Tiron Has Negative Effects on Osteogenic Differentiation via Mitochondrial Dysfunction in Human Periosteum-Derived Cells
Abstract
1. Introduction
2. Results and Discussion
2.1. Treatment of hPDCs with Tiron
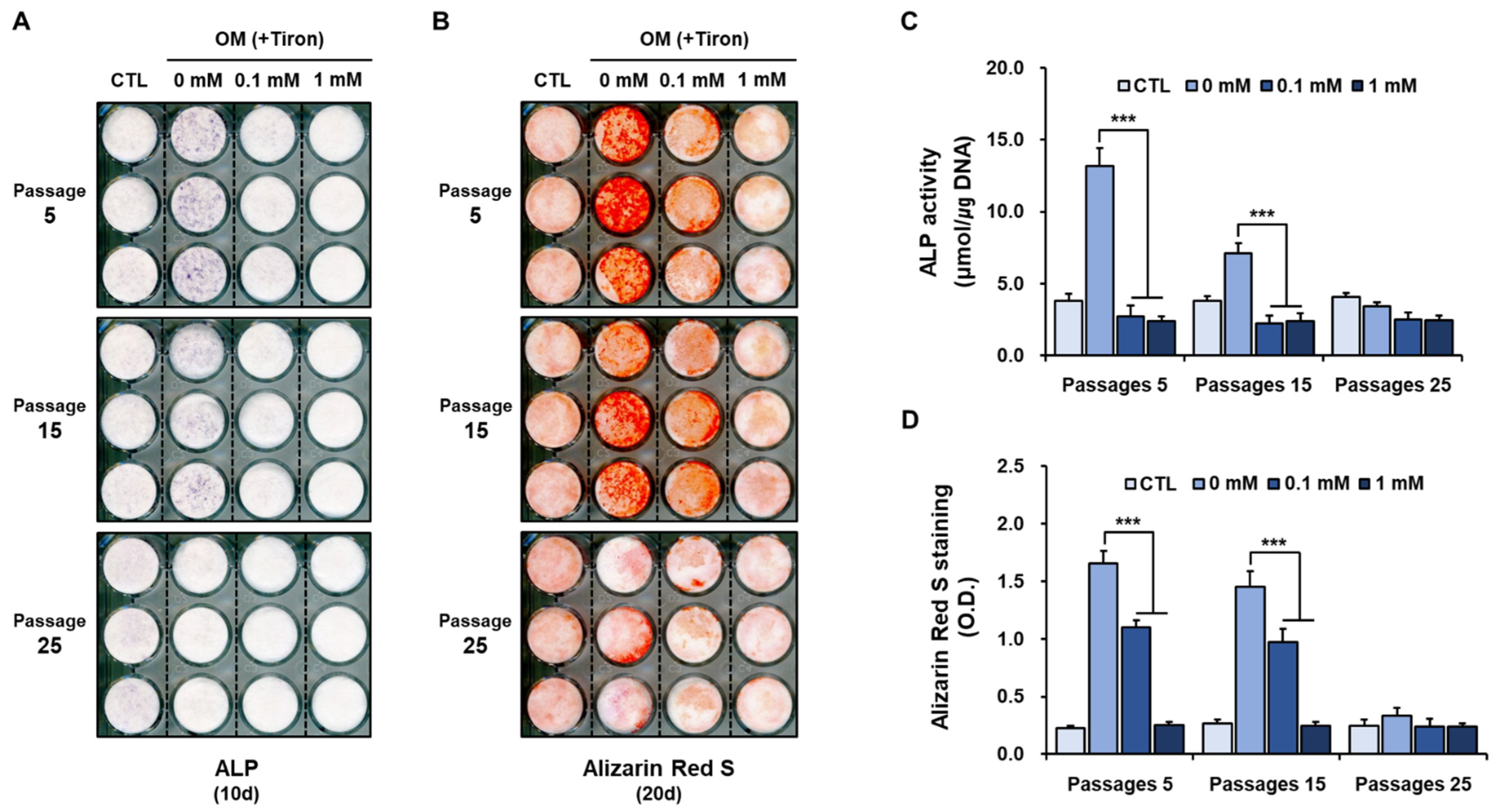
2.2. Effect of Tiron on Osteoblastic Differentiation of hPDCs
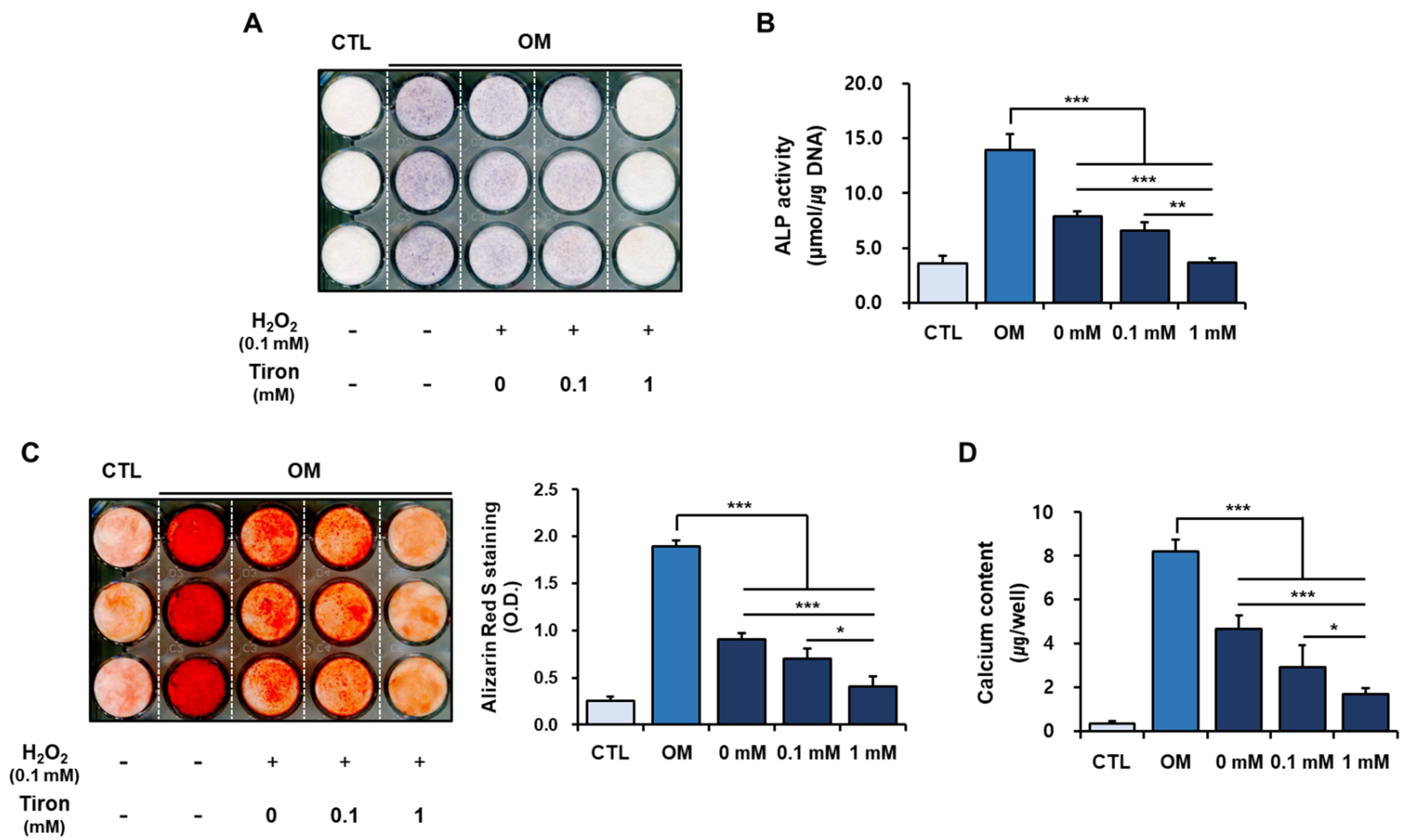
2.3. Effect of Tiron on the Osteoblastic Phenotypes of hPDCs Treated with H2O2
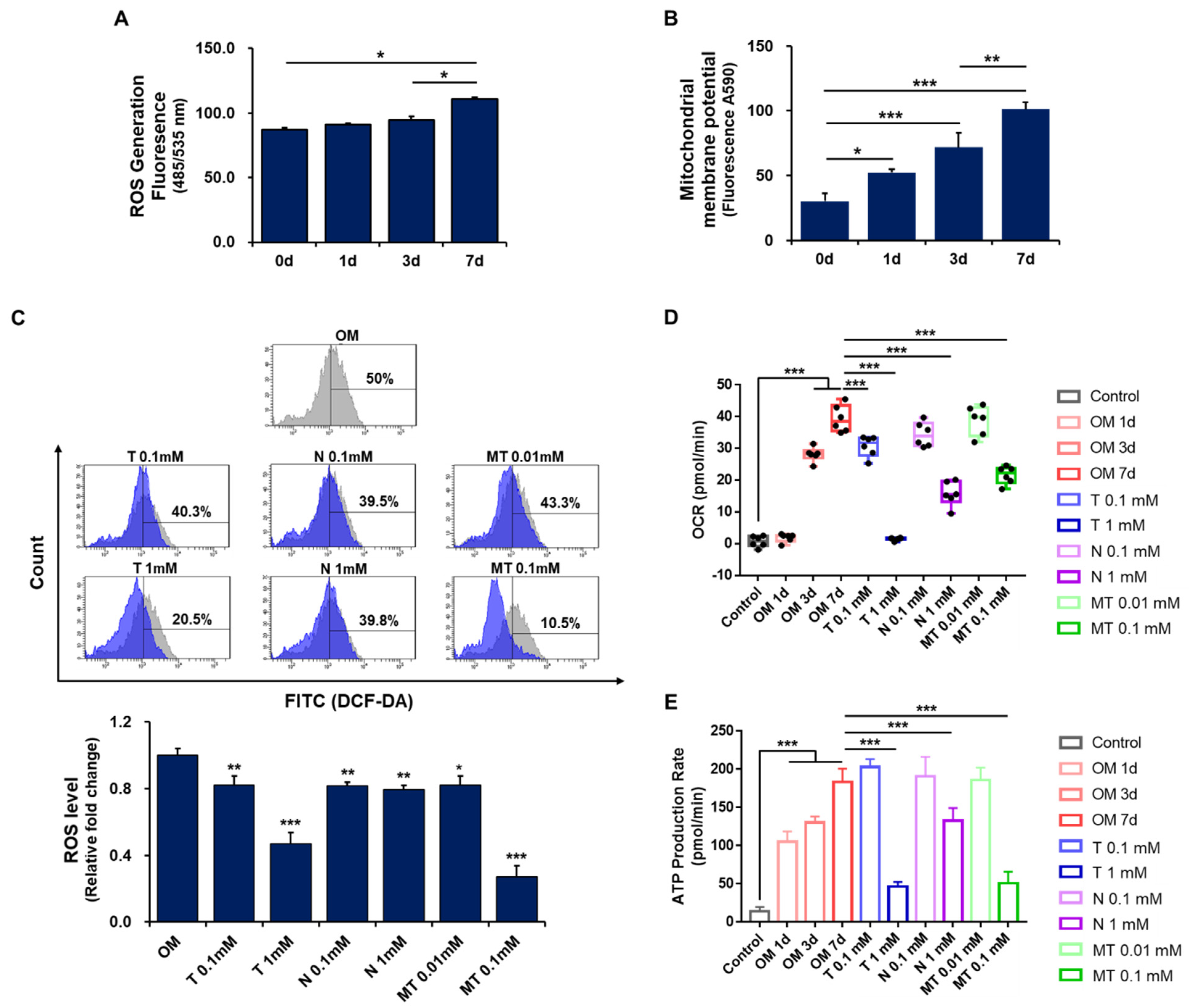
2.4. Effect of Tiron on ROS Generation in Osteoblast-Differentiating hPDCs
2.5. Effect of Antioxidants on Mitochondrial Metabolism in Osteoblast-Differentiating hPDCs
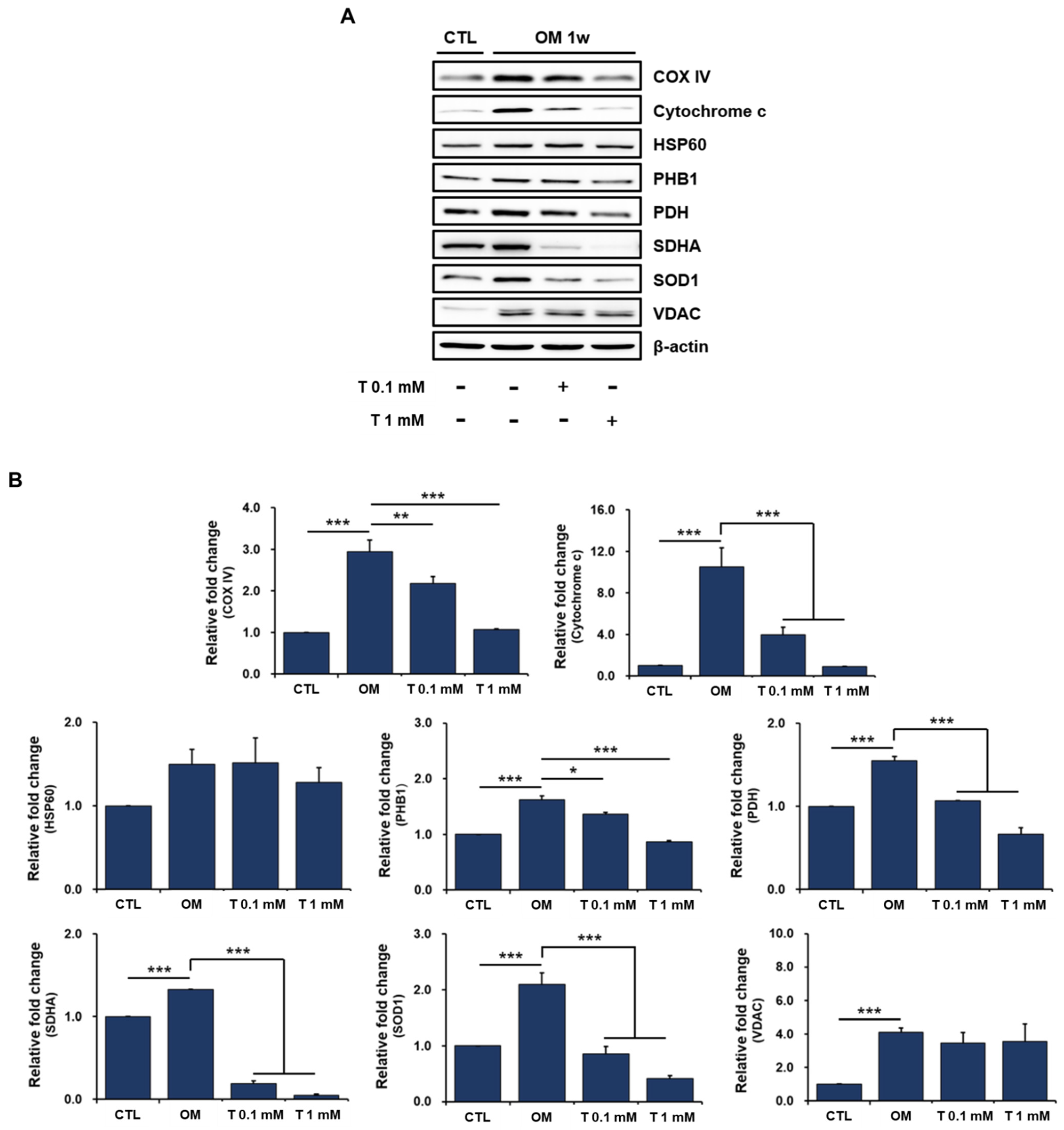
2.6. Effects of Tiron on Mitochondrial Proteins in Osteoblast-Differentiating hPDCs
3. Materials and Methods
3.1. Culture and Differentiation of hPDCs
3.2. Measurement of hPDC Viability in the Presence of Tiron
3.3. Measurement of Mitochondrial Membrane Potential in hPDCs Treated with Tiron
3.4. Senescence-Associated β-Galactosidase Activity and Staining
3.5. Evaluation of Osteoblastic Differentiation of hPDCs Treated with Tiron
3.6. Effects of Tiron on the Osteoblastic Phenotypes of hPDCs Treated with H2O2
3.7. Measurement of ROS in Osteoblast-Differentiating hPDCs
3.8. Measurement of Metabolic Flux and ATP Production in Osteoblast-Differentiating hPDCs
3.9. Western Blot Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pisoschi, A.M.; Pop, A.; Iordache, F.; Stanca, L.; Predoi, G.; Serban, A.I. Oxidative stress mitigation by antioxidants-an overview on their chemistry and influences on health status. Eur. J. Med. Chem. 2021, 209, 112891. [Google Scholar] [CrossRef] [PubMed]
- Akbari, B.; Baghaei-Yazdi, N.; Bahmaie, M.; Mahdavi Abhari, F. The role of plant-derived natural antioxidants in reduction of oxidative stress. BioFactors 2022, 48, 611–633. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.; Koliada, A.; Lushchak, O.; Castillo, M.J. Repurposing drugs to fight aging: The difficult path from bench to bedside. Med. Res. Rev. 2021, 41, 1676–1700. