Complement System Inhibition Modulates the Inflammation Induced by the Venom of Premolis semirufa, an Amazon Rainforest Moth Caterpillar
Abstract
1. Introduction
2. Results
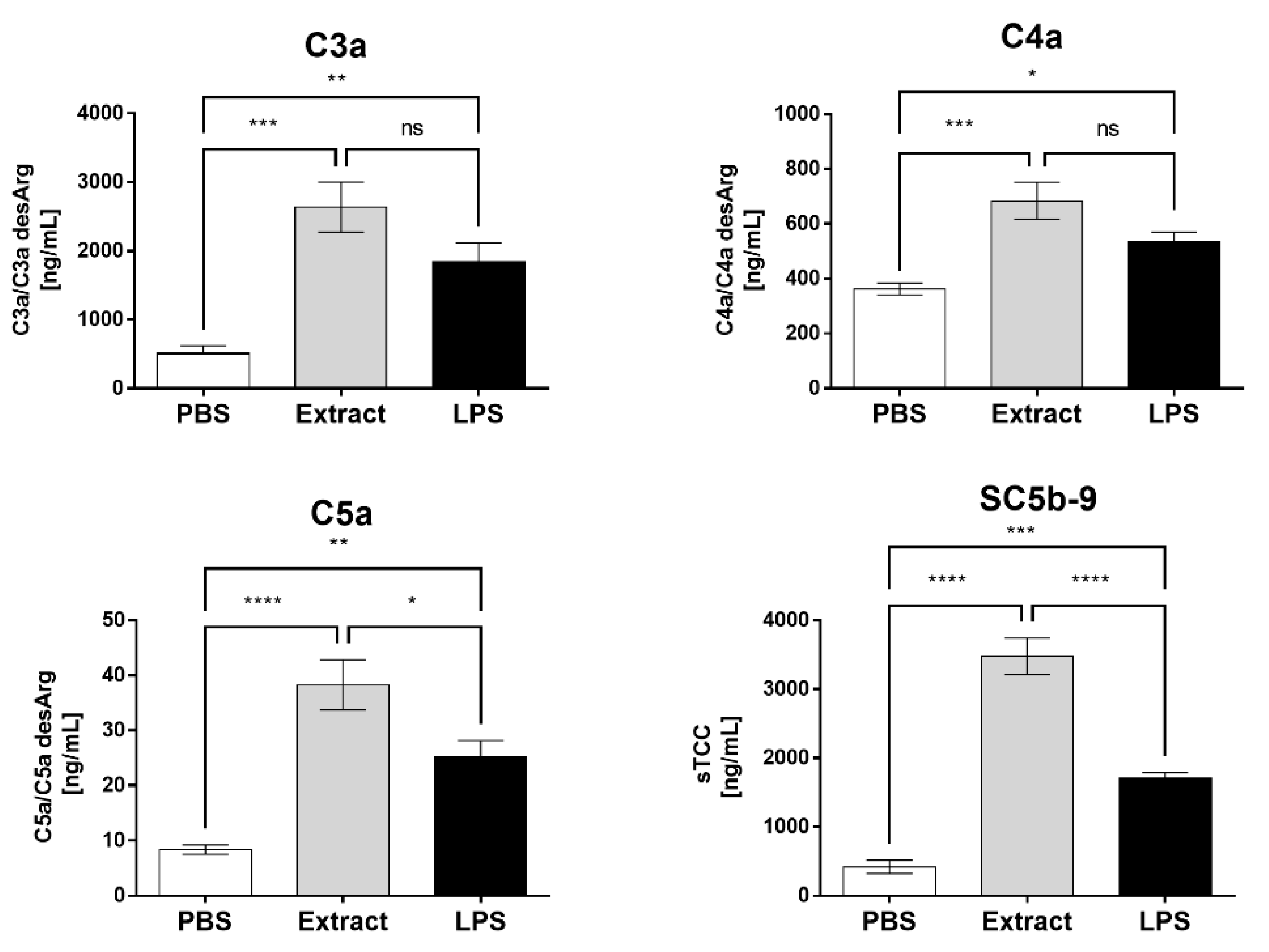
2.1. Pararama Hair Extract Activates the Complement System in Human Blood
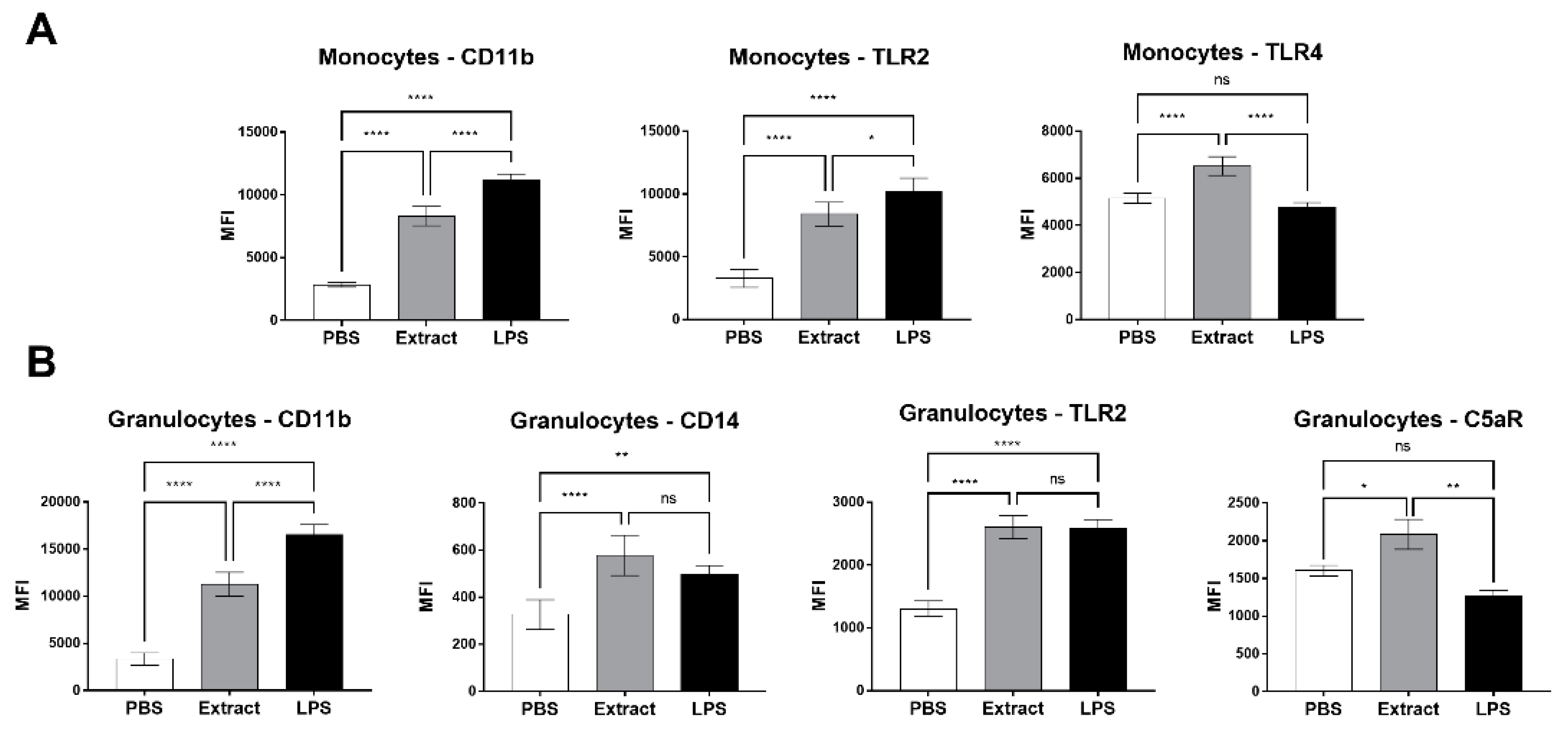
2.2. Pararama Hair Extract Induces Increased Expression of Cell Surface Markers in Human Blood
2.3. Pararama Hair Extract Induces the Production of Cytokines and Chemokines in Human Blood
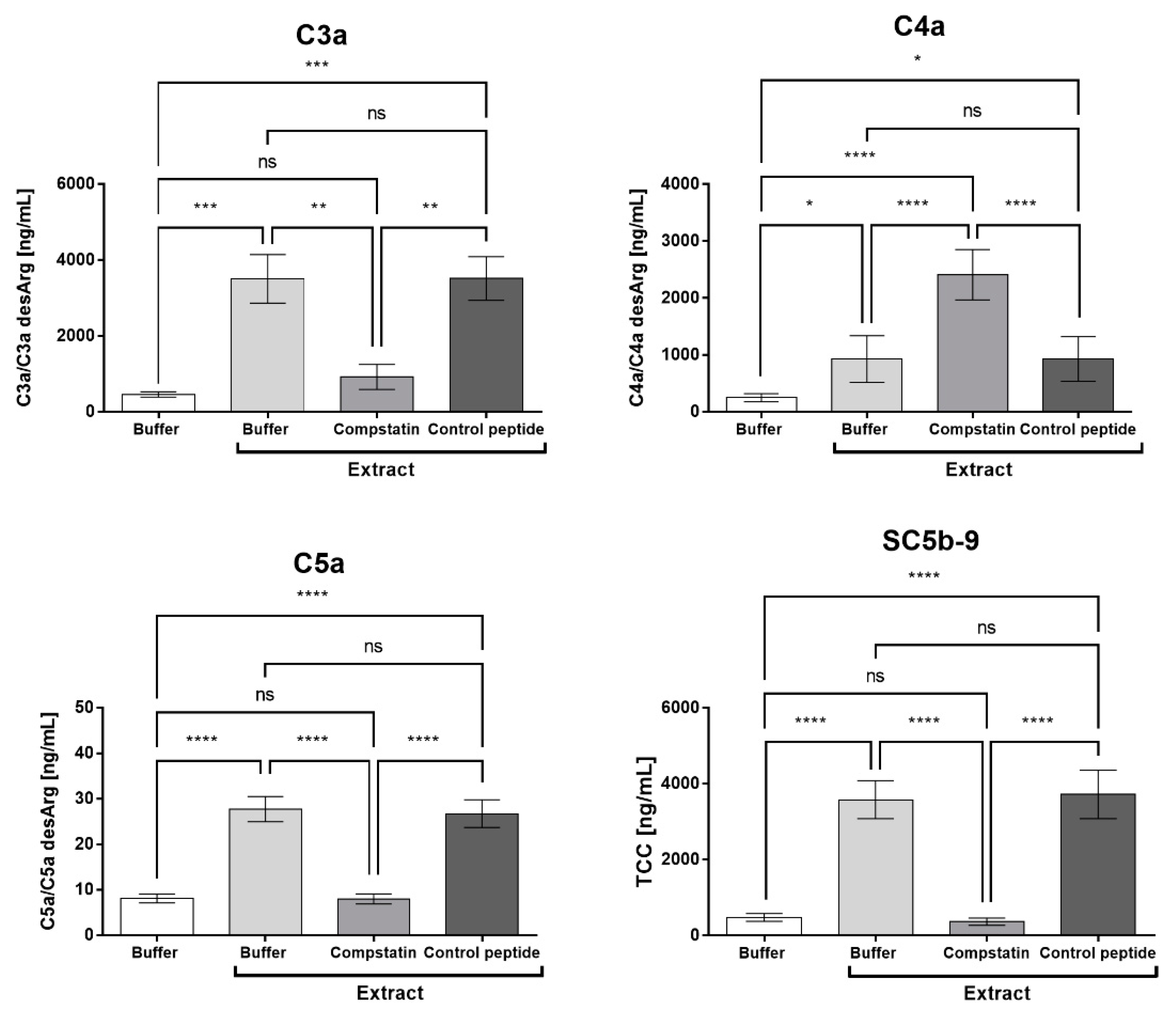
2.4. Compstatin Inhibits C3a, C5a, and TCC Generation Induced by Pararama Hair Extract in Human Blood
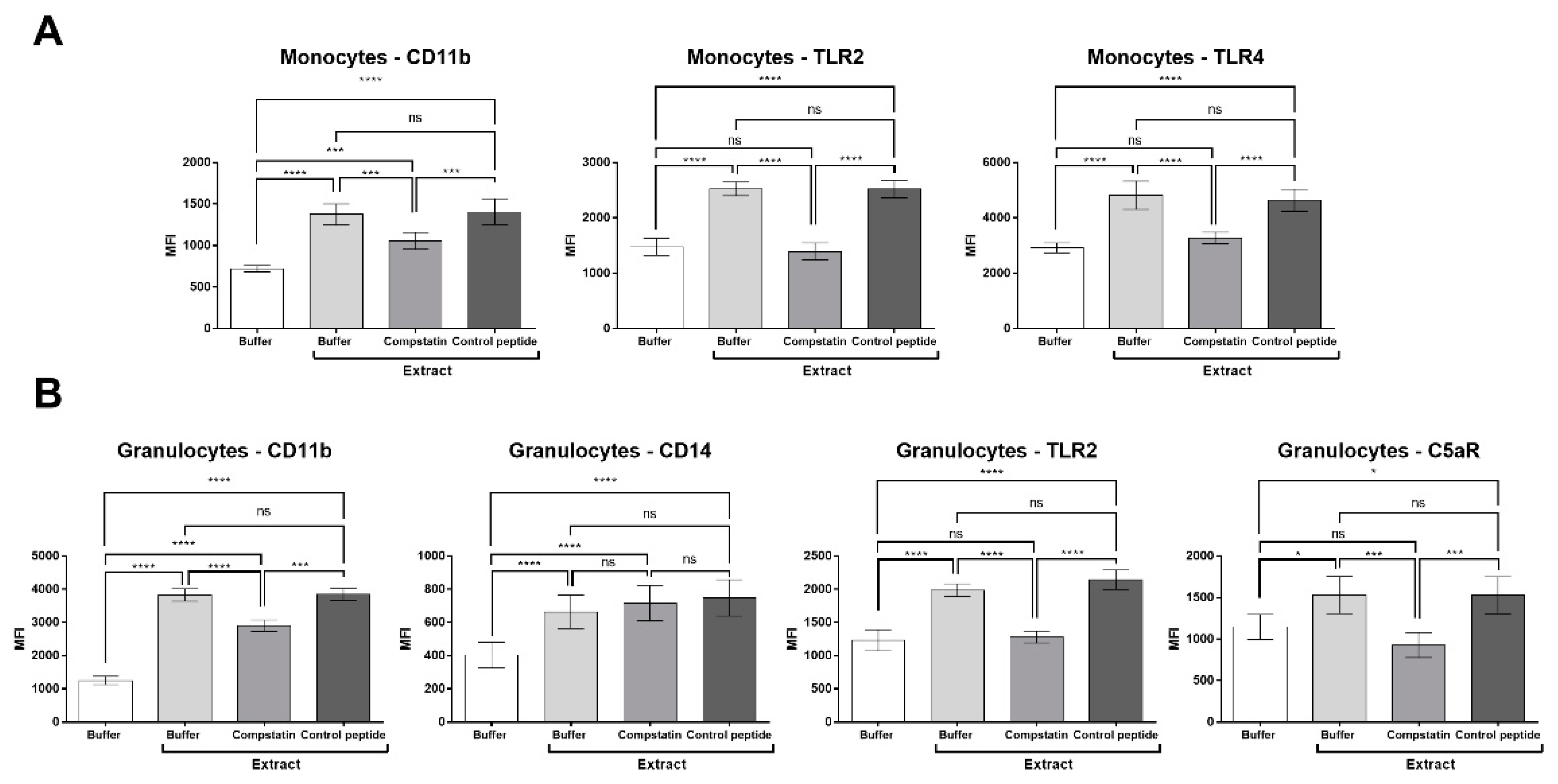
2.5. Compstatin Modulates Surface Cell Marker Expression Induced by Pararama Hair Extract in Granulocytes and Monocytes
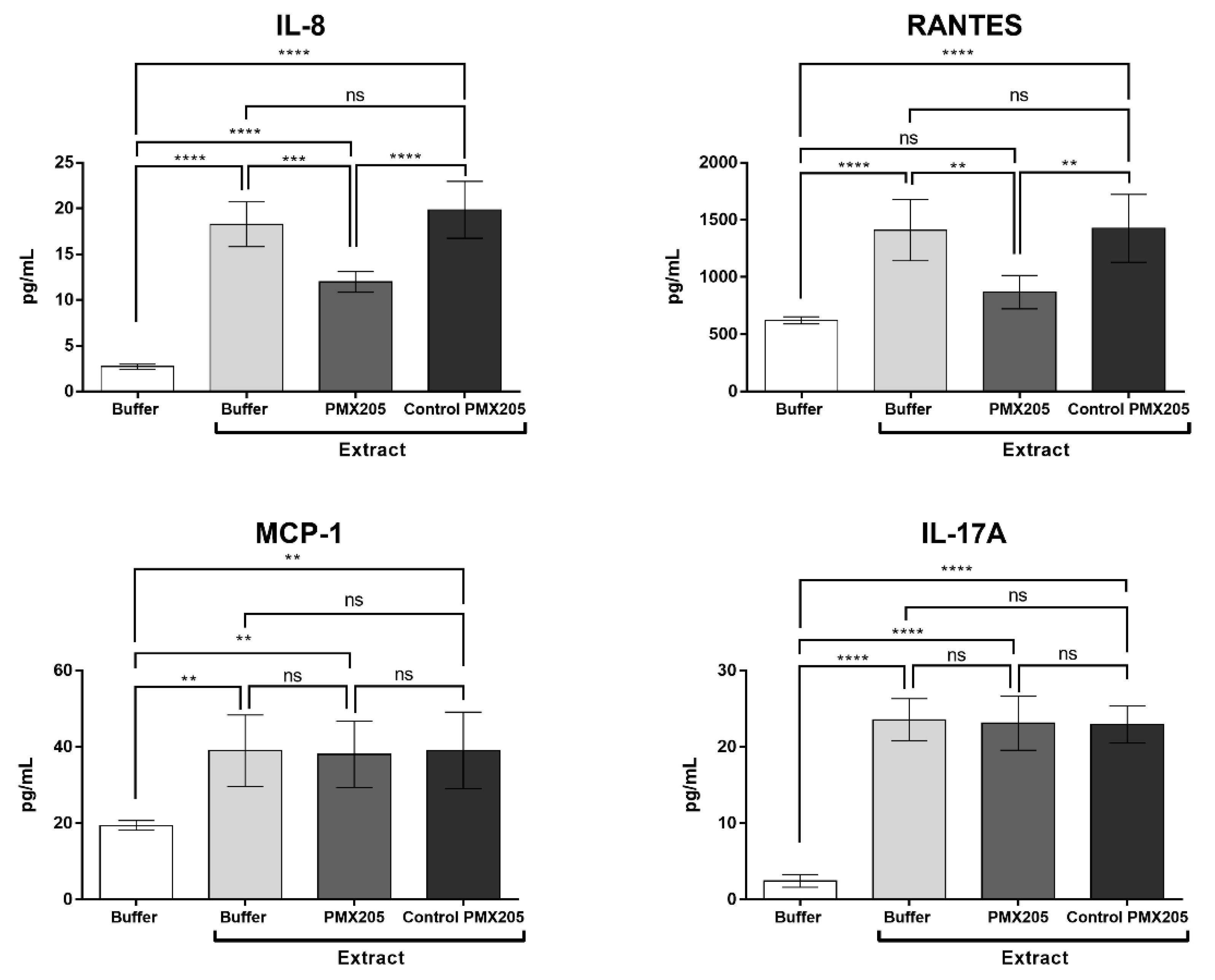
2.6. Compstatin Modulates IL-17, IL-8, RANTES, and MCP-1 Production Induced by Pararama Hair Extract in Human Blood
2.7. C5a is Involved in the Production of IL-8 and RANTES Induced by Pararama Hair Extract in Human Blood
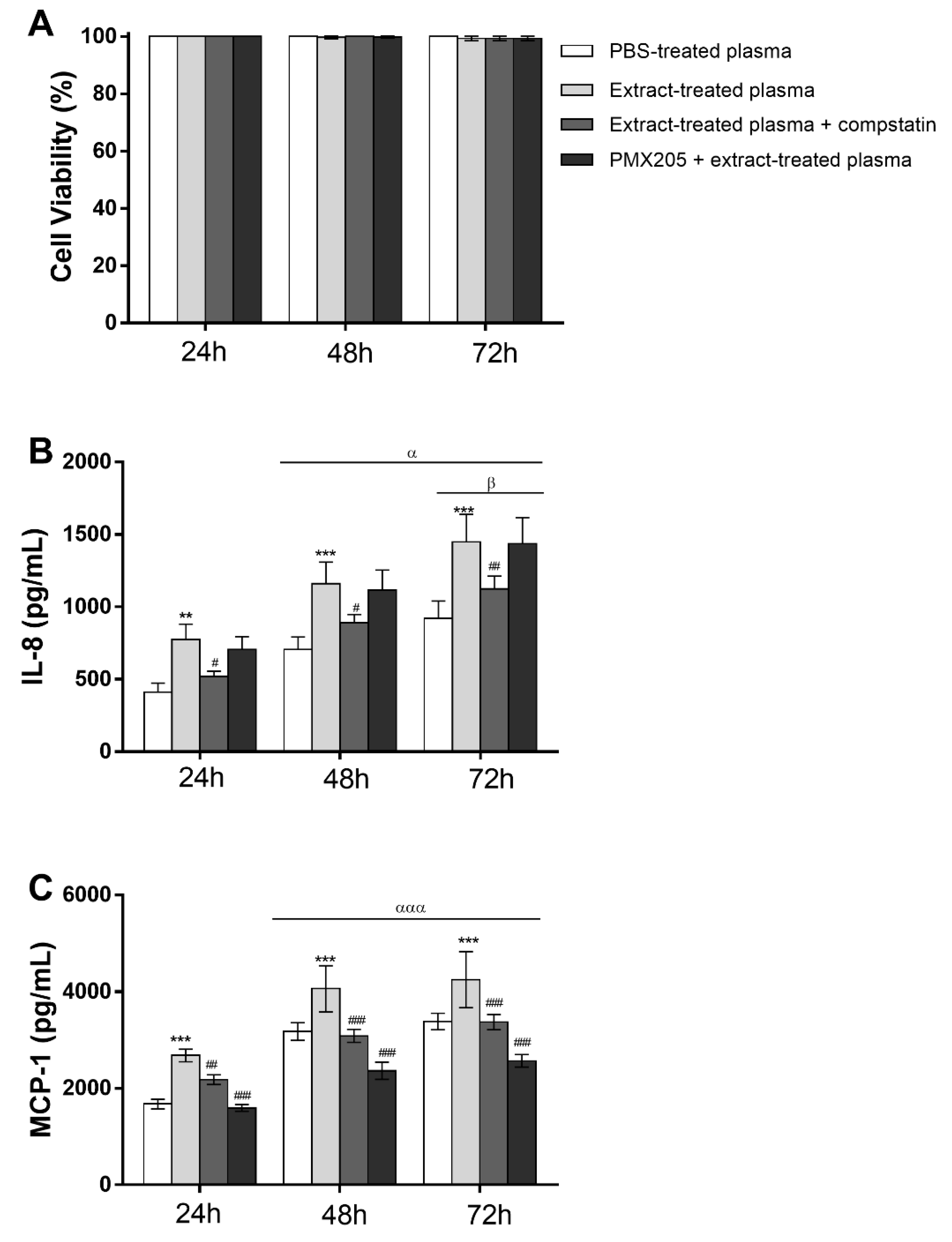
2.8. Pararama Hair Extract Induces IL-8 and MCP-1 Production in Eahy926 Human Endothelial Cells
2.9. Complement Activation Products Induced by Pararama Hair Extract are Involved in IL-8 and MCP-1 Production by Endothelial Cells
3. Discussion
4. Materials and Methods
4.1. Premolis Semirufa Hair Extract
4.2. Human Whole Blood Model
4.3. Blood Sample Treatment
4.4. Complement System Inhibition
4.5. Detection of the Soluble C5b-9 Complex, Anaphylatoxins, Cytokines, and Chemokines in Plasma Samples
4.6. Analysis of Surface Marker Expression in Granulocytes and Monocytes
4.7. Direct and Indirect Action of Pararama Hair Extract on Endothelial Cells
4.8. Endothelial Cell Viability Analysis by MTT Assay
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Costa, R.M. Artropatia da pararamose: Epidemiologia, clínica e modelos experimentais. Ph.D. Thesis, Universidade Federal de São Paulo (UNIFESP)/Escola Paulista de Medicina (EPM), São Paulo, Brazil, 1991. [Google Scholar]
- Costa, R.M.; Silva, N.P.; Leser, P.G.; Andrade, L.E.C.; Junior, A.G.; Atra, E. Activity of bristles from an Amazonian lepidoptera, “Premolis semirufa”, on the human complement system. Rev. Bras. Reumatol. 1995, 35, 143–146. [Google Scholar]
- Dias, L.B.; de Azevedo, M.C. Pararama, a disease caused by moth larvae: Experimental findings. Boletín Oficina Sanit. Panam. OSP 1973, 7, 9–14. [Google Scholar]
- Pidde, G.; Nishiyama, M.Y.; de Oliveira, U.C.; Villas-Boas, I.M.; Paes-Leme, A.F.; Junqueira-de-Azevedo, I.L.; Marques-Porto, R.; Squaiella-Baptistao, C.C.; Tambourgi, D.V. Integrative multiomics analysis of Premolis semirufa caterpillar venom in the search for molecules leading to a joint disease. Sci. Rep. 2021, 11, 1995. [Google Scholar] [CrossRef]
