Chimeric Antigen Receptor T-Cell Therapy: What We Expect Soon
Abstract
1. Introduction
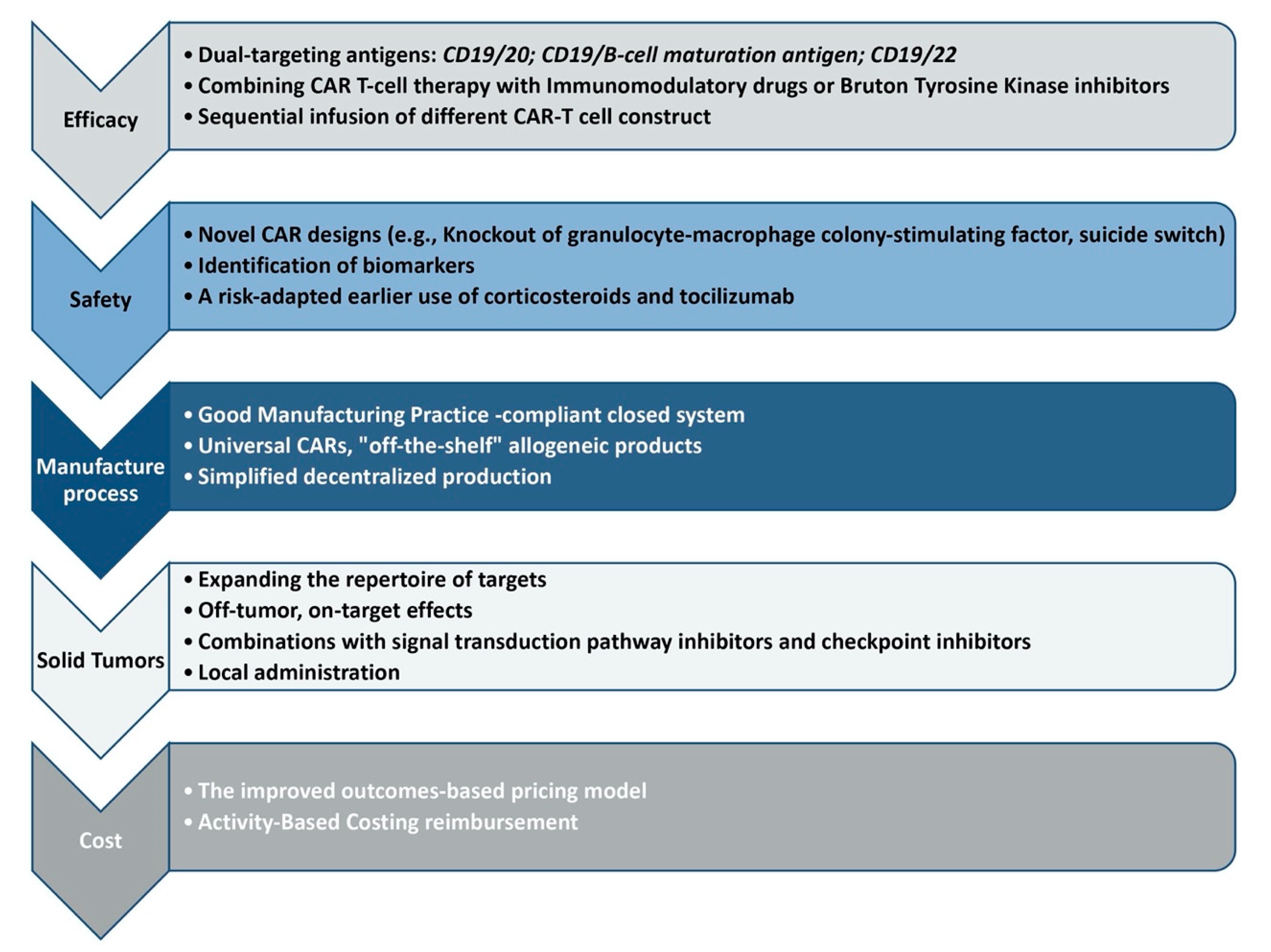
2. Improving Responses
3. Safety Profile
4. Manufacture Process
5. Solid Tumors
6. Cost
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kamb, A.; Go, W.Y. Cancer T-cell therapy: Building the foundation for a cure. F1000Research 2020, 9, 1295. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.; Alati, C.; Canale, F.; Musuraca, G.; Martinelli, G.; Cerchione, C. A Review of Clinical Outcomes of CAR T-Cell Therapies forB-Acute Lymphoblastic Leukemia. Int. J. Mol. Sci. 2021, 22, 2150. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.; Canale, F.A.; Alati, C.; Vincelli, I.D.; Moscato, T.; Porto, G.; Loteta, B.; Naso, V.; Mazza, M.; Nicolini, F.; et al. CART-Cell Therapy: Recent Advances and New Evidence in Multiple Myeloma. Cancers 2021, 13, 2639. [Google Scholar] [CrossRef] [PubMed]
- Schepisi, G.; Cursano, M.C.; Casadei, C.; Menna, C.; Altavilla, A.; Lolli, C.; Cerchione, C.; Paganelli, G.; Santini, D.; Tonini, G.; et al. CAR-T cell therapy: A potential new strategy against prostate cancer. J. Immunother. Cancer 2019, 7, 258. [Google Scholar] [CrossRef]
- FDA Approves Tisagenlecleucel for Adults with Relapsed or Refractory Large B-Cell Lymphoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tisagenlecleucel-adults-relapsed-or-refractory-large-b-cell-lymphoma (accessed on 5 March 2018).
