Detection of TRPM6 and TRPM7 Proteins in Normal and Diseased Cardiac Atrial Tissue and Isolated Cardiomyocytes
Abstract
:1. Introduction
2. Results
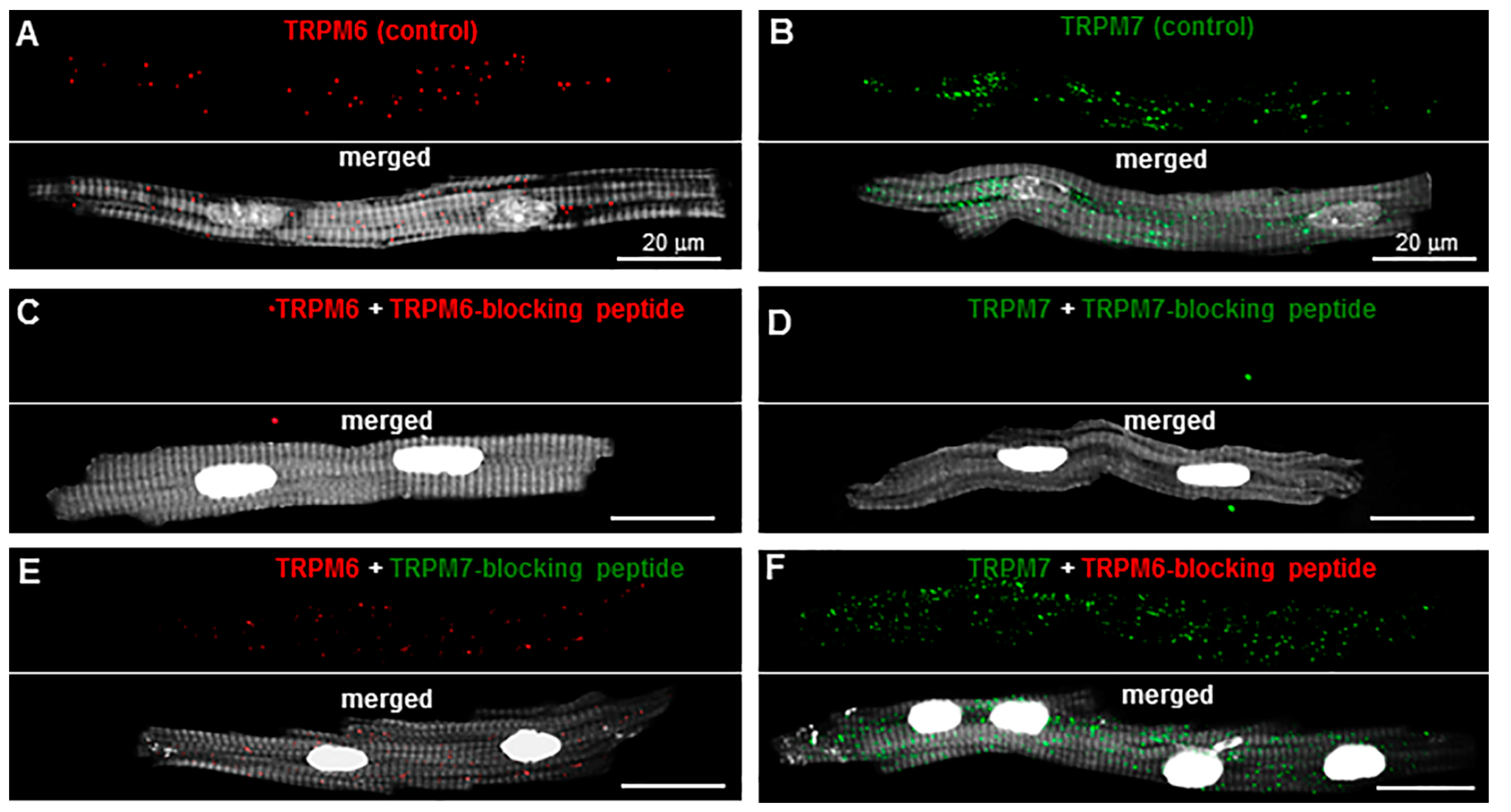
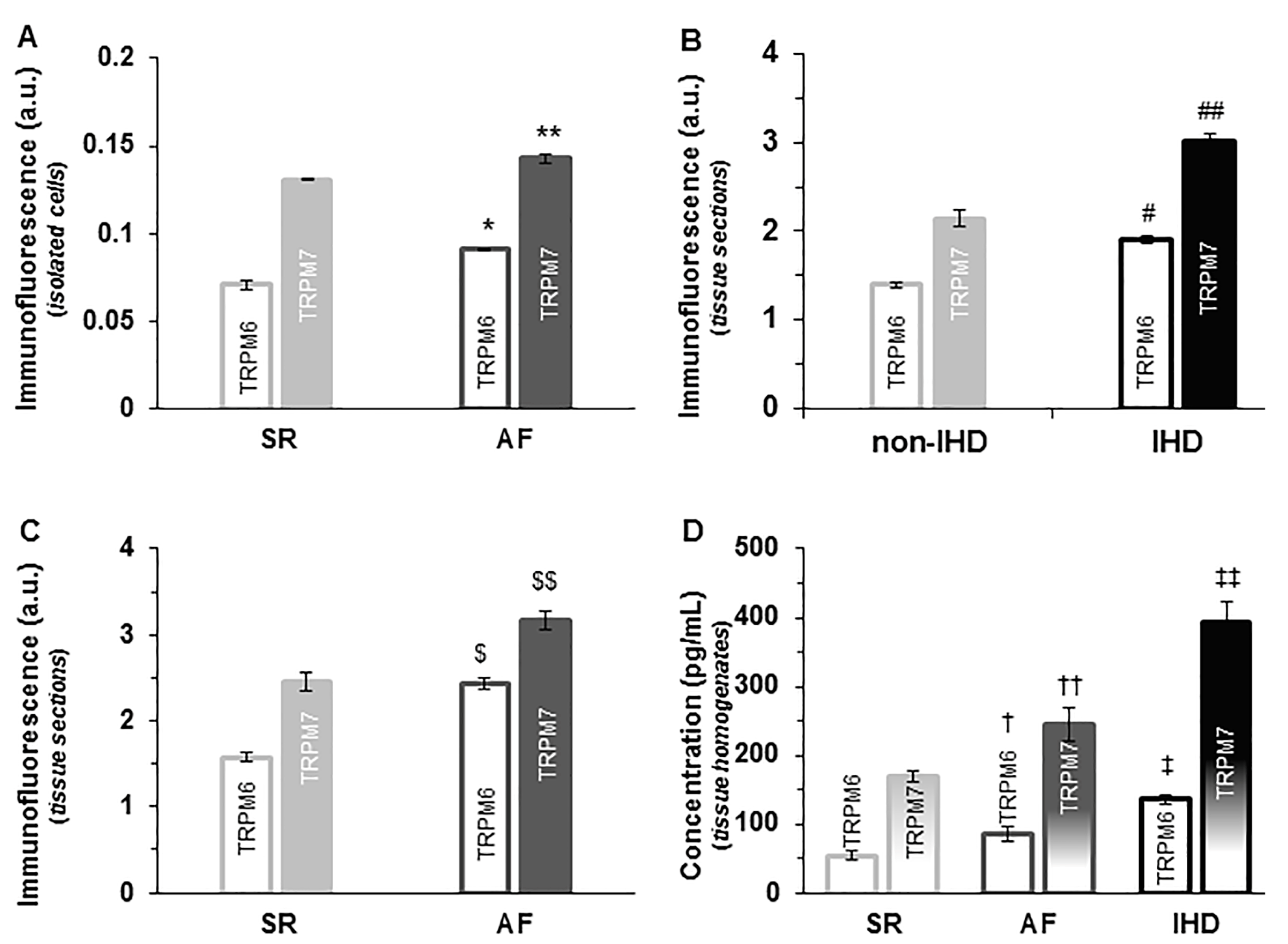
2.1. TRP Detection in Isolated Single Cardiomyocytes
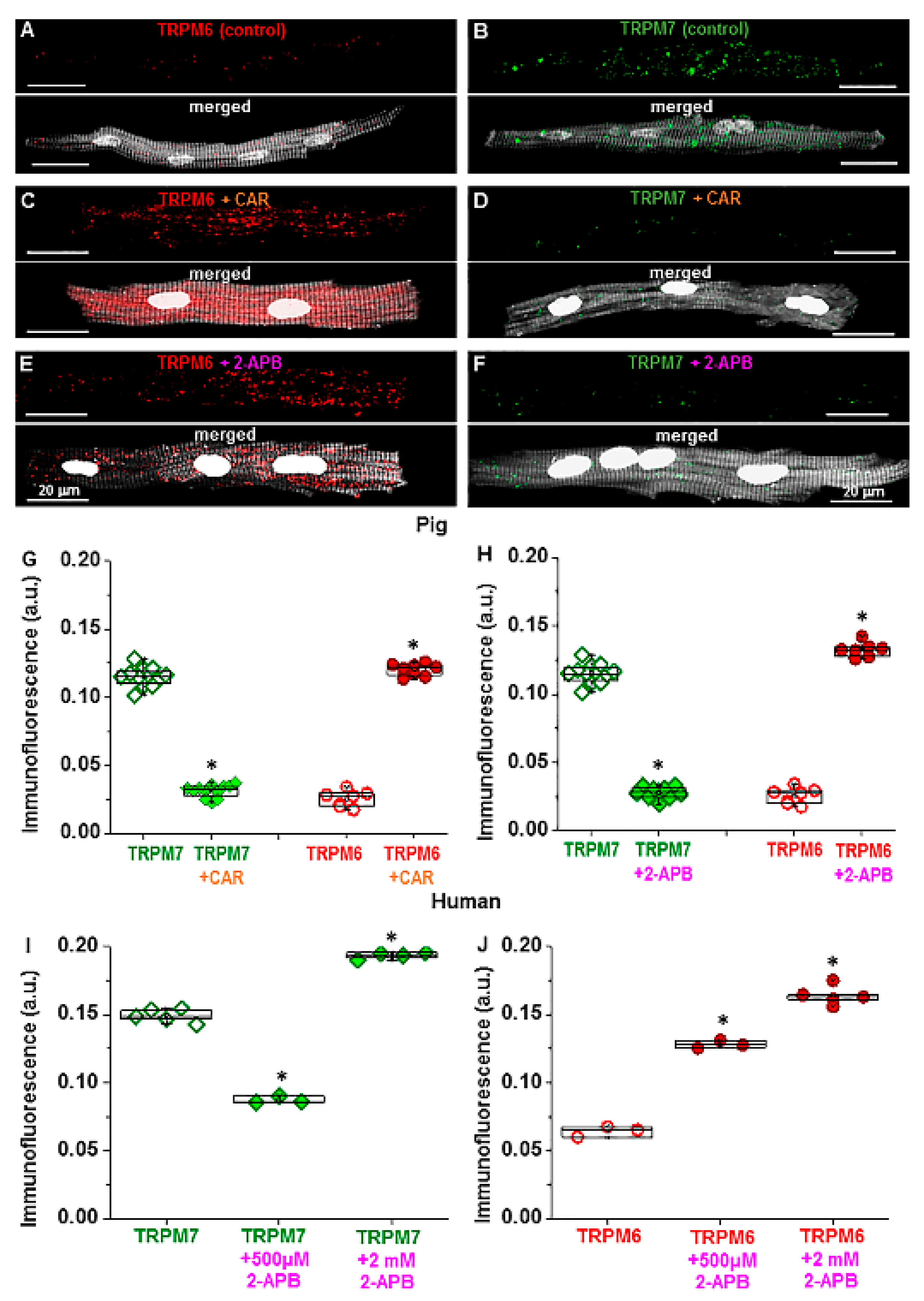
2.2. Sensitivity to Pharmacological Modulation
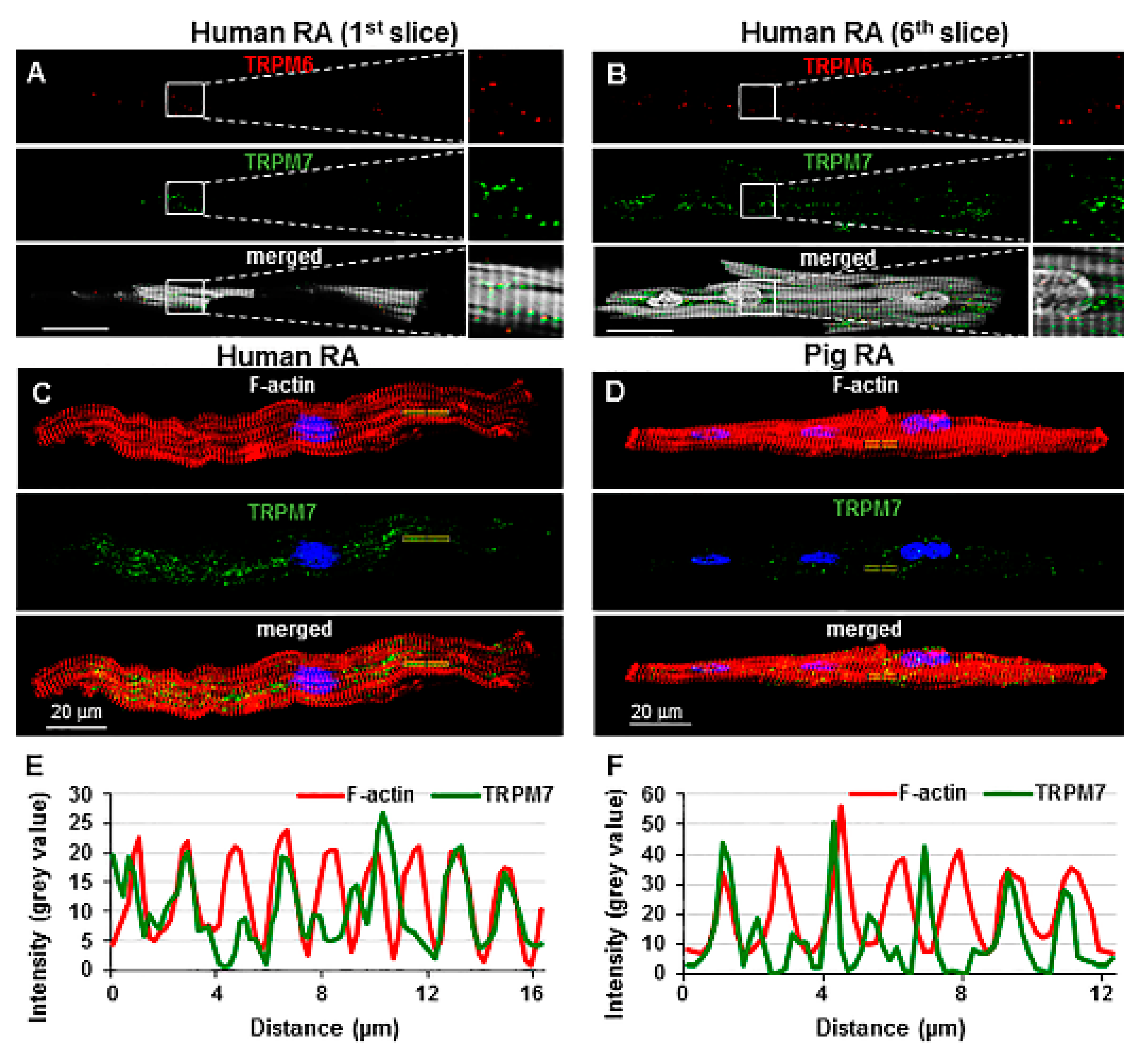
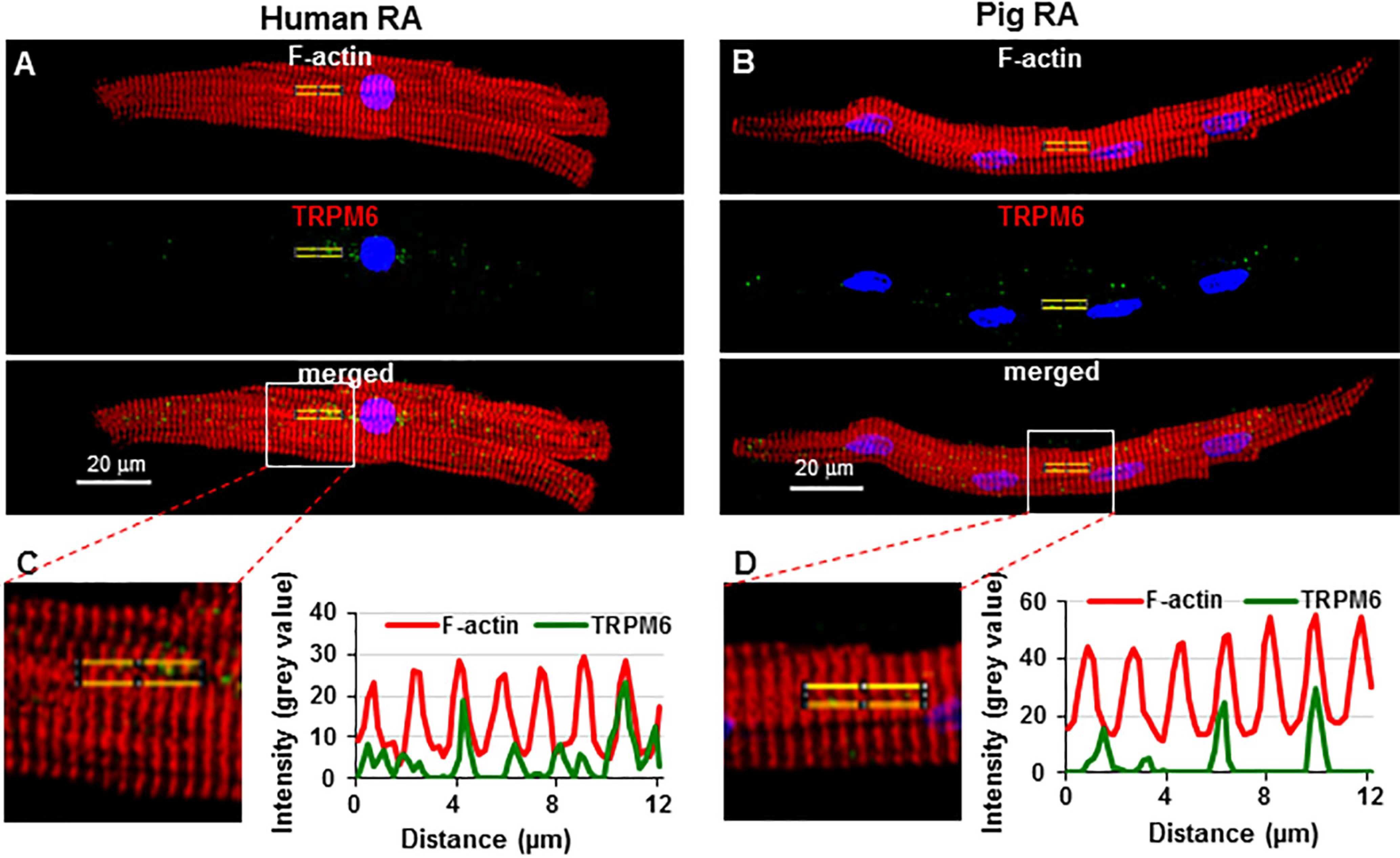
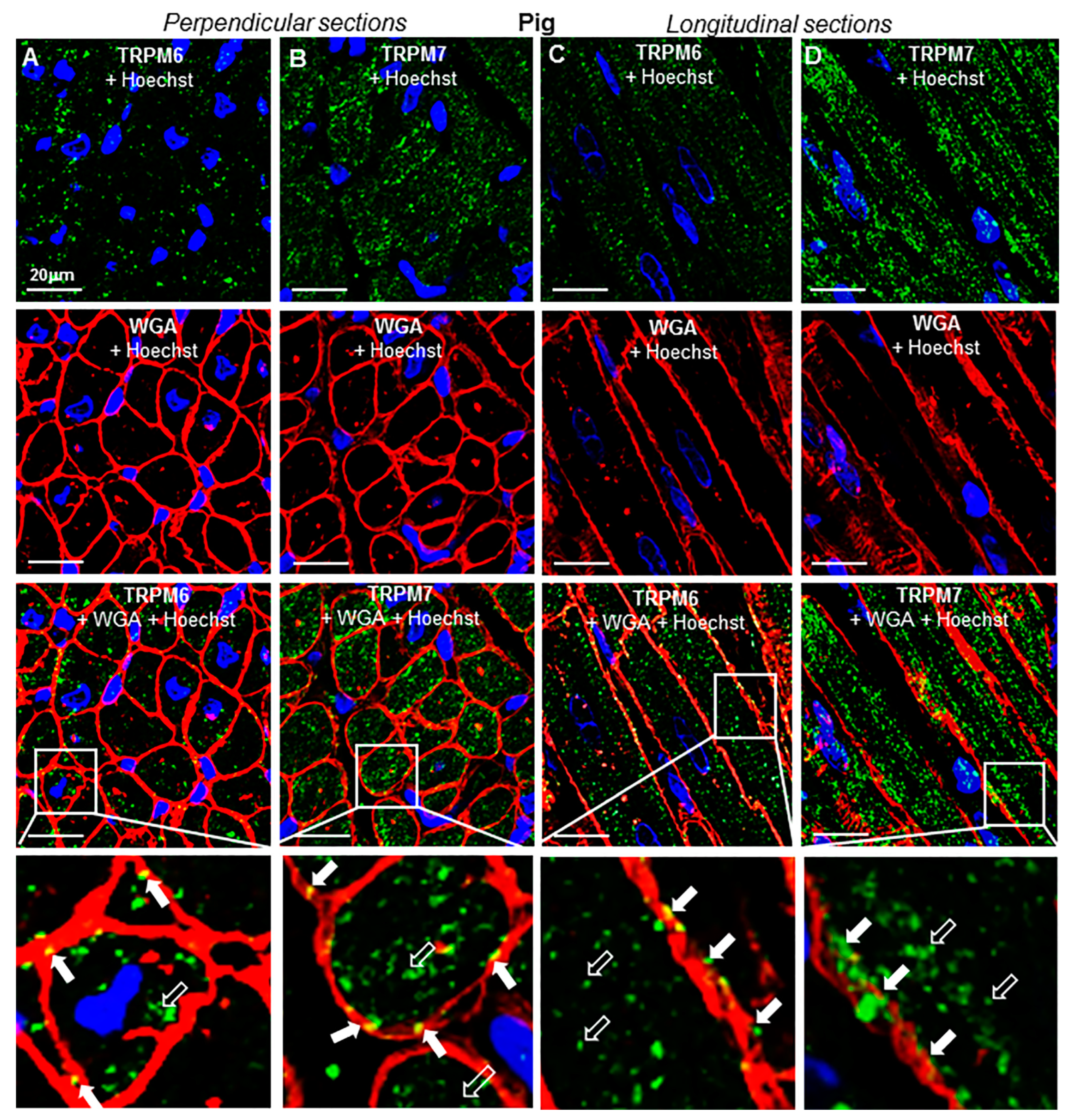
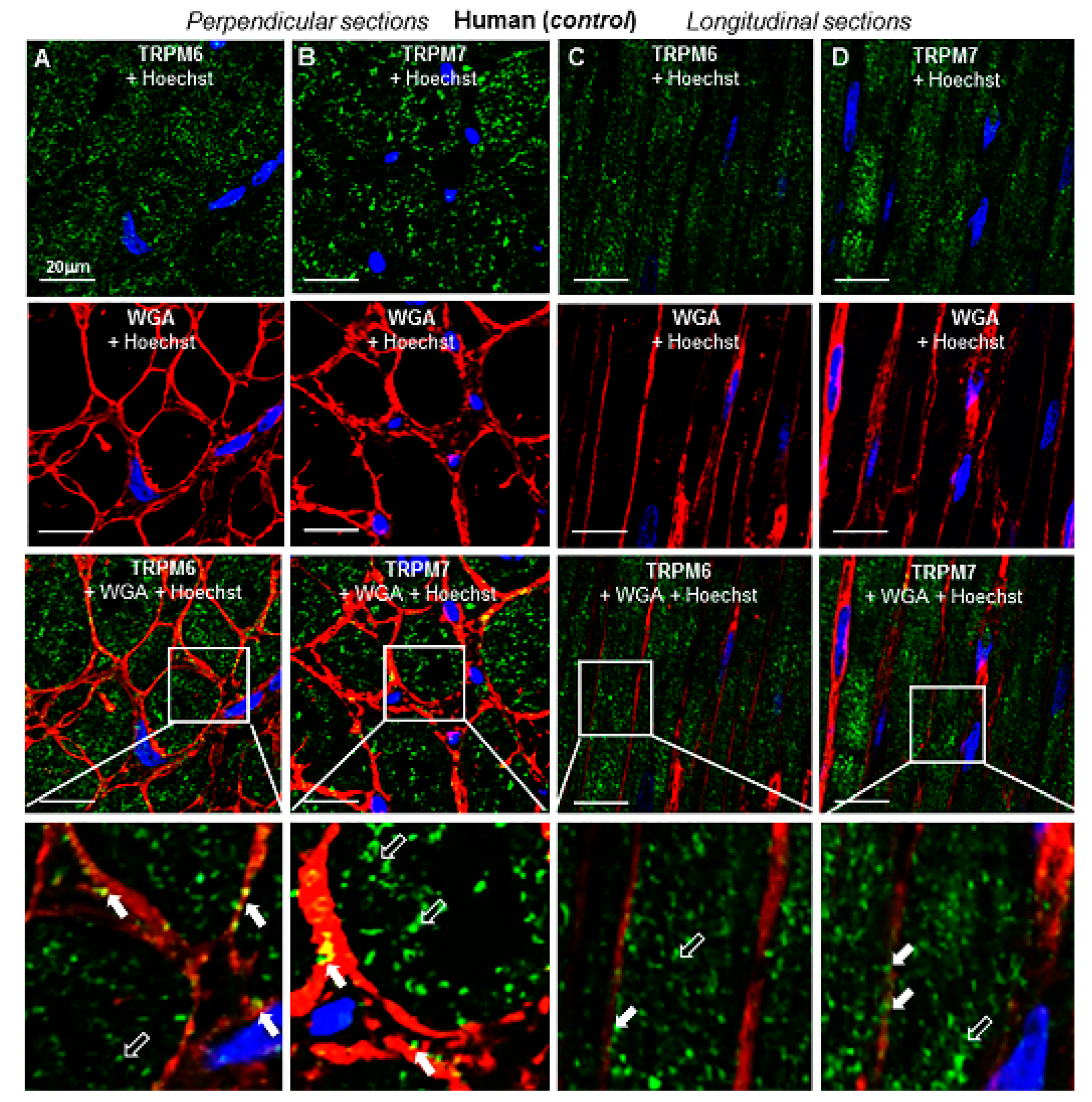
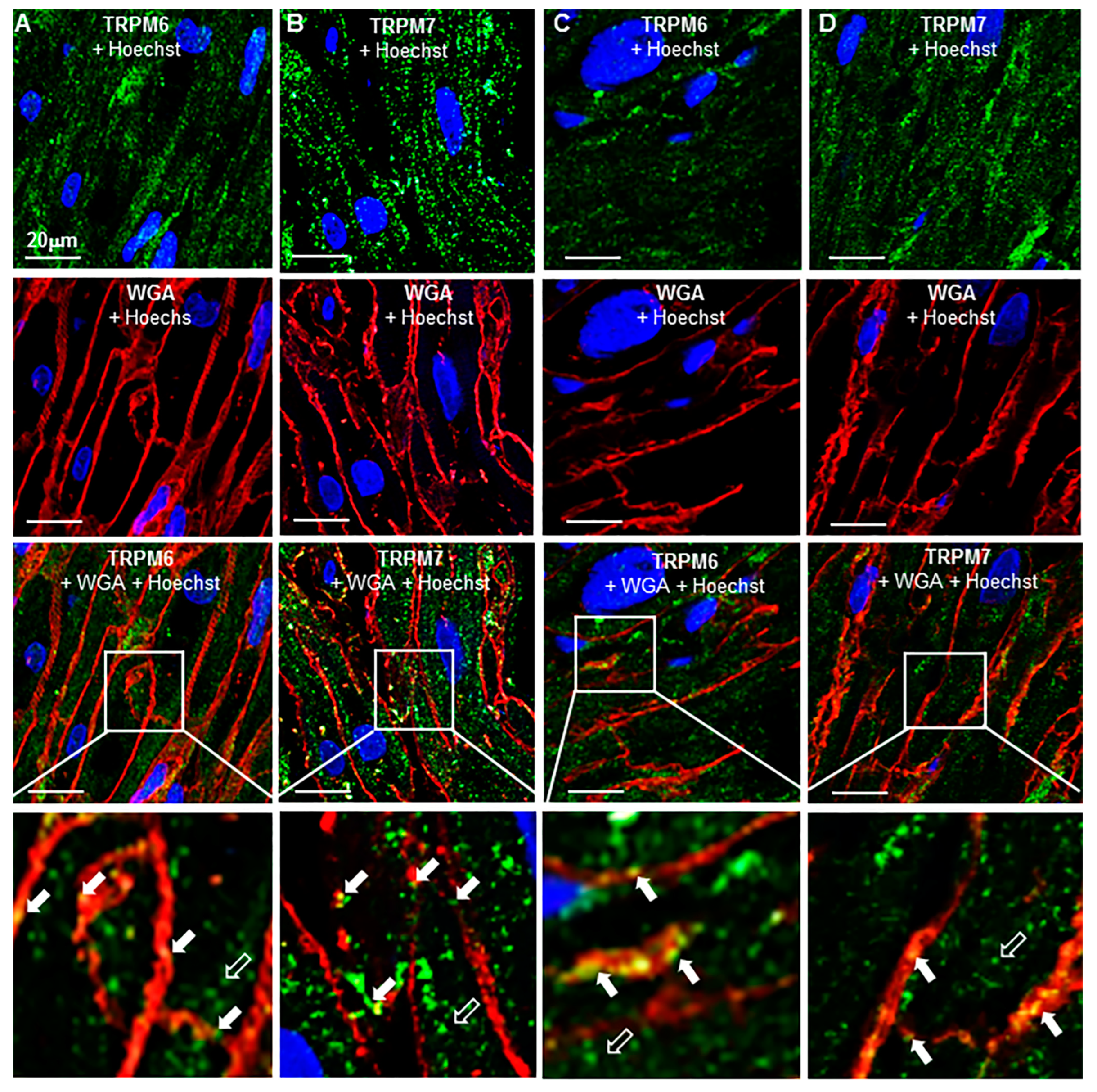
2.3. TRP Detection in Cardiac Tissue and Subcellular Localization
2.4. Change of Expression by Diseases
3. Discussion
4. Materials and Methods
4.1. Acquisition of Cardiac Samples
4.2. Immunofluorescence
4.3. ELISA Assay
4.4. Chemicals
4.5. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zakharov, S.; Smani, T.; Leno, E.; Macianskiene, R.; Mubagwa, K.; Bolotina, V. Monovalent cation (MC) current in cardiac and smooth muscle cells: Regulation by intracellular Mg2+ and inhibition by polycations. Br. J. Pharmacol. 2003, 138, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Gwanyanya, A.; Amuzescu, B.; Zakharov, S.I.; Macianskiene, R.; Sipido, K.R.; Bolotina, V.M.; Vereecke, J.; Mubagwa, K. Magnesium-inhibited, TRPM6/7-like channel in cardiac myocytes: Permeation of divalent cations and pH-mediated regulation. J. Physiol. 2004, 559, 761–776. [Google Scholar] [CrossRef] [Green Version]
