Alterations of the Mucosal Immune Response and Microbial Community of the Skin upon Viral Infection in Rainbow Trout (Oncorhynchus mykiss)
Abstract
1. Introduction
2. Results
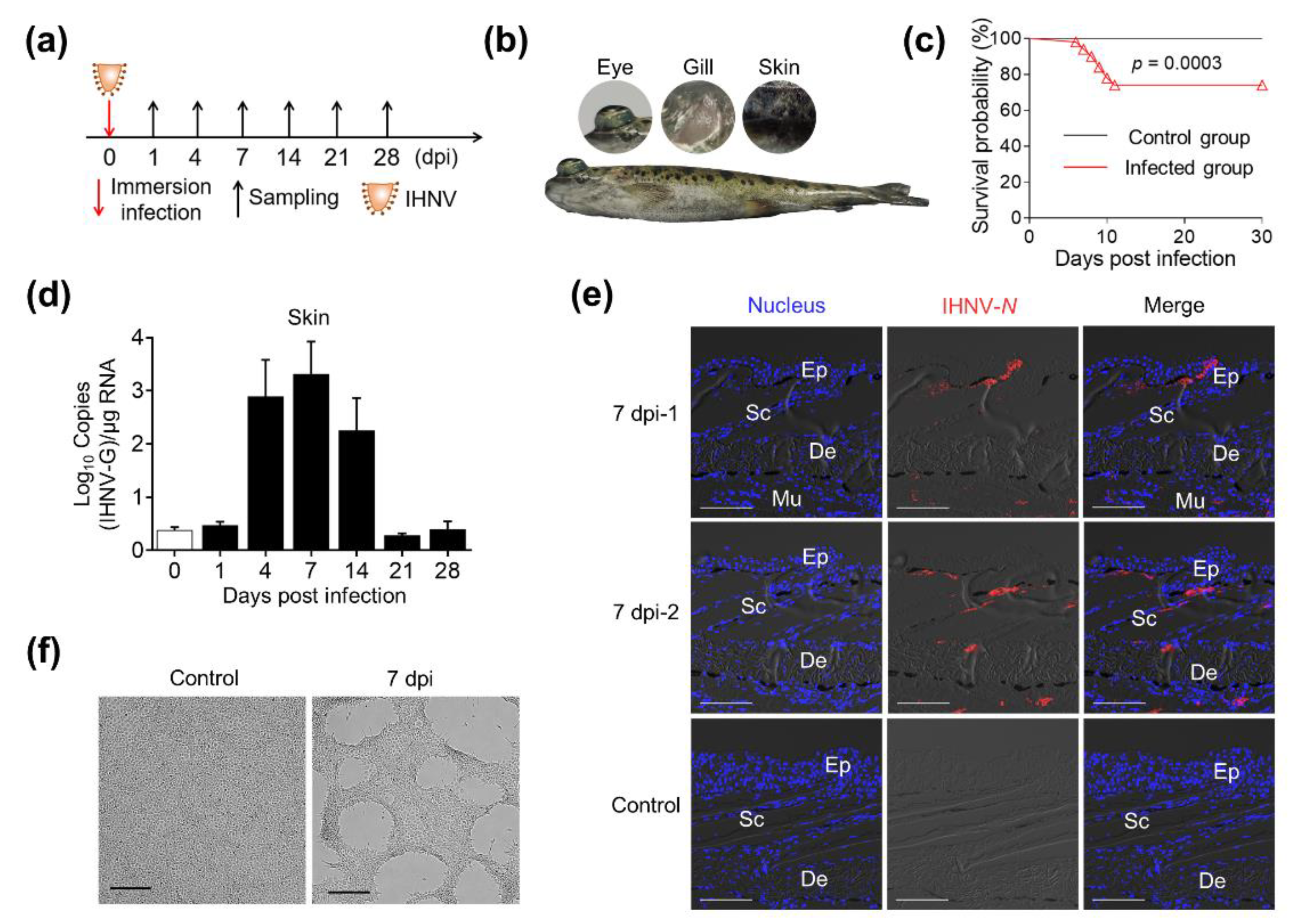
2.1. IHNV Successfully Invaded the Skin of Rainbow Trout
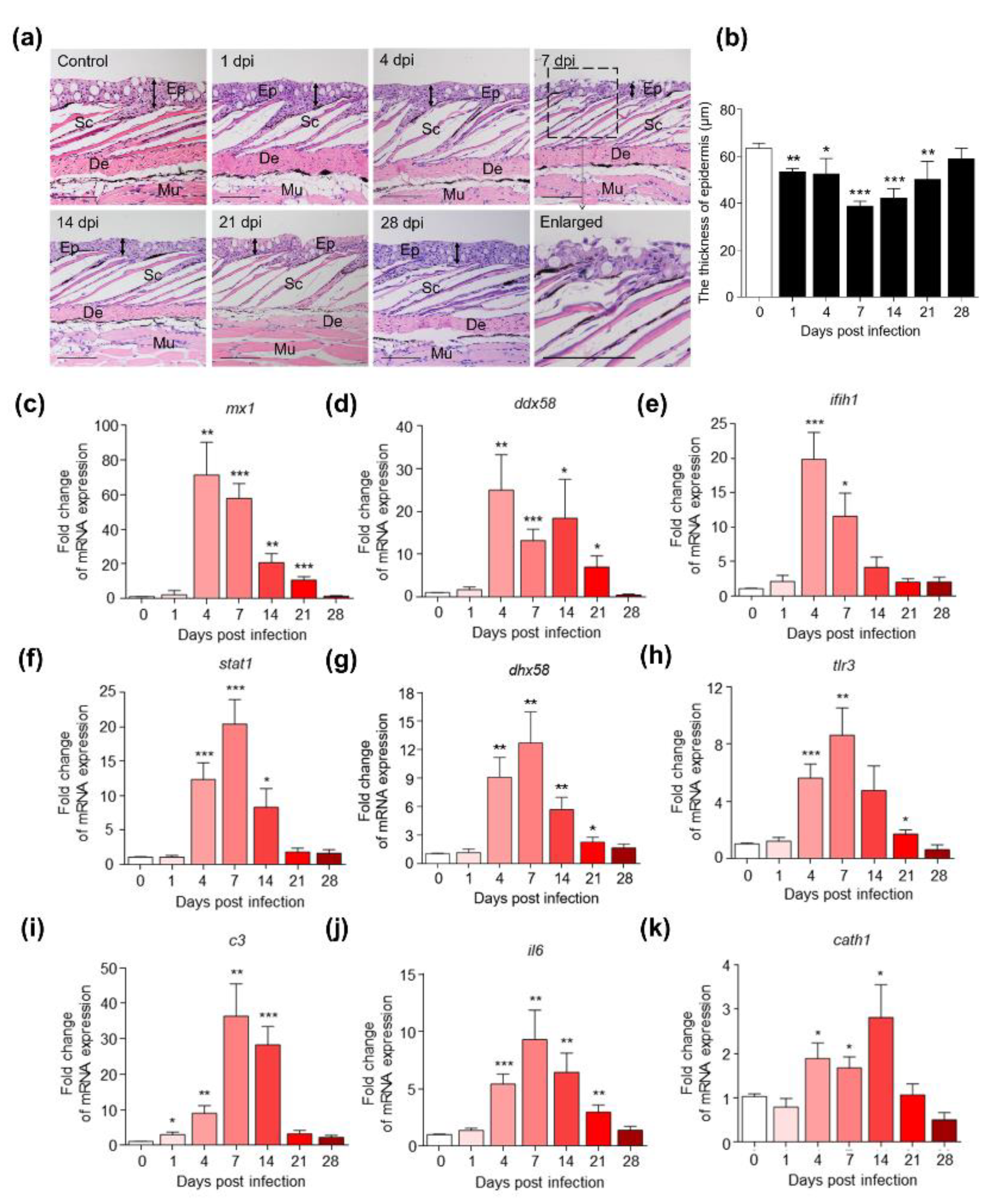
2.2. IHNV Infection Caused Significant Histopathological Changes and Strong Innate Immune Responses in Rainbow Trout Skin
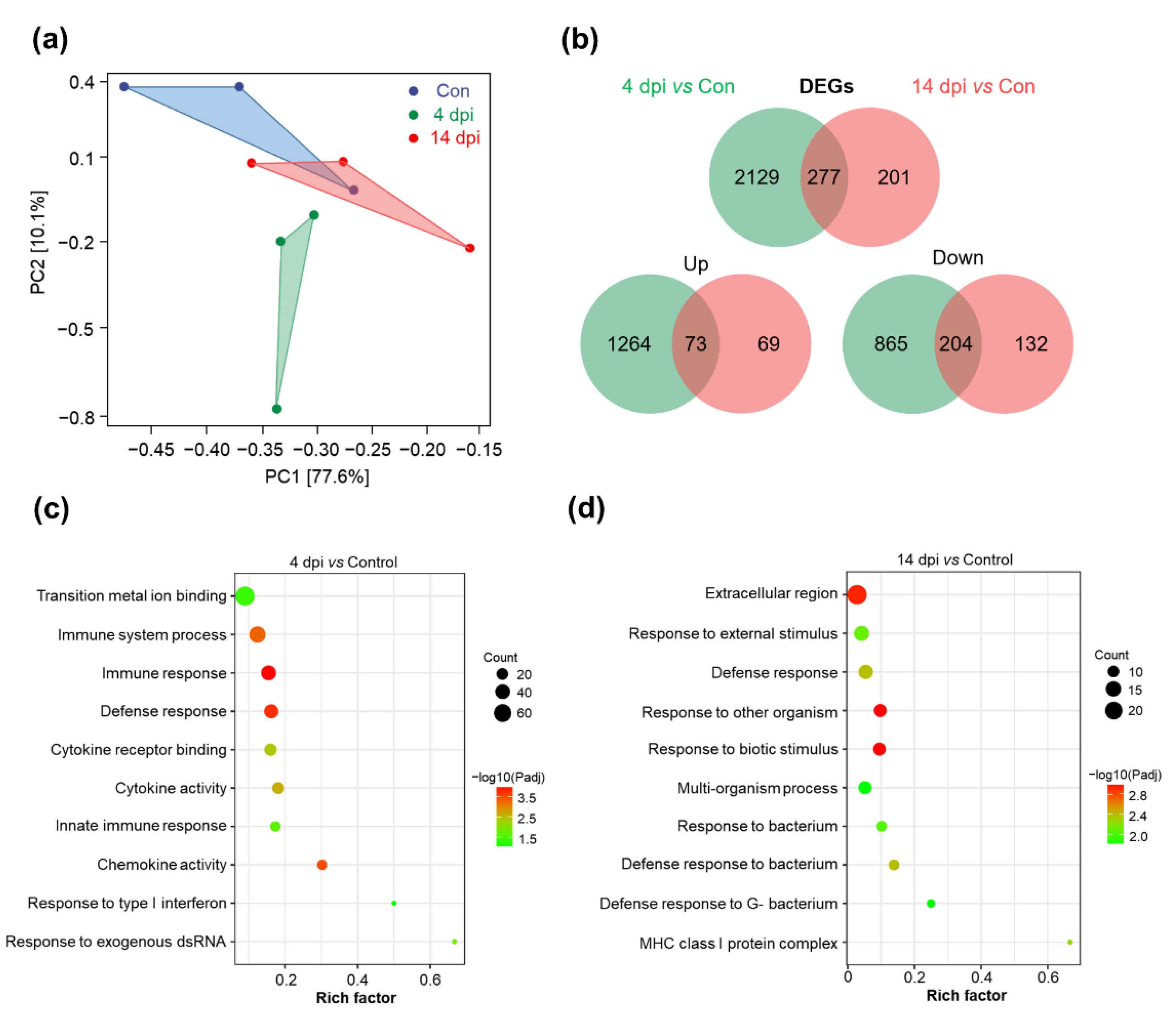
2.3. Transcriptome Profiling in the Skin of Rainbow Trout after Infection with IHNV
2.4. KEGG Pathway and GO Enrichment Analysis of DEGs
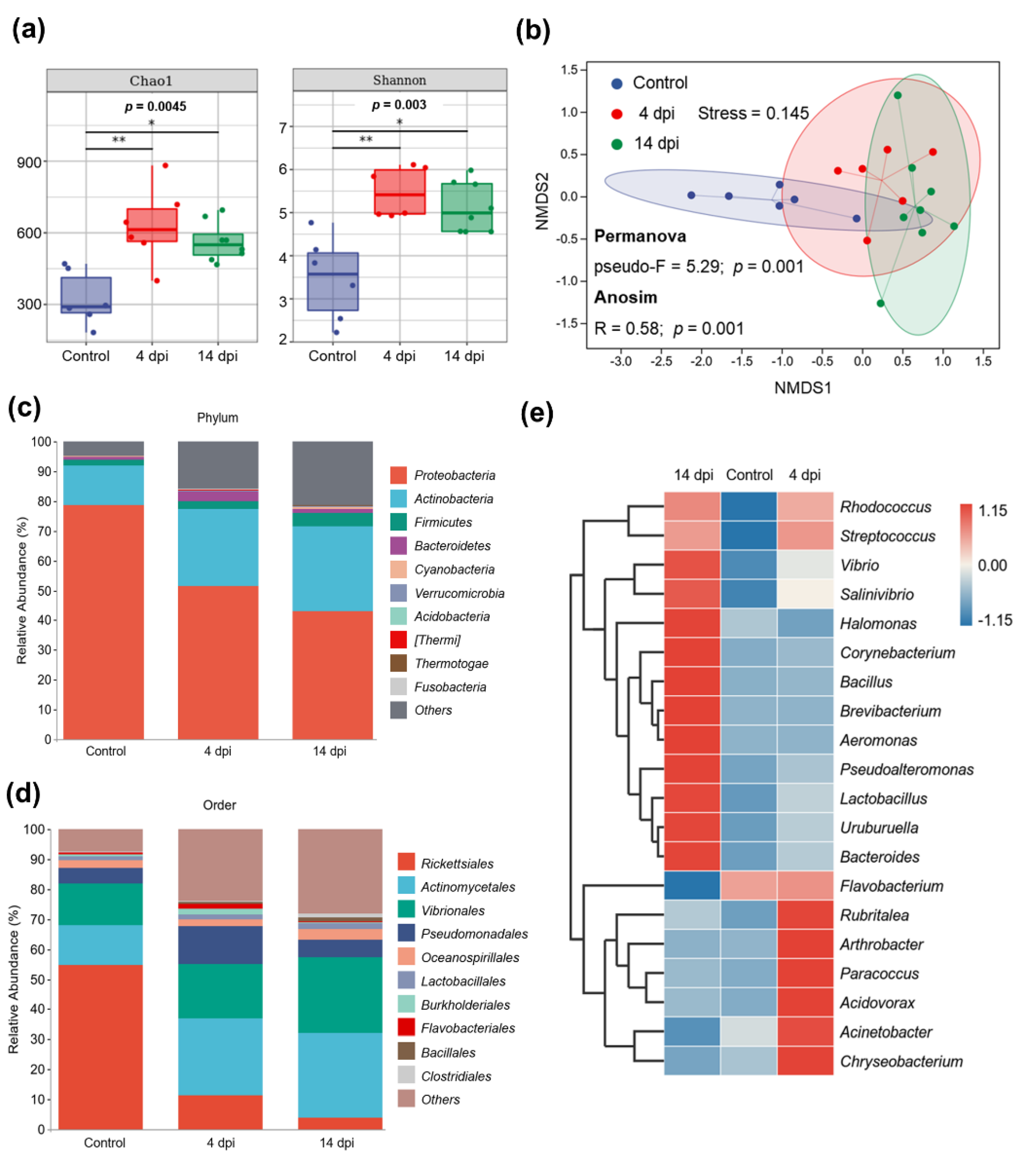
2.5. IHNV Infection Caused Changes in the Composition of Trout Skin Microbial Community
2.6. The Differentially Abundant Taxa Enriched in Skin Microbiota in 4 dpi Group and 14 dpi Group after IHNV Infection
3. Discussion
4. Materials and Methods
4.1. Fish Maintenance
4.2. Cell Culture and IHNV Enrichment
4.3. Sampling and Infection
4.4. Histology, Light Microscopy, and Immunofluorescence Microscopy Studies
4.5. RNA Extraction, cDNA Synthesis and RT-qPCR Analysis
4.6. Plaque Assay of IHNV
4.7. RNA-Seq Library Construction and Transcriptome Analysis
4.8. DNA Amplification, 16S rRNA Sequencing and Bioinformatics Analysis
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ross, A.A.; Hoffmann, A.R.; Neufeld, J.D. The skin microbiome of vertebrates. Microbiome 2019, 7, 1–14. [Google Scholar] [CrossRef]
- Xu, Z.; Parra, D.; Gómez, D.; Salinas, I.; Zhang, Y.-A.; Von Gersdorff Jørgensen, L.; Heinecke, R.D.; Buchmann, K.; LaPatra, S.; Oriol Sunyer, J. Teleost skin, an ancient mucosal surface that elicits gut-like immune responses. Proc. Natl. Acad. Sci. USA 2013, 110, 13097–13102. [Google Scholar] [CrossRef]
- Salinas, I.; Zhang, Y.-A.; Sunyer, J.O. Mucosal immunoglobulins and B cells of teleost fish. Dev. Comp. Immunol. 2011, 35, 1346–1365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, L.; Yu, Y.; Kong, W.; Yin, Y.; Huang, Z.; Zhang, X.; Xu, Z. The Change of Teleost Skin Commensal Microbiota Is Associated With Skin Mucosal Transcriptomic Responses During Parasitic Infection by Ichthyophthirius multifillis. Front. Immunol. 2018, 9, 2972. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-T.; Yu, Y.-Y.; Xu, H.-Y.; Huang, Z.-Y.; Liu, X.; Cao, J.-F.; Meng, K.-F.; Wu, Z.-B.; Han, G.-K.; Zhan, M.-T.; et al. Prevailing Role of Mucosal Igs and B Cells in Teleost Skin Immune Responses to Bacterial Infection. J. Immunol. 2021, 206, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Doane, M.P.; Morris, M.M.; Papudeshi, B.; Allen, L.; Pande, D.; Haggerty, J.M.; Johri, S.; Turnlund, A.C.; Peterson, M.; Kacev, D.; et al. The skin microbiome of elasmobranchs follows phylosymbiosis, but in teleost fishes, the microbiomes converge. Microbiome 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Hooper, L.V.; Littman, D.R.; MacPherson, A.J. Interactions Between the Microbiota and the Immune System. Science 2012, 336, 1268–1273. [Google Scholar] [CrossRef]
