Decay-Accelerating Factor Creates an Organ-Protective Phenotype after Hemorrhage in Conscious Rats
Abstract
1. Introduction
2. Results
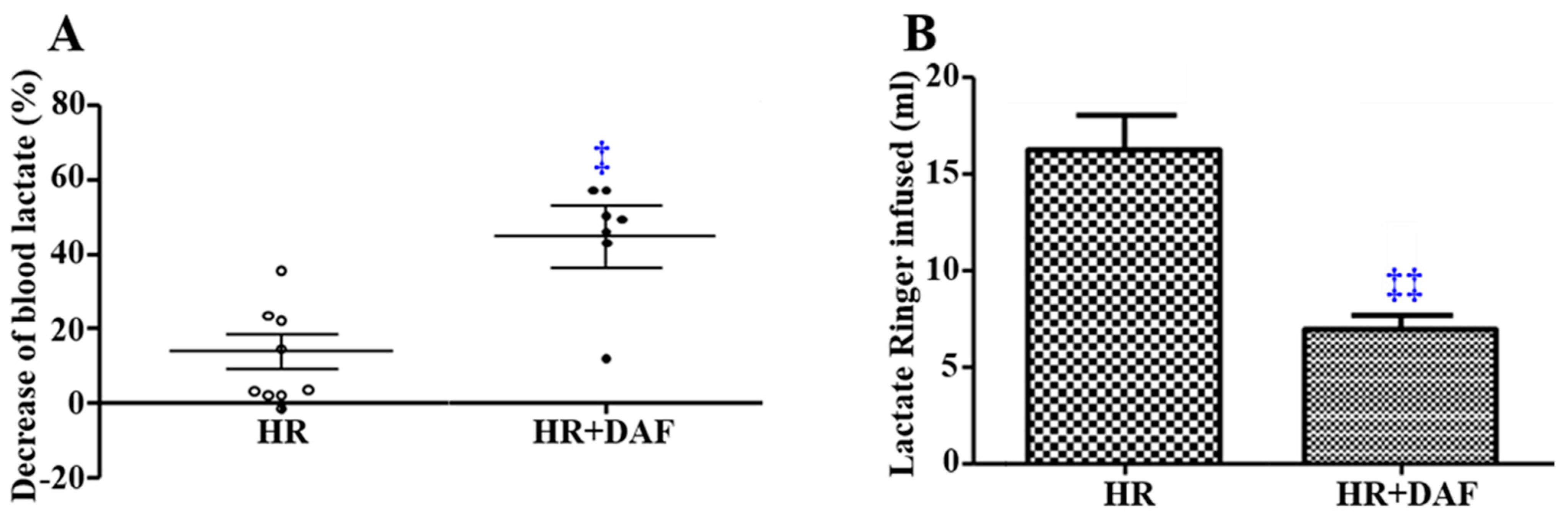
2.1. Effect of Recombinant Human DAF on Hemorrhage/Hypotensive Resuscitation-Induced Acidosis and Fluid Requirements
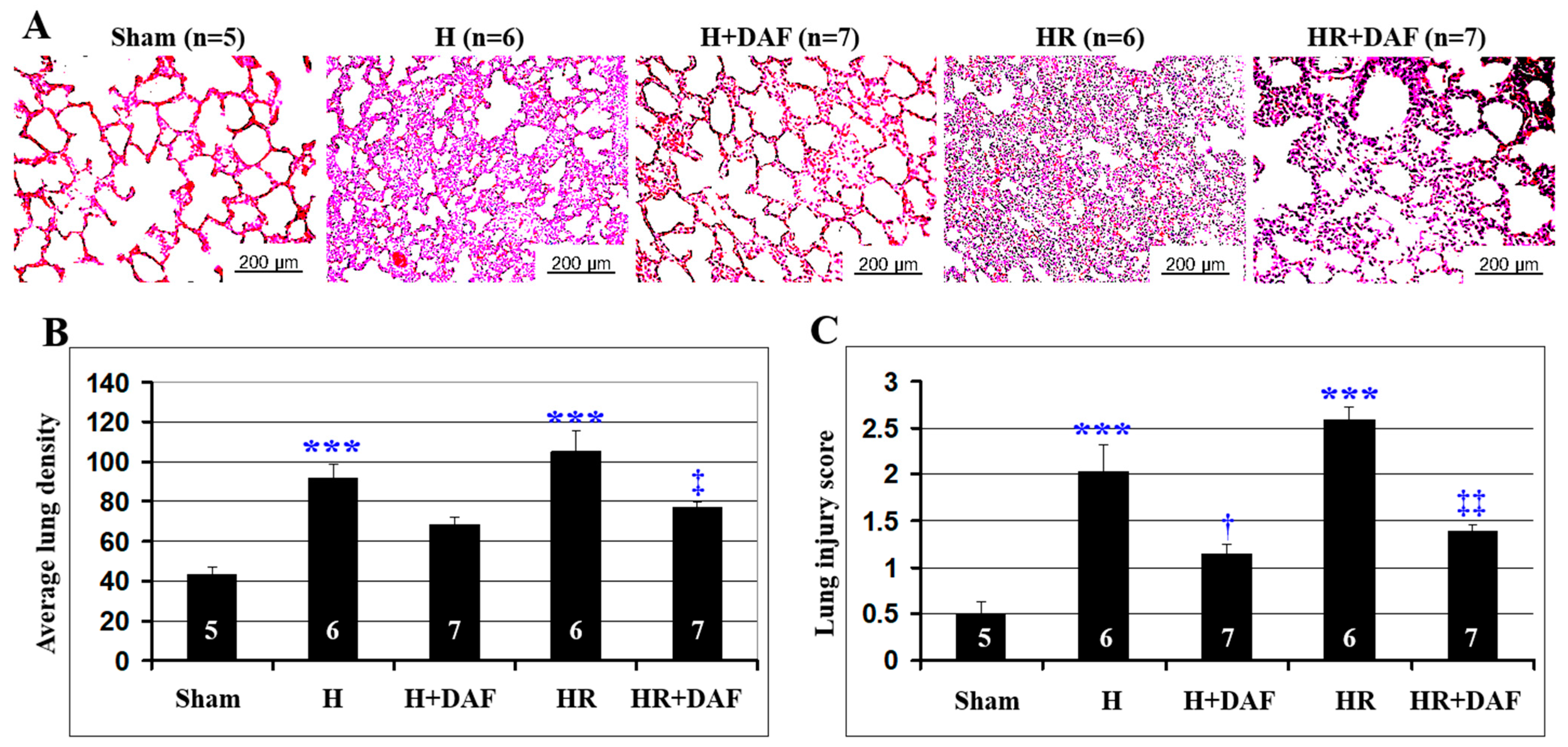
2.2. Effect of DAF on Hemorrhage/Hypotensive Resuscitation-Induced Acute Lung Injury (ALI)
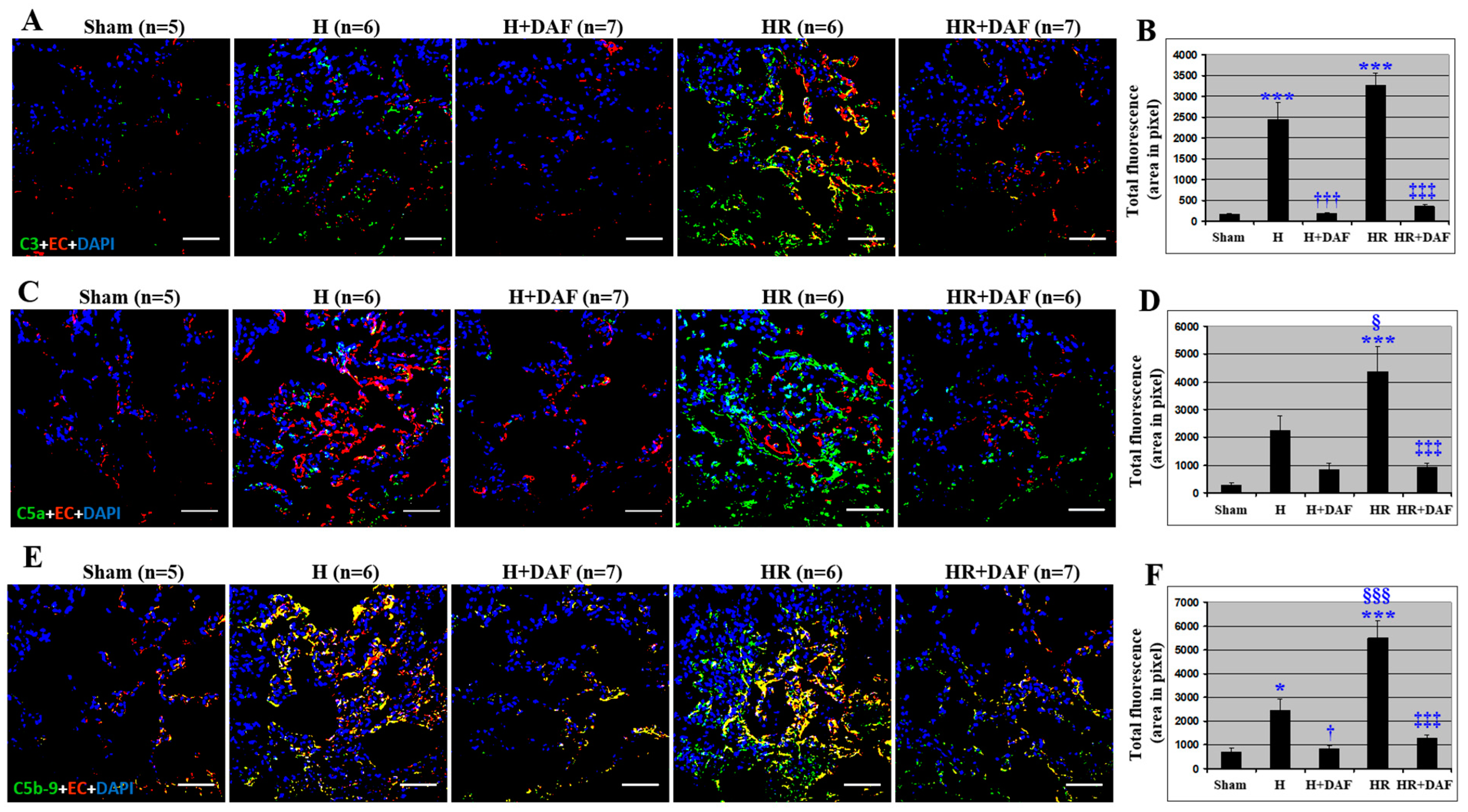
2.3. Effect of DAF on Hemorrhage/Hypotensive Resuscitation-Induced Pulmonary Complement Generation and Deposition
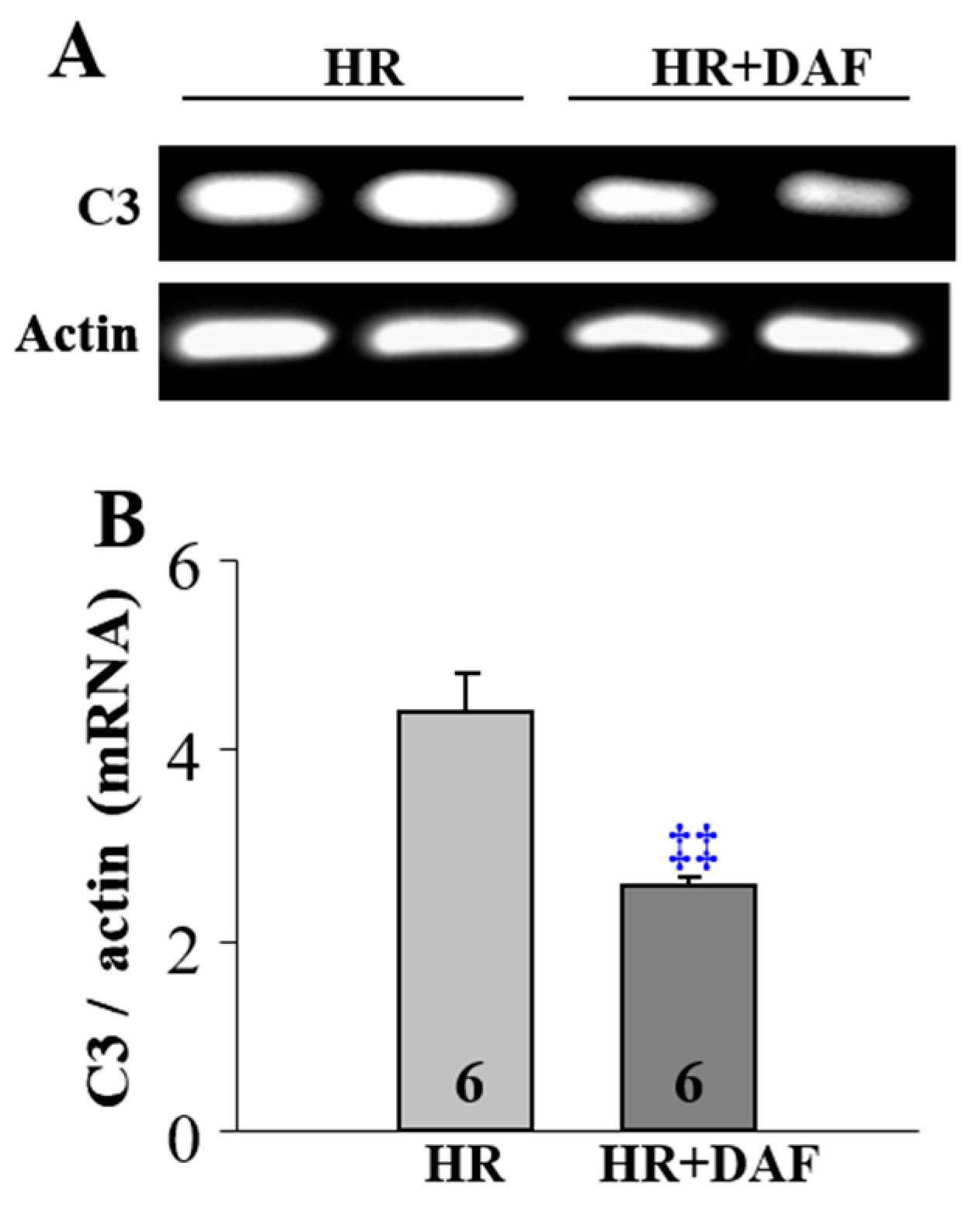
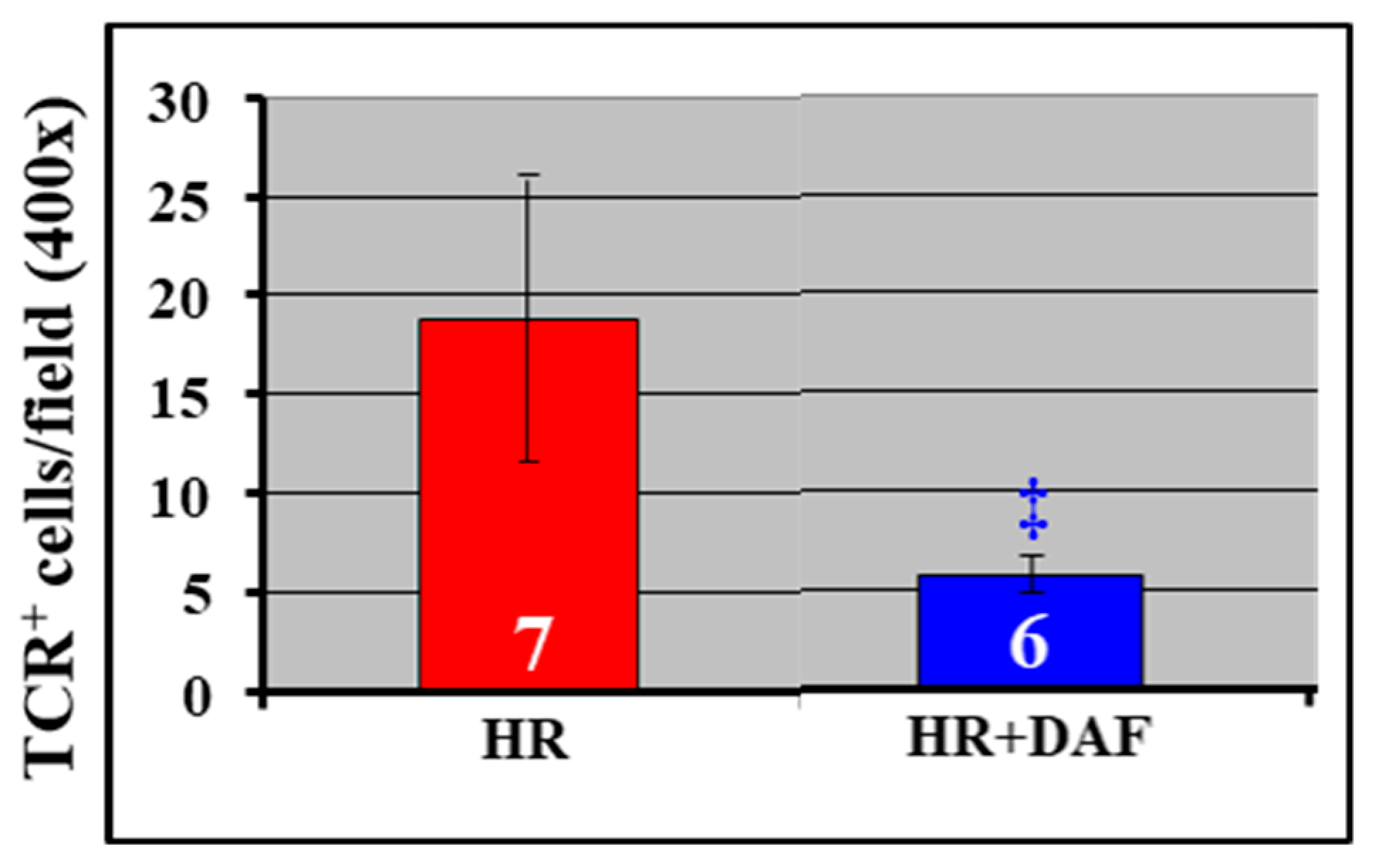
2.4. Effect of DAF on Hemorrhage/Hypotensive Resuscitation-Induced Pulmonary C3 Synthesis and T lymphocyte Infiltration
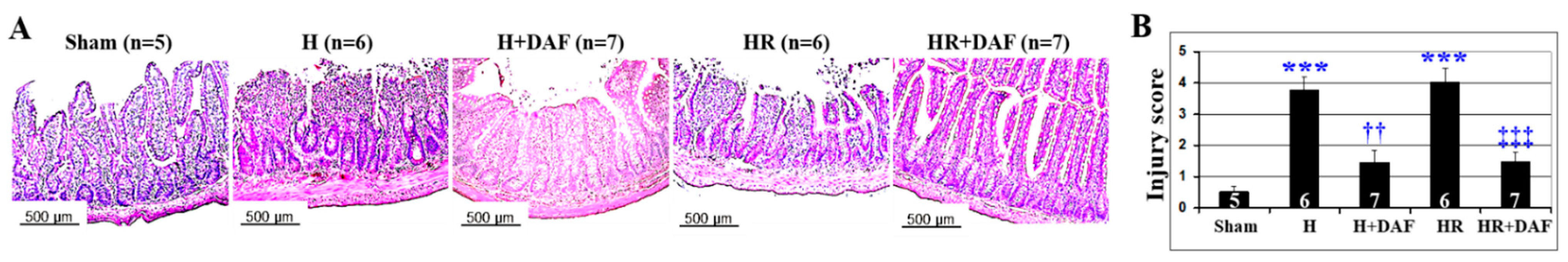
2.5. Effect of DAF on Hemorrhage/Hypotensive Resuscitation-Induced Acute Intestinal Injury
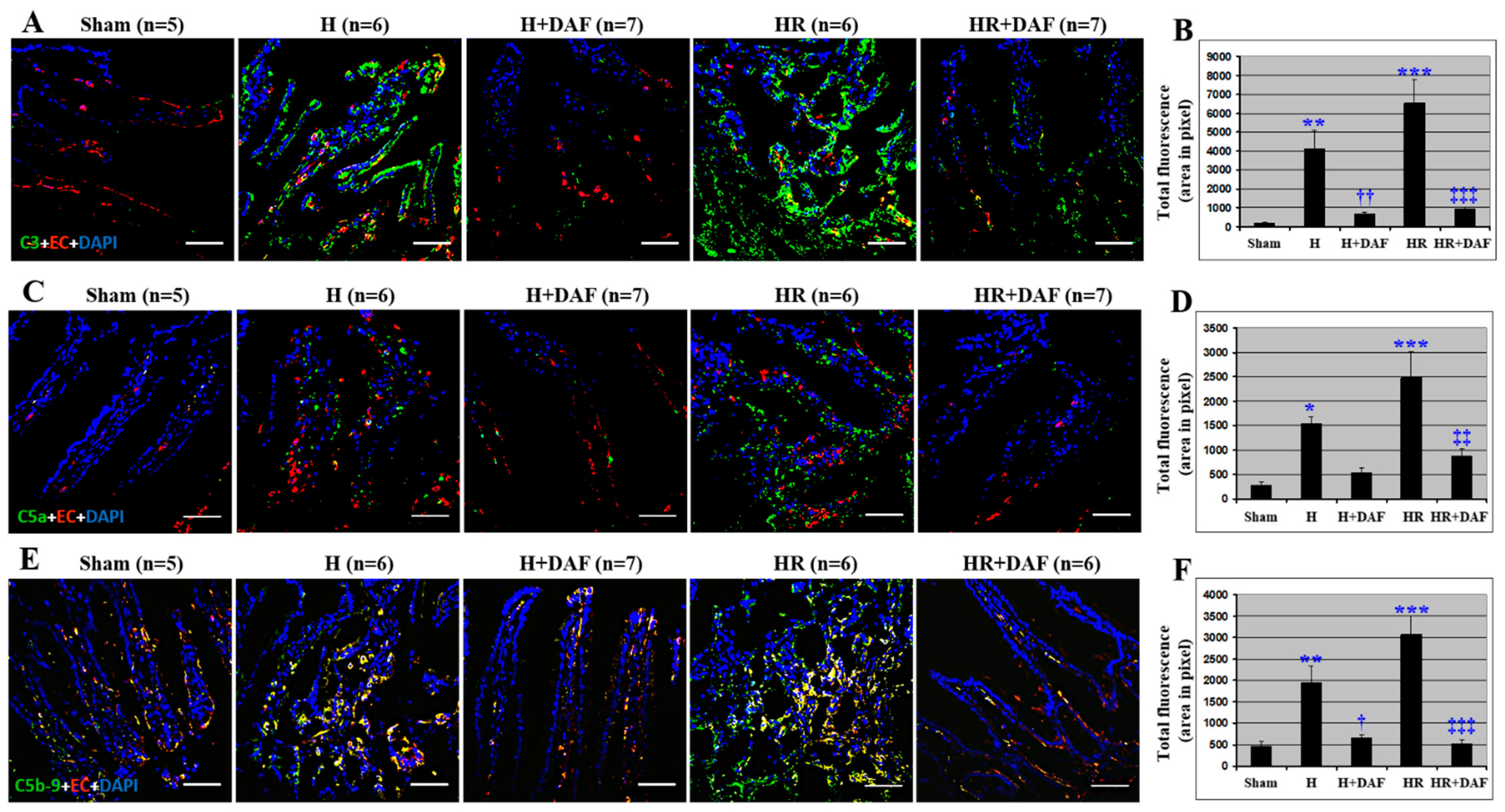
2.6. Effect of DAF on Hemorrhage/Hypotensive Resuscitation-Induced Intestinal Complement Activation and Deposition
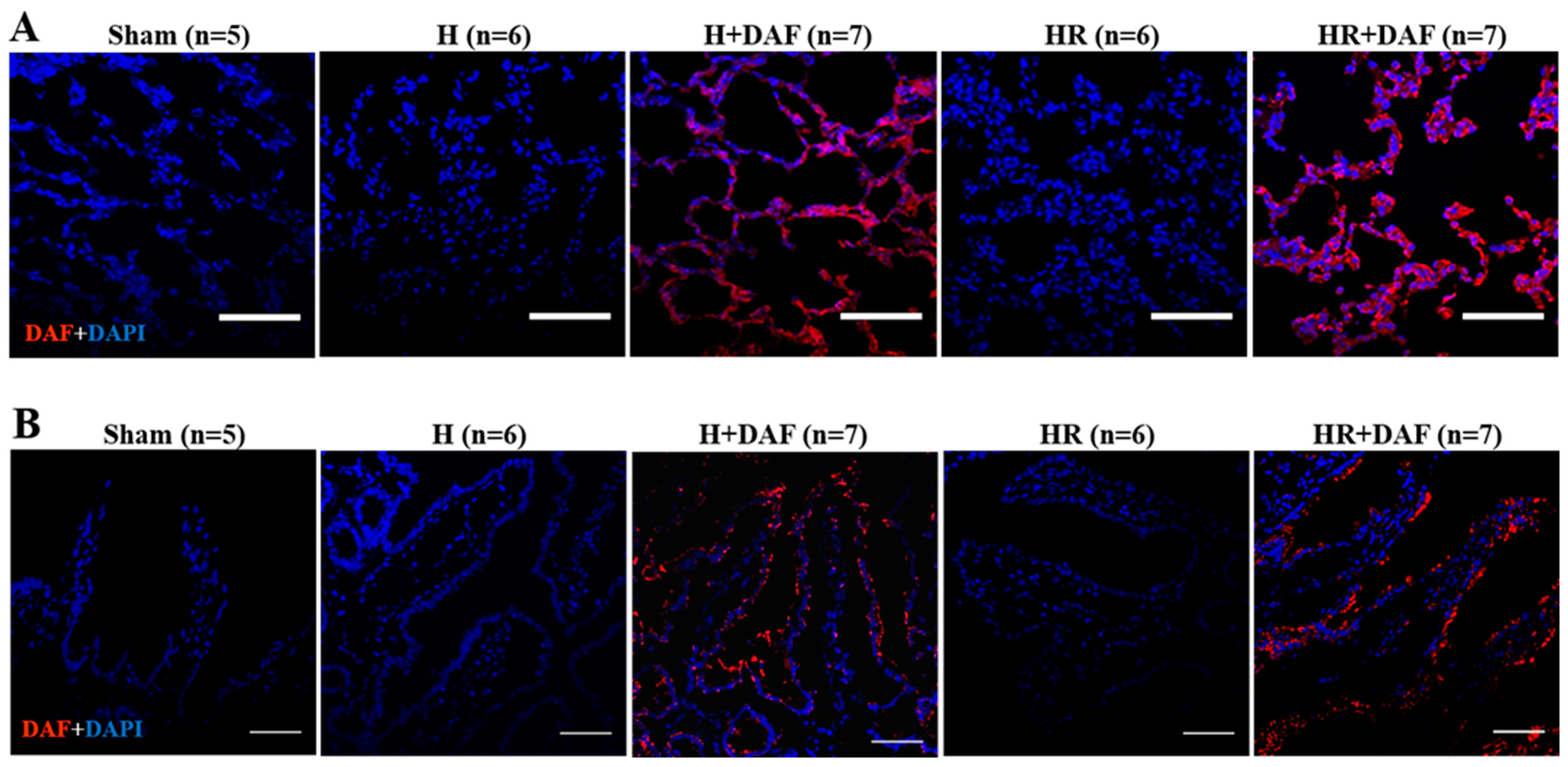
2.7. Deposition of rhDAF in the Lung and Intestinal Tissues
3. Discussion
4. Materials and Methods
4.1. Animal Study
4.2. Reagents
4.3. Surgical Procedures
4.4. Hemorrhagic Shock and Resuscitation
4.5. Tissue Harvest
4.6. Histopathological Evaluation
4.7. Immunofluorescent Staining
4.8. Total RNA Isolation and Reverse-Transcription Polymerase Chain Reaction
4.9. Circulating Lactate Concentration
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lord, J.M.; Midwinter, M.J.; Chen, Y.-F.; Belli, A.; Brohi, K.; Kovacs, E.J.; Koenderman, L.; Kubes, P.; Lilford, R.J. The systemic immune response to trauma: An overview of pathophysiology and treatment. Lancet 2014, 384, 1455–1465. [Google Scholar] [CrossRef]
- Cannon, J.W. Hemorrhagic Shock. N. Engl. J. Med. 2018, 378, 370–379. [Google Scholar] [CrossRef]
- Crandall, C.G.; Rickards, C.A.; Johnson, B.D. Impact of environmental stressors on tolerance to hemorrhage in humans. Am. J. Physiol. Integr. Comp. Physiol. 2019, 316, R88–R100. [Google Scholar] [CrossRef]
- Cavaillon, J.-M.; Annane, D. Compartmentalization of the inflammatory response in sepsis and SIRS. J. Endotoxin Res. 2006, 12, 151–170. [Google Scholar] [CrossRef]
- Peckham, R.M.; Handrigan, M.T.; Bentley, T.B.; Falabella, M.J.; Chrovian, A.D.; Stahl, G.L.; Tsokos, G.C. C5-blocking antibody reduces fluid requirements and improves responsiveness to fluid infusion in hemorrhagic shock managed with hypotensive resuscitation. J. Appl. Physiol. 2007, 102, 673–680. [Google Scholar] [CrossRef][Green Version]
