How Can We Improve Vaccination Response in Old People? Part I: Targeting Immunosenescence of Innate Immunity Cells
Abstract
:1. Introduction
1.1. Trends in Aging: Consequences and Solutions
1.2. The Innate Immune System
2. Immunosenescence of the Innate Immune Cells
2.1. Neutrophils
2.2. Monocytes
2.3. Dendritic Cells
2.4. Natural Killer Cells
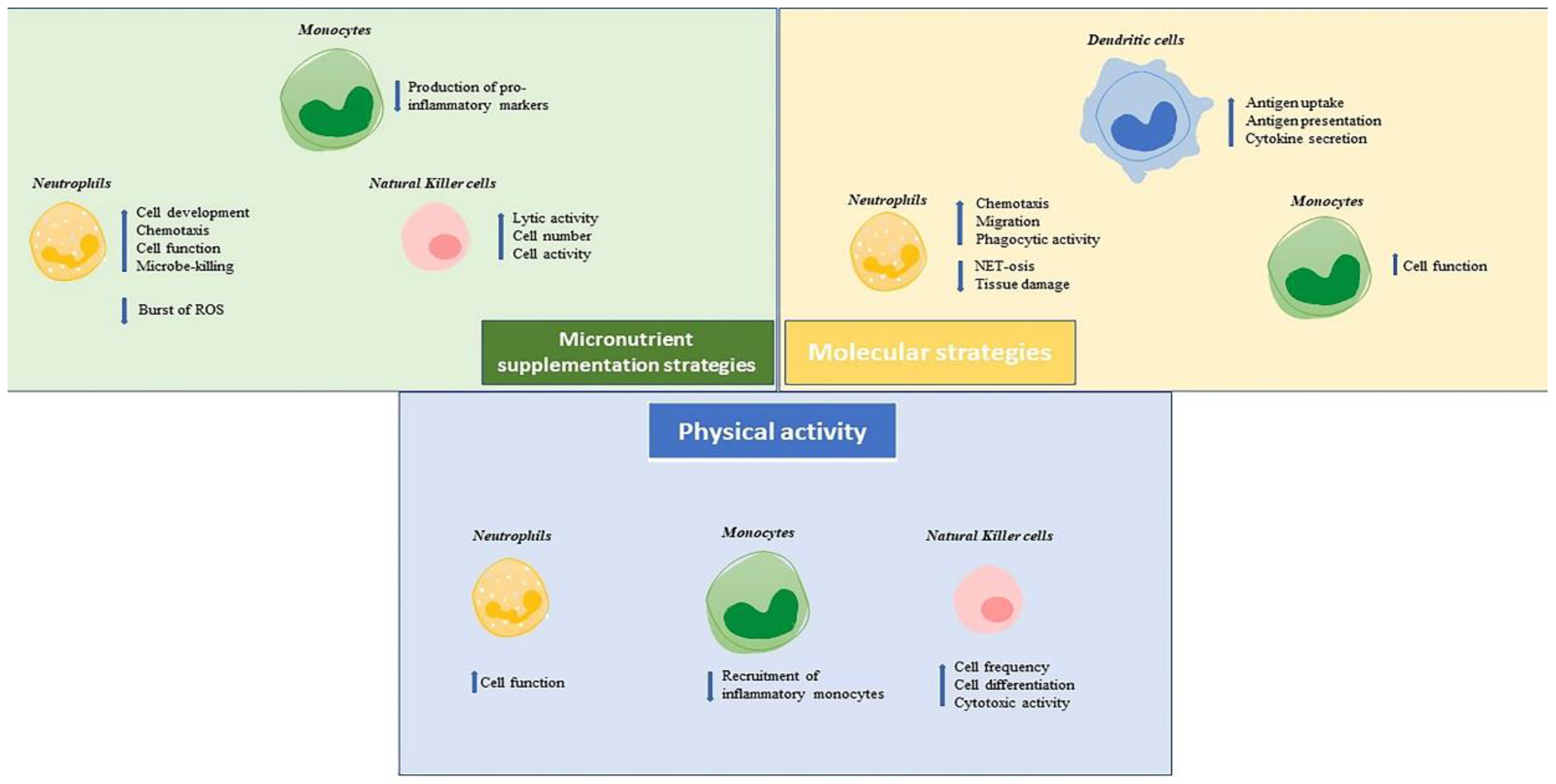
3. Strategies to Reverse Immunosenescence of the Innate Immunity System in Older People
3.1. Neutrophils
3.2. Monocytes
3.3. Natural Killer Cells
3.4. Dendritic Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Ageing and Health. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 27 July 2022).
