TGF-β/Smad Signalling Activation by HTRA1 Regulates the Function of Human Lens Epithelial Cells and Its Mechanism in Posterior Subcapsular Congenital Cataract
Abstract
1. Introduction
2. Results
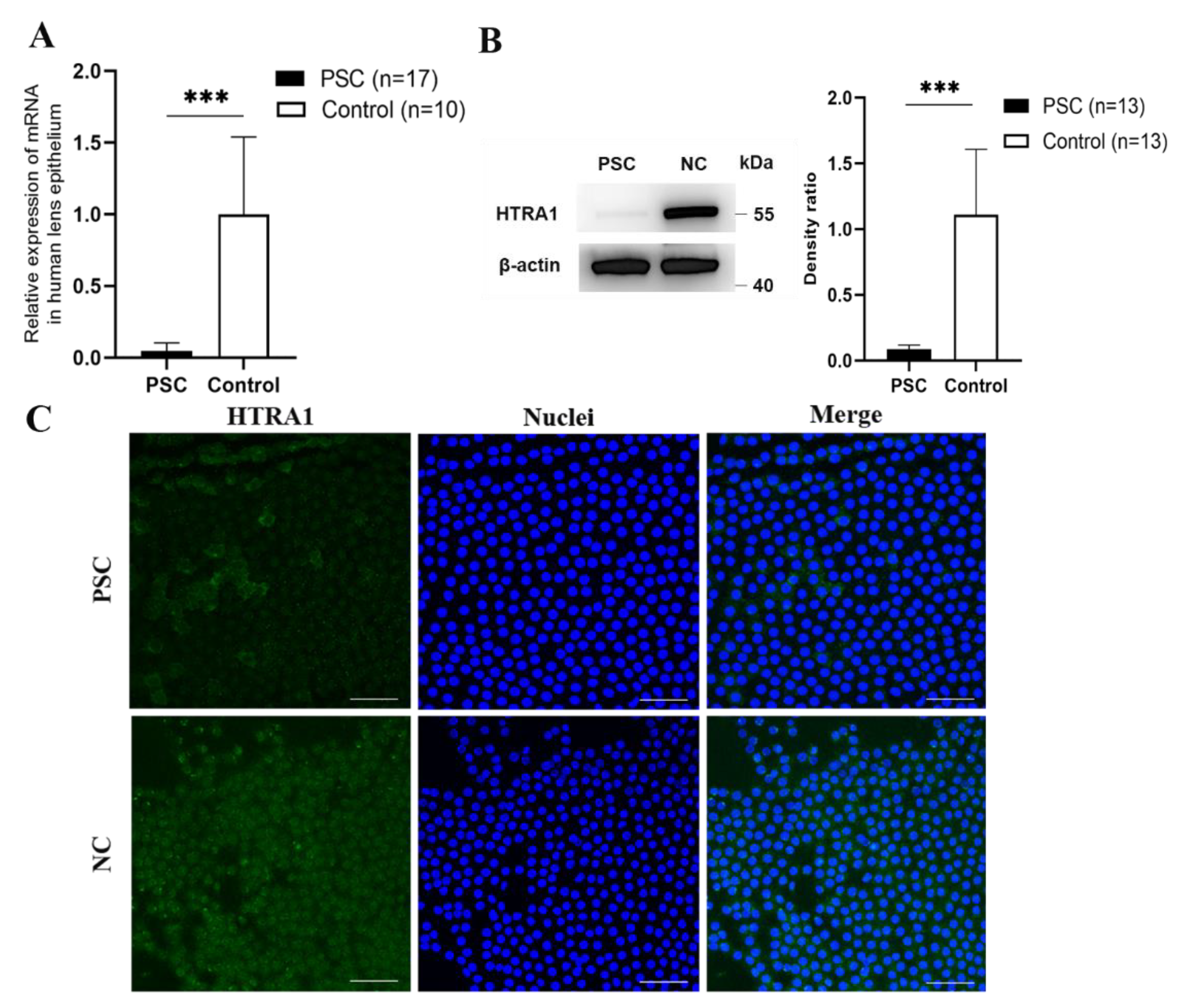
2.1. Downregulation of HTRA1 in Human Lens Epithelial Cells of Subjects with PSC
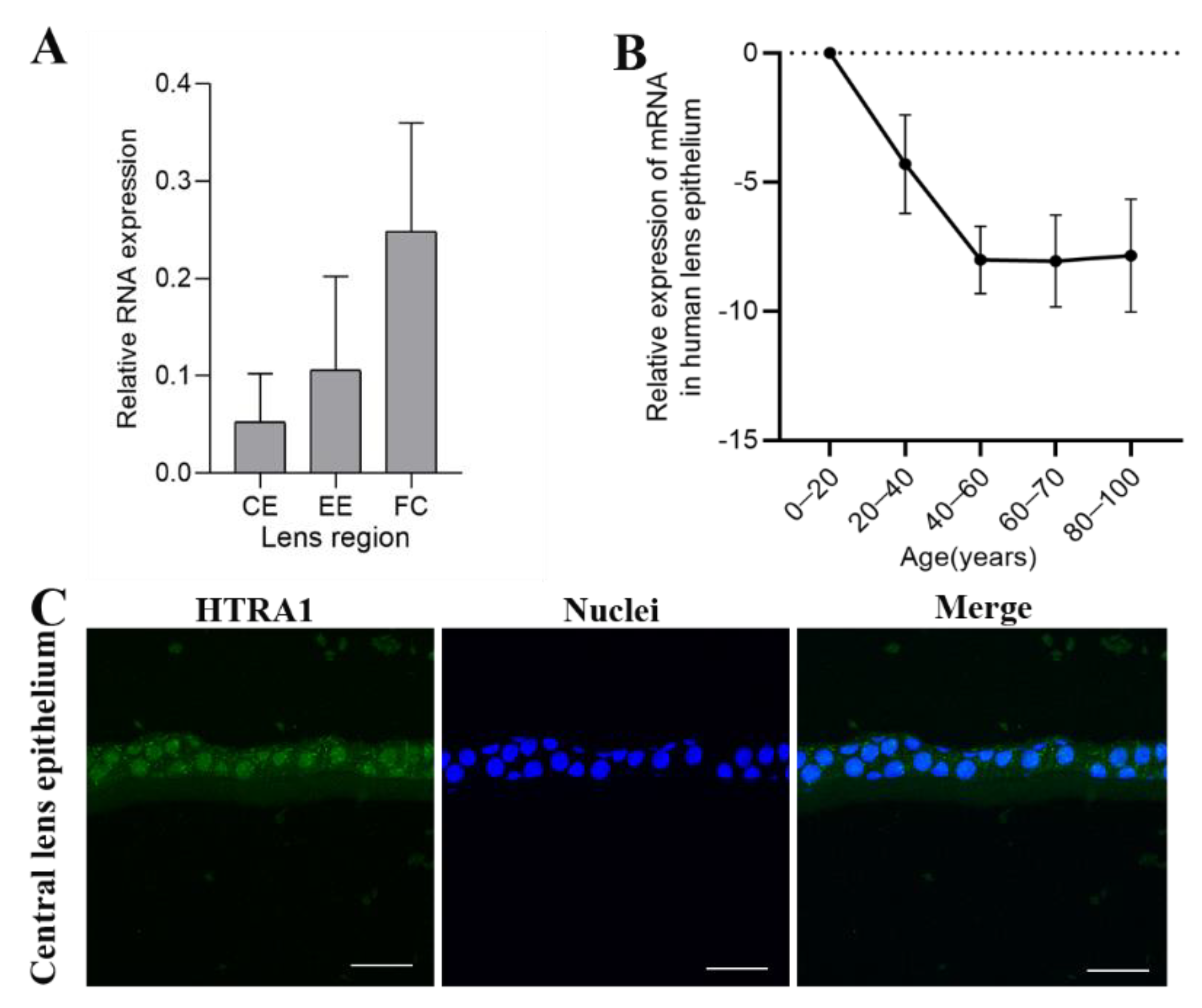
2.2. HTRA1 Expression Levels in the Normal Human Lens at Different Layers and in Different Age Groups
2.3. TGF-β/Smad Signalling Pathway Was Activated in HLECs of PSC Patients
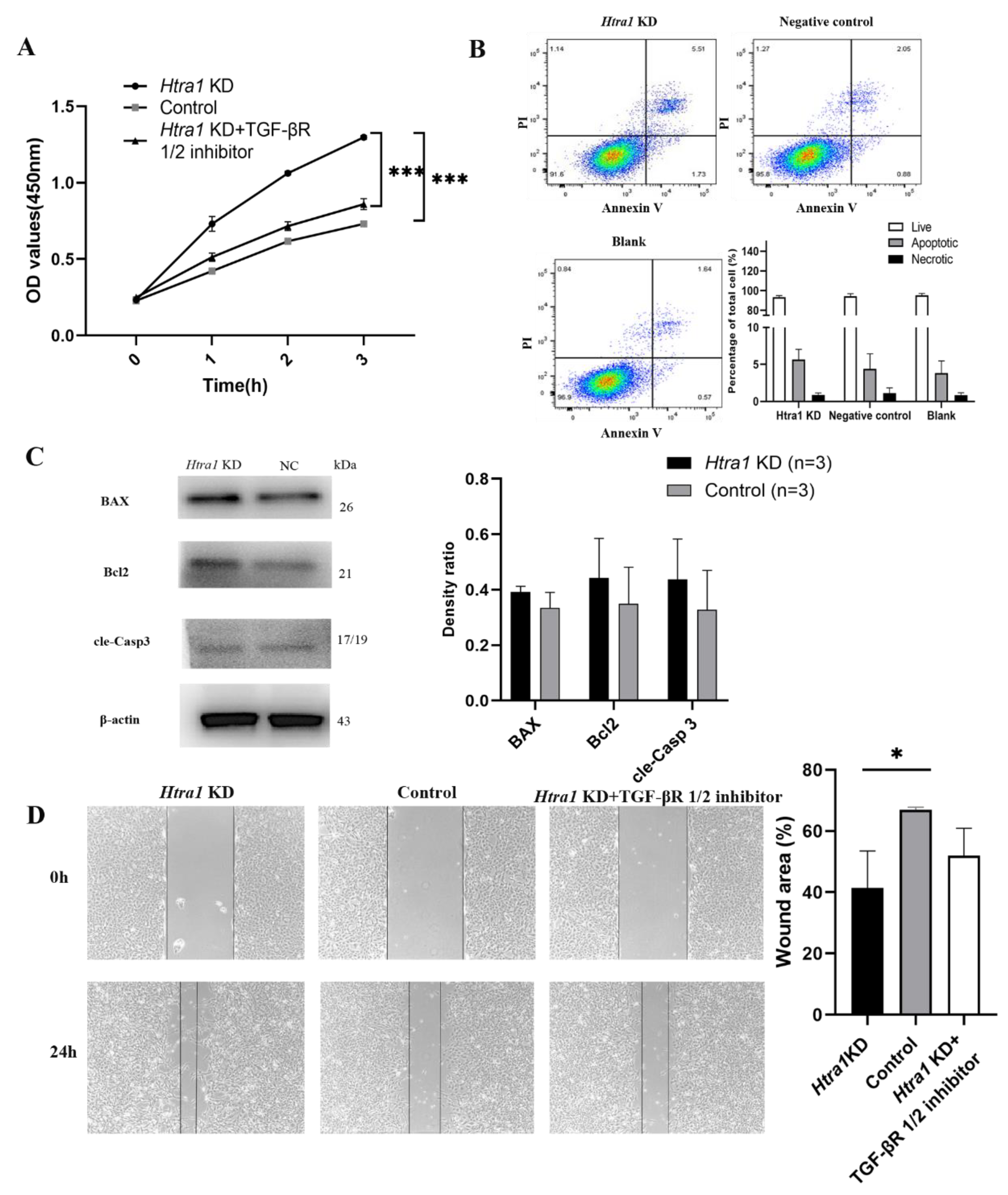
2.4. Downregulation of HTRA1 and Activated TGF-β/Smad Signalling Promotes HLEC Proliferation and Migration
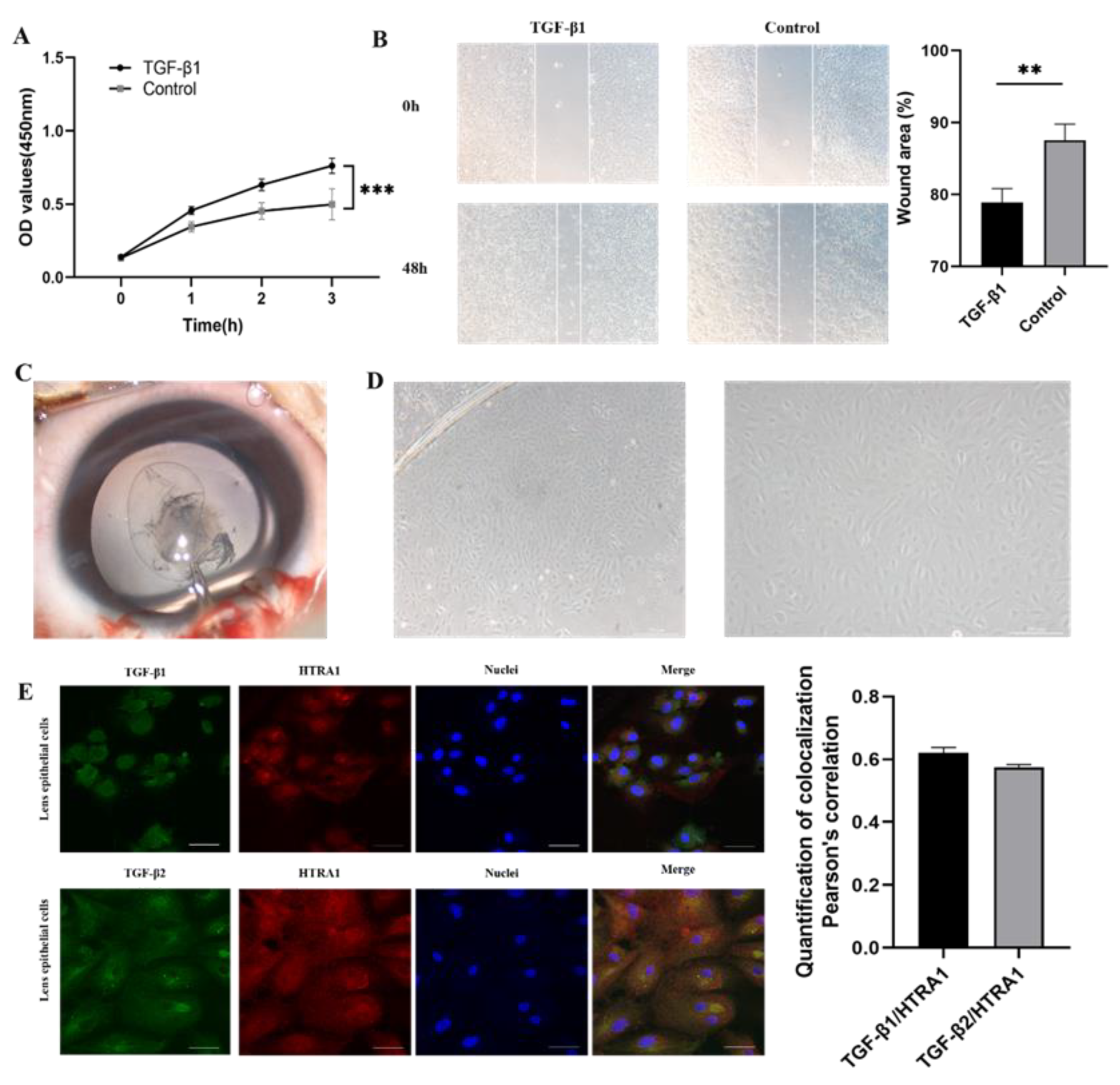
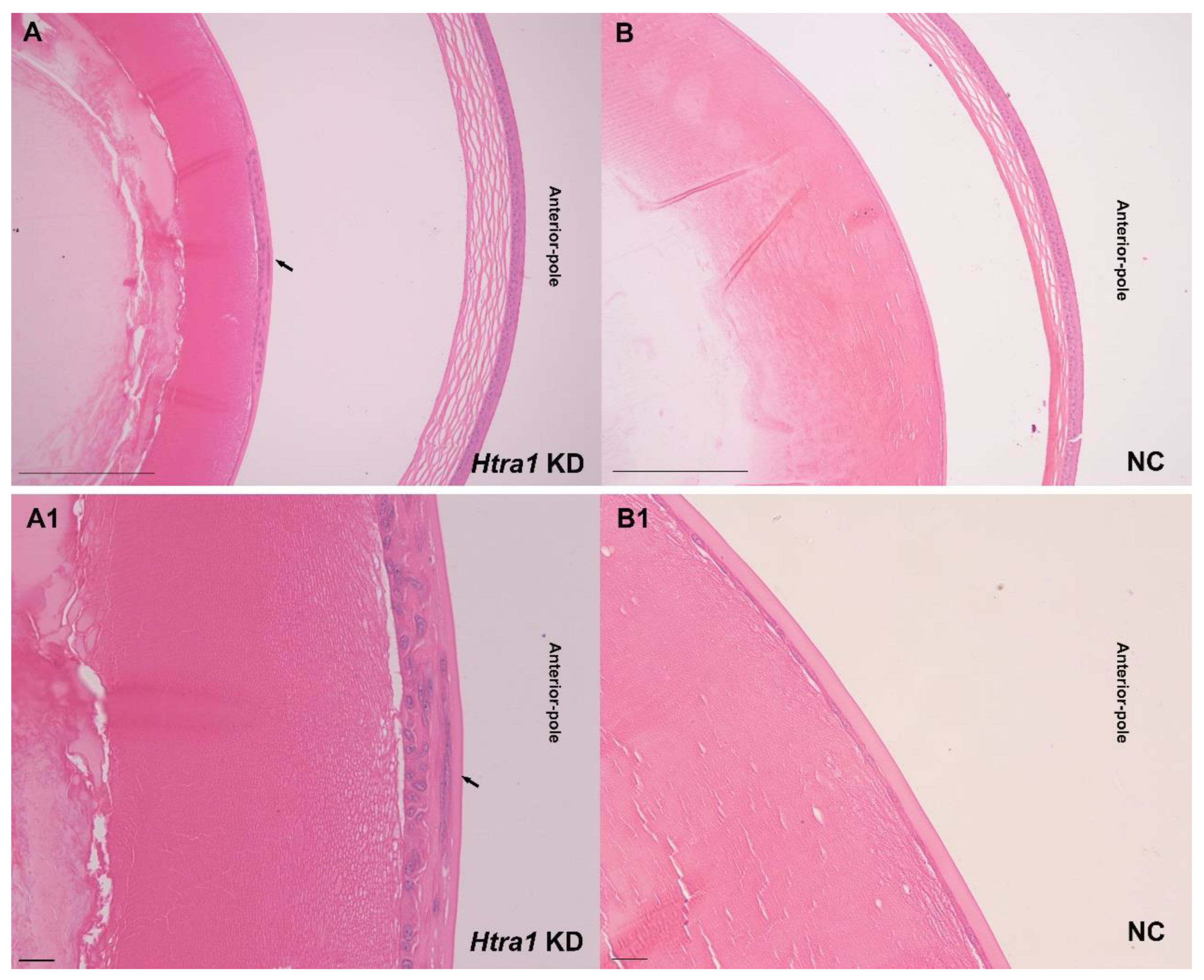
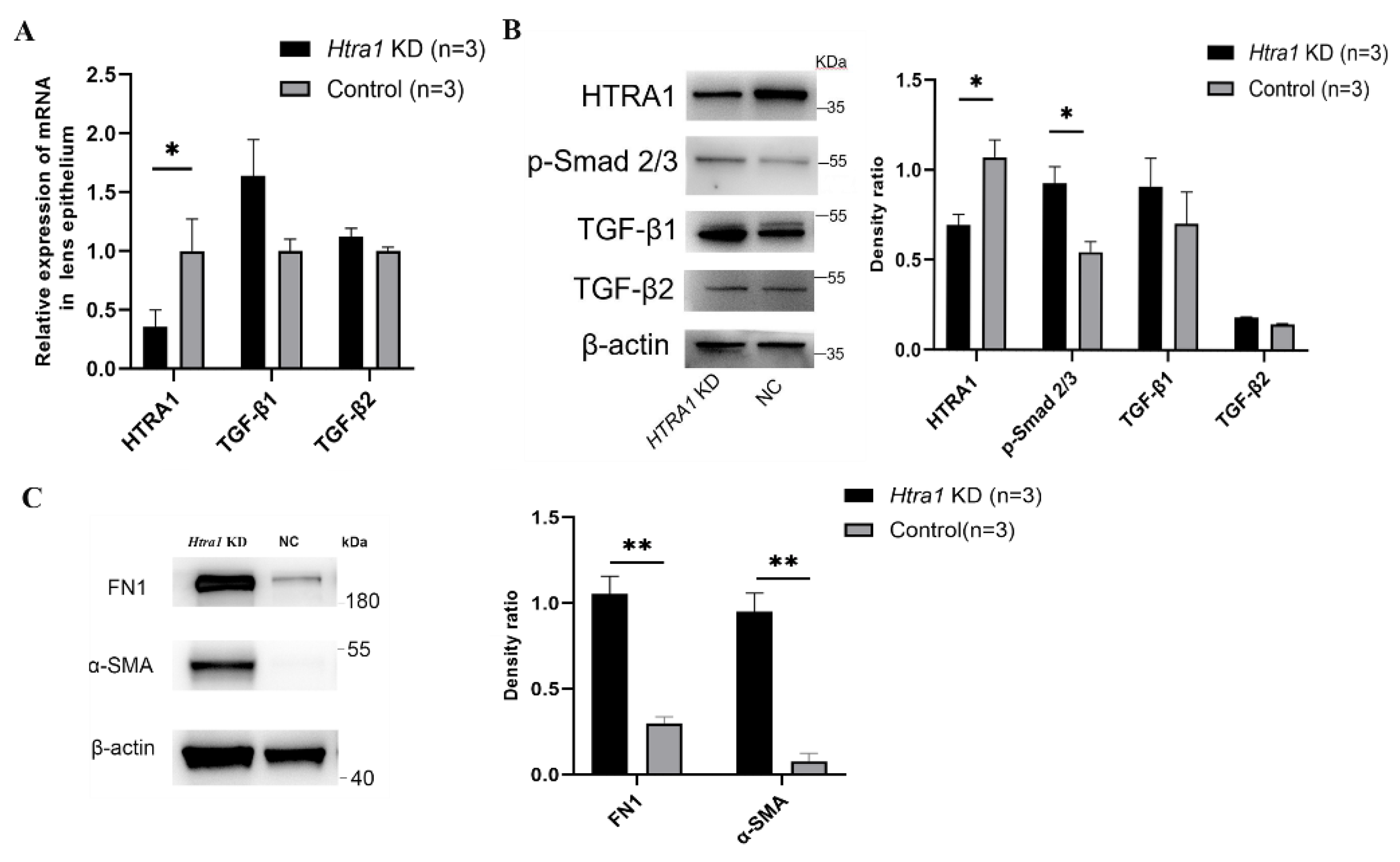
2.5. Downregulation of HTRA1 Directly Activated TGF-β/Smad Signalling in Primary Lens Epithelial Cells
3. Discussion
4. Materials and Methods
4.1. Patients and Tissue Sample Collection and Preservation
4.2. Construction of Recombinant Lentiviral Vectors and Adenoviral Vector
4.3. Cell Line and Primary HLEC Culture
4.4. Lentivirus Transfection
4.5. Mouse Model and Sample Acquirement
4.6. RT-qPCR
4.7. Western Blotting and Immunofluorescence Staining
4.8. Cell Proliferation Assay
4.9. Scratch Healing Assay
4.10. Annexin V/7-AAD Staining and Flow Cytometry
4.11. TGF-β1 and TGF-βR1/2 Inhibitor Treatment
4.12. Haematoxylin and Eosin Staining
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board and Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohammadpour, M.; Shaabani, A.; Sahraian, A.; Momenaei, B.; Tayebi, F.; Bayat, R.; Mirshahi, R. Updates on managements of pediatric cataract. J. Curr. Ophthalmol. 2019, 31, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Churchill, A.; Graw, J. Clinical and experimental advances in congenital and paediatric cataracts. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 1234–1249. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Yan, Y.; Yao, K. Molecular genetics of congenital cataracts. Exp. Eye Res. 2020, 191, 107872. [Google Scholar] [CrossRef]
