Bmp4 Synexpression Gene, Sizzled, Transcription Is Collectively Modulated by Smad1 and Ventx1.1/Ventx2.1 in Early Xenopus Embryos
Abstract
1. Introduction
2. Results
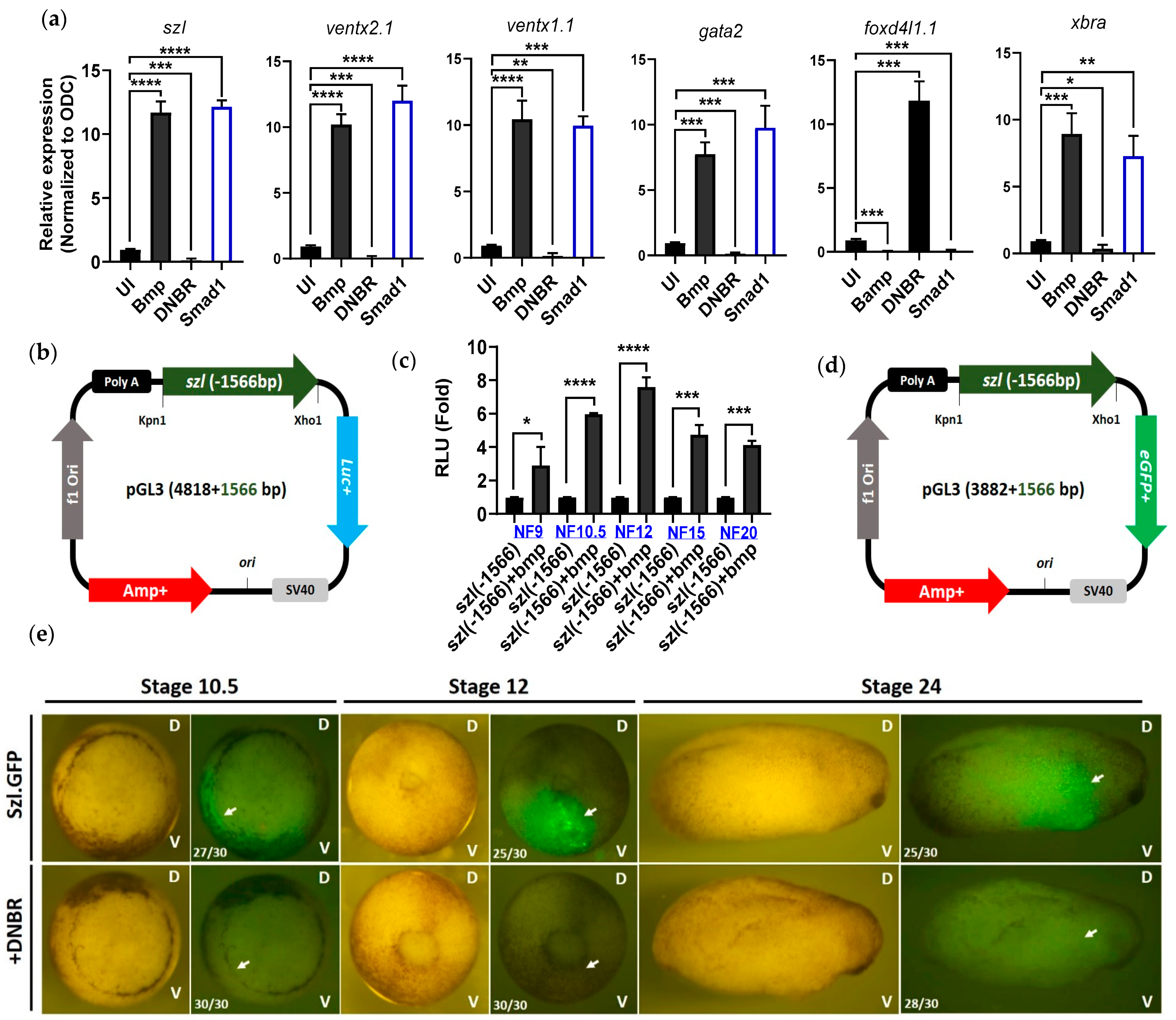
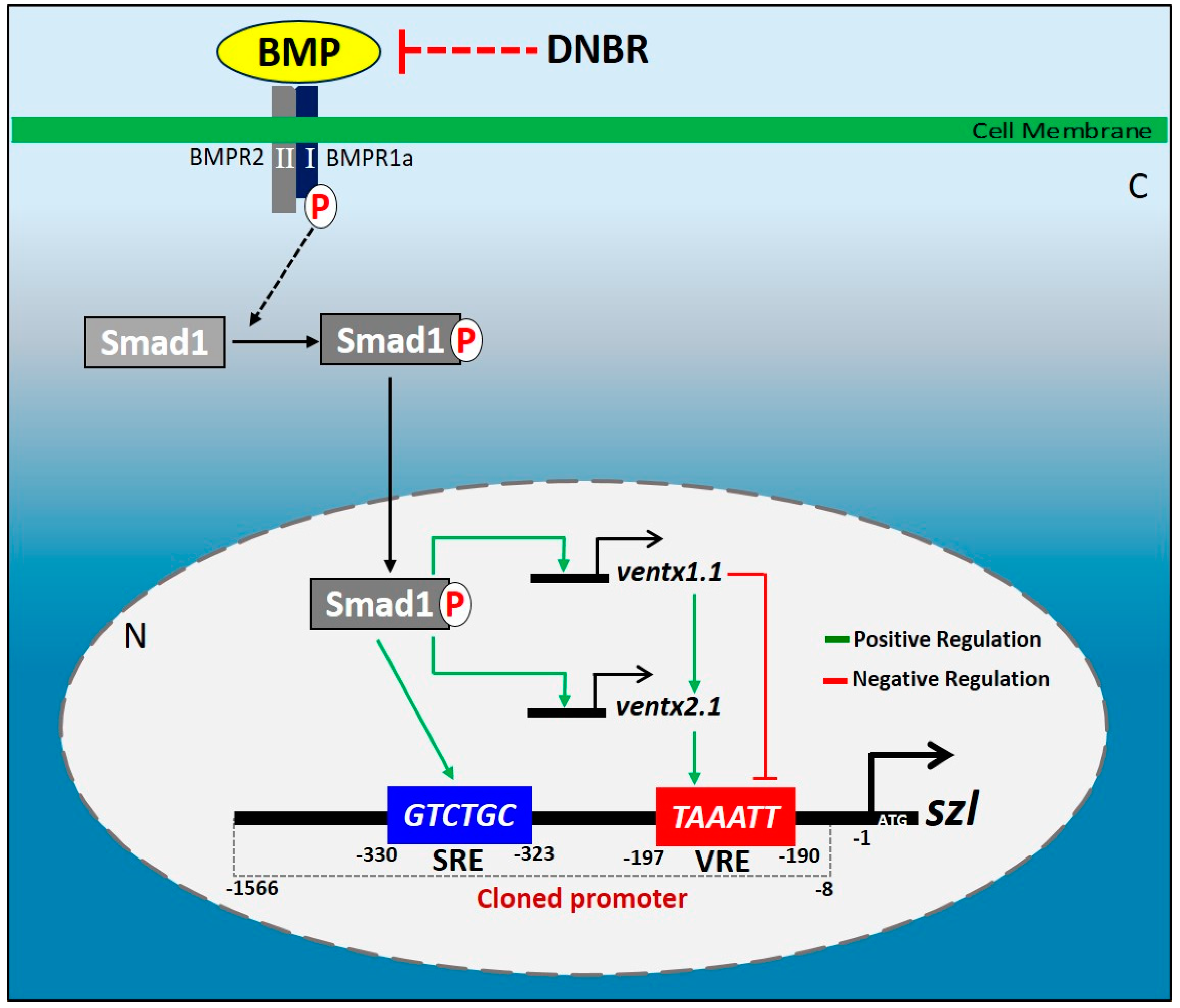
2.1. Isolated Szl(-1566) Promoter Contains the Bmp4 Regulatory Elements Required for Bmp4 Synexpression
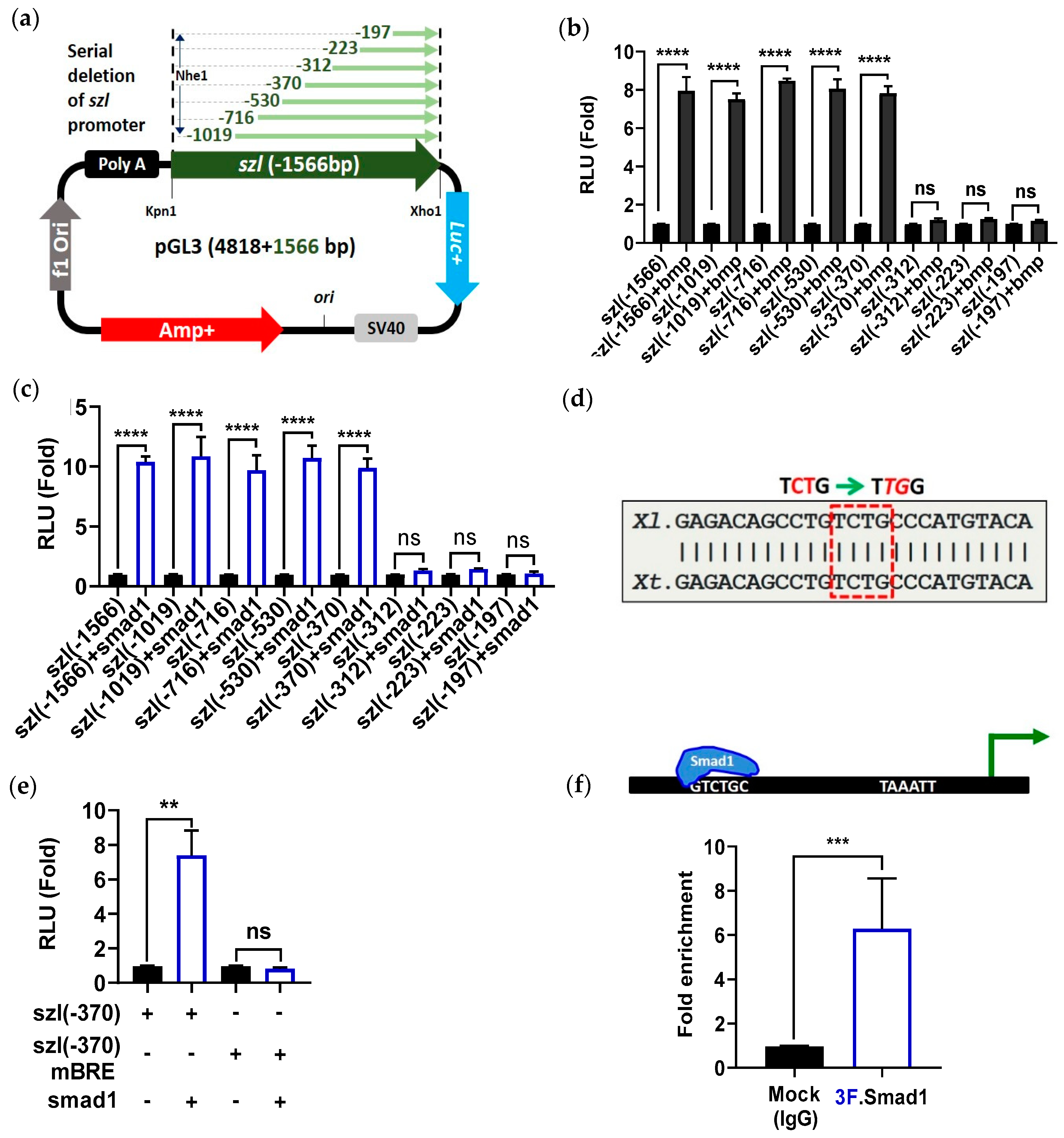
2.2. Direct Smad1 Binding on Bmp4/Smad1 Response Cis-Acting Element (BRE) Site of Szl Promoter Is Essential for Bmp4 Synexpression
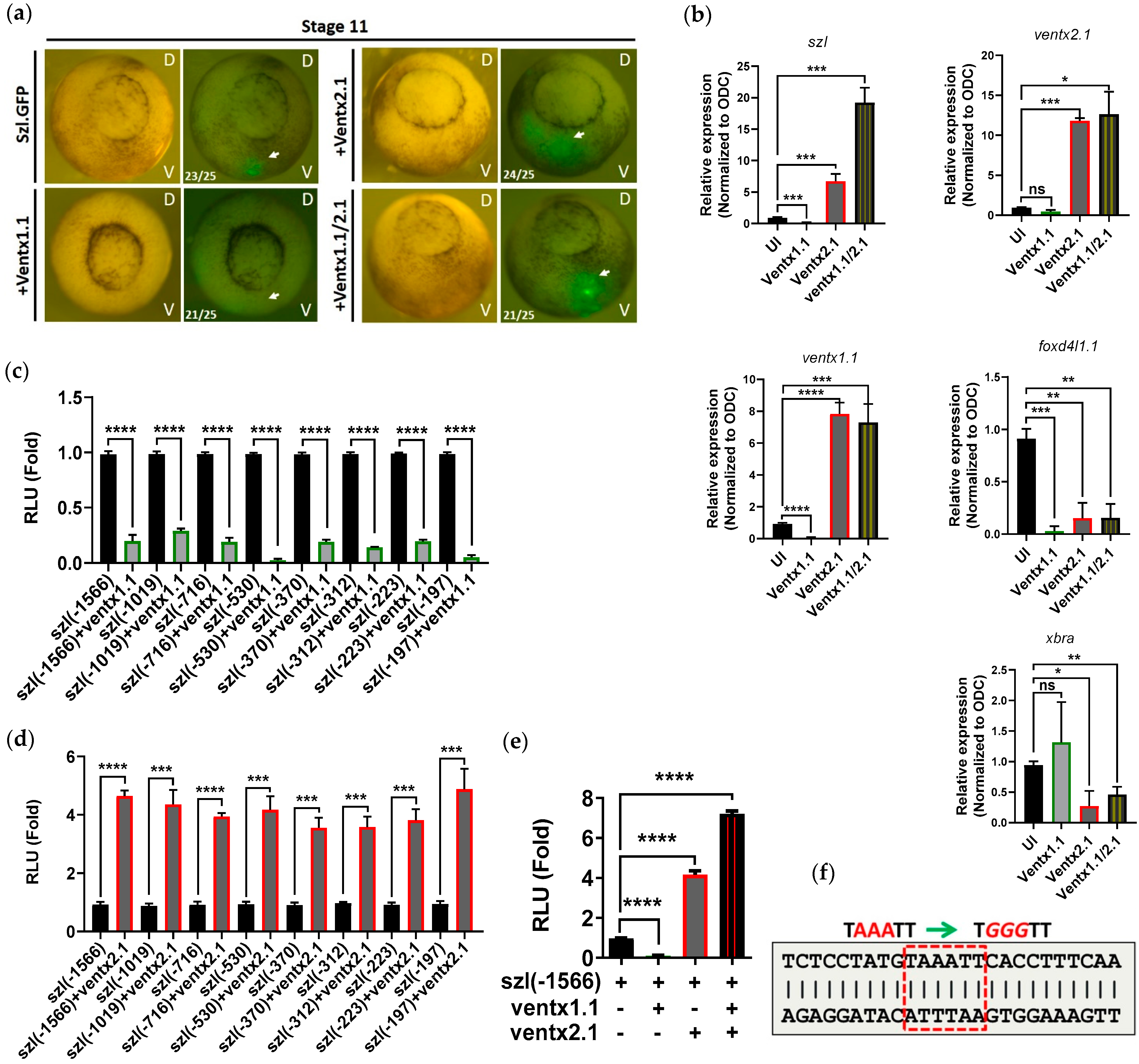
2.3. Two Direct Targets of Bmp4 Signaling, Ventx1.1 and Ventx2.1 Oppositely Modulate but Ventx1.1/2.1 Together Jointly Upregulate Szl Transcription
2.4. Site-Directed Mutagenesis of One VRE Site on the Szl Promoter Abolishes Ventx1.1 as Well as Ventx2.1 Mediated Szl Transcriptional Regulation
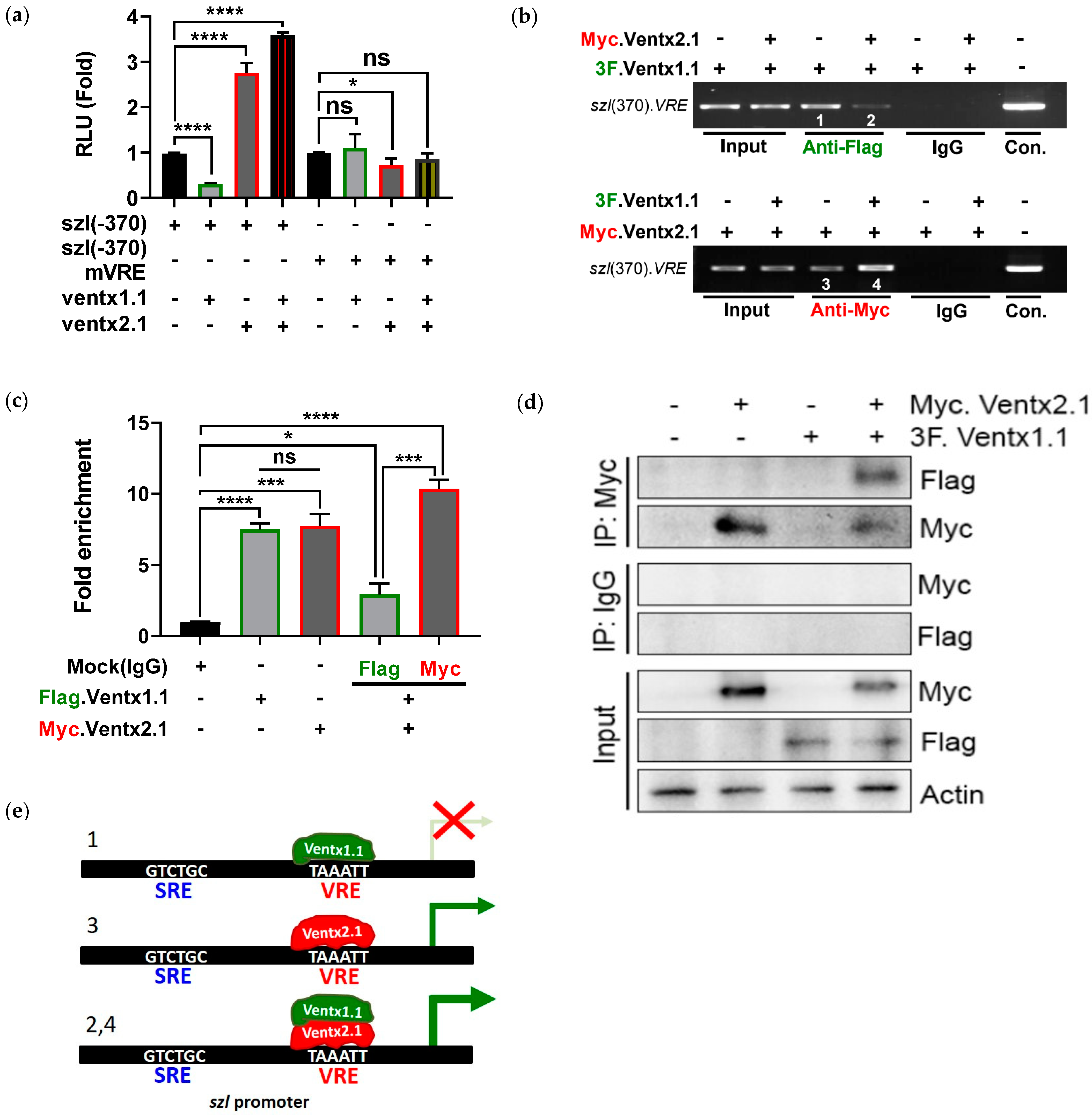
2.5. Smad1 and Ventx1.1/Ventx2.1 Collectively Modulate Szl Transcription
3. Discussion
3.1. Szl Is a Direct Target as Well as a Secondary Response Gene of Bmp4 Signaling
3.2. Ventx1.1 Enhances Ventx2.1’s Stimulatory Effect
3.3. Smad1 and Ventx1.1/Ventx2.1 Collectively Upregulates Szl Expression
4. Materials and Methods
4.1. Ethics Statement
4.2. Nucleic Acids (DNA, RNA) Preparation
4.3. Cloning of Szl, Bmp7.1 and Bambi Promoter Constructs
4.4. Xenopus Embryos Microinjection
4.5. Embryos Animal Cap Explants Culture
4.6. RT-PCR Experiment
4.7. Quantitative RT-PCR (qRT-PCR) Analysis
4.8. Luciferase Reporter Gene Assay
4.9. Site Directed Mutagenesis of Smad1 and Ventx Response Elements (SRE and VRE)
4.10. Chromatin Immunoprecipitation (ChIP) Assay
4.11. Immunoprecipitation