- Campbell, J.C.; Li, Y.; van Amersfoort, E.; Relan, A.; Dubick, M.; Sheppard, F.; Pusateri, A.; Niemeyer, D.; Tsokos, G.C.; Lucca, J.J.D. C1 Inhibitor Limits Organ Injury and Prolongs Survival in Swine Subjected to Battlefield Simulated Injury. Shock 2016, 46 (Suppl. S1), 177–188. [Google Scholar] [CrossRef]
- Chang, R.; Holcomb, J.B. Optimal Fluid Therapy for Traumatic Hemorrhagic Shock. Crit. Care Clin. 2017, 33, 15–36. [Google Scholar] [CrossRef]
- Munoz, C.; Aletti, F.; Govender, K.; Cabrales, P.; Kistler, E.B. Resuscitation After Hemorrhagic Shock in the Microcirculation: Targeting Optimal Oxygen Delivery in the Design of Artificial Blood Substitutes. Front. Med. 2020, 7, 585638. [Google Scholar] [CrossRef]
- Arumugam, T.; Shiels, I.A.; Woodruff, T.; Granger, D.N.; Taylor, S.M. The role of the complement system in ischemia-reperfusion injury. Shock 2004, 21, 401–409. [Google Scholar] [CrossRef]
- Gorsuch, W.B.; Chrysanthou, E.; Schwaeble, W.; Stahl, G.L. The complement system in ischemia–reperfusion injuries. Immunobiology 2012, 217, 1026–1033. [Google Scholar] [CrossRef]
- Hecke, F.; Schmidt, U.; Kola, A.; Bautsch, W.; Klos, A.; Koehl, J. Circulating complement proteins in multiple trauma patients-Correlation with injury severity, development of sepsis, and outcome. Crit. Care Med. 1997, 25, 2015–2024. [Google Scholar] [CrossRef]
- Younger, J.G.; Sasaki, N.; Waite, M.D.; Murray, H.N.; Saleh, E.F.; Ravage, Z.A.; Hirschl, R.B.; Ward, P.A.; Till, G.O. Detrimental effects of complement activation in hemorrhagic shock. J. Appl. Physiol. 2001, 90, 441–446, Erratum in J. Appl. Physiol. 2004, 96, 405. [Google Scholar] [CrossRef]
- Lucca, J.J.D.; Chavko, M.; Dubick, M.A.; Adeeb, S.; Falabella, M.J.; Slack, J.L.; McCarron, R.; Li, Y. Blast-induced moderate neurotrauma (BINT) elicits early complement activation and tumor necrosis factor alpha (TNFα) release in a rat brain. J. Neurol. Sci. 2012, 318, 146–154. [Google Scholar] [CrossRef]
- Li, Y.; Chavko, M.; Slack, J.L.; Liu, B.; McCarron, R.M.; Ross, J.D.; Lucca, J.J.D. Protective effects of decay-accelerating factor on blast-induced neurotrauma in rats. Acta Neuropathol. Commun. 2013, 1, 52. [Google Scholar] [CrossRef]
- Szebeni, J.; Baranyi, L.; Savay, S.; Götze, O.; Alving, C.R.; Bünger, R.; Mongan, P.D. Complement Activation During Hemorrhagic Shock and Resuscitation in Swine. Shock 2003, 20, 347–355. [Google Scholar] [CrossRef]
- Harkin, D.W.; Marron, C.D.; Rother, R.P.; Romaschin, A.; Rubin, B.B.; Lindsay, T.F. C5 complement inhibition attenuates shock and acute lung injury in an experimental model of ruptured abdominal aortic aneurysm. Br. J. Surg. 2005, 92, 1227–1234. [Google Scholar] [CrossRef]
