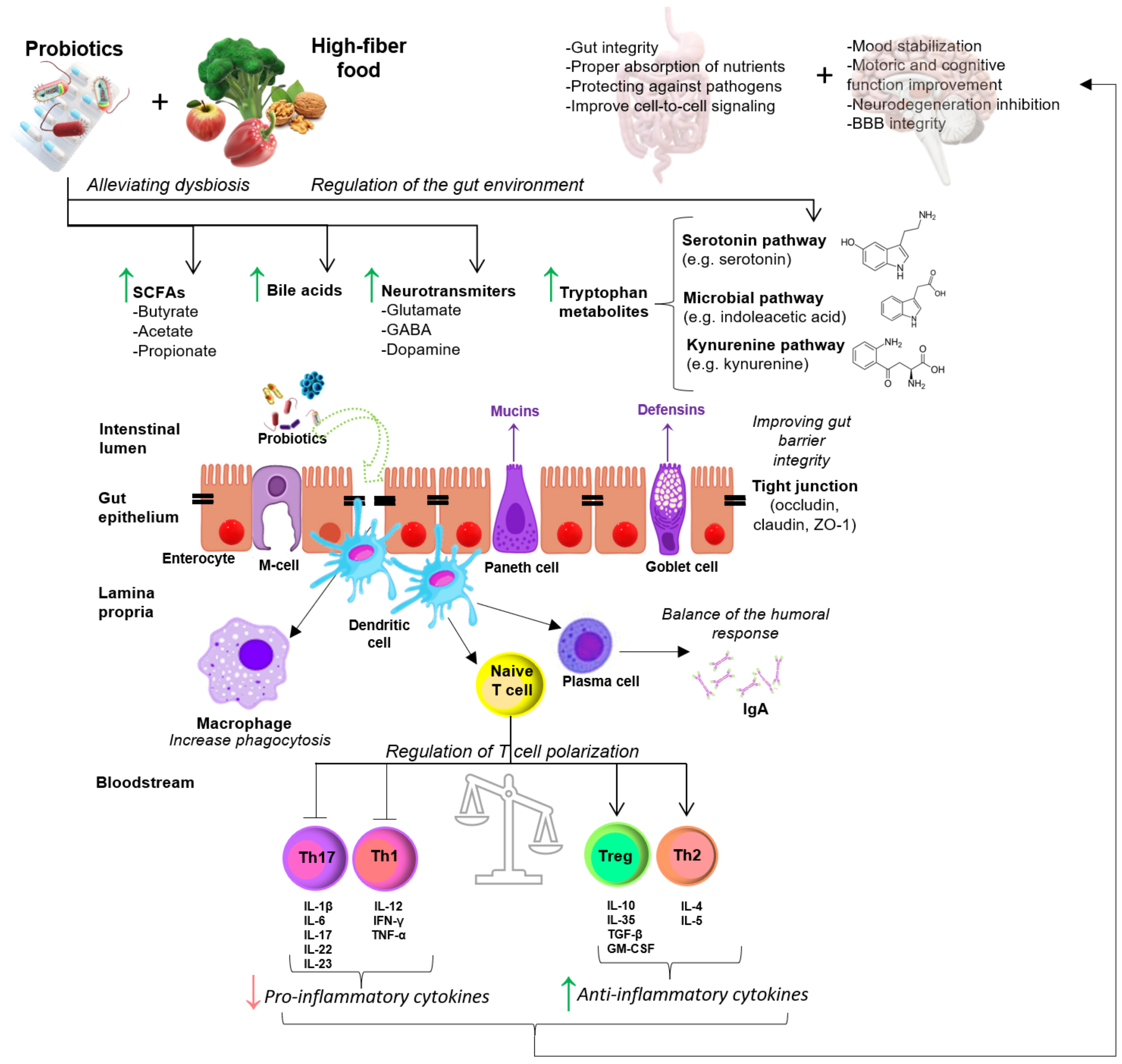
4. Effect of Probiotic Supplementation on EAE/MS
4.1. Animal Studies
4.2. Human Studies
5. Concluding Remarks and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The Human Microbiome Project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.R.; Pop, M.; Deboy, R.T.; Eckburg, P.B.; Turnbaugh, P.J.; Samuel, B.S.; Gordon, J.I.; Relman, D.A.; Fraser-Liggett, C.M.; Nelson, K.E. Metagenomic analysis of the human distal gut microbiome. Science 2006, 312, 1355–1359. [Google Scholar] [CrossRef] [PubMed]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Li, J.; Jia, H.; Cai, X.; Zhong, H.; Feng, Q.; Sunagawa, S.; Arumugam, M.; Kultima, J.R.; Prifti, E.; Nielsen, T.; et al. An integrated catalog of reference genes in the human gut microbiome. Nat. Biotechnol. 2014, 32, 834–841. [Google Scholar] [CrossRef]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.S.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef]
- Nelson, K.E.; Weinstock, G.M.; Highlander, S.K.; Worley, K.C.; Creasy, H.H.; Wortman, J.R.; Rusch, D.B.; Mitreva, M.; Sodergren, E.; Chinwalla, A.T.; et al. A catalog of reference genomes from the human microbiome. Science 2010, 328, 994–999. [Google Scholar] [CrossRef]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Liu, Y.; Alookaran, J.J.; Rhoads, J.M. Probiotics in Autoimmune and Inflammatory Disorders. Nutrients 2018, 10, 1537. [Google Scholar] [CrossRef]
- Giri, P.; Shah, F.; Dwivedi, M. Probiotics and Prebiotics in the Suppression of Autoimmune Diseases; Academic Press: Cambridge, MA, USA, 2022; pp. 161–186. [Google Scholar]
- Yousefi, B.; Eslami, M.; Ghasemian, A.; Kokhaei, P.; Sadeghnejad, A. Probiotics can really cure an autoimmune disease? Gene Rep. 2019, 15, 100364. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Y.; Deng, Y.; Yi, C.; Ding, M.; Liu, J.; Jin, X.; Shen, L.; He, Y.; Wu, X.; et al. Probiotic Supplements: Hope or Hype? Front Microbiol. 2020, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Dash, S.; Syed, Y.A.; Khan, M.R. Understanding the Role of the Gut Microbiome in Brain Development and Its Association with Neurodevelopmental Psychiatric Disorders. Front. Cell Dev. Biol. 2022, 10, 880544. [Google Scholar] [CrossRef]
- Kim, C.S.; Cha, L.; Sim, M.; Jung, S.; Chun, W.Y.; Baik, H.W.; Shin, D.M. Probiotic Supplementation Improves Cognitive Function and Mood with Changes in Gut Microbiota in Community-Dwelling Older Adults: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Dinan, T.G.; Stanton, C.; Cryan, J.F. Psychobiotics: A novel class of psychotropic. Biol. Psychiatry 2013, 74, 720–726. [Google Scholar] [CrossRef]
- Tran, S.M.; Mohajeri, M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients 2021, 13, 732. [Google Scholar] [CrossRef]
- Zhou, L.; Foster, J.A. Psychobiotics and the gut-brain axis: In the pursuit of happiness. Neuropsychiatr. Dis. Treat. 2015, 11, 715–723. [Google Scholar] [CrossRef]
- Magalhães-Guedes, K.T. Psychobiotic Therapy: Method to Reinforce the Immune System. Clin. Psychopharmacol. Neurosci. 2022, 20, 17–25. [Google Scholar] [CrossRef]
- Carabotti, M.; Scirocco, A.; Maselli, M.A.; Severi, C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann. Gastroenterol. 2015, 28, 203–209. [Google Scholar]
- Skonieczna-Żydecka, K.; Marlicz, W.; Misera, A.; Koulaouzidis, A.; Łoniewski, I. Microbiome-The Missing Link in the Gut-Brain Axis: Focus on Its Role in Gastrointestinal and Mental Health. J. Clin. Med. 2018, 7, 521. [Google Scholar] [CrossRef]
- Morais, L.H.; Schreiber, H.L.T.; Mazmanian, S.K. The gut microbiota-brain axis in behaviour and brain disorders. Nat. Rev. Microbiol. 2021, 19, 241–255. [Google Scholar] [CrossRef]
- Breit, S.; Kupferberg, A.; Rogler, G.; Hasler, G. Vagus Nerve as Modulator of the Brain-Gut Axis in Psychiatric and Inflammatory Disorders. Front. Psychiatry 2018, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Toledo, A.R.; Monroy, G.R.; Salazar, F.E.; Lee, J.-Y.; Jain, S.; Yadav, H.; Borlongan, C.V. Gut–Brain Axis as a Pathological and Therapeutic Target for Neurodegenerative Disorders. Int. J. Mol. Sci. 2022, 23, 1184. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Noto, D.; Hoshino, Y.; Mizuno, M.; Miyake, S. Butyrate suppresses demyelination and enhances remyelination. J. Neuroinflamm. 2019, 16, 165. [Google Scholar] [CrossRef]
- Codagnone, M.G.; Spichak, S.; O’Mahony, S.M.; O’Leary, O.F.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Programming Bugs: Microbiota and the Developmental Origins of Brain Health and Disease. Biol. Psychiatry 2019, 85, 150–163. [Google Scholar] [CrossRef]
- Wang, Y.; Kasper, L.H. The role of microbiome in central nervous system disorders. Brain Behav. Immun. 2014, 38, 1–12. [Google Scholar] [CrossRef]
- O’Sullivan, E.; Barrett, E.; Grenham, S.; Fitzgerald, P.; Stanton, C.; Ross, R.P.; Quigley, E.M.; Cryan, J.F.; Dinan, T.G. BDNF expression in the hippocampus of maternally separated rats: Does Bifidobacterium breve 6330 alter BDNF levels? Benef. Microbes 2011, 2, 199–207. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The anxiolytic effect of Bifidobacterium longum NCC3001 involves vagal pathways for gut-brain communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Liu, Y.W.; Liu, W.H.; Wu, C.C.; Juan, Y.C.; Wu, Y.C.; Tsai, H.P.; Wang, S.; Tsai, Y.C. Psychotropic effects of Lactobacillus plantarum PS128 in early life-stressed and naïve adult mice. Brain Res. 2016, 1631, 1–12. [Google Scholar] [CrossRef]