- Partridge, L.; Deelen, J.; Slagboom, P.E. Facing up to the global challenges of ageing. Nature 2018, 561, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Ciabattini, A.; Nardini, C.; Santoro, F.; Garagnani, P.; Franceschi, C.; Medaglini, D. Vaccination in the elderly: The challenge of immune changes with aging. Semin. Immunol. 2018, 40, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Goronzy, J.J.; Weyand, C.M. Understanding immunosenescence to improve responses to vaccines. Nat. Immunol. 2013, 14, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.; Garner-Spitzer, E.; Jasinska, J.; Kollaritsch, H.; Stiasny, K.; Kundi, M.; Wiedermann, U. Age-related differences in humoral and cellular immune responses after primary immunisation: Indications for stratified vaccination schedules. Sci. Rep. 2018, 8, 9825. [Google Scholar] [CrossRef] [PubMed]
- Pinti, M.; De Biasi, S.; Gibellini, L.; Lo Tartaro, D.; De Gaetano, A.; Mattioli, M.; Fidanza, L.; Nasi, M.; Cossarizza, M. Aging of Immune System. In Human Aging; Caruso, C., Candore, G., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 113–128. [Google Scholar]
- Caruso, C.; Vasto, S. Immunity and Aging. In Encyclopedia of Immunobiology; Ratcliffe, M.J.H., Ed.; Academic Press: Cambridge, MA, USA, 2016; Volume 5, pp. 127–132. [Google Scholar]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and Its Hallmarks: How to Oppose Aging Strategically? A Review of Potential Options for Therapeutic Intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef]
- Caruso, C.; Ligotti, M.E.; Accardi, G.; Aiello, A.; Candore, G. An immunologist’s guide to immunosenescence and its treatment. Expert Rev. Clin. Immunol. 2022, 18, 961–981. [Google Scholar] [CrossRef] [PubMed]
- Parkin, J.; Cohen, B. An overview of the immune system. Lancet 2001, 357, 1777–1789. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar]
- Gruenewald, T.L.; Cohen, S.; Matthews, K.A.; Tracy, R.; Seeman, T.E. Association of socioeconomic status with inflammation markers in black and white men and women in the Coronary Artery Risk Development in Young Adults (CARDIA) study. Soc. Sci. Med. 2009, 69, 451–459. [Google Scholar] [CrossRef]
- Liu, R.S.; Aiello, A.E.; Mensah, F.K.; Gasser, C.E.; Rueb, K.; Cordell, B.; Juonala, M.; Wake, M.; Burgner, D.P. Socioeconomic status in childhood and C reactive protein in adulthood: A systematic review and meta-analysis. J. Epidemiol. Community Health 2017, 71, 817–826. [Google Scholar] [CrossRef]
- Yousefzadeh, M.J.; Flores, R.R.; Zhu, Y.; Schmiechen, Z.C.; Brooks, R.W.; Trussoni, C.E.; Cui, Y.; Angelini, L.; Lee, K.A.; McGowan, S.J.; et al. An aged immune system drives senescence and ageing of solid organs. Nature 2021, 594, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Caruso, C.; Aiello, A.; Pawelec, G.; Ligotti, M.E. Vaccination in Old Age: Challenges and Promises. In Human Aging; Caruso, C., Candore, G., Eds.; Academic Press: Cambridge, MA, USA, 2021; pp. 129–153. [Google Scholar]
- Mantovani, A.; Netea, M.G. Trained Innate Immunity, Epigenetics, and COVID-19. N. Engl. J. Med. 2020, 383, 1078–1080. [Google Scholar] [CrossRef] [PubMed]
- Kleinnijenhuis, J.; Quintin, J.; Preijers, F.; Joosten, L.A.; Jacobs, C.; Xavier, R.J.; van der Meer, J.W.; van Crevel, R.; Netea, M.G. BCG-induced trained immunity in NK cells: Role for non-specific protection to infection. Clin. Immunol. 2014, 155, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Van Puffelen, J.H.; Keating, S.T.; Oosterwijk, E.; van der Heijden, A.G.; Netea, M.G.; Joosten, L.A.B.; Vermeulen, S.H. Trained immunity as a molecular mechanism for BCG immunotherapy in bladder cancer. Nat. Rev. Urol. 2020, 17, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- Drew, W.; Wilson, D.V.; Sapey, E. Inflammation and neutrophil immunosenescence in health and disease: Targeted treatments to improve clinical outcomes in the elderly. Exp. Gerontol. 2018, 105, 70–77. [Google Scholar] [CrossRef]
- Lopes, A.B.; Lopes, L.B.; da Silveira Antunes, R.N.; Fukasawa, J.T.; de A Cavaretto, D.; Calamita, Z. Effects of Immunosenescence on the Lower Expression of Surface Molecules in Neutrophils and Lymphocytes. Curr. Aging Sci. 2018, 11, 118–125. [Google Scholar] [CrossRef]
- Bonecchi, R.; Mantovani, A.; Jaillon, S. Chemokines as Regulators of Neutrophils: Focus on Tumors, Therapeutic Targeting, and Immunotherapy. Cancers 2022, 14, 680. [Google Scholar] [CrossRef]
