Abstract
Abiotic stresses, such as drought, salinity, heat, cold, and heavy metals, are associated with global climate change and hamper plant growth and development, affecting crop yields and quality. However, the negative effects of abiotic stresses can be mitigated through exogenous treatments using small biomolecules. For example, the foliar application of melatonin provides the following: it protects the photosynthetic apparatus; it increases the antioxidant defenses, osmoprotectant, and soluble sugar levels; it prevents tissue damage and reduces electrolyte leakage; it improves reactive oxygen species (ROS) scavenging; and it increases biomass, maintains the redox and ion homeostasis, and improves gaseous exchange. Glutathione spray upregulates the glyoxalase system, reduces methylglyoxal (MG) toxicity and oxidative stress, decreases hydrogen peroxide and malondialdehyde accumulation, improves the defense mechanisms, tissue repairs, and nitrogen fixation, and upregulates the phytochelatins. The exogenous application of proline enhances growth and other physiological characteristics, upregulates osmoprotection, protects the integrity of the plasma lemma, reduces lipid peroxidation, increases photosynthetic pigments, phenolic acids, flavonoids, and amino acids, and enhances stress tolerance, carbon fixation, and leaf nitrogen content. The foliar application of glycine betaine improves growth, upregulates osmoprotection and osmoregulation, increases relative water content, net photosynthetic rate, and catalase activity, decreases photorespiration, ion leakage, and lipid peroxidation, protects the oxygen-evolving complex, and prevents chlorosis. Chemical priming has various important advantages over transgenic technology as it is typically more affordable for farmers and safe for plants, people, and animals, while being considered environmentally acceptable. Chemical priming helps to improve the quality and quantity of the yield. This review summarizes and discusses how exogenous melatonin, glutathione, proline, and glycine betaine can help crops combat abiotic stresses.
Keywords:
antioxidants; drought; heat; salinity; heavy metals; abiotic stress; melatonin; glutathione; proline; glycine betaine 1. Introduction
Plants, being sessile in nature, must withstand various abiotic stresses, including drought, salinity, extreme temperatures, and heavy metals, which they accomplish through physiological means rather than behavioral avoidance. These abiotic stresses have severe negative impacts on crop productivity, growth, and development. It is therefore of vital importance to improve plant physiological and biochemical functions by protecting their cellular environments under abiotic stresses to fulfill food security needs [1].
Drought is considered one of the most severe abiotic stresses that causes osmotic imbalance in plants; due to unpredictable rainfall, light intensity, and fluctuating temperatures, the electron transport rate, stomatal conductance, photosynthesis, and carbon dioxide fixation are reduced. Salinity stress is another major threat to crop productivity; exposure to high salinity causes both ionic and osmotic imbalances, resulting in the overproduction of reactive oxygen species (ROS). Extreme temperatures can be too high or too low for normal physiological functions in the plant, limiting agricultural production. Heat stress causes the denaturation of enzymes and protein structures. Cold stress is induced by freezing temperatures, which negatively affect plant growth and yield. Extreme temperatures lead to the overproduction of ROS, which causes lipid peroxidation, protein damage, or cell structure destruction. Heavy metal stress is also an increasing threat to agricultural land and crop productivity. The uptake of heavy metals and metalloids such as cadmium (Cd), lead (Pb), and arsenic (As) from contaminated soil results in overproduction of ROS, oxidative damage, and reduction in plant productivity [2] (Figure 1).
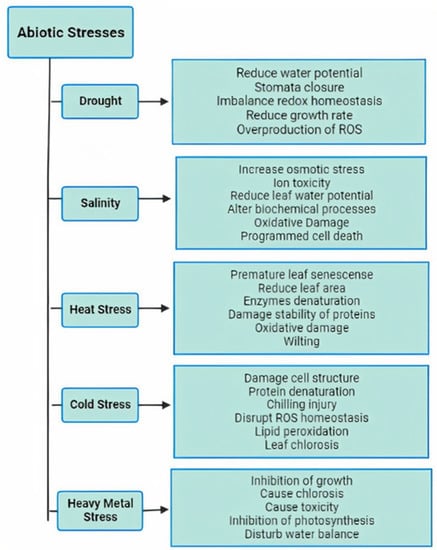
Figure 1.
The negative impacts of abiotic stresses on plants.
Antioxidants are the key elements that protect plants from the oxidative damage caused by abiotic stresses. Melatonin acts as an effective antioxidant against different abiotic stresses and enhances certain physiological activities, including osmotic regulation, growth, germination, photosynthesis, primary and secondary metabolism, anti-senescence, plant hormone regulation, transpiration, ROS scavenging, and antioxidant enzyme activities. For example, seed priming using melatonin enhanced the plant’s ability to scavenge ROS and improved the photosynthetic efficiency in various crops [3,4]. Glutathione is known as one of the most abundant and essential metabolites for combating abiotic stresses as an antioxidant. A study of the exogenous application of glutathione demonstrated an increase in the abiotic stress tolerance of plants by improving the growth parameters, chlorophyll, photosynthesis, and antioxidant enzyme activities [5]. Despite being an osmolyte, proline is also considered an antioxidant. The external application of proline during abiotic stress was shown to increase the growth, photosynthetic activity, endogenous proline content, and antioxidant enzyme activities, which protected the integrity of the plasmalemma, scavenged ROS, and stabilized proteins and other physiological functions of the plants [6]. Glycine betaine is an osmoprotectant that also works as an antioxidant against abiotic stresses. Exogenous glycine betaine played a significant role in the detoxification of ROS, increased photosynthesis and stomatal conductance, decreased photorespiration in certain crops, improved yield and relative membrane permeability, reduced stomatal closure and the accumulation of malondialdehyde and leaf damage, and alleviated Cd stress in various crops [7,8] (Figure 2).
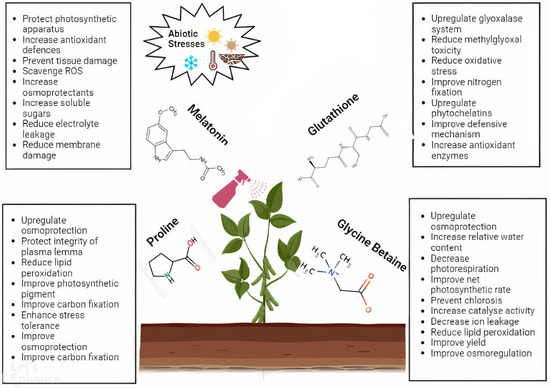
Figure 2.
The external application of melatonin, glutathione, proline, and glycine betaine, along with their contribution for increasing the plant’s ability to withstand abiotic stresses.
Comparing chemical priming to transgenic technology, there are some substantial advantages that are affordable for farmers and safe for plants, humans, and animals while being inexpensive and environment friendly. The exogenous application of melatonin, glutathione, proline, and glycine betaine mitigate the adverse effects of abiotic stresses on plants and provide an alternative to the utilization of transgenic plants.
In this review, we will discuss the beneficial effects of externally applied melatonin, glutathione, proline, and glycine betaine, especially their antioxidant roles in crop plants under various abiotic stresses.
2. Melatonin
2.1. Structure and Function of Melatonin
Melatonin (N–acetyl-5-methoxytryptamine) is a widespread and highly conserved molecule across species that was initially discovered in 1995. It is an indolic compound derived from tryptophan with a very similar structure to indole-3-acetic acid (IAA); likewise, melatonin serves similar functions in metabolic pathways. It has an exceptionally diverse range of activities to the extent that it can be another plant hormone [9]. It acts as an antioxidant, neutralizing the overproduction of ROS under stressful conditions to reduce oxidative damage and improve the growth and development of plants. Various physiological processes are enhanced by melatonin to overcome abiotic stresses, such as stomatal conductance, photosynthesis, root dynamics, carbohydrate assimilation, and water relation. Transpiration, ROS scavenging, and antioxidant enzyme activities, which together serve to alleviate oxidative damage to lipids, proteins, and nucleic acids, are also enhanced by the application of melatonin. For example, seeds pre-soaked in melatonin had increased numbers and a wider opening of stomata, enhanced antioxidant enzyme activities, osmoprotectants, and ROS scavenging activity in many crops [3,10].
Seed germination and seedling development (the growth of roots and shoots) are two vital processes of a plant’s successful lifecycle. Melatonin has been observed to regulate these processes and increase plant stress tolerance. Plant biomass ultimately depends on the amount of assimilated CO2. Some processes are determined by the efficiency of the leaf photochemistry such as the light harvesting, chlorophyll production, photosynthetic electron transfer, and leaf gaseous exchange processes. It was reported that, under stress conditions, the external application of melatonin enhanced the photosynthetic rate in plants [9]. Melatonin is a master phytohormone regulator, especially for auxin levels, and it improves the ability of plants to tolerate stress conditions. Melatonin has been added to the current list of secondary messengers that control the operation of transporter proteins and maintain the ion homeostasis in plants [11]. Melatonin was found to regulate the dark reaction of photosynthesis through carbon fixation at the molecular level by upregulating the activity of various enzymes, such as ribulose bisphosphate carboxylase/oxygenase (RuBisCo), glyceraldehyde-3-phosphate dehydrogenase, phosphoglycerate kinase, phosphoribulokinase, and fructose-bisphosphate aldolase. Chlorophyll fluorescence was significantly increased by external melatonin application [12].
2.2. Melatonin Effect against Drought Stress
Drought stress generally causes an osmotic imbalance in plants, which leads to the disruption of the membrane system, electron transport chain, stomatal conductance, photosynthesis, and carboxylation efficiency; these in turn lead to the over-production of ROS, which ultimately upsets the redox homeostasis, resulting in changes to the physiological and biochemical processes, particularly when the drought stress is prolonged and extreme. The exogenous application of melatonin has been proven to be beneficial against drought stress. It is effective at controlling drought-induced oxidative damage, leaf senescence, and inhibition of photosystems, ultimately improving the crop yield [13].
In Arabidopsis thaliana (Arabidopsis/Thale cress), adding 50 µM of melatonin supplement to the nutrient solution upregulated stress-responsive genes (COR15A, RD22, and KIN1) when grown under drought conditions, raising the level of soluble sugars such as sucrose when grown under drought conditions [14] (Table 1). Pretreating seeds with 100 µM of melatonin improved plant growth, osmoprotectant levels, induced stress-responsive genes, scavenged ROS, and reduced electrolyte leakage in Oryza sativa (Rice) when under drought stress [15]. In Zea mays (Maize), a 1 mM melatonin treatment upregulated photoprotection (increased photosystem PS Ⅱ efficiency), and a foliar application of 100 µM of melatonin increased stomatal conductance, photosynthesis and transpiration rates, cell turgor, water holding capacity, increased enzymatic and non-enzymatic antioxidants, regulated osmotic potential, and scavenged ROS to tolerate drought stress [16,17]. In Triticum aestivum (Wheat), the application of 500 µM of melatonin to the soil regulated photosynthesis, cell turgor, increased water holding capacity, scavenged ROS, and reduced membrane damage; seed pretreatment with 10 or 100 µM of melatonin (dependent on variety) increased germination percentage, increased radicle and plumule lengths, and increased lysine content (a germination-related amino acid) during drought stress [18,19]. The foliar application of 100 µM of melatonin to Fagopyrum tataricum (Tartary Buckwheat) increased osmoprotectants, water status, secondary metabolites, antioxidant enzymes, photosynthetic rate, and ROS scavenging when under drought stress [20]. The foliar or soil application of 1 mM of melatonin to Hordeum vulgare (Barley) increased the endogenous melatonin level, antioxidants, abscisic acid (ABA), water status, photosynthetic rate, and PS Ⅱ efficiency when grown under drought stress [21]. In Glycine max (Soybean), coating seeds with 50 µM of melatonin increased seedling biomass and seedling growth and reduced electrolyte leakage; the foliar or root application of 100 µM of melatonin increased plant growth, flowering, seed yield, gaseous exchange, PS Ⅱ efficiency, and antioxidant enzymes to combat drought stress [22,23]. In Minhot esculenta (Cassava), the application of 100 µM of melatonin to the soil increased peroxidase (POD) activity and scavenging of ROS to reduce the negative effects of drought stress [24]. In Gossypium hirsutum (Cotton) seeds pre-soaked with 100 µM of melatonin developed into plants with increased numbers, increased opening of stomata, enhanced antioxidant enzyme activities, osmoprotection, and ROS scavenging when grown under drought stress [25]. Soil treatment with 10 µM of melatonin in the case of Medicago sativa (Alfalfa) increased chlorophyll content, stomatal conductance, osmoprotection, upregulated nitro-oxidative homeostasis and ROS scavenging, and reduced cellular redox disruption against drought stress [26]. In Malus domestica (Apple), the application of 100 µM of melatonin to the soil increased the water holding capacity, rate of photosynthesis, stomatal regulation, and antioxidants; furthermore, it decreased the electrolyte leakage, ROS production, oxidative damage, and leaf senescence, which helped to avoid the consequences drought stress [27]. Roots of Vitis vinifera (Grape) were pretreated with 100 µM of melatonin and had increased photoprotection, leaf thickness, stomata size, enzymatic and non-enzymatic antioxidants, and reduced oxidative damage when grown under drought stress [4]. Actinidia chinensis (Kiwifruit) treated with 100 µM of melatonin had increased osmoprotectants, protein biosynthesis, photosynthesis, and reduced cell membrane damage against drought stress [12]. The foliar application of 100 µM of melatonin to Carya cathayensis (Chinese hickory) increased photosynthesis, antioxidants, osmoprotectants, and scavenging of ROS under drought stress [13]. The foliar application of 200 µM of melatonin to Solanum lycopersicum (Tomato) increased chlorophyll and antioxidant enzyme contents; a 0.1 mM melatonin application increased photosynthesis, PS Ⅱ efficiency, antioxidants, and reduced toxic substance contents to tolerate drought stress [28,29]. Seeds of Capsicum annuum (Pepper) were pretreated with 50 µM of melatonin and had an increased water holding capacity, endogenous melatonin, increased carotenoids, and increased chlorophyll contents to combat drought stress [30]. In Citrullus lanatus (Watermelon), a 150 µM melatonin pretreatment of the root increased wax accumulation and reduced the ABA level under drought stress [19]. In Cucumis sativus (Cucumber), seeds primed with a nutrient solution supplemented by 100 µM of melatonin had increased germination rates, root growths, chlorophyll, photosynthesis, antioxidant enzymes, and ROS scavenging, whereas a foliar application of 10 µM of melatonin resulted in ROS scavenging and increased drought tolerance [4,31]. In Brassica napus (Rapeseed), seed primed with 500 µM of melatonin had increased chlorophyll, stomatal regulation, cell wall expansion, antioxidant enzymes and osmoprotectants, which operated in tandem with a reduction in oxidative injury to combat drought stress [32]. Foliar applications of 100 µM of melatonin increased photosynthesis, chlorophyll, and osmoprotectants while reducing cell membrane damage and relative conductivity in Dendranthema morifolium (Jinyu Chuju) in order to avoid the consequences of drought stress [33]. In Dracocephalum moldavica (Moldavian balm), foliar applications of 100 µM of melatonin increased plant growth, flowering, antioxidant activities, chlorophyll contents, water holding capacity, and ROS scavenging when grown under drought stress [34]. Foliar applications of 20 µM of melatonin increased photosynthetic contents, water holding capacity, PS Ⅱ efficiency, and ROS scavenging while also reducing leaf senescence in Agrostis stolonifera (Creeping bentgrass) to combat drought stress [35]. In Festuca arundinacea (Tall fescue), an irrigation pretreatment with 20 µM of melatonin increased antioxidant enzyme activities, chlorophyll content, plant growth, and ROS scavenging under drought conditions [36]. In Cynodon dactylon (Bermuda grass), 20 and 100 µM of melatonin was included in irrigation pretreatments, which increased plant growth, chlorophyll content, and antioxidant activities while also inducing stress-responsive genes, improved hormonal regulation, and ROS scavenging to reduce the negative effects of drought stress [14]. Foliar applications of 100 and 300 µM of melatonin to Trigonella foenum-graecum (Fenugreek) increased the levels of endogenous melatonin, secondary metabolites, chlorophyll and antioxidant enzymes, and improved ROS scavenging under drought [37]. In Coffea Arabica (Coffee), the application of 300 µM of melatonin to soil increased photoprotection, gaseous exchange, carboxylation activities, chlorophyll content, and antioxidant enzyme activities to combat drought stress [38]. In Camellia sinensis (Tea), the foliar application of 100 µM of melatonin increased photosynthesis, antioxidant enzymes, and ROS scavenging while reducing glutathione (GSH) and ascorbic acid (AsA) contents when grown under drought stress [39]. In Nicotiana benthamiana (Tobacco), the foliar application of 10 µM of melatonin increased drought tolerance through ROS scavenging and reduced oxidative damage [31].

