The Novel AT2 Receptor Agonist β-Pro7-AngIII Exerts Cardiac and Renal Anti-Fibrotic and Anti-Inflammatory Effects in High Salt-Fed Mice
Abstract
1. Introduction
2. Results
2.1. Blood Pressure and Body Weight
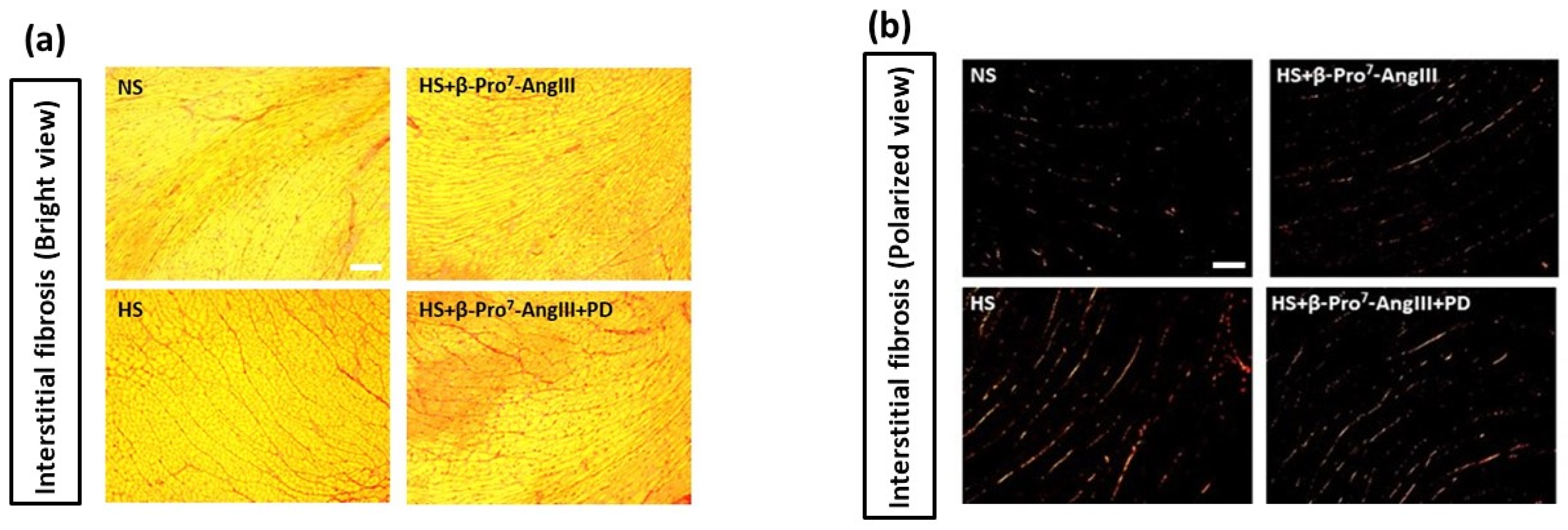
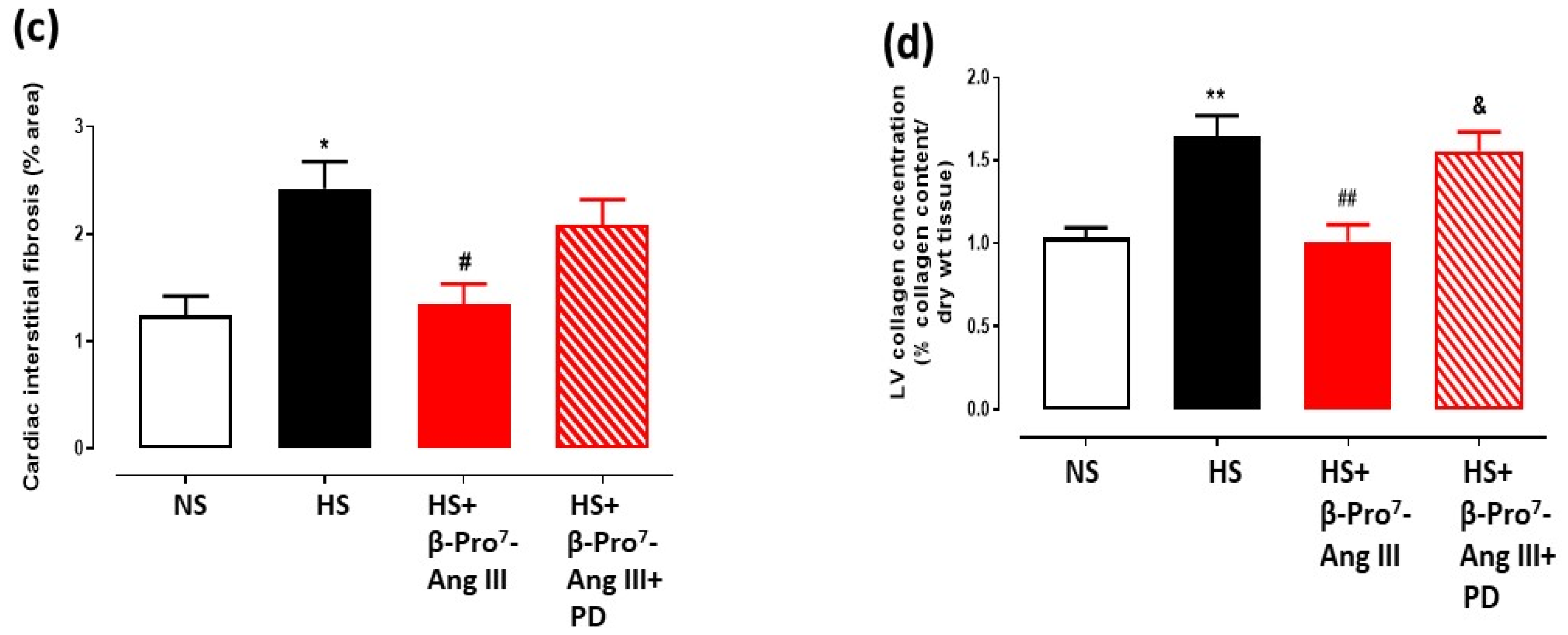
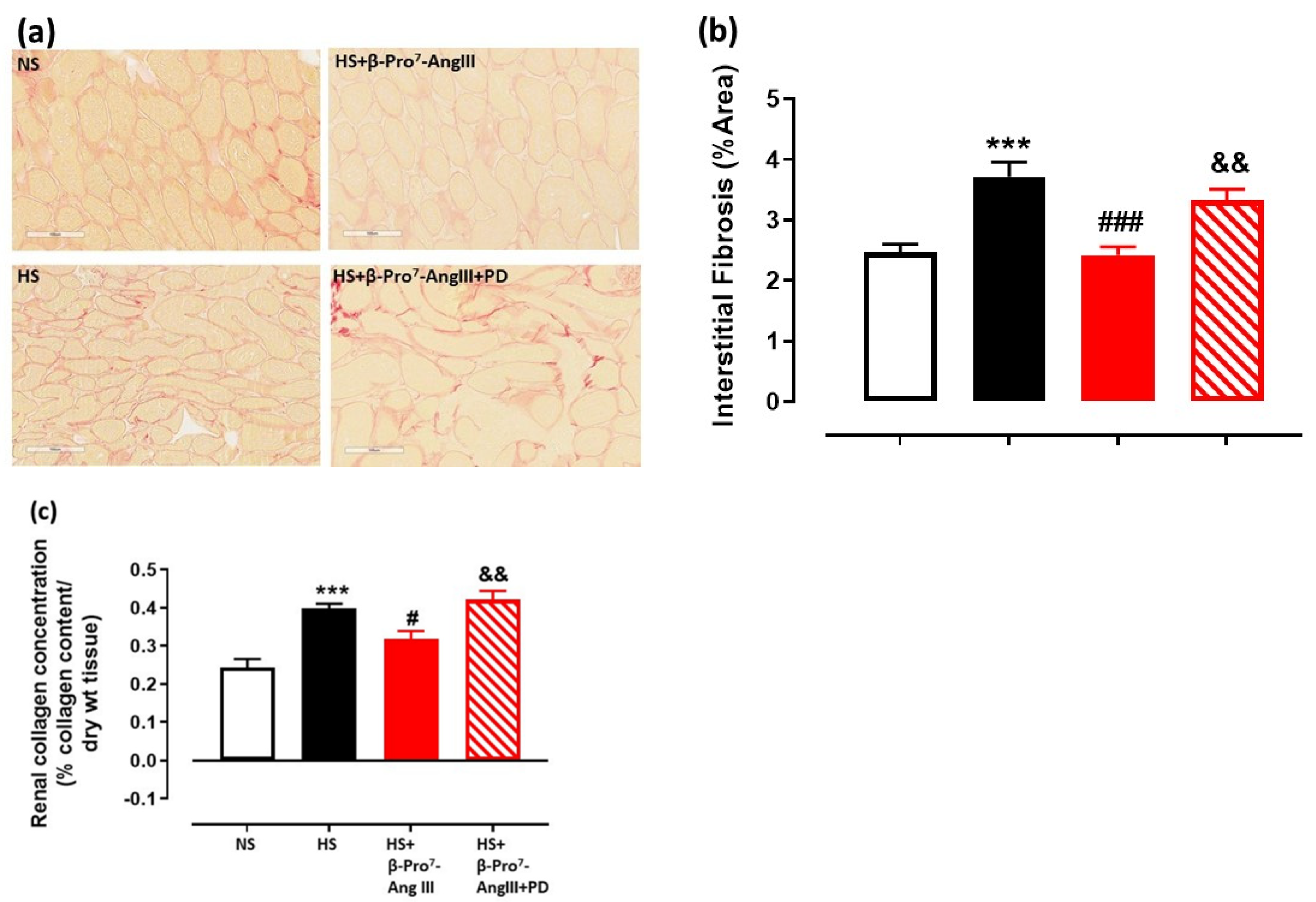
2.2. Cardiac and Renal Fibrosis
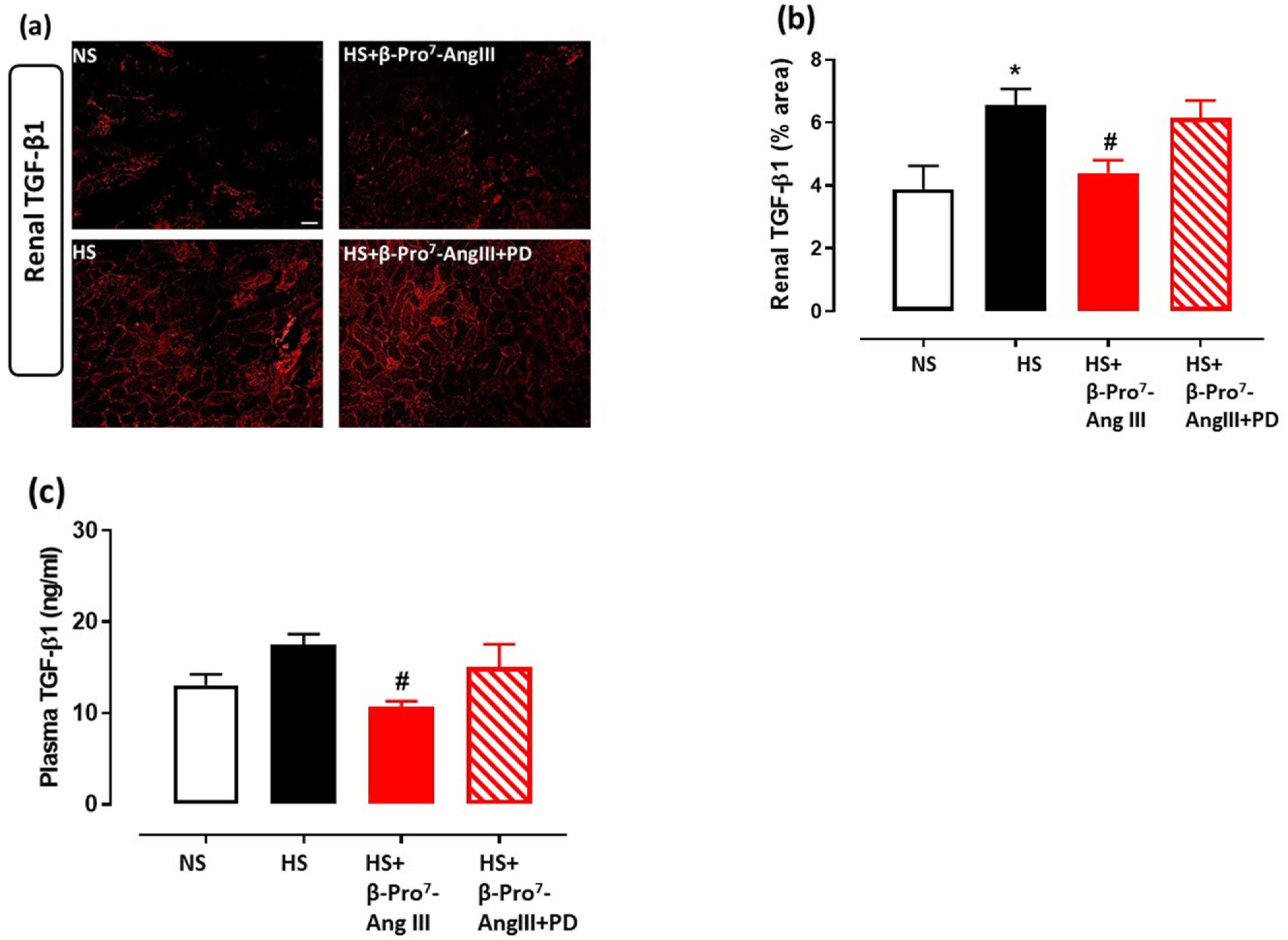
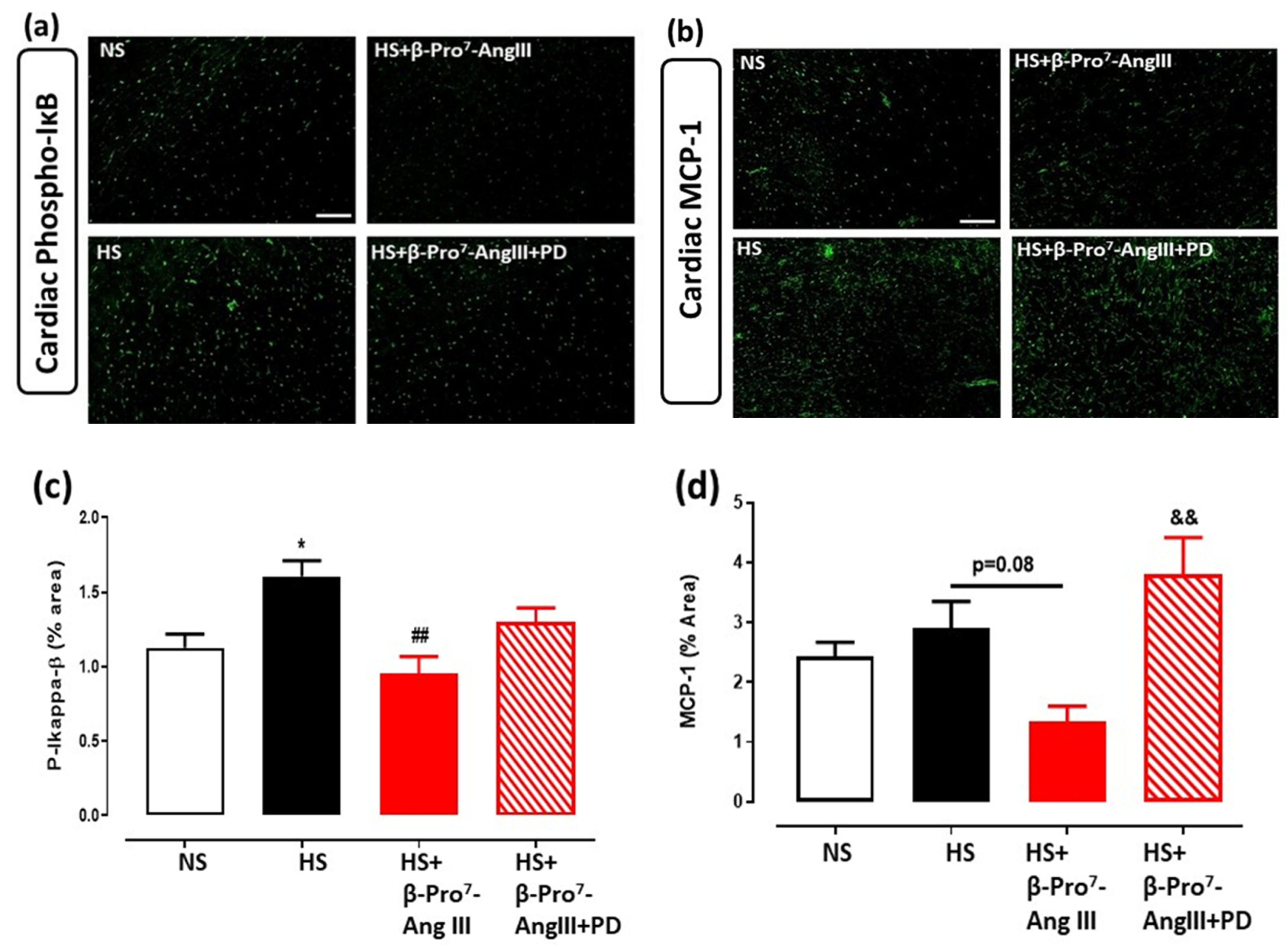
2.3. Cardiac and Renal Inflammation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Animals and Treatments
4.3. Blood Pressure and Body Weight Measurements
4.4. Analysis of Extracellular Matrix (ECM) and Inflammation
4.5. Plasma TGF-β1 Measurement by ELISA
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prabhu, S.D.; Frangogiannis, N.G. The Biological Basis for Cardiac Repair After Myocardial Infarction: From Inflammation to Fibrosis. Circ. Res. 2016, 119, 91–112. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Sillje, H.H.W.; de Boer, R.A. From Inflammation to Fibrosis-Molecular and Cellular Mechanisms of Myocardial Tissue Remodelling and Perspectives on Differential Treatment Opportunities. Curr. Heart Fail. Rep. 2017, 14, 235–250. [Google Scholar] [CrossRef]
- Bujak, M.; Frangogiannis, N.G. The role of TGF-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc. Res. 2007, 74, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Huynh, P.; Chai, Z. Transforming growth factor beta (TGFbeta) and related molecules in chronic kidney disease (CKD). Clin. Sci. 2019, 133, 287–313. [Google Scholar] [CrossRef]
- Tomasek, J.J.; Gabbiani, G.; Hinz, B.; Chaponnier, C.; Brown, R.A. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nat. Rev. Mol. Cell. Biol. 2002, 3, 349–363. [Google Scholar] [CrossRef]
- Schiller, M.; Javelaud, D.; Mauviel, A. TGF-beta-induced SMAD signaling and gene regulation: Consequences for extracellular matrix remodeling and wound healing. J. Dermatol. Sci. 2004, 35, 83–92. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Samuel, C.S.; Widdop, R.E. Preclinical rodent models of cardiac fibrosis. Br. J. Pharmacol. 2022, 179, 882–899. [Google Scholar] [CrossRef]