- Villas-Boas, I.M.; Pidde, G.; Lichtenstein, F.; Ching, A.T.C.; Junqueira-de-Azevedo, I.L.M.; DeOcesano-Pereira, C.; Trufen, C.E.M.; Chudzinski-Tavassi, A.M.; Morais, K.L.P.; Tambourgi, D.V. Human Chondrocyte Activation by Toxins from Premolis semirufa, an Amazon Rainforest Moth Caterpillar: Identifying an Osteoarthritis Signature. Front. Immunol. 2020, 11, 2191. [Google Scholar] [CrossRef]
- Cardoso, A.E.C.; Junior, V.H. Accidents caused by lepidopterans (moth larvae and adult): Study on the epidemiological, clinical and therapeutic aspects Acidentes por Lepidópteros (larvas e adultos de mariposas): Estudo dos aspectos epidemiológicos, clínicos e terapêuticos. An. Bras. Dermatol. 2005, 80, 573–578. [Google Scholar] [CrossRef]
- Villas-Boas, I.M.; Alvarez-Flores, M.P.; Chudzinski-Tavassi, A.M.; Tambourgi, D.V. Envenomation by caterpillars. In Clinical Toxinology; Gopalakrishnakone, P., Faiz, S., Gnanathasan, C., Habib, A., Fernando, R., Yang, C., Eds.; Springer: Dordrecht, The Netherlands, 2018; pp. 429–449. [Google Scholar]
- Villas-Boas, I.M.; Goncalves-de-Andrade, R.M.; Pidde-Queiroz, G.; Assaf, S.L.; Portaro, F.C.; Sant’Anna, O.A.; van den Berg, C.W.; Tambourgi, D.V. Premolis semirufa (Walker, 1856) envenomation, disease affecting rubber tappers of the Amazon: Searching for caterpillar-bristles toxic components. PLoS Negl. Trop. Dis. 2012, 6, e1531. [Google Scholar] [CrossRef]
- Villas-Boas, I.M.; Goncalves-de-Andrade, R.M.; Squaiella-Baptistao, C.C.; Sant’Anna, O.A.; Tambourgi, D.V. Characterization of phenotypes of immune cells and cytokines associated with chronic exposure to Premolis semirufa caterpillar bristles extract. PLoS ONE 2013, 8, e71938. [Google Scholar] [CrossRef]
- Villas-Boas, I.M.; Pidde-Queiroz, G.; Magnoli, F.C.; Goncalves-de-Andrade, R.M.; van den Berg, C.W.; Tambourgi, D.V. A serine protease isolated from the bristles of the Amazonic caterpillar, Premolis semirufa, is a potent complement system activator. PLoS ONE 2015, 10, e0118615. [Google Scholar] [CrossRef]
- Leffler, J.; Bengtsson, A.A.; Blom, A.M. The complement system in systemic lupus erythematosus: An update. Ann. Rheum. Dis. 2014, 73, 1601–1606. [Google Scholar] [CrossRef]
- Chimenti, M.S.; Ballanti, E.; Triggianese, P.; Perricone, R. Vasculitides and the complement system: A comprehensive review. Clin. Rev. Allergy Immunol. 2015, 49, 333–346. [Google Scholar] [CrossRef]
- Ma, R.; Cui, Z.; Liao, Y.-H.; Zhao, M.-H. Complement activation contributes to the injury and outcome of kidney in human anti-glomerular basement membrane disease. J. Clin. Immunol. 2013, 33, 172–178. [Google Scholar] [CrossRef]
- Lim, W. Complement and the antiphospholipid syndrome. Curr. Opin. Hematol. 2011, 18, 361–365. [Google Scholar] [CrossRef]
- Senaldi, G.; Lupoli, S.; Vergani, D.; Black, C.M. Activation of the complement system in systemic sclerosis. Relationship to clinical severity. Arthritis Rheum. 1989, 32, 1262–1267. [Google Scholar] [CrossRef]
- Lahoria, R.; Selcen, D.; Engel, A.G. Microvascular alterations and the role of complement in dermatomyositis. Brain 2016, 139, 1891–1903. [Google Scholar] [CrossRef]