- Gwanyanya, A.; Andriulė, I.; Istrate, B.M.; Easmin, F.; Mubagwa, K.; Mačianskienė, R. Modulation of the cardiac myocytes action potential by the magnesium-sensitive TRPM6 and TRPM7-like current. Int. J. Mol. Sci. 2021, 22, 8744. [Google Scholar] [CrossRef]
- Mačianskienė, R.; Martisiene, I.; Zablockaite, D.; Gendviliene, V. Characterization of Mg2+ regulated TRPM7-like current in human atrial myocytes. J. Biomed. Sci. 2012, 19, 75. [Google Scholar] [CrossRef] [Green Version]
- Macianskiene, R.; Almanaityte, M.; Jekabsone, A.; Mubagwa, K. Modulation of human cardiac TRPM7 current by extracellular acidic pH depends upon extracellular concentrations of divalent cations. PLoS ONE 2017, 12, e0170923. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.H.; Sun, H.Y.; Chen, K.H.; Du, X.L.; Liu, B.; Cheng, L.C.; Li, X.; Jin, M.W.; Li, G.R. Evidence for functional expression of TRPM7 channels in human atrial myocytes. Basic Res. Cardiol. 2012, 107, 282. [Google Scholar] [CrossRef] [Green Version]
- Mubagwa, K.; Gwanyanya, A.; Zakharov, S.; Macianskiene, R. Regulation of cations channels in cardiac and smooth muscle cells by intracellular magnesium. Arch. Bioch. Biophys. 2007, 458, 73–89. [Google Scholar] [CrossRef]
- Du, J.; Xie, J.; Zhang, Z.; Tsujikawa, H.; Fusco, D.; Silverman, D.; Liang, B.; Yue, L. TRPM7-mediated Ca2+ signals confer fibrogenesis in human Atrial Fibrillation. Circ. Res. 2010, 106, 992–1003. [Google Scholar] [CrossRef] [Green Version]
- Yu, Y.; Chen, S.; Xiao, C.; Jia, Y.; Guo, J.; Jiang, J.; Liu, P. TRPM7 is involved in angiotensin II induced cardiac fibrosis development by mediating calcium and magnesium influx. Cell Calcium 2014, 55, 252–260. [Google Scholar] [CrossRef]