- Bavishi, C.; Bangalore, S.; Messerli, F.H. Renin Angiotensin Aldosterone System Inhibitors in Hypertension: Is There Evidence for Benefit Independent of Blood Pressure Reduction? Prog. Cardiovasc. Dis. 2016, 59, 253–261. [Google Scholar] [CrossRef]
- van Vark, L.C.; Bertrand, M.; Akkerhuis, K.M.; Brugts, J.J.; Fox, K.; Mourad, J.J.; Boersma, E. Angiotensin-converting enzyme inhibitors reduce mortality in hypertension: A meta-analysis of randomized clinical trials of renin-angiotensin-aldosterone system inhibitors involving 158,998 patients. Eur. Heart J. 2012, 33, 2088–2097. [Google Scholar] [CrossRef]
- Busche, S.; Gallinat, S.; Bohle, R.M.; Reinecke, A.; Seebeck, J.; Franke, F.; Fink, L.; Zhu, M.; Sumners, C.; Unger, T. Expression of angiotensin AT(1) and AT(2) receptors in adult rat cardiomyocytes after myocardial infarction. A single-cell reverse transcriptase-polymerase chain reaction study. Am. J. Pathol. 2000, 157, 605–611. [Google Scholar] [CrossRef]
- Nio, Y.; Matsubara, H.; Murasawa, S.; Kanasaki, M.; Inada, M. Regulation of gene transcription of angiotensin II receptor subtypes in myocardial infarction. J. Clin. Investig. 1995, 95, 46–54. [Google Scholar] [CrossRef]
- Ozono, R.; Wang, Z.Q.; Moore, A.F.; Inagami, T.; Siragy, H.M.; Carey, R.M. Expression of the subtype 2 angiotensin (AT2) receptor protein in rat kidney. Hypertension 1997, 30, 1238–1246. [Google Scholar] [CrossRef]
- Cao, Z.; Kelly, D.J.; Cox, A.; Casley, D.; Forbes, J.M.; Martinello, P.; Dean, R.; Gilbert, R.E.; Cooper, M.E. Angiotensin type 2 receptor is expressed in the adult rat kidney and promotes cellular proliferation and apoptosis. Kidney Int. 2000, 58, 2437–2451. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Esteban, V.; Suzuki, Y.; Ruperez, M.; Mezzano, S.; Ardiles, L.; Justo, P.; Ortiz, A.; Egido, J. Renal expression of angiotensin type 2 (AT2) receptors during kidney damage. Kidney Int. 2003, 64 (Suppl. S86), S21–S26. [Google Scholar] [CrossRef]
- Bautista, R.; Sanchez, A.; Hernandez, J.; Oyekan, A.; Escalante, B. Angiotensin II type AT(2) receptor mRNA expression and renal vasodilatation are increased in renal failure. Hypertension 2001, 38 Pt 2, 669–673. [Google Scholar] [CrossRef][Green Version]
