Alterations in Primary Carbon Metabolism in Cucumber Infected with Pseudomonas syringae pv lachrymans: Local and Systemic Responses
Abstract
1. Introduction
2. Results
2.1. Respiration Rate
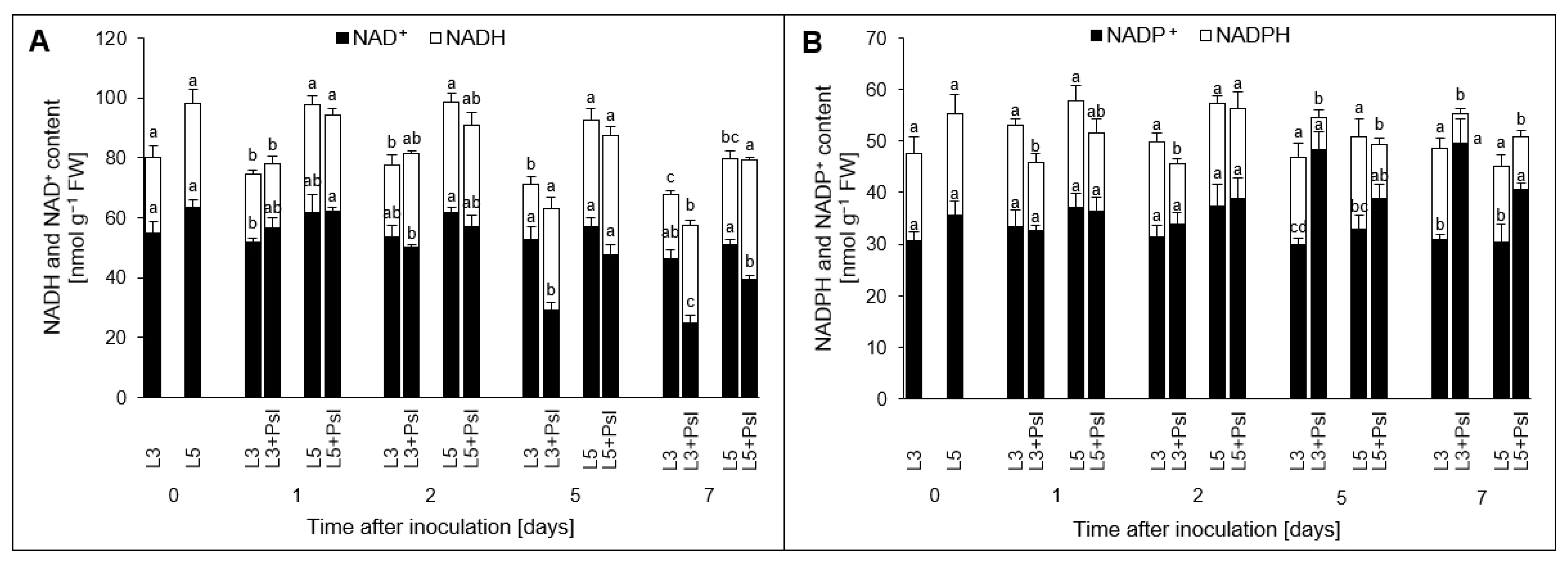
2.2. Nicotinamide Adenine Dinucleotides [(NAD(P)H, NAD(P)+] Contents
2.3. Saccharides and Myo-inositol Contents
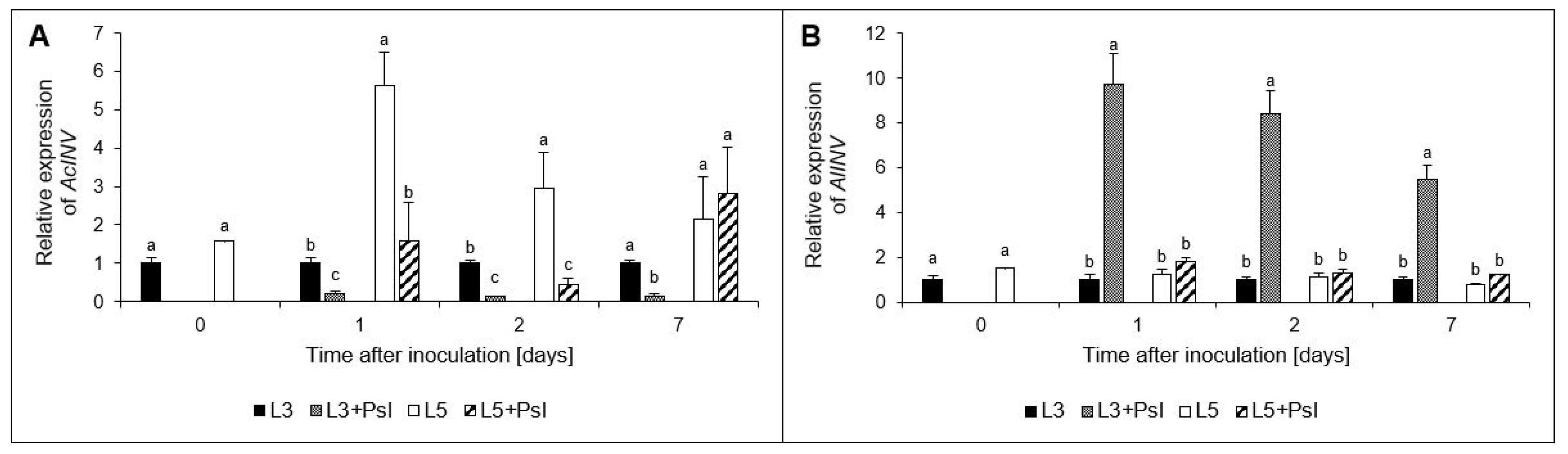
2.4. Acid (AcInv) and Alcaline (AlInv) Invertases Genes Relative Expressions
2.5. Glucose-6-phosphate Content and Glucose-6-phosphate Dehydrogenase (G6PDH) Activity. Hexokinase (HK) and G6PDH Genes Relative Expressions
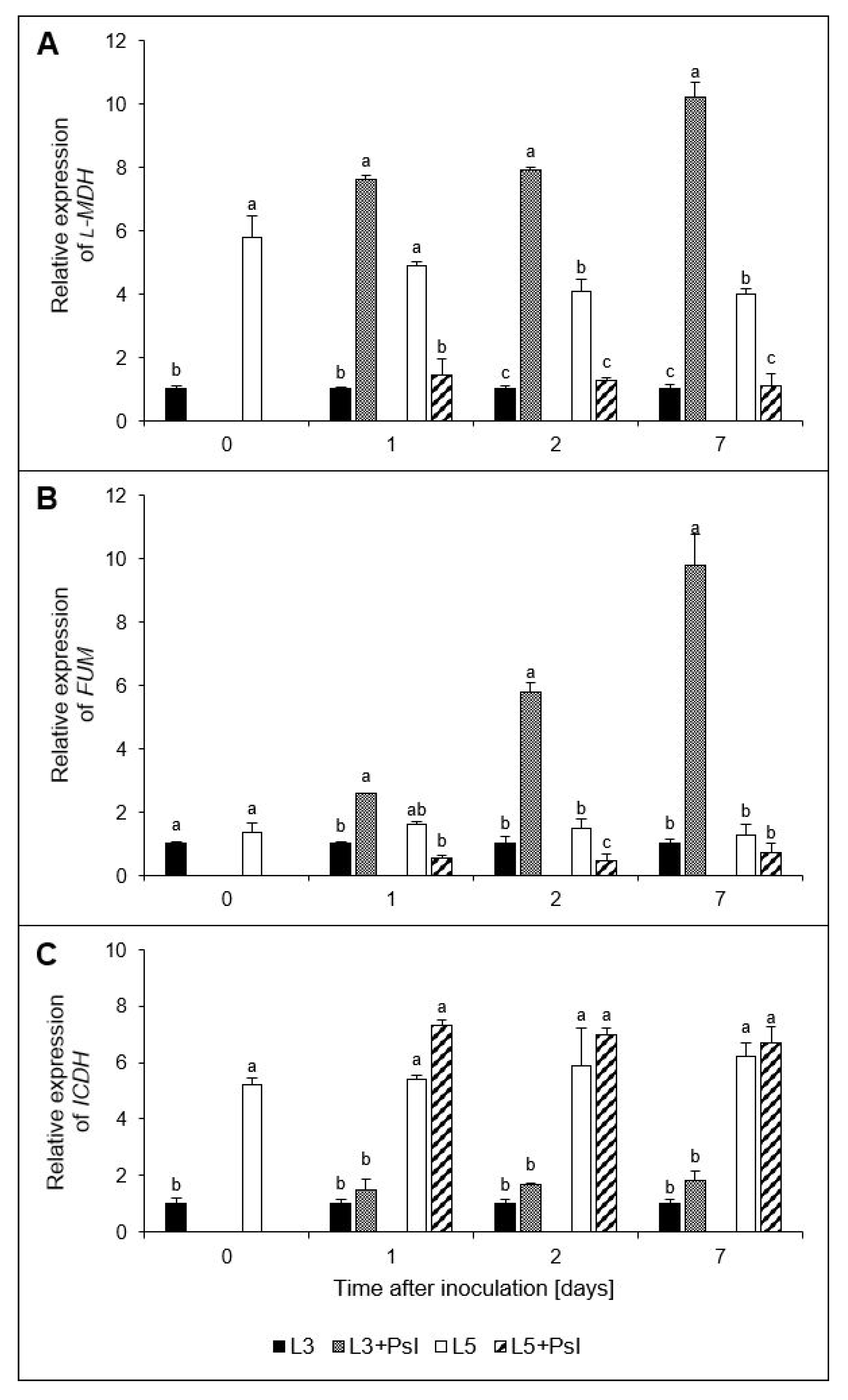
2.6. l-Malic and Oxaloacetic Acids Contents, l-Malate Dehydrogenase (l-MDH) and Fumarase (FUM) Activities
2.7. l-Malate dehydrogenase (l-MDH), Fumarase (FUM), and Isocitrate Dehydrogenase (ICDH) Gene Relative Expressions
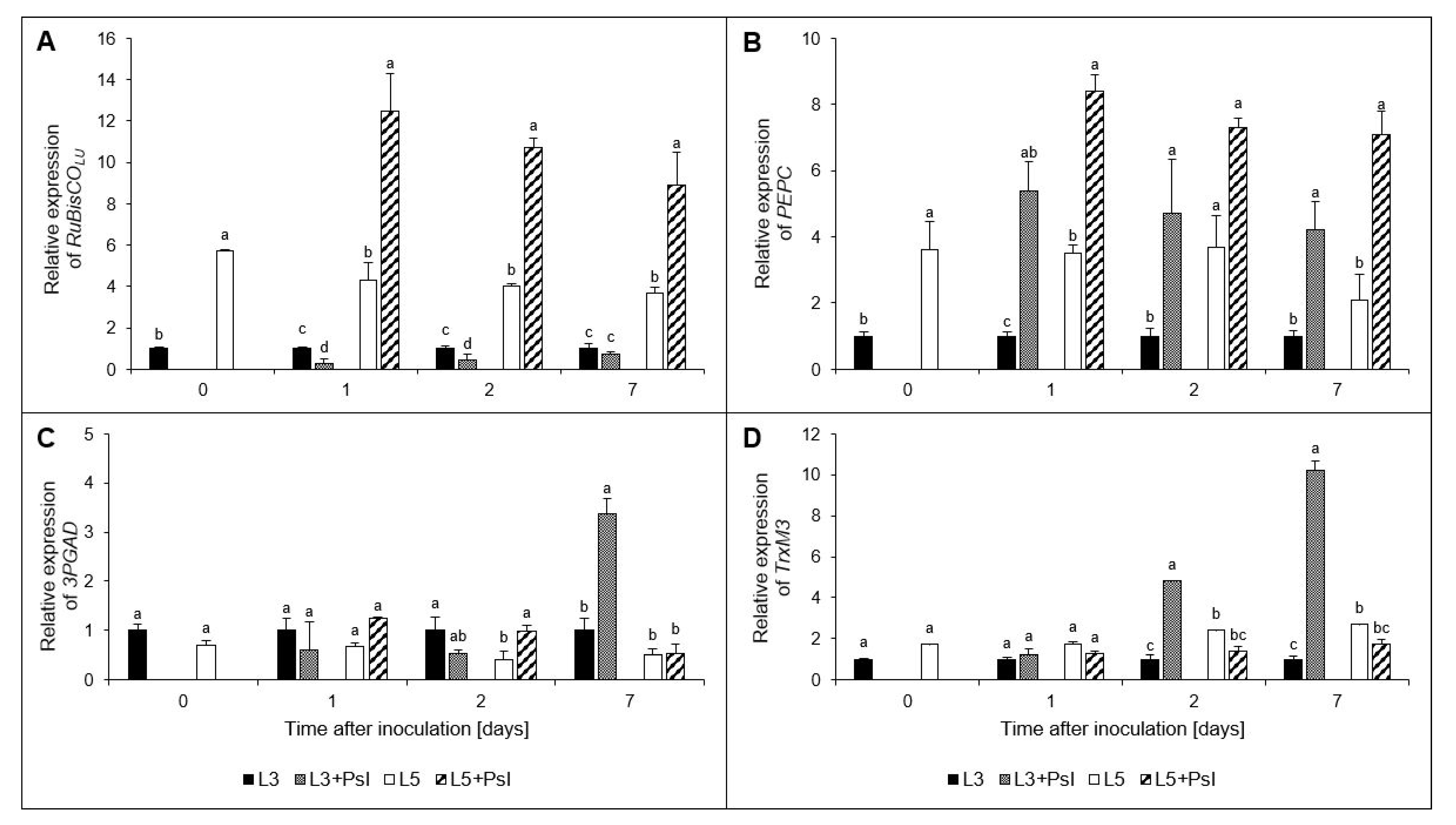
2.8. Ribulose-1,5-bisphosphate Carboxylase/Oxygenase Large Unit (RuBisCOLU), Phosphoenolpyruvate Carboxylase (PEPC), Glyceraldehyde-3-phosphate Dehydrogenase (3PGAD), and Chloroplastic Thioredoxin M3 (TrxM3) Gene Relative Expressions
3. Discussion
3.1. Local and Systemic Changes in the Nicotinamide Adenine Dinucleotides Pool
3.2. Carbohydrate Composition and Metabolism
3.3. Malate- and Oxaloacetate-Related Metabolic Changes
4. Materials and Methods
4.1. Plant Material, Bacterial Infection, and Sample Collection
4.2. Measurements
4.2.1. Respiration Rate Analysis
4.2.2. Determination of Nicotinamide Adenine Dinucleotides
4.2.3. Enzyme Assays
4.2.4. Determination of l-Malic and Oxaloacetic Acids
4.2.5. Quantification of Sucrose, d-Fructose, d-Glucose, and Glucose-6-Phosphate
4.2.6. Determination of Trehalose, Raffinose, and Myo-inositol by GC-MS
4.2.7. Gene Expression Analysis
4.2.8. Protein Determination
4.2.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abscisic acid | ABA |
| Acid invertase | AcINV |
| Alkaline invertase | AlINV |
| Effector-triggered immunity | ETI |
| Fumarase | FUM |
| Glucose-6-phosphate dehydrogenase | G6PDH |
| Glyceraldehyde-3-phosphate dehydrogenase | 3PGAD |
| Hexokinase | HK |
| Hypersensitive response | HR |
| Isocitrate dehydrogenase | ICDH |
| l-Malate dehydrogenase | l-MDH |
| Oxidative pentose phosphate pathway | OPPP |
| Pathogenesis related | PR |
| Pattern-triggered immunity | PTI |
| Phosphoenolpyruvate carboxylase | PEPC |
| Programmed cell death | PCD |
| Pseudomonas syringae pv lachrymans | Psl |
| Ribulose-l,5-bis-phosphate carboxylase/oxygenase large unit | RuBisCOLU |
| Reactive oxygen species | ROS |
| Thioredoxin M3 | TrxM3 |
| Tricarboxylic acid cycle | TCA cycle |
Appendix A
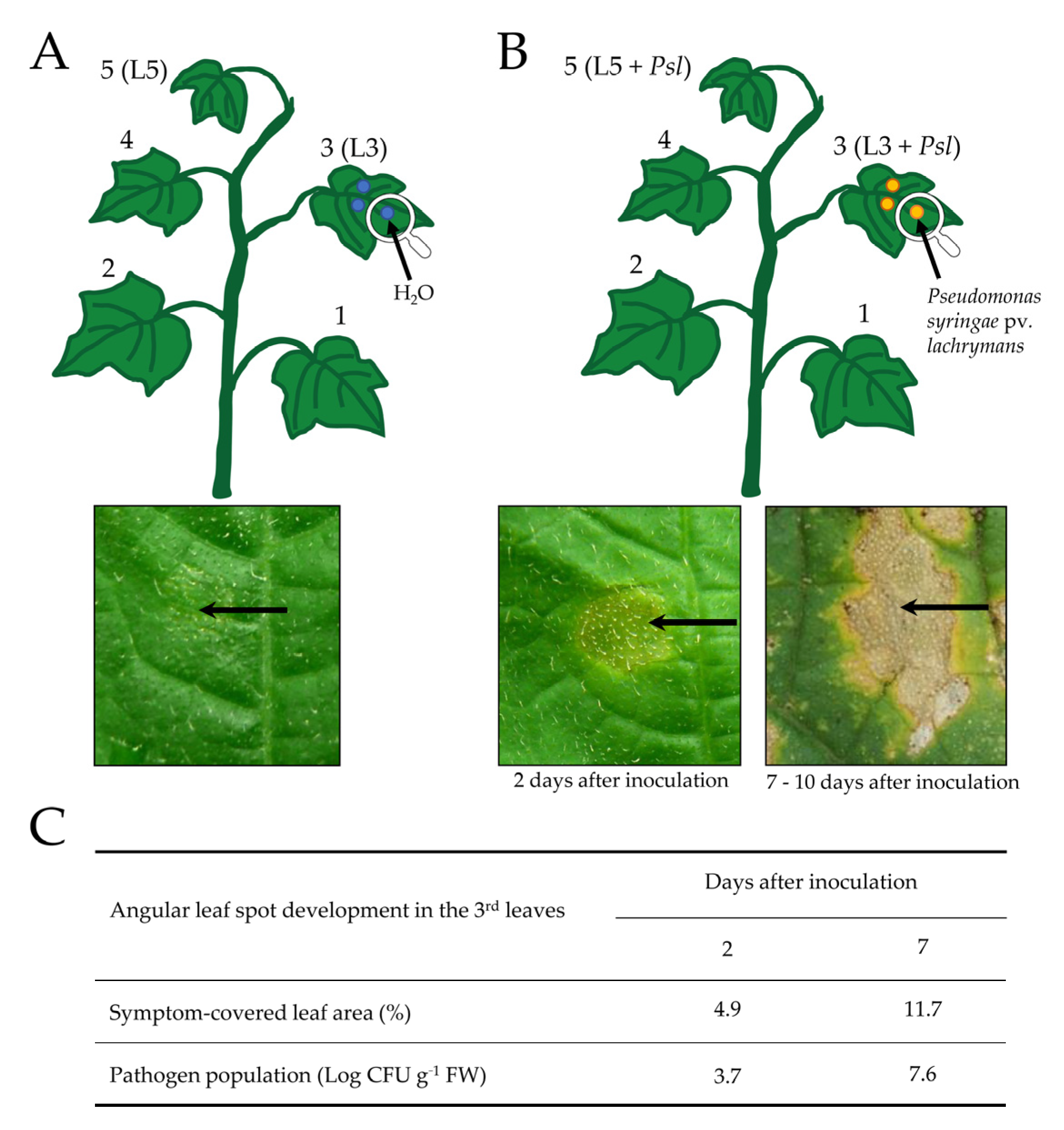
| Redox Ratio | Time [day] | L3 | L3 + Psl | L5 | L5 + Psl |
|---|---|---|---|---|---|
| 0 | 0.5 ± 0.2 (a) | 0.6 ± 0.1 (a) | |||
| 1 | 0.4 ± 0.1 (a) | 0.4 ± 0.1 (a) | 0.6 ± 0.2 (a) | 0.5 ± 0.1 (a) | |
| [NADH]/[NAD+] | 2 | 0.5 ± 0.2 (a) | 0.6 ± 0.2 (a) | 0.6 ± 0.0 (a) | 0.6 ± 0.2 (a) |
| 5 | 0.4 ± 0.0 (c) | 1.2 ± 0.0 (a) | 0.6 ± 0.3 (b) | 0.8 ± 0.1 (b) | |
| 7 | 0.5 ± 0.1 (b) | 1.3 ± 0.4 (a) | 0.6 ± 0.1 (b) | 1.0 ± 0.3 (a) | |
| 0 | 0.6 ± 0.1 (a) | 0.6 ± 0.2 (a) | |||
| 1 | 0.6 ± 0.0 (a) | 0.4 ± 0.1 (a) | 0.6 ± 0.0 (a) | 0.4 ± 0.0 (a) | |
| [NADPH]/[NADP+] | 2 | 0.6 ± 0.1 (a) | 0.4 ± 0.1 (a) | 0.5 ± 0.1 (a) | 0.5 ± 0.1 (a) |
| 5 | 0.6 ± 0.1 (a) | 0.1 ± 0.1 (c) | 0.5 ± 0.0 (a) | 0.3 ± 0.1 (b) | |
| 7 | 0.6 ± 0.2 (a) | 0.1 ± 0.2 (c) | 0.5 ± 0.2 (a) | 0.3 ± 0.0 (b) |
| NCBI Accesion Number | Forward Primer | Reverse Primer |