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- De Robertis, E.M. Evo-devo: Variations on ancestral themes. Cell 2008, 132, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, M.; Kuratani, S.; Takeda, H.; Irie, N. Recapitulation-like developmental transitions of chromatin accessibility in vertebrates. Zool. Lett. 2019, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.M.; Smith, J.C. Inductive signals. Revolving vertebrates. Curr. Biol. 1995, 5, 574–576. [Google Scholar] [CrossRef][Green Version]
- Eivers, E.; Demagny, H.; De Robertis, E.M. Integration of BMP and Wnt signaling via vertebrate Smad1/5/8 and Drosophila Mad. Cytokine Growth Factor Rev. 2009, 20, 357–365. [Google Scholar] [CrossRef]
- Wills, A.; Dickinson, K.; Khokha, M.; Baker, J.C. Bmp signaling is necessary and sufficient for ventrolateral endoderm specification in Xenopus. Dev. Dyn. 2008, 237, 2177–2186. [Google Scholar] [CrossRef]
- Dosch, R.; Gawantka, V.; Delius, H.; Blumenstock, C.; Niehrs, C. Bmp-4 acts as a morphogen in dorsoventral mesoderm patterning in Xenopus. Development 1997, 124, 2325–2334. [Google Scholar] [CrossRef]
- Tucker, J.A.; Mintzer, K.A.; Mullins, M.C. The BMP signaling gradient patterns dorsoventral tissues in a temporally progressive manner along the anteroposterior axis. Dev. Cell 2008, 14, 108–119. [Google Scholar] [CrossRef]
- Hawley, S.H.; Wunnenberg-Stapleton, K.; Hashimoto, C.; Laurent, M.N.; Watabe, T.; Blumberg, B.W.; Cho, K.W. Disruption of BMP signals in embryonic Xenopus ectoderm leads to direct neural induction. Genes Dev. 1995, 9, 2923–2935. [Google Scholar] [CrossRef]
- Marchal, L.; Luxardi, G.; Thome, V.; Kodjabachian, L. BMP inhibition initiates neural induction via FGF signaling and Zic genes. Proc. Natl. Acad. Sci. USA 2009, 106, 17437–17442. [Google Scholar] [CrossRef]