- Esteban, V.; Lorenzo, O.; Ruperez, M.; Suzuki, Y.; Mezzano, S.; Blanco, J.; Kretzler, M.; Sugaya, T.; Egido, J.; Ruiz-Ortega, M. Angiotensin II, via AT1 and AT2 receptors and NF-kappaB pathway, regulates the inflammatory response in unilateral ureteral obstruction. J. Am. Soc. Nephrol. 2004, 15, 1514–1529. [Google Scholar] [CrossRef]
- Rogg, H.; de Gasparo, M.; Graedel, E.; Stulz, P.; Burkart, F.; Eberhard, M.; Erne, P. Angiotensin II-receptor subtypes in human atria and evidence for alterations in patients with cardiac dysfunction. Eur. Heart J. 1996, 17, 1112–1120. [Google Scholar] [CrossRef]
- Tsutsumi, Y.; Matsubara, H.; Ohkubo, N.; Mori, Y.; Nozawa, Y.; Murasawa, S.; Kijima, K.; Maruyama, K.; Masaki, H.; Moriguchi, Y.; et al. Angiotensin II type 2 receptor is upregulated in human heart with interstitial fibrosis, and cardiac fibroblasts are the major cell type for its expression. Circ. Res. 1998, 83, 1035–1046. [Google Scholar] [CrossRef]
- Goette, A.; Arndt, M.; Rocken, C.; Spiess, A.; Staack, T.; Geller, J.C.; Huth, C.; Ansorge, S.; Klein, H.U.; Lendeckel, U. Regulation of angiotensin II receptor subtypes during atrial fibrillation in humans. Circulation 2000, 101, 2678–2681. [Google Scholar] [CrossRef]
- Kaschina, E.; Namsolleck, P.; Unger, T. AT2 receptors in cardiovascular and renal diseases. Pharmacol. Res. 2017, 125 Pt A, 39–47. [Google Scholar] [CrossRef]
- Wang, Y.; Del Borgo, M.; Lee, H.W.; Baraldi, D.; Hirmiz, B.; Gaspari, T.A.; Denton, K.M.; Aguilar, M.I.; Samuel, C.S.; Widdop, R.E. Anti-fibrotic Potential of AT2 Receptor Agonists. Front. Pharmacol. 2017, 8, 564. [Google Scholar] [CrossRef] [PubMed]
- Sumners, C.; Peluso, A.A.; Haugaard, A.H.; Bertelsen, J.B.; Steckelings, U.M. Anti-fibrotic mechanisms of angiotensin AT2 -receptor stimulation. Acta Physiol. 2019, 227, e13280. [Google Scholar] [CrossRef] [PubMed]