Table 1.
Physiological responses of various crops to melatonin treatment against drought stress.
2.3. Melatonin Effect against Salinity Stress
The negative impacts of salinity stress on agricultural production and plant growth include a reduction in the photosynthetic activity and disturbances in the protein and carbohydrate metabolisms. The first sign of salinity is the generation of ROS and their harmful physiological effects, such as protein degradation, lipid peroxidation, and DNA mutation, which result in oxidative damage and the downregulation of carbon dioxide fixation, leading to physiological dysfunction and programmed cell death. Salinity speeds up leaf senescence and reduces cell expansion and crop yield. The exogenous application of melatonin increases relative water content, antioxidant enzyme activity, and improves photosynthetic efficiency to enhance the tolerance against salinity stress [40,41].
Under high salinity conditions, priming Momordica charantia (Bitter melon) seeds with 150 µM of melatonin increased relative water content, antioxidant enzyme activities, stress-responsive gene expression levels, and decreased hydrogen peroxide and malondialdehyde levels [42] (Table 2). In Zea mays (Maize), the pretreatment of seeds with 0.4, 0.8, and 1.6 mM of melatonin improved the shoot and root lengths, rates of germination, fresh and dry weights of the seedlings, potassium ion (K+) contents, antioxidant enzyme activities, and relative water content under high salinity conditions [43]. In Gossypium hirsutum (Cotton), priming the seed with 25 µM of melatonin enhanced the plant’s ability to scavenge ROS and improved photosynthetic efficiency against salt stress [44]. Seed priming with 70 µM of melatonin enhanced photosynthetic pigments, indole-3-acetic acid (IAA) contents, and growth parameters in Triticum aestivum (Wheat) to avoid the consequences of salinity stress [45]. In Ocimum basilicum (Basil), seed priming with 10 µM of melatonin increased the contents of flavonoids and phenolic acids under high salinity conditions [46]. Priming Vicia faba (Faba bean) seeds with 100 or 500 mM of melatonin improved novel protein expressions against salt stress [47]. In Cucumis sativum (Cucumber), seed priming with 1 µM of melatonin enhanced seed germination under saline conditions [43]. The foliar treatment of Arabidopsis thaliana (Arabidopsis/Thale cress) using 10 µM of melatonin induced the antioxidant defense system, scavenged ROS, and upregulated abscisic acid (ABA) responsive genes under salinity stress [48]. The foliar application of Brassica napus (Rapeseed) using 1 µM of melatonin reduced lipid peroxidation and hydrogen peroxide content while also maintaining redox and ion homeostasis against salinity [49]. In Brassica juncea (Mustard greens), the foliar application of 1 µM of melatonin increased leaf length/width, plant height, and stem diameter, and improved gaseous exchange and relative water content; furthermore, it increased the salicylic acid level while reducing the abscisic acid (ABA) level against saline conditions [50]. Seed pretreatment of Cucumis melo (Melon) with 0, 10, and 50 µM of melatonin increased seed germination under high salinity [51]. In Oryza sativa (Rice), irrigating the roots with 0, 10, and 20 µM of melatonin upregulates antioxidants while inhibiting cell death and chlorophyll degradation against salt stress [52]. In a study on the foliar application of Glycine max (Soybean) using 0–100 µM of melatonin, the author noticed increased levels of photosynthesis, cell division, carbohydrates, fatty acids and ascorbate contents in addition to a reduction in the inhibitory effect of salt on gene expressions under high salinity conditions [22]. In Malus hupehensis (Pingyitiancha) seeds pretreated with 0.1 µM of melatonin, there was an increase in photosynthesis and ion homeostasis and a decrease in oxidative damage due to high salt contents [53]. In Solanum lycopersicum (Tomato), irrigating the roots with 100 µM of melatonin increased protein and membrane protection, antioxidants activities, and photosynthesis against salinity [54]. Seeds of Citrullus lanatus (Watermelon) that were pretreated using 50–150 µM of melatonin had increased antioxidant enzymes, photosynthesis, and PS Ⅱ efficiency in addition to reduced stomatal closure and oxidative damage while the plant was under salt stress [55].

Table 2.
Physiological responses of various crops to melatonin treatment against salinity stress.
2.4. Melatonin Effect against Heat Stress
Heat can cause severe damage to plants. High temperatures affect plant growth, reducing the yield and affecting protein stability, which causes enzyme denaturation due to the overproduction of ROS. Heat stress disrupts the fluidity of the membrane, leading to a reduction in photosynthesis, growth, and yield. The exogenous application of melatonin reduces oxidative damage and upregulates heat shock factors to combat heat stress [56].
In Triticum aestivum (Wheat), the application of 20 µM of melatonin to soil application increased the rate of photosynthesis and reduced oxidative damage due to heat stress [56] (Table 3). The foliar application of melatonin on Lolium perenne (Perennial Ryegrass) regulated cytokinin biosynthetic genes, downregulated ABA biosynthetic genes, and enhanced the endogenous melatonin content against heat stress [57]. Seed pretreatment with 100 µM of melatonin enhanced phenolic acid levels, regulated transcript abundances, increased the endogenous melatonin level, and reduced oxidative stress in Solanum lycopersicum (Tomato) when under heat stress [58]. In Arabidopsis thaliana (Arabidopsis/Thale cress), the foliar application of 20 µM of melatonin upregulated heat shock factors to combat heat stress [59]. The application of 100 µM of melatonin to soil increased photosynthesis and reduced oxidative damage in Zea mays (Maize) grown under an elevated temperature [17].

Table 3.
Physiological responses of various crops to melatonin treatment against heat stress.
2.5. Melatonin Effect against Cold Stress
Cold stress is an oxidative stress caused by chilling or freezing temperatures that can negatively affect plant growth and development. Cold stress induces the excessive generation of ROS, thus disrupting ROS homeostasis; this may lead to lipid peroxidation, cell structure damage, protein denaturation, and chilling injury. Cold stress reduces photosynthetic capacity, chloroplast development, and chlorophyll content. The exogenous application of melatonin improves antioxidant enzyme activities and reduces cold-induced oxidative stress [60].
In Triticum aestivum (Wheat), the exogenous application of 100 µM of melatonin improved antioxidant enzyme activities and reduced the oxidative stress caused by low temperatures [61] (Table 4). In Citrullus lanatus (Watermelon), the application of 150 µM of melatonin to soil increased the accumulation of hydrogen peroxide and increased cold stress tolerance [62]. In Solanum lycopersicum (Tomato), seed priming with 100 µM of melatonin improved photosynthesis and reduced oxidative damage due to cold stress [63]. The foliar application of 100 µM of melatonin increased the arabinose, mannose, and propanoic acid contents in Cynodon dactylon (Bermuda grass) when grown under cold stress [64]. In Hordeum vulgare (Barley), treatment with 1 mM of melatonin improved the water status, antioxidant system, and abscisic acid (ABA) level to tolerate cold stress [21]. In Camellia sinensis (Tea plant), the external application of melatonin increased antioxidant enzyme activities and reduced oxidative stress from exposure to low temperatures [65]. In Oryza sativa (Rice), exogenous melatonin improved antioxidant enzyme activities and reduced cold-induced oxidative stress [55]. In Cucumis sativus (Cucumber), the external application of 100 µM of melatonin improved antioxidant enzyme activities and reduced oxidative stress from low temperatures [66].

Table 4.
Physiological responses of various crops to melatonin treatment against cold stress.
2.6. Melatonin Effect against Heavy Metal Stress
Heavy metals are either non-essential or minimally required elements for the normal growth and development of plants; they are found in the soil water of contaminated soils and are readily taken up by the plant, causing oxidative stress and damage to the plant due to the overproduction of ROS. The exogenous application of melatonin improves chlorophyll content, antioxidant enzymes, and ROS scavenging to combat heavy metal stress [67].
In Triticum aestivum (Wheat), the treatment of cadmium (Cd) heavy metal-contaminated soil with 50 µM of melatonin caused an increase in antioxidant enzymes [68] (Table 5). The foliar application of 50 µM of melatonin to Medicago sativa (Alfalfa) growing in heavy metal-containing soil resulted in an increase in ATP-binding cassette-containing (ABC) transporters and a decrease in cadmium (Cd) accumulation [69]. In Solanum lycopersicum (Tomato), seed pretreatment with 100 µM of melatonin increased antioxidants and plant growth and reduced electrolyte leakage and photoinhibition against cadmium (Cd) metal stress [21]. In Nicotiana benthamiana (Tobacco), a foliar application of 15 µM of melatonin increased cell growth and viability while decreasing DNA damage due to lead (Pb) heavy metal contents [53]. Soil treatment with 50 µM of melatonin increased antioxidants and plant biomass in Cyphomandra betacea (Tree tomato) to reduce the negative effects of cadmium (Cd) heavy metal stress [70]. In Glycine max (Soybean), seed priming with 100 mM of melatonin increased photosynthesis and antioxidants under aluminum (Al) heavy metal stress [53]. The foliar application of Brassica oleracea (Red cabbage) with 10 µM of melatonin increased the germination and fresh weight of the plant in the presence of copper (Cu) heavy metal [53]. In Citrullus lanatus (Watermelon), seed priming with 50 mg/L of melatonin increased plant growth, photosynthesis, chlorophyll content, antioxidant enzymes, and ROS scavenging to combat vanadium (V) heavy metal stress [71]. In Zea mays (Maize), treatment of heavy metal-containing soil with 500 µM of melatonin induced additional proteins related to stress reduction during germination [72]. In Cucumis sativus (Cucumber), irrigating cadmium (Cd) metal-containing soil with 100 or 150 µM of melatonin reduced the expressions of stress-inducible genes such as CsHA2 [43]. In Amaranthus viridis (Amaranthus), the foliar application of 400 µM of melatonin decreased the accumulation of lead (Pb) heavy metal in the roots [73].