- Teissier, T.; Boulanger, E.; Cox, L.S. Interconnections between Inflammageing and Immunosenescence during Ageing. Cells 2022, 11, 359. [Google Scholar] [CrossRef]
- Cunha, L.L.; Perazzio, S.F.; Azzi, J.; Cravedi, P.; Riella, L.V. Remodeling of the Immune Response With Aging: Immunosenescence and Its Potential Impact on COVID-19 Immune Response. Front. Immunol. 2020, 11, 1748. [Google Scholar] [CrossRef]
- Oh, S.J.; Lee, J.K.; Shin, O.S. Aging and the Immune System: The Impact of Immunosenescence on Viral Infection, Immunity and Vaccine Immunogenicity. Immune Netw. 2019, 19, e37. [Google Scholar] [CrossRef] [PubMed]
- Bleve, A.; Motta, F.; Durante, B.; Pandolfo, C.; Selmi, C.; Sica, A. Immunosenescence, Inflammaging, and Frailty: Role of Myeloid Cells in Age-Related Diseases. Clin. Rev. Allergy Immunol. 2022; Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Budamagunta, V.; Manohar-Sindhu, S.; Yang, Y.; He, Y.; Traktuev, D.O.; Foster, T.C.; Zhou, D. Senescence-associated hyper-activation to inflammatory stimuli In Vitro. Aging 2021, 13, 19088–19107. [Google Scholar] [CrossRef] [PubMed]
- Palacios-Pedrero, M.Á.; Osterhaus, A.D.M.E.; Becker, T.; Elbahesh, H.; Rimmelzwaan, G.F.; Saletti, G. Aging and Options to Halt Declining Immunity to Virus Infections. Front. Immunol. 2021, 12, 681449. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Douziech, N.; Fortin, C.; Guérard, K.P.; Lesur, O.; Khalil, A.; Dupuis, G. Signal transduction and functional changes in neutrophils with aging. Aging Cell 2004, 3, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Dupuis, G.; Witkowski, J.M.; Larbi, A. The Role of Immunosenescence in the Development of Age-Related Diseases. Rev. Investig. Clin. 2016, 68, 84–91. [Google Scholar]
- Simmons, S.R.; Tchalla, E.Y.I.; Bhalla, M.; Bou Ghanem, E.N. The Age-Driven Decline in Neutrophil Function Contributes to the Reduced Efficacy of the Pneumococcal Conjugate Vaccine in Old Hosts. Front. Cell Infect. Microbiol. 2022, 12, 849224. [Google Scholar] [CrossRef]
- Jackaman, C.; Nelson, D.J. Are macrophages, myeloid derived suppressor cells and neutrophils mediators of local suppression in healthy and cancerous tissues in aging hosts? Exp. Gerontol. 2014, 54, 53–57. [Google Scholar] [CrossRef]
- Mishalian, I.; Bayuh, R.; Levy, L.; Zolotarov, L.; Michaeli, J.; Fridlender, Z.G. Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumor progression. Cancer Immunol. Immunother. 2013, 62, 1745–1756. [Google Scholar] [CrossRef]
- Elder, E.; Krishna, B.; Williamson, J.; Aslam, Y.; Farahi, N.; Wood, A.; Romashova, V.; Roche, K.; Murphy, E.; Chilvers, E.; et al. Monocytes Latently Infected with Human Cytomegalovirus Evade Neutrophil Killing. iScience 2019, 12, 13–26. [Google Scholar] [CrossRef]
- He, Z.; Fahlberg, M.D.; Takahashi, N.; Slisarenko, N.; Rout, N.; Didier, E.S.; Kuroda, M.J. Declining neutrophil production despite increasing G-CSF levels is associated with chronic inflammation in elderly rhesus macaques. J. Leukoc. Biol. 2021, 109, 1033–1043. [Google Scholar] [CrossRef]
- Moss, D.L.; Rappaport, J. The good, the bad and the ugly: G-CSF, ageing and neutrophils-Implications for severe COVID-19. J. Leukoc. Biol. 2021, 109, 1017–1018. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Kawai, T.; Akira, S. Toll-like receptors and innate immunity. Biochem. Biophys. Res. Commun. 2009, 388, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 2011, 34, 637–650. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2021; Online ahead of print. [Google Scholar] [CrossRef]
- De Maeyer, R.P.H.; Chambers, E.S. The impact of ageing on monocytes and macrophages. Immunol. Lett. 2021, 230, 1–10. [Google Scholar] [CrossRef]
- Marzaioli, V.; Canavan, M.; Floudas, A.; Wade, S.C.; Low, C.; Veale, D.J.; Fearon, U. Monocyte-Derived Dendritic Cell Differentiation in Inflammatory Arthritis Is Regulated by the JAK/STAT Axis via NADPH Oxidase Regulation. Front. Immunol. 2020, 11, 1406. [Google Scholar] [CrossRef]
- Wallis, Z.K.; Williams, K.C. Monocytes in HIV and SIV Infection and Aging: Implications for Inflamm-Aging and Accelerated Aging. Viruses 2022, 14, 409. [Google Scholar] [CrossRef]
- Bella, S.D.; Bierti, L.; Presicce, P.; Arienti, R.; Valenti, M.; Saresella, M.; Vergani, C.; Villa, M.L. Peripheral blood dendritic cells and monocytes are differently regulated in the elderly. Clin. Immunol. 2007, 122, 220–228. [Google Scholar] [CrossRef]