- Gwanyanya, A.; Mubagwa, K. Emerging Role of Transient Receptor Potential (TRP) Ion Channels in Cardiac Fibroblast Pathophysiology. Front. Physiol. 2022, 13, 968393. [Google Scholar] [CrossRef]
- Sah, R.; Mesirca, P.; Van den Boogert, M.; Rosen, J.; Mably, J.; Mangoni, M.E.; Clapham, D.E. Ion channel-kinase TRPM7 is required for maintaining cardiac automaticity. Proc. Natl. Acad. Sci. USA 2013, 110, E3037–E3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hof, T.; Chaigne, S.; Récalde, A.; Sallé, L.; Brette, F.; Guinamard, R. Transient receptor potential channels in cardiac health and disease. Nat. Rev. Cardiol. 2019, 16, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Chubanov, V.; Mittermeier, L.; Gudermann, T. Role of kinase-coupled TRP channels in mineral homeostasis. Pharmacol. Ther. 2018, 184, 159–176. [Google Scholar] [CrossRef]
- Tashiro, M.; Inoue, H.; Konishi, M. Modulation of Mg2+ influx and cytoplasmic free Mg2+ concentration in rat ventricular myocytes. J. Physiol. Sci. 2019, 69, 97–102. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Ma, N.; Su, F.; Liu, H.; Mei, J. Increased TRPM6 expression in atrial fibrillation patients contribute to atrial fibrosis. Exp. Mol. Pathol. 2015, 98, 486–490. [Google Scholar] [CrossRef]
- Montell, C. The TRP superfamily of cation channels. Sci. STKE 2005, 2005, re3. [Google Scholar] [CrossRef] [Green Version]
