Abstract
Fish gills are the major osmoregulatory tissue that contact the external water environment and have developed an effective osmoregulatory mechanism to maintain cellular function. Marine medaka (Oryzias melastigma) has the ability to live in both seawater and fresh water environments. The present study performed a seawater (SW) to 50% seawater (SFW) transfer, and the gill samples were used for comparative transcriptomic analysis to study the alteration of hypo-osmotic stress on immune responsive genes in this model organism. The result identified 518 differentiated expressed genes (DEGs) after the SW to SFW transfer. Various pathways such as p53 signaling, forkhead box O signaling, and the cell cycle were enriched. Moreover, the immune system was highlighted as one of the top altered biological processes in the enrichment analysis. Various cytokines, chemokines, and inflammatory genes that participate in the IL-17 signaling pathway were suppressed after the SW to SFW transfer. On the other hand, some immunoglobulin-related genes were up-regulated. The results were further validated by real-time qPCR. Taken together, our study provides additional gill transcriptome information in marine medaka; it also supports the notion that osmotic stress could influence the immune responses in fish gills.
1. Introduction
Osmoregulation is a critical biological mechanism for various cellular functions such as defining the cell shape and maintaining the intracellular osmolality [1]. Euryhaline fish can reside in both fresh water and seawater. The fish encounter totally opposite osmotic challenges in the two media. In fresh water, the fish deal with osmotic water uptake and loss of salts and vice versa in seawater. Fishes develop an osmoregulatory mechanism to regulate fluid and ion homeostasis to maintain a constant body osmolality in their lives. Fish gills are one of the primary ion-osmoregulatory tissues that constitute about 50% of the surface area of the fish interface with the surrounding medium, and thus develop specific osmoregulatory mechanisms [2]. The remodeling of gill tissues upon salinity changes has been reported [3,4].
Marine medaka (Oryzias melastigma) has the ability to live in both seawater and fresh water, which indicates that its gill could modify the ion and water homeostasis for the ion loss and water gain during hypo-osmotic stress. Due to its ability to live in seawater and well-known genome, it became a common fish model to study the marine pollution in the past few years [5,6,7]. Recently, we noticed that an omics paper on this ecotoxicity model was published. The study reported the gill and liver transcriptomics data of the four-month-acclimated fish in different salinities since the embryonic stage [8]. The authors aimed to understand the responses of O. melastigma under chronic salinity stress by various developmental and histochemical parameters and RNA-Seq. However, the result did not address the early hypo-osmotic responses in an adult fish. It is known that the osmoregulatory mechanism can be divided into three phases; namely, the osmosensors, signal transducers, and effectors [9]. Taking the gill as an example, the sensors in the gill cells detect the change of external osmolality and thus lead to the stimulation of various signaling molecules, and finally induce effectors to compensate for the osmotic challenge. The whole process can be completed in seven to fourteen days; and the sensing and transduction can be achieved from a few minutes to six hours. Signaling events such as the phosphorations of MAPK, JNK, and ERK and the upregulation of the osmotic stress transcription factor (Ostf1) were identified [10,11,12,13]. Lastly, the effectors, such as ion transporters, could modulate their mRNA and protein expressions afterwards, which could be achieved in a week [14]. The prolonged osmotic stress in the published study [15] identified the differentially expressed genes (DEGs) in the fish gill and liver raised from different osmotic salinities, and thus, did not cover the early phases of the osmotic regulation that this study is reporting.
Various environmental factors, such as temperature, salinity change, and oxygen levels, are found to affect the immune system in fishes [15]. Gills contain the large mucosal surfaces that protect the fish from pathogen entry [16]; it forms the gill-associated lymphoid tissue that is composed of various immune cells such as lymphocytes, macrophages, and antibody-secreting cells [17,18,19]. Mucus contains microbiota that could help in osmoregulation and protect the host from infection [20,21]. More importantly, the amount of the mucus production can be affected by water hardness and salinities [22], and the mucus production is higher in the gill surrounding area than the skin sites [23]. These findings further suggest the relationship between salinity and gill mucosa barrier in fishes.
Studies have demonstrated that the osmotic stress could influence the immune system in fishes; for example, an increase of plasma immunoglobulin (Ig) M level in seabream, and white blood cell count in rainbow trout were found after hyper-osmotic stress [24,25]. Numerous studies have been done in immune organs such as the kidney and spleen in fishes. However, how the salinity affects such system in gills is not well-known. Different immune proteins such as toll-like receptor 2 (TLR2) and interleukin-1 receptor type 2 (IL-1R2) are highly expressed in gill cells [26], and a report of eel gill has demonstrated that the immune system could be affected by osmotic stress [27]. In this study, we conducted gill transcriptomic analysis from seawater-acclimated marine medaka (control group; SW), and a group that was transferred from seawater to 50% seawater for seven days (hypo-osmotic stress group; SFW) to gain insight into the immune responses in gill of marine medaka upon hypo-osmotic stress.
2. Results
A total of 25,962 genes were identified, in which 24,746 genes were commonly found in both SW and SFW groups (Figure 1A). The sequencing information such as the mapping ratio, and clean read data are summarized in Supplementary File S1. Five hundred and eighteen DEGs were identified after the transfer, including 198 genes which increased their expression, and 320 genes decreased in the gill of the SFW group (Figure 1B, Supplementary File S2). The DEGs were then subjected to GO and KEGG enrichment analysis to understand the biological roles and functions of the DEGs. Enriched GO terms were grouped into the biological process (BP), cellular component (CC), and molecular functions (MF). The top enriched terms in BP (red color) were the cellular process, followed by the metabolic process. In addition, biological adhesion and immune system process were also identified. Furthermore, cell and binding were the top enriched terms in CC (blue) and MF (green), respectively (Figure 1C, Supplementary File S3). The GO relationship network among the enriched terms was further generated and shown in Supplementary File S4.
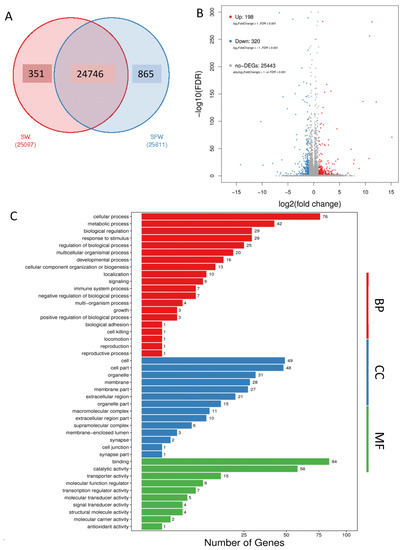
Figure 1.
Identification of differentiated expressed genes (DEGs) in medaka gill after seawater (SW) to 50% seawater transfer (SFW). (A) Venn diagram showing the comparison between SW and SFW. There were 24,746 transcripts identified as common counts. (B) Volcano plot showing 198 up-regulated DEGs and 320 down-regulated DEGs in the SW/SFW group. Red dots indicate the up-regulated genes, while blue represent the down-regulated. Non-DEGs were marked as grey dots. (C) Gene Ontology (GO) of DEGs after the transfer. The enriched GO terms were classified into biological process (BP, red), cellular component (CC, blue), and molecular function (MF, green). The top enriched terms were cellular process in BP; cell in CC; and binding in MF.