Table 5.
Physiological responses of various crops to melatonin treatment against heavy metal stress.
3. Glutathione
3.1. Structure and Function of Glutathione
Glutathione (GSH; γ-glutamyl-cysteinyl-glycine), a low-molecular-weight thiol, is a very crucial metabolite involved in the plant’s antioxidant defense system. The external application of glutathione has been reported to increase growth parameters such as root and shoot lengths, fresh and dry weights, the number of fruits per plant, and fruit weight while enhancing the photosynthetic rate, chlorophyll a and b, and carotenoids; furthermore, glutathione increases antioxidants and osmoprotectants such as endogenous glutathione, total soluble sugars, proteins and polyamines and stimulates gene expressions (e.g., CaAPX1, CaMDHAR1, and CaDHAR1 in Capsicum annuum) and enzymatic activities, decreasing ROS production and the accumulation of malondialdehyde and other toxic substances [5].
It has been demonstrated that glutathione is a potent antioxidant that increases the plant’s resistance against abiotic stresses. Higher glutathione levels facilitate the detoxification of heavy metals, thus preventing any damage to lipids, amino acids, and polysaccharides, as well as any injury to the membranes, photosynthetic machinery, mitochondria, and other organelles, eventually conferring tolerance against abiotic stresses. Glutathione also plays a role in the maintenance of osmotic balance by stimulating osmoprotectants. Externally applied glutathione increases the endogenous glutathione level and improves the ratio of GSH (the reduced form) to GSSG (the oxidized form), enabling the efficient scavenging of excess ROS and therefore reducing oxidative stress and damage to plants. Methylglyoxal detoxification is one of the significant roles that the compound has in protecting the plant from damage by toxic substances. By interacting with other redox systems, glutathione directly or indirectly modulates the stress-responsive transcriptional and post-transcriptional levels [2].
3.2. Glutathione Effect against Drought Stress
Drought stress in plants causes leaf rolling, stunted plant growth, yellowing of leaves and leaf wilting by reduced leaf water potential and turgor pressure, increased stomatal closure, and decreased cell growth. Glutathione application increases plant stress tolerance by increasing antioxidants, abscisic acid, relative water content, and scavenging of ROS, thus improving growth parameters and chlorophyll contents. Exogenous application of glutathione improves chlorophyll content, photosynthesis, relative water content and antioxidant enzyme activities to combat drought stress [5].
In Cicer arietinum (Chickpea), seed soaking with 0.75 mM of glutathione increased the growth parameters, chlorophyll content, photosynthesis, endogenous proline level, and antioxidant enzyme activities under drought conditions [74] (Table 6). In Oryza sativa (Rice), spraying with 0.2 mM of glutathione increased the root and shoot lengths, dry and fresh weights, chlorophyll pigment, relative water content, and antioxidant enzyme activities when grown under drought [5]. The foliar application of glutathione to Brassica napus (Rapeseed) scavenged ROS and reduced oxidative damage due to drought stress [75]. In Triticum aestivum (Wheat), treatment with exogenous glutathione via spraying improved the plant’s tolerance to drought, compared with the untreated cultivar [76]. In Vigna radiata (Mung bean), the exogenous application of glutathione lessened drought-induced oxidative damage by enhancing the capacity of the antioxidant system and glyoxalase activity [77]. Arabidopsis thaliana (Arabidopsis/Thale cress) treated with glutathione via spraying had higher abscisic acid content, was more tolerant against drought stress, and had improved health under drought conditions [78].

Table 6.
Physiological responses of various crops to glutathione treatment against drought stress.
3.3. Glutathione Effect against Salinity Stress
Salinity stress causes nutritional imbalance, inhibition of water uptake, photosynthesis and seed germination, and an overall decrease in crop productivity. Treatment with glutathione can remedy salinity stress by increasing plant growth, dry and fresh weights of roots and shoots, and total yield through the enhancement of water use efficiency, osmoprotectant levels, photosynthetic activities, and stomatal conductance. The exogenous application of glutathione increases water use efficiency, level of osmoprotectants, and antioxidant enzyme activity to combat salinity stress [59].
In Capsicum frutescence (Pepper) experiencing salinity stress, foliar sprays with 0.4 and 0.8 mM of glutathione increased water use efficiency, overall growth, fresh and dry weights of roots and shoots, yield, and the levels of osmoprotectants and antioxidants [79] (Table 7). In Cucumis sativus (Cucumber), soaking seeds in 0.5 mM of glutathione increased growth, fresh and dry weights, relative water content, photosynthetic activities, and stomatal conductance despite salinity stress conditions [80]. In the case of Vicia faba (Faba bean) under salt stress, foliar sprays with 0.5 mM of glutathione increased growth, fresh and dry weights, relative water content, photosynthetic activity, stomatal conductance, and antioxidant enzyme activities [81]. Foliar sprays with 1 mM of glutathione on Triticum aestivum (Wheat) counteracted the effects of salt stress by increasing plant growth, membrane stability, and the accumulation of osmoprotectants [59]. In the case of Glycine max (Soybean) under salt stress, foliar sprays with 1 mM of glutathione increased growth, photosynthesis, membrane stability, soluble sugar contents, and antioxidant enzyme activities [5]. Similarly, in Phaseolus vulgaris (Common bean), foliar sprays of 0.75 mM of glutathione increased the plant length, number and total area of leaves, fresh and dry weights of plant, relative water content, photosynthesis, and soluble sugars to avoid the consequences of salt stress [5]. Arabidopsis thaliana (Arabidopsis/Thale cress) treated with a glutathione foliar spray had higher abscisic acid levels, was more tolerant against salt stress, and had improved overall plant health under the stressful conditions [78]. In Solanum lycopersicum (Tomato), the exogenous application of glutathione reduced lipid peroxidation and improved the plant’s tolerance against salinity stress and oxidative stress [82]. In Oryza sativa (Rice), the spraying of glutathione improved the activities of antioxidant enzymes, reduced ROS accumulation, and reduced ROS-induced DNA damage due to salt stress [83].

Table 7.
Physiological responses of various crops to glutathione treatment against salinity stress.
3.4. Glutathione Effect against Heat Stress
Heat stress negatively impacts plants by reducing the leaf water potential and total leaf area, causing premature leaf senescence and thus affecting the growth and yield of the plant. Glutathione application increases resistance to heat by increasing antioxidant enzyme activities and the scavenging of ROS. The exogenous application of glutathione increases the protection of the plant against heat stress by maintaining relative water content and antioxidant enzyme activity [84].
The treatment of heat-stressed Triticum aestivum (Wheat) with glutathione increased the activities of antioxidant enzymes, therefore increasing the protection of the plant against heat stress [85] (Table 8). In Vigna radiate (Mung bean), pretreating the seeds with glutathione increased antioxidant enzyme activities and therefore enhanced heat stress resistance by decreasing the ROS level [77]. In Cucumis sativus (Cucumber), the external application of glutathione enhanced heat resistance, improved plant growth, chlorophyll content, and photosynthetic rate under heat stress [84]. In Brassica campestris (Mustard), treatment with glutathione maintained the relative water content and increased ROS scavenging and antioxidants during heat stress [86].

Table 8.
Physiological responses of various crops to glutathione treatment against heat stress.
3.5. Glutathione Effect against Cold Stress
Cold stress delays leaf development in plants, prolongs the cell cycle with reduced cell production, stunts growth, and causes leaf chlorosis and a general disruption of the structure and functions of cells and tissues. However, external glutathione applications can reduce the adverse effects of cold stress and cause an increase in the lengths of roots and shoots and fresh and dry weights of the plant by increasing the endogenous glutathione level and decreasing electrolyte leakage and lipid peroxidation compared with untreated plants. The exogenous application of glutathione decreases electrolyte leakage and lipid peroxidation to combat cold stress [60].
In Oryza sativa (Rice), the spraying of 0.5 mM of glutathione increased the lengths of roots and shoots, as well as the fresh and dry weights and the endogenous glutathione level, compared with untreated plants under cold stress [88] (Table 9). In Capsicum annum (Pepper), the spraying of 0.5 mM of glutathione also increased the root and shoot lengths, fresh and dry weights, and endogenous glutathione level of plants subjected to cold stress [60]. The foliar application with glutathione in Cucumis sativus (Cucumber) decreased electrolyte leakage and lipid peroxidation when the plants were placed under cold stress [89]. In Jatropha curcas (Purging nut), the external application of glutathione enhanced the plant’s resistance to low temperatures and enhanced the activities of antioxidant enzymes [90].

Table 9.
Physiological responses of various crops to glutathione treatment against cold stress.
3.6. Glutathione Effect against Heavy Metal Stress
Heavy metal stress generally causes toxic effects on plants, such as inhibition of growth, photosynthesis, and nutrient assimilation, along with altered water balance, chlorosis, and senescence. On the other hand, the application of glutathione has been observed to enhance stress tolerance in plants by increasing ROS scavenging, antioxidant activities, and levels of photosynthetic pigments. The exogenous application of glutathione increases photosynthetic pigments, alleviating oxidative damage to overcome the adverse effects of heavy metal stress [91].
In Triticum aestivum (Wheat), the foliar application of 20 µM of glutathione increased photosynthetic pigments and the endogenous level of glutathione in plants subjected to cadmium (Cd) heavy metal stress [62] (Table 10). In Solanum melongena (Brinjal), seed pretreatment with 1 mM of glutathione mitigated the adverse effects of arsenate (As) heavy metal stress with respect to protein damage [91]. The foliar application of 30 µM of glutathione to Zea mays (Maize) grown in cadmium (Cd) heavy metal-containing soil increased the secondary metabolites and flavonoids in the plants and alleviated oxidative damage [92]. In Lolium multiflorum (Italian ryegrass), the external application of 200 µM of glutathione increased lead (Pb) stress tolerance and the root and shoot biomass compared with untreated plants under the same heavy metal stress conditions [93]. In Hordeum vulgare (Barley), exogenous glutathione improved the antioxidant defense system and photosynthesis and decreased ROS accumulation due to cadmium (Cd) metal stress [94]. Exogenous glutathione applied to Solanum lycopersicum (Tomato) that was subjected to cadmium (Cd) metal stress synchronized the transcript levels of several stress-responsive transcription factors while also improving nitric oxide contents [95]. In Oryza sativa (Rice), treatment with glutathione elevated the endogenous glutathione level, mineral elements, and pigment contents, upregulated phytochelatins, and synchronized antioxidant enzyme activities during cadmium metal stress [96]. In Brassica campestris (Mustard), exogenous glutathione reduced the level of cadmium in roots and leaves as well as the accumulation of ROS, protecting the plant against heavy metal stress [97].

Table 10.
Physiological responses of various crops to glutathione treatment against heavy metal stress.
4. Proline
4.1. Structure and Function of Proline
Due to its cyclic structure and possession of a secondary amino group (the α-amino group) which differentiates it from the other proteinogenic amino acids, proline (Pro) is regarded as one of the most effective osmoprotectants and signaling molecules aside from its crucial role in the primary metabolism both as free amino acids and as a component of proteins. Numerous data sources point to a beneficial correlation between proline accumulation and enhanced plant stress tolerance. Proline is a significant component of the physiological response to stress in many plant species because it can accumulate in the cytosol without harming cellular structures. Due to its capacity to form hydrogen bonds, proline can increase protein stability and safeguard membrane integrity. In addition, proline can also protect cells by enhancing water uptake potential and boosting enzyme activation. Proline is an osmolyte and also a powerful antioxidant molecule, metal chelator, protein stabilizer, ROS scavenger, and inhibitor of programmed cell death [6].
External applications of proline can maintain the turgidity of the cell under stress, protect plants from harmful radiations, increase photosynthetic and transpiration rate, stomatal conductance, and antioxidants, thus increasing the growth and yield of plants even when under abiotic stresses. However, the excessive applications of proline can also impart toxic effects on plants [100].
4.2. Proline Effect against Drought Stress
The exogenous application of proline promotes the uptake of nutrients, increases the concentration of soluble proteins, and enhances the non-enzymatic antioxidant defense system to combat drought stress. In Zea mays (Maize), seed priming with 1 mM of proline increased photosynthetic and transpiration rates and stomatal conductance, while the foliar application of proline promoted the uptake and accumulation of nitrogen, phosphorus, and potassium, and enhanced the tolerance against drought stress [6] (Table 11). In Triticum aestivum (Wheat), the foliar application of 150 ppm of proline reduced the malondialdehyde level and lipid peroxidation caused by drought [101]. In Chenopodium quinoa (Quinoa), the foliar application of proline increased photosynthetic pigments, phenols, free amino acids, plant height, and the dry and fresh weights of roots and shoots compared with untreated plants under drought stress [102]. An external spray of proline in Arabidopsis thaliana (Arabidopsis/Thale cress) helped scavenge ROS and protected the integrity of the plasma lemma during drought stress [100]. In Pisum sativum (Pea), the foliar spray of 4 mM of proline increased the yield and the concentration of soluble proteins, enhancing the non-enzymatic antioxidant defense system compared with untreated plants under the same drought conditions [103].