- Runnels, L.W.; Yue, L.; Clapham, D.E. TRP-PLIK, a bifunctional protein with kinase and ion channel activities. Science 2001, 291, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Voets, T.; Nilius, B.; Hoefs, S.; van der Kemp, A.W.C.M.; Droogmans, G.; Bindels, R.J.M.; Hoenderop, J.G.J. TRPM6 forms the Mg2+ influx channel involved in intestinal and renal Mg2+ absorbtion. J. Biol. Chem. 2004, 279, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Schlingmann, K.P.; Weber, S.; Peters, M.; Nejsum, L.N.; Vitzthum, H.; Klingel, K.; Kratz, M.; Haddad, E.; Ristoff, E.; Dinour, D.; et al. Hypomagnesemia with secondary hypocalcemia is caused by mutations in TRPM6, a new member of the TRPM gene family. Nat. Genet. 2002, 31, 166–170. [Google Scholar] [CrossRef]
- Andriulė, I.; Pangonytė, D.; Almanaitytė, M.; Patamsytė, V.; Kuprytė, M.; Karčiauskas, D.; Mubagwa, K.; Mačianskienė, R. Evidence for the expression of TRPM6 and TRPM7 in cardiomyocytes from all four chamber walls of the human heart. Sci. Rep. 2021, 11, 15445. [Google Scholar] [CrossRef]
- Nilius, B.; Flockerzi, V. Mammalian transient receptor potential (TRP) cation channels. Preface. Handb. Exp. Pharmacol. 2014, 223, v–vi. [Google Scholar] [PubMed]
- Li, M.; Jiang, J.; You, L. Functional characterizarion of homo- and heteromeric channel kinases TRPM6 and TRPM7. J. Gen. Physiol. 2006, 127, 525–537. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.L.; Barszczyk, A.; Turlova, E.; Deurloo, M.; Liu, B.; Yang, B.B.; Rutka, J.T.; Feng, Z.