- Visintin, A.; Mazzoni, A.; Spitzer, J.H.; Wyllie, D.H.; Dower, S.K.; Segal, D.M. Regulation of Toll-like receptors in human monocytes and dendritic cells. J. Immunol. 2001, 166, 249–255. [Google Scholar] [CrossRef]
- Furman, D.; Chang, J.; Lartigue, L.; Bolen, C.R.; Haddad, F.; Gaudilliere, B.; Ganio, E.A.; Fragiadakis, G.K.; Spitzer, M.H.; Douchet, I.; et al. Expression of specific inflammasome gene modules stratifies older individuals into two extreme clinical and immunological states. Nat. Med. 2017, 23, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Wang, X.; Zhang, L.; Chen, S.; Piecychna, M.; Allore, H.; Bockenstedt, L.; Malawista, S.; Bucala, R.; Shaw, A.C.; et al. Age-associated elevation in TLR5 leads to increased inflammatory responses in the elderly. Aging Cell 2012, 11, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Pence, B.D.; Yarbro, J.R. Classical monocytes maintain Ex Vivo glycolytic metabolism and early but not later inflammatory responses in older adults. Immun. Ageing 2019, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, L.M.; Taylor, J.R.; Ding, J.; Lohman, K.; Johnson, C.; Siscovick, D.; Burke, G.; Post, W.; Shea, S.; Jacobs, D.R., Jr.; et al. Age-related variations in the methylome associated with gene expression in human monocytes and T cells. Nat. Commun. 2014, 5, 5366. [Google Scholar] [CrossRef] [PubMed]
- Yarbro, J.R.; Pence, B.D. Classical monocytes from older adults maintain capacity for metabolic compensation during glucose deprivation and lipopolysaccharide stimulation. Mech. Ageing Dev. 2019, 183, 111146. [Google Scholar] [CrossRef]
- Bernshtein, B.; Nachshon, A.; Shnayder, M.; Stern, L.; Avdic, S.; Blyth, E.; Gottlieb, D.; Abendroth, A.; Slobedman, B.; Stern-Ginossar, N.; et al. Profiling the Blood Compartment of Hematopoietic Stem Cell Transplant Patients During Human Cytomegalovirus Reactivation. Front. Cell Infect. Microbiol. 2021, 10, 607470. [Google Scholar] [CrossRef]
- Jackson, S.E.; Chen, K.C.; Groves, I.J.; Sedikides, G.X.; Gandhi, A.; Houldcroft, C.J.; Poole, E.L.; Montanuy, I.; Mason, G.M.; Okecha, G.; et al. Latent Cytomegalovirus-Driven Recruitment of Activated CD4+ T Cells Promotes Virus Reactivation. Front. Immunol. 2021, 12, 657945. [Google Scholar] [CrossRef]
- Puissant-Lubrano, B.; Apoil, P.A.; Guedj, K.; Congy-Jolivet, N.; Roubinet, F.; Guyonnet, S.; Sourdet, S.; Nourhashemi, F.; Blancher, A. Distinct effect of age, sex, and CMV seropositivity on dendritic cells and monocytes in human blood. Immunol. Cell Biol. 2018, 96, 114–120. [Google Scholar] [CrossRef]
- Márquez, E.J.; Chung, C.; Marches, R.; Rossi, R.J.; Nehar-Belaid, D.; Eroglu, A.; Mellert, D.J.; Kuchel, G.A.; Banchereau, J.; Ucar, D. Sexual-dimorphism in human immune system aging. Nat. Commun. 2020, 11, 751. [Google Scholar] [CrossRef]
- Devi, K.S.; Anandasabapathy, N. The origin of DCs and capacity for immunologic tolerance in central and peripheral tissues. Semin. Immunopathol. 2017, 39, 137–152. [Google Scholar] [CrossRef]
- Joffre, O.; Nolte, M.A.; Spörri, R.; Reis e Sousa, C. Inflammatory signals in dendritic cell activation and the induction of adaptive immunity. Immunol. Rev. 2009, 227, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Uematsu, S.; Akira, S. Toll-Like receptors (TLRs) and their ligands. Handb. Exp. Pharmacol. 2008, 183, 1–20. [Google Scholar]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Nizzoli, G.; Krietsch, J.; Weick, A.; Steinfelder, S.; Facciotti, F.; Gruarin, P.; Bianco, A.; Steckel, B.; Moro, M.; Crosti, M.; et al. Human CD1c+ dendritic cells secrete high levels of IL-12 and potently prime cytotoxic T-cell responses. Blood 2013, 122, 932–942. [Google Scholar] [CrossRef]
- Delneste, Y.; Beauvillain, C.; Jeannin, P. Immunité naturelle: Structure et fonction des Toll-like receptors Innate immunity: Structure and function of TLRs. Med. Sci. 2007, 23, 67–73. [Google Scholar]
- Manicassamy, S.; Pulendran, B. Modulation of adaptive immunity with Toll-like receptors. Semin. Immunol. 2009, 21, 185–193. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kawai, T. Toll-like receptor signaling pathways. Front. Immunol. 2014, 5, 461. [Google Scholar] [CrossRef]