- Liu, W.H.; Chuang, H.L.; Huang, Y.T.; Wu, C.C.; Chou, G.T.; Wang, S.; Tsai, Y.C. Alteration of behavior and monoamine levels attributable to Lactobacillus plantarum PS128 in germ-free mice. Behav. Brain Res. 2016, 298, 202–209. [Google Scholar] [CrossRef]
- Erny, D.; Hrabě de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Sarubbo, F.; Cavallucci, V.; Pani, G. The Influence of Gut Microbiota on Neurogenesis: Evidence and Hopes. Cells 2022, 11, 382. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis—A review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; van der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Golden, L.C.; Voskuhl, R. The importance of studying sex differences in disease: The example of multiple sclerosis. J. Neurosci. Res. 2017, 95, 633–643. [Google Scholar] [CrossRef]
- Cree, B.A.C.; Arnold, D.L.; Chataway, J.; Chitnis, T.; Fox, R.J.; Pozo Ramajo, A.; Murphy, N.; Lassmann, H. Secondary Progressive Multiple Sclerosis: New Insights. Neurology 2021, 97, 378–388. [Google Scholar] [CrossRef]
- Silveira, C.; Guedes, R.; Maia, D.; Curral, R.; Coelho, R. Neuropsychiatric Symptoms of Multiple Sclerosis: State of the Art. Psychiatry Investig. 2019, 16, 877–888. [Google Scholar] [CrossRef]
- Ruggieri, S.; Petracca, M.; De Giglio, L.; De Luca, F.; Giannì, C.; Gurreri, F.; Petsas, N.; Tommasin, S.; Pozzilli, C.; Pantano, P. A matter of atrophy: Differential impact of brain and spine damage on disability worsening in multiple sclerosis. J. Neurol. 2021, 268, 4698–4706. [Google Scholar] [CrossRef]
- Gelfand, J.M. Multiple sclerosis: Diagnosis, differential diagnosis, and clinical presentation. Handb. Clin. Neurol. 2014, 122, 269–290. [Google Scholar] [CrossRef]
- Pucak, M.L.; Carroll, K.A.; Kerr, D.A.; Kaplin, A.I. Neuropsychiatric manifestations of depression in multiple sclerosis: Neuroinflammatory, neuroendocrine, and neurotrophic mechanisms in the pathogenesis of immune-mediated depression. Dialogues Clin. Neurosci. 2007, 9, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.D.; Motl, R.; Sandroff, B.M. Depression in multiple sclerosis: Is one approach for its management enough? Mult. Scler. Relat. Disord. 2021, 51, 102904. [Google Scholar] [CrossRef] [PubMed]
- Moore, S. Major depression and multiple sclerosis—A case report. J. Med. Life 2013, 6, 290–291. [Google Scholar] [PubMed]
- Claflin, S.B.; Broadley, S.; Taylor, B.V. The Effect of Disease Modifying Therapies on Disability Progression in Multiple Sclerosis: A Systematic Overview of Meta-Analyses. Front. Neurol. 2018, 9, 1150. [Google Scholar] [CrossRef] [PubMed]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple Sclerosis: Mechanisms and Immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- Chastain, E.M.; Miller, S.D. Molecular mimicry as an inducing trigger for CNS autoimmune demyelinating disease. Immunol. Rev. 2012, 245, 227–238. [Google Scholar] [CrossRef]
- Schreibelt, G.; van Horssen, J.; van Rossum, S.; Dijkstra, C.D.; Drukarch, B.; de Vries, H.E. Therapeutic potential and biological role of endogenous antioxidant enzymes in multiple sclerosis pathology. Brain Res. Rev. 2007, 56, 322–330. [Google Scholar] [CrossRef]
- Acar, G.; Idiman, F.; Idiman, E.; Kirkali, G.; Cakmakçi, H.; Ozakbaş, S. Nitric oxide as an activity marker in multiple sclerosis. J. Neurol. 2003, 250, 588–592. [Google Scholar] [CrossRef]
- Ghonimi, N.A.M.; Elsharkawi, K.A.; Khyal, D.S.M.; Abdelghani, A.A. Serum malondialdehyde as a lipid peroxidation marker in multiple sclerosis patients and its relation to disease characteristics. Mult. Scler. Relat. Disord. 2021, 51, 102941. [Google Scholar] [CrossRef]
- Obradovic, D.; Andjelic, T.; Ninkovic, M.; Dejanovic, B.; Kotur-Stevuljevic, J. Superoxide dismutase (SOD), advanced oxidation protein products (AOPP), and disease-modifying treatment are related to better relapse recovery after corticosteroid treatment in multiple sclerosis. Neurol. Sci. 2021, 42, 3241–3247. [Google Scholar] [CrossRef]
- Choi, I.Y.; Lee, S.P.; Denney, D.R.; Lynch, S.G. Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3 T. Mult. Scler. 2011, 17, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Dendrou, C.A.; Fugger, L.; Friese, M.A. Immunopathology of multiple sclerosis. Nat. Rev. Immunol. 2015, 15, 545–558. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.M.; Lalor, S.J.; Sweeney, C.M.; Tubridy, N.; Mills, K.H. T cells in multiple sclerosis and experimental autoimmune encephalomyelitis. Clin. Exp. Immunol. 2010, 162, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Duarte, J.H.; Veldhoen, M.; Hornsby, E.; Li, Y.; Cua, D.J.; Ahlfors, H.; Wilhelm, C.; Tolaini, M.; Menzel, U.; et al. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat. Immunol. 2011, 12, 255–263. [Google Scholar] [CrossRef]
- El-Behi, M.; Ciric, B.; Dai, H.; Yan, Y.; Cullimore, M.; Safavi, F.; Zhang, G.X.; Dittel, B.N.; Rostami, A. The encephalitogenicity of T(H)17 cells is dependent on IL-1- and IL-23-induced production of the cytokine GM-CSF. Nat. Immunol. 2011, 12, 568–575. [Google Scholar] [CrossRef]
- Constantinescu, C.S.; Farooqi, N.; O’Brien, K.; Gran, B. Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS). Br. J. Pharmacol. 2011, 164, 1079–1106. [Google Scholar] [CrossRef]