- Bosnyak, S.; Jones, E.S.; Christopoulos, A.; Aguilar, M.I.; Thomas, W.G.; Widdop, R.E. Relative affinity of angiotensin peptides and novel ligands at AT1 and AT2 receptors. Clin. Sci. 2011, 121, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Steer, D.L.; Lew, R.A.; Perlmutter, P.; Smith, A.I.; Aguilar, M.I. Beta-amino acids: Versatile peptidomimetics. Curr. Med. Chem. 2002, 9, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Del Borgo, M.; Wang, Y.; Bosnyak, S.; Khan, M.; Walters, P.; Spizzo, I.; Perlmutter, P.; Hilliard, L.; Denton, K.; Aguilar, M.I.; et al. beta-Pro7Ang III is a novel highly selective angiotensin II type 2 receptor (AT2R) agonist, which acts as a vasodepressor agent via the AT2R in conscious spontaneously hypertensive rats. Clin. Sci. 2015, 129, 505–513. [Google Scholar] [CrossRef]
- Hilliard Krause, L.M.; Kemp, B.A.; Tan, A.S.J.; Jones, E.S.; Del Borgo, M.P.; Aguilar, M.I.; Denton, K.M.; Carey, R.M.; Widdop, R.E. Renal functional effects of the highly selective AT2R agonist, beta-Pro7 Ang III, in normotensive rats. Clin. Sci. 2020, 134, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Lattouf, R.; Younes, R.; Lutomski, D.; Naaman, N.; Godeau, G.; Senni, K.; Changotade, S. Picrosirius red staining: A useful tool to appraise collagen networks in normal and pathological tissues. J. Histochem. Cytochem. 2014, 62, 751–758. [Google Scholar] [CrossRef]
- Samuel, C.S.; Unemori, E.N.; Mookerjee, I.; Bathgate, R.A.; Layfield, S.L.; Mak, J.; Tregear, G.W.; Du, X.J. Relaxin modulates cardiac fibroblast proliferation, differentiation, and collagen production and reverses cardiac fibrosis in vivo. Endocrinology 2004, 145, 4125–4133. [Google Scholar] [CrossRef]
- Mack, M.; Yanagita, M. Origin of myofibroblasts and cellular events triggering fibrosis. Kidney Int. 2015, 87, 297–307. [Google Scholar] [CrossRef]
- Liu, Y. Cellular and molecular mechanisms of renal fibrosis. Nat. Rev. Nephrol. 2011, 7, 684–696. [Google Scholar] [CrossRef]
- Grande, M.T.; Lopez-Novoa, J.M. Fibroblast activation and myofibroblast generation in obstructive nephropathy. Nat. Rev. Nephrol. 2009, 5, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Leslie, K.O.; Taatjes, D.J.; Schwarz, J.; vonTurkovich, M.; Low, R.B. Cardiac myofibroblasts express alpha smooth muscle actin during right ventricular pressure overload in the rabbit. Am. J. Pathol. 1991, 139, 207–216. [Google Scholar] [PubMed]
