1. Introduction
The definition of inflammation has been continuously adapted. Recently, it was characterized as a tissue response to an emergent stimulus. This reaction can be macro and/or microscopically identified and there is involvement of a variety of cells. Altogether, these events can lead to necrosis, edema, fibrosis, malignancy and/or infection. An excessive inflammatory response not controlled can evolve into several diseases such as arthritis, osteoporosis [
1], asthma [
2], Alzheimer’s disease [
3], cardiovascular disease [
4], cancer [
5] and obesity [
6]. Thus, the search and development of new anti-inflammatory substances that could reduce or eliminate the inflammatory process continues to be an objective for several groups. Although there is a wide variety of anti-inflammatory drugs, there are concomitantly a wide variety of side effects that can limit their use. Thus, the continuous search for new chemical entities with anti-inflammatory potential and lower incidence of side effects remains a goal for researchers in this area.
Derivatives LASSBio-1524 (1) and LASSBio-1760 (2) have already been described as powerful anti-inflammatory prototypes with action in several acute and chronic models of inflammation [
7,
8,
9]. These two compounds present the
N-acylhydrazone (NAH) subunit, widely described as a privileged subunit [
10], useful for discovering new drug candidates due to its peptidomimetic nature and superior stability to chemical and metabolic hydrolysis [
11].
The bioisosteric relationship of 4-nitrophenyl fragment by 4-phenylboronic acid subunit was previously characterized by comparing the anti-inflammatory profiles of compounds 1 and 2 [
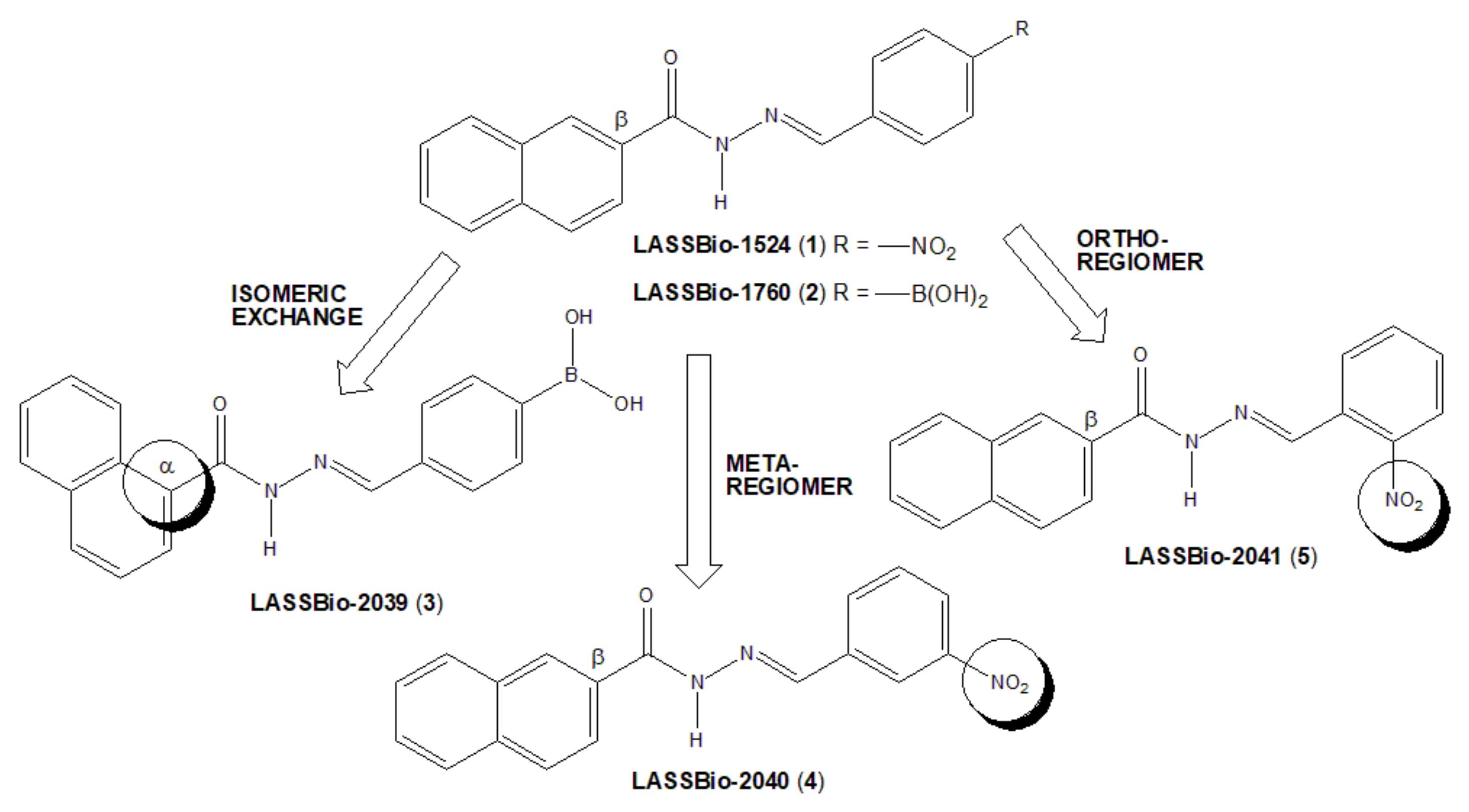
8]. An isomeric exchange was performed in the naphthyl subunit of LASSBio-1760 (2), with NAH linked to position α of the naphthyl subunit to generate LASSBio-2039 (3) (
Figure 1). Additionally, two LASSBio-1524 (1) regioisomers were proposed, with the exchange of the nitro group of LASSBio-1524 from para-position to meta position (LASSBio-2040, 3) or to the ortho position (LASSBio-2041, 5).
The changes introduced in new regioisomeric analogues (
4) and (
5) were purposed to avoid the potential toxicity produced by nitroaromatic derivatives [
12]. The main idea was exchanging the position of the nitro group from a more accessible para-position to a more hindered meta- and ortho-position, respectively, in order to prevent access to the CYP reductase enzyme [
13]. Moreover, it is well-known from the literature that the β-substituted naphthyl group is more susceptible to oxidative metabolism to form toxic metabolites than the corresponding α-substituted naphthyl, as learned from the discovery of β-blocker propranolol from its precursor pronetalol [
14]. So, we proposed the exchange of the β-naphthyl group present in LASSBio-1760 (
2) to the α-naphthyl group in LASSBio-2039 (
3), in order to reduce the potential toxicity of this new drug candidate.
Thus, in this study, we described the synthesis and anti-inflammatory actions of a new small series of naphthyl-N-acylhydrazones (3–5), planned as regioisomeric analogues of LASSBio-1524 (1) and LASSBio-1760 (2).
3. Discussion
In this study, we aimed to evaluate the anti-inflammatory effects of three new molecules (named LASSBio-2039, LASSBio-2040 and LASSBio-2041). The advantage of these structures is based on the presence of a
N-acylhydrazone (NAH) subunit, considered a privileged subunit [
10] used for discovering new drug candidates [
11].