KEGG pathway analysis was further performed to show the functional enrichment from the DEGs. Different enriched terms were spotted: cell growth and death were found in the cellular processes; signal transduction and membrane transport in environmental information processing; folding, sorting, and degradation in genetic information processing; cancers in human diseases; amino acid metabolism and lipid metabolism in metabolism; and immune system and endocrine system in organismal systems (Figure 2). When the data was shown at a higher resolution, more specific pathways such as the IL-17 signaling pathway, alanine, asparate, and glutamate metabolism, FoxO signaling pathway, p53 signaling pathway, and cell cycle were spotted (Figure 3A, Supplementary File S5). Validations of selected genes in the above pathways by real-time qPCR were performed. The relative mRNA expression level of argininosuccinate synthase 1 (ass1), and carbamoyl-phosphate synthetase 2, aspartate transcarbamylase, and dihydroorotase (cad), which are responsible for amino acid and pyrimidine metabolism, were decreased (Figure 3B,C).
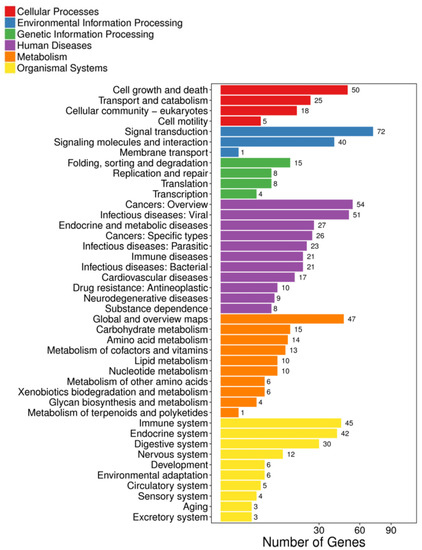
Figure 2.
Pathway classification of DEGs in SW/SFW group. X-axis represents the number of DEGs, and the Y-axis indicates the functional classification of KEGG that has been further classified into seven categories: cellular processed, environmental information processing, genetic information processing, human disease, metabolism, and organismal systems. The signaling transduction under the environmental information processing was enriched by the highest DEGs (72 genes). Immune system (45 genes) was the top enriched term in organismal systems.
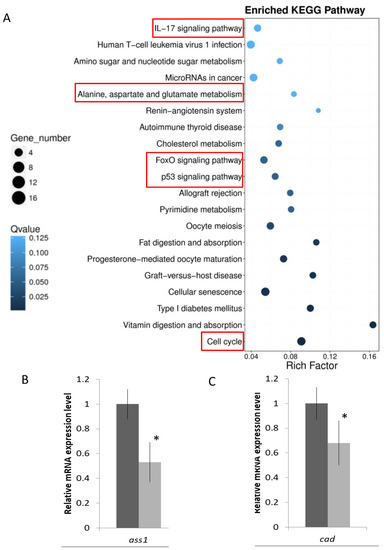
Figure 3.
Enriched KEGG pathways in SW/SFW group. (A) Pathway functional enrichment of DEGs in SW/SFW group. X-axis represents the enrichment factor, while Y-axis indicates the enriched pathways. The color indicated the q-value; the blue color represented the lower value, while the white color indicated the higher value. In addition, the point size indicated the number of DEGs; bigger dots represented more genes. The greater the rich factor, the more significant enrichment. IL17 signaling pathway, alanine, aspartate, and glutamate metabolism, FoxO signaling pathway, p53 signaling pathway, and cell cycle (red boxes) were found to be enriched in the SW/SFW group. (B,C) Validations of the mRNA gene expression levels of two selected alanine and glutamate metabolism related genes (ass and cad) transcripts by real time qPCR. Results matched with the sequencing data. n = 5; mean ± s.e., * indicated p < 0.05.
Genes that participated in the FoxO and p53 pathways that are related to the cell cycle were further validated as well (Figure 4A). The mRNA expressions of S-phase kinase-associated protein 2 (skp2), polo-like kinase 1 (plk1), cyclin E2 (ccne2), and cyclin B1 (ccnb1) were reduced after the transfer (Figure 4B–E). Lastly, we focused on the enriched immune-related IL-17 pathway that includes the core activator protein 1 transcription factor, c-fos, and its downstream effectors such as chemokines, cytokines, anti-microbial, and tissue remodelling. Genes labelled in the green boxes were suppressed after the fresh water transfer, while the one in the red box was the induced one (Figure 5A). The IL-17 signaling pathway involves the activator protein 1 (AP-1) transcription factor, c-fos, which could activate a large number of downstream effectors such as chemokines, cytokines, and inflammatory genes (Figure 4A). The mRNA expression levels of selected genes such as c-fos, il-1β, il1-r2, il8, and mmp9 were decreased after the SW to SFW transfer (Figure 5B–E). Additionally, numerous immunoglobulin-related transcripts were induced upon hypo-osmotic stress in this study. For example, Fc epsilon receptor II (fcer2) and polymeric immunoglobulin receptor (pigr) were upregulated (Figure 5G–H). Lastly, IPA analysis was performed to have an overview of the possible bio-functions and related diseases of the DEGs. Significant changes of the immune-related terms were spotted. It was noticed that the IL-1β was commonly found in the pathways that matched with other analysis (Table 1). The full list of the terms can be referred to in Supplementary File S6.
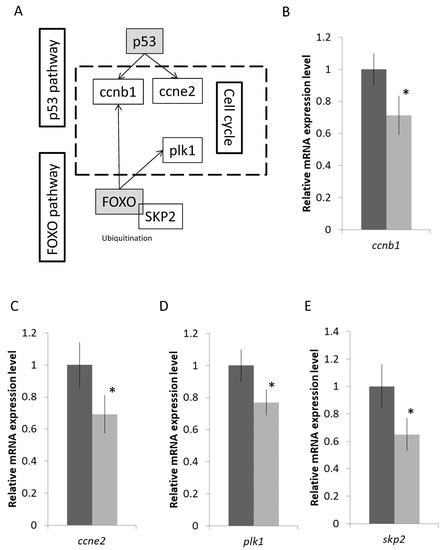
Figure 4.
Hypo-osmotic stress influences the FoxO, p53 pathways, and cell cycle in gill cells. (A) The relationship among FoxO, p53, and cell cycle. (B–E) Validations of the mRNA gene expression levels of the selected transcripts by real time qPCR. Down regulation of the selected transcripts (ccnb1; ccne2; plk1; and skp2) in the pathways. Results matched with the sequencing data. n = 5; mean ± s.e., * indicated p < 0.05.