- Desmouliere, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.; Mente, A.; Yusuf, S. Sodium Intake and Cardiovascular Health. Circ. Res. 2015, 116, 1046–1057. [Google Scholar] [CrossRef]
- Morrison, A.C.; Ness, R.B. Sodium intake and cardiovascular disease. Annu. Rev. Public Health 2011, 32, 71–90. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, D.N.; Katayama, I.A.; Oliveira, I.B.; Rosa, K.T.; Furukawa, L.N.; Coelho, M.S.; Casarini, D.E.; Heimann, J.C. Salt-induced cardiac hypertrophy and interstitial fibrosis are due to a blood pressure-independent mechanism in Wistar rats. J. Nutr. 2010, 140, 1742–1751. [Google Scholar] [CrossRef]
- Yu, H.C.; Burrell, L.M.; Black, M.J.; Wu, L.L.; Dilley, R.J.; Cooper, M.E.; Johnston, C.I. Salt induces myocardial and renal fibrosis in normotensive and hypertensive rats. Circulation 1998, 98, 2621–2628. [Google Scholar] [CrossRef]
- Lal, A.; Veinot, J.P.; Leenen, F.H. Prevention of high salt diet-induced cardiac hypertrophy and fibrosis by spironolactone. Am. J. Hypertens. 2003, 16, 319–323. [Google Scholar] [CrossRef]
- Grobe, J.L.; Mecca, A.P.; Mao, H.; Katovich, M.J. Chronic angiotensin-(1-7) prevents cardiac fibrosis in DOCA-salt model of hypertension. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H2417-23. [Google Scholar] [CrossRef]
- Wang, Y.; Han, L.; Shen, M.; Jones, E.S.; Spizzo, I.; Walton, S.L.; Denton, K.M.; Gaspari, T.A.; Samuel, C.S.; Widdop, R.E. Serelaxin and the AT2 Receptor Agonist CGP42112 Evoked a Similar, Nonadditive, Cardiac Antifibrotic Effect in High Salt-Fed Mice That Were Refractory to Candesartan Cilexetil. ACS Pharmacol. Transl. Sci. 2020, 3, 76–87. [Google Scholar] [CrossRef]
- Hijmans, R.S.; Shrestha, P.; Sarpong, K.A.; Yazdani, S.; El Masri, R.; de Jong, W.H.A.; Navis, G.; Vives, R.R.; van den Born, J. High sodium diet converts renal proteoglycans into pro-inflammatory mediators in rats. PLoS ONE 2017, 12, e0178940. [Google Scholar] [CrossRef] [PubMed]