- Koskella, B.; Hall, L.J.; Metcalf, C.J.E. The microbiome beyond the horizon of ecological and evolutionary theory. Nat. Ecol. Evol. 2017, 1, 1606–1615. [Google Scholar] [CrossRef]
- Robinson, C.J.; Bohannan, B.J.M.; Young, V.B. From Structure to Function: The Ecology of Host-Associated Microbial Communities. Microbiol. Mol. Biol. Rev. 2010, 74, 453–476. [Google Scholar] [CrossRef]
- Schaupp, L.; Muth, S.; Rogell, L.; Kofoed-Branzk, M.; Melchior, F.; Lienenklaus, S.; Ganal-Vonarburg, S.C.; Klein, M.; Guendel, F.; Hain, T.; et al. Microbiota-Induced Type I Interferons Instruct a Poised Basal State of Dendritic Cells. Cell 2020, 181, 1080–1096.e19. [Google Scholar] [CrossRef]
- Stressmann, F.A.; Bernal-Bayard, J.; Perez-Pascual, D.; Audrain, B.; Rendueles, O.; Briolat, V.; Bruchmann, S.; Volant, S.; Ghozlane, A.; Häussler, S.; et al. Mining zebrafish microbiota reveals key community-level resistance against fish pathogen infection. ISME J. 2020, 15, 702–719. [Google Scholar] [CrossRef] [PubMed]
- Hacquard, S.; Garrido-Oter, R.; González, A.; Spaepen, S.; Ackermann, G.; Lebeis, S.; McHardy, A.C.; Dangl, J.L.; Knight, R.; Ley, R.; et al. Microbiota and Host Nutrition across Plant and Animal Kingdoms. Cell Host Microbe 2015, 17, 603–616. [Google Scholar] [CrossRef]
- Kelly, C.; Salinas, I. Under Pressure: Interactions between Commensal Microbiota and the Teleost Immune System. Front. Immunol. 2017, 8, 559. [Google Scholar] [CrossRef] [PubMed]
- Grier, A.; Gill, A.L.; A Kessler, H.; Corbett, A.; Bandyopadhyay, S.; Java, J.; Holden-Wiltse, J.; Falsey, A.R.; Topham, D.J.; Mariani, T.J.; et al. Temporal Dysbiosis of Infant Nasal Microbiota Relative to Respiratory Syncytial Virus Infection. J. Infect. Dis. 2020, 223, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Mazel-Sanchez, B.; Yildiz, S.; Schmolke, M. Ménage à trois: Virus, Host, and Microbiota in Experimental Infection Models. Trends Microbiol. 2019, 27, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Naik, S.; Bouladoux, N.; Wilhelm, C.; Molloy, M.J.; Salcedo, R.; Kastenmuller, W.; Deming, C.; Quinones, M.; Koo, L.; Conlan, S.; et al. Compartmentalized Control of Skin Immunity by Resident Commensals. Science 2012, 337, 1115–1119. [Google Scholar] [CrossRef]
- Nakamura, Y.; Oscherwitz, J.; Cease, K.B.; Chan, S.M.; Muñoz-Planillo, R.; Hasegawa, M.; Villaruz, A.E.; Cheung, G.Y.C.; McGavin, M.J.; Travers, J.B.; et al. Staphylococcus δ-toxin induces allergic skin disease by activating mast cells. Nature 2013, 503, 397–401. [Google Scholar] [CrossRef]
