The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health
Abstract
1. Introduction
2. Age Dependence of the Biological and Social Functions of Androgens in Males
3. Types of Androgens—Synthesis, Structure and Functions
- ○
- DHEA dehydroepiandrosterone (DHEA): 7OH-DHEA and its esters: DHEAS and acyl-DHEA.
- ○
- DHEA is formed from cholesterol via pregnelonone and 17OH-pregnelonone. Its hydrophilic sulfate ester, DHEAS, is the main steroid hormone in the human bloodstream [51]. DHEA binds both (albeit not strongly) the androgen (AR) and estrogen (ER) receptors [52]. Acyl-DHEA can be formed by plasma lipoproteins [53], via lecithin-cholesterol acyltransferase [54], and has been related to DHEA transport into tissues [55]. DHEA affects the regulation of corticosteroids [56,57], but all its functions have not been fully unraveled.
- ○
- T testosterone (T).
- ○
- AcT 17 β-acyl-testosterone esters (AcT).
- ○
- KTs 11-oxo-androgens, such as 11β-hydroxy-testosterone and 11-keto-testosterone (KT).
- ○
- DHT 4,5-dihydro-testosterone (DHT).
- ○
- A4 androstenedione (4-androstenedione, A4).
- ○
- AP ∆16–17 androgenic pheromones: androstenone, androstenols.
- ○
- EA estrogenic androgens (e.g., 5-androstene-3α,17β-diol).
- ○
- Ane androsterone (5α-androsterone, Ane).
- ○
- Other androgen metabolism intermediates and excretion molecular species.
4. Mechanisms of Action of Androgens
4.1. Canonic Androgen Receptor Signaling
4.2. Main Non-Canonic Receptor Signaling (AcT, SARM)
4.3. Cell Membrane-Related Androgen Signaling
- SHBG-mediated signaling. In this case, SHBG is purportedly used as an anchor for membrane AR androgen binding [174]. SHBG is produced in a number of cell types, containing its receptors in the cell surface [175,176]; they can bind the AR agonists, primarily T [175], but can also bind E2 [177]. After the agonist is bound, the SHBG-receptor-agonist complex is internalized, and then activates a membrane-related adenylate kinase, inducing the production of cAMP [176,178]. A number of effects of androgens have been attributed to these mechanisms [179,180].
- Attachment to membrane G-proteins This mechanism is based on the attachment of the AR to membrane G-proteins [170], in a way similar to that of estrogens. The binding of DHT or other agonists to AR in the membrane immediacy may then induce the activation of cytoplasmic protein kinase C, resulting in rapid effects of androgens [181]; in this case the action is also mediated by the AR, albeit not in the nucleus (i.e., not through gene expression).
- Implication of ion channels. This possibility relies on the formation of a complex of membrane G-proteins plus the AR and an agonist (as described in the previous alternative); however, this time, this affects the membrane zinc transporters [182] and the calcium channels, increasing the Ca2+ input [183,184]. The rise in calcium can activate the A- or MAP-kinases, inducing fast (and short-term) effects [185,186]. However, MAP-kinase and Ca2+ are also known modulators of gene expression, thus, AR activation may (in addition) indirectly induce genomic actions through this pathway [175].
- Activation of cytoplasmic AR near the cell membrane. A possible action on the control of membrane receptor-induced signals may result in binding AR close to the cell membrane (perhaps bound to a G-protein), previously activated by androgenic agonists and binding a tyrosine kinase [186] in healthy and tumor cells. Tyrosine kinase is also a critical modulator of the AR function [187].
5. The Varied Physiological Functions of Androgens
5.1. Estrogen Synthesis
5.2. The Complex “Love-Hate” Interactions of Androgens and GC
5.3. Modulation of the Immune Response
5.4. Androgens and Reproduction
- The production of spermatozoa. Androgens elicit the production and maturation of spermatozoa through the activation of the testicle Sertoli cells [255,256,257] and their incorporation to the seminal fluid. The use of large doses (and extended treatments) of T, AcT or DHT (but also E2) partially block the action of FSH [255]. Both FSH and testosterone are needed to produce viable spermatozoa [255,258]; the alteration of this delicate process often destabilizes spermatogenesis, resulting in sterility [259].
- The development of male sexual secondary characteristics. The male secondary sex characteristics are typically induced by T [230], but are generally attributed to DHT [260,261] (rather than T, which obviously also induces them [262]), because of the higher response elicited by DHT, which seem final for this purpose in comparison with the T intervention in a wide panoply of functions and paths. T seems to stimulate the defining characteristics of “maleness”, at least in part, via its conversion by 5α-reductase to DHT [263], where isozymes are considered to be responsible for most of the male-defining physical and behavioral characteristics [264]. The genetic absence of 5α-reductase isoforms may even result in individuals arriving to adolescence with a full female phenotype. However, in many cases, they become fully fertile men with male behavioral orientation, albeit they are initially devoid of secondary male sex characteristics; they often recover/develop the necessary functional and anatomical structures to allow successful mating [265] and impregnation. Nevertheless, sexual development is probably induced by a full collaborative effort of most types of androgens (except AP, AE, and probably natural AcT), and requires the presence of E2 in addition to T and/or DHT [266]; this conjoint action includes successful spermatogenesis [267].
- Interpersonal communication and signaling. This is a process in which intervene the AP, sex-specific pheromone [85,87]. Some of these compounds display neurosteroid functions [215], but they mostly have been related to inter-individual communication as pheromones [87,202,268] and/or odorous markers [205]. They are produced by and secreted from skin apocrine glands and other organs such as the brain, adrenals, ovaries and testes [83,215], and have been detected in circulating boar plasma [269].
- The focussing of brain structure/function (and behavior) towards reproduction. This includes extensive behavioral and social energy (and time) investments [270,271]. T is necessary for mating and the maintenance of a sexual/affective relationship for both sexes [272]. T activates mating relationships [273] through complex mechanisms in which cortisol [274] and reward systems, such as endorphins [275] and oxytocin [276], play important roles. The settling of durable relationships result in a decrease in T [277] and increase in oxytocin [278,279] levels in males. This change may be, in part, justified by the need to temper the aggressiveness and dominance drive elicited by high testosterone [280] or DHT. The varied effects of DHEA [281], T and DHT on sexual drive are well known, but DHEA effects are more extensive in females than in males [282], and those of DHT are practically circumscribed to men due to the direct relationship of DHT with male secondary sex characteristics. In women, DHT levels are normally low [283], rising only in some pathologies [284] and provoking serious metabolic complications in animal models when administered (i.e., PCOS [285]). Consequently, the activation and maintenance of libido is largely centered on T, for both men [286] and women [287]. The implication of KTs in these sex-related processes has not yet been clarified, but we can hypothesize that they may not be critical on this issue, since the highest circulating levels of KTs are found in childhood (in both sexes), i.e., before sexual maturity. In any case, the effects of T on the libido of women correlate with T administration/levels [288] (as in males), irrespective of their already higher and maintained KT levels [289].
- The potentiation of growth and development. This is achieved through gender-related differentiation; this is especially marked on the effects induced on brain organization [290,291], behavior [270,292] and fulfillment of the biological male phenotype [293]. The effects may be quite different with DHEA, which neurosteroid nature [194] and diverse brain effects range from behavior (i.e., aggression [294] or mood [281]) to cognition [281,295]. There is scant information on the possible effects of DHT on the nervous system, but in the brain, T reduction to DHT has been described [296]. Similarly, the known role of KTs on fish neural development has not yet been observed (albeit it is hinted to [297]) in humans.
- The accrual, maintenance and regulation of body (i.e., muscle) protein. Androgens play a critical participation in the regulation of body protein content (including, especially, muscle mass and distribution) [245]. The marked decay of testosterone availability with senescence [298] lowers muscle mass and function [299] down to sarcopenia [300]. The consequences are compounded by the limitation of estrogen production because of insufficient T. The use of T as senolytic helps limit the consequences of this deficiency [301,302]. Androgens are commonly used as drugs for the development, growth and maintenance of body protein, especially muscle mass [245,303]. This is a complex process in which other hormones intervene, such as insulin [304], growth hormones [305] and estrogens [306], and is dependent on the adequate supply of dietary energy and protein. The main androgen agents favoring body protein accrual and maintenance are KTs, T and DHT; the intervention of AcT is also probable because of their unique natural long half-life, but no specific information is available on their effects under physiological conditions. However, there is considerable evidence of the protein sparing/mass-enhancing effects of long-term TRT (T replacement therapy) [307,308], including their use for the treatment of sarcopenia [309,310]. Furthermore, the abuse of AcT as anabolic agents for sport doping, or body muscular build-up (i.e., often for non-health-related purposes) has shown that their overuse indeed results in an exacerbated growth of muscle mass [311,312] partly at the expense of body fat [313]. Their use may also result in the development of dependence [314]; often inducing severe cardiovascular, behavioral and reproductive disorders too [315].
- Energy partition and handling. Androgens are directly implicated in the mechanisms of energy partition and utilization for metabolic function. They participate in the intertwined regulation of energy metabolism with estrogens and other regulatory hormones, such as GH [305,316], insulin [317,318], calciferols [319] and cytokines such as leptin [317], but also favoring an anti-inflammatory vs. inflammatory cytokine distribution [320]. These actions have been essentially described for T and KT, but can also be elicited by AcT-based TRT [321]. Hypoandrogenism is correlated with obesity [322] and it is one of the key MS disorders (and markers) [323]. Consequently, the “recovery” of androgen levels (lost to age and/or MS) may be expected to favor the shedding of excess body fat. In fact, treatments using T decrease adiposity [324,325,326]. However, longer treatments with exogenous AcT may reduce body fat, but not massively [327,328,329,330]. Some estrogens (such as E2 and acyl-E1) are known to lower body fat [9], and hypoandrogenism results in the insufficient availability/circulation of E2 [331] because the lack of T deprives the process of aromatization to E2 of its main substrate. It can be assumed that the “adipolytic” effect of T is probably (or, potentially, mainly) a consequence of the restitution to normalcy of E2 levels [9] elicited by the T administration. Thus, only aromatizable androgens may be expected to significantly influence body fat when used for substitutive androgenization treatments. A low T is correlated with lower insulin sensitivity in men [332,333]; insulin resistance does not affect T but the reverse is true [219], since T lowers insulin resistance [220], and helps maintain glycaemia [334,335]. DHEA also decrease insulin resistance [336,337]. However, DHT (in men) has been found to increase insulin sensitivity [317,338]. The effects on insulin sensitivity/resistance induced by long-term pharmacological treatment with T and AcT are presented and discussed in Section 7.2. Effects on insulin resistance/sensitivity were observed both using T- [325,335,339] and AcT-based [329,330,340] TRTs.
6. Regulation of Testosterone Synthesis and Availability
6.1. Hypothalamus–Hypophysis Axis Regulation of Androgens
6.2. Hormone Availability, Interactions and Turnover
7. Current Pharmacological Utilization of Androgens
7.1. The First Studies: T as Senolytic/Energizer
7.2. The Practical Difficulties of Oral Administration of T
7.3. The Widely Extended Use of T Acyl-Esters in the Treatment of Hypoandrogenism in Men
7.4. Justification, Expectations and Variability of Long-Term “Testosterone” Treatments
7.5. Effectiveness and Insufficient Overall Analysis, of Long-Term Use of AcT for TRT
| Main Effects Observed | ↑ Testosterone | ↑ Glycaemia | ↓ Insulin Resistance | ↑ Insulin Sensitivity | ↓ Body Weight | ↓ Body Fat | ↑ Ipid Oxidation | ↓ Circulating Lipids | ↑ Body Protein | ↑= Muscle-Mass | ↑= Mobility | ↑ Cardiovascular Function | ↓ Liver Steatosis | |
| H | V | T | GLUCOSE | LIPIDS | PROTEIN | SYSTEMS | ||||||||
| T | Tr | [335,339,474,497,498,499,500] | [325,335,339,501] | [325,335,339,501] | [325,335,339] | [325,497] | [324,325,326,497,498,499,502,503] | [110] | [325,335,501,504,505] | [339,497,498] | [479,499,500] | [497,502] | [506,507,508,509,510] | |
| Inj | [388,511,512] | [318] | [318] | [318,512] | [512] | |||||||||
| Or | [513] | |||||||||||||
| TU | Tr | [514] | ||||||||||||
| Inj | [327,494,514,515,516,517,518,519,520,521,522,523] | [328,340,475,514,515,516,524] | [340,515,522] | [329,330,340] | [328,516,517,519,521,522,524,525] | [327,328,329,330,475,516,517,523] | [31,327,328,475,514,515,516,519,524] | [524,526] | [517,523] | |||||
| Or | [523] | [527,528] | ||||||||||||
| TE | Inj | [529,530,531,532] | [533] | [533] | [520,533] | [529,531] | [534,535] | [307,532,536] | [537] | [509,538] | ||||
| TC | Inj | [509,538,539] | ||||||||||||
| UN | ND | [540] | [540,541] | [540,541] | [540,541] | [540,542] | [482] | [463,478,479,541] | ||||||
| Tr | [317,543] | [514] | [317] | [544] | ||||||||||
| Inj | [525,533,545,546] | [339,525,547] | [339,525] | [492,522,525] | [525,526,548] | [328,525] | [549] | [329,515,539,550,551,552,553] | ||||||
| Or | [554] | [528] | ||||||||||||
| Main Effects Observed | ↑= Bone Function | ↑ Kidney Function | ↓ Erectile Ysfunction | ↑ Sexual Function | ↑ Libido | ↓ Fertility Contraception | ↑ Life Expectancy | ↓ Depressive States | ↑ General Well-Being | ↑ Sleep Time | ↑ Hemoglobin | ↑ Hematocrit Hematopoiesis | ↑ Serious Negative Effects | |
| H | V | SYSTEMS | REPRODUCTIVE | LIFE AND BEHAVIOR | BLOOD | NEGATIVE | ||||||||
| T | Tr | [326,497,513,555] | [325,326,473,474] | [325] | [507] | [474] | [473,474,512] | [497] | [500,556] | [311,462] | ||||
| Inj | [388] | [511] | ||||||||||||
| Or | [513 | [462] | ||||||||||||
| TU | Tr | [557] | ||||||||||||
| Inj | [523,558] | [518] | [475,559] | [476,521] | [559] | [507,523] | [476] | [475] | [327,494,519,526] | [494,518,556] | [556] | |||
| Or | [430,528] | [560] | [430] | [500] | ||||||||||
| TE | Inj | [561] | [533,535] | [535] | [438,520] | [538] | [507] | [329] | [529,556] | [556] | ||||
| TC | Inj | [562] | [538,563] | [507] | [549] | [564] | [556] | [556,565] | ||||||
| UN | ND | [482,541] | [482] | [482,566] | [478,541] | [482,567] | [542] | [482,541,542] | [480,508] | |||||
| Tr | [317] | [568] | ||||||||||||
| Inj | [543] | [545] | [545,551,569,570] | [388] | [546,551,563] | [549,571] | [492] | [564] | [565,572,573,574] | [550,565,568,572,575,576,577] | ||||
| Or | [568] | |||||||||||||

7.6. Additional Questions Posed by TRT in Women
8. The Use of Androgens for the Treatment of Functional Disorders
- Patient conditions.First, the real existence of a need for androgenization treatment must be clearly established after an intensive and exhaustive metabolic and hormonal analysis of the subject. Then, the plausibility of carrying out an androgenization treatment must be decided upon, taking into account the individual conditions: sex, age, nutrition or hormonal disorders (especially including metabolic syndrome and its related pathologies, andropause, menopause, old age and hypogonadism). These aspects should be weighed according to the severity of the conditions, time available and health objectives sought. Psychological factors must be considered because of the probable duration of the process and the behavioral/social changes expected.
- Molecular species usedCurrently, practically all androgenization treatments are based in either T or a number of eka-T products, as described in the text, but their mode of action is not the same. This analysis should be open to specific (even combined) treatments including T, AcT, KTs and the fully synthetic drugs available at present (not discussed in this review), tailoring the molecules to the needs of the treatment (and the extent of the risks taken with their use). Evidently, age, sex and metabolic disorders are critical factors to ponder in addition to a solid reason for applying an androgenic supplementation treatment. The eventual addition of other hormones, such as E2, should be also considered.
- Way of administration and doseThis aspect directly depends on the molecule(s) selected, the disorder to be treated, and the real rates of release/inactivation. The problems posed by the different methods of administration are known and should be better specifically known through more extensive and complete checks of the molecular species involved. In general, it is better to use repeated short term procedures (i.e., transdermal applications) to maximize the possibilities of adjustment, evaluation, control or interruption of the treatment as needed.
- Duration of the treatmentTime of treatment is closely related to the two previous points, since most of the procedures used so far rely, essentially, on massive, uninterrupted and prolonged treatment with (usually) a single hormonal agent. However, hormones are extremely sensitive to physiological modulation and are carefully regulated in vivo. Only good feedback of the effects sought by the treatment may provide the sufficient level of safety and effectivity to carry on. This is another reason favoring shorter treatments (preferably repeated after safety interludes), and the continued control of physiological functions, hormone levels and related markers, as well as behavior/patient satisfaction (and compliance) feedback.
- Maintenance of hormonal homeostasisThe administration of a drug with hormonal effects constitutes per se a destabilizing intrusion in the body humoral homeostasis, obviously, irrespective of the nature and quality of that homeostasis. In the case of “testosterone” long-term treatments, often the only guide available (differential effects) can begin to be observable after long saturation (resulting in inactivation) of the physiological control systems, after using high doses and a prolonged time. Only a few studies include periods of “rest”, interspersed after several months of treatment. This is a critical point that needs further (and deeper) development, extending the treatment periods based on limiting the overwhelming damage to the natural biological cycles (i.e., monthly, bi-weekly or even shorter alternate periods), and leaving time and space for the homeostatic systems (essentially the HHG and HHA axes) to re-start and help solve the disorder instead of enduring or fighting the powerful and continuous drug intrusion.
9. Conclusions and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A4 | 4-androstenedione |
| ABP | androgen-binding protein |
| AcT | * acyl-testosterone |
| ACTH | corticotropin-[adrenocorticotropic] hormone |
| Ane | * androsterone [5α-androsterone] |
| AP | * ∆16–17-androgenic pheromones |
| AR | androgen receptor |
| ARE | androgen response element |
| CRH | corticotropin-releasing hormone |
| DHEA | dehydroepiandrosterone |
| DHEAS | dehydroepiandrosterone-sulfate |
| DHT | dihydrotestosterone |
| E1 | estrone |
| E2 | estradiol [17β-estradiol] |
| EA | * estrogenic androgens |
| ER | estrogen receptor |
| FSH | follicle-stimulating hormone |
| GC | glucocorticoid(s) |
| GH | growth hormone |
| GnIH | gonadotropin-inhibitory hormone |
| GnRH | gonadotropin-releasing hormone |
| GR | glucocorticoid receptor |
| HHA | hypothalamus–hypophysis–adrenals (axis) |
| HHG | hypothalamus–hypophysis–gonads (axis) |
| KT | * 11-keto-testosterone |
| KTs | * 11-oxo-androgens in general |
| LH | luteotropic hormone |
| MS | * metabolic syndrome |
| POCS | polycystic ovary syndrome |
| SARM | selective AR modulator |
| SERM | selective ER modulator |
| SHBG | sex-hormone binding globulin |
| T | testosterone |
| TAG | triacyl-glycerol(s) |
| TRP | transient receptor protein |
| TRT | testosterone-replacement therapy |
| 11βHSDH | 11β-hydroxysteroid dehydrogenase |
| * non-standard abbreviations/acronyms. | |
References
- Comitato, R.; Saba, A.; Turrini, A.; Arganini, C.; Virgili, F. Sex hormones and macronutrient metabolism. Crit. Rev. Food Sci. Nutr. 2015, 55, 227–241. [Google Scholar] [CrossRef]
- Giudicelli, Y.; Dieudonne, M.N.; Lacasa, D.; Pasquier, Y.N.; Pecquery, R. Modulation by sex hormones of the membranous transducing system regulating fatty acid mobilization in adipose tissue. Prostaglandins Leukot. Essent. Fat. Acids 1993, 48, 91–100. [Google Scholar] [CrossRef]
- Remesar, X.; Alemany, M. Dietary energy partition: The central role of glucose. Int. J. Mol. Sci. 2020, 21, 7729. [Google Scholar] [CrossRef] [PubMed]
- Stumpf, W.E. Steroid hormones and the cardiovascular system: Direct actions of estradiol, progesterone, testosterone, gluco- and mineralcorticoids, and soltriol (vitamin D) on central nervous regulatory and peripheral tissues. Experientia 1990, 46, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Fietta, P.; Fietta, P.; Delsante, G. Central nervous system effects of natural and synthetic glucocorticoids. Psychiatry Clin. Neurosci. 2009, 63, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Hylemon, P.B. Bile acids are nutrient signaling hormones. Steroids 2014, 86, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Norman, A.W.; Henry, H.L. Vitamin D to 1,25-dihydroxycholecalciferol: Evolution of a steroid hormone. Trends Biochem. Sci. 1979, 4, 14–18. [Google Scholar] [CrossRef]
- Tintut, Y.; Demer, L.L. Potential impact of the steroid hormone, vitamin D, on the vasculature. Am. Heart J. 2021, 239, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Alemany, M. Estrogens and the regulation of glucose metabolism. World J. Diabetes 2021, 12, 1622–1654. [Google Scholar] [CrossRef] [PubMed]
- Barsoum, I.; Hung-Chang Yao, H. The road to maleness: From testis to Wolffian duct. Trends Endocrinol. Metab. 2006, 17, 223–228. [Google Scholar] [CrossRef][Green Version]
- Moos, W.H.; Dykens, J.A.; Nohynek, D.; Rubinchik, E.; Howell, N. Review of the effects of 17 α-estradiol in humans: A less feminizing estrogen with neuroprotective potential. Drug Dev. Res. 2009, 70, 23. [Google Scholar] [CrossRef]
- Van Pelt, R.E.; Gavin, K.M.; Kohrt, W.M. Regulation of body composition and bioenergetics by estrogens. Endocrinol. Metab. Clin. N. Am. 2015, 44, 663–676. [Google Scholar] [CrossRef] [PubMed]
- Turcu, A.F.; Nanba, A.T.; Auchus, R.J. The rise, fall, and resurrection of 11-oxygenated androgens in human physiology and disease. Horm. Res. Paediatr. 2018, 89, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.D.; Wu, Y.Q. Anabolic-androgenic steroids and cardiovascular risk. Chin. Med. J. 2019, 132, 2229–2236. [Google Scholar] [CrossRef]
- Song, S.H.; Sung, S.; Her, Y.S.; Oh, M.; Shin, D.H.; Lee, J.; Baek, J.; Lee, W.S.; Kim, D.S. Misuse of testosterone replacement therapy in men in infertile couples and its influence on infertility treatment. Clin. Exp. Reprod. Med. 2019, 46, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.L.; Casson, P.R.; Toth, M.J. Relationship of androgens to body composition, energy and substrate metabolism and aerobic capacity in healthy, young women. Steroids 2011, 76, 1247–1251. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lopez, M. Central regulation of energy metabolism by estrogens. Mol. Metab. 2018, 15, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Clegg, D.J.; Hevener, A.L. The role of estrogens in control of energy balance and glucose homeostasis. Endocr. Rev. 2013, 34, 309–338. [Google Scholar] [CrossRef]
- Harris, I.D.; Fronczak, C.; Roth, L.; Meacham, R.B. Fertility and the aging male. Rev. Urol. 2011, 13, e184–e190. [Google Scholar] [PubMed]
- Majer, I.M.; Nusselder, W.J.; Mackenbach, J.P.; Kunst, A.E. Life expectancy and life expectancy with disability of normal weight, overweight, and obese smokers and nonsmokers in Europe. Obesity 2011, 19, 1451–1459. [Google Scholar] [CrossRef]
- Konstantinov, V.V.; Deev, A.D.; Kapustina, A.V.; Shestov, D.B.; Timofeeva, T.N.; Lelchuk, I.N.; Balanova, Y.A.; Oganov, R.G. Prevalence of excessive body mass and its relation to mortality from cardiovascular and main chronic noninfectious diseases among urban male population of geographical different regions in Russia. Kardiologiya 2002, 42, 45–49. [Google Scholar] [PubMed]
- Tan, R.S.; Pu, S.J. Impact of obesity on hypogonadism in the andropause. Int. J. Androl. 2002, 25, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Carr, M.C. The emergence of the metabolic syndrome with menopause. J. Clin. Endocrinol. Metab. 2003, 88, 2404–2411. [Google Scholar] [CrossRef] [PubMed]