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- Dias, R.T.; Martin-Hidalgo, D.; Silva, B.M.; Oliveira, P.F.; Alves, M.G. Endogenous and exogenous antioxidants as a tool to ameliorate male infertility induced by reactive oxygen species. Antioxid. Redox Signal. 2020, 33, 767–785. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, F.; Malina, C.; Campbell, K.; Mormino, M.; Fuchs, J.; Vorontsov, E.; Gustafsson, C.M.; Nielsen, J. Absolute yeast mitochondrial proteome quantification reveals trade-off between biosynthesis and energy generation during diauxic shift. Proc. Natl. Acad. Sci. USA 2020, 117, 7524–7535. [Google Scholar] [CrossRef] [PubMed]
- Manoj, K.M. Debunking chemiosmosis and proposing murburn concept as the operative principle for cellular respiration. Biomed. Rev. 2018, 28, 31–48. [Google Scholar] [CrossRef]
- Sun, C.; Liu, X.; Wang, B.; Wang, Z.; Liu, Y.; Di, C.; Si, J.; Li, H.; Wu, Q.; Xu, D.; et al. Endocytosis-mediated mitochondrial transplantation: Transferring normal human astrocytic mitochondria into glioma cells rescues aerobic respiration and enhances radiosensitivity. Theranostics 2019, 9, 3595–3607. [Google Scholar] [CrossRef]
- Forbes, J.M.; Thorburn, D.R. Mitochondrial dysfunction in diabetic kidney disease. Nat. Rev. Nephrol. 2018, 14, 291–312. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, X.; Shen, Q.; Xing, D. Mitochondria-specific drug release and reactive oxygen species burst induced by polyprodrug nanoreactors can enhance chemotherapy. Nat. Commun. 2019, 10, 1–14. [Google Scholar] [CrossRef]
- Gao, J.; Zhou, Q.; Wu, D.; Chen, L. Mitochondrial iron metabolism and its role in diseases. Clin. Chim. Acta 2020, 513, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Che, J.; Yang, J.; Zhao, B.; Zhang, G.; Wang, L.; Peng, S.; Shang, P. The effect of abnormal iron metabolism on osteoporosis. Biol. Trace Elem. Res. 2019, 195, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; Bayir, H.; Belousov, V.; Chang, C.J.; Davies, K.J.A.; Davies, M.J.; Dick, T.P.; Finkel, T.; Forman, H.J.; Janssen-Heininger, Y.; et al. Guidelines for measuring reactive oxygen species and oxidative damage in cells and in vivo. Nat. Metab. 