- Monney, L.; Sabatos, C.A.; Gaglia, J.L.; Ryu, A.; Waldner, H.; Chernova, T.; Manning, S.; Greenfield, E.A.; Coyle, A.J.; Sobel, R.A.; et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 2002, 415, 536–541. [Google Scholar] [CrossRef]
- Cua, D.J.; Sherlock, J.; Chen, Y.; Murphy, C.A.; Joyce, B.; Seymour, B.; Lucian, L.; To, W.; Kwan, S.; Churakova, T.; et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 2003, 421, 744–748. [Google Scholar] [CrossRef]
- Jäger, A.; Dardalhon, V.; Sobel, R.A.; Bettelli, E.; Kuchroo, V.K. Th1, Th17, and Th9 effector cells induce experimental autoimmune encephalomyelitis with different pathological phenotypes. J. Immunol. 2009, 183, 7169–7177. [Google Scholar] [CrossRef]
- Komiyama, Y.; Nakae, S.; Matsuki, T.; Nambu, A.; Ishigame, H.; Kakuta, S.; Sudo, K.; Iwakura, Y. IL-17 Plays an Important Role in the Development of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2006, 177, 566. [Google Scholar] [CrossRef]
- Zozulya, A.L.; Wiendl, H. The role of regulatory T cells in multiple sclerosis. Nat. Clin. Pract. Neurol. 2008, 4, 384–398. [Google Scholar] [CrossRef]
- Kleinewietfeld, M.; Hafler, D.A. Regulatory T cells in autoimmune neuroinflammation. Immunol. Rev. 2014, 259, 231–244. [Google Scholar] [CrossRef]
- Chen, J.; Chia, N.; Kalari, K.R.; Yao, J.Z.; Novotna, M.; Paz Soldan, M.M.; Luckey, D.H.; Marietta, E.V.; Jeraldo, P.R.; Chen, X.; et al. Multiple sclerosis patients have a distinct gut microbiota compared to healthy controls. Sci. Rep. 2016, 6, 28484. [Google Scholar] [CrossRef]
- Tremlett, H.; Fadrosh, D.W.; Faruqi, A.A.; Zhu, F.; Hart, J.; Roalstad, S.; Graves, J.; Lynch, S.; Waubant, E. Gut microbiota in early pediatric multiple sclerosis: A case-control study. Eur. J. Neurol. 2016, 23, 1308–1321. [Google Scholar] [CrossRef]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.W.; et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef]
- Bhargava, P.; Smith, M.D.; Mische, L.; Harrington, E.; Fitzgerald, K.C.; Martin, K.; Kim, S.; Reyes, A.A.; Gonzalez-Cardona, J.; Volsko, C.; et al. Bile acid metabolism is altered in multiple sclerosis and supplementation ameliorates neuroinflammation. J. Clin. Invest. 2020, 130, 3467–3482. [Google Scholar] [CrossRef]
- Duscha, A.; Gisevius, B.; Hirschberg, S.; Yissachar, N.; Stangl, G.I.; Eilers, E.; Bader, V.; Haase, S.; Kaisler, J.; David, C.; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067.e1016–1080.e1016. [Google Scholar] [CrossRef]
- Saresella, M.; Marventano, I.; Barone, M.; La Rosa, F.; Piancone, F.; Mendozzi, L.; d’Arma, A.; Rossi, V.; Pugnetti, L.; Roda, G.; et al. Alterations in Circulating Fatty Acid Are Associated with Gut Microbiota Dysbiosis and Inflammation in Multiple Sclerosis. Front. Immunol. 2020, 11, 1390. [Google Scholar] [CrossRef]
- Camara-Lemarroy, C.R.; Silva, C.; Greenfield, J.; Liu, W.-Q.; Metz, L.M.; Yong, V.W. Biomarkers of intestinal barrier function in multiple sclerosis are associated with disease activity. Mult. Scler. J. 2019, 26, 1340–1350. [Google Scholar] [CrossRef]
- Olsson, A.; Gustavsen, S.; Langkilde, A.R.; Hansen, T.H.; Sellebjerg, F.; Bach Søndergaard, H.; Oturai, A.B. Circulating levels of tight junction proteins in multiple sclerosis: Association with inflammation and disease activity before and after disease modifying therapy. Mult. Scler. Relat. Disord. 2021, 54, 103136. [Google Scholar] [CrossRef]
- Wunsch, M.; Jabari, S.; Voussen, B.; Enders, M.; Srinivasan, S.; Cossais, F.; Wedel, T.; Boettner, M.; Schwarz, A.; Weyer, L.; et al. The enteric nervous system is a potential autoimmune target in multiple sclerosis. Acta Neuropathol. 2017, 134, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Nouri, M.; Bredberg, A.; Weström, B.; Lavasani, S. Intestinal barrier dysfunction develops at the onset of experimental autoimmune encephalomyelitis, and can be induced by adoptive transfer of auto-reactive T cells. PLoS ONE 2014, 9, e106335. [Google Scholar] [CrossRef] [PubMed]
- Secher, T.; Kassem, S.; Benamar, M.; Bernard, I.; Boury, M.; Barreau, F.; Oswald, E.; Saoudi, A. Oral Administration of the Probiotic Strain Escherichia coli Nissle 1917 Reduces Susceptibility to Neuroinflammation and Repairs Experimental Autoimmune Encephalomyelitis-Induced Intestinal Barrier Dysfunction. Front. Immunol. 2017, 8, 1096. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.; Gandhi, R.; Cox, L.M.; Li, N.; von Glehn, F.; Yan, R.; Patel, B.; Mazzola, M.A.; Liu, S.; Glanz, B.L.; et al. Alterations of the human gut microbiome in multiple sclerosis. Nat. Commun. 2016, 7, 12015. [Google Scholar] [CrossRef]
- Kozhieva, M.; Naumova, N.; Alikina, T.; Boyko, A.; Vlassov, V.; Kabilov, M.R. Primary progressive multiple sclerosis in a Russian cohort: Relationship with gut bacterial diversity. BMC Microbiol. 2019, 19, 309. [Google Scholar] [CrossRef]
- Cekanaviciute, E.; Yoo, B.B.; Runia, T.F.; Debelius, J.W.; Singh, S.; Nelson, C.A.; Kanner, R.; Bencosme, Y.; Lee, Y.K.; Hauser, S.L.; et al. Gut bacteria from multiple sclerosis patients modulate human T cells and exacerbate symptoms in mouse models. Proc. Natl. Acad. Sci. USA 2017, 114, 10713–10718. [Google Scholar] [CrossRef]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Tjon, E.; Laghi, L.; Cox, L.M.; Kivisäkk, P.; Pierre, I.V.; Hrishikesh, L.; Gandhi, R.; et al. A probiotic modulates the microbiome and immunity in multiple sclerosis. Ann. Neurol. 2018, 83, 1147–1161. [Google Scholar] [CrossRef]