- Madamanchi, A.; Mullins, M.C.; Umulis, D.M. Diversity and robustness of bone morphogenetic protein pattern formation. Development 2021, 148, dev192344. [Google Scholar] [CrossRef]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010, 147, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Deng, C.; Li, Y.P. TGF-beta and BMP signaling in osteoblast differentiation and bone formation. Int. J. Biol. Sci. 2012, 8, 272–288. [Google Scholar] [CrossRef] [PubMed]
- Karaulanov, E.; Knochel, W.; Niehrs, C. Transcriptional regulation of BMP4 synexpression in transgenic Xenopus. EMBO J. 2004, 23, 844–856. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Li, Z.; Zhang, J.; Xu, F.; Liu, J.; Liu, H. The crystal structure of full-length Sizzled from Xenopus laevis yields insights into Wnt-antagonistic function of secreted Frizzled-related proteins. J. Biol. Chem. 2017, 292, 16055–16069. [Google Scholar] [CrossRef]
- Marom, K.; Fainsod, A.; Steinbeisser, H. Patterning of the mesoderm involves several threshold responses to BMP-4 and Xwnt-8. Mech. Dev. 1999, 87, 33–44. [Google Scholar] [CrossRef]
- Lee, H.X.; Ambrosio, A.L.; Reversade, B.; De Robertis, E.M. Embryonic dorsal-ventral signaling: Secreted frizzled-related proteins as inhibitors of tolloid proteinases. Cell 2006, 124, 147–159. [Google Scholar] [CrossRef]
- Salic, A.N.; Kroll, K.L.; Evans, L.M.; Kirschner, M.W. Sizzled: A secreted Xwnt8 antagonist expressed in the ventral marginal zone of Xenopus embryos. Development 1997, 124, 4739–4748. [Google Scholar] [CrossRef]
- Wei, C.Y.; Wang, H.P.; Zhu, Z.Y.; Sun, Y.H. Transcriptional factors smad1 and smad9 act redundantly to mediate zebrafish ventral specification downstream of smad5. J. Biol. Chem. 2014, 289, 6604–6618. [Google Scholar] [CrossRef]
- Fuentealba, L.C.; Eivers, E.; Ikeda, A.; Hurtado, C.; Kuroda, H.; Pera, E.M.; De Robertis, E.M. Integrating patterning signals: Wnt/GSK3 regulates the duration of the BMP/Smad1 signal. Cell 2007, 131, 980–993. [Google Scholar] [CrossRef]