Table 11.
Physiological responses of various crops to proline treatment against drought stress.
4.3. Proline Effect against Salinity Stress
The exogenous application of proline increases chlorophyll, relative water content, transpiration rate, and stomatal conductance to alleviate salinity stress. In Cucumis melo (Muskmelon), a foliar spray of 10 mM of proline increased the growth, chlorophyll, endogenous proline, and relative water contents of plants experiencing salinity stress [104] (Table 12). In Cucumis sativus (Cucumber), supplementing the nutrient solution with 10 mM of proline increased the growth, endogenous proline content, and antioxidant enzyme activities despite saline conditions [105]. In Glycine max (Soybean), the external application of 25 mM of proline increased the growth, endogenous proline content, antioxidant enzyme activities, and nitrogen fixation compared with untreated plants under salinity stress conditions [106]. In Helianthus annus (Sunflower), foliar sprays of 30 and 60 mM of proline increased the growth, endogenous proline content, antioxidant enzyme activities, and free amino acid contents [107]. In Zea mays (Maize), foliar sprays with 30 mM of proline increased the growth and endogenous proline content under salt stress conditions [108]. Triticum durum (Durum wheat) seeds pretreated with 12 mM of proline saw increased growth, photosynthetic activities, endogenous proline content, and antioxidant enzyme activities in saline conditions [109]. A foliar spray with 30 mM of proline increased the growth, relative water content, gaseous exchange, free amino acids, and proline contents in Sorghum bicolor (Great millet) despite saline conditions [110]. In Oryza sativa (Rice), seeds pretreated with 1, 5, and 10 mM of proline increased the growth, seed germination, and chlorophyll and endogenous proline contents to mitigate the effects of salt stress [111]. In Brassica juncea (Mustard greens), the foliar application of 20 mM of proline improved the yield and salt stress tolerance [112]. The foliar application of 0.8 mM of proline to Capsicum annum (Red pepper) improved antioxidant enzyme activities, photosynthetic and transpiration rates, dry and fresh weights of the plant, and root and shoot lengths under salt stress conditions [113]. The foliar application of proline to Daucus carota (Wild carrot) improved antioxidant enzyme activities under salt stress as well as potassium and calcium levels in the roots and shoots [114]. In Phaseolus vulgaris (Bean), the foliar application of proline improved antioxidant enzymes activities and the endogenous proline level in response to salinity [115]. In Raphanus sativus (Radish), the foliar application of proline improved the transpiration rate, stomatal conductance, photosynthetic pigment contents, and contents of proteins and other nutrients to alleviate salinity stress [116]. The foliar application of proline to Vicia faba (Faba bean) experiencing salinity stress resulted in an improvement in the contents of photosynthetic pigments, soluble carbohydrates, endogenous proline, and free amino acids [6]. The foliar application of 5 µM of proline improved plant growth, photosynthetic rate, chlorophyll content, and yield in salt-stressed Lactuca sativa (Lettuce) [117].

Table 12.
Physiological responses of various crops to proline treatment against salinity stress.
4.4. Proline Effect against Heat Stress
In Abelmoschus esculentus (Okra), the foliar application of proline improved the shoot length, the number of leaves per plant, and free amino acid contents during heat stress [118]. Similarly, in Lactuca sativa (Lettuce), the foliar application of 5µM proline improved plant growth, photosynthetic rate, chlorophyll content and yield [117]. In Vigna radiate (Mung bean), the foliar application of proline improved CO2 assimilation capacity and heat tolerance [119] (Table 13).

Table 13.
Physiological responses of various crops to proline treatment against heat stress.
4.5. Proline Effect against Cold Stress
The foliar application of proline increases antioxidant enzyme activities and the content of phenolic acids, flavonoids, and endogenous level of proline to overcome cold stress. In Citrus reticulata (Mandarin orange), Citrus sinensis (Sweet orange), and Citrus paradise (Grapefruit), foliar applications of proline increased the contents of phenolic acids, flavonoids, and endogenous proline and enhanced antioxidant enzyme activities caused by low temperatures [120]. In Capsicum annum (Red pepper), the foliar application of 24 mM of proline increased the endogenous proline level and antioxidant enzyme activity due to cold stress [6] (Table 14).

Table 14.
Physiological responses of various crops to proline treatment against cold stress.
4.6. Proline Effect against Heavy Metal Stress
The exogenous application of proline enhances oil contents, antioxidant enzyme activity, relative water content, organic osmolyte levels, and reduces the hydrogen peroxide levels to combat heavy metal stress. In Cicer arietinum (Chickpea), the foliar application of proline improved nitrogen fixation, the nitrogen content in leaves, and antioxidant enzyme activities against cadmium (Cd) metal stress [121]. In Olea europaea (Olive), the foliar application of 20 mM of proline enhanced the endogenous proline and oil contents and antioxidant enzyme activities while reducing hydrogen peroxide levels induced by cadmium (Cd) heavy metal exposure [122]. Treatment of Phaseolus vulgaris (Bean) with exogenous proline in the culture medium under selenium (Se) heavy metal improved the relative water content, chlorophyll endogenous proline levels, and antioxidant enzyme activities [123]. In Pisum sativum (Pea), foliar applications with proline enhanced the growth, photosynthetic activities, relative water content, and organic osmolyte levels when exposed to heavy metals [6]. In Poncirus trifoliate (Trifoliate orange), proline supplements in the nutrient solution enhanced protein and cellulose contents despite aluminum (Al) heavy metal exposure [124]. In Solanum melongena (Aubergine/Brinjal), seedling treatments with proline increased the endogenous proline content and antioxidant enzyme activities due to arsenate (As) heavy metal stress [125]. In Triticum aestivum (Wheat), foliar sprays with 80 mM of proline reduced the ROS and increased the plant height, weight, and photosynthetic capacity compared with untreated plants under the same heavy metal exposure [103]. Exogenous proline applied to Zea mays (Maize) improved the defense mechanism and sugar biosynthesis against cadmium (Cd) heavy metal stress [126] (Table 15).

Table 15.
Physiological responses of various crops to proline treatment against heavy metal stress.
5. Glycine Betaine
5.1. Structure and Function of Glycine Betaine
Glycine betaine (N, N, N-trimethyl glycine) is an osmoprotectant that also functions as an antioxidant; it is a dipolar molecule that is electrically neutral at its physiological pH. It plays numerous roles in plant growth and metabolism. By preserving the water balance and structural integrity of proteins, it shields cellular components from various stresses. It is now recognized that glycine betaine is synthesized in both the cytosol and chloroplast, and that the production of the chloroplastic form is positively correlated with stress tolerance, whereas that of the cytosolic form is not [127].
The external application of glycine betaine promotes plant growth and protects the plant from harmful substances; it improves stress tolerance by enhancing the relative water content, level of osmolytes, antioxidants and soluble sugars, thus increasing the number of pods, seeds, and leaves per plant, and promoting the overall plant growth, boosting the fresh and dry weights and ultimately the yield of the plant. Glycine betaine also has a protective effect on the oxygen-evolving complex, photosystem II, and plasma membrane by reducing the production of ROS and malondialdehyde. Exogenous glycine betaine protects RuBisCo from oxidative damage and raises carbon fixation and the net photosynthetic rate, protects membrane integrity, and protects subcellular structures through the detoxification of ROS [128].
5.2. Glycine Betaine Effect against Drought Stress
The exogenous application of glycine betaine improves crop productivity by increasing antioxidant enzymes activity, soluble sugars, and soluble proteins, improving the osmotic adjustment and photosynthetic rate to combat drought stress. In Triticum aestivum (Wheat), the external application of glycine betaine during drought conditions increased the spike length, number of spikelets per spike, number of grains, and leaf turgor potential therefore increasing the total yield; it could have accomplished these effects through an improvement of the stress tolerance index, enhancement in the level of osmolytes, and increase in the relative water content [129,130]. In Zea mays (Maize), the foliar application of 100 mM of glycine betaine enhanced the growth, yield, and antioxidant enzyme activities during drought conditions [131]. Similarly in the case of Solanum lycopersicum (Tomato), the exogenous application of glycine betaine also improved the yield [132]. Treatment with glycine betaine in Pisum sativum (Pea) enhanced the overall growth, numbers of pods and leaves per plant, and the levels of soluble sugars and soluble proteins in the leaves in addition to antioxidant enzyme activities, compared with untreated plants under drought stress conditions [133]. In Nicotiana tabacum (Tobacco), the external application of glycine betaine during drought improved plant growth, osmotic adjustment, photosynthesis, and antioxidant enzyme activities [128]. In Glycine max (Soybean), the foliar application of glycine betaine at a rate of 3 kg/ha increased the seed number when under drought stress compared with untreated plants [134] (Table 16).

Table 16.
Physiological responses of various crops to glycine betaine treatment against drought stress.
5.3. Glycine Betaine Effect against Salinity Stress
The exogenous application of proline reduces ROS production, lipid peroxidation which increases antioxidant enzymes, soluble sugars, and free amino acids to combat salinity stress. In Vigna unguiculata (Cowpea) under salt stress, treatment with glycine betaine increased the soluble sugars and antioxidant enzymes [135]. In Phaseolus vulgaris (Common bean), the external application of glycine betaine when grown under saline conditions increased plant fresh weight, leaf area ratio, relative water content, soluble sugars, and free amino acids [136]. In Oryza sativa (Rice), the foliar application of glycine betaine increased plant height, fresh and dry weights, chlorophyll content, and reduced malondialdehyde accumulation during salinity stress conditions [128]. Treatment of salt-stressed Solanum lycopersicum (Tomato) with glycine betaine increased photosynthesis and stomatal conductance and decreased photorespiration [128]. In Glycine max (Soybean), the external application of glycine betaine reduced ROS and lipid peroxidation due to high salinity and increased antioxidant enzyme activities [137]. In Lolium perenne (Perennial ryegrass), exogenous glycine betaine was capable of increasing fresh weight and relative water content while reducing electrolyte leakage and malondialdehyde accumulation due to salt stress [138]. The external application of glycine betaine to Triticum aestivum (Wheat) growing in high salinity increased the rate of photosynthesis [139] (Table 17).

Table 17.
Physiological responses of various crops to glycine betaine treatment against salinity stress.
5.4. Glycine Betaine Effect against Heat Stress
The exogenous application of glycine betaine increases the accumulation of heat shock proteins, photosynthetic rate, relative membrane permeability, and ROS scavenging activity to overcome heat stress. In Solanum lycopersicum (Tomato) under heat stress, treatment with glycine betaine increased the fruit yield and rate of photosynthesis. The external application of 1 and 5 mM of glycine betaine improved seed germination, increased the expressions of heat shock protein genes, and increased the accumulation of heat shock proteins to alleviate heat stress [128,140]. In Hordeum vulgare (Barley), a 10 mM of glycine betaine treatment increased the heat tolerance of photosystem II and had a protective effect on the oxygen-evolving complex. A 10–50 mM glycine betaine application improved the overall growth, photosynthesis, and water relations while decreasing the ion leakage caused by oxidative damage due to high temperatures [141,142]. In Triticum aestivum (Wheat), treatment with 100 mM of glycine betaine maintained a higher chlorophyll content, photosystem II photochemical activity, net photosynthetic rate, and resulted in the accumulation of endogenous glycine betaine. The application of 50 and 100 mM of glycine betaine also improved the yield and relative membrane permeability during heat stress [7,85]. Saccharum officinarum (Sugarcane) treated with 20 mM of exogenous glycine betaine under heat stress conditions had improved bud sprouting, soluble sugar accumulation, endogenous levels of osmolytes, and decreased hydrogen peroxide production [143]. In Tagetes erecta (Marigold), the external application of 0.5 and 1 mM of glycine betaine improved gaseous exchange and reduced ROS accumulation due to heat stress [144] (Table 18).

Table 18.
Physiological responses of various crops to glycine betaine treatment against heat stress.
5.5. Glycine Betaine Effect against Cold Stress
The exogenous application of glycine betaine increases photosynthesis, osmolality, prevents chlorosis, and reduces lipid peroxidation to combat cold stress. The foliar application of Zea mays (Maize) with the exogenous application of 100 mM of glycine betaine prevented chlorosis and reduced the lipid peroxidation of membranes under cold stress [145]. In Triticum aestivum (Wheat), a 100 mM glycine betaine foliar spray increased osmolality and photosynthesis at low temperatures [146]. In Prunus persica (Peach), the external application of 10 mM of glycine betaine lowered the malondialdehyde content and increased the endogenous glycine betaine level when the plants were under cold stress [147]. In the case of Medicago sativa (Alfalfa) experiencing cold stress, the application of exogenous glycine betaine decreased ion leakage from shoot tissues [148]. In Solanum lycopersicum (Tomato), a foliar spray of 0.1 mM of exogenous glycine betaine increased catalase activity, which reduced hydrogen peroxide accumulation as a result of low temperature stress [149]. The external application of glycine betaine also increased the osmolality and endogenous level of glycine betaine in Hordeum vulgare (Barley) under cold stress conditions [146] (Table 19).

Table 19.
Physiological responses of various crops to glycine betaine treatment against cold stress.
5.6. Glycine Betaine Effect against Heavy Metal Stress
The exogenous application of glycine betaine improves chlorophyll content, antioxidant enzymes activity, reduces lipid peroxidation, and increases gaseous exchange to alleviate heavy metal stress. Spraying 0–100mM of glycine betaine on leaves of Triticum aestivum (Wheat) improved the overall growth and chlorophyll, biomass, and protein contents against chromium (Cr) stress [150]. In Gossypium hirsutum (Cotton), the application of exogenous glycine betaine (1 mM) improved plant growth, antioxidant enzyme activities, and the rate of photosynthesis and gaseous exchange processes, thus alleviating cadmium (Cd) and lead (Pb) stress [151,152]. A foliar application with 0, 50, and 100 mM of glycine betaine also improved plant growth and alleviated Cr stress in Vigna radiata (Mung bean) [153]. In Amaranthus tricolor (Amaranth), the external application of glycine betaine improved photosynthesis and the chlorophyll content in leaves and alleviated Cd stress [154]. In Lolium perenne (Perennial ryegrass), the application of exogenous glycine betaine improved membrane stability, reduced lipid peroxidation, and alleviated Cd stress [155]. In the case of Nicotiana tabacum (Tobacco), treatment with exogenous glycine betaine reduced stomatal closure, malondialdehyde accumulation, and leaf damage, thus alleviating Cd stress [8]. The foliar application of glycine betaine to Cucumis sativus (Cucumber) had a significant protective effect on the chlorophyll content of the plant and alleviated aluminum (Al) stress [156]. In Sorghum bicolor (Millet), treatment with 50–100 mM of glycine betaine improved plant quality and yield and alleviated Cr stress [157]. In Oryza sativa (Rice), the application of exogenous glycine betaine increased the expressions of the glutathione S-transferase (GST) and glutaredoxin (GRX) genes and alleviated arsenic (As) stress [158] (Table 20).