- Abdurasulova, I.N.; Tarasova, E.A.; Nikiforova, I.G.; Il’ves, A.G.; Ivashkova, E.V.; Matsulevich, A.V.; Tatarinov, A.E.; Shangina, L.V.; Ermolenko, E.I.; Klimenko, V.M.; et al. The intestinal microbiota composition in patients with multiple sclerosis receiving different disease-modifying therapies DMT. Zh. Nevrol. Psikhiatr. Im. Korsakova 2018, 118, 62–69. [Google Scholar] [CrossRef]
- Forbes, J.D.; Chen, C.-y.; Knox, N.C.; Marrie, R.-A.; El-Gabalawy, H.; de Kievit, T.; Alfa, M.; Bernstein, C.N.; Van Domselaar, G. A comparative study of the gut microbiota in immune-mediated inflammatory diseases—Does a common dysbiosis exist? Microbiome 2018, 6, 221. [Google Scholar] [CrossRef]
- Oezguen, N.; Yalcinkaya, N.; Kücükali, C.I.; Dahdouli, M.; Hollister, E.B.; Luna, R.A.; Türkoglu, R.; Kürtüncü, M.; Eraksoy, M.; Savidge, T.C.; et al. Microbiota stratification identifies disease-specific alterations in neuro-Behçet’s disease and multiple sclerosis. Clin. Exp. Rheumatol. 2019, 37 (Suppl. S121), 58–66. [Google Scholar]
- Choileáin, S.N.; Kleinewietfeld, M.; Raddassi, K.; Hafler, D.A.; Ruff, W.E.; Longbrake, E.E. CXCR3+ T cells in multiple sclerosis correlate with reduced diversity of the gut microbiome. J. Transl. Autoimmun. 2020, 3, 100032. [Google Scholar] [CrossRef] [PubMed]
- Levi, I.; Gurevich, M.; Perlman, G.; Magalashvili, D.; Menascu, S.; Bar, N.; Godneva, A.; Zahavi, L.; Chermon, D.; Kosower, N.; et al. Potential role of indolelactate and butyrate in multiple sclerosis revealed by integrated microbiome-metabolome analysis. Cell Rep. Med. 2021, 2, 100246. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.M.; Maghzi, A.H.; Liu, S.; Tankou, S.K.; Dhang, F.H.; Willocq, V.; Song, A.; Wasén, C.; Tauhid, S.; Chu, R.; et al. Gut Microbiome in Progressive Multiple Sclerosis. Ann. Neurol. 2021, 89, 1195–1211. [Google Scholar] [CrossRef] [PubMed]
- Luczynski, P.; Whelan, S.O.; O’Sullivan, C.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F. Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. Eur. J. Neurosci. 2016, 44, 2654–2666. [Google Scholar] [CrossRef] [PubMed]
- Ogbonnaya, E.S.; Clarke, G.; Shanahan, F.; Dinan, T.G.; Cryan, J.F.; O’Leary, O.F. Adult Hippocampal Neurogenesis Is Regulated by the Microbiome. Biol. Psychiatry 2015, 78, e7–e9. [Google Scholar] [CrossRef]
- El Aidy, S.; Dinan, T.G.; Cryan, J.F. Gut Microbiota: The Conductor in the Orchestra of Immune-Neuroendocrine Communication. Clin. Ther. 2015, 37, 954–967. [Google Scholar] [CrossRef]
- Hoban, A.E.; Stilling, R.M.; Ryan, F.J.; Shanahan, F.; Dinan, T.G.; Claesson, M.J.; Clarke, G.; Cryan, J.F. Regulation of prefrontal cortex myelination by the microbiota. Transl. Psychiatry 2016, 6, e774. [Google Scholar] [CrossRef]
- Lee, Y.K.; Menezes, J.S.; Umesaki, Y.; Mazmanian, S.K. Proinflammatory T-cell responses to gut microbiota promote experimental autoimmune encephalomyelitis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. S1), 4615–4622. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Ditrio, L.E.; Burroughs, A.R.; Foureau, D.M.; Haque-Begum, S.; Kasper, L.H. Role of gut commensal microflora in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2009, 183, 6041–6050. [Google Scholar] [CrossRef]
- Yamashita, M.; Ukibe, K.; Matsubara, Y.; Hosoya, T.; Sakai, F.; Kon, S.; Arima, Y.; Murakami, M.; Nakagawa, H.; Miyazaki, T. Lactobacillus helveticus SBT2171 Attenuates Experimental Autoimmune Encephalomyelitis in Mice. Front. Microbiol. 2017, 8, 2596. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Ditrio, L.E.; Burroughs, A.R.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. Central nervous system demyelinating disease protection by the human commensal Bacteroides fragilis depends on polysaccharide A expression. J. Immunol. 2010, 185, 4101–4108. [Google Scholar] [CrossRef] [PubMed]
- Shahi, S.K.; Freedman, S.N.; Murra, A.C.; Zarei, K.; Sompallae, R.; Gibson-Corley, K.N.; Karandikar, N.J.; Murray, J.A.; Mangalam, A.K. Prevotella histicola, A Human Gut Commensal, Is as Potent as COPAXONE® in an Animal Model of Multiple Sclerosis. Front. Immunol. 2019, 10, 462. [Google Scholar] [CrossRef] [PubMed]
- Lavasani, S.; Dzhambazov, B.; Nouri, M.; Fåk, F.; Buske, S.; Molin, G.; Thorlacius, H.; Alenfall, J.; Jeppsson, B.; Weström, B. A novel probiotic mixture exerts a therapeutic effect on experimental autoimmune encephalomyelitis mediated by IL-10 producing regulatory T cells. PLoS ONE 2010, 5, e9009. [Google Scholar] [CrossRef]
- He, B.; Hoang, T.K.; Tian, X.; Taylor, C.M.; Blanchard, E.; Luo, M.; Bhattacharjee, M.B.; Freeborn, J.; Park, S.; Couturier, J.; et al. Lactobacillus reuteri Reduces the Severity of Experimental Autoimmune Encephalomyelitis in Mice by Modulating Gut Microbiota. Front. Immunol. 2019, 10, 385. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.K.; Kim, G.C.; Kim, Y.; Hwang, W.; Jash, A.; Sahoo, A.; Kim, J.E.; Nam, J.H.; Im, S.H. Amelioration of experimental autoimmune encephalomyelitis by probiotic mixture is mediated by a shift in T helper cell immune response. Clin. Immunol. 2013, 146, 217–227. [Google Scholar] [CrossRef]