- Gimblet, C.; Meisel, J.S.; Loesche, M.A.; Cole, S.D.; Horwinski, J.; Novais, F.O.; Misic, A.M.; Bradley, C.W.; Beiting, D.P.; Rankin, S.C.; et al. Cutaneous Leishmaniasis Induces a Transmissible Dysbiotic Skin Microbiota that Promotes Skin Inflammation. Cell Host Microbe 2017, 22, 13–24.e4. [Google Scholar] [CrossRef]
- Cámara-Ruiz, M.; Cerezo, I.; Guardiola, F.; García-Beltrán, J.; Balebona, M.; Moriñigo, M.; Esteban, M. Alteration of the Immune Response and the Microbiota of the Skin during a Natural Infection by Vibrio harveyi in European Seabass (Dicentrarchus labrax). Microorganisms 2021, 9, 964. [Google Scholar] [CrossRef]
- Reid, K.M.; Patel, S.; Robinson, A.J.; Bu, L.; Jarungsriapisit, J.; Moore, L.J.; Salinas, I. Salmonid alphavirus infection causes skin dysbiosis in Atlantic salmon (Salmo salar L.) post-smolts. PLoS ONE 2017, 12, e0172856. [Google Scholar] [CrossRef]
- Ali, A.; Rexroad, C.E.; Thorgaard, G.H.; Yao, J.; Salem, M. Characterization of the rainbow trout spleen transcriptome and identification of immune-related genes. Front. Genet. 2014, 5, 348. [Google Scholar] [CrossRef] [PubMed]
- Thorgaard, G.H.; Bailey, G.S.; Williams, D.; Buhler, D.R.; Kaattari, S.L.; Ristow, S.S.; Hansen, J.D.; Winton, J.R.; Bartholomew, J.L.; Nagler, J.J.; et al. Status and opportunities for genomics research with rainbow trout. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 133, 609–646. [Google Scholar] [CrossRef]
- Segner, H.; Sundh, H.; Buchmann, K.; Douxfils, J.; Sundell, K.S.; Mathieu, C.; Ruane, N.; Jutfelt, F.; Toften, H.; Vaughan, L. Health of farmed fish: Its relation to fish welfare and its utility as welfare indicator. Fish Physiol. Biochem. 2012, 38, 85–105. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Soltani, M.; Mardani, K.; Shokrpoor, S.; Hassanzadeh, R.; Ahmadpoor, M.; Rahmati-Holasoo, H.; Meshkini, S. Infectious hematopoietic necrosis virus (IHNV) outbreak in farmed rainbow trout in Iran: Viral isolation, pathological findings, molecular confirmation, and genetic analysis. Virus Res. 2017, 229, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Abbadi, M.; Fusaro, A.; Ceolin, C.; Casarotto, C.; Quartesan, R.; Pozza, M.D.; Cattoli, G.; Toffan, A.; Holmes, E.C.; Panzarin, V. Molecular Evolution and Phylogeography of Co-circulating IHNV and VHSV in Italy. Front. Microbiol. 2016, 7, 1306. [Google Scholar] [CrossRef]
- Wang, C.; Lian, G.; Zhao, L.; Wu, Y.; Li, Y.; Tang, L.; Qiao, X.; Jiang, Y.; Liu, M. Virulence and serological studies of recombinant infectious hematopoietic necrosis virus (IHNV) in rainbow trout. Virus Res. 2016, 220, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Johansson, T.; Einer-Jensen, K.; Batts, W.; Ahrens, P.; Björkblom, C.; Kurath, G.; Bjorklund, H.; Lorenzen, N. Genetic and serological typing of European infectious haematopoietic necrosis virus (IHNV) isolates. Dis. Aquat. Org. 2009, 86, 213–221. [Google Scholar] [CrossRef]