- Kuk, J.L.; Ardern, C.I. Age and sex differences in the clustering of metabolic syndrome factors. Association with mortality risk. Diabetes Care 2010, 33, 2457–2461. [Google Scholar] [CrossRef]
- Forti, P.; Pirazzoli, G.L.; Maltoni, B.; Bianchi, G.; Magalotti, D.; Muscari, A.; Mariani, E.; Ravaglia, G.; Zoli, M. Metabolic syndrome and all-cause mortality in older men and women. Eur. J. Clin. Invest. 2012, 42, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Guarner-Lans, V.; Rubio-Ruiz, M.E.; Pérez-Torres, I.; Baños de MacCarthy, G. Relation of aging and sex hormones to metabolic syndrome and cardiovascular disease. Exp. Gerontol. 2011, 46, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Lehmkuhl, E.; Weickert, M.O. Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clin. Res. Cardiol. 2006, 95, 136–147. [Google Scholar] [CrossRef]
- Korhonen, S.; Hippeläinen, M.; Vanhala, M.; Heinonen, S.; Niskanen, L. The androgenic sex hormone profile is an essential feature of metabolic syndrome in premenopausal women: A controlled community-based study. Fertil. Steril. 2003, 79, 1327–1334. [Google Scholar] [CrossRef]
- Markopoulos, M.C.; Kassi, E.; Alexandraki, K.I.; Mastorakos, G.; Kaltsas, G. Management of endocrine disease: Hyperandrogenism after menopause. Eur. J. Endocrinol. 2015, 172, R79–R91. [Google Scholar] [CrossRef]
- Tsujimura, A.; Miyagawa, Y.; Takezawa, K.; Okuda, H.; Fukuhara, S.; Kiuchi, H.; Takao, T.; Yamamoto, R.; Nishida, M.; Yamauchi-Takihara, K.; et al. Is low testosterone concentration a risk factor for metabolic syndrome in healthy middle-aged men? Urology 2013, 82, 814–819. [Google Scholar] [CrossRef]
- Dimopoulou, C.; Goulis, D.G.; Corona, G.; Maggi, M. The complex association between metabolic syndrome and male hypogonadism. Metab.-Clin. Exp. 2018, 86, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Blouin, K.; Despres, J.P.; Couillard, C.; Tremblay, A.; Prud’homme, D.; Bouchard, C.; Tchernof, A. Contribution of age and declining androgen levels to features of the metabolic syndrome in men. Metab. Clin. Exp. 2005, 54, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Chasland, L.C.; Yeap, B.B.; Maiorana, A.J.; Chan, Y.X.; Maslen, B.A.; Cooke, B.R.; Dembo, L.; Naylor, L.H.; Green, D.J. Testosterone and exercise: Effects on fitness, body composition, and strength in middle-to-older aged men with low-normal serum testosterone levels. Am. J. Physiol. 2021, 320, H1985–H1998. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.T.; Hildreth, K.L.; Pelak, V.S. Effects of testosterone therapy on cognitive function in aging: A systematic review. Cogn. Behav. Neurol. 2016, 29, 122–138. [Google Scholar] [CrossRef]
- Borràs, C.; Gambini, J.; López-Grueso, R.; Pallardó, F.V.; Viña, J. Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochim. Biophys. Acta 2010, 1802, 205–211. [Google Scholar] [CrossRef]
- Grande, G.; Barrachina, F.; Soler-Ventura, A.; Jodar, M.; Mancini, F.; Marana, R.; Chiloiro, S.; Pontecorvi, A.; Oliva, R.; Milardi, D. The role of testosterone in spermatogenesis: Lessons from proteome profiling of human spermatozoa in testosterone deficiency. Front. Endocrinol. 2022, 13, 852661. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, F.; Ye, L.; Zirkin, B.; Chen, H. Steroidogenesis in Leydig cells: Effects of aging and environmental factors. Reproduction 2017, 154, R111–R122. [Google Scholar] [CrossRef]
- Zwain, I.H.; Yen, S.S.C. Dehydroepiandrosterone: Biosynthesis and metabolism in the brain. Endocrinology 1999, 140, 880–887. [Google Scholar] [CrossRef]
- Blouin, K.; Veilleux, A.; Luu-The, V.; Tchernof, A. Androgen metabolism in adipose tissue: Recent advances. Mol. Cell. Endocrinol. 2009, 301, 97–103. [Google Scholar] [CrossRef]
- Karbowska, J.; Kochan, Z. Effect of DHEA on endocrine functions of adipose tissue, the involvement of PPARγ. Biochem. Pharmacol. 2005, 70, 249–257. [Google Scholar] [CrossRef]
- Hu, C.; Rusin, C.G.; Tan, Z.; Guagliardo, N.A.; Barrett, P.Q. Zona glomerulosa cells of the mouse adrenal cortex are intrinsic electrical oscillators. J. Clin. Invest. 2012, 122, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Koldzic-Zivanovic, N.; Tu, H.; Juelich, T.L.; Rady, P.L.; Tyring, S.K.; Hudnall, S.D.; Smith, E.M.; Hughes, T.K. Regulation of adrenal glucocorticoid synthesis by interleukin-10: A preponderance of IL-10 receptor in the adrenal zona fasciculata. Brain Behav. Immun. 2006, 20, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Endoh, A.; Kristiansen, S.B.; Casson, P.R.; Buster, J.E.; Hornsby, P.J. The zona reticularis is the site of biosynthesis of dehydroepiandrosterone and dehydroepiandrosterone sulfate in the adult human adrenal cortex resulting from its low expression of 3β-hydroxysteroid dehydrogenase. J. Clin. Endocrinol. Metab. 1996, 81, 3558–3565. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, T.; Sato, T.; Nemoto, T.; Nagata, S.; Imamichi, Y.; Kitano, T.; Sekiguchi, T.; Uwada, J.; Sayful Islam, M.; Mikami, D.; et al. 11-Ketotestosterone is a major androgen produced in porcine adrenal glands and testes. J. Steroid Biochem. Mol. Biol. 2021, 210, 105847. [Google Scholar] [CrossRef] [PubMed]
- Turcu, A.F.; Auchus, R.J. Clinical significance of 11-oxygenated androgens. Curr. Opin. Endocrinol. Diabetes Obes. 2017, 24, 252–259. [Google Scholar] [CrossRef]
- Meinhardt, U.; Mullis, P.E. The essential role of the aromatase/P450arom. Semin. Reprod. Med. 2002, 20, 277–284. [Google Scholar] [CrossRef]
- Charlier, T.D.; Cornil, C.A.; Ball, G.F.; Balthazart, J. Diversity of mechanisms involved in aromatase regulation and estrogen action in the brain. Biochim. Biophys. Acta 2010, 1800, 1094–1105. [Google Scholar] [CrossRef][Green Version]
- Cornil, C.A.; Charlier, T.D. Rapid behavioural effects of oestrogens and fast regulation of their local synthesis by brain aromatase. J. Neuroendocrinol. 2010, 22, 664–673. [Google Scholar] [CrossRef]
- Hojo, Y.; Murakami, G.; Mukai, H.; Higo, S.; Hatanaka, Y.; Ogiue-Ikeda, M.; Ishii, H.; Kimoto, T.; Kawato, S. Estrogen synthesis in the brain-Role in synaptic plasticity and memory. Mol. Cell. Endocrinol. 2008, 290, 31–43. [Google Scholar] [CrossRef]
- Stocco, C. Tissue physiology and pathology of aromatase. Steroids 2012, 77, 27–35. [Google Scholar] [CrossRef]
- Kroboth, P.D.; Salek, F.S.; Pittenger, A.L.; Fabian, T.J.; Frye, R.F. DHEA and DHEA-S: A review. J. Clin. Pharmacol. 1999, 39, 327–348. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.J.; Geoghegan, T.E.; Prough, R.A.; Michael Miller, K.K. The biological actions of dehydroepiandrosterone involves multiple receptors. Drug Metab. Rev. 2006, 38, 89–116. [Google Scholar] [CrossRef] [PubMed]
- Leszczynski, D.E.; Schafer, R.M.; Perkins, E.G.; Jerrell, J.P.; Kummerow, F.A. Esterificaction of dehydroepiandrosterone by human plasma HDL3. Biochim. Biophys. Acta 1989, 1014, 90–97. [Google Scholar] [CrossRef]
- Lavallée, B.; Provost, P.R.; Bélanger, A. Formation of pregnelonone- and dehydroepiandrosterone-fatty acid esters by lecithin-cholesterol acyltransferase in human plasma high density lipoproteins. Biochim. Biophys. Acta 1996, 1299, 306–312. [Google Scholar] [CrossRef]
- Lavallée, B.; Provost, P.R.; Roy, R.; Gauthier, M.-C.; Bélanger, A. Dehydroepiandrosterone-fatty acid esters in human plasma: Formation, transport and delivery to steroid target tissues. J. Endocrinol. 1996, 150, S119–S124. [Google Scholar] [CrossRef]
- Kalimi, M.; Shafagoj, Y.; Loria, R.; Padgett, D.; Regelson, W. Anti-glucocorticoid effects of dehydroepiandrosterone (DHEA). Mol. Cell. Biochem. 1994, 131, 99–104. [Google Scholar] [CrossRef] [PubMed]
- McNelis, J.C.; Manolopoulos, K.N.; Gathercole, L.L.; Bujalska, I.J.; Stewart, P.M.; Tomlinson, J.W.; Arlt, W. Dehydroepiandrosterone exerts antiglucocorticoid action on human preadipocyte proliferation, differentiation, and glucose uptake. Am. J. Physiol. 2013, 305, E1134–E1144. [Google Scholar] [CrossRef]
- Morales, A. The long and tortuous history of the discovery of testosterone and its clinical application. J. Sex. Med. 2013, 10, 1178–1183. [Google Scholar] [CrossRef]
- Nieschlag, E.; Nieschlag, S. The history of discovery, synthesis and development of testosterone for clinical use. Eur. J. Endocrinol. 2019, 180, R201–R212. [Google Scholar] [CrossRef]
- Vihma, V.; Tikkanen, M.J. Fatty acid esters of steroids: Synthesis and metabolism in lipoproteins and adipose tissue. J. Steroid Biochem. Mol. Biol. 2011, 124, 65–76. [Google Scholar] [CrossRef]
- De la Torre, X.; Segura, J.; Polettini, A.; Montagna, M. Detection of testosterone esters in human plasma. J. Mass Spectrom. 1995, 30, 1393–1404. [Google Scholar] [CrossRef]
- Kanji, S.S.; Kuohung, W.; Labaree, D.C.; Hochberg, R.B. Regiospecific esterification of estrogens by lecithin: Cholesterol acyltransferase. J. Clin. Endocrinol. Metab. 1999, 84, 2481–2488. [Google Scholar] [PubMed]
- Lund-Pero, M.; Jeppson, B.; Arneklo-Nobin, B.; Sjögren, H.O.; Holmgren, K.; Pero, R.W. Non-specific steroidal esterase activity and distribution in human and other mammalian tissues. Clin. Chim. Acta 1994, 224, 9–20. [Google Scholar] [CrossRef]
- Larner, J.M.; Pahuja, S.L.; Brown, V.M.; Hochberg, R.B. Aromatase and testosterone fatty acid esters: The search for a cryptic biosynthetic pathway to estradiol esters. Steroids 1992, 57, 475–479. [Google Scholar] [PubMed]
- Pretorius, E.; Arlt, W.; Storbeck, K.-H. A new dawn for androgens: Novel lessons from 11-oxygenated C19 steroids. Mol. Cell. Endocrinol. 2017, 441, 76–85. [Google Scholar] [CrossRef]
- Rege, J.; Turcu, A.; Kasa-Vubu, J.Z.; Lerario, A.M.; Auchus, G.C.; Auchus, R.J.; Smith, J.M.; White, P.C.; Rainey, W.E. 11-ketotestosterone is the dominant circulating bioactive androgen during normal and premature adrenarche. J. Clin. Endocrinol. Metab. 2018, 103, 4589–4598. [Google Scholar] [CrossRef]
- Imamichi, Y.; Yuhki, K.-I.; Orisaka, M.; Kitano, T.; Mukai, K.; Ushikubi, F.; Taniguchi, T.; Umezawa, A.; Miyamoto, K.; Yazawa, T. 11-Ketotestosterone is a major androgen produced in human gonads. J. Clin. Endocrinol. Metab. 2016, 101, 3582–3591. [Google Scholar] [CrossRef]
- Tsachaki, M.; Meyer, A.; Weger, B.; Kratschmar, D.V.; Tokarz, J.; Adamski, J.; Belting, H.-G.; Affolter, M.; Dickmeis, T.; Odermatt, A. Absence of 11-keto reduction of cortisone and 11-ketotestosterone in the model organism zebrafish. J. Endocrinol. 2017, 232, 323–335. [Google Scholar] [CrossRef][Green Version]
- Turcu, A.F.; Rege, J.; Auchus, R.J.; Rainey, W.E. 11-Oxygenated androgens in health and disease. Nat. Rev. Endocrinol. 2020, 16, 284–296. [Google Scholar] [CrossRef]
- Pretorius, E.; Africander, D.J.; Vlok, M.; Perkins, M.S.; Quanson, J.; Storbeck, K.-H. 11-Ketotestosterone and 11-ketodihydrotestosterone in castration resistant prostate cancer: Potent androgens which can no longer be ignored. PLoS ONE 2016, 11, e0159867. [Google Scholar] [CrossRef]
- Swerdloff, R.S.; Dudley, R.E.; Page, S.T.; Wang, C.; Salameh, W.A. Dihydrotestosterone: Biochemistry, physiology, and clinical implications of elevated blood levels. Endocr. Rev. 2017, 38, 220–254. [Google Scholar] [CrossRef]
- Roy, A.K.; Lavrovsky, Y.; Song, C.S.; Chen, S.; Jung, M.H.; Velu, N.K.; Bi, B.Y.; Chatterjee, B. Regulation of androgen action. Vitam. Horm. 1998, 55, 309–352. [Google Scholar] [CrossRef]
- Fang, H.; Tong, W.; Branham, W.S.; Moland, C.L.; Dial, S.L.; Hong, H.; Xie, Q.; Perkins, R.; Owens, W.; Sheehan, D.M. Study of 202 natural, synthetic, and environmental chemicals for binding to the androgen receptor. Chem. Res. Toxicol. 2003, 16, 1338–1358. [Google Scholar] [CrossRef] [PubMed]
- Auchus, R.J. The backdoor pathway to dihydrotestosterone. Trends Endocrinol. Metab. 2004, 15, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Swerdloff, R.S.; Wang, C. Dihydrotestosterone: A rationale for its use as a non-aromatizable androgen replacement therapeutic agent. Baillière’s Clin. Endocrinol. Metab. 1998, 12, 501–506. [Google Scholar] [CrossRef]
- Sartorius, G.A.; Ly, L.P.; Handelsman, D.J. Male sexual function can be maintained without aromatization: Randomized placebo-controlled trial of dihydrotestosterone (DHT) in healthy, older men for 24 months. J. Sex. Med. 2014, 11, 2562–2570. [Google Scholar] [CrossRef] [PubMed]
- Badawy, M.T.; Sobeh, M.; Xiao, J.; Farag, M.A. Androstenedione (a natural steroid and a drug supplement): A comprehensive review of its consumption, metabolism, health effects, and toxicity with sex differences. Molecules 2021, 26, 6210. [Google Scholar] [CrossRef] [PubMed]
- Flickinger, G.L.; Wu, C.-H.; Touchstone, J.C. Neutral steroid metabolites of dehydroepiandrosterone-7α-3H in human ovarian tissues. Steroids 1968, 11, 389–399. [Google Scholar] [CrossRef]
- Fogle, R.H.; Stanczyk, F.Z.; Zhang, X.; Paulson, R.J. Ovarian androgen production in postmenopausal women. J. Clin. Endocrinol. Metab. 2007, 92, 3040–3043. [Google Scholar] [CrossRef]
- Bloem, L.M.; Storbeck, K.-H.; Schloms, L.; Swart, A.C. 11β-Hydroxyandrostenedione returns to the steroid arena: Biosynthesis, metabolism and function. Molecules 2013, 18, 13228–13244. [Google Scholar] [CrossRef] [PubMed]
- Barnard, L.; du Toit, T.; Swart, A.C. Back where it belongs: 11β-hydroxyandrostenedione compels the re-assessment of C11-oxy androgens in steroidogenesis. Mol. Cell. Endocrinol. 2021, 525, 111189. [Google Scholar] [CrossRef] [PubMed]
- Gower, D.B. 16-Unsaturated C19 steroids a review of their chemistry, biochemistry and possible physiological role. J. Steroid Biochem. 1972, 3, 45–103. [Google Scholar] [CrossRef]
- Weusten, J.J.A.M.; Legemaat, G.; van der Wouw, M.P.M.E.; Smals, A.G.H.; Kloppenborg, P.W.C.; Benraad, T.J. The mechanism of the synthesis of 16-androstenes in human testicular homogenates. J. Steroid Biochem. 1989, 32, 689–694. [Google Scholar] [CrossRef]
- Dufort, I.; Soucy, P.; Lacoste, L.; Luu-The, V. Comparative biosynthetic pathway of androstenol and androgens. J. Steroid Biochem. Mol. Biol. 2001, 77, 223–227. [Google Scholar] [CrossRef]
- Semwal, A.; Kumar, R.; Teotia, U.V.S.; Singh, R. Pheromones and their role as aphrodisiacs: A review. J. Acute Dis. 2013, 2, 253–261. [Google Scholar] [CrossRef]
- Sobel, N.; Brown, W.M. The scented brain: Pheromonal responses in humans. Neuron 2001, 31, 512–514. [Google Scholar] [CrossRef]
- Wyart, C.; Webster, W.W.; Chen, J.H.; Wilson, S.R.; McClary, A.; Khan, R.M.; Sobel, N. Smelling a single component of male sweat alters levels of cortisol in women. J. Neurosci. 2007, 27, 1261–1265. [Google Scholar] [CrossRef]
- Sun, H.Z.; Zang, W.J.; Zhou, B.; Xu, L.; Wu, S.F. DHEA suppresses longitudinal bone growth by acting directly at growth plate through estrogen receptors. Endocrinology 2011, 152, 1423–1433. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Engdahl, C.; Lagerquist, M.K.; Stubelius, A.; Andersson, A.; Studer, E.; Ohlsson, C.; Westberg, L.; Carlsten, H.; Forsblad-d’Elia, H. Role of androgen and estrogen receptors for the action of dehydroepiandrosterone (DHEA). Endocrinology 2014, 155, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Langley, E.; Velazquez-Cruz, R.; Parra-Torres, A.; Enriquez, J. The non-aromatic Δ5-androstenediol derivative of dehydroepiandrosterone acts as an estrogen agonist in neonatal rat osteoblasts through an estrogen receptor α-related mechanism. Endocr. Res. 2019, 44, 87–102. [Google Scholar] [CrossRef] [PubMed]
- Osborne, D.M.; Edinger, K.; Frye, C.A. Chronic administration of androgens with actions at estrogen receptor beta have anti-anxiety and cognitive-enhancing effects in male rats. Age 2009, 31, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Frye, C.A.; Koonce, C.J.; Edinger, K.L.; Osborne, D.M.; Walf, A.A. Androgens with activity at estrogen receptor beta have anxiolytic and cognitive-enhancing effects in male rats and mice. Horm. Behav. 2008, 54, 726–734. [Google Scholar] [CrossRef]
- Wang, S.; Lai, K.; Moy, F.J.; Bhat, A.; Hartman, H.B.; Evans, M.J. The nuclear hormone receptor farnesoid X receptor (FXR) is activated by androsterone. Endocrinology 2006, 147, 4025–4033. [Google Scholar] [CrossRef] [PubMed]
- Maiworm, R.E.; Langthaler, W.U. Influence of androstenol and androsterone on the evalulation of men of varying attractiveness levels. In Chemical Signals in Vertebrates; Doty, R.L., Müller-Schwarze, D., Eds.; Springer: Boston, MA, USA, 1992; Volume 6, pp. 575–579. [Google Scholar]
- Büttner, A.; Thieme, D. Side effects of anabolic androgenic steroids: Pathological findings and structure–activity relationships. Handb. Exp. Pharmacol. 2010, 195, 459–484. [Google Scholar] [CrossRef]
- Sessa, F.; Salerno, M.; di Mizio, G.; Bertozzi, G.; Messina, G.; Tomaiuolo, B.; Pisanelli, D.; Maglietta, F.; Ricci, P.; Pomara, C. Anabolic androgenic steroids: Searching new molecular biomarkers. Front. Pharmacol. 2018, 9, 1321. [Google Scholar] [CrossRef] [PubMed]
- Sebo, Z.L.; Rodeheffer, M.S. Testosterone metabolites differentially regulate obesogenesis and fat distribution. Mol. Metab. 2021, 44, 101141. [Google Scholar] [CrossRef]
- Pingili, A.K.; Jennings, B.L.; Mukherjee, K.; Akroush, W.; Gonzalez, F.J.; Malik, K.U. 6β-Hydroxytestosterone, a metabolite of testosterone generated by CYP1B1, contributes to vascular changes in angiotensin II-induced hypertension in male mice. Biol. Sex Differ. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Clyne, C.; Rubin, G.; Boon, W.C.; Robertson, K.; Britt, K.; Speed, C.; Jones, M. Aromatase. A brief overview. Annu. Rev. Physiol. 2002, 64, 93–127. [Google Scholar] [CrossRef]
- Alexander, S.P.H.; Cidlowski, J.A.; Kelly, E.; Mathie, A.; Peters, J.A.; Veale, E.L.; Armstrong, J.F.; Faccenda, E.; Harding, S.D.; Pawson, A.J.; et al. The concise guide to pharmacology. 2019/20: Nuclear hormone receptors. Br. J. Pharmacol. 2019, 176, S229–S246. [Google Scholar] [CrossRef] [PubMed]
- Bleach, R.; McIlroy, M. The divergent function of androgen receptor in breast cancer; analysis of steroid mediators and tumor intracrinology. Front. Endocrinol. 2018, 9, 594. [Google Scholar] [CrossRef]
- Gao, W.; Bohl, C.E.; Dalton, J.T. Chemistry and structural biology of androgen receptor. Chem. Rev. 2005, 105, 3352–3370. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.Z.; Wardell, S.E.; Burnstein, K.L.; Defranco, D.; Fuller, P.J.; Giguere, V.; Hochberg, R.B.; McKay, L.; Renoir, J.-M.; Weigel, N.L.; et al. International Union of Pharmacology. LXV. The pharmacology and classification of the nuclear receptor superfamily: Glucocorticoid, mineralocorticoid, progesterone, and androgen receptors. Pharmacol. Rev. 2006, 58, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Gao, T.; McPhaul, M.J. Functional activities of the A and B forms of the human androgen receptor in response to androgen receptor agonists and antagonists. Mol. Endocrinol. 1998, 12, 654–663. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Davey, R.A.; Grossmann, M. Androgen receptor structure, function and biology: From bench to bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar] [PubMed]
- Brinkmann, A.O.; Faber, P.W.; van Rooij, H.C.J.; Kuiper, G.G.J.M.; Ris, C.; Klaassen, P.; van der Korput, J.A.G.M.; Voorhorst, M.M.; van Laar, J.H.; Mulder, E.; et al. The human androgen receptor: Domain structure, genomic organization and regulation of expression. J. Steroid Biochem. 1989, 34, 307–310. [Google Scholar] [CrossRef]
- McEwan, I.J. Molecular mechanisms of androgen receptor-mediated gene regulation: Structure-function analysis of the AF-1 domain. Endocr.-Relat. Cancer 2004, 11, 281–293. [Google Scholar] [CrossRef]
- Verrijdt, G. Selective DNA recognition by the androgen receptor as a mechanism for hormone-specific regulation of gene expression. Mol. Genet. Metab. 2003, 78, 175–185. [Google Scholar] [CrossRef]
- Gelmann, E.P. Molecular biology of the androgen receptor. J. Clin. Oncol. 2002, 20, 3001–3015. [Google Scholar] [CrossRef]
- Shaffer, P.L.; Jivan, A.; Dollins, D.E.; Claessens, F.; Gewirth, D.T. Structural basis of androgen receptor binding to selective androgen response elements. Proc. Natl. Acad. Sci. USA 2004, 101, 4758–4763. [Google Scholar] [CrossRef]
- Takane, K.K.; McPhaul, M.J. Functional analysis of the human androgen receptor promoter. Mol. Cell. Endocrinol. 1996, 119, 83–93. [Google Scholar] [CrossRef]
- Xiao, J.; Gong, A.-Y.; Eischeid, A.N.; Chen, D.; Deng, C.; Young, C.Y.F.; Chen, X.-M. miR-141 modulates androgen receptor transcriptional activity in human prostate cancer cells through targeting the small heterodimer partner protein. Prostate 2012, 72, 1514–1522. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Kagechika, H. Androgen receptor modulators: A review of recent patents and reports (2012–2018). Expert Opin. Ther. Pat. 2019, 29, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Verrijdt, G.; Schoenmakers, E.; Haelens, A.; Peeters, B.; Verhoeven, G.; Rombauts, W.; Claessens, F. Change of specificity mutations in androgen-selective enhancers. J. Biol. Chem. 2000, 275, 12298–12305. [Google Scholar] [CrossRef]
- Hunter, I.; Hay, C.W.; Esswein, B.; Watt, K.; McEwan, I.J. Tissue control of androgen action: The ups and downs of androgen receptor expression. Mol. Cell. Endocrinol. 2018, 465, 27–35. [Google Scholar] [CrossRef]
- Jenster, G.; Trapman, J.; Brinkmann, A.O. Nuclear import of the human androgen receptor. Biochem. J. 1993, 293, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Cutress, M.L.; Whitaker, H.C.; Mills, I.G.; Stewart, M.; Neal, D.E. Structural basis for the nuclear import of the human androgen receptor. J. Cell Sci. 2008, 121, 957–968. [Google Scholar] [CrossRef]
- Clinckemalie, L.; Vanderschueren, D.; Boonen, S.; Claessens, F. The hinge region in androgen receptor control. Mol. Cell. Endocrinol. 2012, 358, 1–8. [Google Scholar] [CrossRef]
- Veldscholte, J.; Berrevoets, C.A.; Zegers, N.D.; van der Kwast, T.H.; Grootegoed, J.A.; Mulder, E. Hormone-induced dissociation of the androgen receptor-heat-shock protein complex: Use of a new monoclonal antibody to distinguish transformed from nontransformed receptors. Biochemistry 1992, 31, 7422–7430. [Google Scholar] [CrossRef]
- Claessens, F.; Verrijdt, G.; Schoenmakers, E.; Haelens, A.; Peeters, B.; Verhoeven, G.; Rombauts, W. Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation. J. Steroid Biochem. Mol. Biol. 2001, 76, 23–30. [Google Scholar] [CrossRef]
- Pereira de Jésus-Tran, K.; Côté, P.L.; Cantin, L.; Blanchet, J.; Labrie, F.; Breton, R. Comparison of crystal structures of human androgen receptor ligand-binding domain complexed with various agonists reveals molecular determinants responsible for binding affinity. Protein Sci. 2006, 15, 987–999. [Google Scholar] [CrossRef]
- Askew, E.B.; Gampe, R.T.; Stanley, T.B.; Faggart, J.L.; Wilson, E.M. Modulation of androgen receptor activation function 2 by testosterone and dihydrotestosterone. J. Biol. Chem. 2007, 282, 25801–25816. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.E.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.-L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Dehm, S.M.; Tindall, D.J. Androgen receptor structural and functional elements: Role and regulation in prostate cancer. Mol. Endocrinol. 2007, 21, 2855–2863. [Google Scholar] [CrossRef] [PubMed]
- Azzouni, F.; Godoy, A.; Li, Y.; Mohler, J. The 5α-reductase isozyme family: A review of basic biology and their role in human diseases. Adv. Urol. 2012, 2012, 530121. [Google Scholar] [CrossRef]
- Dart, D.A.; Waxman, J.; Aboagye, E.O.; Bevan, C.L. Visualising androgen receptor activity in male and female mice. PLoS ONE 2013, 8, e71694. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Chodak, G.; Mutchnik, S.; Nakamoto, T.; Chang, C. Immunohistochemical localization of androgen receptors with mono- and polyclonal antibodies to androgen receptor. J. Endocrinol. 1990, 126, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Shibata, N.; Nagai, K.; Morita, Y.; Ujikawa, O.; Ohoka, N.; Hattori, T.; Koyama, R.; Sano, O.; Imaeda, Y.; Nara, H.; et al. Development of protein degradation inducers of androgen receptor by conjugation of androgen receptor ligands and inhibitor of apoptosis protein ligands. J. Med. Chem. 2018, 61, 543–575. [Google Scholar] [CrossRef]
- Li, J.; White, J.T.; Saavedra, H.; Wrabl, J.O.; Motlagh, H.N.; Liu, K.; Sowers, J.; Schroer, T.A.; Thompson, E.B.; Hilser, V.J. Genetically tunable frustration controls allostery in an intrinsically disordered transcription factor. eLife 2017, 6, e30688. [Google Scholar] [CrossRef]
- McEwan, I.J. Intrinsic disorder in the androgen receptor: Identification, characterisation and drugability. Mol. Biosyst. 2012, 8, 82–90. [Google Scholar] [CrossRef]
- Southren, A.L.; Gordon, G.G.; Tochimoto, S.; Pinzon, G.; Lane, D.R.; Stypulkowski, W. Mean plasma concentration, metabolic clearance and basal plasma production rates of testosterone in normal young men and women using a constant infusion procedure: Effect of time of day and plasma concentration on the metabolic clearance rate of testosterone. J. Clin. Endocrinol. 1967, 27, 686–694. [Google Scholar] [CrossRef]
- Vermeulen, A.; Verdonck, L. Studies on the binding of testosterone to human plasma. Steroids 1968, 11, 609–635. [Google Scholar] [CrossRef]
- Philip, A. Steroid Binding to Sex Hormone-Binding Globulin: Studies on Relative Binding of Estradiol and Testosterone, and Characterization of certain previously unrecognized ligands in pregnancy. Ph.D. Thesis, McGill University, Montreal, QC, Canada, 1988. Available online: https://escholarship.mcgill.ca/concern/theses/z890rw87g (accessed on 27 August 2022).