- Santana, A.; Waiswo, M. The genetic and molecular basis of congenital cataract. Arq. Bras. Oftalmol. 2011, 74, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Rechsteiner, D.; Issler, L.; Koller, S.; Lang, E.; Bähr, L.; Feil, S.; Rüegger, C.M.; Kottke, R.; Toelle, S.P.; Zweifel, N.; et al. Genetic Analysis in a Swiss Cohort of Bilateral Congenital Cataract. JAMA Ophthalmol. 2021, 139, 691. [Google Scholar] [CrossRef] [PubMed]
- Messina-Baas, O.; Cuevas-Covarrubias, S.A. Inherited Congenital Cataract: A Guide to Suspect the Genetic Etiology in the Cataract Genesis. Mol. Syndromol. 2017, 8, 58–78. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Long, E.; Lin, H.; Liu, Y. Prevalence and epidemiological characteristics of congenital cataract: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 28564. [Google Scholar] [CrossRef] [PubMed]
- Sheeladevi, S.; Lawrenson, J.G.; Fielder, A.R.; Suttle, C.M. Global prevalence of childhood cataract: A systematic review. Eye 2016, 30, 1160–1169. [Google Scholar] [CrossRef]
- Bell, S.J.; Oluonye, N.; Harding, P.; Moosajee, M. Congenital cataract: A guide to genetic and clinical management. Ther. Adv. Rare Dis. 2020, 1, 1726979822. [Google Scholar] [CrossRef]
- Cao, K.; Wang, J.; Zhang, J.; Yusufu, M.; Jin, S.; Hou, S.; Zhu, G.; Wang, B.; Xiong, Y.; Li, J.; et al. Efficacy and safety of vitrectomy for congenital cataract surgery: A systematic review and meta-analysis based on randomized and controlled trials. Acta Ophthalmol. 2019, 97, 233–239. [Google Scholar] [CrossRef]
- Lin, X.; Li, H.; Yang, T.; Liu, X.; Fan, F.; Zhou, X.; Luo, Y. Transcriptomics Analysis of Lens from Patients with Posterior Subcapsular Congenital Cataract. Genes 2021, 12, 1904. [Google Scholar] [CrossRef]
- Tocharus, J.; Tsuchiya, A.; Kajikawa, M.; Ueta, Y.; Oka, C.; Kawaichi, M. Developmentally regulated expression of mouse HtrA3 and its role as an inhibitor of TGF-β signaling. Dev. Growth Differ. 2004, 46, 257–274. [Google Scholar] [CrossRef] [PubMed]