- Tacchi, L.; Musharrafieh, R.; Larragoite, E.T.; Crossey, K.; Erhardt, E.B.; Martin, S.; LaPatra, S.E.; Salinas, I. Nasal immunity is an ancient arm of the mucosal immune system of vertebrates. Nat. Commun. 2014, 5, 1–11. [Google Scholar] [CrossRef]
- Dixon, P.; Paley, R.; Alegria-Moran, R.; Oidtmann, B. Epidemiological characteristics of infectious hematopoietic necrosis virus (IHNV): A review. Vet. Res. 2016, 47, 1–26. [Google Scholar] [CrossRef]
- Dong, S.; Ding, L.-G.; Cao, J.-F.; Liu, X.; Xu, H.-Y.; Meng, K.-F.; Yu, Y.-Y.; Wang, Q.; Xu, Z. Viral-Infected Change of the Digestive Tract Microbiota Associated With Mucosal Immunity in Teleost Fish. Front. Immunol. 2019, 10, 2878. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Z.; Kong, W.; Dong, F.; Zhang, X.; Zhai, X.; Cheng, G.; Zhan, M.; Cao, J.; Ding, L.; et al. Teleost swim bladder, an ancient air-filled organ that elicits mucosal immune responses. Cell Discov. 2022, 8, 1–18. [Google Scholar] [CrossRef]
- Vendramin, N.; Alencar, A.L.F.; Iburg, T.M.; Dahle, M.K.; Wessel, Ø.; Olsen, A.B.; Rimstad, E.; Olesen, N.J. Piscine orthoreovirus infection in Atlantic salmon (Salmo salar) protects against subsequent challenge with infectious hematopoietic necrosis virus (IHNV). Vet. Res. 2018, 49, 1–12. [Google Scholar] [CrossRef]
- Velazquez-Salinas, L.; Verdugo-Rodriguez, A.; Rodriguez, L.L.; Borca, M.V. The Role of Interleukin 6 During Viral Infections. Front. Microbiol. 2019, 10, 1057. [Google Scholar] [CrossRef]
- Furlan, M.; Rosani, U.; Gambato, S.; Irato, P.; Manfrin, A.; Mardirossian, M.; Venier, P.; Pallavicini, A.; Scocchi, M. Induced expression of cathelicidins in trout (Oncorhynchus mykiss) challenged with four different bacterial pathogens. J. Pept. Sci. 2018, 24, e3089. [Google Scholar] [CrossRef]
- Wu, Z.-B.; Meng, K.-F.; Ding, L.-G.; Wu, S.; Han, G.-K.; Zhai, X.; Sun, R.-H.; Yu, Y.-Y.; Ji, W.; Xu, Z. Dynamic Interaction Between Mucosal Immunity and Microbiota Drives Nose and Pharynx Homeostasis of Common Carp (Cyprinus carpio) after SVCV Infection. Front. Immunol. 2021, 12, 769775. [Google Scholar] [CrossRef]
- Liao, Z.; Su, J. Progresses on three pattern recognition receptor families (TLRs, RLRs and NLRs) in teleost. Dev. Comp. Immunol. 2021, 122, 104131. [Google Scholar] [CrossRef]
- Morimoto, N.; Kono, T.; Sakai, M.; Hikima, J.-I. Inflammasomes in Teleosts: Structures and Mechanisms That Induce Pyroptosis during Bacterial Infection. Int. J. Mol. Sci. 2021, 22, 4389. [Google Scholar] [CrossRef]
- Maier, V.H.; Dorn, K.V.; Gudmundsdottir, B.K.; Gudmundsson, G.H. Characterisation of cathelicidin gene family members in divergent fish species. Mol. Immunol. 2008, 45, 3723–3730. [Google Scholar] [CrossRef]