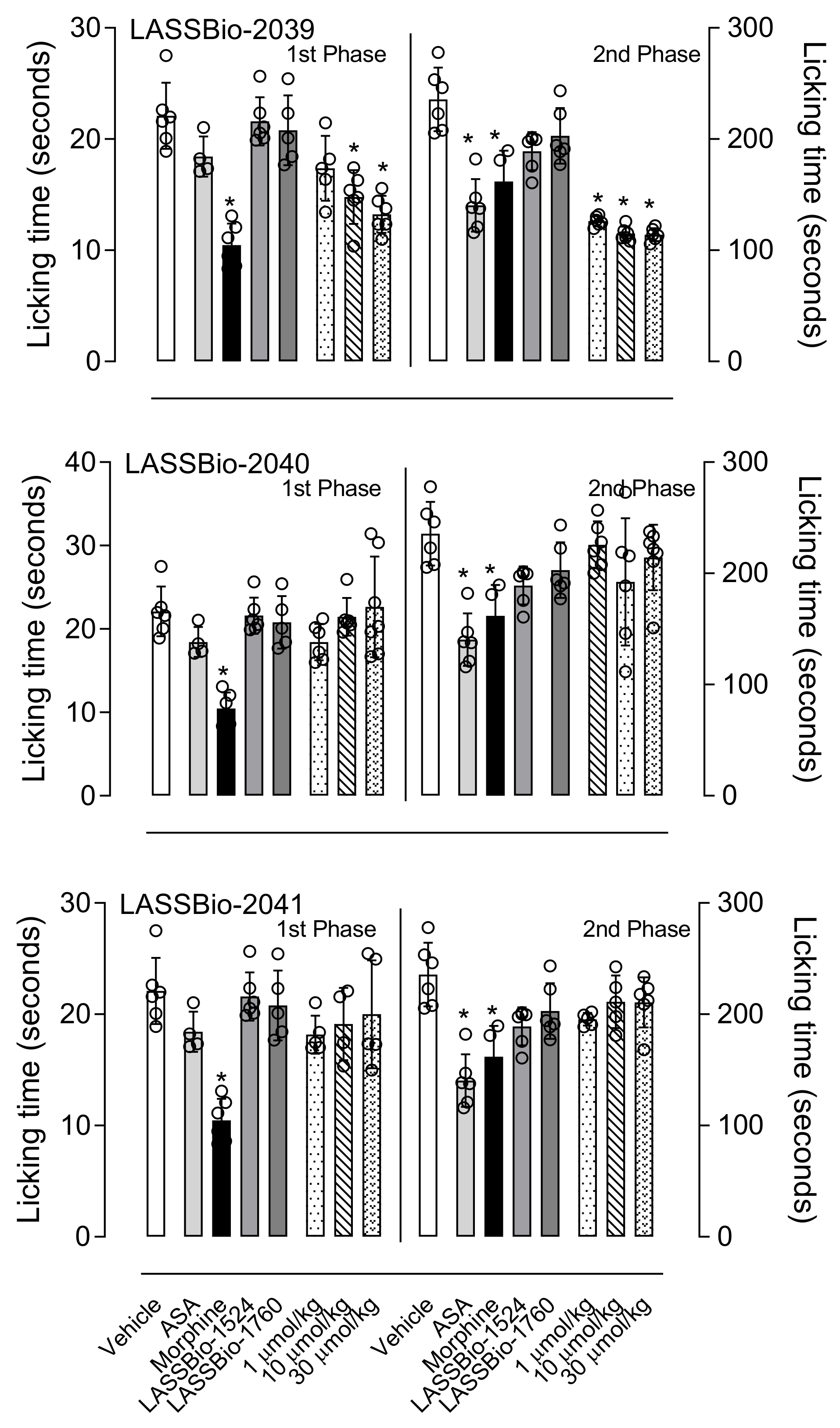
Formalin injection into the hind paw causes a biphasic response, with the first phase (or neurogenic phase) occurring by an activation of nociceptors present in unmyelinated axons and the second phase (or inflammatory phase) occurring due to the release of mediators (i.e., histamine, prostaglandin, and serotonin) that can sensitize sensory neurons [
22]. Drug-mediated reduction in paw licking occurs differently in both phases. Non-steroidal anti-inflammatory drugs act preferentially in the second phase through a peripheral action, whereas opioids act through a central action, inhibiting both phases [
23,
24,
25]. Therefore, this model becomes suitable for the assessment of non-inflammatory or inflammatory pain. Our results showed that pre-treatment with LASSBio-2039 reduced the licking time in both phases and the result in the second phase suggests possible anti-inflammatory activity.
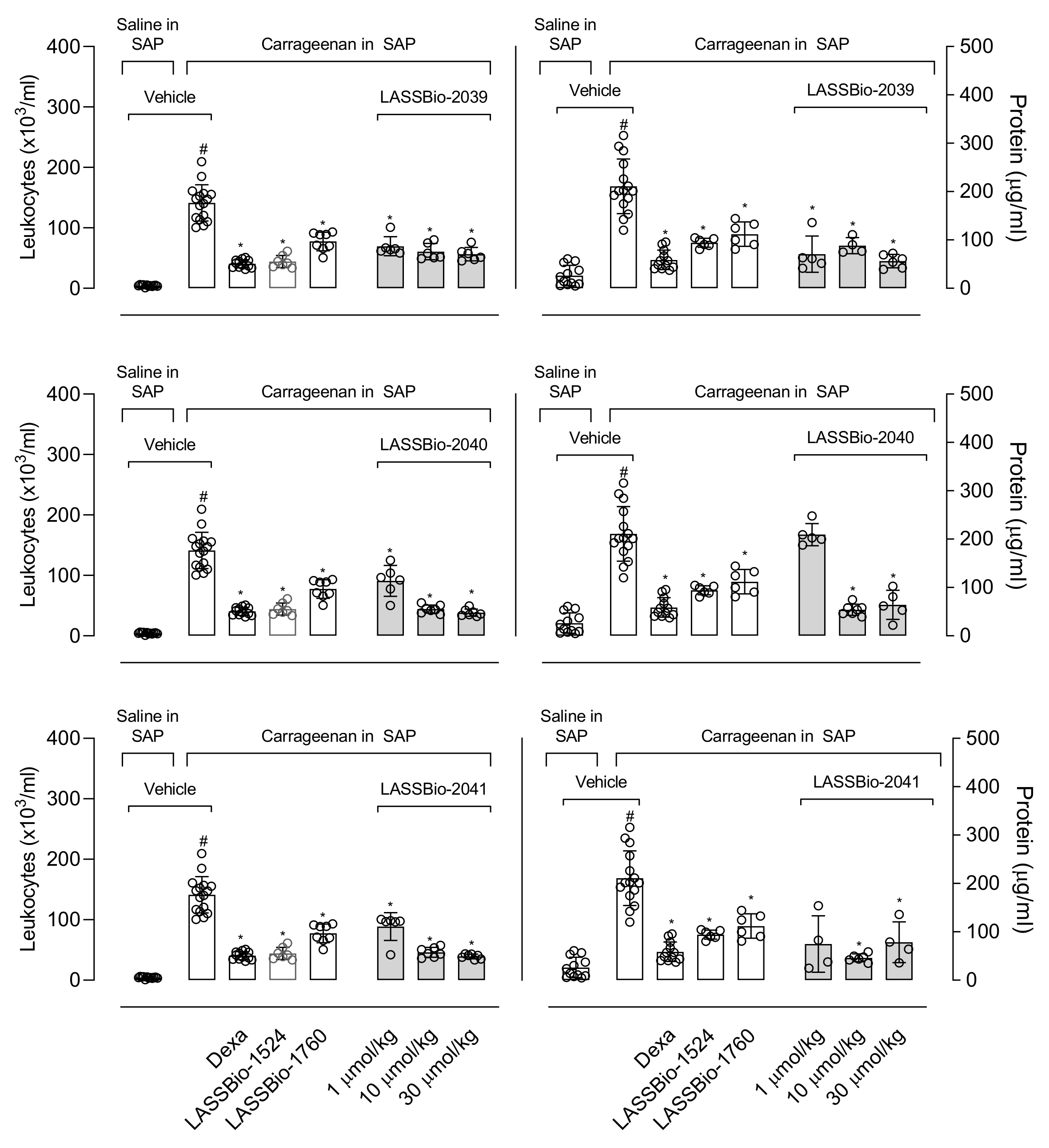
The remarkable effects of LASSBio-2039, even when compared with the original compound, LASSBio-1760, propelled us to continue investigating the anti-inflammatory effects. In this regard, we used the carrageenan-induced inflammation into the subcutaneous air pouch (SAP). This model is suitable for evaluating the local inflammatory response in vivo. Carrageenan injected into the SAP stimulates an inflammatory response with influx of leukocytes, formation of exudate and accumulation of inflammatory mediators [
10]. The migration of cells to the inflammatory site is involved in the development of several diseases. Therefore, inhibiting leukocyte recruitment enables effective control of the inflammatory process [
26]. Pre-treatment with LASSBios caused a significant reduction in leukocyte migration. Some possibilities may be involved with this effect. Among them, the inhibition in the production or release of mediators involved with the chemotaxis process or even an action of LASSBios directly on the cells, preventing them from migrating to the inflammatory site.