- Kakrana, A.; Yang, A.; Anand, D.; Djordjevic, D.; Ramachandruni, D.; Singh, A.; Huang, H.; Ho, J.; Lachke, S.A. iSyTE 2.0: A database for expression-based gene discovery in the eye. Nucleic Acids Res. 2018, 46, D875–D885. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, J.; Rothzerg, E.; Wu, X.; Xu, H.; Zhu, S.; Xu, J. Molecular structure and the role of high-temperature requirement protein 1 in skeletal disorders and cancers. Cell Proliferat. 2020, 53, e12746. [Google Scholar] [CrossRef] [PubMed]
- Tossetta, G.; Fantone, S.; Licini, C.; Marzioni, D.; Mattioli-Belmonte, M. The multifaced role of HtrA1 in the development of joint and skeletal disorders. Bone 2022, 157, 116350. [Google Scholar] [CrossRef]
- Launay, S.; Maubert, E.; Lebeurrier, N.; Tennstaedt, A.; Campioni, M.; Docagne, F.; Gabriel, C.; Dauphinot, L.; Potier, M.C.; Ehrmann, M.; et al. HtrA1-dependent proteolysis of TGF-beta controls both neuronal maturation and developmental survival. Cell Death Differ. 2008, 15, 1408–1416. [Google Scholar] [CrossRef]
- Zhang, L.; Lim, S.L.; Du, H.; Zhang, M.; Kozak, I.; Hannum, G.; Wang, X.; Ouyang, H.; Hughes, G.; Zhao, L.; et al. High Temperature Requirement Factor A1 (HTRA1) Gene Regulates Angiogenesis through Transforming Growth Factor-β Family Member Growth Differentiation Factor 6. J. Biol. Chem. 2012, 287, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Tosi, G.M.; Caldi, E.; Neri, G.; Nuti, E.; Marigliani, D.; Baiocchi, S.; Traversi, C.; Cevenini, G.; Tarantello, A.; Fusco, F.; et al. HTRA1 and TGF-beta1 Concentrations in the Aqueous Humor of Patients with Neovascular Age-Related Macular Degeneration. Investig. Opthalmology Vis. Sci. 2017, 58, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Leung, L.; Saturno, G.; Viros, A.; Smith, D.; Di Leva, G.; Morrison, E.; Niculescu-Duvaz, D.; Lopes, F.; Johnson, L.; et al. Lysyl oxidase drives tumour progression by trapping EGF receptors at the cell surface. Nat. Commun. 2017, 8, 14909. [Google Scholar] [CrossRef]
- Hu, H.; Chen, D.; Wang, Y.; Feng, Y.; Cao, G.; Vaziri, N.D.; Zhao, Y. New insights into TGF-β/Smad signaling in tissue fibrosis. Chem.-Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef]