2022, 4, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Sun, Y.; Pi, C.; Wang, H.; Sun, H.; Yu, X.; Shi, Y.; He, X. Sirt3 attenuates oxidative stress damage and rescues cellular senescence in rat bone marrow mesenchymal stem cells by targeting superoxide dismutase 2. Front. Cell Dev. Biol. 2020, 8, 599376. [Google Scholar] [CrossRef]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef]
- Hu, C.; Zhao, L.; Peng, C.; Li, L. Regulation of the mitochondrial reactive oxygen species: Strategies to control mesenchymal stem cell fates ex vivo and in vivo. J. Cell. Mol. Med. 2018, 22, 5196–5207. [Google Scholar] [CrossRef]
- Wu, S.-H.; Yu, J.-H.; Liao, Y.-T.; Liu, K.-H.; Chiang, E.-R.; Chang, M.-C.; Wang, J.-P. Comparison of the infant and adult adipose-derived mesenchymal stem cells in proliferation, senescence, anti-oxidative ability and differentiation potential. Tissue Eng. Regen. Med. 2022, 19, 589–601. [Google Scholar] [CrossRef]
- van de Vyver, M.; Powrie, Y.S.L.; Smith, C. Targeting stem cells in chronic inflammatory diseases. Rev. New Drug Targets Age-Relat. Disord. 2021, 1286, 163–181. [Google Scholar] [CrossRef]
- Tan, L.; Cao, Z.; Chen, H.; Xie, Y.; Yu, L.; Fu, C.; Zhao, W.; Wang, Y. Curcumin reduces apoptosis and promotes osteogenesis of human periodontal ligament stem cells under oxidative stress in vitro and in vivo. Life Sci. 2021, 270, 119125. [Google Scholar] [CrossRef]
- Zheng, C.-X.; Sui, B.-D.; Qiu, X.-Y.; Hu, C.-H.; Jin, Y. Mitochondrial regulation of stem cells in bone homeostasis. Trends Mol. Med. 2019, 26, 89–104. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.-S.; Wu, R.-W.; Chen, Y.-S.; Ko, J.-Y.; Jahr, H.; Lian, W.-S. Biophysical modulation of the mitochondrial metabolism and redox in bone homeostasis and osteoporosis: How biophysics converts into bioenergetics. Antioxidants 2021, 10, 1394. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.E.; El-Haroun, H.; Kafafy, M.; Mohamed, D. Histological effect of high versus low dose isotretinoin and possible protective role of Tiron on skin of adult male albino rat. Egypt. J. Histol. 2019, 42, 453–466. [Google Scholar] [CrossRef]
