Variation of miRNA Content in Cow Raw Milk Depending on the Dairy Production System
Abstract
1. Introduction
2. Results
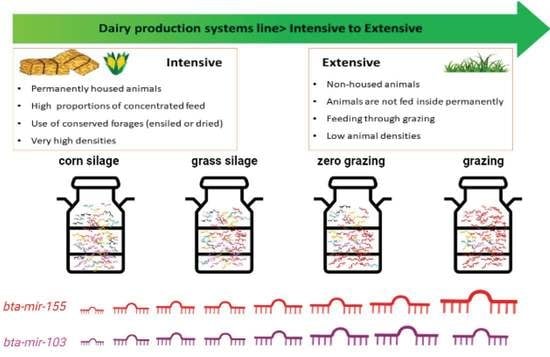
2.1. MiRNAs with Differential Levels in Cow Milk According to Production System
2.2. miRNA Functionality and Pathway Analyses
3. Discussion
4. Materials and Methods
4.1. Study Farms
4.2. Farm Classification
4.3. Sample Collection and Processing
4.4. Total RNA Extraction
4.5. Quantitative Real-Time PCR
4.6. Prediction of Potential Functions and Pathways of Genes Targeted by Milk miRNAs
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ruiz, J.; Herrera, M.P.; Barba, R.; Busqué, J. Definición y Caracterización de La Extensividad En Las Explotaciones Ganaderas En España. Situación de La Ganadería Extensiva En España (I). Minist. Agric. Y Pesca Aliment. Y Medio Ambiente 2017, 1–98. [Google Scholar]
- Vicente Mainar, F.; Martínez-Fernández, A.; Soldado, A.; De la Roza Delgado, B.; Argamentería Gutiérrez, A. Producción Sostenible de Leche de Vaca Mediante El Aprovechamiento de Los Recursos Naturales y Su Impacto Sobre El Medio Ambiente. In 3er Simposio Internacional del Producción Animal; Univ. Autónoma del Estado México: Ciudad de Mexico, Mexico, 2013; pp. 76–89. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Elahi, B.; Cassida, K.A.; Daneshvar, F.; Hernandez-Suarez, J.S.; Abouali, M.; Herman, M.R.; Dawood Al Masraf, S.A.; Harrigan, T. Food Footprint as a Measure of Sustainability for Grazing Dairy Farms. Environ. Manag. 2018, 62, 1073–1088. [Google Scholar] [CrossRef] [PubMed]
- Villar Bonet, A.; Quintana Ruíz, M. “Leche de Pastoreo” y “Leche de Pasto”. Es Urgente Una Figura de Calidad Reconocida Oficialmente. Vaca Pint. 2021, 26, 154–163. [Google Scholar]
- Knaus, W. Perspectives on Pasture versus Indoor Feeding of Dairy Cows. J. Sci. Food Agric. 2016, 96, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Comisión de Normas Sanitarias de la OIE para los Animales Terrestres. In Bienestar Animal y Sistemas de Producción de Vacas Lecheras; World Organisation for Animal Health: Paris, France, 2014; Proyecto de capítulo 7. X; Available online: https://www.woah.org/fileadmin/Home/esp/Internationa_Standard_Setting/docs/pdf/E_TAHSC_Feb_2014_Parte_B.pdf (accessed on 1 May 2022).