- Okroj, M.; Heinegård, D.; Holmdahl, R.; Blom, A.M. Rheumatoid arthritis and the complement system. Ann. Med. 2007, 39, 517–530. [Google Scholar] [CrossRef]
- Silawal, S.; Triebel, J.; Bertsch, T.; Schulze-Tanzil, G. Osteoarthritis and the complement cascade. Clin. Med. Insights Arthritis Musculoskelet. Disord. 2018, 11, 1179544117751430. [Google Scholar] [CrossRef]
- Wang, Q.; Rozelle, A.L.; Lepus, C.M.; Scanzello, C.R.; Song, J.J.; Larsen, D.M.; Crish, J.F.; Bebek, G.; Ritter, S.Y.; Lindstrom, T.M. Identification of a central role for complement in osteoarthritis. Nat. Med. 2011, 17, 1674–1679. [Google Scholar] [CrossRef]
- Abbink, J.J.; Kamp, A.M.; Nuijens, J.H.; Erenberg, A.J.; Swaak, A.J.; Hack, C.E. Relative contribution of contact and complement activation to inflammatory reactions in arthritic joints. Ann. Rheum. Dis. 1992, 51, 1123–1128. [Google Scholar] [CrossRef]
- Guisantes, J.; Tuñon, J.; Subirá, M.; Valtueña, J. Deposits of complement in synovial vessels in Reiter’s syndrome. A role for hydatid antigen? Allergol. Immunopathol. 1983, 11, 273–275. [Google Scholar]
- Hasselbacher, P. Immunoelectrophoretic assay for synovial fluid C3 with correction for synovial fluid globulin. Arthritis Rheum. 1979, 22, 243–250. [Google Scholar] [CrossRef]
- Holers, V.M.; Banda, N.K. Complement in the initiation and evolution of rheumatoid arthritis. Front. Immunol. 2018, 9, 1057. [Google Scholar] [CrossRef]
- Hunder, G.G.; McDuffie, F.C.; Mullen, B. Activation of complement components C3 and factor B in synovial fluids. J. Lab. Clin. Med. 1977, 89, 160–171. [Google Scholar] [PubMed]
- Mellbye, O.; Naes, B.; Munthe, E. Complement and immunoglobulins in synovial fluid from synovectomized patients with rheumatoid arthritis. Ann. Rheum. Dis. 1976, 35, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Naff, G.B.; Byers, P.H. Complement as a mediator of inflammation in acute gouty arthritis. I. Studies on the reaction between human serum complement and sodium urate crystals. J. Lab. Clin. Med. 1973, 81, 747–760. [Google Scholar] [PubMed]
- Pekin, T.J.; Zvaifler, N.J. Hemolytic complement in synovial fluid. J. Clin. Investig. 1964, 43, 1372–1382. [Google Scholar] [CrossRef] [PubMed]
- Russell, I.J.; Mansen, C.; Kolb, L.M.; Kolb, W.P. Activation of the fifth component of human complement (C5) induced by monosodium urate crystals: C5 convertase assembly on the crystal surface. Clin. Immunol. Immunopathol. 1982, 24, 239–250. [Google Scholar] [CrossRef]
- Hedberg, H. The depressed synovial complement activity in adult and juvenile rheumatoid arthritis. Acta Rheumatol. Scand. 1964, 10, 109–127. [Google Scholar]
- Hedberg, H.; Lundh, B.; Laurell, A.-B. Studies of the third component of complement in synovial fluid from arthritic patients. II. Conversion and its relation to total complement. Clin. Exp. Immunol. 1970, 6, 707. [Google Scholar]