- Endemann, D.H.; Touyz, R.M.; Iglarz, M.; Savoia, C.; Schiffrin, E.L. Eplerenone prevents salt-induced vascular remodeling and cardiac fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 2004, 43, 1252–1257. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.N.; Ali, Q.; Samuel, P.; Steckelings, U.M.; Hussain, T. Angiotensin II Type 2 Receptor and Receptor Mas Are Colocalized and Functionally Interdependent in Obese Zucker Rat Kidney. Hypertension 2017, 70, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Lauer, D.; Slavic, S.; Sommerfeld, M.; Thone-Reineke, C.; Sharkovska, Y.; Hallberg, A.; Dahlof, B.; Kintscher, U.; Unger, T.; Steckelings, U.M.; et al. Angiotensin type 2 receptor stimulation ameliorates left ventricular fibrosis and dysfunction via regulation of tissue inhibitor of matrix metalloproteinase 1/matrix metalloproteinase 9 axis and transforming growth factor beta1 in the rat heart. Hypertension 2014, 63, e60-7. [Google Scholar] [CrossRef]
- Kaschina, E.; Grzesiak, A.; Li, J.; Foryst-Ludwig, A.; Timm, M.; Rompe, F.; Sommerfeld, M.; Kemnitz, U.R.; Curato, C.; Namsolleck, P.; et al. Angiotensin II type 2 receptor stimulation: A novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction? Circulation 2008, 118, 2523–2532. [Google Scholar] [CrossRef]
- Rehman, A.; Leibowitz, A.; Yamamoto, N.; Rautureau, Y.; Paradis, P.; Schiffrin, E.L. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 2012, 59, 291–299. [Google Scholar] [CrossRef]
- Koulis, C.; Chow, B.S.; McKelvey, M.; Steckelings, U.M.; Unger, T.; Thallas-Bonke, V.; Thomas, M.C.; Cooper, M.E.; Jandeleit-Dahm, K.A.; Allen, T.J. AT2R agonist, compound 21, is reno-protective against type 1 diabetic nephropathy. Hypertension 2015, 65, 1073–1081. [Google Scholar] [CrossRef]
- Castoldi, G.; di Gioia, C.R.; Bombardi, C.; Maestroni, S.; Carletti, R.; Steckelings, U.M.; Dahlof, B.; Unger, T.; Zerbini, G.; Stella, A. Prevention of diabetic nephropathy by compound 21, selective agonist of angiotensin type 2 receptors, in Zucker diabetic fatty rats. Am. J. Physiol. Renal. Physiol. 2014, 307, F1123-31. [Google Scholar] [CrossRef]
- Gelosa, P.; Pignieri, A.; Fandriks, L.; de Gasparo, M.; Hallberg, A.; Banfi, C.; Castiglioni, L.; Turolo, L.; Guerrini, U.; Tremoli, E.; et al. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: Focus on renal damage. J. Hypertens. 2009, 27, 2444–2451. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Matavelli, L.C.; Huang, J.; Siragy, H.M. Angiotensin AT(2) receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension 2011, 57, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Chow, B.S.; Kocan, M.; Bosnyak, S.; Sarwar, M.; Wigg, B.; Jones, E.S.; Widdop, R.E.; Summers, R.J.; Bathgate, R.A.; Hewitson, T.D.; et al. Relaxin requires the angiotensin II type 2 receptor to abrogate renal interstitial fibrosis. Kidney Int. 2014, 86, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Kain, D.; Amit, U.; Yagil, C.; Landa, N.; Naftali-Shani, N.; Molotski, N.; Aviv, V.; Feinberg, M.S.; Goitein, O.; Kushnir, T.; et al. Macrophages dictate the progression and manifestation of hypertensive heart disease. Int. J. Cardiol. 2016, 203, 381–395. [Google Scholar] [CrossRef]
- Yang, G.H.; Zhou, X.; Ji, W.J.; Liu, J.X.; Sun, J.; Dong, Y.; Jiang, T.M.; Li, Y.M. VEGF-C-mediated cardiac lymphangiogenesis in high salt intake accelerated progression of left ventricular remodeling in spontaneously hypertensive rats. Clin. Exp. Hypertens. 2017, 39, 740–747. [Google Scholar] [CrossRef] [PubMed]
- Behr, T.M.; Willette, R.N.; Coatney, R.W.; Berova, M.; Angermann, C.E.; Anderson, K.; Sackner-Bernstein, J.D.; Barone, F.C. Eprosartan improves cardiac performance, reduces cardiac hypertrophy and mortality and downregulates myocardial monocyte chemoattractant protein-1 and inflammation in hypertensive heart disease. J. Hypertens. 2004, 22, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Dhande, I.; Ali, Q.; Hussain, T. Proximal tubule angiotensin AT2 receptors mediate an anti-inflammatory response via interleukin-10: Role in renoprotection in obese rats. Hypertension 2013, 61, 1218–1226. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Dhande, I.; Gray, E.A.; Ali, Q.; Hussain, T. Prevention of lipopolysaccharide-induced CD11b(+) immune cell infiltration in the kidney: Role of AT(2) receptors. Biosci. Rep. 2019, 39, BSR20190429. [Google Scholar] [CrossRef]
- Fatima, N.; Patel, S.; Hussain, T. Angiotensin AT2 Receptor is Anti-inflammatory and Reno-Protective in Lipopolysaccharide Mice Model: Role of IL-10. Front. Pharmacol. 2021, 12, 600163. [Google Scholar] [CrossRef]