- Pera, E.M.; Ikeda, A.; Eivers, E.; De Robertis, E.M. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev 2003, 17, 3023–3028. [Google Scholar] [CrossRef]
- Lee, H.S.; Lee, S.Y.; Lee, H.; Hwang, Y.S.; Cha, S.W.; Park, S.; Lee, J.Y.; Park, J.B.; Kim, S.; Park, M.J.; et al. Direct response elements of BMP within the PV.1A promoter are essential for its transcriptional regulation during early Xenopus development. PLoS ONE 2011, 6, e22621. [Google Scholar] [CrossRef] [PubMed]
- Stevens, M.L.; Chaturvedi, P.; Rankin, S.A.; Macdonald, M.; Jagannathan, S.; Yukawa, M.; Barski, A.; Zorn, A.M. Genomic integration of Wnt/beta-catenin and BMP/Smad1 signaling coordinates foregut and hindgut transcriptional programs. Development 2017, 144, 1283–1295. [Google Scholar] [PubMed]
- Dale, L.; Howes, G.; Price, B.M.; Smith, J.C. Bone morphogenetic protein 4: A ventralizing factor in early Xenopus development. Development 1992, 115, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Fainsod, A.; Deissler, K.; Yelin, R.; Marom, K.; Epstein, M.; Pillemer, G.; Steinbeisser, H.; Blum, M. The dorsalizing and neural inducing gene follistatin is an antagonist of BMP-4. Mech. Dev. 1997, 63, 39–50. [Google Scholar] [CrossRef]
- Cvekl, A.; Zhang, X. Signaling and Gene Regulatory Networks in Mammalian Lens Development. Trends Genet. 2017, 33, 677–702. [Google Scholar] [CrossRef]
- Lee, H.; Seidl, C.; Sun, R.; Glinka, A.; Niehrs, C. R-spondins are BMP receptor antagonists in Xenopus early embryonic development. Nat. Commun. 2020, 11, 5570. [Google Scholar] [CrossRef]
- Tuazon, F.B.; Wang, X.; Andrade, J.L.; Umulis, D.; Mullins, M.C. Proteolytic Restriction of Chordin Range Underlies BMP Gradient Formation. Cell Rep. 2020, 32, 108039. [Google Scholar] [CrossRef]