- Trouw, L.; Daha, N.; Kurreeman, F.; Böhringer, S.; Goulielmos, G.; Westra, H.; Zhernakova, A.; Franke, L.; Stahl, E.; Levarht, E. Genetic variants in the region of the C1q genes are associated with rheumatoid arthritis. Clin. Exp. Immunol. 2013, 173, 76–83. [Google Scholar] [CrossRef]
- Sturfelt, G.; Truedsson, L. Complement in the immunopathogenesis of rheumatic disease. Nat. Rev. Rheumatol. 2012, 8, 458–468. [Google Scholar] [CrossRef]
- Sun, Y.; Li, C.; Yu, W.; Huo, G.; Zhu, M.; Zhao, P.; Wang, T.; Huang, G.; Xu, A. Complement system deregulation in SAPHO syndrome revealed by proteomic profiling. J. Proteom. 2022, 251, 104399. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, N.; Grässel, S. Involvement of complement peptides C3a and C5a in osteoarthritis pathology. Peptides 2022, 154, 170815. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.P.; Harris, C.L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug. Discov. 2015, 14, 857–877. [Google Scholar] [CrossRef] [PubMed]
- Quigg, R.J. Use of complement inhibitors in tissue injury. Trends Mol. Med. 2002, 8, 430–436. [Google Scholar] [CrossRef]
- Tesser, J.; Kivitz, A.; Fleischmann, R.; Mojcik, C.; Bombara, M.; Burch, F. Safety and efficacy of the humanized anti-C5 antibody h5G1. 1 in patients with rheumatoid arthritis. Arthritis Rheum. 2001, 44, S274. [Google Scholar]
- Mizuno, M. A review of current knowledge of the complement system and the therapeutic opportunities in inflammatory arthritis. Curr. Med. Chem. 2006, 13, 1707–1717. [Google Scholar] [CrossRef]
- Sahu, A.; Kay, B.K.; Lambris, J.D. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J. Immunol. 1996, 157, 884–891. [Google Scholar]
- Lamers, C.; Mastellos, D.C.; Ricklin, D.; Lambris, J.D. Compstatins: The dawn of clinical C3-targeted complement inhibition. Trends Pharmacol. Sci. 2022, 43, 629–640. [Google Scholar] [CrossRef]
- Li, X.X.; Lee, J.D.; Massey, N.L.; Guan, C.; Robertson, A.A.; Clark, R.J.; Woodruff, T.M. Pharmacological characterisation of small molecule C5aR1 inhibitors in human cells reveals biased activities for signalling and function. Biochem. Pharmacol. 2020, 180, 114156. [Google Scholar] [CrossRef] [PubMed]
- March, D.R.; Proctor, L.M.; Stoermer, M.J.; Sbaglia, R.; Abbenante, G.; Reid, R.C.; Woodruff, T.M.; Wadi, K.; Paczkowski, N.; Tyndall, J.D.; et al. Potent cyclic antagonists of the complement C5a receptor on human polymorphonuclear leukocytes. Relationships between structures and activity. Mol. Pharmacol. 2004, 65, 868–879. [Google Scholar] [CrossRef]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697. [Google Scholar] [CrossRef] [PubMed]
- John, T.; Stahel, P.F.; Morgan, S.J.; Schulze-Tanzil, G. Impact of the complement cascade on posttraumatic cartilage inflammation and degradation. Histol. Histopathol. 2007, 22, 781–790. [Google Scholar] [PubMed]
- Honorati, M.C.; Bovara, M.; Cattini, L.; Piacentini, A.; Facchini, A. Contribution of interleukin 17 to human cartilage degradation and synovial inflammation in osteoarthritis. Osteoarthr. Cartil. 2002, 10, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, S.A.; Neseem, N.; Metwally, S.; Farag, S. IL-17 in primary knee osteoarthritis and its relation with severity of the disease. Int. J. Clin. Rheumatol. 2018, 13, 364–369. [Google Scholar] [CrossRef]