|---|---|---|
| L21937 (RuBisCOLU) | ATATCTTGGCAGCATTCC | TTCCATAGCATCGTCCTT |
| AJ417435 (PEPC) | ACTTCGTCTCCGTGATTCATACAT | GGTTTGCTTGCTTCCATTATTTCC |
| LT160744 (3PGAD) | AAGGTGGCAATCAATGGA | GACGACAACAACATCAAGG |
| XM_004144692 (G6PDH) | CTCGGCAAGTTCCTGAATCG | TCAACATCCACTGAAGCATCC |
| XM_004137169 (l-MDH) | CAAGATGGAGGAACAGAAGTAG | TTCGGTGACGGTTGATTG |
| NM_001308925 (ICDH) | CAAGAAGTCTACGAATCTCAGT | CAGAAGTCATCAAGCCAAGA |
| XM_004152189 (FUM) | TGCCACACCTCTAACTCTTG | CAACAGCCGTTCCACCTT |
| XM_004136337 (HK) | ATTCATCCTGAGCACACC | CACACCTCTACGACGATT |
| XM_004136929 (TrxM3) | TTCAATCGCCGTCTTAATCC | GTCCACACCAACTAGCATAA |
| KP727817.1 (AcINV) | ACCATCCGCTTCATTATGC | CGTCGTGCCTTGTATCATC |
| XM_004150438 (AlINV) | ACCATCCGCTTCATTATGC | CGTCGTGCCTTGTATCATC |
| AJ715498 (α-TUB) | GCACTGGTCTTCAAGGAT | GTAAGGCTCAACGACAGA |
| AY372537.1 (UBI-ep) | ACCTTGTGCTCCGTCTCAG | CCTTCTTGTGCTTGTGCTTGAT |
References
- Kopczewski, T.; Kuźniak, E.; Kornaś, A.; Rut, G.; Nosek, M.; Ciereszko, I.; Szczepaniak, L. Local and Systemic Changes in Photosynthetic Parameters and Antioxidant Activity in Cucumber Challenged with Pseudomonas syringae pv lachrymans. Int. J. Mol. Sci. 2020, 21, 6378. [Google Scholar] [CrossRef] [PubMed]
- Piasecka, A.; Jedrzejczak-Rey, N.; Bednarek, P. Secondary Metabolites in Plant Innate Immunity: Conserved Function of Divergent Chemicals. New Phytol. 2015, 206, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Ziv, C.; Zhao, Z.; Gao, Y.G.; Xia, Y. Multifunctional Roles of Plant Cuticle during Plant-Pathogen Interactions. Front. Plant Sci. 2018, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bentham, A.R.; de la Concepcion, J.C.; Mukhi, N.; Zdrzałek, R.; Draeger, M.; Gorenkin, D.; Hughes, R.K.; Banfield, M.J. A Molecular Roadmap to the Plant Immune System. J. Biol. Chem. 2020, 295, 14916–14935. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.E.; Mesarich, C.H.; Thomma, B.P.H.J. Understanding Plant Immunity as a Surveillance System to Detect Invasion. Annu. Rev. Phytopathol. 2015, 53, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Ngou, B.P.M.; Ding, P.; Xin, X.F. PTI-ETI Crosstalk: An Integrative View of Plant Immunity. Curr. Opin. Plant Biol. 2021, 62, 102030. [Google Scholar] [CrossRef] [PubMed]
- Lapin, D.; Van den Ackerveken, G. Susceptibility to Plant Disease: More than a Failure of Host Immunity. Trends Plant Sci. 2013, 18, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth-Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287. [Google Scholar] [CrossRef]
- Schwachtje, J.; Baldwin, I.T. Why Does Herbivore Attack Reconfigure Primary Metabolism? Plant Physiol. 2008, 146, 845–851. [Google Scholar] [CrossRef]
- Xian, L.; Yu, G.; Wei, Y.; Rufian, J.S.; Li, Y.; Zhuang, H.; Xue, H.; Morcillo, R.J.L.; Macho, A.P. A Bacterial Effector Protein Hijacks Plant Metabolism to Support Pathogen Nutrition. Cell Host Microbe 2020, 28, 548–557. [Google Scholar] [CrossRef]
- Berger, S.; Papadopoulos, M.; Schreiber, U.; Kaiser, W.; Roitsch, T. Complex Regulation of Gene Expression, Photosynthesis and Sugar Levels by Pathogen Infection in Tomato. Physiol. Plant. 2004, 122, 419–428. [Google Scholar] [CrossRef]
- Scharte, J.; Schön, H.; Weis, E. Photosynthesis and Carbohydrate Metabolism in Tobacco Leaves during an Incompatible Interaction with Phytophthora nicotianae. Plant Cell Environ. 2005, 28, 1421–1435. [Google Scholar] [CrossRef]