- Schulz, A.R.; Mälzer, J.N.; Domingo, C.; Jürchott, K.; Grützkau, A.; Babel, N.; Nienen, M.; Jelinek, T.; Niedrig, M.; Thiel, A. Low Thymic Activity and Dendritic Cell Numbers Are Associated with the Immune Response to Primary Viral Infection in Elderly Humans. J. Immunol. 2015, 195, 4699–4711. [Google Scholar] [CrossRef]
- Zareian, N.; Aprile, S.; Cristaldi, L.; Ligotti, M.E.; Vasto, S.; Farzaneh, F. Triggering of Toll-like Receptors in Old Individuals. Relevance for Vaccination. Curr. Pharm. Des. 2019, 25, 4163–4167. [Google Scholar] [CrossRef]
- Grolleau-Julius, A.; Harning, E.K.; Abernathy, L.M.; Yung, R.L. Impaired dendritic cell function in aging leads to defective antitumor immunity. Cancer Res. 2008, 68, 6341–6349. [Google Scholar] [CrossRef]
- Orsini, G.; Legitimo, A.; Failli, A.; Massei, F.; Biver, P.; Consolini, R. Enumeration of human peripheral blood dendritic cells throughout the life. Int. Immunol. 2012, 24, 347–356. [Google Scholar] [CrossRef] [PubMed]
- You, J.; Dong, H.; Mann, E.R.; Knight, S.C.; Yaqoob, P. Ageing impairs the T cell response to dendritic cells. Immunobiology 2013, 218, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Stervbo, U.; Meier, S.; Mälzer, J.N.; Baron, U.; Bozzetti, C.; Jürchott, K.; Nienen, M.; Olek, S.; Rachwalik, D.; Schulz, A.R.; et al. Effects of aging on human leukocytes (part I): Immunophenotyping of innate immune cells. Age 2015, 37, 92. [Google Scholar] [CrossRef]
- Agrawal, A.; Agrawal, S.; Cao, J.N.; Su, H.; Osann, K.; Gupta, S. Altered innate immune functioning of dendritic cells in elderly humans: A role of phosphoinositide 3-kinase-signaling pathway. J. Immunol. 2007, 178, 6912–6922. [Google Scholar] [CrossRef] [Green Version]
- Panda, A.; Qian, F.; Mohanty, S.; van Duin, D.; Newman, F.K.; Zhang, L.; Chen, S.; Towle, V.; Belshe, R.B.; Fikrig, E.; et al. Age-associated decrease in TLR function in primary human dendritic cells predicts influenza vaccine response. J. Immunol. 2010, 184, 2518–2527. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Shaheen, E.; Drake, R.R.; Chen, N.; Gravenstein, S.; Deng, Y. Aging is associated with a numerical and functional decline in plasmacytoid dendritic cells, whereas myeloid dendritic cells are relatively unaltered in human peripheral blood. Hum. Immunol. 2009, 70, 777–784. [Google Scholar] [CrossRef]
- Tariq, M.A.; Hazeldine, J.; Lord, J.M. Innate Immunosenescence and Its Impact on Health in Old Age. In The Ageing Immune System and Health; Bueno, V., Lord, J., Jackson, T., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Shaw, A.C. Effects of Aging on Human Toll-Like Receptor Function. In Handbook of Immunosenescence; Fulop, T., Franceschi, C., Hirokawa, K., Pawelec, G., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Crooke, S.N.; Ovsyannikova, I.G.; Poland, G.A.; Kennedy, R.B. Immunosenescence: A systems-level overview of immune cell biology and strategies for improving vaccine responses. Exp. Gerontol. 2019, 124, 110632. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Fuentes, M.; Alarcón, M.; Palomo, I. Immune System Dysfunction in the Elderly. An. Acad. Bras. Cienc. 2017, 89, 285–299. [Google Scholar] [CrossRef]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef]
- Herberman, R.B.; Nunn, M.E.; Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int. J. Cancer 1975, 16, 216–229. [Google Scholar] [CrossRef]
- Orange, J.S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 2013, 132, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Antonangeli, F.; Zingoni, A.; Soriani, A.; Santoni, A. Senescent cells: Living or dying is a matter of NK cells. J. Leukoc. Biol. 2019, 105, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Vitale, M.; Della Chiesa, M.; Carlomagno, S.; Pende, D.; Aricò, M.; Moretta, L.; Moretta, A. NK-dependent DC maturation is mediated by TNFalpha and IFNgamma released upon engagement of the NKp30 triggering receptor. Blood 2005, 106, 566–571. [Google Scholar] [CrossRef]
- Chijioke, O.; Münz, C. Dendritic cell derived cytokines in human natural killer cell differentiation and activation. Front. Immunol. 2013, 4, 365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferlazzo, G.; Tsang, M.L.; Moretta, L.; Melioli, G.; Steinman, R.M.; Münz, C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J. Exp. Med. 2002, 195, 343–351. [Google Scholar] [CrossRef]