- Mimpen, J.Y.; Baldwin, M.J.; Cribbs, A.P.; Philpott, M.; Carr, A.J.; Dakin, S.G.; Snelling, S.J. Interleukin-17A causes osteoarthritis-like transcriptional changes in human osteoarthritis-derived chondrocytes and synovial fibroblasts in vitro. Front. Immunol. 2021, 12, 676173. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Song, J.H.; Yoon, J.H.; Park, Y.G.; Lee, S.W.; Choi, Y.J.; Nam, S.W.; Lee, J.Y.; Park, W.S. TNF-α and TNF-β polymorphisms are associated with susceptibility to osteoarthritis in a Korean population. Korean J. Pathol. 2012, 46, 30. [Google Scholar] [CrossRef]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.-P.; Fahmi, H.J.N.R.R. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat. Rev. Rheumatol. 2011, 7, 33–42. [Google Scholar] [CrossRef]
- Stannus, O.; Jones, G.; Cicuttini, F.; Parameswaran, V.; Quinn, S.; Burgess, J.; Ding, C. Circulating levels of IL-6 and TNF-α are associated with knee radiographic osteoarthritis and knee cartilage loss in older adults. Osteoarthr. Cartil. 2010, 18, 1441–1447. [Google Scholar] [CrossRef]
- Guerne, P.; Carson, D.; Lotz, M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J. Immunol. 1990, 144, 499–505. [Google Scholar]
- Lotz, M.; Terkeltaub, R.; Villiger, P.M. Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J. Immunol. 1992, 148, 466–473. [Google Scholar]
- Alaaeddine, N.; Olee, T.; Hashimoto, S.; Creighton-Achermann, L.; Lotz, M. Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum. 2001, 44, 1633–1643. [Google Scholar] [CrossRef]
- Borzı, R.M.; Pulsatelli, L.; Meliconi, R. Production of the chemokine RANTES by articular chondrocytes and its role in cartilage degradation: Comment on the article by Alaaeddine et al. J. Arthritis Rheum. 2003, 48, 278. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, Ł.A.; Szukiewicz, D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediat. Inflamm. 2014, 2014, 561459. [Google Scholar] [CrossRef]
- Linton, S.M.; Morgan, B.P. Complement activation and inhibition in experimental models of arthritis. Mol. Immunol. 1999, 36, 905–914. [Google Scholar] [CrossRef]
- Zelek, W.M.; Xie, L.; Morgan, B.P.; Harris, C.L. Compendium of current complement therapeutics. Mol. Immunol. 2019, 114, 341–352. [Google Scholar] [CrossRef]
- Chi, Z.-L.; Yoshida, T.; Lambris, J.D.; Iwata, T. Suppression of drusen formation by compstatin, a peptide inhibitor of complement C3 activation, on cynomolgus monkey with early-onset macular degeneration. In Inflammation and Retinal Disease: Complement Biology and Pathology; Springer: Berlin/Heidelberg, Germany, 2010; pp. 127–135. [Google Scholar]
- Dmytrijuk, A.; Robie-Suh, K.; Cohen, M.H.; Rieves, D.; Weiss, K.; Pazdur, R. FDA report: Eculizumab (Soliris®) for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Oncologist 2008, 13, 993–1000. [Google Scholar] [CrossRef]