- Bardin, C.W.; Musto, N.; Gunsalus, G.; Kotite, N.; Cheng, S.L.; Larrea, F.; Becker, R. Extracellular androgen binding proteins. Annu. Rev. Physiol. 1981, 43, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.-E. Dehydroepiandrosterone (DHEA): A fountain of youth? J. Clin. Endocrinol. Metab. 1996, 81, 3147–3151. [Google Scholar] [CrossRef]
- Goodarzi, M.O.; Korenman, S.G. The importance of insulin resistance in polycystic ovary syndrome. Fertil. Steril. 2003, 80, 255–258. [Google Scholar] [CrossRef]
- Ducluzeau, P.H.; Cousin, P.; Malvoisin, E.; Bornet, H.; Vidal, H.; Laville, M.; Pugeat, M. Glucose-to-insulin ratio rather than sex hormone-binding globulin and adiponectin levels is the best predictor of insulin resistance in Nonobese women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2003, 88, 3626–3631. [Google Scholar] [CrossRef]
- Özturan, D.; Morova, T.; Lack, N.A. Androgen receptor-mediated transcription in prostate cancer. Cells 2022, 11, 898. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Lim, B.; Chi, B.H.; Lee, W.; Kim, J.H.; Kyung, Y.S.; You, D.; Kim, C.-S. The curative effect of androgen deprivation therapy alone is insufficient in high-risk prostate cancer. Medicine 2021, 100, e26833. [Google Scholar] [CrossRef]
- Cinar, O.; Turunc, T.; Kazaz, I.O.; Yildirim, O.; Deliktas, H.; Cihan, A.; Gudeloglu, A.; Ure, I.; Deveci, S.; Sahin, B.; et al. Effects of androgen deprivation therapy on cognitive functions in patients with metastatic prostate cancer: A multicentric, prospective study of the Society of Urological Surgery Andrology group. Int. J. Clin. Pract. 2021, 75, e14095. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Filippi, S.; Bianchi, N.; Dicuio, M.; Rastrelli, G.; Concetti, S.; Sforza, A.; Maggi, M. Cardiovascular risks of androgen deprivation therapy for prostate cancer. World J. Men’s Health 2021, 39, 429–443. [Google Scholar] [CrossRef]
- Lee, H.H.; Park, S.; Joung, J.Y.; Kim, S.H.; Lee, H. How does androgen deprivation therapy affect mental health including cognitive dysfunction in patients with prostate cancer? World J. Men’s Health 2021, 39, 598–605. [Google Scholar] [CrossRef]
- Negro-Vilar, A. Selective androgen receptor modulators (SARMs): A novel approach to androgen therapy for the new millennium. J. Clin. Endocrinol. Metab. 1999, 84, 3459–3462. [Google Scholar] [CrossRef] [PubMed]
- Zhi, L.; Martinborough, E. Selective androgen receptor modulators (SARMs). Annu. Rep. Med. Chem. 2001, 36, 169–180. [Google Scholar] [CrossRef]
- Mohler, M.L.; Bohl, C.E.; Jones, A.; Coss, C.C.; Narayanan, R.; He, Y.; Hwang, D.J.; Dalton, J.T.; Miller, D.D. Nonsteroidal selective androgen receptor modulators (SARMS): Dissociating the anabolic and androgenic activities of the androgen receptor for therapeutic benefit. J. Med. Chem. 2009, 52, 3597–3617. [Google Scholar] [CrossRef]
- Bhasin, S.; Jasuja, R. Selective androgen receptor modulators as function promoting therapies. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Wesley Peixoto da Fonseca, G.; Dworatzek, E.; Ebner, N.; von Haehling, S. Selective androgen receptor modulators (SARMs) as pharmacological treatment for muscle wasting in ongoing clinical trials. Expert Opin. Invest. Drugs 2020, 29, 881–891. [Google Scholar] [CrossRef] [PubMed]
- Estébanez-Perpiñá, E.; Bevan, C.L.; McEwan, I.J. Eighty years of targeting androgen receptor activity in prostate cancer: The fight goes on. Cancers 2021, 13, 509. [Google Scholar] [CrossRef] [PubMed]
- Nyquist, M.D.; Ang, L.S.; Corella, A.; Coleman, I.M.; Meers, M.P.; Christiani, A.J.; Pierce, C.; Janssens, D.H.; Meade, H.E.; Bose, A.; et al. Selective androgen receptor modulators activate the canonical prostate cancer androgen receptor program and repress cancer growth. J. Clin. Invest. 2021, 131, e146777. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Harada, S.-I.; Kimmel, D.B.; Bai, C.; Chen, F.; Rutledge, S.J.; Vogel, R.L.; Scafonas, A.; Gentile, M.A.; Nantermet, P.V.; et al. Identification of anabolic selective androgen receptor modulators with reduced activities in reproductive tissues and sebaceous glands. J. Biol. Chem. 2009, 284, 36367–36376. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Duan, M.; Fu, W.; Pang, J.; Tang, Q.; Sun, H.; Xu, L.; Chang, S.; Li, D.; Hou, T. Discovery of novel androgen receptor ligands by structure-based virtual screening and bioassays. Genom. Proteom. Bioinform. 2018, 16, 416–427. [Google Scholar] [CrossRef]
- Forsdahl, G.; Vatne, H.K.; Geisendorfer, T.; Gmeiner, G. Screening of testosterone esters in human plasma. Drug Test. Anal. 2013, 5, 826–833. [Google Scholar] [CrossRef] [PubMed]
- Borg, W.; Shackleton, C.H.L.; Pahuja, S.L.; Hochberg, R.B. Long-lived testosterone esters in the rat. Proc. Natl. Acad. Sci. USA 1995, 92, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.Y.; Zhu, B.T.; Conney, A.H. Effect of clofibrate administration on the esterification and deesterification of steroid hormones by liver and extrahepatic tissues in rats. Biochem. Pharmacol. 2002, 63, 985–992. [Google Scholar] [CrossRef]
- Remesar, X.; Fernández-López, J.A.; Alemany, M. Oleoyl-estrone. Med. Res. Rev. 2012, 32, 1263–1291. [Google Scholar] [CrossRef] [PubMed]
- Bailly, J.; Raab, S.; Clerc, R.; Sebokova, E.; Krust, A.; Chambon, P. The effect of oleoyl-estrone on body weight is mediated via the alpha estrogen receptor and not the beta estrogen receptor. Obes. Rev. 2005, 6, 48. [Google Scholar]
- Grasa, M.M.; Cabot, C.; Esteve, M.; Yubero, P.; Masanés, R.M.; Blay, M.T.; Vilà, R.; López-Martí, J.; Fernández-López, J.A.; Remesar, X.; et al. Daily oral oleoyl-estrone gavage induces a dose-dependent loss of fat in Wistar rats. Obes. Res. 2001, 9, 202–209. [Google Scholar] [CrossRef]
- Sanchis, D.; Balada, F.; Grasa, M.M.; Virgili, J.; Peinado, J.; Monserrat, C.; Fernández-López, J.A.; Remesar, X.; Alemany, M. Oleoyl-estrone induces the loss of body fat in rats. Int. J. Obes. 1996, 20, 588–594. [Google Scholar]
- Badeau, M.; Vihma, V.; Mikkola, T.S.; Tiitinen, A.; Tikkanen, M.J. Estradiol fatty acid esters in adipose tissue and serum of pregnant and pre- and postmenopausal women. J. Clin. Endocrinol. Metab. 2007, 92, 4327–4331. [Google Scholar] [CrossRef][Green Version]
- Mauvais-Jarvis, F. Sex differences in metabolic homeostasis, diabetes, and obesity. Biol. Sex Differ. 2015, 6, 14. [Google Scholar] [CrossRef] [PubMed]
- Vourc’h, C.; Eychenne, B.; Jo, D.-H.; Raulin, J.; Lapous, D.; Baulieu, E.-E.; Robel, P. Δ5-3β-Hydroxysteroid acyl transferase activity in the rat brain. Steroids 1992, 57, 210–215. [Google Scholar] [CrossRef]
- Robel, P.; Bourreau, E.; Corpéchot, C.; Dang, D.C.; Halberg, F.; Clarke, C.; Haug, M.; Schlegel, M.L.; Synguelakis, M.; Vourch, C.; et al. Neuro-steroids: 3β-hydroxy-Δ5-derivatives in rat and monkey brain. J. Steroid Biochem. 1987, 27, 649–655. [Google Scholar] [CrossRef]
- Smith, A.J.; Watson, T.G. The Δ5-3β-hydroxy steroid acyl transferase activities in tissues of the male rat and sheep. Steroids 1997, 62, 422–426. [Google Scholar] [CrossRef]
- Nieschlag, E.; Mauss, J.; Coert, A.; Kićović, P. Plasma androgen levels in men after oral administration of testosterone or testosterone undecanoate. Acta Endocrinol. 1975, 79, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Hammond, J.; Le, Q.; Goodyer, C.; Gelfand, M.; Trifiro, M.; LeBlanc, A. Testosterone-mediated neuroprotection through the androgen receptor in human primary neurons. J. Neurochem. 2001, 77, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Moenter, S.M.; Chu, Z. Rapid nongenomic effects of oestradiol on gonadotrophin-releasing hormone neurones. J. Neuroendocrinol. 2012, 24, 117–121. [Google Scholar] [CrossRef]
- Schwartz, N.; Verma, A.; Bivens, C.B.; Schwartz, Z.; Boyan, B.D. Rapid steroid hormone actions via membrane receptors. Biochim. Biophys. Acta 2016, 1863, 2289–2298. [Google Scholar] [CrossRef] [PubMed]
- Michels, G.; Hoppe, U.C. Rapid actions of androgens. Front. Neuroendocrinol. 2008, 29, 182–198. [Google Scholar] [CrossRef] [PubMed]
- Bauer, E.R.S.; Meyer, H.H.D.; Stahlschmidt-Allner, P.; Sauerwein, H. Application of an androgen receptor assay for the characterisation of the androgenic or antiandrogenic activity of various phenylurea herbicides and their derivatives. Analyst 1998, 123, 2485–2487. [Google Scholar] [CrossRef] [PubMed]
- Shihan, M.; Bulldan, A.; Scheiner-Bobis, G. Non-classical testosterone signaling is mediated by a G-protein-coupled receptor interacting with Gnα11. Biochim. Biophys. Acta 2014, 1843, 1172–1181. [Google Scholar] [CrossRef]
- Bajpai, P.; Koc, E.; Sonpavde, G.; Singh, R.; Singh, K.K. Mitochondrial localization, import, and mitochondrial function of the androgen receptor. J. Biol. Chem. 2019, 294, 6621–6634. [Google Scholar] [CrossRef] [PubMed]
- Yager, J.D.; Chen, J.Q. Mitochondrial estrogen receptors-new insights into specific functions. Trends Endocrinol. Metab. 2007, 18, 89–91. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P. Membrane androgen receptors unrelated to nuclear steroid receptors. Endocrinology 2019, 160, 772–781. [Google Scholar] [CrossRef]
- Ding, V.D.H.; Moller, D.E.; Feeney, W.P.; Didolkar, V.; Nakhla, A.M.; Rhodes, L.; Rosner, W.; Smith, R.G. Sex hormone-binding globulin mediates prostate androgen receptor action via a novel signaling pathway. Endocrinology 1998, 139, 213–218. [Google Scholar] [CrossRef][Green Version]
- Heinlein, C.A.; Chang, C. The roles of androgen receptors and androgen-binding proteins in nongenomic androgen actions. Mol. Endocrinol. 2002, 16, 2181–2187. [Google Scholar] [CrossRef]
- Kahn, S.M.; Hryb, D.J.; Nakhla, A.M.; Romas, N.A.; Rosner, W. Beyond carrier proteins: Sex hormone-binding globulin is synthesized in target cells. J. Endocrinol. 2002, 175, 113–120. [Google Scholar] [CrossRef]
- Fortunati, N.; Fissore, F.; Comba, A.; Becchis, M.; Catalano, M.G.; Fazzari, A.; Berta, L.; Frairia, R. Sex steroid-binding protein and its membrane receptor in estrogen-dependent breast cancer: Biological and pathophysiological impact. Horm. Res. 1996, 45, 202–206. [Google Scholar] [CrossRef]
- Rosner, W.; Hryb, D.J.; Khan, M.S.; Nakhla, A.M.; Romas, N.A. Sex hormone-binding globulin mediates steroid hormone signal transduction at the plasma membrane. J. Steroid Biochem. Mol. Biol. 1999, 69, 481–485. [Google Scholar] [CrossRef]
- Navarro, G.; Xu, W.; Jacobson, D.A.; Wicksteed, B.; Allard, C.; Zhang, G.; de Gendt, K.; Kim, H.S.; Wu, H.; Zhang, H.; et al. Extranuclear actions of the androgen receptor enhance glucose-stimulated insulin secretion in the male. Cell Metab. 2016, 23, 837–851. [Google Scholar] [CrossRef] [PubMed]
- Troncoso, M.F.; Pavez, M.; Wilson, C.; Lagos, D.; Duran, J.; Ramos, S.; Barrientos, G.; Silva, P.; Llanos, P.; Basualto-Alarcón, C.; et al. Testosterone activates glucose metabolism through AMPK and androgen signaling in cardiomyocyte hypertrophy. Biol. Res. 2021, 54, 3. [Google Scholar] [CrossRef] [PubMed]
- Gatson, J.W.; Kaur, P.; Singh, M. Dihydrotestosterone differentially modulates the mitogen-activated protein kinase and the phosphoinositide 3-kinase/akt pathways through the nuclear and novel membrane androgen receptor in C6 cells. Endocrinology 2006, 147, 2028–2034. [Google Scholar] [CrossRef]
- Thomas, P.; Pang, Y.; Dong, J. Membrane androgen receptor characteristics of human ZIP9 (SLC39A) zinc transporter in prostate cancer cells: Androgen-specific activation and involvement of an inhibitory G protein in zinc and MAP kinase signaling. Mol. Cell. Endocrinol. 2017, 447, 23–34. [Google Scholar] [CrossRef]
- Sun, Y.-H.; Gao, X.; Tang, Y.-J.; Xu, C.-L.; Wang, L.-H. Androgens induce increases in intracellular calcium via a G protein-coupled receptor in LNCaP prostate cancer cells. J. Androl. 2006, 27, 671–678. [Google Scholar] [CrossRef] [PubMed]
- Gorczynska, E.; Handelsman, D.J. Androgens rapidly increase the cytosolic calcium concentration in Sertoli cells. Endocrinology 1995, 136, 2052–2059. [Google Scholar] [CrossRef] [PubMed]
- Conde, K.; Fabelo, C.; Krause, W.C.; Propst, R.; Goethel, J.; Fischer, D.; Hur, J.; Meza, C.; Ingraham, H.A.; Wagner, E.J. Testosterone rapidly augments retrograde endocannabinoid signaling in proopiomelanocortin neurons to suppress glutamatergic input from steroidogenic factor 1 neurons via upregulation of diacylglycerol lipase-α. Neuroendocrinology 2017, 105, 341–356. [Google Scholar] [CrossRef]
- Castoria, G.; Auricchio, F.; Migliaccio, A. Extranuclear partners of androgen receptor: At the crossroads of proliferation, migration, and neuritogenesis. FASEB J. 2017, 31, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Dai, B.; Jiang, T.; Xu, K.; Xie, Y.; Kim, O.; Nesheiwat, I.; Kong, X.; Melamed, J.; Handratta, V.D.; et al. Regulation of androgen receptor activity by tyrosine phosphorylation. Cancer Cell 2006, 10, 309–319. [Google Scholar] [CrossRef]
- Xu, X.; de Pergola, G.; Björntorp, P. The effects of androgens on the regulation of lipolysis in adipose precursor cells. Endocrinology 1990, 126, 1229–1234. [Google Scholar] [CrossRef]
- Benten, W.P.M.; Lieberherr, M.; Giese, G.; Wrehlke, C.; Stamm, O.; Sekeris, C.E.; Mossmann, H.; Wunderlich, F. Functional testosterone receptors in plasma membranes of T cells. FASEB J. 1999, 13, 123–133. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.-R.; Chen, K.-H. The utilization of dehydroepiandrosterone as a sexual hormone precursor in premenopausal and postmenopausal women: An overview. Pharmaceuticals 2022, 15, 46. [Google Scholar] [CrossRef]
- Traish, A.M.; Kang, H.P.; Saad, F.; Guay, A.T. Dehydroepiandrosterone (DHEA)—A precursor steroid or an active hormone in human physiology (CME). J. Sex. Med. 2011, 8, 2960–2982. [Google Scholar] [CrossRef]
- Muller, C.; Hennebert, O.; Morfin, R. The native anti-glucocorticoid paradigm. J. Steroid Biochem. Mol. Biol. 2006, 100, 95–105. [Google Scholar] [CrossRef]
- Friess, E.; Schiffelholz, T.; Steckler, T.; Steiger, A. Dehydroepiandrosterone—A neurosteroid. Eur. J. Clin. Invest. 2000, 30, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Baulieu, E.-E.; Robel, P. Dehydroepiandrosterone (DHEA) and dehydroepiandrosterone sulfate (DHEAS) as neuroactive neurosteroids. Proc. Natl. Acad. Sci. USA 1998, 95, 4089–4091. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, I.; Charalampopoulos, I.; Alexaki, V.-I.; Avlonitis, N.; Pediaditakis, I.; Efstathopoulos, P.; Calogeropoulou, T.; Castanas, E.; Gravanis, A. Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis. PLoS Biol. 2011, 9, e1001051. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.D.; Demirgören, S.; Spivak, C.E.; London, E.D. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990, 526, 143–146. [Google Scholar] [CrossRef]
- Prough, R.A.; Clark, B.J.; Klinge, C.M. Novel mechanisms for DHEA action. J. Mol. Endocrinol. 2016, 56, R139–R155. [Google Scholar] [CrossRef]
- Cao, J.; Lu, M.; Yan, W.; Li, L.; Ma, H. Dehydroepiandrosterone alleviates intestinal inflammatory damage via GPR30-mediated Nrf2 activation and NLRP3 inflammasome inhibition in colitis mice. Free. Radic. Biol. Med. 2021, 172, 386–402. [Google Scholar] [CrossRef]
- Dutheil, F.; de Saint Vincent, S.; Pereira, B.; Schmidt, J.; Moustafa, F.; Charkhabi, M.; Bouillon-Minois, J.-B.; Clinchamps, M. DHEA as a biomarker of stress: A systematic review and meta-analysis. Front. Psychiatry 2021, 12, 688367. [Google Scholar] [CrossRef]
- Harada, K.; Hanayama, Y.; Obika, M.; Itoshima, K.; Okada, K.; Otsuka, F. Involvement of serum dehydroepiandrosterone sulfate in erythropoietic activity. Aging Male 2020, 23, 756–763. [Google Scholar] [CrossRef]
- Johnson, M.D.; Bebb, R.A.; Sirrs, S.M. Uses of DHEA in aging and other disease states. Ageing Res. Rev. 2002, 1, 29–41. [Google Scholar] [CrossRef]
- Pause, B.M. Are androgen steroids acting as pheromones in humans? Physiol. Behav. 2004, 83, 21–29. [Google Scholar] [CrossRef]
- Kohl, J.V.; Atzmueller, M.; Fink, B.; Grammer, K. Human pheromones: Integrating neuroendocrinology and ethology. Neuroendocrinol. Lett. 2011, 22, 309–321. [Google Scholar]
- Beier, K.; Ginez, I.; Schaller, H. Localization of steroid hormone receptors in the apocrine sweat glands of the human axilla. Histochem. Cell Biol. 2005, 123, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Body malodours and their topical treatment agents. Int. J. Cosmet. Sci. 2011, 33, 298–311. [Google Scholar] [CrossRef] [PubMed]
- Voznessenskaya, V.V.; Klyuchnikova, M.A.; Wysocki, C.J. Roles of the main olfactory and vomeronasal systems in the detection of androstenone in inbred strains of mice. Curr. Zool. 2010, 56, 813–818. [Google Scholar] [CrossRef]
- Monti-Bloch, L.; Jennings-White, C.; Dolberg, D.S.; Berliner, D.L. The human vomeronasal system. Psychoneuroendocrinology 1994, 19, 673–686. [Google Scholar] [CrossRef]
- Moran, D.T.; Jafek, B.W.; Rowley, J.C. The vomeronasal (Jacobson’s) organ in man: Ultrastructure and frequency of occurence. J. Steroid Biochem. Mol. Biol. 1991, 39, 545–552. [Google Scholar] [CrossRef]
- Bhutta, M.F. Sex and the nose: Human pheromonal responses. J. R. Soc. Med. 2007, 100, 268–274. [Google Scholar] [CrossRef]
- Rodriguez, I.; Greer, C.A.; Mok, M.Y.; Mombaerts, P. A putative pheromone receptor gene expressed in human olfactory mucosa. Nat. Genet. 2000, 26, 18–19. [Google Scholar] [CrossRef]
- Takeda, S.; Kadowaki, S.; Haga, T.; Takaesu, H.; Mitaku, S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002, 520, 97–101. [Google Scholar] [CrossRef]
- Yildirim, E.; Birnbaumer, L. TRPC2: Molecular biology and functional importance. Handb. Exp. Pharmacol. 2007, 179, 53–75. [Google Scholar] [CrossRef]
- Restrepo, D.; Delay, R.; Lin, W.; López, F.; Bacigalupo, J. TRP channels in transduction for responses to odorants and pheromones. In TRP Channels in Sensory Transduction; Madrid, R., Bacigalupo, J., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 111–125. [Google Scholar] [CrossRef]
- Precone, V.; Paolacci, S.; Beccari, T.; Dalla Ragione, L.; Stuppia, L.; Baglivo, M.; Guerri, G.; Manara, E.; Tonini, G.; Herbst, K. Pheromone receptors and their putative ligands: Possible role in humans. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 2140–2150. [Google Scholar]
- Kaminski, R.M.; Marini, H.; Ortinski, P.I.; Vicini, S.; Rogawski, M.A. The pheromone androstenol (5α-androst-16-en-3α-ol) is a neurosteroid positive modulator of GABAA receptors. J. Pharmacol. Exp. Ther. 2006, 317, 694–703. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Huang, X. Endocrine function of pheromones couples fat rationing and nutrient scarcity. Sci. China-Life Sci. 2022, 65, 1267–1269. [Google Scholar] [CrossRef] [PubMed]
- Carrier, N.; Saland, S.K.; Duclot, F.; He, H.; Mercer, R.; Kabbaj, M. The anxiolytic and antidepressant-like effects of testosterone and estrogen in gonadectomized male rats. Biol. Psychiatry 2015, 78, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Ramírez, A.; Morato, T.; Campos, R.; Rubio, I.; Calzada, C.; Méndez, E.; Ceballos, G. Acute effects of testosterone on intracellular Ca2+ kinetics in rat coronary endothelial cells are exerted via aromatization to estrogens. Am. J. Physiol. 2004, 287, H63–H71. [Google Scholar] [CrossRef][Green Version]
- Ottarsdottir, K.; Niisson, A.G.; Heilgren, M.; Lindblad, U.; Daka, B. The association between serum testosterone and insulin resistance: A longitudinal study. Endocr. Connect. 2018, 7, 1491–1500. [Google Scholar] [CrossRef]
- Zitzmann, M. Testosterone deficiency, insulin resistance and the metabolic syndrome. Nat. Rev. Endocrinol. 2009, 5, 673–681. [Google Scholar] [CrossRef]
- Song, D.Z.; Arikawa, E.; Galipeau, D.M.; Yeh, J.N.; Battell, M.L.; Yuen, V.G.; McNeill, J.H. Chronic estrogen treatment modifies insulin-induced insulin resistance and hypertension in ovariectomized rats. Am. J. Hypertens. 2005, 18, 1189–1194. [Google Scholar] [CrossRef][Green Version]
- Corbould, A. Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J. Endocrinol. 2007, 192, 585–594. [Google Scholar] [CrossRef]
- Gray, J.M.; Núñez, A.A.; Siegel, L.I.; Wade, G.N. Effects of testosterone on body weight and adipose tissue: Role of aromatization. Physiol. Behav. 1979, 23, 465–469. [Google Scholar] [CrossRef]
- Geer, E.B.; Islam, J.; Buettner, C. Mechanisms of glucocorticoid-induced insulin resistance focus on adipose tissue function and lipid metabolism. Endocrinol. Metab. Clin. N. Am. 2014, 43, 75–102. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Malacrida, B.; Oieni, J.; Serafini, M.M.; Davin, A.; Galbiati, V.; Corsini, E.; Racchi, M. DHEA modulates the effect of cortisol on RACK1 expression via interference with the splicing of the glucocorticoid receptor. Br. J. Pharmacol. 2015, 172, 2918–2927. [Google Scholar] [CrossRef] [PubMed]
- Welsh, T.H.; Bambino, T.H.; Hsueh, A.J.W. Mechanism of glucocorticoid-induced suppression of testicular androgen biosynthesis in vitro. Biol. Reprod. 1982, 27, 1138–1146. [Google Scholar] [CrossRef]
- Alemany, M. Do the interactions between glucocorticoids and sex hormones regulate the development of the metabolic syndrome? Front. Endocrinol. 2012, 3, 27. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Zhang, Y.; Xin, Y.; Chen, G.; Sun, X.; Chen, Y.; He, B. Dysfunction in Sertoli cells participates in glucocorticoid-induced impairment of spermatogenesis. Mol. Reprod. Dev. 2021, 88, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Turcu, A.F.; Nanba, A.T.; Chomic, R.; Upadhyay, S.K.; Giordano, T.J.; Shields, J.J.; Merke, D.P.; Rainey, W.E.; Auchus, R.J. Adrenal-derived 11-oxygenated 19-carbon steroids are the dominant androgens in classic 21-hydroxylase deficiency. Eur. J. Endocrinol. 2016, 174, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Stárka, L.; Dušková, M.; Vítků, J. 11-Keto-testosterone and other androgens of adrenal origin. Physiol. Res. 2020, 69, S187–S192. [Google Scholar] [CrossRef]
- Gomez-Sanchez, E.P.; Gomez-Sanchez, C.E. 11β-hydroxysteroid dehydrogenases: A growing multi-tasking family. Mol. Cell. Endocrinol. 2021, 526, 111210. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J.; Cooper, E.R.; Heather, A.K. Bioactivity of 11 keto and hydroxy androgens in yeast and mammalian host cells. J. Steroid Biochem. Mol. Biol. 2022, 218, 106049. [Google Scholar] [CrossRef] [PubMed]
- Mindnich, R.; Möller, G.; Adamski, J. The role of 17 beta-hydroxysteroid dehydrogenases. Mol. Cell. Endocrinol. 2004, 218, 7–20. [Google Scholar] [CrossRef]
- Draper, N.; Stewart, P.M. 11β-Hydroxysteroid dehydrogenase and the pre-receptor regulation of corticosteroid hormone action. J. Endocrinol. 2005, 186, 251–271. [Google Scholar] [CrossRef] [PubMed]
- Gibney, J.; Wolthers, T.; Johannsson, G.; Umpleby, A.M.; Ho, K.K.Y. Growth hormone and testosterone interact positively to enhance protein and energy metabolism in hypopituitary men. Am. J. Physiol. 2005, 289, E266–E271. [Google Scholar] [CrossRef]
- Kraemer, W.J.; Ratamess, N.A.; Hymer, W.C.; Nindl, B.C.; Fragala, M.S. Growth hormone(s), testosterone, insulin-like growth factors, and cortisol: Roles and integration for cellular development and growth with exercise. Front. Endocrinol. 2020, 11, 33. [Google Scholar] [CrossRef]
- Mazziotti, G.; Giustina, A. Glucocorticoids and the regulation of growth hormone secretion. Nat. Rev. Endocrinol. 2013, 9, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Lam, T.; Poljak, A.; McLean, M.; Bahl, N.; Ho, K.K.Y.; Birzniece, V. Testosterone prevents protein loss via the hepatic urea cycle in human. Eur. J. Endocrinol. 2017, 176, 489–496. [Google Scholar] [CrossRef][Green Version]
- Nasiri, M.; Nikolaou, N.; Parajes, S.; Krone, N.P.; Valsamakis, G.; Mastorakos, G.; Hughes, B.; Taylor, A.; Bujalska, I.J.; Gathercole, L.L.; et al. 5α-Reductase type 2 regulates glucocorticoid action and metabolic phenotype in human hepatocytes. Endocrinology 2015, 156, 2863–2871. [Google Scholar] [CrossRef] [PubMed]
- Qin, D.D.; Rizak, J.; Feng, X.L.; Yang, S.C.; Lu, L.B.; Pan, L.; Yin, Y.; Hu, X.T. Prolonged secretion of cortisol as a possible mechanism underlying stress and depressive behaviour. Sci. Rep. 2016, 6, 30187. [Google Scholar] [CrossRef]
- Zunszain, P.A.; Anacker, C.; Cattaneo, A.; Carvalho, L.A.; Pariante, C.M. Glucocorticoids, cytokines and brain abnormalities in depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2011, 35, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.C.; Yu, J.; Bi, J.H.; Qi, H.M.; Di, W.J.; Wu, L.; Wang, L.; Zha, J.M.; Lv, S.; Zhang, F.; et al. Glucocorticoids transcriptionally regulate miR-27b expression promoting body fat accumulation via suppressing the browning of white adipose tissue. Diabetes 2015, 64, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Holt, H.B.; Wild, S.H.; Postle, A.D.; Zhang, J.; Koster, G.; Umpleby, M.; Shojaee-Moradie, F.; Dewbury, K.; Wood, P.J.; Phillips, D.I.; et al. Cortisol clearance and associations with insulin sensitivity, body fat and fatty liver in middle-aged men. Diabetologia 2007, 50, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Burt, M.G.; Gibney, J.; Ho, K.K.Y. Protein metabolism in glucocorticoid excess: Study in Cushing’s syndrome and the effect of treatment. Am. J. Physiol. 2007, 292, E1426–E1432. [Google Scholar] [CrossRef] [PubMed]
- Griggs, R.C.; Kingston, W.; Jozefowicz, R.F.; Herr, B.E.; Forbes, G.; Halliday, D. Effect of testosterone on muscle mass and muscle protein synthesis. J. Appl. Physiol. 1989, 66, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.X.; Jiao, H.C.; Zhao, J.P.; Wang, X.J.; Lin, H. Glucocorticoids enhance muscle proteolysis through a myostatin-dependent pathway at the early stage. PLoS ONE 2016, 11, e0156225. [Google Scholar] [CrossRef] [PubMed]
- Tiao, G.; Fagan, J.; Roegner, V.; Lieberman, M.; Wang, J.J.; Fischer, J.E.; Hasselgren, P.-O. Energy-ubiquitin-dependent muscle proteolysis during sepsis in rats is regulated by glucocorticoids. J. Clin. Invest. 1996, 97, 339–348. [Google Scholar] [CrossRef]
- Bouman, A.; Schipper, M.; Heineman, M.J.; Faas, M.M. Gender difference in the non-specific and specific immune response in humans. Am. J. Reprod. Immunol. 2004, 52, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Boonekamp, J.J.; Ros, A.H.F.; Verhulst, S. Immune activation suppresses plasma testosterone level: A meta-analysis. Biol. Lett. 2008, 4, 741–744. [Google Scholar] [CrossRef]
- Trigunaite, A.; Dimo, J.; Jørgensen, T.N. Suppressive effects of androgens on the immune system. Cell. Immunol. 2015, 294, 87–94. [Google Scholar] [CrossRef]
- Cain, D.W.; Cidlowski, J.A. Immune regulation by glucocorticoids. Nat. Rev. Immunol. 2017, 17, 233–247. [Google Scholar] [CrossRef]
- Franchimont, D. Overview of the actions of glucocorticoids on the immune response. A good model to characterize new pathways of immunosuppression for new treatment strategies. Ann. N. Y. Acad. Sci. 2004, 1024, 124–137. [Google Scholar] [CrossRef] [PubMed]