- Mohamed, S.A.; El-Kashef, D.H.; Nader, M.A. Tiron alleviates MPTP-induced Parkinsonism in mice via activation of Keap-1/Nrf2 pathway. J. Biochem. Mol. Toxicol. 2021, 35, e22685. [Google Scholar] [CrossRef]
- Morgan, A.; Ibrahim, M.A.; Galal, M.K.; Ogaly, H.A.; Abd-Elsalam, R.M. Innovative perception on using Tiron to modulate the hepatotoxicity induced by titanium dioxide nanoparticles in male rats. Biomed. Pharmacother. 2018, 103, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Khaled, S.; Makled, M.N.; Nader, M.A. Tiron protects against nicotine-induced lung and liver injury through antioxidant and anti-inflammatory actions in rats in vivo. Life Sci. 2020, 260, 118426. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, S.Y.; Lee, H.-Y.; Lee, J.H.; Rho, G.-J.; Lee, H.-J.; Lee, H.-C.; Byun, J.-H.; Oh, S.H. Oxygen-releasing microparticles for cell survival and differentiation ability under hypoxia for effective bone regeneration. Biomacromolecules 2019, 20, 1087–1097. [Google Scholar] [CrossRef]
- Foster, N.C. An Investigation of Protocols for 2D and 3D Models of Human Embryonic Stem Cell Chondrogenic Differentiation and Tissue Engineering. Ph.D. Thesis, Keele University, Keele, UK, 2020. [Google Scholar]
- Fan, X.-L.; Zhang, Y.; Li, X.; Fu, Q.-L. Mechanisms underlying the protective effects of mesenchymal stem cell-based therapy. Cell. Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef]
- Song, K.; Yang, G.-M.; Han, J.; Gil, M.; Dayem, A.A.; Kim, K.; Lim, K.M.; Kang, G.-H.; Kim, S.; Jang, S.B. Modulation of osteogenic differentiation of adipose-derived stromal cells by co-treatment with 3, 4’-dihydroxyflavone, U0126, and n-acetyl cysteine. Int. J. Stem Cells 2022, 15, 334–345. [Google Scholar] [CrossRef]
- Zhou, T.; Yan, Y.; Zhao, C.; Xu, Y.; Wang, Q.; Xu, N. Resveratrol improves osteogenic differentiation of senescent bone mesenchymal stem cells through inhibiting endogenous reactive oxygen species production via AMPK activation. Redox Rep. 2019, 24, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, L.; Sun, Q.; Cai, H.; Tan, W.