The production of inflammatory mediators also stimulates the increase in vascular permeability. This step causes the passage of fluids and plasma proteins from the bloodstream to the tissue [
27]. Our data showed that all three substances reduced the protein present in the exudate. This effect suggests that LASSBios may be acting by inhibiting the increase in vascular permeability, preventing the proteins from passing into the tissue. According to Lampugnani and collaborators [
28], mediators induce the formation of radial stress fibers and the contraction of actomyosin, and this can result in the retraction of the intercellular junction, allowing the passage of fluids and plasma proteins from plasma to tissues. So, our results could suggest a direct effect against cells located in vascular lineage prevent them to contract, thus reducing protein leakage to tissue.
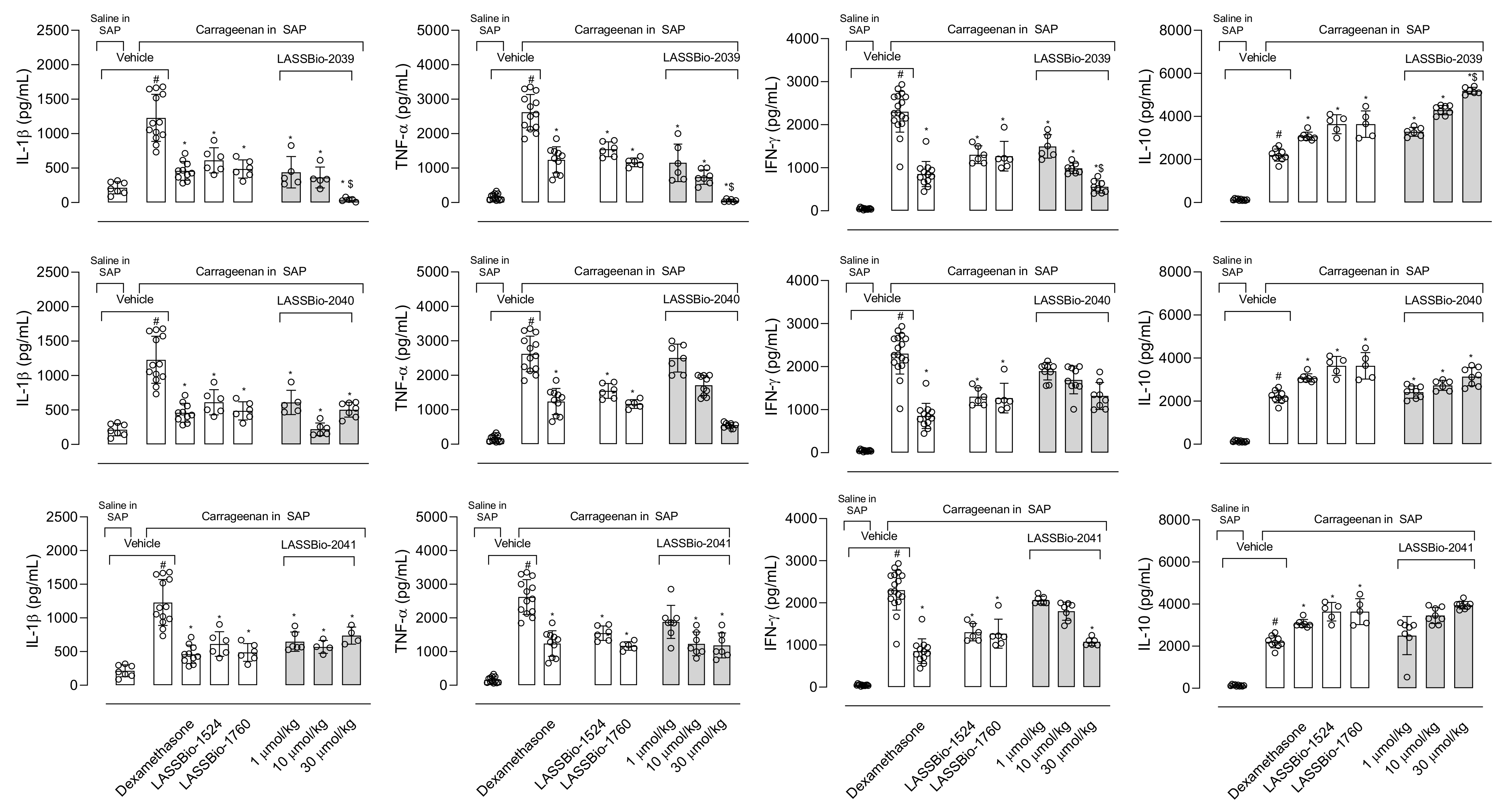
Interleukin-1β (IL-1β) participates in the inflammatory response through the activation of several molecules such as the cyclooxygenase-2 (COX-2) enzyme, nitric oxide (NO) and endothelial adhesion molecules that will act in the maintenance of inflammatory response [
29,
30]. Pre-treatment mice with LASSBios reduced the production of the cytokine in the exudate. The inhibition could lead to a decrease in the migration of cells to the inflammatory site and, therefore, a decrease in the arrival of activated cells, producing other mediators and recruiting more cells. This hypothesis is in accordance with Theofilis and collaborators [
31] who found that cytokines can influence the expression of adhesion molecules that participate in the process of leukocyte diapedesis. Thus, controlling the production of this cytokine brings benefits for the treatment of various inflammatory diseases [
32].
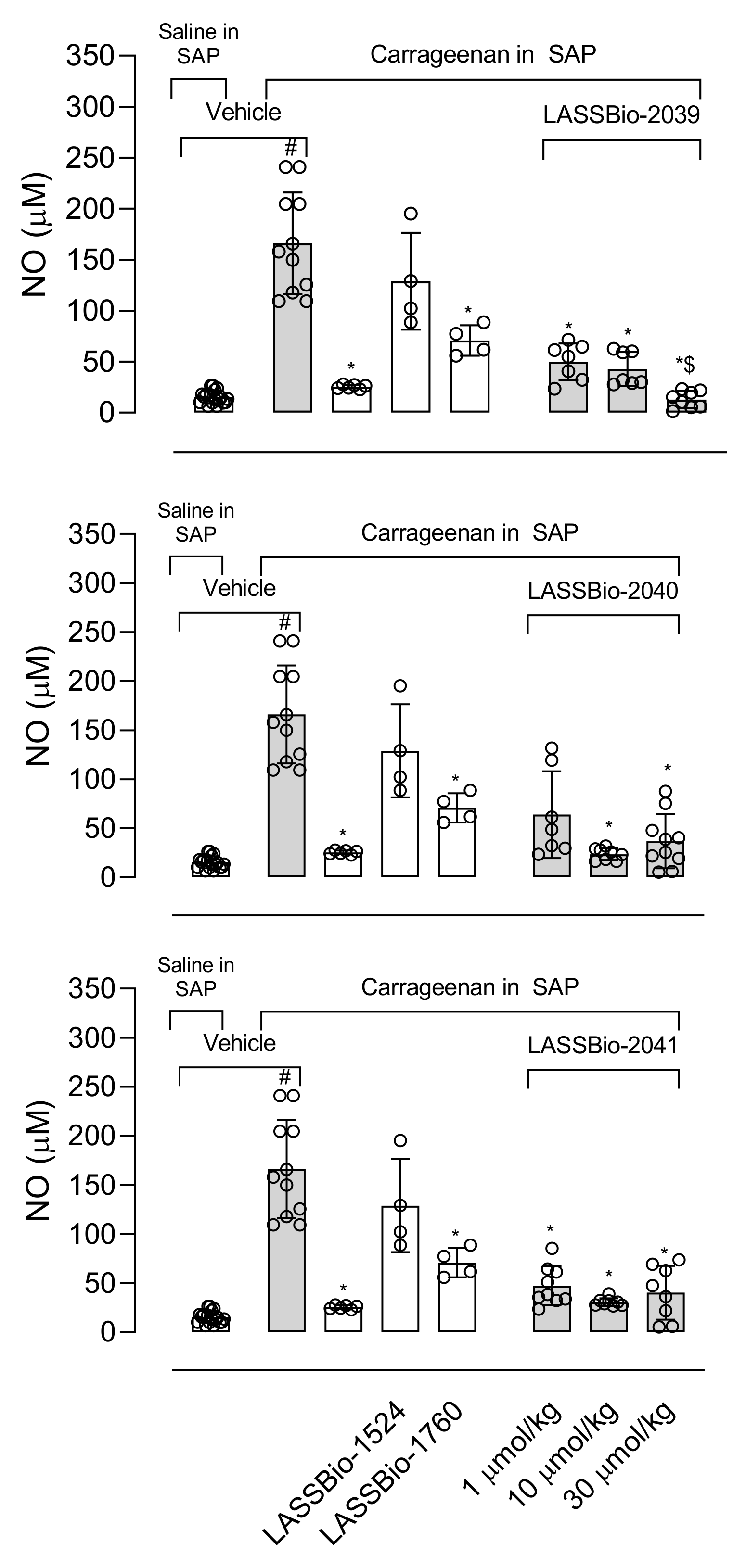
The production of NO plays a fundamental role in the development and maintenance of the inflammatory process. The increase in iNOS (inducible nitric oxide synthase enzyme) expression also occurs in response to activation by lipopolysaccharide (LPS) and the NO produced is one of the key molecules in the pathogenesis of several diseases, so the inhibition of this mediator becomes the target of several anti-inflammatory proposals [
33]. Our results show that LASSBios significantly reduced the concentration of NO in the inflammatory exudate. It could be that once there was a reduction in the number of leukocytes, there would also be a reduction in the production of NO. However, the percentage of reduction in NO concentration is not proportional to the reduction in the number of cells that migrated. In this sense our hypothesis is that it may be that the inhibition observed in NO production could be due to the direct effect of LASSBios on the cells, either by inhibiting the production or the activity of the iNOS.