- Machado Xavier, A.; Olimpio Anunciato, A.K.; Rosado Rosenstock, T.; Glezer, I. Gene expression control by glucocorticoid receptors during innate immune responses. Front. Endocrinol. 2016, 7, 31. [Google Scholar] [CrossRef]
- Oppong, E.; Cato, A.C.B. Effects of glucocorticoids in the immune system. Adv. Exp. Med. Biol. 2015, 872, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H.; Cheng, J. FSH and testosterone signaling in Sertoli cells. Reproduction 2005, 130, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H. Non-classical actions of testosterone and spermatogenesis. Phil. Trans. R. Soc. B 2010, 365, 1557–1569. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, F.; Tsiampali, C.; Chaliasos, N.; Tsounapi, P.; Takenaka, A.; Sofikitis, N. The Sertoli cell as the orchestra conductor of spermatogenesis: Spermatogenic cells dance to the tune of testosterone. Hormones 2015, 14, 479–503. [Google Scholar] [CrossRef]
- Ramaswamy, S.; Weinbauer, G.F. Endocrine control of spermatogenesis: Role of FSH and LH/ testosterone. Spermatogenesis 2014, 4, e996025. [Google Scholar] [CrossRef] [PubMed]
- Dierich, A.; Sairam, M.R.; Monaco, L.; Fimia, G.M.; Gansmuller, A.; LeMeur, M.; Sassone-Corsi, P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. USA 1998, 95, 13612–13617. [Google Scholar] [CrossRef]
- Wilson, J.D.; Griffin, J.E.; Leshin, M.; George, F.W. Role of gonadal hormones in development of the sexual phenotypes. Hum. Genet. 1981, 58, 78–84. [Google Scholar] [CrossRef]
- Marchetti, P.M.; Barth, J.H. Clinical biochemistry of dihydrotestosterone. Ann. Clin. Biochem. 2013, 50, 95–107. [Google Scholar] [CrossRef]
- McGinnis, M.Y.; Dreifuss, R.M. Evidence for a role of testosterone-androgen receptor interactions in mediating masculine sexual behavior in male rats. Endocrinology 1989, 124, 618–626. [Google Scholar] [CrossRef]
- Sinisi, A.A.; Pasquali, D.; Notaro, A.; Bellastella, A. Sexual differentiation. J. Endocrinol. Invest. 2003, 26, 23–28. [Google Scholar]
- Okeigwe, I.; Kuohung, W. 5-Alpha reductase deficiency: A 40-year retrospective review. Curr. Opin. Endocrinol. Diabetes Obes. 2014, 21, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.-J.; Imperato-Mcginley, J.; Zhu, Y.-S.; Rosenwaks, Z. The effect of 5α-reductase-2 deficiency on human fertility. Fertil. Steril. 2014, 101, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Schulster, M.; Bernie, A.M.; Ramasamy, R. The role of estradiol in male reproductive function. Asian J. Androl. 2016, 18, 435–440. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.; Robertson, K.M.; Jones, M.E.; Simpson, E.R. Estrogen and Spermatogenesis. Endocr. Rev. 2001, 22, 289–318. [Google Scholar] [CrossRef] [PubMed]
- Banner, A.; Frumin, I.; Shamay-Tsoory, S.G. Androstadienone, a chemosignal found in human sweat, increases individualistic behavior and decreases cooperative responses in men. Chem. Senses 2018, 43, 189–196. [Google Scholar] [CrossRef]
- Bone, C.; Anderson, C.; Lou, Y.; Squires, E.J. The characterization of androstenone transport in boar plasma. J. Steroid Biochem. Mol. Biol. 2019, 185, 218–224. [Google Scholar] [CrossRef]
- Brain, P.F. Androgens and human behaviour: A complex relationship. Behav. Brain Sci. 1998, 21, 363–364. [Google Scholar] [CrossRef]
- Simmons, Z.L.; Roney, J.R. Variation in CAG repeat length of the androgen receptor gene predicts variables associated with intrasexual competitiveness in human males. Horm. Behav. 2011, 60, 306–312. [Google Scholar] [CrossRef]
- Sherwin, B.B. A comparative analysis of the role of androgen in human male and female sexual behavior: Behavioral specificity, critical thresholds, and sensitivity. Psychobiology 1988, 16, 416–425. [Google Scholar] [CrossRef]
- Roney, J.R.; Gettler, L.T. The role of testosterone in human romantic relationships. Curr. Opin. Psychol. 2015, 1, 81–86. [Google Scholar] [CrossRef]
- Ketay, S.; Welker, K.M.; Slatcher, R.B. The roles of testosterone and cortisol in friendship formation. Psychoneuroendocrinology 2017, 76, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Ponzi, D.; Dandy, M. The influence of endogenous opioids on the relationship between testosterone and romantic bonding. Hum. Nat. 2019, 30, 98–116. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Sheng, F.; Belkaya, N.; Platt, M.L. Oxytocin and testosterone administration amplify viewing preferences for sexual images in male rhesus macaques. Philos. Trans. R. Soc. B 2022, 377, 20210133. [Google Scholar] [CrossRef] [PubMed]
- Grebe, N.M.; Sarafin, R.E.; Strenth, C.R.; Zilioli, S. Pair-bonding, fatherhood, and the role of testosterone: A meta-analytic review. Neurosci. Biobehav. Rev. 2019, 98, 221–233. [Google Scholar] [CrossRef]
- Bakermans-Kranenburg, M.J.; Verhees, M.W.F.T.; Lotz, A.M.; Alyousefi-van Dijk, K.; van IJzendoorn, M.H. Is paternal oxytocin an oxymoron? Oxytocin, vasopressin, testosterone, oestradiol and cortisol in emerging fatherhood. Phil. Trans. R. Soc. B 2022, 377. [Google Scholar] [CrossRef]
- Gettler, L.T.; Kuo, P.X.; Sarma, M.S.; Trumble, B.C.; Burke Lefever, J.E.; Braungart-Rieker, J.M. Fathers’ oxytocin responses to first holding their newborns: Interactions with testosterone reactivity to predict later parenting behavior and father-infant bonds. Dev. Psychobiol. 2021, 63, 1384–1398. [Google Scholar] [CrossRef]
- Mazur, A.; Booth, A. Testosterone and dominance in men. Behav. Brain Sci. 1998, 21, 353–397. [Google Scholar] [CrossRef]
- Pluchino, N.; Drakopoulos, P.; Bianchi-Demicheli, F.; Wenger, J.M.; Petignat, P.; Genazzani, A.R. Neurobiology of DHEA and effects on sexuality, mood and cognition. J. Steroid Biochem. Mol. Biol. 2015, 145, 273–280. [Google Scholar] [CrossRef]
- Spark, R.F. Dehydroepiandrosterone: A springboard hormone for female sexuality. Fertil. Steril. 2002, 77, 19–25. [Google Scholar] [CrossRef]
- Rothman, M.S.; Carlson, N.E.; Xu, M.; Wang, C.; Swerdloff, R.; Lee, P.; Goh, V.H.H.; Ridgway, E.C.; Wierman, M.E. Reexamination of testosterone, dihydrotestosterone, estradiol and estrone levels across the menstrual cycle and in postmenopausal women measured by liquid chromatography–tandem mass spectrometry. Steroids 2011, 76, 177–182. [Google Scholar] [CrossRef]
- Chrousos, G.P.; Peck, G.L.; Gross, E.G.; Cutler, G.B.; Loriaux, D.L. Adrenal function in women with idiopathic acne. J. Invest. Dermatol. 1982, 78, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Mannerås, L.; Cajander, S.; Holmäng, A.; Seleskovic, Z.; Lystig, T.; Lönn, M.; Stener-Victorin, E. A new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndrome. Endocrinology 2007, 148, 3781–3791. [Google Scholar] [CrossRef]
- Rastrelli, G.; Corona, G.; Maggi, M. Testosterone and sexual function in men. Maturitas 2018, 112, 46–52. [Google Scholar] [CrossRef]
- Davis, S.R.; Tran, J. Testosterone influences libido and well being in women. Trends Endocrinol. Metab. 2001, 12, 33–37. [Google Scholar] [CrossRef]
- Wåhlin-Jacobsen, S.; Tønnes Pedersen, A.; Kristensen, E.; Læssøe, N.C.; Lundqvist, M.; Cohen, A.S.; Hougaard, D.M.; Giraldi, A. Is there a correlation between androgens and sexual desire in women? J. Sex. Med. 2015, 12, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Caron, P.; Turcotte, V.; Guillemette, C. A quantitative analysis of total and free 11-oxygenated androgens and its application to human serum and plasma specimens using liquid-chromatography tandem mass spectrometry. J. Chromatogr. A 2021, 1650, 462228. [Google Scholar] [CrossRef] [PubMed]
- Hulshoff Pol, H.E.; Cohen-Kettenis, P.T.; van Haren, N.E.M.; Peper, J.S.; Brans, R.G.H.; Cahn, W.; Schnack, H.G.; Gooren, L.J.G.; Kahn, R.S. Changing your sex changes your brain: Influences of testosterone and estrogen on adult human brain structure. Eur. J. Endocrinol. 2006, 155, S107–S114. [Google Scholar] [CrossRef]
- Lombardo, M.V.; Ashwin, E.; Auyeung, B.; Chakrabarti, B.; Taylor, K.; Hackett, G.; Bullmore, E.T.; Baron-Cohen, S. Fetal testosterone influences sexually dimorphic gray matter in the human brain. J. Neurosci. 2012, 32, 674–680. [Google Scholar] [CrossRef]
- Celec, P.; Ostatníková, D.; Hodosy, J. On the effects of testosterone on brain behavioral functions. Front. Neurosci. 2015, 9, 12. [Google Scholar] [CrossRef]
- Savic, I.; Frisen, L.; Manzouri, A.; Nordenstrom, A.; Lindén Hirschberg, A. Role of testosterone and Y chromosome genes for the masculinization of the human brain. Hum. Brain Mapp. 2017, 38, 1801–1814. [Google Scholar] [CrossRef]
- Soma, K.K.; Rendon, N.M.; Boonstra, R.; Albers, H.E.; Demas, G.E. DHEA effects on brain and behavior: Insights from comparative studies of aggression. J. Steroid Biochem. Mol. Biol. 2015, 145, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Maggio, M.; de Vita, F.; Fisichella, A.; Colizzi, E.; Provenzano, S.; Lauretani, F.; Luci, M.; Ceresini, G.; dall’Aglio, E.; Caffarra, P.; et al. DHEA and cognitive function in the elderly. J. Steroid Biochem. Mol. Biol. 2015, 145, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Stoffel-Wagner, B. Neurosteroid metabolism in the human brain. Eur. J. Endocrinol. 2001, 145, 669–680. [Google Scholar] [CrossRef] [PubMed]
- Cumberland, A.L.; Hirst, J.J.; Badoer, E.; Wudy, S.A.; Greaves, R.F.; Zacharin, M.; Walker, D.W. The enigma of the adrenarche: Identifying the early life mechanisms and possible role in postnatal brain development. Int. J. Mol. Sci. 2021, 22, 4296. [Google Scholar] [CrossRef] [PubMed]
- Zirkin, B.R.; Tenover, J.L. Aging and declining testosterone: Past, present, and hopes for the future. J. Androl. 2012, 33, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Yuki, A.; Otsuka, R.; Kozakai, R.; Kitamura, I.; Okura, T.; Ando, F.; Shimokata, H. Relationship between low free testosterone levels and loss of muscle mass. Sci. Rep. 2013, 3, 1818. [Google Scholar] [CrossRef] [PubMed]
- Moctezuma-Velazquez, C.; Low, G.; Mourtzakis, M.; Ma, M.; Burak, K.W.; Tandon, P.; Montano-Loza, A.J. Association between low testosterone levels and sarcopenia in cirrhosis: A cross-sectional study. Ann. Hepatol. 2018, 17, 615–623. [Google Scholar] [CrossRef]
- Rodrigues dos Santos, M.; Storer, T.W. Testosterone treatment as a function-promoting therapy in sarcopenia associated with aging and chronic disease. Endocrinol. Metab. Clin. N. Am. 2022, 51, 187–204. [Google Scholar] [CrossRef]
- Sinclair, M.; Grossmann, M.; Hoermann, R.; Angus, P.W.; Gow, P.J. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: A randomised controlled trial. J. Hepatol. 2016, 65, 906–913. [Google Scholar] [CrossRef]
- Urban, R.J.; Bodenburg, Y.H.; Gilkison, C.; Foxworth, J.; Coggan, A.R.; Wolfe, R.R.; Ferrando, A. Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. Am. J. Physiol. 1995, 269, E820–E826. [Google Scholar] [CrossRef]
- Pal, M.; Khan, J.; Kumar, R.; Surolia, A.; Gupta, S. Testosterone supplementation improves insulin responsiveness in HFD fed male T2DM mice and potentiates insulin signaling in the skeletal muscle and C2C12 myocyte cell line. PLoS ONE 2019, 14, e0224162. [Google Scholar] [CrossRef]
- Giannoulis, M.G.; Jackson, N.; Shojaee-Moradie, F.; Nair, K.S.; Sonksen, P.H.; Martin, F.C.; Umpleby, A.M. The effects of growth hormone and/or testosterone on whole body protein kinetics and skeletal muscle gene expression in healthy elderly men: A randomized controlled trial. J. Clin. Endocrinol. Metab. 2008, 93, 3066–3074. [Google Scholar] [CrossRef] [PubMed]
- Gharahdaghi, N.; Phillips, B.E.; Szewczyk, N.J.; Smith, K.; Wilkinson, D.J.; Atherton, P.J. Links between testosterone, oestrogen, and the growth hormone/insulin-like growth factor axis and resistance exercise muscle adaptations. Front. Physiol. 2021, 11, 621226. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Sheffield-Moore, M.; Yeckel, C.W.; Gilkison, C.; Jiang, J.; Achacosa, A.; Lieberman, S.A.; Tipton, K.; Wolfe, R.R.; Urban, R.J. Testosterone administration to older men improves muscle function: Molecular and physiological mechanisms. Am. J. Physiol. 2002, 282, E601–E607. [Google Scholar] [CrossRef] [PubMed]
- Kruse, R.; Petersson, S.J.; Christensen, L.L.; Kristensen, J.M.; Sabaratnam, R.; Ørtenblad, N.; Andersen, M.; Højlund, K. Effect of long-term testosterone therapy on molecular regulators of skeletal muscle mass and fibre-type distribution in aging men with subnormal testosterone. Metab.-Clin. Exp. 2020, 112, 154347. [Google Scholar] [CrossRef] [PubMed]
- Kovacheva, E.L.; Hikim, A.P.S.; Shen, R.Q.; Sinha, I.; Sinha-Hikim, I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology 2010, 151, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Wendowski, O.; Redshaw, Z.; Mutungi, G. Dihydrotestosterone treatment rescues the decline in protein synthesis as a result of sarcopenia in isolated mouse skeletal muscle fibres. J. Cachexia Sarcopenia Muscle 2017, 8, 48–56. [Google Scholar] [CrossRef]
- Basaria, S.; Coviello, A.D.; Travison, T.G.; Storer, T.W.; Farwell, W.R.; Jette, A.M.; Eder, R.; Tennstedt, S.; Ulloor, J.; Zhang, A.; et al. Adverse events associated with testosterone administration. N. Engl. J. Med. 2010, 363, 109–122. [Google Scholar] [CrossRef]
- Evans, N.A. Current concepts in anabolic-androgenic steroids. Am. J. Sport. Med. 2004, 32, 534–542. [Google Scholar] [CrossRef]
- Nordström, A.; Hogström, G.; Eriksson, A.; Bonnerud, P.; Tegner, Y.; Malm, C. Higher muscle mass but lower gynoid fat mass in athletes using anabolic androgenic steroids. J. Strength Cond. Res. 2012, 26, 246–250. [Google Scholar] [CrossRef]
- Kanayama, G.; Brower, K.J.; Wood, R.I.; Hudson, J.I.; Pope Jr, H.G. Anabolic-androgenic steroid dependence: An emerging disorder. Addiction 2009, 104, 1966–1978. [Google Scholar] [CrossRef] [PubMed]
- Nottin, S.; Nguyen, L.D.; Terbah, M.; Obert, P. Cardiovascular effects of androgenic anabolic steroids in male bodybuilders determined by tissue Doppler imaging. Am. J. Cardiol. 2006, 97, 912–915. [Google Scholar] [CrossRef] [PubMed]
- Mauras, N.; Rini, A.; Welch, S.; Sager, B.; Murphy, S.P. Synergistic effects of testosterone and growth hormone on protein metabolism and body composition in prepubertal boys. Metabolism 2003, 52, 964–969. [Google Scholar] [CrossRef]
- Simon, D.; Charles, M.-A.; Lahlou, N.; Nahoul, K.; Oppert, J.-M.; Gouault-Heilmann, M.; Lemort, N.; Thibult, N.; Joubert, E.; Balkau, B.; et al. Androgen therapy improves insulin sensitivity and decreases leptin level in healthy adult men with low plasma total testosterone. Diabetes Care 2001, 24, 2149. [Google Scholar] [CrossRef] [PubMed]
- Dhindsa, S.; Ghanim, H.; Batra, M.; Kuhadiya, N.D.; Abuaysheh, S.; Sandhu, S.; Green, K.; Makdissi, A.; Hejna, J.; Chaudhuri, A.; et al. Insulin resistance and inflammation in hypogonadotropic hypogonadism and their reduction after testosterone replacement in men with type 2 diabetes. Diabetes Care 2016, 39, 82–91. [Google Scholar] [CrossRef]
- D’Andrea, S.; Martorella, A.; Coccia, F.; Castellini, C.; Minaldi, E.; Totaro, M.; Parisi, A.; Francavilla, F.; Francavilla, S.; Barbonetti, A. Relationship of vitamin D status with testosterone levels: A systematic review and meta-analysis. Endocrine 2021, 72, 49–61. [Google Scholar] [CrossRef]
- Bianchi, V.E. The anti inflammatory effects of testosterone. J. Endocr. Soc. 2019, 3, 91–107. [Google Scholar] [CrossRef]
- Srinivas-Shankar, U.; Wu, F.C.W. Drug insight: Testosterone preparations. Nat. Clin. Pract. Urol. 2006, 3, 653–665. [Google Scholar] [CrossRef] [PubMed]
- Fui, M.N.T.; Hoermann, R.; Cheung, A.S.; Gianatti, E.J.; Zajac, J.D.; Grossmann, M. Obesity and age as dominant correlates of low testosterone in men irrespective of diabetes status. Andrology 2013, 1, 906–912. [Google Scholar]
- Haring, R.; Völzke, H.; Felix, S.B.; Schipf, S.; Dörr, M.; Rosskopf, D.; Nauck, M.; Schöfl, C.; Wallaschofski, H. Prediction of metabolic syndrome by low serum testosterone levels in men results from the study of health in Pomerania. Diabetes 2009, 58, 2027–2031. [Google Scholar] [CrossRef]
- Frederiksen, L.; Højlund, K.; Hougaard, D.M.; Mosbech, T.H.; Larsen, R.; Flyvbjerg, A.; Frystyk, J.; Brixen, K.; Andersen, M. Testosterone therapy decreases subcutaneous fat and adiponectin in aging men. Eur. J. Endocrinol. 2012, 166, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.H.; Arver, S.; Behre, H.M.; Buvat, J.; Meuleman, E.; Moncada, I.; Morales, A.M.; Volterrani, M.; Yellowlees, A.; Howell, J.D.; et al. Testosterone replacement in hypogonadal men with type 2 diabetes and/or metabolic syndrome (the TIMES2 Study). Diabetes Care 2011, 34, 828–837. [Google Scholar] [CrossRef]
- Steidle, C.; Schwartz, S.; Jacoby, K.; Sebree, T.; Smith, T.; Bachand, R. AA2500 Testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J. Clin. Endocrinol. Metab. 2003, 88, 2673–2681. [Google Scholar] [CrossRef] [PubMed]
- Canguven, O.; Talib, R.A.; El Ansari, W.; Yassin, D.J.; Salman, M.; Al-Ansari, A. Testosterone therapy has positive effects on anthropometric measures, metabolic syndrome components (obesity, lipid profile, Diabetes Mellitus control), blood indices, liver enzymes, and prostate health indicators in elderly hypogonadal men. Andrologia 2017, 49, e12768. [Google Scholar] [CrossRef]
- Haider, A.; Saad, F.; Doros, G.; Gooren, L. Hypogonadal obese men with and without diabetes mellitus type 2 lose weight and show improvement in cardiovascular risk factors when treated with testosterone: An observational study. Obes. Res. Clin. Pract. 2014, 8, e339–e349. [Google Scholar] [CrossRef]
- Mårin, P.; Holmang, S.; Jonsson, L.; Sjostrom, L.; Kvist, H.; Holm, G.; Lindstedt, G.; Björntorp, P. The effects of testosterone treatment on body-composition and metabolism in middle-aged obese men. Int. J. Obes. 1992, 16, 991–997. [Google Scholar]
- Reddy, K.C.O.; Yadav, S.B. Effect of testosterone replacement therapy on insulin sensitivity and body composition in congenital hypogonadism: A prospective longitudinal follow-up study. J. Postgrad. Med. 2021, 67, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Rochira, V.; Balestrieri, A.; Madeo, B.; Zirilli, L.; Granata, A.R.M.; Carani, C. Osteoporosis and male age-related hypogonadism: Role of sex steroids on bone (patho)physiology. Eur. J. Endocrinol. 2006, 154, 175–185. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Thomas, M.C.; Panagiotopoulos, S.; Sharpe, K.; MacIsaac, R.J.; Clarke, S.; Zajac, J.D.; Jerums, G. Low testosterone levels are common and associated with insulin resistance in men with diabetes. J. Clin. Endocrinol. Metab. 2008, 93, 1834–1840. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M.; Saad, F.; Guay, A. The dark side of testosterone deficiency: II. Type 2 diabetes and insulin resistance. J. Androl. 2009, 30, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Gupta, S. Testosterone supplementation improves glucose homeostasis despite increasing hepatic insulin resistance in male mouse model of type 2 diabetes mellitus. Nutr. Diabetes 2016, 6, e236. [Google Scholar] [CrossRef]
- Heufelder, A.E.; Saad, F.; Bunck, M.C.; Gooren, L. Fifty-two-week treatment with diet and exercise plus transdermal testosterone reverses the metabolic syndrome and improves glycemic control in men with newly diagnosed type 2 diabetes and subnormal plasma testosterone. J. Androl. 2009, 30, 726–733. [Google Scholar] [CrossRef]
- Weiss, E.P.; Villareal, D.T.; Fontana, L.; Han, D.H.; Holloszy, J.O. Dehydroepiandrosterone (DHEA) replacement decreases insulin resistance and lowers inflammatory cytokines in aging humans. Aging-US 2011, 3, 533–542. [Google Scholar] [CrossRef]
- Li, L.L.; Yao, Y.; Zhao, J.L.; Cao, J.; Ma, H.T. Dehydroepiandrosterone protects against hepatic glycolipid metabolic disorder and insulin resistance induced by high fat via activation of AMPK-PGC-1 alpha-NRF-1 and IRS1-AKT-GLUT2 signaling pathways. Int. J. Obes. 2020, 44, 1075–1086. [Google Scholar] [CrossRef]
- Mårin, P.; Holmäng, S.; Gustafsson, C.; Jönsson, L.; Kvist, H.; Elander, A.; Eldh, J.; Sjöström, L.; Holm, G.; Björntorp, P. Androgen treatment of abdominally obese men. Obes. Res. 1993, 1, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Groti, K.; Žuran, I.; Antonič, B.; Foršnarič, L.; Pfeifer, M. The impact of testosterone replacement therapy on glycemic control, vascular function, and components of the metabolic syndrome in obese hypogonadal men with type 2 diabetes. Aging Male 2018, 21, 158–169. [Google Scholar] [CrossRef]
- Caliber, M.; Saad, F. Testosterone therapy for prevention and reversal of type 2 diabetes in men with low testosterone. Curr. Opin. Pharmacol. 2021, 58, 83–89. [Google Scholar] [CrossRef]
- Grishkovskaya, I.; Avvakumov, G.V.; Hammond, G.L.; Catalano, M.G.; Muller, Y.A. Steroid ligands bind human sex hormone-binding globulin in specific orientations and produce distinct changes in protein conformation. J. Biol. Chem. 2002, 277, 32086–32093. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, A.; Schwanz, H.A.; Spencer, D.J.; Bhasin, S.; Hamilton, J.A.; Jayaram, B.; Goldman, A.L.; Krishna, M.; Krishnan, M.; Shah, A.; et al. Allosterically coupled multisite binding of testosterone to human serum albumin. Endocrinology 2021, 162, bgaa199. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, W.Y.; de Veer, S.J.; Swedberg, J.E.; Hong, E.J.; Reid, J.C.; Walsh, T.P.; Hooper, J.D.; Hammond, G.L.; Clements, J.A.; Harris, J.M. Selective cleavage of human sex hormone-binding globulin by kallikrein-related peptidases and effects on androgen action in LNCaP Prostate cancer cells. Endocrinology 2012, 153, 3179–3189. [Google Scholar] [CrossRef]
- Round, P.; Das, S.; Wu, T.S.; Wahala, K.; Van Petegem, F.; Hammond, G.L. Molecular interactions between sex hormone-binding globulin and nonsteroidal ligands that enhance androgen activity. J. Biol. Chem. 2020, 295, 1202–1211. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Urban, R.J.; Dufau, M.L. Evidence that androgen negative feedback regulates hypothalamic gonadotropin-releasing hormone impulse strength and the burst-like secretion of biologically active luteinizing hormone in men. J. Clin. Endocrinol. Metab. 1992, 74, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Ryan, G.E.; Bohaczuk, S.C.; Cassin, J.; Witham, E.A.; Shojaei, S.; Ho, E.V.; Thackray, V.G.; Mellon, P.L. Androgen receptor positively regulates gonadotropin-releasing hormone receptor in pituitary gonadotropes. Mol. Cell. Endocrinol. 2021, 530, 111286. [Google Scholar] [CrossRef] [PubMed]
- Krsmanovic, L.Z.; Hu, L.; Leung, P.-K.; Feng, H.; Catt, K.J. The hypothalamic GnRH pulse generator: Multiple regulatory mechanisms. Trends Endocrinol. Metab. 2009, 20, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Popa, G. A portal circulation from the pituitary to the hypothalamic region. J. Anat. 1930, 65, 88–91. [Google Scholar]
- Ramakrishnappa, N.; Rajamahendran, R.; Lin, Y.-M.; Leung, P.C.K. GnRH in non-hypothalamic reproductive tissues. Anim. Reprod. Sci. 2005, 88, 95–113. [Google Scholar] [CrossRef]
- George, J.T.; Hendrikse, M.; Veldhuis, J.D.; Clarke, I.J.; Anderson, R.A.; Millar, R.P. Effect of gonadotropin-inhibitory hormone on luteinizing hormone secretion in humans. Clin. Endocrinol. 2017, 86, 731–738. [Google Scholar] [CrossRef]
- Koike, K.; Miyake, A.; Aono, T.; Sakamoto, T.; Ohmichi, M.; Yamaguchi, M.; Tanizaw, O. Effect of prolactin on the secretion of hypothalamic GnRH and pituitary gonadotropins. Horm. Res. 1991, 35, 5–12. [Google Scholar] [CrossRef]
- Breen, K.M.; Davis, T.L.; Doro, L.C.; Nett, T.M.; Oakley, A.E.; Padmanabhan, V.; Rispoli, L.A.; Wagenmaker, E.R.; Karsch, F.J. Insight into the neuroendocrine site and cellular mechanism by which cortisol suppresses pituitary responsiveness to gonadotropin-releasing hormone. Endocrinology 2008, 149, 767–773. [Google Scholar] [CrossRef]
- Gore, A.C.; Attardi, B.; DeFranco, D.B. Glucocorticoid repression of the reproductive axis: Effects on GnRH and gonadotropin subunit mRNA levels. Mol. Cell. Endocrinol. 2006, 256, 40–48. [Google Scholar] [CrossRef]
- Whirledge, S.; Cidlowski, J.A. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010, 35, 109–125. [Google Scholar] [PubMed]
- Calogero, A.E.; Gallucci, W.T.; Gold, P.W.; Chrousos, G.P. Multiple feedback regulatory loops upon rat hypothalamic corticotropin-releasing hormone secretion. Potential clinical implications. J. Clin. Invest. 1988, 82, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Dallman, M.F.; Akana, S.F.; Jacobson, L.; Levin, N.; Cascio, C.S.; Shinsako, J. Characterization of corticosterone feedback regulation of ACTH secretion. Ann. N. Y. Acad. Sci. 1987, 512, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Christensen, A.K. Morphometric analysis of Leydig cells in the normal rat testis. J. Cell Biol. 1980, 84, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Luu-The, V.; Bélanger, A.; Labrie, F. Androgen biosynthetic pathways in the human prostate. Best Pract. Res. Clin. Endocrinol. Metab. 2008, 22, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Turcu, A.; Smith, J.M.; Auchus, R.; Rainey, W.E. Adrenal androgens and androgen precursors-definition, synthesis, regulation and physiologic actions. Compr. Physiol. 2014, 4, 1369–1381. [Google Scholar] [CrossRef]
- Wen, X.; Li, D.; Tozer, A.J.; Docherty, S.M.; Iles, R.K. Estradiol, progesterone, testosterone profiles in human follicular fluid and cultured granulosa cells from luteinized pre-ovulatory follicles. Reprod. Biol. Endocrinol. 2010, 8, 117. [Google Scholar] [CrossRef]
- Hagen, F.S.; Arguelles, C.; Sui, L.M.; Zhang, W.; Seidel, P.R.; Conroy, S.C.; Petra, P.H. Mammalian expression of the human sex steroid-binding protein of plasma (SBP or SHBG) and testis (ABP)-characterization of the recombinant protein. FEBS Lett. 1992, 299, 23–27. [Google Scholar] [CrossRef][Green Version]
- Hansson, V.; Weddington, S.C.; Naess, O.; Attramadal, A.; French, F.S.; Kotite, N.; Nayfeh, S.N. Testicular androgen binding protein (ABP): A parameter of Sertoli cell secretory function. Curr. Top. Mol. Endocrinol. 1975, 2, 323–336. [Google Scholar] [CrossRef]
- Schock, H.W.; Herbert, Z.; Sigusch, H.; Figulla, H.R.; Jirikowski, G.F.; Lotze, U. Expression of androgen-binding protein (ABP) in human cardiac myocytes. Horm. Metab. Res. 2006, 38, 225–229. [Google Scholar] [CrossRef]
- Hansson, V.; Ritzen, M.E.; French, F.S.; Weddington, S.C.; Nayfeh, S.N. Testicular androgen-binding protein (ABP): Comparison of ABP in rabbit testis and epididymis with a similar androgen-binding protein (TeBG) in rabbit serum. Mol. Cell. Endocrinol. 1975, 3, 1–20. [Google Scholar] [CrossRef]
- Karn, R.C.; Yazdanifar, G.; Pezer, Z.; Boursot, P.; Laukaitis, C.M. Androgen-binding protein (ABP) evolutionary history: Has positive selection caused fixation of different paralogs in different taxa of the genus Mus? Genome Biol. Evol. 2021, 13, evab220. [Google Scholar] [CrossRef] [PubMed]
- Kirchhoff, J.; Soffie, M.; Rousseau, G.G. Differences in the steroid-binding site specificities of rat prostate androgen receptor and epididymal androgen-binding protein (ABP). J. Steroid Biochem. 1979, 10, 487–497. [Google Scholar] [CrossRef]