- FDA Approves Axicabtagene Ciloleucel for Large B-Cell Lymphoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-axicabtagene-ciloleucel-large-b-cell-lymphoma (accessed on 9 August 2022).
- First Two CAR-T Cell Medicines Recommended for Approval in the European Union. Available online: https://www.ema.europa.eu/en/news/first-two-car-t-cell-medicines-recommended-approval-european-union (accessed on 9 August 2022).
- FDA Approves Lisocabtagene Maraleucel for Relapsed or Refractory Large B-Cell Lymphoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-lisocabtagene-maraleucel-relapsed-or-refractory-large-b-cell-lymphoma (accessed on 9 August 2022).
- FDA Approves Idecabtagene Vicleucel for Multiple Myeloma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-idecabtagene-vicleucel-multiple-myeloma (accessed on 9 August 2022).
- Chen, Y.; Nagarajan, C.; Tan, M.S.; Martinelli, G.; Cerchione, C. BCMA-targeting approaches for treatment of multiple myeloma. Panminerva Medica 2021, 63, 28–36. [Google Scholar] [CrossRef]
- Juan, M. CAR T cells targeting options in the fight against multiple myeloma. Panminerva Medica 2021, 63, 37–45. [Google Scholar] [CrossRef]
- FDA Approves Brexucabtagene Autoleucel for Relapsed or Refractory Mantle Cell Lymphoma. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-brexucabtagene-autoleucel-relapsed-or-refractory-mantle-cell-lymphoma (accessed on 9 August 2022).
- Shah, B.D.; Ghobadi, A.; Oluwole, O.O.; Logan, A.; Boissel, N.; Cassaday, R.D.; Forcade, E.; Bishop, M.R.; Topp, M.S.; Tzachanis, D.; et al. Phase 2 results of the ZUMA-3 study evaluating KTE-X19, an anti-CD19 chimeric antigen receptor (CAR) T-cell therapy, in adult patients (pts) with relapsed/refractory B-cell acute lymphoblastic leukemia (R/R B-ALL). J. Clin. Oncol. 2021, 39, 7002. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324. [Google Scholar] [CrossRef]
- Frigault, M.J.; O’Donnell, E.; Raje, N.S.; Cook, D.; Yee, A.; Rosenblatt, J.; Gibson, C.; Logan, E.; Avigan, D.; Bishop, M.R.; et al. Phase 1 Study of CART-ddBCMA, a CAR-T therapy utilizing a novel synthetic binding domain, for the treatment of subjects with relapsed and refractory multiple myeloma. J. Clin. Oncol. 2021, 39, 8015. [Google Scholar] [CrossRef]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2019, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobson, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- Abramson, J.S.; Palomba, M.L.; Gordon, L.I.; Lunning, M.A.; Wang, M.; Arnason, J.; Mehta, A.; Purev, E.; Maloney, D.G.; Andreadis, C.; et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): A multicentre seamless design study. Lancet 2020, 396, 839–852. [Google Scholar] [CrossRef]
- Maude, S.L.; Laetsch, T.W.; Buechner, J.; Rives, S.; Boyer, M.; Bittencourt, H.; Bader, P.; Verneris, M.R.; Stefanski, H.E.; Myers, G.D.; et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 439–448. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Neelapu, S.S.; Rossi, M.J.M.; Jacobson, C.A.; Locke, F.L.; Miklos, D.B.; Reagan, P.M.; Rodig, S.J.; Lekakis, L.J.; Flinn, I.W.; Zheng, L.; et al. CD19-Loss with Preservation of Other B Cell Lineage Features in Patients with Large B Cell Lymphoma Who Relapsed Post-Axi-Cel. Blood 2019, 134, 203. [Google Scholar] [CrossRef]