- Maeno, M.; Mead, P.E.; Kelley, C.; Xu, R.H.; Kung, H.F.; Suzuki, A.; Ueno, N.; Zon, L.I. The role of BMP-4 and GATA-2 in the induction and differentiation of hematopoietic mesoderm in Xenopus laevis. Blood 1996, 88, 1965–1972. [Google Scholar] [CrossRef]
- Suzuki, A.; Thies, R.S.; Yamaji, N.; Song, J.J.; Wozney, J.M.; Murakami, K.; Ueno, N. A truncated bone morphogenetic protein receptor affects dorsal-ventral patterning in the early Xenopus embryo. Proc. Natl. Acad. Sci. USA 1994, 91, 10255–10259. [Google Scholar] [CrossRef]
- Xu, R.H.; Kim, J.; Taira, M.; Zhan, S.; Sredni, D.; Kung, H.F. A dominant negative bone morphogenetic protein 4 receptor causes neuralization in Xenopus ectoderm. Biochem. Biophys. Res. Commun. 1995, 212, 212–219. [Google Scholar] [CrossRef]
- Inomata, H.; Shibata, T.; Haraguchi, T.; Sasai, Y. Scaling of dorsal-ventral patterning by embryo size-dependent degradation of Spemann’s organizer signals. Cell 2013, 153, 1296–1311. [Google Scholar] [CrossRef] [PubMed]
- Itoh, K.; Sokol, S.Y. Axis determination by inhibition of Wnt signaling in Xenopus. Genes. Dev. 1999, 13, 2328–2336. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Saito, H.; Yamamura, K.; Suzuki, N. Reduced bone morphogenetic protein receptor type 1A signaling in neural-crest-derived cells causes facial dysmorphism. Dis. Model Mech. 2012, 5, 948–955. [Google Scholar] [PubMed]
- Wilkinson, D.G.; Bhatt, S.; Herrmann, B.G. Expression pattern of the mouse T gene and its role in mesoderm formation. Nature 1990, 343, 657–659. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Cao, Y.; Zhao, C.; Postlethwait, J.; Meng, A. An SP1-like transcription factor Spr2 acts downstream of Fgf signaling to mediate mesoderm induction. EMBO J. 2003, 22, 6078–6088. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Umair, Z.; Yoon, J.; Lee, U.; Kim, S.C.; Park, J.B.; Lee, J.Y.; Kim, J. Xbra and Smad-1 cooperate to activate the transcription of neural repressor ventx1.1 in Xenopus embryos. Sci. Rep. 2018, 8, 11391. [Google Scholar] [CrossRef]