- Walker, W.H. Testosterone signaling and the regulation of spermatogenesis. Spermatogenesis 2011, 1, 116–120. [Google Scholar] [CrossRef]
- Walker, W.H. Androgen actions in the testis and the regulation of spermatogenesis. Adv. Exp. Med. Biol. 2021, 1288, 175–203. [Google Scholar] [CrossRef]
- Soules, M.R.; Steiner, R.A.; Clifton, D.K.; Cohen, N.L.; Aksel, S.; Bremner, W.J. Progesterone modulation of pulsatile luteinizing hormone secretion in normal women. J. Clin. Endocrinol. Metab. 1984, 58, 378–383. [Google Scholar] [CrossRef]
- Veldhuis, J.D.; Dufau, M.L. Steroidal regulation of biologically active luteinizing hormone secretion in men and women. Hum. Reprod. 1993, 8, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-Q.; Zhang, W.-D.; Yuan, B.; Zhang, J.-B. Advances in the regulation of mammalian follicle-stimulating hormone secretion. Animals 2021, 11, 1134. [Google Scholar] [CrossRef]
- Navarro, V.M.; Castellano, J.M.; Fernández-Fernández, R.; Tovar, S.; Roa, J.; Mayen, A.; Barreiro, M.L.; Casanueva, F.F.; Aguilar, E.; Diéguez, C.; et al. Effects of KiSS-1 peptide, the natural ligand of GPR54, on follicle-stimulating hormone secretion in the rat. Endocrinology 2005, 146, 1689–1697. [Google Scholar] [CrossRef]
- Ying, S.-Y. Inhibins, activins, and follistatins: Gonadal proteins modulating the secretion of follicle-stimulating hormone. Endocr. Rev. 1988, 9, 267–293. [Google Scholar] [CrossRef]
- Zhang, H.; Basit, A.; Busch, D.; Yabut, K.; Bhatt, D.K.; Drozdzik, M.; Ostrowski, M.; Li, A.; Collins, C.; Oswald, S.; et al. Quantitative characterization of UDP-glucuronosyltransferase 2B17 in human liver and intestine and its role in testosterone first -pass metabolism. Biochem. Pharmacol. 2018, 156, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Galetin, A.; Houston, J.B. Intestinal and hepatic metabolic activity of five cytochrome P450 enzymes: Impact on prediction of first-pass metabolism. J. Pharmacol. Exp. Ther. 2006, 318, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Vierhapper, H.; Nowotny, P.; Waldhäusl, W. Determination of testosterone production rates in men and women using stable isotope/dilution and mass spectrometry. J. Clin. Endocrinol. Metab. 1997, 82, 1492–1496. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grinspon, R.P.; Bergadá, I.; Rey, R.A. Male hypogonadism and disorders of sex development. Front. Endocrinol. 2020, 11, 211. [Google Scholar] [CrossRef] [PubMed]
- Jarow, J.P.; Zirkin, B.R. The androgen microenvironment of the human testis and hormonal control of spermatogenesis. Ann. N. Y. Acad. Sci. USA 2005, 1061, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.M.; McKinnell, C.; Kivlin, C.; Fisher, J.S. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction 2003, 125, 769–784. [Google Scholar] [CrossRef] [PubMed]
- Takihara, H.; Cosentino, M.J.; Sakatoku, J.; Cockett, A.T.K. Significance of testicular size measurement in andrology: II. Correlation of testicular size with testicular function. J. Urol. 1987, 137, 416–419. [Google Scholar] [CrossRef]
- Dhindsa, S.; Prabhakar, S.; Sethi, M.; Bandyopadhyay, A.; Chaudhuri, A.; Dandona, P. Frequent occurrence of hypogonadotropic hypogonadism in type 2 diabetes. J. Clin. Endocrinol. Metab. 2004, 89, 5462–5468. [Google Scholar] [CrossRef] [PubMed]
- Bakhtyukov, A.A.; Derkach, K.V.; Dar’in, D.V.; Sharova, T.S.; Shpakov, A.O. Decrease in the basal and luteinizing hormone receptor agonist-stimulated testosterone production in aging male rats. Adv. Gerontol. 2019, 9, 179–185. [Google Scholar] [CrossRef]
- Kaplan, S.A.; Meehan, A.G.; Shah, A. The age related decrease in testosterone is significantly exacerbated in obese men with the metabolic syndrome. What are the implications for the relatively high incidence of erectile dysfunction observed in these men? J. Urol. 2006, 176, 1524–1527. [Google Scholar] [CrossRef] [PubMed]
- Midzak, A.S.; Chen, H.L.; Papadopoulos, V.; Zirkin, B.R. Leydig cell aging and the mechanisms of reduced testosterone synthesis. Mol. Cell. Endocrinol. 2009, 299, 23–31. [Google Scholar] [CrossRef]
- Corona, G.; Monami, M.; Rastrelli, G.; Aversa, A.; Tishova, Y.; Saad, F.; Lenzi, A.; Forti, G.; Mannucci, E.; Maggi, M. Testosterone and metabolic syndrome: A meta-analysis study. J. Sex. Med. 2011, 8, 272–283. [Google Scholar] [CrossRef]
- Liang, J.; Peng, Q.; Yang, X.; Yang, C. The association between serum testosterone levels and metabolic syndrome among women. Diabetol. Metab. Syndr. 2021, 13, 26. [Google Scholar] [CrossRef]
- Saad, F.; Gooren, L. The role of testosterone in the metabolic syndrome: A review. J. Steroid Biochem. Mol. Biol. 2009, 114, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J.; Conway, A.J.; Boylan, L.M. Suppression of human spermatogenesis by testosterone implants. J. Clin. Endocrinol. Metab. 1992, 75, 1326–1332. [Google Scholar] [CrossRef]
- McBride, J.A.; Coward, R.M. Recovery of spermatogenesis following testosterone replacement therapy or anabolic-androgenic steroid use. Asian J. Androl. 2016, 18, 373–380. [Google Scholar] [CrossRef]
- Crosnoe, L.E.; Grober, E.; Ohl, D.; Kim, E.D. Exogenous testosterone: A preventable cause of male infertility. Transl. Androl. Urol. 2013, 2, 106–113. [Google Scholar] [CrossRef]
- Patel, A.S.; Leong, J.Y.; Ramos, L.; Ramasamy, R. Testosterone is a contraceptive and should not be used in men who desire fertility. World J. Men’s Health 2019, 37, 45–54. [Google Scholar] [CrossRef]
- Gill-Sharma, M.K. Testosterone retention mechanism in Sertoli cells: A biochemical perspective. Open Biochem. J. 2018, 12, 103–112. [Google Scholar] [CrossRef]
- Labrie, F. All sex steroids are made intracellularly in peripheral tissues by the mechanisms of intracrinology after menopause. J. Steroid Biochem. Mol. Biol. 2015, 145, 133–138. [Google Scholar] [CrossRef]
- Zhao, D.; Guallar, E.; Ouyang, P.; Subramanya, V.; Vaidya, D.; Ndumele, C.E.; Lima, J.A.; Allison, M.A.; Shah, S.J.; Bertoni, A.G.; et al. Endogenous sex hormones and incident cardiovascular disease in post-menopausal women. J. Am. Coll. Cardiol. 2018, 71, 2555–2566. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.B. The pathogenesis of polycystic ovary syndrome: Lessons from ovarian stimulation studies. J. Endocrinol. Invest. 1998, 21, 567–579. [Google Scholar] [CrossRef] [PubMed]
- Panidis, D.; Farmakiotis, D.; Rousso, D.; Katsikis, I.; Kourtis, A.; Diamanti-Kandarakis, E. Serum luteinizing hormone levels are markedly increased and significantly correlated with Δ4-androstenedione levels in lean women with polycystic ovary syndrome. Fertil. Steril. 2005, 84, 538–540. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, B.; Price, C.A. Insulin alters the effects of follicle stimulating hormone on aromatase in bovine granulosa cells in vitro. Steroids 2001, 66, 511–519. [Google Scholar] [CrossRef]
- Hsueh, A.J.W.; Erickson, G.F. Glucocorticoid inhibition of FSH-induced estrogen production in cultured rat granulosa cells. Steroids 1978, 32, 639–648. [Google Scholar] [CrossRef]
- Brown Séquard, C.E. Note on the effects produced on man by subcutaneous injections of a liquid obtained from the testicles of animals. Lancet 1889, 134, 105–107. [Google Scholar] [CrossRef]
- Freeman, E.R.; Bloom, D.A.; McGuire, E.J. A brief history of testosterone. J. Urol. 2001, 165, 371–373. [Google Scholar] [CrossRef] [PubMed]
- Joubert, S.W. Rejuvenation: Steinach vs. Voronoff. S. Afr. Med. J. 1928, 2, 8–10. [Google Scholar]
- Stanley, L.L. An analysis of one thousand testicular substance implantations. Endocrinology 1922, 6, 787–794. [Google Scholar] [CrossRef]
- Borrell, M. Brown-Séquard’s organotherapy and its appearance in America at the end of the nineteenth century. Bull. Hist. Med. 1976, 50, 309–320. [Google Scholar]
- David, K.; Dingemanse, E.; Freud, J.; Laqueur, E. Über krystallinisches männliches Hormon aus Hoden (Testosteron), wirksamer als aus Harn oder aus Cholesterin bereitetes Androsteron [About a crystalline male hormone from testicles (testosterone), more effective than androsterone prepared from urine or cholesterol]. Hoppe-Seyler Z. Physiol. Chem. 1935, 233, 281–283. [Google Scholar] [CrossRef]
- Butenandt, A.; Hanisch, G. Über Testosteron. Umwandlung des Dehydro-androsterons in Androstendiol und Testosteron; ein Weg zur Darstellung des Testosterons aus Cholesterin [About testosterone. conversion of dehydro-androsterone into androstenediol and testosterone; a way of preparing testosterone from cholesterol]. Hoppe-Seyler Z. Physiol. Chem. 1935, 237, 89–97. [Google Scholar] [CrossRef]
- Shinohara, Y.; Baba, S.; Kasuya, Y. Absorption, metabolism, and excretion of oral testosterone in humans by mass fragmentography. J. Clin. Endocrinol. Metab. 1980, 51, 1459–1462. [Google Scholar] [CrossRef] [PubMed]
- Farthing, M.J.; Vinson, G.P.; Edwards, C.R.; Dawson, A.M. Testosterone metabolism by the rat gastrointestinal tract, in vitro and in vivo. Gut 1982, 23, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, T.; Charman, W.N.A.; Stella, V.J. The effect of drug lipophilicity and lipid vehicles on the lymphatic absorption of various testosterone esters. Int. J. Pharm. 1985, 24, 173–184. [Google Scholar] [CrossRef]
- Birzniece, V. Hepatic actions of androgens in the regulation of metabolism. Curr. Opin. Endocrinol. Diabetes Obes. 2018, 25, 201–208. [Google Scholar] [CrossRef]
- Sohlenius-Sternbeck, A.-K.; Orzechowski, A. Characterization of the rates of testosterone metabolism to various products and of glutathione transferase and sulfotransferase activities in rat intestine and comparison to the corresponding hepatic and renal drug-metabolizing enzymes. Chem.-Biol. Interact. 2004, 148, 49–56. [Google Scholar] [CrossRef]
- Trane, I.; Sager, G.; Sveberg Dietrichs, E.; Westrheim Ravna, A. Molecular modeling study of the testosterone metabolizing enzyme UDP-glucuronosyltransferase 2B17. Bioorganic Med. Chem. 2021, 36, 116060. [Google Scholar] [CrossRef]
- Li, X.; Cheng, W.; Shang, H.; Wei, H.; Deng, C. The interplay between androgen and gut microbiota: Is there a microbiota-gut-testis axis. Reprod. Sci. 2022, 29, 1674–1684. [Google Scholar] [CrossRef]
- Korenman, S.G.; Wilson, H.; Lipsett, M.B. Testosterone production rates in normal adults. J. Clin. Invest. 1963, 42, 1753–1760. [Google Scholar] [CrossRef]
- Horton, R.; Shinsako, J.; Forsham, P.H. Testosterone production and metabolic clearance rates with volumes of distributon in normal adult men and women. Acta Endocrinol. 1965, 48, 446–458. [Google Scholar] [CrossRef] [PubMed]
- Southren, A.L.; Tochimoto, S.; Carmody, N.C.; Isurugi, K. Plasma production rates of testosterone in normal adult men and women and in patients with the syndrome of feminizing testes. J. Clin. Endocrinol. Metab. 1965, 25, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Knapstein, P.; Wortmann, W.; Krämer, C. Metabolism and plasma half life of [14C]testosterone plus [3H]testosterone [35S]sulfate perfused through human liver or injected intravenously. Hoppe-Seyler Z. Physiol. Chem. 1972, 353, 1447–1453. [Google Scholar] [CrossRef] [PubMed]
- Shoskes, J.J.; Wilson, M.K.; Spinner, M.L. Pharmacology of testosterone replacement therapy preparations. Transl. Androl. Urol. 2016, 5, 834–843. [Google Scholar] [CrossRef]
- Porst, H. Intramuscular testosterone treatment in elderly men: Evidence of memory decline and altered brain function. J. Sex. Med. 2008, 5, 534. [Google Scholar]
- Täuber, U.; Schröder, K.; Düsterberg, B.; Matthes, H. Absolute bioavailability of testosterone after oral administration of testosterone-undecanoate and testosterone. Eur. J. Drug Metab. Pharmacokinet. 1986, 11, 145–149. [Google Scholar] [CrossRef]
- Zgair, A.; Dawood, Y.; Ibrahem, S.M.; Back, H.-m.; Kagan, L.; Gershkovich, P.; Lee, J.B. Predicting intestinal and hepatic first-pass metabolism of orally administered testosterone undecanoate. Appl. Sci. 2020, 10, 7283. [Google Scholar] [CrossRef]
- Wang, C.; Ilani, N.; Arver, S.; McLachlan, R.I.; Soulis, T.; Watkinson, A. Efficacy and safety of the 2% formulation of testosterone topical solution applied to the axillae in androgen-deficient men. Clin. Endocrinol. 2011, 75, 836–843. [Google Scholar] [CrossRef]
- Tajbakhsh, M.; Saeedi, M.; Morteza-Semnani, K.; Akbari, J.; Nokhodchi, A. Innovation of testosome as a green formulation for the transdermal delivery of testosterone enanthate. J. Drug Deliv. Sci. Technol. 2020, 57, 101685. [Google Scholar] [CrossRef]
- Handelsman, D.J.; Conway, A.J.; Boylan, L.M. Pharmacokinetics and pharmacodynamics of testosterone pellets in man. J. Clin. Endocrinol. Metab. 1990, 71, 216–222. [Google Scholar] [CrossRef]
- Kesler, D.J.; Christensen, D.C.; Wallace, M.E. The effect of esterification on the release of testosterone and estradiol from silicone implants. Drug Dev. Ind. Pharm. 1996, 22, 275–279. [Google Scholar] [CrossRef]
- Spratt, D.I.; Stewart, I.I.; Savage, C.; Craig, W.; Spack, N.P.; Chandler, D.W.; Spratt, L.V.; Eimicke, T.; Olshan, J.S. Subcutaneous injection of testosterone is an effective and preferred alternative to intramuscular injection: Demonstration in female-to-male transgender patients. J. Clin. Endocrinol. Metab. 2017, 102, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Arver, S.; Dobs, A.S.; Meikle, A.W.; Caramelli, K.E.; Rajaram, L.; Sanders, S.W.; Mazer, N.A. Long-term efficacy and safety of a permeation-enhanced testosterone transdermal system in hypogonadal men. Clin. Endocrinol. 1997, 47, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Korbonits, M.; Slawik, M.; Cullen, D.; Ross, R.J.; Stalla, G.; Schneider, H.; Reincke, M.; Bouloux, P.M.; Grossman, A.B. A comparison of a novel testosterone bioadhesive buccal system, Striant, with a testosterone adhesive patch in hypogonadal males. J. Clin. Endocrinol. Metab. 2004, 89, 2039–2043. [Google Scholar] [CrossRef]
- Hattori, K.; Igarashi, M.; Itoh, M.; Tomisawa, H.; Tateishi, M. Specific induction by glucocorticoids of steroid esterase in rat hepatic microsomes and its release into serum. Biochem. Pharmacol. 1992, 43, 1921–1927. [Google Scholar] [CrossRef]
- Coert, A.; Geelen, J.; de Visser, J.; van der Vies, J. The pharmacology and metabolism of testosterone undecanoate (TU), a new orally active androgen. Acta Endocrinol. 1975, 79, 789–800. [Google Scholar] [CrossRef]
- Skakkebaek, N.E.; Bancroft, J.; Davidson, D.W.; Warner, P. Androgen replacement with oral testosterone undecanoate in hypogonadal men: A double blind controlled study. Clin. Endocrinol. 1981, 14, 49–61. [Google Scholar] [CrossRef]
- Edelstein, D.; Basaria, S. Testosterone undecanoate in the treatment of male hypogonadism. Expert Opin. Pharmacother. 2010, 11, 2095–2106. [Google Scholar] [CrossRef]
- Nieschlag, E.; Cüppers, H.J.; Wiegelmann, W.; Wickings, E.J. Bioavailability and LH-suppressing effect of different testosterone preparations in normal and hypogonadal men. Horm. Res. 1976, 7, 138–145. [Google Scholar] [CrossRef]
- Frisch, F.; Sumida, K.D. Temporal effects of testosterone propionate injections on serum lipoprotein concentrations in rats. Med. Sci. Sport. Exerc. 1999, 31, 664–669. [Google Scholar] [CrossRef]
- Sadowska-Krepa, E.; Klapcinska, B.; Jagsz, S.; Sobczak, A.; Chrapusta, S.J.; Chalimoniuk, M.; Grieb, P.; Poprzecki, S.; Langfort, J. High-dose testosterone propionate treatment reverses the effects of endurance training on myocardial antioxidant defenses in adolescent male rats. Cardiovasc. Toxicol. 2011, 11, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, Í.; Krause Neto, W.; dos Santos Portella Amorim, L.; Moraes Munhoz Ortiz, V.; Lopes Geraldo, V.; da Silva Ferreira, G.H.; Torres de Lima, J.; Alves Ribeiro Massoni, A.; Massensini Oliveira, B.; Anaruma, C.A.; et al. Previous short-term use of testosterone propionate enhances muscle hypertrophy in Wistar rats submitted to ladder-based resistance training. Tissue Cell 2022, 75, 101741. [Google Scholar] [CrossRef] [PubMed]
- Whitsel, E.A.; Boyko, E.J.; Matsumoto, A.M.; Anawalt, B.D.; Siscovick, D.S. Intramuscular testosterone esters and plasma lipids in hypogonadal men: A meta-analysis. Am. J. Med. 2001, 111, 261–269. [Google Scholar] [CrossRef]
- Kishimoto, Y. Fatty acid esters of testosterone in rat brain: Identification, distribution, and some properties of enzymes which synthesize and hydrolyze the esters. Arch. Biochem. Biophys. 1973, 159, 528–542. [Google Scholar] [CrossRef]
- Steinberger, E.; Smith, K.D. Effect of chronic administration of testosterone enanthate on sperm production and plasma testosterone, follicle-stimulating hormone, and luteinizing hormone levels: A preliminary evaluation of a possible male contraceptive. Fertil. Steril. 1977, 28, 1320–1328. [Google Scholar] [CrossRef]
- Snyder, P.J.; Lawrence, D.A. Treatment of male hypogonadism with testosterone enanthate. J. Clin. Endocrinol. Metab. 1980, 51, 1335–1339. [Google Scholar] [CrossRef]
- Bjelic, M.M.; Stojkov, N.J.; Radovic, S.M.; Baburski, A.Z.; Janjic, M.M.; Kostic, T.S.; Andric, S.A. Prolonged in vivo administration of testosterone-enanthate, the widely used and abused anabolic androgenic steroid, disturbs prolactin and cAMP signaling in Leydig cells of adult rats. J. Steroid Biochem. Mol. Biol. 2015, 149, 58–69. [Google Scholar] [CrossRef]
- Al-Futaisi, A.M.; Al-Zakwani, I.S.; Almahrezi, A.M.; Morris, D.J. Subcutaneous administration of testosterone. A pilot study report. Saudi Med. J. 2006, 27, 1843–1846. [Google Scholar]
- Nankin, H.R. Hormone kinetics after intramuscular testosterone cypionate. Fertil. Steril. 1987, 47, 1004–1009. [Google Scholar] [CrossRef]
- Schürmeyer, T.; Nieschlag, E. Comparative pharmacokinetics of testosterone enanthate and testosterone cyclohexanecarboxylate as assessed by serum and salivary testosterone levels in normal men. Int. J. Androl. 1984, 7, 181–187. [Google Scholar] [CrossRef]
- Brady, B.M.; Amory, J.K.; Perheentupa, A.; Zitzmann, M.; Hay, C.J.; Apter, D.; Anderson, R.A.; Bremner, W.J.; Pollanen, P.; Nieschlag, E.; et al. A multicentre study investigating subcutaneous etonogestrel implants with injectable testosterone decanoate as a potential long-acting male contraceptive. Hum. Reprod. 2006, 21, 285–294. [Google Scholar] [CrossRef]
- Al-Aubody, N.M.; Al-Diwan, M.A. Histopathological changes of liver, kidneys and heart of male rats caused by abusing of Sustanon® 250. Basrah J. Vet. Res. 2018, 17, 457–471. [Google Scholar]
- Jones, T.H. Testosterone replacement therapy. Br. J. Hosp. Med. 2007, 68, 547–553. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.Q.; Liang, X.W.; Wu, W.X.; Liu, M.L.; Song, S.X.; Cheng, L.F.; Bo, L.W.; Xiong, C.L.; Wang, X.H.; Liu, X.Z.; et al. Multicenter contraceptive efficacy trial of injectable testosterone undecanoate in Chinese men. J. Clin. Endocrinol. Metab. 2009, 94, 1910–1915. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.; Azad, S. Delayed oleoma formation with injection of oil-suspended testosterone: A case report and review of pathogenesis. SAGE Open Med. Case Rep. 2022, 10, 2050313x221086318. [Google Scholar] [CrossRef]
- Narayana, N.S.; Ly, L.P.; Jayadev, V.; Fennell, C.; Savkovic, S.; Conway, A.J.; Handelsman, D.J. Optimal injection interval for testosterone undecanoate treatment of hypogonadal and transgender men. Endocr. Connect. 2021, 10, 758–766. [Google Scholar] [CrossRef] [PubMed]
- Gooren, L.J.G.; Bunck, M.C.M. Transdermal testosterone delivery: Testosterone patch and gel. World J. Urol. 2003, 21, 316–319. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E. Testosterone treatment comes of age: New options for hypogonadal men. Clin. Endocrinol. 2006, 65, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Belkoff, L.; Brock, G.; Carrara, D.; Neijber, A.; Ando, M.; Mitchel, J. Efficacy and safety of testosterone replacement gel for treating hypogonadism in men: Phase III open-label studies. Andrologia 2018, 50, e12801. [Google Scholar] [CrossRef]
- Kamal, N.S.; Alayoubi, A.; Elfakhri, K.H.; Ibrahim, S.; Seggel, M.; Ashraf, M.; Zidan, A. Effects of formulation variables on the in vitro performance of testosterone transdermal gel. Int. J. Pharm. 2020, 590, 119951. [Google Scholar] [CrossRef] [PubMed]
- Retzler, J.; Smith, A.B.; Goncalves, A.S.O.; Whitty, J.A. Preferences for the administration of testosterone gel: Evidence from a discrete choice experiment. Patient Prefer. Adherence 2019, 13, 657–664. [Google Scholar] [CrossRef]
- Puiu, A.A.; Radke, S.; Votinov, M.; Habel, U.; Herpertz-Dahlmann, B.; Turetsky, B.; Konrad, K. Serum testosterone and cortisol concentrations after single-dose administration of 100-mg transdermal testosterone in healthy men. Front. Pharmacol. 2019, 10, 1397. [Google Scholar] [CrossRef] [PubMed]
- Rolf, C.; Nieschlag, E. Potential adverse effects of long-term testosterone therapy. Baillière’s Clin. Endocrinol. Metab. 1998, 12, 521–534. [Google Scholar] [CrossRef]
- Miller, M.G.; Rogol, A.D.; ZumBrunnen, T.L. Secondary exposure to testosterone from patients receiving replacement therapy with transdermal testosterone gels. Curr. Med. Res. Opin. 2012, 28, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Gronski, M.A.; Grober, E.D.; Gottesman, I.S.; Ormsby, R.W.; Bryson, N. Efficacy of nasal testosterone gel (natesto) stratified by baseline endogenous testosterone levels. J. Endocr. Soc. 2019, 3, 1652–1662. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Morley, J.E.; Niehoff, M.L.; Mattern, C. Delivery of testosterone to the brain by intranasal administration: Comparison to intravenous testosterone. J. Drug Target. 2009, 17, 91–97. [Google Scholar] [CrossRef]
- Dobs, A.S.; Hoover, D.R.; Chen, M.-C.; Allen, R. Pharmacokinetic characteristics, efficacy, and safety of buccal testosterone in hypogonadal males: A pilot study1. J. Clin. Endocrinol. Metab. 1998, 83, 33–39. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Narukawa, T.; Soh, J.; Kanemitsu, N.; Harikai, S.; Ukimura, O. Efficacy of combined treatment of intramuscular testosterone injection and testosterone ointment application for late-onset hypogonadism: An open-labeled, randomized, crossover study. Aging Male 2021, 23, 1059–1065. [Google Scholar] [CrossRef]
- Albert, S.G.; Morley, J.E. Testosterone therapy, association with age, initiation and mode of therapy with cardiovascular events: A systematic review. Clin. Endocrinol. 2016, 85, 436–443. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.; Wu, F.C.W.; Jones, T.H.; Jackson, G.; Behre, H.M.; Hackett, G.; Martin-Morales, A.; Balercia, G.; Dobs, A.S.; Arver, S.T.E.; et al. Testosterone treatment is not associated with increased risk of adverse cardiovascular events: Results from the Registry of Hypogonadism in Men (RHYME). Int. J. Clin. Pract. 2016, 70, 843–852. [Google Scholar] [CrossRef] [PubMed]
- McCullough, D.; Webb, R.; Enright, K.J.; Lane, K.E.; McVeigh, J.; Stewart, C.E.; Davies, I.G. How the love of muscle can break a heart: Impact of anabolic androgenic steroids on skeletal muscle hypertrophy, metabolic and cardiovascular health. Rev. Endocr. Metab. Disord. 2021, 22, 389–405. [Google Scholar] [CrossRef]
- Onasanya, O.; Iyer, G.; Lucas, E.; Lin, D.; Singh, S.; Alexander, G.C. Association between exogenous testosterone and cardiovascular events: An overview of systematic reviews. Lancet Diabetes Endocrinol. 2016, 4, 943–956. [Google Scholar] [CrossRef]
- Zelleroth, S.; Nylander, E.; Ortenblad, A.; Stam, F.; Nyberg, F.; Gronbladh, A.; Hallberg, M. Structurally different anabolic androgenic steroids reduce neurite outgrowth and neuronal viability in primary rat cortical cell cultures. J. Steroid Biochem. Mol. Biol. 2021, 210, 105863. [Google Scholar] [CrossRef] [PubMed]
- Hauger, L.E.; Havnes, I.A.; Jørstad, M.L.; Bjørnebekk, A. Anabolic androgenic steroids, antisocial personality traits, aggression and violence. Drug Alcohol Depend. 2021, 221, 108604. [Google Scholar] [CrossRef] [PubMed]
- Corona, G.; Rastrelli, G.; Marchiani, S.; Filippi, S.; Morelli, A.; Sarchielli, E.; Sforza, A.; Vignozzi, L.; Maggi, M. Consequences of anabolic-androgenic steroid abuse in males; sexual and reproductive perspective. World J. Men’s Health 2022, 40, 165–179. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Vilar Neto, J.; da Silva, C.A.; Bruno da Silva, C.A.; Pinto, D.V.; de Sá Roriz Caminha, J.; de Matos, R.S.; Nunes, J.C.C.; Alves, F.R.; Magalhães, S.C.; de Francesco Daher, E. Anabolic androgenic steroid-induced hypogonadism, a reversible condition in male individuals? A systematic review. Andrologia 2021, 53, e14062. [Google Scholar] [CrossRef]
- Falqueto, H.; Rodrigues dos Santos, M.; Manfredi, L.H. Anabolic-androgenic steroids and exercise training: Breaking the myths and dealing with better outcome in sarcopenia. Front. Physiol. 2022, 13, 838526. [Google Scholar] [CrossRef] [PubMed]
- Stancampiano, M.R.; Lucas-Herald, A.K.; Bryce, J.; Russo, G.; Barera, G.; Balsamo, A.; Baronio, F.; Bertelloni, S.; Valiani, M.; Cools, M.; et al. Testosterone therapy and its monitoring in adolescent boys with hypogonadism: Results of an international survey from the I-DSD registry. Sex. Dev. 2021, 15, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Travison, T.G.; Morley, J.E.; Araujo, A.B.; O’Donnell, A.B.; McKinlay, J.B. The relationship between libido and testosterone levels in aging men. J. Clin. Endocrinol. Metab. 2006, 91, 2509–2513. [Google Scholar] [CrossRef]
- Amano, T.; Imao, T.; Takemae, K.; Iwamoto, T.; Yamakawa, K.; Baba, K.; Nakanome, M.; Sugimori, H.; Tanaka, T.; Yoshida, K.; et al. Profile of serum testosterone levels after application of testosterone ointment (Glowmin) and its clinical efficacy in late-onset hypogonadism patients. J. Sex. Med. 2008, 5, 1727–1736. [Google Scholar] [CrossRef]
- Snyder, P.J.; Bhasin, S.; Cunningham, G.R.; Matsumoto, A.M.; Stephens-Shields, A.J.; Cauley, J.A.; Gill, T.M.; Barrett-Connor, E.; Swerdloff, R.S.; Wang, C.; et al. Effects of testosterone treatment in older men. N. Engl. J. Med. 2016, 374, 611–624. [Google Scholar] [CrossRef] [PubMed]
- Yassin, D.J.; Doros, G.; Hammerer, P.G.; Yassin, A.A. Long-term testosterone treatment in elderly men with hypogonadism and erectile dysfunction reduces obesity parameters and improves metabolic syndrome and health-related quality of life. J. Sex. Med. 2014, 11, 1567–1576. [Google Scholar] [CrossRef]
- Giltay, E.J.; Tishova, Y.A.; Mskhalaya, G.J.; Gooren, L.J.G.; Saad, F.; Kalinchenko, S.Y. Effects of testosterone supplementation on depressive symptoms and sexual dysfunction in hypogonadal men with the metabolic syndrome. J. Sex. Med. 2010, 7, 2572–2582. [Google Scholar] [CrossRef]
- Bhasin, S.; Seidman, S. Testosterone treatment of depressive disorders in men. JAMA Psychiatry 2019, 76, 9–10. [Google Scholar] [CrossRef]