- Marshall, C.J.; Beck, M.R.; Garrett, K.; Barrell, G.K.; Al-Marashdeh, O.; Gregorini, P. Grazing Dairy Cows with Low Milk Urea Nitrogen Breeding Values Excrete Less Urinary Urea Nitrogen. Sci. Total Environ. 2020, 739, 139994. [Google Scholar] [CrossRef]
- Lopez, C.; Briard-Bion, V.; Ménard, O. Polar Lipids, Sphingomyelin and Long-Chain Unsaturated Fatty Acids from the Milk Fat Globule Membrane Are Increased in Milks Produced by Cows Fed Fresh Pasture Based Diet during Spring. Food Res. Int. 2014, 58, 59–68. [Google Scholar] [CrossRef]
- Veskoukis, A.S.; Kerasioti, E.; Sidiropoulos, K.; Maragou, I.; Skaperda, Z.O.I.; Kouretas, D. Nutritional Habits and Free Grazing Regimen of Productive Animals along with Specific Ingredients Are Influential Factors for the Antioxidant Properties of Milk: From Farm to Market. Biomed. Rep. 2020, 13, 31–36. [Google Scholar] [CrossRef]
- Heard, J.W.; Hannah, M.C.; Ho, C.K.M.; Wales, W.J. Predicting Immediate Marginal Milk Responses and Evaluating the Economics of Two-Variable Input Tactical Feeding Decisions in Grazing Dairy Cows. Animals 2021, 11, 1920. [Google Scholar] [CrossRef]
- Soder, K.J.; Rotz, C.A. Economic and Environmental Impact of Four Levels of Concentrate Supplementation in Grazing Dairy Herds. J. Dairy Sci. 2001, 84, 2560–2572. [Google Scholar] [CrossRef]
- Dartt, B.A.; Lloyd, J.W.; Radke, B.R.; Black, J.R.; Kaneene, J.B. A Comparison of Profitability and Economic Efficiencies between Management-Intensive Grazing and Conventionally Managed Dairies in Michigan. J. Dairy Sci. 1999, 82, 2412–2420. [Google Scholar] [CrossRef]
- Nakajima, N.; Yayota, M. Grazing and Cattle Health: A Nutritional, Physiological, and Immunological Status Perspective. Anim. Behav. Manag. 2019, 55, 143–153. [Google Scholar]
- Croissant, A.E.; Washburn, S.P.; Dean, L.L.; Drake, M.A. Chemical Properties and Consumer Perception of Fluid Milk from Conventional and Pasture-Based Production Systems. J. Dairy Sci. 2007, 90, 4942–4953. [Google Scholar] [CrossRef] [PubMed]
- Stampa, E.; Schipmann-Schwarze, C.; Hamm, U. Consumer Perceptions, Preferences, and Behavior Regarding Pasture-Raised Livestock Products: A Review. Food Qual. Prefer. 2020, 82, 103872. [Google Scholar] [CrossRef]
- He, L.; Hannon, G.J. MicroRNAs: Small RNAs with a Big Role in Gene Regulation. Nat. Rev. Genet. 2004, 5, 522–531. [Google Scholar] [CrossRef]
- Zhou, G.; Zhou, Y.; Chen, X. New Insight into Inter-Kingdom Communication: Horizontal Transfer of Mobile Small RNAs. Front. Microbiol. 2017, 8, 768. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, L.; Li, M.; Guo, C.; Yao, J.; Mi, S. Exosome and Exosomal MicroRNA: Trafficking, Sorting, and Function. Genom. Proteom. Bioinforma 2015, 13, 17–24. [Google Scholar] [CrossRef]
- del Pozo-Acebo, L.; López de las Hazas, M.; Margollés, A.; Dávalos, A.; García-Ruiz, A. Eating MicroRNAs: Pharmacological Opportunities for Cross-kingdom Regulation and Implications in Host Gene and Gut Microbiota Modulation. Br. J. Pharmacol. 2021, 178, 2218–2245. [Google Scholar] [CrossRef]