- Rother, R.P.; Rollins, S.A.; Mojcik, C.F.; Brodsky, R.A.; Bell, L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat. Biotechnol. 2007, 25, 1256–1264. [Google Scholar] [CrossRef]
- Kulasekararaj, A.G.; Hill, A.; Rottinghaus, S.T.; Langemeijer, S.; Wells, R.; Gonzalez-Fernandez, F.A.; Gaya, A.; Lee, J.W.; Gutierrez, E.O.; Piatek, C.I. Ravulizumab (ALXN1210) vs. eculizumab in C5-inhibitor—Experienced adult patients with PNH: The 302 study. Blood 2019, 133, 540–549. [Google Scholar] [CrossRef]
- Hillmen, P.; Szer, J.; Weitz, I.; Röth, A.; Höchsmann, B.; Panse, J.; Usuki, K.; Griffin, M.; Kiladjian, J.-J.; de Castro, C.; et al. Pegcetacoplan versus eculizumab in paroxysmal nocturnal hemoglobinuria. N. Engl. J. Med. 2021, 384, 1028–1037. [Google Scholar] [CrossRef]
- Hoy, S.M. Pegcetacoplan: First Approval. Drugs 2021, 81, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. First approval of a complement C3 inhibitor opens up autoimmune and inflammatory opportunities. Nat. Rev. Drug Discov. 2021, 20, 496. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Hasturk, H.; Lambris, J.D.; Contributing, A. C3-targeted therapy in periodontal disease: Moving closer to the clinic. Trends Immunol. 2021, 42, 856–864. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, H.; Hajishengallis, G.; Lambris, J.D.; Mastellos, D.C.; Yancopoulou, D. Phase IIa clinical trial of complement C3 inhibitor AMY-101 in adults with periodontal inflammation. J. Clin. Investig. 2021, 131, e152973. [Google Scholar] [CrossRef] [PubMed]
- Mastaglio, S.; Ruggeri, A.; Risitano, A.M.; Angelillo, P.; Yancopoulou, D.; Mastellos, D.C.; Huber-Lang, M.; Piemontese, S.; Assanelli, A.; Garlanda, C. The first case of COVID-19 treated with the complement C3 inhibitor AMY-101. Clin. Immunol. 2020, 215, 108450. [Google Scholar] [CrossRef] [PubMed]
- Risitano, A.M.; Mastellos, D.C.; Huber-Lang, M.; Yancopoulou, D.; Garlanda, C.; Ciceri, F.; Lambris, J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020, 20, 343–344. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Lee, J.D.; Clark, R.J.; Noakes, P.G.; Taylor, S.M.; Woodruff, T.M. Preclinical Pharmacokinetics of Complement C5a Receptor Antagonists PMX53 and PMX205 in Mice. ACS Omega 2020, 5, 2345–2354. [Google Scholar] [CrossRef]
- Vergunst, C.; Gerlag, D.; Dinant, H.; Schulz, L.; Vinkenoog, M.; Smeets, T.; Sanders, M.; Reedquist, K.; Tak, P. Blocking the receptor for C5a in patients with rheumatoid arthritis does not reduce synovial inflammation. Rheumatology 2007, 46, 1773–1778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kimura, Y.; Fang, C.; Zhou, L.; Sfyroera, G.; Lambris, J.D.; Wetsel, R.A.; Miwa, T.; Song, W.-C. Regulation of Toll-like receptor—Mediated inflammatory response by complement in vivo. Blood 2007, 110, 228–236. [Google Scholar] [CrossRef]