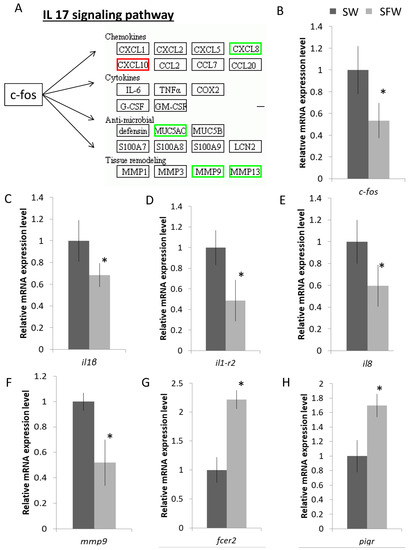
Figure 5.
Hypo-osmotic stress influences the immune system in gill cells. (A) IL-17 signaling pathway was enriched in the SW/SFW group; green indicated the reduced transcripts, while red indicated the up-regulated transcript. The transcription factor, c-fos, could induce various down-stream targets. (B–H) Validations of the mRNA gene expression levels of the selected transcripts by real time qPCR. Down-regulation of the selected immune-related genes (transcription factor c-fos; interleukin-related genes (il1-r2; il1b; and il8); tissue remodelling gene, mmp9) and up-regulation of the selected immunoglobulin-related receptors (fcer2; and pigr) after the transfer. Results matched with the sequencing data. n = 5; mean ± s.e., * indicated p < 0.05.

Table 1.
IPA analysis (disease and bio-function) of the DEGs in gill upon hypo-osmotic stress. Selected terms were shown.
3. Discussion
Our findings identified various biological processes upon hypo-tonic stress in marine medaka gill. The enrichment of cell-cycle-related genes have been identified in other fishes under osmotic stress [28,29,30]. A study in fish esophageal cells showed the occurrence of cell proliferation or cell death during the osmotic challenges [31]. In addition, the shift of the seawater or freshwater type of gill mitochondria-rich cells upon osmotic stress were achieved by cellular proliferation and apoptosis that are controlled by the cell cycle regulators [32]. FoxO transcription factors are known as critical regulators of the cellular stress response and act as the redox regulators [33]. The induction of the FoxO signaling pathway during osmotic stress was reported in oyster [34]. Additionally, it has been suggested to play roles in extending the life span in C. elegans [35]. FOXO proteins are regulated by the ubiquitination of SKP2 and control the cell-cycle-related genes such as PLK1 and CCNB1 [36,37,38]. Here, various cell-cycle-related genes were down-regulated, which further supported the notion that several cell cycle regulators could play roles in modifying cell proliferation, differentiation, and survival during osmotic stress [39]. Furthermore, the p53 pathway is closely related to cell death and cell cycle regulations. A hyper-tonic stress experiment on climbing perch could induce apoptosis and p53 protein expression in gill [32]. Moreover, all these pathways are found to be closely related to hypoxia-induced cell cycle arrest [40]. Studies in killifish and hagfish have demonstrated the relationship between salinity acclimation ability and the hypoxia condition. Changes of plasma osmolality and ion flux rates in gills were found in fishes under hypoxia [41,42]. Furthermore, hypoxia could induce the immune response in fish gill. A study in large yellow croaker showed changes of mRNA expression levels of various chemokines and their receptors via a transcriptome study after hypoxia stress [43]. All these findings further support the notion of the relationships between salinity stress, immune response, and hypoxia.
Fish gills, besides their osmoregulatory function, play important roles in immune response. Since fishes are continuously exposed to the external medium rich in microbiota, they develop various mucosa-associated lymphoid tissues (MALT) to protect themselves from infection. There are four major types of MALT, including the gill, gut, skin, and nasopharynx [44]. The mucosal immune system is composed of innate and adaptive immune cells and molecules to protect the host against pathogens. Mucosal B and T cells and the presence of specialized mucosal antibodies have been identified in teleost fish [45]. B cells, plasma cells and the Igs form the mucosal barriers [46], in which the B cells contribute to only about 1% of total gill leukocytes in the rainbow trout [47]. On the other hand, T cells contribute to 10–20% of the lymphoid cells in gill-associated lymphoid tissue, [48].
Osmotic stress affects immune responses. Osmoregulatory cytokines, such as interleukins (ILs), were reported to play roles in the osmotic stress signaling network in fishes to regulate epithelial responses to salinity changes [49,50,51]. ILs are the inflammatory cytokines and have been reported to respond to osmotic stress [27]. IL-17 belongs to the pro-inflammatory cytokines that are produced by helper T-cells and could induce varies cytokines and chemokines, such as IL-1β and IL-8, suggesting its roles in immune response. Furthermore, IL-17 signaling is found to play important roles in the host’s metabolic activity and participates directly in the induction of metabolism-related genes [52]. In addition, the IL-17 signaling pathway is involved in tissue repair and tumorigenesis [53]. It is known that when the tissue is damaged, the immune Th17 cells produce IL-17 and protect the mucous membranes and epithelial tissues against infection. Additionally, the Th17 cells expand at the mucosal surface to clear the invading microorganisms [54,55]. Furthermore, Th17-cell-associated cytokines are involved in wound healing by modulating the mucosal surfaces [56]. The IL-17 signaling pathway involves the core transcription factor, c-Fos, that could activate effectors such as chemokines, cytokines, and inflammatory genes [57,58]. Among the cytokines’ families, the IL-1 family plays a critical role in the innate immune response, in which the IL-1 receptor is responsible for the fundamental inflammatory response [59]. IL-1β is the most studied member in the IL-1 family. It is a pro-inflammatory cytokine that is secreted by macrophages and could further modify the nature of immune cells [60]. Although osmotic stress (hyper-osmolality) has been recognized as a pro-inflammatory stress to the corneal epithelium [61], the relationship between the immune response and osmoregulation is still not well-described. Nevertheless, some in vitro mammalian cell line studies have demonstrated that osmotic stress could stimulate the IL signaling pathway. For example, a study using the human limbal epithelial cell lines showed a higher IL-1β and IL-8 concentrations and mRNA expressions in the hyperosmolar culture medium-cultured cells [62]. Furthermore, another hyperosmotic stress study in the human corneal epithelia cells showed the induction of IL-1 [61]. Our reduced mRNA expression levels of il1β and il8 under hypo-osmotic stress matched with those reports. Regarding the studies in fishes, a study in tilapia found that acute hypo-osmotic stress could suppress the immune response as indicated by lymphopenia and neutrophilia [63]. Another hypo-osmotic stress study in seabream demonstrated the functional alternations of inflammatory reactions along the brain-gut axis, with the involvement of IL-1β [64]. Moreover, an in vitro study using the renal masses in spotted scat also identified the changes of mRNA expression levels of various pro-inflammatory cytokines, including the il-1β upon osmotic stress [65].