-P.; Sun, H.S. Inhibition of TRPM7 by carvacrol suppresses glioblastoma cell proliferation, migration and invasion. Oncotarget 2015, 6, 16321–16340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chokshi, R.; Fruasaha, P.; Kozak, J.A. 2-aminoethyl diphenyl borinate (2-APB) inhibits TRPM7 channels through an intracellular acidification mechanism. Channels 2012, 6, 362–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rössig, A.; Hill, K.; Nörenberg, W.; Weidenbach, S.; Zierler, S.; Schaefer, M.; Gudermann, T.; Chubanov, V. Pharmacological agents selectively acting on the channel moieties of TRPM6 and TRPM7. Cell Calcium 2022, 106, 102640. [Google Scholar] [CrossRef]
- Kühn, F.; Kühn, C.; Lückhoff, A. Different Principles of ADP-Ribose-Mediated Activation and Opposite Roles of the NUDT9 Homology Domain in the TRPM2 Orthologs of Man and Sea Anemone. Front. Physiol. 2017, 8, 879. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Saotome, K.; McGoldrick, L.L.; Sobolevsky, A.I. Structural bases of TRP channel TRPV6 allosteric modulation by 2-APB. Nat. Commun. 2018, 9, 2465. [Google Scholar] [CrossRef]
- Niu, C.; Sun, X.; Hu, F.; Tang, X.; Wang, K. Molecular determinants for the chemical activation of the warmth-sensitive TRPV3 channel by the natural monoterpenoid carvacrol. J. Biol. Chem. 2022, 298, 101706. [Google Scholar] [CrossRef]
- Oancea, E.; Wolfe, J.T.; Clapham, D.E. Functional TRPM7 channels accumulate at the plasma membrane in response to fluid flow. Circ. Res. 2006, 98, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Everaerts, W.; Vriens, J.; Owsianik, G.; Appendino, G.; Voets, T.; De Ridder, D.; Nilius, B. Functional characterization of transient receptor potential channels in mouse urothelial cells. Am. J. Physiol. Ren. Physiol. 2010, 298, F692–F701. [Google Scholar] [CrossRef]