- Lee, J.D.; Kumar, V.; Fung, J.N.; Ruitenberg, M.J.; Noakes, P.G.; Woodruff, T.M. Pharmacological inhibition of complement C5a-C5a1 receptor signalling ameliorates disease pathology in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. Br. J. Pharmacol. 2017, 174, 689–699. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Costantini, K.J.; Crane, J.W.; Atkin, J.D.; Monk, P.N.; Taylor, S.M.; Noakes, P.G. The complement factor C5a contributes to pathology in a rat model of amyotrophic lateral sclerosis. J. Immunol. 2008, 181, 8727–8734. [Google Scholar] [CrossRef]
- Woodruff, T.M.; Crane, J.W.; Proctor, L.M.; Buller, K.M.; Shek, A.B.; de Vos, K.; Pollitt, S.; Williams, H.M.; Shiels, I.A.; Monk, P.N.; et al. Therapeutic activity of C5a receptor antagonists in a rat model of neurodegeneration. FASEB J. 2006, 20, 1407–1417. [Google Scholar] [CrossRef] [PubMed]
- Biggins, P.J.; Brennan, F.H.; Taylor, S.M.; Woodruff, T.M.; Ruitenberg, M.J. The alternative receptor for complement component 5a, C5aR2, conveys neuroprotection in traumatic spinal cord injury. J. Neurotrauma 2017, 34, 2075–2085. [Google Scholar] [CrossRef]
- Brennan, F.H.; Gordon, R.; Lao, H.W.; Biggins, P.J.; Taylor, S.M.; Franklin, R.J.; Woodruff, T.M.; Ruitenberg, M.J. The complement receptor C5aR controls acute inflammation and astrogliosis following spinal cord injury. J. Neurosci. 2015, 35, 6517–6531. [Google Scholar] [CrossRef] [PubMed]
- Ager, R.R.; Fonseca, M.I.; Chu, S.; Sanderson, S.D.; Taylor, S.M.; Woodruff, T.M.; Tenner, A.J. Microglial C5aR (CD88) expression correlates with amyloid-β deposition in murine models of Alzheimer’s disease. J. Neurochem. 2010, 113, 389–401. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, M.I.; Ager, R.R.; Chu, S.-H.; Yazan, O.; Sanderson, S.D.; LaFerla, F.M.; Taylor, S.M.; Woodruff, T.M.; Tenner, A.J. Treatment with a C5aR antagonist decreases pathology and enhances behavioral performance in murine models of Alzheimer’s disease. J. Immunol. 2009, 183, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Silva de França, F.; Villas-Boas, I.M.; Cogliati, B.; Woodruff, T.M.; da Silva Reis, E.; Lambris, J.D.; Tambourgi, D.V. C5a-C5aR1 axis activation drives envenomation immunopathology by the snake naja annulifera. Front. Immunol. 2021, 12, 1138. [Google Scholar] [CrossRef] [PubMed]
- Mai, J.; Virtue, A.; Shen, J.; Wang, H.; Yang, X.-F. An evolving new paradigm: Endothelial cells—Conditional innate immune cells. J. Hematol. Oncol. 2013, 6, 61. [Google Scholar] [CrossRef] [PubMed]
- Trepels, T.; Zeiher, A.M.; Fichtlscherer, S. The endothelium and inflammation. Endothelium 2006, 13, 423–429. [Google Scholar] [CrossRef]
- Till, G.O. Therapeutic Interventions in the complement System. Arch. Pathol. Lab. Med. 2001, 125, 708. [Google Scholar] [CrossRef]
- Dobrina, A.; Pausa, M.; Fischetti, F.; Bulla, R.; Vecile, E.; Ferrero, E.; Mantovani, A.; Tedesco, F. Cytolytically inactive terminal complement complex causes transendothelial migration of polymorphonuclear leukocytes in vitro and in vivo. Blood 2002, 99, 185–192. [Google Scholar] [CrossRef]