- Mestre, L.; Carrillo-Salinas, F.J.; Feliú, A.; Mecha, M.; Alonso, G.; Espejo, C.; Calvo-Barreiro, L.; Luque-García, J.L.; Estevez, H.; Villar, L.M.; et al. How oral probiotics affect the severity of an experimental model of progressive multiple sclerosis? Bringing commensal bacteria into the neurodegenerative process. Gut Microbes 2020, 12, 1813532. [Google Scholar] [CrossRef]
- Raheem, A.; Liang, L.; Zhang, G.; Cui, S. Modulatory Effects of Probiotics During Pathogenic Infections With Emphasis on Immune Regulation. Front. Immunol. 2021, 12, 616713. [Google Scholar] [CrossRef]
- Yokote, H.; Miyake, S.; Croxford, J.L.; Oki, S.; Mizusawa, H.; Yamamura, T. NKT cell-dependent amelioration of a mouse model of multiple sclerosis by altering gut flora. Am. J. Pathol. 2008, 173, 1714–1723. [Google Scholar] [CrossRef]
- Dargahi, N.; Matsoukas, J.; Apostolopoulos, V. Streptococcus thermophilus ST285 Alters Pro-Inflammatory to Anti-Inflammatory Cytokine Secretion against Multiple Sclerosis Peptide in Mice. Brain Sci. 2020, 10, 126. [Google Scholar] [CrossRef]
- Salehipour, Z.; Haghmorad, D.; Sankian, M.; Rastin, M.; Nosratabadi, R.; Soltan Dallal, M.M.; Tabasi, N.; Khazaee, M.; Nasiraii, L.R.; Mahmoudi, M. Bifidobacterium animalis in combination with human origin of Lactobacillus plantarum ameliorate neuroinflammation in experimental model of multiple sclerosis by altering CD4+ T cell subset balance. Biomed. Pharmacother. 2017, 95, 1535–1548. [Google Scholar] [CrossRef]
- Sokovic, S.; Mihajlovic, S.; Radojevic, D.; Popović, D.; Djokic, J.; Stanisavljević, S.; Lazarević, M.; Miljković, D.; Ruas-Madiedo, P.; Golić, N.; et al. Characterization of pH resistance and the proteolytic activity of GABA producing Lactobacillus brevis BGZLS10-17 in preparation of fermented milk beverage and the effects on the symptoms of the experimental autoimmune encephalomyelitis. J. Serb. Chem. Soc. 2019, 85, 94. [Google Scholar] [CrossRef]
- Abdurasulova, I.N.; Matsulevich, A.V.; Tarasova, E.A.; Kudryavtsev, I.V.; Serebrjakova, M.K.; Ermolenko, E.I.; Bisaga, G.N.; Klimenko, V.M.; Suvorov, A.N. Enterococcus faecium strain L-3 and glatiramer acetate ameliorate experimental allergic encephalomyelitis in rats by affecting different populations of immune cells. Benef. Microbes 2016, 7, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Suzuki, T.; Kaji, R.; Serata, M.; Nagata, T.; Ando, M.; Iizuka, R.; Tsujibe, S.; Murakami, J.; Kiyoshima-Shibata, J.; et al. Probiotic upregulation of peripheral IL-17 responses does not exacerbate neurological symptoms in experimental autoimmune encephalomyelitis mouse models. Immunopharmacol. Immunotoxicol. 2012, 34, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Gharehkhani Digehsara, S.; Name, N.; Esfandiari, B.; Karim, E.; Taheri, S.; Tajabadi-Ebrahimi, M.; Arasteh, J. Effects of Lactobacillus casei Strain T2 (IBRC-M10783) on the Modulation of Th17/Treg and Evaluation of miR-155, miR-25, and IDO-1 Expression in a Cuprizone-Induced C57BL/6 Mouse Model of Demyelination. Inflammation 2021, 44, 334–343. [Google Scholar] [CrossRef]
- Tankou, S.K.; Regev, K.; Healy, B.C.; Cox, L.M.; Tjon, E.; Kivisakk, P.; Vanande, I.P.; Cook, S.; Gandhi, R.; Glanz, B.; et al. Investigation of probiotics in multiple sclerosis. Mult. Scler. 2018, 24, 58–63. [Google Scholar] [CrossRef]
- Cristofori, F.; Dargenio, V.N.; Dargenio, C.; Miniello, V.L.; Barone, M.; Francavilla, R. Anti-Inflammatory and Immunomodulatory Effects of Probiotics in Gut Inflammation: A Door to the Body. Front. Immunol. 2021, 12, 578386. [Google Scholar] [CrossRef]
- Li, H.; Wang, P.; Huang, L.; Li, P.; Zhang, D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol. Motil. 2019, 31, e13677. [Google Scholar] [CrossRef]
- Kouchaki, E.; Tamtaji, O.R.; Salami, M.; Bahmani, F.; Daneshvar Kakhaki, R.; Akbari, E.; Tajabadi-Ebrahimi, M.; Jafari, P.; Asemi, Z. Clinical and metabolic response to probiotic supplementation in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Clin. Nutr. 2017, 36, 1245–1249. [Google Scholar] [CrossRef]
- Mohammadi, A.A.; Jazayeri, S.; Khosravi-Darani, K.; Solati, Z.; Mohammadpour, N.; Asemi, Z.; Adab, Z.; Djalali, M.; Tehrani-Doost, M.; Hosseini, M.; et al. The effects of probiotics on mental health and hypothalamic–pituitary–adrenal axis: A randomized, double-blind, placebo-controlled trial in petrochemical workers. Nutritional. Neurosci. 2016, 19, 387–395. [Google Scholar] [CrossRef]
- Salami, M.; Kouchaki, E.; Asemi, Z.; Tamtaji, O.R. How probiotic bacteria influence the motor and mental behaviors as well as immunological and oxidative biomarkers in multiple sclerosis? A double blind clinical trial. J. Funct. Foods 2019, 52, 8–13. [Google Scholar] [CrossRef]
- Rahimlou, M.; Hosseini, S.A.; Majdinasab, N.; Haghighizadeh, M.H.; Husain, D. Effects of long-term administration of Multi-Strain Probiotic on circulating levels of BDNF, NGF, IL-6 and mental health in patients with multiple sclerosis: A randomized, double-blind, placebo-controlled trial. Nutr. Neurosci. 2022, 25, 411–422. [Google Scholar] [CrossRef]
- Tamtaji, O.R.; Kouchaki, E.; Salami, M.; Aghadavod, E.; Akbari, E.; Tajabadi-Ebrahimi, M.; Asemi, Z. The Effects of Probiotic Supplementation on Gene Expression Related to Inflammation, Insulin, and Lipids in Patients with Multiple Sclerosis: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Coll. Nutr. 2017, 36, 660–665. [Google Scholar] [CrossRef]