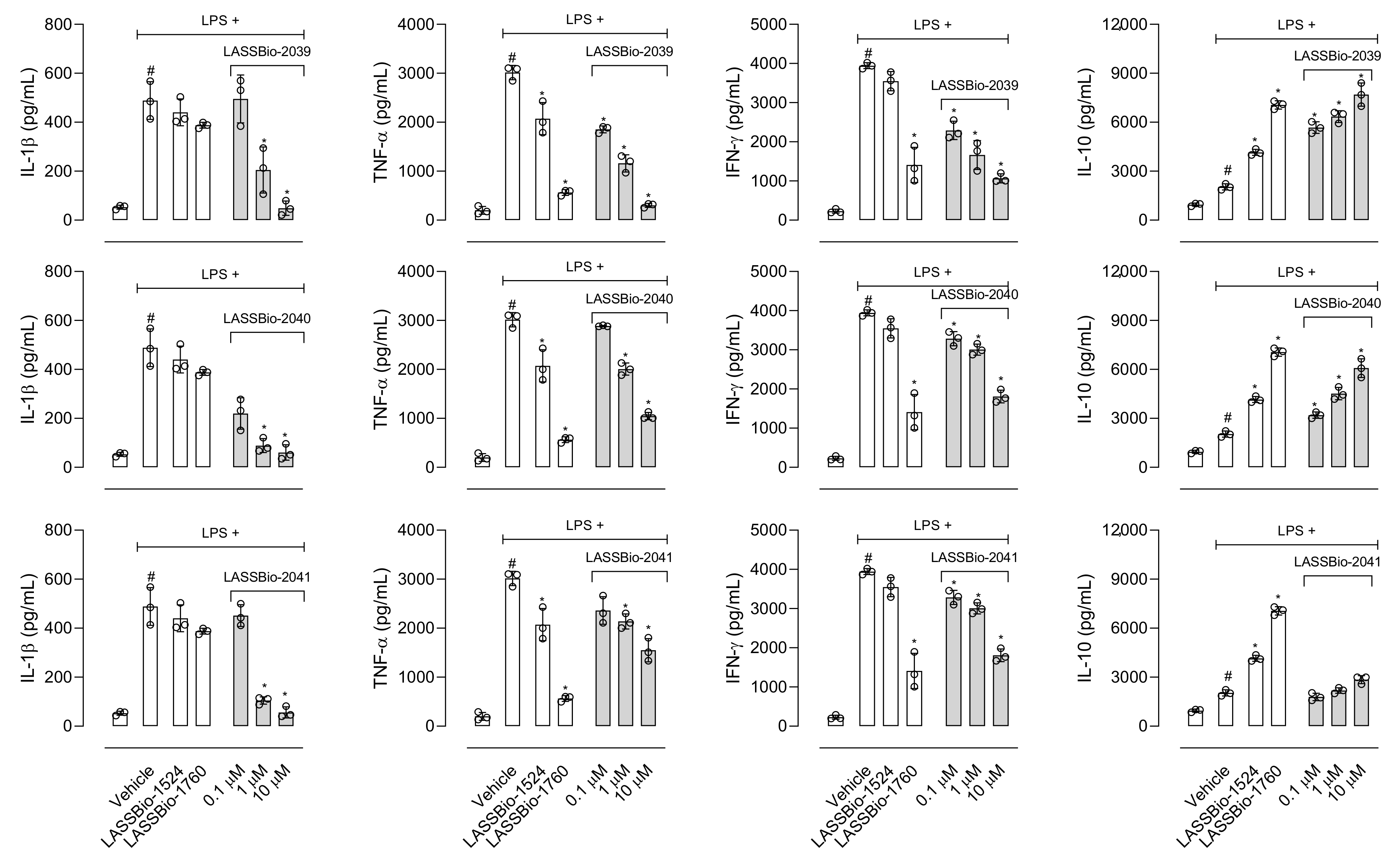
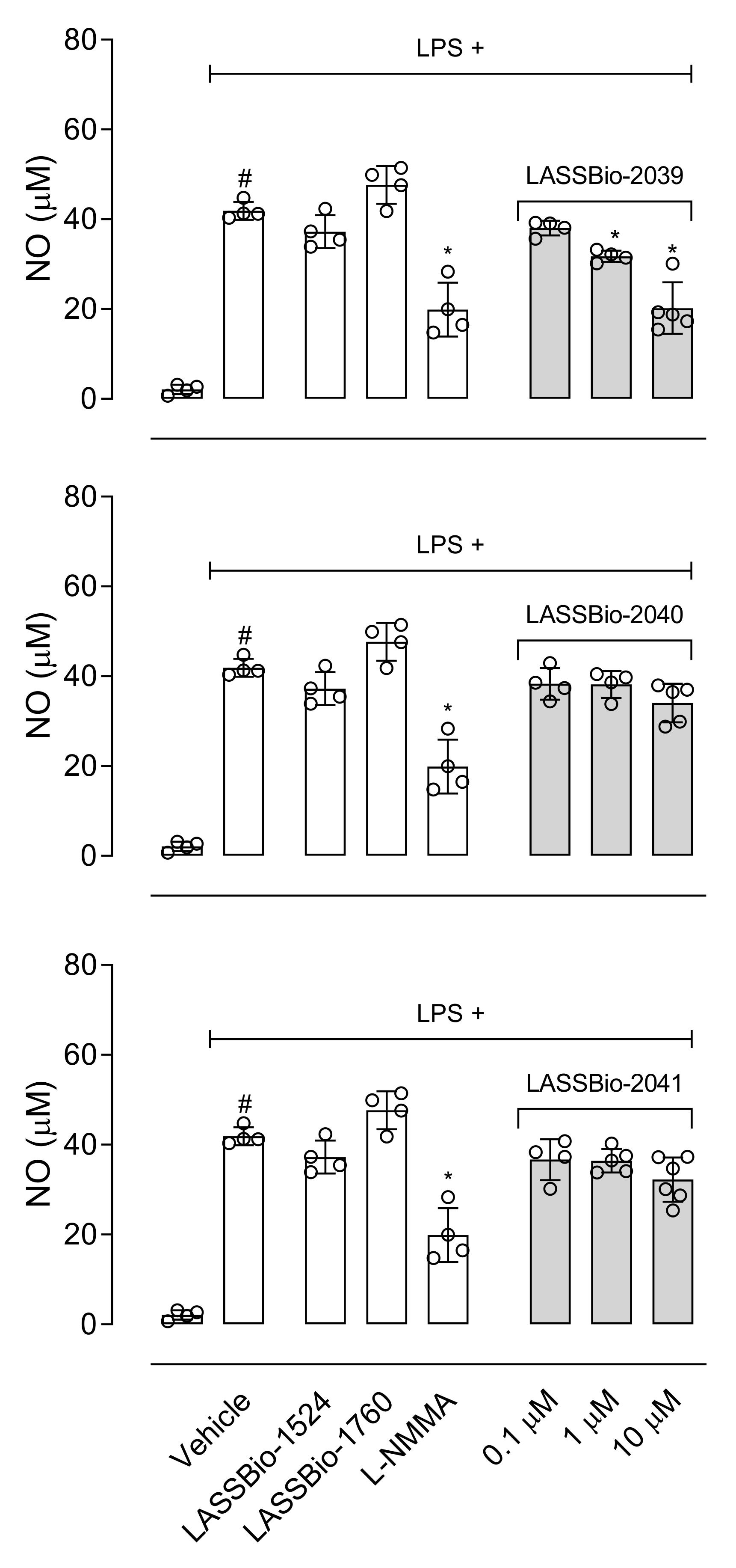
Among the substances that activate macrophages is LPS, which activates Toll-like receptor triggering production of a series of mediators [
34,
35]. Our data show that all LASSBios reduced IL-1β production by LPS-activated cells. Our next step aimed to analyze the effects of LASSBios on NO production in LPS-activated macrophages. Our data indicate that only LASSBio-2039 reduced the NO production by activated macrophages. After 8 h of activation with LPS, there is a peak in the expression of the iNOS enzyme and that, despite this, the production of NO and this production remains for 24 h [
36]. So, we evaluated the effect of LASSBios on NO production after 8 h of LPS activation; however, none of the LASSBios affected NO production. These results together suggest that the action of inhibiting the production of NO caused by LASSBio-2039 can happen through the reduction in the expression of iNOS and not by a reduction in the activity of this enzyme.