Additionally, chemokines play roles in the immunoregulatory and inflammatory processes [66]. The chemokine IL-8 has been reported as the osmo-responsive gene in human epithelial cells [62]. Its mRNA expression level was reduced in gill upon hypo-osmotic stress (Figure 4B,F). Ig are glycoproteins that have antibody activity for immune response. Receptors interacting with the Ig play critical immune reactions in vertebrates [67]. For example, fcer2 is classified as the low-affinity receptor for IgE, which plays roles in allergy reaction and modulating the IgE serum levels [68,69]. Although it is known that the IgE is unique in mammals [70], fcer is identified in the genome of bony fishes such as zebrafish (ENSDARG00000103276.3) and medaka (ENSORLG00000016096.2). The reason for its presence in fishes and corresponding functions are not well-understood. A study in zebrafish demonstrated a cross-reactivity of antibodies against human FcεRIγ and human IgE on the zebrafish high-affinity IgE-like receptor (fcer1) [71]. More importantly, stimulation of FcεRI receptor by IgG and other immune mediators is found in mammals [72,73]. This may suggest that the medaka fcer may be responsive to other Ig that requires further investigation. In terms of evolution, pigr members represent a major step in Fc receptor evolution. They are not found in cartilagous fish and are suggested to have first appeared within bony fishes [67]. In fish, pigr bind with various Ig [74] and is highly expressed in fish gill [75]. Our results marked the increased mRNA expression levels of these two receptors which further supports the notion that osmotic stress could influence the immune responses. All these results support the notion that osmotic stress could influence the immune response.
Lastly, various metabolomics studies in fishes have identified that osmotic stress could alter metabolic pathways. A transcriptomic study identified numerous enriched metabolic pathways in eel gill after fresh water to seawater transfer [29]. A catfish study suggested that hyper-osmotic stress could alter amino acid metabolism [76], while glutamate metabolism was enriched in tonguefish [77]. We demonstrated the reduced mRNA expression of ass1 and cad, which are responsible for amino acid and pyrimidine metabolism after the transfer. Moreover, various studies in aquatic lives have identified the changes of amino acid metabolism and immunity together during osmotic challenges. For example, a study in crab found that hyper-osmotic stress could affect energy metabolism, amino acid metabolism, and immunity [78], while glucose metabolism and the immune system were enriched in prawn upon hypo-tonic stress [79].
This study is limited in that we did not demonstrate the presence of the immune cell (Th cell) in the gills via immunohistochemical CD4 staining [80]. The condition of the fish before and during the transfer experiment showed no infections or other obvious abnormalities. Regarding the presence of the immune cells in medaka gills, studies have identified the immune-related proteins, such as B lymphocyte-induced maturation protein-1 and Hepatitis A virus cellular receptor 2, in gills that are responsible for maturation of lymphoid cells [81] and as a T helper 1-specific cell surface protein [82], which support the presence of immune cells in gills.
To summarize, osmotic stress could not only influence the typical well-known water/ion homeostasis and cell-cycle-related genes, but could also modulate the immune system via IL-17 signaling pathway in medaka gill. The underlying osmoregulatory mechanisms among fish ILs, Ig, and their relative receptors must be further studied to fill the immune-osmotic stress knowledge gap in fishes.
4. Materials and Methods
4.1. Fish Maintenance and Experimental Setup
Six-month-old medaka (O. melastigma) was maintained in artificial seawater (Sea Treasure, Tokyo, Japan) at 26 °C. Fifty fish were kept in a 20 L tank for more than one week prior to the transfer experiment. Twenty-five fish were transferred to 50% seawater for seven days as the SFW transfer group. The remaining twenty-five fish were the control group that had gone through the seawater-to-seawater transfer (SW). The density of the SW was measured and confirmed by the refractometer at 1.02 g/cm2. The 50% seawater was prepared by diluting the seawater with aerated fresh water that reached the density at 1.01 g/cm2. One-third of the rearing water in tanks was changed every three days to maintain good water quality. The tanks were constantly aerated, and the fresh water was prepared by de-chlorinating tap water for at least a week. Fish were fed with brine shrimp (Brine Shrimp Direct, Ogden, UT, USA) once a day and commercial fish feed twice a day (Marubeni, Tokyo, Japan).
4.2. Fish Sampling
Twenty medaka from each group were anesthetized by the MS222 (Sigma-Aldrich, Lanham, MD, USA). A pair of gills was collected, and their total RNA was extracted by the TRIZOL reagent (Life Technologies, Carlsbad, CA, USA) for transcriptome sequencing. Briefly, five gills from five individuals were pooled as one biological sample (4 replicates from each condition) for RNA sequencing. The experiments were performed using protocols approved by Kyushu University, Fukuoka, Japan (A19-165-1).
4.3. Library Construction and Illumina RNA-seq
For library construction, RNA concentrations were measured using a Qubit RNA Assay Kit on a Qubit 2.0 Fluorometer (Life Technologies, Carlsbad, CA, USA). We used 300 ng total RNA with an RNA Integrity Number > 8 for the library construction (Agilent Technologies, Santa Clara, CA, USA), followed by the qualification using the Agilent 2100 Bioanalyzer system. cDNA libraries were prepared by the TruSeq Stranded mRNA LT Sample Prep Kit (Illumina, San Diego, CA, USA), and the index codes were ligated afterwards to identify the individual samples. Lastly, mRNA was purified from total RNA using poly-T oligo-attached magnetic beads (Illumina, San Diego, CA, USA) before fragmentation. cDNAs were synthesized by applying random oligonucleotides and SuperScript II with DNA polymerase I and RNase H treatment. Overhangs were then blunted by treatment with exonuclease/polymerase, and the 3′-end adenylation and ligation to Illumina PE adaptor oligonucleotides were then performed. DNA fragments with adaptor molecules on both ends were enriched by the Illumina PCR Primer Cocktail with 15 PCR cycle. Products were then purified by the AMPure XP system and quantified by the Agilent Bioanalyzer 2100 system and were then sequenced by the BGISEQ-500 platform. Sequence reads were filtered by the SOAPunke software (v1.5.2, Beijing Genomics Institute, Shenzhen, China) to remove reads with adaptors, > 0.1% unknown bases (N), and low-quality reads (percentage of base that quality is lesser than 20 is greater than 50% in a read). Sequencing reads were mapped to the reference genome using Bowtie2 (v2.2.5, Johns Hopkins University, Baltimore, MD, USA) [83], and the gene expression level was calculated by the RSME software package [84]. Clustering analysis was done by the Mfuzz software (v2.34.0, University of Algarve, Faro, Portugal) with the parameters of c = 12; m = 1.25 [85]. The SW raw data used in this study was extracted from our previously published data [20], and the SFW-transferred fish in this study were obtained from the same patch of the fish. The DEGs between SW/SFW were detected by the PossionDis with fold change ≥ 2.0 and FDR ≤ 0.001 [86]. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were then performed by using phyper, a function of R. The false discovery rate (FDR) smaller than 0.01 was defined as significant enriched. The QIAGEN’s Ingenuity® Pathway Analysis Release (2019) (IPA, QIAGEN Redwood City, CA, USA, www.qiagen.com/ingenuity, accessed on 3 September, 2020), was used to delineate the disease and biological functions of the DEGs after the transfer. The parameter was set as the default as described [87].