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. The role of oxidative stress in the pathogenesis of multiple sclerosis: The need for effective antioxidant therapy. J. Neurol. 2004, 251, 261–268. [Google Scholar] [CrossRef]
| Subjects | Altered Genera in MS | Study |
|---|---|---|
| RRMS (n = 20) CTR (n = 40) | ↑: Streptococcus, Eggerthella ↓: Faecalibacterium, Prevotella, Anaerostipes | Miyake et al., Japan (2015) [66] |
| RRMS (n = 60) CTR (n = 43) | ↑: Akkermansia, Methanobrevibacter ↓: Butyricimonas, Collinsella, Slackia, Prevotella | Jangi et al., USA (2016) [75] |
| RRMS (n = 71) CTR (n = 71) | ↑: Akkermansia, Acinetobacter, Calcoaceticus ↓: Parabacteroides | Cekanaviciute et al., USA (2017) [77] |
| RRMS (n = 9) CTR (n = 13) | ↑: Lactobacillus ↓: Akkermansia, Blautia | Tankou et al., USA (2018) [78] |
| RRMS (n = 17) CTR (n = 17) | ↑: atypical E coli, Enterobacter sp. ↓: E. coli | Abdurasulva et al., Russia (2018) [79] |
| RRMS (n = 19) CTR (n = 23) | ↑: Actinomyces, Eggerthella, Anaerofustis, Clostridia XIII, Clostridium III, Faecalicoccus, Streptococcus ↓: Butyricicoccus, Faecalibacterium, Dialister, Gemmiger, Lachnospiraceae, Subdolibacterium | Forbes et al., Canada (2018) [80] |
| RRMS (n = 13) CTR (n = 14) | ↑: None ↓: Prevotella | Oezguen et al., USA (2019) [81] |
| RRMS (n = 26) CTR (n = 39) | ↑: Bacteroidetes ↓: Coprococcus, Firmicutes, Paraprevotella, Ruminococcaceae | Choileáin et al., USA (2020) [82] |
| RRMS (n = 26) SPMS (n = 12) CTR (n = 38) | ↑: Akkermansia in SPMS, Streptococcus in RRMS, Collinsella in RRMS and SPMS ↓: Coprococcus, Roseburia in RRMS and SPMS, Lachnospira in RRMS | Saresella et al., Italy (2020) [69] |
| RRMS (n = 129) CTR (n = 58) | ↑: Lawsonella ↓: Faecalibacterium prausnitzii, Bacteroides fragiils, Eubacterium rectale, Butyrivibrio, Clostridium, Coprococcus, Roseburia | Levi et al., Israel (2021) [83] |
| RRMS (n = 199) Progressive MS (n = 44) CTR (n = 40) | ↑: Clostridium, Bacteroides, Gemella, Akkermansia in RRMS and progressive MS ↓: Prevotella and Dorea in RRMS and progressive MS | Cox et al., USA (2021) [84] |
| Model | Intervention | Duration | Measurements | Major Findings | Study |
|---|---|---|---|---|---|
| PLP-induced EAE in SJL/J female mice; MOG-induced EAE in C57BL/6 female mice (7 weeks old, n = 15 per group) | Administration groups (G): G1: Control (saline/peptone, orally) G2: L. casei strain Shirota (orally, once daily, 0.6–1.2 × 109 CFU) | 50 days | -Evaluation of neurological symptoms; -Histopathological changes in the spinal cord; -mRNA and protein level: IL-10, IL-17A, and IFN-γ; -Cytometric analysis of cell surface antigens: anti-CD3, anti-CD4, anti-CD8, and anti-CD25. Material: inguinal lymph nodes (ILN) and spleen. | -Improved neurological symptoms in the PLP model; -Slightly increased IL-10 level in ILN; -The enhanced percentage of CD4+/CD25+ (Tregs) in ILN and spleen; -Increased level of CD3+/CD8+ (Tcyt) in the spleen; -Elevated concentration of IL-17A and IL-10 in ILN. | Kobayashi et al., (2012) Japan [104] |
| MOG-induced EAE in C57BL/6 female mice (6–8 weeks old, n = 10 per group) | G1: Control (PBS, orally) G2: IRT5 probiotics powder: L. casei, L. acidophilus, L. reuteni, B. bifidum, and S. thermophilus (orally, once daily, 1 × 108 CFU of each strain, final 5 × 108 CFU) | 30 days | -Clinical condition and symptoms using hematoxylin and eosin test staining; -mRNA level: IL-1β, IL-2, IL-4, IL-6, IL-10, IL-12, IL-17A, and TGF-β; -Cytometric analysis of cell surface antigens: anti-B220, anti-Gr1, anti-CD11b, anti-CD11c, and anti-CD4; intracellular cytokines: anti-IL-12, anti-IL-10, anti-IL-17, anti-IFN-γ, anti-Foxp3, and anti-TNF-α. Material: spinal cord. | -Inhibited development and progression of EAE; -Delayed onset of EAE; -Suppressed EAE incidence; -Decreased the clinical symptoms of EAE; -Reduced lymphocyte infiltration in the spinal cord; -Decreased levels of Gr1+ or/and CD11b+ monocyte and CD4+ T cells in the spinal cord; -Suppressed expression levels of pathogenic cytokines: IL-1β, IL-2, IFN-γ, TNF-α, and IL-17; -Enhanced production of IL-10 in CD4+ T cells and CD11c+ dendritic cells; -Slightly increased level of B220+ B cells; -Mitigated Th1/Th17 polarization while inducing IL-10+ producing CD4+ T cells in draining lymph nodes; -Down-regulated expression levels of IL-6, IFN-γ and TNF-α at mRNA level by CD4+ T cells; -Enhanced generation of CD4+/FoxP3+ Tregs at the site of inflammation. | Kwon et al., (2013) Republic of Korea [96] |
| EGM-induced EAE in male Wistar rats (3 months old, total n = 122 per 4 groups) | G1: Control (saline, subcutaneously) G2: Control (saline, intragastric) G3: Glatiramer acetate (GA) (subcutaneously, 4 mg/kg/day) G4: E. faecium L3 (intragastrically, 8 CFU/mL) | 28 days | -Blood cell phenotyping by flow cytometry: anti-CD3, anti-CD4, anti-CD8, anti-CD16, anti-CD25, anti-FoxP3, anti-CD45RA; -Evaluation of neurological symptoms. Material: spinal cord and whole blood | -Decreased severity and disease duration of EAE animals; -Reduced number of (CD4+/CD25+/FoxP3+) Tregs and NK cells. | Abdurasulova et al., (2016) Russia [103] |