- Xie, J.; Obiefuna, V.; Hodgkinson, J.W.; McAllister, M.; Belosevic, M. Teleost antimicrobial peptide hepcidin contributes to host defense of goldfish (Carassius auratus L.) against Trypanosoma carassii. Dev. Comp. Immunol. 2019, 94, 11–15. [Google Scholar] [CrossRef]
- Sha, Z.-X.; Wang, Q.-L.; Liu, Y.; Chen, S.-L. Identification and expression analysis of goose-type lysozyme in half-smooth tongue sole (Cynoglossus semilaevis). Fish Shellfish Immunol. 2012, 32, 914–921. [Google Scholar] [CrossRef]
- Zhou, C.; Lin, H.; Huang, Z.; Wang, J.; Wang, Y.; Yu, W. Cloning and expression analysis of c-type lysozyme gene in golden pompano, Trachinotus ovatus. Fish Shellfish Immunol. 2016, 54, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Raida, M.K.; Buchmann, K. Innate immune response in rainbow trout (Oncorhynchus mykiss) against primary and secondary infections with Yersinia ruckeri O1. Dev. Comp. Immunol. 2009, 33, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Shen, X.; Zheng, Y.; Guo, P.; Gu, Z.; Gao, Y.; Zhao, X.; Cheng, H.; Xu, J.; Chen, X.; et al. Identification, expression patterns, evolutionary characteristics and recombinant protein activities analysis of CD209 gene from Megalobrama amblycephala. Fish Shellfish Immunol. 2022, 126, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Sun, L. Tongue Sole CD209: A Pattern-Recognition Receptor that Binds a Broad Range of Microbes and Promotes Phagocytosis. Int. J. Mol. Sci. 2017, 18, 1848. [Google Scholar] [CrossRef]
- Larsen, A.; Tao, Z.; Bullard, S.A.; Arias, C.R. Diversity of the skin microbiota of fishes: Evidence for host species specificity. FEMS Microbiol. Ecol. 2013, 85, 483–494. [Google Scholar] [CrossRef]
- Sehnal, L.; Brammer-Robbins, E.; Wormington, A.M.; Blaha, L.; Bisesi, J.; Larkin, I.; Martyniuk, C.J.; Simonin, M.; Adamovsky, O. Microbiome Composition and Function in Aquatic Vertebrates: Small Organisms Making Big Impacts on Aquatic Animal Health. Front. Microbiol. 2021, 12, 567408. [Google Scholar] [CrossRef]
- Declercq, A.M.; Haesebrouck, F.; Van den Broeck, W.; Bossier, P.; Decostere, A. Columnaris disease in fish: A review with emphasis on bacterium-host interactions. Vet. Res. 2013, 44, 27. [Google Scholar] [CrossRef]
- Li, S.; Chai, J.; Knupp, C.; Nicolas, P.; Wang, D.; Cao, Y.; Deng, F.; Chen, F.; Lu, T.; Loch, T.P. Phenotypic and Genetic Characterization of Flavobacterium psychrophilum Recovered from Diseased Salmonids in China. Microbiol. Spectr. 2021, 9, e0033021. [Google Scholar] [CrossRef]
- Zamora, L.; I Vela, A.; Palacios, M.A.; Domínguez, L.; Fernández-Garayzábal, J.F. First isolation and characterization of Chryseobacterium shigense from rainbow trout. BMC Vet. Res. 2012, 8, 77. [Google Scholar] [CrossRef]