- Harkin, D.W.; Romaschin, A.; Taylor, S.M.; Rubin, B.B.; Lindsay, T.F. Complement c5a receptor antagonist attenuates multiple organ injury in a model of ruptured abdominal aortic aneurysm. J. Vasc. Surg. 2004, 39, 196–206. [Google Scholar] [CrossRef]
- Horstick, G.; Kempf, T.; Lauterbach, M.; Bhakdi, S.; Kopacz, L.; Heimann, A.; Malzahn, M.; Horstick, M.; Meyer, J.; Kempski, O. C1-esterase-inhibitor treatment at early reperfusion of hemorrhagic shock reduces mesentry leukocyte adhesion and rolling. Microcirculation 2001, 8, 427–433. [Google Scholar] [CrossRef]
- Spain, D.A.; Fruchterman, T.M.; Matheson, P.J.; Wilson, M.A.; Martin, A.W.; Garrison, R.N. Complement Activation Mediates Intestinal Injury after Resuscitation from Hemorrhagic Shock. J. Trauma Inj. Infect. Crit. Care 1999, 46, 224–233. [Google Scholar] [CrossRef]
- Lucca, J.J.D.; Simovic, M.; Li, Y.; Moratz, C.; Falabella, M.; Tsokos, G.C. Decay-Accelerating Factor Mitigates Controlled Hemorrhage-Instigated Intestinal and Lung Tissue Damage and Hyperkalemia in Swine. J. Trauma Inj. Infect. Crit. Care 2011, 71 (Suppl. S1), S151–S160. [Google Scholar] [CrossRef]
- Lucca, J.J.D.; Li, Y.; Simovic, M.; Pusateri, A.E.; Falabella, M.; Dubick, M.A.; Tsokos, G.C. Effects of C1 Inhibitor on Tissue Damage in a Porcine Model of Controlled Hemorrhage. Shock 2012, 38, 82–91. [Google Scholar] [CrossRef]
- Yang, Z.; Simovic, M.O.; Edsall, P.R.; Liu, B.; Cancio, T.S.; Batchinsky, A.I.; Cancio, L.C.; Li, Y. HMGB1 Inhibition to Ameliorate Organ Failure and Increase Survival in Trauma. Biomolecules 2022, 12, 101. [Google Scholar] [CrossRef]
- Yang, Z.; Nunn, M.A.; Le, T.D.; Simovic, M.O.; Edsall, P.R.; Liu, B.; Barr, J.L.; Lund, B.J.; Hill-Pryor, C.D.; Pusateri, A.E.; et al. Immunopathology of Terminal Complement Activation and Complement C5 Blockade Creating a Pro-survival and Organ-protective Phenotype in Trauma. Br. J. Pharmacol. 2022. [Google Scholar] [CrossRef]
- NAEMT. TCCC Guidelines for Medical Personnel. wwwnaemtorg. 2018. Available online: https://www.naemt.org/docs/default-source/education-documents/tccc/tccc-mp/guidelines/tccc-guidelines-for-medical-personnel-180801.pdf?sfvrsn=13fc892_2 (accessed on 22 August 2022).
- Butler, F.K.; Bennett, B.; Wedmore, C.I. Tactical Combat Casualty Care and Wilderness Medicine: Advancing Trauma Care in Austere Environments. Emerg. Med. Clin. North Am. 2017, 35, 391–407. [Google Scholar] [CrossRef]
- Brenner, M.; Stein, D.M.; Hu, P.F.; Aarabi, B.; Sheth, K.; Scalea, T.M. Traditional systolic blood pressure targets underestimate hypotension-induced secondary brain injury. J. Trauma Acute Care Surg. 2012, 72, 1135–1139. [Google Scholar] [CrossRef]