- Zempleni, J.; Baier, S.R.; Howard, K.M.; Cui, J. Gene Regulation by Dietary MicroRNAs. Can. J. Physiol. Pharmacol. 2015, 93, 1097–1102. [Google Scholar] [CrossRef]
- Weber, J.A.; Baxter, D.H.; Zhang, S.; Huang, D.Y.; Huang, K.H.; Lee, M.J.; Galas, D.J.; Wang, K. The MicroRNA Spectrum in 12 Body Fluids. Clin. Chem. 2010, 56, 1733–1741. [Google Scholar] [CrossRef]
- Carrillo-Lozano, E.; Sebastián-Valles, F.; Knott-Torcal, C. Circulating Micrornas in Breast Milk and Their Potential Impact on the Infant. Nutrients 2020, 12, 3066. [Google Scholar] [CrossRef]
- Tzelos, T.; Ho, W.; Charmana, V.I.; Lee, S.; Donadeu, F.X. MiRNAs in Milk Can Be Used towards Early Prediction of Mammary Gland Inflammation in Cattle. Sci. Rep. 2022, 12, 5131. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.C.; Fujikawa, T.; Maemura, T.; Ando, T.; Kitahara, G.; Endo, Y.; Yamato, O.; Koiwa, M.; Kubota, C.; Miura, N. Inflammation-Related MicroRNA Expression Level in the Bovine Milk Is Affected by Mastitis. PLoS ONE 2017, 12, e0177182. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, H.; Jin, X.; Lo, L.; Liu, J. Expression Profiles of MicroRNAs from Lactating and Non-Lactating Bovine Mammary Glands and Identification of MiRNA Related to Lactation. BMC Genom. 2012, 13, 731. [Google Scholar] [CrossRef] [PubMed]
- Colitti, M.; Sgorlon, S.; Licastro, D.; Stefanon, B. Differential Expression of MiRNAs in Milk Exosomes of Cows Subjected to Group Relocation. Res. Vet. Sci. 2019, 122, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Shibata, M.; Hayashi, M.; Oe, M.; Ojima, K. Differences in Circulating MicroRNAs between Grazing and Grain-Fed Wagyu Cattle Are Associated with Altered Expression of Intramuscular MicroRNA, the Potential Target PTEN, and Lipogenic Genes. PLoS ONE 2016, 11, e0162496. [Google Scholar] [CrossRef]
- Li, R.; Beaudoin, F.; Ammah, A.A.; Bissonnette, N.; Benchaar, C.; Zhao, X.; Lei, C.; Ibeagha-Awemu, E.M. Deep Sequencing Shows MicroRNA Involvement in Bovine Mammary Gland Adaptation to Diets Supplemented with Linseed Oil or Safflower Oil. BMC Genom. 2015, 16, 884. [Google Scholar] [CrossRef]
- Mobuchon, L.; Le Guillou, S.; Marthey, S.; Laubier, J.; Laloë, D.; Bes, S.; Le Provost, F.; Leroux, C. Sunflower Oil Supplementation Affects the Expression of MiR-20a-5p and MiR-142-5p in the Lactating Bovine Mammary Gland. PLoS ONE 2017, 12, e0185511. [Google Scholar] [CrossRef]
- Billa, P.A.; Faulconnier, Y.; Ye, T.; Chervet, M.; Le Provost, F.; Pires, J.A.A.; Leroux, C. Deep RNA-Seq Reveals MiRNome Differences in Mammary Tissue of Lactating Holstein and Montbéliarde Cows. BMC Genom. 2019, 20, 621. [Google Scholar] [CrossRef]
- Chen, X.; Gao, C.; Li, H.; Huang, L.; Sun, Q.; Dong, Y.; Tian, C.; Gao, S.; Dong, H.; Guan, D.; et al. Identification and Characterization of MicroRNAs in Raw Milk during Different Periods of Lactation, Commercial Fluid, and Powdered Milk Products. Cell Res. 2010, 20, 1128–1137. [Google Scholar] [CrossRef]