| MOG-induced EAE in C57BL/6 female mice (8–10 weeks old, n = 8 per group) | G1: Control (saline, orally) G2: L. plantarum (intragastric, once daily, 1 × 109 CFU) G3: B. animalis (intragastric, once daily, 1 × 109 CFU) G4: both probiotics | 22 days | -Clinical score evaluation; -Body weight control; -Histopathology of the spinal cord; -Evaluate the proliferative activity of isolated splenic T cells using a Brdu assay; -Determination of Tregs by flow cytometry using anti-CD4, anti-CD25, and anti-FoxP3; -Protein level: IL-4, IL-6, IL-10, IL-17, IFN-γ, TGF-β; -mRNA level: FoxP3, T-bet, GATA3, and RORγt. Material: spinal cord, spleen, brain, and peripheral lymph nodes. | -Induced polarization of CD4+ T cells toward anti-inflammatory Tregs (CD4+/CD25+/Foxp3+); -Suppressed autoreactive T cells proliferation; -Inhibited leukocyte infiltration into CNS; -Ameliorated EAE condition by favoring Th2 and Treg differentiation; -Inhibited differentiation of Th1 and Th17 cells; -Increased level of IL-6, IL-17, IFN-γ, and diminished concentration of IL-4, IL10 and TGF-β in splenocytes and lymph nodes. | Salehipour et al., (2017) Iran [101] |
| MOG-induced EAE in C57BL/6 female mice (8–12 weeks old, n = 30–40 per group) | G1: Control (PBS, orally) G2: E.coli Nissle 1917 (ECN) (orally, one daily, 1 × 108 CFU) G3: archetypal E.coli strain MG1655 (orally, one daily, 1 × 108 CFU) | 30 days | -In vivo and ex vivo intestinal permeability assessment; -mRNA level: ZO-1, claudin-8, IL-6, Reg3β, and Reg3γ; -Protein level: IFN-β, IL-17, GM-CSF. Material: serum, ileum, colon, brain, spinal cord, and lymph nodes. | -ECN reduced the severity of EAE; -ECN treatment protects from EAE-mediated alteration of the intestinal barrier function; -Reduced migration of CD4+ T cells from the periphery to the CNS during the acute phase; -Increased production of IL-10 by MOG-specific CD4+ T cells. | Secher et al., (2017) France [74] |
| PLP-induced EAE in HLA-DR3.DQ8 double transgenic and C57BL/6, both male and female mice (8–12 weeks old, n = 4–8 mice per group) | G1: Control (PBS, orally) G2: TSB media (orally) G3: P. histicola (orally, one daily, 108 CFU) G4: Copaxone® (GA) (subcutaneously, 100 μg every day) G5: Copaxone®+ P. histicola | 14 days | -Evaluation of clinical EAE scores; -Clinical condition and symptoms using hematoxylin and eosin test staining; -Evaluation of gut microbiota composition; -Cytometric analysis of cell surface antigens: anti-CD4 and anti-CD25, intracellular expression of FoxP3+ and IL-10; Material: fecal pellets, brain, and spinal cord. | -Significantly reduced severity score and delayed onset of disease; -Increased number of CD4+/FoxP3+ Tregs in periphery and gut; -Reduced frequency of IFN-y and IL-17-producing CD4+ T cells in the CNS; -P. histicola, together with Copaxone®, more effectively suppressed disease compared to either treatment alone. | Shahi et al., (2019) USA [93] |
| SCH-induced EAE in female Dark Agouti (DA) rats (8–10 weeks old, n = 5 per group) | G1:Control (MRS Broth, orally, medium for Lactobacillus spp.) G2: L. brevis BGZLS10-17 (high GABA-producing strain) (subcutaneously, one daily, 1 × 108 CFU) | 30 days | -Neurological symptoms assessment. Material: spinal cord. | -Ameliorated severity score of EAE model (G2) after L. brevis intake. | Sokovic Bajic et al., (2019) Serbia [102] |
| MBP-induced EAE in female SJL/J mice (6–9 weeks old, n = 3 per group) | G1: Control (medium, orally) G2: S. thermophilus 285 (orally, one daily, 1 × 108 CFU) | 14 days | -Cytokine level analysis: IL-1β, IL-2, IL-4, IL-5, IL-6, IL-10, GM-CSF, TNF-α, and IFN-γ using Bioplex system. Material: spleen. | -Increased level of IL-4, IL-5 and IL-10 cytokines and diminished levels of IL-1β and IFN-y. | Dargahi et al., (2020) Australia [100] |
| TMEV-infected susceptible female SJL/J mice (6–8 weeks old, n = 5–10 per group) | G1: Sham mice G2: Sham mice + Vivomixx (orally, 3 × 108 CFU) G3: TMEV-mice G4: TMEV-mice + Vivomixx Vivomixx (L. paracasei, L. plantarum, L. acidophilus, L. delbruckeii subspecies bulgaricus, B. longum, B. infantis, B. breve, and S. thermophilus). | 15 days | -Assessment of the motor functions; -Measurement of bacteria-derived SCFAs; -mRNA level: IL-1β, IL-6, TNF-α, IL-4 and IL-10 in spinal cord; -Estimation of the level of Tregs and Bregs population; -Microglial morphology; -Cytometric analysis of cell surface antigens: anti-CD4, anti-CD8, and anti-CD39; -Identification of the gut microbiota community changes. Material: plasma, brain, spinal cord, spleen, and mesenteric lymph nodes. | -The increased abundance of Bcteroidetes, Actinobacteria, and Tenericutes; -Improved motor disability; -Reduced microgliosis, astrogliosis, and leukocyte infiltration; -The enhanced presence of Bregs (CD19+/CD5+/CD1dhigh) in the CNS; -Diminished IL-1b and IL-6 gene expression in spinal cord; -Promoted IL-10 gene expression; -Increased plasma level of butyrate and acetate levels; -Restricted IL-17 production by Th17-polarized CD4+ T cells from mesenteric lymph nodes. | Mestre et al., (2020) Spain [97] |
| Cuprizone-induced mouse model of demyelination in C57BL/6 female mice (8–10 weeks old) | G1: Control G2: Cuprizone control G3: Probiotic control G4: L. casei (oral administration, 1 × 109 CFU) for 4 weeks, then cuprizone for 4 weeks G5: Cuprizone for 4 weeks, then L. casei for 4 weeks G6: Cuprizone for 4 weeks, then L. casei for 4 weeks with vitamin D3 (20 IU per day) | 28 days | -Assessment of the motor behaviors; -Y-maze test for spatial memory and learning; -mRNA expression: IDO-1, miR-155, and miR-25; -Protein level: IL-17 and TGF-β. Material: brain, blood. | -L. casei ameliorated the CPZ-induced motor impairment; -Decreased the mRNA expression of IFN-γ, IDO-1, and miR-155; -Increased serum level of TGF-β and miR-25; -L. casei can shift responses from Th17 to Tregs; -Reduced pro-inflammatory cytokines; -Diminished demyelinating symptoms. | Gharehkhani Digehsara et al., (2020) Iran [105] |