- The Brain Trauma Foundation. The American Association of Neurological Surgeons. The Joint Section on Neurotrauma and Critical Care. Hypotension. J. Neurotrauma. 2000, 17, 591–595. [Google Scholar]
- Baranyi, L.; Szebeni, J.; Bentley, T.B.; Esser, D.; Smith, R.; Purger, D.; Alving, C.R. Complement inhibitor APT070 dramatically reduces the need for resuscitation and improves survival in controlled isobaric rat hemorrhage model. In Proceedings of the Combat Casualty Care in Ground Based Tactical Situations: Trauma Technology and Emergency Medical Procedures, St. Pete Beach, FL, USA, 16–18 August 2004. RTO-MP-HFM-109. [Google Scholar]
- Lucca, J.J.D.; Li, Y.; Simovic, M.O.; Slack, J.L.; Cap, A.; Falabella, M.J.; Dubick, M.; Lebeda, F.; Tsokos, G.C. Decay-accelerating factor limits hemorrhage-instigated tissue injury and improves resuscitation clinical parameters. J. Surg. Res. 2013, 179, 153–167. [Google Scholar] [CrossRef]
- Tremoleda, J.L.; Kerton, A.; Gsell, W. Anaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: Considerations on experimental outcomes and animal welfare. EJNMMI Res. 2012, 2, 44. [Google Scholar] [CrossRef]
- Tremoleda, J.L.; Watts, S.A.; Reynolds, P.S.; Thiemermann, C.; Brohi, K. Modeling Acute Traumatic Hemorrhagic Shock Injury: Challenges and Guidelines for Preclinical Studies. Shock 2017, 48, 610–623. [Google Scholar] [CrossRef]
- Cai, C.; Gill, R.; Eum, H.-A.; Cao, Z.; Loughran, P.A.; Darwiche, S.; Edmonds, R.D.; Menzel, C.L.; Billiar, T.R. Complement factor 3 deficiency attenuates hemorrhagic shock-related hepatic injury and systemic inflammatory response syndrome. Am. J. Physiol. Integr. Comp. Physiol. 2010, 299, R1175–R1182. [Google Scholar] [CrossRef]
- Brodbeck, W.G.; Kuttner-Kondo, L.; Mold, C.; Medof, M.E. Structure/function studies of human decay-accelerating factor. Immunology 2000, 101, 104–111. [Google Scholar] [CrossRef]
- Todd, S.R.; Malinoski, D.; Muller, P.J.; Schreiber, M.A. Lactated Ringer’s is Superior to Normal Saline in the Resuscitation of Uncontrolled Hemorrhagic Shock. J. Trauma Inj. Infect. Crit. Care 2007, 62, 636–639. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Liu, B.; Dixon, A.; Cancio, L.; Dubick, M.; Lucca, J.D. Early complementopathy predicts the outcomes of patients with trauma. Trauma Surg. Acute Care Open 2019, 4, e000217. [Google Scholar] [CrossRef]
- Ganter, M.T.; Brohi, K.; Cohen, M.J.; Shaffer, L.A.; Walsh, M.C.; Stahl, G.L.; Pittet, J.-F. Role of the alternative pathway in the early complement activation following major trauma. Shock 2007, 28, 29–34. [Google Scholar] [CrossRef]