- Paul, S.; Lal, G. The Molecular Mechanism of Natural Killer Cells Function and Its Importance in Cancer Immunotherapy. Front. Immunol. 2017, 8, 1124. [Google Scholar] [CrossRef]
- Romagnani, C.; Juelke, K.; Falco, M.; Morandi, B.; D’Agostino, A.; Costa, R.; Ratto, G.; Forte, G.; Carrega, P.; Lui, G.; et al. CD56brightCD16-killer Ig-like receptor- NK cells display longer telomeres and acquire features of CD56dim NK cells upon activation. J. Immunol. 2007, 178, 4947–4955. [Google Scholar] [CrossRef]
- Michel, T.; Poli, A.; Cuapio, A.; Briquemont, B.; Iserentant, G.; Ollert, M.; Zimmer, J. Human CD56bright NK Cells: An Update. J. Immunol. 2016, 196, 2923–2931. [Google Scholar] [CrossRef]
- Yang, C.; Siebert, J.R.; Burns, R.; Gerbec, Z.J.; Bonacci, B.; Rymaszewski, A.; Rau, M.; Riese, M.J.; Rao, S.; Carlson, K.S.; et al. Heterogeneity of human bone marrow and blood natural killer cells defined by single-cell transcriptome. Nat. Commun. 2019, 10, 3931. [Google Scholar] [CrossRef]
- Solana, R.; Campos, C.; Pera, A.; Tarazona, R. Shaping of NK cell subsets by aging. Curr. Opin. Immunol. 2014, 29, 56–61. [Google Scholar] [CrossRef]
- Brillantes, M.; Beaulieu, A.M. Memory and Memory-Like NK Cell Responses to Microbial Pathogens. Front. Cell Infect. Microbiol. 2020, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Tarannum, M.; Romee, R. Cytokine-induced memory-like natural killer cells for cancer immunotherapy. Stem. Cell Res. Ther. 2021, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, J.M.; Larbi, A.; Le Page, A.; Fülöp, T. Natural Killer Cells, Aging, and Vaccination. Interdiscip. Top. Gerontol. Geriatr. 2020, 43, 18–35. [Google Scholar] [PubMed]
- Cox, A.; Cevik, H.; Feldman, H.A.; Canaday, L.M.; Lakes, N.; Waggoner, S.N. Targeting natural killer cells to enhance vaccine responses. Trends Pharmacol. Sci. 2021, 42, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Vidal, S.M.; Khakoo, S.I.; Biron, C.A. Natural killer cell responses during viral infections: Flexibility and conditioning of innate immunity by experience. Curr. Opin. Virol. 2011, 1, 497–512. [Google Scholar] [CrossRef] [PubMed]
- Brauning, A.; Rae, M.; Zhu, G.; Fulton, E.; Admasu, T.D.; Stolzing, A.; Sharma, A. Aging of the Immune System: Focus on Natural Killer Cells Phenotype and Functions. Cells 2022, 11, 1017. [Google Scholar] [CrossRef]
- Solana, C.; Tarazona, R.; Solana, R. Immunosenescence of Natural Killer Cells, Inflammation, and Alzheimer’s Disease. Int. J. Alzheimers Dis. 2018, 2018, 3128758. [Google Scholar] [CrossRef]
- Bulut, O.; Kilic, G.; Domínguez-Andrés, J.; Netea, M.G. Overcoming immune dysfunction in the elderly: Trained immunity as a novel approach. Int. Immunol. 2020, 32, 741–753. [Google Scholar] [CrossRef]
- Hazeldine, J.; Hampson, P.; Lord, J.M. Reduced release and binding of perforin at the immunological synapse underlies the age-related decline in natural killer cell cytotoxicity. Aging Cell 2012, 11, 751–759. [Google Scholar] [CrossRef]
- Lutz, C.T.; Karapetyan, A.; Al-Attar, A.; Shelton, B.J.; Holt, K.J.; Tucker, J.H.; Presnell, S.R. Human NK cells proliferate and die in vivo more rapidly than T cells in healthy young and elderly adults. J. Immunol. 2011, 186, 4590–4598. [Google Scholar] [CrossRef]
- Ligotti, M.E.; Aiello, A.; Accardi, G.; Aprile, S.; Bonura, F.; Bulati, M.; Gervasi, F.; Giammanco, G.M.; Pojero, F.; Zareian, N.; et al. Analysis of T and NK cell subsets in the Sicilian population from young to supercentenarian: The role of age and gender. Clin. Exp. Immunol. 2021, 205, 198–212. [Google Scholar] [CrossRef]
- Gounder, S.S.; Abdullah, B.J.J.; Radzuanb, N.E.I.B.M.; Zain, F.D.B.M.; Sait, N.B.M.; Chua, C.; Subramani, B. Effect of Aging on NK Cell Population and Their Proliferation at Ex Vivo Culture Condition. Anal. Cell Pathol. 2018, 2018, 7871814. [Google Scholar] [CrossRef] [PubMed]
- Pera, A.; Campos, C.; López, N.; Hassouneh, F.; Alonso, C.; Tarazona, R.; Solana, R. Immunosenescence: Implications for response to infection and vaccination in older people. Maturitas 2015, 82, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Le Garff-Tavernier, M.; Béziat, V.; Decocq, J.; Siguret, V.; Gandjbakhch, F.; Pautas, E.; Debré, P.; Merle-Beral, H.; Vieillard, V. Human NK cells display major phenotypic and functional changes over the life span. Aging Cell 2010, 9, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Solana, R.; Tarazona, R.; Gayoso, I.; Lesur, O.; Dupuis, G.; Fulop, T. Innate immunosenescence: Effect of aging on cells and receptors of the innate immune system in humans. Semin. Immunol. 2012, 24, 331–341. [Google Scholar] [CrossRef]