Table 20.
Physiological responses of various crops to glycine betaine treatment against heavy metal stress.
Author Contributions
M.K. wrote the manuscript and created the figures; H.M.R. and H.-M.L. conceived the idea and proofread the manuscript; N.A., M.U., S.N., F.S. and S.A. collected and reviewed the literature about proline and glycine betaine; I.A.R., R.M.A. and Q.U.Z. collected and reviewed the literature about glutathione, and melatonin. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Hong Kong Research Grants Council’s Area of Excellence Scheme (AoE/M-403/16) and Lo Kwee-Seong Biomedical Research Fund to H.-M.L.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Jee Yan Chu for copy-editing the manuscript and Man-Wah Li for proofreading the manuscript. Any opinions, findings, conclusions, or recommendations expressed in this publication do not reflect the views of the Government of the Hong Kong Special Administrative Region or Innovation and Technology Commission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, N.A.; Munné-Bosch, S. Nanofertilizer Use for Sustainable Agriculture: Advantages and Limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Nahar, K.; Hossain, M.S.; Anee, T.I.; Parvin, K.; Fujita, M. Nitric Oxide Pretreatment Enhances Antioxidant Defense and Glyoxalase Systems to Confer PEG-Induced Oxidative Stress in Rapeseed. J. Plant Interact. 2017, 12, 323–331. [Google Scholar] [CrossRef]
- Imran, M.; Latif Khan, A.; Shahzad, R.; Aaqil Khan, M.; Bilal, S.; Khan, A.; Kang, S.-M.; Lee, I.-J. Exogenous Melatonin Induces Drought Stress Tolerance by Promoting Plant Growth and Antioxidant Defence System of Soybean Plants. AoB Plants 2021, 13, plab026. [Google Scholar] [CrossRef]
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.S.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-Induced Water Stress Tolerance in Plants: Recent Advances. Antioxidants 2020, 9, 809. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.S.; Wong, S.K.; Ismail, N.H.; Zengin, G.; Duangjai, A.; Saokaew, S.; Phisalprapa, P.; Tan, K.W.; Goh, B.H.; Tang, S.Y. Mitigation of Environmental Stress-Impacts in Plants: Role of Sole and Combinatory Exogenous Application of Glutathione. Front. Plant Sci. 2021, 12, 791205. [Google Scholar] [CrossRef] [PubMed]
- Hosseinifard, M.; Stefaniak, S.; Ghorbani Javid, M.; Soltani, E.; Wojtyla, Ł.; Garnczarska, M. Contribution of Exogenous Proline to Abiotic Stresses Tolerance in Plants: A Review. Int. J. Mol. Sci. 2022, 23, 5186. [Google Scholar] [CrossRef]
- Chowdhury, A.R.; Ghosh, M.; Lal, M.; Pal, A.; Hazra, K.; Acharya, S.; Chaurasiya, A.; Pathak, S. Foliar Spray of Synthetic Osmolytes Alleviates Terminal Heat Stress in Late-Sown Wheat. Int. J. Plant Prod. 2020, 14, 321–333. [Google Scholar] [CrossRef]
- He, X.; Richmond, M.E.; Williams, D.V.; Zheng, W.; Wu, F. Exogenous Glycinebetaine Reduces Cadmium Uptake and Mitigates Cadmium Toxicity in Two Tobacco Genotypes Differing in Cadmium Tolerance. Int. J. Mol. Sci. 2019, 20, 1612. [Google Scholar] [CrossRef]
- Huang, X.; Tanveer, M.; Min, Y.; Shabala, S. Melatonin as a Regulator of Plant Ionic Homeostasis: Implications for Abiotic Stress Tolerance. J. Exp. Bot. 2022, 73, 5886–5902. [Google Scholar] [CrossRef]
- Godoy, F.; Olivos-Hernández, K.; Stange, C.; Handford, M. Abiotic Stress in Crop Species: Improving Tolerance by Applying Plant Metabolites. Plants 2021, 10, 186. [Google Scholar] [CrossRef]
- Kong, M.; Sheng, T.; Liang, J.; Ali, Q.; Gu, Q.; Wu, H.; Chen, J.; Liu, J.; Gao, X. Melatonin and Its Homologs Induce Immune Responses via Receptors TrP47363-TrP13076 in Nicotiana Benthamiana. Front. Plant Sci. 2021, 12, 1197. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Ni, Z.; Xia, H.; Xie, Y.; Lv, X.; Wang, J.; Lin, L.; Deng, Q.; Luo, X. Exogenous Melatonin Promotes Biomass Accumulation and Photosynthesis of Kiwifruit Seedlings under Drought Stress. Sci. Hortic. 2019, 246, 34–43. [Google Scholar] [CrossRef]
- Sharma, A.; Wang, J.; Xu, D.; Tao, S.; Chong, S.; Yan, D.; Li, Z.; Yuan, H.; Zheng, B. Melatonin Regulates the Functional Components of Photosynthesis, Antioxidant System, Gene Expression, and Metabolic Pathways to Induce Drought Resistance in Grafted Carya Cathayensis Plants. Sci. Total Environ. 2020, 713, 136675. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.-X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative Physiological, Metabolomic, and Transcriptomic Analyses Reveal Mechanisms of Improved Abiotic Stress Resistance in Bermudagrass [Cynodon dactylon (L). Pers.] by Exogenous Melatonin. J. Exp. Bot. 2015, 66, 681–694. [Google Scholar] [CrossRef]
- Lee, H.-J.; Back, K. 2-Hydroxymelatonin Promotes the Resistance of Rice Plant to Multiple Simultaneous Abiotic Stresses (Combined Cold and Drought). J. Pineal Res. 2016, 61, 303–316. [Google Scholar] [CrossRef]
- Fleta-Soriano, E.; Díaz, L.; Bonet, E.; Munné-Bosch, S. Melatonin May Exert a Protective Role against Drought Stress in Maize. J. Agron. Crop Sci. 2017, 203, 286–294. [Google Scholar] [CrossRef]
- Ye, J.; Wang, S.; Deng, X.; Yin, L.; Xiong, B.; Wang, X. Melatonin Increased Maize (Zea mays L.) Seedling Drought Tolerance by Alleviating Drought-Induced Photosynthetic Inhibition and Oxidative Damage. Acta Physiol. Plant. 2016, 38, 48. [Google Scholar] [CrossRef]
- Cui, G.; Zhao, X.; Liu, S.; Sun, F.; Zhang, C.; Xi, Y. Beneficial Effects of Melatonin in Overcoming Drought Stress in Wheat Seedlings. Plant Physiol. Biochem. 2017, 118, 138–149. [Google Scholar] [CrossRef]
- Li, D.; Zhang, D.; Wang, H.; Li, H.; Song, S.; Li, H.; Li, R. Effects of Melatonin on Germination and Amino Acid Content in Different Wheat Varieties Seeds under Polyethylene Glycol Stress. bioRxiv 2019, 710954. [Google Scholar]
- Hossain, Md.S.; Li, J.; Sikdar, A.; Hasanuzzaman, M.; Uzizerimana, F.; Muhammad, I.; Yuan, Y.; Zhang, C.; Wang, C.; Feng, B. Exogenous Melatonin Modulates the Physiological and Biochemical Mechanisms of Drought Tolerance in Tartary Buckwheat (Fagopyrum tataricum (L.) Gaertn). Molecules 2020, 25, 2828. [Google Scholar] [CrossRef]
- Li, M.; Hasan, M.K.; Li, C.; Ahammed, G.J.; Xia, X.; Shi, K.; Zhou, Y.; Reiter, R.J.; Yu, J.; Xu, M. Melatonin Mediates Selenium-induced Tolerance to Cadmium Stress in Tomato Plants. J. Pineal Res. 2016, 61, 291–302. [Google Scholar] [CrossRef]
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S. Melatonin Enhances Plant Growth and Abiotic Stress Tolerance in Soybean Plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; He, S.; Zhan, Y.; Qin, B.; Jin, X.; Wang, M.; Zhang, Y.; Hu, G.; Teng, Z.; Wu, Y. Exogenous Melatonin Reduces the Inhibitory Effect of Osmotic Stress on Photosynthesis in Soybean. PLoS ONE 2019, 14, e0226542. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Tie, W.; Fu, L.; Yan, Y.; Liu, G.; Yan, W.; Li, Y.; Wu, C.; Zhang, J.; Hu, W. Strand-Specific RNA-Seq Based Identification and Functional Prediction of Drought-Responsive LncRNAs in Cassava. BMC Genomics 2019, 20, 214. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Xiao, S.; Zhang, Z.; Zhang, Y.; Sun, H.; Zhang, K.; Wang, X.; Bai, Z.; Li, C.; Liu, L. Melatonin Improves the Germination Rate of Cotton Seeds under Drought Stress by Opening Pores in the Seed Coat. PeerJ 2020, 8, e9450. [Google Scholar] [CrossRef]
- Antoniou, C.; Chatzimichail, G.; Xenofontos, R.; Pavlou, J.J.; Panagiotou, E.; Christou, A.; Fotopoulos, V. Melatonin Systemically Ameliorates Drought Stress-induced Damage in M Edicago Sativa Plants by Modulating Nitro-oxidative Homeostasis and Proline Metabolism. J. Pineal Res. 2017, 62, e12401. [Google Scholar] [CrossRef]
- Liang, B.; Ma, C.; Zhang, Z.; Wei, Z.; Gao, T.; Zhao, Q.; Ma, F.; Li, C. Long-Term Exogenous Application of Melatonin Improves Nutrient Uptake Fluxes in Apple Plants under Moderate Drought Stress. Environ. Exp. Bot. 2018, 155, 650–661. [Google Scholar] [CrossRef]
- Liu, J.; Wang, W.; Wang, L.; Sun, Y. Exogenous Melatonin Improves Seedling Health Index and Drought Tolerance in Tomato. Plant Growth Regul. 2015, 77, 317–326. [Google Scholar] [CrossRef]
- Karaca, P.; Cekic, F.Ö. Exogenous Melatonin-Stimulated Defense Responses in Tomato Plants Treated with Polyethylene Glycol. Int. J. Veg. Sci. 2019, 25, 601–609. [Google Scholar] [CrossRef]
- Kaya, A.; Doganlar, Z.B. Melatonin Improves the Multiple Stress Tolerance in Pepper (Capsicum annuum). Sci. Hortic. 2019, 256, 108509. [Google Scholar] [CrossRef]
- Lee, H.-J.; Back, K. 2-Hydroxymelatonin Confers Tolerance against Combined Cold and Drought Stress in Tobacco, Tomato, and Cucumber as a Potent Anti-Stress Compound in the Evolution of Land Plants. Melatonin Res. 2019, 2, 35–46. [Google Scholar] [CrossRef]
- Khan, M.N.; Zhang, J.; Luo, T.; Liu, J.; Rizwan, M.; Fahad, S.; Xu, Z.; Hu, L. Seed Priming with Melatonin Coping Drought Stress in Rapeseed by Regulating Reactive Oxygen Species Detoxification: Antioxidant Defense System, Osmotic Adjustment, Stomatal Traits and Chloroplast Ultrastructure Perseveration. Ind. Crops Prod. 2019, 140, 111597. [Google Scholar] [CrossRef]
- Wu, Y.; Lian, H.; Mu, X.; Wang, X.; Zhang, Y. Effects of Foliar Spraying Exogenous Melatonin on Physiological and Biochemical Characteristics of Dendranthema Morifolium’Chuju’seedlings under Drought Stress. Acta Bot. Boreali-Occident. Sin. 2016, 36, 2241–2246. [Google Scholar]
- Kabiri, R.; Hatami, A.; Oloumi, H.; Naghizadeh, M.; Nasibi, F.; Tahmasebi, Z. Foliar Application of Melatonin Induces Tolerance to Drought Stress in Moldavian Balm Plants (Dracocephalum moldavica) through Regulating the Antioxidant System. Folia Hortic. 2018, 30, 155. [Google Scholar] [CrossRef]
- Ma, X.; Zhang, J.; Burgess, P.; Rossi, S.; Huang, B. Interactive Effects of Melatonin and Cytokinin on Alleviating Drought-Induced Leaf Senescence in Creeping Bentgrass (Agrostis stolonifera). Environ. Exp. Bot. 2018, 145, 1–11. [Google Scholar] [CrossRef]
- Alam, M.N.; Wang, Y.; Chan, Z. Physiological and Biochemical Analyses Reveal Drought Tolerance in Cool-Season Tall Fescue (Festuca arundinacea) Turf Grass with the Application of Melatonin. Crop Pasture Sci. 2018, 69, 1041–1049. [Google Scholar] [CrossRef]
- Zamani, Z.; Amiri, H.; Ismaili, A. Improving Drought Stress Tolerance in Fenugreek (Trigonella Foenum-Graecum) by Exogenous Melatonin. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2020, 154, 643–655. [Google Scholar] [CrossRef]