- Burk, A.-M.; Martin, M.; Flierl, M.A.; Rittirsch, D.; Helm, M.; Lampl, L.; Bruckner, U.; Stahl, G.; Blom, A.M.; Perl, M.; et al. Early Complementopathy After Multiple Injuries in Humans. Shock 2012, 37, 348–354. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Chavko, M.; Liu, B.; Aderemi, O.A.; Simovic, M.O.; Dubick, M.A.; Cancio, L.C. Complement inhibition ameliorates blast-induced acute lung injury in rats: Potential role of complement in intracellular HMGB1-mediated inflammation. PLoS ONE 2018, 13, e0202594. [Google Scholar] [CrossRef]
- Majde, J.A. Animal Models for Hemorrhage and Resuscitation Research. J. Trauma Inj. Infect. Crit. Care 2003, 54, S100–S105. [Google Scholar] [CrossRef]
- Cole, E.; Gillespie, S.; Vulliamy, P.; Brohi, K.; Akkad, H.; Apostolidou, K.; Ardley, R.; Aylwin, C.; Bassford, C.; Bonner, S.; et al. Multiple organ dysfunction after trauma. Br. J. Surg. 2020, 107, 402–412. [Google Scholar] [CrossRef]
- Gentile, L.F.; Cuenca, A.G.; Efron, P.A.; Ang, D.; Bihorac, A.; McKinley, B.A.; Moldawer, L.L.; Moore, F.A. Persistent inflammation and immunosuppression: A common syndrome and new horizon for surgical intensive care. J. Trauma Acute Care Surg. 2012, 72, 1491–1501. [Google Scholar] [CrossRef]
- Huber-Lang, M.; Lambris, J.; Ward, P.A. Innate immune responses to trauma. Nat. Immunol. 2018, 19, 327–341. [Google Scholar] [CrossRef]
- Huber-Lang, M.S.; Ignatius, A.; Köhl, J.; Mannes, M.; Braun, C.K. Complement in trauma—Traumatised complement? J. Cereb. Blood Flow Metab. 2020, 178, 2863–2879. [Google Scholar] [CrossRef]
- Watters, J.M.; Tieu, B.H.; Todd, S.R.; Jackson, T.; Muller, P.J.; Malinoski, D.; Schreiber, M.A. Fluid Resuscitation Increases Inflammatory Gene Transcription After Traumatic Injury. J. Trauma Inj. Infect. Crit. Care 2006, 61, 300–309. [Google Scholar] [CrossRef]
- Lukacik, P.; Roversi, P.; White, J.; Esser, D.; Smith, G.P.; Billington, J.; Williams, P.A.; Rudd, P.M.; Wormald, M.R.; Harvey, D.J.; et al. Complement regulation at the molecular level: The structure of decay-accelerating factor. Proc. Natl. Acad. Sci. USA 2004, 101, 1279–1284. [Google Scholar] [CrossRef]
- Harris, C.L.; Spiller, B.; Morgan, P. Human and rodent decay-accelerating factors (CD55) are not species restricted in their complement-inhibiting activities. Immunology 2000, 100, 462–470. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Y.; Lucca, S.L.D.; Simovic, M.; Tsokos, G.C.; Lucca, J.J.D. Decay accelerating factor (CD55) protects neuronal cells from chemical hypoxia-induced injury. J. Neuroinflammation 2010, 7, 24. [Google Scholar] [CrossRef]