- Samur, M.K.; Fulciniti, M.; Aktas Samur, A.; Bazarbachi, A.H.; Tai, Y.-T.; Prabhala, R.; Alonso, A.; Sperling, A.S.; Campbell, T.; Petrocca, F.; et al. Biallelic loss of BCMA as a resistance mechanism to CAR T cell therapy in a patient with multiple myeloma. Nat. Commun. 2021, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Deng, B.; Yin, Z.; Lin, Y.; An, L.; Liu, D.; Pan, J.; Yu, X.; Chen, B.; Wu, T.; et al. Combination of CD19 and CD22 CAR-T cell therapy in relapsed B-cell acute lymphoblastic leukemia after allogeneic transplantation. Am. J. Hematol. 2021, 96, 671–679. [Google Scholar] [CrossRef]
- Spiegel, J.Y.; Patel, S.; Muffly, L.; Hossain, N.M.; Oak, J.; Baird, J.H.; Frank, M.J.; Shiraz, P.; Sahaf, B.; Craig, J.; et al. CAR T cells with dual targeting of CD19 and CD22 in adult patients with recurrent or refractory B cell malignancies: A phase 1 trial. Nat. Med. 2021, 27, 1419–1431. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, Y.; Hong, R.; Zhao, H.; Wei, G.; Wu, W.; Xu, H.; Cui, J.; Zhang, Y.; Chang, A.H.; et al. A retrospective comparison of CD19 single and CD19/CD22 bispecific targeted chimeric antigen receptor T cell therapy in patients with relapsed/refractory acute lymphoblastic leukemia. Blood Cancer J. 2020, 10, 1–3. [Google Scholar] [CrossRef]
- Liang, A.; Ye, S.; Li, P.; Huang, J.; Zhu, S.; Yao, X.; Zhou, L.; Xu, Y.; Zhu, J.; Zheng, C.; et al. Safety and efficacy of a novel an-ti-CD20 chimeric antigen receptor (CAR)-T cell therapy in relapsed/refractory (r/r) B-cell non-Hodgkin lymphoma (B-NHL) patients after failing CD19 CAR-T therapy. J. Clin. Oncol. 2021, 39 (Suppl. S15), 2508. [Google Scholar] [CrossRef]
- Shah, N.N.; Johnson, B.D.; Schneider, D.; Zhu, F.; Szabo, A.; Keever-Taylor, C.A.; Krueger, W.; Worden, A.A.; Kadan, M.J.; Yim, S.; et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: A phase 1 dose escalation and expansion trial. Nat. Med. 2020, 26, 1569–1575. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Qu, S.; Shang, J.; Shi, X.; Kang, L.; Xu, N.; Zhu, M.; Zhou, J.; Jin, S.; Yao, W.; et al. Sequential CD19 and BCMA-specific CAR T-cell treatment elicits sustained remission of relapsed and/or refractory myeloma. Cancer Med. 2020, 10, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Killock, D. Anti-CD22 CAR T cells in ALL. Nat. Rev. Clin. Oncol. 2020, 17, 391. [Google Scholar] [CrossRef] [PubMed]
- Baird, J.H.; Frank, M.J.; Craig, J.; Patel, S.; Spiegel, J.Y.; Sahaf, B.; Oak, J.S.; Younes, S.F.; Ozawa, M.G.; Yang, E.; et al. CD22-directed CAR T-cell therapy induces complete remissions in CD19-directed CAR–refractory large B-cell lymphoma. Blood 2021, 137, 2321–2325. [Google Scholar] [CrossRef]
- BCMA-Specific CAR T-Cells Combined with a Gamma Secretase Inhibitor (JSMD194) to Treat Relapsed or Persistent Multiple Myeloma. Available online: https://clinicaltrials.gov/ct2/show/NCT03502577 (accessed on 9 August 2022).