4.4. Reverse Transcription and Quantitative Real-Time PCR
Total RNA was extracted from another new set of transfer experiments with the same setting as mentioned. Five fish (n = 5) were used in each group (SW control, and SFW transferred group) for sampling. Purified gill RNA with a ratio of 1.8–2.0 at A260/A280 was used to synthesize cDNA using SuperScript VILO Master Mix (Invitrogen; Life Technologies) by PTC-200 Peltier Thermal Cycler (MJ Research, St. Bruno, QC, Canada). Real-time qPCR was performed by using the Power SYBR Green PCR Master Mix (Applied Biosystems, Waltham, MA, USA) and QuantStudio 3 Real-Time PCR System (Applied Biosystems). Primer sequences were tabulated in Table 2. The relative expression ratio was calculated according to the method described by Pfaffl [88] and the data were normalized using the expression levels of 18S-ribosomal RNA. The existence of primer–dimers and secondary products was checked using melting curve analysis. All data are represented as mean ± s.e. Statistical significance was tested using Student’s t-test or one-way analysis (ANOVA), followed by Tukey’s HSD test. Groups were considered significantly different if p < 0.05. Normality test was performed using the Kolmogorov–Smirnov normality test in SPSS software.

Table 2.
qPCR primers used in the study.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms232012417/s1.
Author Contributions
Conceptualization; funding acquisition; writing-original draft; writing- review & editing: R.L., J.L., K.P.L. and W.K.F.T.; Investigation; formal analysis; methodology; resources; visualization: R.L., J.L., C.T.L., K.P.L. and W.K.F.T. Methodology; resources; software: X.L. and T.F.C.; Supervision: T.F.C., K.P.L. and W.K.F.T. All authors have read and agreed to the published version of the manuscript.
Funding
The osmoregulatory works in WKFT group are partially supported by the Cooperative Program of Atmosphere and Ocean Research Institute, The University of Tokyo (123-2018, 127-2019, 127-2022), Japan; and the Individual Research Collaboration Project, National Institute of Basic Biology, Japan (17-314, 18-319). This study is supported by the National Natural Science Foundation of China (No. 82160282) to KPL.
Institutional Review Board Statement
All methods were performed in accordance with the relevant guidelines and regulations based on the Kyushu University, Japan and were approved (A19-165-1).
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequencing data from this study have been submitted to the NCBI BioProject (https://www.ncbi.nlm.nih.gov/bioproject, accessed on 10 August 2022) under the accession number PRJNA588335. Other data supporting the result of this article are included within the article and the Supplementary Files S1–S6.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Hwang, P.P.; Lee, T.H.; Lin, L.Y. Ion regulation in fish gills: Recent progress in the cellular and molecular mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R28–R47. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The multifunctional fish gill: Dominant site of gas exchange, osmoregulation, acid-base regulation, and excretion of nitrogenous waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef]
- Mizuhira, V.; Amakawa, T.; Yamashina, S.; Shirai, N.; Utida, S. Electron microscopic studies on the localization of sodium ions and sodium-potassium-activated adenosinetriphosphatase in chloride cells of eel gills. Exp. Cell Res. 1970, 59, 346–348. [Google Scholar] [CrossRef]
- Wong, C.K.C.; Chan, D.K.O. Effects of cortisol on chloride cells in the gill epithelium of Japanese eel, Anguilla japonica. J. Endocrinol. 2001, 168, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, B.Y.; Han, J.; Jeong, C.B.; Hwang, D.S.; Lee, M.C.; Kang, H.M.; Kim, D.H.; Lee, D.; Kim, J.; et al. The genome of the marine medaka Oryzias melastigma. Mol. Ecol. Resour. 2018, 18, 656–665. [Google Scholar] [CrossRef]
- Lai, K.P.; Li, J.W.; Wang, S.Y.; Chiu, J.M.Y.; Tse, A.; Lau, K.; Lok, S.; Au, D.W.T.; Tse, W.K.F.; Wong, C.K.C.; et al. Tissue-specific transcriptome assemblies of the marine medaka Oryzias melastigma and comparative analysis with the freshwater medaka Oryzias latipes. BMC Genom. 2015, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.P.; Gong, Z.; Tse, W.K.F. Zebrafish as the toxicant screening model: Transgenic and omics approaches. Aquat. Toxicol. 2021, 234, 105813. [Google Scholar] [CrossRef]
- Liang, P.; Saqib, H.S.A.; Lin, Z.; Zheng, R.; Qiu, Y.; Xie, Y.; Ma, D.; Shen, Y. RNA-seq analyses of Marine Medaka (Oryzias melastigma) reveals salinity responsive transcriptomes in the gills and livers. Aquat. Toxicol. Amst. Neth. 2021, 240, 105970. [Google Scholar] [CrossRef] [PubMed]
- Kultz, D. The combinatorial nature of osmosensing in fishes. Physiol. Bethesda Md. 2012, 27, 259–275. [Google Scholar] [CrossRef]
- Tse, W.K.F.; Au, D.W.T.; Wong, C.K.C. Effect of osmotic shrinkage and hormones on the expression of Na+/H+ exchanger-1, Na+/K+/2Cl(-) cotransporter and Na+/K+-ATPase in gill pavement cells of freshwater adapted Japanese eel, Anguilla japonica. J. Exp. Biol. 2007, 210, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Tse, W.K.F.; Chow, S.C.; Wong, C.K.C. The cloning of eel osmotic stress transcription factor and the regulation of its expression in primary gill cell culture. J. Exp. Biol. 2008, 211, 1964–1968. [Google Scholar] [CrossRef]
- Tse, W.K.F.; Lai, K.P.; Takei, Y. Medaka osmotic stress transcription factor 1b (Ostf1b/TSC22D3-2) triggers hyperosmotic responses of different ion transporters in medaka gill and human embryonic kidney cells via the JNK signalling pathway. Int. J. Biochem. Cell Biol. 2011, 43, 1764–1775. [Google Scholar] [CrossRef]
- Chow, S.C.; Wong, C.K.C. Regulatory function of hyperosmotic stress-induced signaling cascades in the expression of transcription factors and osmolyte transporters in freshwater Japanese eel primary gill cell culture. J. Exp. Biol. 2011, 214, 1264–1270. [Google Scholar] [CrossRef][Green Version]