| Subjects | Sex Ratio (M/F) | BMI (kg × m−2) | Average Age ± SD | Probiotic Bacteria | Dosage (CFU g−1) | Administration | Major Findings | Limitations | Study |
|---|---|---|---|---|---|---|---|---|---|
| RRMS (n = 40) (EDSS ≤ 4.5) Including: Placebo group (n = 20) and probiotic group (n = 20) | No data. | Placebo group: 24.7 ± 3.7 Probiotic group: 25.6 ± 4.6 | Placebo group: 34.9 ± 8.9 Probiotic group: 32.8 ± 9.2 | L. acidophilus, L. casei, B. bifidum, and L. fermentum Placebo group: starch | 2 × 109 | Orally, once a day for 3 months | -Down-regulated gene expression of IL-8 and TNF-α in PBMCs compared with the placebo group. | -Lack of information about microbiota changes; -Small sample size; -No confirmation of changes in the proteins level of studied molecules (only gene expression results); -Lack of diet control. | Double-blind RCT Tamtaji et al., (2017) Iran [113] |
| RRMS (n = 60) (EDSS ≤ 4.5) Including: Placebo group (n = 30) and probiotic group (n = 30) | Placebo group: 5/25 Probiotic group: 5/25 | Placebo group: 24.7 ± 3.3 Probiotic group: 25.4 ± 4.0 | Placebo group: 33.8 ± 8.9 Probiotic group: 34.4 ± 9.2 | L. acidophilus, L. casei, B. bifidum, and L. fermentum Placebo group: starch | 2 × 109 | Orally, once a day for 3 months | -Improved EDSS, BDI, GHQ-28, and DASS scales; -Decreased serum insulin level; -Increased quantitative insulin sensitivity check index and HDL-cholesterol levels; -Diminished levels of hs CRP, plasma NO metabolites, and MDA. | Double-blind RCT Kouchaki et al., (2017) Iran [109] | |
| Control group (CTR) (n = 13) RRMS on GA (n = 7) or untreated (n = 2) | No data. | CTR: 25.8 ± 4.1 MS: 31.1 ± 5.6 | CTR: 35 ± 14 MS: 50 ± 10 | VSL3 probiotics powder consisting of Lactobacillus (L. paracasei, L. plantarum, L. acidophilus, and L. delbruckeii subspecies bulgaricus), Bifidobacterium (B. longum, B. infantis, and B. breve) and S. thermophilus Brand name: Visbiome (USA) or Vivomixx (Europe). | 3 × 1011 | Orally, twice daily for 2 months. | -Diminished level of CD14+CD16+ and enhanced frequency of CD8+ T cells in MS patients; -Decreased MFI of HLA-DR on CD45+/LIN−/CD11c+ in MS patients; -The relative level of Th1 and Th17 cells were trending down in both controls and MS patients. | -Very small study and control group; -RRMS subjects (n = 2) were treated with glatiramer acetate during supplementation; -The subjects enrolled in this study were not on a dietary restriction; -No information about the gender of the subjects. | Clinical Trial Tankou et al., (2018) USA [106] |
| RRMS (n = 48) (EDSS ≤ 4.5) Including: Placebo group (n = 24) and probiotic group (n = 24) | Placebo group: 8/18 Probiotic group: 6/18 | Placebo group: 24.5 ± 0.63 Probiotic group: 24.7 ± 0.55 | Placebo group: 36.5 ± 1.44 Probiotic group: 34.8 ± 1.06 | B. infantis, B. lactis, L. reuteri, L. casei, L. plantarum and L. fermentum Placebo group: maltodextrin | 2 × 109 | Orally, once daily for 4 months. | -Markedly improves mental health parameters: BDI, GHQ-28, and DASS; -Reduced levels of hs-CRP, NO, and MDA; -Improved insulin resistance and lipid metabolism. -Decreased EDSS parameter. | -There is no information about the potential changes in bacterial strains. | Double-blind RCT Salami et al., (2019) Iran [111] |
| RRMS (n = 70) (EDSS ≤ 4.5) Including: Placebo group (n = 35) and probiotic group (n = 35) | Placebo group: 12/21 Probiotic group: 6/26 | Placebo group: 24.55 ± 3.51 Probiotic group: 25.48 ± 4.54 | Placebo group: 39.9 ± 8.76 Probiotic group: 42.15 ± 11.98 | Protein probiotics powder consisting of the following: B. subtilis, B. bifidum, B. breve, B. infantis, B. longum, L. acidophilus, L. bulgaricus, L. casei, L. plantarum, L. rhamnosus, L. helveticus, L. salivarius, L. lactis, and 0S. thermophilus. Placebo group: maltodextrin | 2 × 109 | Orally, twice daily for 6 months. | -Greater improvement in mental health parameters: GHQ-28, BDI, FSS, PRI. | Double-blind RCT Rahimlou et al., (2020) Iran [112] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dziedzic, A.; Saluk, J. Probiotics and Commensal Gut Microbiota as the Effective Alternative Therapy for Multiple Sclerosis Patients Treatment. Int. J. Mol. Sci. 2022, 23, 14478. https://doi.org/10.3390/ijms232214478
Dziedzic A, Saluk J. Probiotics and Commensal Gut Microbiota as the Effective Alternative Therapy for Multiple Sclerosis Patients Treatment. International Journal of Molecular Sciences. 2022; 23(22):14478. https://doi.org/10.3390/ijms232214478
Chicago/Turabian StyleDziedzic, Angela, and Joanna Saluk. 2022. "Probiotics and Commensal Gut Microbiota as the Effective Alternative Therapy for Multiple Sclerosis Patients Treatment" International Journal of Molecular Sciences 23, no. 22: 14478. https://doi.org/10.3390/ijms232214478
APA StyleDziedzic, A., & Saluk, J. (2022). Probiotics and Commensal Gut Microbiota as the Effective Alternative Therapy for Multiple Sclerosis Patients Treatment. International Journal of Molecular Sciences, 23(22), 14478. https://doi.org/10.3390/ijms232214478