- Finney, O.C.; Brakke, H.M.; Rawlings-Rhea, S.; Hicks, R.; Doolittle, D.; Lopez, M.; Futrell, R.B.; Orentas, R.J.; Li, D.; Gardner, R.A.; et al. CD19 CAR T cell product and disease attributes predict leukemia remission durability. J. Clin. Investig. 2019, 129, 2123–2132. [Google Scholar] [CrossRef]
- Sommermeyer, D.; Hudecek, M.; Kosasih, P.L.; Gogishvili, T.; Maloney, D.G.; Turtle, C.J.; Riddell, S.R. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 2015, 30, 492–500. [Google Scholar] [CrossRef]
- Leblay, N.; Maity, R.; Barakat, E.; Mc Culloch, M.S.; Duggan, F.P.; Jimenez-Zepeda, V.; Bahlis, N.J.; Neri, P. Cite-Seq Profiling of T Cells in Multiple Myeloma Patients Undergoing BCMA Targeting CAR-T or Bites Immunotherapy. Blood 2020, 136, 11–12. [Google Scholar] [CrossRef]
- Works, M.; Soni, N.; Hauskins, C.; Sierra, C.; Baturevych, A.; Jones, J.C.; Curtis, W.; Carlson, P.; Johnstone, T.G.; Kugler, D.; et al. Anti–B-cell Maturation Antigen Chimeric Antigen Receptor T cell Function against Multiple Myeloma Is Enhanced in the Presence of Lenalidomide. Mol. Cancer Ther. 2019, 18, 2246–2257. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, S.; Rotiroti, M.C.; Attianese, G.M.P.G.; Arcangeli, S.; Zhang, R.; Banerjee, P.; Galletti, G.; McManus, S.; Mazza, M.; Nicolini, F.; et al. Lenalidomide enhances CD23.CAR T cell therapy in chronic lymphocytic leukemia. Leuk. Lymphoma 2022, 63, 1566–1579. [Google Scholar] [CrossRef]
- Qin, J.S.; Johnstone, T.G.; Baturevych, A.; Hause, R.J.; Ragan, S.P.; Clouser, C.R.; Jones, J.C.; Ponce, R.; Krejsa, C.M.; Salmon, R.A.; et al. Antitumor Potency of an Anti-CD19 Chimeric Antigen Receptor T-Cell Therapy, Lisocabtagene Maraleucel in Combination with Ibrutinib or Acalabrutinib. J. Immunother. 2020, 43, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Legut, M.; Dolton, G.; Mian, A.A.; Ottmann, O.G.; Sewell, A.K. CRISPR-mediated TCR replacement generates superior anticancer transgenic T cells. Blood 2018, 131, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Liu, D.-Y.; Sun, R.-J.; Zhang, J.-P.; Zhou, J.-R.; Wei, Z.-J.; Xiong, M.; Cao, X.-Y.; Lu, Y.; Yang, J.-F.; et al. Integrating CAR T-Cell Therapy and Transplantation: Comparisons of Safety and Long-Term Efficacy of Allogeneic Hematopoietic Stem Cell Transplantation After CAR T-Cell or Chemotherapy-Based Complete Remission in B-Cell Acute Lymphoblastic Leukemia. Front. Immunol. 2021, 12, 605766. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Huang, H. How to Combine the Two Landmark Treatment Methods—Allogeneic Hematopoietic Stem Cell Transplantation and Chimeric Antigen Receptor T Cell Therapy Together to Cure High-Risk B Cell Acute Lymphoblastic Leukemia? Front. Immunol. 2020, 11, 611710. [Google Scholar] [CrossRef]
- Jacoby, E. The role of allogeneic HSCT after CAR T cells for acute lymphoblastic leukemia. Bone Marrow Transplant. 2019, 54, 810–814. [Google Scholar] [CrossRef]
- Lu, P.; Lu, X.-A.; Zhang, X.; Xiong, M.; Zhang, J.; Zhou, X.; Qi, F.; Yang, J.; He, T. Which is better in CD19 CAR-T treatment of r/r B-ALL, CD28 or 4-1BB? A parallel trial under the same manufacturing process. J. Clin. Oncol. 2018, 36, 3041. [Google Scholar] [CrossRef]
- DeAngelo, D.; Ghobadi, A.; Park, J.; Dinner, S.; Mannis, G.; Lunning, M.; Khaled, S.; Fathi, A.; Gojo, I.; Wang, E.; et al. Clinical outcomes for the phase 2, single-arm, multicenter trial of JCAR015 in adult B-ALL (ROCKET study). J. Immunother. Cancer 2017, 5 (Suppl. S2). [Google Scholar]