- Gayoso, I.; Sanchez-Correa, B.; Campos, C.; Alonso, C.; Pera, A.; Casado, J.G.; Morgado, S.; Tarazona, R.; Solana, R. Immunosenescence of human natural killer cells. J. Innate Immun. 2011, 3, 337–343. [Google Scholar] [CrossRef]
- Hokland, M.; Kuppen, P.J. Natural killer cells: From “disturbing” background to central players of immune responses. Mol. Immunol. 2005, 42, 381–383. [Google Scholar] [CrossRef]
- Maggini, S.; Pierre, A.; Calder, P.C. Immune Function and Micronutrient Requirements Change over the Life Course. Nutrients 2018, 10, 1531. [Google Scholar] [CrossRef]
- Yaqoob, P. Ageing alters the impact of nutrition on immune function. Proc. Nutr Soc. 2017, 76, 347–351. [Google Scholar] [CrossRef]
- Calder, P.C. Nutrition and immunity: Lessons for COVID-19. Nutr. Diabetes 2021, 11, 19. [Google Scholar] [CrossRef]
- Nakaya, H.I.; Hagan, T.; Duraisingham, S.S.; Lee, E.K.; Kwissa, M.; Rouphael, N.; Frasca, D.; Gersten, M.; Mehta, A.K.; Gaujoux, R.; et al. Systems Analysis of Immunity to Influenza Vaccination across Multiple Years and in Diverse Populations Reveals Shared Molecular Signatures. Immunity 2015, 43, 1186–1198. [Google Scholar] [CrossRef] [PubMed]
- Albright, J.M.; Dunn, R.C.; Shults, J.A.; Boe, D.M.; Afshar, M.; Kovacs, E.J. Advanced Age Alters Monocyte and Macrophage Responses. Antioxid. Redox Signal. 2016, 25, 805–815. [Google Scholar] [CrossRef] [PubMed]
- Chambers, E.S.; Vukmanovic-Stejic, M.; Shih, B.B.; Trahair, H.; Subramanian, P.; Devine, O.P.; Glanville, J.; Gilroy, D.; Rustin, M.H.A.; Freeman, T.C.; et al. Recruitment of inflammatory monocytes by senescent fibroblasts inhibits antigen-specific tissue immunity during human aging. Nat. Aging 2021, 1, 101–113. [Google Scholar] [CrossRef]
- Morandi, F.; Yazdanifar, M.; Cocco, C.; Bertaina, A.; Airoldi, I. Engineering the Bridge between Innate and Adaptive Immunity for Cancer Immunotherapy: Focus on γδ T and NK Cells. Cells 2020, 9, 1757. [Google Scholar] [CrossRef]
- Llavero, F.; Alejo, L.B.; Fiuza-Luces, C.; López Soto, A.; Valenzuela, P.L.; Castillo-García, A.; Morales, J.S.; Fernández, D.; Aldazabal, I.P.; Ramírez, M.; et al. Exercise training effects on natural killer cells: A preliminary proteomics and systems biology approach. Exerc. Immunol. Rev. 2021, 27, 125–141. [Google Scholar]
- Rumpf, C.; Proschinger, S.; Schenk, A.; Bloch, W.; Lampit, A.; Javelle, F.; Zimmer, P. The Effect of Acute Physical Exercise on NK-Cell Cytolytic Activity: A Systematic Review and Meta-Analysis. Sports Med. 2021, 51, 519–530. [Google Scholar] [CrossRef]
- Ito, T.; Wang, Y.H.; Liu, Y.J. Plasmacytoid dendritic cell precursors/type I interferon-producing cells sense viral infection by Toll-like receptor (TLR) 7 and TLR9. Springer Semin. Immunopathol. 2005, 26, 221–229. [Google Scholar] [CrossRef]
- Schreibelt, G.; Tel, J.; Sliepen, K.H.; Benitez-Ribas, D.; Figdor, C.G.; Adema, G.J.; de Vries, I.J. Toll-like receptor expression and function in human dendritic cell subsets: Implications for dendritic cell-based anti-cancer immunotherapy. Cancer Immunol. Immunother. 2010, 59, 1573–1582. [Google Scholar] [CrossRef]
- Nouri, Y.; Weinkove, R.; Perret, R. T-cell intrinsic Toll-like receptor signaling: Implications for cancer immunotherapy and CAR T-cells. J. Immunother. Cancer 2021, 9, e003065. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y. Toll-like receptors and immune regulation: Their direct and indirect modulation on regulatory CD4+ CD25+ T cells. Immunology 2007, 122, 149–156. [Google Scholar] [CrossRef]
- Hua, Z.; Hou, B. TLR signaling in B-cell development and activation. Cell Mol. Immunol. 2013, 10, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.X.; Tseng, J.C.; Yu, G.Y.; Luo, Y.; Huang, C.F.; Hong, Y.R.; Chuang, T.H. Recent Advances in the Development of Toll-like Receptor Agonist-Based Vaccine Adjuvants for Infectious Diseases. Pharmaceutics 2022, 14, 423. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Laupèze, B.; Di Pasquale, A.; Hergli, N.; Collignon, C.; Garçon, N. Adjuvant system AS01: Helping to overcome the challenges of modern vaccines. Expert Rev. Vaccines 2017, 16, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Didierlaurent, A.M.; Morel, S.; Lockman, L.; Giannini, S.L.; Bisteau, M.; Carlsen, H.; Kielland, A.; Vosters, O.; Vanderheyde, N.; Schiavetti, F.; et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J. Immunol. 2009, 183, 6186–6197. [Google Scholar] [CrossRef] [PubMed]