- Kubo, E.; Shibata, T.; Singh, D.; Sasaki, H. Roles of TGF-β and FGF Signals in the Lens: Tropomyosin Regulation for Posterior Capsule Opacity. Int. J. Mol. Sci. 2018, 19, 3093. [Google Scholar] [CrossRef] [PubMed]
- de Iongh, R.; Gordon-Thomson, C.; Chamberlain, C.G.; Hales, A.M.; Avoy, J.W.M.C. TGFβ Receptor Expression in Lens: Implications for Differentiation and Cataractogenesis. Exp. Eye Res. 2001, 72, 649–659. [Google Scholar] [CrossRef] [PubMed]
- de Iongh, R.U.; Lovicu, F.J.; Overbeek, P.A.; Schneider, M.D.; Joya, J.; Hardeman, E.D.; Mcavoy, J.W. Requirement for TGFbeta receptor signaling during terminal lens fiber differentiation. Development 2001, 128, 3995–4010. [Google Scholar] [CrossRef] [PubMed]
- Wernecke, L.; Keckeis, S.; Reichhart, N.; Strauß, O.; Salchow, D.J. Epithelial-Mesenchymal Transdifferentiation in Pediatric Lens Epithelial Cells. Investig. Opthalmology Vis. Sci. 2018, 59, 5785. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Guo, D.; Wu, Q.; Liu, D.; Bi, H. Zinc Chloride Inhibits Human Lens Epithelial Cell Migration and Proliferation Involved in TGF-β1 and TNF-α Signaling Pathways in HLE B-3 Cells. Biol. Trace Elem. Res. 2014, 159, 425–433. [Google Scholar] [CrossRef]
- Srinivasan, Y.; Lovicu, F.J.; Overbeek, P.A. Lens-specific expression of transforming growth factor beta1 in transgenic mice causes anterior subcapsular cataracts. J. Clin. Investig. 1998, 101, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Flügel-Koch, C.; Ohlmann, A.; Piatigorsky, J.; Tamm, E.R. Disruption of anterior segment development by TGF-β1 overexpression in the eyes of transgenic mice. Dev. Dynam. 2002, 225, 111–125. [Google Scholar] [CrossRef] [PubMed]
- Banh, A.; Deschamps, P.A.; Gauldie, J.; Overbeek, P.A.; Sivak, J.G.; West-Mays, J.A. Lens-Specific Expression of TGF-β Induces Anterior Subcapsular Cataract Formation in the Absence of Smad3. Investig. Opthalmology Vis. Sci. 2006, 47, 3450. [Google Scholar] [CrossRef]