- Pera, E.M.; De Robertis, E.M. A direct screen for secreted proteins in Xenopus embryos identifies distinct activities for the Wnt antagonists Crescent and Frzb-1. Mech. Dev. 2000, 96, 183–195. [Google Scholar] [CrossRef]
- Onai, T.; Matsuo-Takasaki, M.; Inomata, H.; Aramaki, T.; Matsumura, M.; Yakura, R.; Sasai, N.; Sasai, Y. XTsh3 is an essential enhancing factor of canonical Wnt signaling in Xenopus axial determination. EMBO J. 2007, 26, 2350–2360. [Google Scholar] [CrossRef]
- Eivers, E.; Fuentealba, L.C.; De Robertis, E.M. Integrating positional information at the level of Smad1/5/8. Curr. Opin. Genet. Dev. 2008, 18, 304–310. [Google Scholar] [CrossRef]
- Rastegar, S.; Friedle, H.; Frommer, G.; Knochel, W. Transcriptional regulation of Xvent homeobox genes. Mech. Dev. 1999, 81, 139–149. [Google Scholar] [CrossRef]
- Friedle, H.; Knochel, W. Cooperative interaction of Xvent-2 and GATA-2 in the activation of the ventral homeobox gene Xvent-1B. J. Biol. Chem. 2002, 277, 23872–23881. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Dai, K.R.; Tang, T.T. The role of Smads and related transcription factors in the signal transduction of bone morphogenetic protein inducing bone formation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2003, 17, 359–362. [Google Scholar] [PubMed]
- Henningfeld, K.A.; Friedle, H.; Rastegar, S.; Knochel, W. Autoregulation of Xvent-2B; direct interaction and functional cooperation of Xvent-2 and Smad1. J. Biol. Chem. 2002, 277, 2097–2103. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.-S.; Chae, J.-P.; Kim, D.-S.; Park, K.-M.; Bae, Y.-C.; Park, M.-J. Screening of Interacting Proteins with PV. 1 as Downstream Factors of BMP Signal. Korean J. Anat. 2007, 203–210. [Google Scholar]