- Wallis, C.J.D.; Lo, K.; Lee, Y.; Krakowsky, Y.; Garbens, A.; Satkunasivam, R.; Herschorn, S.; Kodama, R.T.; Cheung, P.; Narod, S.A.; et al. Survival and cardiovascular events in men treated with testosterone replacement therapy: An intention-to-treat observational cohort study. Lancet Diabetes Endocrinol. 2016, 4, 498–506. [Google Scholar] [CrossRef]
- Loo, S.Y.; Chen, B.Y.; Yu, O.H.Y.; Azoulay, L.; Renoux, C. Testosterone replacement therapy and the risk of stroke in men: A systematic review. Maturitas 2017, 106, 31–37. [Google Scholar] [CrossRef]
- Finkle, W.D.; Greenland, S.; Ridgeway, G.K.; Adams, J.L.; Frasco, M.A.; Cook, M.B.; Fraument, J.F.; Hoover, R.N. Increased risk of non-fatal myocardial infarction following testosterone therapy prescription in men. PLoS ONE 2014, 9, e85805. [Google Scholar] [CrossRef]
- Bhasin, S.; Brito, J.P.; Cunningham, G.R.; Hayes, F.J.; Hodis, H.N.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Wu, F.C.; Yialamas, M.A. Testosterone therapy in men with hypogonadism: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2018, 103, 1715–1744. [Google Scholar] [CrossRef]
- Bhasin, S. Testosterone replacement in aging men: An evidence-based patient-centric perspective. J. Clin. Invest. 2021, 131, e146607. [Google Scholar] [CrossRef]
- Abildgaard, J.; Petersen, J.H.; Bang, A.K.; Aksglaede, L.; Christiansen, P.; Juul, A.; Jørgensen, N. Long-term testosterone undecanoate treatment in the elderly testosterone deficient male: An observational cohort study. Andrology 2022, 10, 322–332. [Google Scholar] [CrossRef]
- Forsdahl, G.; Erceg, D.; Geisendorfer, T.; Turkalj, M.; Plavec, D.; Thevis, M.; Tretzel, L.; Gmeiner, G. Detection of testosterone esters in blood. Drug Test. Anal. 2015, 7, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Solheim, S.A.; Levernæs, M.C.S.; Mørkeberg, J.; Juul, A.; Upners, E.N.; Nordsborg, N.B.; Dehnes, Y. Stability and detectability of testosterone esters in dried blood spots after intramuscular injections. Drug Test. Anal. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wahjoepramono, E.J.; Asih, P.R.; Aniwiyanti, V.; Taddei, K.; Dhaliwal, S.S.; Fuller, S.J.; Foster, J.; Carruthers, M.; Verdile, G.; Sohrabi, H.R.; et al. The effects of testosterone supplementation on cognitive functioning in older men. CNS Neurol. Disord.-Drug Targets 2016, 15, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Kaweski, S. Anti-aging medicine: Hormone replacement therapy in men. Plast. Reconstr. Surg. 2004, 113, 1506–1510. [Google Scholar] [CrossRef]
- Cherrier, M.M.; Matsumoto, A.M.; Amory, J.K.; Ahmed, S.; Bremner, W.; Peskind, E.R.; Raskind, M.A.; Johnson, M.; Craft, S. The role of aromatization in testosterone supplementation. Effects on cognition in older men. Neurology 2005, 64, 290–296. [Google Scholar] [CrossRef]
- Nelson, J.A.; Strauss, L.; Skowronski, M.; Ciufo, R.; Novak, R.; McFadden, E.R. Effect of long-term salmeterol treatment on exercise-induced asthma. N. Engl. J. Med. 1998, 339, 141–146. [Google Scholar] [CrossRef]
- Kamp, G.A. High dose growth hormone treatment induces acceleration of skeletal maturation and an earlier onset of puberty in children with idiopathic short stature. Arch. Dis. Child. 2002, 87, 215–220. [Google Scholar] [CrossRef]
- Alemany, M.; Fernández-López, J.A.; Petrobelli, A.; Granada, M.; Foz, M.; Remesar, X. Pérdida de peso en un paciente con obesidad mórbida en tratamiento con oleoil-estrona (Weight loss in a patient with morbid obesity under treatment with oleoyl-estrone). Med. Clínica 2003, 121, 496–499. [Google Scholar] [CrossRef]
- Liu, P.Y.; Yee, B.; Wishart, S.M.; Jimenez, M.; Jung, D.G.; Grunstein, R.R.; Handelsman, D.J. The short-term effects of high-dose testosterone on sleep, breathing, and function in older men. J. Clin. Endocrinol. Metab. 2003, 88, 3605–3613. [Google Scholar] [CrossRef]
- Mayo, A.; Macintyre, H.; Wallace, A.M.; Ahmed, S.F. Transdermal testosterone application: Pharmacokinetics and effects on pubertal status, short-term growth, and bone turnover. J. Clin. Endocrinol. Metab. 2004, 89, 681–687. [Google Scholar] [CrossRef][Green Version]
- Tan, W.S.; Low, W.Y.; Ng, C.J.; Tan, W.K.; Tong, S.F.; Ho, C.; Khoo, E.M.; Lee, G.; Lee, B.C.; Lee, V.; et al. Efficacy and safety of long-acting intramuscular testosterone undecanoate in aging men: A randomised controlled study. Br. J. Urol. Int. 2013, 111, 1130–1140. [Google Scholar] [CrossRef] [PubMed]
- Partsch, C.-J.; Weinbauer, G.F.; Fang, R.; Nieschlag, E. Injectable testosterone undecanoate has more favourable pharmacokinetics and pharmacodynamics than testosterone enanthate. Eur. J. Endocrinol. 1995, 132, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Gooren, L.J.G. A ten-year safety study of the oral androgen testosterone undecanoate. J. Androl. 1994, 15, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Peachey, H.; Berlin, J.A.; Hannoush, P.; Haddad, G.; Dlewati, A.; Santanna, J.; Loh, L.; Lenrow, D.A.; Holmes, J.H.; et al. Effects of testosterone replacement in hypogonadal men. J. Clin. Endocrinol. Metab. 2000, 85, 2670–2677. [Google Scholar] [CrossRef] [PubMed]
- Magnussen, L.V.; Glintborg, D.; Hermann, P.; Hougaard, D.M.; Højlund, K.; Andersen, M. Effect of testosterone on insulin sensitivity, oxidative metabolism and body composition in aging men with type 2 diabetes on metformin monotherapy. Diabetes Obes. Metab. 2016, 18, 980–989. [Google Scholar] [CrossRef]
- Allan, C.A.; Strauss, B.J.G.; Burger, H.G.; Forbes, E.A.; McLachlan, R.I. Testosterone therapy prevents gain in visceral adipose tissue and loss of skeletal muscle in nonobese aging men. J. Clin. Endocrinol. Metab. 2008, 93, 139–146. [Google Scholar] [CrossRef]
- Barnouin, Y.; Armamento-Villareal, R.; Celli, A.; Jiang, B.; Paudyal, A.; Nambi, V.; Bryant, M.S.; Marcelli, M.; Garcia, J.M.; Qualls, C.; et al. Testosterone replacement therapy added to intensive lifestyle intervention in older men with obesity and hypogonadism. J. Clin. Endocrinol. Metab. 2021, 106, E1096–E1110. [Google Scholar] [CrossRef]
- Frederiksen, L.; Højlund, K.; Hougaard, D.M.; Brixen, K.; Andersen, M. Testosterone therapy increased muscle mass and lipid oxidation in aging men. Age 2012, 34, 145–156. [Google Scholar] [CrossRef]
- Magnussen, L.V.; Hvid, L.G.; Hermann, A.P.; Hougaard, D.M.; Gram, B.; Caserotti, P.; Andersen, M.S. Testosterone therapy preserves muscle strength and power in aging men with type 2 diabetes-a randomized controlled trial. Andrology 2017, 5, 946–953. [Google Scholar] [CrossRef]
- Howell, S.J.; Radford, J.A.; Adams, J.E.; Smets, E.M.A.; Warburton, R.; Shalet, S.M. Randomized placebo-controlled trial of testosterone replacement in men with mild Leydig cell insufficiency following cytotoxic chemotherapy. Clin. Endocrinol. 2001, 55, 315–324. [Google Scholar] [CrossRef]
- Snyder, P.J.; Peachey, H.; Berlin, J.A.; Rader, D.; Usher, D.; Loh, L.; Hannoush, P.; Dlewati, A.; Holmes, J.H.; Santanna, J.; et al. Effect of transdermal testosterone treatment on serum lipid and apolipoprotein levels in men more than 65 years of age. Am. J. Med. 2001, 111, 255–260. [Google Scholar] [CrossRef]
- Kenny, A.M.; Prestwood, K.M.; Gruman, C.A.; Fabregas, G.; Biskup, B.; Mansoor, G. Effects of transdermal testosterone on lipids and vascular reactivity in older men with low bioavailable testosterone levels. J. Gerontol. A 2002, 57, M460–M465. [Google Scholar] [CrossRef] [PubMed]
- English, K.M.; Steeds, R.P.; Jones, T.H.; Diver, M.J.; Channer, K.S. Low-dose transdermal testosterone therapy improves angina threshold in men with chronic stable angina. Circulation 2000, 102, 1906–1911. [Google Scholar] [CrossRef] [PubMed]
- Quang, L.M.; Kalhan, A. Cardiovascular benefits and risks of testosterone replacement therapy in hypogonadal men with type 2 diabetes mellitus and/or the metabolic syndrome: A systematic review. Br. J. Diabetes 2018, 18, 141–146. [Google Scholar] [CrossRef]
- Chasland, L.C.; Green, D.J.; Schlaich, M.P.; Maiorana, A.J.; Cooke, B.R.; Cox, K.L.; Naylor, L.H.; Yeap, B.B. Effects of testosterone treatment, with and without exercise training, on ambulatory blood pressure in middle-aged and older men. Clin. Endocrinol. 2021, 95, 176–186. [Google Scholar] [CrossRef]
- Shores, M.M.; Walsh, T.J.; Korpak, A.; Krakauer, C.; Forsberg, C.W.; Fox, A.E.; Moore, K.P.; Heckbert, S.R.; Thompson, M.L.; Smith, N.L.; et al. Association between testosterone treatment and risk of incident cardiovascular events among US male veterans with low testosterone levels and multiple medical comorbidities. J. Am. Heart Assoc. 2021, 10, e020562. [Google Scholar] [CrossRef]
- Budoff, M.J.; Ellenberg, S.S.; Lewis, C.E.; Mohler, E.R., III; Wenger, N.K.; Bhasin, S.; Barrett-Connor, E.; Swerdloff, R.S.; Stephens-Shields, A.; Cauley, J.A.; et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. J. Am. Med. Assoc. 2017, 317, 708–716. [Google Scholar] [CrossRef]
- Drinka, P.J.; Jochen, A.L.; Cuisinier, M.; Bloom, R.; Rudman, I.; Rudman, D. Polycythemia as a complication of testosterone replacement therapy in nursing home men with low testosterone levels. J. Am. Geriatr. Soc. 1995, 43, 899–901. [Google Scholar] [CrossRef]
- Malkin, C.J.; Pugh, P.J.; Morris, P.D.; Kerry, K.E.; Jones, R.D.; Jones, T.H.; Channer, K.S. Testosterone replacement in hypogonadal men with angina improves ischaemic threshold and quality of life. Heart 2004, 90, 871–876. [Google Scholar] [CrossRef]
- Aversa, A.; Isidori, A.M.; de Martino, M.U.; Caprio, M.; Fabbrini, E.; Rocchietti-March, M.; Frajese, G.; Fabbri, A. Androgens and penile erection: Evidence for a direct relationship between free testosterone and cavernous vasodilation in men with erectile dysfunction. Clin. Endocrinol. 2000, 53, 517–522. [Google Scholar] [CrossRef]
- Aversa, A.; Bruzziches, R.; Francomano, D.; Rosano, G.; Isidori, A.M.; Lenzi, A.; Spera, G. Effects of testosterone undecanoate on cardiovascular risk factors and atherosclerosis in middle-aged men with late-onset hypogonadism and metabolic syndrome: Results form a 24-month, randomized, double-blind, placebo-controlled study. J. Sex. Med. 2010, 7, 3495–3503. [Google Scholar] [CrossRef]
- Antonic, K.G.; Antonic, B.; Zuran, I.; Pfeifer, M. Testosterone treatment longer than 1 year shows more effects on functional hypogonadism and related metabolic, vascular, diabetic and obesity parameters (results of the 2-year clinical trial). Aging Male 2021, 23, 1442–1454. [Google Scholar] [CrossRef] [PubMed]
- Kato, Y.; Shigehara, K.; Nakashima, K.; Iijima, M.; Kawagushi, S.; Nohara, T.; Izumi, K.; Kadono, Y.; Konaka, H.; Namiki, M.; et al. The five-year effects of testosterone replacement therapy on lipid profile and glucose tolerance among hypogonadal men in Japan: A case control study. Aging Male 2020, 23, 23–28. [Google Scholar] [CrossRef]
- Yassin, A.A.; Alwani, M.; Talib, R.; Almehmadi, Y.; Nettleship, J.E.; Alrumaihi, K.; Albaba, B.; Kelly, D.M.; Saad, F. Long-term testosterone therapy improves liver parameters and steatosis in hypogonadal men: A prospective controlled registry study. Aging Male 2021, 23, 1553–1563. [Google Scholar] [CrossRef]
- Alwani, M.; Al-Zoubi, R.M.; Al-Qudimat, A.; Yassin, A.; Aboumarzouk, O.; Al-Rumaihi, K.; Talib, R.; Al-Ansari, A. The impact of long-term testosterone therapy (TTh) in renal function (RF) among hypogonadal men: An observational cohort study. Ann. Med. Surg. 2021, 69, 102748. [Google Scholar] [CrossRef] [PubMed]
- Saad, F.; Gooren, L.; Haider, A.; Yassin, A. An exploratory study of the effects of 12 month administration of the novel long-acting testosterone undecanoate on measures of sexual function and the metabolic syndrome. Arch. Androl. 2007, 53, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Young, N.R.; Baker, H.W.G.; Liu, G.D.; Seeman, E. Body-composition and muscle strength in healthy-men receiving testosterone enanthate for contraception. J. Clin. Endocrinol. Metab. 1993, 77, 1028–1032. [Google Scholar] [PubMed]
- Hackett, G.; Cole, N.; Bhartia, M.; Kennedy, D.; Raju, J.; Wilkinson, P.; Saghir, A. The response to testosterone undecanoate in men with type 2 diabetes is dependent on achieving threshold serum levels (the BLAST study). Int. J. Clin. Pract. 2014, 68, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Kalinchenko, S.Y.; Tishova, Y.A.; Mskhalaya, G.J.; Gooren, L.J.G.; Giltay, E.J.; Saad, F. Effects of testosterone supplementation on markers of the metabolic syndrome and inflammation in hypogonadal men with the metabolic syndrome: The double-blinded placebo-controlled Moscow study. Clin. Endocrinol. 2010, 73, 602–612. [Google Scholar] [CrossRef]
- Deng, Q.; Rasool, R.U.; Russell, R.M.; Natesan, R.; Asangani, I.A. Targeting androgen regulation of TMPRSS2 and ACE2 as a therapeutic strategy to combat COVID-19. iScience 2021, 24, 102254. [Google Scholar] [CrossRef]
- Haider, A.; Yassin, A.; Haider, K.S.; Doros, G.; Saad, F.; Rosano, G.M.C. Men with testosterone deficiency and a history of cardiovascular diseases benefit from long-term testosterone therapy: Observational, real-life data from a registry study. Vasc. Health Risk Manag. 2016, 12, 251–261. [Google Scholar] [CrossRef]
- Francomano, D.; Lenzi, A.; Aversa, A. Effects of five-year treatment with testosterone undecanoate on metabolic and hormonal parameters in ageing men with metabolic syndrome. Int. J. Endocrinol. 2014, 2014, 527470. [Google Scholar] [CrossRef]
- Mathur, A.; Malkin, C.; Saeed, B.; Muthusamy, R.; Jones, T.H.; Channer, K. Long-term benefits of testosterone replacement therapy on angina threshold and atheroma in men. Eur. J. Endocrinol. 2009, 161, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Wittert, G.A.; Chapman, I.M.; Haren, M.T.; Mackintosh, S.; Coates, P.; Morley, J.E. Oral testosterone supplementation increases muscle and decreases fat mass in healthy elderly males with low–normal gonadal status. J. Gerontol. A 2003, 58, M618–M625. [Google Scholar] [CrossRef]
- Boyanov, M.A.; Boneva, Z.; Christov, V.G. Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male 2003, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Perry, H.M., III; Kaiser, F.E.; Kraenzle, D.; Jensen, J.; Houston, K.; Mattammal, M.; Perry, H.M., Jr. Effects of testosterone replacement therapy in old hypogonadal males: A preliminary study. J. Am. Geriatr. Soc. 1993, 41, 149–152. [Google Scholar] [CrossRef]
- Hall, G.M.; Larbre, J.P.; Spector, T.D.; Perry, L.A.; da Silva, J.A.P. A randomized trial of testosterone therapy in males with rheumatoid arthritis. Br. J. Rheumatol. 1996, 35, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Page, S.T.; Amory, J.K.; Bowman, F.D.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J.; Tenover, J.L. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J. Clin. Endocrinol. Metab. 2005, 90, 1502–1510. [Google Scholar] [CrossRef]
- Casaburi, R.; Bhasin, S.; Cosentino, L.; Porszasz, J.; Somfay, A.; Lewis, M.I.; Fournier, M.; Storer, T.W. Effects of testosterone and resistance training in men with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004, 170, 870–878. [Google Scholar] [CrossRef] [PubMed]
- Giagulli, V.A.; Carbone, M.D.; Ramunni, M.I.; Licchelli, B.; de Pergola, G.; Sabbà, C.; Guastamacchia, E.; Triggiani, V. Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology 2015, 3, 1094–1103. [Google Scholar] [CrossRef]
- Ferrando, A.A.; Sheffield-Moore, M.; Paddon-Jones, D.; Wolfe, R.R.; Urban, R.J. Differential anabolic effects of testosterone and amino acid feeding in older men. J. Clin. Endocrinol. Metab. 2003, 88, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Svartberg, J.; Aasebø, U.; Hjalmarsen, A.; Sundsfjord, J.; Jorde, R. Testosterone treatment improves body composition and sexual function in men with COPD, in a 6-month randomized controlled trial. Respir. Med. 2004, 98, 906–913. [Google Scholar] [CrossRef]
- Sheffield-Moore, M.; Dillon, E.L.; Casperson, S.L.; Gilkison, C.R.; Paddon-Jones, D.; Durham, W.J.; Grady, J.J.; Urban, R.J. A randomized pilot study of monthly cycled testosterone replacement or continuous testosterone replacement versus placebo in older men. J. Clin. Endocrinol. Metab. 2011, 96, E1831–E1837. [Google Scholar] [CrossRef]
- Amory, J.K.; Chansky, H.A.; Chansky, K.L.; Camuso, M.R.; Hoey, C.T.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J. Preoperative supraphysiological testosterone in older men undergoing knee replacement surgery. J. Am. Geriatr. Soc. 2002, 50, 1698–1701. [Google Scholar] [CrossRef]
- Cheetham, T.C.; An, J.J.; Jacobsen, S.J.; Niu, F.; Sidney, S.; Quesenberry, C.P.; VanDenEeden, S.K. Association of testosterone replacement with cardiovascular outcomes among men with androgen deficiency. JAMA Intern. Med. 2017, 177, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Jaffe, M.D. Effect of testosterone cypionate on postexercise ST segment depression. Br. Heart J. 1977, 39, 1217–1222. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Zhao, Y.L.; Yang, Y.F.; Wang, X.; Nie, M.; Wu, X.Y.; Mao, J.F. Metabolic effects of testosterone replacement therapy in patients with type 2 diabetes mellitus or metabolic syndrome: A meta-analysis. Int. J. Endocrinol. 2020, 2020, 4732021. [Google Scholar] [CrossRef] [PubMed]
- Hackett, G.I. Testosterone replacement therapy and mortality in older men. Drug Saf. 2016, 39, 117–130. [Google Scholar] [CrossRef]
- Fernandez-Balsells, M.M.; Murad, M.H.; Lane, M.; Lampropulos, J.F.; Albuquerque, F.; Mullan, R.J.; Agrwal, N.; Elamin, M.B.; Gallegos-Orozco, J.F.; Wang, A.T.; et al. Adverse effects of testosterone therapy in adult men: A systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2010, 95, 2560–2575. [Google Scholar] [CrossRef] [PubMed]
- Snyder, P.J.; Peachey, H.; Hannoush, P.; Berlin, J.A.; Loh, L.; Holmes, J.H.; Dlewati, A.; Staley, J.; Santanna, J.; Kapoor, S.C.; et al. Effect of testosterone treatment on bone mineral density in men over 65 years of age1. J. Clin. Endocrinol. Metab. 1999, 84, 1966–1972. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, H.; Benoit, K.; Wang, W.; Motsko, S. Association between use of exogenous testosterone therapy and risk of venous thrombotic events among exogenous testosterone treated and untreated men with hypogonadism. J. Urol. 2016, 195, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Hackett, G.; Cole, N.; Saghir, A.; Jones, P.; Strange, R.C.; Ramachandran, S. Testosterone replacement therapy: Improved sexual desire and erectile function in men with type 2 diabetes following a 30-week randomized placebo-controlled study. Andrology 2017, 5, 905–913. [Google Scholar] [CrossRef] [PubMed]
- Hackett, G.; Heald, A.H.; Sinclair, A.; Jones, P.W.; Strange, R.C.; Ramachandran, S. Serum testosterone, testosterone replacement therapy and all-cause mortality in men with type 2 diabetes: Retrospective consideration of the impact of PDE5 inhibitors and statins. Int. J. Clin. Pract. 2016, 70, 244–253. [Google Scholar] [CrossRef]
- Wittert, G.; Bracken, K.; Robledo, K.P.; Grossmann, M.; Yeap, B.B.; Handelsman, D.J.; Stuckey, B.; Conway, A.; Inder, W.; McLachlan, R.; et al. Testosterone treatment to prevent or revert type 2 diabetes in men enrolled in a lifestyle programme (T4DM): A randomised, double-blind, placebo-controlled, 2-year, phase 3b trial. Lancet Diabetes Endocrinol. 2021, 9, 32–45. [Google Scholar] [CrossRef]
- Malkin, C.J.; Pugh, P.J.; Jones, R.D.; Kapoor, D.; Channer, K.S.; Jones, T.H. The effect of testosterone replacement on endogenous inflammatory cytokines and lipid profiles in hypogonadal men. J. Clin. Endocrinol. Metab. 2004, 89, 3313–3318. [Google Scholar] [CrossRef]
- Crawford, B.A.L.; Liu, P.Y.; Kean, M.T.; Bleasel, J.F.; Handelsman, D.J. Randomized placebo-controlled trial of androgen effects on muscle and bone in men requiring long-term systemic glucocorticoid treatment. J. Clin. Endocrinol. Metab. 2003, 88, 3167–3176. [Google Scholar] [CrossRef] [PubMed]
- Baillargeon, J.; Urban, R.J.; Kuo, Y.-F.; Ottenbacher, K.J.; Raji, M.A.; Du, F.; Lin, Y.-l.; Goodwin, J.S. Risk of myocardial infarction in older men receiving testosterone therapy. Ann. Pharmacother. 2014, 48, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B.; Nieschlag, E. Testosterone therapy in the aging male. Aging Male 2007, 10, 139–153. [Google Scholar] [CrossRef]
- Hackett, G. Metabolic effects of testosterone therapy in men with type 2 diabetes and metabolic syndrome. Sex. Med. Rev. 2019, 7, 476–490. [Google Scholar] [CrossRef]
- Anderson, J.L.; May, H.T.; Lappé, D.L.; Bair, T.; Le, V.; Carlquist, J.F.; Muhlestein, J.B. Impact of testosterone replacement therapy on myocardial infarction, stroke, and death in men with low testosterone concentrations in an integrated health care system. Am. J. Cardiol. 2016, 117, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Weng, X. Therapeutic effect of andriol on serum lipids and apolipoproteins in elderly male coronary heart disease patients. Chin. Med. Sci. J. 1992, 7, 137–141. [Google Scholar] [PubMed]
- Aversa, A.; Isidori, A.M.; Spera, G.; Lenzi, A.; Fabbri, A. Androgens improve cavernous vasodilation and response to sildenafil in patients with erectile dysfunction. Clin. Endocrinol. 2003, 58, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Calof, O.M.; Singh, A.B.; Lee, M.L.; Kenny, A.M.; Urban, R.J.; Tenover, J.L.; Bhasin, S. Adverse events associated with testosterone replacement in middle-aged and older men: A meta-analysis of randomized, placebo-controlled trials. J. Gerontol. 2005, 60, 1451–1457. [Google Scholar] [CrossRef] [PubMed]
- Muraleedharan, V.; Marsh, H.; Kapoor, D.; Channer, K.S.; Jones, T.H. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur. J. Endocrinol. 2013, 169, 725–733. [Google Scholar] [CrossRef] [PubMed]
- Ng Tang Fui, M.; Hoermann, R.; Bracken, K.; Handelsman, D.J.; Inder, W.J.; Stuckey, B.G.A.; Yeap, B.B.; Ghasem-Zadeh, A.; Robledo, K.P.; Jesudason, D.; et al. Effect of testosterone treatment on bone microarchitecture and bone mineral density in men: A 2-year RCT. J. Clin. Endocrinol. Metab. 2021, 106, E3143–E3158. [Google Scholar] [CrossRef]
- Garcia, J.A.; Sanchez, P.E.; Fraile, C.; Escovar, P. Testosterone undecanoate improves erectile dysfunction in hypogonadal men with the metabolic syndrome refractory to treatment with phosphodiesterase type 5 inhibitors alone. Andrologia 2011, 43, 293–296. [Google Scholar] [CrossRef]
- Emmelot-Vonk, M.H.; Verhaar, H.J.J.; Nakhai-Pour, H.R.; Grobbee, D.E.; van der Schouw, Y.T. Effect of testosterone supplementation on sexual functioning in aging men: A 6-month randomized controlled trial. Int. J. Impot. Res. 2009, 21, 129–138. [Google Scholar] [CrossRef]
- Amory, J.K.; Watts, N.B.; Easley, K.A.; Sutton, P.R.; Anawalt, B.D.; Matsumoto, A.M.; Bremner, W.J.; Tenover, J.L. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J. Clin. Endocrinol. Metab. 2004, 89, 503–510. [Google Scholar] [CrossRef]
- Colleluori, G.; Aguirre, L.; Napoli, N.; Qualls, C.; Villareal, D.T.; Armamento-Villareal, R. Testosterone therapy effects on bone mass and turnover in hypogonadal men with type 2 diabetes. J. Clin. Endocrinol. Metab. 2021, 106, E3058–E3068. [Google Scholar] [CrossRef]
- Shores, M.M.; Smith, N.L.; Forsberg, C.W.; Anawalt, B.D.; Matsumoto, A.M. Testosterone treatment and mortality in men with low testosterone levels. J. Clin. Endocrinol. Metab. 2012, 97, 2050–2058. [Google Scholar] [CrossRef]
- Sih, R.; Morley, J.E.; Kaiser, F.E.; Perry, H.M.; Patrick, P.; Ross, C. Testosterone replacement in older hypogonadal men: A 12-month randomized controlled trial. J. Clin. Endocrinol. Metab. 1997, 82, 1661–1667. [Google Scholar] [CrossRef] [PubMed]
- Poirier-Blanchette, L.; Koolian, M.; Blair Schwartz, C. Testosterone replacement therapy causing extensive portal and mesenteric vein thrombosis. Am. J. Med. 2021, 134, E426–E427. [Google Scholar] [CrossRef] [PubMed]
- Steinkampf, M.P.; Malizia, B.A. Male infertility—A common side effect of testosterone therapy. Ala. BME Newsl. Rep. 2011, 26, 1–3. [Google Scholar]
- Amore, M.; Innamorati, M.; Costi, S.; Sher, L.; Girardi, P.; Pompili, M. Partial androgen deficiency, depression, and testosterone supplementation in aging men. Int. J. Endocrinol. 2012, 2012, 280724. [Google Scholar] [CrossRef]
- Vigen, R.; O’Donnell, C.I.; Baron, A.E.; Grunwald, G.K.; Maddox, T.M.; Bradley, S.M.; Barqawi, A.; Woning, G.; Wierman, M.E.; Plomondon, M.E.; et al. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. J. Am. Med. Assoc. 2013, 310, 1829–1836. [Google Scholar] [CrossRef]
- Matthiesson, K.L.; Amory, J.K.; Berger, R.; Ugoni, A.; McLachlan, R.I.; Bremner, W.J. Novel male hormonal contraceptive combinations: The hormonal and spermatogenic effects of testosterone and levonorgestrel combined with a 5α-reductase inhibitor or gonadotropin-releasing hormone antagonist. J. Clin. Endocrinol. Metab. 2005, 90, 91–97. [Google Scholar] [CrossRef]
- Windfeld-Mathiasen, J.; Dalhoff, K.P.; Andersen, J.T.; Klemp, M.; Horwitz, A.; Horwitz, H. Male fertility before and after androgen abuse. J. Clin. Endocrinol. Metab. 2021, 106, 442–449. [Google Scholar] [CrossRef]
- Tan, R.S.; Pu, S.J. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male 2003, 6, 13–17. [Google Scholar] [CrossRef]
- Loo, S.Y.; Azoulay, L.; Nie, R.; dell’Aniello, S.; Yu, O.H.Y.; Renoux, C. Cardiovascular and cerebrovascular safety of testosterone replacement therapy among aging men with low testosterone levels: A cohort study. Am. J. Med. 2019, 132, 1069–1077. [Google Scholar] [CrossRef]
- Jones, S.D.; Dukovac, T.; Sangkum, P.; Yafi, F.A.; Hellstrom, W.J.G. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex. Med. Rev. 2015, 3, 101–112. [Google Scholar] [CrossRef]
- Tenover, J.S. Effects of testosterone supplementation in the aging male. J. Clin. Endocrinol. Metab. 1992, 75, 1092–1098. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M.; Hoermann, R.; Wittert, G.; Yeap, B.B. Effects of testosterone treatment on glucose metabolism and symptoms in men with type 2 diabetes and the metabolic syndrome: A systematic review and meta-analysis of randomized controlled clinical trials. Clin. Endocrinol. 2015, 83, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Laouali, N.; Brailly-Tabard, S.; Helmer, C.; Ancelin, M.L.; Tzourio, C.; Singh-Manoux, A.; Dugravot, A.; Elbaz, A.; Guiochon-Mantel, A.; Canonico, M. Testosterone and all-cause mortality in older men: The role of metabolic syndrome. J. Endocr. Soc. 2018, 2, 322–335. [Google Scholar] [CrossRef]
- Martínez, C.; Suissa, S.; Rietbrock, S.; Katholing, A.; Freedman, B.; Cohen, A.T.; Handelsman, D.J. Testosterone treatment and risk of venous thromboembolism: Population based case-control study. Br. Med. J. 2016, 355, i5968. [Google Scholar] [CrossRef] [PubMed]
- FDA Approved Drug Products: TLANDO (Testosterone Undecanoate) Capsules, for Oral Use. Full Prescribing Information; Report Number. 4959091; FDA: Bethesda, MD, USA, 2022. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2022/208088s000lbl.pdf (accessed on 27 August 2022).