- Valencik, M.L.; Zhang, D.; Punske, B.; Hu, P.; McDonald, J.A.; Litwin, S.E. Integrin activation in the heart: A link between electrical and contractile dysfunction? Circ. Res. 2006, 99, 1403–1410. [Google Scholar] [CrossRef] [Green Version]
- Clark, K.; Middelbeek, J.; Lasonder, E.; Dulyaninova, N.G.; Morrice, N.A.; Ryazanov, A.G.; Bresnick, A.R.; Figdor, C.G.; van Leeuwen, F.N. TRPM7 regulates myosin IIA filament stability and protein localization by heavy chain phosphorylation. J. Mol. Biol. 2008, 378, 790–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, Z.; Inoue, K.; Sun, H.; Leng, T.; Feng, X.; Zhu, L.; Xiong, Z.G. TRPM7 regulates vascular endothelial cell adhesion and tube formation. Am. J. Physiol. Cell Physiol. 2015, 308, C308–C318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, L.T.; Liu, W.; Chen, H.C.; González-Pagán, O.; Habas, R.; Runnels, L.W. TRPM7 regulates polarized cell movements. Biochem. J. 2011, 434, 513–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laovitthayanggoon, S.; Henderson, C.J.; McCluskey, C.; MacDonald, M.; Tate, R.J.; Grant, M.H.; Currie, S. Cobalt Administration Causes Reduced Contractility with Parallel Increases in TRPC6 and TRPM7 Transporter Protein Expression in Adult Rat Hearts. Cardiovasc. Toxicol. 2019, 19, 276–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morillo, C.A.; Klein, G.J.; Jones, D.L.; Guiraudon, C.M. Chronic rapid atrial pacing. Structural, functional, and electrophysiological characteristics of a new model of sustained atrial fibrillation. Circulation 1995, 91, 1588–1595. [Google Scholar] [CrossRef]