- Tse, W.K.F.; Au, D.W.T.; Wong, C.K.C. Characterization of ion channel and transporter mRNA expressions in isolated gill chloride and pavement cells of seawater acclimating eels. Biochem. Biophys. Res. Commun. 2006, 346, 1181–1190. [Google Scholar] [CrossRef]
- Sudhagar, A.; Kumar, G.; El-Matbouli, M. Transcriptome Analysis Based on RNA-Seq in Understanding Pathogenic Mechanisms of Diseases and the Immune System of Fish: A Comprehensive Review. Int. J. Mol. Sci. 2018, 19, 245. [Google Scholar] [CrossRef]
- Salinas, I.; Zhang, Y.-A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, N.M.S.; Taverne-Thiele, J.J.; Barnes, A.C.; van Muiswinkel, W.B.; Ellis, A.E.; Rombout, J.H.W.M. The gill is a major organ for antibody secreting cell production following direct immersion of sea bass (Dicentrarchus labrax L.) in a Photobacterium damselae ssp. piscicida bacterin: An ontogenetic study. Fish Shellfish Immunol. 2001, 11, 65–74. [Google Scholar] [CrossRef]
- Grove, S.; Johansen, R.; Reitan, L.J.; Press, C.M. Immune- and enzyme histochemical characterisation of leukocyte populations within lymphoid and mucosal tissues of Atlantic halibut (Hippoglossus hippoglossus). Fish Shellfish Immunol. 2006, 20, 693–708. [Google Scholar] [CrossRef] [PubMed]
- Mulero, I.; Sepulcre, M.P.; Roca, F.J.; Meseguer, J.; García-Ayala, A.; Mulero, V. Characterization of macrophages from the bony fish gilthead seabream using an antibody against the macrophage colony-stimulating factor receptor. Dev. Comp. Immunol. 2008, 32, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.P.; Zhu, P.; Boncan, D.A.T.; Yang, L.; Leung, C.C.T.; Ho, J.C.H.; Lin, X.; Chan, T.F.; Kong, R.Y.C.; Tse, W.K.F. Integrated Omics Approaches Revealed the Osmotic Stress-Responsive Genes and Microbiota in Gill of Marine Medaka. mSystems 2022, 7, e0004722. [Google Scholar] [CrossRef] [PubMed]
- Shephard, K.L. Functions for fish mucus. Rev. Fish Biol. Fish. 1994, 4, 401–429. [Google Scholar] [CrossRef]
- Roberts, S.D.; Powell, M.D. Comparative ionic flux and gill mucous cell histochemistry: Effects of salinity and disease status in Atlantic salmon (Salmo salar L.). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 134, 525–537. [Google Scholar] [CrossRef]
- Ringo, E.; Wesmajervi, M.S.; Bendiksen, H.R.; Berg, A.; Olsen, R.E.; Johnsen, T.; Mikkelsen, H.; Seppola, M.; Strom, E.; Holzapfel, W. Identification and characterization of Carnobacteria isolated from fish intestine. Syst. Appl. Microbiol. 2001, 24, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, A.; Laiz-Carrion, R.; Del Rio, M.P.; Meseguer, J.; Mancera, J.M.; Esteban, M.A. Salinity influences the humoral immune parameters of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2005, 18, 255–261. [Google Scholar] [CrossRef]
- Taylor, J.F.; Needham, M.P.; North, B.P.; Morgan, A.; Thompson, K.; Migaud, H. The influence of ploidy on saltwater adaptation, acute stress response and immune function following seawater transfer in non-smolting rainbow trout. Gen. Comp. Endocrinol. 2007, 152, 314–325. [Google Scholar] [CrossRef]
- Tse, W.K.F.; Sun, J.; Zhang, H.M.; Lai, K.P.; Gu, J.; Qiu, J.W.; Wong, C.K.C. iTRAQ-based quantitative proteomic analysis reveals acute hypo-osmotic responsive proteins in the gills of the Japanese eel (Anguilla japonica). J. Proteom. 2014, 105, 133–143. [Google Scholar] [CrossRef]
- Gu, J.; Dai, S.; Liu, H.; Cao, Q.; Yin, S.; Lai, K.P.; Tse, W.K.F.; Wong, C.K.C.; Shi, H. Identification of immune-related genes in gill cells of Japanese eels (Anguilla japonica) in adaptation to water salinity changes. Fish Shellfish Immunol. 2018, 73, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Ma, A.; Huang, Z.; Wang, X.; Liu, Z.; Xia, D.; Yang, S.; Zhao, T. Comparative transcriptomic analysis reveals mechanisms of divergence in osmotic regulation of the turbot Scophthalmus maximus. Fish Physiol. Biochem. 2020, 46, 1519–1536. [Google Scholar] [CrossRef]
- Lai, K.P.; Li, J.W.; Gu, J.; Chan, T.F.; Tse, W.K.; Wong, C.K. Transcriptomic analysis reveals specific osmoregulatory adaptive responses in gill mitochondria-rich cells and pavement cells of the Japanese eel. BMC Genom. 2015, 16, 1072. [Google Scholar] [CrossRef]
- Gao, J.; Xu, G.; Xu, P. Gills full-length transcriptomic analysis of osmoregulatory adaptive responses to salinity stress in Coilia nasus. Ecotoxicol. Env. Saf. 2021, 226, 112848. [Google Scholar] [CrossRef]
- Takahashi, H.; Prunet, P.; Kitahashi, T.; Kajimura, S.; Hirano, T.; Grau, E.G.; Sakamoto, T. Prolactin receptor and proliferating/apoptotic cells in esophagus of the Mozambique tilapia (Oreochromis mossambicus) in fresh water and in seawater. Gen. Comp. Endocrinol. 2007, 152, 326–331. [Google Scholar] [CrossRef]
- Ching, B.; Chen, X.L.; Yong, J.H.; Wilson, J.M.; Hiong, K.C.; Sim, E.W.; Wong, W.P.; Lam, S.H.; Chew, S.F.; Ip, Y.K. Increases in apoptosis, caspase activity and expression of p53 and bax, and the transition between two types of mitochondrion-rich cells, in the gills of the climbing perch, Anabas testudineus, during a progressive acclimation from freshwater to seawater. Front. Physiol. 2013, 4, 135. [Google Scholar] [CrossRef]
- Klotz, L.O.; Sánchez-Ramos, C.; Prieto-Arroyo, I.; Urbánek, P.; Steinbrenner, H.; Monsalve, M. Redox regulation of FoxO transcription factors. Redox Biol. 2015, 6, 51–72. [Google Scholar] [CrossRef]
- Xiao, S.; Wong, N.K.; Li, J.; Lin, Y.; Zhang, Y.; Ma, H.; Mo, R.; Zhang, Y.; Yu, Z. Analysis of in situ Transcriptomes Reveals Divergent Adaptive Response to Hyper- and Hypo-Salinity in the Hong Kong Oyster, Crassostrea hongkongensis. Front. Physiol. 2018, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.N.; Corkins, M.E.; Li, J.C.; Singh, K.; Parsons, S.; Tucey, T.M.; Sorkaç, A.; Huang, H.; Dimitriadi, M.; Sinclair, D.A.; et al. C. elegans lifespan extension by osmotic stress requires FUdR, base excision repair, FOXO, and sirtuins. Mech. Ageing Dev. 2016, 154, 30–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Qin, H.; Du, W.; Shen, Y.W.; Lee, W.H.; Riggs, A.D.; Liu, C.P. Inhibition of S-phase kinase-associated protein 2 (Skp2) reprograms and converts diabetogenic T cells to Foxp3+ regulatory T cells. Proc. Natl. Acad. Sci. USA 2012, 109, 9493–9498. [Google Scholar] [CrossRef]