- Ilardi, P.; Fernández, J.; Avendaño-Herrera, R. Chryseobacterium piscicola sp. nov., isolated from diseased salmonid fish. Int. J. Syst. Evol. Microbiol. 2009, 59, 3001–3005. [Google Scholar] [CrossRef]
- Ilardi, P.; Abad, J.; Rintamäki, P.; Bernardet, J.F.; Avendaño-Herrera, R. Phenotypic, serological and molecular evidence of Chryseobacterium piscicola in farmed Atlantic salmon, Salmo salar L., in Finland. J. Fish Dis. 2010, 33, 179–181. [Google Scholar] [CrossRef]
- Ina-Salwany, M.Y.; Al-Saari, N.; Mohamad, A.; Mursidi, F.A.; Mohd-Aris, A.; Amal, M.N.A.; Kasai, H.; Mino, S.; Sawabe, T.; Zamri-Saad, M. Vibriosis in Fish: A Review on Disease Development and Prevention. J. Aquat. Anim. Health 2019, 31, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Li, X.; Xue, M.; Zhang, M.; Liu, W.; Fan, Y.; Chen, X.; Chu, Z.; Gong, F.; Zeng, L.; et al. Vibrio metschnikovii, a Potential Pathogen in Freshwater-Cultured Hybrid Sturgeon. Animals 2022, 12, 1101. [Google Scholar] [CrossRef] [PubMed]
- Olsen, A.; Birkbeck, T.; Nilsen, H.; MacPherson, H.; Wangel, C.; Myklebust, C.; Laidler, L.; Aarflot, L.; Thoen, E.; Nygard, S.; et al. Vaccine-associated systemic Rhodococcus erythropolis infection in farmed Atlantic salmon Salmo salar. Dis. Aquat. Org. 2006, 72, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Avendaño-Herrera, R.; Balboa, S.; Doce, A.; Ilardi, P.; Lovera, P.; Toranzo, A.E.; Romalde, J.L. Pseudo-membranes on internal organs associated with Rhodococcus qingshengii infection in Atlantic salmon (Salmo salar). Veter-Microbiol. 2011, 147, 200–204. [Google Scholar] [CrossRef]
- Speare, D.J.; Brocklebank, J.; Macnair, N.; Claveau, R.; Backman, S.; Ewan, E.P.; A Bernard, K. Eastern Canada. Granulomatous nephritis associated with Rhodococcus sp. infection in Atlantic salmon (Salmo salar). Can. Vet. J. 1992, 33, 192. [Google Scholar] [PubMed]
- Backman, S.; Ferguson, H.W.; Prescott, J.F.; Wilcock, B.P. Progressive Panophthalmitis in Chinook Salmon, Oncorhynchus-Tshawytscha (Walbaum)-a Case-Report. J. Fish Dis. 1990, 13, 345–353. [Google Scholar] [CrossRef]
- Algammal, A.M.; Mabrok, M.; Sivaramasamy, E.; Youssef, F.M.; Atwa, M.H.; El-Kholy, A.W.; Hetta, H.F.; Hozzein, W.N. Emerging MDR-Pseudomonas aeruginosa in fish commonly harbor oprL and toxA virulence genes and bla(TEM), bla(CTX-M), and tetA antibiotic-resistance genes. Sci. Rep. 2020, 10, 15961. [Google Scholar] [CrossRef]