- Sander, V.; Reversade, B.; De Robertis, E.M. The opposing homeobox genes Goosecoid and Vent1/2 self-regulate Xenopus patterning. EMBO J. 2007, 26, 2955–2965. [Google Scholar] [PubMed]
- Hwang, Y.S.; Lee, H.S.; Roh, D.H.; Cha, S.; Lee, S.Y.; Seo, J.J.; Kim, J.; Park, M.J. Active repression of organizer genes by C-terminal domain of PV.1. Biochem. Biophys. Res. Commun. 2003, 308, 79–86. [Google Scholar] [PubMed]
- Hwang, Y.S.; Seo, J.J.; Cha, S.W.; Lee, H.S.; Lee, S.Y.; Roh, D.H.; Kung Hf, H.F.; Kim, J.; Ja Park, M. Antimorphic PV.1 causes secondary axis by inducing ectopic organizer. Biochem. Biophys. Res. Commun. 2002, 292, 1081–1086. [Google Scholar]
- Umair, Z.; Kumar, S.; Kim, D.H.; Rafiq, K.; Kumar, V.; Kim, S.; Park, J.B.; Lee, J.Y.; Lee, U.; Kim, J. Ventx1.1 as a Direct Repressor of Early Neural Gene zic3 in Xenopus laevis. Mol. Cells 2018, 41, 1061–1071. [Google Scholar]
- Yoon, J.; Kim, J.H.; Lee, S.Y.; Kim, S.; Park, J.B.; Lee, J.Y.; Kim, J.P.V. 1 induced by FGF-Xbra functions as a repressor of neurogenesis in Xenopus embryos. BMB Rep. 2014, 47, 673–678. [Google Scholar]
- Xu, R.H.; Ault, K.T.; Kim, J.; Park, M.J.; Hwang, Y.S.; Peng, Y.; Sredni, D.; Kung, H. Opposite effects of FGF and BMP-4 on embryonic blood formation: Roles of PV.1 and GATA-2. Dev. Biol. 1999, 208, 352–361. [Google Scholar]
- Kumar, S.; Umair, Z.; Kumar, V.; Lee, U.; Choi, S.C.; Kim, J. Ventx1.1 competes with a transcriptional activator Xcad2 to regulate negatively its own expression. BMB Rep. 2019, 52, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Umair, Z.; Kumar, V.; Kumar, S.; Lee, U.; Kim, J. Foxd4l1.1 negatively regulates transcription of neural repressor ventx1.1 during neuroectoderm formation in Xenopus embryos. Sci. Rep. 2020, 10, 16780. [Google Scholar] [CrossRef] [PubMed]