- Nasta, S.D.; Hughes, M.E.; Namoglu, E.C.; Landsburg, D.J.; Chong, E.A.; Barta, S.K.; Frey, N.V.; Gerson, J.N.; Maity, A.; Plastaras, J.; et al. A Characterization of Bridging Therapies Leading up to Commercial CAR T-Cell Therapy. Blood 2019, 134, 4108. [Google Scholar] [CrossRef]
- Lee, D.W.; Santomasso, B.D.; Locke, F.L.; Ghobadi, A.; Turtle, C.J.; Brudno, J.N.; Maus, M.V.; Park, J.H.; Mead, E.; Pavletic, S.; et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated with Immune Effector Cells. Biol. Blood Marrow Transplant. 2019, 25, 625–638. [Google Scholar] [CrossRef]
- Siegler, E.L.; Kenderian, S.S. Neurotoxicity and Cytokine Release Syndrome After Chimeric Antigen Receptor T Cell Therapy: Insights into Mechanisms and Novel Therapies. Front. Immunol. 2020, 11, 1973. [Google Scholar] [CrossRef]
- Hong, R.; Hu, Y.; Huang, H. Biomarkers for Chimeric Antigen Receptor T Cell Therapy in Acute Lymphoblastic Leukemia: Prospects for Personalized Management and Prognostic Prediction. Front. Immunol. 2021, 12, 627764. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Marsal, J.; Huang, C.-Y.; Lo, M.; Kambhampati, S.; Kennedy, V.E.; Arora, S.; Wolf, J.L.; Martin, T.G.; Wong, S.W.; et al. Early Time-to-Tocilizumab after B Cell Maturation Antigen-Directed Chimeric Antigen Receptor T Cell Therapy in Myeloma. Transplant. Cell. Ther. 2021, 27, 477.e1–477.e7. [Google Scholar] [CrossRef] [PubMed]
- Munugala, N.; Dashkevych, U.; Husnain, M. Role of anakinra in the management of icans after CAR T-cell therapy for lymphoma. J. Clin. Oncol. 2022, 40, e19506. [Google Scholar] [CrossRef]
- Eyquem, J.; Mansilla-Soto, J.; Giavridis, T.; van der Stegen, S.J.C.; Hamieh, M.; Cunanan, K.M.; Odak, A.; Gönen, M.; Sadelain, M. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017, 543, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Duchateau, P.; Depil, S.; Poirot, L.; Valton, J. Granulocyte–macrophage colony-stimulating factor inactivation in CAR T-cells prevents monocyte-dependent release of key cytokine release syndrome mediators. J. Biol. Chem. 2019, 294, 5430–5437. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.A.; Savoldo, B.; Torrano, V.; Ballard, B.; Zhang, H.; Dakhova, O.; Liu, E.; Carrum, G.; Kamble, R.T.; Gee, A.P.; et al. Clinical responses with T lymphocytes targeting malignancy-associated k light chains. J. Clin. Investig. 2016, 126, 2588–2596. [Google Scholar] [CrossRef]
- Kim, M.Y.; Yu, K.-R.; Kenderian, S.S.; Ruella, M.; Chen, S.; Shin, T.-H.; Aljanahi, A.A.; Schreeder, D.; Klichinsky, M.; Shestova, O.; et al. Genetic Inactivation of CD33 in Hematopoietic Stem Cells to Enable CAR T Cell Immunotherapy for Acute Myeloid Leukemia. Cell 2018, 173, 1439–1453.e19. [Google Scholar] [CrossRef]
- Yang, J.; He, J.; Zhang, X.; Wang, Z.; Zhang, Y.; Cai, S.; Sun, Z.; Ye, X.; He, Y.; Shen, L.; et al. A Feasibility and Safety Study of a New CD19-Directed Fast CAR-T Therapy for Refractory and Relapsed B Cell Acute Lymphoblastic Leukemia. Blood 2019, 134 (Suppl. S1), 825. [Google Scholar] [CrossRef]
- Jackson, Z.; Roe, A.; Sharma, A.A.; Lopes, F.B.T.P.; Talla, A.; Kleinsorge-Block, S.; Zamborsky, K.; Schiavone, J.; Manjappa, S.; Schauner, R.; et al. Automated Manufacture of Autologous CD19 CAR-T Cells for Treatment of Non-hodgkin Lymphoma. Front. Immunol. 2020, 11, 1941. [Google Scholar] [CrossRef]