- Campos, C.N.; Ávila, R.G.; de Souza, K.R.D.; Azevedo, L.M.; Alves, J.D. Melatonin Reduces Oxidative Stress and Promotes Drought Tolerance in Young Coffea arabica L. Plants. Agric. Water Manag. 2019, 211, 37–47. [Google Scholar] [CrossRef]
- Li, J.; Yang, Y.; Sun, K.; Chen, Y.; Chen, X.; Li, X. Exogenous Melatonin Enhances Cold, Salt and Drought Stress Tolerance by Improving Antioxidant Defense in Tea Plant (Camellia sinensis (L.) O. Kuntze). Molecules 2019, 24, 1826. [Google Scholar] [CrossRef]
- Mosaad, I.S.; Serag, A.H.; Moustafa-Farag, M.; Seadh, A.K. Effect of Exogenous Proline Application on Maize Yield and the Optimum Rate of Mineral Nitrogen under Salinity Stress. J. Plant Nutr. 2020, 43, 354–370. [Google Scholar] [CrossRef]
- Elkelish, A.A.; Soliman, M.H.; Alhaithloul, H.A.; El-Esawi, M.A. Selenium Protects Wheat Seedlings against Salt Stress-Mediated Oxidative Damage by up-Regulating Antioxidants and Osmolytes Metabolism. Plant Physiol. Biochem. 2019, 137, 144–153. [Google Scholar] [CrossRef]
- Sheikhalipour, M.; Mohammadi, S.A.; Esmaielpour, B.; Zareei, E.; Kulak, M.; Ali, S.; Nouraein, M.; Bahrami, M.K.; Gohari, G.; Fotopoulos, V. Exogenous Melatonin Increases Salt Tolerance in Bitter Melon by Regulating Ionic Balance, Antioxidant System and Secondary Metabolism-Related Genes. BMC Plant Biol. 2022, 22, 380. [Google Scholar] [CrossRef]
- Rajora, N.; Vats, S.; Raturi, G.; Thakral, V.; Kaur, S.; Rachappanavar, V.; Kumar, M.; Kesarwani, A.K.; Sonah, H.; Sharma, T.R. Seed Priming with Melatonin: A Promising Approach to Combat Abiotic Stress in Plants. Plant Stress 2022, 4, 100071. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, L.; Wang, Z.; Feng, G.; Gao, Q.; Li, X. Induction of Low Temperature Tolerance in Wheat by Pre-Soaking and Parental Treatment with Melatonin. Molecules 2021, 26, 1192. [Google Scholar] [CrossRef] [PubMed]
- Talaat, N.B. Polyamine and Nitrogen Metabolism Regulation by Melatonin and Salicylic Acid Combined Treatment as a Repressor for Salt Toxicity in Wheat (Triticum aestivum L.) Plants. Plant Growth Regul. 2021, 95, 315–329. [Google Scholar] [CrossRef]
- Bahcesular, B.; Yildirim, E.D.; Karaçocuk, M.; Kulak, M.; Karaman, S. Seed Priming with Melatonin Effects on Growth, Essential Oil Compounds and Antioxidant Activity of Basil (Ocimum basilicum L.) under Salinity Stress. Ind. Crops Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Dawood, M.G.; El-Awadi, M.E. Alleviation of Salinity Stress on Vicia faba L. Plants via Seed Priming with Melatonin. Acta Biológica Colomb. 2015, 20, 223–235. [Google Scholar] [CrossRef]
- Shukla, M.R.; Bajwa, V.S.; Freixas-Coutin, J.A.; Saxena, P.K. Salt Stress in Arabidopsis Thaliana Seedlings: Role of Indoleamines in Stress Alleviation. Melatonin Res. 2021, 4, 70–83. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, Y.; Yu, X.; Kiprotich, F.; Han, H.; Guan, R.; Wang, R.; Shen, W. Nitric Oxide Is Required for Melatonin-Enhanced Tolerance against Salinity Stress in Rapeseed (Brassica napus L.) Seedlings. Int. J. Mol. Sci. 2018, 19, 1912. [Google Scholar] [CrossRef]
- Park, H.-S.; Kazerooni, E.A.; Kang, S.-M.; Al-Sadi, A.M.; Lee, I.-J. Melatonin Enhances the Tolerance and Recovery Mechanisms in Brassica juncea (L.) Czern. under Saline Conditions. Front. Plant Sci. 2021, 12, 593717. [Google Scholar] [CrossRef]
- Castañares, J.L.; Bouzo, C.A. Effect of Exogenous Melatonin on Seed Germination and Seedling Growth in Melon (Cucumis melo L.) under Salt Stress. Hortic. Plant J. 2019, 5, 79–87. [Google Scholar] [CrossRef]
- Liang, C.; Zheng, G.; Li, W.; Wang, Y.; Hu, B.; Wang, H.; Wu, H.; Qian, Y.; Zhu, X.; Tan, D. Melatonin Delays Leaf Senescence and Enhances Salt Stress Tolerance in Rice. J. Pineal Res. 2015, 59, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Moustafa-Farag, M.; Elkelish, A.; Dafea, M.; Khan, M.; Arnao, M.B.; Abdelhamid, M.T.; El-Ezz, A.A.; Almoneafy, A.; Mahmoud, A.; Awad, M. Role of Melatonin in Plant Tolerance to Soil Stressors: Salinity, PH and Heavy Metals. Molecules 2020, 25, 5359. [Google Scholar] [CrossRef]
- Martinez, V.; Nieves-Cordones, M.; Lopez-Delacalle, M.; Rodenas, R.; Mestre, T.C.; Garcia-Sanchez, F.; Rubio, F.; Nortes, P.A.; Mittler, R.; Rivero, R.M. Tolerance to Stress Combination in Tomato Plants: New Insights in the Protective Role of Melatonin. Molecules 2018, 23, 535. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous Melatonin Confers Salt Stress Tolerance to Watermelon by Improving Photosynthesis and Redox Homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Iqbal, N.; Fatma, M.; Gautam, H.; Umar, S.; Sofo, A.; D’ippolito, I.; Khan, N.A. The Crosstalk of Melatonin and Hydrogen Sulfide Determines Photosynthetic Performance by Regulation of Carbohydrate Metabolism in Wheat under Heat Stress. Plants 2021, 10, 1778. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, Y.; Zhang, X.; Du, H.; Xu, B.; Huang, B. Melatonin Suppression of Heat-Induced Leaf Senescence Involves Changes in Abscisic Acid and Cytokinin Biosynthesis and Signaling Pathways in Perennial Ryegrass (Lolium perenne L.). Environ. Exp. Bot. 2017, 138, 36–45. [Google Scholar] [CrossRef]
- Jahan, M.S.; Shu, S.; Wang, Y.; Chen, Z.; He, M.; Tao, M.; Sun, J.; Guo, S. Melatonin Alleviates Heat-Induced Damage of Tomato Seedlings by Balancing Redox Homeostasis and Modulating Polyamine and Nitric Oxide Biosynthesis. BMC Plant Biol. 2019, 19, 414. [Google Scholar] [CrossRef]
- ur Rehman, H.; Alharby, H.F.; Bamagoos, A.A.; Abdelhamid, M.T.; Rady, M.M. Sequenced Application of Glutathione as an Antioxidant with an Organic Biostimulant Improves Physiological and Metabolic Adaptation to Salinity in Wheat. Plant Physiol. Biochem. 2021, 158, 43–52. [Google Scholar] [CrossRef]
- Yao, M.; Ge, W.; Zhou, Q.; Zhou, X.; Luo, M.; Zhao, Y.; Wei, B.; Ji, S. Exogenous Glutathione Alleviates Chilling Injury in Postharvest Bell Pepper by Modulating the Ascorbate-Glutathione (AsA-GSH) Cycle. Food Chem. 2021, 352, 129458. [Google Scholar] [CrossRef]
- Khan, A.; Numan, M.; Khan, A.L.; Lee, I.-J.; Imran, M.; Asaf, S.; Al-Harrasi, A. Melatonin: Awakening the Defense Mechanisms during Plant Oxidative Stress. Plants 2020, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Guo, Y.; Lan, Z.; Xu, K.; Chang, J.; Ahammed, G.J.; Ma, J.; Wei, C.; Zhang, X. Methyl Jasmonate Mediates Melatonin-Induced Cold Tolerance of Grafted Watermelon Plants. Hortic. Res. 2021, 8, 57. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Wang, M.; Liu, B.; Zhang, S. Exogenous Melatonin Mitigates Photoinhibition by Accelerating Non-Photochemical Quenching in Tomato Seedlings Exposed to Moderate Light during Chilling. Front. Plant Sci. 2017, 8, 244. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Hu, Z.; Xie, Y.; Chan, Z.; Chen, K.; Amombo, E.; Chen, L.; Fu, J. Alleviation of Cold Damage to Photosystem II and Metabolisms by Melatonin in Bermudagrass. Front. Plant Sci. 2015, 6, 925. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wei, J.-P.; Scott, E.R.; Liu, J.-W.; Guo, S.; Li, Y.; Zhang, L.; Han, W.-Y. Exogenous Melatonin Alleviates Cold Stress by Promoting Antioxidant Defense and Redox Homeostasis in Camellia sinensis L. Molecules 2018, 23, 165. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Fu, X.; Han, L.; Xu, C.; Liu, C.; Bi, H.; Ai, X. Nitric Oxide Functions as a Downstream Signal for Melatonin-Induced Cold Tolerance in Cucumber Seedlings. Front. Plant Sci. 2021, 12, 1432. [Google Scholar] [CrossRef]
- Tiwari, G.; Duraivadivel, P.; Sharma, S. 1-Aminocyclopropane-1-Carboxylic Acid Deaminase Producing Beneficial Rhizobacteria Ameliorate the Biomass Characters of Panicum Maximum Jacq. by Mitigating Drought and Salt Stress. Sci. Rep. 2018, 8, 17513. [Google Scholar] [CrossRef]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous Melatonin Confers Cadmium Tolerance by Counterbalancing the Hydrogen Peroxide Homeostasis in Wheat Seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef]
- Gu, Q.; Chen, Z.; Yu, X.; Cui, W.; Pan, J.; Zhao, G.; Xu, S.; Wang, R.; Shen, W. Melatonin Confers Plant Tolerance against Cadmium Stress via the Decrease of Cadmium Accumulation and Reestablishment of MicroRNA-Mediated Redox Homeostasis. Plant Sci. 2017, 261, 28–37. [Google Scholar] [CrossRef]
- Lin, L.; Li, J.; Chen, F.; Liao, M.; Tang, Y.; Liang, D.; Xia, H.; Lai, Y.; Wang, X.; Chen, C. Effects of Melatonin on the Growth and Cadmium Characteristics of Cyphomandra Betacea Seedlings. Environ. Monit. Assess. 2018, 190, 119. [Google Scholar] [CrossRef]
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin Pretreatment Improves Vanadium Stress Tolerance of Watermelon Seedlings by Reducing Vanadium Concentration in the Leaves and Regulating Melatonin Biosynthesis and Antioxidant-Related Gene Expression. J. Plant Physiol. 2018, 220, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Kołodziejczyk, I.; Dzitko, K.; Szewczyk, R.; Posmyk, M.M. Exogenous Melatonin Expediently Modifies Proteome of Maize (Zea mays L.) Embryo during Seed Germination. Acta Physiol. Plant. 2016, 38, 146. [Google Scholar] [CrossRef]
- David, O.; Jolayemi, O.; Akomolafe, G.; Adegoke, M. Lead Sequestration, Immobilization and Xylem Cavitation in Melatonin-Primed Amaranthus Cruentus. Plant Physiol. Rep. 2021, 26, 162–171. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Sofy, M.R. Role of Ascorbic Acid, Glutathione and Proline Applied as Singly or in Sequence Combination in Improving Chickpea Plant through Physiological Change and Antioxidant Defense under Different Levels of Irrigation Intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Fujita, M. Selenium Pretreatment Upregulates the Antioxidant Defense and Methylglyoxal Detoxification System and Confers Enhanced Tolerance to Drought Stress in Rapeseed Seedlings. Biol. Trace Elem. Res. 2011, 143, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Jaiswal, J.P.; Misra, S.; Tripathi, B.N.; Prasad, M. A Comprehensive Study on Dehydration-Induced Antioxidative Responses during Germination of Indian Bread Wheat (Triticum aestivum L. Em Thell) Cultivars Collected from Different Agroclimatic Zones. Physiol. Mol. Biol. Plants 2012, 18, 217–228. [Google Scholar] [CrossRef]
- Nahar, K.; Hasanuzzaman, M.; Alam, M.; Fujita, M. Glutathione-Induced Drought Stress Tolerance in Mung Bean: Coordinated Roles of the Antioxidant Defence and Methylglyoxal Detoxification Systems. AoB Plants 2015, 7, plv069. [Google Scholar] [CrossRef]