- Baldi, A.; De Luca, A.; Morini, M.; Battista, T.; Felsani, A.; Baldi, F.; Catricalà, C.; Amantea, A.; Noonan, D.M.; Albini, A.; et al. The HtrA1 serine protease is down-regulated during human melanoma progression and represses growth of metastatic melanoma cells. Oncogene 2002, 21, 6684–6688. [Google Scholar] [CrossRef]
- Zurawa-Janicka, D.; Skorko-Glonek, J.; Lipinska, B. HtrA proteins as targets in therapy of cancer and other diseases. Expert. Opin. Ther. Targets 2010, 14, 665–679. [Google Scholar] [CrossRef]
- Wang, N.; Eckert, K.A.; Zomorrodi, A.R.; Xin, P.; Pan, W.; Shearer, D.A.; Weisz, J.; Maranus, C.D.; Clawson, G.A. Down-regulation of HtrA1 activates the epithelial-mesenchymal transition and ATM DNA damage response pathways. PLoS ONE 2012, 7, e39446. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.; Staub, J.; Hu, S.; Erickson-Johnson, M.R.; Couch, F.J.; Smith, D.I.; Crowl, R.M.; Kaufmann, S.H.; Shridhar, V. A candidate tumor suppressor HtrA1 is downregulated in ovarian cancer. Oncogene 2004, 23, 1636–1644. [Google Scholar] [CrossRef] [PubMed]
- Zurawa-Janicka, D.; Kobiela, J.; Galczynska, N.; Stefaniak, T.; Lipinska, B.; Lachinski, A.; Skorko-Glonek, J.; Narkiewicz, J.; Proczko-Markuszewska, M.; Sledzinski, Z. Changes in expression of human serine protease HtrA1, HtrA2 and HtrA3 genes in benign and malignant thyroid tumors. Oncol. Rep. 2012, 28, 1838–1844. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, M.; Cui, L.; Ren, Y.; Zhang, J.; Chen, J.; Jia, L.; Zhang, J.; Yang, J.; Chen, G.; et al. Characterization of a novel HDAC/RXR/HtrA1 signaling axis as a novel target to overcome cisplatin resistance in human non-small cell lung cancer. Mol. Cancer 2020, 19, 134. [Google Scholar] [CrossRef]
- Su, J.; Li, H. RAC1 overexpression promotes the proliferation, migration and epithelial-mesenchymal transition of lens epithelial cells. Int. J. Clin. Exp. Pathol. 2015, 8, 10760–10767. [Google Scholar]
- Chien, J.; Ota, T.; Aletti, G.; Shridhar, R.; Boccellino, M.; Quagliuolo, L.; Baldi, A.; Shridhar, V. Serine protease HtrA1 associates with microtubules and inhibits cell migration. Mol. Cell. Biol. 2009, 29, 4177–4187. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhu, H.; Cao, M.; Lu, C.; Bao, S.; Pan, Y. HtrA1 suppresses the growth of pancreatic cancer cells by modulating Notch-1 expression. Braz. J. Med. Biol. Res. 2019, 52, e7718. [Google Scholar] [CrossRef] [PubMed]