- Le Guillou, S.; Leduc, A.; Laubier, J.; Barbey, S.; Rossignol, M.N.; Lefebvre, R.; Marthey, S.; Laloë, D.; Le Provost, F. Characterization of Holstein and Normande Whole Milk MiRNomes Highlights Breed Specificities. Sci. Rep. 2019, 9, 20345. [Google Scholar] [CrossRef]
- Guan, J.; Long, K.; Ma, J.; Zhang, J.; He, D.; Jin, L.; Tang, Q.; Jiang, A.; Wang, X.; Hu, Y.; et al. Comparative Analysis of the MicroRNA Transcriptome between Yak and Cattle Provides Insight into High-Altitude Adaptation. PeerJ 2017, 2017, e3959. [Google Scholar] [CrossRef] [PubMed]
- Bauman, D.E.; Harvatine, K.J.; Lock, A.L. Nutrigenomics, Rumen-Derived Bioactive Fatty Acids, and the Regulation of Milk Fat Synthesis. Annu. Rev. Nutr. 2011, 31, 299–319. [Google Scholar] [CrossRef] [PubMed]
- Padovani, M.; Lavigne, J.A.; Chandramouli, G.V.R.; Perkins, S.N.; Barrett, J.C.; Hursting, S.D.; Bennett, L.M.; Berrigan, D. Distinct Effects of Calorie Restriction and Exercise on Mammary Gland Gene Expression in C57BL/6 Mice. Cancer Prev. Res. 2010, 12, 1076–1087. [Google Scholar] [CrossRef]
- Tao, S.; Bubolz, J.W.; do Amaral, B.C.; Thompson, I.M.; Hayen, M.J.; Johnson, S.E.; Dahl, G.E. Effect of Heat Stress during the Dry Period on Mammary Gland Development. J. Dairy Sci. 2011, 94, 5976–5986. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Kosaka, N.; Shimizu, T.; Sekine, K.; Ochiya, T.; Takase, M. Bovine Milk Contains MicroRNA and Messenger RNA That Are Stable under Degradative Conditions. J. Dairy Sci. 2012, 95, 4831–4841. [Google Scholar] [CrossRef] [PubMed]
- Buschmann, D.; Haberberger, A.; Kirchner, B.; Spornraft, M.; Riedmaier, I.; Schelling, G.; Pfaffl, M.W. Toward Reliable Biomarker Signatures in the Age of Liquid Biopsies—How to Standardize the Small RNA-Seq Workflow. Nucleic Acids Res. 2016, 44, 5995–6018. [Google Scholar] [CrossRef] [PubMed]
- Abou el qassim, L. Variaciones de Los Perfiles de MicroARN En La Leche Cruda de Vaca Según El Sistema de Alimentación. Master’s Thesis, Universidad de Zaragoza, Zaragoza, Spain, 2017. [Google Scholar]
- Abou el qassim, L.; Alonso, J.; Zhao, K.; Le Guillou, S.; Diez, J.; Vicente, F.; Fernández-Sanjurjo, M.; Iglesias-Gutiérrez, E.; Guan, L.L.; Royo, L.J. Differences in the MicroRNAs Levels of Raw Milk from Dairy Cattle Raised under Extensive or Intensive Production Systems. Servicio Regional de Investigación y Desarrollo Agroalimentario (SERIDA): Villaviciosa, Spain, 2022; Manuscript under review. [Google Scholar]
- Muroya, S.; Ogasawara, H.; Hojito, M. Grazing Affects Exosomal Circulating MicroRNAs in Cattle. PLoS ONE 2015, 10, e0136475. [Google Scholar] [CrossRef] [PubMed]
- Muroya, S.; Ogasawara, H.; Nohara, K.; Oe, M.; Ojima, K.; Hojito, M. Coordinated Alteration of MRNA-MicroRNA Transcriptomes Associated with Exosomes and Fatty Acid Metabolism in Adipose Tissue and Skeletal Muscle in Grazing Cattle. Asian-Australasian J. Anim. Sci. 2020, 33, 1824–1836. [Google Scholar] [CrossRef]