- Bonfig, K.B.; Schreiber, U.; Gabler, A.; Roitsch, T.; Berger, S. Infection with Virulent and Avirulent P. Syringae Strains Differentially Affects Photosynthesis and Sink Metabolism in Arabidopsis Leaves. Planta 2006, 225, 1–12. [Google Scholar] [CrossRef]
- Swarbrick, P.J.; Schulze-Lefert, P.; Scholes, J.D. Metabolic Consequences of Susceptibility and Resistance (Race-Specific and Broad-Spectrum) in Barley Leaves Challenged with Powdery Mildew. Plant Cell Environ. 2006, 29, 1061–1076. [Google Scholar] [CrossRef]
- Murray, D.C.; Walters, D.R. Increased Photosynthesis and Resistance to Rust Infection in Upper, Uninfected Leaves of Rusted Broad Bean (Vicia faba L.). New Phytol. 1992, 120, 235–242. [Google Scholar] [CrossRef]
- Xu, Q.; Tang, C.; Wang, X.; Sun, S.; Zhao, J.; Kang, Z.; Wang, X. An Effector Protein of the Wheat Stripe Rust Fungus Targets Chloroplasts and Suppresses Chloroplast Function. Nat. Commun. 2019, 10, 5571. [Google Scholar] [CrossRef]
- Paul, M.J.; Foyer, C.H. Sink Regulation of Photosynthesis. J. Exp. Bot. 2001, 52, 1383–1400. [Google Scholar] [CrossRef]
- Roitsch, T.; González, M.C. Function and Regulation of Plant Invertases: Sweet Sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Jeandet, P.; Formela-Luboińska, M.; Labudda, M.; Morkunas, I. The Role of Sugars in Plant Responses to Stress and Their Regulatory Function during Development. Int. J. Mol. Sci. 2022, 23, 5161. [Google Scholar] [CrossRef]
- Bolouri Moghaddam, M.R.; Van Den Ende, W. Sweet Immunity in the Plant Circadian Regulatory Network. J. Exp. Bot. 2013, 64, 1439–1449. [Google Scholar] [CrossRef]
- Formela-Luboińska, M.; Chadzinikolau, T.; Drzewiecka, K.; Jeleń, H.; Bocianowski, J.; Kęsy, J.; Labudda, M.; Jeandet, P.; Morkunas, I. The Role of Sugars in the Regulation of the Level of Endogenous Signaling Molecules during Defense Response of Yellow Lupine to Fusarium oxysporum. Int. J. Mol. Sci. 2020, 21, 4133. [Google Scholar] [CrossRef]
- Herbers, K.; Meuwly, P.; Métraux, J.P.; Sonnewald, U. Salicylic Acid-Independent Induction of Pathogenesis-Related Protein Transcripts by Sugars Is Dependent on Leaf Developmental Stage. FEBS Lett. 1996, 397, 239–244. [Google Scholar] [CrossRef]
- Liu, Y.H.; Song, Y.H.; Ruan, Y.L. Sugar Conundrum in Plant-Pathogen Interactions: Roles of Invertase and Sugar Transporters Depend on Pathosystems. J. Exp. Bot. 2022, 73, 1910–1925. [Google Scholar] [CrossRef]
- Less, H.; Angelovici, R.; Tzin, V.; Galili, G. Coordinated Gene Networks Regulating Arabidopsis Plant Metabolism in Response to Various Stresses and Nutritional Cues. Plant Cell 2011, 23, 1264–1271. [Google Scholar] [CrossRef]
- Rojas, C.M.; Senthil-Kumar, M.; Tzin, V.; Mysore, K.S. Regulation of Primary Plant Metabolism during Plant-Pathogen Interactions and Its Contribution to Plant Defense. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef]
- Camañes, G.; Scalschi, L.; Vicedo, B.; González-Bosch, C.; García-Agustín, P. An Untargeted Global Metabolomic Analysis Reveals the Biochemical Changes Underlying Basal Resistance and Priming in Solanum lycopersicum, and Identifies 1-Methyltryptophan as a Metabolite Involved in Plant Responses to botrytis Cinerea and Pseudomonas syringae. Plant J. 2015, 84, 125–139. [Google Scholar] [CrossRef]
- Zhu, L.; Zhou, Y.; Li, X.; Zhao, J.; Guo, N.; Xing, H. Metabolomics Analysis of Soybean Hypocotyls in Response to Phytophthora Sojae Infection. Front. Plant Sci. 2018, 9, 1530. [Google Scholar] [CrossRef]
- Noctor, G. Metabolic Signalling in Defence and Stress: The Central Roles of Soluble Redox Couples. Plant Cell Environ. 2006, 29, 409–425. [Google Scholar] [CrossRef]