- Jones, E.S.; Del Borgo, M.P.; Kirsch, J.F.; Clayton, D.; Bosnyak, S.; Welungoda, I.; Hausler, N.; Unabia, S.; Perlmutter, P.; Thomas, W.G.; et al. A single beta-amino acid substitution to angiotensin II confers AT2 receptor selectivity and vascular function. Hypertension 2011, 57, 570–576. [Google Scholar] [CrossRef]
- Chow, B.S.; Chew, E.G.; Zhao, C.; Bathgate, R.A.; Hewitson, T.D.; Samuel, C.S. Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: The additional involvement of iNOS. PLoS ONE 2012, 7, e42714. [Google Scholar] [CrossRef]
- Hossain, M.A.; Samuel, C.S.; Binder, C.; Hewitson, T.D.; Tregear, G.W.; Wade, J.D.; Bathgate, R.A. The chemically synthesized human relaxin-2 analog, B-R13/17K H2, is an RXFP1 antagonist. Amino Acids 2010, 39, 409–416. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yodgee, J.; Del Borgo, M.; Spizzo, I.; Nguyen, L.; Aguilar, M.-I.; Denton, K.M.; Samuel, C.S.; Widdop, R.E. The Novel AT2 Receptor Agonist β-Pro7-AngIII Exerts Cardiac and Renal Anti-Fibrotic and Anti-Inflammatory Effects in High Salt-Fed Mice. Int. J. Mol. Sci. 2022, 23, 14039. https://doi.org/10.3390/ijms232214039
Wang Y, Yodgee J, Del Borgo M, Spizzo I, Nguyen L, Aguilar M-I, Denton KM, Samuel CS, Widdop RE. The Novel AT2 Receptor Agonist β-Pro7-AngIII Exerts Cardiac and Renal Anti-Fibrotic and Anti-Inflammatory Effects in High Salt-Fed Mice. International Journal of Molecular Sciences. 2022; 23(22):14039. https://doi.org/10.3390/ijms232214039
Chicago/Turabian StyleWang, Yan, Jonathan Yodgee, Mark Del Borgo, Iresha Spizzo, Levi Nguyen, Marie-Isabel Aguilar, Kate M. Denton, Chrishan S. Samuel, and Robert E. Widdop. 2022. "The Novel AT2 Receptor Agonist β-Pro7-AngIII Exerts Cardiac and Renal Anti-Fibrotic and Anti-Inflammatory Effects in High Salt-Fed Mice" International Journal of Molecular Sciences 23, no. 22: 14039. https://doi.org/10.3390/ijms232214039
APA StyleWang, Y., Yodgee, J., Del Borgo, M., Spizzo, I., Nguyen, L., Aguilar, M.-I., Denton, K. M., Samuel, C. S., & Widdop, R. E. (2022). The Novel AT2 Receptor Agonist β-Pro7-AngIII Exerts Cardiac and Renal Anti-Fibrotic and Anti-Inflammatory Effects in High Salt-Fed Mice. International Journal of Molecular Sciences, 23(22), 14039. https://doi.org/10.3390/ijms232214039