- Kilgore, K.S.; Schmid, E.; Shanley, T.P.; Flory, C.M.; Maheswari, V.; Tramontini, N.L.; Cohen, H.; Ward, P.A.; Friedl, H.P.; Warren, J.S. Sublytic concentrations of the membrane attack complex of complement induce endothelial interleukin-8 and monocyte chemoattractant protein-1 through nuclear factor-kappa B activation. Am. J. Pathol. 1997, 150, 2019. [Google Scholar] [PubMed]
- Foreman, K.E.; Vaporciyan, A.A.; Bonish, B.K.; Jones, M.L.; Johnson, K.J.; Glovsky, M.M.; Eddy, S.M.; Ward, P.A. C5a-induced expression of P-selectin in endothelial cells. J. Clin. Investig. 1994, 94, 1147–1155. [Google Scholar] [CrossRef] [PubMed]
- Murphy, H.; Shayman, J.; Till, G.; Mahrougui, M.; Owens, C.; Ryan, U.; Ward, P. Superoxide responses of endothelial cells to C5a and TNF-alpha: Divergent signal transduction pathways. Am. J. Physiol. Lung Cell. Mol. Physiol. 1992, 263, L51–L59. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-H.; Tsai, I.-J.; Chang, C.-J.; Chuang, Y.-H.; Hsu, H.-Y.; Chiang, B.-L. The interaction between circulating complement proteins and cutaneous microvascular endothelial cells in the development of childhood Henoch-Schönlein purpura. PLoS ONE 2015, 10, e0120411. [Google Scholar] [CrossRef] [PubMed]
- Mollnes, T.E.; Brekke, O.-L.; Fung, M.; Fure, H.; Christiansen, D.; Bergseth, G.; Videm, V.; Lappegård, K.T.; Köhl, J.; Lambris, J.D. Essential role of the C5a receptor in E coli–induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood 2002, 100, 1869–1877. [Google Scholar]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Method. 1983, 65, 55–63. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrili, J.J.M.; Villas-Boas, I.M.; Pidde, G.; Squaiella-Baptistão, C.C.; Woodruff, T.M.; Tambourgi, D.V. Complement System Inhibition Modulates the Inflammation Induced by the Venom of Premolis semirufa, an Amazon Rainforest Moth Caterpillar. Int. J. Mol. Sci. 2022, 23, 13333. https://doi.org/10.3390/ijms232113333
Gabrili JJM, Villas-Boas IM, Pidde G, Squaiella-Baptistão CC, Woodruff TM, Tambourgi DV. Complement System Inhibition Modulates the Inflammation Induced by the Venom of Premolis semirufa, an Amazon Rainforest Moth Caterpillar. International Journal of Molecular Sciences. 2022; 23(21):13333. https://doi.org/10.3390/ijms232113333
Chicago/Turabian StyleGabrili, Joel J. M., Isadora Maria Villas-Boas, Giselle Pidde, Carla Cristina Squaiella-Baptistão, Trent M. Woodruff, and Denise V. Tambourgi. 2022. "Complement System Inhibition Modulates the Inflammation Induced by the Venom of Premolis semirufa, an Amazon Rainforest Moth Caterpillar" International Journal of Molecular Sciences 23, no. 21: 13333. https://doi.org/10.3390/ijms232113333
APA StyleGabrili, J. J. M., Villas-Boas, I. M., Pidde, G., Squaiella-Baptistão, C. C., Woodruff, T. M., & Tambourgi, D. V. (2022). Complement System Inhibition Modulates the Inflammation Induced by the Venom of Premolis semirufa, an Amazon Rainforest Moth Caterpillar. International Journal of Molecular Sciences, 23(21), 13333. https://doi.org/10.3390/ijms232113333