- Champion, C.R. Heplisav-B: A Hepatitis B Vaccine With a Novel Adjuvant. Ann. Pharmacother. 2021, 55, 783–791. [Google Scholar] [CrossRef]
- Wells, J.W.; Cowled, C.J.; Farzaneh, F.; Noble, A. Combined triggering of dendritic cell receptors results in synergistic activation and potent cytotoxic immunity. J. Immunol. 2008, 181, 3422–3431. [Google Scholar] [CrossRef]
- Tye, G.J.; Ioannou, K.; Amofah, E.; Quartey-Papafio, R.; Westrop, S.J.; Krishnamurthy, P.; Noble, A.; Harrison, P.M.; Gaensler, K.M.L.; Barber, L.D.; et al. The combined molecular adjuvant CASAC enhances the CD8+ T cell response to a tumor-associated self-antigen in aged, immunosenescent mice. Immun. Ageing 2015, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Gambino, C.M.; Vasto, S.; Ioannou, K.; Candore, G.; Caruso, C.; Farzaneh, F.; Accardi, G.; Caruso, C. (Eds.) Triggering of Toll-Like Receptors in the Elderly. A Pilot Study Relevant for Vaccination. In Updates in Pathobiology: Causality and Chance in Ageing, Age-Related Diseases and Longevity; Palermo University Press: Palermo, Italy, 2017. [Google Scholar]
| Cell | Phenotype | Changes | References |
|---|---|---|---|
| Neutrophils | CD16+ | Reduced response to chemotactic signals; Reduced apoptosis; Impaired signal transduction; Decreased superoxide production; Decreased MHC-expression; Reduced recruitment into lipid rafts. | [20,21,22,23,25,29,30] |
| Monocytes | Classical (CD14++/CD16−) Intermediate (CD14++/CD16+) Non-classical (CD14+/CD16++) | Reduced absolute number and frequency of classical monocytes, increased presence of non-classical and intermediate monocytes; Reduced phagocytosis and chemotaxis; Decreased MHC expression and signalling; Decreased ROS and cytokine production; Altered TLR expression (decreased except for TLR5) and compromised function. | [25,44,45,46,47,48,49] |
| Dendritic cells | Myeloid (CD11c+/CD123−) Plasmacytoid (CD11c−/CD123+) | Decline in function and (or not) number of pDCs Decline in the number of mDCs Impaired migration Decreased maturation and antigen presentation; Reduced phagocytosis in mDCs; Altered TLR expression and signalling in pDCs; Altered CD80 and CD86 expression. | [23,43,57,64,65,66,67,68,69,70,71,72] |
| Natural Killer cells | Cytotoxic (CD56lo/CD16+) Secreting-cytokines (CD56hi/CD16−) | Decreased fraction of CD56hi NK subset, expansion of cytotoxic NK subset; Decreased cytokine production by CD56hi NK; Decreased lytic capacity of CD56lo NK. | [86,97,98,100,102,103] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aiello, A.; Ligotti, M.E.; Garnica, M.; Accardi, G.; Calabrò, A.; Pojero, F.; Arasanz, H.; Bocanegra, A.; Blanco, E.; Chocarro, L.; et al. How Can We Improve Vaccination Response in Old People? Part I: Targeting Immunosenescence of Innate Immunity Cells. Int. J. Mol. Sci. 2022, 23, 9880. https://doi.org/10.3390/ijms23179880
Aiello A, Ligotti ME, Garnica M, Accardi G, Calabrò A, Pojero F, Arasanz H, Bocanegra A, Blanco E, Chocarro L, et al. How Can We Improve Vaccination Response in Old People? Part I: Targeting Immunosenescence of Innate Immunity Cells. International Journal of Molecular Sciences. 2022; 23(17):9880. https://doi.org/10.3390/ijms23179880
Chicago/Turabian StyleAiello, Anna, Mattia Emanuela Ligotti, Maider Garnica, Giulia Accardi, Anna Calabrò, Fanny Pojero, Hugo Arasanz, Ana Bocanegra, Ester Blanco, Luisa Chocarro, and et al. 2022. "How Can We Improve Vaccination Response in Old People? Part I: Targeting Immunosenescence of Innate Immunity Cells" International Journal of Molecular Sciences 23, no. 17: 9880. https://doi.org/10.3390/ijms23179880
APA StyleAiello, A., Ligotti, M. E., Garnica, M., Accardi, G., Calabrò, A., Pojero, F., Arasanz, H., Bocanegra, A., Blanco, E., Chocarro, L., Echaide, M., Fernandez-Rubio, L., Ramos, P., Piñeiro-Hermida, S., Kochan, G., Zareian, N., Farzaneh, F., Escors, D., Caruso, C., & Candore, G. (2022). How Can We Improve Vaccination Response in Old People? Part I: Targeting Immunosenescence of Innate Immunity Cells. International Journal of Molecular Sciences, 23(17), 9880. https://doi.org/10.3390/ijms23179880