- Bijakowski, C.; Vadon-Le Goff, S.; Delolme, F.; Bourhis, J.M.; Lecorche, P.; Ruggiero, F.; Becker-Pauly, C.; Yiallouros, I.; Stocker, W.; Dive, V.; et al. Sizzled is unique among secreted frizzled-related proteins for its ability to specifically inhibit bone morphogenetic protein-1 (BMP-1)/tolloid-like proteinases. J. Biol. Chem. 2012, 287, 33581–33593. [Google Scholar] [CrossRef] [PubMed]
- Blythe, S.A.; Reid, C.D.; Kessler, D.S.; Klein, P.S. Chromatin immunoprecipitation in early Xenopus laevis embryos. Dev. Dyn. 2009, 238, 1422–1432. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, Z.U.; Tayyaba, F.; Lee, U.; Kim, J. Bmp4 Synexpression Gene, Sizzled, Transcription Is Collectively Modulated by Smad1 and Ventx1.1/Ventx2.1 in Early Xenopus Embryos. Int. J. Mol. Sci. 2022, 23, 13335. https://doi.org/10.3390/ijms232113335
Rehman ZU, Tayyaba F, Lee U, Kim J. Bmp4 Synexpression Gene, Sizzled, Transcription Is Collectively Modulated by Smad1 and Ventx1.1/Ventx2.1 in Early Xenopus Embryos. International Journal of Molecular Sciences. 2022; 23(21):13335. https://doi.org/10.3390/ijms232113335
Chicago/Turabian StyleRehman, Zia Ur, Faryal Tayyaba, Unjoo Lee, and Jaebong Kim. 2022. "Bmp4 Synexpression Gene, Sizzled, Transcription Is Collectively Modulated by Smad1 and Ventx1.1/Ventx2.1 in Early Xenopus Embryos" International Journal of Molecular Sciences 23, no. 21: 13335. https://doi.org/10.3390/ijms232113335
APA StyleRehman, Z. U., Tayyaba, F., Lee, U., & Kim, J. (2022). Bmp4 Synexpression Gene, Sizzled, Transcription Is Collectively Modulated by Smad1 and Ventx1.1/Ventx2.1 in Early Xenopus Embryos. International Journal of Molecular Sciences, 23(21), 13335. https://doi.org/10.3390/ijms232113335