- Gakière, B.; Fernie, A.R.; Pétriacq, P. More to NAD + than Meets the Eye: A Regulator of Metabolic Pools and Gene Expression in Arabidopsis. Free Radic. Biol. Med. 2018, 122, 86–95. [Google Scholar] [CrossRef]
- Dutilleul, C.; Lelarge, C.; Prioul, J.L.; De Paepe, R.; Foyer, C.H.; Noctor, G. Mitochondria-Driven Changes in Leaf NAD Status Exert a Crucial Influence on the Control of Nitrate Assimilation and the Integration of Carbon and Nitrogen Metabolism. Plant Physiol. 2005, 139, 64–78. [Google Scholar] [CrossRef]
- Zhang, X.; Mou, Z. Extracellular Pyridine Nucleotides Induce PR Gene Expression and Disease Resistance in Arabidopsis. Plant J. 2009, 57, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, V.; Tsers, I. Plant Susceptible Responses: The Underestimated Side of Plant–Pathogen Interactions. Biol. Rev. 2022, 97, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Ward, J.L.; Forcat, S.; Beckmann, M.; Bennett, M.; Miller, S.J.; Baker, J.M.; Hawkins, N.D.; Vermeer, C.P.; Lu, C.; Lin, W.; et al. The Metabolic Transition during Disease Following Infection of Arabidopsis thaliana by Pseudomonas syringae pv. tomato. Plant J. 2010, 63, 443–457. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, X.; Zeng, Y.; Liu, P. Metabolic Profiling Reveals Local and Systemic Responses of Kiwifruit to Pseudomonas syringae pv. actinidiae. Plant Direct 2020, 4, 1–13. [Google Scholar] [CrossRef]
- Berens, M.L.; Wolinska, K.W.; Spaepen, S.; Ziegler, J.; Nobori, T.; Nair, A.; Krüler, V.; Winkelmüller, T.M.; Wang, Y.; Mine, A.; et al. Balancing Trade-Offs between Biotic and Abiotic Stress Responses through Leaf Age-Dependent Variation in Stress Hormone Cross-Talk. Proc. Natl. Acad. Sci. USA 2019, 116, 2364–2373. [Google Scholar] [CrossRef]
- Schwachtje, J.; Fischer, A.; Erban, A.; Kopka, J. Primed Primary Metabolism in Systemic Leaves: A Functional Systems Analysis. Sci. Rep. 2018, 8, 216. [Google Scholar] [CrossRef]
- Berger, S.; Sinha, A.K.; Roitsch, T. Plant Physiology Meets Phytopathology: Plant Primary Metabolism and Plant-Pathogen Interactions. J. Exp. Bot. 2007, 58, 4019–4026. [Google Scholar] [CrossRef]
- Jones, A.M.E.; Thomas, V.; Bennett, M.H.; Mansfield, J.; Grant, M. Modifications to the Arabidopsis Defense Proteome Occur Prior to Significant Transcriptional Change in Response to Inoculation with Pseudomonas Syringae. Plant Physiol. 2006, 142, 1603–1620. [Google Scholar] [CrossRef]
- Allwood, J.W.; Williams, A.; Uthe, H.; van Dam, N.M.; Luis, L.A.J.; Grant, M.R.; Pétriacq, P. Unravelling Plant Responses to Stress—The Importance of Targeted and Untargeted Metabolomics. Metabolites 2021, 11, 558. [Google Scholar] [CrossRef]
- Noctor, G.; Queval, G.; Gakière, B. NAD (P) Synthesis and Pyridine Nucleotide Cycling in Plants and Their Potential Importance in Stress Conditions. J. Exp. Bot. 2006, 57, 1603–1620. [Google Scholar] [CrossRef]
- Smith, E.N.; Schwarzländer, M.; Ratcliffe, R.G.; Kruger, N.J. Shining a Light on NAD- and NADP-Based Metabolism in Plants. Trends Plant Sci. 2021, 26, 1072–1086. [Google Scholar] [CrossRef]
- Pétriacq, P.; de Bont, L.; Tcherkez, G.; Gakière, B. NAD: Not Just a Pawn on the Board of Plant-Pathogen Interactions. Plant Signal. Behav. 2013, 8, 7–17. [Google Scholar] [CrossRef]
- Chojak-Koźniewska, J.; Kuźniak, E.; Linkiewicz, A.; Sowa, S. Primary Carbon Metabolism-Related Changes in Cucumber Exposed to Single and Sequential Treatments with Salt Stress and Bacterial Infection. Plant Physiol. Biochem. 2018, 123, 160–169. [Google Scholar] [CrossRef]