- Huang, H.; Tindall, D.J. Regulation of FOXO protein stability via ubiquitination and proteasome degradation. Biochim. Et. Biophys. Acta 2011, 1813, 1961–1964. [Google Scholar] [CrossRef]
- Ke, Z.B.; Cai, H.; Wu, Y.P.; Lin, Y.Z.; Li, X.D.; Huang, J.B.; Sun, X.L.; Zheng, Q.S.; Xue, X.Y.; Wei, Y.; et al. Identification of key genes and pathways in benign prostatic hyperplasia. J. Cell Physiol. 2019, 234, 19942–19950. [Google Scholar] [CrossRef]
- Brennan, R.S.; Galvez, F.; Whitehead, A. Reciprocal osmotic challenges reveal mechanisms of divergence in phenotypic plasticity in the killifish Fundulus heteroclitus. J. Exp. Biol. 2015, 218, 1212–1222. [Google Scholar] [CrossRef]
- Li, C.; Liu, Z.; Wu, G.; Zang, Z.; Zhang, J.Q.; Li, X.; Tao, J.; Shen, M.; Liu, H. FOXO1 mediates hypoxia-induced G0/G1 arrest in ovarian somatic granulosa cells by activating the TP53INP1-p53-CDKN1A pathway. Development 2021, 148, dev199453. [Google Scholar] [CrossRef] [PubMed]
- Giacomin, M.; Dal Pont, G.; Eom, J.; Schulte, P.M.; Wood, C.M. The effects of salinity and hypoxia exposure on oxygen consumption, ventilation, diffusive water exchange and ionoregulation in the Pacific hagfish (Eptatretus stoutii). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 232, 47–59. [Google Scholar] [CrossRef]
- Giacomin, M.; Bryant, H.J.; Val, A.L.; Schulte, P.M.; Wood, C.M. The osmorespiratory compromise: Physiological responses and tolerance to hypoxia are affected by salinity acclimation in the euryhaline Atlantic killifish (Fundulus heteroclitus). J. Exp. Biol. 2019, 222, jeb.206599. [Google Scholar] [CrossRef]
- Mu, Y.; Li, W.; Wei, Z.; He, L.; Zhang, W.; Chen, X. Transcriptome analysis reveals molecular strategies in gills and heart of large yellow croaker (Larimichthys crocea) under hypoxia stress. Fish Shellfish Immunol. 2020, 104, 304–313. [Google Scholar] [CrossRef]
- Salinas, I. The Mucosal Immune System of Teleost Fish. Biology 2015, 4, 525–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.A.; Salinas, I.; Oriol Sunyer, J. Recent findings on the structure and function of teleost IgT. Fish Shellfish Immunol. 2011, 31, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.A.; Salinas, I.; Li, J.; Parra, D.; Bjork, S.; Xu, Z.; LaPatra, S.E.; Bartholomew, J.; Sunyer, J.O. IgT, a primitive immunoglobulin class specialized in mucosal immunity. Nat. Immunol. 2010, 11, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Gomez, F.; Greene, W.; Rego, K.; Hansen, J.D.; Costa, G.; Kataria, P.; Bromage, E.S. Discovery and Characterization of Secretory IgD in Rainbow Trout: Secretory IgD Is Produced through a Novel Splicing Mechanism. J. Immunol. 2012, 188, 1341. [Google Scholar] [CrossRef]
- Boardman, T.; Warner, C.; Ramirez-Gomez, F.; Matrisciano, J.; Bromage, E. Characterization of an anti-rainbow trout (Oncorhynchus mykiss) CD3ε monoclonal antibody. Vet. Immunol. Immunopathol. 2012, 145, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.E.; Gayle, M.A.; Slack, J.L.; Alderson, M.R.; Bird, T.A.; Giri, J.G.; Colotta, F.; Re, F.; Mantovani, A.; Shanebeck, K. Interleukin 1 signaling occurs exclusively via the type I receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 6155–6159. [Google Scholar] [CrossRef]
- Loitsch, S.M.; von Mallinckrodt, C.; Kippenberger, S.; Steinhilber, D.; Wagner, T.O.F.; Bargon, J. Reactive Oxygen Intermediates Are Involved in IL-8 Production Induced by Hyperosmotic Stress in Human Bronchial Epithelial Cells. Biochem. Biophys. Res. Commun. 2000, 276, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Bekeredjian-Ding, I.; Inamura, S.; Giese, T.; Moll, H.; Endres, S.; Sing, A.; Zähringer, U.; Hartmann, G. Staphylococcus aureus Protein A Triggers T Cell-Independent B Cell Proliferation by Sensitizing B Cells for TLR2 Ligands. J. Immunol. 2007, 178, 2803–2812. [Google Scholar] [CrossRef]
- Okamura, Y.; Miyanishi, H.; Kinoshita, M.; Kono, T.; Sakai, M.; Hikima, J.I. A defective interleukin-17 receptor A1 causes weight loss and intestinal metabolism-related gene downregulation in Japanese medaka, Oryzias latipes. Sci. Rep. 2021, 11, 12099. [Google Scholar] [CrossRef]
- Zepp, J.A.; Zhao, J.; Liu, C.; Bulek, K.; Wu, L.; Chen, X.; Hao, Y.; Wang, Z.; Wang, X.; Ouyang, W.; et al. IL-17A-Induced PLET1 Expression Contributes to Tissue Repair and Colon Tumorigenesis. J. Immunol. Baltim. Md. 1950 2017, 199, 3849–3857. [Google Scholar] [CrossRef]
- Khader, S.A.; Gaffen, S.L.; Kolls, J.K. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol. 2009, 2, 403–411. [Google Scholar] [CrossRef]
- Brockmann, L.; Giannou, A.D.; Gagliani, N.; Huber, S. Regulation of T(H)17 Cells and Associated Cytokines in Wound Healing, Tissue Regeneration, and Carcinogenesis. Int. J. Mol. Sci. 2017, 18, 1033. [Google Scholar] [CrossRef]
- Jiang, P.; Zheng, C.; Xiang, Y.; Malik, S.; Su, D.; Xu, G.; Zhang, M. The involvement of TH17 cells in the pathogenesis of IBD. Cytokine Growth Factor Rev. 2022. [Google Scholar] [CrossRef]
- Chiu, R.; Boyle, W.J.; Meek, J.; Smeal, T.; Hunter, T.; Karin, M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell 1988, 54, 541–552. [Google Scholar] [CrossRef]
- McGeachy, M.J.; Cua, D.J.; Gaffen, S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity 2019, 50, 892–906. [Google Scholar] [CrossRef]
- Dinarello, C.A. Immunological and Inflammatory Functions of the Interleukin-1 Family. Annu. Rev. Immunol. 2009, 27, 519–550. [Google Scholar] [CrossRef]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Shi, X.; Chen, X.; Wu, H.; Zhang, H.; Xie, J.; Yang, X.; Gou, Z.; Ye, J. 17-β-estradiol inhibits hyperosmolarity-induced proinflammatory cytokine elevation via the p38 MAPK pathway in human corneal epithelial cells. Mol. Vis. 2012, 18, 1115–1122. [Google Scholar]