- Zhu, B.; Chen, Y.; Xu, F.; Shen, X.; Chen, X.; Lv, J.; Zhang, S. Androgens impair β-cell function in a mouse model of polycystic ovary syndrome by activating endoplasmic reticulum stress. Endocr. Connect. 2021, 10, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, A.; Kaji, T.; Imaizumi, J.; Shirakawa, A.; Suga, K.; Nakagawa, R.; Maeda, K.; Irahara, M.; Iwasa, T. Transgender man receiving testosterone treatment became pregnant and delivered a girl: A case report. J. Obstet. Gynaecol. Res. 2022, 48, 866–868. [Google Scholar] [CrossRef]
- Nieschlag, E. Use of testosterone alone as hormonal male contraceptive. Basic Clin. Androl. 2012, 22, 136–140. [Google Scholar] [CrossRef]
- Thirumalai, A.; Page, S.T. Recent developments in male contraception. Drugs 2019, 79, 11–20. [Google Scholar] [CrossRef]
- World Health Organization. Task Force on Methods for the Regulation of Male Fertility. Contraceptive efficacy of testosterone-induced azoospermia and oligozoospermia in normal men. Fertil. Steril. 1996, 65, 821–829. [Google Scholar] [CrossRef]
- Gava, G.; Meriggiola, M.C. Update on male hormonal contraception. Ther. Adv. Endocrinol. Metab. 2019, 10, 2042018819834846. [Google Scholar] [CrossRef]
- Guo, W.; Bachman, E.; Li, M.; Roy, C.N.; Blusztajn, J.; Wong, S.; Chan, S.Y.; Serra, C.; Jasuja, R.; Travison, T.G.; et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell 2013, 12, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Bachman, E.; Travison, T.G.; Basaria, S.; Davda, M.N.; Guo, W.; Li, M.; Westfall, J.C.; Bae, H.; Gordeuk, V.; Bhasin, S. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: Evidence for a new erythropoietin/hemoglobin set point. J. Gerontol. A 2014, 69, 725–735. [Google Scholar] [CrossRef]
- Zitzmann, M.; Cremers, J.F.; Krallmann, C.; Kliesch, S. The HEAT-Registry (HEmatopoietic Affection by Testosterone): Comparison of a transdermal gel vs long-acting intramuscular testosterone undecanoate in hypogonadal men. Aging Male 2022, 25, 134–144. [Google Scholar] [CrossRef] [PubMed]
- Rochira, V.; Zirilli, L.; Madeo, B.; Maffei, L.; Carani, C. Testosterone action on erythropoiesis does not require its aromatization to estrogen: Insights from the testosterone and estrogen treatment of two aromatase-deficient men. J. Steroid Biochem. Mol. Biol. 2009, 113, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Molinari, P.F.; Rosenkrantz, H. Erythropoietic activity and androgenic implications of 29 testosterone derivatives in orchiectomized rats. J. Lab. Clin. Med. 1971, 78, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Kochakian, C.D.; Welder, A.A. Anabolic-androgenic steroids: In cell culture. In Vitro Cellular and Developmental Biology. Animal 1993, 29, 433–438. [Google Scholar] [CrossRef]
- Bélanger, C.; Hould, F.S.; Lebel, S.; Biron, S.; Brochu, G.; Tchernof, A. Omental and subcutaneous adipose tissue steroid levels in obese men. Steroids 2006, 71, 674–682. [Google Scholar] [CrossRef]
- Sheng, J.; Kimmelman, J.; Zarin, D. Citation-Impaired Clinical Trialing. The Scientist Digest, 1 July 2022; 9–10. [Google Scholar]
- Osterberg, E.C.; Bernie, A.M.; Ramasamy, R. Risks of testosterone replacement therapy in men. Indian J. Urol. 2014, 30, 2–7. [Google Scholar] [CrossRef]
- Khodamoradi, K.; Khosravizadeh, Z.; Parmar, M.; Kuchakulla, M.; Ramasamy, R.; Arora, H. Exogenous testosterone replacement therapy versus raising endogenous testosterone levels: Current and future prospects. Fertil. Steril. Rev. 2021, 2, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Westfield, G.; Kaiser, U.B.; Lamb, D.J.; Ramasamy, R. Short-acting testosterone: More physiologic? Front. Endocrinol. 2020, 11, 572465. [Google Scholar] [CrossRef]
- Bolour, S.; Braunstein, G. Testosterone therapy in women: A review. Int. J. Impot. Res. 2005, 17, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Dunsmoor-Su, R.; Fuller, A.; Voedisch, A. Testosterone therapy in women: A clinical challenge. Obstet. Gynecol. 2021, 138, 809–812. [Google Scholar] [CrossRef] [PubMed]
- Glaser, R.; Dimitrakakis, C. Testosterone therapy in women: Myths and misconceptions. Maturitas 2013, 74, 230–234. [Google Scholar] [CrossRef]
- Somboonporn, W.; Davis, S.R. Testosterone effects on the breast: Implications for testosterone therapy for women. Endocr. Rev. 2004, 25, 374–388. [Google Scholar] [CrossRef]
- Pasquali, R.; Zanotti, L.; Fanelli, F.; Mezzullo, M.; Fazzini, A.; Labate, A.M.M.; Repaci, A.; Ribichini, D.; Gambineri, A. Defining hyperandrogenism in women with polycystic ovary syndrome: A challenging perspective. J. Clin. Endocrinol. Metab. 2016, 101, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Zaman, A.; Rothman, M.S. Postmenopausal hyperandrogenism: Evaluation and treatment strategies. Endocrinol. Metab. Clin. N. Am. 2021, 50, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Cussons, A.J.; Stuckey, B.G.A.; Walsh, J.P.; Burke, V.; Norman, R.J. Polycystic ovarian syndrome: Marked differences between endocrinologists and gynaecologists in diagnosis and management. Clin. Endocrinol. 2005, 62, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Abbott, D.H.; Dumesic, D.A.; Levine, J.E. Hyperandrogenic origins of polycystic ovary syndrome-implications for pathophysiology and therapy. Expert Rev. Endocrinol. Metab. 2019, 14, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Diamanti-Kandarakis, E.; Dunaif, A. Insulin resistance and the polycystic ovary syndrome revisited: An update on mechanisms and implications. Endocr. Rev. 2012, 33, 981–1030. [Google Scholar] [CrossRef]
- Jeanes, Y.M.; Reeves, S. Metabolic consequences of obesity and insulin resistance in polycystic ovary syndrome: Diagnostic and methodological challenges. Nutr. Res. Rev. 2017, 30, 97–105. [Google Scholar] [CrossRef]
- Markopoulos, M.C.; Rizos, D.; Valsamakis, G.; Deligeoroglou, E.; Grigoriou, O.; Chrousos, G.P.; Creatsas, G.; Mastorakos, G. Hyperandrogenism in women with polycystic ovary syndrome persists after menopause. J. Clin. Endocrinol. Metab. 2011, 96, 623–631. [Google Scholar] [CrossRef]
- Burger, H.G. Androgen production in women. Fertil. Steril. 2002, 77, 3–5. [Google Scholar] [CrossRef]
- Foth, D.; Römer, T. Postmenopausal hyperandrogenemia (android obesity, insulin resistance, diabetes mellitus) and therapeutic consequences. In Menopause Andropause. Hormone Replacement Therapy through the Ages. New Cognition and Therapy Concepts, 1st ed.; Fischl, F.H., Ed.; Krause & Pachernegg: Gablitz, Austria, 2001; pp. 159–163. [Google Scholar]
- Gilling-Smith, C.; Willis, D.S.; Beard, R.W.; Franks, S. Hypersecretion of androstenedione by isolated thecal cells from polycystic ovaries. J. Clin. Endocrinol. Metab. 1994, 79, 1158–1165. [Google Scholar]
- Kumar, A.; Woods, K.S.; Bartolucci, A.A.; Azziz, R. Prevalence of adrenal androgen excess in patients with the polycystic ovary syndrome (PCOS). Clin. Endocrinol. 2005, 62, 644–649. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.C.; Edman, C.D.; Hemsell, D.L.; Porter, J.C.; Siiteri, P.K. Effect of obesity on conversion of plasma androstenedione to estrone in postmenopausal women with and without endometrial cancer. Am. J. Obstet. Gynecol. 1978, 130, 448–455. [Google Scholar] [CrossRef]
- Judd, H.L.; Judd, G.E.; Lucas, W.E.; Yen, S.S.C. Endocrine function of the postmenopausal ovary: Concentration of androgens and estrogens in ovarian and peripheral vein blood. J. Clin. Endocrinol. Metab. 1974, 39, 1020–1024. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, M.W.; Kempegowda, P.; Jenkinson, C.; Taylor, A.E.; Quanson, J.L.; Storbeck, K.-H.; Arlt, W. 11-Oxygenated C19 steroids are the predominant androgens in polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 2017, 102, 840–848. [Google Scholar] [CrossRef]
- Gonzales, G.F.; Góñez, C.; Villena, A. Adrenopause or decline of serum adrenal androgens with age in women living at sea level or at high altitude. J. Endocrinol. 2002, 173, 95–102. [Google Scholar] [CrossRef]
- Pandey, S.; Srinivas, M.; Agashe, S.; Joshi, J.; Galvankar, P.; Prakasam, C.P.; Vaidya, R. Menopause and metabolic syndrome: A study of 498 urban women from western India. J. Midlife Health 2010, 1, 63–69. [Google Scholar] [CrossRef]
- Golden, S.H.; Ding, J.; Szklo, M.; Schmidt, M.I.; Duncan, B.B.; Dobs, A. Glucose and insulin components of the metabolic syndrome are associated with hyperandrogenism in postmenopausal women: The Atherosclerosis Risk in Communities Study. Am. J. Epidemiol. 2004, 160, 540–548. [Google Scholar] [CrossRef]
- Alpañés, M.; González-Casbas, J.M.; Sánchez, J.; Pián, H.; Escobar-Morreale, H.F. Management of postmenopausal virilization. J. Clin. Endocrinol. Metab. 2012, 97, 2584–2588. [Google Scholar] [CrossRef]
- Brind, J.; Strain, G.; Miller, L.; Zumoff, B.; Vogelman, J.; Orentreich, N. Obese men have elevated plasma levels of estrone sulfate. Int. J. Obes. 1990, 14, 483–486. [Google Scholar]
- Remesar, X.; Tang, V.; Ferrer, E.; Torregrosa, C.; Virgili, J.; Masanés, R.M.; Fernández-López, J.A.; Alemany, M. Estrone in food: A factor influencing the development of obesity? Eur. J. Nutr. 1999, 38, 247–253. [Google Scholar] [CrossRef]
- Glintborg, D.; Hermann, A.P.; Brusgaard, K.; Hangaard, J.; Hagen, C.; Andersen, M. Significantly higher adrenocorticotropin-stimulated cortisol and 17-hydroxyprogesterone levels in 337 consecutive, premenopausal, caucasian, hirsute patients compared with healthy controls. J. Clin. Endocrinol. Metab. 2005, 90, 1347–1353. [Google Scholar] [CrossRef][Green Version]
- Cagnacci, A.; Cannoletta, M.; Caretto, S.; Zanin, R.; Xholli, A.; Volpe, A. Increased cortisol level: A possible link between climacteric symptoms and cardiovascular risk factors. Menopause 2011, 18, 273–278. [Google Scholar] [CrossRef]
- Genazzani, A.R.; Pluchino, N.; Begliuomini, S.; Stomati, M.; Bernardi, F.; Pierv, M.; Casarosa, E.; Palumbo, M.; Genazzani, A.D.; Luisi, M. Long-term low-dose oral administration of dehydroepiandrosterone modulates adrenal response to adrenocorticotropic hormone in early and late postmenopausal women. Gynecol. Endocrinol. 2006, 22, 627–635. [Google Scholar] [CrossRef]
- Anagnostis, P.; Bosdou, J.K.; Vaitsi, K.; Goulis, D.G.; Lambrinoudaki, I. Estrogen and bones after menopause: A reappraisal of data and future perspectives. Hormones 2021, 20, 13–21. [Google Scholar] [CrossRef]
- Heiss, C.J.; Sanborn, C.F.; Nichols, D.L.; Bonnick, S.L.; Alford, B.B. Associations of body fat distribution, circulating sex hormones, and bone density in postmenopausal women. J. Clin. Endocrinol. Metab. 1995, 80, 1591–1596. [Google Scholar] [PubMed]
- Altindag, O.; Altindag, A.; Asoglu, M.; Gunes, M.; Soran, N.; Deveci, Z. Relation of cortisol levels and bone mineral density among premenopausal women with major depression. Int. J. Clin. Pract. 2007, 61, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ren, H.; Shen, G.; Qiu, T.; Liang, D.; Yang, Z.; Yao, Z.; Tang, J.; Jiang, X.; Wei, Q. Animal models for glucocorticoid-induced postmenopausal osteoporosis: An updated review. Biomed. Pharmacother. 2016, 84, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, R. The menopause and osteoporosis. Obstet. Gynecol. 1996, 87, 16S–19S. [Google Scholar] [CrossRef]
- Tella, S.H.; Gallagher, J.C. Prevention and treatment of postmenopausal osteoporosis. J. Steroid Biochem. Mol. Biol. 2014, 142, 155–170. [Google Scholar] [CrossRef] [PubMed]
- Blain, H.; Carrière, I.; Favier, F.; Jeandel, C.; Papoz, L. Body weight change since menopause and percentage body fat mass are predictors of subsequent bone mineral density change of the proximal femur in women aged 75 years and older: Results of a 5 year prospective study. Calcif. Tissue Int. 2004, 75, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Marzullo, P.; Mele, C.; Mai, S.; Nardone, A.; Scacchi, M.; Aimaretti, G. Obesity and bone loss at menopause: The role of sclerostin. Diagnostics 2021, 11, 1914. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-H.; Teng, M.; Huo, M. Association of possible sarcopenic obesity with osteoporosis and fragility fractures in postmenopausal women. Arch. Osteoporos. 2022, 17, 65. [Google Scholar] [CrossRef]
- Li, J.; Chen, X.; Lu, L.Y.; Yu, X.J. The relationship between bone marrow adipose tissue and bone metabolism in postmenopausal osteoporosis. Cytokine Growth Factor Rev. 2020, 52, 88–98. [Google Scholar] [CrossRef]
- Abdi, F.; Mobedi, H.; Bayat, F.; Mosaffa, N.; Dolatian, M.; Tehranif, F.R. The effects of transdermal estrogen delivery on bone mineral density in postmenopausal women: A meta-analysis. Iran. J. Pharm. Res. 2017, 16, 380–389. [Google Scholar]
- Khastgir, G.; Studd, J.; Holland, N.; Alaghband-Zadeh, J.; Fox, S.; Chow, J. Anabolic effect of estrogen replacement on bone in postmenopausal women with osteoporosis: Histomorphometric evidence in a longitudinal study. J. Clin. Endocrinol. Metab. 2001, 86, 289–295. [Google Scholar] [CrossRef]
- Bonomo, S.M.; Rigamonti, A.E.; Giunta, M.; Galimberti, D.; Guaita, A.; Gagliano, M.G.; Müller, E.E.; Cella, S.G. Menopausal transition: A possible risk factor for brain pathologic events. Neurobiol. Aging 2009, 30, 71–80. [Google Scholar] [CrossRef]
- Van Geel, T.A.C.M.; Geusens, P.P.; Winkens, B.; Sels, J.P.J.E.; Dinant, G.J. Measures of bioavailable serum testosterone and estradiol and their relationships with muscle mass, muscle strength and bone mineral density in postmenopausal women: A cross-sectional study. Eur. J. Endocrinol. 2009, 160, 681–687. [Google Scholar] [CrossRef]
- Panzer, C.; Guay, A. Testosterone replacement therapy in naturally and surgically menopausal women (CME). J. Sex. Med. 2009, 6, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Iellamo, F.; Volterrani, M.; Caminiti, G.; Karam, R.; Massaro, R.; Fini, M.; Collins, P.; Rosano Giuseppe, M.C. Testosterone therapy in women with chronic heart failure. J. Am. Coll. Cardiol. 2010, 56, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Flöter, A.; Nathorst-Böös, J.; Carlström, K.; von Schoultz, B. Addition of testosterone to estrogen replacement therapy in oophorectomized women: Effects on sexuality and well-being. Climacteric 2002, 5, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Lunenfeld, B. Testosterone deficiency and the metabolic syndrome. Aging Male 2007, 10, 53–56. [Google Scholar] [CrossRef] [PubMed]
- Hansen Viuff, M.; Just, J.; Brun, S.; Vrist Dam, T.; Hansen, M.; Melgaard, L.; Hougaard, D.M.; Lappe, M.; Højbjerg Gravholt, C. Women with Turner syndrome are both estrogen and androgen deficient: The impact of hormone replacement therapy. J. Clin. Endocrinol. Metab. 2022, 107, 1983–1993. [Google Scholar] [CrossRef]
- Tanabe, M.; Akehil, Y.; Nomiyama, T.; Murakami, J.; Yanase, T. Total testosterone is the most valuable indicator of metabolic syndrome among various testosterone values in middle-aged Japanese men. Endocr. J. 2015, 62, 123–132. [Google Scholar] [CrossRef]
- Maestripieri, D.; Mayhew, J.; Carlson, C.L.; Hoffman, C.L.; Radtke, J.M. One-male harems and female social dynamics in Guinea baboons. Folia Primatol. 2007, 78, 56–68. [Google Scholar] [CrossRef]
- Teichroeb, J.A.; Jack, K.M. Alpha male replacements in nonhuman primates: Variability in processes, outcomes, and terminology. Am. J. Primatol. 2017, 79, e22674. [Google Scholar] [CrossRef]
- Beehner, J.C.; Bergman, T.J.; Cheney, D.L.; Seyfarth, R.M.; Whitten, P.L. Testosterone predicts future dominance rank and mating activity among male Chacma baboons. Behav. Ecol. Sociobiol. 2006, 59, 469–479. [Google Scholar] [CrossRef]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone therapy in adult men with androgen deficiency syndromes: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2006, 91, 1995–2010. [Google Scholar] [CrossRef]
- Bhasin, S.; Cunningham, G.R.; Hayes, F.J.; Matsumoto, A.M.; Snyder, P.J.; Swerdloff, R.S.; Montori, V.M. Testosterone therapy in men with androgen deficiency Syndromes: An Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 2010, 95, 2536–2559. [Google Scholar] [CrossRef]
- Dimopoulou, C.; Ceausu, I.; Depypere, H.; Lambrinoudaki, I.; Mueck, A.; Pérez-López, F.R.; Rees, M.; van der Schouw, Y.T.; Senturk, L.M.; Simonsini, T.; et al. EMAS position statement: Testosterone replacement therapy in the aging male. Maturitas 2016, 84, 94–99. [Google Scholar] [CrossRef]
- Pelzman, D.L.; Hwang, K. Testosterone therapy: Where do the latest guidelines agree and differ? Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Handelsman, D.J. Global trends in testosterone prescribing, 2000–2011: Expanding the spectrum of prescription drug misuse. Med. J. Aust. 2013, 199, 548–551. [Google Scholar] [CrossRef] [PubMed]
- Wierman, M.E.; Basson, R.; Davis, S.R.; Khosla, S.; Miller, K.K.; Rosner, W.; Santoro, N. Androgen therapy in women: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2006, 91, 3697–3710. [Google Scholar] [CrossRef] [PubMed]
- Parish, S.J.; Simon, J.A.; Davis, S.R.; Giraldi, A.; Goldstein, I.; Goldstein, S.W.; Kim, N.N.; Kingsberg, S.A.; Morgentaler, A.; Nappi, R.E.; et al. International Society for the Study of Women’s Sexual Health clinical practice guideline for the use of systemic testosterone for hypoactive sexual desire disorder in women. J. Women’s Health 2021, 30, 474–491. [Google Scholar] [CrossRef]
- Davis, S.R.; Baber, R.; Panay, N.; Bitzer, J.; Perez, S.C.; Islam, R.M.; Kaunitz, A.M.; Kingsbergg, S.A.; Lambrinoudaki, I.; Liu, J.; et al. Global consensus position statement on the use of testosterone therapy for women. Maturitas 2019, 128, 89–93. [Google Scholar] [CrossRef]
- Handelsman, D.J. Androgen misuse and abuse. Endocr. Rev. 2021, 42, 457–501. [Google Scholar] [CrossRef]
- Kanayama, G.; Pope, H.G. Illicit use of androgens and other hormones. Curr. Opin. Endocrinol. Diabetes Obes. 2012, 19, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.B.; Meacham, R.B. Androgen replacement therapy in the aging male. Rev. Urol. 2003, 5, 216–226. [Google Scholar]
- Morgentaler, A.; Zitzmann, M.; Traish, A.M.; Fox, A.W.; Jones, T.H.; Maggi, M.; Arver, S.; Aversa, A.; Chan, J.C.N.; Dobs, A.S.; et al. Fundamental concepts regarding testosterone deficiency and treatment. Mayo Clin. Proc. 2016, 91, 881–896. [Google Scholar] [CrossRef] [PubMed]
- Grossmann, M. Testosterone and glucose metabolism in men: Current concepts and controversies. J. Endocrinol. 2014, 220, R37–R55. [Google Scholar] [CrossRef]
- Brodsky, I.G.; Balagopal, P.; Nair, K.S. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men. A clinical research center study. J. Clin. Endocrinol. Metab. 1996, 81, 3469–3475. [Google Scholar] [CrossRef]
- Wang, C.; Cunningham, G.; Dobs, A.; Iranmanesh, A.; Matsumoto, A.M.; Snyder, P.J.; Weber, T.; Berman, N.; Hull, L.; Swerdloff, R.S. Long-term testosterone gel (AndroGel) treatment maintains beneficial effects on sexual function and mood, lean and fat mass, and bone mineral density in hypogonadal men. J. Clin. Endocrinol. Metab. 2004, 89, 2085–2098. [Google Scholar] [CrossRef]
- Straftis, A.A.; Gray, P.B. Sex, energy, well-being and low testosterone: An exploratory survey of U. S. men’s experiences on prescription testosterone. Int. J. Environ. Res. Public Health 2019, 16, 3261. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Werstuck, G.H. The effect of testosterone on cardiovascular disease and cardiovascular risk factors in men: A review of clinical and preclinical data. CJC Open 2021, 3, 1238–1248. [Google Scholar] [CrossRef]
- Bahrke, M.S.; Yesalis, C.E.; Wright, J.E. Psychological and behavioural effects of endogenous testosterone and anabolic-androgenic steroids. Sport. Med. 1996, 22, 367–390. [Google Scholar] [CrossRef]