- Mhamdi, A.; Mauve, C.; Houda, G.; Saindrenan, P.; Hodges, M.; Noctor, G. Cytosolic NADP-Dependent Isocitrate Dehydrogenase Contributes to Redox Homeostasis and the Regulation of Pathogen Responses in Arabidopsis Leaves. Plant. Cell Environ. 2010, 33, 1112–1123. [Google Scholar] [CrossRef]
- Henry, E.; Fung, N.; Liu, J.; Drakakaki, G.; Coaker, G. Beyond Glycolysis: GAPDHs Are Multi-Functional Enzymes Involved in Regulation of ROS, Autophagy, and Plant Immune Responses. PLoS Genet. 2015, 11, e1005199. [Google Scholar] [CrossRef]
- Frommer, W.B.; Sonnewald, U. Molecular Analysis of Carbon Partitioning in Solanaceous Species. J. Exp. Bot. 1995, 46, 587–607. [Google Scholar] [CrossRef]
- Biemelt, S.; Sonnewald, U. Plant-Microbe Interactions to Probe Regulation of Plant Carbon Metabolism. J. Plant Physiol. 2006, 163, 307–318. [Google Scholar] [CrossRef]
- Skłodowska, M.; Naliwajski, M.; Wielanek, M.; Gajewska, E.; Kuźniak, E. Arbutin- and Benzotiadiazole-Mediated Cucumber Response to Pseudomonas syringae Pv. lachrymans Infection in Carbohydrate Metabolism. Sci. Hortic. 2015, 192, 200–210. [Google Scholar] [CrossRef]
- Roitsch, T.; Balibrea, M.E.; Hofmann, M.; Proels, R.; Sinha, A.K. Extracellular Invertase: Key Metabolic Enzyme and PR Protein. J. Exp. Bot. 2003, 54, 513–524. [Google Scholar] [CrossRef]
- Sonnewald, S.; Priller, J.P.R.; Schuster, J.; Glickmann, E.; Hajirezaei, R.; Siebig, S.; Mudgett, M.B.; Sonnewald, U. Regulation of Cell Wall-Bound Invertase in Pepper Leaves by Xanthomonas campestris pv. vesicatoria Type Three Effectors. PLoS ONE 2012, 7, e51763. [Google Scholar] [CrossRef]
- Sutton, P.N.; Gilbert, M.J.; Williams, L.E.; Hall, J.L. Powdery Mildew Infection of Wheat Leaves Changes Host Solute Transport and Invertase Activity. Physiol. Plant 2007, 129, 787–795. [Google Scholar] [CrossRef]
- Chen, L.Q.; Hou, B.H.; Lalonde, S.; Takanaga, H.; Hartung, M.L.; Qu, X.Q.; Guo, W.J.; Kim, J.G.; Underwood, W.; Chaudhuri, B.; et al. Sugar Transporters for Intercellular Exchange and Nutrition of Pathogens. Nature 2010, 468, 527–532. [Google Scholar] [CrossRef]
- Rolland, F.; Baena-Gonzalez, E.; Sheen, J. Sugar Sensing and Signaling in Plants: Conserved and Novel Mechanisms. Annu. Rev. Plant Biol. 2006, 57, 675–709. [Google Scholar] [CrossRef]
- Häffner, E.; Konietzki, S.; Diederichsen, E. Keeping Control: The Role of Senescence and Development in Plant Pathogenesis and Defense. Plants 2015, 4, 449–488. [Google Scholar] [CrossRef]
- Battaglia, M.E.; Martin, M.V.; Lechner, L.; Martínez-Noël, G.M.A.; Salerno, G.L. The Riddle of Mitochondrial Alkaline/Neutral Invertases: A Novel Arabidopsis Isoform Mainly Present in Reproductive Tissues and Involved in Root ROS Production. PLoS ONE 2017, 12, e0185286. [Google Scholar] [CrossRef]
- Vargas, W.A.; Salerno, G.L. The Cinderella Story of Sucrose Hydrolysis: Alkaline/Neutral Invertases, from Cyanobacteria to Unforeseen Roles in Plant Cytosol and Organelles. Plant Sci. 2010, 178, 1–8. [Google Scholar] [CrossRef]
- Xiang, L.; Le Roy, K.; Bolouri-Moghaddam, M.R.; Vanhaecke, M.; Lammens, W.; Rolland, F.; Van Den Ende, W. Exploring the Neutral Invertase-Oxidative Stress Defence Connection in Arabidopsis Thaliana. J. Exp. Bot. 2011, 62, 3849–3862. [Google Scholar] [CrossRef]
- Lecompte, F.; Nicot, P.C.; Ripoll, J.; Abro, M.A.; Raimbault, A.K.; Lopez-Lauri, F.; Bertin, N. Reduced Susceptibility of Tomato Stem to the Necrotrophic Fungus Botrytis Cinerea Is Associated with a Specific Adjustment of Fructose Content in the Host Sugar Pool. Ann. Bot. 2017, 119, 931–943. [Google Scholar] [CrossRef]