- Tudisco, R.; Morittu, V.M.; Addi, L.; Moniello, G.; Grossi, M.; Musco, N.; Grazioli, R.; Mastellone, V.; Pero, M.E.; Lombardi, P.; et al. Influence of Pasture on Stearoyl-CoA Desaturase and MiRNA 103 Expression in Goat Milk: Preliminary Results. Animals 2019, 9, 606. [Google Scholar] [CrossRef]
- Lin, X.; Luo, J.; Zhang, L.; Wang, W.; Gou, D. MiR-103 Controls Milk Fat Accumulation in Goat (Capra Hircus) Mammary Gland during Lactation. PLoS ONE 2013, 8, e79258. [Google Scholar] [CrossRef]
- De La Torre-Santos, S.; Royo, L.J.; Martínez-Fernández, A.; Menéndez-Miranda, M.; Rosa-García, R.; Vicente, F. Influence of the Type of Silage in the Dairy Cow Ration, with or without Grazing, on the Fatty Acid and Antioxidant Profiles of Milk. Dairy 2021, 2, 716–728. [Google Scholar] [CrossRef]
- Sanh, M.V.; Wiktorsson, H.; Ly, L.V. Effects of Natural Grass Forage to Concentrate Ratios and Feeding Principles on Milk Production and Performance of Crossbred Lactating Cows. Asian-Australasian J. Anim. Sci. 2002, 15, 650–657. [Google Scholar] [CrossRef]
- Avril-Sassen, S.; Goldstein, L.D.; Stingl, J.; Blenkiron, C.; Le Quesne, J.; Spiteri, I.; Karagavriilidou, K.; Watson, C.J.; Tavaré, S.; Miska, E.A.; et al. Characterisation of MicroRNA Expression in Post-Natal Mouse Mammary Gland Development. BMC Genom. 2009, 10, 548. [Google Scholar] [CrossRef] [PubMed]
- Vallanat, B.; Anderson, S.P.; Brown-Borg, H.M.; Ren, H.; Kersten, S.; Jonnalagadda, S.; Srinivasan, R.; Corton, J.C. Analysis of the Heat Shock Response in Mouse Liver Reveals Transcriptional Dependence on the Nuclear Receptor Peroxisome Proliferator-Activated Receptor α (PPARα). BMC Genom. 2010, 11, 16. [Google Scholar] [CrossRef]
- Pedernera, M.; Celi, P.; García, S.C.; Salvin, H.E.; Barchia, I.; Fulkerson, W.J. Effect of Diet, Energy Balance and Milk Production on Oxidative Stress in Early-Lactating Dairy Cows Grazing Pasture. Vet. J. 2010, 186, 352–357. [Google Scholar] [CrossRef]
- Braghieri, A.; Pacelli, C.; De Rosa, G.; Girolami, A.; De Palo, P.; Napolitano, F. Podolian Beef Production on Pasture and in Confinement. Animal 2011, 5, 927–937. [Google Scholar] [CrossRef]
- Celi, P. Biomarkers of Oxidative Stress in Ruminant Medicine. Immunopharmacol. Immunotoxicol. 2011, 33, 233–240. [Google Scholar] [CrossRef]
- Di Grigoli, A.; Di Trana, A.; Alabiso, M.; Maniaci, G.; Giorgio, D.; Bonanno, A. Effects of Grazing on the Behaviour, Oxidative and Immune Status, and Production of Organic Dairy Cows. Animals 2019, 9, 371. [Google Scholar] [CrossRef]
- Vicente Mainar, F.; Morales-Almaraz, E.; Martínez-Fernández, A. El Uso de Forrajes Para La Mejora de Producción y de Calidad de La Leche de Vacuno Lechero. Ganadería 2012, 82, 30–33. [Google Scholar]
- Tomé-Carneiro, J.; Larrosa, M.; Yáñez-Gascón, M.J.; Dávalos, A.; Gil-Zamorano, J.; Gonzálvez, M.; García-Almagro, F.; Ruiz Ros, J.A.; Tomás-Barberán, F.; Espín, J.C.; et al. One-Year Supplementation with a Grape Extract Containing Resveratrol Modulates Inflammatory-Related MicroRNAs and Cytokines Expression in Peripheral Blood Mononuclear Cells of Type 2 Diabetes and Hypertensive Patients with Coronary Artery Disease. Pharmacol. Res. 2013, 72, 69–82. [Google Scholar] [CrossRef]