- Globus, O.; Evron, T.; Caspi, M.; Siman-Tov, R.; Rosin-Arbesfeld, R. High-Temperature Requirement A1 (Htra1)—A Novel Regulator of Canonical Wnt Signaling. Sci. Rep. 2017, 7, 17995. [Google Scholar] [CrossRef] [PubMed]
- Oka, C.; Tsujimoto, R.; Kajikawa, M.; Koshiba-Takeuchi, K.; Ina, J.; Yano, M.; Tsuchiya, A.; Ueta, Y.; Soma, A.; Kanda, H.; et al. HtrA1 serine protease inhibits signaling mediated by Tgfbeta family proteins. Development 2004, 131, 1041–1053. [Google Scholar] [CrossRef]
- Poulsen, E.T.; Nielsen, N.S.; Scavenius, C.; Mogensen, E.H.; Risør, M.W.; Runager, K.; Lukassen, M.V.; Rasmussen, C.B.; Christiansen, G.; Richner, M.; et al. The serine protease HtrA1 cleaves misfolded transforming growth factor β–induced protein (TGFBIp) and induces amyloid formation. J. Biol. Chem. 2019, 294, 11817–11828. [Google Scholar] [CrossRef]
- Truebestein, L.; Tennstaedt, A.; Mönig, T.; Krojer, T.; Canellas, F.; Kaiser, M.; Clausen, T.; Ehrmann, M. Substrate-induced remodeling of the active site regulates human HTRA1 activity. Nat. Struct. Mol. Biol. 2011, 18, 386–388. [Google Scholar] [CrossRef]
- Massagué, J.; Wotton, D. Transcriptional control by the TGF-beta/Smad signaling system. EMBO J. 2000, 19, 1745–1754. [Google Scholar] [CrossRef]
- Li, X.; Ban, G.; Al-Shameri, B.; He, X.; Liang, D.; Chen, W. High-temperature Requirement Protein A1 Regulates Odontoblastic Differentiation of Dental Pulp Cells via the Transforming Growth Factor Beta 1/Smad Signaling Pathway. J. Endodont. 2018, 44, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Robertson, J.V.; Nathu, Z.; Najjar, A.; Dwivedi, D.; Gauldie, J.; West-Mays, J.A. Adenoviral gene transfer of bioactive TGFbeta1 to the rodent eye as a novel model for anterior subcapsular cataract. Mol. Vis. 2007, 13, 457–469. [Google Scholar]