- Benjamin, R.; Graham, C.; Yallop, D.; Jozwik, A.; Mirci-Danicar, O.C.; Lucchini, G.; Pinner, D.; Jain, N.; Kantarjian, H.; Boissel, N.; et al. Genome-edited, donor-derived allogeneic anti-CD19 chimeric antigen receptor T cells in paediatric and adult B-cell acute lymphoblastic leukaemia: Results of two phase 1 studies. Lancet 2020, 396, 1885–1894. [Google Scholar] [CrossRef]
- Townsend, M.H.; Bennion, K.; Robison, R.A.; O’Neill, K.L. Paving the way towards universal treatment with allogenic T cells. Immunol. Res. 2020, 68, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Qasim, W. Allogeneic CAR T cell therapies for leukemia. Am. J. Hematol. 2019, 94, S50–S54. [Google Scholar] [CrossRef] [PubMed]
- Poirot, L.; Philip, B.; Schiffer-Mannioui, C.; Le Clerre, D.; Chion-Sotinel, I.; Derniame, S.; Potrel, P.; Bas, C.; Lemaire, L.; Galetto, R.; et al. Multiplex Genome-Edited T-cell Manufacturing Platform for “Off-the-Shelf” Adoptive T-cell Immunotherapies. Cancer Res. 2015, 75, 3853–3864. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.; Wickman, E.; DeRenzo, C.; Gottschalk, S. CAR T Cell Therapy for Solid Tumors: Bright Future or Dark Reality? Mol. Ther. 2020, 28, 2320–2339. [Google Scholar] [CrossRef] [PubMed]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Nishio, N.; Diaconu, I.; Liu, H.; Cerullo, V.; Caruana, I.; Hoyos, V.; Bouchier-Hayes, L.; Savoldo, B.; Dotti, G. Armed Oncolytic Virus Enhances Immune Functions of Chimeric Antigen Receptor–Modified T Cells in Solid Tumors. Cancer Res. 2014, 74, 5195–5205. [Google Scholar] [CrossRef]
- Mc Granahan, N.; Swanton, C. Clonal heterogeneity and tumor evolution: Past, present, and the future. Cell 2017, 168, 613–662. [Google Scholar] [CrossRef]
- Finotello, F.; Trajanoski, Z. Quantifying tumor-infiltrating immune cells from transcriptomics data. Cancer Immunol. Immunother. 2018, 67, 1031–1040. [Google Scholar] [CrossRef]
- Li, T.; Fu, J.; Zeng, Z.; Cohen, D.; Li, J.; Chen, Q.; Li, B.; Liu, X.S. TIMER2.0 for analysis of tumor-infiltrating immune cells. Nucleic Acids Res. 2020, 48, W509–W514. [Google Scholar] [CrossRef]
- Mistichelli, J. Diagnosis Related Groups (DRGs) and the Prospective Payment System: Forecasting Social Implications; Georgetown University: Washington, DC, USA, 1984. [Google Scholar]
- Fetter, R.B.; Freeman, J.L. Diagnosis-related groups: Product line management within hospitals. Acad. Manag. Rev. 1986, 11, 41–54. [Google Scholar] [CrossRef]
- Martino, M.; Console, G.; Russo, L.; Meliado’, A.; Meliambro, N.; Moscato, T.; Irrera, G.; Messina, G.; Pontari, A.; Morabito, F. Autologous Stem Cell Transplantation in Patients with Multiple Myeloma: An Activity-based Costing Analysis, Comparing a Total Inpatient Model Versus an Early Discharge Model. Clin. Lymphoma Myeloma Leuk. 2017, 17, 506–512. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.; Kaplan, R.S. The promise--and peril--of integrated cost systems. Harv. Bus. Rev. 1998, 76, 109–119. [Google Scholar] [PubMed]
| Name | General Description | Therapeutic Indications |
|---|---|---|
| Tisagenlecleucel | Immunocellular therapy containing tisagenlecleucel, autologous T cells genetically modified ex vivo using a lentiviral vector encoding an anti-CD19 chimeric antigen receptor. | Pediatric and young adult patients up to and including 25 years of age with B-cell acute lymphoblastic leukemia that is refractory, in relapse post-transplant, or in second or later relapse. Adult patients with R/R diffuse large B-cell lymphoma after two or more lines of systemic therapy. |