- Avadhanula, V.; Rodriguez, C.A.; DeVincenzo, J.P.; Wang, Y.; Webby, R.J.; Ulett, G.C.; Adderson, E.E. Respiratory Viruses Augment the Adhesion of Bacterial Pathogens to Respiratory Epithelium in a Viral Species- and Cell Type-Dependent Manner. J. Virol. 2006, 80, 1629–1636. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a Chimera-Checked 16S rRNA Gene Database and Workbench Compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- White, J.R.; Nagarajan, N.; Pop, M. Statistical Methods for Detecting Differentially Abundant Features in Clinical Metagenomic Samples. PLoS Comput. Biol. 2009, 5, e1000352. [Google Scholar] [CrossRef] [PubMed]

| Gene Name | Accession Number | Description | 4 dpi vs. Control | 14 dpi vs. Control | ||||
|---|---|---|---|---|---|---|---|---|
| Fpkm (vs. Control) | FC | p (adj) | Fpkm (vs. Control) | FC | p (adj) | |||
| hamp | XM_021595153.2 | hepcidin | 30.77 ± 5.72 | 42.18 | 9.01 × 10−5 | 690.8 ± 379.0 | 717.01 | 0.031 |
| saa5 | XM_021586448.2 | serum amyloid A-5 protein | 44.86 ± 17.66 | 53.91 | 6.88 × 10−4 | 470.7 ± 262.7 | 468.26 | 0.038 |
| cd209 | NM_001124633.1 | CD209 molecule | 11.72 ± 2.31 | 14.82 | 2.90 × 10−3 | 88.64 ± 27.78 | 88.42 | 2.38 × 10−4 |
| cath | NM_001124463.1 | cathelicidin antimicrobial peptide | 4.27 ± 0.81 | 5.15 | 3.79 × 10−5 | 14.37 ± 4.37 | 13.20 | 0.002 |
| lyz2 | NM_001124716.1 | lysozyme II | 4.81 ± 2.63 | 5.69 | 0.343 ns | 15.51 ± 2.96 | 15.38 | 3.73 × 10−5 |
| vig1 | NM_001124253.1 | viperin | 345.20 ± 39.88 | 430.66 | 1.47 × 10−43 | 85.26 ± 65.47 | 85.83 | 0.366 ns |
| mx2 | NM_001124751.1 | interferon-induced GTP-binding protein Mx2 | 83.21 ± 13.71 | 101.76 | 2.55 × 10−31 | 14.58 ± 6.55 | 14.27 | 0.208 ns |
| trim39 | XM_021573556.2 | E3 ubiquitin-protein ligase TRIM39-like | 74.67 ± 1.67 | 96.74 | 1.21 × 10−15 | 20.68 ± 5.72 | 20.90 | 5.14 × 10−5 |
| ifnar2 | XM_021578985.2 | interferon alpha/beta receptor 2-like | 62.86 ± 11.18 | 76.09 | 5.39 × 10−28 | 22.2 ± 9.6 | 21.53 | 0.111 ns |
| irf7 | XM_021600499.2 | interferon regulatory factor 7 | 56.75 ± 12.38 | 71.40 | 2.89 × 10−19 | 24.63 ± 12.14 | 24.91 | 0.190 ns |
| irf8 | XM_036956322.1 | interferon regulatory factor 8 | 37.54 ± 2.30 | 48.23 | 6.93 × 10−24 | 8.7 ± 4.88 | 8.80 | 0.464 ns |
| gig2e | XM_036944105.1 | grass carp reovirus (GCRV)-induced gene 2e | 40.28 ± 15.07 | 46.59 | 2.25 × 10−4 | 11.12 ± 4.62 | 10.58 | 0.205 ns |
| ddx58 | XM_036973405.1 | DEAD (Asp-Glu-Ala-Asp) box polypeptide 58 | 25.37 ± 0.80 | 31.83 | 7.00 × 10−21 | 7.67 ± 3.25 | 7.51 | 0.289 ns |
| stat1b | XM_021579196.2 | signal transducer and activator of transcription 1b | 12.61 ± 0.39 | 16.13 | 1.34 × 10−14 | 3.81 ± 1.05 | 3.75 | 0.207 ns |
| dhx58 | XM_036938249.1 | DEXH (Asp-Glu-X-His) box polypeptide 58 | 10.06 ± 1.49 | 13.00 | 1.76 × 10−5 | 2.69 ± 1.53 | 2.66 | 0.761 ns |
| ccl19a.1 | XM_021605155.2 | C-C motif chemokine 19a.1 | 174.20 ± 47.88 | 221.68 | 5.49 × 10−14 | 182.3 ± 135.1 | 188.58 | 0.274 ns |
| tlr3 | NM_001124578.1 | toll-like receptor 3 | 6.45 ± 0.82 | 8.08 | 7.58 × 10−9 | 3.7 ± 0.71 | 3.62 | 0.039 |