- Chen, J.-H.; Jiang, H.-W.; Hsieh, E.-J.; Chen, H.-Y.; Chien, C.-T.; Hsieh, H.-L.; Lin, T.-P. Drought and Salt Stress Tolerance of an Arabidopsis Glutathione S-Transferase U17 Knockout Mutant Are Attributed to the Combined Effect of Glutathione and Abscisic Acid. Plant Physiol. 2012, 158, 340–351. [Google Scholar] [CrossRef]
- Al-Elwany, O.A.; Mohamed, G.F.; Abdurrahman, H.A.; LATEF, A.A.A. Exogenous Glutathione-Mediated Tolerance to Deficit Irrigation in Salt-Affected Capsicum frutescence (L.) Plants Is Connected with Higher Antioxidant Content and Ionic Homeostasis. Not. Bot. Hortic. Agrobot. Cluj Napoca 2020, 48, 1957–1979. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Semida, W.M.; Rady, M.M.; Mohamed, G.F.; Hemida, K.A.; Alhammad, B.A.; Hassan, M.M.; Shami, A. Sequential Application of Antioxidants Rectifies Ion Imbalance and Strengthens Antioxidant Systems in Salt-Stressed Cucumber. Plants 2020, 9, 1783. [Google Scholar] [CrossRef]
- Semida, W.M.; El-Mageed, A.; Taia, A.; Abdalla, R.M.; Hemida, K.A.; Howladar, S.; Leilah, A.A.; Rady, M.O. Sequential Antioxidants Foliar Application Can Alleviate Negative Consequences of Salinity Stress in Vicia faba L. Plants 2021, 10, 914. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wen, Z.; Zhang, J.; Chen, X.; Cui, J.; Xu, W.; Liu, H. Exogenous Glutathione Alleviates Salt-Induced Oxidative Stress in Tomato Seedlings by Regulating Glutathione Metabolism, Redox Status, and the Antioxidant System. Sci. Hortic. 2017, 220, 90–101. [Google Scholar] [CrossRef]
- El-Shabrawi, H.; Kumar, B.; Kaul, T.; Reddy, M.K.; Singla-Pareek, S.L.; Sopory, S.K. Redox Homeostasis, Antioxidant Defense, and Methylglyoxal Detoxification as Markers for Salt Tolerance in Pokkali Rice. Protoplasma 2010, 245, 85–96. [Google Scholar] [CrossRef]
- Ding, X.; Jiang, Y.; He, L.; Zhou, Q.; Yu, J.; Hui, D.; Huang, D. Exogenous Glutathione Improves High Root-Zone Temperature Tolerance by Modulating Photosynthesis, Antioxidant and Osmolytes Systems in Cucumber Seedlings. Sci. Rep. 2016, 6, 35424. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cai, J.; Liu, F.; Dai, T.; Cao, W.; Wollenweber, B.; Jiang, D. Multiple Heat Priming Enhances Thermo-Tolerance to a Later High Temperature Stress via Improving Subcellular Antioxidant Activities in Wheat Seedlings. Plant Physiol. Biochem. 2014, 74, 185–192. [Google Scholar] [CrossRef]
- Zou, M.; Yuan, L.; Zhu, S.; Liu, S.; Ge, J.; Wang, C. Response of Osmotic Adjustment and Ascorbate-Glutathione Cycle to Heat Stress in a Heat-Sensitive and a Heat-Tolerant Genotype of Wucai (Brassica campestris L.). Sci. Hortic. 2016, 211, 87–94. [Google Scholar] [CrossRef]
- Almeselmani, M.; Deshmukh, P.; Sairam, R. High Temperature Stress Tolerance in Wheat Genotypes: Role of Antioxidant Defence Enzymes. Acta Agron. Hung. 2009, 57, 1–14. [Google Scholar] [CrossRef]
- Park, S.-I.; Kim, J.-J.; Kim, H.-S.; Kim, Y.-S.; Yoon, H.-S. Enhanced Glutathione Content Improves Lateral Root Development and Grain Yield in Rice Plants. Plant Mol. Biol. 2021, 105, 365–383. [Google Scholar] [CrossRef]
- Lukatkin, A.S.; Anjum, N.A. Control of Cucumber (Cucumis sativus L.) Tolerance to Chilling Stress—Evaluating the Role of Ascorbic Acid and Glutathione. Front. Environ. Sci. 2014, 2, 62. [Google Scholar] [CrossRef]
- Li, Z.-G.; Yuan, L.-X.; Wang, Q.-L.; Ding, Z.-L.; Dong, C.-Y. Combined Action of Antioxidant Defense System and Osmolytes in Chilling Shock-Induced Chilling Tolerance in Jatropha Curcas Seedlings. Acta Physiol. Plant. 2013, 35, 2127–2136. [Google Scholar] [CrossRef]
- Alamri, S.; Kushwaha, B.K.; Singh, V.P.; Siddiqui, M.H.; Al-Amri, A.A.; Alsubaie, Q.D.; Ali, H.M. Ascorbate and Glutathione Independently Alleviate Arsenate Toxicity in Brinjal but Both Require Endogenous Nitric Oxide. Physiol. Plant. 2021, 173, 276–286. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Lin, K.; Chen, H.; Qi, Z.; Liu, B.; Cao, F.; Chen, H.; Wu, F. Metabolome Analysis Revealed the Mechanism of Exogenous Glutathione to Alleviate Cadmium Stress in Maize (Zea mays L.) Seedlings. Plants 2021, 10, 105. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Rahman, A.; Mahmud, J.A.; Alharby, H.F.; Fujita, M. Exogenous Glutathione Attenuates Lead-Induced Oxidative Stress in Wheat by Improving Antioxidant Defense and Physiological Mechanisms. J. Plant Interact. 2018, 13, 203–212. [Google Scholar] [CrossRef]
- Wang, F.; Chen, F.; Cai, Y.; Zhang, G.; Wu, F. Modulation of Exogenous Glutathione in Ultrastructure and Photosynthetic Performance against Cd Stress in the Two Barley Genotypes Differing in Cd Tolerance. Biol. Trace Elem. Res. 2011, 144, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Liu, C.; Wang, F.; Ahammed, G.J.; Zhou, J.; Xu, M.-X.; Yu, J.-Q.; Xia, X.-J. Glutathione-Mediated Regulation of Nitric Oxide, S-Nitrosothiol and Redox Homeostasis Confers Cadmium Tolerance by Inducing Transcription Factors and Stress Response Genes in Tomato. Chemosphere 2016, 161, 536–545. [Google Scholar] [CrossRef]
- Cao, F.; Cai, Y.; Liu, L.; Zhang, M.; He, X.; Zhang, G.; Wu, F. Differences in Photosynthesis, Yield and Grain Cadmium Accumulation as Affected by Exogenous Cadmium and Glutathione in the Two Rice Genotypes. Plant Growth Regul. 2015, 75, 715–723. [Google Scholar] [CrossRef]
- Anjum, N.A.; Umar, S.; Ahmad, A.; Iqbal, M.; Khan, N.A. Sulphur Protects Mustard (Brassica campestris L.) from Cadmium Toxicity by Improving Leaf Ascorbate and Glutathione. Plant Growth Regul. 2008, 54, 271–279. [Google Scholar] [CrossRef]
- Chen, F.; Wang, F.; Wu, F.; Mao, W.; Zhang, G.; Zhou, M. Modulation of Exogenous Glutathione in Antioxidant Defense System against Cd Stress in the Two Barley Genotypes Differing in Cd Tolerance. Plant Physiol. Biochem. 2010, 48, 663–672. [Google Scholar] [CrossRef]
- Cai, Y.; Cao, F.; Cheng, W.; Zhang, G.; Wu, F. Modulation of Exogenous Glutathione in Phytochelatins and Photosynthetic Performance against Cd Stress in the Two Rice Genotypes Differing in Cd Tolerance. Biol. Trace Elem. Res. 2011, 143, 1159–1173. [Google Scholar] [CrossRef]
- Ilyas, N.; Amjid, M.W.; Saleem, M.A.; Khan, W.; Wattoo, F.M.; Rana, R.M.; Maqsood, R.H.; Zahid, A.; Shah, G.A.; Anwar, A. Quantitative Trait Loci (QTL) Mapping for Physiological and Biochemical Attributes in a Pasban90/Frontana Recombinant Inbred Lines (RILs) Population of Wheat (Triticum Aestivum) under Salt Stress Condition. Saudi J. Biol. Sci. 2020, 27, 341–351. [Google Scholar] [CrossRef]
- Farooq, M.; Nawaz, A.; Chaudhry, M.; Indrasti, R.; Rehman, A. Improving Resistance against Terminal Drought in Bread Wheat by Exogenous Application of Proline and Gamma-aminobutyric Acid. J. Agron. Crop Sci. 2017, 203, 464–472. [Google Scholar] [CrossRef]
- Elewa, T.A.; Sadak, M.S.; Saad, A.M. Proline Treatment Improves Physiological Responses in Quinoa Plants under Drought Stress. Biosci. Res. 2017, 14, 21–33. [Google Scholar]
- Zouari, M.; Hassena, A.B.; Trabelsi, L.; Rouina, B.B.; Decou, R.; Labrousse, P. Exogenous Proline-Mediated Abiotic Stress Tolerance in Plants: Possible Mechanisms. In Osmoprotectant-Mediated Abiotic Stress Tolerance in Plants; Springer: Berlin/Heidelberg, Germany, 2019; pp. 99–121. [Google Scholar]
- Kaya, C.; Tuna, A.L.; Ashraf, M.; Altunlu, H. Improved Salt Tolerance of Melon (Cucumis melo L.) by the Addition of Proline and Potassium Nitrate. Environ. Exp. Bot. 2007, 60, 397–403. [Google Scholar] [CrossRef]
- Huang, Y.; Bie, Z.; Liu, Z.; Zhen, A.; Wang, W. Protective Role of Proline against Salt Stress Is Partially Related to the Improvement of Water Status and Peroxidase Enzyme Activity in Cucumber. Soil Sci. Plant Nutr. 2009, 55, 698–704. [Google Scholar] [CrossRef]
- Sabagh, A.E.; Sorour, S.; Ragab, A.; Saneoka, H.; Islam, M. The Effect of Exogenous Application of Proline and Glycine Betaineon the Nodule Activity of Soybean under Saline Condition. J. Agric. Biotechnol. 2017, 2, 01–05. [Google Scholar]
- Iqbal, A.; Iftikhar, I.; Nawaz, H.; Nawaz, M. Role of Proline to Induce Salinity Tolerance in Sunflower (Helianthus annusl). Sci. Technol. Dev. 2014, 33, 88–93. [Google Scholar]
- de Freitas, P.A.F.; de Souza Miranda, R.; Marques, E.C.; Prisco, J.T.; Gomes-Filho, E. Salt Tolerance Induced by Exogenous Proline in Maize Is Related to Low Oxidative Damage and Favorable Ionic Homeostasis. J. Plant Growth Regul. 2018, 37, 911–924. [Google Scholar]
- Rady, M.M.; Kuşvuran, A.; Alharby, H.F.; Alzahrani, Y.; Kuşvuran, S. Pretreatment with Proline or an Organic Bio-Stimulant Induces Salt Tolerance in Wheat Plants by Improving Antioxidant Redox State and Enzymatic Activities and Reducing the Oxidative Stress. J. Plant Growth Regul. 2019, 38, 449–462. [Google Scholar]
- de Freitas, P.A.F.; de Carvalho, H.H.; Costa, J.H.; Miranda, R.d.S.; Saraiva, K.D.d.C.; de Oliveira, F.D.B.; Coelho, D.G.; Prisco, J.T.; Gomes-Filho, E. Salt Acclimation in Sorghum Plants by Exogenous Proline: Physiological and Biochemical Changes and Regulation of Proline Metabolism. Plant Cell Rep. 2019, 38, 403–416. [Google Scholar] [CrossRef]
- El Moukhtari, A.; Cabassa-Hourton, C.; Farissi, M.; Savouré, A. How Does Proline Treatment Promote Salt Stress Tolerance during Crop Plant Development? Front. Plant Sci. 2020, 11, 1127. [Google Scholar] [CrossRef]
- Wani, A.; Ahmad, A.; Hayat, S.; Tahir, I. Is Foliar Spray of Proline Sufficient for Mitigation of Salt Stress in Brassica Juncea Cultivars? Environ. Sci. Pollut. Res. 2016, 23, 13413–13423. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Kharal, M.A.; Ahmad, M.; Abbasi, G.H.; Nazli, F.; Hussain, A.; Akhtar, M.F.-Z. Inducing Salinity Tolerance in Red Pepper (Capsicum annuum L.) through Exogenous Application of Proline and L-Tryptophan. Soil Environ. 2018, 37, 160–168. [Google Scholar]
- Qirat, M.; Shahbaz, M.; Perveen, S. Beneficial Role of Foliar-Applied Proline on Carrot (Daucus carota L.) under Saline Conditions. Pak J Bot 2018, 50, 1735–1744. [Google Scholar]
- Abdelhamid, M.T.; Rady, M.M.; Osman, A.S.; Abdalla, M.A. Exogenous Application of Proline Alleviates Salt-Induced Oxidative Stress in Phaseolus vulgaris L. Plants. J. Hortic. Sci. Biotechnol. 2013, 88, 439–446. [Google Scholar] [CrossRef]
- Shaddad, M. The Effect of Proline Application on the Physiology Ofraphanus Sativus Plants Grown under Salinity Stress. Biol. Plant. 1990, 32, 104–112. [Google Scholar] [CrossRef]
- Orsini, F.; Pennisi, G.; Mancarella, S.; Al Nayef, M.; Sanoubar, R.; Nicola, S.; Gianquinto, G. Hydroponic Lettuce Yields Are Improved under Salt Stress by Utilizing White Plastic Film and Exogenous Applications of Proline. Sci. Hortic. 2018, 233, 283–293. [Google Scholar] [CrossRef]