- Li, D.-Q.; Luo, L.; Chen, Z.; Kim, H.-S.; Song, X.J.; Pflugfelder, S.C. JNK and ERK MAP kinases mediate induction of IL-1β, TNF-α and IL-8 following hyperosmolar stress in human limbal epithelial cells. Exp. Eye Res. 2006, 82, 588–596. [Google Scholar] [CrossRef]
- Choi, K.; Cope, W.G.; Harms, C.A.; Law, J.M. Rapid decreases in salinity, but not increases, lead to immune dysregulation in Nile tilapia, Oreochromis niloticus (L.). J. Fish Dis. 2013, 36, 389–399. [Google Scholar] [CrossRef]
- Lin, G.; Li, S.; Huang, J.; Gao, D.; Lu, J. Hypoosmotic stress induced functional alternations of intestinal barrier integrity, inflammatory reactions, and neurotransmission along gut-brain axis in the yellowfin seabream (Acanthopagrus latus). Fish Physiol. Biochem. 2021, 47, 1725–1738. [Google Scholar] [CrossRef]
- Su, M.; Zhang, R.; Liu, N.; Zhang, J. Modulation of inflammatory response by cortisol in the kidney of spotted scat (Scatophagus argus) in vitro under different osmotic stresses. Fish Shellfish Immunol. 2020, 104, 46–54. [Google Scholar] [CrossRef]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key Players in Innate and Adaptive Immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef]
- Akula, S.; Mohammadamin, S.; Hellman, L. Fc receptors for immunoglobulins and their appearance during vertebrate evolution. PLoS ONE 2014, 9, e96903. [Google Scholar] [CrossRef]
- Sutton, B.J.; Davies, A.M. Structure and dynamics of IgE-receptor interactions: FcεRI and CD23/FcεRII. Immunol. Rev. 2015, 268, 222–235. [Google Scholar] [CrossRef]
- Engeroff, P.; Vogel, M. The role of CD23 in the regulation of allergic responses. Allergy 2021, 76, 1981–1989. [Google Scholar] [CrossRef]
- Warr, G.W.; Magor, K.E.; Higgins, D.A. IgY: Clues to the origins of modern antibodies. Immunol. Today 1995, 16, 392–398. [Google Scholar] [CrossRef]
- Da’as, S.; Teh, E.M.; Dobson, J.T.; Nasrallah, G.K.; McBride, E.R.; Wang, H.; Neuberg, D.S.; Marshall, J.S.; Lin, T.J.; Berman, J.N. Zebrafish mast cells possess an FcɛRI-like receptor and participate in innate and adaptive immune responses. Dev. Comp. Immunol. 2011, 35, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Mehlhop, P.D.; van de Rijn, M.; Goldberg, A.B.; Brewer, J.P.; Kurup, V.P.; Martin, T.R.; Oettgen, H.C. Allergen-induced bronchial hyperreactivity and eosinophilic inflammation occur in the absence of IgE in a mouse model of asthma. Proc. Natl. Acad. Sci. USA 1997, 94, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Katz, H.R.; Raizman, M.B.; Gartner, C.S.; Scott, H.C.; Benson, A.C.; Austen, K.F. Secretory granule mediator release and generation of oxidative metabolites of arachidonic acid via Fc-IgG receptor bridging in mouse mast cells. J. Immunol. Baltim. Md. 1950 1992, 148, 868–871. [Google Scholar]
- Kong, X.; Wang, L.; Pei, C.; Zhang, J.; Zhao, X.; Li, L. Comparison of polymeric immunoglobulin receptor between fish and mammals. Vet. Immunol. Immunopathol. 2018, 202, 63–69. [Google Scholar] [CrossRef]
- Xu, G.; Zhan, W.; Ding, B.; Sheng, X. Molecular cloning and expression analysis of polymeric immunoglobulin receptor in flounder (Paralichthys olivaceus). Fish Shellfish Immunol. 2013, 35, 653–660. [Google Scholar] [CrossRef]
- Banerjee, B.; Bhuyan, G.; Saha, N. Influence of environmental hypertonicity on the induction of ureogenesis and amino acid metabolism in air-breathing walking catfish (Clarias batrachus, Bloch). Indian J. Exp. Biol. 2014, 52, 728–738. [Google Scholar]
- Jiang, W.; Tian, X.; Fang, Z.; Li, L.; Dong, S.; Li, H.; Zhao, K. Metabolic responses in the gills of tongue sole (Cynoglossus semilaevis) exposed to salinity stress using NMR-based metabolomics. Sci. Total Environ. 2019, 653, 465–474. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, J.; Zhu, L.; Chen, A.; Cheng, Y. Label-free quantification proteomics analysis reveals acute hyper-osmotic responsive proteins in the gills of Chinese mitten crab (Eriocheir sinensis). Comp. Biochem. Physiol. Part D Genom. Proteom. 2022, 43, 101009. [Google Scholar] [CrossRef]
- Liu, B.; Gao, Q.; Liu, B.; Sun, C.; Song, C.; Liu, M.; Zhou, Q.; Zheng, X.; Liu, X. Response of microbiota and immune function to different hypotonic stress levels in giant freshwater prawn Macrobrachium rosenbergii post-larvae. Sci. Total Environ. 2022, 844, 157258. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, J.; Lao, H.; Yin, Z.; He, W.; Weng, S.; Yu, X.; Chan, S.; He, J. Molecular cloning and expression analysis of the ASC gene from mandarin fish and its regulation of NF-kappaB activation. Dev. Comp. Immunol. 2008, 32, 391–399. [Google Scholar] [CrossRef]
- Zhao, H.; Zhang, X.; Cheng, N.; Duan, J.; Wang, J.; Nagahama, Y.; Zhong, X.; Zhou, Q.; Wang, Y. Identification and expression profiles of prdm1 in medaka Oryzias latipes. Mol. Biol. Rep. 2014, 41, 617–626. [Google Scholar] [CrossRef]
- Nibona, E.; Xu, G.; Wu, K.; Shen, H.; Zhang, R.; Ke, X.; Al Hafiz, A.; Wang, Z.; Qi, C.; Zhao, H. Identification, characterization, expression profiles of OlHavcr2 in medaka (Oryzias latipes). Gen. Comp. Endocrinol. 2019, 277, 30–37. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Futschik, M.E. Mfuzz: A software package for soft clustering of microarray data. Bioinformation 2007, 2, 5–7. [Google Scholar] [CrossRef] [PubMed]
- Audic, S.; Claverie, J.M. The significance of digital gene expression profiles. Genome Res. 1997, 7, 986–995. [Google Scholar] [CrossRef]
- Lau, M.C.; Kwong, E.M.; Lai, K.P.; Li, J.W.; Ho, J.C.; Chan, T.F.; Wong, C.K.; Jiang, Y.J.; Tse, W.K. Pathogenesis of POLR1C-dependent Type 3 Treacher Collins Syndrome revealed by a zebrafish model. Biochim. Et. Biophys. Acta 2016, 1862, 1147–1158. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).