-S. Porous chitosan derivative scaffolds affect proliferation and osteogenesis of mesenchymal stem cell via reducing intracellular ROS. Carbohydr. Polym. 2020, 237, 116108. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yan, J.; He, F.; Zhong, D.; Yang, H.; Pei, M.; Luo, Z.-P. Mechanical stretch induces antioxidant responses and osteogenic differentiation in human mesenchymal stem cells through activation of the AMPK-SIRT1 signaling pathway. Free Radic. Biol. Med. 2018, 126, 187–201. [Google Scholar] [CrossRef] [PubMed]
- Elfawy, H.A.; Das, B. Crosstalk between mitochondrial dysfunction, oxidative stress, and age related neurodegenerative disease: Etiologies and therapeutic strategies. Life Sci. 2019, 218, 165–184. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Mills, K.; le Cessie, S.; Noordam, R.; van Heemst, D. Ageing, age-related diseases and oxidative stress: What to do next? Ageing Res. Rev. 2020, 57, 100982. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Liu, Y.; Wong, N.-K.; Xiao, J.; So, K.-F. Oxidative stress in stem cell aging. Cell Transplant. 2017, 26, 1483–1495. [Google Scholar] [CrossRef]
- Al-Azab, M.; Safi, M.; Idiiatullina, E.; Al-Shaebi, F.; Zaky, M.Y. Aging of mesenchymal stem cell: Machinery, markers, and strategies of fighting. Cell. Mol. Biol. Lett. 2022, 27, 1–40. [Google Scholar] [CrossRef]
- Park, B.-W.; Kang, E.-J.; Byun, J.-H.; Son, M.-G.; Kim, H.-J.; Hah, Y.-S.; Kim, T.-H.; Kumar, B.M.; Ock, S.-A.; Rho, G.-J. In vitro and in vivo osteogenesis of human mesenchymal stem cells derived from skin, bone marrow and dental follicle tissues. Differentiation 2012, 83, 249–259. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, B.-W.; Kang, Y.-H.; Byun, S.-H.; Hwang, S.-C.; Kim, D.R.; Woo, D.K.; Byun, J.-H. Lin28a enhances in vitro osteoblastic differentiation of human periosteum-derived cells. Cell Biochem. Funct. 2017, 35, 497–509. [Google Scholar] [CrossRef]
- Guo, X.; Wang, J.; Zou, W.; Wei, W.; Guan, X.; Liu, J. Exploring microenvironment strategies to delay mesenchymal stem cell senescence. Stem Cells Dev. 2022, 31, 38–52. [Google Scholar] [CrossRef]