- De La Torre-Santos, S. Identificación de Biomarcadores Específicos Para Autentificar El Origen y El Sistema de Alimentación Del Vacuno Lechero. Ph.D. Thesis, Universidad de Zaragoza, Zaragoza, Spain, 2021. [Google Scholar]
- Lamarche, A.; Martin, B.; Hauwuy, A.; Coulon, J.B.; Poutrel, B. Evolution of Milk Somatic Cell Count of Cows Grazing an Alpine Pasture According to the Infection of Udder by Pathogens. Anim. Res. 2000, 49, 45–54. [Google Scholar] [CrossRef]
- Bishop, S.C.; Woolliams, J.A. Genomics and Disease Resistance Studies in Livestock. Livest. Sci. 2014, 166, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Luoreng, Z.M.; Wang, X.P.; Mei, C.G.; Zan, L. Sen Comparison of MicroRNA Profiles between Bovine Mammary Glands Infected with Staphylococcus Aureus and Escherichia Coli. Int. J. Biol. Sci. 2018, 14, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Liu, H.T. MAPK Signal Pathways in the Regulation of Cell Proliferation in Mammalian Cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef]
- Cross, T.G.; Scheel-Toellner, D.; Henriquez, N.V.; Deacon, E.; Salmon, M.; Lord, J.M. Serine/Threonine Protein Kinases and Apoptosis. Exp. Cell Res. 2000, 256, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liang, G.; Wang, B.; Sun, H.; Liu, J.; Guan, L.L. Systematic MicroRNAome Profiling Reveals the Roles of MicroRNAs in Milk Protein Metabolism and Quality: Insights on Low-Quality Forage Utilization. Sci. Rep. 2016, 6, 21194. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting Effective MicroRNA Target Sites in Mammalian MRNAs. Elife 2015, 4, e05005. [Google Scholar] [CrossRef]
- Dennis, G.; Sherman, B.T.; Hosack, D.A.; Yang, J.; Gao, W.; Lane, H.C.; Lempicki, R.A. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol. 2003, 4, R60. [Google Scholar] [CrossRef]
- Vlachos, I.S.; Zagganas, K.; Paraskevopoulou, M.D.; Georgakilas, G.; Karagkouni, D.; Vergoulis, T.; Dalamagas, T.; Hatzigeorgiou, A.G. DIANA-MiRPath v3.0: Deciphering MicroRNA Function with Experimental Support. Nucleic Acids Res. 2015, 43, W460–W466. [Google Scholar] [CrossRef]


| KEGG Signaling Pathway | Biomarker | No. of Target Genes | p-Value | |||
|---|---|---|---|---|---|---|
| bta-miR-103 | bta-miR-155 | bta-miR-532 | bta-miR-7863 | |||
| AMPK 1 | x | x | x | 38 | 4.6 × 10−7 | |
| MAPK | x | x | x | x | 67 | 3.2 × 10−6 |
| PI3K-Akt 2 | x | x | x | 27 | 5.6 × 10−5 | |
| Oxytocin | x | x | x | 38 | 1.1 × 10−4 | |
| Prolactin | x | x | 25 | 1.2 × 10−4 | ||
| Insulin | x | x | 35 | 1.4 × 10−4 | ||
| Ras 3 | x | x | x | 53 | 1.6 × 10−4 | |
| Growth hormone synthesis, secretion and action | x | x | 31 | 1.8 × 10−4 | ||
| TGF-beta 4 | x | x | 25 | 6.2 × 10−4 | ||
| Calcium | x | x | 52 | 7.3 × 10−4 | ||
| Glucagon | x | 24 | 6.0 × 10−3 | |||
| Lipid and atherosclerosis | x | x | 43 | 2.5 × 10−2 | ||
| cGMP-PKG 5 | x | 32 | 3.3 × 10−2 | |||