| il4i1 | XM_036961927.1 | interleukin 4 induced 1 | 56.01 ± 7.00 | 69.92 | 9.62 × 10−27 | 23.91 ± 14.47 | 23.75 | 0.348 ns |
| c1q1 | XM_021624859.2 | complement C1q-like protein 2 | 12.45 ± 4.76 | 15.30 | 0.004 | 137.5 ± 58.09 | 135.74 | 0.008 |
| c4b | NM_001124385.1 | complement C4-B precursor | 44.67 ± 21.22 | 56.24 | 0.011 | 31.71 ± 11.96 | 29.90 | 0.018 |
| Category | Pathway Terms | Number of DEGs | p (adj) | ||
|---|---|---|---|---|---|
| Up | Down | Total | |||
| Immune system | NOD-like receptor signaling pathway | 38 | 6 | 44 | 0.01590 |
| C-type lectin receptor signaling pathway | 17 | 14 | 31 | 0.01590 | |
| Toll-like receptor signaling pathway | 21 | 8 | 29 | 0.03047 | |
| Signaling molecules and interaction | Cytokine-cytokine receptor interaction | 44 | 15 | 59 | 0.00128 |
| ECM-receptor interaction | 2 | 25 | 27 | 0.00506 | |
| Cellular community | Focal adhesion | 8 | 43 | 51 | 0.03047 |
| Cell growth and death | Necroptosis | 40 | 1 | 41 | 0.02875 |
| Folding, sorting and degradation | Protein processing in endoplasmic reticulum | 28 | 11 | 39 | 0.02728 |
| Proteasome | 34 | 0 | 34 | 6.43 × 10−14 | |
| Energy metabolism | Oxidative phosphorylation | 35 | 0 | 35 | 0.00018 |
| Category | Pathway Terms | Number of DEGs | p (adj) | ||
|---|---|---|---|---|---|
| Up | Down | Total | |||
| Signaling molecules and interaction | ECM-receptor interaction | 1 | 17 | 18 | 2.72 × 10−10 |
| Cellular community | Focal adhesion | 0 | 23 | 23 | 3.01 × 10−7 |
| Folding, sorting and degradation | Proteasome | 7 | 0 | 7 | 0.00298 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhan, M.; Huang, Z.; Cheng, G.; Yu, Y.; Su, J.; Xu, Z. Alterations of the Mucosal Immune Response and Microbial Community of the Skin upon Viral Infection in Rainbow Trout (Oncorhynchus mykiss). Int. J. Mol. Sci. 2022, 23, 14037. https://doi.org/10.3390/ijms232214037
Zhan M, Huang Z, Cheng G, Yu Y, Su J, Xu Z. Alterations of the Mucosal Immune Response and Microbial Community of the Skin upon Viral Infection in Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences. 2022; 23(22):14037. https://doi.org/10.3390/ijms232214037
Chicago/Turabian StyleZhan, Mengting, Zhenyu Huang, Gaofeng Cheng, Yongyao Yu, Jianguo Su, and Zhen Xu. 2022. "Alterations of the Mucosal Immune Response and Microbial Community of the Skin upon Viral Infection in Rainbow Trout (Oncorhynchus mykiss)" International Journal of Molecular Sciences 23, no. 22: 14037. https://doi.org/10.3390/ijms232214037
APA StyleZhan, M., Huang, Z., Cheng, G., Yu, Y., Su, J., & Xu, Z. (2022). Alterations of the Mucosal Immune Response and Microbial Community of the Skin upon Viral Infection in Rainbow Trout (Oncorhynchus mykiss). International Journal of Molecular Sciences, 23(22), 14037. https://doi.org/10.3390/ijms232214037