- Liao, N.; Shi, Y.; Zhang, C.; Zheng, Y.; Wang, Y.; Zhao, B.; Zeng, Y.; Liu, X.; Liu, J. Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res. Ther. 2019, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ma, J.; Du, Y.; Miao, J.; Chen, N. Human amnion-derived mesenchymal stem cells protect human bone marrow mesenchymal stem cells against oxidative stress-mediated dysfunction via ERK1/2 MAPK signaling. Mol. Cells 2016, 39, 186. [Google Scholar] [PubMed]
- Honma, M.; Ikebuchi, Y.; Suzuki, H. Mechanisms of RANKL delivery to the osteoclast precursor cell surface. J. Bone Miner. Metab. 2020, 39, 27–33. [Google Scholar] [CrossRef] [PubMed]
- AlQranei, M.S.; Senbanjo, L.T.; Aljohani, H.; Hamza, T.; Chellaiah, M.A. Lipopolysaccharide- TLR-4 axis regulates osteoclastogenesis independent of RANKL/RANK signaling. BMC Immunol. 2021, 22, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Li, F.; Dang, L.; Liang, C.; Lu, A.; Zhang, G. RANKL/RANK System-based mechanism for breast cancer bone metastasis and related therapeutic strategies. Front. Cell Dev. Biol. 2020, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Nugud, A.; Sandeep, D.; El-Serafi, A.T. Two faces of the coin: Minireview for dissecting the role of reactive oxygen species in stem cell potency and lineage commitment. J. Adv. Res. 2018, 14, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.; Rocha-Ferreira, E.; Fleiss, B.; Nijboer, C.H.; Gressens, P.; Mallard, C.; Hagberg, H. Neuroprotection offered by mesenchymal stem cells in perinatal brain injury: Role of mitochondria, inflammation, and reactive oxygen species. J. Neurochem. 2021, 158, 59–73. [Google Scholar] [CrossRef]
- Mandal, S.; Gamit, N.; Varier, L.; Dharmarajan, A.; Warrier, S. Inhibition of breast cancer stem-like cells by a triterpenoid, ursolic acid, via activation of Wnt antagonist, sFRP4 and suppression of miRNA-499a-5p. Life Sci. 2021, 265, 118854. [Google Scholar] [CrossRef]
- Hu, Q.; Khanna, P.; Wong, B.S.E.; Heng, Z.S.L.; Subhramanyam, C.S.; Thanga, L.Z.; Tan, S.W.S.; Baeg, G.H. Oxidative stress promotes exit from the stem cell state and spontaneous neuronal differentiation. Oncotarget 2018, 9, 4223. [Google Scholar] [CrossRef]