- Takahashi, K.; Igarashi, T.; Miyake, K.; Kobayashi, M.; Katakai, Y.; Hayashita-Kinoh, H.; Fujimoto, C.; Kameya, S.; Takahashi, H.; Okada, T. Amount of Green Fluorescent Protein in the Anterior Chamber after Intravitreal Injection of Triple-Mutated Self-Complementary AAV2 Vectors is Not Affected by Previous Vitrectomy Surgery. J. Nippon. Med. Sch. 2021, 88, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Krieglstein, K.; Strelau, J.; Schober, A.; Sullivan, A.; Unsicker, K. TGF-beta and the regulation of neuron survival and death. J. Physiol. Paris 2002, 96, 25–30. [Google Scholar] [CrossRef]
- Hori, K.; Matsuda, A.; Ebihara, N.; Imai, K.; Mori, K.; Funaki, T.; Watanabe, Y.; Nakatani, S.; Okada, K.; Matsuo, O.; et al. Involvement of plasminogen activator inhibitor-1 in the pathogenesis of atopic cataracts. Investig. Opthalmology Vis. Sci. 2012, 53, 1846–1851. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xiao, W.; Chen, W.; Liu, X.; Wu, M.; Bo, Q.; Luo, Y.; Ye, S.; Cao, Y.; Liu, Y. MicroRNA-26a and -26b inhibit lens fibrosis and cataract by negatively regulating Jagged-1/Notch signaling pathway. Cell Death Differ. 2017, 24, 1431–1442. [Google Scholar] [CrossRef]
- Thiery, J.P.; Acloque, H.; Huang, R.Y.; Nieto, M.A. Epithelial-mesenchymal transitions in development and disease. Cell 2009, 139, 871–890. [Google Scholar] [CrossRef]
- Lovicu, F.J.; Shin, E.H.; Mcavoy, J.W. Fibrosis in the lens. Sprouty regulation of TGFβ-signaling prevents lens EMT leading to cataract. Exp. Eye Res. 2016, 142, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Nathu, Z.; Dwivedi, D.J.; Reddan, J.R.; Sheardown, H.; Margetts, P.J.; West-Mays, J.A. Temporal changes in MMP mRNA expression in the lens epithelium during anterior subcapsular cataract formation. Exp. Eye Res. 2009, 88, 323–330. [Google Scholar] [CrossRef] [PubMed]
- de Iongh, R.U.; Wederell, E.; Lovicu, F.J.; Mcavoy, J.W. Transforming growth factor-beta-induced epithelial-mesenchymal transition in the lens: A model for cataract formation. Cells Tissues Organs 2005, 179, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.K.; Vaughan, D.E. PAI-1 in tissue fibrosis. J. Cell. Physiol. 2012, 227, 493–507. [Google Scholar] [CrossRef] [PubMed]