After obtaining positive results in reducing the production of inflammatory mediators in vitro, we choose to evaluate whether incubation with LASSBios would influence the process of cell migration. Our assay is advantageous because it mimics the process of cell migration in vivo, in addition to being considered a simple and inexpensive technique to analyze this process [
37]. Only incubation with LASSBio-2039 inhibited the macrophage migration process in vitro. This result could be explained by the role of LASSBios in the inhibition of NO that is involved with the SRC-FAK pathway. Macrophages activated with LPS initiate the synthesis of a series of mediators, including the iNOS enzyme responsible for the synthesis of NO. The NO produced actively participates in the SRC-FAK cascade (steroid receptor co-activator-focal adhesion kinase). Studies indicate that this cascade is linked to the process of macrophage mobility, influencing their migratory capacity [
38,
39]. By understanding the importance of the SRC-FAK cascade in the migration process and how this cascade is highly dependent on NO, our hypothesis is that the effect caused by LASSBio-2039 to reduce cell migration may be occurring due to the reduction caused in the production of NO.
In 2011, LASSBio-1524 was synthesized with the aim of being an inhibitor of the IKK-β enzyme and this was confirmed from structure-based drug design trials. IKK-β is an important enzyme that participates in the activation of the signaling pathway of the nuclear transcription factor kappa B (Nf-κB), the gene responsible for the transcription of several mediators and enzymes that participate in the development and maintenance of the inflammatory process [
7]. In 2016, it was observed not only LASSBio-1524, but also LASSbio-1760 pronounced anti-inflammatory activity through reduced cell migration, reducing NO and TNF-α production. A reduction in the expression of phosphorylated Nf-κB suggested that the anti-inflammatory effects of these compounds occur through the inhibition of the Nf-κB signaling pathway [
8]. The structural modifications performed for the synthesis of LASSBio-2039, LASSBio-2040 and LASSBio-2041 did not influence the anti-inflammatory activity presented by these compounds. When comparing all three new LASSBios with LASSBio-1524 or LASSBio-1760, only the LASSBio-2039 presented a significant effect when compared with the original molecule (LASSBio-1760). As the molecular target of LASSBio-1524 and LASSBio-1760 is the enzyme that participates in the activation of the Nf-κB pathway, we can assume that the LASSBios tested in this study also act on the same molecular target. This signaling pathway can be activated by the cytokine IL-1β and, once activated, initiates the synthesis of iNOS responsible for producing NO. We observed a reduction in the production of the cytokine, which may have decreased the activation of the Nf-κB signaling pathway, which may cause a reduction in leukocyte migration, in NO, in vascular permeability and a consequent reduction in the concentration of protein in the inflammatory exudate.
The modifications carried out in all three new substances evaluated in this study demonstrated an absence of toxic effects observed in nitroaromatic derivatives [