| Mineral absorption | x | 13 | 8.3 × 10−2 | |||
| Lysine degradation | x | 14 | 9.7 × 10−2 | |||
| Biological Process | Biomarker | No. of Target Genes | p-Value | |||
|---|---|---|---|---|---|---|
| bta-miR-103 | bta-miR-155 | bta-miR-532 | bta-miR-7863 | |||
| Growth regulation | x | 11 | 2.5 × 10−3 | |||
| Transport | x | 249 | 1.4 × 10−2 | |||
| Ion transport | x | 79 | 1.6 × 10−2 | |||
| Calcium transport | x | 15 | 1.7 × 10−2 | |||
| Amino- acid transport | x | 7 | 2.4 × 10−2 | |||
| Protein transport | x | x | 67 | 2.7 × 10−2 | ||
| Differentiation | x | 45 | 6.0 × 10−2 | |||
| Sodium transport | x | 13 | 9.5 × 10−2 | |||
| Molecular Function | Biomarker | No. of Target Genes | p-Value | |||
|---|---|---|---|---|---|---|
| bta-miR-103 | bta-miR-155 | bta-miR-532 | bta-miR-7863 | |||
| Transferase | x | x | x | x | 279 | 1.4 × 10−20 |
| Activator | x | x | x | 64 | 1.3 × 10−8 | |
| Ion channel | x | x | 65 | 3.0 × 10−8 | ||
| Serine/threonine-protein kinase | x | x | x | x | 55 | 7.1 × 10−8 |
| Developmental protein | x | x | x | 64 | 3.0 × 10−6 | |
| Glycosyltransferase | x | 31 | 2.5 × 10−3 | |||
| Hydrolase | x | x | x | 182 | 2.8 × 10−3 | |
| Growth factor | x | 17 | 9.0 × 10−3 | |||
| Protein phosphatase | x | x | 19 | 1.3 × 10−2 | ||
| Guanine-nucleotide releasing factor | x | x | 16 | 1.6 × 10−2 | ||
| Calcium channel | x | 8 | 4.0 × 10−2 | |||
| Potassium channel | x | 12 | 4.8 × 10−2 | |||
| Ration Composition | |||||
|---|---|---|---|---|---|
| Production System | Grazing | Fresh Grass in the Stable | Grass Silage | Corn Silage | Concentrated Feed |
| Grazing (n = 44) | + | - | - | - | + |
| + | + | - | - | + | |
| + | - | + | - | + | |
| + | - | + | - | + | |
| + | - | - | - | + | |
| + | + | + | - | + | |
| + | - | + | + | + | |
| + | - | + | + | + | |
| Zero grazing (n = 13) | - | + | + | - | + |
| - | + | - | - | + | |
| - | + | + | - | + | |
| - | + | - | - | + | |
| Grass silage (n = 10) | - | - | + | - | + |
| Corn silage (n = 45) | - | - | + | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou el qassim, L.; Le Guillou, S.; Royo, L.J. Variation of miRNA Content in Cow Raw Milk Depending on the Dairy Production System. Int. J. Mol. Sci. 2022, 23, 11681. https://doi.org/10.3390/ijms231911681
Abou el qassim L, Le Guillou S, Royo LJ. Variation of miRNA Content in Cow Raw Milk Depending on the Dairy Production System. International Journal of Molecular Sciences. 2022; 23(19):11681. https://doi.org/10.3390/ijms231911681
Chicago/Turabian StyleAbou el qassim, Loubna, Sandrine Le Guillou, and Luis J. Royo. 2022. "Variation of miRNA Content in Cow Raw Milk Depending on the Dairy Production System" International Journal of Molecular Sciences 23, no. 19: 11681. https://doi.org/10.3390/ijms231911681
APA StyleAbou el qassim, L., Le Guillou, S., & Royo, L. J. (2022). Variation of miRNA Content in Cow Raw Milk Depending on the Dairy Production System. International Journal of Molecular Sciences, 23(19), 11681. https://doi.org/10.3390/ijms231911681