- Murwantoko; Yano, M.; Ueta, Y.; Murasaki, A.; Kanda, H.; Oka, C.; Kawaichi, M. Binding of proteins to the PDZ domain regulates proteolytic activity of HtrA1 serine protease. Biochem. J. 2004, 381, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Grau, S.; Richards, P.J.; Kerr, B.; Hughes, C.; Caterson, B.; Williams, A.S.; Junker, U.; Jones, S.A.; Clausen, T.; Ehrmann, M. The role of human HtrA1 in arthritic disease. J. Biol. Chem. 2006, 281, 6124–6129. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Han, X.; Zhao, Z.; Lu, Y.; Yang, J. Functional analysis of deleterious EPHA2 SNPs in lens epithelial cells. Mol. Vis. 2021, 27, 403–414. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Yang, T.; Liu, X.; Fan, F.; Zhou, X.; Li, H.; Luo, Y. TGF-β/Smad Signalling Activation by HTRA1 Regulates the Function of Human Lens Epithelial Cells and Its Mechanism in Posterior Subcapsular Congenital Cataract. Int. J. Mol. Sci. 2022, 23, 14431. https://doi.org/10.3390/ijms232214431
Lin X, Yang T, Liu X, Fan F, Zhou X, Li H, Luo Y. TGF-β/Smad Signalling Activation by HTRA1 Regulates the Function of Human Lens Epithelial Cells and Its Mechanism in Posterior Subcapsular Congenital Cataract. International Journal of Molecular Sciences. 2022; 23(22):14431. https://doi.org/10.3390/ijms232214431
Chicago/Turabian StyleLin, Xiaolei, Tianke Yang, Xin Liu, Fan Fan, Xiyue Zhou, Hongzhe Li, and Yi Luo. 2022. "TGF-β/Smad Signalling Activation by HTRA1 Regulates the Function of Human Lens Epithelial Cells and Its Mechanism in Posterior Subcapsular Congenital Cataract" International Journal of Molecular Sciences 23, no. 22: 14431. https://doi.org/10.3390/ijms232214431
APA StyleLin, X., Yang, T., Liu, X., Fan, F., Zhou, X., Li, H., & Luo, Y. (2022). TGF-β/Smad Signalling Activation by HTRA1 Regulates the Function of Human Lens Epithelial Cells and Its Mechanism in Posterior Subcapsular Congenital Cataract. International Journal of Molecular Sciences, 23(22), 14431. https://doi.org/10.3390/ijms232214431