12]. These changes lead to changes in the position of the nitro group to a meta-and ortho-position (in LASSBios-2040 and 2041, respectively). It is also known that the presence of β-substituted naphthyl group confers susceptibility to metabolism with the formation of toxic metabolites [
14]. So, the exchange of this group by a α-naphthyl group (in LASSBio-2039) may have influenced this
N-acylhydrazone to act differently from the others, indicating LASSBio-2039 a pronounced activity in relation to LASSBio-2040 and LASSBio-2041. It is worth noting that the modification carried out in LASSBio-2039 in comparison to LASSBio-1760 resulted in a more pronounced effect, indicating that the addition of the α-naphthyl group resulted in an increase in activity.
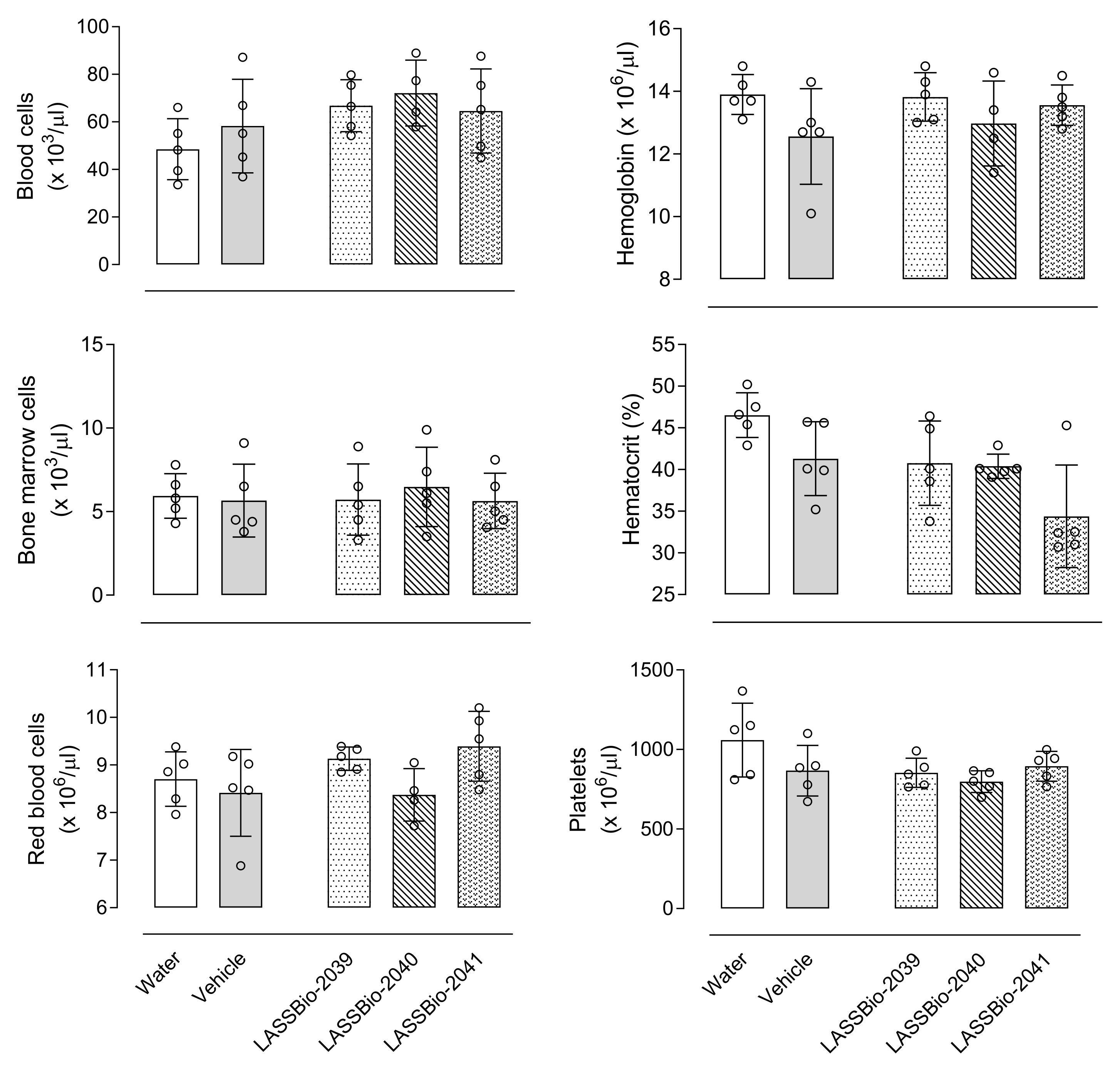
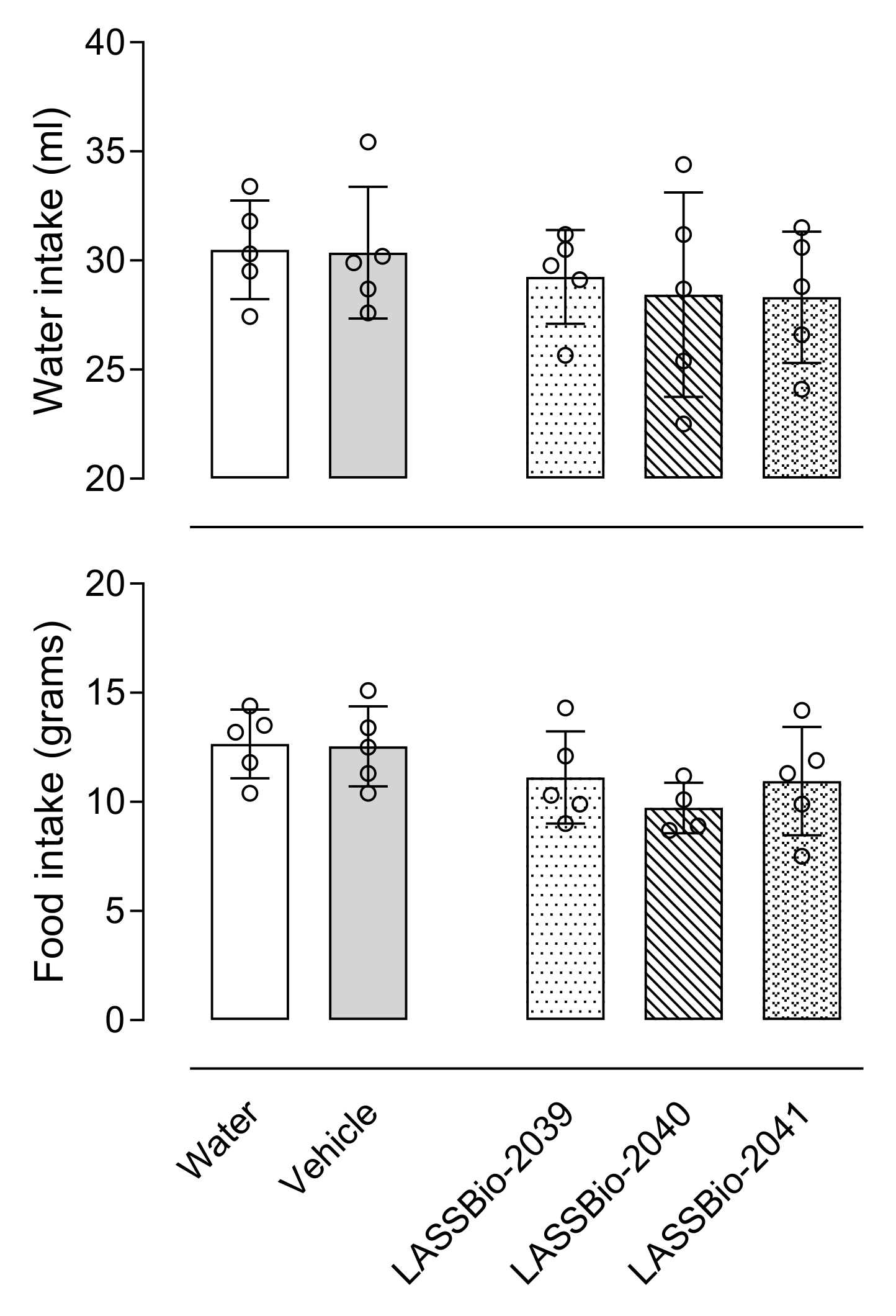
It is also important to emphasize that unlike what is observed with anti-inflammatory drugs already on the market, the substances evaluated in this study did not demonstrate toxic effects, either for changes in the blood count, bone marrow, organs, or behavior, which makes them promising substances for testing in chronic disease models as yet untreated.
Taken together, the data demonstrated in this study suggest LASSBio-2039 with a significant anti-inflammatory profile in pre-clinical models of acute inflammation could be developed as a new substance that can be used as a source for the synthesis of new prototypes.
4. Materials and Methods
4.1. Chemistry
Reagents and solvents were purchased from commercial suppliers and have not been purified. Melting points were determined in a Quimis Q340.23 apparatus and are uncorrected. 1H-NMR and 13C-NMR spectra were recorded on Bruker AC-200, Bruker DRX-300 and Varian MR-400 (coupling constant (J) values were given in Hertz). Infrared spectra (IR) were carried out in the spectrophotometer apparatus Fourier transform IR Nicolet 6700 FT-IR using tablets of potassium bromide (KBr). Purity of the final product was determined by high-performance liquid chromatography (HPLC) on Shimadzu LC-20AD with Kromasil 100–5 C18 column (4.6 mm × 250 mm), and Detector SPD-M20A (diode array). Analyte quantification was performed using a standardized wavelength, 254 nm, and acetonitrile and water 60% were used as the mobile phase.
Synthetic methodologies used to prepare methyl 1-naphthoate have been carefully described in previously published studies [
15,
16]. Moreover, 2-naphthohydrazide was prepared as previously described by Cordeiro et al. [
8,
9]. All the spectroscopical data can be accessed in the
Supplementary Material.
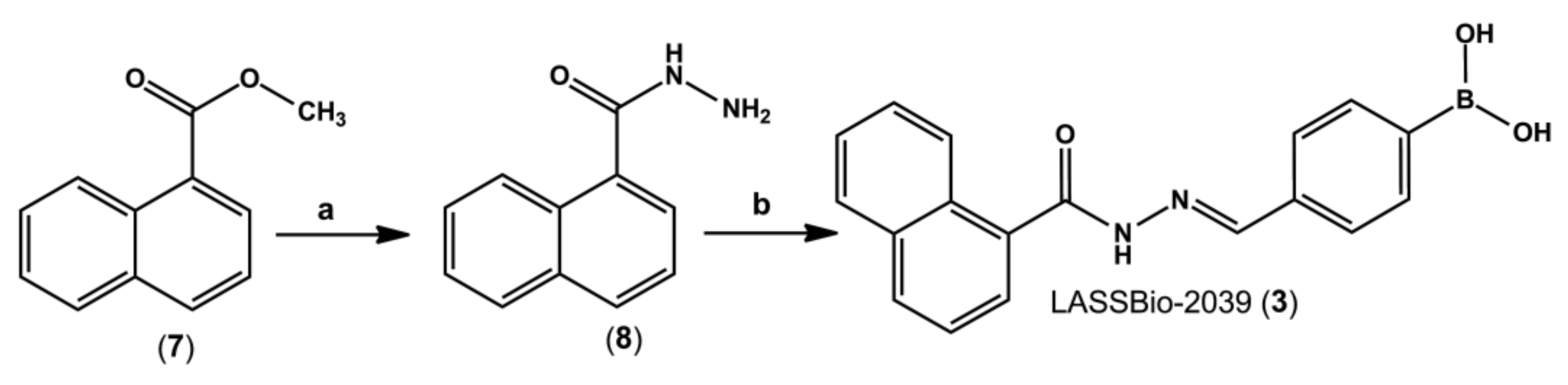
4.2. Synthesis of 1-Naphthohydrazide (8)
Methyl 1-naphthoate (7) (1.4 g, 7.5 mmol) was solubilized in 50 mL of absolute ethanol in a round bottom flask. Then, 5 equivalents of hydrazine hydrate 100% (1.88 mL, 37.6 mmol) were slowly added under magnetic stirring. The mixture was refluxed for 6 h, then volume was partially reduced under reduced pressure. To the flask was added crushed ice and 10 mL of cold water, with precipitate formation. 1-naphthohydrazide (8) (11.6 g, 83%) was isolated by vacuum filtration in a Büchner funnel.