| Axicabtageneciloleucel | A CD19-directed genetically modified autologous T-cell immunotherapy. T cells are genetically modified ex vivo by retroviral transduction to express a chimeric antigen receptor comprising a murine anti-CD19 single-chain variable fragment linked to the CD28 co-stimulatory domain and CD3-zeta signaling domain. | After two or more lines of systemic therapy, adult patients with R/R diffuse large B-cell lymphoma and primary mediastinal large B-cell lymphoma. |
| Lisocabtagenemaraleucel | Anti-CD19 single-chain variable fragment (scFv) targeting domain for antigen specificity, a transmembrane domain, a 4-1BB costimulatory domain hypothesized to increase T-cell proliferation and persistence, and a CD3-zeta T-cell activation domain. | After two or more lines of systemic therapy, adult patients with R/R large B-cell lymphoma, including diffuse large B-cell lymphoma, not otherwise specified, high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma, and follicular lymphoma grade 3B. |
| Brexucabtageneautoleucel | Autologous peripheral blood T-lymphocytes (PBTL) that have been transduced with a retroviral vector expressing a chimeric antigen receptor (CAR) consisting of an anti-CD19 single-chain variable fragment (scFv) coupled to the zeta chain of the T-cell receptor (TCR)/CD3 complex (CD3 zeta) and the costimulatory signaling domain CD28. | Treatment of adult patients with R/R mantle cell lymphoma. |
| Idecabtagenevicleucel | Anti B-Cell maturation antigen (BCMA) scFv fused to the CD137 (4-1BB) co-stimulatory and CD3ζ signaling domains. | Adult patients with R/R multiple myeloma after four or more prior lines of therapy, including an immunomodulatory agent, a proteasome inhibitor, and an anti-CD38 monoclonal antibody. |
| Ciltacabtageneautoleucel | BCMA-targeted T-cell therapies are directed against two BCMA epitopes (VH1 and VH2) to confer improved affinity for BCMA-expressing cells. | Not authorized. Trials ongoing in R/R multiple myeloma to both immunomodulatory agents and proteasome inhibitors, or with at least three prior lines of therapy and previously exposed to anti-CD38 monoclonal antibody. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martino, M.; Naso, V.; Loteta, B.; Canale, F.A.; Pugliese, M.; Alati, C.; Musuraca, G.; Nappi, D.; Gaimari, A.; Nicolini, F.; et al. Chimeric Antigen Receptor T-Cell Therapy: What We Expect Soon. Int. J. Mol. Sci. 2022, 23, 13332. https://doi.org/10.3390/ijms232113332
Martino M, Naso V, Loteta B, Canale FA, Pugliese M, Alati C, Musuraca G, Nappi D, Gaimari A, Nicolini F, et al. Chimeric Antigen Receptor T-Cell Therapy: What We Expect Soon. International Journal of Molecular Sciences. 2022; 23(21):13332. https://doi.org/10.3390/ijms232113332
Chicago/Turabian StyleMartino, Massimo, Virginia Naso, Barbara Loteta, Filippo Antonio Canale, Marta Pugliese, Caterina Alati, Gerardo Musuraca, Davide Nappi, Anna Gaimari, Fabio Nicolini, and et al. 2022. "Chimeric Antigen Receptor T-Cell Therapy: What We Expect Soon" International Journal of Molecular Sciences 23, no. 21: 13332. https://doi.org/10.3390/ijms232113332
APA StyleMartino, M., Naso, V., Loteta, B., Canale, F. A., Pugliese, M., Alati, C., Musuraca, G., Nappi, D., Gaimari, A., Nicolini, F., Mazza, M., Bravaccini, S., Derudas, D., Martinelli, G., & Cerchione, C. (2022). Chimeric Antigen Receptor T-Cell Therapy: What We Expect Soon. International Journal of Molecular Sciences, 23(21), 13332. https://doi.org/10.3390/ijms232113332