- Hussain, R.; Ayyub, C.M.; Shaheen, M.R.; Rashid, S.; Nafees, M.; Ali, S.; Butt, M.; Ali, M.; Maqsood, A.; Fiaz, S. Regulation of Osmotic Balance and Increased Antioxidant Activities under Heat Stress in Abelmoschus esculentus L. Triggered by Exogenous Proline Application. Agronomy 2021, 11, 685. [Google Scholar] [CrossRef]
- Priya, M.; Sharma, L.; Singh, I.; Bains, T.; Siddique, K.H.; Bindumadhava, H.; Nair, R.M.; Nayyar, H. Securing Reproductive Function in Mungbean Grown under High Temperature Environment with Exogenous Application of Proline. Plant Physiol. Biochem. 2019, 140, 136–150. [Google Scholar] [CrossRef]
- Mohammadrezakhani, S.; Hajilou, J.; Rezanejad, F.; Zaare-Nahandi, F. Assessment of Exogenous Application of Proline on Antioxidant Compounds in Three Citrus Species under Low Temperature Stress. J. Plant Interact. 2019, 14, 347–358. [Google Scholar] [CrossRef]
- Alyemeni, M.N.; Hayat, Q.; Hayat, S.; Faizan, M.; Faraz, A. Exogenous Proline Application Enhances the Efficiency of Nitrogen Fixation and Assimilation in Chickpea Plants Exposed to Cadmium. Legume Res. Int. J. 2016, 39, 221–227. [Google Scholar] [CrossRef]
- Zouari, M.; Ahmed, C.B.; Elloumi, N.; Bellassoued, K.; Delmail, D.; Labrousse, P.; Abdallah, F.B.; Rouina, B.B. Impact of Proline Application on Cadmium Accumulation, Mineral Nutrition and Enzymatic Antioxidant Defense System of Olea europaea L. Cv Chemlali Exposed to Cadmium Stress. Ecotoxicol. Environ. Saf. 2016, 128, 195–205. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, M.; Sharma, S.; Kaur, N.; Pathania, D.; Bhandhari, K.; Kaushal, N.; Kaur, R.; Singh, K.; Srivastava, A.; Nayyar, H. Exogenous Proline Application Reduces Phytotoxic Effects of Selenium by Minimising Oxidative Stress and Improves Growth in Bean (Phaseolus vulgaris L.) Seedlings. Biol. Trace Elem. Res. 2011, 140, 354–367. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Riaz, M.; Jiang, C. Exogenous Application of Proline Alleviates B-Deficiency-Induced Injury While Aggravates Aluminum Toxicity in Trifoliate Orange Seedlings. Sci. Hortic. 2020, 268, 109372. [Google Scholar] [CrossRef]
- Singh, M.; Singh, V.P.; Dubey, G.; Prasad, S.M. Exogenous Proline Application Ameliorates Toxic Effects of Arsenate in Solanum Melongena L. Seedlings. Ecotoxicol. Environ. Saf. 2015, 117, 164–173. [Google Scholar] [CrossRef]
- Karalija, E.; Selović, A. The Effect of Hydro and Proline Seed Priming on Growth, Proline and Sugar Content, and Antioxidant Activity of Maize under Cadmium Stress. Environ. Sci. Pollut. Res. 2018, 25, 33370–33380. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M.; Siddique, K.H. Role of Glycine Betaine in the Thermotolerance of Plants. Agronomy 2022, 12, 276. [Google Scholar] [CrossRef]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and Temporal Profile of Glycine Betaine Accumulation in Plants under Abiotic Stresses. Front. Plant Sci. 2019, 10, 230. [Google Scholar] [CrossRef]
- Gupta, N.; Thind, S.K.; Bains, N.S. Glycine Betaine Application Modifies Biochemical Attributes of Osmotic Adjustment in Drought Stressed Wheat. Plant Growth Regul. 2014, 72, 221–228. [Google Scholar] [CrossRef]
- Raza, M.; Saleem, M.; Shah, G.; Khan, I.; Raza, A. Exogenous Application of Glycinebetaine and Potassium for Improving Water Relations and Grain Yield of Wheat under Drought. J. Soil Sci. Plant Nutr. 2014, 14, 348–364. [Google Scholar] [CrossRef]
- Anjum, S.; Farooq, M.; Wang, L.C.; Xue, L.; Wang, S.; Wang, L.; Zhang, S.; Chen, M. Gas Exchange and Chlorophyll Synthesis of Maize Cultivars Are Enhanced by Exogenously-Applied Glycinebetaine under Drought Conditions. Plant Soil Environ. 2011, 57, 326–331. [Google Scholar] [CrossRef]
- Jokinen, K.; Somersalo, S.; Mäkelä, P.; Urbano, P.; Rojo, C.; González, J.; Soler, J.; Usano, M.; Moure, J.; Moya, M. Glycinebetaine from Sugar Beet Enhances the Yield of ‘Field-Grown’Tomatoes. In VI International Symposium on Processing Tomato & Workshop on Irrigation & Fertigation of Processing Tomato; ISHS: Leuven, Belgium, 1998; pp. 233–236. [Google Scholar]
- Osman, H.S. Enhancing Antioxidant–Yield Relationship of Pea Plant under Drought at Different Growth Stages by Exogenously Applied Glycine Betaine and Proline. Ann. Agric. Sci. 2015, 60, 389–402. [Google Scholar] [CrossRef]
- Agboma, P.; Sinclair, T.; Jokinen, K.; Peltonen-Sainio, P.; Pehu, E. An Evaluation of the Effect of Exogenous Glycinebetaine on the Growth and Yield of Soybean: Timing of Application, Watering Regimes and Cultivars. Field Crops Res. 1997, 54, 51–64. [Google Scholar] [CrossRef]
- Manaf, H.H. Beneficial Effects of Exogenous Selenium, Glycine Betaine and Seaweed Extract on Salt Stressed Cowpea Plant. Ann. Agric. Sci. 2016, 61, 41–48. [Google Scholar] [CrossRef]
- Osman, H.S.; Salim, B.B. Influence of Exogenous Application of Some Phytoprotectants on Growth, Yield and Pod Quality of Snap Bean under NaCl Salinity. Ann. Agric. Sci. 2016, 61, 1–13. [Google Scholar] [CrossRef][Green Version]
- Malekzadeh, P. Influence of Exogenous Application of Glycinebetaine on Antioxidative System and Growth of Salt-Stressed Soybean Seedlings (Glycine max L.). Physiol. Mol. Biol. Plants 2015, 21, 225–232. [Google Scholar] [CrossRef]
- Hu, L.; Hu, T.; Zhang, X.; Pang, H.; Fu, J. Exogenous Glycine Betaine Ameliorates the Adverse Effect of Salt Stress on Perennial Ryegrass. J. Am. Soc. Hortic. Sci. 2012, 137, 38–46. [Google Scholar] [CrossRef]
- Rajasekaran, L.; Kriedemann, P.; Aspinall, D.; Paleg, L.G. Physiological Significance of Proline and Glycinebetaine: Maintaining Photosynthesis during NaCl Stress in Wheat. Photosynthetica 1998, 34, 357–366. [Google Scholar] [CrossRef]
- Li, S.; Li, F.; Wang, J.; Zhang, W.; Meng, Q.; Chen, T.H.; Murata, N.; Yang, X. Glycinebetaine Enhances the Tolerance of Tomato Plants to High Temperature during Germination of Seeds and Growth of Seedlings. Plant Cell Environ. 2011, 34, 1931–1943. [Google Scholar] [CrossRef]
- Oukarroum, A.; El Madidi, S.; Strasser, R. Exogenous Glycine Betaine and Proline Play a Protective Role in Heat-Stressed Barley Leaves (Hordeum vulgare L.): A Chlorophyll a Fluorescence Study. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2012, 146, 1037–1043. [Google Scholar]
- Wahid, A.; Shabbir, A. Induction of Heat Stress Tolerance in Barley Seedlings by Pre-Sowing Seed Treatment with Glycinebetaine. Plant Growth Regul. 2005, 46, 133–141. [Google Scholar] [CrossRef]
- Rasheed, R.; Wahid, A.; Farooq, M.; Hussain, I.; Basra, S. Role of Proline and Glycinebetaine Pretreatments in Improving Heat Tolerance of Sprouting Sugarcane (Saccharum sp.) Buds. Plant Growth Regul. 2011, 65, 35–45. [Google Scholar] [CrossRef]
- Sorwong, A.; Sakhonwasee, S. Foliar Application of Glycine Betaine Mitigates the Effect of Heat Stress in Three Marigold (Tagetes erecta) Cultivars. Hortic. J. 2015, 84, 161–171. [Google Scholar] [CrossRef]
- Chen, W.; Li, P.; Chen, T. Glycinebetaine Increases Chilling Tolerance and Reduces Chilling-induced Lipid Peroxidation in Zea mays L. Plant Cell Environ. 2000, 23, 609–618. [Google Scholar] [CrossRef]
- Allard, F.; Houde, M.; Kröl, M.; Ivanov, A.; Huner, N.P.; Sarhan, F. Betaine Improves Freezing Tolerance in Wheat. Plant Cell Physiol. 1998, 39, 1194–1202. [Google Scholar] [CrossRef]
- Shan, T.; Jin, P.; Zhang, Y.; Huang, Y.; Wang, X.; Zheng, Y. Exogenous Glycine Betaine Treatment Enhances Chilling Tolerance of Peach Fruit during Cold Storage. Postharvest Biol. Technol. 2016, 114, 104–110. [Google Scholar] [CrossRef]
- Zhao, Y.; Aspinall, D.; Paleg, L. Protection of Membrane Integrity in Medicago sativa L. by Glycinebetaine against the Effects of Freezing. J. Plant Physiol. 1992, 140, 541–543. [Google Scholar] [CrossRef]
- Park, E.-J.; Jeknic, Z.; Chen, T.H. Exogenous Application of Glycinebetaine Increases Chilling Tolerance in Tomato Plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef]
- Ali, S.; Chaudhary, A.; Rizwan, M.; Anwar, H.T.; Adrees, M.; Farid, M.; Irshad, M.K.; Hayat, T.; Anjum, S.A. Alleviation of Chromium Toxicity by Glycinebetaine Is Related to Elevated Antioxidant Enzymes and Suppressed Chromium Uptake and Oxidative Stress in Wheat (Triticum aestivum L.). Environ. Sci. Pollut. Res. 2015, 22, 10669–10678. [Google Scholar] [CrossRef]
- Farooq, M.; Ali, S.; Hameed, A.; Bharwana, S.; Rizwan, M.; Ishaque, W.; Farid, M.; Mahmood, K.; Iqbal, Z. Cadmium Stress in Cotton Seedlings: Physiological, Photosynthesis and Oxidative Damages Alleviated by Glycinebetaine. South Afr. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- Ali, S.; Abbas, Z.; Seleiman, M.F.; Rizwan, M.; YavaŞ, İ.; Alhammad, B.A.; Shami, A.; Hasanuzzaman, M.; Kalderis, D. Glycine Betaine Accumulation, Significance and Interests for Heavy Metal Tolerance in Plants. Plants 2020, 9, 896. [Google Scholar] [CrossRef]
- Jabeen, N.; Abbas, Z.; Iqbal, M.; Rizwan, M.; Jabbar, A.; Farid, M.; Ali, S.; Ibrahim, M.; Abbas, F. Glycinebetaine Mediates Chromium Tolerance in Mung Bean through Lowering of Cr Uptake and Improved Antioxidant System. Arch. Agron. Soil Sci. 2016, 62, 648–662. [Google Scholar] [CrossRef]
- Fan, W.; Zhang, M.; Zhang, H.; Zhang, P. Improved Tolerance to Various Abiotic Stresses in Transgenic Sweet Potato (Ipomoea Batatas) Expressing Spinach Betaine Aldehyde Dehydrogenase. PLoS ONE 2012, 7, e37344. [Google Scholar] [CrossRef] [PubMed]
- Ali, Q.; Anwar, F.; Ashraf, M.; Saari, N.; Perveen, R. Ameliorating Effects of Exogenously Applied Proline on Seed Composition, Seed Oil Quality and Oil Antioxidant Activity of Maize (Zea mays L.) under Drought Stress. Int. J. Mol. Sci. 2013, 14, 818–835. [Google Scholar] [CrossRef] [PubMed]
- Stepien, P. Effects of the Exogenous Glycinebetaine on Photosynthetic Apparatus in Cucumber Leaves Challenging Al Stress. In Proceedings of the 18 th International Conference on Heavy Metals in the Environment, Ghent, Belgium, 12–15 September 2016. [Google Scholar]
- Kumar, P.; Tokas, J.; Singal, H. Amelioration of Chromium VI Toxicity in Sorghum (Sorghum bicolor L.) Using Glycine Betaine. Sci. Rep. 2019, 9, 16020. [Google Scholar] [CrossRef]
- Dubey, A.K.; Kumar, N.; Ranjan, R.; Gautam, A.; Pande, V.; Sanyal, I.; Mallick, S. Application of Glycine Reduces Arsenic Accumulation and Toxicity in Oryza sativa L. by Reducing the Expression of Silicon Transporter Genes. Ecotoxicol. Environ. Saf. 2018, 148, 410–417. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).