1H-NMR (300 MHz, DMSO-d6) δ 9.71 (s, 1H, NH), 8.23–8.21 (m, 1H), 8.02–7.96 (m, 2H), 7.58–7.51 (m, 4H), 4.62 (s, 3H, NH2); 13C-NMR (75 MHz, DMSO-d6) δ 168.00, 133.35, 133.12, 130.0, 129.96, 128.18, 126.64, 126.23, 125.42, 125.35, 124.99. IR (KBr, cm−1) 3276 (ʋ N-H) 1645 (ν C=O).
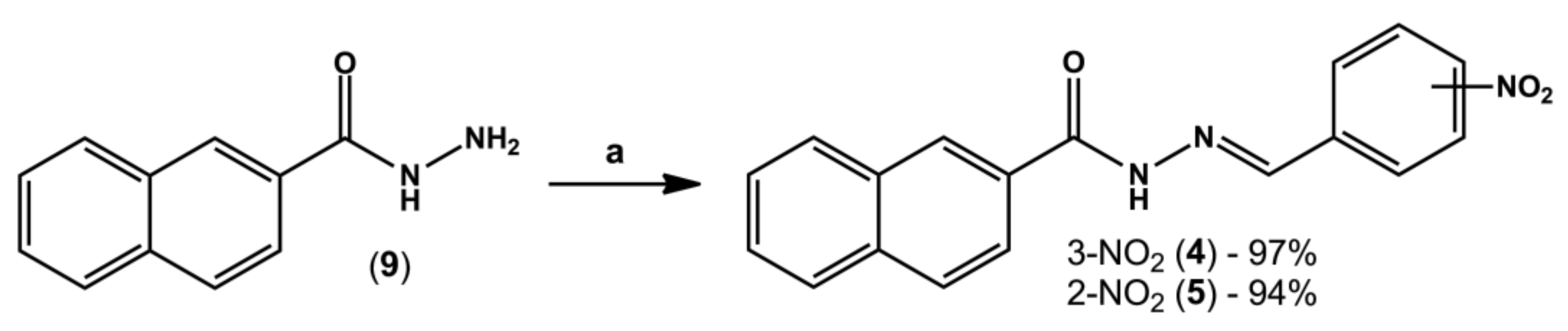
4.3. General Procedure for the Synthesis of N-Acylhydrazone Derivatives (4) and (5)
In a round-bottom flask containing equimolar amounts of hydrazide (8 or 9, 0.3 g, 1.6 mmol) and the desired aromatic aldehyde (0.24 g, 1.6 mmol), three drops of 20% aq. HCl and 20 mL of absolute ethanol were added. The mixture was stirred at room temperature for three hours; however, in a few minutes intense formation of precipitates was already visible. The volume of ethanol was partially reduced under reduced pressure and then crushed ice and cold water were added to the flask. Finally, the solid was collected by vacuum filtration in a Büchner funnel.
4-((E)-(1-naphthoylimino)methyl)phenylboronic acid (LASSBio-2039, 3): White amorphous solid; 92%; m.p. - °C. 1H-NMR (300 MHz, DMSO-d6) δ 12.07 (s, NH), 8.36 (s, 1H), 8.21 (s, 2H, BOH2), 8.10 (d, J = 7.8 Hz, 1H), 8.04–8.01 (m, 1H), 7.90 (d, J = 7.9 Hz, 2H), 7.78–7.72(m, 3H), 7.64–7.59 (m, 4H). 13C-NMR (75 MHz, DMSO-d6) δ 170.73, 164.78, 147.90, 136.38, 135.62, 134.56, 133.17, 132.79, 130.55, 129.96, 128.39, 127.13, 126.49, 126.14, 125.91, 125.02. IR (KBr, cm−1). Purity (HPLC): 99.5%.
(E)-N-(3-nitrobenzylidene)-2-naphthohydrazide (LASSBio-2040, 4): White amorphous solid; 97%; m.p. -°C. 1H-NMR (500 MHz, DMSO-d6) δ 12.32 (s, 1H), 8.61 (s, 1H), 8.57 (s, 2H), 8.27 (d, J = 8.0 Hz, 1H), 8.18 (d, J = 7.6 Hz, 1H), 8.10–8.06 (m, 2H), 8.02–8.00 (m, 2H), 7.76 (t, J = 7.9 Hz, 1H), 7.67–7.61 (m, 2H). 13C-NMR (126 MHz, DMSO-d6) δ 163.49, 148.28, 145.38, 136.26, 134.49, 133.49, 132.09, 130.54, 130.44, 129.02, 128.09, 127.78, 127.04, 124.36, 120.97. IR (KBr, cm−1). Purity (HPLC): 99.2%.
(E)-N-(2-nitrobenzylidene)-2-naphthohydrazide (LASSBio-2041, 5): White amorphous solid; 94%; m.p. -°C. 1H-NMR (300 MHz, DMSO-d6) δ 12.41 (s, 1H), 8.93 (s, 1H), 8.59 (s, 1H), 8.18 (d, J = 7.2 Hz, 1H), 8.11–8.00 (m, 5H), 7.84 (t, J = 7.7 Hz, 1H), 7.71–7.63 (m, 3H). 13C-NMR (75 MHz, DMSO-d6) δ 163.43, 148.3, 142.99, 134.52, 133.82, 132.09, 130.75, 130.29, 129.02, 128.81, 128.4, 128.24, 128.1, 128.02, 127.77, 127.02, 124.75, 124.38. IR (KBr, cm−1). Purity (HPLC): 98.8%.
4.4. Animals
Swiss Webster mice (25–30 g) were kindly donated by Instituto Vital Brazil (Niterói, Rio de Janeiro, Brazil). Mice were maintained in a room in a light-dark cycle of 12 h, 22 ± 2 °C from 60% to 80% humidity and with food and water provided ad libitum. Animals were acclimatized to the laboratory conditions for at least 1h before each test and were used only once throughout the experiments. All protocols were conducted in accordance with the Guidelines on Ethical Standards for Investigation of Experimental Pain in Animals [
40] and followed the principles and guidelines adopted by the National Council for the Control of Animal Experimentation (CONCEA), approved by the Ethical Committee for Animal Research (# 31/19 and 34/19). All experimental protocols were performed during the light phase. Animal numbers per group were kept at a minimum and at the end of each experiment mice were sacrificed by ketamine/xylazine overdose.
4.5. Drugs, Reagents and Treatments
Acetylsalicylic acid (ASA), dexamethasone, L-NMMA (L-NG-monomethyl arginine), Ara-C (cytosine arabinoside), MTT (3-(4,5-dimethyl-ltiazol-2-yl)-2,5-diphenyltetrazole) and lipopolysaccharide were purchased from Sigma Aldrich (St. Louis, MO, USA). Ethanol and formalin were purchased from Merck Inc. (São Paulo, Brazil). Cytokines kits were purchased from BD Biosciences (Franklin Lakes, NJ, USA), protein kit (Kit Pierce BCA ™ Protein Assay) was purchased from ThermoFisher Scientific, Inc. (Waltham, MA, USA). Morphine sulfate was kindly provided by Cristália (São Paulo, Brazil).