- Parkinson, A.B.; Evans, N.A. Anabolic androgenic steroids: A survey of 500 users. Med. Sci. Sport. Exerc. 2006, 38, 644–651. [Google Scholar] [CrossRef] [PubMed]
- Nieschlag, E.; Vorona, E. Mechanisms in endocrinology: Medical consequences of doping with anabolic androgenic steroids: Effects on reproductive functions. Eur. J. Endocrinol. 2015, 173, R47–R58. [Google Scholar] [CrossRef] [PubMed]
- Graf, C.E.; Pichard, C.; Herrmann, F.R.; Sieber, C.C.; Zekry, D.; Genton, L. Prevalence of low muscle mass according to body mass index in older adults. Nutrition 2017, 34, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.X.; Karagounis, L.G.; Ng, T.P.; Carre, C.; Narang, V.; Wong, G.; Ying Tan, C.T.; Zin Nyunt, M.S.; Gao, Q.; Abel, B.; et al. Systemic and metabolic signature of sarcopenia in community-dwelling older adults. J. Gerontol. A 2020, 75, 309–317. [Google Scholar] [CrossRef]
- Papadopoulou, S.K. Sarcopenia: A contemporary health problem among older adult populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-Y.; Shin, S. Association of sarcopenia with osteopenia and osteoporosis in community-dwelling older Korean adults: A cross-sectional study. J. Clin. Med. 2022, 11, 129. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Mackenzie, T.A.; Barre, L.K.; Lopez-Jimenez, F.; Bartels, S.J. Sarcopenia, sarcopenic obesity and mortality in older adults: Results from the National Health and Nutrition Examination Survey III. Eur. J. Clin. Nutr. 2014, 68, 1001–1007. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Rodriguez, D.; Marco, E.; Cruz-Jentoft, A.J. Defining sarcopenia: Some caveats and challenges. Curr. Opin. Clin. Nutr. Metab. Care 2020, 23, 127–132. [Google Scholar] [CrossRef]
- Basile, G.; Sardella, A. From cognitive to motor impairment and from sarcopenia to cognitive impairment: A bidirectional pathway towards frailty and disability. Aging Clin. Exp. Res. 2021, 33, 469–478. [Google Scholar] [CrossRef]
- Park, S.W.; Gooodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; de Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive loss of skeletal muscle mass in older adults with type 2 diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef]
- Tong, Q.; Wang, X.; Sheng, Y.; Chen, S.; Lai, B.; Lv, R.; Yu, J. Metabolic syndrome and its association with components of sarcopenia in older community-dwelling Chinese. J. Biomed. Res. 2022, 36, 120–126. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Gray, S.R.; Pell, J.P.; Ho, F.K.; Celis-Morales, C. The joint association of sarcopenia and frailty with incidence and mortality health outcomes: A prospective study. Clin. Nutr. 2021, 40, 2427–2434. [Google Scholar] [CrossRef]
- Thompson, M.Q.; Yu, S.; Tucker, G.R.; Adams, R.J.; Cesari, M.; Theou, O.; Visvanathan, R. Frailty and sarcopenia in combination are more predictive of mortality than either condition alone. Maturitas 2021, 144, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Murton, A.J. Muscle protein turnover in the elderly and its potential contribution to the development of sarcopenia. Proc. Nutr. Soc. 2015, 74, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Batsis, J.A.; Petersen, C.L.; Crow, R.S.; Cook, S.B.; Stevens, C.J.; Seo, L.M.; Brooks, E.; Mackenzie, T.A. Weight change and risk of the foundation of National Institute of Health Sarcopenia-defined low lean mass: Data from the National Health and Nutrition examination surveys 1999-2004. Clin. Nutr. 2020, 39, 2463–2470. [Google Scholar] [CrossRef]
- Khadra, D.; Itani, L.; Chebaro, Y.; Obeid, M.; Jaber, M.; Ghanem, R.; Ayton, A.; Kreidieh, D.; Masri, D.E.; Kimura, A.; et al. Association between sarcopenic obesity and metabolic syndrome in adults: A systematic review and meta-analysis. Curr. Cardiol. Rev. 2020, 16, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Delegge, M. Body composition (sarcopenia) in obese patients. J. Parenter. Enter. Nutr. 2011, 35, 21S–28S. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Lee, H.Y.; Kim, Y.S.; Park, I.; Ahn, H.K.; Cho, E.K.; Jeong, Y.M.; Kim, J.H. Prognostic significance of cachexia score assessed by CT in male patients with small cell lung cancer. Eur. J. Cancer Care 2018, 27, e12695. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Felipe, A.; López-Soriano, F.J. Molecular mechanisms involved in muscle wasting in cancer and ageing: Cachexia versus sarcopenia. Int. J. Biochem. Cell Biol. 2005, 37, 1084–1104. [Google Scholar] [CrossRef]
- Chapman, I.M.; Visvanathan, R.; Hammond, A.J.; Morley, J.E.; Field, J.B.; Tai, K.; Belobrajdic, D.P.; Chen, R.Y.; Horowitz, M. Effect of testosterone and a nutritional supplement, alone and in combination, on hospital admissions in undernourished older men and women. Am. J. Clin. Nutr. 2009, 89, 880–889. [Google Scholar] [CrossRef]
- Ikizler, T.A.; Cano, N.J.; Franch, H.; Fouque, D.; Himmelfarb, J.; Kalantar-Zadeh, K.; Kuhlmann, M.K.; Stenvinkel, P.; Terwee, P.; Teta, D.; et al. Prevention and treatment of protein energy wasting in chronic kidney disease patients: A consensus statement by the International Society of Renal Nutrition and Metabolism. Kidney Int. 2013, 84, 1096–1107. [Google Scholar] [CrossRef]
- Visvanathan, R.; Piantadosi, C.; Lange, K.; Naganathan, V.; Hunter, P.; Cameron, I.D.; Chapman, I. The randomized control trial of the effects of testosterone and a nutritional supplement on hospital admissions in undernourished, community dwelling, older people. J. Nutr. Health Aging 2016, 20, 769–779. [Google Scholar] [CrossRef]
- Rhee, H.; Navaratnam, A.; Oleinikova, I.; Gilroy, D.; Scuderi, Y.; Heathcote, P.; Nguyen, T.; Wood, S.; Ho, K.K.Y. A novel liver-targeted testosterone therapy for sarcopenia in androgen deprived men with prostate cancer. J. Endocr. Soc. 2021, 5, bvab116. [Google Scholar] [CrossRef]
- Burney, B.O.; Hayes, T.G.; Smiechowska, J.; Cardwell, G.; Papusha, V.; Bhargava, P.; Konda, B.; Auchus, R.J.; Garcia, J.M. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J. Clin. Endocrinol. Metab. 2012, 97, E700–E709. [Google Scholar] [CrossRef]
- Gullett, N.P.; Hebbar, G.; Ziegler, T.R. Update on clinical trials of growth factors and anabolic steroids in cachexia and wasting. American J. Clin. Nutr. 2010, 91, 1143S–1147S. [Google Scholar] [CrossRef]
- Laughlin, G.A.; Barrett-Connor, E.; Bergstrom, J. Low serum testosterone and mortality in older men. J. Clin. Endocrinol. Metab. 2008, 93, 68–75. [Google Scholar] [CrossRef]
- Gray, A.; Feldman, H.A.; Mckinlay, J.B.; Longcope, C. Age, disease, and changing sex hormone levels in middle-aged men: Results of the Massachusetts Male Aging Study. J. Clin. Endocrinol. Metab. 1991, 73, 1016–1025. [Google Scholar] [CrossRef]
- Morley, J.E.; Kaiser, F.E.; Perry, H.M.; Patrick, P.; Morley, P.M.K.; Stauber, P.M.; Vellas, B.; Baumgartner, R.N.; Garry, P.J. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism 1997, 46, 410–413. [Google Scholar] [CrossRef]
- Vaninetti, S.; Baccarelli, A.; Romoli, R.; Fanelli, M.; Faglia, G.; Spada, A. Effect of aging on serum gonadotropin levels in healthy subjects and patients with nonfunctioning pituitary adenomas. Eur. J. Endocrinol. 2000, 142, 144–149. [Google Scholar] [CrossRef][Green Version]
- Archer, D.F. Lower doses of oral estrogen and progestogens as treatment for postmenopausal women. Semin. Reprod. Med. 2005, 23, 188–195. [Google Scholar] [CrossRef]
- Irwig, M.S. Testosterone therapy for transgender men. Lancet Diabetes Endocrinol. 2017, 5, 301–311. [Google Scholar] [CrossRef]
- Siqueira Cunha, F.; Domenice, S.; Palma Sircili, M.H.; Bilharinho de Mendonca, B.; Frade Costa, E.M. Low estrogen doses normalize testosterone and estradiol levels to the female range in transgender women. Clinics 2018, 73, e86. [Google Scholar] [CrossRef]
- Tangpricha, V.; den Heijer, M. Oestrogen and anti-androgen therapy for transgender women. Lancet Diabetes Endocrinol. 2017, 5, 291–300. [Google Scholar] [CrossRef]
- Pappas, I.I.; Craig, W.Y.; Spratt, L.V.; Spratt, D.I. Efficacy of sex steroid therapy without progestin or GnRH agonist for gonadal suppression in adult transgender patients. J. Clin. Endocrinol. Metab. 2021, 106, E1290–E1300. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.M.; Manzouri, A.H.; Dhejne, C.; Bergstrom, K.; Arver, S.; Feusner, J.D.; Savic-Berglund, I. Testosterone effects on the brain in transgender men. Cereb. Cortex 2018, 28, 1582–1596. [Google Scholar] [CrossRef]
- Christensen, L.L.; Glintborg, D.; Tauberg Kristensen, T.; Diederichsen, A.; T’Sjoen, G.; Frystyk, J.; Skovsager Andersen, M. Masculinising testosterone treatment and effects on preclinical cardiovascular disease, muscle strength and power, aggression, physical fitness and respiratory function in transgender men: Protocol for a 10-year, prospective, observational cohort study in Denmark at the Body Identity Clinic (BIC). BMJ Open 2020, 10, e045714. [Google Scholar] [CrossRef]
- Hodges-Simeon, C.R.; Grail, G.P.O.; Albert, G.; Groll, M.D.; Stepp, C.E.; Carré, J.M.; Arnocky, S.A. Testosterone therapy masculinizes speech and gender presentation in transgender men. Sci. Rep. 2021, 11, 3494. [Google Scholar] [CrossRef]
- Irwig, M.S.; Childs, K.; Hancock, A.B. Effects of testosterone on the transgender male voice. Andrology 2017, 5, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.J.; Liang, J.J.; Jolly, D.; Weinand, J.D.; Safer, J.D. Exogenous testosterone does not induce or exacerbate the metabolic features associated with PCOS among transgender men. Endocr. Pract. 2018, 24, 565–572. [Google Scholar] [CrossRef]
- Iannantuoni, F.; Salazar, J.D.; de Marañón, A.; Martínez Bañuls, C.; López-Doménech, S.; Rocha, M.; Hurtado-Murillo, F.; Morillas, C.; Gómez-Balaguer, M.; Víctor, V.M. Testosterone administration increases leukocyte-endothelium interactions and inflammation in transgender men. Fertil. Steril. 2021, 115, 483–489. [Google Scholar] [CrossRef]
- Gulanski, B.I.; Flannery, C.A.; Peter, P.R.; Leone, C.A.; Stachenfeld, N.S. Compromised endothelial function in transgender men taking testosterone. Clin. Endocrinol. 2020, 92, 138–144. [Google Scholar] [CrossRef]
- Kirisawa, T.; Ichihara, K.; Sakai, Y.; Morooka, D.; Iyoki, T.; Masumori, N. Physical and psychological effects of gender-affirming hormonal treatment using intramuscular testosterone enanthate in Japanese transgender men. Sex. Med. 2021, 9, 100306. [Google Scholar] [CrossRef]
- Chan, K.J.; Jolly, D.; Liang, J.J.; Weinand, J.D.; Safer, J.D. Estrogen levels do not rise with testosterone treatment for transgender men. Endocr. Pract. 2018, 24, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Nolan, B.J.; Zwickl, S.; Wong, A.F.Q.; Locke, P.; Simpson, S.; Li, L.; Zajac, J.D.; Cheung, A.S. Testosterone concentrations and prescription patterns of 1% testosterone gel in transgender and gender diverse individuals. Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221083512. [Google Scholar] [CrossRef] [PubMed]
- Hevener, A.L.; Clegg, D.J.; Mauvais-Jarvis, F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol. Cell. Endocrinol. 2015, 418, 306–321. [Google Scholar] [CrossRef] [PubMed]
- Rubinow, K.B. Estrogens and body weight regulation in men. Adv. Exp. Med. Biol. 2017, 1043, 285–313. [Google Scholar] [CrossRef] [PubMed]
- Ting, W.J.; Huang, C.Y.; Jiang, C.H.; Lin, Y.M.; Chung, L.C.; Shen, C.Y.; Pai, P.; Lin, K.H.; Viswanadha, V.P.; Liao, S.C. Treatment with 17β-estradiol reduced body weight and the risk of cardiovascular disease in a high-fat diet-induced animal model of obesity. Int. J. Mol. Sci. 2017, 18, 629. [Google Scholar] [CrossRef]
- Bendale, D.S.; Karpe, P.A.; Chhabra, R.; Shete, S.P.; Shah, H.; Tikoo, K. 17-β Oestradiol prevents cardiovascular dysfunction in post-menopausal metabolic syndrome by affecting SIRT1/AMPK/H3 acetylation. Br. J. Pharmacol. 2013, 170, 779–795. [Google Scholar] [CrossRef] [PubMed]
- Mikkola, T.S.; Tuomikoski, P.; Lyytinen, H.; Korhonen, P.; Hoti, F.; Vattulainen, P.; Gissler, M.; Ylikorkala, O. Estradiol-based postmenopausal hormone therapy and risk of cardiovascular and all-cause mortality. Menopause 2015, 22, 976–983. [Google Scholar] [CrossRef]
- Maher, A.C.; Akhtar, M.; Tarnopolsky, M.A. Men supplemented with 17β-estradiol have increased beta-oxidation capacity in skeletal muscle. Physiol. Genom. 2010, 42, 342–347. [Google Scholar] [CrossRef]
- Torres, M.J.; Kew, K.A.; Ryan, T.E.; Pennington, E.R.; Lin, C.T.; Buddo, K.A.; Fix, A.M.; Smith, C.A.; Gilliam, L.A.; Karvinen, S.; et al. 17β-Estradiol directly lowers mitochondrial membrane microviscosity and improves bioenergetic function in skeletal muscle. Cell Metab. 2018, 27, 167–179. [Google Scholar] [CrossRef]
- De Vanna, M.; Carlino, D.; Aguglia, E. Neuroprotection by estradiol: Molecular mechanism of action and implications for neuropsychopharmacology. Int. J. Psychiatry Clin. Pract. 2003, 7, 81–92. [Google Scholar] [CrossRef]
- Elzer, J.G.; Muhammad, S.; Wintermantel, T.M.; Regnier-Vigouroux, A.; Ludwig, J.; Schütz, G.; Schwaninger, M. Neuronal estrogen receptor-α mediates neuroprotection by 17β-estradiol. J. Cereb. Blood Flow Metab. 2010, 30, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Groeger, M.; Plesnila, N. The neuroprotective effect of 17β-estradiol is independent of its antioxidative properties. Brain Res. 2014, 1589, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Imtiaz, B.; Tolppanen, A.M.; Solomon, A.; Soininen, H.; Kivipelto, M. Estradiol and cognition in the cardiovascular risk factors, Aging and Dementia (CAIDE) Cohort Study. J. Alzheimer’s Dis. 2017, 56, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Lebesgue, D.; Chevaleyre, V.; Zukin, R.S.; Etgen, A.M. Estradiol rescues neurons from global ischemia-induced cell death: Multiple cellular pathways of neuroprotection. Steroids 2009, 74, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Galmés-Pascual, B.M.; Nadal-Casellas, A.; Bauzá-Thorbrugge, M.; Sbert-Roig, M.; García-Palmer, F.J.; Proenza, A.M.; Gianotti, M.; Lladó, I. 17β-estradiol improves hepatic mitochondrial biogenesis and function through PGC1B. J. Endocrinol. 2017, 232, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Klinge, C.M. Estrogenic control of mitochondrial function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef]
- Lee, M.Y.; Jung, S.C.; Lee, J.H.; Han, H.J. Estradiol-17β protects against hypoxia-induced hepatocyte injury through ER-mediated upregulation of Bcl-2 as well as ER-independent antioxidant effects. Cell Res. 2008, 18, 491–499. [Google Scholar] [CrossRef][Green Version]
- Shwaery, G.T.; Vita, J.A.; Keaney, J.F. Antioxidant protection of LDL by physiologic concentrations of estrogens is specific for 17-beta-estradiol. Atherosclerosis 1998, 138, 255–262. [Google Scholar] [CrossRef]
- Kim, K.G.; Park, Y.S. Oestradiol-17β is a local factor inducing the early stage of spermatogenesis in mouse testes. Andrologia 2018, 50, e12905. [Google Scholar] [CrossRef] [PubMed]
- Tao, K.; Sun, Y.; Chao, Y.; Xing, L.; Leng, L.; Zhou, D.; Zhu, W.; Fan, L. β-Estradiol promotes the growth of primary human fetal spermatogonial stem cells via the induction of stem cell factor in Sertoli cells. J. Assist. Reprod. Genet. 2021, 38, 2481–2490. [Google Scholar] [CrossRef]
- Kumar, R.; Balhuizen, A.; Amisten, S.; Lundquist, I.; Salehi, A. Insulinotropic and antidiabetic effects of 17β-estradiol and the GPR30 agonist G-1 on human pancreatic islets. Endocrinology 2011, 152, 2568–2579. [Google Scholar] [CrossRef]
- Kapoor, D.; Goodwin, E.; Channer, K.S.; Jones, T.H. Testosterone replacement therapy improves insulin resistance, glycaemic control, visceral adiposity and hypercholesterolaemia in hypogonadal men with type 2 diabetes. Eur. J. Endocrinol. 2006, 154, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Yeap, B.B.; Chubb, S.A.P.; Hyde, Z.; Jamrozik, K.; Hankey, G.J.; Flicker, L.; Norman, P.E. Lower serum testosterone is independently associated with insulin resistance in non-diabetic older men: The health in men study. Eur. J. Endocrinol. 2009, 161, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.M.; Kelly, D.M.; Jones, T.H. Testosterone and insulin resistance in the metabolic syndrome and T2DM in men. Nat. Rev. Endocrinol. 2013, 9, 479–493. [Google Scholar] [CrossRef]
- Cheung, A.S.; Hoermann, R.; Dupuis, P.; Joon, D.L.; Zajac, J.D.; Grossmann, M. Relationships between insulin resistance and frailty with body composition and testosterone in men undergoing androgen deprivation therapy for prostate cancer. Eur. J. Endocrinol. 2016, 175, 229–237. [Google Scholar] [CrossRef]
- Faiman, C.; Winter, J.S.D. Diurnal cycles in plasma FSH, testosterone and cortisol in men. J. Clin. Endocrinol. Metab. 1971, 33, 186–192. [Google Scholar] [CrossRef]
- Bodenheemer, S.; Winter, J.S.D.; Faiman, C. Diurnal rhythms of serum gonadotropins, testosterone, estradiol and cortisol in blind men. J. Clin. Endocrinol. Metab. 1973, 37, 472–475. [Google Scholar] [CrossRef] [PubMed]
- Barbería, J.M.; Giner, J.; Cortés-Gallegos, V. Diurnal variations of plasma testosterone in men. Steroids 1973, 22, 615–626. [Google Scholar] [CrossRef]
- Kanabar, R.; Mazur, A.; Plum, A.; Schmied, J. Correlates of testosterone change as men age. Aging Male 2022, 25, 29–40. [Google Scholar] [CrossRef]
- Smith, R.P.; Coward, R.M.; Kovac, J.R.; Lipshultz, L.I. The evidence for seasonal variations of testosterone in men. Maturitas 2013, 74, 208–212. [Google Scholar] [CrossRef]
- Doering, C.H.; Kraemer, H.C.; Brodie, H.K.H.; Hamburg, D.A. A cycle of plasma testosterone in the human male. J. Clin. Endocrinol. Metab. 1975, 40, 492–500. [Google Scholar] [CrossRef]
- Graupner, H. Elixiere des Lebens-von Hormonen und Vitaminen; Wegweiser-Verlag: Berlin, Germany, 1939. [Google Scholar]
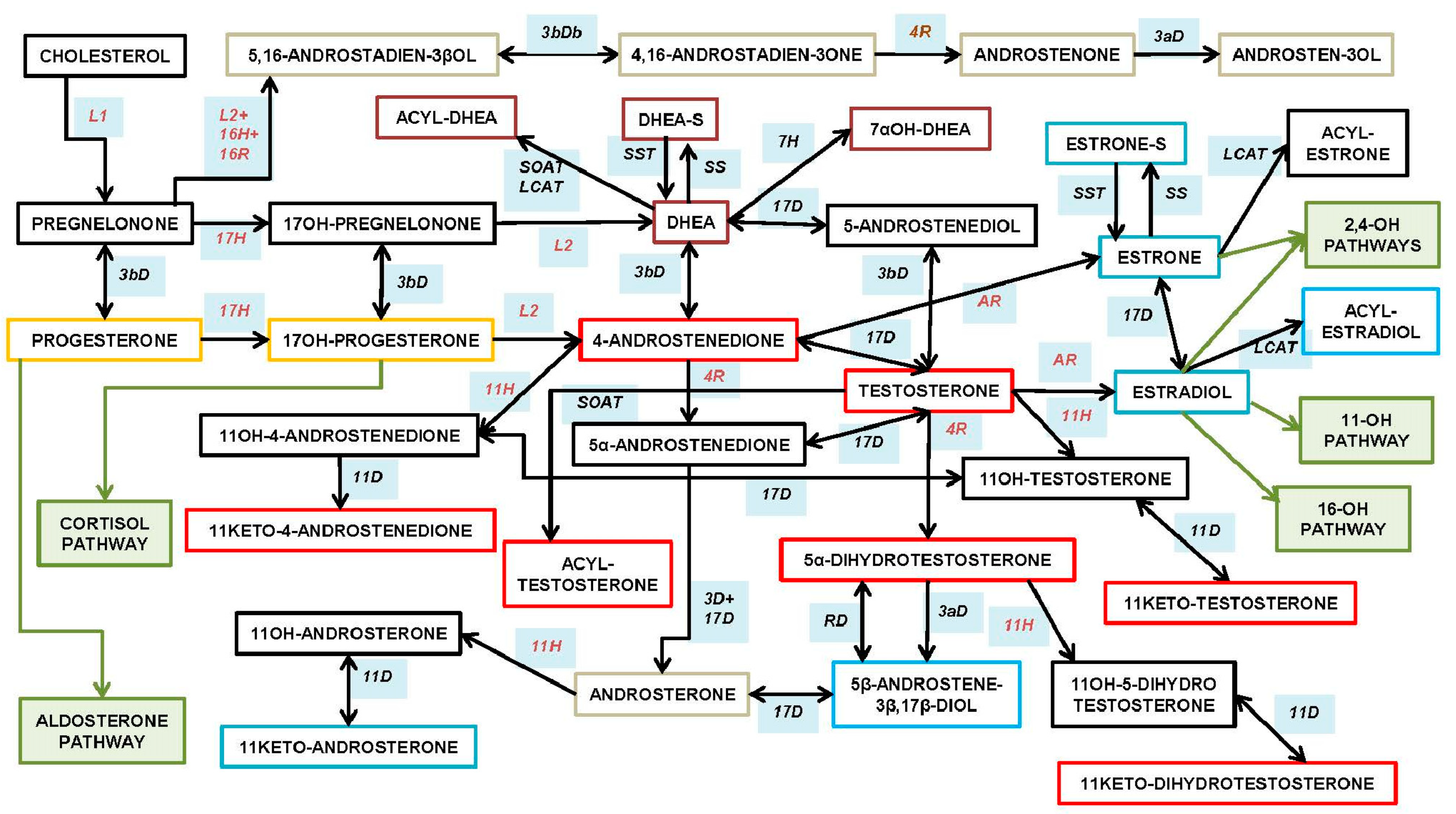
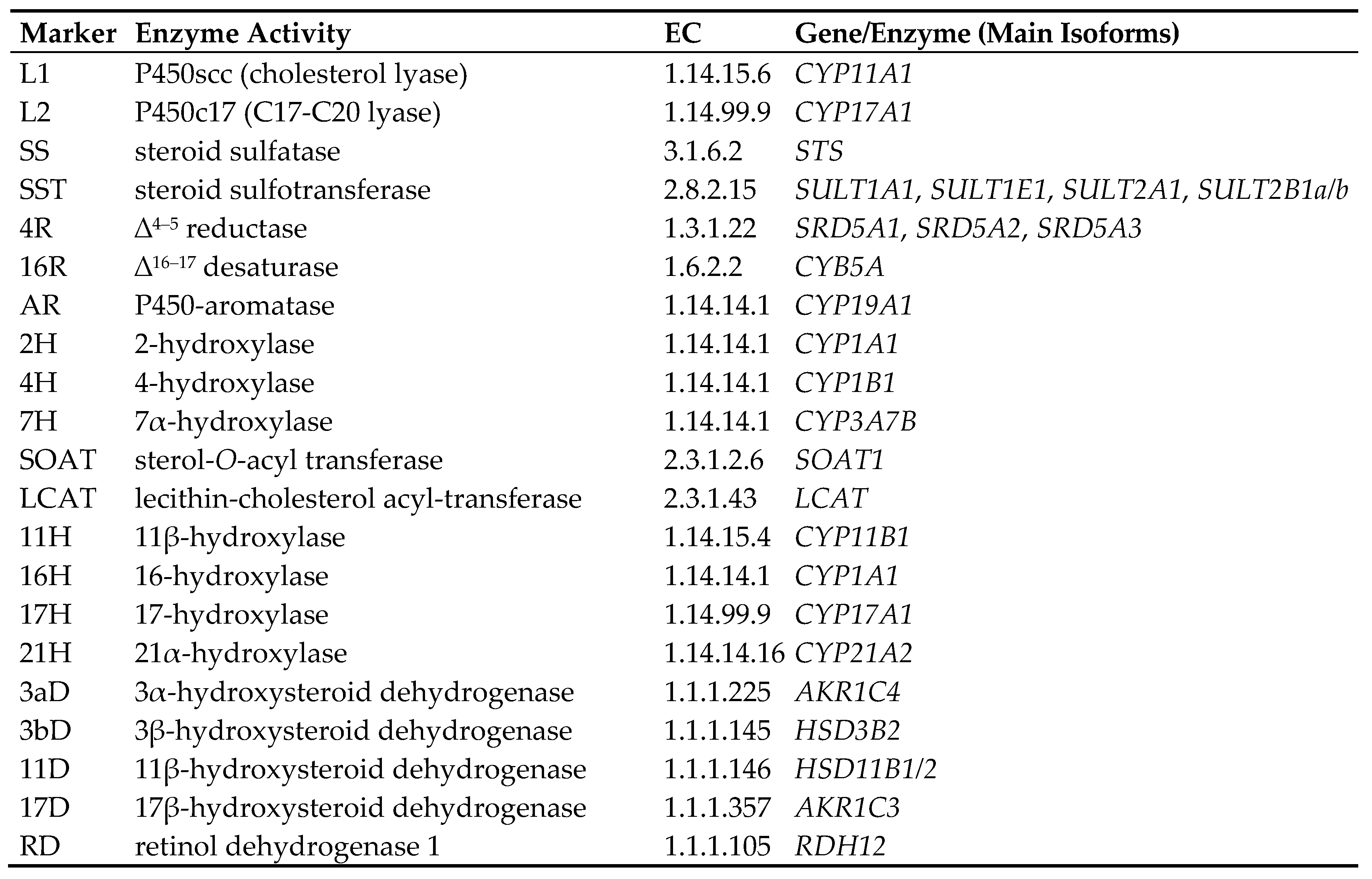
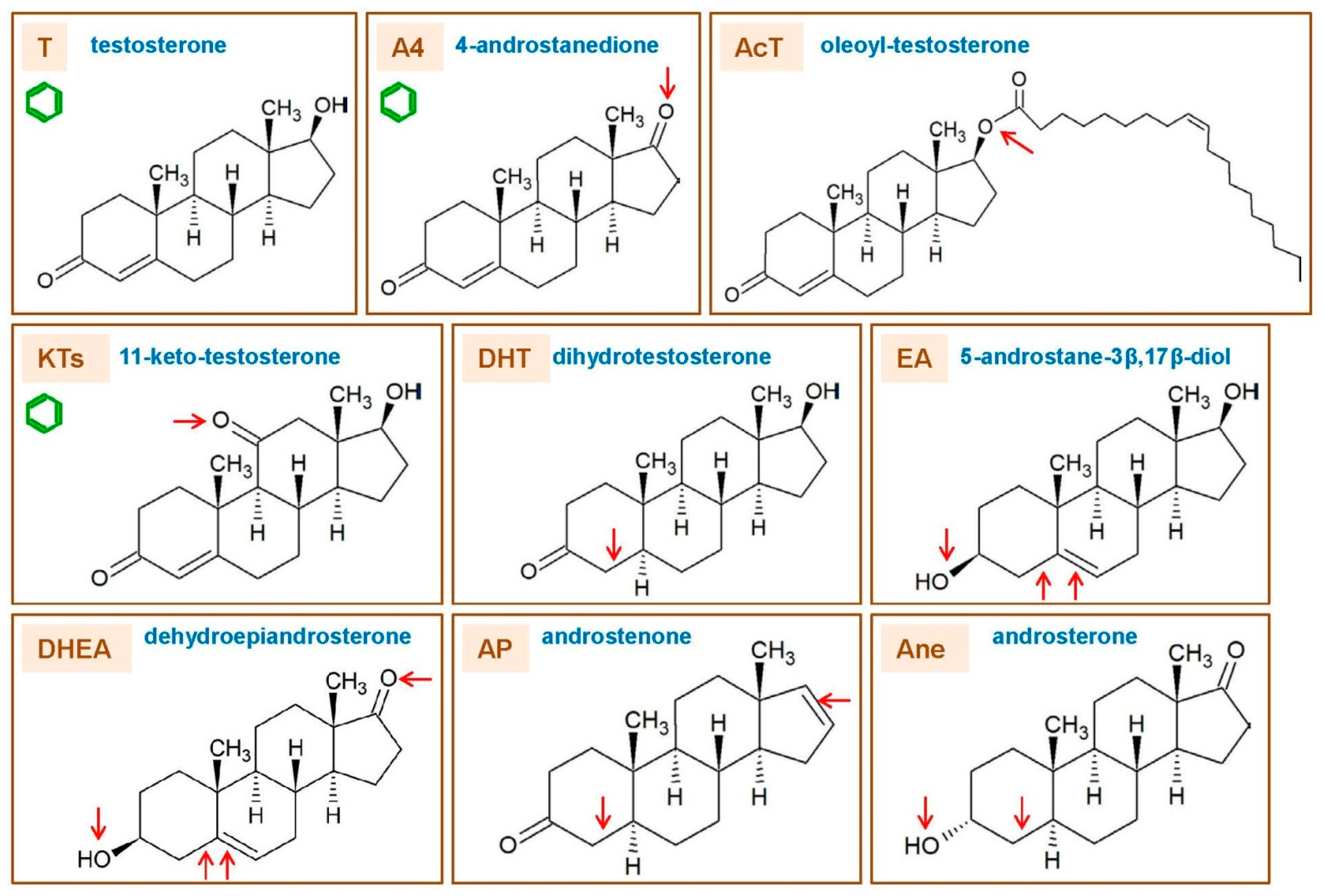

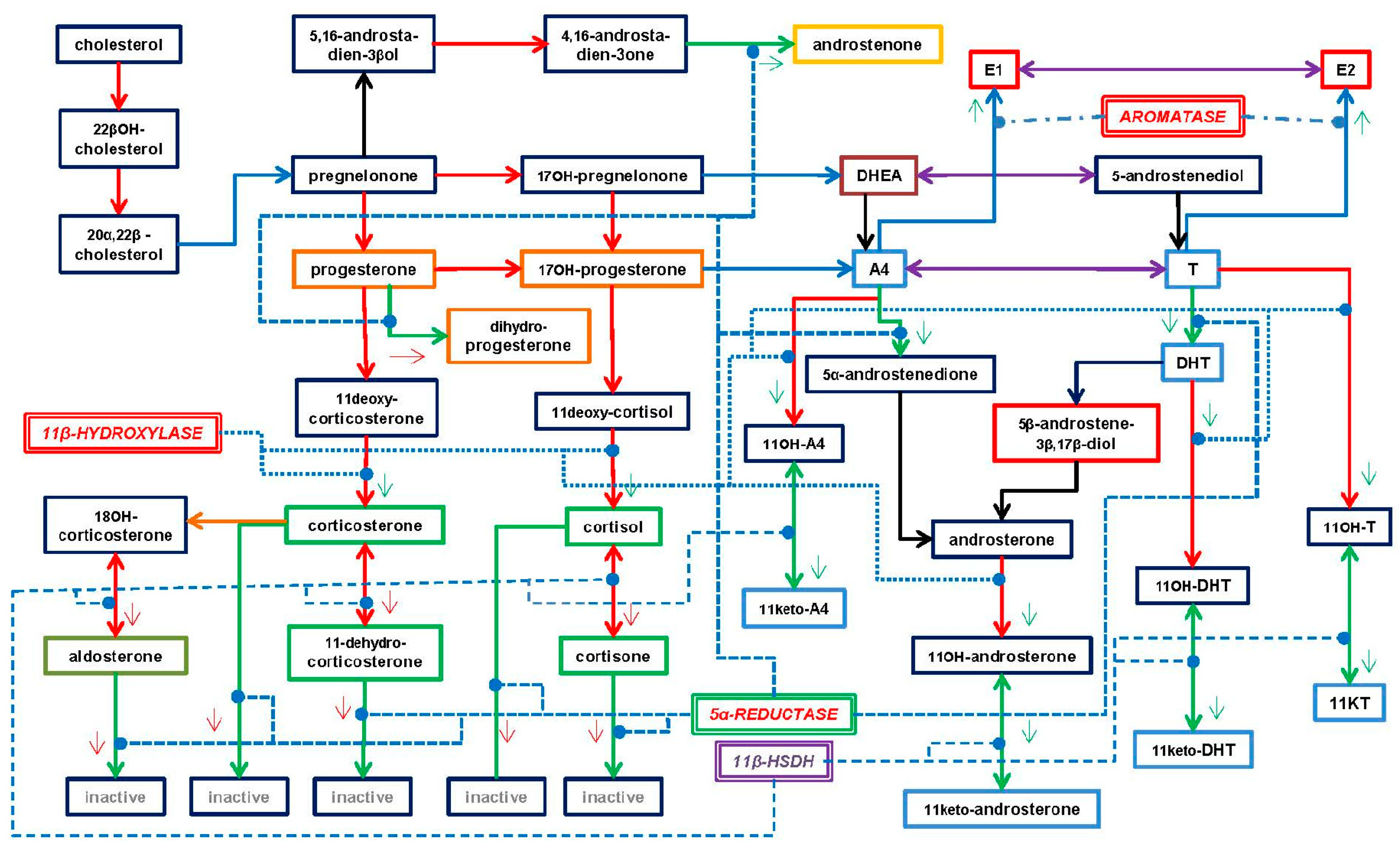
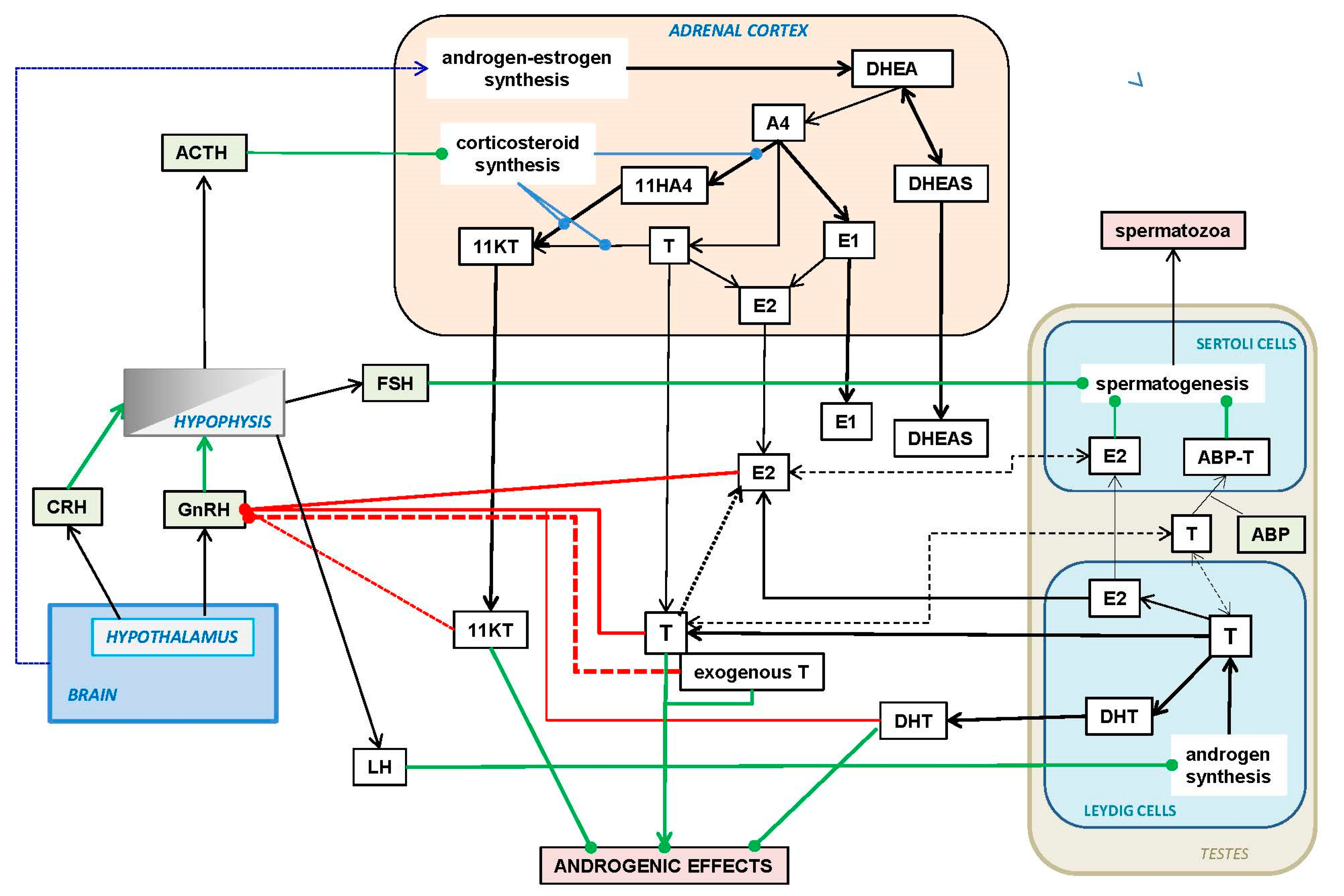
| DHEA | T | AcT | KT | DHT | A4 | AP | |
|---|---|---|---|---|---|---|---|
| Gender influences effects | M~F | M > F | ~ | M~F | M > F | M~F | M > (F) |
| Binds to AR | (+) | ++ | ~ | ++ | +++ | + | (−) |
| Binds to ER | + | − | − | − | − | − | − |
| Binds to SHBG | (+) 1 | + | − | (−) | ++ | − | ~ |
| Male secondary sex effects | − | (+) | ~ | − | +++ | − | − |
| Aromatase substrate in vivo | − | + | − | + | − | + | − |
| Activates sexual development | + | + | (+) | + | + | + | − |
| Increases libido | + F>M | +.M&F | ~ | − | + M | ~ | ~ |
| Enhances muscle mass | − | + | (+) | + | + | − | − |
| Low levels are obesogenic | + M&F | + M | (+) M | ~ | ~ | + M&F | ~ |
| Lowers insulin resistance | + | + | (+) | − | + | − 2 | − |
| Anti-GC effects | ++ | − 3 | ~ | (+) 4 | ~ | ~ | ~ |
| Pheromone effects | − | − | − | − | − | − | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alemany, M. The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health. Int. J. Mol. Sci. 2022, 23, 11952. https://doi.org/10.3390/ijms231911952
Alemany M. The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health. International Journal of Molecular Sciences. 2022; 23(19):11952. https://doi.org/10.3390/ijms231911952
Chicago/Turabian StyleAlemany, Marià. 2022. "The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health" International Journal of Molecular Sciences 23, no. 19: 11952. https://doi.org/10.3390/ijms231911952
APA StyleAlemany, M. (2022). The Roles of Androgens in Humans: Biology, Metabolic Regulation and Health. International Journal of Molecular Sciences, 23(19), 11952. https://doi.org/10.3390/ijms231911952