- Allessie, M.; Ausm, J.; Schotten, U. Electrical, contractile and structural remodeling during atrial fibrillation. Cardiovasc. Res. 2002, 54, 230–246. [Google Scholar] [CrossRef]
- Borgers, M.; Thoné, F.; Wouters, L.; Ausma, J.; Shivalkar, B.; Flameng, W. Structural correlates of regional myocardial dysfunction in patients with critical coronary artery stenosis: Chronic hibernation? Cardiovasc. Pathol. 1993, 2, 237–245. [Google Scholar] [CrossRef]
- Dragún, M.; Gažová, A.; Kyselovič, J.; Hulman, M.; Máťuš, M. TRP Channels Expression Profile in Human End-Stage Heart Failure. Medicina 2019, 55, 380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.J.; Zhou, Y.M.; Tang, Y.H.; Cao, F. Increased expression of transient receptor potential melastatin 7 in mouse cardiac fibroblasts post myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi 2008, 36, 641–645. [Google Scholar]
- Zhong, H.; Wang, T.; Lian, G.; Xu, C.; Wang, H.; Xie, L. TRPM7 regulates angiotensin II-induced sinoatrial node fibrosis in sick sinus syndrome rats by mediating Smad signaling. Heart Vessels 2018, 33, 1094–1105. [Google Scholar] [CrossRef] [PubMed]
- Bensley, J.G.; De Matteo, R.; Harding, R.; Black, M.J. Three-dimentional direct measurement of cardiomyocytes volume, nuclearity, and ploidy in thick histological sections. Sci. Rep. 2016, 6, 23756. [Google Scholar] [CrossRef] [PubMed]