- Lu, X.; Li, Y.; Simovic, M.O.; Peckham, R.; Wang, Y.; Tsokos, G.C.; Lucca, J.J.D. Decay-Accelerating Factor Attenuates C-Reactive Protein-Potentiated Tissue Injury After Mesenteric Ischemia/Reperfusion. J. Surg. Res. 2011, 167, e103–e115. [Google Scholar] [CrossRef]
- Weeks, C.; Moratz, C.; Zacharia, A.; Stracener, C.; Egan, R.; Peckham, R.; Moore, F.D.; Tsokos, G.C. Decay-accelerating factor attenuates remote ischemia–reperfusion-initiated organ damage. Clin. Immunol. 2007, 124, 311–327. [Google Scholar] [CrossRef]
- Yang, Z.; Aderemi, O.A.; Zhao, Q.; Edsall, P.R.; Simovic, M.O.; Lund, B.J.; Espinoza, M.D.; Woodson, A.M.; Li, Y.; Cancio, L.C. Early Complement and Fibrinolytic Activation in a Rat Model of Blast-Induced Multi-Organ Damage. Mil. Med. 2019, 184, 282–290. [Google Scholar] [CrossRef]
- Lee, D.M.; Friend, D.S.; Gurish, M.F.; Benoist, C.; Mathis, D.; Brenner, M.B. Mast Cells: A Cellular Link Between Autoantibodies and Inflammatory Arthritis. Science 2002, 297, 1689–1692. [Google Scholar] [CrossRef]
- Mannes, M.; Schmidt, C.Q.; Nilsson, B.; Ekdahl, K.N.; Huber-Lang, M. Complement as driver of systemic inflammation and organ failure in trauma, burn, and sepsis. Semin. Immunopathol. 2021, 43, 773–788. [Google Scholar] [CrossRef]
- Karasu, E.; Nilsson, B.; Köhl, J.; Lambris, J.; Huber-Lang, M. Targeting Complement Pathways in Polytrauma- and Sepsis-Induced Multiple-Organ Dysfunction. Front. Immunol. 2019, 10, 543. [Google Scholar] [CrossRef]
- Chang, J.C. TTP-like syndrome: Novel concept and molecular pathogenesis of endotheliopathy-associated vascular microthrombotic disease. Thromb. J. 2018, 16, 20. [Google Scholar] [CrossRef]
- Chang, J.C. Disseminated intravascular coagulation: New identity as endotheliopathy-associated vascular microthrombotic disease based on in vivo hemostasis and endothelial molecular pathogenesis. Thromb. J. 2020, 18, 25. [Google Scholar] [CrossRef]
- Hallek, M. Signaling the end of chronic lymphocytic leukemia: New frontline treatment strategies. Hematol. Am. Soc. Hematol. Educ. Program 2013, 2013, 138–150, Erratum in Hematol. Am. Soc. Hematol. Educ. Program 2014, 2014, 604. [Google Scholar] [CrossRef][Green Version]
- Ostrowski, S.R.; Johansson, P.I. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J. Trauma Acute Care Surg. 2012, 73, 60–66. [Google Scholar] [CrossRef]
- Haywood-Watson, R.J.; Holcomb, J.B.; Gonzalez, E.A.; Peng, Z.; Pati, S.; Park, P.W.; Wang, W.; Zaske, A.M.; Menge, T.; Kozar, R.A. Modulation of Syndecan-1 Shedding after Hemorrhagic Shock and Resuscitation. PLoS ONE 2011, 6, e23530. [Google Scholar] [CrossRef]