- Sang, W.M.; Park, S.H.; Oh, M.K.; Wang, J.H. Outcomes of human umbilical cord blood-derived mesenchymal stem cells in enhancing tendon-graft healing in anterior cruciate ligment reconstruction: An exploratory study. Knee Surg. Relat. Res. 2021, 33, 1–10. [Google Scholar]
- Cavaliere, G.; Trinchese, G.; Penna, E.; Cimmino, F.; Pirozzi, C.; Lama, A.; Annunziata, C.; Catapano, A.; Mattace Raso, G.; Meli, R.; et al. High-fat diet induces neuroinflammation and mitochondrial impairment in mice cerebral cortex and synaptic fraction. Front. Cell. Neurosci. 2019, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Kasapoğlu, I.; Seli, E. Mitochondrial dysfunction and ovarian aging. Endocrinology 2020, 161. [Google Scholar] [CrossRef] [PubMed]
- Luciani, A.; Schumann, A.; Berquez, M.; Chen, Z.; Nieri, D.; Failli, M.; Debaix, H.; Festa, B.P.; Tokonami, N.; Raimondi, A. Impaired mitophagy links mitochondrial disease to epithelial stress in methylmalonyl-CoA mutase deficiency. Nat. Commun. 2020, 11, 1–21. [Google Scholar]
- Hu, C.; Fan, L.; Cen, P.; Chen, E.; Jiang, Z.; Li, L. Energy metabolism plays a critical role in stem cell maintenance and differentiation. Int. J. Mol. Sci. 2016, 17, 253. [Google Scholar] [CrossRef] [PubMed]
- Bahat, A.; Gross, A. Mitochondrial plasticity in cell fate regulation. J. Biol. Chem. 2019, 294, 13852–13863. [Google Scholar] [CrossRef]
- Payandeh, Z.; Tazehkand, A.P.; Barati, G.; Pouremamali, F.; Kahroba, H.; Baradaran, B.; Samadi, N. Role of Nrf2 and mitochondria in cancer stem cells; in carcinogenesis, tumor progression, and chemoresistance. Biochimie 2020, 179, 32–45. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; De Pinto, V.; Zweckstetter, M.; Raviv, Z.; Keinan, N.; Arbel, N. VDAC, a multi-functional mitochondrial protein regulating cell life and death. Mol. Asp. Med. 2010, 31, 227–285. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, G.; Lee, J.; Lee, Y.; Kim, J.-H. Secretome of stem cells: Roles of extracellular vesicles in diseases, stemness, differentiation, and reprogramming. Tissue Eng. Regen. Med. 2021, 19, 1–15. [Google Scholar] [CrossRef]