| Patient Data | SR | IHD | AF |
|---|---|---|---|
| Age range (years) | 29–79 | 54–87 | 40–80 |
| Mean age (years) ± SEM | 53.0 ± 11.76 | 65.9 ± 9.59 | 63.2 ± 11.11 |
| Female, n (%) | 3 (17.7) | 8 (26.7) | 6 (35.3) |
| Male, n (%) | 14 (82.4) | 22 (73.3) | 11 (64.7) |
| Total, n (%) | 17 (100) | 30 (100) | 17 (100) |
| Surgical intervention | |||
| Valve surgery, n (%) | 12 (70.6) | 0 (0) | 13 (76.5) |
| CABG surgery, n (%) | 0 (0) | 14 (46.7) | 2 (11.8) |
| CABG and Valve surgery, n (%) | 0 (0) | 11 (36.7) | 1 (5.9) |
| Transplantation, n (%) | 5 (29.4) | 5 (16.7) | 1 (5.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andriulė, I.; Pangonytė, D.; Gwanyanya, A.; Karčiauskas, D.; Mubagwa, K.; Mačianskienė, R. Detection of TRPM6 and TRPM7 Proteins in Normal and Diseased Cardiac Atrial Tissue and Isolated Cardiomyocytes. Int. J. Mol. Sci. 2022, 23, 14860. https://doi.org/10.3390/ijms232314860
Andriulė I, Pangonytė D, Gwanyanya A, Karčiauskas D, Mubagwa K, Mačianskienė R. Detection of TRPM6 and TRPM7 Proteins in Normal and Diseased Cardiac Atrial Tissue and Isolated Cardiomyocytes. International Journal of Molecular Sciences. 2022; 23(23):14860. https://doi.org/10.3390/ijms232314860
Chicago/Turabian StyleAndriulė, Inga, Dalia Pangonytė, Asfree Gwanyanya, Dainius Karčiauskas, Kanigula Mubagwa, and Regina Mačianskienė. 2022. "Detection of TRPM6 and TRPM7 Proteins in Normal and Diseased Cardiac Atrial Tissue and Isolated Cardiomyocytes" International Journal of Molecular Sciences 23, no. 23: 14860. https://doi.org/10.3390/ijms232314860