LASSBio-1524, LASSBio-1760, LASSBio-2039, LASSBio-2040 and LASSBio-2041 were dissolved in dimethylsulfoxide (DMSO) to prepare 100 µmol/mL stock solutions. For use, solutions were prepared from each stock solution using tween as vehicle. Doses of 0.1 to 10 µmol/kg (final volume of 0.1 mL per animal) were administered by gavage and final tween percentage did not exceed 1%. Acetylsalicylic acid, morphine, dexamethasone, and L-NMMA were used as references drugs. The doses of ASA, morphine, dexamethasone, and L-NMMA were chosen based on previous results obtained by our group when it was calculated the ED50 or IC50, the dose/concentration caused a 50% reduction in the effect in each procedure. The control group was given vehicle (Tween 80, Isofar, Rio de Janeiro, Brazil). All drugs and LASSBios were diluted just before their use. Data for LASSBio-1524 and LASSBio-1760 are original, independent duplicates of past results and have not been published previously.
4.6. Cell Culture
The mouse monocyte macrophage J774.A1 (ATCC # TIB-67) was grown in RPMI medium supplemented with 10% fetal bovine serum (from now on, named as RPMI) and kept in a 5% CO2 incubator at 37 °C. An exchange of RPMI was carried out until cells reached 90% confluence and exponential growth. On the day of assays, cells were collected by scraping bottles and adhered in 96- or 12-well culture plates (2 × 106 cells/mL).
4.7. In Vitro Toxicity Test (Cell Viability)
In 96-well plates, J774.A1 cells (10
5/well, final volume of 200 µL) were adhered at 37 °C, 5% CO
2. After 30 min incubation with LASSBio-1524 or LASSBio-1760 (30 µM), LASSBio-2039, LASSBio-2040 or LASSBio-2041 (0.1, 1 or 10 µM), LPS (1 µg/mL) was added to some groups. After 24 h of incubation (at 37 °C, 5% CO
2), supernatant was changed and MTT solution (5 mg/mL, 100 µL/well) was added. After 4 h of incubation (at 37 °C, 5% CO
2), supernatants were discarded and DMSO (100 µL/well) was added to solubilize the MTT-formazan crystals formed [
41]. Absorbance was measured at a wavelength of 570 nm. Control groups were composed by cells which received only RPMI plus DMSO.
4.8. In vivo Toxicity Test
Different groups of animals received an oral administration of 100 µmol/kg of LASSBios. After 24 h, mice were euthanized with ketamine (50 mg/kg)/xylazine (20 mg/kg). Sample of blood was collected. The femur was removed, the ends were cut, and the bone marrow was washed with 1 mL of saline (NaCl 0.9%) and collected. Both samples of blood and bone marrow were submitted to a complete blood hemogram and cell count, respectively, in an automatic cell counter (PocH-100iV Diff, Sysmex, Kobe, Japan). Signs of acute toxicity, such as behavioral parameters (i.e., convulsion, hyperactivity, sedation, grooming, loss of righting reflexes, or increased or decreased respiration), as well as food and water intake, were observed over a 5-day period after a single oral dose of each substance (100 µmol/kg) administered to a group of ten animals of both sexes. After this period, the animals were sacrificed by ketamine/xylazine overdose, and their stomachs were removed. An incision was made along the great curvature, and the presence of ulcers or perforations and degree of hyperemia was observed and counted.
4.9. Formalin-Induced Paw Licking Model
The method was similar to previously described by Hunskaar and Hole [
23,
24] with modifications [
42]. Briefly, mice received an intraplantar injection of formalin (20 µL, 2.5%) in one hind paw. Immediately they were individually placed in a box and the sum of the times each one remained licking the formalin-injected paw was recorded with the aid of a stopwatch at intervals of 5 min (first phase) or 15–30 min (second phase). Mice were pretreated with vehicle, ASA (1100 µmol/kg), morphine (15 µmol/kg), LASSBio-1524 or LASSBio-1760 (30 µmol/kg), LASSBios (1, 10 or 30 µmol/kg) for 1 h before formalin injection.
4.10. Carrageenan-Induced Inflammation into the Subcutaneous Air Pouch (SAP)
The protocol was based in Raymundo et al. [
43]. A subcutaneous air pouch was induced in the mice’s back through an injection of 10 mL of sterile air. After 3 days, a new injection of 7 mL of sterile air was performed on the animals’ backs. On the 6th day, the animals were orally treated with vehicle, LASSBios (1, 10 or 30 µmol/kg), LASSBio-1524 or LASSBio-1760 (30 µmol/kg) or dexamethasone (6.5 µmol/kg) and after 60 min mice received an injection of saline or carrageenan (0.5%, 1 mL) into the SAP. After 24 h, the animals were euthanized, and the SAP washed with 1 mL of saline. The exudate was collected for leukocyte count and centrifuged at 1500 r.p.m., for 10 min, 4 °C. The supernatant was collected and stored at −20 °C for several dosages (see below).
4.11. Quantification of Proteins and Cytokines
To perform the quantification of proteins in the exudate obtained in the BAS the BCA Protein Assay Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA) was used. Quantification of cytokines was performed in the exudate collected from BAS and in the supernatant of J774.A1 cells, using an immunoenzymatic assay method (ELISA) with specific ELISA kits (BD OptEIATM Set mouse, B&D, Albuquerque, NM, USA). Protocols were carried out according to the manufacturer’s instructions.
4.12. Quantification of Nitric Oxide (NO) Production
When produced in biological fluids, NO interacts with hemoglobin and decays to nitrate (NO
3−) and when its production occurs in vitro it interacts with oxygen decaying to nitrite (NO
2−). As the technique does not quantify NO
3−, it is necessary to convert the nitrate generated after NO production in vivo to nitrite. The protocol for converting nitrate to nitrite was described by Bartholomew [
44] with adaptations made by Raymundo et al. [
43].
Both the supernatant collected in the NO
3− to NO
2− conversion protocol and that collected from cell cultures were mixed, in equal parts, with the Griess reagent [
45]. The absorbance was read in a microplate reader (FlexStation, Molecular Devices, San Jose, CA, USA) at 540 nm. The sodium nitrite concentrations were calculated using a standard sodium nitrite curve.
4.13. Inducible Nitric Oxide (iNOS) Synthase Activity and NO-Scavenger Activity Assays
J774.A1 cells were plated in 96 well-plated and incubated with vehicle or lipopolysaccharide (LPS, 1 µg/mL). After 8 h incubation, different concentrations (0.1, 1 or 10 µM) of each LASSBio were added to different groups. Twenty-four hours after LPS activation, the supernatants were collected to NO measurement.
The NO donor, S-nitroso-N-acetyl-DL-penicillamine (SNAP, at 1 mM) was incubated with vehicle or LASSBios (10 µM) for 12 h at 37 °C. After incubation, an aliquot of 0.1 mL was used for nitrite measurement as previously cited.
4.14. Cell Migration In Vitro
To assess the effect of LASSBios on cell migration in vitro, J774.A1 cells were plated at 1 × 106 cells per well in 12-well plates (in a final volume of 2 mL) and after 3 h a healing was made in the well with the aid of a P20 tip. The wells were washed with RPMI to remove non-adherent cells. In order to inhibit cell proliferation, the anti-mitotic cytosine Arabinoside (AraC; 10−5 M, Sigma-Aldrich, USA) was added to wells. The cells were treated with LASSBios (0.1, 1 or 10 µM) and immediately after treatment and after 24 h, photographs of the wells were performed using an EvosM500 microscope (ThermoFisher). The healing area was measured with the aid of the ImageJ software. To obtain the results, three independent experiments were carried out.
4.15. Statistical Analysis
The experimental groups of the in vivo models were composed of 6 to 12 animals selected at random. The in vitro experiments were repeated at least three times on different days (and with a different cell lot) and each experimental group was carried out in triplicate. The results were expressed as mean ± standard deviation (S.D.) and through the analysis of variance test (ANOVA), statistical significance was calculated followed by the Bonferroni post-test with the aid of the GraphPad Prisma 8.02 software. p values less than 0.05 (* p < 0.05) were considered significant.