- Fu, B.M.; Tarbell, J.M. Mechano-sensing and transduction by endothelial surface glycocalyx: Composition, structure, and function. WIREs Syst. Biol. Med. 2013, 5, 381–390. [Google Scholar] [CrossRef]
- Johansson, P.I.; Stensballe, J.; Rasmussen, L.S.; Ostrowski, S.R. A High Admission Syndecan-1 Level, A Marker of Endothelial Glycocalyx Degradation, Is Associated With Inflammation, Protein C Depletion, Fibrinolysis, and Increased Mortality in Trauma Patients. Ann. Surg. 2011, 254, 194–200. [Google Scholar] [CrossRef]
- Rahbar, E.; Cardenas, J.C.; Baimukanova, G.; Usadi, B.; Bruhn, R.; Pati, S.; Ostrowski, S.R.; I Johansson, P.; Holcomb, J.B.; E Wade, C. Endothelial glycocalyx shedding and vascular permeability in severely injured trauma patients. J. Transl. Med. 2015, 13, 117. [Google Scholar] [CrossRef]
- Denk, S.; Neher, M.D.; Messerer, D.A.C.; Wiegner, R.; Nilsson, B.; Rittirsch, D.; Nilsson-Ekdahl, K.; Weckbach, S.; Ignatius, A.; Kalbitz, M.; et al. Complement C5a Functions as a Master Switch for the pH Balance in Neutrophils Exerting Fundamental Immunometabolic Effects. J. Immunol. 2017, 198, 4846–4854. [Google Scholar] [CrossRef]
- Kulkarni, H.S.; Elvington, M.L.; Perng, Y.-C.; Liszewski, M.K.; Byers, D.E.; Farkouh, C.; Yusen, R.D.; Lenschow, D.J.; Brody, S.L.; Atkinson, J.P. Intracellular C3 Protects Human Airway Epithelial Cells from Stress-associated Cell Death. Am. J. Respir. Cell Mol. Biol. 2019, 60, 144–157. [Google Scholar] [CrossRef]
- Strainic, M.G.; Liu, J.; Huang, D.; An, F.; Lalli, P.N.; Muqim, N.; Shapiro, V.S.; Dubyak, G.R.; Heeger, P.S.; Medof, M.E. Locally Produced Complement Fragments C5a and C3a Provide Both Costimulatory and Survival Signals to Naive CD4+ T Cells. Immunity 2008, 28, 425–435. [Google Scholar] [CrossRef]
- Markiewski, M.M.; Lambris, J.D. The Role of Complement in Inflammatory Diseases From Behind the Scenes into the Spotlight. Am. J. Pathol. 2007, 171, 715–727. [Google Scholar] [CrossRef]
- Alenghat, F.J.; Golan, D.E. Membrane Protein Dynamics and Functional Implications in Mammalian Cells. Curr. Top. Membr. 2013, 72, 89–120. [Google Scholar] [CrossRef]
- Bösch, F.; Angele, M.K.; Chaudry, I.H. Gender differences in trauma, shock and sepsis. Mil. Med. Res. 2018, 5, 35. [Google Scholar] [CrossRef]
- Yu, H.-P.; Chaudry, I.H. The role of estrogen and receptor agonists in maintaining organ function after trauma-hemorrhage. Shock 2009, 31, 227–237. [Google Scholar] [CrossRef]
- Doucet, D.; Badami, C.; Palange, D.; Bonitz, R.P.; Lu, Q.; Xu, D.; Kannan, K.B.; Colorado, I.; Feinman, R.; Deitch, E.A. Estrogen Receptor Hormone Agonists Limit Trauma Hemorrhage Shock-Induced Gut and Lung Injury in Rats. PLoS ONE 2010, 5, e9421. [Google Scholar] [CrossRef]