- Paulina Aguilera-Alvarado, G.; Sanchez-Nieto, S. Plant Hexokinases Are Multifaceted Proteins. Plant Cell Physiol. 2017, 58, 1151–1160. [Google Scholar] [CrossRef]
- Chojak-Koźniewska, J.; Linkiewicz, A.; Sowa, S.; Radzioch, M.A.; Kuźniak, E. Interactive Effects of Salt Stress and Pseudomonas Syringae Pv. Lachrymans Infection in Cucumber: Involvement of Antioxidant Enzymes, Abscisic Acid and Salicylic Acid. Environ. Exp. Bot. 2017, 136, 9–20. [Google Scholar] [CrossRef]
- Scharte, J.; Schön, H.; Tjaden, Z.; Weis, E.; Schaewen, A. Von Isoenzyme Replacement of Glucose-6-Phosphate Dehydrogenase in the Cytosol Improves Stress Tolerance in Plants. Proc. Natl. Acad. Sci. USA 2009, 106, 8061–8066. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Guerriero, G.; Vona, V.; Di Martino Rigano, V.; Carfagna, S.; Rigano, C. Glutamate Synthase Activities and Protein Changes in Relation to Nitrogen Nutrition in Barley: The Dependence on Different Plastidic Glucose-6P Dehydrogenase Isoforms. J. Exp. Bot. 2005, 56, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Lunn, J.E.; Delorge, I.; Figueroa, C.M.; Van Dijck, P.; Stitt, M. Trehalose Metabolism in Plants. Plant J. 2014, 79, 544–567. [Google Scholar] [CrossRef] [PubMed]
- Piazza, A.; Zimaro, T.; Garavaglia, B.S.; Ficarra, F.A.; Thomas, L.; Marondedze, C.; Feil, R.; Lunn, J.E.; Gehring, C.; Ottado, J.; et al. The Dual Nature of Trehalose in Citrus Canker Disease: A Virulence Factor for Xanthomonas citri subsp. citri and a Trigger for Plant Defence Responses. J. Exp. Bot. 2015, 66, 2795–2811. [Google Scholar] [CrossRef] [PubMed]
- MacIntyre, A.M.; Meline, V.; Gorman, Z.; Augustine, S.P.; Dye, C.J.; Hamilton, C.D.; Iyer-Pascuzzi, A.S.; Kolomiets, M.V.; McCulloh, K.A.; Allen, C. Trehalose Increases Tomato Drought Tolerance, Induces Defenses, and Increases Resistance to Bacterial Wilt Disease. PLoS ONE 2022, 17, e0266254. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, O.; Béthencourt, L.; Quero, A.; Sangwan, R.S.; Clément Christophe, C. Trehalose and Plant Stress Responses: Friend or Foe? Trends Plant Sci. 2010, 15, 409–417. [Google Scholar] [CrossRef]
- Freeman, B.C.; Chen, C.; Beattie, G.A. Identification of the Trehalose Biosynthetic Loci of Pseudomonas Syringae and Their Contribution to Fitnes in the Phyllosphere. Environ. Microbiol. 2010, 12, 1486–1497. [Google Scholar]
- Van Den Ende, W.; Valluru, R. Sucrose, Sucrosyl Oligosaccharides, and Oxidative Stress: Scavenging and Salvaging? J. Exp. Bot. 2009, 60, 9–18. [Google Scholar] [CrossRef]
- Nishizawa, A.; Yabuta, Y.; Shigeoka, S. Galactinol and Raffinose Constitute a Novel Function to Protect Plants from Oxidative Damage. Plant Physiol. 2008, 147, 1251–1263. [Google Scholar] [CrossRef]
- Wang, X.; Li, S.; Zhang, X.; Gao, L.; Ruan, Y.L.; Tian, Y.; Ma, S. From Raffinose Family Oligosaccharides to Sucrose and Hexoses: Gene Expression Profiles Underlying Host-to-Nematode Carbon Delivery in Cucumis Sativus Roots. Front. Plant Sci. 2022, 13, 823382. [Google Scholar] [CrossRef]
- Karner, U.; Peterbauer, T.; Raboy, V.; Jones, D.A.; Hedley, C.L.; Richter, A. Myo-Inositol and Sucrose Concentrations Affect the Accumulation of Raffinose Family Oligosaccharides in Seeds. J. Exp. Bot. 2004, 55, 1981–1987. [Google Scholar] [CrossRef]