- Park, B.-W.; Hah, Y.-S.; Kim, D.R.; Kim, J.-R.; Byun, J.-H. Osteogenic phenotypes and mineralization of cultured human periosteal-derived cells. Arch. Oral Biol. 2007, 52, 983–989. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, J. Bone tissue regeneration—Application of mesenchymal stem cells and cellular and molecular mechanisms. Curr. Stem Cell Res. Ther. 2017, 12, 357–364. [Google Scholar] [CrossRef]
- Yoon, D.-K.; Park, J.-S.; Rho, G.-J.; Lee, H.-J.; Sung, I.-Y.; Son, J.-H.; Park, B.-W.; Kang, Y.-H.; Byun, S.-H.; Hwang, S.-C.; et al. The involvement of histone methylation in osteoblastic differentiation of human periosteum-derived cells cultured in vitro under hypoxic conditions. Cell Biochem. Funct. 2017, 35, 441–452. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-H.; Koh, E.-B.; Seo, Y.-J.; Oh, H.-S.; Won, J.-Y.; Hwang, S.-C.; Byun, J.-H. Tiron Has Negative Effects on Osteogenic Differentiation via Mitochondrial Dysfunction in Human Periosteum-Derived Cells. Int. J. Mol. Sci. 2022, 23, 14040. https://doi.org/10.3390/ijms232214040
Park J-H, Koh E-B, Seo Y-J, Oh H-S, Won J-Y, Hwang S-C, Byun J-H. Tiron Has Negative Effects on Osteogenic Differentiation via Mitochondrial Dysfunction in Human Periosteum-Derived Cells. International Journal of Molecular Sciences. 2022; 23(22):14040. https://doi.org/10.3390/ijms232214040
Chicago/Turabian StylePark, Jin-Ho, Eun-Byeol Koh, Young-Jin Seo, Hye-Seong Oh, Ju-Yeong Won, Sun-Chul Hwang, and June-Ho Byun. 2022. "Tiron Has Negative Effects on Osteogenic Differentiation via Mitochondrial Dysfunction in Human Periosteum-Derived Cells" International Journal of Molecular Sciences 23, no. 22: 14040. https://doi.org/10.3390/ijms232214040
APA StylePark, J.-H., Koh, E.-B., Seo, Y.-J., Oh, H.-S., Won, J.-Y., Hwang, S.-C., & Byun, J.-H. (2022). Tiron Has Negative Effects on Osteogenic Differentiation via Mitochondrial Dysfunction in Human Periosteum-Derived Cells. International Journal of Molecular Sciences, 23(22), 14040. https://doi.org/10.3390/ijms232214040