- Suzuki, T.; Yu, H.-P.; Hsieh, Y.-C.; Choudhry, M.A.; Bland, K.I.; Chaudry, I.H. Estrogen-mediated activation of non-genomic pathway improves macrophages cytokine production following trauma-hemorrhage. J. Cell. Physiol. 2008, 214, 662–672. [Google Scholar] [CrossRef]
- Marcolini, E.G.; Albrecht, J.S.; Sethuraman, K.N.; Napolitano, L.M. Gender Disparities in Trauma Care: How Sex Determines Treatment, Behavior, and Outcome. Anesthesiol. Clin. 2019, 37, 107–117. [Google Scholar] [CrossRef]
- Fremont, R.D.; Koyama, T.; Calfee, C.S.; Wu, W.; Dossett, L.A.; Bossert, F.R.; Mitchell, D.; Wickersham, N.; Bernard, G.R.; Matthay, M.A.; et al. Acute Lung Injury in Patients With Traumatic Injuries: Utility of a Panel of Biomarkers for Diagnosis and Pathogenesis. J. Trauma Inj. Infect. Crit. Care 2010, 68, 1121–1127. [Google Scholar] [CrossRef]
- Hassoun, H.T.; Kone, B.C.; Mercer, D.W.; Moody, F.G.; Weisbrodt, N.W.; Moore, F.A. Post-injury multiple organ failure: The role of the gut. Shock 2001, 15, 1–10. [Google Scholar] [CrossRef]
- Handrigan, M.T.; Bentley, T.B.; Oliver, J.D.; Tabaku, L.S.; Burge, J.R.; Atkins, J.L. Choice of fluid influences outcome in prolonged hypotensive resuscitation after hemorrhage in awake rats. Shock 2005, 23, 337–343. [Google Scholar] [CrossRef]
- Jensen, E.C. Quantitative Analysis of Histological Staining and Fluorescence Using ImageJ. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2013, 296, 378–381. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simovic, M.O.; Falabella, M.J.; Le, T.D.; DalleLucca, J.J.; Li, Y. Decay-Accelerating Factor Creates an Organ-Protective Phenotype after Hemorrhage in Conscious Rats. Int. J. Mol. Sci. 2022, 23, 13563. https://doi.org/10.3390/ijms232113563
Simovic MO, Falabella MJ, Le TD, DalleLucca JJ, Li Y. Decay-Accelerating Factor Creates an Organ-Protective Phenotype after Hemorrhage in Conscious Rats. International Journal of Molecular Sciences. 2022; 23(21):13563. https://doi.org/10.3390/ijms232113563
Chicago/Turabian StyleSimovic, Milomir O., Michael J. Falabella, Tuan D. Le, Jurandir J. DalleLucca, and Yansong Li. 2022. "Decay-Accelerating Factor Creates an Organ-Protective Phenotype after Hemorrhage in Conscious Rats" International Journal of Molecular Sciences 23, no. 21: 13563. https://doi.org/10.3390/ijms232113563
APA StyleSimovic, M. O., Falabella, M. J., Le, T. D., DalleLucca, J. J., & Li, Y. (2022). Decay-Accelerating Factor Creates an Organ-Protective Phenotype after Hemorrhage in Conscious Rats. International Journal of Molecular Sciences, 23(21), 13563. https://doi.org/10.3390/ijms232113563