- Keller, R.; Brearley, C.A.; Trethewey, R.N.; Müller-Röber, B. Reduced Inositol Content and Altered Morphology in Transgenic Potato Plants Inhibited for 1D-Myo-Inositol 3-Phosphate Synthase. Plant J. 1998, 16, 403–410. [Google Scholar] [CrossRef]
- Valluru, R.; Van den Ende, W. Myo-Inositol and beyond—Emerging Networks under Stress. Plant Sci. 2011, 181, 387–400. [Google Scholar] [CrossRef]
- Murphy, A.M.; Otto, B.; Brearley, C.A.; Carr, J.P.; Hanke, D.E. A Role for Inositol Hexakisphosphate in the Maintenance of Basal Resistance to Plant Pathogens. Plant J. 2008, 56, 638–652. [Google Scholar] [CrossRef]
- Hu, L.; Zhou, K.; Ren, G.; Yang, S.; Liu, Y.; Zhang, Z.; Li, Y.; Gong, X.; Ma, F. Myo-Inositol Mediates Reactive Oxygen Species-Induced Programmed Cell Death via Salicylic Acid-Dependent and Ethylene-Dependent Pathways in Apple. Hortic. Res. 2020, 7, 1–13. [Google Scholar] [CrossRef]
- Geigenberger, P.; Kolbe, A.; Tiessen, A. Redox Regulation of Carbon Storage and Partitioning in Response to Light and Sugars. J. Exp. Bot. 2005, 56, 1469–1479. [Google Scholar] [CrossRef]
- Lehmann, M.M.; Rinne, K.T.; Blessing, C.; Siegwolf, R.T.W.; Buchmann, N.; Werner, R.A. Malate as a Key Carbon Source of Leaf Dark-Respired CO2 across Different Environmental Conditions in Potato Plants. J. Exp. Bot. 2015, 66, 5769–5781. [Google Scholar] [CrossRef]
- Aubry, S.; Brown, N.J.; Hibberd, J.M. The Role of Proteins in C3 Plants Prior to Their Recruitment into the C4 Pathway. J. Exp. Bot. 2011, 62, 3049–3059. [Google Scholar] [CrossRef]
- Doubnerová, V.; Ryšlavá, H. What Can Enzymes of C4 Photosynthesis Do for C3 Plants under Stress? Plant Sci. 2011, 180, 575–583. [Google Scholar] [CrossRef]
- Müller, K.; Doubnerová, V.; Synková, H.; Čeřovská, N.; Ryšlavá, H. Regulation of Phosphoenolpyruvate Carboxylase in PVYNTN-Infected Tobacco Plants. Biol. Chem. 2009, 390, 245–251. [Google Scholar] [CrossRef]
- Sweetlove, L.J.; Beard, K.F.M.; Nunes-Nesi, A.; Fernie, A.R.; Ratcliffe, R.G. Not Just a Circle: Flux Modes in the Plant TCA Cycle. Trends Plant Sci. 2010, 15, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Manning, I.; Heldt, H.W. On the Function of Mitochondrial Metabolism during Photosynthesis in Spinach (Spinacia oleracea L.) Leaves: Partitioning between Respiration and Export of Redox Equivalents and Precursors for Nitrate Assimilation Products. Plant Physiol. 1993, 103, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Selinski, J.; Scheibe, R. Malate Valves: Old Shuttles with New Perspectives. Plant Biol. 2019, 21, 21–30. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.M.; Neale, H.C.; Geilfus, C.M.; Jackson, R.W.; Arnold, D.L.; Preston, G.M. Early Changes in Apoplast Composition Associated with Defence and Disease in Interactions between Phaseolus vulgaris and the Halo Blight Pathogen Pseudomonas syringae Pv. Phaseolicola. Plant Cell Environ. 2016, 39, 2172–2184. [Google Scholar] [CrossRef] [PubMed]
- Mellgren, E.M.; Kloek, A.P.; Kunkel, B.N. Mqo, a Tricarboxylic Acid Cycle Enzyme, Is Required for Virulence of Pseudomonas syringae Pv. Tomato Strain DC3000 on Arabidopsis thaliana. J. Bacteriol. 2009, 191, 3132–3141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gibon, Y.; Larher, F. Cycling Assay for Nicotinamide Adenine Dinucleotides: NaCl Precipitation and Ethanol Solubilization of the Reduced Tetrazolium. Anal. Biochem. 1997, 251, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Bergmeyer, H.U.; Gawehn, K.; Grassi, L.M. Enzymes as Biochemical Reagents. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 425–556. ISBN 978-0-12-091302-2425. [Google Scholar]
- Asahi, T.; Nishimura, M. Regulatory Function of Malate Dehydrogenase Isoenzymes in the Cotyledons of Mung Bean. J. Biochem. 1973, 73, 217–225. [Google Scholar] [CrossRef]
- Sgherri, C.; Quartacci, M.F.; Izzo, R.; Navari-Izzo, F. Relation between Lipoic Acid and Cell Redox Status in Wheat Grown in Excess Copper. Plant Physiol. Biochem. 2002, 40, 591–597. [Google Scholar] [CrossRef]
- Chen, L.S.; Lin, Q.; Nose, A. A Comparative Study on Diurnal Changes in Metabolite Levels in the Leaves of Three Crassulacean Acid Metabolism (CAM) Species, Ananas comosus, Kalanchoë daigremontiana and K. pinnata. J. Exp. Bot. 2002, 53, 341–350. [Google Scholar] [CrossRef]
- Wahlefeld, A.W. Oxaloacetate, U.V. Spectrophotometric Determination. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 1604–1608. ISBN 978-0-12-091302-2. [Google Scholar]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gocławski, J.; Sekulska-Nalewajko, J.; Kuźniak, E. Neural Network Segmentation of Images from Stained Cucurbits Leaves with Colour Symptoms of Biotic and Abiotic Stresses. Int. J. Appl. Math. Comput. Sci. 2012, 22, 669–684. [Google Scholar] [CrossRef]
- Jacob, C.; Panchal, S.; Melotto, M. Surface Inoculation and Quantification of Pseudomonas syringae Population in the Arabidopsis Leaf Apoplast. Bio-Protocol 2017, 7, 1–9. [Google Scholar] [CrossRef]



| Time (day) | L3 | L3 + Psl | L5 | L5 + Psl |
|---|---|---|---|---|
| 0 | 1.976 ± 0.269 (b) | 2.374 ± 0.212 (b) | ||
| 2 | 2.300 ± 0.354 (b) | 3.589 ± 0.219 (a) | 2.450 ± 0.272 (b) | 2.344 ± 0.265 (b) |
| 7 | 1.956 ± 0.235 (b) | 2.926 ± 0.266 (a) | 2.494 ± 0.306 (ab) | 2.400 ± 0.267 (ab) |
| Metabolite | Time (day) | L3 | L3 + Psl | L5 | L5 + Psl |
|---|---|---|---|---|---|
| Raffinose | 0 | 1.0 ± 0.1 (a) | 0.2 ± 0.1 (b) | ||
| 2 | 1.0 ± 0.2 (c) | 1.9 ± 0.1 (b) | 0.1 ± 0.02 (d) | 2.8 ± 0.1 (a) | |
| 7 | 1.0 ± 0.2 (c) | 4.0 ± 0.3 (a) | 0.5 ± 0.1 (d) | 3.0 ± 0.1 (b) | |
| Trehalose | 0 | - | - | ||
| 2 | - | 0.6 ± 0.2 (a) | - | 0.3 ± 0.1 (b) | |
| 7 | 0.4 ± 0.1 (b) | 0.8 ± 0.2 (a) | 0.4 ± 0.1 (b) | 0.7 ± 0.1 (a) | |
| Myo-inositol | 0 | 3.5 ± 0.1 (b) | 6.9 ± 0.2 (a) | ||
| 2 | 3.7 ± 0.5 (b) | 8.4 ± 0.6 (a) | 6.5 ± 0.1 (ab) | 6.0 ± 0.5 (ab) | |
| 7 | 3.8 ± 0.5 (b) | 7.3 ± 0.4 (a) | 6.2 ± 0.7 (ab) | 6.2 ± 1.0 (ab) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopczewski, T.; Kuźniak, E.; Ciereszko, I.; Kornaś, A. Alterations in Primary Carbon Metabolism in Cucumber Infected with Pseudomonas syringae pv lachrymans: Local and Systemic Responses. Int. J. Mol. Sci. 2022, 23, 12418. https://doi.org/10.3390/ijms232012418
Kopczewski T, Kuźniak E, Ciereszko I, Kornaś A. Alterations in Primary Carbon Metabolism in Cucumber Infected with Pseudomonas syringae pv lachrymans: Local and Systemic Responses. International Journal of Molecular Sciences. 2022; 23(20):12418. https://doi.org/10.3390/ijms232012418
Chicago/Turabian StyleKopczewski, Tomasz, Elżbieta Kuźniak, Iwona Ciereszko, and Andrzej Kornaś. 2022. "Alterations in Primary Carbon Metabolism in Cucumber Infected with Pseudomonas syringae pv lachrymans: Local and Systemic Responses" International Journal of Molecular Sciences 23, no. 20: 12418. https://doi.org/10.3390/ijms232012418
APA StyleKopczewski, T., Kuźniak, E., Ciereszko, I., & Kornaś, A. (2022). Alterations in Primary Carbon Metabolism in Cucumber Infected with Pseudomonas syringae pv lachrymans: Local and Systemic Responses. International Journal of Molecular Sciences, 23(20), 12418. https://doi.org/10.3390/ijms232012418









